In Western society, acupuncture is becoming a popular alternative for medical treatment—as evidenced by the 15 million Americans who have reported treatment with that procedure in a single year. The World Health Organization (WHO) reported that there are approximately 10,000 acupuncture specialists in the United States, and an estimated 3,000 practicing acupuncturists are physicians [1]. A scientific understanding of the neuroanatomical regions involved in acupuncture treatment has not been completely developed but will likely be necessary for the increased acceptance of acupuncture by the Western medical community.
With the advent of functional magnetic resonance imaging (fMRI), it is possible to noninvasively measure brain activation in response to a stimulus (for a review, see [2]–[5]). fMRI exploits the magnetic differences in the blood when it is oxygenated (active state) or deoxygenated (resting state) [2]–[6]. These magnetic variations in the blood are brought about by changes in cerebral blood flow. The use of new imaging technology to reveal the anatomic components involved in acupuncture allows a scientifically based approach for understanding acupuncture and sham treatment. Previous studies that have looked at the effects of acupuncture with functional imaging methods have met with mixed success [7]–[9]. Robust scientific evidence demonstrating an effect in the brain following acupuncture will provide momentum and support to the investigation of the mechanisms behind acupuncture treatment.
Methods
All functional brain activation studies investigating acupuncture effects on the brain were conducted at the Center for Advanced Magnetic Resonance Imaging, which houses a research-dedicated 3 Tesla (3T) whole-body imaging system. A total of 28 normal, young, healthy, right-handed volunteers participated in this study. Prior to entering the magnet, the subject was interviewed by the acupuncturist, which included a questionnaire about past acupuncture treatment and a measure of the pulse.
The protocol employed a simple block design. All subjects were naïve to acupuncture and were told they would receive acupuncture treatments in their lower leg. Some subjects were apprehensive about the needles. However, their fears were reduced once they were shown the fineness (34 G × 25 mm) of the needles (Seirin) used in the study. The single-use, sterile, stainless steel needles were slightly magnetic in the 3T environment. A hospital sheet was taped across the opening of the magnet for safety purposes. The sheet blocked the subject from overtly visualizing any of the acupuncture treatments, which would contaminate the functional imaging results. The acupuncturist was in the magnet room for the entire functional imaging session. Instructions about when to insert or remove the acupuncture needle were given via headphones connected only to the acupuncturist. During the rest period, and when not placing the needles, the acupuncturist sat quietly in a wooden chair until instructed to act.
Before imaging, the order of the needle placement (called needling) was randomized, and a list was given to the acupuncturist. Typically, three experiments with different block lengths and acupuncture-point stimulation were performed on each subject. The data in this study were from a larger investigation of the temporal changes in the blood-flow response induced by acupuncture. As such, different subjects were used to compose the data for each acupuncture point; no one subject had all three acupuncture points stimulated by the 5-min block design. However, they may have had two of the three points stimulated. No needle was present at the control period, and the active condition was when the needle was placed at a specific acupuncture point. Throughout the entire experiment, the subject was instructed to relax and to not think about anything. To reduce the motion involved in having a needle placed, the acupuncturist would lightly touch the subject before applying the needle. In three subjects, the motion effects were so large and synchronized with the needle placement that the data were not useable and were discarded from the study. The acupuncture points used in the study were 1) Bladder 60 (BL60) for visual stimulation; 2) Kidney 3 (K3) for auditory stimulation; and 3) Spleen 6 (SP6) for the sham point because it is not considered to have a visual or auditory treatment component but instead affects the digestive and immune systems. The BL60 and K3 points were located on the lateral and medial portion of the ankle, respectively. The SP6 point was located in a slightly more superior portion of the leg than the BL60 and K3 points. Since the hypothesis of the study was to explore the involvement of the visual and auditory cortex and not the somatosensory cortex, this difference in the control stimuli was acceptable.
fMRI Data Acquisition
Inside the circularly polarized transmit/receive head coil, the subject's head was placed in a vacuum pillow in order to reduce head motion during the study. Once the subject was completely situated on the table and the headphones were in place, the pillow was evacuated to form a rigid cast. The pillow evenly distributed the weight of the head, reducing motion related to discomfort and allowing the subject to remain in the magnet for a longer period of time. Localizer scans were performed to aid in the identification of the anterior commisure—the posterior commisure plane to which all scans were oriented [10]. A susceptibility weighted single-shot echo-planar imaging sequence was used to obtain the functional data. The imaging parameters were TR = 3 s, TE = 30 ms, matrix = 64 × 64, FOV = 220 mm; 32 slices that were 3 mm thick with zero gap and an optimized flip angle of 82° were used. Four volumes (12 s) were collected at the beginning of each run and discarded to allow the MR signal to reach steady-state.
A total of 500 volumes were collected during the experiment for analysis, and each block consisted of 100 volumes. Three resting and two active blocks were acquired in an alternating pattern starting with rest. The total imaging time per run was 25 min. Once the functional imaging session was completed, a high-resolution anatomic scan was acquired for localizing the activations from the functional data. The three-dimensional (3-D) MP-RAGE sequence had parameters of TR = 2.1 s, TE = 4.38 ms, flip angle of 15°, matrix size of 256 × 256, FOV = 220 mm, and 160 1-mm-thick partitions oriented in the same plane as the functional data.
fMRI Data Analysis
All of the functional data were analyzed individually and as a group using SPM99 (http://www.fil.ion.ucl.ac.uk/spm). The data were preprocessed using a slice-timing correction, 3-D motion correction, and 3-D spatial smoothing using a 6-mm Gaussian kernel. For the individual analysis, the data were investigated with a physiologically corrected statistical model of the experiment in the original brain space. For the group analysis, the smoothed and motion-corrected scans were normalized using the Montreal Neurological Institute template image (MNI305) supplied with SPM-99. This template conforms to the space defined by the ICBM, the National Institutes of Health P-20 project, and closely approximates the space described in the atlas of Talairach and Tournoux [10]. These data were then explored with the same physiologically corrected statistical model.
The hypothesis for this experiment was that acupuncture at a given site (BL60 or K3) would increase the MR signal intensity in the appropriate cortical regions, whereas the control point (SP6) would not. In order to interrogate just the cortical regions of interest, a mask was created that included the visual cortex and auditory cortex. The purpose was to eliminate any spurious activation in brain regions that were not involved in the experiment and to address the multiple comparisons problem. By decreasing the number of voxels (volume pixels) involved in the statistical analysis, a less conservative threshold could be set. Using SPM99 and 12 subjects in each group, the statistical threshold corresponded to a Z score of 3.0. The statistical maps were then merged with a high-resolution anatomic data set that had been normalized to a standard brain template in order to localize the activations. The merged maps were generated for each acupuncture point.
Results
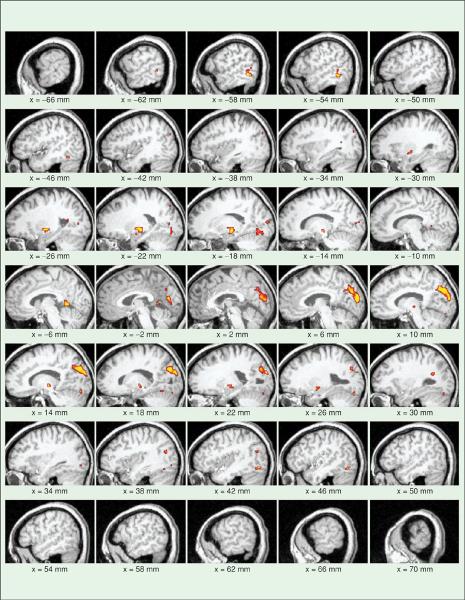
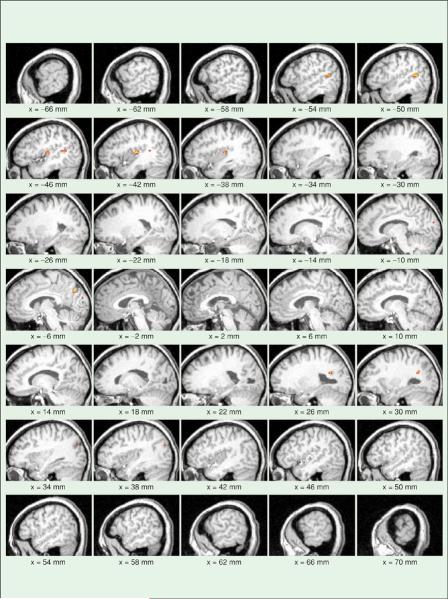
Both positive and negative correlations were explored during the data analysis. However, only positive correlations demonstrated any significant results. The activation maps generated from visual and auditory acupuncture point stimulation demonstrated increased activation (increased blood flow) in the appropriate associated cortices. This was evident in the single subject and group analysis. Since the population response to acupuncture is more interesting, only the group data will be shown. Figure 1 demonstrates the activation pattern obtained from a group analysis of 12 subjects undergoing stimulation of BL60. Note the strong activation patterns in the visual cortex and the absence of any patterns in the auditory cortex. In Figure 2, there is the opposite effect; there are strong activations in the auditory cortex (unilateral, right-sided) but none in the visual areas due to the stimulation of the K3 point. Finally, stimulation of the sham acupuncture point, SP6, did not result in activation in either the occipital or auditory cortex (Figure 3). The activations present are in the cerebellum and in the basal ganglia, which may be associated with digestive function or somatosensory stimulation. The same stimulation paradigm and analysis were used for these data, yet no activations were evident in the regions of interest.
Fig. 1.
Functional imaging maps demonstrating activation in the visual cortex in response to treatment with acupuncture at the point Bladder 60, which is associated with visual healing. The colored regions represent areas of the brain that have a significant increase in the MR signal synchronized with the delivery of acupuncture. Note that there is significant activation in the visual cortex compared to the auditory cortex. These maps were generated with a mask to limit analysis to the visual and auditory cortices.
Fig. 2.
Similar activation maps are shown; however, acupuncture is applied to the Kidney 3 point, which is associated with auditory treatment. Note the different pattern in the activation. It is limited to the auditory cortex and is much more focal than the visual activation in Figure 1; this may be due to the relative potency of the points or the interpretation of the point location.
Fig. 3.
Activation maps of the control condition are presented here. In this case, a real acupuncture point is stimulated, Spleen 6, that has treatment effects for the digestive system. This is independent of the auditory and visual cortex as demonstrated by the lack of activation in these anatomic areas. These maps show that the act of acupuncture does not invoke the auditory or visual systems in the experimental setup used. More specifically, the maps shown in Figures 1 and 2 are a result of the acupuncture point stimulated.
Discussion
Functional magnetic resonance imaging is an ideal method to noninvasively explore the whole brain for cortical activation related to acupuncture. The results from this study demonstrate that fMRI can identify specific cortical regions associated with acupuncture stimulation. The choice of acupuncture stimulation using visual or auditory points simplifies the interpretation of the results because the anatomic correlates are well defined. The magnitude and extent of the activations were different for the auditory and the visual stimulation as demonstrated in Figures 1 and 2; this may be due to the relative potency of the acupuncture points used, the overall imbalance associated with these systems, and the interpretation of the acupuncture point by the acupuncturist. Nevertheless, these data reflect significant blood-flow changes within the appropriate regions of the brain in response to stimulation of acupuncture points in the lower leg.
In addition, the stimulation of a sham point—an actual acupuncture point having nothing to do with audition or vision—resulted in no activation changes in the previously defined anatomic regions. With the appropriate experimental design, a clearer understanding of the neural substrate associated with acupuncture is likely to provide considerable insights into acupuncture treatment. This type of scientific approach to investigating and validating alternative medicine techniques is critical for the widespread acceptance of these methods.
Biographies

Todd B. Parrish received his Ph.D. in biophysical sciences from the University of Minnesota in 1998 and his B.S. and M.S. in biomedical engineering from Case Western Reserve University, Cleveland, Ohio, in 1988. Since 1996, he has been on the faculty at Northwestern University, Chicago, Illinois, where he is currently an associate professor of biomedical engineering and radiology. Parrish is the director of the Center for Advanced MRI where he participates in a wide range of neuroimaging topics, including data acquisition, image processing, statistical analysis, and basic cerebrovascular physiology.

Alissa Schaeffer is currently a master's degree student in the Fulton School of Engineering at Arizona State University. She received her bachelor's degree in biomedical engineering from Vanderbilt University, Nashville, Tennessee, in 2000. She worked in the neuroimaging research group of Dr. Todd Parrish at Northwestern University, Illinois, from 2001–2003. Her academic focus is in the area of neural engineering.

Madelyn Catanese is a state-licensed, board-certified acupuncturist. She received her B.A. from Dominican University. Following her interest in alternative medicine she attended The Midwest College of Oriental Medicine and completed a three-year course of studies and internship in Oriental Medicine and Acupuncture. Catanese works with health care professionals and lectures on the benefits of acupuncture as a healing modality. She maintains a private acupuncture practice in Mundelein, Illinois, treating a wide range of illnesses.

Mary J. Rogel practices acupuncture full time in Chicago, Illinois. She obtained her acupuncture certificate in 1986 from the Midwest Center for the Study of Oriental Medicine (now the Midwest College of Oriental Medicine). Prior to becoming an acupuncturist, she earned a Ph.D. in social psychology from the University of Chicago, Illinois, in 1976 and spent ten years teaching and doing research. She has continued doing research consultation and program evaluation on health-related topics, although most of her time is devoted to her private practice.
References
- [1].Consensus Statement 107. vol. 15. NIH; Bethesda, MD: Nov. 3–5, 1997. Acupuncture. no. 5 [Online]. Available: http://consensus.nih.gov/cons/107/107_statement.pdf. [Google Scholar]
- [2].Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 1990;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- [3].Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Nat. Acad. Sci. USA. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Nat. Acad. Sci. USA. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Parrish T. Functional MR imaging. Magn. Resonance Imaging Clin. N. America. 1999;7(4):765–782. [PubMed] [Google Scholar]
- [6].Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim. Biophys. Acta. 1982;714:265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- [7].Cho ZH, Chung SC, Jones JP, Park JB, Park HJ, Lee HJ, Wong EK, Min BI. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc. Nat. Acad. Sci. USA. 1998;95(5):2670–2673. doi: 10.1073/pnas.95.5.2670. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [8].Gareus IK, Lacour M, Schulte AC, Hennig J. Is there a BOLD response of the visual cortex on stimulation of the vision-related acupoint GB 37? J. Magn. Reson. Imaging. 2002;15(3):227–232. doi: 10.1002/jmri.10059. [DOI] [PubMed] [Google Scholar]
- [9].Li G, Cheung RT, Ma QY, Yang ES. Visual cortical activations on fMRI upon stimulation of the vision-implicated acupoints. Neuroreport. 2003;14(5):669–673. doi: 10.1097/00001756-200304150-00002. [DOI] [PubMed] [Google Scholar]
- [10].Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brian: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers, Inc.; New York: 1988. [Google Scholar]