Abstract
Tamoxifen induces important changes in serum lipid profiles in some women; however, little information is available to predict which women will experience improved lipid profiles during tamoxifen therapy. As part of a multicenter prospective observational trial in 176 breast cancer patients, we tested the hypothesis that tamoxifen-induced lipid changes were associated with genetic variants in candidate target genes (CYP2D6, ESR1, and ESR2). Tamoxifen lowered low-density lipoprotein cholesterol (P<0.0001) by 23.5mg/dl (13.5–33.5mg/dl) and increased triglycerides (P=0.006). In postmenopausal women, the ESR1-XbaI and ESR2-02 genotypes were associated with tamoxifen-induced changes in total cholesterol (P=0.03; GG vs GA/AA) and triglycerides (P=0.01; gene–dose effect), respectively. In premenopausal women, the ESR1-XbaI genotypes were associated with tamoxifen-induced changes in triglycerides (P=0.002; gene–dose effect) and high-density lipoprotein (P=0.004; gene–dose effect). Our results suggest that estrogen receptor genotyping may be useful in predicting which women would benefit more from tamoxifen.
The use of exogenous estrogen to prevent cardiovascular disease in women has been a subject of controversy since data from the Women’s Health Initiative trial suggested that postmenopausal women might experience a greater number of cardiovascular events when treated with hormone replacement therapy.1 There is large interindividual variability in the response to estrogens, and Herrington et al.2 have suggested that this may be due in part to genetic variants in the estrogen receptors (ERs). As the Women’s Health Initiative study made prospective study of the effects of estrogens difficult and as the selective estrogen response modifier tamoxifen acts as an exogenous estrogen on serum lipids, we elected to study the effects of tamoxifen on serum lipids and to test the hypothesis that genetic variants are associated with variability in response.
Tamoxifen is a selective ER modulator widely used in the treatment of ER- or progesterone receptor-positive breast cancer, and it is the only agent approved for the prevention of the disease.3–6 As a selective ER modulator, tamoxifen has both estrogenic and anti-estrogenic effects depending on the target tissue. Tamoxifen effects on serum lipid concentration are largely similar to those of estrogen.7,8
Tamoxifen is documented to cause a reduction in serum total and low-density lipoprotein (LDL), but data on high-density lipoprotein (HDL) and triglycerides have not been consistent.9–14 The effects of tamoxifen vary from patient to patient, and this variability is specifically seen in the response of lipid parameters.11,14,15 The causes of this variability are not completely understood, but may be, in part, due to inherited genetic differences in the cellular receptors.16 Tamoxifen acts via binding and modulation of the ERs, which are members of the nuclear steroid receptor superfamily. Two subsets of human ERs have been identified: ER-α and ER-β. These receptors are products of different genes, designated ESR1 and ESR2, respectively.17–19 The expression of these receptors is influenced by genetic polymorphisms, as is the case for other members of the nuclear receptor superfamily.16 Single-nucleotide polymorphisms (SNPs) in the genes coding for the ERs may therefore explain some of the variability seen in the response to tamoxifen, including changes in lipid profile. The interaction of the ERs with each other may also be important, as studies have suggested that ER-β modulates some of the responses resulting from activation of ER-α targets.20
Herrington et al.2 have showed an association between HDL-cholesterol levels and polymorphisms in ER-α gene related to exogenous estrogen. They reported a significantly higher HDL response to estrogen therapy compared with placebo of postmenopausal women who carry the ER-α IVIS-401C/C genotype vs variant genotypes. Similarly, other investigators have reported an association between ER genotype and baseline concentration of LDL-cholesterol in Japanese school children, who were not exposed to any medications.21
In 2000, we initiated a prospective, multi-institutional cohort clinical trial to study SNPs in multiple candidate genes and non-tumoral effects of tamoxifen in women at high risk for or with newly diagnosed breast cancer. From this cohort, we have reported previously an association of cytochrome P450 2D6 (CYP2D6) genotypes and tamoxifen metabolite concentrations in breast cancer patients treated with tamoxifen.22–24 Endoxifen, an active metabolite of tamoxifen, is 30- to 100-fold more potent than tamoxifen as an anti-estrogen in the suppression of estrogen-responsive genes and cell proliferation.22 CYP2D6 genotypes may therefore be associated with non-tumoral responses of tamoxifen such as lipid profile change.
We report here the results of the analysis of this prospective cohort in which we tested the hypothesis that lipid profile changes in breast cancer patients on tamoxifen therapy are associated with candidate polymorphisms in ER-α, ER-β, or CYP2D6.
RESULTS
Subjects
The baseline characteristics of the 176 subjects who qualified for the lipid substudy overall, and the 134 subjects used for the genotype–phenotype analyses are shown in Table 1. Our cohort was composed mostly of Caucasian women (92%). As dictated by the protocol, the use of lipid-altering medication was no different at baseline compared with 4 months after initiation of tamoxifen treatment. Among the 134 samples analyzed for genotype–phenotype association, seven were African Americans, one Arabic, one Hispanic, and one Asian (Table 1). Their lipids were not different from the Caucasian lipid samples.
Table 1.
Baseline characteristics of patients in study
| Entire cohort | Genotype–phenotype cohort | |
|---|---|---|
| Characteristics | (N=176) | (N=134)a |
| Age (years), mean (range) | 55 (32–82) | 50 (33–78) |
| Premenopausal | 49 (32–63) | 48 (34–63) |
| Perimenopausal | 53 (45–60) | 63 (39–80) |
| Postmenopausal | 64 (39–82) | |
| BMI, mean (SD) | 27 (8) | 27 (6) |
| Race/ethnicityb | ||
| African Americans | 8 (4)c | 7 (5) |
| Caucasians | 161 (92) | 125 (93) |
| Othersc | 7 (4) | 3 (2) |
| Menopausal statusb | ||
| Premenopausal | 55 (31) | 53 (39) |
| Perimenopausal | 20 (11) | 0 (0) |
| Postmenopausal | 101 (57) | 81 (61) |
| Patients on lipid-lowering medications b | ||
| At baseline | 22 | 0 |
| At 4 months | 22 | 0 |
BMI, base metabolic index; N, number in each category.
Genotype–phenotype cohort refers to the 136 patients for the genotype–lipid association analysis who were neither perimenopausal nor on lipid-lowering medications.
Number in group (%).
Others consist of two patients of Arabic, one of Hispanic, one of Asian, and two of unknown ancestry, and race/ethnicity percentages may not add up to 100 because of rounding.
Lipid concentrations independent of genotypes
In the analysis of the primary cohort of 176 women (N = 176) for the lipid substudy, the mean baseline total cholesterol was lower in premenopausal women compared with postmenopausal women (211 vs 225 mg/dl, P=0.02). There was a statistically nonsignificant trend toward lower mean baseline LDL in the premenopausal group compared with the postmenopausal group (122 vs 135 mg/dl, P=0.09). The mean triglyceride concentration was also lower (124 vs 159 mg/dl, P=0.09) and HDL-cholesterol was higher in the premenopausal group (65 vs 60 mg/dl, P=0.03) compared with their postmenopausal counterparts.
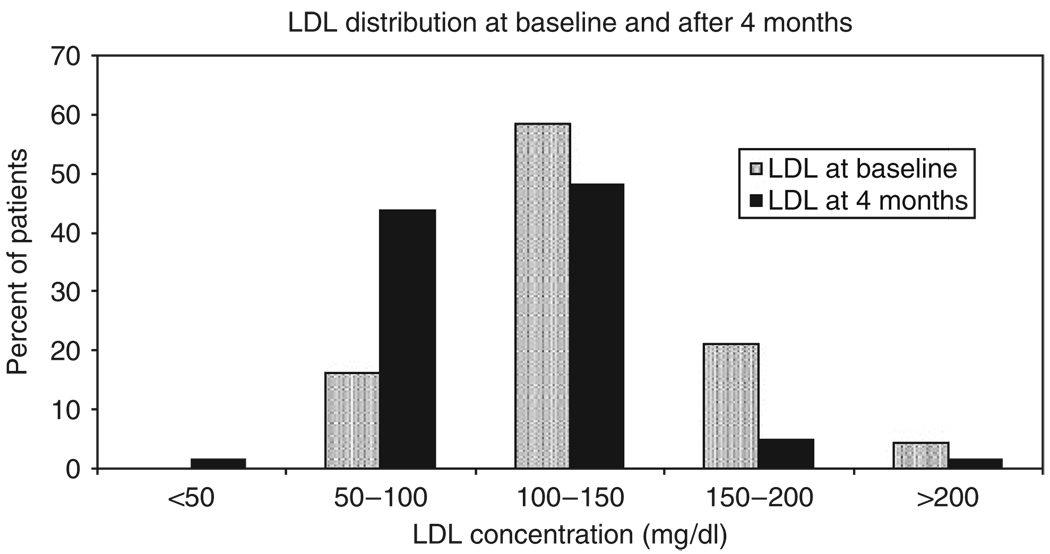
After 4 months of tamoxifen treatment, there was a mean decrease in (−) 17.7 mg/dl (P≤0.0001) and (−) 23.5 mg/dl (P≤0.0001) in serum total and LDL-cholesterol concentration, respectively (Table 2). The triglyceride concentration increased by a mean of (+) 24.4 mg/dl (P=0.006), but the HDL was not significantly changed compared with baseline (P=0.54, Table 2). Overall, our data show a shift of the LDL distribution toward lower concentrations after 4 months of tamoxifen (Figure 1).
Table 2.
Change in lipid concentrations after 4 months of tamoxifen treatment
| All subjects (N=176) |
Premenopausal (N=55) |
Postmenopausal (N=101) |
||||
|---|---|---|---|---|---|---|
| Lipid particle | Mean change (95% CI)a | P-valueb | Mean change (95% CI) | P-value | Mean change (95% CI) | P-value |
| T-Chol | −17.7 (−24.7, 10.7) | <0.0001 | −12.0 (−20, −4) | 0.004 | −24.1 (−30, −18) | <0.0001 |
| TG | 24.4 (−4.4, 44.4) | 0.006 | 41.3 (11.2, 71.5) | 0.009 | 17.4 (−4.6, 39.4) | 0.14 |
| HDL | 0.5 (−0.5, 1.5) | 0.54 | 0.7 (−2.1, 3.5) | 0.60 | −0.01 (−2.0, 2.0) | 0.99 |
| LDL | −23.5 (−13.5, 33.5) | <0.0001 | −18.7 (−26.3, 11.1) | <0.0001 | −26.5 (−31.1, −20.9) | <0.0001 |
CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; T-Chol, total cholesterol; TG, triglycerides.
Mean change in lipid particle concentration is shown in mg/dl with 95% CI given in parentheses.
P-values are calculated for difference between baseline and lipid concentrations following 4 months of tamoxifen treatment.
Figure 1.
The distribution of serum cholesterol at baseline and after 4 months of tamoxifen. The plot shows the distribution of serum LDL-cholesterol at baseline and after 4 months of tamoxifen treatment in 176 breast cancer patients. The baseline distribution is shown in gray bars, whereas the black bar represents the distribution following 4 months of tamoxifen treatment. The percentage of patients is on the y axis and LDL concentration (mg/dl) is shown on the x axis. The LDL categories were selected arbitrarily and no test statistics were conducted.
With regard to menopausal status, there was a decrease in total cholesterol of (−) 12 mg/dl (P=0.004) and (−) 24.1 mg/dl (P≤0.0001) for pre- and postmenopausal women, respectively. When analyzed by menopausal status, the triglyceride changed by (+) 41.3 mg/dl (P=0.009) and (+) 17.4 mg/dl (P=0.14) in pre- and postmenopausal women, respectively, compared with baseline. The mean triglyceride concentration in the premenopausal group had a trend toward lower values compared with the postmenopausal group (P=0.09, Table 2).
With regard to the tamoxifen metabolite concentrations, there was no association between the lipid concentrations and the metabolite concentrations in the cohort of women (n= 134) who were not on any antilipidemic drugs.
Lipid concentrations and genotypes
As described in Methods, 134 women who were not taking lipid-lowering medications and in whom menstrual status was clear were included in the genotype–phenotype analysis.
Serum lipid concentrations and ER-α PvuII genotypes
Associations between polymorphisms in ER-α and serum lipid concentrations were analyzed. For ER-α PvuII, there were no obvious or statistically significant associations between polymorphisms and any of the lipid concentrations, either at baseline or after 4 months of tamoxifen (data not shown).
Serum lipid concentrations and ER-α XbaI genotypes
At baseline, serum triglyceride and HDL-cholesterol concentrations were similar regardless of ER-α XbaI genotype, but the mean total and LDL-cholesterol concentrations differed according to ER-α XbaI polymorphism (Table 3a). The mean baseline total serum cholesterol concentrations were 223, 214, and 244 mg/dl for the AA, AG, and GG genotypes of ER-a XbaI, respectively (P=0.017 for comparison of GG vs AA/AG), and mean LDL-cholesterol levels were 135, 125, and 150 mg/dl, for the three genotypes, respectively (P=0.03). In subset analyses according to menstrual status, these differences remained statistically significant for premenopausal, but not postmenopausal women (Table 3a). Although there were trends toward an association between this ESR1 variant and effects on lipids in postmenopausal that are consistent with the work of Herrington et al.2 and others when hormone replacement therapy was studied, these did not reach statistical significance. Premenopausal women who carried the GG genotype had a mean baseline serum cholesterol concentration of 244mg/dl compared with 206mg/dl in women with the AA/AG alleles (P=0.04), and mean LDL-cholesterol levels of 148 mg/dl for GG compared with 120 and 118mg/dl for AA and AG, respectively (P=0.04, Table 3a).
Table 3.
| Table 3a Mean baseline lipid concentrations according to ER-α XbaI genotype and menopausal status | |||||
|---|---|---|---|---|---|
| ER-α XbaI genotype | Lipid particle | AA (n=50) | AG (n=59) | GG (n=19) | P-valuea |
| All women (N=128)b | Total cholesterol | 223 (215, 231)c | 214 (206, 222) | 244 (228, 260) | 0.017 |
| Triglycerides | 131 (109, 153) | 132 (110, 154) | 142 (102, 182) | 0.63 | |
| HDL-cholesterol | 61 (57, 65) | 63 (59, 67) | 65 (57, 73) | 0.49 | |
| LDL-cholesterol | 135 (128, 142) | 125 (117, 133) | 150 (136, 164) | 0.03 | |
| Premenopausal women (N=53) | Total cholesterol | 206 (184, 229) | 206 (192, 220) | 244 (212, 276) | 0.04 |
| Triglycerides | 119 (67, 171) | 113 (83, 157) | 142 (66, 218) | 0.53 | |
| HDL-cholesterol | 61 (54, 70) | 66 (59, 71) | 67 (55, 79) | 0.61 | |
| LDL-cholesterol | 120 (101, 137) | 118 (105, 129) | 148 (122, 174) | 0.04 | |
| Postmenopausal women (N=81) | Total cholesterol | 230 (218, 242) | 222 (208, 236) | 243 (221, 265) | 0.16 |
| Triglycerides | 135 (112, 160) | 152 (124, 180) | 142 (100, 184) | 0.94 | |
| HDL-cholesterol | 61 (57, 65) | 60 (54, 66) | 64 (54, 74) | 0.54 | |
| LDL-cholesterol | 141 (131, 151) | 131 (119, 143) | 151 (131, 171) | 0.20 | |
| Table 3b Association of total cholesterol concentration change at 4 months with ER-α XbaI genotypes (postmenopausal group) | ||||
|---|---|---|---|---|
| ER-α XbaI genotype |
||||
| Lipid particle | AA (n=36) | AG (n=29) | GG (n=12) | P-valuea |
| Total cholesterol | −22 (−30, −14)b | −20 (−30, −10) | −40 (−58, −22) | 0.03 |
| Triglycerides | 17 (−19, 53) | 33 (−9, 74) | 29 (−35, 93) | 0.07 |
| HDL-cholesterol | 0 (−3.4, −3.4) | 1.3 (−2.7, 5.3) | −1.2 (−7.2, 4.8) | 0.19 |
| LDL-cholesterol | −25 (−33, −17) | −23 (−33, −13) | −40 (−56, −24) | 0.08 |
CI, confidence interval; ER-α, estrogen receptor alpha; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P-values are calculated for the differences between the AA/AG and the GG alleles.
Number of ER-α genotypes available.
Mean baseline lipid particle concentration (95% CI).
CI, confidence interval; ER-α, estrogen receptor alpha; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P-values are calculated for the differences between baseline and 4 months concentration for the AA/AG vs the GG alleles.
Change in serum lipid particle concentration is shown in mg/dl with 95% CI.
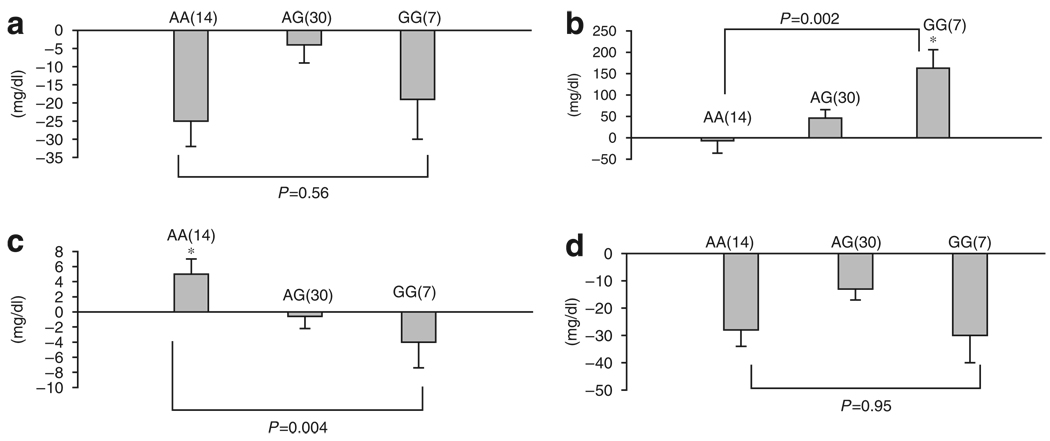
Following 4 months of tamoxifen treatment, we observed a statistically significant association between the change in total cholesterol and ER-α XbaI in postmenopausal (Table 3b) but not premenopausal women (Table 3b and Figure 2a). The changes in total cholesterol concentration were (−) 22 mg/dl, (−) 20 mg/dl, and (−) 40 mg/dl for the AA, AG, and GG genotypes in ER-α XbaI, respectively, with a statistically significant difference for the AA/AG versus GG genotypes (P=0.03, Table 3b). Although, as shown in Table 2, total serum cholesterol decreased with tamoxifen in premenopausal women (P=0.004), no obvious or statistically significant tamoxifen-induced changes in total cholesterol were observed according to ER-α XbaI genotype in this menstrual subgroup (Figure 2a).
Figure 2.
Response of serum lipid particle to tamoxifen in premenopausal women according to ER-α XbaI genotype. (a) Total cholesterol, (b) triglycerides, (c) HDL-cholesterol, and (d) LDL-cholesterol. The bars show the mean change in serum lipid concentration (mg/dl), whereas the error bars are the SE of the means. The y axis indicates the direction of the change in lipid concentration. The number of subjects in each genotype group is in parentheses against each bar. *P-value for gene–dose effect.
In contrast to the results seen with total cholesterol, no statistically significant association between ER-α XbaI and tamoxifen-induced changes in triglycerides, LDL- or HDL-cholesterol were observed in postmenopausal women (Table 3b). However, in premenopausal women, tamoxifen treatment was associated with a statistically significant correlation between ER-α XbaI polymorphism and changes in triglycerides and HDL concentrations (P=0.004, Figure 2). There was a mean change in triglyceride of (−) 7mg/dl, (+) 46mg/dl, and (+) 163 mg/dl for women who carry the AA, AG, and GG genotypes, respectively (P=0.002 for gene–dose effect, Figure 2b). Similarly, the change in serum HDL-cholesterol was observed to be associated with the ER-α XbaI polymorphism (Figure 2c). HDL concentration changed by (+) 5mg/dl, (−) 0.6mg/dl, and (−) 4 mg/dl for the AA, AG, and GG genotypes, respectively (P=0.004 for gene–dose effect).
Serum lipid levels and ER-β genotypes
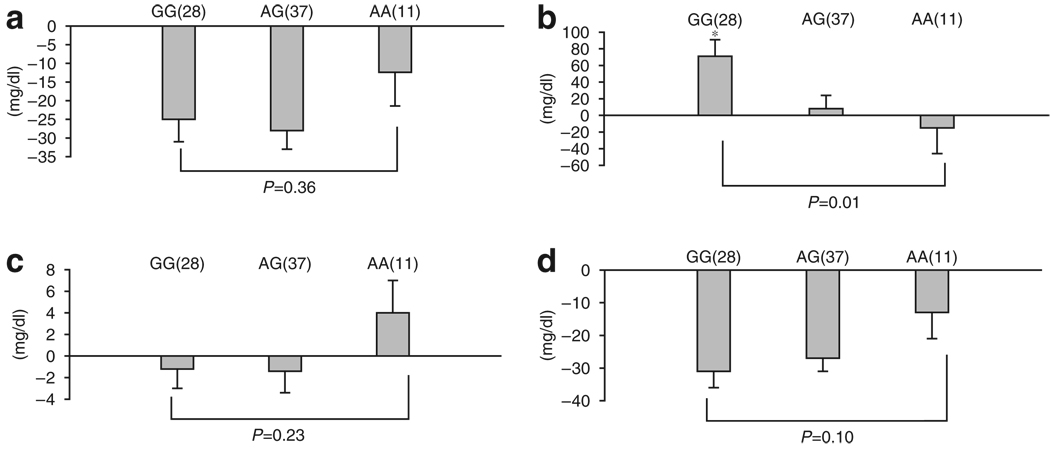
In postmenopausal women, changes in triglyceride levels, but not total, HDL-, or LDL-cholesterol levels, were associated with ER-β (ESR2-02 rs498693) genotypes (Figure 3). After 4 months of tamoxifen in these subjects, triglyceride levels were (+) 71 mg/dl, (+) 8 mg/dl, and (−) 15 mg/dl from baseline for the GG, AG, and AA genotypes in ESR2-02, respectively (P=0.01 for gene—dose effect, Figure 3b). Although postmenopausal women had a mean change in HDL-cholesterol of (−) 1.2 mg/dl, (−) 1.4 mg/dl, and (+) 4 mg/dl for the, GG, AG, and AA genotypes, respectively, the difference was not statistically significant (P=0.23 for gene–dose effect, Figure 3c). Likewise, changes in total cholesterol and LDL-cholesterol were not statistically significantly associated with ER-β genotype (Figure 3a and d).
Figure 3.
Response of lipid concentration to tamoxifen in postmenopausal women according to ER-β (ESR2-02) polymorphism. (a) Total cholesterol, (b) triglycerides, (c) HDL-cholesterol, and (d) LDL-cholesterol. The bars show the mean change in serum lipid concentration (mg/dl), whereas the error bars are the SE of the means. The y axis indicates the direction and magnitude of the change in serum lipid concentration. The number of subjects in each genotype group is in parentheses against each bar. *P-value for gene–dose effect.
In premenopausal women, the change in triglyceride was (+) 97 mg/dl, (+) 37 mg/dl, and (+) 7 mg/dl for the GG, AG, and AA genotypes in ESR2-02, respectively (P=0.04 for gene–dose effect; data not shown). No other association between ER-β polymorphisms and lipid level changes was detected in these younger subjects (data not shown).
Serum lipid concentration and CYP2D6 genotypes
We have reported previously that tamoxifen is metabolized to a highly abundant, potent anti-estrogenic metabolite, endoxifen (4-hydroxy-N-desmethyl-tamoxifen) by CYP2D6.24 Endoxifen concentrations are lowered by genetic variants in CYP2D6 and by pharmacologic inhibitors of CYP2D6.24 A recently published report suggests that breast cancer patients treated with adjuvant tamoxifen who carry the *4 genotype of CYP2D6 have poorer outcomes than those with wild-type CYP2D6.25 However, we did not observe a statistically significant association between CYP2D6 genotype and the change in lipid particle concentration in response to tamoxifen treatment (data not shown).
Tamoxifen and LDL subfractions
LDL consists of heterogenous particles and small, dense LDL particles are regarded as an independent risk factor for coronary artery disease.26 A small non-human primate study has suggested that tamoxifen may adversely affect LDL particles.27 In a preliminary subset of 50 patients, we explored the effects of tamoxifen on LDL subfraction diameter and composition, but none were observed (data not shown). The mean LDL subfraction diameter at baseline was 268.0 Å compared with 266.8 Å after 4 months of tamoxifen treatment (P=0.47). These data are consistent with published data indicating no change in small, dense LDL particle after treatment with hormone replacement therapies.
Power analysis
With 176 subjects, we have 99% power to detect the observed decreases (Table 2) in total cholesterol and LDL, and 91% power to detect the observed increase in triglycerides with 5% type I error. The power calculation was based on an empirical t-test distribution from a repeated measure two-way analysis of variance (ANOVA) model (see Methods).
When subjects who were taking lipid-altering medications or whose menopausal status was unclear were excluded, 134 subjects remained. On the basis of the observed effect size (Table 3a), we have 87% power to detect the effect of ER-α XbaI on the total cholesterol, and 71% power to detect its effect on the LDL. The type I error was justified by multiple SNPs in an empirical t-test distribution from a two-way ANOVA model (see Methods).
Using these 134 samples, based on the observed effect size (Figure 2 and Figure 3), we have 74 and 70% power to detect the effect of ER-α XbaI on the triglycerides and HDL, respectively, among premenopausal women, and we have 65% power to detect ESR2-02 effects on the triglycerides. The type I error was justified by multiple SNPs and menopause groups in an empirical t-test distribution from a two-way ANOVA model (see Methods).
According to data from the 50 subjects, we were able to analyze for more specific lipid subfractions. The maximum effect size of any possible genotype on the change in subfraction distribution after 4 months of tamoxifen treatment did not exceed 0.15, where the effect size is defined as the change in lipid concentration brought about by tamoxifen, divided by its SD. It follows that none of these changes are consistent or large enough to be clinically meaningful and that a study of more than 2,000 patients would be required to detect a change with 80% power.
DISCUSSION
In this prospective study, we observed that serum lipid concentrations in women at baseline and after taking tamoxifen for the treatment of breast cancer were associated with germline ER genotypes and presumably with the estrogen agonistic effects of tamoxifen on the ERs. Overall, the response of serum lipid concentrations to tamoxifen in our cohort was consistent with data reported previously.9,28
Tamoxifen treatment was associated with significant reductions in both total and LDL-cholesterol but, unlike exogenous estrogen, with no change in HDL-cholesterol. Conversely, triglyceride concentration increased significantly when compared with baseline. A greater reduction in serum total cholesterol concentration after 4 months of tamoxifen was observed in postmenopausal women when compared with premenopausal women, but this difference did not achieve statistical significance (P=0.06). The association observed between ER genotypes and the response of serum lipid particle concentrations was different in premenopausal women compared with the postmenopausal group. In postmenopausal women, we observed a significant association between ER-α XbaI and the change in total cholesterol in response to tamoxifen treatment. Although this association was not seen with premenopausal women, the baseline total cholesterol concentration was found to be lower in women with the AA/AG compared with GG genotypes of the ER-α XbaI polymorphism. Similarly, a previous study reported an association between ER-α XbaI and the serum cholesterol concentration in Japanese school-age children.21 These differences in the experiences of pre- and postmenopausal women with regard to baseline cholesterol and the response to tamoxifen treatment lead us to speculate that the association may be related to the higher concentrations of estrogen in premenopausal compared with postmenopausal women.
The differences seen with regard to menopausal status and ER genotypes were particularly evident in the case of serum triglycerides. We observed a group of women who had a reduction in serum triglyceride concentration in response to tamoxifen. Postmenopausal women with the AA genotype of ER-β (ESR2-02) had a mean reduction of (−) 15 mg/dl in triglyceride concentration compared with an increase of 71 mg/dl in women with the GG genotype. In premenopausal women, although there was no reduction in the mean triglyceride concentration in women with the AA genotype of ESR2-02, there was an increase of only 7 mg/dl compared with 97 mg/dl in women with the GG genotype. It is possible that endogenous circulating and tissue concentrations of estrogen in premenopausal women would dampen the agonistic effects of tamoxifen and its metabolites, whereas these effects would be more evident in the setting of lower estrogen concentrations in postmenopausal women. In both groups of women, the change in HDL-cholesterol was in a direction that was predictable based on changes seen in the serum triglycerides. These genetic associations are biologically plausible, as the changes in serum triglycerides and HDL seen with specific ER variants are in a consistent direction.
Although hormone replacement therapy and exogenous estrogen have been shown to alter the serum concentrations of HDL, this has never been reported with tamoxifen. It follows that although the mechanism of action of tamoxifen is similar and probably mediated through at least ERα, there are also subtle differences between its action and that of estrogen itself. In this study, hints of these differences are also present. These may reflect the activity of different estrogenic and anti-estrogenic metabolites. Consistent with an estrogenic effect, GG XbaI genotype was associated with a greater lowering of total cholesterol and a greater increase in triglycerides on tamoxifen therapy. In contrast, there was a trend toward greater reduction in HDL levels, presumably through another mechanism.
Although we have previously reported the effect of CYP2D6 genotype on endoxifen concentration,24 no association was detected between this genotype and lipid particle concentration. This observation suggests that endoxifen, being anti-estrogenic, may not play a role in the effects of tamoxifen or its other metabolites on the observed changes in lipid concentrations.
Previous studies of the association between genetic variation in ER-α and serum lipid concentrations have yielded inconsistent data. For instance, Herrington et al.2 have shown an association between ER-α PvuII and the response of serum HDL-cholesterol to estrogen therapy. After annual follow-up for 3.2 years, they observed that women with the CC genotype had a significantly greater increase in HDL-cholesterol in response to estrogen therapy compared with the CT/TT genotypes. However, Almeida et al.29 did not observe a similar association in postmenopausal women on estrogen therapy after 4 months of treatment. Although we detected associations between ER-α XbaI polymorphisms and tamoxifen-induced lipid changes, we observed none with ER-α PvuII.
Almeida et al.30 have recently reported an association between ER-β and the response of serum lipids in 472 women unexposed or exposed to estrogen therapy for at least 4 months. They found that LDL-cholesterol concentrations were associated with ER-β 1082 G-A (rs1256049) in pre- or postmenopausal women if they received estrogen. In our tamoxifen cohort, we found a statistically significant association between ER-β (ESR2-02) and the triglyceride change after 4 months of treatment in pre- and postmenopausal women.
The biochemical mechanisms underlying these associations are of interest, as XbaI is an intronic SNP in ER-α and ESR2-02 is located in the 3′ untranslated region of ER-β. It is possible, as others have suggested, that SNPs that do not cause amino-acid change may alter gene expression, affect the folding of mRNA, or be in linkage disequilibrium with a functional site.2,30 Moreover, the role of polymorphisms in genes that code for the coregulator proteins of the ERs and that may alter their binding to ligands such as tamoxifen is not clear. Other investigators have shown that tamoxifen interferes with synthesis of cholesterol in hepatic cells by inhibition of the conversion of precursor lipids31 and that tamoxifen is a potent inhibitor of sterol Δ8-isomerase, a cholesterol synthetic enzyme.32 Although our data suggest that the mechanism of tamoxifen’s lipid-lowering effect may be mediated by ERs, it is not known if these receptors influence Δ-isomerase activity, or whether other downstream signaling mechanisms are involved. These remain important subjects for future research.
The clinical significance of our findings may be substantial, as cardiovascular disease is the leading cause of death in both men and women, and many women with breast cancer now live more than 10 years after treatment. Recent data suggest that lower lipid concentrations are associated with better coronary heart outcomes compared with previously accepted practice standards.33 However, two recently reported prospective studies, the Women’s Health Initiative1 and the Heart and Estrogen/Progesterone Replacement Study,34 have provided data that challenge the widely held belief in a cardioprotective role for estrogens. Contrary to results regarding estrogen administration, most, but not all studies of tamoxifen, have shown a positive cardioprotective effect, and none have shown a deleterious effect.4,35,36 It is of note that a large study on the effects of another selective ER modulator, raloxifene,37 on cardiovascular outcomes showed no effect, further illustrating the complexity of the interaction between ER-active agents and overall cardiovascular effects. This complexity emphasizes the value of future research in this area as do a number of limitations of this study. These include the absence of data on hormone concentrations in these women that would allow more careful mechanistic determinations of relationships between estrogen, androgen, and progesterone concentration and effect, and the absence of data on breast cancer or cardiovascular outcomes that are not possible in a study of 1-year duration.
These data may also be relevant in the choice between tamoxifen and aromatase inhibitors in postmenopausal women being treated for breast cancer. Recently, several studies have suggested a modest advantage in reduction of breast cancer-related events for aromatase inhibitors versus tamoxifen.38–42 Of concern, a small excess of coronary artery events has been a worrisome, although not universal, finding in the adjuvant trials of aromatase inhibitors versus tamoxifen. As these agents are purely anti-estrogenic and are associated with less beneficial lipid profiles compared with tamoxifen,43 it might be advantageous to identify individuals, based on ER genotypes, who are more likely to experience overall global positive effects with one class of drugs versus the other.
We conclude that ER genotypes are associated with changes in serum lipid concentrations in breast cancer patients receiving adjuvant tamoxifen treatment. These changes were importantly affected by menopausal status. Functional studies of polymorphism in the ERs are needed to elucidate the biologic mechanisms that these data point to. These findings require replication in other cohorts treated with tamoxifen and, if validated, the genetic testing of ER variants findings may play a role in the prediction of women who will experience optimal changes in serum cholesterol and its subfractions during tamoxifen therapy.
METHODS
Subjects and study design
Subjects in this study are part of a prospective, multi-institutional observational open-label clinical registry for which women 18 years and older who were initiating treatment with tamoxifen were eligible. The study design has already been described in detail and is listed on http://www.clinicaltrials.gov (the trial identifier is NCT00228930).24 Participants were enrolled if they had newly diagnosed, non-metastatic breast cancer or if they were at high risk for developing breast cancer and about to start tamoxifen. Patients were recruited to the cohort from the breast cancer clinics at the University of Michigan Comprehensive Cancer Center and the Lombardi Comprehensive Cancer Center at Georgetown University. Patients enrolled at the Indiana University School of Medicine site did not have serum samples collected for conventional analysis of cholesterol, LDL, HDL, and triglycerides, but were part of a separate study designed to examine the effects of tamoxifen on lipid subfractions.
All enrollees had to have completed all appropriate primary surgery, radiation, and adjuvant chemotherapy. Pretreatment medical histories, medication lists, physical examinations, and laboratory samples were obtained for each subject at baseline and 4, 8, and 12 months after the start of tamoxifen treatment. Patients were excluded from enrollment for the following reasons: if they had been given tamoxifen concurrently with adjuvant chemotherapy or if they were taking other adjuvant endocrine therapies; if they were pregnant or lactating; or if they were on chronic corticosteroid or megestrol acetate therapies. The Institutional Review Board at each study site approved the study, and all subjects gave their written informed consent.
This study describes the results of the substudy of associations between candidate genetic polymorphisms in ER-α, ER-β, and CYP2D6 and tamoxifen-induced lipid changes. Overall, 290 patients were enrolled into the registry from April 2001 to January 2006. Of these, 176 patients had completed 4 months of tamoxifen and had baseline and serial lipid levels that were appropriately collected and processed at the University of Michigan and Georgetown University. Patients enrolled into the registry from Indiana University were not included in these analyses of lipid concentrations. Therefore, this study includes 176 subjects who were enrolled at the University of Michigan and Georgetown University, and who had completed sufficient follow-up with appropriate specimen collection to be evaluable.
Participants were classified as either premenopausal, perimenopausal, or postmenopausal based on history. Women were classified as premenopausal if they were having regular menstrual periods. They were classified as postmenopausal if they had been amenorrheic for 12 consecutive months, had undergone bilateral oophorectomy, or had undergone hysterectomy without bilateral oophorectomy and were older than 60 years. Perimenopausal women were those who did not fit either category.
Patients who were recorded as taking medications known to alter lipid profiles, other than tamoxifen, were accrued into the primary cohort of 176 subjects for the lipid substudy, provided that there was no change in the dose of the lipid-altering medication during the study. The 176 subjects were included in the analysis of baseline lipid concentration and change from baseline to 4 months independent of genotype analysis. To minimize the confounding effects of lipid-altering medications on the analyses of genotype association with lipid level changes, women who changed therapy with such medications were excluded from the study and women taking stable doses of antilipidemic drugs (22 subjects) were excluded from our assessment of the effects of tamoxifen on serum lipid concentrations overall, and from the specific analysis of genetic associations as a result. In addition, women classified as perimenopausal (20 subjects), for whom menstrual status and ovarian function are uncertain, were excluded from the genotype–phenotype analyses, reducing the number of subjects for the genotype association analyses to 134 patients.
Sample collection
Ten milliliters of blood was collected at baseline in heparinized vacutainer tubes (Becton-Dickinson, Franklin Lakes, NJ) and separated for genotyping and collection of plasma. At each follow-up visit, blood (5 ml) was collected in heparinized containers and plasma was separated within 60 min by centrifugation at 2,060 g. Venous blood samples for lipid analysis were collected after an overnight fast immediately before and after 4 months of tamoxifen therapy. All blood samples (whole blood and plasma) were shipped in cryogenic vials (Corning, Cambridge, MA) to the Division of Clinical Pharmacology at Indiana University and were stored at −80°C until analyses were performed.
Genotyping
Candidate genes
On the basis of previous studies by our group and others, we chose to conduct analyses of serum lipid concentrations at baseline and after 4 months of tamoxifen and to correlate these concentrations with genetic variants in three candidate genes: ERα (ESR1), ERβ (ESR2), and CYP2D6. We specifically looked at the following SNPs: ESR1_PvuII, ESR1_XbaI, ESR2_01, ESR2_02, and CYP2D6 *1, *4, and *6. Genotyping data can be found on the Pharmacogenetics and Pharmacogenomics Knowledge Base website (http://www.pharmgkb.org) with accession IDs CYP2D6 (PS204849, PS204850, PS204858, PS204859, PS204873, PS204874, PS204875, PS204901, PS204991, and PS204996), ESR1 (PS204992 and PS204997), and ESR2 (PS205000 and PS203537, PS203538 and PS204999).
Sequencing
>Genomic DNA was extracted from whole blood samples using the QIAamp midi kit (Qiagen, Valencia, CA). ER-α genotype was determined using the method described by Herrington et al.2 with minor modifications. Genomic DNA was amplified using the primers CTGCCACCCTATCTGTATCTTTTCCTATTCTCC (forward) and TCTTTCTCTGCC ACCCTGGCGTCGATTATCTGA (reverse) at final concentrations of 200 nm. Genotyping for two ER-β SNPs (ESR2-01: rs#1256049; ESR2-02: rs#4986938) was performed by TaqMan assays as described previously by the National Cancer Institute’s Cancer Genome Anatomy Project (CGAP) (http://cgap.nci.nih.gov/). The ESR2-01 primers were TTTGTGGAGCTCAGCCTGTTC (forward) and CATCATTAACACCTCCATCCAACA (reverse), and the probes were FAM-CAAGTGCGGCTCT-MGB and VIC-ACCAAGTACGGCTCT-MGB. The ESR2-02 primers were CCTGGCCCTGAGGTGAACT (forward) and GCCCAGGCTCCTGACACA (reverse), and the probes were FAM-AGGTCACAGGCTGAA-MGB and VIC-TCACAAGCTGAAGCG-MGB. Screening for CYP2D6 alleles *1, *4, and *6 was performed by endonuclease-specific mutation analysis, using a 4.7-kb fragment containing all nine exons that was amplified from the genomic DNA using an expanded long-template polymerase chain reaction, and this was used as a template to determine specific genetic variants, as described previously.44,45 In addition, we used the AmpliChip CYP450 Test to test for 33 CYP2D6 alleles (i.e., *1 to *10AB, *11, *14A, *14B, *15, *17, *19, *20, *25, *26, *29 to *31, *35, *36, *40, *41, *1xN, *2xN, *4xN, *10xN, *17xN, *35xN, and *41xN). The AmpliChip CYP450 Test microarray contains more than 15,000 different oligonucleotide probes, which can be used to analyze both sense and antisense strands of an amplified target DNA sample.
Measurement of serum lipids
Triglycerides, total cholesterol, and HDL-cholesterol were analyzed at the clinical laboratories at Georgetown University Medical Center and the University of Michigan Health System using standard methods. All test centers met the Clinical Laboratory Improvement Amendments (CLIA) standards. LDL-cholesterol was calculated using the Friedewald equation.46,47 Lipid data set can be found on http://www.pharmgk-b.org with the following accession ID: PS206340. Lipid subfraction measurements were performed at Children’s Hospital Oakland Research Institute (Oakland, CA). Levels of LDL, intermediate-density lipoprotein, and lipoprotein(a) peak particle size of the whole plasma were determined using non-denaturing 2–14% polyacrylamide gradient gel electrophoresis, as described previously.48
Statistical analysis
In univariate analysis, means and SD were calculated for all continuous variables, and frequencies for categorical variables are reported. The Hardy–Weinberg equilibrium tests were reported for each genotype. A repeated measure two-way ANOVA model (time/menopause) with interaction was implemented to compare each baseline lipid to its month 4 level among different menopause groups. Its analyses are reported in Table 2. A two-way ANOVA (menopause/genotype) model with interaction was implemented to analyze either a baseline lipid or a lipid change from baseline to the fourth month. These analyses are reported in Table 3a and b and Figure 2 and Figure 3. Three genetic associations were tested: dominant, recessive, and gene–dose effect. The genotypic association analyses were carried out on the 134 subjects who were neither perimenopausal nor were taking concomitant lipid-lowering medications. A bootstrap resampling algorithm was employed to adjust for the assumptions of non-normality and unequal variance, and this takes into account the correlations between the predictors (genotypic and clinical).49 The family-wise type I error was controlled for each phenotype (lipid), by generating empirical distributions of the t-statistic using a bootstrap approach (i.e., by randomly permuting the phenotype). It considered multiple tests among SNPs and menopause strata. P-values of less than 5% were considered to be statistically significant. The bootstrap resampling algorithm was implemented using SAS (version 3.2.2, SAS Institute Inc., 100 SAS Campus Dr, Cary, NC 27513), and the P-values were calculated from 50,000 sets of bootstrap samples.
ACKNOWLEDGMENTS
The description of the study design can be found on http://www.clinicaltrials.gov (NCT00228930). The study was supported in part by a Pharmacogenetics Research Network Grant no. U-01 GM61373 (DAF), which supports the Consortium on Breast Cancer Pharmacogenomics (COBRA), a Clinical Pharmacology training Grant 5T32-GM08425 (NN and MR) from the National Institute of General Medical Sciences, National Institutes of Health (Bethesda, MD), Damon Runyon–Lilly Clinical Investigator Award CI-3 from the Damon Runyon Cancer Research Foundation (VS), Indiana University GCRC Grant M01RR00750 from the National Institutes of Health (Bethesda, MD), University of Michigan GCRC Grant M01-RR00042 from the National Institutes of Health (Bethesda, MD), and by the Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale (DFH).
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Anderson GL, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 2.Herrington DM, et al. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N. Engl. J. Med. 2002;346:967–974. doi: 10.1056/NEJMoa012952. [DOI] [PubMed] [Google Scholar]
- 3.Furr BJ, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol. Ther. 1984;25:127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- 4.Costantino JP, Kuller LH, Ives DG, Fisher B, Dignam J. Coronary heart disease mortality and adjuvant tamoxifen therapy. J. Natl. Cancer Inst. 1997;89:776–782. doi: 10.1093/jnci/89.11.776. [DOI] [PubMed] [Google Scholar]
- 5.Center for Drug Evaluation and Research. < http://www.FDA.GOV/CDER/NEWS/TAMOXIFEN/DEFAULT.htm>.
- 6.Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN. Chemoprevention of breast cancer: a summary of the evidence for the US Preventive Services Task Force. Ann. Intern. Med. 2002;137:59–69. doi: 10.7326/0003-4819-137-1-200207020-00017. [DOI] [PubMed] [Google Scholar]
- 7.Diel P. Tissue-specific estrogenic response and molecular mechanisms. Toxicol. Lett. 2002;127:217–224. doi: 10.1016/s0378-4274(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 8.Katzenellenbogen BS, Katzenellenbogen JA. Biomedicine. Defining the ‘S’ in SERMs. Science. 2002;295:2380–2381. doi: 10.1126/science.1070442. [DOI] [PubMed] [Google Scholar]
- 9.Love RR, Wiebe DA, Feyzi JM, Newcomb PA, Chappell RJ. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J. Natl. Cancer Inst. 1994;86:1534–1539. doi: 10.1093/jnci/86.20.1534. [DOI] [PubMed] [Google Scholar]
- 10.Dziewulska-Bokiniec A, Wojtacki J, Skokowski J, Kortas B. The effect of tamoxifen treatment on serum cholesterol fractions in breast cancer women. Neoplasma. 1994;41:13–16. [PubMed] [Google Scholar]
- 11.Thangaraju M, Kumar K, Gandhirajan R, Sachdanandam P. Effect of tamoxifen on plasma lipids and lipoproteins in postmenopausal women with breast cancer. Cancer. 1994;73:659–663. doi: 10.1002/1097-0142(19940201)73:3<659::aid-cncr2820730325>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Grey AB, Stapleton JP, Evans MC, Reid IR. The effect of the anti-estrogen tamoxifen on cardiovascular risk factors in normal postmenopausal women. J. Clin. Endocrinol. Metab. 1995;80:3191–3195. doi: 10.1210/jcem.80.11.7593425. [DOI] [PubMed] [Google Scholar]
- 13.Vrbanec D, Reiner Z, Belev B, Plestina S. Changes in serum lipid and lipoprotein levels in postmenopausal patients with node-positive breast cancer treated with tamoxifen. Tumori. 1998;84:687–690. doi: 10.1177/030089169808400615. [DOI] [PubMed] [Google Scholar]
- 14.Sharma D, Sharma U, Bhatnagar VB, Singh VS. A study of the effect of tamoxifen on serum lipoprotein profiles in premenopausal and postmenopausal women with breast carcinoma and associated risk of cardiovascular disease. Indian J. Med. Sci. 2001;55:359–365. [PubMed] [Google Scholar]
- 15.Osborne CK. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 16.Massart F. Human races and pharmacogenomics of effective bone treatments. Gynecol. Endocrinol. 2005;20:36–44. doi: 10.1080/09513590400019437. [DOI] [PubMed] [Google Scholar]
- 17.Green S, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 18.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuiper GG, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 20.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi T, Hashimoto N, Kawasaki T, Uchiyama M. Association of serum low-density lipoprotein metabolism with oestrogen receptor gene polymorphisms in healthy children. Acta Paediatr. 2000;89:42–45. doi: 10.1080/080352500750029040. [DOI] [PubMed] [Google Scholar]
- 22.Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother. Pharmacol. 2005;55:471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 23.Johnson MD, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res. Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 25.Goetz MP, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J. Clin. Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 26.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109 suppl. 1:III2–III7. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- 27.Manning JM, et al. Effects of hormone replacement modalities on low density lipoprotein composition and distribution in ovariectomized cynomolgus monkeys. Atherosclerosis. 1996;121:217–229. doi: 10.1016/0021-9150(95)05723-4. [DOI] [PubMed] [Google Scholar]
- 28.Ilanchezhian S, Thangaraju M, Sachdanandam P. Plasma lipids and lipoprotein alterations in tamoxifen-treated breast cancer women in relation to the menopausal status. Cancer Biochem. Biophys. 1995;15:83–90. [PubMed] [Google Scholar]
- 29.Almeida S, Fiegenbaum M, de Andrade FM, Osorio-Wender MC, Hutz MH. ESR1 and APOE gene polymorphisms, serum lipids, and hormonal replacement therapy. Maturitas. 2006;54:119–126. doi: 10.1016/j.maturitas.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Almeida S, Franken N, Zandona MR, Osorio-Wender MC, Hutz MH. Estrogen receptor 2 and progesterone receptor gene polymorphisms and lipid levels in women with different hormonal status. Pharmacogenomics J. 2005;5:30–34. doi: 10.1038/sj.tpj.6500272. [DOI] [PubMed] [Google Scholar]
- 31.Holleran AL, Lindenthal B, Aldaghlas TA, Kelleher JK. Effect of tamoxifen on cholesterol synthesis in HepG2 cells and cultured rat hepatocytes. Metabolism. 1998;47:1504–1513. doi: 10.1016/s0026-0495(98)90078-6. [DOI] [PubMed] [Google Scholar]
- 32.Gylling H, Pyrhonen S, Mantyla E, Maenpaa H, Kangas L, Miettinen TA. Tamoxifen and toremifene lower serum cholesterol by inhibition of delta 8-cholesterol conversion to lathosterol in women with breast cancer. J. Clin. Oncol. 1995;13:2900–2905. doi: 10.1200/JCO.1995.13.12.2900. [DOI] [PubMed] [Google Scholar]
- 33.Cannon CP, et al. Intensive vs moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 34.Grady D, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 35.Bradbury BD, Lash TL, Kaye JA, Jick SS. Tamoxifen-treated breast carcinoma patients and the risk of acute myocardial infarction and newly-diagnosed angina. Cancer. 2005;103:1114–1121. doi: 10.1002/cncr.20900. [DOI] [PubMed] [Google Scholar]
- 36.Fisher B, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 37.Barrett-Connor E, et al. Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N. Engl. J. Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 38.Thurlimann B, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N. Engl. J. Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 39.Winer EP, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J. Clin. Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 40.Howell A, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 41.Goss PE, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J. Natl. Cancer Inst. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 42.Coombes RC, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N. Engl. J. Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 43.Bundred NJ. The effects of aromatase inhibitors on lipids and thrombosis. Br. J. Cancer. 2005;93 suppl. 1:S23–S27. doi: 10.1038/sj.bjc.6602692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desta Z, Kerbusch T, Flockhart DA. Effect of clarithromycin on the pharmacokinetics and pharmacodynamics of pimozide in healthy poor and extensive metabolizers of cytochrome P450 2D6 (CYP2D6) Clin. Pharmacol. Ther. 1999;65:10–20. doi: 10.1016/S0009-9236(99)70117-7. [DOI] [PubMed] [Google Scholar]
- 45.Hersberger M, Marti-Jaun J, Rentsch K, Hanseler E. Rapid detection of the CYP2D6*3, CYP2D6*4, and CYP2D6*6 alleles by tetra-primer PCR and of the CYP2D6*5 allele by multiplex long PCR. Clin. Chem. 2000;46(Part 1):1072–1077. [PubMed] [Google Scholar]
- 46.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 47.Warnick GR, Knopp RH, Fitzpatrick V, Branson L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990;36:15–19. [PubMed] [Google Scholar]
- 48.Krauss RM. Detection and quantification of LDL subfractions. Curr. Opin. Lipdol. 1992;3:377–383. [Google Scholar]
- 49.Efron B, Tibshirani JR. An Introduction to the Bootstrap. New York, NY: Chapman & Hall/CRC; 1993. [Google Scholar]