Abstract
Objective
To determine if the risk of death or moderate/severe disability in term infants with hypoxic-ischemic encephalopathy increases with relatively high esophageal or skin temperature occurring between 6 and 78 hours following birth.
Patients and Methods
This is an observational secondary study within the NICHD Neonatal Research Network randomized trial comparing whole body cooling and usual care (control) for term infants with hypoxic-ischemic encephalopathy. Esophageal and skin temperatures were recorded serially for 72 hours. Each infant’s temperatures for each site were rank ordered. The high temperature was defined for each infant as the mean of all temperature measurements in the upper quartile. The low temperature was similarly defined as the mean of the lower quartile. Outcome was related to temperature in three logistic regressions for the high, median and low temperature at each temperature site for each group adjusting for level of encephalopathy, gender, gestational age and race.
Results
In control infants the mean esophageal temperature was 37.2±0.7°C over the 72 hours and 63, 22 and 8% of all temperatures were > 37, > 37.5 and > 38°C, respectively. For skin temperature the mean was 36.5±0.8°C and 12, 5 and 2% of all temperatures were > 37, > 37.5 and > 38°C, respectively. The odds of death or disability were increased 3.6–4 fold for each centigrade increase in the highest quartile of skin or esophageal temperature. There were no associations between temperature and outcome in the cooled group.
Conclusion
Relatively high temperatures during usual care following hypoxia-ischemia were associated with increased risk of adverse outcome. The results may reflect underlying brain injury and/or adverse effects of temperature on outcome.
Introduction
Perinatal hypoxia-ischemia represents the etiology for newborn encephalopathy in up to 30% of affected infants, and can result in death, cerebral palsy, mental impairment and seizures.1, 2 Management of infants with HIE has been limited to supportive intensive care without any brain oriented specific therapy. This approach is changing with the recognition that brain temperature during and/or following hypoxia-ischemia may modulate the extent of injury. A small reduction in brain temperature (as little as 2°C) of neonatal animals attenuates damaging processes involved in the pathogenesis of brain injury (e.g., energy depletion, excitotoxicity, nitric oxide production, apoptosis) and attenuates the extent of clinical and histological brain injury.3 Conversely a small increase in brain temperature of neonatal animals during and/or following hypoxia-ischemia exacerbates the extent of injury.4, 5
In humans, a beneficial effect of reducing brain temperature has been obtained from two large randomized clinical trials in near-term and term infants with hypoxia-ischemia.6, 7 In contrast, harmful effects of raised temperature on human brain are uncertain and extremely complex. Observations that intra-partum maternal fever is associated with an increased risk of seizures8, neonatal encephalopathy,9 and subsequent cerebral palsy in term infants have little direct information on the extent, timing, and duration of raised temperature and possible associated brain injury.10 Brain injury in adults may raise brain temperature, and temperature of the brain and body may be either concordant or discordant.11 In human neonates heat flux measurements to indirectly derive brain temperature support the potential for dissociation of brain and body temperature.12 Our randomized trial of whole body hypothermia provided an opportunity to examine the hypothesis that raised body temperature is associated with worse outcome among term infants with encephalopathy presumed due to hypoxia-ischemia.
Methods
Study infants
This is an observational study using data from the NICHD Neonatal Research Network randomized trial comparing whole body hypothermia and current usual care representing a control group.7 The trial was performed after informed consent was obtained. Eligibility criteria included a gestational age ≥ 36 weeks gestation, a post-natal age ≤ 6 hours and sequential fulfillment of specific physiologic and/or clinical criteria (acute perinatal event, acidemia, low Apgars, need for ventilation) followed by demonstration of a moderate or severe encephalopathy using a modified Sarnat criteria.
Temperature control
Control infants were initially servo-controlled using a radiant warmer to maintain abdominal skin temperature between 36.5 – 37.0°C and maintain core temperature. Subsequent adjustments of the servo-control in response to relatively high or low axilla temperature were made according to guidelines within each participating Network NICUs. All infants had an esophageal temperature probe positioned in the lower third of the esophagus with subsequent verification of position by x-ray. Esophageal temperatures were recorded using either a Blanketrol II Hyper-Hypothermia system (Cincinnati Sub-Zero) in the monitoring mode, or an independent temperature monitoring unit (Mon-a therm, Mallinckrodt). Esophageal temperatures were not used in patient management in the randomized controlled trial because this did not represent usual care. Control infants had esophageal and skin temperature recorded at 4 hour intervals for 72 hours (total of 19 values per patient) during which whole body hypothermia was performed in the experimental group.
Each Network center was surveyed to identify hospital specific care practices for temperature regulation in near-term and term infants with hypoxic-ischemic encephalopathy during the randomized trial (July 2000 to May 2003). Network Research Coordinators identified whether such infants were routinely nursed under a radiant warmer or incubator, whether and how temperature was servo controlled, acceptable skin and axilla temperature range, and what information was documented in the medical record.
Infants randomized to whole body cooling were positioned on a cooling-heating blanket which was attached to a Blanketrol II Hyper-Hypothermia system (Cincinnati Sub-Zero, Cincinnati, OH). The automatic control mode was used to maintain an esophageal temperature at 33.5°C for 72 hours followed by rewarming and subsequent temperature regulation by local practice (see Reference 7 for details). Esophageal and skin temperatures were recorded at 15 minute intervals during the first 4 hours of cooling, at hourly intervals until 12 hours of cooling, and at 4 hour intervals until 72 hours of cooling. Temperatures between 2 and 72 hours (26 values per patient) were used in this analysis and represented temperatures after equilibration during cooling.
Outcome
The primary outcome was death or moderate/severe disability at 18–22 months of age. Trained assessors blinded to treatment group assessed outcome using standardized assessments. Disability was pre-defined as either severe or moderate. Severe disability included any of the following: Bayley II Mental Developmental Index (MDI) < 70, Gross Motor Functional Classification System (GMFCS) level of 3–5, blindness, or a hearing deficit with amplification. Criteria for moderate disability were an MDI of 70–84 with any of the following: GMFCS level 2, persistent seizure disorder, or hearing deficit without amplification.
Data Analysis
To analyze the relationship between temperature and outcome, temperature values were derived representing the extremes and midpoint of the multiple temperatures recorded for each infant. Esophageal and skin temperatures for each infant were rank ordered. For each infant we defined his or her high temperature as the mean temperature within the highest quartile, and the low temperature as the mean of the lowest quartile. Each infant’s high temperature, as just defined, was used in a logistic regression analysis to relate temperature to the outcome described above within each group separately (hypothermic and control). Separate analyses were performed for the high temperature of esophageal and skin sites and each regression was adjusted for level of encephalopathy, gender, race and gestational age. Similar analyses were performed for the low and median temperature. The duration of a raised temperature was related to the outcome by adjusted logistic regressions using the total hours and longest consecutive hours above threshold temperatures for esophageal and skin temperature. Threshold temperatures were explored by examining esophageal temperatures > 37.5°C and skin temperatures >37°C by increments of 0.5°C for each temperature site. Of the 106 control infants, 7 were excluded from these analyses due to missing temperature (n = 4) or outcome (n = 3) data. Of 102 cooled infants, 5 were excluded due to missing temperature (n = 3), missing level of encephalopathy (n = 1) or protocol violation (n = 1). Associations between temperature and outcome are expressed as an odds ratio (OR) and 95% confidence interval (CI). Results are expressed as a mean ± SD where appropriate.
Results
Maternal and infant characteristics of the 99 control and 97 cooled infants analyzed for this study were similar to the entire study cohort (n = 208).7 At least one intra-partum complication (fetal heart rate decelerations, cord prolapse, uterine rupture, maternal pyrexia, shoulder dystocia and maternal hemorrhage) occurred in 86 and 89% of mothers of control and cooled infants, respectively. Transfer from birth hospital to Network centers occurred in 41 and 47% of control and cooled infants, respectively. Intubation was performed in 92 and 95% of control and cooled infants, respectively, and the cord pH and base deficits were 6.8±0.2 and 20±9mM/L and 6.9±0.2 and 19±7mM/L, respectively. Characteristics that were adjusted for in regressions were as follows: maternal race (black/white/other) was similar in the control (37/31/31%) and cooled (31/40/29%) groups; male gender constituted 64 and 49% of control and cooled infants, respectively; gestational age was 39±2 weeks in each group; and moderate and severe encephalopathy occurred in 63 and 37% of control infants and in 69 and 31% of cooled infants.
As verified in the Coordinator survey, control infants were initially cared for under a radiant warmer with servo control of the abdominal skin temperature. The initial servo set point temperature was 36.5°C in 15 of the 20 hospitals participating in the study (4 network sites enrolled infants from 2 hospitals). The remaining 5 hospitals used initial servo set point temperatures of 36°C (n=3), 36.6°C (n=1), and 36–36.5°C (n=1). Temperatures from the axilla and skin were monitored as part of usual care at all hospitals. The minimum and maximum acceptable temperatures were 36.0 and 37.5°C, respectively, and ranges of up to 1°C were acceptable within some hospitals. The frequency that temperature was recorded ranged from one to four hours.
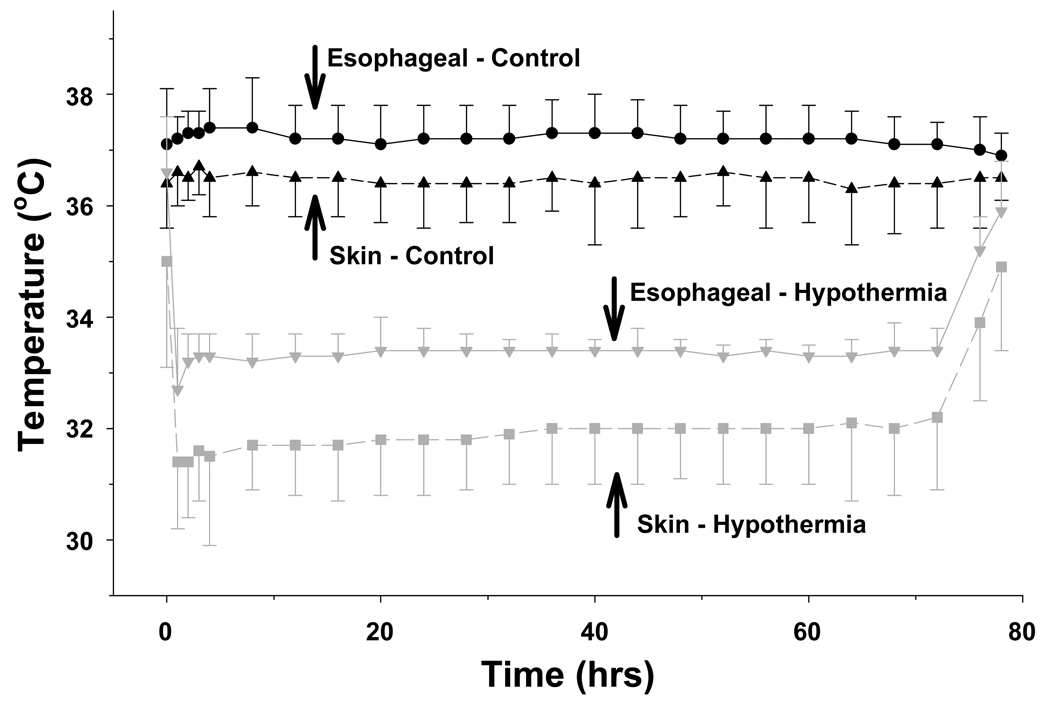
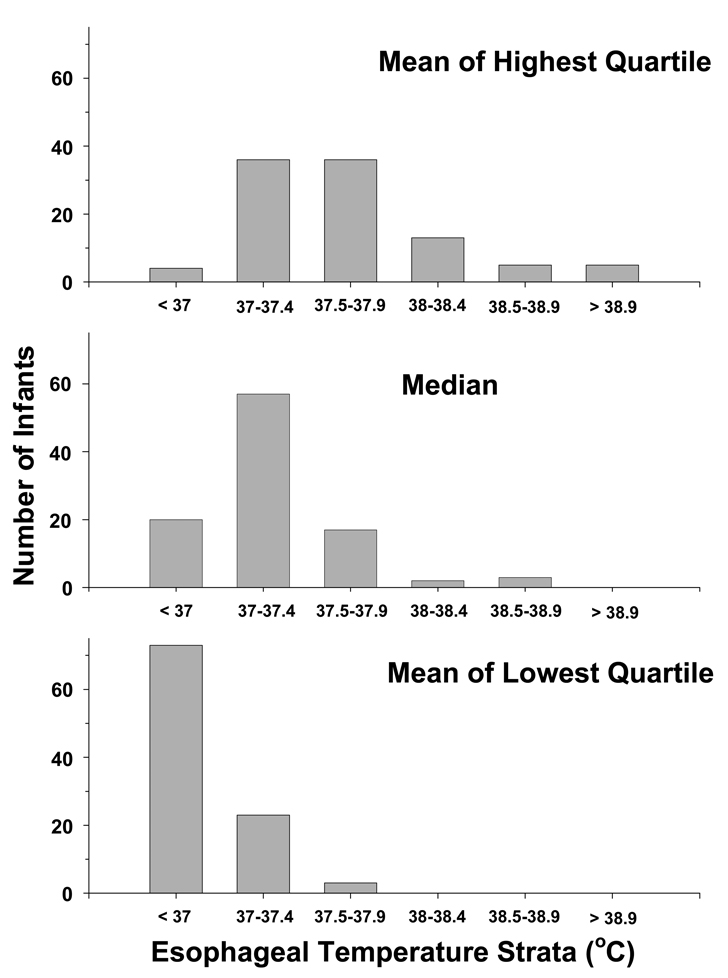
Figure I indicates the esophageal and skin temperature for each group during the 72 hour study period. The mean esophageal temperature for all control infants was 37.2 ± 0.7°C over the 72 hour intervention with 25th and 75th percentiles of 36.9°C and 37.5°C. In control infants 63%, 22% and 8% of all esophageal temperature values (n = 1690, 191 missing values) were > 37, > 37.5 and > 38°C, respectively. Corresponding values for skin temperatures (n = 1731, 150 missing values) were a mean temperature of 36.5°C ±0.8°C with 12%, 5% and 2% of values > 37, > 37.5 and > 38°C, respectively. The correlation between esophageal and skin temperature for the control infants was 0.36 (p<.0001). The distribution of control infants among the high, median and low esophageal temperatures are indicated in Figure II. The high esophageal temperatures (mean of the highest quartile) ranged from a minimum of 36.5°C to a maximum of 40°C and 23 infants had a high temperature ≥ 38°C. The median value ranged from 36.3°C to 38.9°C and 5 infants had a median temperature > 38°C. The low temperatures (mean of the lowest quartile) ranged from 33.8°C to 37.8°C and none had a low temperature > 38°C. Overlap is present among the high, low and median temperatures reflecting that some infant’s high temperatures are equal or less than other infant’s low temperatures.
Figure I.
Esophageal and skin temperatures of both groups are plotted over the 72 hour interval of whole body cooling and subsequent rewarming. Symbols represent the mean and standard deviation. Control infants are represented by black symbols using circles for esophageal and triangles for skin temperature. Whole body cooling infants are represented by gray symbols using inverted triangles for esophageal and squares for skin temperature. Time 0 hours represents the initiation of body cooling in the intervention group.
Figure II.
The distribution of control infants is plotted among strata of esophageal temperature for the average (avg) of the highest quartile (top panel), median (middle panel), and average of the lowest quartile (lower panel) of temperature. The strata are in 0.5°C increments to facilitate viewing the distribution of the data.
Logistic regressions relating each of the three esophageal temperature measurements to the primary outcome indicate significant associations for the high and median temperature (Table I). For the mean of the highest quartile of esophageal temperature the odds of death or moderate/severe disability was increased 4 fold, and the odds of death alone was increased 6.2 fold for each centigrade increase. For the median esophageal temperature the odds of death alone was increased 5.9 fold for each centigrade increase. There was no association between the mean of the lowest quartile and the primary outcome or its components.
Table 1.
Odds Ratios Relating Esophageal Temperature to Adverse Outcomes for Control Infants
| Esophageal Temperature (°C) |
Death or Disability N = 99 |
Death N = 99 |
Disability N = 65 |
|---|---|---|---|
| Highest Quartile |
4.0* (1.5 – 11.2) |
6.2 (2.1 – 17.9) |
1.8 (0.4 – 8.2) |
| Median | 3.2 (0.9 – 11.2) |
5.9 (1.5 – 22.7) |
1.0 (0.2 – 5.1) |
| Lowest Quartile |
1.5 (0.6 – 3.5) |
1.4 (0.6 – 3.3) |
1.1 (0.3 – 3.5) |
Each regression represents values from each control infant using the mean of the highest quartile (top row), median (middle row) and mean of the lowest quartile of temperature (bottom row) for death or disability, death alone and disability alone. The number of infants in each analysis is indicated by the N.
Results are odds ratio per °C increase (95% confidence interval) adjusted for level of encephalopathy, gender, gestational age, and race
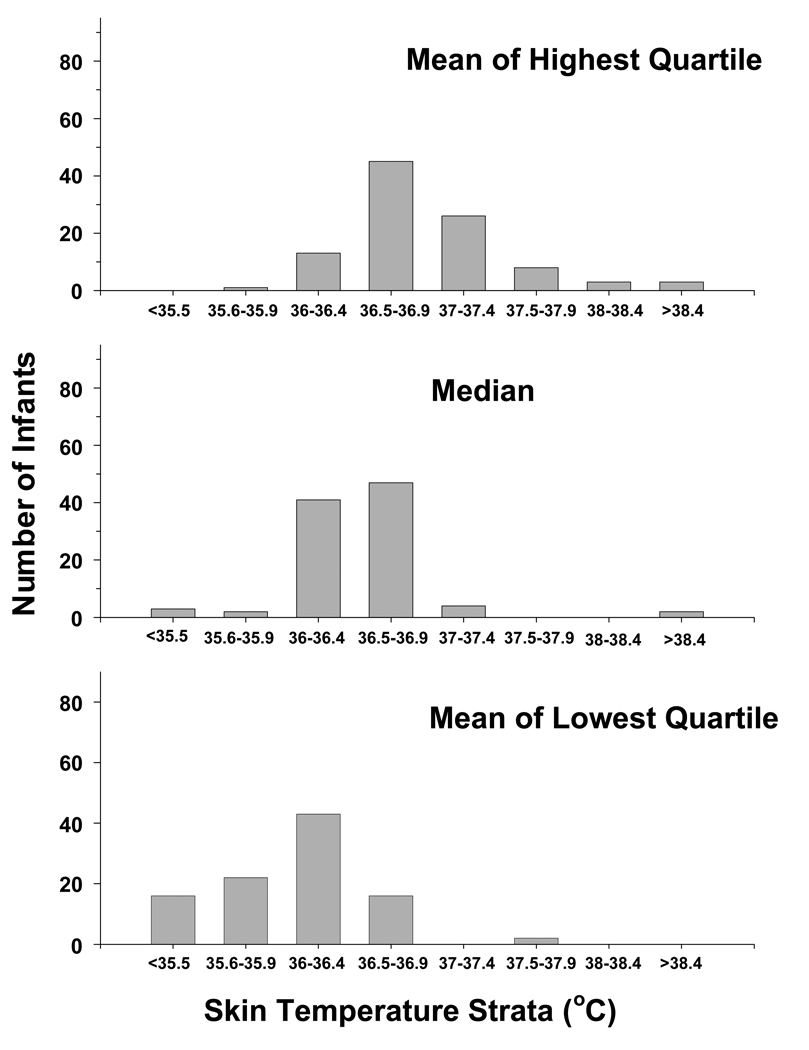
The frequency of control infants among high, median and low skin temperatures is displayed in Figure III. The high temperatures (mean of the highest quartile) ranged from a minimum of 35.7°C to a maximum of 39.3°C and 6 infants had a high temperature > 38°C. The median value ranged from 34.3°C to 38.6°C and 2 infants had a median temperature > 38°C. The low temperatures (mean of the lowest quartile) ranged from 32.1°C to 37.9°C and no temperature was > 38°C. Logistic regressions relating each of the three skin temperature measurements to the primary outcome indicate significant associations for the high temperature (Table II). The mean of the highest quartile of skin temperature was associated with a 3.6 and 3.2 fold increase in death or moderate/severe disability, or death alone, respectively for each centigrade increase.
Figure III.
The distribution of control infants is plotted among strata of skin temperature for the average (avg) of the highest quartile (top panel), median (middle panel), and average of the lowest quartile (lower panel) of temperature. The strata are in 1.0°C increments to facilitate viewing the distribution of the data
Table 2.
Odds Ratios Relating Skin Temperature to Adverse Outcomes for Control Infants
| Skin Temperature (°C) |
Death or Disability N = 99 |
Death N = 99 |
Disability N = 65 |
|---|---|---|---|
| Highest Quartile |
3.6* (1.3 – 10.4) |
3.2 (1.2 – 8.3) |
2.9 (0.7 – 11.6) |
| Median | 1.4 (0.5 – 3.6) |
1.8 (0.7 – 5.0) |
1.0 (0.3 – 3.5) |
| Lowest Quartile |
1.2 (0.7 – 2.3) |
1.2 (0.7 – 2.3) |
1.2 (0.5 – 2.8) |
Each regression represents values from each control infant using the mean of the highest quartile (top row), median (middle row) and mean of the lowest quartile of temperature (bottom row) for death or disability, death alone and disability alone. The number of infants in each analysis is indicated by the N.
Results are odds ratio per °C increase (95% confidence interval) adjusted for level of encephalopathy, gender, gestational age, and race
Duration of raised temperature was also examined. Death or moderate/severe disability was associated with the total hours of esophageal temperature > 38°C (OR 1.13, 95% CI 1.04–1.24) and the longest consecutive hours with values > 38°C (OR 1.16, 95% CI 1.04–1.29). Death or moderate/severe disability was associated with the longest consecutive hours of skin temperature > 37.5°C (OR 1.25, 95% CI 1.03–1.51) but not with total hours above this threshold temperature (OR 1.09, 95% CI 0.99–1.20). There were 22 infants with at least 2 consecutive esophageal temperatures > 38°C (> 4 consecutive hours) and the median duration for each infant ranged from 8–28 hours with an overall median value of 12 hours for all 22 infants. Approximately half of the esophageal temperatures > 38°C occurred in the first 24 hours of the intervention interval with the remainder occurring equally among the second and third day.
The mean esophageal temperature for cooled infants was 33.3 ± 0.4°C over the 72 hour intervention. The distribution of temperatures among cooled infants was narrower than the control group with the 25th and 75th percentiles of 33.2°C and 33.5°C; 89.7% of all esophageal temperatures (total 2334 measurements, 188 missing values) were between 33.1 and 34.0°C inclusive. The narrower temperature distribution of cooled compared to control infants resulted in greater overlap between predefined high (range 33.2–34.4°C), low (range 29.6–33.6°C) and median temperatures (range 30.2–33.8°C) of this group. The mean skin temperature was 31.8±1.1°C over the 72 hour intervention with the 25th and 75th percentiles of 31.3°C and 32.2°C. The correlation between esophageal and skin temperature for the cooled infants was 0.69 (p<.0001). No associations were found between esophageal or skin temperatures and death or moderate/severe disability in the logistic regression analysis of the cooled group.
Discussion
There are two principal findings of this observational study. First, broad ranges of esophageal and skin temperature were observed among control infants given usual care in contemporary Neonatal Intensive Care Unit environments. Second, within this range relatively high esophageal and skin temperatures were associated with an increase in the odds of death, and death or moderate/severe disability in analyses controlling for degree of encephalopathy as well as gender, race and gestational age. The risk of death or moderate/severe disability was increased 3.6–4 fold for every 1°C increase in the mean of the highest quartile of skin or esophageal temperature. The risk of death alone was increased 5.9 fold for every 1°C increase in the median esophageal temperature. The risk of death or moderate/severe disability was increased with duration of raised temperature but the odds ratios were less prominent.
The association between raised temperature and death or disability has at least 3 equally plausible explanations; brain injury raised body temperature, raised body temperature results in brain injury, or raised temperature is a marker for an underlying process of which a component is encephalopathy. These possibilities are not mutually exclusive. Similar observations have been made in adults with stroke in whom there were associations between hyperthermia, initial stroke severity, infarct size and mortality.13–15
In support of brain injury resulting in raised body temperature are the observations that the extent and region of brain lesions triggers mechanisms involved in the pathogenesis of elevated temperature. For example, IL-6 protein is expressed in neurons and microglia within hours of focal ischemia and expression is predominantly up-regulated in the penumbra with less expression in the contralateral cortex associated with spreading depression.16 IL-6 is an endogenous pyrogen based on systemic or central intraventricular application of this cytokine evoking fever.17, 18 Knockout of IL-6 in mice have demonstrated that IL-6 expression within brain is necessary for febrile responses to exogenous (lipopolysaccharide) and downfield endogenous (IL-1β) pyrogens.19 Site specific injury associated with elevated temperature has been assessed by correlating thermoregulatory response and histological injury in rodents undergoing fluid percussion injury.20 This model is associated with post-traumatic hyperthermia, a blunting or loss of circadian rhythm in temperature, and diffuse brain injury involving cortex, thalamus and hippocampus. Animals with post-traumatic hyperthermia had similar extents of cell loss to animals without hyperthermia but were distinguished by infiltration of astrocytes and microglia in four areas of the brain involved in temperature control (ventromedial preoptic nucleus, paraventricular nucleus, suprachiasmatic nucleus, and anterior perifornical nucleus). Localization of pathology in animal data share interesting parallels with specific patterns of injury in term infants on MRI following neonatal encephalopathy. Of the multiple observed injury patterns, injury predominated by changes in the basal ganglia and thalamus was associated with the most severe neonatal signs and the greatest impairment of motor and cognitive function at 30 months of age.21
Alternatively there is experimental support for raised temperature resulting in brain injury. Elevated intra-ischemic temperature exacerbates the extent of neuronal damage in adult dogs and rodents.22–26 Similar observations have been reported in 7 day rat pups in whom an increase in brain temperature of 1–2°C during hypoxia-ischemia aggravated behavioral deficits and neuronal injury compared to normothermic animals.4 There is less data to demonstrate that an elevation of temperature remote from brain ischemia exacerbates injury. In adult rats 3 hours of hyperthermia (39–40°C) initiated at 24 hours following brain ischemia increased ischemic neurons of the CA-1 sector by 2.5 fold compared to 38°C.27 In 10 day rat pups an increase in core body temperature (37.5°C compared to 36.0°C) for four hours immediately following hypoxia-ischemia increased the extent of neuronal injury.5 Intra-ischemic hyperthermia acts through several mechanisms to worsen brain injury including enhanced release of neurotransmitters, exaggerated oxygen radical production, greater blood-brain barrier breakdown, enhanced inflammatory response, impaired recovery of energy metabolism and protein synthesis and worsening of cyto-skeletal proteolysis.28–30 It remains unclear to what extent these same processes are activated when temperature is raised remote from a hypoxic-ischemic event.
There is little direct evidence to support raised temperature representing a marker of an as yet undiagnosed underlying process. Of newborns with encephalopathy, 69% have only ante-partum risk factors without evidence of intra-partum impaired gas exchange (ref 2). Understanding the causal path to newborn encephalopathy may involve intra-partum events alone, or interactions among multiple ante-partum or ante-and intra-partum variables. Inflammation modulating the effects of acute hypoxia-ischemia represents a potential example of these interactions (ref 31, new); in this context fever could be a marker for the underlying process and its severity. Given these considerations, the current study used enrollment criteria that reflected evidence of intra-partum events (ref 7).
Thermoregulatory practices for the term infant are largely extrapolated from the preterm infant in whom neonatal mortality was lower if infants were nursed in warmer compared to cooler environmental temperature. 31, 32 Differences in survival reflect the relationship between temperature and oxygen consumption, and thermal regulatory practices have evolved to care for infants in environments that minimize oxygen consumption. In preterm infants oxygen consumption increases with changes in skin temperature before core temperature is altered, and servo control of skin temperature can be used to minimize oxygen consumption more effectively than servo control of core temperature.33 Servo control of abdominal skin temperature using a radiant warmer and initial set point of 36.5°C approximates conditions associated with a minimal metabolic rate in preterm infants34 and is readily used in term infants in the absence of other available data. However, it is unclear how oxygen consumption would be best regulated in term infants, and whether the skin or axilla temperature needed to minimize oxygen consumption and reduce adverse outcomes are the same in healthy term infants and those with hypoxic-ischemic encephalopathy. Although bedside providers were not shielded from the temperature display, esophageal temperature was not used to guide clinical care since it is rarely measured in newborns. Simultaneous esophageal and axilla temperatures were not recorded and other documentation of changes in the automatic control set point or other measures to limit the extent of elevated temperature were not systematically collected. Other etiologies for raised temperature such as infection are unlikely since there were only 2 infants in each group with positive blood cultures.35
There were no associations between high, low and median temperatures of infants undergoing whole body cooling and outcome. This may reflect that the majority of esophageal temperatures were within a relatively narrow range (90% of the 2334 values were between 33.1–34.0°C). Circulation of water through 2 blankets simultaneously (a pediatric size for the infant to lie on and a suspended adult size) in conjunction with the automatic control mode of the Blanketrol device contributed to maintaining 90% of all esophageal temperatures within 0.5°C of the set point7.
The results of this study should be viewed as hypothesis generating. This was an observational secondary study that demonstrated an association between elevated temperature and death or moderate/severe disability. The association could be attributable to the severity of the brain lesion, specific adverse effects of an elevated temperature or both. Whether this association is causal is unclear. A randomized controlled trial would be needed to determine if prevention of elevated esophageal or skin temperatures associated with adverse outcomes in our study would reduce death or moderate/severe disability for infants cared for without active cooling.
Acknowledgments
Supported in part by grants: U10 HD34216, U10 HD27853, U10 HD27871, U10 HD40461, U10 HD40689, U10 HD27856, U10 HD27904, U10 HD40498, U10 HD40521, U01 HD36790, U10 HD21385, U10 HD27880, U10 HD27851, U10 HD 21373 and GCRCs: M01 RR 08084, M01 RR 00125, M01 RR 00750, M01 RR 00070, M01 RR 0039-43, M01RR 00039, 5 M01 RR00044
Footnotes
The Hypothermia Study Group
Case Western Reserve University Rainbow Children’s Hospital Principal Investigator: Avroy A. Fanaroff, MD; Co-PI: Michele C. Walsh, MD; Study Coordinator: Nancy Newman, BA; RN; Follow Up Principal Investigator: DeeAnne Wilson-Costello, MD; Follow Up Coordinator: Bonnie Siner, RN. Brown University Women & Infant's Hospital Principal Investigator: William Oh, MD; Study Coordinator: Angelita Hensman, BSN, RNC; Follow Up Principal Investigator: Betty Vohr, MD; Follow Up Coordinator: Lucy Noel, RN. Duke University Principal Investigator: C. Michael Cotten, MD; Study Coordinator: Kathy Auten, BS; Follow Up Principal Investigator: Ricki Goldstein, MD; Follow Up Coordinator: Melody Lohmeyer, RN. Emory University Grady Memorial Hospital and Crawford Long Hospital Principal Investigator: Barbara J. Stoll, MD; Co-PI: Lucky Jain, MD; Study Coordinator: Ellen Hale, RN, BS. Indiana University Riley Hospital for Children and Methodist Hospital Principal Investigator: James A. Lemons, MD; Study Coordinators: Diana Dawn Appel, RN BSN, Lucy Miller, RN, BSN; Follow Up Principal Investigator: Anna Dusick, MD; Follow Up Coordinator: Leslie Richard, RN. Stanford University Principal Investigator: David K. Stevenson, MD; Co-PI: Krisa VanMeurs, MD; Study Coordinator: M. Bethany Ball, BS, CCRC; Follow Up Principal Investigator: Susan R. Hintz, MD. University of Alabama at Birmingham University Hospital-UAB Principal Investigator: Waldemar A. Carlo, MD; Study Coordinator: Monica Collins, RN, BSN, Shirley Cosby, RN, BSN; Follow Up Principal Investigator: Myriam Peralta-Carcelen, MD; Follow Up Coordinator: Vivien Phillips, RN, BSN. University of Cincinnati The University Hospital, Cincinnati Children’s Hospital Medical Center; Principal Investigator: Edward F. Donovan, MD; Study Coordinators: Cathy Grisby, BSN, Barb Alexander, RN, Jody Shively, RN, Holly Mincey, RN; Follow Up Principal Investigator: Jean Steichen, MD; Follow Up Coordinator: Teresa Gratton, PA. University of California-San Diego UCSD Medical Center and Sharp Mary Birch Hospital for Women Principal Investigators: Neil N. Finer, MD; Co-PI: David Kaegi, MD; Study Coordinators: Chris Henderson, CRTT, Wade Rich, RRT-NPS, Kathy Arnell, RN; Follow Up Principal Investigator: Yvonne E. Vaucher, MD, MPH; Follow Up Coordinator: Martha Fuller, RN, MSN. University of Miami Principal Investigator: Shahnaz Duara, MD; Study Coordinator: Ruth Everett, BSN; Follow Up Principal Investigator: Charles R. Bauer, MD. University of Rochester Golisano Children's Hospital at Strong Principal Investigator: Ronnie Guillet, MD; PhD; Study Coordinator: Linda Reubens, RN; Follow Up Principal Investigator: Gary Myers, MD; Follow Up Coordinator: Diane Hust, RN. The University of Texas Southwestern Medical Center at Dallas: Parkland Hospital Principal Investigator: Abbot R. Laptook, MD; Study Coordinators: Susie Madison, RN, Gay Hensley, RN, Nancy Miller, RN; Follow Up Principal Investigator: Roy Heyne, MD, Sue Broyles, MD; Follow Up Coordinator: Jackie Hickman, RN. University of Texas – Houston Memorial Hermann Children’s Hospital Principal Investigator: Jon E. Tyson, MD, MPH; Study Coordinator: Georgia McDavid, RN, Esther G. Akpa, RN, BSN, Claudia Y. Franco, RN, BNS, MSN, NNP, Patty A. Cluff, RN, Anna E. Lis, RN, BSN; Follow-Up Principal Investigators: Brenda H. Morris, MD, Pamela J. Bradt, MD, MPH. Wayne State University Hutzel Women's Hospital & Children's Hospital of Michigan Principal Investigator: Seetha Shankaran, MD; Study Coordinators: Rebecca Bara, RN, BSN, Geraldine Muran, RN, BSN; Follow Up Principal Investigator: Yvette Johnson, MD; Follow Up Coordinator: Debbie Kennedy, RN. Yale University New Haven Children's Hospital Principal Investigator: Richard A. Ehrenkranz, M.D. Study Coordinator: Patricia Gettner, RN; Follow Up Coordinator: Elaine Romano, RN.
NICHD Neonatal Research Steering Committee
Brown University William Oh, MD; Case Western University Avroy A. Fanaroff, MD; Duke University Ronald N. Goldberg, MD; Emory University Barbara J. Stoll, MD; Indiana University James A. Lemons, MD; Stanford University David K. Stevenson, M.D.; University of Alabama at Birmingham Waldemar A. Carlo, MD; University of Cincinnati Edward F. Donovan, MD; University of California-San Diego Neil N. Finer, MD; University of Miami Shahnaz Duara, MD; University of Rochester Dale L. Phelps, MD; University of Texas – Dallas Abbot R. Laptook, MD; University of Texas – Houston Jon E. Tyson, MD, MPH; Wake Forest University T. Michael O’Shea, MD, MPH; Wayne State University Seetha Shankaran, MD; Yale University Richard A. Ehrenkranz, MD, Chair, Alan Jobe, University of Cincinnati
Data Coordinating Center: RTI International
Principal Investigator: W. Kenneth Poole, PhD; Coordinators: Betty Hastings and Carolyn M. Petrie, MS
National Institute of Child Health and Human Development
Program Scientist: Rosemary D. Higgins, MD, Linda L. Wright, MD; Coordinator: Elizabeth McClure, MEd
Data Safety and Monitoring Committee
Children’s National Medical Center Gordon Avery, MD; Columbia University Mary D’Alton, MD; RTI International W. Kenneth Poole, PhD (ex officio); University of Virginia John C. Fletcher, Ph.D. (deceased); University of Washington Christine A. Gleason, MD; University of Pittsburgh Carol Redmond, Ph.D.
References
- 1.Paneth N. The causes of cerebral palsy. Recent evidence. Clin Invest Med. 1993;16(2):95–102. [PubMed] [Google Scholar]
- 2.Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317(7172):1554–1558. doi: 10.1136/bmj.317.7172.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laptook AR, Corbett RJ. The effects of temperature on hypoxic-ischemic brain injury. Clin Perinatol. 2002;29(4):623–649. doi: 10.1016/s0095-5108(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 4.Mishima K, Ikeda T, Yoshikawa T, Aoo N, Egashira N, Xia YX, et al. Effects of hypothermia and hyperthermia on attentional and spatial learning deficits following neonatal hypoxia-ischemic insult in rats. Behav Brain Res. 2004;151(1–2):209–217. doi: 10.1016/j.bbr.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011(1):48–57. doi: 10.1016/j.brainres.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman E, Lang J, Richardson DK, Frigoletto FD, Heffner LJ, Cohen A. Intrapartum maternal fever and neonatal outcome. Pediatrics. 2000;105(1 Pt 1):8–13. doi: 10.1542/peds.105.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Impey L, Greenwood C, MacQuillan K, Reynolds M, Sheil O. Fever in labour and neonatal encephalopathy: a prospective cohort study. Bjog. 2001;108(6):594–597. doi: 10.1111/j.1471-0528.2001.00145.x. [DOI] [PubMed] [Google Scholar]
- 10.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278(3):207–211. [PubMed] [Google Scholar]
- 11.Mellergard P, Nordstrom CH. Intracerebral temperature in neurosurgical patients. Neurosurgery. 1991;28(5):709–713. [PubMed] [Google Scholar]
- 12.Simbruner G, Nanz S, Fleischhacker E, Derganc M. Brain temperature discriminates between neonates with damaged, hypoperfused, and normal brains. Am J Perinatol. 1994;11(2):137–143. doi: 10.1055/s-2007-994574. [DOI] [PubMed] [Google Scholar]
- 13.Hajat C, Hajat S, Sharma P. Effects of poststroke pyrexia on stroke outcome : a meta-analysis of studies in patients. Stroke. 2000;31(2):410–414. doi: 10.1161/01.str.31.2.410. [DOI] [PubMed] [Google Scholar]
- 14.Rossi S, Roncati Zanier I, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psych. 2001;71:448–454. doi: 10.1136/jnnp.71.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347(8999):422–425. doi: 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, Tanaka K, Nogawa S, Nagata E, Daisuke I, Dembo T, et al. Temporal profile and cellular localization of interleukin-6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1256–1262. doi: 10.1097/00004647-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA, Cannon JG, Mancilla J, Bishai I, Lees J, Coceani F. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Res. 1991;562(2):199–206. doi: 10.1016/0006-8993(91)90622-3. [DOI] [PubMed] [Google Scholar]
- 18.Lesnikov VA, Efremov OM, Korneva EA, Van Damme J, Billiau A. Fever produced by intrahypothalamic injection of interleukin-1 and interleukin-6. Cytokine. 1991;3(3):195–198. doi: 10.1016/1043-4666(91)90016-7. [DOI] [PubMed] [Google Scholar]
- 19.Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 beta: a study on IL-6-deficient mice. J Exp Med. 1996;183(1):311–316. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson HJ, Hoover RC, Tkacs NC, Saatman KE, McIntosh TK. Development of posttraumatic hypothermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J Cereb Blood Flow Metab. 2005;25:163–176. doi: 10.1038/sj.jcbfm.9600008. [DOI] [PubMed] [Google Scholar]
- 21.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146(4):453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 22.Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7(6):729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- 23.Minamisawa H, Smith ML, Siesjo BK. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol. 1990;28(1):26–33. doi: 10.1002/ana.410280107. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich WD, Busto R, Valdes I, Loor Y. Effects of normothermic versus mild hyperthermic forebrain ischemia in rats. Stroke. 1990;21(9):1318–1325. doi: 10.1161/01.str.21.9.1318. [DOI] [PubMed] [Google Scholar]
- 25.Reglodi D, Somogyvari-Vigh A, Maderdrut JL, Vigh S, Arimura A. Postischemic spontaneous hyperthermia and its effects in middle cerebral artery occlusion in the rat. Exp Neurol. 2000;163(2):399–407. doi: 10.1006/exnr.2000.7367. [DOI] [PubMed] [Google Scholar]
- 26.Wass CT, Lanier WL, Hofer RE, Scheithauer BW, Andrews AG. Temperature changes of> or = 1 degree C alter functional neurologic outcome and histopathology in a canine model of complete cerebral ischemia. Anesthesiology. 1995;83(2):325–335. doi: 10.1097/00000542-199508000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Baena RC, Busto R, Dietrich WD, Globus MY, Ginsberg MD. Hyperthermia delayed by 24 hours aggravates neuronal damage in rat hippocampus following global ischemia. Neurology. 1997;48(3):768–773. doi: 10.1212/wnl.48.3.768. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda H, Tomimatsu T, Watanabe N, Mu JW, Kohzuki M, Endo M, et al. Post-ischemic hypothermia blocks caspase-3 activation in the newborn rat brain after hypoxia-ischemia. Brain Res. 2001;910(1–2):187–191. doi: 10.1016/s0006-8993(01)02659-2. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Murata T, Jiang L, Power GG. Hypothermia prevents metabolic and cerebral flow responses to hypoxia in the fetal sheep. J Soc Gynecol Invest. 2000;7(No 1):45–50. doi: 10.1016/s1071-5576(99)00068-4. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg MD, Busto R. Combating hyperthermia in acute stroke: a significant clinical concern. Stroke. 1998;29(2):529–534. doi: 10.1161/01.str.29.2.529. [DOI] [PubMed] [Google Scholar]
- 31.Silverman WA, Fertig JW, Berger AP. The influence of the thermal environment upon the survival of newly born premature infants. Pediatrics. 1958;22(5):876–886. [PubMed] [Google Scholar]
- 32.Buetow KC, Klein SW. Effect of Maintenance of "Normal" Skin Temperature on Survival of Infants of Low Birth Weight. Pediatrics. 1964;34:163–170. [PubMed] [Google Scholar]
- 33.Silverman WA, Sinclair JC, Agate FJ., Jr The oxygen cost of minor changes in heat balance of small newborn infants. Acta Paediatr Scand. 1966;55(3):294–300. doi: 10.1111/j.1651-2227.1966.tb17657.x. [DOI] [PubMed] [Google Scholar]
- 34.Sedin G. The Thermal Environment of the Newborn Infant. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin's Neonatal-Perinatal Medicine of the Fetus and Infant. 3th ed. Philadelphia, PA: Mosby Elsevier; 2006. pp. 585–596. [Google Scholar]
- 35.Shankaran S. The NICHD Neonatal Research Network. Progress Since 2004: NICHD NRN Whole Body Hypothermia for the HIE Trial; From Proceedings of Hot Topics '05 in Neonatology. [Google Scholar]