Abstract
Stress-activated MAP kinases (MAPKs), comprised of JNK and p38, play prominent roles in the innate and adaptive immune systems. Activation of MAPKs is mediated by a three-tiered kinase module comprised of MAPK kinase kinases (MAP3Ks), MAPK kinases (MAP2Ks) and MAPKs through sequential protein phosphorylation. Activated MAPKs, in turn, phosphorylate transcription factors and other targets to regulate gene transcription and immune responses. Recent studies have provided new insight into the upstream and downstream components of the MAPK pathway that facilitate the activation and propagation of MAPK signaling in immune responses. Moreover, MAPK activity is negatively regulated by MAPK phosphatases (MKPs), a group of dual-specificity phosphatases that dephosphorylate and inactivate the MAPKs. Here we discuss the recent advances in our understanding of these regulatory processes in MAPK signaling with a focus on their impacts on immune function.
Keywords: Kinase, phosphatase, signaling, receptor, immunity
1. Introduction
The primary function of the immune system is to protect the organism from invading pathogens. To perform this function, the mammalian immune system has developed two components: innate immunity and adaptive immunity. Both arms of immunity recognize invading pathogens as non-self, although they utilize different receptor systems. In adaptive immunity, T and B lymphocytes recognize non-self through antigen-specific receptors, such as T cell receptors (TCRs) and immunoglobulins. These receptors are generated by gene rearrangement, which allows the recognition of a vast number of different antigens. In contrast, innate immune cells, including macrophages and dendritic cells (DCs), utilize an evolutionarily conserved receptor system of pattern recognition. Such pattern-recognition receptors (PRRs), which include Toll-like receptors (TLRs), NOD-like receptors (NLRs) proteins and RIG-I like receptors (RLRs), are germline encoded and do not undergo gene rearrangement [1]. In addition to the antigen receptors and PRRs that are essential to prime adaptive and innate immunity, immune cells also receive a plethora of signals delivered through other receptor systems. For example, for a productive adaptive response to occur, T cells need to be activated by TCRs, co-stimulatory signals and immunoregulatory cytokines such as IL-12 and IL-4, which are known as signals 1, 2, and 3, respectively [2]. Likewise, innate immunity is under the control of cytokine receptors, ITAM-coupled receptors and tyrosine kinase receptors that together with PRRs, dictate the ultimate cellular responses to infectious agents [3–5].
While pathogen recognition begins at the receptor level, it is the signaling components downstream of each receptor and the way they interact with each other that ultimately determine the specific transcriptional response and immunological outcome. Among the central pathways activated in immune cells are the MAP kinases (MAPKs), a family of serine/threonine kinases. The four well characterized subfamilies of MAPKs include: the extracellular signal-regulated kinases (ERK1/2), c-Jun NH2-terminal kinases (JNK-1/2/3), p38 (p38α/β/γ/δ) and ERK5. Among them, JNK and p38 can be activated by cellular stresses and are collectively known as stress-activated MAPKs. MAPKs contain the signature sequence –TXY–, where T and Y are threonine and tyrosine, and X is glutamate, proline or glycine, in ERK, JNK or p38, respectively [6]. Phosphorylation of both the threonine and tyrosine within this signature sequence is required for MAPK activation. Phosphorylation of MAPKs is achieved via a signaling cascade involving a MAPK kinase (MAPKK or MAP2K) that is responsible for phosphorylation of the appropriate MAPK, and a MAPK kinase kinase (MAPKKK or MAP3K) that phosphorylates and activates MAPKK (Fig. 1) [6]. There are a total of approximately 20 MAP3Ks in mammalian cells, with each of them receiving and integrating specific upstream signals [7, 8]. The main MAP2Ks mediating JNK activation are MKK4 and MKK7, whereas p38 can be activated by MKK3 and MKK6, as well as MAP2K-independent pathways such as TAB1 and ZAP70 [9]. Upon activation, MAPKs regulate key cellular events in the cytoplasm by phosphorylation of membrane-associated and cytoplasmic proteins including other kinases and cytoskeletal elements. Activated MAPKs also translocate to the nucleus to phosphorylate transcription factors such as c-Jun, c-Fos, Elk-1 and c-Myc, which coordinate the expression of downstream target genes [6].
Figure 1. Organization of the MAPK cascade in the immune system.
Stimulation of receptors in the immune system results in the activation of the three-tiered kinase module comprised of MAP3K, MAP2K and MAPK through sequential protein phosphorylation. Activated MAPKs are dephosphorylated by MKPs. Certain MAP3Ks such as TAK1 and MEKK3 also contribute to the activation of the IKK/NF-κB pathway. Although not depicted here, p38 can be activated by MAP2K-independent mechanisms.
Negative regulation of MAPK activities is effected primarily by MAPK phosphatases (MKPs), also known as dual specificity phosphatases (DUSPs), a group of approximately 10 phosphatases that dephosphorylate the MAPKs on both the threonine and tyrosine residues in the signature sequence –TXY– [10–12]. These MKPs have unique and overlapping substrate specificity toward MAPKs, and localize to either cytoplasm or nucleus or both [10, 12]. Some of the MKPs are ubiquitously expressed whereas others are more restricted. Despite the high level of redundancy, recent work has revealed that the immunoregulatory functions of MKPs are rather unique.
One of the earliest work highlighting a role of the stress-activated MAPK pathways in immune responses was a report by Su et al that described JNK-mediated integration of TCR and co-stimulation signals [13]. This was followed by a number of studies that employed mouse genetic approaches to address the immunoregulatory functions of MAPK signaling by deleting various molecules at the MAPK and MAP2K levels, including JNK1, JNK2, MKK3, MKK4, MKK6, MKK7, and individual members of the p38 family. These studies have led to important insight into the molecular mechanisms of immune regulation, and readers are encouraged to read excellent reviews on how MAPKs contribute to the function of the immune system [6, 14, 15].
Despite these studies, however, key questions remained: How do immune cells recognize a plethora of stimuli to properly activate the MAPK modules? Once activated, how is MAPK signaling propagated to effect gene regulation and immune reaction, and how is MAPK activation terminated to avoid exuberant immune responses? Recent studies have provided important answers to these questions, and our review will focus on the stimuli and mechanisms that regulate MAPKs in immune cells. First, we will discuss how MAP3Ks integrate upstream signals from multiple receptors in the innate and adaptive immune systems. Second, we will discuss how downstream signaling molecules bridge MAPK activation to immune responses. Third, we will discuss the function and regulation of MKPs in the feedback modulation of MAPK activities. Given the promises of the drug inhibitors targeting the MAPK pathway in inflammatory diseases, understanding the signaling mechanisms in MAPK regulation is not only insightful from a scientific point of view but also has the potential to be translated into new therapeutic strategies. However, for mechanistic studies, pharmacological inhibitors are well known to cause non-specific or non-physiological effects [16, 17]. Therefore, we will mainly discuss the conclusions obtained from mouse genetic systems, with a focus on studies published during the last five years.
2. MAP3Ks integrate various upstream signals to induce MAPK activation
A total of 21 kinases have been shown to function as MAP3K, generally having the capacity to activate the MAP kinase pathways after overexpression in cell lines [7, 8]. Notably, overexpression of MAP3Ks can sometimes result in their artificial activation and provoke physiologically irrelevant interactions. The presence of a large number of MAP3Ks suggests that multiple MAP3Ks may be required for any stimulus. Indeed, analyses of MAP3K-deficient mouse embryonic fibroblasts (MEFs) showed that TNF-α induced JNK activation was affected in those lacking TPL-2, ASK1, TAK1, MLK3, MEKK1 or MEKK3 [7]. Even in Drosophila cells whose genetic redundancy is considerably less pronounced than mammalian cells, maximal p38 activation in response to NaCl required four MAP3Ks: MEKK1, TAK1, ASK1, and MLK [18]. It is unclear why so many MAPKs are involved in these cellular responses.
In contrast, for certain immune stimuli, only one or very few MAP3Ks are required to mediate MAPK activation. Moreover, one MAP3K can integrate signals from multiple immunoreceptors. Particularly striking is the involvement of transforming growth factor-β-activating kinase 1 (TAK1) in transducing signals from diverse receptor systems in both the innate and adaptive immunity, and its ability to activate the downstream JNK and p38 as well as the NF-κB pathways [19]. Several other MAP3Ks have also been endowed with physiological functions in integrating upstream receptor signals in the immune system (Fig. 2). Here we discuss our current understanding on the function and regulation of MAP3Ks in integrating immune signals.
Figure 2. Integration of multiple receptor signals by the MAP3Ks in innate and adaptive immune systems.
Different MAP3Ks are involved in the activation of innate immune cells (A), T cells (B) and B cells (C).
2.1 TAK1 in multiple innate and adaptive immune receptor signaling
TAK1 (MAP3K7) is arguably the most widely utilized MAP3K in the immune system. TAK1 was initially identified as a kinase mediating TGF-β-induced transcriptional regulation [20], and subsequent experiments showed that it is also involved in the IL-1 and TNF-α signaling pathway [21, 22]. The essential role of TAK1 in innate immune responses was first demonstrated in Drosophila [23, 24]. The TAK1 null mutants exhibited defective NF-κB activation and failed to produce antibacterial peptides, resulting in a high susceptibility to Gram-negative bacterial infection [23]. Further, TAK1 is required for the activation of I-κB kinase (IKK) and JNK in Drosophila cells [24]. Although germline deletion of TAK1 in mice resulted in defective vascular development and early embryonic lethality [25], the use of TAK1−/− MEFs provided strong genetic evidence for a role of TAK1 in the mammalian innate immune system. TAK1 was found to transduce signals from a variety of receptors, including TLRs, TNFR and IL-1R, leading to the activation of JNK, p38 and NF-κB (Fig. 2A) [26, 27]. Notably, although JNK activation by proinflammatory cytokines was completely abolished in TAK1-deficient cells, the IKK activity was mostly but not completely blocked, suggesting that a TAK1-independent mechanism may contribute to the residual activation of IKK. TAK1 also mediates intracellular sensor NOD-like receptor NOD1/NOD2 pathways [28, 29], but TLR8-induced activation of NF-κB and JNK is independent of TAK1 [30]. Taken together, TAK1 mediates an evolutionarily conserved pathway for the activation of MAPK and NF-κB pathways in multiple innate immune receptor systems (Fig. 2A).
The involvement of TAK1 in antigen receptor signaling was initially identified based on biochemical studies and RNAi knockdown of TAK1 in Jurkat cells [31]. Subsequently, mice with T cell specific deletion of TAK1 were independently generated and analyzed by three groups [32–34]. Using CD4-Cre mice to ablate TAK1 function during late thymocyte development stage, we found that numbers of mature thymocytes and peripheral T cells were drastically reduced in TAK1 deficient mice, suggesting that TAK1 is essential for thymic development and survival. Using LCK-Cre mice to delete TAK1 at an earlier developmental stage, Sato et al and Liu et al reached similar conclusions [33, 34]. Notably, thymic Foxp3+ regulatory T cells were preferentially ablated in the absence of TAK1, suggesting a selective requirement for TAK1 in the development of this subset of cells [32, 33]. Once T cells are matured in the thymus, they are released into the peripheral lymphoid organs including the spleen and lymph nodes where they circulate as naïve T cells until their encounter with antigens, which induce the functional activation of T cells and their differentiation into effector cells. In naïve T cells, TAK1 mediates two key signals for their survival and activation [32]. First, IL-7 is a major cytokine in maintaining survival of naïve T cells; in the absence of TAK1, IL-7 induced T cell survival was ablated. Also, TAK1 was required in mediating TCR-induced cellular responses including cell cycle progression and production of IL-2. Both basal and TCR-induced activation of NF-κB and JNK were diminished in TAK1-deficient naïve T cells. These results indicate that TAK1 integrates IL-7 and TCR signaling to induce downstream NF-κB and JNK activation in naïve T cells. TAK1 appears to have a distinct function in previously activated effector T cells in that it mediates TCR-induced JNK but not NF-κB activation. In addition, TAK1 deficiency was incompatible with long-term survival of effector T cells or their proliferative response to γc-containing cytokine stimulation [32]. Collectively, these results indicate that TAK1 can integrate TCR and cytokine signals in both naïve and effector T cells, with activation of distinct downstream signaling pathways and cellular responses (Fig. 2B) [32].
In order to analyze a role for TAK1 in B cell biology, Sato et al used CD19-Cre mice to delete TAK1 selectively in B cells. Deficiency of TAK1 impaired the development of a subset of B cells, known as B1 B cells, but not splenic follicular and marginal zone B cells. Inactivation of TAK1 decreased the proliferative response of B cells to TLR, BCR and CD40 stimulation, indicating that TAK1 is involved in the signaling pathways used by BCR, TLR and CD40. Defective humoral immune response was observed in TAK1 deficient mice, indicating the functional importance of TAK1 in B cell response in vivo. Notably, while JNK activation was affected in TAK1-dificient B cells in response to TLR or BCR stimulation, defective NF-κB activation was observed only in TAK1-dificient B cells following TLR but not BCR stimulation. This conclusion is in disagreement with the requirement of TAK1 for BCR-induced NF-κB activation in DT40 B cell line [35]. One explanation for the discrepancy was the presence of a truncated TAK1 protein in the studies of Sato et al [27]. Indeed, using independently generated B cell-specific TAK1-deficient mice, Schuman et al recently found that TAK1 was critical for BCR-mediated IKK and NF-κB activation. TAK1 deficiency also resulted in an increase of BCR-induced apoptosis. In addition, lack of TAK1 led to a marked reduction of multiple B cell types including follicular, marginal and B1 B cell numbers. Thus, TAK1 plays a comparable role in mediating antigen receptor-induced NF-κB and MAPK activation between T and B cells (Fig. 2C) [36].
As a member of the MAP3K family, TAK1 is unique in that its activation requires its binding proteins TAB1, TAB2 and TAB3 [19, 37]. TAB2 and TAB3 contain ubiquitin-binding domains that bind lysine 63 (K63) polyubiquitin chains assembled on various signaling proteins [38]. Mutations of the ubiquitin-binding domains abolished the ability of TAB2 and TAB3 to bind polyubiquitin chains, and their ability to activate TAK1, indicating the importance of the ubiquitin pathway in activating TAK1 [39]. TNF-α stimulation triggers the assembly of the K63 polyubiquitin chains on the protein kinase RIP1, which serve as a scaffold to recruit the TAK1 complex through TAB2 and TAB3, as well as the IKK complex through the regulatory submit NEMO (IKKγ), allowing TAK1 to phosphorylate and activate IKK [38, 40]. Similarly, K63-linked polyubiquitination of IRAK-1 and Bcl10 are required to recruit NEMO and the IKK complex in the IL-1/TLR and TCR pathways, respectively [41, 42]. In addition, assembly of K63-linked polyubiquitination chains on the E3 ligases such as TRAF6 themselves has been implicated in the activation of TAK1 and IKK [37], although TCR-induced NF-κB activation is not defective in TRAF6-deficient T cells [43]. Whereas the molecular nature of how K63-linked ubiquitination affects the phosphorylation cascade remains to be identified, it is clear that the this process has an important role in the activation of TAK1 and the downstream IKK/NF-κB pathway [37, 44]. In contrast, how MAP2Ks are activated by TAK1 is less understand, although Bcl10 has been shown to serve as a scaffold protein to assemble a TAK1-MKK7-JNK2 complex to facilitate JNK2 activation in T cells [45].
2.2 MEKK1 in Th2 cell differentiation and B cell function
MEKK1 (MAP3K1) was among the first MAP3Ks identified. MEKK1 has been reported to activate ERK, JNK, p38 as well as NF-κB in vitro [46–48], although more recent studies suggest that the function of MEKK1 in the immune system may be restricted to the stress-activated MAPKs. MEKK1−/− T cells showed reduced JNK activity, and upon TCR engagement, produced significantly higher amount of Th2 cell cytokines including IL-4, IL-5, IL-10, and IL-13 [49]. This skewed Th2 differentiation was associated with the increased expression levels of JunB and c-Jun, transcription factors required for IL-4 expression. The MEKK1-JNK pathway was found to be essential for the phosphorylation and activation of Itch, a ubiquitin ligase, which in turn targeted JunB and c-Jun for proteosomal degradation [49]. Moreover, Th2 cells lacking the MEKK1 kinase activity or deficient in JNK1 or Itch could not be tolerized [50]. These studies identify a signaling pathway comprised of MEKK1, JNK1 and Itch that suppresses Th2 cell cytokine production and is essential to establish Th2 cell-mediated tolerance induction (Fig. 2B).
Additionally, MEKK1−/− mice had defective germinal center formation and diminished production of antibodies recognizing thymus-dependent antigens. These were due to B cell intrinsic defects, because MEKK1 is necessary for CD40-mediated activation of JNK and p38 and AP-1 transcription factors. In addition, MEKK1 deficient B cells were hypoproliferative after CD40 stimulation, associated with reduced expression of cyclin D2 [51]. MEKK1 also contributed to the activation of JNK and p38 induced by B cell-activating factor (BAFF). In contrast, MEKK1 had little or no contribution to CD40-induced activation of ERK or IKK [51]. Thus, MEKK1 is involved in CD40- and BAFF-induced activation of JNK and p38 and cellular responses in B cells (Fig. 2C).
Using CD40-induced MEKK1 activation as a model, Matsuzawa et al proposed a two-stage signaling mechanism for the activation of MAP3Ks following cytokine and immune receptor stimulation [52]. Upon CD40 ligation, TRAF2 and TRAF3, together with ubiquitin-conjugating enzyme Ubc13 and cellular inhibitor of apoptosis proteins 1 and 2 (c-IAP1/2), are recruited to B cell membrane, followed by the recruitment of NEMO and MEKK1 [52]. The assembly of the membrane-anchored multicomponent signaling complex, while required for MEKK1 activation, does not activate the kinase. Instead, it is the subsequent release of the MEKK1-associated complex into the cytosol, mediated by the proteasomal degradation of TRAF3, that activates MEKK1 and the downstream JNK and p38. Notably, activation of TAK1 by CD40 appears to follow a similar two-step mechanism [52], but whether such a mechanism applies to the activation of other MAP3Ks in immune responses remains to be determined.
2.3 MEKK4 in Th1 cell-mediated immune response
MEKK4, also known as MAP3K4 or MTK1, stimulates JNK and p38 pathways when overexpressed [53, 54]. Loss of MEKK4 function resulted in highly penetrant neural tube defects associated with greatly elevated apoptosis of neuroepithelial cells [55, 56]. The immunoregulatory function of MEKK4 has been mainly studied in T cells. Naïve T cells lacking MEKK4 activated MAPKs normally in response to TCR stimulation, suggesting that MEKK4 is dispensable for T cell activation. However, T cells deficient in MEKK4 produced diminished levels of IFN-γ during Th1 cell differentiation. Moreover, MEKK4−/− Th1 cells exhibited lower activation of p38 but not JNK. Consistently, wild-type cells treated with a p38 inhibitor produced decreased IFN-γ. Collectively, these studies indicate the MEKK4, by facilitating p38 activation, contributes to Th1 cell cytokine production (Fig. 2B) [57].
MEKK4 has been proposed to function downstream of stress-inducible GADD45 proteins (GADD45α/β/γ) to mediate JNK/p38 activation and apoptosis [58], although considerable controversy remained [59, 60]. Given a role for GADD45β and GADD45γ in mediating IFN-γ production and Th1 cell function [61, 62], we tested whether MEKK4 functions downstream of GADD45 protein in T cell responses. Whereas exogenous GADD45β or GADD45γ was capable of promoting IFN-γ production in wild-type cells, MEKK4 deficiency completely abrogated the function of GADD45β and GADD45γ in IFN-γ upregulation [57]. Interestingly, expression of GADD45β and GADD45γ are mediated by TCR and IL-12/STAT4 pathways, respectively. By integrating TCR and cytokine signals, the GADD45/MEKK4/p38 pathway is an important regulator of Th1 cell differentiation [57]. Recent biochemical analysis reveals the molecular mechanisms of MEKK4 activation by GADD45 proteins [63]. MEKK4 has an extensive N-terminal noncatalytic domain that binds to the C-terminal kinase domain, thereby inhibiting the kinase activity. This N-C intramolecular interaction is disrupted by the binding of GADD45 to the MEKK4 N-terminal GADD45-binding site [64]. GADD45 binding also induces MEKK4 dimerization, which is essential for the trans autophosphorylation of MEKK4 at threonine 1493 in the kinase activation loop. Therefore, GADD45 binding induces MEKK4 N-C dissociation, dimerization and autophosphorylation, leading to the activation of the kinase catalytic domain [63].
2.4 MEKK3 in innate and adaptive immune responses
MEKK3 (MAP3K3) shows significant homology with another MAP3K, MEKK2 (MAP3K2), and both molecules are capable of activating ERK and JNK upon overexpression [65]. Deletion of MEKK3 (MAP3K3) in mice resulted in early embryonic development due to defective blood vessel formation [66]. MEKK3-deficient MEFs showed delayed NF-κB, JNK and p38 activation in response to TLR4 and IL-1 stimulation. In addition, TNFα-induced activation of NF-κB was defective in MEKK3 deficient MEFs [67, 68]. Moreover, TLR8-mediated NF-κB and JNK activation was completely abolished in MEKK3−/− MEFs but was unaffected by the lack of TAK1 (Fig. 2A) [30]. It appears that MEKK3 and TAK1 can both mediate MAPK and NF-κB activation in innate immune responses, but their relative contributions in response to a particular stimulus require further detailed investigation.
T cells deficient in MEKK3 were generated by two independent groups using the Lck-Cre deletion system [69, 70]. Yang et al reported that T cells deficient in MEKK3 developed normally in the thymus, and in response to TCR stimulation, showed no defects in their proliferate [69]. However, MEKK3 deficiency resulted in a substantial reduction in peripheral T cell numbers. In an adoptive mixed transfer system, MEKK3-deficient T cells were severely impaired in lymphopenia-induced cell proliferation and survival, associated with attenuated ERK signaling. Therefore, MEKK3 plays a unique role in T cell homeostasis by transducing signals from the interaction between TCR and self peptide/MHC ligands (Fig. 2B) [69]. In contrast, Shinohara et al reported that T cells lacking MEKK3 were defective in TCR and cytokine-induced responses associated with impaired activation of NF-κB [70]. Activation of MAPK, however, was affected in these T cells (Fig. 2B). The discrepancy between these two studies is not clear. As compared with TAK1, MEKK1 and MEKK4, mechanisms of MEKK3 activation are less understood. However, phosphorylation at serine 526 is crucial for the activation of MEKK3 [71].
2.5 Other MAP3Ks in immune responses
Functions of several other MAP3Ks have also been genetically defined, although their regulations are not well understood. ASK1 is activated by intracellular reactive oxygen species and contributes to TLR4-induced activation of p38 but not of JNK or NF-κB [72]. ASK1-deficient mice produced reduced levels of proinflammatory cytokines and were resistant to LPS-induced septic shock. However, activation of p38 in DCs stimulated via TLR2, TLR3 or TLR9 did not require ASK1 (Fig. 2A) [72]. In addition, analysis of ASK1−/− MEFs indicated that only the sustained phase of JNK and p38 activation triggered by TNF-α depended on ASK1 [73]. Therefore, ASK1 coupling to p38 and JNK varies in a cell-type and receptor specific manner.
In the adaptive immune system, T cells deficient in MEKK2 (MAP3K2) exhibited increased JNK activation but normal regulation of ERK and p38. Such T cells were hyperproliferative to TCR stimulation and produced increased IL-2 and IFN-γ. Moreover, MEKK2−/− thymocytes were more susceptible than wild-type thymocytes to TCR-induced cell death. These results suggest that MEKK2 is involved in negatively controlling the strength of TCR signaling (Fig. 2B) [74].
3. Propagation of MAPK signaling by downstream pathways
MAPKs mediate inflammatory responses mainly by activating gene expression. A group of sequence-specific transcription factors known as AP-1 are conventional substrates for JNK and p38. For example, c-Jun and ATF2 are well characterized substrates for JNK and p38, respectively. In addition, JNK and p38 can phosphorylate a plethora of intracellular proteins, including transcriptional coregulators, cytoskeletal proteins, translational machine components, and other signaling proteins, which in turn regulate various physiological processes [6]. Recent studies have revealed novel signaling mechanisms that are essential to propagate MAPK-dependent responses in the immune system. One mechanism involves p38-mediated phosphorylation and activation of a group of downstream protein kinases collectively known as MAPK-activated protein kinases (MKs). Those functionally subordinate to p38 include MAPK-activated protein kinases MK2 and MK3 (also called MAPKAP-K2 and MAPKAP-K3), mitogen- and stress-activated kinases MSK1 and MSK2, and MAPK-interacting kinases MNK1 and MNK2. Once phosphorylated by p38, these MKs can act on other kinases and transcription factors, thus forming a multilayered protein kinase cascade downstream of p38 that controls a broad range of cellular functions. Another mechanism of MAPK regulation involves JNK-mediated phosphorylation of the ubiquitin ligase Itch [49], which defines a new mode of the crosstalk between the phosphorylation and ubiquitination systems.
3.1 p38-dependent MK cascade in TLR responses
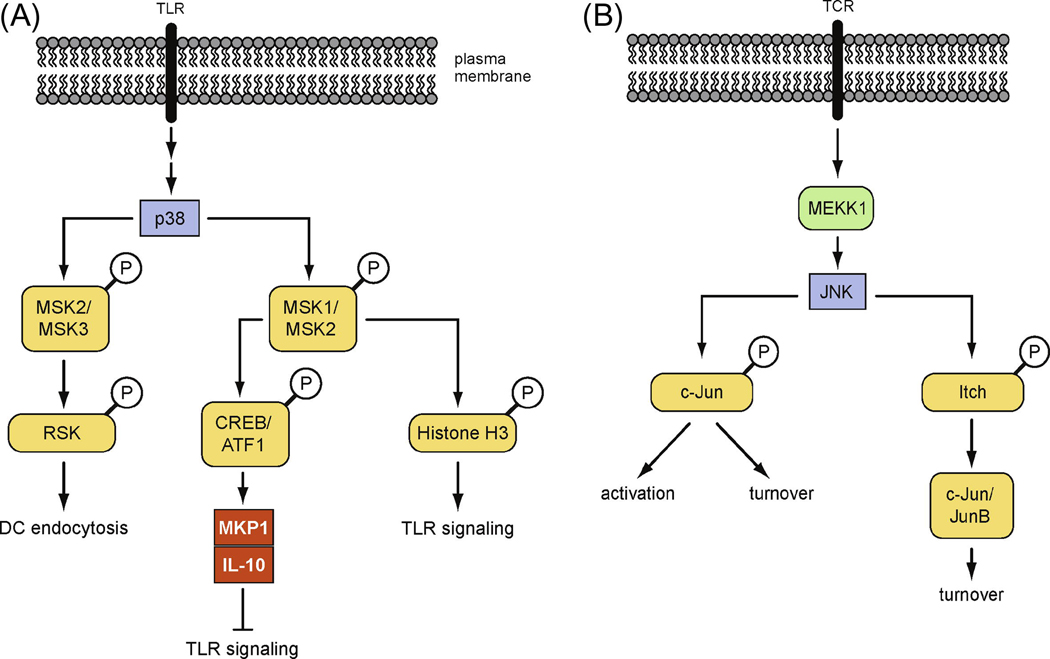
The p90 ribosomal S6 kinases (Rsk) can activate a wide variety of substrates linked to the control of transcription, cell proliferation, cytoskeleton rearrangement, glycogen metabolism and cell survival [75]. Two specific and structurally different inhibitors of Rsk suppressed TLR-induced endocytosis, thus defining a specific requirement for Rsk in TLR responses in DCs.
Although Rsk was originally thought to be activated exclusively by the ERK pathway, activation of Rsk in DCs was found to be mediated by both p38 and ERK. p38 did not activate Rsk directly, but through the intermediates MK2 and MK3. In support of this model, DCs deficient in both MK2 and MK3 lacked the phosphorylation of Rsk at Ser386 and were defective in LPS-stimulated endocytic response [76]. These studies identify a new configuration of the MAPK system consisting of p38 – MK2/MK3 – Rsk that facilitates TLR-induced endocytosis. Interestingly, such a pathway is functional specifically in DCs but not in other cell types examined (Fig. 3A) [76].
Figure 3. Propagation of MAPK signaling.
MKs and Itch have been implicated in transducing signals from activated p38 (A) and JNK (B), respectively, leading to diverse immune responses.
Another group of p38-dependent MKs includes two related kinases MSK1 and MSK2. MSK1 and MSK2 can phosphorylate transcription factors CREB and ATF1 and histone H3 molecule in response to mitogens and cellular stress [77, 78]. Combined deletion of MSK1 and MSK2 in mice identified these kinases as key components of negative feedback mechanisms of TLR responses. MSKs limited TLR–driven inflammation by inducing the activation of CREB and ATF1 and the expression of negative regulators of TLR signaling including MKP-1 and IL-10 [79]. Furthermore, deletion of p38α in myeloid cells also revealed an unexpected negative role for p38α in restraining TLR-induced inflammatory responses. A subset of p38 target genes, including MKP-1 and IL-10, is strictly dependent upon MSKs for their expression following TLR stimulation [80, 81]. These results define a pathway consisting of p38 – MSK1/2 – CREB/ATF1 in limiting TLR induced inflammation (Fig. 3A). In addition to phosphorylating CREB and ATF1, MSKs can induce chromatin remodeling by phosphorylating histone H3 following p38 activation. This marks cytokine promoters for increased NF-κB recruitment and may facilitate expression of certain cytokine genes (Fig. 3A) [82].
3.2 JNK-mediated regulation of the ubiquitin pathway in T cell responses
Extracellular stimuli often affect ubiquitin-dependent proteolysis by inducible target protein phosphorylation, which confers recognition by E3 ligases. JNK-dependent phosphorylation of c-Jun decreases c-Jun ubiquitination and enhances its stability, although under resting conditions, JNK can facilitate c-Jun for degradation [83–86]. This is in addition to the traditional mode of regulation that involves JNK-dependent phosphorylation of the transcriptional activation domain of c-Jun, directly leading to the enhanced AP-1 activity. Yet, Gao et al identified a new substrate for JNK that modulates AP-1 activity in T cell responses. As described above, T cells lacking MEKK1 activity showed reduced JNK activation but increased protein levels of c-Jun and JunB. These cells produced increased levels of Th2 cytokines, a phenotype similar as Itchy T cells. Indeed, JNK was found to phosphorylate the E3 ubiquitin ligase Itch, leading to its functional activation and consequently, accelerated degradation of c-Jun and JunB. These studies demonstrate that the effect of JNK on c-Jun and JunB ubiquitination and turnover in T cells is exerted by means of Itch, whose catalytic activity is strongly modulated in response to T cell activation through JNK-dependent phosphorylation [49]. Therefore, JNK can modulate AP-1 activity through multiple mechanisms (Fig. 3B).
4. Negative regulation of MAPKs by MKPs
The magnitude and duration of JNK and p38 signal transduction are critical determinants of its biological effects. Activation of MAPKs occurs within minutes in response to most stimuli and is transient. This suggests that MAPKs function as a biological switch that must be downregulated, both under basal conditions and during adaptation. Inhibition of MAPK activity is effected primarily by MAPK phosphatases (MKPs), a group of approximately 10 dual-specificity phosphatases that dephosphorylate the regulatory threonine and tyrosine residues of their target MAPK [11, 12, 87]. Genetic analyses have revealed that three members of the MKP family, MKP-1, MKP-2 and DUSP2, play important and distinct functions in innate immunity (Fig. 4).
Figure 4. Inactivation of MAPK signaling by MKPs in innate immunity.
4.1 MKP-1, a key negative regulator of TLR responses
MKP-1, the founding member of the MKP family, was initially identified as an immediate-early response gene induced by growth factors and stress. MKP-1 localizes to the nucleus through its amino terminus and preferentially dephosphorylates activated p38 and JNK relative to ERK [88]. Using MKP-1 deficient mice, we and three other groups have independently reported that MKP-1 is a key negative regulator of TLR signaling and innate immune responses [89–92]. In response to TLR stimulation, MKP-1−/− macrophages exhibited increased and prolonged activation of p38 and JNK, and produced excessive amounts of cytokines, including TNF-α, IL-6 and IL-10. Transcriptional profiling revealed that MKP-1 controlled a significant fraction of LPS-induced genes, including the innate cytokines as well as chemokines CCL3, CCL4, and CXCL2 [90]. Moreover, mice deficient in MKP-1 showed greatly elevated susceptibility to endotoxic shock and autoimmune arthritis, associated with elevated production of proinflammatory cytokines and mediators [89–92]. These studies indicate that the MKP-1 is a key negative regulator of innate immunity.
Given the pivotal role of MKP-1 in innate immunity, there is growing interest in exploring how its gene expression and activity are regulated. In macrophages responding to TLR stimulation, there was a strong and rapid induction of MKP-1 mRNA and protein abundance, peaking at one hour after stimulation [89, 91, 93–95]. The induction of MKP-1 correlated with a decline in the activities of JNK and p38, which is consistent with a role for MKP-1 in the inactivation of these MAPKs as a feedback mechanism to restrain excessive inflammation. MKP-1 was also induced by multiple immunosuppressive agents, including glucocorticoids and anti-inflammatory cytokines, and this induction partially mediates the inhibitory effects of these agents on MAPK activation and inflammation [96]. Conversely, proinflammatory stimuli such as IFN-γ attenuated MKP-1 expression to facilitate their positive effects on MAPK activation [91]. Therefore, it appears that the induction of MKP-1 represents a common mechanism by which immunomodulatory agents “fine tune” innate immune responses [97].
Posttranslational modifications of MKP-1 also play an important role in regulating its activity and stability. These modifications include ERK-dependent phosphorylation of MKP-1 C-terminus [98–100], and reactive oxygen species (ROS)-induced oxidation of the catalytic cysteine residue [101, 102]. Cao et al. have recently identified another mode of posttranslational modification of MKP-1 that does not depend upon protein stability or intrinsic phosphatase activity [102, 103]. LPS activated p300-dependent acetylation of MKP-1 on lysine 57 within its substrate-binding domain. Acetylation of MKP-1 enhanced its interaction with p38 and thus the dephosphorylation toward its substrate, leading to the termination of MAPK signaling. Collectively, these studies suggest that transcriptional and post-transcriptional regulation of MKP-1 is an important control mechanism in innate immunity.
4.2 Regulation of both innate and adaptive immunity by MKP-5
MKP-5 is localized in both the cytoplasm and the nucleus and has been shown to dephosphorylate both JNK and p38 in vitro. MKP-5 was upregulated in macrophages responding to LPS stimulation, and overexpression of MKP-5 decreased the activity of AP-1 transcription factors [104]. To investigate the immunoregulatory function of MKP-5, Zhang et al generated MKP-5 deficient mice, which developed normally but showed defects in both innate and adaptive immunity. After LPS challenge, macrophages deficient in MKP-5 exhibited increased JNK activity, but unaltered ERK or p38 activity, suggesting a role for MKP-1 to preferentially dephosphorylate JNK in vivo. MKP-5-deficient cells produced enhanced levels of proinflammatory cytokines during innate immune responses and had increased ability to serve as antigen presenting cells to prime activation of antigen-specific T cells. Injection of LPS into MKP-5−/− mice also resulted in two fold higher levels of TNF-α in the serum, which was less profound than those of MKP-1−/− mice, probably reflecting the limited substrate specificity of MKP-5 on JNK but not on p38 or ERK. T cells lacking MKP-5 proliferated poorly upon activation, which likely contributed to the reduced incidence and severity of disease in a model of autoimmune brain inflammation, experimental autoimmune encephalomyelitis (EAE). However, MKP-5−/− T cells produced increase levels of effector cytokines including IFN-γ and IL-4, which led to much more robust and rapidly fatal immune responses to secondary infection with lymphocytic choriomeningitis virus (LCMV) [104]. Therefore, MKP-5 regulates both innate and adaptive immunity.
4.3. DUSP2, a positive regulator of innate immune responses
DUSP2 (PAC-1) is a nuclear MKP whose expression is mainly restricted to immune cells and tissues. DUSP2 is the most highly induced MKP transcript in activated human leukocytes [105]. In contrast to MKP-1−/− and MKP-5−/− cells, DUSP2−/− innate immune cells show reduced function. In response to LPS stimulation, DUSP2−/− macrophages produced diminished TNF-α, IL-6 and IL-12 and decreased PGE2 and nitric oxide levels. Moreover, DUSP2−/− mice had reduced inflammatory responses in a serum transfer model of rheumatoid arthritis. DUSP2 deficiency resulted in increased JNK activity but surprisingly, decreased ERK and p38 activities in LPS-activated macrophages. This was associated with impaired transcriptional activities of Elk-1 and NFAT-AP1, two main nuclear targets of the MAPK pathways. Significantly, pharmacological inhibition of JNK reversed the impaired activation of ERK in DUSP2-deficient cells, indicating that in the absence of DUSP2, the enhanced JNK activity mediates inhibition of ERK. Indeed, there are precedents for cross-talk amongst the MAPKs, as both p38 and JNK can negatively regulate ERK [106, 107]. Therefore, DUSP2 appears to suppress JNK-mediated inhibition of ERK signaling in macrophages to positively regulate innate immunity [105].
5. Concluding remarks
One of the central functions of the stress-activated MAPKs is to orchestrate the immune response. Pharmacological inhibition of the p38 and JNK pathways has proven effective in treating or alleviating various inflammatory conditions [108–110]. However, the toxicity and undesired adverse effects of such inhibitors are recognized as well, which may arise from perturbed cross-regulatory signaling or self-limiting mechanisms that rely on JNK and p38 activities [111]. In this review, we discuss several new modes of MAPK regulation that may lead to the development of more selective approaches for clinical intervention. Since JNK and p38 are activated by a large number of immune receptors, specific inhibition of a signaling module or a regulatory target that functions either upstream or downstream of JNK and p38 likely offers a more efficacious and selective anti-inflammatory strategy. The mechanisms that are responsible for the termination of MAPK activity are also important for our understanding of the role of MAPKs in normal physiology and disease, and the development of new therapeutic strategies. Research into these areas will continue to generate new insights into mechanisms of immune regulation and strategies of therapeutic intervention.
ACKNOWLEDGMENTS
The authors acknowledge the entire Chi laboratory for stimulating discussions, and Betsy Williford for help with art work for the figures. This work was supported by US National Institutes of Health R01 NS064599 and Cancer Center Support Grant CA021765, National Multiple Sclerosis grant RG4180-A-1, the Hartwell Foundation Individual Biomedical Research Award, Cancer Research Institute Investigator Award, and the American Lebanese Syrian Associated Charities (to H.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol. 2008;8:816–822. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X, Chen J, Wang L, Ivashkiv LB. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J Leukoc Biol. 2007;82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 6.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 7.Symons A, Beinke S, Ley SC. MAP kinase kinase kinases and innate immunity. Trends Immunol. 2006;27:40–48. doi: 10.1016/j.it.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Winter-Vann AM, Johnson GL. Integrated activation of MAP3Ks balances cell fate in response to stress. J Cell Biochem. 2007;102:848–858. doi: 10.1002/jcb.21522. [DOI] [PubMed] [Google Scholar]
- 9.Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- 10.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 11.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 13.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 14.Dong C, Davis RJ, Flavell RA. MAP kinases in immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 15.Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 16.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bain J, Plater L, Elliott M, Shpiro N, Hastie J, McLauchlan H, Klevernic I, Arthur S, Alessi D, Cohen P. The selectivity of protein kinase inhibitors; a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang ZH, Zhou Y, Yu MC, Silverman N, Ge BX. Regulation of Drosophila p38 activation by specific MAP2 kinase and MAP3 kinase in response to different stimuli. Cell Signal. 2006;18:441–448. doi: 10.1016/j.cellsig.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 21.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 22.Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- 23.Vidal S, Khush RS, Leulier F, Tzou P, Nakamura M, Lemaitre B. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev. 2001;15:1900–1912. doi: 10.1101/gad.203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, Maniatis T. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278:48928–48934. doi: 10.1074/jbc.M304802200. [DOI] [PubMed] [Google Scholar]
- 25.Jadrich JL, O'Connor MB, Coucouvanis E. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133:1529–1541. doi: 10.1242/dev.02333. [DOI] [PubMed] [Google Scholar]
- 26.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. Embo J. 2008;27:373–383. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Omori E, Matsumoto K, Nunez G, Ninomiya-Tsuji J. TAK1 is a central mediator of NOD2 signaling in epidermal cells. J Biol Chem. 2008;283:137–144. doi: 10.1074/jbc.M704746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin J, Yao J, Cui G, Xiao H, Kim TW, Fraczek J, Wightman P, Sato S, Akira S, Puel A, Casanova JL, Su B, Li X. TLR8-mediated NF-kappaB and JNK activation are TAK1-independent and MEKK3-dependent. J Biol Chem. 2006;281:21013–21021. doi: 10.1074/jbc.M512908200. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 32.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 33.Sato S, Sanjo H, Tsujimura T, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Takeuchi O, Akira S. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int Immunol. 2006;18:1405–1411. doi: 10.1093/intimm/dxl082. [DOI] [PubMed] [Google Scholar]
- 34.Liu HH, Xie M, Schneider MD, Chen ZJ. Essential role of TAK1 in thymocyte development and activation. Proc Natl Acad Sci U S A. 2006;103:11677–11682. doi: 10.1073/pnas.0603089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinohara H, Yasuda T, Aiba Y, Sanjo H, Hamadate M, Watarai H, Sakurai H, Kurosaki T. PKC beta regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J Exp Med. 2005;202:1423–1431. doi: 10.1084/jem.20051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuman J, Chen Y, Podd A, Yu M, Liu HH, Wen R, Chen ZJ, Wang D. A critical role of TAK1 in B cell receptor-mediated NF-{kappa}B activation. Blood. 2009 doi: 10.1182/blood-2008-08-176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 38.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 39.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 41.Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol. 2008;28:3538–3547. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CJ, Ashwell JD. NEMO recognition of ubiquitinated Bcl10 is required for T cell receptor-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:3023–3028. doi: 10.1073/pnas.0712313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, Choi Y. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- 44.Shambharkar PB, Blonska M, Pappu BP, Li H, You Y, Sakurai H, Darnay BG, Hara H, Penninger J, Lin X. Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways. Embo J. 2007;26:1794–1805. doi: 10.1038/sj.emboj.7601622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blonska M, Pappu BP, Matsumoto R, Li H, Su B, Wang D, Lin X. The CARMA1-Bcl10 signaling complex selectively regulates JNK2 kinase in the T cell receptor-signaling pathway. Immunity. 2007;26:55–66. doi: 10.1016/j.immuni.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia Y, Makris C, Su B, Li E, Yang J, Nemerow GR, Karin M. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc Natl Acad Sci U S A. 2009;97:5243–5248. doi: 10.1073/pnas.97.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, Fanger GR, Henson P, Johnson GL. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc Natl Acad Sci U S A. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yujiri T, Sather S, Fanger GR, Johnson GL. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 49.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 50.Venuprasad K, Elly C, Gao M, Salek-Ardakani S, Harada Y, Luo JL, Yang C, Croft M, Inoue K, Karin M, Liu YC. Convergence of Itch-induced ubiquitination with MEKK1-JNK signaling in Th2 tolerance and airway inflammation. J Clin Invest. 2006;116:1117–1126. doi: 10.1172/JCI26858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallagher E, Enzler T, Matsuzawa A, Anzelon-Mills A, Otero D, Holzer R, Janssen E, Gao M, Karin M. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol. 2007;8:57–63. doi: 10.1038/ni1421. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzawa A, Tseng PH, Vallabhapurapu S, Luo JL, Zhang W, Wang H, Vignali DA, Gallagher E, Karin M. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 2008;321:663–668. doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerwins P, Blank JL, Johnson GL. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J Biol Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 54.Takekawa M, Posas F, Saito H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. Embo J. 1997;16:4973–4982. doi: 10.1093/emboj/16.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chi H, Sarkisian MR, Rakic P, Flavell RA. Loss of mitogen-activated protein kinase kinase kinase 4 (MEKK4) results in enhanced apoptosis and defective neural tube development. Proc Natl Acad Sci U S A. 2005;102:3846–3851. doi: 10.1073/pnas.0500026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abell AN, Rivera-Perez JA, Cuevas BD, Uhlik MT, Sather S, Johnson NL, Minton SK, Lauder JM, Winter-Vann AM, Nakamura K, Magnuson T, Vaillancourt RR, Heasley LE, Johnson GL. Ablation of MEKK4 kinase activity causes neurulation and skeletal patterning defects in the mouse embryo. Mol Cell Biol. 2005;25:8948–8959. doi: 10.1128/MCB.25.20.8948-8959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chi H, Lu B, Takekawa M, Davis RJ, Flavell RA. GADD45beta/GADD45gamma and MEKK4 comprise a genetic pathway mediating STAT4-independent IFNgamma production in T cells. EMBO J. 2004;23:1576–1586. doi: 10.1038/sj.emboj.7600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95:521–530. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Gorospe M, Holbrook NJ. gadd45 is not required for activation of c-Jun N-terminal kinase or p38 during acute stress. J Biol Chem. 1999;274:29599–29602. doi: 10.1074/jbc.274.42.29599. [DOI] [PubMed] [Google Scholar]
- 60.Shaulian E, Karin M. Stress-induced JNK activation is independent of Gadd45 induction. J Biol Chem. 1999;274:29595–29598. doi: 10.1074/jbc.274.42.29595. [DOI] [PubMed] [Google Scholar]
- 61.Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–590. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 62.Lu B, Ferrandino AF, Flavell RA. Gadd45[beta] is important for perpetuating cognate and inflammatory signals in T cells. Nat Immunol. 2004;5:38–44. doi: 10.1038/ni1020. [DOI] [PubMed] [Google Scholar]
- 63.Miyake Z, Takekawa M, Ge Q, Saito H. Activation of MTK1/MEKK4 by GADD45 through induced N-C dissociation and dimerization-mediated trans autophosphorylation of the MTK1 kinase domain. Mol Cell Biol. 2007;27:2765–2776. doi: 10.1128/MCB.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mita H, Tsutsui J, Takekawa M, Witten EA, Saito H. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol Cell Biol. 2002;22:4544–4555. doi: 10.1128/MCB.22.13.4544-4555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blank JL, Gerwins P, Elliott EM, Sather S, Johnson GL. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J Biol Chem. 1996;271:5361–5368. doi: 10.1074/jbc.271.10.5361. [DOI] [PubMed] [Google Scholar]
- 66.Yang J, Boerm M, McCarty M, Bucana C, Fidler IJ, Zhuang Y, Su B. Mekk3 is essential for early embryonic cardiovascular development. Nat Genet. 2000;24:309–313. doi: 10.1038/73550. [DOI] [PubMed] [Google Scholar]
- 67.Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, Liao W, Chen Z, Liu Z, Su B. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Chang X, Facchinetti V, Zhuang Y, Su B. MEKK3 is essential for lymphopenia-induced T cell proliferation and survival. J Immunol. 2009;182:3597–3608. doi: 10.4049/jimmunol.0803738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinohara H, Yamasaki S, Maeda S, Saito T, Kurosaki T. Regulation of NF-kappaB-dependent T cell activation and development by MEKK3. Int Immunol. 2009;21:393–401. doi: 10.1093/intimm/dxp007. [DOI] [PubMed] [Google Scholar]
- 71.Zhang D, Facchinetti V, Wang X, Huang Q, Qin J, Su B. Identification of MEKK2/3 serine phosphorylation site targeted by the Toll-like receptor and stress pathways. Embo J. 2006;25:97–107. doi: 10.1038/sj.emboj.7600913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 73.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo Z, Clydesdale G, Cheng J, Kim K, Gan L, McConkey DJ, Ullrich SE, Zhuang Y, Su B. Disruption of Mekk2 in mice reveals an unexpected role for MEKK2 in modulating T-cell receptor signal transduction. Mol Cell Biol. 2002;22:5761–5768. doi: 10.1128/MCB.22.16.5761-5768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hauge C, Frodin M. RSK and MSK in MAP kinase signalling. J Cell Sci. 2006;119:3021–3023. doi: 10.1242/jcs.02950. [DOI] [PubMed] [Google Scholar]
- 76.Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat Immunol. 2007;8:1227–1235. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]
- 77.Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. Embo J. 2003;22:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, Wingate A, Monk CE, Toth R, Santos SG, Iversen L, Arthur JS. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028–1036. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 80.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, Park JM. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 83.Musti AM, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- 84.Fuchs SY, Dolan L, Davis RJ, Ronai Z. Phosphorylation-dependent targeting of c-Jun ubiquitination by Jun N-kinase. Oncogene. 1996;13:1531–1535. [PubMed] [Google Scholar]
- 85.Nateri AS, Riera-Sans L, Da Costa C, Behrens A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science. 2004;303:1374–1378. doi: 10.1126/science.1092880. [DOI] [PubMed] [Google Scholar]
- 86.Laine A, Ronai Z. Ubiquitin chains in the ladder of MAPK signaling. Sci STKE. 2005;2005:re5. doi: 10.1126/stke.2812005re5. [DOI] [PubMed] [Google Scholar]
- 87.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 88.Franklin CC, Kraft AS. Conditional Expression of the Mitogen-activated Protein Kinase (MAPK) Phosphatase MKP-1 Preferentially Inhibits p38 MAPK and Stress-activated Protein Kinase in U937 Cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 89.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 93.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem. 2005;280:8101–8108. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]
- 95.Hu JH, Chen T, Zhuang ZH, Kong L, Yu MC, Liu Y, Zang JW, Ge BX. Feedback control of MKP-1 expression by p38. Cell Signal. 2007;19:393–400. doi: 10.1016/j.cellsig.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 96.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chi H, Bennett AM, Flavell RA. Mitogen-activated protein kinase phosphatase-1 (MKP-1): a critical regulator of innate immune responses. J Organ Dysfunct. 2007;3:72–81. [Google Scholar]
- 98.Brondello JM, Pouysségur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 99.Lin YW, Chuang SM, Yang JL. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem. 2003;278:21534–21541. doi: 10.1074/jbc.M301854200. [DOI] [PubMed] [Google Scholar]
- 100.Lin YW, Yang JL. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J Biol Chem. 2006;281:915–926. doi: 10.1074/jbc.M508720200. [DOI] [PubMed] [Google Scholar]
- 101.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 102.Chi H, Flavell RA. Acetylation of MKP-1 and the control of inflammation. Sci Signal. 2008;1:pe44. doi: 10.1126/scisignal.141pe44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205:1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD, Flavell RA, Dong DC. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature. 2004;430:793–797. doi: 10.1038/nature02764. [DOI] [PubMed] [Google Scholar]
- 105.Jeffrey KL, Brummer T, Rolph MS, Liu SM, Callejas NA, Grumont RJ, Gillieron C, Mackay F, Grey S, Camps M, Rommel C, Gerondakis SD, Mackay CR. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat Immunol. 2006;7:274–283. doi: 10.1038/ni1310. [DOI] [PubMed] [Google Scholar]
- 106.Shen YH, Godlewski J, Zhu J, Sathyanarayana P, Leaner V, Birrer MJ, Rana A, Tzivion G. Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J Biol Chem. 2003;278:26715–26721. doi: 10.1074/jbc.M303264200. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H, Shi X, Hampong M, Blanis L, Pelech S. Stress-induced inhibition of ERK1 and ERK2 by direct interaction with p38 MAP kinase. J Biol Chem. 2001;276:6905–6908. doi: 10.1074/jbc.C000917200. [DOI] [PubMed] [Google Scholar]
- 108.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 109.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 110.Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- 111.Dambach DM. Potential adverse effects associated with inhibition of p38alpha/beta MAP kinases. Curr Top Med Chem. 2005;5:929–939. doi: 10.2174/1568026054985911. [DOI] [PubMed] [Google Scholar]