Abstract
A variety of neuropharmacological strategies are being pursued in the search for an effective treatment for methamphetamine addiction. In this study, we investigated the safety and potential efficacy of aripiprazole, an antipsychotic agent acting on both dopamine and serotonin systems. We conducted a double-blind inpatient clinical pharmacology study to assess potential interactions between intravenous (IV) methamphetamine (15mg and 30mg) and oral aripiprazole (15mg). In addition, the effects of aripiprazole treatment on abstinence related craving and cue-induced craving were evaluated. Participants included non-treatment seeking, methamphetamine dependent patients (N=16), 18-45 years of age, currently using methamphetamine. Following baseline methamphetamine dosing (15mg and 30mg), participants received 2 weeks of treatment with aripiprazole (n=8) or placebo (n=8). Participants then completed cue exposure sessions using neutral and methamphetamine-related cues. Methamphetamine dosing (15mg and 30mg) was then repeated. Aripiprazole treatment had no effect on cue-induced methamphetamine craving, or on daily baseline craving assessed over the course of medication treatment, though aripiprazole treatment was associated with increased craving independent of methamphetamine dosing. Aripiprazole treatment was associated with significantly higher ratings on ARCI subscales reflecting euphoria and amphetamine-like effects following methamphetamine dosing. Aripiprazole was treatment was also associated with significant reductions in ratings of “bad effects” and reductions on the ARCI subscale for sedation effects following methamphetamine dosing. Aripiprazole treatment reduced the increase in systolic blood pressure following methamphetamine dosing, but had no other effects on cardiovascular responses to methamphetamine. Aripiprazole treatment did not alter the pharmacokinetics of methamphetamine. These findings indicate that aripiprazole treatment appears to be safe in volunteers with methamphetamine dependence, though the finding that aripiprazole increased some of the rewarding and stimulatory effects produced by acute methamphetamine suggests that 15mg aripiprazole is unlikely to be efficacious for the treatment of methamphetamine dependence. Further research with lower doses of aripiprazole, possibly using study designs aimed at evaluating efficacy for relapse prevention, are needed before ruling out aripiprazole as a treatment for methamphetamine dependence.
Introduction
In 2006 it was estimated that 4.5% of Americans used methamphetamine at some point in their lives, that 1.3 million used methamphetamine in the previous year, and that more than 512,000 used methamphetamine in the previous month (SAMHSA 2006). In some regions of the country, methamphetamine is the most commonly abused illegal drug and methamphetamine abuse poses a major criminal justice concern.
Currently available treatments for methamphetamine dependence are marginally effective, at best. In a recent study evaluating optimal behavioral treatments (a combination of contingency management and cognitive behavioral therapy), abstinence from methamphetamine could be documented on 60% of study visits (Rawson et al 2006). Other less-intensive commonly used treatments such as counseling are less effective (Rawson et al 2004).
One way to improve the efficacy of available behavioral treatments is to employ adjunctive medication treatments. This approach has recently yielded some success for the development treatments for cocaine dependence (Carroll et al 2004; Dackis et al 2005). Although bupropion holds promise as an adjunctive medication for the treatment of methamphetamine dependence (Elkashef et al 2007), other more effective options are needed.
Methamphetamine dependence is associated with long-lasting neurobiological changes that may be relevant for selecting potential medication treatments. These include reductions in dopamine transporter (DAT) expression (Johanson et al 2006; McCann et al 1998; Sekine et al 2001; Volkow et al 2001b) and DA D2 receptor binding availability (Volkow et al 2001a; Volkow et al 1993). Pre-clinical and post-mortem human data are consistent with these findings and further suggest that chronic methamphetamine exposure is associated with reduced DA levels in striatum (Wilson et al 1996).
Medications that compensate for these neurobiological abnormalities are attractive candidates for evaluation as treatments for methamphetamine dependence. We selected aripiprazole for evaluation as an adjunctive treatment based on its partial agonist activity at the DA D2 receptor (Ozdemir et al 2002). Partial agonists function as agonists under conditions of low neurotransmitter availability, but as antagonists under conditions of high neurotransmitter availability. In recently abstinent methamphetamine users, DA availability is hypothesized to be low (Volkow et al 1998), whereas methamphetamine use greatly enhances synaptic DA concentrations. Thus aripiprazole is hypothesized to normalize the function of brain monoamine systems, yielding a range of possible beneficial impacts for methamphetamine users.
Two previous studies of the effects of aripiprazole examined effects of aripiprazole on the discriminative stimulus and subjective effects produced by low doses of oral amphetamine in healthy volunteers (Lile et al 2005; Stoops et al 2006). Both found that aripiprazole significantly attenuated the discriminative stimulus and positive subjective effects produced by amphetamine, lending additional credence to the current study's rationale.
We conducted this phase I randomized, double-blind, placebo-controlled inpatient study to assess the effects of methamphetamine administration in acutely abstinent chronic methamphetamine users during treatment with aripiprazole or placebo. We hypothesized that aripiprazole treatment would reduce the positive subjective effects of methamphetamine. In addition, we hypothesized that aripiprazole treatment alone would be well tolerated and that aripiprazole treatment would not adversely exacerbate the cardiovascular effects of methamphetamine.
Materials and Methods
Subjects
Sixteen subjects completed the entire study (n=8 placebo and n=8 aripiprazole). The study was conducted at the University of California, Los Angeles (UCLA) and New York University (NYU). Participants were recruited through advertisements and were compensated for their time. All participants met DSM IV-TR criteria for methamphetamine dependence and were not seeking treatment at the time of screening or admission. Participants were between 18 and 45 years of age, were in otherwise good health, and had normal physical examinations, EKGs, and clinical laboratory assessments. Exclusion criteria included history of asthma, pregnancy, and prior adverse reaction to methamphetamine or aripiprazole, history of seizure disorder, head trauma, dependence on other drugs aside from nicotine, or other axis one psychiatric disorder. Concomitant use of psychotropic medications or medications interacting with aripiprazole were not allowed. The institutional review boards at UCLA and NYU (Medical School and main campus) approved the study. All participants give informed consent after having potential risks fully explained to them. The demographics of participants at the two sites were similar, though participants from the New York site tended to use methamphetamine intermittently whereas those from the Los Angeles site were more frequent users.
Study Design
The study employed a double-blind, placebo-controlled, parallel group design. Following admission to the inpatient clinical research center (CRC), participants received a series of 3 masked doses of methamphetamine IV (placebo-0mg, 15mg, and 30mg, randomized with the constraint that the 15mg dose precede the 30mg dose) for an initial safety and baseline response evaluation. The methamphetamine doses were separated by 2 days. Participants were then randomized to receive oral aripiprazole (15 mg) or placebo once daily at 7:30am. Following 10 days of treatment, participants received a second series of 3 doses of methamphetamine and placebo on days 10, 12 and 15 of aripiprazole treatment following a similar constrained randomization schedule to evaluate the effects of methamphetamine during treatment with aripiprazole/placebo. Subjects not completing all study activities were replaced.
Drugs
Aripiprazole tablets were encapsulated in gelatin capsules by research pharmacies at each site. Placebo was prepared similarly. The 15mg dose was selected because this dose has been shown to be well tolerated and effective for other neuropsychiatric disorders (e.g. bipolar disorder and schizophrenia), and was the effective dose used by Rush and colleagues (Lile et al., 2005; Stoops et al., 2006). A NIDA contractor provided sterile methamphetamine solution for human use and an equal volume of sterile saline solution was used as the placebo. The doses selected for study (15mg and 30mg) were chosen based on prior research in our labs showing they are safe to administer to methamphetamine-dependent volunteers and because they produced significant elevations in positive subjective effects (e.g., Newton et al 2006a; Newton et al 2005; Newton et al 2006b). MA and saline were provided from the pharmacy in indistinguishable syringes to maintain the blind. Both were dosed using a syringe pump over 2 minutes. This study was performed under an IND from the FDA. Methamphetamine was administered intravenously over 2-min using a syringe pump with a physician in attendance. Heart rate and blood pressure were monitored frequently to ensure safety.
Acute Methamphetamine Testing Procedures
Subjective effects data were collected using computerized visual analog rating scales (VAS) and the Addiction Research Center Inventory (ARCI, Haertzen 1965; Haertzen 1966). VAS data were collected 15 min before and 3, 6, 10, 15, 30, 45, 60, 90, 180, 210, 240, 300, 360, 420, and 480 min following each methamphetamine injection. VAS ratings were obtained for “high,” “any drug effect,” “desire for methamphetamine,” “stimulated,” “depressed,” “anxious,” “good effects,” “bad effects,” “like methamphetamine,” and “likely to use” on a continuous scale digitized between 0 to 100. The ARCI was administered prior to and 30 min following each injection of methamphetamine or saline. The Brief Psychiatric Rating Scale (BPRS, Overall and Gorham 1962) was completed 60 min following each methamphetamine or saline injection Plasma samples for methamphetamine pharmacokinetic (PK) analyses were collected at min −5, 5, 15, 30, 60, 90, 120, 240, 360, 480 and hr 12. 20, 24, 36 and 48 after infusion and analyzed using validated methods including liquid chromatography with either electrospray ionization and atmospheric pressure chemical ionization and tandem mass spectrometry (methamphetamine quantitation limit = 2.0 ng/ml) Craving and Mood symptoms were monitored throughout the screening and medication treatment periods using the Brief Substance Craving Scale (BSCS, Elkashef et al 2005), the Beck Depression Inventory (BDI, Beck et al 1996), the Brief Symptom Inventory (BSI, Derogatis 1982), and the Profile of Mood States (POMS, McNair et al 1992). Akathisia, a potential side effect of aripiprazole, was assessed using the Barnes rating scale (Barnes 1989).
On Day 1, prior to receiving the first dose of treatment medication, and again on Day 9 of treatment, cue-induced craving for methamphetamine was assessed. Before and after cue presentation, subjective effects were measured using a VAS including items: “desire for methamphetamine,” “depressed,” “anxious,” “stimulated,” “likely to use methamphetamine,” and “methamphetamine-like effect”. In addition, all subjects completed the BSCS to further characterize craving. The cue procedures were performed between 11:00 AM and 1:00 PM. Each cue procedure started with baseline VAS and BSCS assessments (-10 min) followed by a 10 min relaxation period with baseline heart rate (HR) and blood pressure (BP) recordings. Cue presentation included 5 min of methamphetamine paraphernalia viewing and handling followed by 10 min of video viewing. The methamphetamine-paraphernalia including pipe stems, a lighter, and a small plastic bag containing white powder designed to simulate methamphetamine crystals. Participants handled and inspected these items for 5 min after which they viewed a 10 min long video segment of actors appearing to use methamphetamine (by 3 distinct routes of administration: smoking, snorting and intravenous administration). The neutral cues consisted of pinecones, shells and rocks that were handled and inspected for 5 min followed by viewing a 10 min long video segment of an aquarium and items found in nature. The VAS measures were assessed immediately after completion of the cue procedures and again 10 min later. BP and HR were assessed in 5 min intervals (min -10 to min 30) before, during, and after cue presentation. There was a 20 min rest period between the neutral and methamphetamine cue sessions, which were conducted in random order.
Data Analysis
For each subject and dose of methamphetamine, a repeated measures model was constructed to determine the effect of medication treatment on maximum change and the maximum change-by-methamphetamine dose interaction. Then, analyses were performed separately for each methamphetamine dose level and the least square mean for the maximum change was calculated using ANOVA models. Statistics from models analyzing each methamphetamine dose level are reported, with the significance level set at p<.05. The ARCI, BDI, BSI, and Barnes Akathisia data were analyzed using statistical models including terms for pre-dose rating and treatment. For plasma methamphetamine PK analyses, Cmax and T max were calculated by computerized WinNonlin algorithm (Pharsight Corporation, Mt. View, CA), and AUC was calculated using a linear trapezoidal rule up to the Cmax time point and a logarithmic trapezoidal rule thereafter.
Results
Demographics
Completing participants included 16 methamphetamine-dependent volunteers. Their gender, age, and preferred route of methamphetamine administration are shown in Table 1. Participants in aripiprazole versus placebo groups did not differ significantly along any of the demographic variable, though there was a trend for greater days of methamphetamine use in the aripiprazole treated group.
Table 1.
shows participants' demographic and clinical characteristics.
| Aripiprazole (N=8) |
Placebo (N=8) |
||
|---|---|---|---|
| Gender (N) | Male | 8 | 7 |
| Female | 0 | 1 | |
| *Ethnicity (N) | White | 7 | 5 |
| Hispanic or Latino | 1 | 2 | |
| African American | 1 | 2 | |
| Age | 32.5±6.4 | 28.3+7.9 | |
| Education (yrs) | 14.6+1.8 | 14.6+1.8 | |
| ASI Drug | 0.15+0.07 | 0.076+0.08 | |
| BDI | 11.3±10.9 | 8.8±7.5 | |
| POMS | 47.1±44.7 | 23.0+21.7 | |
| MA use last 30 Days | 20.4+9.4 | 10.3+9.9** | |
Some participants reported more than one race category and this accounts for the extra numbers shown
p<.06
Aripiprazole Treatment
Mean ratings on the Barnes Akathisia scale on day 12 of treatment were 0.0 (± 0.0) for the placebo treated group and 1.6 (±2.61) for the aripiprazole treated group (p=NS). None of the placebo-treated participants had akathisia symptoms at any time during the study, whereas 2 of the aripiprazole-treated participants had symptoms of akathisia.
Depression, mood and psychiatric symptoms, indexed by the BDI, POMS and BSI, did not differ between the groups at baseline (Table 1) and there were no significant effects of aripiprazole treatment on subsequent measures. The mean BDI score after 10 to 14 days of treatment was 4.88 (±3.83) in the placebo-treated group and 11.0 (±8.40) in the aripiprazole-treated group (t=1.875, p=.08). The mean BSI score after 10 to 14 days of treatment in the placebo-treated group was 17.4 (±11.0) and 21.9 (+29.3) in the aripiprazole-treated group (p=NS). POMS total score, as well as subscores for tension, anger, depression, fatigue, vigor, and confusion also did not differ between the groups (data not shown).
Methamphetamine Treatment
Physician-completed BPRS ratings indicated that methamphetamine treatment was not associated with the development of psychotic symptoms. The BPRS scores prior to and following randomization are shown in Table 2. There were no differences between the placebo and aripiprazole treated groups. The saline/methamphetamine contrast was slightly greater following randomization to study medication (aripiprazole/placebo) for both groups (p=.051 for the placebo-treated group and p<.01 for the aripiprazole-treated group).
Table 2.
shows participants' pre- and post-randomization BPRS scores following saline and methamphetamine 30mg.
| BPRS Total Score | Aripiprazole (N=8) |
Placebo (N=8) |
|---|---|---|
| Pre-randomization saline | 27.5 (7.93) | 28.6 (9.24) |
|
Pre-randomization methamphetamine (30mg) |
27.0 (2.51) | 26.9 (4.41) |
| Post-randomization saline | 24.9 (1.13) | 25.8 (1.91) |
|
Post-randomization methamphetamine (30mg) |
27.5 (2.14)* | 27.9 (2.02)** |
Saline-methamphetamine contrast greater in the aripiprazole treated group (p<.01)
Saline-methamphetamine contrast greater in the placebo treated group (p=.051)
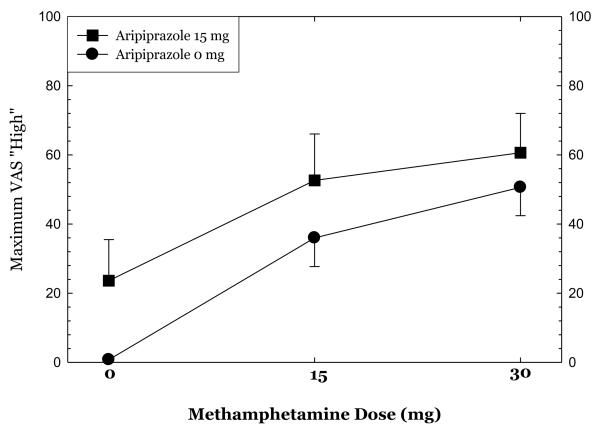
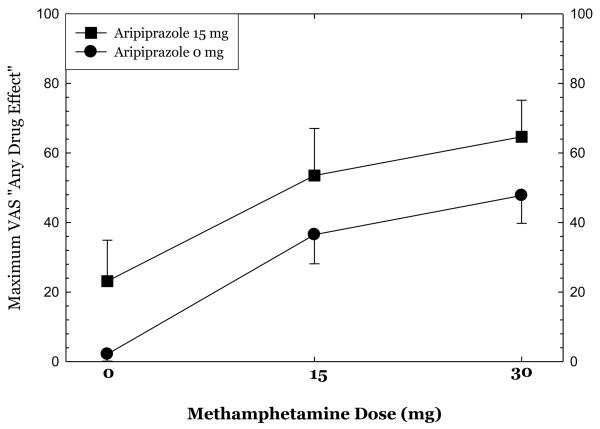
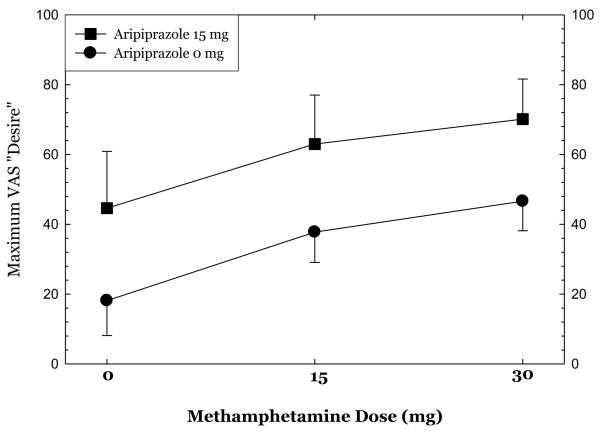
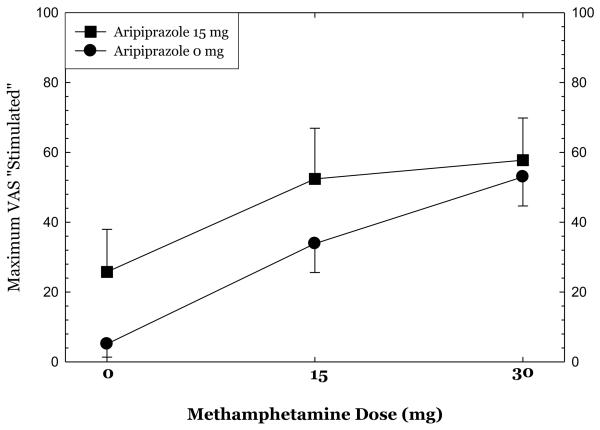
Methamphetamine administration produced substantial increases in ratings of “high,” “any drug effect,” “desire for methamphetamine,” and “stimulated” (Figures 1-4). Overall, the group receiving aripiprazole appeared to exhibit enhanced methamphetamine-induced positive subjective effects, though differences between the groups did not reach statistical significance for any of these positive affect measures. For ratings of “desire methamphetamine” there was a significant main effect of treatment (p<0.05) but no treatment by methamphetamine dose interaction, indicating that across all doses of methamphetamine (including saline) aripiprazole treatment tended to increase ratings of “desire”. Aripiprazole treatment was also associated with a small but statistically significant reduction in “bad effects” ratings (data not shown, p<.05).
Figures 1-4.
show maximum change from pre-dose baseline in subjective effects ratings following administration of 0mg, 15mg, and 30mg methamphetamine in the post-randomization condition only. Participants rated subjective effects on a scale ranging from 0 (not at all) to 100 (most ever).
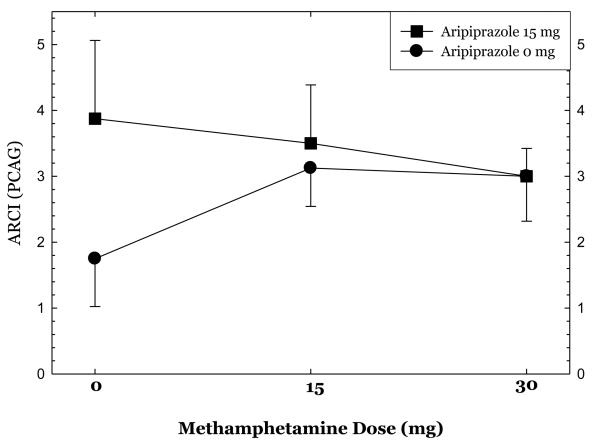
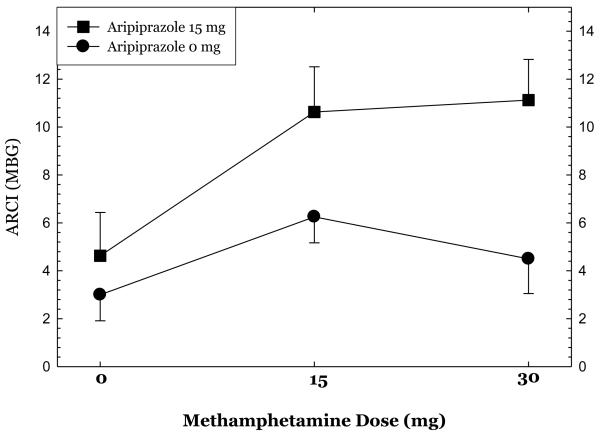
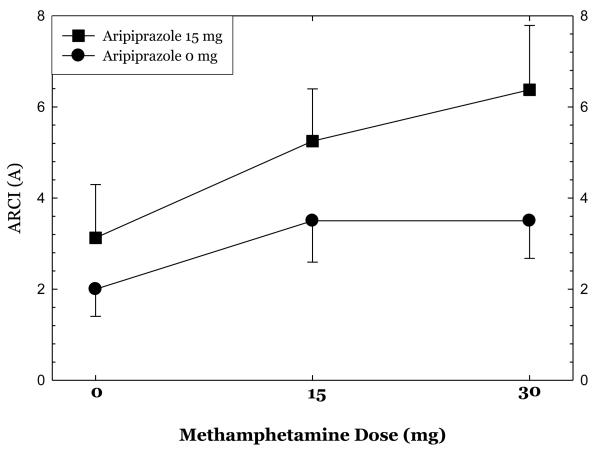
Ratings on selected ARCI subscales at each dose of methamphetamine are shown in Figures 5, 6, & 7. Aripiprazole treatment was associated significant reductions in ratings on the pentobarbital, chlorpromazine, alcohol group (PCAG) subscale reflecting sedation effects (p<.02), increases in ratings on the morphine-benzedrine group (MBG) subscale reflecting euphoria (p<.002), and a trend towards increases in ratings on the amphetamine (A) subscale reflecting stimulation (p<.10).
Figures 5-7.
show ratings on selected Addiction Research Center Inventory (ARCI) subscales collected 30 minutes following administration of 0mg, 15mg, and 30mg methamphetamine dosing in the post-randomization condition only.
Heart Rate, Blood Pressure and Methamphetamine Pharmacokinetics
Methamphetamine (30mg) increased diastolic blood pressure (mmHg) to a similar degree after treatment with placebo (24±8) as compared to aripiprazole (22±5)(p=NS). Similarly, methamphetamine (30mg) increased heart rate (bpm) to a similar extent during treatment with placebo (29±12) versus aripiprazole (27±6(p=NS). However, methamphetamine (30mg) increased systolic blood pressure to a lesser degree after treatment with aripiprazole (29±6) as compared to placebo (43±11) (p<0.05). There were no statistically significant differences between treatment groups in the methamphetamine PK parameters for Cmax, Tmax, and AUC.
Daily and Cue-induced Methamphetamine Craving
Craving assessments with the BSCS indicated a moderate level of craving (approximate range: 3-6 points out a max of 12) among study participants, which remained stable across the duration of their inpatient hospitalization (25 days). Analysis of the craving ratings during medication treatment revealed no significant effect of aripiprazole.
Exposure to methamphetamine cues induced moderate increases in craving. Analyses of testing on Day 9 following randomization to study medication (aripiprazole/placebo) revealed no significant effects of medication treatment on cue-induced methamphetamine craving. Analysis revealed main effects of treatment on VAS measures “anxious” (p<0.01), “nervous” (p<0.1) and “irritable” (p<0.05), which were higher in the group receiving aripiprazole both pre- and post-cue exposure. There was no effect of aripiprazole treatment, or cue exposure, or on HR or BP.
Adverse Events
Most participants had at least one adverse event (AE) following randomization to study medication (aripiprazole/placebo). Six of 8 placebo-treated participants had at least one AE and 7 of 8 aripiprazole-treated participants had at least one such AE (p=NS). One participant in the placebo-treated group had an AE rated as “severe” as did 2 participants in the aripiprazole-treated group (p=NS). The most common AEs following randomization to study mediation occurring in at least 3 participants were fatigue (n=3), psychiatric disorders (anxiety, n=7, disturbance in attention, n=3, and restlessness, n=3), central nervous system disorders (headache, n=8, insomnia, n=7, and tremor, n=6) predominating and gastrointestinal disorders (diarrhea, n=3). These tended to be equally distributed between the treatment groups, except for tremor, which was greater in the aripiprazole-treated group (n=4) compared to the placebo-treated group (n=2) (chi-square =.667, df=1, p=NS) and restlessness, which was greater in the aripiprazole-treated group (n=3) compared to the placebo-treated group (n=0) (chi-square =3.00, df=1, p=.083).
Discussion
Overall, aripiprazole treatment had only moderate effects on the subjective responses produced by methamphetamine administration. Aripiprazole treatment was associated with statistically significant reductions in ratings of “bad effects” and significantly higher ratings for “desire for methamphetamine”. Further examination of these data revealed that the magnitude of change in ratings of “bad effects” was extremely small and not clinically significant. Ratings of “desire for methamphetamine” were higher across all methamphetamine treatment doses as well as prior to the infusion, and thus reflect group differences rather than craving induced by methamphetamine or interactions between aripiprazole treatment and methamphetamine dosing. The pre-existing differences between the groups in recent MA use may also have contributed to these findings. Robust findings were seen using the ARCI, as aripiprazole treatment was associated with significant reductions in the PCAG subscale score and increases in the MBG and (at a trend level) A subscale scores. Recently, Haney and colleagues (2007) presented data showing that aripiprazole treatment increased cocaine self-administration in a human laboratory study (International Society for the Study of Drugs as Reinforcers, Quebec, Canada, June 2007). This is consistent with our finding that aripiprazole increased ratings on several measures of stimulant effects.
These findings suggest that aripiprazole is unlikely to be beneficial for the treatment of methamphetamine dependence, and could possibly worsen outcomes. Indeed, a clinical trial was halted recently when it was discovered that participants receiving 15mg aripiprazole were at least 2 times more likely to provide a positive amphetamine urine test then participants receiving placebo, indicating more frequent amphetamine use in the aripiprazole-treated group (Tiihonen et al 2007). However, the dose of aripiprazole used in all stimulant studies to date was limited to 15 mg, indicating the need to test a broader range of doses.
The results obtained in this study diverge substantially from those obtained previously (Lile et al 2005; Stoops et al 2006). Both found that aripiprazole significantly attenuated the effects produced by amphetamine, a finding not replicated here. Several differences in the studies' designs (e.g. drug, dose, dosing duration, and route of administration) may account for this discrepancy.
A number of findings from this study, both research and clinically oriented, suggest that aripiprazole treatment may be associated with moderately aversive side effects in methamphetamine dependent patients. Adverse event ratings, though not statistically significant, indicated that aripiprazole treatment was associated with more symptoms of akathisia, tremor and restlessness. Moreover, a main effect of treatment on the VAS measures “anxious,” “nervous,” and “irritable” during the methamphetamine cue tests revealed that subjects in the aripiprazole group were experiencing unpleasant psychological side effects. Finally, methamphetamine-induced increases in BPRS measures were more pronounced post-randomization in the aripiprazole group. The reasons for these effects are not clear, but might be due to lower DA D2 receptor levels (Volkow et al 2001a; Volkow et al 1993) or lower endogenous DA levels (Wilson et al 1996) seen in heavy methamphetamine abusers. Further studies on medication tolerability, including a broader aripiprazole dose range, could address this clinically important question.
The primary limitations of this study include a small sample size, the use of a single dose level of aripiprazole, and the omission of behavioral measures of reinforcing effects of methamphetamine. The sample size (n=8 for aripiprazole and placebo) precludes detection of any but statistically large effects. The earlier studies (Lile, Stoops, and colleagues) included a smaller number (n=6), but they used a within-subjects design, which is statistically more powerful. The use of several dose levels of aripiprazole would have allowed determination of the dose-dependency of any observed effects of aripiprazole. There were baseline differences between the aripiprazole and placebo treated groups, with the aripiprazole-treated group using methamphetamine on nearly twice as many days as did the placebo-treated group. It is unlikely that this baseline difference accounted for the finding that aripiprazole treatment was associated in increases in stimulant-like effects following methamphetamine dosing, as more frequent use would, if anything, likely be associated with greater tolerance to the stimulant effects of methamphetamine. Finally, treatment medications are intended to reduce drug-taking behavior rather than to modify subjective effects, so it would be preferable to include behavioral measures of reinforcement (i.e. drug self-administration or the multiple-choice procedure, Fischman and Schuster 1982; Griffiths et al 1993). These limitations aside, further research with lower doses of aripiprazole, possibly using study designs aimed at evaluating efficacy for relapse prevention, are needed before ruling out aripiprazole as a treatment for methamphetamine dependence.
Acknowledgements
The authors thank Ellie DeCandia, RN, Timothy Fong, M.D., Roger Donovick, M.D. and Gilles Fleury, M.D. for medical supervision of participants during this study. This work was supported by the National Institutes of Health (RR-00865 to UCLA, M01RR00096 to NYU, and contract NO1DA-3-8838).
Footnotes
The authors declare no conflicts of interest in regards to this manuscript.
References
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, et al. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. The brief symptom inventory (BSI): Administration, scording and procedures manual-I. Johns Hopkins University and School of Medicine; Baltimore: 1982. [Google Scholar]
- Elkashef A, Holmes TH, Bloch DA, Shoptaw S, Kampman K, Reid MS, et al. Retrospective analyses of pooled data from CREST I and CREST II trials for treatment of cocaine dependence. Addiction. 2005;100(Suppl 1):91–101. doi: 10.1111/j.1360-0443.2005.00986.x. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: An efficient approach for investigating drug reinforcement in humans. Behavioural Pharmacology. 1993;4:3–13. [PubMed] [Google Scholar]
- Haertzen CA. Addiction Research Center Inventory (ARCI): development of a general drug estimation scale. J Nerv Ment Dis. 1965;141:300–307. doi: 10.1097/00005053-196509000-00006. [DOI] [PubMed] [Google Scholar]
- Haertzen CA. Development of scales based on patterns of drug effects, using the addiction Research Center Inventory (ARCI) Psychol Rep. 1966;18:163–194. doi: 10.2466/pr0.1966.18.1.163. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PE, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus and subject-rated effects of D-amphetamine in humans. Neuropsychopharmacology. 2005;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of mood states manual (rev.) Educational and Industrial Testing Service; San Diego: 1992. [Google Scholar]
- Newton TF, De La Garza R, 2nd, Fong T, Chiang N, Holmes TH, Bloch DA, et al. A comprehensive assessment of the safety of intravenous methamphetamine administration during treatment with selegiline. Pharmacol Biochem Behav. 2006a doi: 10.1016/j.pbb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, et al. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, Fong T, Wallace CL, Li SH, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006b;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Ozdemir V, Fourie J, Ozdener F. Aripiprazole (Otsuka Pharmaceutical Co) Curr Opin Investig Drugs. 2002;3:113–120. [PubMed] [Google Scholar]
- Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA . In: Results from the 2005 National Survey on Drug Use and Health: National Findings. Administration SAMHS, editor. Rockville, MD.: 2006. (Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06-4194). [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Rush CR. A low dose of aripiprazole attenuates the subject-rated effects of d-amphetamine. Drug Alcohol Depend. 2006 doi: 10.1016/j.drugalcdep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Fohr J, Tuomola P, Kuikanmaki O, Vorma H, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature medicine. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]