Abstract
Acute kidney injury (AKI) activates pathways of cell death and cell proliferation. Although seemingly discrete and unrelated mechanisms, these pathways can now be shown to be connected and even to be controlled by similar pathways. The dependence of the severity of renal-cell injury on cell cycle pathways can be used to control and perhaps to prevent acute kidney injury. This review is written to address the correlation between cellular life and death in kidney tubules, especially in acute kidney injury.
Keywords: apoptosis, cisplatin, cyclin-dependent kinase, necrosis
In a recent study of adult hospital admissions, increases in serum creatinine (0.5 mg per 100 ml) were reported in more than 14% of adult admissions, and were associated with a 6.5-fold increased mortality.1 Despite our increased understanding of the incidence and consequences of acute kidney injury (AKI), morbidity and mortality associated with this syndrome in critically ill patients have remained above 50%.2 AKI is a heterogeneous syndrome, and the possible contributing causes are similarly heterogeneous, ranging from inflammation and inflammatory cytokines3–11 to members of cell death pathways12–15 and mitogen-activated protein kinases.16 There is agreement that the cell death that accompanies AKI contributes significantly to the severity of the syndrome. In addition, we have shown that both apoptotic cell death in vitro and necrotic tubular cell death in vivo are associated with cisplatin cytotoxicity and are dependent on the activation of certain enzymes of the cell cycle (see below). It is therefore appropriate to discuss possible correlations between these two pathways.
KIDNEY CELL DEATH
In multicellular organisms, it is fairly obvious that without life there can be no death. However, this is less obvious on a cellular level. Several types of cell death have been characterized in eukaryotes, primarily by morphological criteria. For tubular cell death in AKI and for cultured kidney cells, the processes of necrosis and apoptosis are most apparent (Figure 1). The morphological differences between these two types of cell death were first described in 1972,17 and the pathways culminating in these different morphologies are active avenues of investigation.18 In the in vitro model of AKI, the form of death observed in kidney cell cultures after cisplatin administration is initially apoptosis, but eventually these cells seem to be necrotic, a morphology characterized as `secondary necrosis.' In rodent models of AKI, both necrotic and apoptotic cells are found, with necrosis primarily found in the S3 segment of proximal tubules, whereas apoptosis occurs in distal tubules. The overall contribution of these two morphologically distinct forms of cell death to that observed in tubules is difficult to determine. First, apoptotic cells in vivo are rapidly engulfed by neighboring cells, whereas necrotic cells are usually not removed efficiently, making direct comparison inaccurate; second, as is found in vitro, apoptotic cells can progress into `secondary necrotic' cells, with death arising from apoptosis, but morphologically similar to necrosis. A significant in vivo distinction between these two morphologies is that necrotic cells lyse, resulting in inflammation, whereas apoptotic cells can be removed before lysis. Inflammation is a major complication of AKI, and Reeves et al.19 found that tumor necrosis factor, a primary contributor to nephrotoxicity, is produced by kidney cells in vivo after cisplatin injection. At the same time, however, experimental evidences linking the apoptotic and necrotic forms of cell death are emerging. It is known that apoptosis is an active process, requiring energy and following distinct metabolic pathways, but similarly, necrotic cell death may also be regulated by a set of signal transduction pathways and catabolic mechanisms and is as well controlled and programmed as apoptosis.20,21 Of particular relevance in kidney cell death, using cultured mouse proximal tubular cells, Lieberthal et al.22 showed that cell death characterized either as primarily apoptosis or as primarily necrosis could be induced by the same agent (cisplatin), showing a possible interrelationship between these two processes. Similarly, activation of a cell-cycle enzyme-dependent pathway common to both cell death pathways can be inferred by our results showing that cyclin-dependent kinase 2 (Cdk2) inhibition can protect both cisplatin-induced apoptosis in vitro and necrosis in vivo (see below). Two other major forms of cell death, that is cornification and autophagy, have been defined by the Nomenclature Committee on Cell Death,23 although it is not certain how much these two forms of cell death contribute to AKI. Specifically, cornification occurs exclusively in the epidermis, whereas autophagy has a clear role in pro-survival pathways, but its role in cell death is less clear,24 and in AKI, autophagy was reported to be cytoprotective.25
Figure 1. Kidney cell death in vivo and in vitro.
Photomicrograph sections of periodic acid-schiff–stained mouse kidney (a and b) showing the corticomedullary junction on the left and the cortex region on the right of each micrograph. Photomicrograph of cultured mouse kidney cells (TKPTS) with Nomarski imaging (c and d) or fixed cells stained with 4′,6′-diamidino-2-phenylindole (DAPI) (e and f). Tissues and cells were from untreated mice and cultures (a, c, and e), from mice 3 days after 20 mg/kg cisplatin injection (b), or from cultures 1 day after the addition of 25 μM cisplatin.
Many of the molecular pathways leading to apoptotic cell death have been extensively studied and elaborated, whereas those leading to cell death by necrosis are not yet as clear. This discussion, therefore, will focus on several aspects of the apoptotic pathways, keeping in mind that these molecular events could be shared by other forms of cell death. A more detailed discussion of apoptosis focused on cisplatin-induced AKI has recently been published.26 Cellular death by apoptosis (Figure 2) proceeds in an orderly fashion and is an active process requiring specific proteins. Apoptotic pathways were first described in the nematode Caenorhabditis elegans27 in which the role of proteases (now called caspases) in cell death was shown. Structurally similar enzymes were soon isolated from human and murine cells.28 Caspases are members of a family of approximately 13 cysteine proteases that fall into two groups, `initiator' and `executioner' caspases. Caspase activation is generally considered to be a `point of no return' in cell death pathways, and even prevention of caspase activity usually just results in diversion into cell death pathways that are independent of these proteases.29–31 However, recently, metabolic roles for active caspases that do not result in cell death have been observed,32 so that caspase activation per se does not determine cell death, and its proapoptotic activity is likely to be dependent on other cellular events. The cascades of cell death are initiated by primarily two origins, either an intrinsic pathway that can start from cytoplasmic events such as endoplasmic reticulum damage and nuclear events such as DNA damage, or an extrinsic pathway that communicates through cell surface death receptors such as the tumor necrosis factor receptor. After initiation, many of the death pathways require disruption of the outer membrane of mitochondria and release of mitochondrial proteins, such as cytochrome c.33 These internal mitochondrial proteins are usually important for various mitochondrial functions, but after release into the cytoplasm, they can activate caspases and DNases. The interaction of the death pathways with the mitochondrial membrane often is mediated by a family of proteins that can either facilitate or interrupt the pathway. Proapoptotic family members, such as Bax and Bak, are believed to form pores in the outer mitochondrial membrane thereby causing release, but antiapoptotic family members, such as Bcl-2 and Bcl-xL, antagonize pore formation.34 Depending on the combination of family members participating in the response, mitochondrial membrane damage can be either alleviated or worsened. One of the still unanswered questions is the mechanism by which a signal initiated by cellular stress is communicated to pro- and antiapoptotic proteins to control the mitochondrial pore. After cytochrome c enters the cytoplasm, it induces a conformational change in Apaf-1 and, together with procaspase-9, forms a heptameric structure (the `apoptosome'),35 activating the initiator protease, caspase-9. Downstream targets of caspase-9 are executioner proteases, caspase-3 and -7. Other pro-apoptotic factors can also be liberated from the mitochondria after outer membrane permeabilization. These proteins include AIF (apoptosis-inducing factor36) and endonuclease G,37 which translocate to the nucleus to participate in cell death that can be independent of caspase activation.
Figure 2. Simplified version of apoptotic cell death pathways.
Extrinsic, receptor-mediated pathway illustrated on the left and intrinsic pathway illustrated on the right. Both pathways depicted converge on the activation of caspase-3, but other caspase-independent pathways also exist.
Although most of the proteins, including proteases, DNases, and activators of pro-death molecules, were first described because of their apoptotic function, almost all are now known to have vital functions unrelated to cell death pathways.38 The roles of `death' proteins in cellular life, and the role of a metabolic protein, cytochrome c, in death pathways, illustrate the conservation in evolution resulting in seemingly unrelated and possibly conflicting functions for proteins. We now provide substantial evidence that a protein originally identified as necessary for cell division, Cdk2, is required for pathways of both apoptosis and necrosis in the kidney.
CELL CYCLE EVENTS
When we talk about cell death in relation to the kidney, its importance is obvious, especially in the setting of AKI. But less than 1% of kidney tubular cells are proliferating, and this small percentage declines even further with age.39 Several years ago, it was noted that in response to injury, many normally quiescent kidney cells enter the cell cycle (Figure 3). This process begins shortly after injury, in which both necrotic cells and replicating cells line the injured proximal tubules. There are increases in nuclear proliferating cell nuclear antigen (PCNA),40–42 incorporation of 3H-thymidine and 5-bromo-2-deoxyuridine into nuclear DNA, and induction of mRNA for `immediate-early' genes, for example c-fos, c-jun, and egr-1,43,44 whose expression is frequently associated with the entry of cells from quiescence into the cell cycle. It is clear that damage of cells that results in cell death also results in cell proliferation. It is reasonable to assume that these events occur because of cell stress and/or cell death, but it is not certain the extent to which they contribute to injury or to protection and recovery.
Figure 3. Cell-cycle analysis of kidney after cisplatin injection.
(a) Immunodetection of nuclear BrdU incorporation 4 days after injection and (b) that of nuclear PCNA localization 1 day after injection. Sections were from p21(−/−) mice. BrdU, 5-bromo-2-deoxyuridine.
Cell division is a carefully synchronized sequence of events in which genomic DNA is replicated, usually followed by equal separation of the genome into two similar cells. The cell division cycle was initially divided into two phases: mitotic phase and interphase, which is the period between mitoses. In 1953, James Watson and Francis Crick elegantly described their model for DNA structure and suggested a mechanism for replication by noting that `it has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material.'45 At about the same time, Howard and Pelc,46 working with Vicia faba root tips, found that radioactive phosphorus was incorporated into DNA only during a distinct segment of the interphase, which was separated in time from the beginning to the end of mitosis. This observation ultimately led to the division of the cell cycle into four phases: the mitotic phase M, the DNA synthetic phase S, and the pre- and post-DNA synthetic phases, G1 and G2, respectively.
An important aspect of the cycle is the necessity for it to proceed only in one direction, ensuring that only one DNA synthetic phase occurs for each mitotic phase. The first set of controls for this system was discovered by Tim Hunt and Tom Evans47 at the 1982 Physiology course at Woods Hole. They reported a protein, later identified as cyclin B, that was continuously synthesized after fertilization of sea urchin eggs and then almost completely degraded about 10 min before the eggs divided. Characterization of other `cyclic' proteins steadily accumulated. These observations established that some cell cycle regulatory proteins are periodically synthesized and degraded during the cycle, which helps maintain unidirectional progression. It was shown that cyclin D is synthesized in early G1 and degraded as cells exit G1 and enter S phase. Cyclin E is synthesized in late G1 and is degraded in S, and cyclin A is synthesized in S and is degraded as cells progress through S and exit G2. Cyclin B is synthesized in late G2 and degraded during late M phase, before the cells exit mitosis. The cyclins were found to act as heterodimers in combination with Cdk subunits, which had been found several years previously in yeast48,49 and, by homology, in humans.50 The Cdk subunit is a serine/threonine protein kinase51 that is inactive unless associated with a cyclin. The binding of the cyclin to its Cdk confers basal kinase activity;52,53 full activity is dependent on its phosphorylation at certain residues and its dephosphorylation at other residues.54 In vertebrates, several different cyclins and Cdk partners are sequentially present and active throughout the cell cycle, with cyclin D pairing with Cdk4/6, cyclin E with Cdk2, cyclin A with Cdk2 and then with Cdk1, and cyclin B with Cdk1. The observations that different cyclin-Cdk pairings caused sequential activation of different Cdks at various stages of the cell cycle and experiments with antibody injection, antisense and dominant-negative repression55 of these proteins resulted in the view of the cycle that these different cyclins and Cdks were essential for cell cycle progression. This would be a more complex system than is observed in yeast, in which only one Cdk is active during the cycle.
However, recent work using mouse knockout techniques reveals a much different story, in which most of the cyclins, with the exception of cyclin A2 and cyclin B1 (there are two cyclin A and three cyclin B genes known), and Cdk2, -4, and -6, with the exception of Cdk1, can be individually knocked out without a major phenotype. For the Cdks, even a triple knockout of Cdk2, -4, and -6 does not prevent cell cycle progression in mouse embryo fibroblasts. Rather than being essential for cell cycle activity, these Cdks and cyclins were found to be important for specific cellular functions and/or specific organogenesis. For example, cyclin E was found important for cells to enter the cycle after quiescence, Cdk2 was found essential for male and female fertility, inferring a requirement during meiosis, Cdk4 for pancreatic β cells, anterior pituitary cells, and Leydig cell development, and Cdk6 for complete erythroid differentiation. Double knockouts of Cdks were found to be important in different organogeneses: Cdk4 plus Cdk6 for hematopoietic development and Cdk2 plus Cdk4 for cardiomyocyte proliferation. This emerging picture has prompted Hunt and co-workers56 to propose a `threshold' model of cell cycle control in which different localizations and increasing Cdk activity during the cell cycle control progression from G1 phase through mitosis. A similar model in fission yeast was promoted 10 years previously by Stern and Nurse.57 Recently, Philipp Kaldis58 found that genetic substitution of Cdk1 by Cdk2 did not restore the viability of early mouse embryos, even though Cdk2 was expressed from the Cdk1 locus. This has shown the importance of molecular specificity of the cyclin-Cdk heterodimer, at least for Cdk1 and Cdk2, or alternately, in keeping with a threshold model, that Cdk2 may not have as high an intrinsic kinase activity as does Cdk1. Regardless of the degree to which the Cdks and cyclins are redundant, there is agreement that the kinase activity of the Cdks and possibly other protein kinases either permits or prevents progression of the cell cycle from one event to another, but for most of the substrates, it is not clear how this control occurs. The complexity of the process was recently revealed by Dephoure et al.,59 who identified over 14,000 phosphorylation events involving more than 3600 proteins for one round of the cell cycle.
In addition to the orderly progression from one cell cycle stage to the next, several protein effectors respond to DNA damage, incomplete DNA synthesis, and successful completion of mitosis. The idea of `checkpoints' throughout the cell cycle was initially proposed by Hartwell and Weinert,60 after their finding that some yeast mutants failed to arrest the cell cycle in response to radiation. A similar DNA damage checkpoint was found in human cells involving the accumulation of the p53 protein61 in response to damaged DNA in which cells were prevented from cell cycle progression. These checkpoint effectors, which also go under the name of cyclin-dependent kinase inhibitors, primarily control the entry of cells into S and M phases both by inhibiting and by stimulating Cdk activity. In humans and other vertebrates, there are two families of these proteins. The first protein characterized from these families was p21, a 21-kDa protein62 that is constitutively expressed at low levels. The family also contains p2763–66 and p57.67,68 The p21 cDNA was cloned simultaneously by three groups, each with a different assay for its presence, revealing the spectrum of activities of the p21 protein. The separate criteria were induction by p53,69 cyclin kinase inhibition,70,71 and induction of senescence.72 Overexpression of p21 has other effects on cell fate, ranging from cell cycle interruption62,71,73–75 to activation,76 from terminal differentiation77–82 to inhibition of regeneration83 and induction of cellular senescence.84 The events controlled by p21 are likely the product of the cell type and the circumstances of its induction. In normal replicative cycles, p21 and p27 are bound to Cdk2, repressing progression into late G1 and S. As the abundance and the activity of the primary G1 cyclin-Cdk (cyclin D-Cdk4/6) rises, this complex binds to p21/27, effectively reducing the amount of p21/27 available for binding and inhibiting cyclin-Cdk2. In late G1, active Cdk2 phosphorylates p21/27, starting the process of p21/27 ubiquitination and proteasomal degradation.85 It is remarkable that in the combined absences of Cdk4, Cdk6, and Cdk2, the coordination of p21/27 titration, inhibition, and degradation can be accomplished solely by Cdk1. Perhaps in view of these knockout results, our view of this level of control will have to be modified.
A second family of small-molecular-weight proteins, the `INK4' family (inhibitors of Cdk4), ranging from 14 to 19 kDa, bind the kinase subunit of the cyclin D heteroduplex (Cdk4/6), and arrest the cell cycle in the G1 phase.73,86–89 The p16INK4A-p15INK4B locus occupies a small region (35 kb) of the human genome and is deleted in a wide spectrum of tumors including melanoma, pancreatic adeno-carcinoma, glioblastoma, certain leukemias, non-small-cell lung cancer, and bladder carcinoma (reviewed in Kim and Sharpless90). Other members of this family have not been associated with tumor suppression and although differentially expressed during development, their functions may be redundant.
The relevance of the cell cycle and its controls is particularly important in cultured cells and models of AKI using these in vitro methods. However, most cells in kidney in vivo are quiescent and enter the cell cycle only after stimulation, such as that induced by stress. The distinction between cycling and quiescent (and senescent) cells is actively being investigated.91 Comparisons between quiescent and cycling cells in vitro rely on quiescence being induced by cell cycle inhibitors, withdrawal of growth factors, and so on. Similarly, comparisons between in vivo and in vitro models of AKI rely on comparing quiescent and actively dividing cells. These comparisons can result in generalizations being based on `apples versus oranges' logic. For example, as described above, cisplatin-induced toxicity results primarily in apoptotic cell death in vitro but necrotic cell death in vivo. We argue that this difference merely reflects the environment and cell cycle characteristics of the stressed cells and not the activation of different cell death pathways having no relevant comparison. But regardless of the accuracy of the comparisons, the underlying mechanism(s) of cytotoxicity affecting cell death will provide insights into the prevention and cure of AKI.
CYCLIN INHIBITOR INDUCTION AND ITS INFLUENCE IN AKI
When cells of the injured kidney enter the cell cycle, there is a rapid induction of p21, but not other family members, in several models of AKI (Figure 4a), such as cisplatin exposure, ischemia–reperfusion, and ureteral obstruction.92 The p21 protein was localized to both distal and proximal tubule cells by immunohistochemistry. We studied the effect(s) of p21 induction in AKI by comparing wild-type p21(+/+) mice with mice homozygous for a p21 gene deletion. 5-bromo-2-deoxyuridine incorporation into nuclear DNA and increases of PCNA content were much higher after AKI in kidney cells of p21(−/−) mice, compared with p21(+/+) mice. The picture that emerged was that stressing the kidney caused cells to enter the cell cycle, whereas at the same time, the stress induced a protein identified as a cell cycle inhibitor. But another role of p21 was also apparent: p21 induction ameliorated AKI.40,41 Following either cisplatin administration or ischemia–reperfusion, compared with their p21(+/+) littermates, p21(−/−) mice developed more severe morphological damage (Figure 4b,c), displayed a more rapid onset of the physiological signs of AKI (Figure 4d), and had a higher mortality (Figure 4e). Miyaji et al.93 speculated that p21 induction could contribute to acquired resistance from cisplatin-induced AKI. Similarly, Nath and co-workers94 found that LLC-PK1 kidney cells were resistant to several apoptotic stimuli by heme oxygenase-1 overexpression. They proposed that this resistance is conferred by p21 upregulation. These studies were recently extended by Nath et al.95 using a rat proximal tubule cell culture in which blocking the apoptotic effects of hemin was correlated with p21 induction.
Figure 4. Expression and consequences of p21 expression in vivo after AKI.

Northern blot analysis of p21 mRNA transcripts in rat kidney cells (a). Control, untreated kidney. Ischemia, kidney collected from rats after renal ischemia, in which rats were made ischemic for 50 min and killed after reflow of 0 and 30 min, 1, 2, 4, 24, and 48 h. Obstruction, kidney obtained from rats after 24 h unilateral and or bilateral ureteral obstruction. Cisplatin, kidney obtained from rats after treatment with 7.5 mg/kg cisplatin for 6, 12, 24, and 72 h. All lanes had the same amount of mRNA, as assessed by hybridization to probe specific for constitutively expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (not shown). Immunochemical localization of p21 protein in kidney after 50 min of renal ischemia. Sections represent kidney from untreated mice (b) and from mice 2 days after ischemia (c). Urea nitrogen levels (d) in serum (blood urea nitrogen (BUN)) after cisplatin administration. Values, in mg per 100 ml, at each time point represent means (±s.e.) of at least six mice. Statistically significant differences (P<.05) are indicated. Kaplan–Meier survival curve comparing the survival of p21(+/+) and p21(−/−) mice after 50 min of renal ischemia (e). AKI, acute kidney injury.
Microarray analysis of mRNA before and up to 3 days after cisplatin administration revealed that p21 is the most highly upregulated gene after exposure (Price, PM et al., unpublished results). As p21 is induced to such high levels after AKI, it was not apparent whether expression before AKI would protect any more than the endogenous gene activation. However, using cultured kidney proximal tubule cells and adenoviral transduction, we expressed p21 before cisplatin was administered and found that it protected from apoptosis96 (Figure 5a–c). This type of induction is technically difficult in vivo, so we approached the problem of the in vivo model by first determining the mechanism of this protection. p21 binds to many proteins, but its simple structure is conducive to fragmentation, and we showed that only the Cdk2-binding moiety of p21 was protective97 (Figure 5d–h). This Cdk2 dependence was further shown by Cdk2 inhibitory drugs and by dominant-negative Cdk2 transduction. One of the drugs, purvalanol, was used in vivo, and it also protected from cisplatin nephrotoxicity.98 For the final proof that cisplatin cytotoxicity and nephrotoxicity was dependent on Cdk2, we relied on transgenics that expressed either p21 or dominant-negative Cdk2 under the control of a proximal tubule-specific testosterone-inducible promoter, which was obtained from Curt Sigmund. Induction of either transgene protected from cisplatin nephrotoxicity (Figure 6). These findings showed an unexpected relationship between cell cycle, that is cell `life' and cell death pathways: pathways of cell death are dependent on a cell cycle protein. These findings also highlight a similarity between the cell death pathways of apoptosis and necrosis. As both cisplatin-induced necrosis and apoptosis were dependent on Cdk2, at the least this enzyme activity is in common, even though the substrate(s) in these death pathways can be different and remains to be determined.
Figure 5. Fluorescence-activated cell sorter (FACS) analysis and light microscopy of TKPTS cells of the effect of different domains of p21 on the protection of cisplatin-induced cell death.
Analyses were performed to quantify cell death of TKPTS cells without or with 25 μM cisplatin in the absence or presence of adenovirus encoding p21 full-length or truncations as green fluorescent protein (GFP) fusion protein. For FACS analysis, TKPTS cells were harvested by trypsinization and collected by centrifugation. Cells were fixed and treated with RNase A and propidium iodide and analyzed using FACSCalibur. For each culture condition, 105 cells were analyzed. The parts of the FACS analyses representing different phases of the cell cycle are indicated. Cells in the phase of sub-G1/G0 are classified as the apoptotic fraction. TKPTS cells were photographed using Hoffman optics before harvesting. (a) Untreated cells. (b) Cells treated for 24 h with cisplatin. (c) Cells treated with full-length p21 adenovirus for 24 h and then for 24 h with cisplatin. (d) Cells treated with amino-terminal p21 adenovirus for 24 h and then for 24 h with cisplatin. (e) Cells treated with carboxy-terminal p21 adenovirus for 24 h and then for 24 h with cisplatin. (f) Cells treated with p21 amino acids 1–45 adenovirus for 24 h and then for 24 h with cisplatin. (g) Cells treated with p21 amino acids 38–91 adenovirus for 24 h and then for 24 h with cisplatin. (h) Representation of various p21 constructions with different protein-interacting domains. Adenovirus encoding full-length, amino-terminal, carboxy-terminal, amino acids 1–45, and amino acids 38–91 of p21 as green fluorescent protein (GFP) fusion proteins with or without different functional domains is shown. cdk2: cdk2-binding domain; PCNA: PCNA-binding domain; procasp-3: procaspase-3-binding domain; NLS: nuclear localization sequence.
Figure 6. Effect of cdk2 inhibition either by p21 or by dominant-negative cdk2 (DN-cdk2) expression on cisplatin-induced AKI.

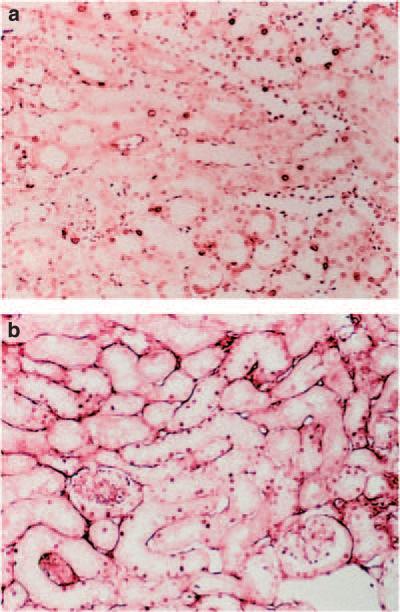
The p21 and DN-cdk2 cDNA transgenes were constructed as GFP fusion proteins and were expressed using a testosterone-inducible kidney androgen protein (KAP2) promoter. Relative functional damage 1, 2, and 3 days after 20 mg/kg cisplatin represented as blood urea nitrogen (BUN) (a) and creatinine (b) in the serum. Relative morphological damage 3 days after 20 mg/kg cisplatin shown as photomicrograph sections of periodic acid-schiff–stained mouse kidney from wild-type (c) and testosterone-induced p21-GFP transgenic (d). The corticomedullary junction is on the right and the cortex region is on the left of each micrograph. AKI, acute kidney injury; GFP, green fluorescent protein.
The use of cell cycle inhibitors to ameliorate AKI, in which cell cycle activity should be beneficial to replace damaged cells to restore lost function, seems to be counter-intuitive. In fact, growth factors such as erythropoietin, hepatocyte growth factor, and insulin-like growth factor-1 have been used to lessen injury and/or accelerate recovery from AKI in rodents (cited in Jo et al.99). The mechanism of the protective effect by these agents has not been determined, but could be a combination of increased proliferation of resident or bone marrow endothelial progenitor cells, or reduced inflammatory response to tissue damage. It is possible that a combination of treatments, in which Cdk2 inhibition can be used to lessen cell death, and growth factor used to stimulate recovery, will eventually be most effective in AKI.
OTHER POSSIBLE CONNECTIONS OF CDK/CELL CYCLE ACTIVITY AND RENAL DISEASES
A recent review focused on the use of Cdk inhibitors to treat renal diseases.100 It is not surprising that most of these diseases are associated with kidney cell proliferation, and it is encouraging that these inhibitors show promise in a wide variety of kidney-specific syndromes. The first instance of the use of a Cdk inhibitor to treat a renal disease was in 1997, for the treatment of mesangial proliferative glomerulonephritis.101 This pioneering investigation soon was expanded to include antiproliferative actions on podocytes in experimental crescentic glomerulonephritis102 and collapsing glomerulopathy.103 The targeting of podocytes infected with human immunodeficiency virus-1 using Cdk inhibition was also effective to promote podocyte differentiation and limit proliferation.104–106 Recently, Cdk inhibition was found useful to treat murine polycystic kidney disease,107 although the long-term use of the particular drug used (roscovitine) was shown to have deleterious side effects in a phase 2 trial in IgA nephropathy.108
The finding that p21 but not other cyclin kinase family members are induced in cortical tubules after AKI is most likely a recapitulation of developmental expression. We reported that in a 12.5-day mouse embryo kidney, p21 expression is highest in comma- and S-shaped bodies, and even at E18.5, the expression is still high in S-shaped bodies of the outer cortex; there was little if any expression in the medulla or glomeruli.109 This is in marked contrast to the developmental expression of p27, which was primarily localized to the visceral epithelial cells of differentiating glomeruli in human fetal kidney.110 As inhibitors of Cdk2, both proteins should be equally cytoprotective after AKI, similar to pharmacologic Cdk2 inhibitors,98 but their beneficial effects could be altered by other functions of these proteins.
FUTURE DIRECTIONS
As an approach to understand the mechanism of Cdk2 dependence, we are starting to identify the substrates of Cdk2 that could be related to cell death pathways. A known substrate of Cdk2 is the retinoblastoma protein, pRb, a repressor of E2F transcription factors. Phosphorylation of pRb by Cdk2 dissociates it from its inhibitory interaction with E2F, and E2F-mediated transcription is activated, primarily during the G1–S transition of the cell cycle. A member of the E2F family, E2F-1, which is associated with both cell cycle stimulation and apoptosis, was upregulated after cisplatin treatment, both in vitro and in vivo.111 A knockout for this gene provided significant protection on both renal function and renal morphology in mice. Although E2F-1 is not a direct substrate of Cdk2, it is activated by Cdk2 and it affects both cell cycle and cell death pathways with relevance in AKI. Although protection is afforded in vitro by E2F-1 interference and in vivo by E2F-1 knockout, protection is not as complete as by Cdk2 inhibition, suggesting that other substrates exist.
Ultimately, a complete understanding of the interactions of cell cycle proteins with cell death pathways will likely begin from determinations of Cdk2 substrates. Several recently developed methodologies,112,113 as well as mass spectrometry,114 are natural candidates to explore this direction. We published that Cdk2 is localized in both the nucleus and cytoplasm in cultured kidney cells115 (Figure 7), confirming an earlier finding of Shankland.116 Furthermore, cisplatin-induced apoptosis in enucleated cells was prevented by Cdk2 inhibition. We infer from this that a Cdk2 substrate, essential to cell death pathways, is localized in the cytoplasm.
Figure 7. Subcellular localization of cdk2-green fluorescent protein (GFP) fusion protein.
Colocalization of cdk2-GFP (green) and nuclear PCNA (red) appears as yellow.
At the same time that it becomes possible to ameliorate AKI, it is important to keep in mind that recovery from the injury should not be compromised, nor, as in the case of cisplatin-induced AKI, interfering in the extrarenal function of the drug. As was pointed out by Pabla and Dong,26 renoprotective strategies for cisplatin should not be at the expense of the antitumor efficacy of the cisplatin therapy. In the case of Cdk inhibitors as protectors against cisplatin-induced AKI, the known activities of these inhibitors should actually augment rather than reduce the effectiveness of chemotherapy, as at least 10 of these drugs are in clinical trials as cancer chemotherapeutics.117–119
CONCLUSION
AKI results in cell cycle activation, which promotes injury by Cdk2 induction and prevents injury by p21 induction. Drugs that mimic the action of p21 to inhibit Cdk2 activity can be used to protect from cisplatin-induced nephrotoxicity. The actual mechanism of the relationship of Cdk2 with cell death and whether we can interfere in this mechanism to improve human health presents an important problem. We will eventually understand how these cell cycle activities control the cell death pathways and be able to use this knowledge to prevent and treat AKI. A paraphrased comment by the physicist Ernest Rutherford will then be appropriate: `All of science is either impossible or trivial. It is impossible until you understand it, and then it becomes trivial.'
ACKNOWLEDGMENTS
We thank Zheng Dong (Medical College of Georgia and Charlie Norwood VA Medical Center, Augusta, Georgia, USA) and Philipp Kaldis (Institute of Molecular and Cell Biology, Proteos, Singapore) for reviewing the manuscript before submission. This work was supported in part by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-54471) and a VA Merit Review and with resources and the use of facilities at the John L McClellan Memorial Veterans' Hospital (Little Rock, AR, USA).
Footnotes
DISCLOSURE All the authors declared no competing interests.
REFERENCES
- 1.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 3.Okusa M. The inflammatory cascade in acute ischemic renal failure. Nephron. 2002;90:133–138. doi: 10.1159/000049032. [DOI] [PubMed] [Google Scholar]
- 4.Ramesh G, Reeves WB. TNF-α mediates cytokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day YJ, Huang L, Ye H, et al. Renal ischemia–reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–F731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 6.Oh D-J, Dursun B, He Z, et al. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol. 2008;294:F264–F271. doi: 10.1152/ajprenal.00204.2007. [DOI] [PubMed] [Google Scholar]
- 7.Del Rio M, Imam A, DeLeon MG, et al. The death domain of kidney ankyrin interacts with Fas and promotes Fas-mediated cell death in renal epithelia. J Am Soc Nephrol. 2004;15:41–51. doi: 10.1097/01.asn.0000104840.04124.5c. [DOI] [PubMed] [Google Scholar]
- 8.Hamar P, Song E, Kokeny G, et al. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2004;101:14883–14888. doi: 10.1073/pnas.0406421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 10.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 11.Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang M, Pabla N, Murphy RF, et al. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem. 2007;282:2636–2645. doi: 10.1074/jbc.M606928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Q, Dong G, Yang T, et al. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol. 2007;293:F1282–F1291. doi: 10.1152/ajprenal.00230.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushal GP, Kaushal V, Hong X, et al. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001;60:1726–1736. doi: 10.1046/j.1523-1755.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 15.Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther. 2002;302:8–17. doi: 10.1124/jpet.302.1.8. [DOI] [PubMed] [Google Scholar]
- 16.DiMari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol Renal Physiol. 1999;277:F195–F203. doi: 10.1152/ajprenal.1999.277.2.F195. [DOI] [PubMed] [Google Scholar]
- 17.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Ramesh G, Norbury CC, et al. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor produced by renal parenchymal cells. Kidney Int. 2007;72:37–44. doi: 10.1038/sj.ki.5002242. [DOI] [PubMed] [Google Scholar]
- 20.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-characterized form of cell demise: signaling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Lieberthal W, Triaca V, Levine JS. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs necrosis. Am J Physiol Renal Physiol. 1998;70:F700–F708. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- 23.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendation of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Periyasamy-Thandavan S, Jiang M, Wei Q, et al. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–640. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 26.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 27.Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Shaman S, Ledoux S, et al. The C elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 29.Hitomi J, Christofferson DE, Ng A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gouch NR. Roads to death city. Sci Signal. 2009;2:ec3. [Google Scholar]
- 31.Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE. 2006;2006:pe44. doi: 10.1126/stke.3582006pe44. [DOI] [PubMed] [Google Scholar]
- 32.Yi CH, Yuan J. The Jekyll and Hyde functions of caspases. Dev Cell. 2009;16:21–34. doi: 10.1016/j.devcel.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Kim CN, Yang J, et al. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 34.Bormer C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615–647. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 36.Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 37.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 38.Galluzzi L, Joza N, Tasdemir E, et al. No death without life: vital functions of apoptotic effectors. Cell Death Differ. 2008;15:1113–1123. doi: 10.1038/cdd.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt R, Cantley LG. The impact of aging on kidney repair. Am J Physiol Renal Physiol. 2008;294:F1265–F1272. doi: 10.1152/ajprenal.00543.2007. [DOI] [PubMed] [Google Scholar]
- 40.Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1997;101:777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Megyesi J, Andrade L, Vieira J, et al. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int. 2001;60:2164–2172. doi: 10.1046/j.1523-1755.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 42.Witzgall R, Brown D, Schwarz C, et al. Localization of proliferating cell nuclear antigen, vimentin, c-fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safirstein R, Price PM, Saggi SJ, et al. Changes in gene expression after temporary renal ischemia. Kidney Int. 1990;37:1515–1521. doi: 10.1038/ki.1990.143. [DOI] [PubMed] [Google Scholar]
- 44.Ouellette AJ, Malt RA, Sukhatme VP, et al. Expression of two `immediate early' genes, Egr-1 and c-fos, in response to renal ischemia and during renal hypertrophy. J Clin Invest. 1990;85:766–771. doi: 10.1172/JCI114502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson JD, Crick FHC. Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 46.Howard A, Pelc SR. Synthesis of DNA in normal and irradiated cells and its relation to chromosome breakage. Heredity Suppl. 1953;6:261–273. [Google Scholar]
- 47.Evans T, Rosenthal ET, Youngbloom J, et al. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 48.Hartwell LH, Culotti J, Pringle JR, et al. Genetic control of the cell division cycle in yeast. Science. 1974;183:46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- 49.Nurse P, Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981;292:558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- 50.Lee MG, Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987;327:31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- 51.Lorincz AT, Reed SI. Primary structure homology between the product of yeast division control gene CDC28 and vertebrate oncogenes. Nature. 1984;307:183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- 52.Jeffrey PD, Russo AA, Polyak K, et al. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 53.Connell-Crowley L, Solomon MJ, Wei N, et al. Phosphorylation-independent activation of human cyclin-dependent kinase 2 by cyclin A in vitro. Mol Biol Cell. 1993;4:79–92. doi: 10.1091/mbc.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fisher RP, Morgan DO. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994;78:713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 55.Van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 56.Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol. 2008;9:910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- 57.Stern B, Nurse P. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet. 1996;12:345–350. [PubMed] [Google Scholar]
- 58.Satyanarayana A, Berthet C, Lopez-Molina J, et al. Genetic substitution of Cdk1 by Cdk2 leads to embryonic lethality and loss of meiotic function of Cdk2. Development. 2008;135:3389–3340. doi: 10.1242/dev.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dephoure N, Zhou C, Villén J, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartwell L, Weinert T. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 61.Kastan MB, Onyerkwere O, Sidransky D, et al. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;53:6304–6311. [PubMed] [Google Scholar]
- 62.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- 63.Polyak K, Kato J, Solomon MJ, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-B and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 64.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin/cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 65.Slingerland JM, Hengst L, Pan C-H, et al. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor-arrested epithelial cells. Mol Cell Biol. 1994;14:3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koff A, Ohtsuki M, Polyak K, et al. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-β. Science. 1993;260:536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- 67.Lee M-H, Reynisdóttir I, Massagué J. Cloning of p57KIP2 a cyclin-dependent kinase inhibitor with unique domain structure tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 68.Matsuoka S, Edwards MC, Bai C, et al. p57KIP2 a structurally distinct member of the p21CIP1 Cdk inhibitor family is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- 69.El-Deiry WS, Tokino T, Velculescu VE, et al. WAF-1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 70.Harper JW, Adami GR, Wei N, et al. The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 71.Gu Y, Turck CW, Morgan DO. Inhibition of cdk2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- 72.Noda AF, Ning Y, Venable S, et al. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 73.Guan K-L, Jenkins CW, Li Y, et al. Growth suppression of p18,a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 74.Duli V, Kaufmann WK, Wilson S, et al. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 75.El-Deiry WS, Harper JW, O'Conner PM, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 76.Cheng M, Olivier P, Diehl JA, et al. The p21Cip1 and p27Kip1 CDK `inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Halevy O, Novitch BG, Spicer DB, et al. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by myoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 78.Parker SB, Eichele G, Zhang P, et al. p53-Independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 79.Skapek SX, Rhee J, Spicer DB, et al. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- 80.Steinman RA, Hoffman B, Iro A, et al. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994;9:3389–3396. [PubMed] [Google Scholar]
- 81.Jiang H, Lin J, Su ZZ, et al. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene. 1994;9:3397–3406. [PubMed] [Google Scholar]
- 82.Macleod KF, Sherry N, Hannon G, et al. p53-Dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 83.Zhang W, Grasso L, McClain CD, et al. p53-independent induction of WAF1/CIP1 in human leukemia cells is correlated with growth arrest accompanying monocyte/macrophage differentiation. Cancer Res. 1995;55:668–674. [PubMed] [Google Scholar]
- 84.Wu H, Wade M, Krall L, et al. Targeted in vivo expression of the cyclin-dependent kinase inhibitor p21 halts hepatocyte cell-cycle progression, postnatal liver development, and regeneration. Genes Dev. 1996;10:245–260. doi: 10.1101/gad.10.3.245. [DOI] [PubMed] [Google Scholar]
- 85.Zhu H, Nie L, Maki CG. Cdk2-dependent inhibition of p21 stability via a C-terminal cyclin-binding motif. J Biol Chem. 2005;280:29282–29288. doi: 10.1074/jbc.M407352200. [DOI] [PubMed] [Google Scholar]
- 86.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 87.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 88.Hirai H, Roussel MF, Kato J, et al. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan FKM, Zhang J, Cheng L, et al. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol Cell Biol. 1995;15:2682–2688. doi: 10.1128/mcb.15.5.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by transcriptional repressor HES1. Science. 2008;321:1095–1100. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Megyesi J, Udvarhelyi N, Safirstein RL, et al. p 53-independent activation of transcription of p21WAF1/CIP1/SDI1 after acute renal failure. Am J Physiol. 1995;271:F1211–F1216. doi: 10.1152/ajprenal.1996.271.6.F1211. [DOI] [PubMed] [Google Scholar]
- 93.Miyaji T, Kato A, Yasuda H, et al. Role of the increase in p 21 in cisplatin ciinduced acute renal failure in rats. J Am Soc Nephrol. 2001;12:900–908. doi: 10.1681/ASN.V125900. [DOI] [PubMed] [Google Scholar]
- 94.Inguaggiato P, Gonzalez-Michaca L, Croatt AJ, et al. Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int. 2001;60:2181–2191. doi: 10.1046/j.1523-1755.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez-Michaca L, Farrugia G, Croatt AJ, et al. Heme: a determinant of life and death in tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F370–F377. doi: 10.1152/ajprenal.00300.2003. [DOI] [PubMed] [Google Scholar]
- 96.Price PM, Safirstein RL, Megyesi J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am J Physiol Renal Physiol. 2004;286:F378–F384. doi: 10.1152/ajprenal.00192.2003. [DOI] [PubMed] [Google Scholar]
- 97.Yu F, Megyesi J, Safirstein RL, et al. Identification of the functional domain of p 21WAF1/Cip1 that protects from cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2005;289:F514–F520. doi: 10.1152/ajprenal.00101.2005. [DOI] [PubMed] [Google Scholar]
- 98.Price PM, Yu F, Kaldis P, et al. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J Am Soc Nephrol. 2006;17:2434–2442. doi: 10.1681/ASN.2006020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 100.Obligado SH, Ibraghimov-Beskrovnaya O, Zuk A, et al. CDK/GSK-3 inhibitors as therapeutic agents for parenchymal renal diseases. Kidney Int. 2008;73:684–690. doi: 10.1038/sj.ki.5002731. [DOI] [PubMed] [Google Scholar]
- 101.Pippin JW, Qu Q, Meijer L, et al. Direct in vivo inhibition of the nuclear cell cycle cascade in experimental mesangial proliferative glomerulonephritis with Roscovitine, a novel cyclin-dependent kinase antagonist. J Clin Invest. 1997;100:2512–2520. doi: 10.1172/JCI119793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Griffin SV, Krofft RD, Pippin JW, et al. Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int. 2005;67:977–986. doi: 10.1111/j.1523-1755.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 103.Gherardi D, D'Agati V, Chu TH, et al. Reversal of collapsing glomerulopathy in mice with the cyclin-dependent kinase inhibitor CYC 202. J Am Soc Nephrol. 2004;15:1212–1222. doi: 10.1097/01.asn.0000124672.41036.f4. [DOI] [PubMed] [Google Scholar]
- 104.Nelson PJ, Gelman IH, Klotman PE. Suppression of HIV-1 expression by inhibitors of cyclin-dependent kinases promotes differentiation of infected podocytes. J Am Soc Nephrol. 2001;12:2827–2831. doi: 10.1681/ASN.V12122827. [DOI] [PubMed] [Google Scholar]
- 105.Nelson PJ, Sunamoto M, Husain M, et al. HIV-1 expression induces cyclin D1 expression and pRb phosphorylation in infected podocytes: cell-cycle mechanisms contributing to the proliferative phenotype in HIV-associated nephropathy. BMC Microbiol. 2002;2:26. doi: 10.1186/1471-2180-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nelson PJ, D'Agati VD, Gries JM, et al. Amelioration of nephropathy in mice expressing HIV-1 genes by the cyclin-dependent kinase inhibitor flavopiridol. J Antimicrob Chemother. 2003;51:921–929. doi: 10.1093/jac/dkg175. [DOI] [PubMed] [Google Scholar]
- 107.Bukanov NO, Smith LA, Klinger KW, et al. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 108.Nelson PJ, Shankland SJ. Therapeutics in renal disease: the road ahead for antiproliferative targets. Nephron Exp Nephrol. 2006;103:e6–e15. doi: 10.1159/000090138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Price PM, Megyesi J, Udvarhelyi N, et al. Induction of terminal differentiation in kidney tubules by the p21 cyclin-dependent kinase inhibitor. J Am Soc Nephrol. 1996;7:1603. (abstract) [Google Scholar]
- 110.Combs HL, Shankland SJ, Setzer SV, et al. Expression of the cyclin kinase inhibitor, p27kip1, in developing and mature human kidney. Kidney Int. 1998;53:892–896. doi: 10.1111/j.1523-1755.1998.00842.x. [DOI] [PubMed] [Google Scholar]
- 111.Yu F, Megyesi J, Safirstein RL, et al. Involvement of the cdk2-e2f1 pathway in cisplatin cytotoxicity in vitro and in vivo. Am J Physiol Renal Physiol. 2007;293:F52–F59. doi: 10.1152/ajprenal.00119.2007. [DOI] [PubMed] [Google Scholar]
- 112.Ubersax JA, Woodbury EL, Quang PN, et al. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425:859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- 113.Zhang C, Kenski DM, Paulson JL, et al. A second-site suppressor strategy for chemical genetic analysis of diverse protein kinases. Nat Methods. 2005;2:435–441. doi: 10.1038/nmeth764. [DOI] [PubMed] [Google Scholar]
- 114.Archambault V, Chang EJ, Drapkin BJ, et al. Targeted proteomic study of the cyclin-cdk module. Mol Cell. 2004;14:699–711. doi: 10.1016/j.molcel.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 115.Yu F, Megyesi J, Price PM. Cytoplasmic initiation of cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2008;295:F44–F52. doi: 10.1152/ajprenal.00593.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hiromura K, Pippin JW, Blonski MJ, et al. The subcellular localization of cyclin dependent kinase 2 determines the fate of mesangial cells: role in apoptosis and differentiation. Oncogene. 2002;21:1750–1758. doi: 10.1038/sj.onc.1205238. [DOI] [PubMed] [Google Scholar]
- 117.Hunt T. You never know: cdk inhibitors as anti-cancer drugs. Cell Cycle. 2008;7:3789–3790. doi: 10.4161/cc.7.24.7515. [DOI] [PubMed] [Google Scholar]
- 118.Perez de Castro I, de Carcer G, Montoya G, et al. Emerging cancer therapeutic opportunities by inhibiting mitotic kinases. Curr Opin Pharmacol. 2008;8:375–383. doi: 10.1016/j.coph.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 119.Bettayeb K, Sallam H, Ferandin Y, et al. N-&-N, a new class of cell death-inducing kinase inhibitors derived from the purine roscovitine. Mol Cancer Ther. 2008;7:2713–2724. doi: 10.1158/1535-7163.MCT-08-0080. [DOI] [PubMed] [Google Scholar]