Abstract
Mitochondrial dysfunction, oxidative stress and reductions in thiamine-dependent enzymes have been implicated in multiple neurological disorders including Alzheimer's disease (AD). Experimental thiamine deficiency (TD) is an established model for reducing the activities of thiamine-dependent enzymes in brain. TD diminishes thiamine dependent enzymes throughout the brain, but produces a time-dependent selective neuronal loss, glial activation, inflammation, abnormalities in oxidative metabolism and clusters of degenerating neurites in only specific thalamic regions. The present studies tested how TD alters brain pathology in Tg19959 transgenic mice over expressing a double mutant form of the amyloid precursor protein (APP). TD exacerbated amyloid plaque pathology in transgenic mice and enlarged the area occupied by plaques in cortex, hippocampus and thalamus by 50%, 200% and 200%, respectively. TD increased Aβ1–42 levels by about three-fold, β-CTF (C99) levels by 33% and β-secretase (BACE1) protein levels by 43%. TD induced inflammation in areas of plaque formation. Thus, the induction of mild impairment of oxidative metabolism, oxidative stress and inflammation induced by TD alters metabolism of APP and/or Aβ and promotes accumulation of plaques independent of neuron loss or neuritic clusters.
Keywords: Mild oxidative impairment, Thiamine deficiency, Energy metabolism, Alzheimer’s disease, Aβ peptide, KGDHC, mitochondria
1. Introduction
Alzheimer’s Disease (AD) is characterized by plaques, tangles and cognitive deficits. In addition, numerous thiamine (vitamin B1) dependent processes are diminished in brains from AD patients [25]. Reductions in brain glucose metabolism and increased oxidative stress invariably occur in both AD and thiamine deficiency (TD). Both conditions cause irreversible cognitive impairment; their behavioral consequences overlap but are not identical. Thiamine-dependent enzymes are positioned at critical and arguably rate-limiting steps in brain metabolism [25]. Key enzymes of the pentose shunt (transketolase), the citric acid cycle (e.g., the alpha-ketoglutarate dehydrogenase complex; KGDHC) and the link of glycolysis and the citric acid cycle (e.g., the pyruvate dehydrogenase complex; PDHC) are thiamine dependent and diminished by 50% or more in AD. The reductions are highly correlated (r=0.77) to the clinical dementia rating of patients before death compared to the correlations with plaques or tangles (r=0.2) [5,27]. Thus, the reduction in thiamine-dependent processes can be readily related to the decline in metabolism with AD. The current studies tested whether thiamine deficiency could also modulate one of the classical pathological markers of AD, the amyloid plaques.
Thiamine deficiency (TD) models the mild impairment of oxidative metabolism and reduction in thiamin-dependent processes that accompanies AD. TD induces chronic mild impairment of oxidative metabolism and promotes selective changes in oxidative stress and inflammation that lead to neuronal loss in specific brain regions. As in human AD [6,28], experimental TD reduces thiamine-dependent enzyme activities in multiple brain regions, including those that do not show detectable neuronal loss [6]. However, other TD-induced alterations including markers of inflammation and oxidative stress are very region specific and limited to the area of neuron loss [9,36], The most sensitive brain area is the submedial thalamic nucleus (SmTN) [35]. Numerous markers of oxidative stress occur in this region including hydroxynonenal, iNOS, eNOS, nNOS, hemeoxygenase-1, ICAM-1, CD40L, CD40, microglial and astrocytes activation (summarized in [36]), TNFα, IL-1β and IL-6 [34]. However, only TNFα is altered in cortex [34]. In addition, TD induces endoplasmic reticulum (ER) stress in a region specific manner suggesting that altered processing of proteins is occurring [54]. TD also includes multiple behavioral deficits including memory deficits and changes in motor performance that are due to central cholinergic deficits [24]. Thus, the TD model allows us to test if plaques would form only in regions of neuronal loss that exhibit excessive oxidative stress and inflammation (i.e., the SmTN), or throughout the brain, or both.
We previously demonstrated in wild type mice and in a line of transgenics expressing low levels of the Alzheimer’s APP gene (twice that of endogenous mouse homologue) that TD causes the accumulation of clusters of dystrophic neurites that are highly reminiscent of the morphology of amyloid plaques, except that the centers of lesions were formed from necrotic debris rather than being formed from Aβ [10–12]. The lack of amyoid plaque formation is at least partially attributable to the fact that in wild type mice, murine Aβ is much less aggregatable than human Aβ [7] and that, with regard to the transgenics, we now know that at least four-fold over-expression of APP is required in order to render a transgenic mouse plaque-competent [3]. The observation that TD induces novel neuritic clusters in the absence of amyloid pathology raises the intriguing possibility that impaired metabolism and oxidative stress might initiate a pathogenetic series of events wherein neuritic clusters are formed independent from, or upstream of, amyloid pathology. Brain injury may also alter processing of APP [2]. Traumatic brain injury increases oxidative stress (isoprostanes) and accelerates Aβ accumulation [50]. In order to test the hypothesis that the TD model of mild impairment of oxidative metabolism can modulate both neuritic and Aβ pathology, plaque-competent Tg19959 transgenic mice were made TD, and the results of those studies are reported herein.
2. Materials and Methods
2.1. Animals
Tg19959 mice were produced by pronuclear microinjection of (FVB·129S6F1) embryos with a cosmid insert containing APP695 with two familial AD mutations (KM670/671NL and V717F) under the control of the hamster PrP promoter [14]. This Tg19959 line derives from the same transgene construct as TgCRND8 (APP695, K670N/M671L+V717F) and has similar levels of APP holoprotein expression and associated pathology but is maintained on a different genetic background [21]. Male Tg19959 mice were backcrossed to (C57/B6SJL) F1 female breeders. Genotypes of the offspring were determined by PCR analysis of tail DNA. The animals were housed with constant temperature (22 ± 2°C), humidity (50 ± 5 %) and illumination (12 h light/dark cycles) with food and water provided ad libitum. The Institutional Animal Care and Use Committee of Weill Medical College of Cornell University approved all procedures with the animals.
A total of 23 Tg19959 mice at 60 days were used in this study. The control group was comprised of four males and eight females. In the TD group there were three males and eight females. In the studies by Li et al. [37], no difference in plaque load was observed between males and females of the same transgenic mice (Tg19959 mice). Plaque load in Tg2576 mice does not vary with gender either, although males are more sensitive to alterations in APOE genotype [56]. Other studies suggest sex differences in Tg mice carrying APP with Swedish and the presenilin-1 A246 mutation. Female mice accumulate amyloid at an earlier age and show increased amyloid deposits in the hippocampus compared to age-matched males [52].
2.2. Treatment
Thiamine deficiency was induced in Tg19959 mice as per our previous studies [13,35]. Mice received a thiamine deficient diet (ICN Nutrition Biomedicals, Cleveland, OH) ad libitum, and daily intraperitoneal injections of the thiamine pyrophosphokinase inhibitor [38], pyrithiamine hydrobromide (Sigma Chemical Co.; St. Louis, MO) (0.5 mg/kg body weight) in normal saline for 10 days. Control animals received a thiamine containing diet (ICN Nutrition Biomedicals; Cleveland, OH) ad libitum and daily intraperitoneal injections of normal saline.
2.3. Tissue preparation
Control and TD Tg19959 mice were sacrificed 24 h after the treatment with a lethal intraperitoneal dose of pentobarbitone sodium (200 mg/kg; i.p.; Abbott Laboratories, North Chicago, IL) and perfused with 100 ml of normal saline using a pump (Masterflex, Model 7518-00, Cole-Parmer Instrument Company, Barrington, IL) at 5ml/min. The brains were removed and bisected in the mid-sagittal plane. One-half was post-fixed in 4% paraformaldehyde for a minimum of 48 h and other half was snap frozen in liquid nitrogen and stored at −80°C until analysis.
2.4. Immunoblot analysis
Hemi-brain powders were homogenized in cold lysate buffer (50 mM Tris-HCl pH 7.2, 0.4 % Triton X-100, 1mM DTT , 0.2 mM EGTA, , 50 µM Leupeptin), 40 µg of protein were electrophoresed through 10–20% Tris-Tricine gels, transferred onto nitrocellulose membranes (Pierce Chemical Company, Rockford, IL). Membrane blots were blocked with 50% Odyssey blocking buffer, 50% Tris-buffered saline (TBS) overnight at 4°C. After blocking the membranes were incubated with primary antibodies, APP C-terminal specific polyclonal antibody G369 to epitopes 645–694 of APP695 (1:1000), monoclonal antibody 6E10 against 1–16 residues of human Aβ (1:1000; Signet laboratories, Dedham, MA) or β-actin (1:10,000; Sigma) for 120 min at room temperature. Subsequently, blots were washed with TBS plus 0.1% triton, and incubated at room temperature for one hour with secondary Odyssey Goat anti-Rabbit IRDye 680 antibody and Odyssey goat anti-Mouse IRDye 800 antibody (1:10,000; LICOR Biosciences, Lincoln, NB) and analyzed using the Odyssey infrared imaging program (LI-COR Biotechnology, Nebraska).
The methods for BACE1 immunoblot was performed as described [51]. Briefly, hemi-brain powders were homogenized in cold lysate buffer (60 mM Tris, 10% glycerol, 5% SDS), 15 µg of protein were run on 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA). Membrane blots were blocked with 50% Odyssey blocking buffer, 50% TBS overnight at 4°C. After blocking the membranes were incubated with primary antibodies, BACE1 (1:1000; PA1-757; Affinity Bioreagents, Golden, CO) or β-actin (1:10,000; Sigma) for 120 min at room temperature. Subsequent secondary detection methods are same as above.
2.5. ELISA analysis
Brain powder from hemi-brains were weighed and dissolved in Tissue Homogenization Buffer (250 mM Sucrose, 20 mM Tris Base, 1 mM EDTA, 1 mM EGTA with Protease Inhibitors) weight/volume 1/10 (i.e. 100 mg/1ml) and then homogenized. 200 µl of the 10% w/v were mixed with 440 µl of cold formic acid, sonicated and centrifuged at 100,000g × 1 hr at 4°C. At the end of the centrifugation, 210 µl of the supernatant were mixed with 4 ml of formic acid neutralization solution (1M Tris Base, 0.5 M Na2HPO4, 0.05% NaN3). Aβ levels were then evaluated using ELISA Kit (Signet laboratories, Dedham, MA) using manufacturer's instruction with samples diluted 1:10 (Aβ40) or 1:20 (Aβ42).
2.6. Thioflavine S staining and quantitation of amyloid plaques
To demonstrate the fibrillar Aβ deposition thioflavine S staining was used. Briefly, sections were washed with distilled water for 5 min and stained in freshly prepared and filtered aqueous 0.5% thioflavine S solution for 5 min. These sections were treated with 70% ethanol, hydrated for 5 min and mounted with cytoseal. Sections were visualized and images were captured in Nikon Eclipse 80i microscope with a digital camera attached to the computer (Nikon, Melville, NY) and saved as Tagged image format files. Computer-aided quantification of Aβ deposits was performed using the MetaMorph 6.1 software (Universal Imaging Corp, Downington, PA). Three sections per mice were analyzed. Plaque counts and percentage occupied by the thioflavine S positive plaques were quantified in the cortex, hippocampus and thalamus. The region of interest was drawn manually under 4 × magnification and threshold was kept constant throughout the analysis. The analysis was performed in a blinded fashion.
2.7 Congo red staining
Congo red staining was performed following the method of Puchtler et al., [45] with slight modification. Briefly, Sections were washed with distilled water and stained with filtered Congo red solution for 10 minutes. Then sections were differentiated quickly with alkaline alcohol solution and washed with tap water. Then sections were counterstained with Harris heamatoxylin solution for 30 seconds and washed with tap water, dipped in ammonia water and rinsed in tap water. Finally, sections were dehydrated with 95% and 100% alcohol, cleared with xylene and mounted.
2.8. Immunohistochemistry
Serial sagittal sections (40 µm) thick were cut with a sliding microtome (Microm Lab GmbH, Walldorf, Germany) and processed as free floating sections. The staining protocol employed a modified ABC immunohistochemistry procedure [13,35]. Briefly, sections were washed with 0.1 M potassium phosphate buffered saline (PBS, pH 7.4) and incubated in 1% H2O2 for 30 min to quench the endogenous peroxidase. Then sections were treated with 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, MO) for 15 min and blocked with 2% bovine serum albumin (BSA) in PBS for 1 hr. Sections were incubated with mouse monoclonal anti-NeuN or rabbit anti-malondialdehyde antibody (Chemicon, Temecula, CA; 1:1000), or monoclonal antibodies 6E10 and 4G8 against 1–16 and 17–24 residues of human Aβ (1:1000; Signet laboratories, Dedham, MA) in PBS containing 1% BSA (Sigma Chemical Co., St. Louis, MO), rabbit anti-APP C terminal G369 antibody (1:1000), or rabbit anti-phospho Ser 396 tau antibody (1:500; Invitrogen, Carlsbad, CA) overnight at room temperature. After rinsing in PBS, sections were incubated with biotinylated anti-mouse or anti-rabbit (Vector Laboratories Inc., Burlingame, CA; 1:200 in PBS containing 0.25% BSA) for one hr. Sections were then incubated in avidin-biotin-peroxidase complex for one hour (Vector Laboratories Inc., Burlingame, CA; 1:100 in PBS), rinsed in PBS and developed in 0.05% 3,3’-diaminobenzidine (DAB) (Vector Laboratories Inc., Burlingame, CA) and 0.003% H2O2 in PBS.
Double immunofluorescence was performed to demonstrate the astroglial or TNF-α activation. Briefly, free floating sections were incubated with freshly prepared 0.5% (w/v) thioflavine S (Sigma Chemical Co., St. Louis, MO) solution for 5 min. Sections were washed twice with 70% ethanol and distilled water and incubated in PBS for 10 min. Sections were blocked with 1% BSA and incubated with rabbit anti-GFAP (DAKO, Carpinteria, CA) or goat anti-TNF-α (R&D systems, Minneapolis, MN) antibody in PBS containing 0.25% BSA (Sigma Chemical Co., St. Louis, MO) overnight at room temperature. After rinsing in PBS, sections were incubated with Rhodamine Red conjugated anti-rabbit or anti-goat IgG (Jackson ImmunoResearch Laboratories Inc, West Grove, PA) in PBS containing 0.25% BSA for one hour.
2.9. KGDHC activity assay
KGDHC activity was measured by monitoring the conversion of NAD to NADH with a 96 well fluorometric plate reader (Molecular Devices; Sunnyvale, CA, USA). The reaction mixture (pH 7.0) contained the following (mM): MgCl2 (1), CaCl2 (1), EDTA (0.5), TPP (0.3), DTT (1), NAD (1), Coenzyme A (0.163), α-ketoglutarate (1) and Tris–HCl (50) buffer and 0.1% Triton X-100. KGDHC activities (KG-dependent production of NADH) were assayed immediately as described previously [29]. One unit of enzyme activity was defined as the amount of enzyme catalyzing the production of 1 nmole of NADH or NADPH/min per mg protein.
2.10. Statistical Analysis
All the values are means ± SEM. P < 0.05 was considered significant. Statistical significance of group differences was tested by 2-tailed Students t test or one way ANOVA analysis. SPSS (SPSS Co., Chicago, IL) software was used to perform statistical analysis.
3. Results
3.1. TD decreased the KGDHC activity and induced selective neuronal loss in Tg19959 mice
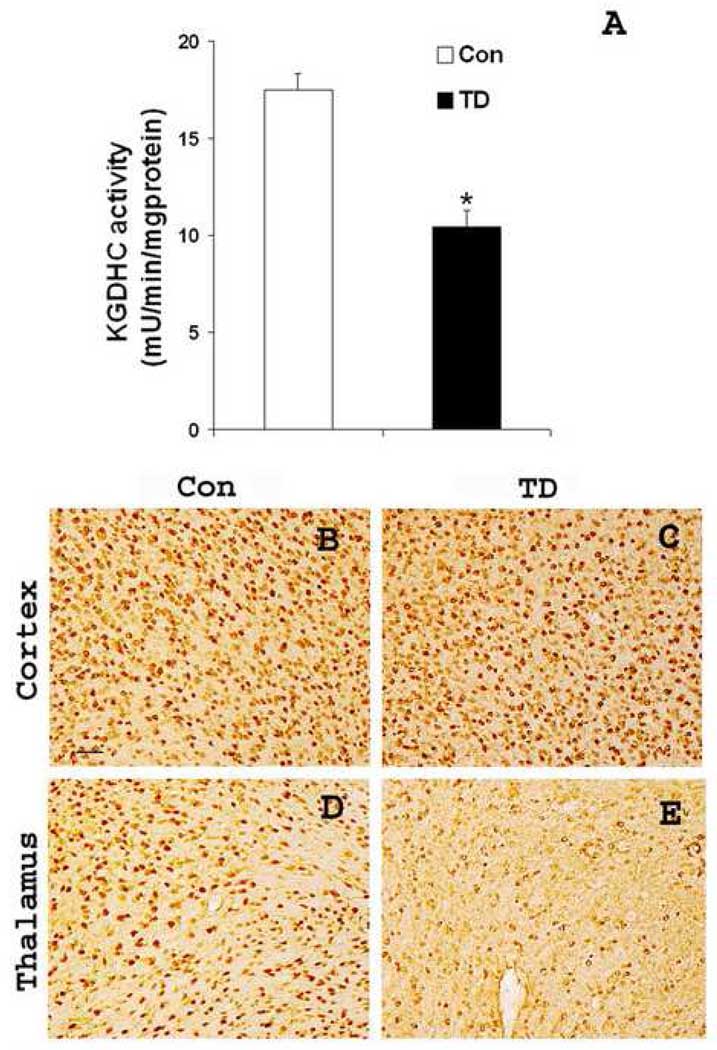
To test whether the general response to TD was altered by the presence of mutant human APP, the activity of KGDHC activity and neuronal loss was determined in control and TD Tg19959 mice. KGDHC activity in brains from TD Tg19959 mice was decreased to a level that was 42% of the activity of controls (p<0.01; Fig. 1A). Neuronal loss was assessed with NeuN antibody staining of sections from control and TD brains. The brain region in which the earliest TD-induced neuron loss occurs is the sub-medial thalamic nucleus of Tg19959 mice. TD caused a marked loss of NeuN immunoreactivity in the thalami of TD-treated mice as compared to control (Fig. 1E). The loss of neurons was still restricted to the thalamus as TD brains did not show any neuronal loss in cortex (Fig.1C). The results indicate that the TD-induced loss of KGDHC activity and neurons is similar in Tg19959 mice as previously observed in wild type C57BL/6 mice [35,36].
Fig. 1. TD reduced KGDHC activity and caused selective neuronal loss.
Sixty days old Tg19959 mice were made TD for 10 days and compared to appropriate control animals (n=4). A. KGDHC activity was measured in control and TD mice brains. KGDHC was measured on the “whole hemi-brain” that was not used for immunocytochemistry. Each value represents the mean ± S.E.M of three separate experiments done in triplicate.* p<0.01, TD vs control. B–E, shows the representative sagittal brain sections from control and TD brains through cortex and submedial thalamic nucleus stained for NeuN antibody. TD brains showed selective neuronal loss in thalamus region (E). Scale bars: B–E, 100 µm.
3.2. TD elevated markers of oxidative stress and induced neuritic clusters in Tg19959 mice
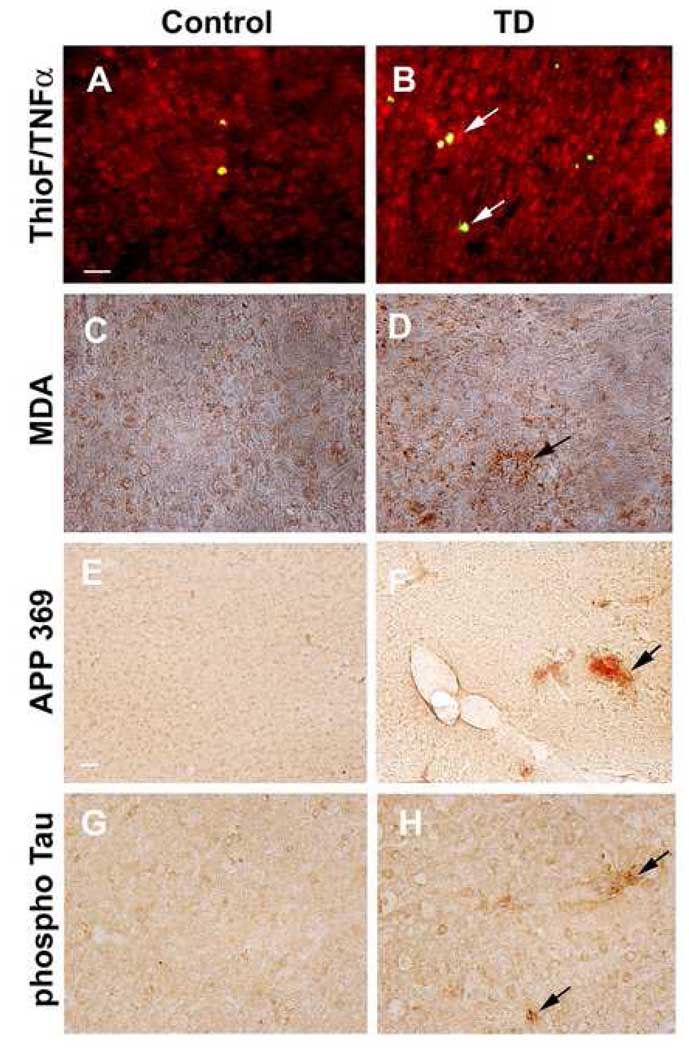
To study whether TD induces oxidative stress, we analyzed control and TD brains for proinflammatory cytokine, TNF-α (or) lipid peroxidation marker, malondialdehyde immunoreactivity. TD brains showed elevated levels of TNF-α and anti-malondialdehyde immunoreactivity (Fig. 2B, D) compared to control (Fig.2A, C). Previous studies of TD in C57BL/6 mice wild type mice revealed accumulation of APP immunoreactivity in dystrophic neuritic clusters [11,12]. We tested for neuritic clusters by examining APP 369 immunoreactivity in the control and TD brains of Tg19959 mice. Analysis of TD brain showed dystrophic neuritic clusters with accumulation of APP immunoreactivity. The clusters were limited to the vulnerable region of the thalamus (Fig 2E, F) and were not apparent in cortex. Further we characterized these neuritic clusters to determine whether they are hyperphosphorylated tau [41]. Brain sections were stained with anti-phospho Ser 396 tau antibody. These dystrophic neurites were positive for phospho Ser 396 tau antibody (Fig. 2H).
Fig. 2. TD induced oxidative stress and neuritic clusters.
Sixty days old Tg19959 mice were made TD for 10 days and compared to appropriate control animals (n=4). A–D shows the representative sagittal brain sections from control and TD brains, stained with thioflavin S and anti-TNF-α or anti-malondialdehyde antibody. Arrows show the elevated levels of TNF-α and malondialdehyde staining in the cortex region of TD brains. E–H show the representative sections from control and TD brains and stained for anti-APP 369 or anti-phospho Ser 396 tau antibody. Arrow shows the accumulation of APP and phospho tau in abnormal neurites in TD brain. Scale bars: A–D; G–H, 100 µm, E–F, 200 µm.
3.3. TD induced glial activation in Tg19959 mice
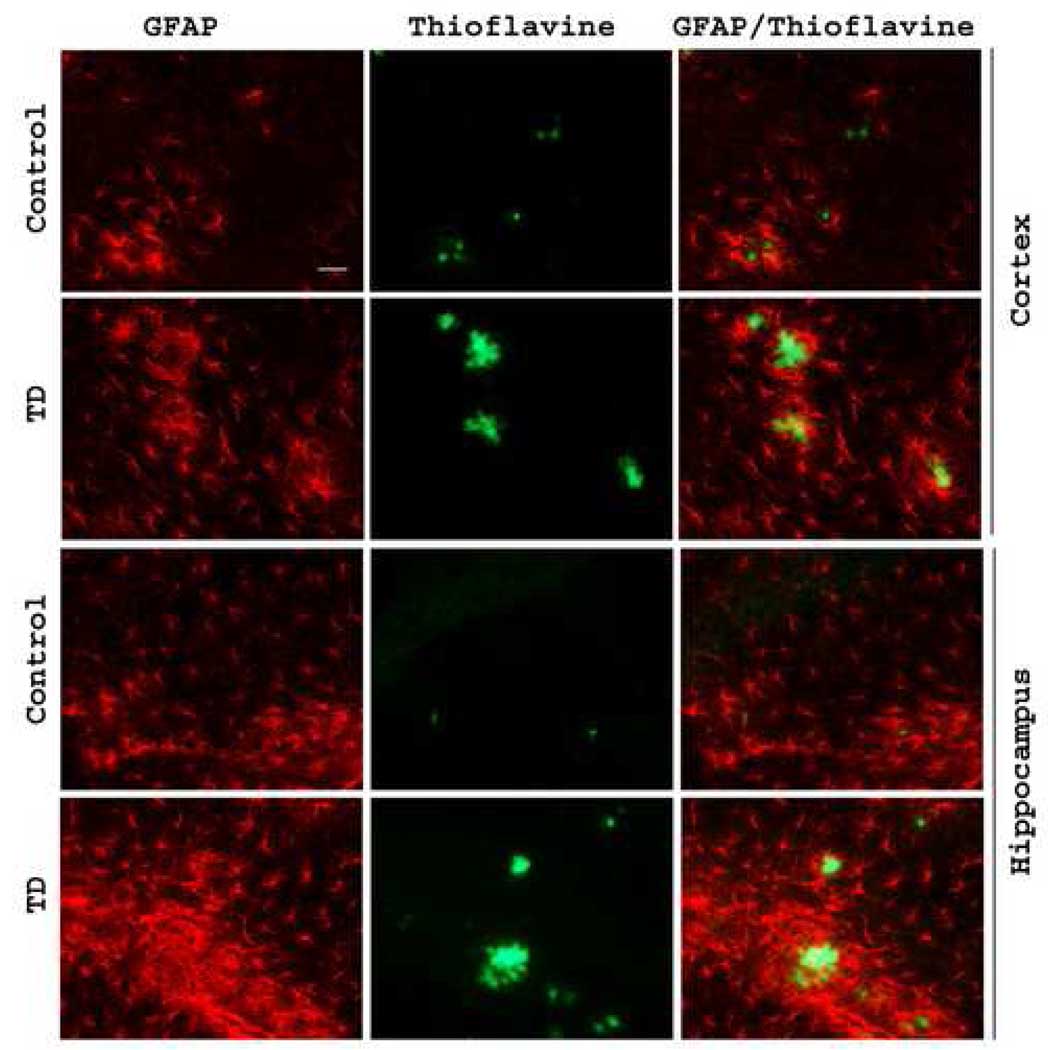
Inflammation has been implicated in AD and TD. Previous studies with plaque non-competent mice revealed that TD-associated inflammatory responses were typically limited to the region of neuron loss (i.e., SmTN). To test whether TD-associated inflammation was detectable in other brain regions when plaques were present, astrocyte activation was studied in 60 days old Tg 19959 mice using glial-fibrillary associated protein (GFAP) immunoreactivity as a marker. Immunostaining with GFAP revealed close proximity of activated astrocytes to amyloid plaques in Tg19959 mice. TD was associated with increased GFAP immunoreactivity in cortex and hippocampus compared to the control group (Fig. 3). Thus, the inflammatory response is different in the Tg19959 mice than in the C57BL/6 mice.
Fig. 3. TD induced astroglial activation.
Sixty days old Tg19959 mice made TD for 10 days. Representative brain sections from control and TD brains in cortex and hippocampus stained with an antibody directed against GFAP to assess astrocyte activation. These results are typical of two independent experiments (n=6). Scale bar, 50 µm.
3.4. TD accelerated formation of amyloid plaques
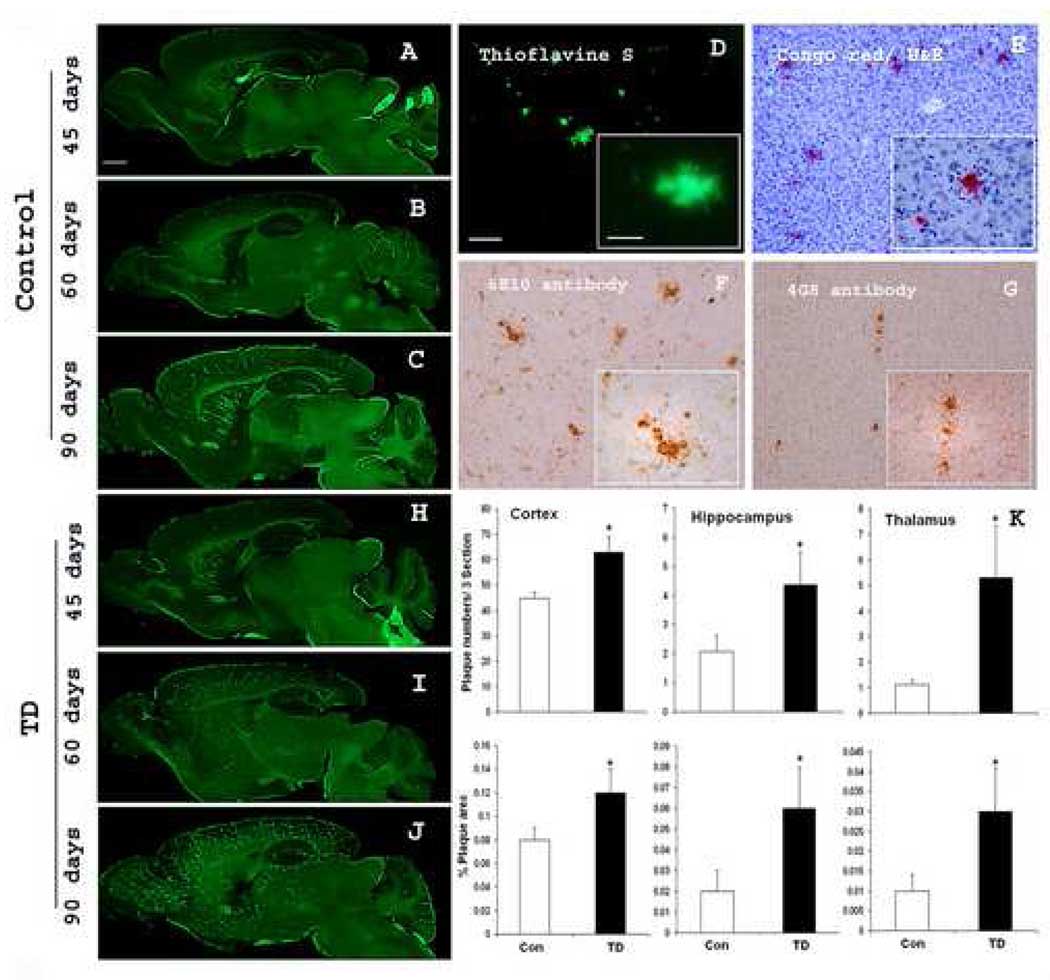
Although TD-induced neuronal loss and formation of neuritic clusters were restricted to the SmTN, TD exacerbated plaque formation throughout the brain. In initial studies, we characterized the time course and regional distribution of plaque formation in the Tg19959 mice to determine the appropriate time to induce TD. The Tg19959 mice showed an age-dependent accumulation of amyloid plaques. Thioflavine S positive plaques started to appear as early as 45 days. However, very few thioflavine S positive plaques occurred and they were mainly in cortex (Fig. 4A). In 60 day-old mice, plaques appeared in cortex, hippocampus, and striatum, but the density was still low (Fig. 4B). At 90 days, plaques were present in cortex, olfactory bulb, striatum, and hippocampus at high densities in comparison to the thalamus where only occasional plaques were observed (Fig. 4C). The deposits that stained positive for thioflavine S also stained with Congo red (Fig. 4D, E). Diffuse plaques stained with the Aβ42 -specific antibodies, as well as the monoclonal antibodies 6E10 (human-Aβ-domain-specific) and 4G8 (pan-species-Aβ) (Fig. 4F, G). The results from the time course study suggested that 60 days of age would be an appropriate time to test the possible effects of TD on plaque formation.
Fig. 4. TD accelerates deposition of amyloid plaques.
A–C show representative sagittal brain sections from 45, 60 and 90 days old Tg19959 mice stained with thioflavine S. D–G illustrates the profiles of amyloid plaques by different staining methods or antibodies: thioflavine S (D) and congo red (E), 6E10 antibody (F) and 4G8 antibody (G). H–J show representative sagittal brain sections from 45, 60 and 90 days old Tg19959 mice made TD for 10 days stained with thioflavine S. (K) shows the plaque counts and % area occupied by plaques quantified from the cortex, hippocampus and thalamus region. A statistical comparison of the sexes within groups did not find any sex differences. In controls, we compared males and females and found no differences (p>0.05). In TD we compared males and females and found no differences (p>0.05). The statistical comparisons were done both by one way ANOVA and Student t test. Data represent means ± SEM; Control (n=9) and TD (n=10) from 2–3 independent experiments. Scale bars: A–C and H–J, 500 µm; D–G, 100 µm; Insets 20 µm.
TD accelerated the accumulation of amyloid plaques in Tg19959 mice at 45, 60 and 90 days (Fig. 4H, I, J). In 60 day-old mice, plaques were present in controls at a low enough density to easily detect the increase. Ten days of TD markedly increased thioflavine S positive plaques in 60 day-old Tg19959 mice (Fig. 4I). The changes in plaque number and in percentage area occupied by plaques were quantified (Fig. 4K). In 60-day-old mice, ten days of TD significantly increased the average number of compact plaques in cortex from 44.8 ± 5.2 to 62.8 ± 6.4, hippocampus from 2.08 ± 0.55 to 4.36 ± 1.12 and thalamus from 1.11 ± 0.17 to 5.3 ± 2.03. TD also increased the percentage area occupied by plaques in cortex from 0.08 ± 0.01 to 0.12 ± 0.02, hippocampus from 0.02 ± 0.01 to 0.06 ± 0.02 and thalamus from 0.01 ± 0.004 to 0.03 ± 0.0107 (Fig. 4K). Non-transgenic, age-matched animals did not show any amyloid plaques (data not shown).
3.5. TD increased levels of total and insoluble Aβ protein in Tg19959 mice
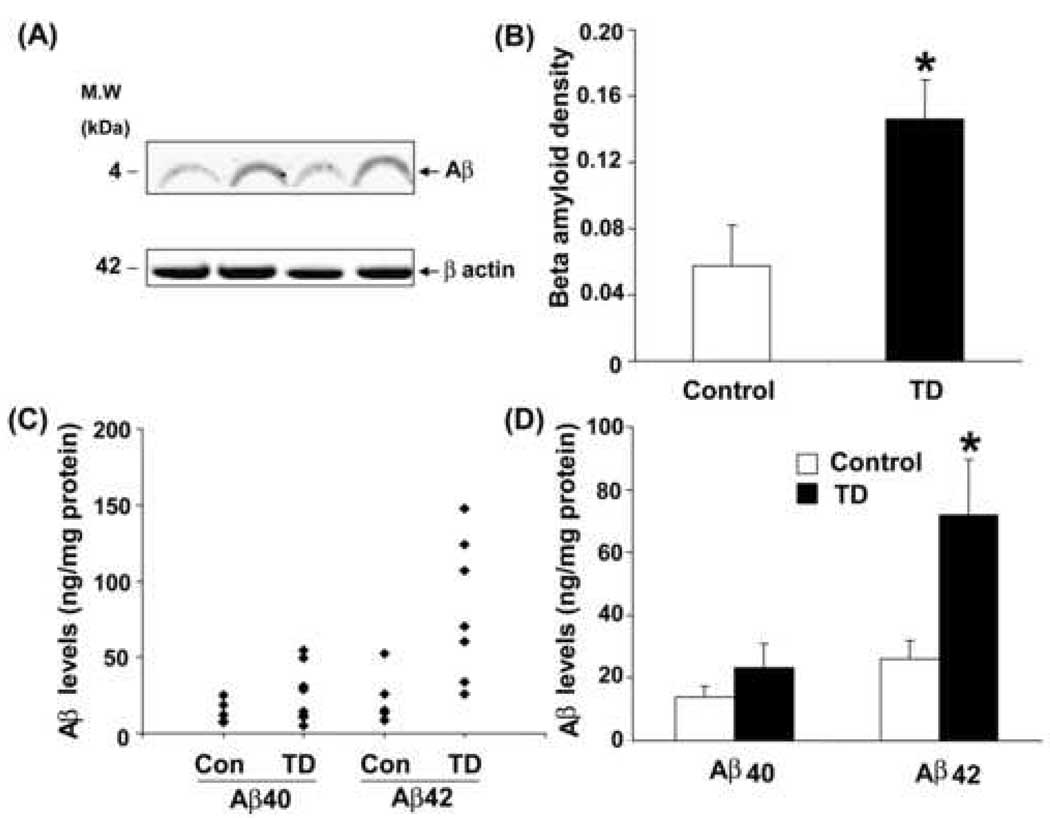
We performed Western blot and ELISA analyses to evaluate the Aβ̣ peptide levels. Whole hemi-brain homogenates from the control and TD Tg19959 mice were separated using SDS-PAGE and probed with APP 6E10 antibody (directed against 1–17 amino acids of human Aβ) (Fig. 5A). Visual inspection of the ~4kDa bands revealed a TD-associated increase in Aβ levels compared to control. Quantification of Aβ levels revealed a significant ~2 fold increase in TD compared to control (Fig. 5B).
Fig. 5. TD increases Aβ protein and Aβ-peptide1–42 levels.
Aβ protein and Aβ1–42 levels were determined in hemi-brain samples from 60 days old Tg 19959 mice made TD for 10 days and appropriate controls. Panel (A) shows a representative Western blot probed with 6E10 antibody, an antibody specific against 1–17 amino acids of Aβ and visualized by LICOR IR dyes. Panel (B) shows the results of Aβ band intensity quantified, and normalized against β-actin. The values represent the results from two independent experiments. Data represent mean ± SEM; Control and TD (n=5). (C) Aβ levels in whole brain extract were plotted for each animal both control and TD. Levels of Aβ1–42 were significantly increased in formic acid soluble extracts of TD (n=8) brains compared to control (n=9). (D) Mean Aβ1–40 and Aβ1–42 levels in control and TD. The values represent the results from two independent experiments.* p<0.03, TD vs control.
Aβ peptides were assessed in formic acid extracted brain fractions from control and TD brains. TD was associated with an increase in the insoluble Aβ42 levels to about three-fold when compared with levels observed in the control animals (p<0.03; Fig. 5A, B). Insoluble Aβ40 levels tended toward an increase as compared to control (1.65-fold), but the difference did not reach statistical significance (Fig. 5C, D).
3.6. TD altered APP CTFs in Tg19959 mice
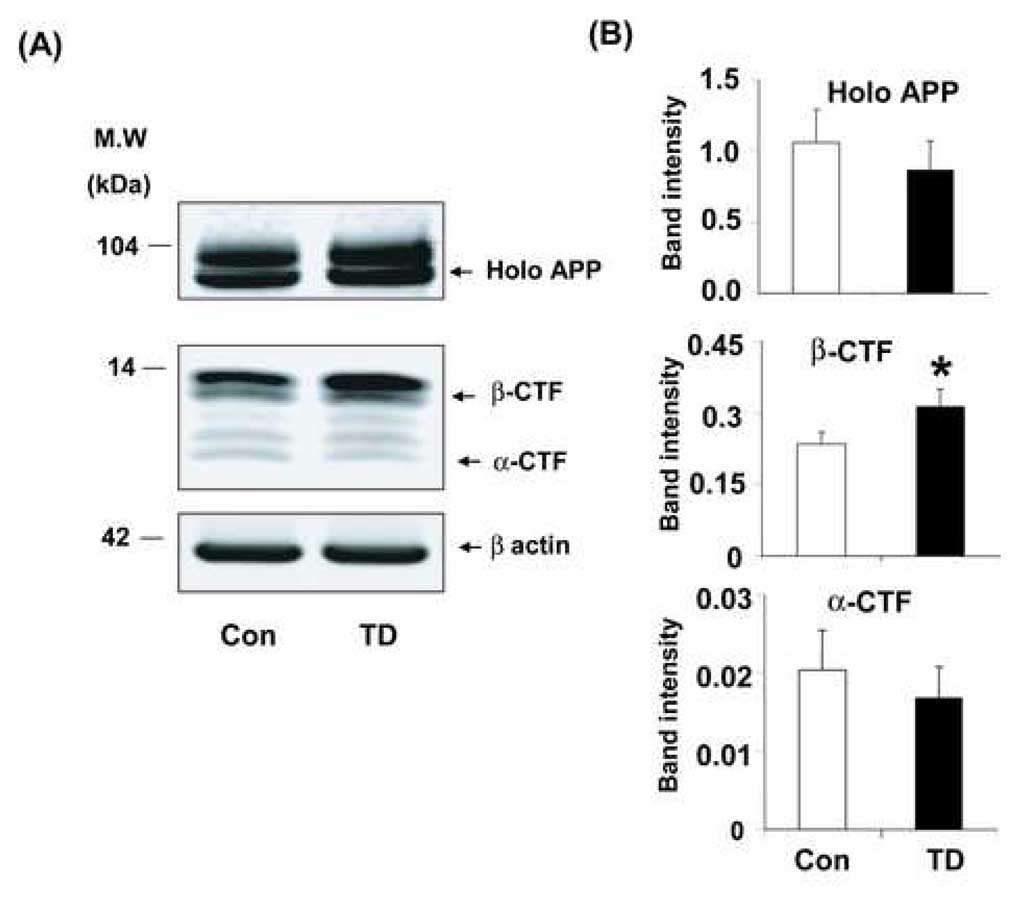
Subsequent studies tested whether the increase in plaque formation was due to altered processing of APP. Whole hemi-brain extracts from sixty-day-old Tg 19959 control and TD mice were analyzed by Western blot using APP G369 antibody (against the APP cytoplasmic tail) to reveal the full-length holo APP and bands corresponding to β-CTF (C99) and α-CTF (C83). The bands that are observed in Fig 6A, in the controls are as expected in the literature [8,19,30,47]. To establish whether our identification of the β -CTFs bands was correct and agreed with the literature, brain lysates from the control Tg19959 were run in parallel with C100 standard as a positive control. After separation, both were probed with G369 antibody (an antibody specific against both APP holo protein and C-terminal fragments) and 6E10 antibody (specific against 1–17 amino acids of Aβ), and visualized by LICOR IR dyes. Overlapping colors confirmed the identity of the β-CTF bands. To further test the identity of the APP CTF, lysates were prepared from whole brain (control Tg19959 mice) using multiple lysate buffers. APP CTF’s were immunoprecipitated using G369 and then probed with G369 or 6E10 antibody and visualized with LICOR IR dyes. The overlap of 6E10 and G369 bands confirmed the assignment of the β-CTF bands in Fig. 6A. Similar results were observed with each of the lysate buffers. Since the results agree with those in the literature, the data are not presented.
Fig. 6. TD increased APP C-terminal fragments in Tg19959 mice.
Sixty days old Tg19959 mice that were made TD for 10 days were compared to appropriate control animals. Panel (A) shows representative Western blots probed with G369 antibody, an antibody specific against both APP holo protein and C-terminal fragments. Panel (B) shows the densitometric analysis of APP holo protein and C-terminal fragments band intensity. The values represent the results from three independent experiments. Data represent mean ± SEM; Control (n=9) and TD (n=8). * p<0.05, TD vs control.
Western blot analysis revealed that TD altered the processing of APP. Although TD reduced the levels of high molecular weight APP species (holo APP) by 18%, the difference was not significant (Fig. 6B). However, TD increased the levels of low molecular weight APP species (12–14kDa) by 33% (p<0.02). This fragment corresponds to the APP C-terminal fragments β-CTF (C99) (Fig. 6A, B). Although, α-CTF (C83) levels showed a corresponding (18%) decrease, the decline was not statistically significant (Fig. 6A, B).
3.7. TD elevated BACE1 levels in Tg19959 mice
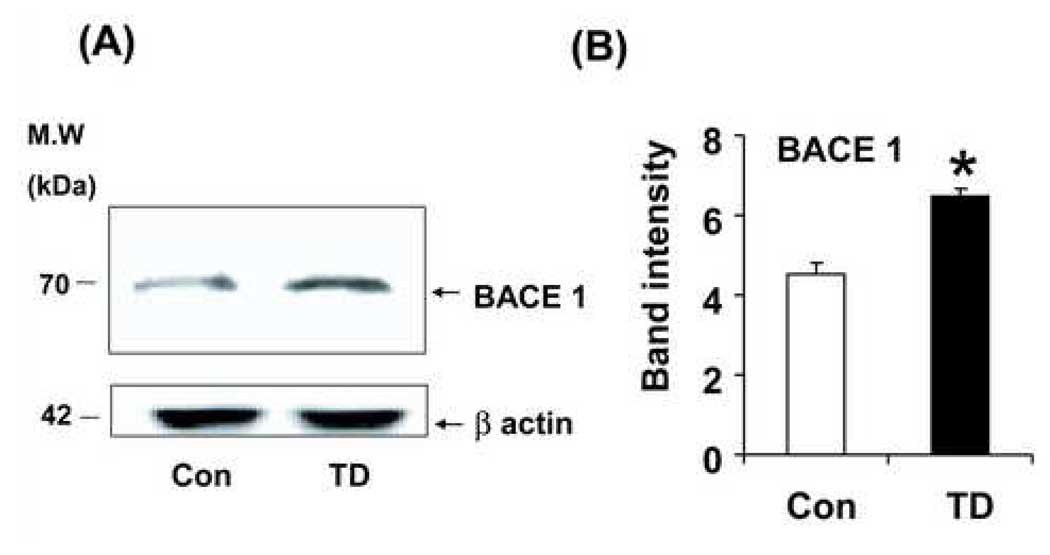
Further, to determine the increase in β-CTF levels is mediated by BACE1, whole hemi-brain extracts from sixty-day-old Tg19959 control and TD mice were analyzed by Western blot using BACE1 antibody. Ten days of TD significantly (43%; P<0.001) increased BACE1 protein levels (Fig. 7A, B).
Fig. 7. TD increased BACE1 levels in Tg19959 mice.
Whole hemi-brain extracts from sixty-day-old Tg 19959 control and TD mice were analyzed by Western blot using BACE1 antibody (PA1-757; Affinity Bioreagents). PA1-757 recognizes the C terminal region of both human and mouse BACE1 and recognize a band at ~70 kDa [51]. Ten days of TD caused a significant (43%; P<0.001) increase in BACE1 protein levels Panel (A) shows representative Western blots probed with BACE1 antibody. Panel (B) shows the densitometric analysis of BACE1 band intensity. The values represent the results from three independent blots of the same samples. Values represent mean ± SEM; Control (n=5) and TD (n=5). * p<0.001, TD vs control.
4. Discussion
Previous studies reveal that TD can mimic many of the reductions in thiamine- dependent processes that accompany AD. Although changes in thiamine-dependent processes can feasibly underlie the reductions in metabolism in AD, previous studies had not directly linked the changes to the pathological hallmarks of the disease. The current study indicates that thiamine deficiency exacerbates plaque formation and alters metabolism of APP and/or Aβ in the Tg19959 plaque competent mice. The results confirm in the Tg19959 mice that, as in the wild type mice, TD reduces KGDHC by 42%, causes selective loss of NeuN immunoreactivity in specific brain regions while other cell types are either activated or unaffected [36]. NeuN expression does not necessarily indicate neuronal number and integrity. However, in our previous studies in TD we found that the reduction in NeuN staining in SmTN (thalamus) correlated to changes in Nissl staining [35] and reflected the loss of NeuN protein levels as analyzed by Western blot [34]. Further, TD elevates markers of oxidative stress markers and induces region specific increases in neuritic clusters [11].
The highly selective nature of TD-induced neuronal damage made it surprising that TD exacerbated plaque formation in the cortex and other regions outside of the SmTN in which there is no neuronal loss and little oxidative stress/inflammation or neurodegeneration [36]. Other experimental paradigms linking oxidative stress to aggravation of Alzheimer-like pathology have been reported recently, and they also reveal changes throughout the brain. Partial knockout of one allele of manganese superoxide dismutase (MnSOD) mice crossed with Tg19959 mice elevated protein carbonyl levels, elevated Aβ levels by 25% in whole brain and increased plaque burden in cortex and hippocampus [37]. Inhibition of energy metabolism by insulin, 2-deoxyglucose, 3-nitropropionic acid, or kainic acid enhances plaque pathology in Tg2576 transgenic mice, and the increase is associated with a 200% increase in Aβ1–40 [51]. On the other hand, a cross of PS/APP double transgenic mice (Tg2576) with mice bearing disrupted iNOS alleles reduces the levels of protein tyrosine nitration products, concentration of Aβ, and cerebral amyloid plaque burden [43]. Our previous studies show that TD reduces thiamine-dependent enzymes throughout the brain while most markers of oxidative stress and inflammation only occur in the SmTN, the region showing neuronal loss [36]. In wild type mice, the only marker of oxidative stress/inflammation that we observed outside the SmTN is elevated TNFα in cortex [34]. In the current studies with Tg 19959 mice, TD increases the lipid peroxidation marker, malondialdehye and proinflammatory cytokine, TNF-α levels. Activated astrocytes observed in cortex in the current study appear to be secondary to the appearance of plaques.
The TD-induced amyloid plaques in thalamus are surprising and may provide insight into plaque formation. Human AD typically leaves the thalamus relatively intact. Tg19959 mice share the AD-like, thalamus-sparing pattern. Previous studies of TD in C57BL/6 mice wild type mice revealed accumulation of APP immunoreactivity in dystrophic neuritic clusters in the thalamus [11,12]. Double immunofluoresence histochemistry revealed co-localization of APP-like protein and neurofilament H in the abnormal neurites [10]. In subsequent studies, double immunofluorescence using antibody 369 and neurofilament 160 kd-immunoreactivities was observed in the neuritic clusters [35]. The results support the suggestion that the clusters are degenerating neurons. The induction of plaques by TD in a region that never has plaques in Tg19959 mice and is the only region that exhibits TD-induced neuritic clusters suggests novel interactions of degenerating neurites and plaque formation. Thus, one possible reason for the plaques in the thalamus is that the neuritic lesions induced by TD promote the plaque formation. There is some precedent for such interactions for tangle formation and in the present studies these neuritic clusters stain positive for phosphorylated tau. Several papers suggest that Aβ can induce degenerating neurites and thus tangle formation but not vice versa [31,32,44]. However, in the current experiments degenerating neurites appear to promote the accumulation of Aβ and phosphorylated tau. The data suggests the possibility that oxidative injury induces dystrophy and that the amyloidosis developed as sequelae to the dystrophic neuritis. However, if so, the data would demonstrate induction of amyloid pathology by upstream neuritic pathology. Oxidative, “Aβ-independent” pathways to Alzheimer’s pathology have been previously proposed [22,23,26]. Injury induces changes in metabolism of APP and/or Aβ [2]. Traumatic brain injury increases oxidative stress (isoprostanes) and accelerates Aβ accumulation [50], and the injury induced by traumatic brain injury can be reversed by the antioxidant vitamin E [16]. An alternative explanation of the current data is that the induction of neuritic clusters and plaques occurs by independent mechanisms. Future experiments could compare the temporal appearance of the neuritic lesions and plaques to test whether neuritic clusters lead to the plaques or if the two pathologies reflect independent processes.
The induction of tangles by Aβ1–42 in other models suggests that tangles could be induced by TD. Tangles occur in brains of thiamine deficient humans with the Wernicke Korsakoff Syndrome, a severe form of thiamine deficiency seen most often in chronic alcoholics [17,33]. The current studies show that TD increases tau phosphorylation. Tangles pathology can be achieved by expressing one of the mutant, “tangle-competent” forms of human tau that underlie human tauopathy, such as fronto-temporal dementia [46]. The TD Tg19959 mice do not have human tau. Since the production of AD-like changes in mice requires the presence of human tau, these studies were beyond the scope of the current experiments.
Comparison with other models which show that impaired metabolism and oxidative stress modulates metabolism of APP and/or Aβ may provide some insight into the mechanism underlying the TD-induced changes in the current experiments. One key aspect of the new data reported here is that that TD elevates brain Aβ levels and increases plaque burden. The three fold increase in Aβ1–42 is greater than that seen in the MnSOD partial knockouts (25%) [37] or following inhibition of energy metabolism in Tg2576 transgenic mice [51] and occurs relatively in a short time. The TD-induced changes in metabolism of APP and/or Aβ suggest a possible mechanism for the increase in Aβ detected by ELISA and in plaques (see Fig 5D; 4K). APP proteolytic products arise from the coordinated action of α-, β- and γ-secretases [22]. In the amyloidogenic pathway, APP is proteolytically cleaved by β-secretase (formally known as BACE, for β-APP-site cleaving enzyme) to release an N-terminal, soluble APP-β (sAPP-β) fragment and a retained, cell-associated amyloidogenic C-terminal fragment (CTF) known as β-CTF or C99. In the non-amyloidogenic pathway, APP is cleaved by α-secretase in the middle of the Aβ sequence to release N-terminal, soluble APP- α (sAPP- α) fragment and a retained non-amyloidogenic C-terminal fragment (CTF) known as α-CTF or C83. Western blot analysis of brain homogenates using antibody G369 revealed full-length holoAPP and bands corresponding to β-CTF (C99) and α-CTF (C83). TD reduced full length holo APP levels and C83 levels although the differences were not significantly different. In contrast, TD increased β-CTF (C99) and Aβ1–42. These findings indicate that one mechanism underlying the increase in Aβ levels following TD may be the increase in levels of the immediate precursor of Aβ, namely β-CTF (C99). Impairment of the electron transport chain promotes production of amyloidogenic fragments [20]. The current data indicates impairing other aspects of energy metabolism may also stimulate production of amyloidogenic fragments.
The increase in β-CTF levels may be due either to increased co-compartmentalization of BACE-1 and APP or to increased levels of BACE. A variety of oxidants, such as 4-hydroxynonenal (HNE), are known to up-regulate BACE-1 expression and activity in differentiated neuronal NT2 cells [48]. In addition, acute inhibition of energy metabolism by insulin, 2-deoxyglucose, 3-nitropropionic acid and kainic acid in C57BL/6 wild type and APP transgenic mice (Tg2576) elevate BACE-1 levels and activity and increase levels of Aβ [51]. Our previous studies show that TD elevates markers of oxidative stress, nitric oxide synthase (NOS), intracellular adhesion molecule-1 (ICAM-1) in early stages and induces HNE and hemeoxygenase-1 (HO-1) in late stages in wild type C57BL/6 mice [36]. Thus, the current results are consistent with an elevated BACE-1, in which the activator is impaired metabolism or an inflammatory signal such as TNF-α. The 43% increase in BACE-1 levels (Fig. 7B) is consistent with this possibility. The increase in TNF-α in cortex of TD mice can regulate β-amyloid deposition and BACE expression [55]. However, other factors may have a large impact. Altered proteasomal activity may alter clearance of the peptides [18]. The much larger increase in Aβ42 than Aβ40 suggests additional factors may be important. TD may enhance nidus and aggregation of soluble Aβ in interstitial fluids. Since we did not distinguish soluble from insoluble Aβ we cannot assess which fraction is most affected by treatment. In addition, reduced Aβ clearance and/or degradation, or accelerated Aβ deposition, via undetected damage in cortex and hippocampus could account for the exacerbation of plaque formation following TD.
Another possible mechanism is activation of BACE by caspase 3. Recently, Tesco et al., have shown that caspase activation increases the BACE and C99 levels. Also the levels of GGA3, an adaptor protein involved in BACE trafficking are decreased and inversely correlated with increased levels of BACE in AD [49]. Inhibition of caspase abolishes brain trauma-induced increases in Aβ peptide [1]. TD can lead to activation of caspases. TD-induced stress may lead to the inactivation of C-Jun N-terminal kinase 1 (JNK1) and the loss of its cellular protective function from programmed cell death [53]. Recent results suggest that TD triggers apoptosis by activating caspase-3 mediated signaling pathway which leads to neuronal loss [15]. Also, TD induces endoplasmic reticulum (ER) stress in neurons and blocking the activation of caspase-12, an ER-anchored caspase, alleviates TD-induced neuronal death [54]. Although, these changes are largely restricted to the areas with neuron death, the studies were not done in plaque competent mice.
Several indirect lines of evidence suggest that thiamine may alter protein processing. Critical components of protein processing occur in the endoplasmic reticulum (ER), trans-Golgi Network and Golgi apparatus where thiamine-processing enzymes also occur. Thiamine pyrophosphatase is a trans-Golgi marker enzyme [40,42]. Recent studies indicate that the thiamine dependent enzyme transketolase is localized around the endoplasmic reticulum (ER) [4]. The ER is involved in post-translational protein processing and transport. The accumulation of unfolded proteins leads to ER stress. TD induces ER stress in thalamus [54]. Electron microscopy also reveals differences in brain ER: enlarged lumens, deposition of vesicles and an irregular packing pattern of rough ER [54]. Thus, TD may induce a general problem in protein processing. We have chosen to examine plaque formation and changes in metabolism of APP and/or Aβ because of its critical role in AD.
The integrity of thiamine-dependent biochemical pathways is critical to normal memory formation and retrieval [24,39]. The current study suggests that thiamine-sensitive pathways are also likely to be important in the pathology of AD and could promote plaque formation. TD plaque-forming transgenic mice may be useful for screening novel thiamine derivatives for their activities as attenuators of plaque accumulation, especially since dramatic changes occur within a ten day period. Multiple nutritional factors (e.g., cholesterol, caloric intake) have been implicated in accelerating the onset of AD, and we would predict that thiamine status and the consequent changes in metabolism and oxidative stress represents another factor that can modulate the pathology in AD.
Acknowledgements
The authors thank Julian Moore for assistance with the pictures.
This work was supported by NIH grants: AG14600, AG11921, AG10491, and AG14930
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
None of the authors have a financial interest in publication of the manuscript
REFERENCES
- 1.Abrahamson EE, Ikonomovic MD, Ciallella JR, Hope CE, Paljug WR, Isanski BA, Flood DG, Clark RS, DeKosky ST. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: implications for clinical outcome. Exp Neurol. 2006;197(2):437–450. doi: 10.1016/j.expneurol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Beeson JG, Shelton ER, Chan HW, Gage FH. Age and damage induced changes in amyloid protein precursor immunohistochemistry in the rat brain. J Comp Neurol. 1994;342(1):69–77. doi: 10.1002/cne.903420108. [DOI] [PubMed] [Google Scholar]
- 3.Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19(4):939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 4.Boren J, Ramos-Montoya A, Bosch KS, Vreeling H, Jonker A, Centelles JJ, Cascante M, Frederiks WM. In situ localization of transketolase activity in epithelial cells of different rat tissues and subcellularly in liver parenchymal cells. J Histochem Cytochem. 2006;54(2):191–199. doi: 10.1369/jhc.5A6745.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57(5):695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 6.Bubber P, Ke ZJ, Gibson GE. Tricarboxylic acid cycle enzymes following thiamine deficiency. Neurochem Int. 2004;45(7):1021–1028. doi: 10.1016/j.neuint.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265(5177):1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 8.Buxbaum JD, Thinakaran G, Koliatsos V, O'Callahan J, Slunt HH, Price DL, Sisodia SS. Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J Neurosci. 1998;18(23):9629–9637. doi: 10.1523/JNEUROSCI.18-23-09629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol. 1999;58(9):946–958. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Calingasan NY, Gandy SE, Baker H, Sheu KF, Kim KS, Wisniewski HM, Gibson GE. Accumulation of amyloid precursor protein-like immunoreactivity in rat brain in response to thiamine deficiency. Brain Res. 1995;677(1):50–60. doi: 10.1016/0006-8993(95)00136-e. [DOI] [PubMed] [Google Scholar]
- 11.Calingasan NY, Gandy SE, Baker H, Sheu KF, Smith JD, Lamb BT, Gearhart JD, Buxbaum JD, Harper C, Selkoe DJ, Price DL, Sisodia SS, Gibson GE. Novel neuritic clusters with accumulations of amyloid precursor protein and amyloid precursor-like protein 2 immunoreactivity in brain regions damaged by thiamine deficiency. Am J Pathol. 1996;149(3):1063–1071. [PMC free article] [PubMed] [Google Scholar]
- 12.Calingasan NY, Gandy SE, Gibson GE. Thiamine deficiency alters APP but not presenilin-1 immunoreactivity in vulnerable brain regions. Neuroreport. 1997;8(11):2631–2634. doi: 10.1097/00001756-199707280-00041. [DOI] [PubMed] [Google Scholar]
- 13.Calingasan NY, Huang PL, Chun HS, Fabian A, Gibson GE. Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J Neuropathol Exp Neurol. 2000;59(3):207–217. doi: 10.1093/jnen/59.3.207. [DOI] [PubMed] [Google Scholar]
- 14.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276(24):21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 15.Chornyy S, Parkhomenko J, Chorna N. Thiamine deficiency caused by thiamine antagonists triggers upregulation of apoptosis inducing factor gene expression and leads to caspase 3-mediated apoptosis in neuronally differentiated rat PC-12 cells. Acta biochim Pol. 2007;54(2):315–322. [PMC free article] [PubMed] [Google Scholar]
- 16.Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, Trojanowski JQ, Lee VM, McIntosh TK, Pratico D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004;90(3):758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- 17.Cullen KM, Halliday GM. Neurofibrillary tangles in chronic alcoholics. Neuropathol Appl Neurobiol. 1995;21(4):312–318. doi: 10.1111/j.1365-2990.1995.tb01065.x. [DOI] [PubMed] [Google Scholar]
- 18.de Vrij FM, Fischer DF, van Leeuwen FW, Hol EM. Protein quality control in Alzheimer's disease by the ubiquitin proteasome system. Prog Neurobiol. 2004;74(5):249–270. doi: 10.1016/j.pneurobio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Estus S, Golde TE, Kunishita T, Blades D, Lowery D, Eisen M, Usiak M, Qu XM, Tabira T, Greenberg BD. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992;255(5045):726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- 20.Gabuzda D, Busciglio J, Chen LB, Matsudaira P, Yankner BA. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem. 1994;269(18):13623–13628. [PubMed] [Google Scholar]
- 21.Gallant M, Rak M, Szeghalmi A, Del Bigio MR, Westaway D, Yang J, Julian R, Gough KM. Focally elevated creatine detected in amyloid precursor protein (APP) transgenic mice and Alzheimer disease brain tissue. J Biol Chem. 2006;281(1):5–8. doi: 10.1074/jbc.C500244200. [DOI] [PubMed] [Google Scholar]
- 22.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115(5):1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandy S, Doeven MK, Poolman B. Alzheimer disease: presenilin springs a leak. Nat Med. 2006;12(10):1121–1123. doi: 10.1038/nm1006-1121. [DOI] [PubMed] [Google Scholar]
- 24.Gibson G, Barclay L, Blass J. The role of the cholinergic system in thiamin deficiency. Ann N Y Acad Sci. 1982;378:382–403. doi: 10.1111/j.1749-6632.1982.tb31213.x. [DOI] [PubMed] [Google Scholar]
- 25.Gibson G, Blass J. Thiamine-Dependent Processes and Treatment Strategies in Neurodegenerations. Antioxid Redox Signal. 2007;9(10):1605–1619. doi: 10.1089/ars.2007.1766. [DOI] [PubMed] [Google Scholar]
- 26.Gibson GE, Blass JP, Beal MF, Bunik V. The alpha-ketoglutarate-dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration. Mol Neurobiol. 2005;31(1–3):43–63. doi: 10.1385/MN:31:1-3:043. [DOI] [PubMed] [Google Scholar]
- 27.Gibson GE, Haroutunian V, Zhang H, Park LC, Shi Q, Lesser M, Mohs RC, Sheu RK, Blass JP. Mitochondrial damage in Alzheimer's disease varies with apolipoprotein E genotype. Ann Neurol. 2000;48(3):297–303. [PubMed] [Google Scholar]
- 28.Gibson GE, Huang HM. Mitochondrial enzymes and endoplasmic reticulum calcium stores as targets of oxidative stress in neurodegenerative diseases. J Bioenerg Biomembr. 2004;36(4):335–340. doi: 10.1023/B:JOBB.0000041764.45552.f3. [DOI] [PubMed] [Google Scholar]
- 29.Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch Neurol. 1988;45(8):836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- 30.Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- 31.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 32.Gotz J, Ittner LM, Schonrock N. Alzheimer's disease and frontotemporal dementia: prospects of a tailored therapy? Med J Aust. 2006;185(7):381–384. doi: 10.5694/j.1326-5377.2006.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 33.Halliday G, Cullen K, Harding A. Neuropathological correlates of memory dysfunction in the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 1994;2:245–251. [PubMed] [Google Scholar]
- 34.Karuppagounder S, Shi Q, Xu H, Gibson GE. Changes in Inflammatory Processes Associated With Selective Vulnerability Following Mild Impairment of Oxidative Metabolism. Neurobiol Dis. 2007;26:353–362. doi: 10.1016/j.nbd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ke ZJ, DeGiorgio LA, Volpe BT, Gibson GE. Reversal of thiamine deficiency-induced neurodegeneration. J Neuropathol Exp Neurol. 2003;62(2):195–207. doi: 10.1093/jnen/62.2.195. [DOI] [PubMed] [Google Scholar]
- 36.Ke ZJ, Gibson GE. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem Int. 2004;45(2–3):361–369. doi: 10.1016/j.neuint.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004;89(5):1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu JY, Timm DE, Hurley TD. Pyrithiamine as a substrate for thiamine pyrophosphokinase. J Biol Chem. 2006;281(10):6601–6607. doi: 10.1074/jbc.M510951200. [DOI] [PubMed] [Google Scholar]
- 39.Mair RG. On the role of thalamic pathology in diencephalic amnesia. Rev Neurosci. 1994;5(2):105–140. doi: 10.1515/revneuro.1994.5.2.105. [DOI] [PubMed] [Google Scholar]
- 40.McGuinness MP, Orth JM. Gonocytes of male rats resume migratory activity postnatally. Eur J Cell Biol. 1992;59(1):196–210. [PubMed] [Google Scholar]
- 41.Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li QX, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2(6):e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morre DJ, Lawrence J, Safranski K, Hammond T, Morre DM. Experimental basis for separation of membrane vesicles by preparative free-flow electrophoresis. Journal of chromatography. 1994;668(1):201–213. doi: 10.1016/0021-9673(94)80110-X. [DOI] [PubMed] [Google Scholar]
- 43.Nathan C, Calingasan N, Nezezon J, Ding A, Lucia MS, La Perle K, Fuortes M, Lin M, Ehrt S, Kwon NS, Chen J, Vodovotz Y, Kipiani K, Beal MF. Protection from Alzheimer's-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202(9):1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 45.Puchtler H, Sweat F. Congo red as a stain for fluorescence microscopy of amyloid. J Histochem Cytochem. 1965;13(8):693–694. doi: 10.1177/13.8.693. [DOI] [PubMed] [Google Scholar]
- 46.Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25(46):10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santiard-Baron D, Langui D, Delehedde M, Delatour B, Schombert B, Touchet N, Tremp G, Paul MF, Blanchard V, Sergeant N, Delacourte A, Duyckaerts C, Pradier L, Mercken L. Expression of human FE65 in amyloid precursor protein transgenic mice is associated with a reduction in beta-amyloid load. J Neurochem. 2005;93(2):330–338. doi: 10.1111/j.1471-4159.2005.03026.x. [DOI] [PubMed] [Google Scholar]
- 48.Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10(3):279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- 49.Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54(5):721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci. 2002;22(2):446–454. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velliquette RA, O'Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis. J Neurosci. 2005;25(47):10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Tanila H, Puolivali J, Kadish I, van Groen T. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol Dis. 2003;14(3):318–327. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Wang JJ, Hua Z, Fentress HM, Singleton CK. JNK1 is inactivated during thiamine deficiency-induced apoptosis in human neuroblastoma cells. J Nutr Biochem. 2000;11(4):208–215. doi: 10.1016/s0955-2863(00)00067-x. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Wang B, Fan Z, Shi X, Ke ZJ, Luo J. Thiamine deficiency induces endoplasmic reticulum stress in neurons. Neuroscience. 2007;144(3):1045–1056. doi: 10.1016/j.neuroscience.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170(2):680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao J, Petanceska SS, Montine TJ, Holtzman DM, Schmidt SD, Parker CA, Callahan MJ, Lipinski WJ, Bisgaier CL, Turner BA, Nixon RA, Martins RN, Ouimet C, Smith JD, Davies P, Laska E, Ehrlich ME, Walker LC, Mathews PM, Gandy S. Aging, gender and APOE isotype modulate metabolism of Alzheimer's Abeta peptides and F-isoprostanes in the absence of detectable amyloid deposits. J Neurochem. 2004;90(4):1011–1018. doi: 10.1111/j.1471-4159.2004.02532.x. [DOI] [PubMed] [Google Scholar]