Abstract
Indoles and alcohols can be coupled in a dehydrogenative process catalyzed by tetrapropylammonium perruthenate (TPAP). This efficient approach to indolylamides proceeds in a single flask under mild conditions. By employing substituted indoles and alkyl, branched, or benzylic alcohols, a variety of indolylamides can be formed. Aryl indolylamides can be functionalized through an additional dehydrogenative coupling to furnish elaborated polycyclic heterocycles similar to biologically active structures previously reported.
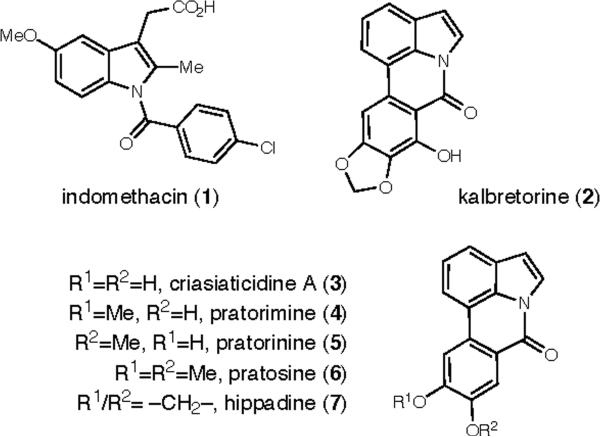
Amides are important, abundant, compounds which are utilized in a broad range of chemical disciplines.1 Given the ubiquity of this stable functional group and its central place in proteins and polymers, amide formation remains a fundamental transformation in organic synthesis.2 The formation of amides by the oxidation of the acyl C-H of aldehydes is an attractive, direct method3 that circumvents the need for coupling agents and prerequisite oxidation. Of particular interest is the potential application of this method toward the synthesis of indolylamides. In addition to their function as protected carboxylate derivatives,4 indolylamides are desirable synthetic targets. N-Acyl indoles are present in numerous biologically active molecules (Figure 1) such as the non-steroid anti-inflammatory agent indomethacin (1),5 members of the pyrrolophenanthridone class of natural products6 isolated from the Amaryllidaceae family (2-7),7 and 6H-Isoindolo[2,1-a]indol-6-ones (vide infra).
Figure 1.
Bioactive Indolylamides
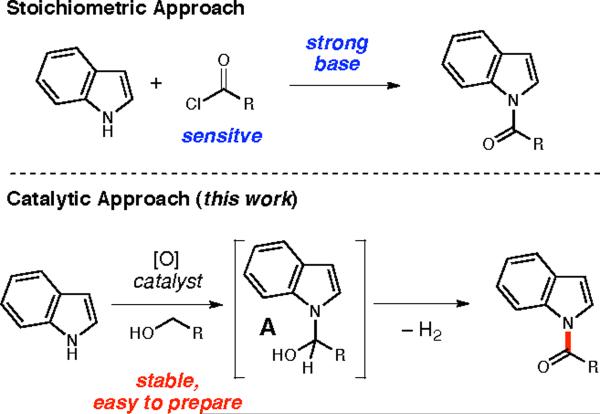
The dehydrogenative coupling of indoles and alcohols8 (Figure 2) is an efficient approach to these synthetically useful N-acylated indole structures. Furthermore, a strategy that accesses higher oxidation states in a single operation (i.e., alcohol to amide) simultaneously exploits the advantages of tandem processes and facilitates the functionalization of challenging substrates. Indoles and pyrroles were attractive targets for this process due to the stability of the aminal intermediate (A).9,10 Herein we report the dehydrogenative coupling of indoles and alcohols which does not require the use of strong hydride or alkyllithium bases, and also obviates the need for sensitive or less accessible acid chloride or anhydride acylating agents.11 In addition, this process utilizes starting materials in the readily-available alcohol oxidation state. To the best of our knowledge this transformation represents the first intermolecular dehydrogenative coupling of aromatic amines and alcohols.
Figure 2.
Dehydrogenative Coupling of Indoles and Alcohols
Our previous work, utilizing nucleophilic N-heterocyclic carbene (NHC) catalysts in tandem oxidation reactions,12 led us to investigate the acylation potential of that process with respect to amines.13 With indole as the nucleophile, we were pleased to observe the formation of the N-acylated product 10a under reaction conditions employing various azolium salts and oxidants (results not shown). The reaction was found to also proceed in the absence of the nucleophilic catalyst (Table 1, entry 1), presumably due to the formation and oxidation of an aminal intermediate, similar to A, which results from indole acting as a nucleophile and attacking the aldehyde in place of the heterocyclic catalyst.14 This reaction occurs specifically at the nitrogen atom of indole. Previous reports indicating nucleophilicity at the C3 position of the heterocycle generally require activation through Lewis acid15 or other catalyst.16 The divergence of the reactivity in this system is a particularly interesting observation and further mechanistic investigations are being carried out to elucidate the causes for this reactivity. A screen of oxidants and solvents (entries 1-4) revealed that the catalytic (5 mol %) oxidant tetrapropylammonium perruthenate (TPAP) in combination with 4-methylmorpholine-N-oxide (NMO)17 allowed this oxidation to take place at ambient temperature,18 albeit in similarly poor yields. Acetonitrile was found to be a more optimal solvent for this transformation than dichloromethanes.
Table 1.
N-Acylation of Indole with Hydrocinnamaldehyde
![]()
| entry | oxidant | additive | 9a:8 | solventa | yield (%)b |
|---|---|---|---|---|---|
| 1 | MnO2 | none | 10:1 | toluenec | 12 |
| 2 | PCC | none | 10:1 | toluenec | 16 |
| 3 | TPAP/NMO | 4 Å mol. sieves | 10:1 | CH2Cl2 | 4 |
| 4 | TPAP/NMO | 4 Å mol. sieves | 10:1 | CH3CN | 15 |
| 5 | TPAP/NMO | 4 Å mol. sieves | 5:1 | CH3CN | 36 |
| 6 | TPAP/NMO | 4 Å mol. sieves | 1:1 | CH3CN | 18 |
| 7 | TPAP/NMO | 4 Å mol. sieves | 1:1 | CH3CNd | 81(74) |
at 25 °C with 9a at 0.33 M.
yields calculated by GC (isolated yield in parentheses).
at 100 °C
9a at 0.6 M
The concentration of the nucleophilic indole 9a had a significant effect on the results of this oxidation (entries 4-7). When the concentration was greater than 1.5 M (entries 4, 5), lower yields were observed, while concentrations in the range of 0.5 M to 1.5 M provided the highest yields (entry 7). Further dilution (entry 6) had a negative effect on the yield of the N-acylated heterocycle.
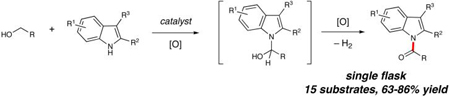
Because TPAP is known to oxidize alcohols to aldehydes, we explored the use of 3-phenyl-1-propanol (11) as a coupling partner. After initial oxidation to an aldehyde, formation of the aminal via nucleophilic addition of the indole derivative allowed further oxidation to the N-acyl heterocycle (Table 2, entry 1). This oxidative process is viable using a variety of indole derivatives. 5-Substituted indoles (Table 2, entries 2-4) were suitable substrates. The more nucleophilic 9b gave the highest yield, while an electron withdrawing group at this position decreased the yield (entry 4). 3-Substituted indoles (entries 6,7) yielded acylated product, but the more encumbered aminal resulting from the combination of 2-substituted or 7-substituted indoles and the alcohol were not capable of undergoing this oxidation (entries 8,9), most likely due to the bulky nature of the oxidizing agent. Indoles were found to be superior to pyrrole, indazole, and 7-aza-indole, all of which resulted in no acylation. Carbazole was also investigated and gave poor results (<40% yield of acylated product).
Table 2.
Dehydrogenative Coupling of Indolesa
![]()
| entry | indole | R1 | R2 | R3 | yield (%)b |
|---|---|---|---|---|---|
| 1 | 9a | H | H | H | 81 |
| 2 | 9b | 5-OMe | H | H | 86 |
| 3 | 9c | 5-Br | H | H | 76 |
| 4 | 9d | 5-CO2Me | H | H | 73 |
| 5 | 9e | 4-Br | H | H | 74 |
| 6 | 9f | H | H | CO2Me | 70 |
| 7 | 9g | H | H | Me | 63 |
| 8 | 9h | 7-Me | H | H | 0 |
| 9 | 9i | H | Me | H | 0 |
See Supporting Information for full experimental details.
Isolated yield.
Variation of the acylating alcohol shows steric interactions influencing the oxidation of the aminal intermediate. Unbranched alkyl alcohols (Table 3, entries 1-4) gave the best results, while increased substitution at the α-position decreased the yield (entries 6, 7). This process tolerates the presence of silyl ethers (entry 4) and olefins (entries 3, 5). The sterically demanding substrate derived from 2,2-dimethyl-1-propanol was afforded in low yield (entry 8). The use of a benzylic alcohol provided the N-acyl product as well (entry 9).
Table 3.
Dehydrogenative Coupling with Alcoholsa

| entry | R | product | yield (%)b |
|---|---|---|---|
| 1 | 10a | 81 | |
| 2 | 10j | 77 | |
| 3 | 10k | 71 | |
| 4 | 10l | 64 | |
| 5 | 10m | 73 | |
| 6 | 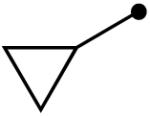 |
10n | 65 |
| 7 | 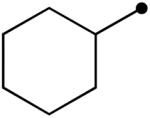 |
10o | 69 |
| 8 | 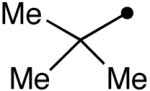 |
10p | 34 |
| 9 | 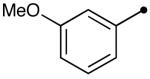 |
10q | 83 |
See Supporting Information for full experimental details.
Isolated yield.
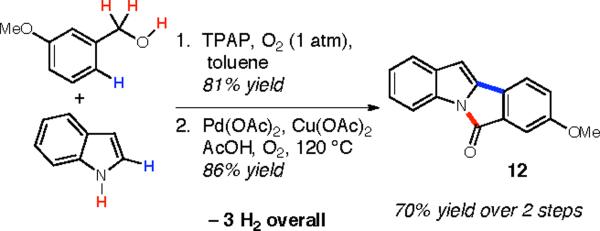
A modification of the procedure using TPAP and molecular oxygen as the oxidative system19 gave the product in comparable yields (Scheme 1). This green oxidative coupling20 of alcohols and indole derivatives allows for the further elaboration of substrates such as 10q. This process was also effective for other alcohols, as 10a and 10m were successfully obtained in 72 and 66% yields respectively using molecular oxygen as the oxidant.21
Scheme 1.
Synthesis of Bioactive Tetracycles
The direct acylation of indole using a benzaldehyde derivative yields acylated indole 10q, which, when treated with palladium(II) acetate (employing the conditions of DeBoef22), cyclizes to form tetracyclic compound 12 from the sequential dehydrogenation of indole and a simple benzyl alcohol (Scheme 1). 6H-Isoindolo[2,1-a]indol-6-ones of this type have been shown to mimic melatonin23 and batracyclin.24 These compounds possess several modes of biological activity,25 including binding to MT3 melatonin receptors, topisomerase inhibition, cytotoxicity, and antiproliferative activity against L1210 leukemia cells.
N-Acylated heterocycles can be synthesized from aromatic amines and alcohols or aldehydes. A variety of indole derivatives and acylating agents, restricted by steric interactions about the resultant aminal, can be used in this process. The oxidation is carried out with a catalytic oxidant (TPAP), which can be used in low catalyst loadings (< 5 mol %) to efficiently provide these useful substrates from readily available starting materials in an environmentally friendly manner. This method has been applied to the preparation of potentially bioactive 6H-isoindolo[2,1-a]indol-6-one frameworks. Further investigations of the mechanism and new applications of this dehydrogenative coupling process are underway and will be reported in due course.
Supplementary Material
Acknowledgment
Research support was generously provided by NIH/NIGMS (GM73072), Abbott Laboratories, Amgen, AstraZeneca, GlaxoSmithKline, the Sloan Foundation, and Boehringer-Ingelheim. B. E. M. was supported by a GAANN Fellowship.
Footnotes
Supporting Information Available: Experimental procedures and spectral data for all new compounds, (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Humphrey JM, Chamberlin AR. Chem. Rev. 1997;97:2243–2266. doi: 10.1021/cr950005s. [DOI] [PubMed] [Google Scholar]
- 2.Larock RC. Comprehensive Organic Transformations. 2nd ed. Wiley-VCH; New York: 1999. [Google Scholar]
- 3.(a) Yoo WJ, Li CJ. J. Am. Chem. Soc. 2006;128:13064–13065. doi: 10.1021/ja064315b. [DOI] [PubMed] [Google Scholar]; (b) Suto Y, Yamagiwa N, Torisawa Y. Tetrahedron Lett. 2008;49:5732–5735. [Google Scholar]; (c) Fang C, Qian WX, Bao WL. Synlett. 2008:2529–2531. [Google Scholar]; (d) Colombeau L, Traore T, Compain P, Martin OR. J. Org. Chem. 2008;73:8647–8650. doi: 10.1021/jo801626v. [DOI] [PubMed] [Google Scholar]; (e) Chan J, Baucorn KD, Murry JA. J. Am. Chem. Soc. 2007;129:14106–14107. doi: 10.1021/ja073872a. [DOI] [PubMed] [Google Scholar]; (f) The oxidative amidation of aldehydes has been recently reviewed: Ekoue-Kovi K, Wolf C. Chem.-Eur. J. 2008;14:6302–6315. doi: 10.1002/chem.200800353..
- 4.For examples of carboxylic acids protected as indolylamides, see (a) ref. 9a De Oliveira Baptista MJV, Barrett AGM, Barton DHR, Girijavallabhan M, Jennings RC, Kelly J, Papadimitriou VJ, Turner JV, Usher NA. J. Chem. Soc., Perkin Trans. 1977;1:1477–1500. doi: 10.1039/p19770001477.. Barrett AGM, Dhanak D. Tetrahedron Lett. 1987;28:3327–3330.. Barrett AGM, Bezuidenhoudt BCB, Dhanak D, Gasiecki AF, Howell AR, Lee AC, Russell MA. J. Org. Chem. 1989;54:3321–3324.. Buller MJ, Gilley CB, Nguyen B, Olshansky L, Fraga B, Kobayashi Y. Synlett. 2008:2244–2248.. Isaacson J, Loo M, Kobayashi Y. Org. Lett. 2008;10:1461–1463. doi: 10.1021/ol800245m.. Linda P, Stener A, Cipiciani A, Savelli G. J. Heterocycl. Chem. 1983;20:247–248..
- 5.Shen TY, Lucas S, Sarett LH, Rosegray A, Nuss GW, Willett JD, Ellis RL, Holly FW, Matzuk AR, Wilson AN, Winter CA, Windholz TB, Risley EA, Stammer CH, Holtz WJ, Witzel BE. J. Am. Chem. Soc. 1963;85:488–489. [Google Scholar]
- 6.(a) Chattopadhyay S, Chattopadhyay U, Mathur PP, Saini KS, Ghosal S. Planta Med. 1983;49:252–254. doi: 10.1055/s-2007-969864. [DOI] [PubMed] [Google Scholar]; (b) Ghosal S, Lochan R, Ashutosh, Kumar Y, Srivastava RS. Phytochemistry. 1985;24:1825–1828. [Google Scholar]
- 7.(a) Ghosal S, Saini KS, Frahm AW. Phytochemistry. 1983;22:2305–2309. [Google Scholar]; (b) Ghosal S, Lochan R, Ashutosh, Kumar Y, Srivastava RS. Phytochemistry. 1985;24:1825–1828. [Google Scholar]; (c) Maddry JA, Joshi BS, Ali AA, Newton MG, Pelletier SW. Tetrahedron Lett. 1985;26:4301–4302. [Google Scholar]; (d) Min BS, Gao JJ, Nakamura N, Kim YH, Hattori M. Chem Pharm Bull. 2001;49:1217–1219. doi: 10.1248/cpb.49.1217. [DOI] [PubMed] [Google Scholar]
- 8.For synthesis of amides via coupling of amines and alcohols, see Gunanathan C, Ben-David Y, Milstein D. Science. 2007;317:790–792. doi: 10.1126/science.1145295.. Nordstrøm LU, Vogt H, Madsen R. J. Am. Chem. Soc. 2008;130:17672–17673. doi: 10.1021/ja808129p.. Zweifel T, Naubron J-V, Grützmacher H. Angew. Chem., Int. Ed. 2009;48:559–563. doi: 10.1002/anie.200804757.. Reddy KR, Maheswari CU, Venkateshwar M, Kantam ML. Eur. J. Org. Chem. 2008:3619–3622..
- 9.(a) Arai E, Tokuyama H, Linsell MS, Fukuyama T. Tetrahedron Lett. 1998;39:71–74. [Google Scholar]; (b) Evans DA, Borg G, Scheidt KA. Angew. Chem., Int. Ed. 2002;41:3188–3191. doi: 10.1002/1521-3773(20020902)41:17<3188::AID-ANIE3188>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]; (c) Dixon DJ, Scott MS, Luckhurst CA. Synlett. 2003:2317–2320. [Google Scholar]
- 10.For oxidation of aminals derived from intramolecular scaffolds with chromium-based oxidants, see: Node M, Nagasawa H, Fuji K. J. Org. Chem. 1990;55:517–521..
- 11.Acylation of indoles with carboxylic acids has been reported with heat and extended reaction times: Terashima M, Fujioka M. Heterocycles. 1982;19:91–92.. Additionally, acylation has been reported utilizing DCC as a coupling agent: Bremner JB, Samosorn S, Ambrus JI. Synthesis. 2004:2653–2658..
- 12.(a) Maki BE, Chan A, Phillips EM, Scheidt KA. Org Lett. 2007;9:371–374. doi: 10.1021/ol062940f. [DOI] [PubMed] [Google Scholar]; (b) Maki BE, Scheidt KA. Org. Lett. 2008;10:4331–4334. doi: 10.1021/ol8018488. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Maki BE, Chan A, Phillips EM, Scheidt KA. Tetrahedron. 2009 doi: 10.1016/j.tet.2008.10.033. In press, DOI: 10.1016/j.tet.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For examples of amidation of aldehydes using NHCs, see: Chan A, Scheidt KA. Org. Lett. 2005;7:905–908. doi: 10.1021/ol050100f.. Vora HU, Rovis T. J. Am. Chem. Soc. 2007;129:13796–13797. doi: 10.1021/ja0764052.. Bode JW, Sohn SS. J. Am. Chem. Soc. 2007;129:13798–13799. doi: 10.1021/ja0768136.. Wong FT, Patra PK, Seayad J, Zhang Y, Ying JY. Org. Lett. 2008;10:2333–2336. doi: 10.1021/ol8004276..
- 14.Although the possibility exists for coupling of the indole with a substrate in the carboxylic acid oxidation state (see ref. 11), significant amounts of the carboxylic acid were not observed with prolonged exposure of 3-phenyl-1-propanol or hydrocinnamaldehyde to oxidation conditions in the absence of indole.
- 15.(a) Soueldan M, Collin J, Gil R. Tetrahedron Lett. 2006;47:5467–5470. [Google Scholar]; (b) Dong HM, Lu HH, Lu LQ, Chen CB, Xiao WJ. Adv. Synth. Catal. 2007;349:1597–1603. [Google Scholar]
- 16.(a) Török B, Abid M, London G, Esquibel J, Török M, Mhadgut SC, Yan P, Prakash GKS. Angew. Chem., Int. Ed. 2005;44:3086–3089. doi: 10.1002/anie.200462877. [DOI] [PubMed] [Google Scholar]; (b) Li HM, Wang YQ, Deng L. Org. Lett. 2006;8:4063–4065. doi: 10.1021/ol061552a. [DOI] [PubMed] [Google Scholar]
- 17.Ley SV, Norman J, Griffith WP, Marsden SP. Synthesis. 1994:639–666. [Google Scholar]
- 18.Optimal results were observed with utilization of TPAP freshly prepared via the method of Bailey AJ, Griffith WP, Mostafa SI, Sherwood PA. Inorg Chem. 1993;32:268–271..
- 19.Marko IE, Giles PR, Tsukazaki M, Chelle-Regnaut I, Urch CJ, Brown SM. J. Am. Chem. Soc. 1997;119:12661–12662. [Google Scholar]
- 20.For reviews of green oxidations, see: Sheldon RA, Arends I, Ten Brink GJ, Dijksman A. Acc. Chem. Res. 2002;35:774–781. doi: 10.1021/ar010075n.. Adam W, Saha-Moller CR, Ganeshpure PA. Chem. Rev. 2001;101:3499–3548. doi: 10.1021/cr000019k..
- 21.See supporting information for full procedural and experimental details.
- 22.Dwight TA, Rue NR, Charyk D, Josselyn R, DeBoef B. Org. Lett. 2007;9:3137–3139. doi: 10.1021/ol071308z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boussard MF, Truche S, Rousseau-Rojas A, Briss S, Descamps S, Droual M, Wierzbicki M, Ferry G, Audinot V, Delagrange P, Boutin JA. Eur. J. Med. Chem. 2006;41:306–320. doi: 10.1016/j.ejmech.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Guillaumel J, Leonce S, Pierre A, Renard P, Pfeiffer B, Arimondo PB, Monneret C. Eur. J. Med. Chem. 2006;41:379–386. doi: 10.1016/j.ejmech.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 25.For reports on biological activity of compounds with closely related 6H-isoindolo[2,1-a]indole skeletons, see: Kozikowski AP, Ma D, Brewer J, Sun S, Costa E, Romeo E, Guidotti A. J. Med. Chem. 1993;36:2908–2920. doi: 10.1021/jm00072a010.. Faust R, Garratt PJ, Jones R, Yeh LK, Tsotinis A, Panoussopoulou M, Calogeropoulou T, Teh MT, Sugden D. J. Med. Chem. 2000;43:1050–1061. doi: 10.1021/jm980684+.. Trotter BW, Quigley AG, Lumma WC, Sisko JT, Walsh ES, Hamann CS, Robinson RG, Bhimnathwala H, Kolodin DG, Zheng W, Buser CA, Huber HE, Lobell RB, Kohl NE, Williams TM, Graham SL, Dinsmore CJ. Bioorg. Med. Chem. Lett. 2001;11:865–869. doi: 10.1016/s0960-894x(01)00061-0.. Ikegashira K, Oka T, Hirashima S, Noji S, Yamanaka H, Hara Y, Adachi T, Tsuruha JI, Doi S, Hase Y, Noguchi T, Ando I, Ogura N, Ikeda S, Hashimoto H. J. Med. Chem. 2006;49:6950–6953. doi: 10.1021/jm0610245..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.