Abstract
Tumors of the adult central nervous system are among the most common and most chemoresistant neoplasms. Malignant tumors of the brain and spinal cord collectively account for approximately 1.3% of all cancers and 2.2% of all cancer-related deaths. Novel pharmacological approaches to nervous system tumors are urgently needed. This review presents the current approaches and challenges to successful pharmacotherapy of adults with malignant tumors of the central nervous system and discusses novel approaches aimed at overcoming these challenges.
Keywords: brain tumors, gliomas, spinal cord tumors, cancer chemotherapy, multidrug resistance
1. Introduction
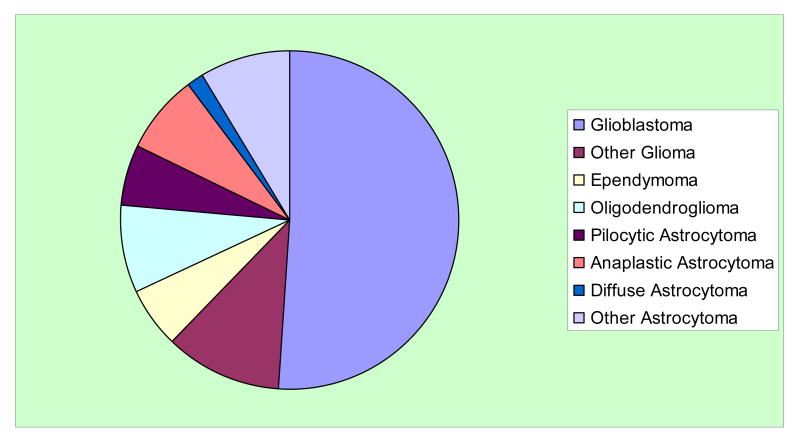
Tumors of the central nervous system (CNS) are both common and therapeutically difficult. They are problematic across the entire age spectrum. The most common malignant tumors of the CNS are derived from glial cells and include oligodendrogliomas, astrocytomas, and glioblastomas (Fig. 1). The American Cancer Society estimates that 21,810 malignant tumors of the brain or spinal cord will be diagnosed during 2008 in the US. Approximately 13,070 people will die from these malignant tumors. This type of cancer accounts for approximately 1.3% of all cancers and 2.2% of all cancer-related deaths (American Cancer Society, 2008).
Fig. 1.
Fractional incidence of subtypes of nervous system gliomas (Central Brain Tumor Registry of the United States, 2007).
Clearly, new approaches are critically needed for malignant tumors of the CNS. This review discusses current approaches and challenges to the pharmacotherapy of malignant tumors of the brain and spinal cord in adults and presents novel approaches aimed at overcoming these challenges.
Pharmacoresistance of brain tumors is clearly multifactorial. Brain tumor resistance mechanisms include multidrug resistance factor 1 and P-glycoprotein (MDR1/P-gp), O6-methylguanine methyltransferase (MGMT), multidrug resistance protein (MRP), metallothioneins, glutathione and glutathione-S-transferases (GSH/GSTs), dihydrofolate reductase (DHFR), protein kinase C (PKC), and topoisomerase-IIα. Aberrant drug transport with MDR1 is bidirectional (decreased influx and increased efflux); with MRP, it is unidirectional (increased efflux only). GSH/GST has been approached in preclinical models using the GSH synthesis inhibitor, buthionine sulfoximine (BSO), and using genetic manipulation of the drug-specific GST isoform. Topoisomerase-IIα is a marker for cell turnover and associated with atypical MDR phenotype. MGMT is suppressed by p53. Metallothioneins inactivate platinum compounds by forming a complex with them. PKC isoforms play three distinct roles in brain tumors: they allow them to arrest in G2 to repair DNA; when overexpressed, they prevent apoptosis; they alter expression of MDR1, P-gp, GST, and MGMT. Altered VEGF expression and alterations in chromosomes 1P and 19q are also implicated in resistance/susceptibility (Bredel and Zentner, 2002; Calatazzolo et al., 2005).
The tumor suppressor p33ING1 has growth-inhibitory and pro-apoptotic effects involving recruitment of p53. p33ING1 also plays a role in DNA repair through interaction with proliferating cell nuclear antigen (PCNA). Various malignant brain tumor cell lines were examined for their sensitivity to cisplatin, doxorubicin, etoposide and the antimitotic agents vincristine and paclitaxel. In general, ING1 levels were higher in glioma cell lines than in normal control cells. Comparing glioma cell lines, p33ING1 gene expression correlated significantly (p = 0.028) with resistance to vincristine (r2 = 0.87; Tallen et al., 2003). Higher glioblastoma cell plating density in vitro results in higher resistance to chemotherapeutic drugs. This suggests that there are autocrine or paracrine factors that facilitate resistance (Ng et al., 2007).
For many years, blood-brain barrier (BBB) penetration was the single most important obstacle facing chemotherapy for brain tumors. Significant progress has now been made in this regard (Gururangen and Friedman, 2002). The hypothesis has been advanced that tumor hypoxia mediates and perpetuates the transformation of more benign tumors to more malignant ones by selecting those cells for survival that express hypoxia-tolerant proteins [e.g., hypoxia-inducible factor (HIF)] and that enhance angiogenesis (Jensen, 2006).
The role of steroids in modulating BBB and chemoresistance of brain tumors is controversial. Steroids modulate P-gp function, are a P-gp substrate, and decrease chemotherapeutic agent uptake into the CNS. However, dexamethasone has also been reported to induce chemoresistance in brain tumor cells (Zhang et al., 2006).
Often, drug concentrations are higher in tumor than in surrounding normal tissue, implying a more permeable BBB in the former, and leading to the concept of the blood-tumor barrier. The BBB has been defined on anatomic, molecular, and electrophysical bases. No one of these alone accounts completely for the behavior of the BBB relative to uptake and distribution of chemotherapeutic agents into the CNS. There are apparently at least two drug binding sites on P-gp; one binds drugs that inhibit transport via the other site, but does not transport them. The other binds and transports drugs. Knowing to which site a drug binds helps determine its effect on chemotherapeutic resistance. It is not clear what the role of other resistance proteins (e.g., MRPs) is in chemoresistant primary brain tumors. Attempts to open up the BBB to let drugs in include osmotic methods (intraarterial mannitol) and bradykinin analogue administration (raising intracellular Ca++ and thereby opening tight junctions). Early human trials of the latter gave mixed results. Use of verapamil or cyclosporine or analogues to circumvent P-gp has proven to be disappointing in CNS tumors in vivo. Irradiation seems to decrease P-gp expression and seems to work synergistically with P-gp inhibitors. Adjunctive, simultaneous irradiation and chemotherapy may be useful for this reason (Bart et al., 2000).
In addition to attempts to overcome chemoresistance, brain tumor research has been aimed at designing novel methods for predicting for a given tumor which available therapies are most likely to be effective and which are most likely to meet resistance. Diffusion imaging and, more recently, sodium MRI have demonstrated their distinct abilities to detect therapy-induced alterations in tumor cellularity, which has been demonstrated to be indicative of therapeutic efficacy. More importantly, both imaging modalities detect tumor response much earlier than traditional methodologies that rely on macroscopic volumetric changes (Schepkin et al., 2006). Proteomics holds the promise of allowing a molecular understanding of the resistance of CNS tumors to chemotherapy and individualization of therapy (Valera et al., 2007a; Valera et al., 2007b). The potential already exists to use microarray and informatics technology to discern patterns of gene expression associated with chemoresistance and to discover novel mechanisms and effectors of such resistance (Bredel et al., 2004).
2. Gliomas and glioblastomas
Glial neoplasms represent 0.5-1% of all cancers in most Western countries. Malignant gliomas are among the most devastating cancers, leading to death in most cases. Techniques to circumvent the resistance mechanisms to chemotherapy in gliomas are being evaluated. Tyrosine kinase inhibitors are among the latest agents to show activity in malignant primary brain tumors. Radioimmunotherapy remains an area of active research (Desjardins et al., 2005). Proposed new strategies for improving therapy for glioblastoma include: temozolomide, an alkylating agent; molecular profiling by microarray and related techniques; anti-angiogenic drugs and strategies; anti-oncogene signaling drugs and strategies (e.g., anti-EGFR kinase). Pharmacotherapies for gliomas and glioblastoma are grouped by mechanism and target of action and summarized in Table 1.
Table 1. Pharmacotherapy used or proposed for adults with tumors of the central nervous system (CNS).
| Drug Target and/or Mechanism(s) of Action | Agent(s) | Tumors of the Adult CNS for Which Agents Are Proposed or Used |
|---|---|---|
| Multidrug resistance | MRP antisense constructs p16 p14ARF Methionine-free diet Scutellaria baicalensis extracts |
Glioblastoma, oligodendroglioma |
| Verapamil | Alters drug efflux in brain vascular endothelial cells but not glial tumor cells | |
| Signaling effectors | Erlotinib Gefitinib Nelfinavir TRAIL HGF and c-Met antisense constructs |
Glioblastoma (adjunctive) |
| DNA modification, damage, and repair | Temozolomide BCNU CCNU Procarbazine Cyclophosphamide Carboplatin Etoposide Camptothecan Doxorubicin Ape-1 antisense constructs |
Glioblastoma, anaplastic astrocytoma, low-grade glioma, oligodendroglioma, CNS lymphoma, spinal ependymoma (esp. etoposide), |
| Targeted (including immune-based) therapy | Tyrosinase-related peptide-targeted cytotoxic T lymphocytes | Glioblastoma |
| Receptor ligand-toxin fusion proteins | CNS lymphoma | |
| Rituximab | ||
| Adhesion and invasion; angiogenesis | cyclic pentapeptide EMD 121974 | Glioblastoma |
| Apoptosis effectors | Bcl-XL antisense constructs Curcumin Ruthenium complex analogue of cisplatin HSV thymidine kinase + gancyclovir Cyclosporin A Transglutaminase 2 inhibitors |
Malignant glioma, glioblastoma |
| Hormone receptor ligands | Pegvisomant Octreotide Lanreotide BIM-23745 Chimeric somatostatin-dopamine molecules Selective estrogen receptor modulators Aromatase inhibitors |
Pituitary adenomas |
| Neurotransmitter receptor ligands | Cabergoline Bromocriptine Quinagolide Chimeric somatostatin-dopamine molecules |
Pituitary adenomas |
Therapeutic challenges include loss of 1p heterozygosity, a marker for chemoresistance (Carpentier, 2005). Glioma therapy can be individualized based on expression (as measured by RT-PCR) of various proteins known to be associated with resistance to particular agents (Tanaka et al., 2000). Proteasome inhibitors may be useful for adjunctive therapy in overcoming resistance to chemotherapeutic agents. They work through enhancement of production of proteins from alternate apoptosis pathways, enhancement of nuclear translocation of mutant p53, and phosphorylation of p53. The last of these is not critical in the astrocytoma model; however, nuclear translocation of p53 is critical (Ceruti et al., 2006).
One study of glial tumors looked at histologic type, stage/grade, and chemoresistance profile. A large percentage of glioblastomas displayed extreme drug resistance to paclitaxel (69%, n = 35), SN38 (75%, n = 28), and vincristine (38%, n = 29). The majority of Grade II/III astrocytomas displayed extreme drug resistance to carboplatin (67%, n = 6), cisplatin (60%, n = 10), and paclitaxel (60%, n = 10). In a similar fashion, oligodendrogliomas displayed extreme drug resistance to vincristine (60%, n = 5) and paclitaxel (50% n = 6). Most meningiomas displayed extreme drug resistance to vincristine (75%, n = 8), dacarbazine (63%, n = 8), and 4-hydroperoxy-cyclophosphamide (50%, n = 8; Haroun et al., 2002). Continuous oral low-dose chemotherapy with methotrexate and cyclophosphamide has modest activity in heavily pretreated patients with breast cancer. However, attempts to make this work for refractory glioblastoma have failed (Herrlinger et al., 2005).
In a phase II study, paclitaxel and topotecan with granulocyte colony stimulating factor (G-CSF) bone marrow support exhibits modest activity in adults with recurrent or refractory glioblastoma and anaplastic astrocytoma. The significant hematotoxicity encountered, however, was thought by the authors to preclude further investigation of this combination in patients with high grade brain tumors (Pipas et al., 2005).
Determining in real time the efficacy of glioma therapy is made easier and quicker by diffusion MRI. Diffusion MRI results predict relapse and correlate with survival and with development of chemoresistance (Lee et al., 2006). In vitro determination of whether or not a patient's glioma demonstrates extreme resistance to a particular drug holds predictive value for long-term survival and time to recurrence after treatment in vivo with that drug (Parker et al., 2004).
Recurrent glioblastoma multiforme occurs so quickly that it is thought to be the result of growth of chemoresistant tumor stem cells. Prolonged exposure to anthracyclines like doxorubicin appears to kill recurrent glioblastoma cells. Aberrant doxorubicin efflux appears not to be a factor in the requirement for longer exposure (Eramo et al., 2006).
Multifocal glioblastoma recurrence is reported to occur more frequently after tamoxifen treatment. Multifocal tumor recurrence was found in 16 of 49 such patients. Compared to tumors that remained local, multifocal tumor recurrences were characterized by a significantly longer median time to tumor progression (41 vs. 23 weeks, Breslow test: p = 0.0123). Multifocal tumor recurrences were mainly observed after an initial response to the study treatment (81%), whereas local regrowth was more often associated with initial treatment failure, i.e. progressive disease (64%). This implies that multifocal recurrence is the result of tamoxifen resistance (Puchner et al., 2004).
2.1. Multidrug resistance
In 27 primary and 17 secondary glioblastomas and their astrocytic precursor tumors, Tews et al. studied the immunohistochemical expression profile of P-gp, MRP, lung resistance protein (LRP), metallothionein, and topoisomerase IIα. Glial tumor cells in all glioblastomas showed constant up-regulation of LRP, MRP, and topoisomerase II alpha. P-gp was found in 90% of the primary and 60% of the secondary glioblastomas. In precursor tumors, these drug resistance-related factors were expressed in varying proportions. Metallothionein, also found in normal and activated astrocytes, was retained in all neoplastic phenotypes. Furthermore, metallothionein, P-gp, LRP, and topoisomerase II alpha were strongly expressed by normal and neoplastic vessels which may confer impaired penetration of therapeutic agents through the blood-brain and blood-tumor barrier. However, the expression profiles of drug resistance-related proteins neither differed between primary and secondary glioblastomas nor revealed any correlation to precursor or recurrent tumors (Tews et al., 2000).
MDR in gliomas is constitutively expressed and not drug-inducible. P-gp is more abundant in vascular endothelial cells in gliomas than in glioma cells themselves; it is also not drug-inducible (Rieger et al., 2000). Expression of proteins associated with chemoresistance (P-gp, MGMT, MRP1, LRP) is very heterogeneous in CNS tumors. P-gp and MRP1 are most frequently seen in capillary endothelium, while LRP appears only in tumor cells. MGMT is prevalent but not correlated with histopathological grade of astrocytomas and oligodendrogliomas (Andersson et al., 2004). Brain capillary endothelial cells demonstrate increased expression of P-gp and increased efflux of calcium after irradiation. They also demonstrate verapamil-resistant drug efflux. Glioma cells themselves do not demonstrate P-gp overexpression (Andersson et al., 2002).
ABCC4 and 5 (formerly MRP4 and 5) are the highest expressed MRPs in gliomas and expression in astrocytomas is far higher than in oligodendrogliomas. Organic anion uptake transporters are expressed (mRNA-detectible), but only OATP1A2 and 2B1 are detectible by protein immunofluorescence (Bronger et al., 2005). MRP3 is expressed in some gliomas. Antisense to MRP3 increases sensitivity of these cells to some, but not all, chemotherapeutic agents. MRP1 and 3 are co-expressed in some gliomas (Haga et al., 2001).
The expression of MRP was detected in 9 of 11 glioblastomas and 3 of 6 anaplastic astrocytomas and correlated with chemoresistance. Sensitivity to adriamycin, VP-16 and cisplatin was significantly increased in MRP antisense-treated cells as compared with the sense-treated cells (Mohri et al., 2000).
The INK4a gene products, p16 and p14ARF, have been suggested as potential regulators of glioma chemo- and radiosensitivity. Ectopic p16 sensitized U87 glioma cells towards treatment with vincristine and possibly also BCNU by approximately 1.5 to 2-fold, and towards ionizing radiation by a factor of 1.5. p14ARF expression was found to render U87 cells 2-fold more radioresistant than controls (Simon et al., 2006).
Methionine depletion (via methionine-free diet) depletes both ATP and GSH and thereby renders gliomas that are MDR- and/or GSH-dependent for chemoresistance sensitive to chemotherapy (Poirson-Bichat et al., 2000). A Chinese herb in the mint family, Scutellaria baicalensis, exhibits activity in vitro against multidrug resistant glioblastoma cells. Extracts of this plant are rich in flavonoids (Scheck et al., 2006).
2.2. Signal transduction
Several low molecular weight signal transduction inhibitors have been examined in preclinical and clinical malignant glioma trials. The efficacy of these agents as monotherapies has been modest, at best; however, small subsets of patients who harbor specific genetic changes in their tumors may display favorable clinical responses to defined small molecule inhibitors. Multitargeted kinase inhibitors or combinations of agents targeting different mitogenic pathways may overcome the resistance of tumors to single-agent targeted therapies. Well designed studies of small molecule kinase inhibitors must include assessment of safety, drug delivery, target inhibition, and correlative biomarkers to define mechanisms of response or resistance to these agents. Predictive biomarkers should enrich for patients most likely to respond in future clinical trials (Sathornsumetee and Rich, 2006).
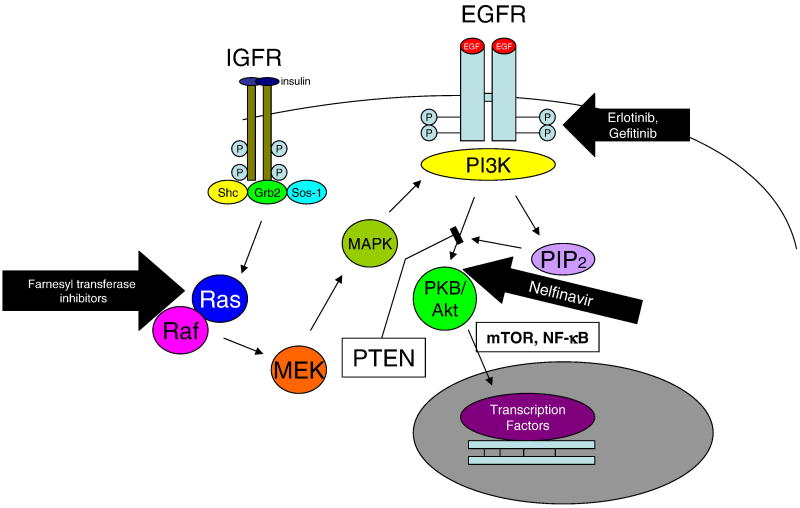
EGFR Akt pathway signaling (Fig. 2) appears to be important for oncogenesis in glioblastoma multiforme. EGFR gene amplification is present in 50% of glioblastomas and mutation is present in 40% of amplified EGFR genes. Mutation results in constitutive activation and up-regulation of the antiapoptotic protein, Bcl-XL. Inhibitors of EGFR signaling enhance radiosensitivity of glioblastomas; however, up-regulation of IGFR confers resistance to inhibitiors of EGFR signaling because of IGFR-mediated RAS/RAF-MAPK-PI3K signaling. One can antagonize RAS with farnesyl transferase inhibitors to overcome this resistance. Inhibition of PDGF-BB inhibits cell growth and proliferation uniquely in chemoresistant glioma cell lines and not in non-resistant gliomas. Additive inhibition again uniquely in chemoresistant gliomas is obtained with inhibitors of the PI3K/Akt pathway (Servidei et al., 2006).
Fig. 2.
EGFR signaling through the PI3K/Akt pathway. Erlotinib and gelfitinib interfere with autophosphorylation of EGFR. In tumors that exhibit upregulation of IGFR, signaling of that receptor through Ras/Raf can bypass the block and generate sufficient activated PI3K to signal through Akt; these tumors are therefore resistant to erlotinib and gelfitinib. Adjunctive treatment with farnesyl transferase inhibitors or nelfinavir can overcome this resistance.
EGFR expression is an independent predictor of prognosis in astrocytoma and glioblastoma multiforme. Erlotinib is an EGFR tyrosine kinase inhibitor. Responsiveness of glioblastoma to erlotinib correlates with EGFR expression and inversely with levels of phosphorylated PKB/Akt (Haas-Kogan et al., 2005). Gefitinib, another EGFR tyrosine kinase inhibitor, is effective in lung cancer but not glioblastoma. This may relate to the fact that the lung cancer EGFR tyrosine kinase domain has characteristic mutations. These do not occur in the glioblastoma, anaplastic oligodendroglioma, or low grade glioma EGFR (Chakravarti et al., 2002; Chakravarti et al., 2004; Marie et al., 2005).
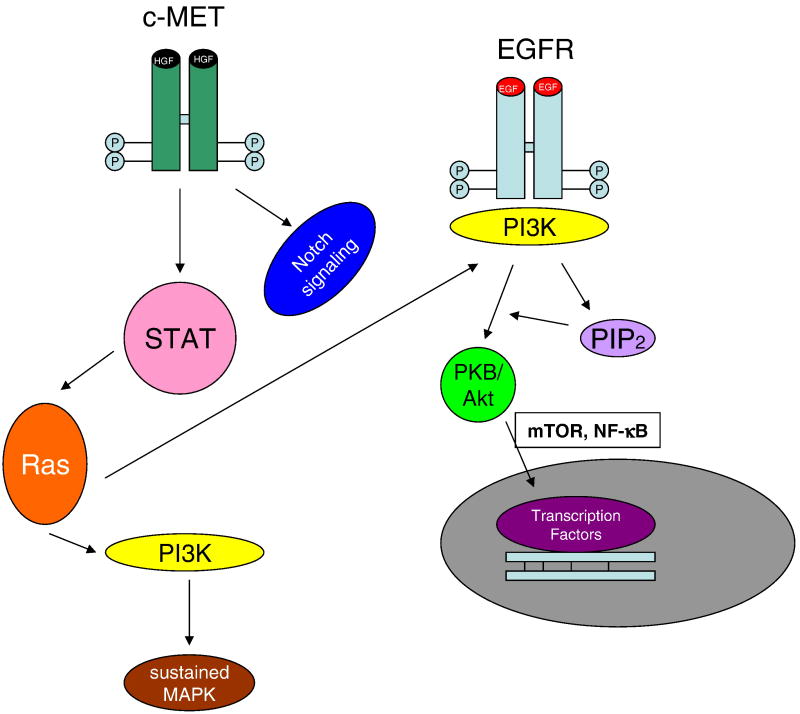
In glioblastoma multiforme, the EGFR is frequently truncated, forming EGFRvIII. A study examining the differential phosphorylation profiles of EGFR and EGFRvIII indicates that when EGFRvIII is expressed, the PI3K pathway predominates over the MAPK and STAT3 pathways. In particular, the phosphorylation site of the c-Met receptor is very responsive to EGFRvIII expression, indicating that c-Met activation and EGFR activation are in different pathways (see Fig. 3), and cross-activation of c-Met occurs when EGFRvIII is expressed. Adjunctive use of EGFR inhibitors and c-Met inhibitors shows promise in preclinical testing of glioblastoma lines as a potential therapeutic strategy (Huang et al., 2007).
Fig. 3.
c-MET signaling pathways are both independent of and overlapping with EGFR signaling.
Expression of p16, a cyclin-dependent kinase inhibitor, is frequently decreased or absent in glioblastomas. Alteration of expression of p16 (including homozygous mutation, deletion, and decreased protein levels) is frequently associated with increased sensitivity to antimetabolite chemotherapeutic agents, but no alteration of sensitivity to alkylating agents, antibiotics, topoisomerase inhibitors and antimicrotubule agents. That is, the tumors remain relatively refractory to the latter groups of agents (Iwadate et al., 2000).
Many glioblastomas are PTEN-deficient and PTEN deficiency, in turn, enhances phosphorylation of Akt and signaling through the antiapoptotic PI3K pathway. Nelfinavir is a protease inhibitor that inhibits signaling through Akt (Fig. 2). Nelfinavir renders glioblastoma cells more sensitive to radiation therapy. Induction of PTEN expression does so as well, but combining the two is no more effective than either alone, implying that they work through the same pathway. Nelfinavir enhances temozolomide effectiveness, as well (Jiang et al., 2007).
mTOR and pAkt play a large role in the chemoresistance signaling in glioblastomas. Migrating glioblastoma multiforme cells are less prone to autophagy and inhibition of the mTOR pathway (PTEN/Akt/PI3K/mTOR/NF-κB) induces autophagy. Temozolomide induces autophagy as well (Lefranc and Kiss, 2006). Resistance of glioma cells to treatment and local metastasis of glioma cells relates to alteration of tumor cell adhesion, modification of extracellular matrix, secretion of proteases by tumor cells, modification of actin cytoskeleton, and signaling via PI3K, mTOR, NF-κB, and autophagy (Lefranc et al., 2005).
Hypoxia contributes to the malignancy and chemo- and radioresistance of gliomas. Efforts have been aimed at altering hypoxia-induced resistance factors, but none has yet been therapeutically efficacious (Knisely and Rockwell, 2002). Adjunctive inhibition of VEGF and tyrosine kinases is a promising approach in in vitro (U87) and animal xenograft studies (Avramis et al., 2002).
TNFα-Related Apoptosis-Inducing Ligand (TRAIL) has been suggested as a therapeutic agent for cancer. However, gliomas are often resistant to TRAIL. TRAIL-resistant gliomas harbor chromosomal deletions in the regions containing the genes for caspase-8, FADD, Bid, Smac/DIABLO, DR4, and DR5 (Li et al., 2006).
HGF (scatter factor) and c-Met (its receptor) are increasingly expressed during transformation from glia to glioblastoma. HGF induces metastatic potential, angiogenesis, and chemoresistance. Downstream effectors identified by microarray include amplified in breast cancer 1, DEAD/H box helicase 21, and polycystic kidney disease 1. Antisense to these factors inhibited somewhat chemoresistance mediated by HGF by a mechanism that appeared not to involve Akt or NF-κB (Ma et al., 2006).
2.3. DNA modification, damage, and repair
BCNU is a potent chemotherapeutic agent for brain tumors. However, acquired resistance to this drug has become a serious problem for the treatment of patients. The adenoviral transfer of antisense RNA and ribozyme down-regulated the transcription and expression of MGMT in vitro. It also conferred sensitivity to BCNU in vitro and in vivo. However, the effect was minimal. These data suggest that incomplete depletion of MGMT is not sufficient to overcome the resistance and that additional optimization will be required for the complete reversion of drug resistance (Manome et al., 2002). C6 rat glioma cells rendered resistant to antitumor nitrosoureas like BCNU have a protein expression profile that suggests a more aggressive, less differentiated behavior (Nakagawa et al., 2006). BCNU plus DNA-alkyltransferase inhibitor is not an effective regimen for nitrosourea-resistant glioma (Quinn et al., 2002). Acidosis enhances toxicity of some chemotherapeutic agents and prevents toxicity of others against glioblastomas. Nitrosoureas are more effective in acidotic conditions (Reichert et al., 2002).
Methylation of the promoter for MGMT is a predictor of responsiveness of gliomas to alkylating agents (Esteller et al., 2000). Brain tumor lines resistant to the combination of BCNU and O-Benzylguanine (a combination meant to tie up the repair enzyme that would allow cells to recover from BCNU) have a mutant repair enzyme that is not able to bind O-Benzylguanine. Heterozygotes express only the mutant enzyme, perhaps because epigenetic methylation silences the normal allele (Bobola et al., 2004). This can be verified in individual cases by methylation-specific PCR on resected tumor tissue which allows identification of patients more likely to respond favourably to the treatment (Paus et al., 2007). Glioma cells that are resistant to BCNU typically have overrepresentation of chromosome 22q12.3-13.32, implying that this chromosome region contains genes important for BCNU resistance (Hank et al., 2006). Resistance of gliomas to alkylators and recurrence of disease after therapy with alkylators are also proportional to the activity in these tumors of apurinic/apyrimidinic endonuclease, a DNA repair enzyme that cleaves DNA at the abasic sites formed by alkylating chemotherapy and radiation (Bobola et al., 2004).
DNA damage triggers up-regulation of p53 and consequent apoptosis/cell cycle arrest (Bacolod et al., 2004). Glioma resistance to temozolomide is not highly related to MGMT but correlates most with p53 status. Wildtype p53 tumors with robust p21-mediated cell cycle arrest are most sensitive. In tumors with non-functional p53, in general, even O-benzylguanine cannot augment the activity of temozolomide (Bocangel et al., 2002).
Loss of heterozygosity at 1p and 19q is correlated with oligodendroglial phenotype and responsiveness to chemo- and radiotherapy in glioblastomas. Loss of heterozygosity at 1p and 19q predicts better response to temozolomide for patients with malignant gliomas, especially in patients with methylation of the MGMT promoter (Ishii et al., 2007).
Oral temozolomide has demonstrable activity in glioma. A recent trial conducted under the auspices of the European Organization for the Research and Treatment of Cancer and National Cancer Institute of Canada Clinical Trials Group has defined a role for temozolomide in the initial management of glioblastoma. A companion correlative tumor-biology study has identified epigenetic silencing of the promoter of the gene that encodes MGMT in tumor specimens as a strong and independent prognostic factor for survival among patients with a newly diagnosed glioblastoma, as well as a predictor of survival benefit from chemoradiotherapy with temozolomide (Mason and Cairncross, 2005).
Patients with temozolomide-refractory anaplastic astrocytoma were administered cyclophosphamide (750 mg/m2/day on 2 consecutive days every 4 weeks for a total of 2-12 cycles). They demonstrated a 22% partial response rate and a 40% stable disease rate, indicating that cyclophosphamide has modest effectiveness with tolerable toxicity in this clinical situation (Chamberlain et al., 2006). In a phase II trial of temozolomide plus BCNU, a combination that includes inhibition of the repair of BCNU-induced DNA damage, patients with anaplastic glioma had only a modest response and very significant toxicity, largely myelosuppression (Chang et al., 2004). Some of the resistance of gliomas to temozolomide/BCNU results from activation of the Fanconi anemia-type DNA repair mechanism (Chen et al., 2007).
Oligodendrogliomas are generally more chemoresponsive and carry a better prognosis than glioblastomas and anaplastic gliomas. From a genetic standpoint, they often have deletions of one chromosome 1p and/or 19q. Poor prognosis is conversely related to homozygous deletions of the CDKN2A gene, mutation of the PTEN gene, and amplification of the EGFR gene (Reifenberger et al., 2003). Adjunctive therapy strategies for glioblastoma have been proposed that use temozolomide along with viruses that either downregulate DNA repair enzymes (adenovirus) or exploit up-regulation of DNA repair enzymes in orchestrating their own replication and the cell's consequent lysis (herpesvirus; Jiang et al., 2006). Loss of chromosome 1p/19q predicts better and longer response to temozolomide, longer progression-free survival, and longer overall survival for patients with low-grade gliomas (149 consecutive patients; Kaloshi et al., 2007).
Temozolomide affords only modest improvement in survival of patients with more benign gliomas (Stupp et al., 2001). Low grade gliomas grow more slowly and regrow more slowly after temozolomide treatment both before and after cessation of treatment if they have deletions of 1p and/or 19q and if they do not overexpress p53. However, with or without the deletions and/or p53, all low grade gliomas regrow after stopping temozolomide, raising the question of optimal duration of treatment (Ricard et al., 2007). Unlike the case for temozolomide, there is no relationship between 1p36 region aberrations and response of gliomas to procarbazine, CCNU, or vincristine. Seventeen % responded; 50% were free of progression at 6 months and 21% were free of progression at 1 year (Triebels et al., 2004).
A critical metareview of temozolomide studies suggests that this drug affords limited efficacy (prolongation of survival by a few weeks over procarbazine) in infiltrative, malignant brain tumors, but is more effective in low-grade gliomas (Nagasubramanian and Dolan, 2003). Glioblastoma that is resistant to conventional dose schedules of temozolomide may respond, at least temporarily, to “metronomic” temozolomide – low doses at frequent intervals. Only tolerable toxicity is noted with this regimen. Several stable disease patients were noted in this study (Kong et al., 2006).
A study of a single patient's primary glioma and two subsequent recurrences revealed that, sometimes, the recurrence is less chromosomally aberrant and more chemosensitive than the primary tumor. In fact, in this particular case, despite methylation of the promoter of MGMT, the second recurrence was more sensitive to temozolomide than the primary or the first recurrence (Spiegl-Kreinecker et al., 2007). Temozolomide was effective in 7/28 patients with oligodendroglial tumors that recurred after first-line therapy with procarbazine, CCNU, and vincristine (van den Bent, 2003).
Carboplatin was examined as a second-line therapy for oligodendroglial tumors that had failed first-line chemo- and radiation therapy. Doses that resulted in a median survival of 16 months, with some partial responses and some stable disease, produced severe myelotoxicity. Carboplatin demonstrates only modest efficacy in this situation (Soffietti et al., 2004). BBR3464 is a cisplatin analogue with multiple platinum-based nuclei and a putatively different DNA binding mechanism from cisplatin. It is effective in model systems vs. astrocytoma cells that are cisplatin-resistant (Servidei et al., 2001).
Addition of an inhibitor of mismatch repair or PARP enhances temozolomide efficacy against gliomas. Tumors that already have defective mismatch repair enzymes are most altered by PARP inhibitors (Tentori et al., 2002). PARP-1 and -2 have been shown to play a role in DNA repair after sublethal irradiation of cancer cells. Selective PARP inhibitors diminish resistance to chemotherapy. The PARP-1-selective inihibitor, CEP-8983 enhances responsiveness of glioblastomas to temozolomide and irinotecan in vitro and a prodrug of CEP-8983 (CEP-9722) administered systemically enhances responsiveness of xenografted tumors in vivo to temozolomide or irinotecan. In the in vivo model, myelotoxicity of temozolomide or irinotecan was not enhanced by the CEP-9722 (Miknyoczki et al., 2007).
Development of resistance to topoisomerase I inhibitors sometimes results in collateral resistance to topoisomerase II inhibitors and sometimes results in enhanced sensitivity to topoisomerase II inhibitors. Development of resistance to topoisomerase I inhibitors is accompanied by down-regulation of topoisomerase I and up-regulation of topoisomerase II, but also by up-regulation of P-gp. Clinical protocols combining a topoisomerase I and a topoisomerase II inhibitor should be considered with caution because antagonistic effects have been observed with combinations of camptothecin and doxorubicin (Pavillard et al., 2001).
Activity of apurinic endonuclease-1 (Ape-1/Ref-1) correlates with resistance of gliomas to alkylating agents. Antisense constructs to Ape1 enhance sensitivity to alkylating agents and increase the content of apurinic sites of DNA. In addition, exposure of glioma cells to reactive oxygen species (ROS) enhances Ape-1 activity, diminishes apurinic sites, and enhances resistance to alkylating agents of gliomas. This is of particular interest given the high ROS environment of most gliomas, especially after radiation therapy (Silber et al., 2002).
All multidrug resistant glioblastomas tested have extremely high expression levels of the mismatch-repair gene hMSH2; most sensitive glioblastomas have low or no expression of hMSH2 (Rellecke et al., 2004). However, others looking at different tumors other than glioblastoma do not observe this correlation (Rovin, 2005).
2.4. Immuno- and targeted therapy
Gliomas secrete immunosuppressive cytokines and therefore impair cytotoxic T-lymphocyte-based therapies. Gliomas also induce production of Fas ligand by microglia; this in turn induces apoptosis in T cells (Badie et al., 2001). Microarray comparison between native and chemoresistant U87 cells reveals significant up-regulation of IL-1β, IL-8, and VEGF in chemoresistant cells. Transfection of native cells with IL-1β gene results in resistance to apoptosis induction (Morandi et al., 2006).
Targeting tumor-associated antigens with dendritic cells may be an effective way to combat glioblastoma (Yu et al., 2006). Tyrosinase-related peptide (TRP) is expressed on the surface of glioma cells and is targeted by endogenous cytotoxic T-cells. After immunotherapy, TRP levels of cells left go down and the tumors become more sensitive to temozolomide and carboplatin. Transfection of glioma cells with TRP makes them more resistant to temozolomide. TRP appears to regulate chemosensitivity without altering known conventional mediators of chemoresistance (Liu et al., 2005).
The endothelium of blood vessels in glioblastomas is different from that in normal brain. The blood vessels are morphologically different (veil-like rather than plump and round), and they constitutively elaborate growth factors like endothelin-1, interleukin-8, VEGF. They are resistant to antimitotic agents and antiapoptotic agents, even though they replicate more slowly than normal endothelial cells. They migrate better and more than normal endothelial cells in the brain. These characteristics could be targeted to effect devascularization of glioblastomas (Charalambous et al., 2006).
Fusion proteins constructed of ligands for glioblastoma-unique receptors linked to toxins, also show promise. Novel delivery systems are needed for these relatively large molecules. Convection-enhanced delivery, a bulk flow method, has been proposed in this regard (Liu et al., 2003).
2.5. Adhesion, invasion, and angiogenesis
In 9L rat gliosarcoma, chemoresistance selection results in more invasive cells as well (Saito et al., 2004). In one glioma line, acquisition of chemotherapeutic resistance was associated with decreased N-CAM and increased integrin expression, resulting in increased adhesion and decreased invasiveness (Hikawa et al., 2000). Transfection with N-CAM has also been shown in to decrease invasiveness of glioma cells. Furthermore, down-regulation of N-CAM in glioma cells makes them more endothelial-adhesive and more invasive (Blaheta et al., 2006).
Multidrug resistance and angiogenic potential were co-segregated in cells in tumorospheres of human glioblastomas. Tumor stem cells were isolated and were similarly drug-resistant but less angiogenic (Salmaggi et al., 2006). Anti-angiogenic, cyclic pentapeptide EMD 121974 inhibits growth of brain implants of U87 glioblastoma cells but not the analogous subcutaneous implants (MacDonald et al., 2001). Expression of strathmin, a microtubule-associated protein, correlates with 1p integrity and poor outcome or chemoresistance of malignant gliomas. Deleted 1p and deficient strathmin all auger well for susceptibility to procarbazine of malignant gliomas, especially oligodendrogliomas (Ngo et al., 2007).
2.6. Apoptosis modulation
Given that known endogenous modulators of apoptosis induction and enactment are expressed in gliomas, it is not surprising that some known and putative chemotherapeutic agents alter the activity or expression of these proteins. It is similarly not surprising that the expression of these proteins can determine the responsiveness of a given glioma to a particular chemotherapeutic agent.
Bcl-XL plays a role in chemoresistance of gliomas. Antisense-mediated reduction in Bcl-XL levels enhances susceptibility to apoptosis-induction by paclitaxel in gliomas (Guensberg et al., 2002). BCNU-sensitive glioma cells translocate Bax to the nucleolus upon BCNU treatment, while BCNU-resistant glioma cells do not. The authors suggest that this implies that Bax translocation is causally related to induction of apoptosis (Joy et al., 2000); but it is also possible that it is a consequence, rather than a cause, of apoptosis induction.
Curcumin decreases resistance to chemotherapy and radiation therapy of malignant gliomas. It is hypothesized that this relates to inhibition of NF-κB-mediated transcription. It correlates with decreased Bcl-2, IAP family peptides, and DNA repair enzyme expression (Dhandapani et al., 2007). A ruthenium complex analogue of cisplatin is cytotoxic to C6 glioma cells and not to normal glia. Interestingly, it seems to have better anti-tumor activity in vivo than in vitro when tested against other tumors. It is thought to be anti-metastatic; however, it is directly cytotoxic to C6 cells in vitro (Djinovic et al., 2004).
Expression of herpes simplex virus type 1-thymidine kinase, followed by gancyclovir treatment, induced apoptosis in short-term human glioma cell cultures derived from surgical biopsies, including three that are resistant to the chemotherapeutic drug CCNU. Expression of murine Fas ligand also induced cell death in four of the five cell cultures studied. One cell culture that was resistant to CCNU was also resistant to apoptosis induced by murine Fas ligand expression (Maleniak et al., 2001).
Treatment of glioblastomas with transglutaminase 2 inhibitors decreases cell adhesion and promotes cell death in response to chemotherapy. Levels of survivin, phosphorylated Akt, phosphorylated Bad, and phosphorylated GSK decrease, and levels of Bim increase (Yuan et al., 2005). Cyclosporin A treatment of glioma cells in vitro results in accumulation of waf1/cip1, even in the absence of p53, and subsequent cell death (Zupanska et al., 2005).
3. Pituitary tumors
Most pituitary tumors of adulthood are benign. As a consequence of this, many are asymptomatic enough that they never come to medical attention. Autopsy studies suggest that 15-20% of people develop adenomatous tissue in the pituitary. However, only 1,300 people annually in the U.S. are diagnosed as having a pituitary tumor. Approximately 7% of all tumors of the brain are pituitary tumors and symptoms of these tumors generally arise from their endocrinological effects (American Cancer Society, 2006).
Somatostatin analogues are used in acromegaly, not only because they decrease growth hormone (GH) and insulin-like growth factor (IGF) secretion, but also because they shrink the GH-producing mass. These two effects are dissociable pharmacologically (Gola et al., 2006). There is heterogeneous expression of somatostatin receptors SSTR2 and SSTR5 in pituitary tumors in acromegalic patients. Bispecific agonists are therefore more efficacious than any one specificity agonist (Saveanu et al., 2001). In patients with acromegaly refractory to somatostain analogues, pegvisomant, a GH receptor antagonist, normalizes IGF-I levels and reduces risk factors for cardiovascular disease. Its effect on size of tumor varies, but in many, the tumor size is stabilized or decreased (Colao et al., 2006).
Pituitary adenomas with mutations in the STTR can demonstrate, not only altered binding of somatostatin, but also altered G-protein binding specificity and consequent conversion of MAPK stimulatory signaling into MAPK inhibitory signaling. A patient has been described with two mutations, one of the STTR that would theoretically be responsive to higher somatostatin concentrations and the other that results in constitutive activation of adenyl cyclase that renders these higher somatostatin concentrations ineffective (Ballare et al., 2001).
About a third of acromegalic patients are resistant to the currently commercially available somatostatin analogs octreotide and lanreotide. Such resistance is related to an overall reduction of SSTR density or to a differentiated expression of SSTR subtypes. There are five known SSTR subtypes. SSTR2 and SSTR5 are usually expressed in GH-secreting pituitary tumors, and both octreotide and lanreotide bind preferentially to SSTR2 and, to a lesser extent, to SSTR5. Somatostatin analogue inhibitory effects on GH secretion and tumor cell proliferation can occur together or be dissociated events, depending on the tumor expression of SSTR subtypes involved in each mechanism. The development of specific somatostatin receptor ligand subtype analogs, mainly for SSTR5, of SSTR2-SSTR5 bispecific compounds, and of “universal” analogs with high affinity for SSTR1, 2, 3, and 5 showed promising, albeit preliminary, results for the treatment of resistant somatotropic adenomas (Bronstein, 2006).
The role of SSTR1 in mediating the inhibitory effect of somatostatin on GH-secreting pituitary tumors has been recently demonstrated. SSTR1 was present in 56.25% (9/16) of the GH-secreting adenomas examined; in all GH-secreting pituitary tumors that expressed SSTR1, BIM-23745, a selective SSTR1 agonist, significantly inhibited GH secretion in vitro, and when the SSTR1 receptor subtype was present in tumors from patients resistant to octreotide-LAR or lanreotide therapy, BIM-23745 was able to inhibit the in vitro GH secretion (Matrone et al., 2004).
Prolactinomas are 40% of all pituitary adenomas, with an incidence of 100/million adults (Gillam et al., 2006). Dopaminergic drugs, including the commonly used drug, cabergoline, are effective in prolactinomas of the pituitary (Colao et al., 2002). Surgery was preferred mode of treatment until the 1980s when bromocryptine became available (Gillam et al, 2006). Cabergoline does not merely decrease secretion of prolactin; it also decreases tumor mass (Colao et al., 2002). However, in some tumors, decreased tumor size does not necessarily imply decreased prolactin production (di Sarno et al., 2001). In a study of 110 patients, primary treatment with cabergoline was better than bromocriptine followed by cabergoline regardless of whether or not the patient responded to the initial bromocriptine treatment (Colao et al., 2000). Recent data challenge the notion that lifelong therapy is needed. Challenges include dopamine-refractory tumors, tumors and pregnancy, malignant prolactinomas. Asymptomatic patients do not necessarily need treatment (Gillam et al., 2006).
Chimeric somatostatin-dopamine molecules suppressed GH and prolactin production more than octreotide or single receptor-interacting molecules. These chimeric molecules bound to both the somatostatin receptors and dopamine receptors of various types (Jaquet et al., 2005).
Resistance to dopamine agonists occurs in a subset of patients with prolactin-secreting pituitary tumors. The resistance is mediated by loss of pituitary D2 receptors and occurs in both microadenomas and macroadenomas. Cabergoline is the most effective drug; trans-sphenoidal pituitary surgery should be reserved for patients who are intolerant of medical therapy, or in whom this has failed. Radiation therapy has a limited role in treatment of resistant prolactinomas and should be reserved for patients in whom medical and surgical therapy has failed (Olafsdottir and Schlechte, 2006).
Quinagolide is a dopaminergic agonist should be prescribed as first-line treatment for patients presenting with bromocriptine-resistant prolactinoma. An anti-tumoral effect was noted in 30.8% of patients and occurred within an interval of less than 2 years in 80% of cases at a dose of 300 microg/d. Normalization of the plasma prolactin concentration was obtained in 44% of cases and occurred in less than one year in 80% of patients at a dose of 300 μg/d (Rohmer et al., 2000).
In pituitary adenomas, failure to normalize prolactin levels is seen in 24% of those treated with bromocriptine, 13% of those treated with pergolide, and 11% of those treated with cabergoline. Failure to achieve at least a 50% reduction in tumor size occurs in about one-third of those treated with bromocriptine and 10-15% of those treated with pergolide or cabergoline. Studies of in vitro cell preparations show that the D2 receptors of resistant tumors are decreased in number but have normal affinity. Treatment approaches for resistant patients include switching to another dopamine agonist and raising the dose of the drug as long as there are continued response to the dose increases and no adverse effects. Transsphenoidal surgery can also be done. Reduction of endogenous estrogen, use of selective estrogen receptor modulators, and aromatase inhibitors are potential experimental approaches (Molitch, 2003; Molitch, 2005; Hamilton et al., 2005; Jan et al., 2007).
Pituitary tumor-transforming gene is a novel potential target for drug therapy of pituitary and other endocrine tumors. Its mechanism of action is complex and only partially understood, but includes enhanced expression of p53 and Bax, mediation of chromatid separation during mitosis, and interactions with VEGF and bFGF. In pituitary tumors, although pituitary tumor-transforming gene expression alone does not predict tumor behavior, nuclear pituitary tumor-transforming gene product levels are one of several features that predict aggressive tumor behavior. Expression of pituitary tumor-transforming gene appears to correlate best with poor outcome in pituitary tumors that produce active hormonal substances, rather than in non-functional tumors, and with recurrence of tumor (Salehi et al., 2008).
4. CNS lymphoma
Chemotherapeutic regimens that include high-dose methotrexate are the mainstay of treatment for primary CNS lymphoma. A recent Japanese study followed 112 patients with primary CNS lymphoma each treated with one of four different regimens over a period of 24 years. The treatment regimens studied were: whole-brain irradiation alone; methotrexate, vincristine, and predonisolone; cyclophosphamide, pirarubicin, etoposide, vincristine, procarbazine, prednisone, and methotrexate; and rituximab, methotrexate, pirarubicin, procarbazine, and prednisone combined-modality therapy. The median progression-free survival duration was 16 months, and the median overall survival duration was 24 months. The 2- and 5-year actuarial probability of survival was 52.4 +/- 4.8% [95% confidence intervals (CI)] and 30.2 +/- 4.8% (95% CI), respectively. Better overall survival duration correlated with treatment with the cyclophosphamide, pirarubicin, etoposide, vincristine, procarbazine, prednisone, and methotrexate protocol, better Karnofsky performance status, higher methotrexate dose and concomitant use of whole brain irradiation. The best overall survival duration and tolerability of side effects were obtained with regimens including 20-30 Gy whole brain irradiation and 500 mg/m2 of methotrexate (Yamanaka et al., 2008).
For recurrent primary CNS lymphoma, treatment with temozolomide plus rituximab gives 14 months median survival and 7.7 months progression-free survival. The overall response rate is 53%. Without treatment, the median survival is 2 months (Enting et al., 2004). In CNS lymphoma, good prognosis is associated with high tumor mutation frequency, and poor prognosis is associated with absence of the VH4 gene family members (Pels et al., 2005).
5. Spinal cord tumors
Spinal cord tumors are 5-10% of CNS malignancies. Treatment for most of these relatively uncommon tumors is surgical and/or radiotherapeutic, and pharmacotherapy is infrequently indicated. As a consequence, little is known of potential biochemical targets and signaling pathways in these neoplasms (Henson, 2001).
Tumors of the spinal cord are most frequently cervical or thoracic. Astrocytomas are only infrequently resectable, while ependymomas are often resectable. Long-term survival is therefore more common with ependymomas, and mild neurological deficits are frequent (Nishio et al., 2000).
Spinal ependymomas most commonly occur as intramedullary tumors throughout the spinal axis. In the lumbosacral region, ependymomas are most commonly associated with the conus medullaris and cauda equina, but can also occur extradurally in the sacrum, presacral tissues, or subcutaneous tissues over the sacrum. These two tumor locations produce different management concerns. Intradural ependymomas, especially those in the lumbosacral region, are now recognized for their potential to spread throughout the CNS, whereas extradural tumors elicit more concern for their association with extraneural metastases. Despite the risk for local recurrence and CNS dissemination, the prognosis for intradural lumbosacral ependymomas is good, with a greater than 90% 10-year patient survival in most series. The prognosis for extradural ependymomas does not appear to be as good. Much depends on extradural tumor location, however; the outlook is better for dorsal sacral tumors than presacral tumors (Fassett and Schmidt, 2003).
A significant fraction of adults with spinal ependymoma whose tumors relapse in the primary site can be salvaged with additional radiation therapy (Kocak et al., 2004). Recurrence of spinal ependymoma after surgery and radiation is generally refractory to chemotherapy. Oral etoposide is well tolerated and, although not curative, effects stabilization of disease in some patients (Chamberlain, 2002).
Meningioma, schwannoma, and ependymoma accounted for 70% of intradural spinal tumours in one series. Complete macroscopic excision was achieved in 84% of extramedullary and 54% of intramedullary tumours. Cerebrospinal fluid leak (10%) and meningitis (7%) were the most common complications. Ninety-six percent of patients with extramedullary tumours improved neurologically or remained unchanged. In the intramedullary group, 82% remained unchanged or improved after treatment. Pre-operative functional status was a predictor of good post-operative function for intra- and extramedullary tumours and for intramedullary tumours a good post-operative neurological function score predicted long-term survival (Jenkinson et al., 2006).
6. Conclusions
For decades, tumors of the nervous system have represented some of the most pharmacologically intractable clinical problems known. Malignant brain and spinal cord tumors are problematic because of both resistance to pharmacological attack and the high cost of damage to adjacent normal tissue. Novel approaches to tumors of the nervous system are based on the expanding understanding of their biology and the relationship of these features to the biology of the host and cells of origin. Pharmacological success in the conquest of such tumors will come only from the application of such knowledge in the development of targeted, individualized therapies. The evaluation of new therapies developed in this context depends critically upon stratification of intervention and analysis by molecular subtypes within and between tumor and host tissue types (Rogers et al., 2008; Mason and Cairncross, 2008).
Acknowledgments
The author is indebted to Jennifer Anstey for expert technical and secretarial assistance in preparation of this manuscript.
Abbreviations
- BBB
blood-brain barrier
- BCNU
1,3-bis-(2-chloroethyl)-nitrosourea
- BSO
buthionine sulfoximine
- CNS
central nervous system
- DIABLO
direct inhibitor-of-apoptosis protein-binding protein with low pI
- DNA
deoxyribonucleic acid
- DHFR
dihydrofolate reductase
- EGFR
epidermal growth factor receptor
- FADD
fas-associated protein with death domain
- G-CSF
granulocyte colony stimulating factor
- GH
growth hormone
- GSH
reduced glutathione
- GST
glutathione-S-transferase
- HIF
hypoxia-inducible factor
- IAP
inhibitor of apoptosis protein
- IGFR
insulin-like growth factor
- IL
interleukin
- LRP
lung resistance protein
- MAPK
MAP kinase
- MDR1
multidrug resistance factor 1
- MGMT
O6-methylguanine methyltransferase
- MRI
magnetic resonance imaging
- MRP
multidrug resistance protein
- mTOR
mammalian target of rapamycin
- N-CAM
neural cell adhesion molecule
- PARP
poly ADP ribose polymerase
- PCNA
proliferating cell nuclear antigen
- PDGF
platelet-derived growth factor
- P-gp
P-glycoprotein
- PI3K
phosphatidyl-inositol-3-kinase
- PKC
protein kinase C
- PTEN
phosphatase and tensin homolog
- ROS
reactive oxygen species
- RT-PCR
real-time polymerase chain reaction
- Smac
second mitochondria-derived activator of caspase
- SSTR
somatostatin receptor
- TRAIL
TNFα-related apoptosis-inducing ligand
- TRP
tyrosine-related peptide
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Cancer Society. What are the key statistics about pituitary tumors? 2006 http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_pituitary_tumors_61.asp?sitearea=
- American Cancer Society. What are the key statistics about brain and spinal cord tumors in adults? 2008 http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_brain_and_spinal_cord_tumors_3.asp?sitearea=
- Andersson U, Grankvist K, Bergenheim AT, Behnam-Motlagh P, Hedman H, Henriksson R. Rapid induction of long-lasting drug efflux activity in brain vascular endothelial cells but not malignant glioma following irradiation. Medical Oncology (Northwood, London, England) 2002;19:1–9. doi: 10.1385/MO:19:1:1. [DOI] [PubMed] [Google Scholar]
- Andersson U, Malmer B, Bergenheim AT, Brannstrom T, Henriksson R. Heterogeneity in the expression of markers for drug resistance in brain tumors. Clinical Neuropathology. 2004;23:21–27. [PubMed] [Google Scholar]
- Avramis IA, Christodoulopoulos G, Suzuki A, Laug WE, Gonzalez-Gomez I, McNamara G, et al. In vitro and in vivo evaluations of the tyrosine kinase inhibitor NSC 680410 against human leukemia and glioblastoma cell lines. Cancer Chemotherapy and Pharmacology. 2002;50:479–489. doi: 10.1007/s00280-002-0507-6. [DOI] [PubMed] [Google Scholar]
- Bacolod MD, Johnson SP, Pegg AE, Dolan ME, Moschel RC, Bullock NS, et al. Brain tumor cell lines resistant to O6-benzylguanine/1,3-bis(2-chloroethyl)-1-nitrosourea chemotherapy have O6-alkylguanine-DNA alkyltransferase mutations. Molecular Cancer Therapeutics. 2004;3:1127–1135. [PubMed] [Google Scholar]
- Badie B, Schartner J, Prabakaran S, Paul J, Vorpahl J. Expression of fas ligand by microglia: Possible role in glioma immune evasion. Journal of Neuroimmunology. 2001;120:19–24. doi: 10.1016/s0165-5728(01)00361-7. [DOI] [PubMed] [Google Scholar]
- Ballare E, Persani L, Lania AG, Filopanti M, Giammona E, Corbetta S, et al. Mutation of somatostatin receptor type 5 in an acromegalic patient resistant to somatostatin analog treatment. The Journal of Clinical Endocrinology and Metabolism. 2001;86:3809–3814. doi: 10.1210/jcem.86.8.7787. [DOI] [PubMed] [Google Scholar]
- Bart J, Groen HJ, Hendrikse NH, van der Graaf WT, Vaalburg W, de Vries EG. The blood-brain barrier and oncology: New insights into function and modulation. Cancer Treatment Reviews. 2000;26:449–462. doi: 10.1053/ctrv.2000.0194. [DOI] [PubMed] [Google Scholar]
- Blaheta RA, Daher FH, Michaelis M, Hasenberg C, Weich EM, Jonas D, et al. Chemoresistance induces enhanced adhesion and transendothelial penetration of neuroblastoma cells by down-regulating NCAM surface expression. BMC Cancer. 2006;6:294. doi: 10.1186/1471-2407-6-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobola MS, Emond MJ, Blank A, Meade EH, Kolstoe DD, Berger MS, et al. Apurinic endonuclease activity in adult gliomas and time to tumor progression after alkylating agent-based chemotherapy and after radiotherapy. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2004;10:7875–7883. doi: 10.1158/1078-0432.CCR-04-1161. [DOI] [PubMed] [Google Scholar]
- Bocangel DB, Finkelstein S, Schold SC, Bhakat KK, Mitra S, Kokkinakis DM. Multifaceted resistance of gliomas to temozolomide. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2002;8:2725–2734. [PubMed] [Google Scholar]
- Bredel M, Zentner J. Brain-tumour drug resistance: The bare essentials. The Lancet Oncology. 2002;3:397–406. doi: 10.1016/s1470-2045(02)00786-6. [DOI] [PubMed] [Google Scholar]
- Bredel M, Bredel C, Sikic BI. Genomics-based hypothesis generation: A novel approach to unravelling drug resistance in brain tumours? The Lancet Oncology. 2004;5:89–100. doi: 10.1016/S1470-2045(04)01382-8. [DOI] [PubMed] [Google Scholar]
- Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Research. 2005;65:11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- Bronstein MD. Acromegaly: Molecular expression of somatostatin receptor subtypes and treatment outcome. Frontiers of Hormone Research. 2006;35:129–134. doi: 10.1159/000094315. [DOI] [PubMed] [Google Scholar]
- Calatozzolo C, Gelati M, Ciusani E, Sciacca FL, Pollo B, Cajola L, et al. Expression of drug resistance proteins pgp, MRP1, MRP3, MRP5 and GST-pi in human glioma. Journal of Neuro-Oncology. 2005;74:113–121. doi: 10.1007/s11060-004-6152-7. [DOI] [PubMed] [Google Scholar]
- Carpentier AF. Neuro-oncology: The growing role of chemotherapy in glioma. Lancet Neurology. 2005;4:4–5. doi: 10.1016/S1474-4422(04)00944-5. [DOI] [PubMed] [Google Scholar]
- Central Brain Tumor Registry of the United States. Primary brain tumors in the United States statistical report. 2007 http://www.cbtrus.org/reports//2007-2008/2007report.pdf.
- Ceruti S, Mazzola A, Abbracchio MP. Proteasome inhibitors potentiate etoposide-induced cell death in human astrocytoma cells bearing a mutated p53 isoform. The Journal of Pharmacology and Experimental Therapeutics. 2006;319:1424–1434. doi: 10.1124/jpet.106.109397. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Dicker A, Mehta M. The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: A review of preclinical and correlative clinical data. International Journal of Radiation Oncology, Biology, Physics. 2004;58:927–931. doi: 10.1016/j.ijrobp.2003.09.092. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Chakladar A, Delaney MA, Latham DE, Loeffler JS. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Research. 2002;62:4307–4315. [PubMed] [Google Scholar]
- Chamberlain MC. Etoposide for recurrent spinal cord ependymoma. Neurology. 2002;58:1310–1311. doi: 10.1212/wnl.58.8.1310. [DOI] [PubMed] [Google Scholar]
- Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with cyclophosphamide for recurrent temozolomide-refractory anaplastic astrocytoma. Cancer. 2006;106:172–179. doi: 10.1002/cncr.21582. [DOI] [PubMed] [Google Scholar]
- Chang SM, Prados MD, Yung WK, Fine H, Junck L, Greenberg H, et al. Phase II study of neoadjuvant 1, 3-bis (2-chloroethyl)-1-nitrosourea and temozolomide for newly diagnosed anaplastic glioma: A North American brain tumor consortium trial. Cancer. 2004;100:1712–1716. doi: 10.1002/cncr.20157. [DOI] [PubMed] [Google Scholar]
- Charalambous C, Chen TC, Hofman FM. Characteristics of tumor-associated endothelial cells derived from glioblastoma multiforme. Neurosurgical Focus. 2006;20:E22. doi: 10.3171/foc.2006.20.4.e22. [DOI] [PubMed] [Google Scholar]
- Chen CC, Taniguchi T, D'Andrea A. The fanconi anemia (FA) pathway confers glioma resistance to DNA alkylating agents. Journal of Molecular Medicine (Berlin, Germany) 2007;85:497–509. doi: 10.1007/s00109-006-0153-2. [DOI] [PubMed] [Google Scholar]
- Colao A, di Sarno A, Landi ML, Scavuzzo F, Cappabianca P, Pivonello R, et al. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: A prospective study in 110 patients. The Journal of Clinical Endocrinology and Metabolism. 2000;85:2247–2252. doi: 10.1210/jcem.85.6.6657. [DOI] [PubMed] [Google Scholar]
- Colao A, di Sarno A, Pivonello R, di Somma C, Lombardi G. Dopamine receptor antagonists for treating prolactinoma. Expert Opin Investigat Drugs. 2002;11:787–800. doi: 10.1517/13543784.11.6.787. [DOI] [PubMed] [Google Scholar]
- Colao A, Pivonello R, Auriemma RS, De Martino MC, Bidlingmaier M, Briganti F, et al. Efficacy of 12-month treatment with the GH receptor antagonist pegvisomant in patients with acromegaly resistant to long-term, high-dose somatostatin analog treatment: Effect on IGF-I levels, tumor mass, hypertension and glucose tolerance. European Journal of Endocrinology / European Federation of Endocrine Societies. 2006;154:467–477. doi: 10.1530/eje.1.02112. [DOI] [PubMed] [Google Scholar]
- Desjardins A, Rich JN, Quinn JA, Vredenburgh J, Gururangan S, Sathornsumetee S, et al. Chemotherapy and novel therapeutic approaches in malignant glioma. Frontiers in Bioscience : A Journal and Virtual Library. 2005;10:2645–2668. doi: 10.2741/1727. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. Journal of Neurochemistry. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- di Sarno A, Landi ML, Cappabianca P, Di Salle F, Rossi FW, Pivonello R, et al. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: Prevalence, clinical definition, and therapeutic strategy. The Journal of Clinical Endocrinology and Metabolism. 2001;86:5256–5261. doi: 10.1210/jcem.86.11.8054. [DOI] [PubMed] [Google Scholar]
- Djinovic V, Momcilovic M, Grguric-Sipka S, Trajkovic V, Mostarica Stojkovic M, Miljkovic D, et al. Novel ruthenium complex K2[ru(dmgly)Cl4].2H2O is toxic to C6 astrocytoma cell line, but not to primary rat astrocytes. Journal of Inorganic Biochemistry. 2004;98:2168–2173. doi: 10.1016/j.jinorgbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Enting RH, Demopoulos A, DeAngelis LM, Abrey LE. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63:901–903. doi: 10.1212/01.wnl.0000137050.43114.42. [DOI] [PubMed] [Google Scholar]
- Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death and Differentiation. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. The New England Journal of Medicine. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- Fassett DR, Schmidt MH. Lumbosacral ependymomas: A review of the management of intradural and extradural tumors. Neurosurgical Focus. 2003;15:E13. doi: 10.3171/foc.2003.15.5.13. [DOI] [PubMed] [Google Scholar]
- Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocrine Reviews. 2006;27:485–534. doi: 10.1210/er.2005-9998. [DOI] [PubMed] [Google Scholar]
- Gola M, Bonadonna S, Mazziotti G, Amato G, Giustina A. Resistance to somatostatin analogs in acromegaly: An evolving concept? Journal of Endocrinological Investigation. 2006;29:86–93. doi: 10.1007/BF03349183. [DOI] [PubMed] [Google Scholar]
- Guensberg P, Wacheck V, Lucas T, Monia B, Pehamberger H, Eichler HG, et al. Bcl-xL antisense oligonucleotides chemosensitize human glioblastoma cells. Chemotherapy. 2002;48:189–195. doi: 10.1159/000063873. [DOI] [PubMed] [Google Scholar]
- Gururangan S, Friedman HS. Innovations in design and delivery of chemotherapy for brain tumors. Neuroimaging Clinics of North America. 2002;12:583–597. doi: 10.1016/s1052-5149(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, Arvold ND, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. Journal of the National Cancer Institute. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- Haga S, Hinoshita E, Ikezaki K, Fukui M, Scheffer GL, Uchiumi T, et al. Involvement of the multidrug resistance protein 3 in drug sensitivity and its expression in human glioma. Japanese Journal of Cancer Research : Gann. 2001;92:211–219. doi: 10.1111/j.1349-7006.2001.tb01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DK, Vance ML, Boulos PT, Laws ER. Surgical outcomes in hyporesponsive prolactinomas: Analysis of patients with resistance or intolerance to dopamine agonists. Pituitary. 2005;8:53–60. doi: 10.1007/s11102-005-5086-1. [DOI] [PubMed] [Google Scholar]
- Hank NC, Shapiro JR, Scheck AC. Over-representation of specific regions of chromosome 22 in cells from human glioma correlate with resistance to 1,3-bis(2-chloroethyl)-1-nitrosourea. BMC Cancer. 2006;6:2. doi: 10.1186/1471-2407-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroun RI, Clatterbuck RE, Gibbons MC, Burger PC, Parker R, Fruehauf JP, et al. Extreme drug resistance in primary brain tumors: In vitro analysis of 64 resection specimens. Journal of Neuro-Oncology. 2002;58:115–123. doi: 10.1023/a:1016049111941. [DOI] [PubMed] [Google Scholar]
- Henson JW. Spinal cord gliomas. Curr Opin Neurol. 2001;14:679–682. doi: 10.1097/00019052-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Herrlinger U, Rieger J, Steinbach JP, Nagele T, Dichgans J, Weller M. UKT-04 trial of continuous metronomic low-dose chemotherapy with methotrexate and cyclophosphamide for recurrent glioblastoma. Journal of Neuro-Oncology. 2005;71:295–299. doi: 10.1007/s11060-004-1726-y. [DOI] [PubMed] [Google Scholar]
- Hikawa T, Mori T, Abe T, Hori S. The ability in adhesion and invasion of drug-resistant human glioma cells. Journal of Experimental & Clinical Cancer Research : CR. 2000;19:357–362. [PubMed] [Google Scholar]
- Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12867–12872. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D, Natsume A, Wakabayashi T, Hatano H, Asano Y, Takeuchi H, et al. Efficacy of temozolomide is correlated with 1p loss and methylation of the deoxyribonucleic acid repair gene MGMT in malignant gliomas. Neurologia Medico-Chirurgica. 2007;47:341–9. doi: 10.2176/nmc.47.341. discussion 350. [DOI] [PubMed] [Google Scholar]
- Iwadate Y, Mochizuki S, Fujimoto S, Namba H, Sakiyama S, Tagawa M, et al. Alteration of CDKN2/p16 in human astrocytic tumors is related with increased susceptibility to antimetabolite anticancer agents. International Journal of Oncology. 2000;17:501–505. doi: 10.3892/ijo.17.3.501. [DOI] [PubMed] [Google Scholar]
- Jan M, Dufour H, Brue T, Jaquet P. Prolactinoma surgery. Annales d'Endocrinologie. 2007;68:118–119. doi: 10.1016/j.ando.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Jaquet P, Gunz G, Saveanu A, Dufour H, Taylor J, Dong J, et al. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. European Journal of Endocrinology / European Federation of Endocrine Societies. 2005;153:135–141. doi: 10.1530/eje.1.01950. [DOI] [PubMed] [Google Scholar]
- Jenkinson MD, Simpson C, Nicholas RS, Miles J, Findlay GF, Pigott TJ. Outcome predictors and complications in the management of intradural spinal tumours. European Spine Journal : Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2006;15:203–210. doi: 10.1007/s00586-005-0902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RL. Hypoxia in the tumorigenesis of gliomas and as a potential target for therapeutic measures. Neurosurgical Focus. 2006;20:E24. doi: 10.3171/foc.2006.20.4.16. [DOI] [PubMed] [Google Scholar]
- Jiang H, Alonso MM, Gomez-Manzano C, Piao Y, Fueyo J. Oncolytic viruses and DNA-repair machinery: Overcoming chemoresistance of gliomas. Expert Review of Anticancer Therapy. 2006;6:1585–1592. doi: 10.1586/14737140.6.11.1585. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Pore N, Cerniglia GJ, Mick R, Georgescu MM, Bernhard EJ, et al. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer Research. 2007;67:4467–4473. doi: 10.1158/0008-5472.CAN-06-3398. [DOI] [PubMed] [Google Scholar]
- Joy A, Panicker S, Shapiro JR. Altered nuclear localization of bax protein in BCNU-resistant glioma cells. Journal of Neuro-Oncology. 2000;49:117–129. doi: 10.1023/a:1026574123273. [DOI] [PubMed] [Google Scholar]
- Kaloshi G, Benouaich-Amiel A, Diakite F, Taillibert S, Lejeune J, Laigle-Donadey F, et al. Temozolomide for low-grade gliomas: Predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- Knisely JP, Rockwell S. Importance of hypoxia in the biology and treatment of brain tumors. Neuroimaging Clinics of North America. 2002;12:525–536. doi: 10.1016/s1052-5149(02)00032-1. [DOI] [PubMed] [Google Scholar]
- Kocak Z, Garipagaoglu M, Adli M, Uzal MC, Kurtman C. Spinal cord ependymomas in adults: Analysis of 15 cases. Journal of Experimental & Clinical Cancer Research : CR. 2004;23:201–206. [PubMed] [Google Scholar]
- Kong DS, Lee JI, Kim WS, Son MJ, Lim do H, Kim ST, et al. A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma. Oncology Reports. 2006;16:1117–1121. [PubMed] [Google Scholar]
- Lee KC, Hall DE, Hoff BA, Moffat BA, Sharma S, Chenevert TL, et al. Dynamic imaging of emerging resistance during cancer therapy. Cancer Research. 2006;66:4687–4692. doi: 10.1158/0008-5472.CAN-05-3205. [DOI] [PubMed] [Google Scholar]
- Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: Special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- Lefranc F, Kiss R. Autophagy, the trojan horse to combat glioblastomas. Neurosurgical Focus. 2006;20:E7. doi: 10.3171/foc.2006.20.4.4. [DOI] [PubMed] [Google Scholar]
- Li YC, Tzeng CC, Song JH, Tsia FJ, Hsieh LJ, Liao SJ, et al. Genomic alterations in human malignant glioma cells associate with the cell resistance to the combination treatment with tumor necrosis factor-related apoptosis-inducing ligand and chemotherapy. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2006;12:2716–2729. doi: 10.1158/1078-0432.CCR-05-1980. [DOI] [PubMed] [Google Scholar]
- Liu G, Akasaki Y, Khong HT, Wheeler CJ, Das A, Black KL, et al. Cytotoxic T cell targeting of TRP-2 sensitizes human malignant glioma to chemotherapy. Oncogene. 2005;24:5226–5234. doi: 10.1038/sj.onc.1208519. [DOI] [PubMed] [Google Scholar]
- Liu TF, Cohen KA, Willingham MC, Tatter SB, Puri RK, Frankel AE. Combination fusion protein therapy of refractory brain tumors: Demonstration of efficacy in cell culture. Journal of Neuro-Oncology. 2003;65:77–85. doi: 10.1023/a:1026286214901. [DOI] [PubMed] [Google Scholar]
- Ma Y, Yuan RQ, Fan S, Hu C, Goldberg ID, Laterra JJ, et al. Identification of genes that modulate sensitivity of U373MG glioblastoma cells to cis-platinum. Anti-Cancer Drugs. 2006;17:733–751. doi: 10.1097/01.cad.0000217429.67455.18. [DOI] [PubMed] [Google Scholar]
- MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48:151–157. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- Maleniak TC, Darling JL, Lowenstein PR, Castro MG. Adenovirus-mediated expression of HSV1-TK or fas ligand induces cell death in primary human glioma-derived cell cultures that are resistant to the chemotherapeutic agent CCNU. Cancer Gene Therapy. 2001;8:589–598. doi: 10.1038/sj.cgt.7700348. [DOI] [PubMed] [Google Scholar]
- Manome Y, Yoshinaga H, Watanabe M, Ohno T. Adenoviral transfer of antisenses or ribozyme to O6-methylguanine-DNA methyltransferase mRNA in brain-tumor model resistant to chloroethyl-nitrosourea. Anticancer Research. 2002;22:2029–2036. [PubMed] [Google Scholar]
- Marie Y, Carpentier AF, Omuro AM, Sanson M, Thillet J, Hoang-Xuan K, et al. EGFR tyrosine kinase domain mutations in human gliomas. Neurology. 2005;64:1444–1445. doi: 10.1212/01.WNL.0000158654.07080.B0. [DOI] [PubMed] [Google Scholar]
- Mason WP, Cairncross JG. Drug insight: Temozolomide as a treatment for malignant glioma--impact of a recent trial. Nature Clinical Practice Neurology. 2005;1:88–95. doi: 10.1038/ncpneuro0045. [DOI] [PubMed] [Google Scholar]
- Mason WP, Cairncross JG. Invited article: The expanding impact of molecular biology on the diagnosis and treatment of gliomas. Neurology. 2008;71:365–373. doi: 10.1212/01.wnl.0000319721.98502.1b. [DOI] [PubMed] [Google Scholar]
- Matrone C, Pivonello R, Colao A, Cappabianca P, Cavallo LM, Del Basso De Caro ML, et al. Expression and function of somatostatin receptor subtype 1 in human growth hormone secreting pituitary tumors deriving from patients partially responsive or resistant to long-term treatment with somatostatin analogs. Neuroendocrinology. 2004;79:142–148. doi: 10.1159/000077272. [DOI] [PubMed] [Google Scholar]
- Miknyoczki S, Chang H, Grobelny J, Pritchard S, Worrell C, McGann N, et al. The selective poly(ADP-ribose) polymerase-1(2) inhibitor, CEP-8983, increases the sensitivity of chemoresistant tumor cells to temozolomide and irinotecan but does not potentiate myelotoxicity. Molecular Cancer Therapeutics. 2007;6:2290–2302. doi: 10.1158/1535-7163.MCT-07-0062. [DOI] [PubMed] [Google Scholar]
- Mohri M, Nitta H, Yamashita J. Expression of multidrug resistance-associated protein (MRP) in human gliomas. Journal of Neuro-Oncology. 2000;49:105–115. doi: 10.1023/a:1026528926482. [DOI] [PubMed] [Google Scholar]
- Molitch ME. Dopamine resistance of prolactinomas. Pituitary. 2003;6:19–27. doi: 10.1023/a:1026225625897. [DOI] [PubMed] [Google Scholar]
- Molitch ME. Pharmacologic resistance in prolactinoma patients. Pituitary. 2005;8:43–52. doi: 10.1007/s11102-005-5085-2. [DOI] [PubMed] [Google Scholar]
- Morandi E, Zingaretti C, Chiozzotto D, Severini C, Semeria A, Horn W, et al. A cDNA-microarray analysis of camptothecin resistance in glioblastoma cell lines. Cancer Letters. 2006;231:74–86. doi: 10.1016/j.canlet.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Nagasubramanian R, Dolan ME. Temozolomide: Realizing the promise and potential. Current Opinion in Oncology. 2003;15:412–418. doi: 10.1097/00001622-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kubota T, Ido K, Sakuma T, Matsuda K. Gene expression profiles of 1-(4-amino-2-methyl-5-pyrimidinyl)-methyl-3-(2-chloroethyl)-3-nitrosourea (ACNU)-resistant C6 rat glioma cells. Journal of Neuro-Oncology. 2006;79:271–279. doi: 10.1007/s11060-006-9143-z. [DOI] [PubMed] [Google Scholar]
- Ng WH, Wan GQ, Too HP. Higher glioblastoma tumour burden reduces efficacy of chemotherapeutic agents: In vitro evidence. Journal of Clinical Neuroscience : Official Journal of the Neurosurgical Society of Australasia. 2007;14:261–266. doi: 10.1016/j.jocn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Ngo TT, Peng T, Liang XJ, Akeju O, Pastorino S, Zhang W, et al. The 1p-encoded protein stathmin and resistance of malignant gliomas to nitrosoureas. Journal of the National Cancer Institute. 2007;99:639–652. doi: 10.1093/jnci/djk135. [DOI] [PubMed] [Google Scholar]
- Nishio S, Morioka T, Fujii K, Inamura T, Fukui M. Spinal cord gliomas: Management and outcome with reference to adjuvant therapy. Journal of Clinical Neuroscience : Official Journal of the Neurosurgical Society of Australasia. 2000;7:20–23. doi: 10.1054/jocn.1999.0128. [DOI] [PubMed] [Google Scholar]
- Olafsdottir A, Schlechte J. Management of resistant prolactinomas. Nature Clinical Practice Endocrinology & Metabolism. 2006;2:552–561. doi: 10.1038/ncpendmet0290. [DOI] [PubMed] [Google Scholar]
- Parker RJ, Fruehauf JP, Mehta R, Filka E, Cloughesy T. A prospective blinded study of the predictive value of an extreme drug resistance assay in patients receiving CPT-11 for recurrent glioma. Journal of Neuro-Oncology. 2004;66:365–375. doi: 10.1023/b:neon.0000014549.77646.f6. [DOI] [PubMed] [Google Scholar]
- Paus C, Murat A, Stupp R, Regli L, Hegi M. Role of MGMT and clinical applications in brain tumours. [Role de la MGMT et implications cliniques dans les tumeurs cerebrales]. Bulletin Du Cancer. 2007;94:769–773. [PubMed] [Google Scholar]
- Pavillard V, Kherfellah D, Richard S, Robert J, Montaudon D. Effects of the combination of camptothecin and doxorubicin or etoposide on rat glioma cells and camptothecin-resistant variants. British Journal of Cancer. 2001;85:1077–1083. doi: 10.1054/bjoc.2001.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pels H, Montesinos-Rongen M, Schaller C, Schlegel U, Schmidt-Wolf IG, Wiestler OD, et al. VH gene analysis of primary CNS lymphomas. Journal of the Neurological Sciences. 2005;228:143–147. doi: 10.1016/j.jns.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Pipas JM, Meyer LP, Rhodes CH, Cromwell LD, McDonnell CE, Kingman LS, et al. A phase II trial of paclitaxel and topotecan with filgrastim in patients with recurrent or refractory glioblastoma multiforme or anaplastic astrocytoma. Journal of Neuro-Oncology. 2005;71:301–305. doi: 10.1007/s11060-004-2026-2. [DOI] [PubMed] [Google Scholar]
- Poirson-Bichat F, Goncalves RA, Miccoli L, Dutrillaux B, Poupon MF. Methionine depletion enhances the antitumoral efficacy of cytotoxic agents in drug-resistant human tumor xenografts. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2000;6:643–653. [PubMed] [Google Scholar]
- Puchner MJ, Giese A, Lohmann F, Cristante L. High-dose tamoxifen treatment increases the incidence of multifocal tumor recurrences in glioblastoma patients. Anticancer Research. 2004;24:4195–4203. [PubMed] [Google Scholar]
- Quinn JA, Pluda J, Dolan ME, Delaney S, Kaplan R, Rich JN, et al. Phase II trial of carmustine plus O(6)-benzylguanine for patients with nitrosourea-resistant recurrent or progressive malignant glioma. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2002;20:2277–2283. doi: 10.1200/JCO.2002.09.084. [DOI] [PubMed] [Google Scholar]
- Reichert M, Steinbach JP, Supra P, Weller M. Modulation of growth and radiochemosensitivity of human malignant glioma cells by acidosis. Cancer. 2002;95:1113–1119. doi: 10.1002/cncr.10767. [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Louis DN. Oligodendroglioma: Toward molecular definitions in diagnostic neuro-oncology. Journal of Neuropathology and Experimental Neurology. 2003;62:111–126. doi: 10.1093/jnen/62.2.111. [DOI] [PubMed] [Google Scholar]
- Rellecke P, Kuchelmeister K, Schachenmayr W, Schlegel J. Mismatch repair protein hMSH2 in primary drug resistance in in vitro human malignant gliomas. Journal of Neurosurgery. 2004;101:653–658. doi: 10.3171/jns.2004.101.4.0653. [DOI] [PubMed] [Google Scholar]
- Ricard D, Kaloshi G, Amiel-Benouaich A, Lejeune J, Marie Y, Mandonnet E, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Annals of Neurology. 2007;61:484–490. doi: 10.1002/ana.21125. [DOI] [PubMed] [Google Scholar]
- Rieger L, Rieger J, Winter S, Streffer J, Esser P, Dichgans J, et al. Evidence for a constitutive, verapamil-sensitive, non-P-glycoprotein multidrug resistance phenotype in malignant glioma that is unaltered by radiochemotherapy in vivo. Acta Neuropathologica. 2000;99:555–562. doi: 10.1007/s004010051160. [DOI] [PubMed] [Google Scholar]
- Rogers D, Nylander KD, Mi Z, Hu T, Schor NF. Molecular predictors of human nervous system cancer responsiveness to enediyne chemotherapy. Cancer Chemotherapy and Pharmacology. 2008;62:699–706. doi: 10.1007/s00280-008-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer V, Freneau E, Morange I, Simonetta C. Efficacy of quinagolide in resistance to dopamine agonists: Results of a multicenter study. club de l'hypophyse. Annales d'Endocrinologie. 2000;61:411–417. [PubMed] [Google Scholar]
- Rovin RA. Drug resistance. Journal of Neurosurgery. 2005;102:1170. 1170–1. doi: 10.3171/jns.2005.102.6.1170. author reply. [DOI] [PubMed] [Google Scholar]
- Saito R, Bringas J, Mirek H, Berger MS, Bankiewicz KS. Invasive phenotype observed in 1,3-bis(2-chloroethyl)-1-nitrosourea-resistant sublines of 9L rat glioma cells: A tumor model mimicking a recurrent malignant glioma. Journal of Neurosurgery. 2004;101:826–831. doi: 10.3171/jns.2004.101.5.0826. [DOI] [PubMed] [Google Scholar]
- Salehi F, Kovacs K, Scheithauer BW, Lloyd RV, Cusimano M. Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update. Endocrine-Related Cancer. 2008;15:721–743. doi: 10.1677/ERC-08-0012. [DOI] [PubMed] [Google Scholar]
- Salmaggi A, Boiardi A, Gelati M, Russo A, Calatozzolo C, Ciusani E, et al. Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype. Glia. 2006;54:850–860. doi: 10.1002/glia.20414. [DOI] [PubMed] [Google Scholar]
- Sathornsumetee S, Rich JN. New treatment strategies for malignant gliomas. Expert Review of Anticancer Therapy. 2006;6:1087–1104. doi: 10.1586/14737140.6.7.1087. [DOI] [PubMed] [Google Scholar]
- Saveanu A, Gunz G, Dufour H, Caron P, Fina F, Ouafik L, et al. Bim-23244, a somatostatin receptor subtype 2- and 5-selective analog with enhanced efficacy in suppressing growth hormone (GH) from octreotide-resistant human GH-secreting adenomas. The Journal of Clinical Endocrinology and Metabolism. 2001;86:140–145. doi: 10.1210/jcem.86.1.7099. [DOI] [PubMed] [Google Scholar]
- Scheck AC, Perry K, Hank NC, Clark WD. Anticancer activity of extracts derived from the mature roots of scutellaria baicalensis on human malignant brain tumor cells. BMC Complementary and Alternative Medicine. 2006;6:27. doi: 10.1186/1472-6882-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepkin VD, Lee KC, Kuszpit K, Muthuswami M, Johnson TD, Chenevert TL, et al. Proton and sodium MRI assessment of emerging tumor chemotherapeutic resistance. NMR in Biomedicine. 2006;19:1035–1042. doi: 10.1002/nbm.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servidei T, Ferlini C, Riccardi A, Meco D, Scambia G, Segni G, et al. The novel trinuclear platinum complex BBR3464 induces a cellular response different from cisplatin. European Journal of Cancer (Oxford, England : 1990) 2001;37:930–938. doi: 10.1016/s0959-8049(01)00061-2. [DOI] [PubMed] [Google Scholar]
- Servidei T, Riccardi A, Sanguinetti M, Dominici C, Riccardi R. Increased sensitivity to the platelet-derived growth factor (PDGF) receptor inhibitor STI571 in chemoresistant glioma cells is associated with enhanced PDGF-BB-mediated signaling and STI571-induced akt inactivation. Journal of Cellular Physiology. 2006;208:220–228. doi: 10.1002/jcp.20659. [DOI] [PubMed] [Google Scholar]
- Silber JR, Bobola MS, Blank A, Schoeler KD, Haroldson PD, Huynh MB, et al. The apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2002;8:3008–3018. [PubMed] [Google Scholar]
- Simon M, Voss D, Park-Simon TW, Mahlberg R, Koster G. Role of p16 and p14ARF in radio- and chemosensitivity of malignant gliomas. Oncology Reports. 2006;16:127–132. [PubMed] [Google Scholar]
- Soffietti R, Nobile M, Ruda R, Borgognone M, Costanza A, Laguzzi E, et al. Second-line treatment with carboplatin for recurrent or progressive oligodendroglial tumors after PCV (procarbazine, lomustine, and vincristine) chemotherapy: A phase II study. Cancer. 2004;100:807–813. doi: 10.1002/cncr.20042. [DOI] [PubMed] [Google Scholar]
- Spiegl-Kreinecker S, Pirker C, Marosi C, Buchroithner J, Pichler J, Silye R, et al. Dynamics of chemosensitivity and chromosomal instability in recurrent glioblastoma. British Journal of Cancer. 2007;96:960–969. doi: 10.1038/sj.bjc.6603652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. The Lancet Oncology. 2001;2:552–560. doi: 10.1016/S1470-2045(01)00489-2. [DOI] [PubMed] [Google Scholar]
- Tallen G, Riabowol K, Wolff JE. Expression of p33ING1 mRNA and chemosensitivity in brain tumor cells. Anticancer Research. 2003;23:1631–1635. [PubMed] [Google Scholar]
- Tanaka S, Kamitani H, Amin MR, Watanabe T, Oka H, Fujii K, et al. Preliminary individual adjuvant therapy for gliomas based on the results of molecular biological analyses for drug-resistance genes. Journal of Neuro-Oncology. 2000;46:157–171. doi: 10.1023/a:1006399903635. [DOI] [PubMed] [Google Scholar]
- Tentori L, Portarena I, Torino F, Scerrati M, Navarra P, Graziani G. Poly(ADP-ribose) polymerase inhibitor increases growth inhibition and reduces G(2)/M cell accumulation induced by temozolomide in malignant glioma cells. Glia. 2002;40:44–54. doi: 10.1002/glia.10113. [DOI] [PubMed] [Google Scholar]
- Tews DS, Nissen A, Kulgen C, Gaumann AK. Drug resistance-associated factors in primary and secondary glioblastomas and their precursor tumors. Journal of Neuro-Oncology. 2000;50:227–237. doi: 10.1023/a:1006491405010. [DOI] [PubMed] [Google Scholar]
- Triebels VH, Taphoorn MJ, Brandes AA, Menten J, Frenay M, Tosoni A, et al. Salvage PCV chemotherapy for temozolomide-resistant oligodendrogliomas. Neurology. 2004;63:904–906. doi: 10.1212/01.wnl.0000137049.65631.db. [DOI] [PubMed] [Google Scholar]
- Valera ET, Machado HR, Scrideli CA, Lucio-Eterovic AK, Tone LG. Drug-resistance in central nervous system tumors: From the traditional cell-resistance model to the genetically driven approaches on therapy. Current Pharmaceutical Biotechnology. 2007a;8:105–113. doi: 10.2174/138920107780487500. [DOI] [PubMed] [Google Scholar]
- Valera ET, Lucio-Eterovic AK, Neder L, Scrideli CA, Machado HR, Carlotti-Junior CG, et al. Quantitative PCR analysis of the expression profile of genes related to multiple drug resistance in tumors of the central nervous system. Journal of Neuro-Oncology. 2007b;85:1–10. doi: 10.1007/s11060-007-9382-7. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ, Chinot O, Boogerd W, Bravo Marques J, Taphoorn MJ, Kros JM, et al. Second-line chemotherapy with temozolomide in recurrent oligodendroglioma after PCV (procarbazine, lomustine and vincristine) chemotherapy: EORTC brain tumor group phase II study 26972. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO. 2003;14:599–602. doi: 10.1093/annonc/mdg157. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Morii K, Shinbo Y, Homma J, Sano M, Tsuchiya N, et al. Results of treatment of 112 cases of primary CNS lymphoma. Jpn J Clin Oncol. 2008;38:373–380. doi: 10.1093/jjco/hyn027. [DOI] [PubMed] [Google Scholar]
- Yu JS, Luptrawan A, Black KL, Liu G. Mahaley clinical research award: Chemosensitization of glioma through dendritic cell vaccination. Clinical Neurosurgery. 2006;53:345–351. [PubMed] [Google Scholar]
- Yuan L, Choi K, Khosla C, Zheng X, Higashikubo R, Chicoine MR, et al. Tissue transglutaminase 2 inhibition promotes cell death and chemosensitivity in glioblastomas. Molecular Cancer Therapeutics. 2005;4:1293–1302. doi: 10.1158/1535-7163.MCT-04-0328. [DOI] [PubMed] [Google Scholar]
- Zhang C, Beckermann B, Kallifatidis G, Liu Z, Rittgen W, Edler L, et al. Corticosteroids induce chemotherapy resistance in the majority of tumour cells from bone, brain, breast, cervix, melanoma and neuroblastoma. International Journal of Oncology. 2006;29:1295–1301. [PubMed] [Google Scholar]
- Zupanska A, Dziembowska M, Ellert-Miklaszewska A, Gaweda-Walerych K, Kaminska B. Cyclosporine a induces growth arrest or programmed cell death of human glioma cells. Neurochemistry International. 2005;47:430–441. doi: 10.1016/j.neuint.2005.05.010. [DOI] [PubMed] [Google Scholar]