EXECUTIVE SUMMARY
Identity, Physical and Chemical Properties, Analytical Methods
A compendium is provided of aluminium compounds used in industrial settings, and as pharmaceuticals, food additives, cosmetics and as other household products. Most aluminium compounds are solids exhibiting high melting points. The solubility of aluminium salts is governed by pH, because the aluminium(III)-cation (Al3+) has a strong affinity for the hydroxide ion, which promotes precipitation. Like Mg2+ and Ca2+ ions, Al3+ in most situations seeks out complexing agents with oxygen-atom donor sites such as carboxylate and phosphate groups, including in biological systems. Aluminium oxides, hydroxides and oxyhydroxides occur in numerous crystallographic forms, which exhibit different surface properties. Few compounds of aluminium are classified in Annex 1 of the European Economic Union Council (EEC) Directive 67/1548, with aluminium powder and sodium aluminium fluoride (cryolite) as examples of exceptions, as well as compounds in which the anion renders them reactive such as aluminium phosphide. And finally, the more recent analytical methods available for the study of chemical speciation in solids and solution, and for quantitative analysis, have been applied to the determination of aluminium and the identification of its various forms.
Sources of Human Exposure
Aluminium and its compounds comprise about 8% of the Earth’s surface; aluminium occurs naturally in silicates, cryolite, and bauxite rock. Natural processes account for most of the redistribution of aluminium in the environment. Acidic precipitation mobilizes aluminium from natural sources, and direct anthropogenic releases of aluminium compounds associated with industrial processes occur mainly to air. Certain uses lead to the presence of aluminium in drinking water and foodstuffs.
Bauxite is the most important raw material used in the production of aluminium. Bauxite is refined to produce alumina from which aluminium metal is recovered by electrolytic reduction; aluminium is also recycled from scrap. Aluminium hydroxide is produced from bauxite. In 2004, primary aluminium was being produced in 41 countries, the largest producers being China, Russia, Canada and the United States. In that year, worldwide production of primary aluminium, alumina and aluminium hydroxide reached about 30, 63, and 5 million tonnes per annum, respectively. More than 7 million tonnes of aluminium is recovered annually from recycled old scrap.
The largest markets for aluminium metal and its alloys are in transportation, building and construction, packaging and in electrical equipment. Transportation uses are one of the fastest growing areas for aluminium use. Aluminium powders are used in pigments and paints, fuel additives, explosives and propellants. Aluminium oxides are used as food additives and in the manufacture of, for example, abrasives, refractories, ceramics, electrical insulators, catalysts, paper, spark plugs, light bulbs, artificial gems, alloys, glass and heat resistant fibres. Aluminium hydroxide is used widely in pharmaceutical and personal care products. Food related uses of aluminium compounds include preservatives, fillers, colouring agents, anti-caking agents, emulsifiers and baking powders; soy-based infant formula can contain aluminium. Natural aluminium minerals especially bentonite and zeolite are used in water purification, sugar refining, brewing and paper industries.
Aluminium has not been classified with respect to carcinogenicity; however, “aluminium production” has been classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC) (for further explanation, please see Effects on Humans, Effects from Occupational Exposure, Cancer). Occupational limits exist in several countries for exposures to aluminium dust and aluminium oxide. For non-occupational environments, limits have been set for intake in foods and drinking water; the latter are based on aesthetic or practical, rather than health, considerations.
Environmental Levels and Human Exposure
Aluminium may be designated as crustal in origin, and thus surface soils at uncontaminated sites constitute a source of soluble aluminium species in surface water and aluminium-containing particulates in sediments and ambient-air aerosols. Not surprisingly, the latter are present extensively in air samples in agricultural communities and when road dust is extensive. Environmental acidification is known to mobilize aluminium from land to aquatic environments. Interestingly, aluminium levels and its various forms (species) are often similar in source water and after its treatment with potassium alum as a flocculent during drinking water purification.
Workers in the aluminium production and user industries, as well as aluminium welders, experience considerable exposures to the metal and/or its compounds. In absence of occupational exposures and chronic use of aluminium-containing antacids and buffered aspirin, food is the major intake source of aluminium, followed by drinking water. When considering bioavailability, namely the fraction that is actually taken up into the blood stream, food is again the primary uptake source for individuals not occupationally exposed. However, chronic use of antacids, buffered aspirins and other medical preparations would likely constitute the major uptake source, even when exposed at work.
Kinetics and Metabolism
Humans
The use of 26Al as a tracer and accelerator mass spectrometry has enabled safe studies of aluminium toxicokinetics with real exposure-relevant doses in humans. Aluminium bioavailability from occupational inhalation exposure is ~ 2% whereas oral aluminium bioavailability from water has been reported to be 0.1 to 0.4%. Oral aluminium bioavailability is increased by citrate, acidic pH, and uraemia and may be decreased by silicon-containing compounds. Oral aluminium bioavailability is also inversely related to iron status.
Oral aluminium bioavailability is greater from water than from aluminium hydroxide or sucralfate. Oral aluminium bioavailability from aluminium hydroxide is ≤ 0.1%, and is less with higher doses. Increased oral aluminium absorption has been suggested in Alzheimer’s disease (AD) and Down’s subjects. Oral aluminium bioavailability from the diet has been estimated to be ~ 0.1 to 0.3%, based on daily aluminium intake and urinary elimination. Results of a few studies with a controlled diet and tea are consistent with this estimate.
Steady state serum to whole blood aluminium concentrations are ~ equal. Slightly > 90% of plasma aluminium is associated with transferrin (Tf), ~ 7 to 8% with citrate, and < 1% with phosphate and hydroxide. Normal plasma aluminium concentration is believed to be 1 to 2 μg/L. Normal tissue aluminium concentrations are greater in lung (due to entrapment of particles from the environment) than bone than soft tissues. Approximately 60, 25, 10, 3 and 1% of the aluminium body burden is in the bone, lung, muscle, liver and brain, respectively. Higher concentrations are seen in uraemia and higher still in dialysis encephalopathy.
Tissue aluminium concentration increases with age. Some studies have reported that the aluminium concentration in the bulk brain samples, neurofibrillary tangles (NFT) and plaques was higher in AD subjects than controls. Other studies have found no difference. Hair aluminium concentration has been described but its value as an indicator of aluminium body burden has not been demonstrated.
Greater than 95% of aluminium is eliminated by the kidney; ~ 2% in bile. Occupational aluminium exposure increases urinary more than plasma aluminium concentration above their normal levels. Depending on the type and route of exposure, aluminium clearance has been characterized as having multiple half-times and are estimated in hours, days, and years. Most of the Al was eliminated within the first week; the terminal half-life probably represents < 1% of the injected aluminium.
Biological monitoring of human aluminium exposure has been conducted with urine, which is thought to indicate recent exposure, and plasma, which is thought to better reflect the aluminium body burden and long-term exposure. However, neither is a very good predictor of the aluminium body burden, which is better estimated by bone aluminium, the desferrioxamine challenge test, or combined measurement of serum iPTH (parathyroid hormone) and the desferrioxamine test.
Serum aluminium > 30 μg/L in dialysis patients has been associated with osteomalacia and related disorders and > 80 μg/L associated with encephalopathy. Up to 5 mg/kg of desferrioxamine once or twice weekly has been shown to be safe and effective for long-term treatment of aluminium overload.
Animals
In studies of animals, pulmonary deposition of fly ash was 2 to 12% and was inversely related to particle size. Oral aluminium bioavailability from water appears to be ~ 0.3%. The very limited data available suggest oral aluminium bioavailability from food is less than from water.
Oral aluminium bioavailability is increased by citrate, and to a lesser extent, other carboxylic acids, increased solubility of the aluminium species, acidic pH, uraemia, increased dose of soluble aluminium species, and perhaps fluoride. Oral aluminium bioavailability is decreased by silicon-containing compounds. Oral aluminium bioavailability is also inversely related to iron, calcium and sodium status.
Absorption of aluminium from the gastrointestinal tract (GI) appears to be primarily in the distal intestine. There is evidence supporting several mechanisms of intestinal aluminium absorption, including sodium transport processes, an interaction with calcium uptake, and paracellular diffusion. Aluminium penetration of the skin is very shallow. Aluminium may be able to enter the brain from the nasal cavity by a direct route, bypassing systemic circulation, but convincing evidence is lacking. Absorption of aluminium from intramuscularly (i.m.) injected aluminium hydroxide and aluminiun phosphate adjuvants is significant, and may eventually be complete. Tissue aluminium concentration increases with age.
The volume of distribution (Vd) of aluminium is initially consistent with the blood volume, and then increases with time. Steady state serum to whole blood aluminium concentrations are ~ equal. Greater than 90% of serum aluminium is bound to Tf. Although aluminium has been reported in many intracellular compartments, concentrations were often greater in the nucleus. Ferritin can incorporate aluminium.
Following i.v. injection, ~ 0.001 to 0.01% of the aluminium dose enters each gram of brain and ~ 100-fold more each gram of bone. Brain aluminium uptake across the blood-brain barrier (BBB) may be mediated by Tf-receptor mediated endocytosis (TfR-ME) and a Tf-independent mechanism that may transport aluminium citrate. There appears to be a transporter that effluxes aluminium from the brain into blood. Aluminium distributes into the placenta, foetus, milk, hair, and can be quantified in all tissues and fluids. Greater than 95% of aluminium is eliminated by the kidney, probably by glomerular filtration. Less than 2% appears in bile.
Aluminium clearance is characterized by multiple half-lives (t½), suggesting multiple compartments. The terminal t½ from the lung is ~ 100 days and from the brain and other soft tissues > 100 days. Prolonged aluminium residence in the bone may account for the prolonged t½ observed in most organs, including the brain.
There are no published reports of physiologically based pharmacokinetic (PBPK) modelling of aluminium. A few models have been developed that incorporate the reported results of toxicokinetic studies with aluminium.
Effects on Laboratory Mammals and In Vitro Test Systems
Regardless of the duration of exposure, the toxicity attributed to aluminium is dependent upon the physiochemical properties (solubility, pH, bioavailability, etc.), type of aluminium preparation, route of administration, and physiological status (presence of renal dysfunction). Following oral exposure, aluminium distributes throughout the organism with accumulation in bone, kidneys and brain being of concern to humans with evidence of renal dysfunction, anemia or neurobehavioural alterations reported after excessive doses. The presence of aluminium in vaccines was found to be associated with macrophagic myofasciitis (MMF) at the site of i.m. injection. The toxicity of aluminium is affected by chelating agents and ligands although the mechanisms underlying toxicity remain unknown. However, it should be noted that only at excessive concentrations of aluminium are toxic manifestations seen and, hence aluminium is considered to possess a “low” potential for producing adverse effects.
Oral administration of aluminium did not affect reproductive capacity in males or females. Exposure to aluminium during gestation did not affect maternal health or development of the foetuses and neonates. Further, there was no evidence of teratogenic alterations in the foetuses of mothers fed dietary aluminium. Maternal dietary exposure to excessive amounts of aluminium during gestation and lactation resulted in neurobehavioural abnormalities in mouse offspring. At physiological concentrations the reproductive system does not appear to be a target for aluminium-induced effects; and if there is exposure during pregnancy, the growth and development of offspring of metal-treated mothers is not adversely affected.
The form of aluminium most often presented to tissues outside of the blood stream is expected to be bound to Tf. In brain, aluminium is prone to dissociate from Tf as a soluble citrate salt. Most cells of the central nervous system (CNS) express Tf receptor, and thus receptor-mediated uptake would be one mechanism by which aluminium could enter cells of the brain. Free flow endocytosis of aluminium citrate could be an alternative route of uptake. As outlined in Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, In Vivo Models, Neuropathology, there is at least one example of human pathology which is consistent with this mode of tissue exposure. Choroid plexus epithelia, cortical glia, and cortical neurons of patients exhibiting dialysis associated encephalopathy (DAE) develop intracellular argentophylllic granules that are lysosome-derived and intracytoplasmic. Uptake of aluminium-Tf complexes via receptor-mediated endocytosis would be expected to produce just such pathology. Whether aluminium, of any amount or speciation, escapes these compartments to impact on intracellular processes in humans is unknown. If relatively high doses produce pathology of such a distinctive nature, then it is reasonable to presume that lower doses of aluminium would follow similar pathways into the nervous system of humans.
In the studies of animals, it is important to note that a few reports have documented a pathologic accumulation of aluminium in intracellular lysosome-derived structures. Aluminium accumulation in lysosome-like cytoplasmic granules of retinal neurons in rats exposed to very high doses of aluminium was reported (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, In Vivo Models, Rodent Models of Aluminium Toxicity by Direct Injection). Severe atrophy of the retina and loss of photoreceptors was also noted. Similarly, another study noted intracellular accumulations of aluminium in the brain of rats feed diets high in aluminium. For CNS it seems likely that the mode of delivery to the tissue is through Tf-mediated uptake. From animal studies and the clear association of aluminium exposure and DAE, it is clear that high levels of aluminium in CNS can lead to neurotoxicity. From the current literature it remains difficult to assess what a concentration of aluminium in serum (chronic levels) correlates with neurotoxicity. The effects of aluminium on the developing nervous system have also not been thoroughly addressed.
In regards to mechanisms by which aluminium could play a role in AD, there are both direct and indirect modes of potential action. In a direct mode, aluminium could potentiate the aggregation of molecules known to form pathologic lesions in AD. There is evidence that aluminium can promote the aggregation of β-amyloid peptide in vitro. However, whether aluminium would dissociate from Tf at an appreciable rate and bind β-amyloid peptide in vivo is unclear. One study found no association between AD-like pathology and long-term ingestion of aluminium. Indeed in this study of older patients, the incidence of AD-associated pathology in patients with DAE was no different from controls. Although these studies would suggest that there is little direct evidence for an association between AD and aluminium, a study of transgenic mice that produce Alzheimer-type amyloid pathology noted that mice feed diets high in aluminium showed increased levels of amyloid (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, Alzheimer’s Disease). Moreover, it is well established in the rabbit that exposure to aluminium induces the formation of filamentous structures containing cytoplasmic neurofilament protein (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, Motor Neuron Disease). Therefore, it is difficult to determine how a life-time of exposure to aluminium might influence the development of Alzheimer-type pathology by affecting the folding or clearance of “at-risk” proteins such as β-amyloid, tau, and α-synuclein.
Apart from the potential that aluminium might interact directly with molecules implicated in AD and related neurodegenerative disorders, studies in animals have revealed potential mechanisms by which aluminium might indirectly impact on the function of the nervous system. In Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, Alzheimer’s Disease, studies are described that reported aluminium may affect levels of cholesterol, which has been suggested in numerous studies as a potential modulator or Alzheimer-type amyloid formation. Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, In Vivo Models, Rodent Models of Aluminium Toxicity by Direct Injection describes several studies that have reported elevated levels of markers of oxidative stress in animals exposed to aluminium. These studies suggest potential mechanisms by which long-term exposure to aluminium could be deleterious and could synergistically worsen cognitive abilities in individuals that have pathologic abnormalities associated with AD.
However, there has not been strong evidence from animal studies that aluminium directly modulates cognitive function. As described in Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity,Behavioural Studies of Laboratory Animals Exposed to Aluminium, there have been several studies that have examined the cognitive abilities of mice and rats exposed to aluminium. For the most part, these studies did not report profound cognitive impairment even when exposed to very high levels of aluminium. Therefore, it seems unlikely that aluminium might lower the threshold for AD by blunting cognitive ability of adults.
Outside of the nervous system, the data regarding the potential for alumimium to cause abnormalities is mixed. There is clear evidence that sustained exposure to high levels of aluminium can cause bone abnormalities. Aluminium is clearly deposited in bone at sites of new growth. Bones in animals exposed to aluminium may show increased weakness and increased brittleness. Deficiencies in calcium or magnesium may exacerbate the effects of aluminium. Aluminium overload leads to PTH suppression and with regards to the bone, may be associated with altered calcium homeostasis.
Aluminium may also have negative effects on hematopoiesis. However, these effects are relatively mild unless animals are deficient in iron. In this latter setting, there will be increased levels of free Tf, which can then bind aluminium and compete for Tf receptor; further limiting the amount of iron available for erythrogenesis. Aluminium may also interfere with the metabolism of other metals. On this latter point, the strongest data, meaning most reproducible, suggest that aluminium exposure can lead to increased excretion of phosphorous.
From the present data, however, it is difficult to determine what level of exposure poses a risk for human health or which systems are most vulnerable. Based on projections from studies in dogs, individuals with sustained aluminium levels in serum that are 10-fold higher than the average range, or 1-2 μg/L, may be at increased risk for bone abnormalities. The exposure levels at which other systems might be affected are more difficult to project, particularly when trying to assess risk for late-onset illnesses.
Although not reported in every study, the majority of studies that utilized high doses of aluminium reported significant reductions in weight gain, particularly in studies initiated in young animals. The physiologic basis for this outcome is unclear, but it was reported that animals exposed to high doses of aluminium in drinking water consumed less food. Whether general effects of aluminium on metabolic processes depress metabolism or reduce nutritional efficiency remains to be resolved.
Experimental aluminium inhalation has been shown to produce effects interpreted as alveolar proteinosis and lipid pneumonia. Inhalation of aluminium had some protective effect against quartz dust-induced fibrosis in some, but not all, studies. Intratracheal aluminium instillation produced nodular fibrosis. Aluminium is used as an adjuvant in vaccines and hyposensitization treatments to precipitate toxins and toxoids, enhance their antigenic properties and reduce their rate of absorption and elimination. Aluminium can produce aluminium-species-dependent dermal irritation.
Experimental animal studies have failed to demonstrate carcinogenicity attributed solely to aluminium compounds. Often the response reported is associated with a tissue response to a foreign body rather than a direct effect of aluminium exposure. This appeared to be consistent across various routes of exposure from inhalation to intraperitoneal (i.p.) injection.
In agreement with their non-carcinogenic activity, aluminium compounds failed to show positive results in most short-term mutagenic assays and animal experiments to determine genotoxic potential of aluminium compounds lead to contradictory results with suggestions of an anti-genotoxic potential.
There is little reported for aluminium compounds in the way of immunotoxicity. There may be an altered immune response to challenge following excess aluminium exposure and this may be influenced by the health and hormonal status of the dam with increased susceptibility to bacterial infection seen in pregnancy.
Effects on Humans
Occupational exposure
Occupational exposure to aluminium occurs during the refining of the primary metal and in secondary industries that use aluminium products. Several studies have reported adverse respiratory tract effects in aluminium industry employees. Asthma-like symptoms, known as potroom asthma, have been the most intensely investigated respiratory effect. Wheezing, dyspnea, and impaired lung function (typically assessed by measuring forced expiratory volume (FEV1) and forced volume capacity (FVC)) are the primary features of this disorder. Several cross-sectional, case-control and longitudinal studies have demonstrated increased frequency of adverse pulmonary effects in potroom workers as compared to non-exposed workers. The cause of potroom asthma has not been fully elucidated, but job specific exposure measurements based on personal sampling data and analysis of plasma levels suggests that exposure to fluorides may be an important determinant. There is some evidence to support that individuals with hay fever and individuals with elevated eosinophil counts are at increased risk of developing potroom asthma. Other studies did not find an association between allergic status and the development of symptoms. The respiratory problems documented in potroom aluminium workers are generally associated with toxic chemicals other than aluminium in the workplace. In contrast, exposure to aluminium powder is thought to be directly correlated with the development of pulmonary fibrosis in aluminium industry workers.
Adverse neurological outcomes as a result of occupational aluminium exposure have also been extensively investigated. Aluminium exposure in these studies was estimated in a number of different ways including; exposure grading for different job categories, determination of total body burden of aluminium, number of years working in the aluminium industry, and ever v.s. never worked in the aluminium industry. Occupational aluminium exposure was significantly correlated with a variety of neuropyschiatric symptoms including; loss of coordination, loss of memory, and problems with balance. Studies which specifically examined the relationship between AD and occupational aluminium exposure did not show any significant correlation. However, these studies are limited by methodological issues.
The occurrence of contact dermatitis and irritant dermatitis was reported in workers exposed to aluminium alloys and aluminium dust.
Several epidemiological studies have reported an increased risk of developing lung cancer or bladder cancer for workers in the aluminium industry, however, in all of these studies the risk has been attributed to the exposure to the PAHs generated during aluminium production rather than from exposure to aluminium compounds. Studies investigating the effects of occupational exposure to aluminium are limited by many methodological issues. Rarely is a worker exposed solely to aluminium containing compounds and exposure information is often not adequate to rule out other toxic substances as the cause of the observed effect. Small sample sizes, misclassification bias, selection of inappropriate comparison groups, and lack of information to control for confounding factors are common weaknesses in these occupational studies.
Changes typical of foreign body reaction, alveolar proteinosis and wall thickening, diffuse pulmonary fibrosis and interstitial emphysema, and some nodule formation but not to the extent of fibrosis caused by quartz dust were associated with occupational exposure in the aluminium industry. This was most severe in Germany during World War II, where industrial environments were heavily contaminated with airborne aluminium flake powder. Lower aluminium exposures contribute to Shaver’s disease, a pulmonary fibrosis seen in workers in bauxite refining or exposed to finely divided aluminium powders; and caused pneumoconiosis, fibrosis, and some cases of asthma.
Only one case-control study examined associations between genotype and the development of asthma for workers employed in a potroom. However this study with very low power did not find any association.
No reliable epidemiological studies exist to reach any conclusion on an association between occupational exposure to aluminium and fertility or developmental effects. No clear results have been obtained on gene-environment interactions.
Non-occupational exposure
The neurotoxic properties of aluminium are well established; however, the evidence surrounding the potential association between aluminium and neurological disorders in humans is much less clear. Aluminium exposure from drinking water has been extensively investigated in relation to the development of neurological disorders, including AD, due to the proposed enhanced bioavailability of aluminium in this form. The data surrounding this association is difficult to interpret due to the large variation in study designs and the highly variable quality of these studies. The majority, but not all, of epidemiological studies identified, reported a positive association between aluminium levels in drinking water and risk of cognitive impairment dementia, or AD. There is some evidence to suggest silica in drinking water is protective against the development of dementia. Fluoride has also been identified as having a potential protective effect. Many of the studies which have investigated the relationship between aluminium in drinking water supplies and the risk of developing AD are limited by methodological issues. These issues include: lack of individual exposure information, poor disease ascertainment, failure to adjust for important confounding factors, and small sample sizes. A recent study conducted in France is methodologically superior to the other studies conducted to date. The finding of a significant positive relationship between drinking water aluminium levels and the development of AD in this large prospective study, together with the finding of a positive relationship in a number of less methodologically sound studies, suggests that the association between aluminium and AD should be further investigated.
Regular consumers of antacids represent a unique subpopulation with heavy exposure to aluminium. A significantly elevated odds ratio for AD for regular antacid consumers compared to non-regular users was found; however, when only aluminium containing acids were analyzed there was no significant association. Other studies have not found a significant association between antacid use and AD. Little is known about the impact of aluminium-containing antacids in human pregnancy and lactation.
Evidence surrounding the relationship between aluminium in food and the risk of AD is very minimal. This may be a result of the difficulty in obtaining accurate exposure information in dietary studies. One small case control study found a positive relationship between the consumption of foods containing high levels of aluminium and the risk of developing AD. These results have not been confirmed in a larger investigation.
There is a large body of literature, mostly in the form of clinical reports, which documents the adverse effects of non-occupational aluminium exposure in individuals with impaired renal function. These patients are typically exposed to aluminium through dialysate fluid or medicinal sources. Anaemia, bone disease, and dialysis encephalopathy are the most commonly reported complications of aluminium exposure in this population.
Contact sensitivity to aluminium is very rare. Sensitization has occurred after injection of aluminium-adjuvant containing vaccines and pollen extracts, resulting in persistent granuloma at the injection site. These effects are much more frequent with aluminium hydroxide than aluminium phosphate adjuvants and more commonly seen following subcutaneous (s.c.) than i.m. injection. Less common is sensitivity during continuous application of aluminium-containing antiperspirants, topical aluminium application, and occupational exposure to aluminium dust and filings which result in recurrent eczema.
Only a few epidemiological studies with no clear results have been undertaken of the possible carcinogenic risks (such as breast cancer) of antiperspirants.
The exact genetic effects of Tf (a major transport protein for both iron and aluminium) itself or its interaction with aluminium remains unclear and has led to contradictory results.
As a result of inadvertent human poisoning with excessive amounts of aluminium, there are reports of damage to bone and CNS as target organs. Further, the administration of aluminium-containing vaccines for extended time periods was found to be associated with the development of MMF at the injection site. In the past, individuals with impaired renal function receiving dialysis were reported to be at greater risk for aluminium intoxication associated with contaminated replacement fluids. However, this incidence has diminished markedly in recent years with the use of non-contaminated fluid and replacement of high-dose antacid therapy with alternatives. Although infants and children may be at higher risk for toxicity due to aluminium, a causal relationship was not confirmed. Hence, it should be noted that only at excessive concentrations of aluminium are toxic manifestations seen in human sensitive subpopulations.
Conclusions
This report synthesizes data from relevant studies on potential health effects of exposure to aluminium to quantify risk using the four-step process specified by the National Research Council: 1) hazard identification, 2) exposure assessment, 3) dose-response assessment, and 4) risk characterization.
Hazard identification qualitatively identifies adverse effects by route of exposure, and determines whether those effects are likely in humans at some level of exposure, perhaps much greater than exposure levels experienced in the population of interest. It is important to note that the identification of effects that can be caused by aluminium says nothing about how likely those effects are at exposure levels in human populations. That probability depends on the level of exposure and the dose-response relationship. This report classified the weight of evidence for each exposure pathway and health effect as strong, modest, limited, or having no clear evidence (see Table 25). We concluded that there is strong evidence that aluminium can cause irritation following exposure via either inhalation or injection. Modest evidence of an effect exists for reproductive toxicity following oral exposure, for neurological toxicity following either oral or injection exposure, and for bone toxicity following injection exposure. All other effects were judged to be supported by either limited evidence or no clear evidence at all. Exposure assessment, dose-response assessment, and risk characterization were conducted for those effects for which the evidence was judged to be either strong or modest. The remainder of this section describes our findings for the general population, subpopulations at special risk, and occupationally-exposed populations.
Table 25.
Strength of evidence for health effects.
| Exposure Pathwaya | |||||
|---|---|---|---|---|---|
| Health Endpointb | Inhalation | Oral | Dermal | Injection | |
| 1 | Acute toxicity | ||||
| 2 | Irritation | Strong | Limited | Limited | Strong |
| 3 | Corrosivity | ||||
| 4 | Sensitization | ||||
| 5 | Repeated dose toxicity | ||||
| 6 | Mutagenicity | Limited | Limited | ||
| 7 | Carcinogenicity | No clear evidence | No clear evidence | ||
| 8 | Reproductive toxicity | Limited | Modest | No clear evidence | |
| 9a | Other – Neurological Toxicity | Limited | Modest | Modest | |
| 9b | Other – Bone Toxicity | No clear evidence | Modest | ||
| 9c | Other – Metabolism | Limited | Limited | ||
The absence of an entry indicates that, effectively, there are no data for the exposure pathway / toxicity endpoint combination.
Health endpoint categories are taken from European Commission (2003).
General population
Exposure assessment quantified aluminium intake and uptake (i.e., absorption of aluminium into systemic circulation) for a variety of pathways (see Table 26). For the general population, intake of aluminium from food (7.2 mg/day for females and 8.6 mg/day for males) dominated that from drinking water (0.16 mg/day) and inhalation exposure (0.06 mg/day). Antacids and buffered aspirin can contribute on the order of thousands of mg/day to aluminium intake. Relative contributions to uptake are ranked similarly to these intake contributions. However, because inhaled aluminium is approximately seven times more bioavailable than aluminium in drinking water, the contribution of inhaled aluminium to uptake (1.7 × 10-5 mg/kg b.w./day) exceeds the corresponding contribution from drinking water (6.9 × 10-6 mg/kg b.w./day). Uptake of aluminium in food is approximately 1 × 10-4 mg/kg b.w./day. Aluminium uptakes from antacids and buffered aspirin amount to 3.1 × 10-1 and 4.3 × 10-2 mg/kg b.w./day, respectively.
Table 26.
Intake and uptake of aluminium.
| Source | Concentration in mediuma | Daily intake mg/daya,b | Bodyweight normalized intake mg/kg b.w/dayc | Bioavailabilitya,b % | Bodyweight normalized uptake (mg/kg b.w./day) |
|---|---|---|---|---|---|
| Food – females | 10 to 400 μg/g food | 7.2 | 0.10 | 0.1 | 1.0 × 10-4 |
| Food – males | 8.6 | 0.12 | 0.1 | 1.2 × 10-4 | |
| Drinking waterd | 100 μg/L | 0.16 | 0.0023 | 0.3 | 6.9 × 10-6 |
| Ambient air – general population | 0.6-7.0 μg/m3 (PM10) | 0.06 | 0.00086 | 2.0 | 1.7 × 10-5 |
| Ambient air – occupational exposure | 1 to 6 mg/m3 “total” | 21 | 0.3 | 2.0 | 6.0 × 10-3 |
| Antacids | 12 to 32 g/L; or 110 to 174 mg/tablet | 7,200 | 100 | 0.3 | 3.1 × 10-1 |
| Buffered aspirin | Not available | 1,000 | 14 | 0.3 | 4.3 × 10-2 |
| Anti-perspirantse | 25% by weight | ? | ? | ? | ? |
Relevant exposure levels of concern for the general population identified as part of dose response assessment included: irritation following inhalation (50 mg/m3), neurological effects due to drinking water exposure (100 μg aluminium/L water), reproductive toxicity due to oral intake (400 mg/kg-b.w./day), and irritation following injection (1 injection). We characterized risk (see Table 27) by calculating a margin of exposure, or MOE (the exposure level of concern divided by actual exposure), for each of these pathway-endpoint combinations. The MOE values were large for local irritation following inhalation (7000) and reproductive toxicity associated with oral intake (2900). For irritation following injection, the MOE is less than unity, although the severity of this endpoint is limited. For neurological effects associated with drinking water exposure, the MOE may be as small as unity. The evidence supporting this effect, however, comes from studies that have a number of methodological limitations, a finding that suggests the causal nature of the association is uncertain.
Table 27.
Comparison between effects at which aluminium induces adverse health effects and levels of human exposure.
| General Population | Occupational Population | ||||
|---|---|---|---|---|---|
| Pathway and Endpoint | Exposure Level of Concern | Exposure | MOEa | Exposure | MOEa |
| Inhalation | |||||
| Irritation | 50 mg/m3b | 0.007 mg/m3 | 7000 | 6 mg/m3 | 8 |
| Oral | |||||
| Neurological (AD) | ≥100 μg/L waterc | 100 μg/L | ≥1 | N/A | |
| Reproductive Toxicity | 400 mg/kg b.w./dayd | 0.14 mg/kg b.w./daye | 2900 | N/A | |
| Injection | |||||
| Irritation | One injectionf | < 1 | N/A | ||
| Neurological | One injectionf | Large | N/A | ||
| Bone toxicity | Largeg | Large | N/A | ||
The MOE is the ratio of the exposure level of concern to the exposure level. These values are rounded to one significant figure.
Based on the occupational cohort studies described in Evaluation of Human Health Risks, Health Effects, Dose Response, Inhalation Exposure.
Based on the findings of the Rondeau et al. (2000) study.
At the reproductive exposure level of concern (400 mg/kg/day), frank toxic effects have been noted; it is possible that other perhaps less severe effects will be observed at lower exposure levels in the future. In this case, the MOE would be decreased.
Estimated intake of aluminium in food is 8.6 mg/day for males and 7.2 mg/day for females 14 years and older (see Table 16 and accompanying text) and using 60 kg as the body mass.
The amount of aluminium in one injection is not known. However, a single injection has been shown to be sufficient to cause irritation due to aluminium, hence resulting in a MOE value less than 1.0. On the other hand, for injections with typical aluminium content, neurological effects have only been observed following daily injections lasting for many months. We are unaware of members of the general population who would have to receive injections containing aluminium for this duration. Hence, the MOE is “large.” We recognize that in some highly unusual circumstances (e.g., contamination of dialysis fluid, which in the past was more common, or contamination of i.v. fluid), the possibility for aluminium toxicity as a result of exposure via injection remains.
Bone toxicity has been identified in dialysis patients, possibly as a result of aluminium contamination of dialysis solution administered i.v. or via i.p. injection, and/or the use of aluminium phosphate binders. In any case, the level of exposure in this circumstance far exceeds the exposure that might be associated with a typical injection (e.g., for vaccination).
Subpopulations at special risk
Individuals with impaired renal function do not clear aluminium as effectively as healthy individuals. This population can also be exposed to extremely high levels of aluminium that are administered inadvertently via their intravenous feeds. This route of exposure may be particularly significant because it bypasses the barrier imposed by GI absorption characteristics. Infants, especially those born pre-term, are also vulnerable to aluminium exposure due to immaturity of the GI wall, the BBB, and the renal system. In addition to their added susceptibility due to compromised renal function, patients on dialysis may be subject to higher aluminium exposure levels if dialysis or intravenous fluid becomes contaminated, a problem that was more common in the past. Although not explicitly quantified, the susceptibility of these populations suggests that the exposure level of concern is less than it is for the general population. At the same time, some sensitive populations may have been exposed to very high aluminium exposures in the past. Because of the substantial quantities of injected fluids received by dialysis patients and their increased susceptibility, the MOE for this pathway for this population may be less than unity.
Occupationally-exposed populations
Occupational populations can be exposed to airborne concentrations of aluminium exceeding concentrations to which the general population is exposed by approximately three orders of magnitude (see Table 26). Aluminium intake resulting from these exposures is estimated to be 21 mg/day, compared to 0.06 mg/day for the general population, with uptake for occupationally exposed individuals amounting to 6 × 10-3 mg/kg b.w./day, compared to 1.7 × 10-5 for the general population (Table 26). The resulting margin of exposure for occupationally exposed populations is approximately 8, compared to 7000 for general population exposure to airborne aluminium (see Table 27).
Research Needs
The following research needs were identified as important research requirements to further improve risk assessments on aluminium:
Studies should be conducted to quantify peak and cumulative air-borne aluminium exposure of workers in the aluminium industry and to characterize aluminium-containing aerosols in terms of particle composition and size. Concomitant assessments of the bioavailability of the inhaled aerosols are crucial.
In many occupational studies of aluminium workers, it was not known whether respiratory tract illness was due to exposure to aluminium or other substances. There have been very few studies of neurological effects of occupational exposure via inhalation to aluminium and aluminium compounds (as measured in serum), and it is not known if the very specific neurological deficits observed lead to more severe illness such as AD. Therefore, large-scale, longitudinal, studies of occupational exposure to aluminium and aluminium compounds via inhalation, with precise methods of exposure measurement, are needed to assess the risks of respiratory tract disease and neurological effects due to aluminium and aluminium compounds.
Further studies are needed to settle the debate over the link between aluminium and aluminium in drinking water and neurological disorders and congnitive impairment. Ideally, individual level data on drinking water exposure as well as other relevant risk factors would be obtained; in the absence of this, replication of the Rondeau et al. (2000) analysis in other study populations, with the ability to control for important confounders and effect modifiers, is needed to assess this potential risk.
IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
Identity
The focus of this document is on aluminium metal, aluminium oxide and aluminium hydroxide; however, in order to more fully understand their toxicity and related human health effects, other pertinent studies involving aluminium compounds were reviewed. The basis for this is that the chemistry and biochemistry of the aluminium ion (Al3+) dominate the pathways that lead to toxic outcomes. Most aluminium compounds currently used in industry, pharmaceuticals, food additives, cosmetics and other household products are identified in this section (see Tables 1 and 2). Many of the compounds listed in these tables have been studied in health-related research and are featured in the critical assessments detailed in subsequent sections of this risk assessment document.
Table 1.
| Compound[CAS No]c | Chemical Formula | Common Synonyms |
|---|---|---|
| Aluminium [7429-90-5] | Al | - |
| Aluminium alkyls | [R3Al]2, R3Al2X3, [R2AlX]2, [RAlX2]2, [RnAlX3-n]2 with R=alkyl groups and X=halides | Trialkylaluminium compounds; alkylaluminium halides |
| Aluminium alkoxides [555-75-9; ethoxide] [556-91-2; tert-butoxide] [555-31-7; isopropoxide] |
Al(OR)3 R=alkyl group | Aluminium t-alkoxides |
| Aluminium antimonide [25152-52-7] | AlSb | - |
| Aluminium basic acetate [142-03-0] [8000-61-1] | Al(OH)(CH3CO2−)2 | Aluminium bis(acetato-0) hydroxy; aluminium diacetate |
| Aluminium borate [11121-16-7] | Al2O3•B2O3 | Mineral: eremeyevite or jeremejevite |
| Aluminium borohydride [16962-07-5] | Al(BH4)3 | - |
| Aluminium bromide [7727-15-3] | Al(Br)3 | Aluminium tribromide |
| Aluminium calcium hydride [16941-10-9] | Ca(AlH4−)2 | - |
| Aluminium carbide [1299-86-1] | Al4C3 | - |
| Aluminium chlorate [15477-33-5] | Al(ClO3−)3 | - |
| Aluminium chloride [7446-70-0] | AlCl3 | Aluminium trichloride; trichloroaluminium |
| Aluminium chloride hexahydrate [7748-13-6] | AlCl3•6H2O | Hydrated aluminium chloride |
| Aluminium fatty-acid salts: [688-37-9], oleate; [555-35-1], palmitate; [637-12-7], stearate; [645-17-0], linoleate |
Al(FA)3 | Fatty acid (FA), aluminium salt |
| Aluminium fluoride [7784-18-1] | AlF3 | Aluminium trifluoride |
| Aluminium hexafluorosilicate [17099-70-6] | Al2(SiF6)3 | Aluminium flurosilicate; aluminium silicofluoride |
| Aluminium hydride [7784-21-6] | AlH3 | - |
| Aluminium hydroxide [21645-51-2] | Al(OH)3 | Aldrox; alumina hydrate; gibbsite |
| Aluminium hypophosphite [7784-22-7] | Al(H2PO2−)3 | - |
| Aluminium iodide [7784-23-8] | AlI3 | Aluminium triiodide |
| Aluminium lactate [18917-91-4] | Al[CH3(OH)CO2−]3 | Aluctyl |
| Aluminium lithium hydride [16853-85-3] | Li(AlH4−) | Lithium aluminium hydride; lithium tetrahydroaluminate |
| Aluminium magnesium silicate [12511-31-8] | MgAl2(SiO44−)2 | Magnesium aluminium silicate; colerainite and other mineral forms |
| Aluminium nitrate [13473-90-0] | Al(NO3−)3 | - |
| Aluminium nitride [24304-00-5] | AlN | - |
| Aluminium oxalate [814-87-9] | Al2(C2O42−)3 | Aluminium salt |
| Aluminium oxide [1344-28-1] | Al2O3 | Aloxite; alumina; α-alumina (corundum) |
| Aluminium phosphate [7784-30-7] | Al(PO4) | Aluminium orthophosphate; phosphoric acid, aluminium salt |
| Aluminium phosphide [20859-73-8] | AlP | Aluminium monophosphide; trade names: celphos, phostoxin, quickphos |
| Aluminium potassium sulphate [10043-67-1] | KAl(SO42−)2 | Alum |
| Aluminium potassium sulphate dodecahydrate [7784-24-9] | KAl(SO42−)2•12H2O | Potassium alum |
| Aluminium selenide [7784-24-9] | Al2Se3 | - |
| Aluminium silicate [12141-46-7] | Al2SiO5•nH2O | Aluminium silicate n-hydrate |
| Aluminium sodium sulphate [10102-71-3] | NaAl(SO42−)2 | Sodium alum; soda alum |
| Aluminium sodium sulphate dodecahydrate [10102-71-3] | NaAl(SO42−)2•12H2O | Hydrated sodium alum |
| Aluminium sulphate [10043-01-3] | Al2(SO42−)3 | Sulphuric acid, aluminium salt; cake alum |
| Aluminium sulphate octadecahydrate [7784-31-8] | Al2(SO42−)3•18H2O | |
| Aluminium sulphide [1302-81-4] | Al2S3 | - |
| Aluminium tartrate [815-78-1] | Al(C4H4O6−)3 | - |
| Aluminium terachloroaluminate [7784-16-9] | NaAlCl4 | Sodium chloroaluminate |
| Aluminium thiocyanite [538-17-0] | Al(CNS)3 | - |
| Ammonium hexafluoroaluminate [7784-19-2] | (NH4+)3(AlF63−) | Ammonium cryolite; ammonium aluminium fluoride |
| Ammonium tetrachloroaluminate [7784-14-7] | (NH4+)(AlCl4−) | Aluminium ammonium chloride; ammonium chloroaluminate |
| Calcium aluminosilicate [1327-39-5] | CaAl2S2O8, Ca2Al2SiO7 | - |
| Cryolite [15096-52-3] | Na3(AlF63−) | Sodium aluminium fluoride; trisodium hexafluoroaluminate (3-) |
| Dihydrobis (2-methoxyethanolate-O,O) aluminate (1-) sodium [22722-98-1] | NaAlH2(C3H7O2−)2 | Sodium bis(methoxyethoxy) aluminium hydride; vitride (R) T reducing agent |
| Fosetyl aluminium [39148-24-8] | Al(CH3OPHO2−)3 | Aluminium tris(ethyl hydrogen phosphonate); phosphonic acid, monoethyl ester; efosite aluminium |
| Hydrated magnesium-aluminium-iron silicate [1318-00-9] | - | Vermiculite |
| Indium gallium aluminium phosphide [108424-49-3; 108730-13-8] | InGaAlP | - |
| Potassium aluminate [1302-63-2] | K2Al2O4 | Aluminium potassium oxide |
| Quanidenium aluminium sulphate hexahydrate [10199-21-0] | N3H6+ [Al(SO42−)2]•6H2O | - |
| Sodium aluminate [1302-42-7] | NaAlO2 | Aluminium sodium dioxide |
| Tris(8-hydroxyquinoline) aluminium [2085-33-8] | Al(C9H6NO−)3 | Aluminium tris(8-hydroxyquinoline) |
Major sources: Lie (1990/1991); The Merck Index (2001); Office of the Federal Register (2003); ChemFinder.com (http://chemfinder.cambridgesoft.com).
See Table 2 for non-industrial compounds.
Chemical Abstracts Service (CAS)
Table 2.
Chemical identity of aluminium and its compounds used in pharmaceuticals, food additives, cosmetics and household productsa, b.
| Compound [CAS No] | Chemical Formula | Common Synonyms |
|---|---|---|
| Acetylglycerrhetinic acid, aluminium salt [29728-34-5] | AlC96H141O15 | Glycyrrhetic acid, aluminium salt; almacet |
| Aluminium [7429-90-5] | Al | Aluminium powder or foil |
| Aluminium acetate [8006-13-1] | Al(CH3CO2−)3 | - |
| Aluminium ammonium sulphate [7784-25-0] anhydrous [7784-26-1] dodecahydrate |
NH4Al(SO42−)2 NH4Al(SO42)2•12H20 |
Alum, ammonium |
| Aluminium basic acetate [142-03-0] [8000-61-1] | Al(OH)(CH3CO2−)2 | Aluminium subacetate; bis(acetato-O) hydroxyaluminium |
| Aluminium benzoate [555-32-8] | Al(C7H6O2−)3 | Aluminium tribenzoate |
| Aluminium bis(acetylsalicylate) [23413-80-1] | Al(OH)(C9H7O4−)2 | Aluminium diaspirin |
| Aluminium bromohydrate [39431-98-6] | Al2(OH)5Br | Dialuminium bromide pentahydroxide |
| Aluminium butyrate [2269-22-9] | Al(C4H9O−)3 | Aluminium sec-butoxide |
| Aluminium carbonate basic [1339-92-0] | Al(OH)(CO32−) | Basic aluminium carbonate |
| Aluminium chloride hexahydrate [7748-13-6] | AlCl3•6H2O | Hydrated aluminium chloride |
| Aluminium chlorohydrex [53026-85-0] [68953-68-4] | - | Aluminium chlorohydroxy propyleneglycol complexes |
| Aluminium citrate [31142-56-0] | (NH4+)5[Al3(H-1Cit)3(OH)(H2O)[NO3−]•6H2O | |
| Aluminium di(2-ethylhexoate) [1336-25-0] | Al(OH)(C7H14CO22−)2 | 2-Ethylexanoic acid aluminium salt |
| Aluminium fatty-acid saltsc | Al(FA)3 | FA, aluminium salt |
| Aluminium glycinate [13682-92-3] | Al(OH)(CH2NH2CO22−) | Dihydroxy aluminium aminoacetate |
| Aluminium hexaurea sulphate triiodide [15304-14-0] | Al[CO(NH2)2]6[SO4I3] | - |
| Aluminium hydroxide [21645-51-2] | Al(OH)3 | Alumina hydrate |
| Aluminium hydroxychloride [1327-41-9] | Al2(OH)5Cl•2H20 | Aluminium chlorhydroxide; basic aluminium chloride |
| Aluminium lactate [18917-91-4] | Al[CH3(OH)CO2−]3 | Aluctyl |
| Aluminium magnesium silicate [12511-31-8] | MgAl2(SiO44−)2 | Magnesium alumino-silicate |
| Aluminium metal silicates Ca [1327-39-5]; Na [1344-00-9]; Na and Ca [1344-01-0] | Na12[(AlO2)12(SiO2)12]•27H2O (sodium) | - |
| Aluminium methanedisulphonate [52667-15-9] | Al2(CH2S2O62−)3 | Methionic acid, aluminium salt |
| Aluminium nicotinate | - | Nicalex |
| Aluminium nitrate [13473-90-0] | Al(NO3-)3 | - |
| Aluminium phenolsulphonate [1300-35-2] | Al(C6H5OSO3−)3 | Aluminium tris(hydroxybenzene-sulphonate) |
| Aluminium phosphate [7784-30-7] | Al(PO4) | Aluminium orthophosphate; phosphoric acid, aluminium salt |
| Aluminium potassium silicate [1327-44-2] anhydrous | Al2O3K2O•6SiO2 | Potassium aluminium silicate |
| [12001-26-2] hydrated | KAl2(AlSiO3O10)(OH)2 | Mica; soapstone |
| Aluminium potassium sulphate [10043-67-1] | KAl(SO42−)2 | Potassium alum |
| Aluminium potassium sulphate dodecahydrate [7784-24-9] | KAl(SO42−)2•12H2O | |
| Aluminium silicate [12141-46-7] anhydrous | Al2SiO5 | Sillimanite; andalusite |
| [1332-58-7] hydrated | Al2O3SiO2•2H2O | China clay; kaolin |
| Aluminium sodium carbonate hexitol complex | - | Alexitol sodium; sodium polyhydroxoxyaluminium monocarbonate hexitol complex |
| Aluminium sodium sulphate [10102-71-3] | NaAl(SO42−)2 | Sodium alum; soda alum |
| Aluminium sodium sulphate dodecahydrate [10102-71-3] | NaAl(SO42−)2•12H2O | |
| Aluminium sulphate [10043-01-3] | Al2(SO42−)3 | Sulphuric acid, aluminium salt; Cake alum; anti-infective |
| Aluminium sulphate octadecahydrate [7784-31-8] | Al2(SO42−)3•18H2O | |
| Basic aluminium clofibrate [24818-79-9] | Al(OH)(C9H10ClOHCO2−)2 | Aluminium 2-(4-chlorophenoxy)-2-methylpropanate |
| Basic aluminium salicylates [not available] | (C7H5O3)nAl(OH)3-n•xH20 | Aluminium salicylates, basic |
| Basic aluminium-magnesium carbonate tetrahydrate [66827-12-1] | Al2Mg6(OH)14(CO3)2•4H20 | Almagate; almax |
| Basic aluminium-magnesium sulphate dihydrate [74978-16-8] | Al5Mg10(OH)31(SO4)2•2H20 | Magaldrate; magnesium aluminate hydrate |
| Basic sodium aluminium phosphate [7785-88-8] | Na8Al2(OH)2(PO4)4 | Kasal phosphate |
| Bismuth aluminate [12284-76-3] | Bi2(Al2O4)3 | Aluminium bismuth oxide |
| Dihydroxy aluminium sodium carbonate [539-68-4] | NaAl(OH)2(CO32−) | [Carbonato(1)-O]di-hydroxyaluminium monosodium salt; Aluminium sodium carbonate hydroxide |
| Dihydroxyaluminium allentoinate [5579-81-7] | Al(OH)2[C4H5N4O4−] | Aldoxia |
| Dihydroxyaluminium acetylsalicylate [53230-06-1] | Al(OH)2(C9H7O4−) | Dihydroxyaluminium aspirin |
| Polyhydroxyaluminium acetylsalicylate [9014-67-9] | Al2O3[C6H5OCOCH3−]5 | Aloxiprin |
| Sodium aluminium chlorohydroxy lactate [97660-24-7] [8038-93-5] | - | Aluminium chlorohydroxy lactate sodium complexes |
| Sucrose octakissulphate aluminium salt [54182-58-0] | R-(CH2OSO3−)8[Al2(OH)5+ ]8 R = sucrose |
Sucralfate |
Major sources: Lie (1990/1991); The Merck Index (2001); Office of the Federal Register (2003); ChemFinder.com (http://chemfinder.cambridgesoft.com).
See Table 1 for list of compounds used industrially
Some specific FA are identified in Table 1
Tables 1 and 2 indicate that the primary identification of aluminium compounds is by the CAS Registry Number. Other numbering systems are not as widely accepted and are thus not as useful. For example, European Inventory of Existing Commercial Substances (EINECS) numbers are available for aluminium (013-001-00-6), aluminium oxide (215-691-6) and aluminium hydroxide (244-492-7) through the International Uniform Chemical Information Database. However, most of the chemicals listed in Tables 1 and 2 are indicated as not having been assigned such a number (ESIS, 2007). Exceptions are those compounds that exhibit high toxicity or are widely used, such as aluminium phosphide (EINECS # 015-004-00-8) and cryolite (15096-52-3). Note that for the three substances that form the focus for this review, the common names assigned in the tables are the same as the EINECS names.
Purity/Impurities, Additives
Most of the substances listed in Tables 1 and 2 are generally available in high purity and thus impurities are not an issue from a risk assessment perspective. However, it is clear that for many of the aluminium compounds, the degree of hydration can vary. Recently, the presence of a thin surface coating of ultrafine particles of sodium fluoride on aluminium oxide particulates has been demonstrated for aerosols collected in an aluminium refinery (Höflich et al., 2005; L’vov et al., 2005).
Physical and Chemical Properties
Properties of aluminium metal
Aluminium is a ubiquitous element in nature and as the metal that has gained industrial and commercial use based upon certain physical and chemical properties such as low specific gravity, high tensile strength, ductility, malleability, reflectivity, corrosion resistance, and high electrical conductivity. Aluminium alloys are light, strong and readily machined into shapes (IPCS,1997; see Sources of Human Exposure, Anthropogenic Sources, Uses for listings of industrial and non-industrial uses).
In spite of aluminium being highly electropositive (i.e., readily forming positive ions), it is resistant to corrosion because of the formation of a hard, tough surface film of its oxide (Cotton & Wilkinson, 1980). Fresh aluminium surfaces achieve this by reacting with water or molecular oxygen. Hydrothermal oxidation of aluminium powders at 150-250°C and water vapour pressures of 500-4500 kPa suggest that surface-adsorbed water oxidizes the aluminium with the release of molecular hydrogen and the formation of aluminium hydroxyoxides on the particle surface (Tikhov et al., 2003). Similarly, thermogravimetric studies of aluminium powders have shown that oxidation with molecular oxygen generates surface layers composed of various aluminium oxide polymorphs, specifically the γ-, θ-, and α- forms depending on the temperature (Trunov et al., 2005) (see also Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation). Furthermore, aluminium metal is soluble in dilute mineral acids, but is inactivated (passivated) by concentrated nitric acid; it is attacked by hot alkali hydroxides (Cotton & Wilkinson, 1980).
Properties of aluminium compounds
Table 3 summarizes the available physico-chemical properties of the compounds. Most of the aluminium compounds are solids exhibiting high melting points; some are liquids. No gaseous substances were identified. Only a few of the compounds sublimate, namely anhydrous aluminium chloride and fluoride, aluminium nitride and sulphide, as well as the complex with 8-hydroxyquinoline. Most of the substances are white or colourless.
Table 3.
| Compound [CAS RN] | Molecular Mass | Physical State | Melting Point (°C) | Boiling Point (°C) | Density (in g) | Solubility (at °C) | Comments | |
|---|---|---|---|---|---|---|---|---|
| Water (g/l) | Organic Solvents | |||||||
| Acetylglycerrhetinic acid, aluminium salt [29728-34-5] | 1562.12 | wh pwdr | 286-290 | - | - | insol | sol | - |
| Aluminium [7429-90-5] | 26.98 | silvery-wh metal; malleable, ductile | 660.1 | 2327 | 2.70 | insol | insol | water and air sensitive; dissolves in alkali or acid |
| Aluminium acetylsalicylate [23413-80-1] | 402.29 | amp wh pwdr, granules | - | - | - | insol | sol | dissolves in alkali or acid |
| Aluminium alkyls | variable | col liquids | 6 (triisobutyl) | 194 (1 atm) (triethyl) | 0.832 (triethyl) | d | sol (anhydr) | may self-ignite in air |
| Alkylaluminium halides | variable | low-melting solids or col liquids | 32 (dichloroethyl) | 127 (0.066 atm) (chlorodiethyl) | 0.961 (chlorodiethyl) | d | sol (anhydr) | sensitive to air |
| Aluminium alkoxides[555-75-9;ethoxide] [556-91-2;tert-butoxide] [555-31-7;isopropoxide] |
162.16 (ethoxide) | wh cryst (ethoxide) | 140 (ethoxide) | 200 (0.01 atm) (ethoxide) | 1.142 (ethoxide) | d | sol (anhydr) | some analogs sublime (tert-butoxide) |
| Aluminium antimonide [25152-52-7] | 148.74 | solid | 1050 | - | - | d | insol (exptd) | has electrical properties |
| Aluminium basic acetate [142-03-0] [8000-61-1] |
162.08 | wh amorph pwdr | - | - | - | sol (undried) insol (dried) | insol (exptd) | - |
| Aluminium benzoate [555-32-8] | 390.32 | wh cryst pwdr | - | - | - | v sl. sol | - | - |
| Aluminium borate [11121-16-7] | variable | needles | 1050-1440 | - | - | insol | insol (exptd) | occurs as mineral in nature |
| Aluminium borohydride [16962-07-5] | 71.51 | liquid | -64.5 | 44.5 | - | d | - | reacts vigorously with water or acid liberating hydrogen; ignites in air |
| Aluminium bromide [7727-15-3] | 266.69 | wh-yl-rd hygrosc lumps | 97 | 250-270 | 3.21 | d | sol | fumes strongly in air; reacts violently with water |
| Aluminium calcium hydride [16941-10-9] | 102.11 | slate-grey mass | - | - | - | d | generally insol (anhydr) | reacts vigorously with water or acid liberating hydrogen |
| Aluminium carbide [1299-86-1] | 143.96 | yl/gr hexagonal cryst/pwdr | 2100 | d ≥ 2200 | 2.36 | d | insol | releases methane with water |
| Aluminium chlorate [15477-33-5] anhydr [7784-15-8] nonahydr | 277.34 439.47 | deliq cryst (nonahydr) | d | - | - | v sol | - | releases ClO2 |
| Aluminium chloride [7446-70-0] | 133.34 | wh/col pwdr | 190 (2.5 atm) | subl 177.8 | 2.44 | 7 | sol (anhydr) | reacts violently with water; strong irritant |
| Aluminium chloride hexahydrate [7748-13-6] | 241.43 | col/wh | d 100 | - | 2.40 | sol | sol (alcs) | deliq |
| Aluminium fatty-acid salts: [688-37-9],oleate; [555-35-1], palmitate; [637-12-7], stearate; [645-17-0], linoleate |
877.41 (stearate) | wh/yl pwdr or mass | 103 (stearate) | - | 1.010 (stearate) | insol | sol | - |
| Aluminium fluoride [7784-18-1] | 83.98 | hexag cryst | 250 | subl 1272 | 2.88 | 5.6 | insol | hydrolyzed by steam |
| Aluminium glycenate [13682-92-3] | 135.05 | fine pwdr | - | - | - | insol | - | forms suspension in water |
| Aluminium hexafluorosilicate [17099-70-6] | 480.19 | hexag cryst (nonahydr) | d > 1000 (nonahydr) | - | - | sol (nonahydr) | insol (exptd) | minerals (topaz: Al2SiO4(OH,F)2) |
| Aluminium hydroxide [21645-51-2] | 78.00 | wh amorph pwdr | d 300 (-H2O) | - | 2.42 | insol | insol | absorbs CO2 |
| Aluminium hypophosphite [7784-22-7] | 221.95 | cryst pwdr | d 220 | - | - | insol | insol (exptd) | releases phosphine on heating |
| Aluminium iodide [7784-23-8] | 407.70 | wh leaflets | 191 | 360 | 3.98 | d | sol | fumes in moist air; reacts strongly with water; hexhydrate is water sol |
| Aluminium lactate [18917-91-4] | 294.19 | wh/yl pwdr | > 300 | - | - | sol | - | irritant |
| Aluminium lithium hydride [16853-85-3] | 37.95 | wh cryst pwdr, lumps | d 125 | - | 0.92 | d | sol | decomp in air; may ignite; reacts with alcohol |
| Aluminium magnesium silicate [12511-31-8] | 262.43 | - | - | - | - | insol (exptd) | insol (exptd) | mineral |
| Aluminium nitrate [13473-90-0] | 213.00 | deliq cryst (nonahydr) | 73 (nonahydr) | d 135 (nonahydr) | - | v sol | sol (alc); insol | - |
| Aluminium nitride [24304-00-5] | 40.99 | hexag wh cryst | >2200 | subl 2000 | 3.26 | d | - | gives ammonia with water |
| Aluminium oxalate [814-87-9] | 318.02 | wh pwdr | - | - | - | insol | insol | - |
| Aluminium oxide [1344-28-1] | 101.96 | wh cryst pwdr | 2015 | 2980 | 3.97 | insol | insol | mineral; hygrosc |
| Aluminium phosphate [7784-30-7] | 121.95 | wh pwdr | > 1500 | - | 2.56 | insol | insol | - |
| Aluminium phosphide [20859-73-8] | 57.96 | drk gr/drk yl cryst | > 1000 | - | 2.40 | d | - | gives phosphine with water |
| Aluminium potassium sulfate [10043-67-1] | 258.19 | wh pwdr | - | - | - | sl sol | - | hygrosc |
| Aluminium potassium sulfate dodecahydrate [7784-24-9] | 474.39 | col cryst | 92.5 | water loss: 200 (-12H2O) | 1.76 | sol | insol | - |
| Aluminium selenide [7784-24-9] | 290.84 | yl/br pwdr | - | - | 3.44 | decomp | insol (exptd) | unstable in air |
| Aluminium silicate [12141-46-7] | 162.05 | wh cryst | 1545 (ptrans) | >1545 | 3.25 | insol | insol | mineral |
| Aluminium sodium sulfate dodecahydrate [10102-71-3] | 458.28 | col cryst/ wh pwdr | 61 | - | 1.68 | sol | insol | - |
| Aluminium sulfate [10043-01-3] | 342.15 | wh cryst / pwdr | d 770 | - | 2.71 | sol | insol | - |
| Aluminium sulfate octadecahydrate [7784-31-8] | 666.43 | col cryst | d 86.5 (-H2O) | - | 1.69 | sol | insol | - |
| Aluminium sulfide [1302-81-4] | 150.16 | yl/gr lumps | 1100 | subl 1500 | 2.02 | d | insol | reaction with water gives H2S |
| Ammonium hexafluoroaluminate [7784-19-2] | 195.09 | cryst | stable ≥ 100 | - | 1.78 | sol | - | does not attack glass |
| Ammonium tetrachloroaluminate [7784-14-7] | 186.83 | - | 304 | - | - | sol | sol (ether) | - |
| Calcium aluminosilicate [1327-39-5] | 278.21 | wh cryst | 1551 | - | 2.77 | insol (exptd) | insol (exptd) | mineral form |
| [1327-39-5] | 274.20 | col cryst snow-wh | 1590 | - | 3.05 | insol (exptd) | insol (exptd) | mineral form |
| Cryolite [15096-52-3] | 209.94 | semi-opaque mass | 1000 | - | 2.95 | - | - | natural form is rd/br/bl |
| Dihydrobis (2- methoxyethanolate-0,0) aluminate (1-) sodium [22722-98-1] | 202.16 | viscous liqd | - | 396-402 d ≥ 205 | 1.04 | d | sol | flash pt 4°C |
| Fosetyl aluminium [39148-24-8] | 354.10 | wh cryst | > 300 | - | - | sol | sl sol | - |
| Potassium aluminate [1302-63-2] | 196.16 | hard lustr cryst | - | - | - | sol | insol (alc) | sol’n strongly alkaline |
| Sodium aluminate [1302-42-7] | 81.97 | wh amorph pwdr | 1650 | - | - | sol | insol (alc) | hygrosc; sol’n strongly alkaline |
| Tris(8-hydroxyquinoline) aluminium [2085-33-8] | 459.43 | lt yl complex | 330-340 | subl > 230 | - | - | - | luminesces |
Major sources: Lie (1990/1991); The Merck Index (2001); Office of the Federal Register (2003); ChemFinder.com (http://chemfinder.cambridgesoft.com); Science Citation Index Expanded
Abbreviations: alc= alcohol, amorph= amorphous, anhydr= anhydrous, bl= black, blu= blue, br=brown, col= colorless, cryst= crystal, d/decomp= decomposes, deliq= deliquescent, exptd= expected, gr= green, hexag= hexagonal, hydr= hydrate, hygrosc= hygroscopic, insol= insoluble, ptrans= phase transition, liqd= liquid, lt= light, lustr= luster, pwdr= powder, rd= red, sl.= slightly, sol= soluable, sol’n= solution, subl= sublime, v= very, wh= white, yl= yellow.
The water solubility of aluminium compounds is limited except for its salts, namely the chloride, nitrate, sulphate and chlorate (often as a corresponding hydrate). Salts of low molecular organic acids also have some water solubility (e.g., acetate, benzoate and lactate), as do salts containing aluminium anion complexes (e.g., ammonium hexafluoroaluminate and tetrachloroaluminate; sodium and potassium aluminate.) As explained in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation, pH is often a factor that can limit solubility in water. Solubility of inorganic aluminium compounds in organic solvents is limited to those which are anhydrous such as the bromides, chlorides, and iodides. Aluminium alkyls, alkyl halides, alkoxides and complexes of long-chain FAs and of high molecular mass organic ligands exhibit solubility in organic solvents.
Aluminium metal, aluminium oxide and aluminium hydroxide are nearly insoluble in water and organic solvents, while freshly prepared aluminium metal surfaces do react with water to form an inert protective coating. By contrast, powdered aluminium can react with water to yield hydrogen gas (see below Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation).
In terms of chemical reactivity, the following compounds are notable for their reactions with water: aluminium alkyls, alkyl halides, hydrides; the anhydrous halides (namely bromide, chloride and iodide); and the carbide, chlorate, nitride and phosphide. Explosive gases are released on contact with water, specifically hydrogen (H2) from the hydrides and methane (CH4) from the carbide. Release of toxic gases on hydration can also occur, that is chlorine dioxide (ClO2) from the chlorate; ammonia (NH3) from the nitride; phosphine (PH3) from the phosphide; and hydrogen sulphide (H2S) from the sulphide.
As described in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation, the Al3+ ion has a very high affinity for the hydroxide ion, even at relatively low pH values. This is consistent with the Class A (Hard) cation reactivity classification of Al3+, that is, it strongly prefers oxygen-containing organic ligands over those with nitrogen or sulphur as the donor atom. Its affinity for the halide anions increases in the order I−<Br−<Cl−≪F− (Nieboer & Fletcher, 1996; Nieboer et al., 1999; Nieboer & Richardson; 1980). This reactivity classification is consistent with the stability or instability patterns towards water outlined for the aluminium compounds listed in Tables 1-3. As a Class A (Hard) cation, the chemistry of Al3+ resembles that of Mg2+, Ca2+, Na+ and K+. In fact, it may be viewed as a super Ca2+ or Mg2+ ion (Nieboer et al., 1999; Nieboer & Richardson, 1980), thereby often inhibiting the biological roles of these essential divalent cations, for example on the surface tissues of fish gills (Reid et al., 1991; Wilkinson et al., 1993).
In biological systems, Al3+, like Mg2+ and Ca2+, seeks out carboxylate and phosphate groups linked to macromolecules (i.e., proteins, RNA and DNA) or as constituents of low-molecular-mass ligands such as amino acids, nucleotides, citrate, phytates, lactate, carbonate, phosphate and sulphate (Harris, 1992). Because of the small size of the unhydrated Al3+, it can also bond to the phenolic group of the amino acid tyrosine in proteins. Most of the Al3+ in human serum is bound to the protein Tf (see Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Transport in Blood), which is a recognized carrier of trivalent metal ions, especially Fe3+ (Barker et al., 1990; Harris, 1992; Harris et al., 1996). Involvement of tyrosine phenolate groups in the Fe3+-Tf complex is well established (DaSilva & Williams, 1991). Under certain instances, such as in its citrate complex, Al3+ can also bind to a deprotonated alcohol group (Feng et al., 1990).
Chemical and morphological speciation
Formally, the definition of elemental speciation is limited to a chemical perspective; thus a chemical species is defined as: “a specific form of a chemical element, such as a molecular or complex structure or oxidation state” (Caruso et al., 2003; Templeton et al., 2000). However, Nieboer et al. (1999; 2005) subscribe to a broader working definition of “speciation”, that is: “an interdisciplinary field of activity concerned with all dimensions of the occurrence and measurement of an element in separately identifiable forms (i.e., chemical, physical or morphological)”. The former and more restrictive definition is employed below for the solution chemistry of Al3+, while the latter is helpful when considering the reactivity of aluminium oxide and aluminium hydroxide solids.
The strong dependence of Al3+ speciation on pH is illustrated by typical distribution curves in Figures 1 and 2.
Figure 1.
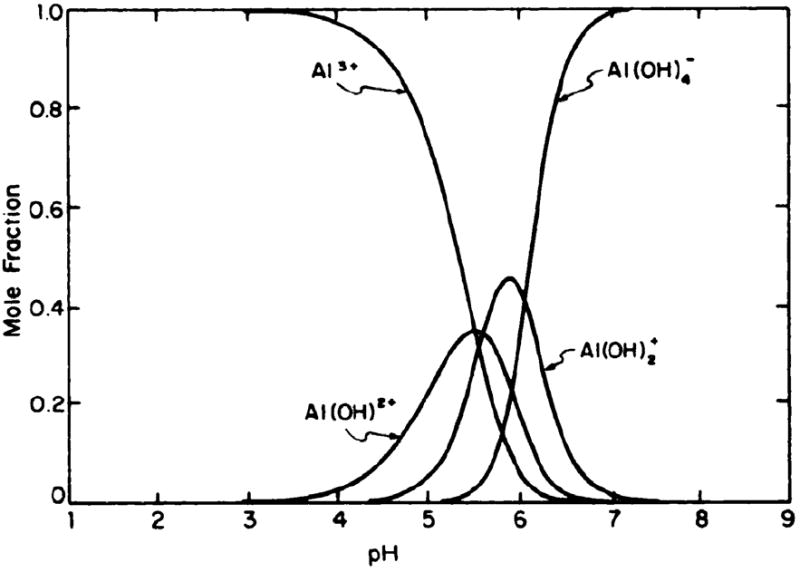
Distribution of soluble, mononuclear aluminium ion species in aqueous solutions. Ordinate: mole fraction of aluminium ion occurring as each designated species. At any pH the individual mole fractions sum to unity. Reprinted with permission from Martin (1986). Copyright 1986 American Association for Clinical Chemistry.
Figure 2.
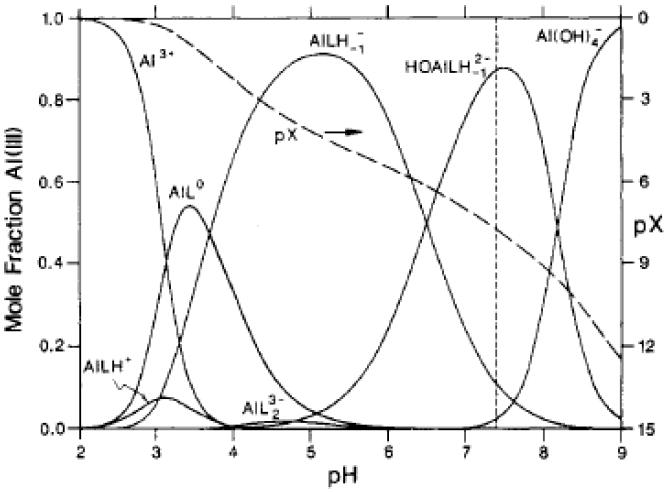
Mole fraction of Al(III) v.s. pH distribution curves (solids) for a solution containing 0.1 mM citrate, 3 mM Ca2+, and 1 pM total Al(III). The distribution is only very weakly dependent on the concentrations of the three components. The dashed line labelled pX refers to the scale on the right, where pX= -log(mole fraction of free Al3+). Thus we have, at pH 7.4, pX = 7.7. Since we also have pAl = pX - log [Al(III)], for 1 μM total Al(III), pAl = 7.4 + 6.0 = 13.7. Reprinted with permission from Martin (1994). Copyright 1994 American Chemical Society. Note that LH denotes the citrate tri-anion, with all three of its three carboxylate groups deprotonated; LH-1 denotes the tetra-anion of citrate, which involves a further loss of a proton from its lone alcohol functional group. Also pAl= -log (concentration of free Al3+) and Al(III) represents Al3+ in all of its forms.
From Figure 1, it is clear that, at physiological pH of 7.4, little or no free (hydrated) Al3+ exists in aqueous solution; the anion Al(OH)4− predominates. The distribution curve in Figure 2 illustrates the competition between hydroxide and citrate as ligand molecules. Under the conditions indicated in the legend to Figure 2, the competition with the OH− is suppressed by the citrate tetra-anion. At physiologic pH, the AlLH-1(OH)2- complex dominates.
The formation of aluminium fluoride complexes in fluoridated drinking water has been debated extensively. Fluoridation of municipal drinking water supplies is a common practice for the prevention of dental caries; fluoride is added at a concentration of around 50 μmol/L (1 mg/L), corresponding to a pF (that is a −log[F]) of 4.3. Further, the pH of municipal water supplies is typically 8.0 ± 0.4 (e.g., Nieboer et al., 1995). Consequently, and with reference to Figure 3, the distribution curves depicted for pH 7.5 are the most relevant. Thus, again, Al(OH)4− is the dominant form in which Al3+ occurs, with little evidence for complexation with the fluoride ion.
Figure 3.
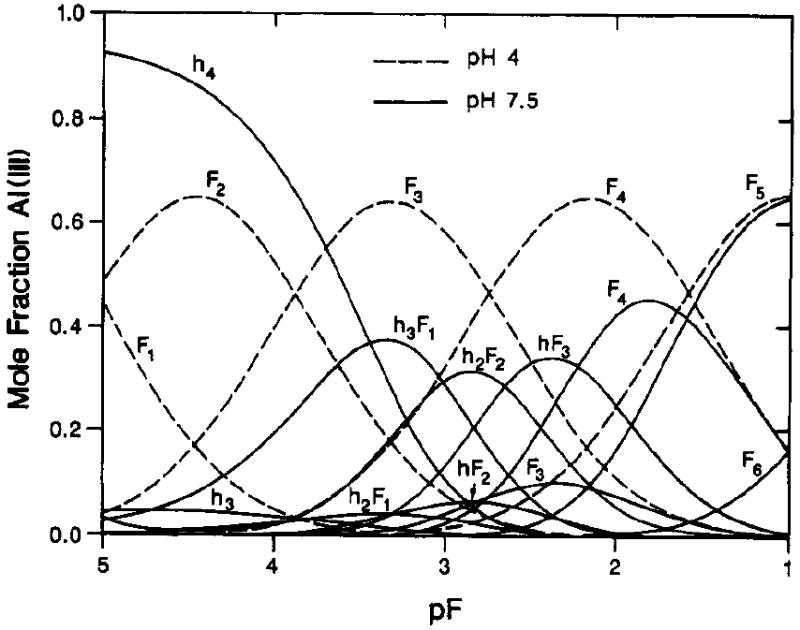
Mole fraction of total Al(II1) v.s. pF = -log [F-], where [F-] is the ambient fluoride molar concentration; for fluoride complexes of aluminium at two pH values, dashed curves for pH 4 and solid curves for pH 7.5. Symbols on curves designate number of fluoride (F) or hydroxy groups (h) bound to Al(III). Thus h4 represents Al(OH)4-, F4, AlF4-, and hF3, (HO)AlF3-. Reprinted with permission from Martin (1994). Copyright 1994 American Chemical Society.
As reviewed elsewhere (see, for example, Baes & Mesmer, 1976; Cotton & Wilkinson, 1980; Nieboer et al., 1995; Stumm & Morgan, 1996; Teagarden et al., 1981), at relatively high concentrations of Al3+ (≥ 100 μg/L) in the pH range 5.3 to 6.5, polymerization occurs and results in the formation of polynuclear species such as Al13O4(OH)247+.
In Human Exposure, Environmental Levels, Water, and Human Exposure, General Population Exposures, Drinking Water, the various operationally defined forms of aluminium in surface and drinking water are discussed, including complexes of natural organic ligands.
The reactivity of aluminium powders depends on their morphology (size, shape and surface area), bulk density and aluminium content. For example, Ilyin et al. (2002), have demonstrated that nanoparticles of fine aluminium powders exhibit maximal values of oxidation (combustion) rates compared to microparticles, and this occurred at lower temperatures. Not surprisingly, because of their thinness and corresponding high surface area, aluminium flake powders (see Sources of Human Exposure, Anthropogenic Sources, Uses, Aluminium Powders) also are relatively reactive. Trunov et al. (2005) and Meda et al. (2004) have reported similar findings. Consequently fine and ultrafine aluminium powders show better promise as propellant additives than do the more conventional-sized (of the order of 10μm) aluminium powders. Interestingly, the combustion products of nano-sized aluminium powders are also different, such as a higher proportion of low-temperature aluminium oxide polymorphs (Meda et al., 2004) (see below) and product morphology. Ilyin et al. (2002) demonstrated that combustion of spherical micro-sized aluminium powders resulted in spherical products, while spherical fine powders produced submicron needles. As explained in the next paragraph, this is important for human exposure characterization.
The size of aerosols is important in terms of where they are deposited in the respiratory system and where they exert their toxic effects. In the workplace it is now common to consider three health-related aerosol fractions (Nieboer et al., 2005; Oller & Bates, 2005; Vincent, 1995). The inhalable aerosol fraction corresponds to the total amount of airborne particulates that enters the body through the nose and/or mouth during breathing (aerodynamic diameters (dae) of ≤ 100μm). The thoracic aerosol fraction penetrates the tracheoalveolar region of the lung (dae < 28μm), while the respirable aerosol fraction (dae < 10μm) penetrates the alveolar region of the lung (includes the respiratory bronchioles, the alveolar ducts and sacs). For outdoor aeorosols, it has been customary to measure the particulate matter (PM)10 fraction, which corresponds to the thoracic fraction but with particles larger than dae=10μm excluded (Nieboer et al., 2005; Vincent, 2005). For a description of these differences, the reader is referred to the review by Vincent (2005) in which the various aerosol fractions criteria are depicted graphically as a function of aerodynamic diameter. More recently, exposure to fine particles (dae<2.5μm) has become the focus in relation to increased rates of cardiovascular and respiratory diseases (Dockery et al., 1993; Englert, 2004; Pope et al., 2002). Ultrafine particles (dae < 100 nm) are also gaining in importance. Fine and ultrafine particles are included in the respirable and PM10 fractions. It is clear from the material presented in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Metal and the previous paragraph that, depending on the exact industrial process involved, aluminium powder workers have the potential of being exposed to some or all of the aerosol fractions discussed. This also appears to be the situation in aluminium refinery workers (Höflich et al., 2005; Skaugset et al., 2005; Thomassen et al., 2006).
Aluminium oxide (Al2O3) occurs in two major forms. α-Al2O3 [corundum; CAS No 1302-74-5] constitutes a high temperature form and is formed on heating aluminium hydroxide, Al(OH)3, at a temperature of 1000 °C or above. It is very hard and resistant to hydration and attack by acids; it occurs in nature as corundum (Cotton & Wilkinson 1980). γ-Al2O3 is generated at 500°C and readily takes up water and dissolves in acids. Other minor forms generated when heating Al(OH)3 include: χ-, κ-, δ-, θ-Al2O3 (Pearson, 1992; Trunov et al., 2005). Recent studies suggest that the structure of the surface layers of α-Al2O3 depends on hydration: on the surface of a highly polished single crystal, the aluminium atom (more correctly Al3+ ions) are exposed, while in the ideal (unrelaxed) structure the oxygen atoms (actually oxide anions, O2-) are at the surface. On hydration, the latter are overlaid by a semi-ordered absorbed water layer with the presence of extensive hydrogen bonding and hydroxyl groups resulting in a relaxed structure. In fact, the hydrated surface structure appears to be in between that of the ideal α-Al2O3 surface and that of γ-Al(OH)3. The Al3+ ions in the Al-terminated surface are strong Lewis acids and react strongly with water, while surface hydroxyl groups are Lewis bases that interact with metal ions (Eng et al., 2000). Surface reactivity constitutes the basis for the use of aluminium oxide and hydroxide as industrial catalysts, absorbents and chromatography packing materials (i.e., as stationary phases). Not surprisingly, the reactivity of solid Al2O3 depends on its specific crystal structure and hydrophilic/hydrophobic surface properties, and thus the degree of surface hydration (Pearson, 1992).
Gibbsite, γ-Al(OH)3 [CAS No 14762-49-3] is one of the three minerals that make up bauxite ore; the others being Al2O3 and the oxyhydroxides boehmite [α-Al2O3 • H2O or α-AlO(OH)] and diaspore [β- Al2O3 • H2O or β-AlO(OH)]. Of the latter, diaspore is the high temperature/high pressure form (Pearson, 1992). Gibsite has three structural polymorphs, namely bayerite [α-Al(OH3); CAS No 20257-20-9], nordstandite [β-Al(OH3); CAS No 13840-05-6] and doyleite which is rather rare (Mineralogy Database, 2006). On heating the Al(OH)3, polymorphs follow a different Al2O3 transition sequence in reaching the high temperature α-Al2O3 form (Pearson, 1992).
In simple terms, the surface layer of a metal binary compound has exposed metal ions (and anions) with reduced coordination number, which can behave as Lewis acids (or bases). At the solid-solution interface, proton association and dissociation can lead to pH-dependent surface charges and complexation. This is further complicated by specific adsorption of cations or anions right at the solid/solution interface, with some ordering of counter-ions in a more diffuse layer at points further into the solution (Stumm & Morgan, 1996). A zero point of charge occurs, which is pH dependent. It is also influenced by the extent of specific ion adsorption. In addition to surface electrostatic and complexation reactions, hydrogen-bonding and London-van der Waals forces can be involved in the adsorption of surfactants, non-polar organic solutes, polymers and polyelectrolytes (Stumm & Morgan, 1996).
Adsorption capacity is central to some of the major uses of Al2O3, Al(OH)3 and other aluminium compounds (e.g., aluminium phosphate, AlPO4). Adsorption of antigens onto Al(OH)3 and AlPO4 constitutes the basis for their use as vaccine adjuvents (Gupta, 1998). At neutral pH, gels of these compounds have different charges, the phosphate being negative and the hydroxide positive. This is important, relative to the charge borne by the antigen at physiological pH. The various forces described are optimized by adjustment of the pH and ionic strength of the medium, temperature, particle size of the adsorbent, and the surface area of the latter (Gupta, 1998). Similarly, the natural hydrophilic surface characteristic of Al2O3 is central to its use as solid-phases in chromatography. More recently, stable surface coatings have been developed which render the Al2O3 surface hydrophobic, which makes it even more versatile in chromatographic applications. Al2O3 and the aluminium oxyhydroxides are used to remove moisture from gases such as argon, alkanes, and sulphur dioxide. They are also used to remove hydrogen fluoride (HF) from air by adsorption; fluoride ions can also be removed effectively by them from water. Fluoride is adsorbed on to alumina at low pH values and can be desorbed on increasing the pH. Clearly, the inhalation of particulates of Al2O3 and related oxyhydroxides in aluminium smelting operations may constitute a delivery vehicle for adsorbed HF (Höflich et al., 2005; L’vov et al., 2005). Finally, the use of finely divided aluminium metal, Al(OH)3, aluminium potassium silicate and other aluminium compounds to generate dye (colour) lakes (see Table 2) stems from the surface adsorptive forces/capacities described.
Classification
Of the three substances reviewed in detail in this report, only aluminium powder is classified in Annex 1 of the European Economic Union Council Directive 67/548. Indeed, very few of the compounds listed in Tables 1 and 3 are classified; those that are listed are recognized as hazardous and are widely used, such as cryolite and aluminium phosphide (see Identity, Physical and Chemical Properties, Analytical Methods, Identity).
Although aluminium (EINECS # 013-001-00-6) is not included in a priority list, it is considered hazardous in powdered form. Aluminium powder is classified as: F; R 10,15 and the following risk (R) and safety (S) phrases have been assigned:
| R 10: | flammable |
| R 15: | contact with water liberates extremely flammable gasses |
| S 2: | keep out of reach of children |
| S 7/8: | keep container tightly closed and dry |
| S 43: | in case of fire, ‘never use water’ |
Cryolite is on the EEC Priority List #3 and has the risk classification: T; R20/22-N; R48/23/25-Xn; R51-53. Assigned risk (R) and safety (S) phrases are:
| R 20/22: | harmful by inhalation if swallowed |
| R 48/23/25 toxic: | danger of serious damage to health by prolonged exposure through inhalation and if swallowed |
| R 51/53: | toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment |
| S1/2: | keep locked up and out of reach of children |
| S 22: | do not breathe dust |
| S 37: | wear suitable gloves |
| S 45: | in case of accident, or if you feel unwell, seek medical advice immediately (show the label where possible) |
| S 61: | avoid release to the environment; refer to special instructions/ safety data sheets |
Aluminium phosphide is not listed on a priority list and has the classification of: F; R15/29-T; R28,32-N; R50. The risk and safety phrases are:
| R 15/29: | contact with water liberates toxic, extremely flammable gas |
| R 28: | very toxic if swallowed |
| R 32: | contact with acids liberates very toxic gas |
| R 50: | very toxic to aquatic organisms |
| S1/2: | keep locked up and out of reach of children |
| S 3/9/14: | keep in a cool, well-ventilated place away from…(incompatible materials to be indicated by the manufacturer) |
| S 30: | never add water to this product |
| S 36/37: | wear suitable protective clothing and gloves |
| S 45: | in case of accident, or if you feel unwell, seek medical advice immediately (show the label where possible) |
| S 61: | avoid release to the environment; refer to special instructions/ safety data sheets. |
Analytical Methods
As emphasized previously (ATSDR, 1999; IPCS, 1997; Nieboer et al., 1995; Savory et al., 1988; Savory & Wills, 1988; Wilhelm & Ohnesorge, 1990), inadvertent contamination during collection, storage, handling, sample preparation and analysis of body fluids and other specimens can introduce considerable uncertainty in the determination of aluminium at the ultratrace levels (≤ 1μmol/L or ≤ 1μmol/kg). It is therefore essential that authors provide documented proof that adequate quality control and assurance measures were in place during the study for which analytical results are reported.
The analytical chemistry of aluminium has been critically reviewed (Savory et al., 1988; Savory & Wills, 1988; Taylor & Walker, 1992) and extensively summarized (ATSDR, 1999; IPCS, 1997). Electrothermal atomic absorption spectrometry (EAAS), also referred to as graphite furnace atomic absorption spectrometry, has traditionally been the method of choice for biological samples and aqueous media. The newest technical development, namely inductively-coupled plasma mass spectrometry (ICP-MS), is now a powerful alternative because of the added capabilities of multi-elemental determinations and speed (Marchante-Gayon et al., 1999; Rodushkin & Odman, 2001; Trentini et al., 1993; Zhu et al., 2005). Optimization of methods and protocols for specific applications will continue for ICP-MS as has been done for EAAS (e.g., for aluminium in bone, (Liang et al., 1991); aluminium in cerebrospinal fluid (CSF), (Johnson & Treble, 1992); aluminium in plasma and urine by transversely heated EAAS, (Bradley & Leung, 1994); effects of modifiers and chloride on the determination of aluminium (Tang et al., 1995). Ion exchange, ion chromatography, and high performance liquid chromatography have been employed in sorting out aluminium speciation in surface water, drinking water and soil extracts (e.g., Mitrovic & Milacic, 2000; Schintu et al., 2000; Drabek et al., 2005).
Localization of aluminium in tissues by energy-dispersive x-ray analysis and electron-probe microanalysis (see Savory & Wills, 1988 for references) has largely replaced the earlier use of histochemical staining employing dyes such as aluminon (Buchanan et al., 1981; Clark & Krueger, 1985; Smith & McClure, 1982).
Limitations of the latter approach have recently been demonstrated for localizing aluminium in maize root tissue (Eticha et al., 2005), although Ruster et al. (2002) recommended this technique for quantifying deposition of aluminium in bone. In 1985, Verbueken et al. critically reviewed studies that examined the localization of aluminium in histological sections, namely, by electron probe X-ray microanalysis, secondary ion mass-spectrometry and laser microprobe mass analysis (LAMMA). These techniques are used in conjunction with scanning electron microscopy (SEM) or transmission electron microscopy (TEM); thus the microanalytical results are correlated with tissue structure. Further details about these and related microanalytical techniques are outlined by Ortner et al. (1998). Verbueken et al. (1985) concluded that all three techniques are helpful in localizing aluminium in tissues, but LAMMA provides the greatest sensitivity (i.e., can detect lower concentrations). Recently, accumulation of aluminium was detected in newly-formed lamellar bone after implantation of titanium plates containing 6% aluminium employing energy dispersive X-ray spectrometry (EDXS) in conjunction with SEM. Similarly, the use of micro-beam proton-induced X-ray emission (PIXE μbeam) confirmed that aluminium leaked diffusely from a titanium-aluminium-vanadium alloy dental implant into the surrounding bone, while vanadium did not (Passi et al., 2002).
SEM, TEM, and SEM coupled with EDXS permit the determination of elements, including aluminium, in micrometre-size particles (Ortner et al., 1998). This approach has been employed to characterize micrometre-sized alumina (Al2O3 or related oxyhydroxides) wear debris from artificial hip joints in wear simulations (Tipper et al., 2002), and in workplace aluminium-containing aerosol particles (Höflich et al., 2000; 2005; L’vov et al., 2005; Nieboer et al., 2005; Rollin et al., 1996; Weinbrüch et al., 2002).
Ellis et al. (1988) illustrated that in vivo monitoring of skeletal aluminium burden in patients with renal failure using neutron activation analysis (NAA) was possible. However, the research reactor required is not widely available. The promise of a portable instrument for an accelerator-based in vivo procedure for detecting aluminium body burden by NAA has recently been reported (Comsa et al., 2004).
26Al is a rare radioactive isotope of aluminium and is produced in particle accelerators by bombarding a magnesium target with deuterons. It is radioactive and has a long t½ (716,000 years) and can thus be detected radiometrically or by mass spectrometry (Priest, 1994). Whole-body counting is possible and increases the versatility of this technique. It has been employed in determining the human toxicokinetics, and the tissue distribution, bioavailability and GI uptake of aluminium (Priest, 2004).
SOURCES OF HUMAN EXPOSURE
Natural Occurrence
Aluminium and its compounds are major constituents of the Earth’s crust, comprising up to about 8% of the Earth’s surface. It is the third most abundant element (after oxygen and silicon) and the most abundant metallic element, and is found in combination with oxygen, fluorine, silicon, sulphur and other species; it does not occur naturally in the elemental state (ATSDR, 1999; Brusewitz, 1984; Wagner, 1999). Naturally occurring aluminium is present in silicates such as feldspars and micas, complexed with sodium and fluorine as cryolite, and in bauxite rock (comprising hydrous aluminium oxides, aluminium hydroxides and impurities such as free silica) (IPCS, 1997).
Anthropogenic Sources
Aluminium is released and dispersed in the environment by natural processes and from human activity. Natural processes account for most of the redistribution of aluminium in the environment (ATSDR, 1999; IPCS, 1997; Wagner, 1999) as a result of the weathering of rocks and minerals in which it is present. Mobilization from natural sources can, however, also result from the deposition of acidic precipitation (IPCS, 1997; Wagner, 1999). Direct anthropogenic releases of aluminium compounds occur primarily to air and these are associated with industrial processes. Thus, the mining and processing of aluminium ores and the production of aluminium metal, alloys and compounds can lead to the release of aluminium compounds into the environment. The use of aluminium and its compounds in processing, packaging and storage of food products, and as flocculants in the treatment of drinking-water may contribute to its presence in drinking-water and food stuffs (ATSDR, 1999).
Production levels and processes
Aluminium
Bauxite, a naturally occurring, heterogeneous material, is the most important raw material used in the production of aluminium (ATSDR, 1999; Dinman, 1983). Bauxite is made up primarily of one or more aluminium hydroxide minerals together with various mixtures of silica, iron oxide, titania, aluminium silicates, and other impurities in minor or trace amounts. The commercial sources of bauxite consist mainly of gibbsite or boehmite. Bauxite is extracted by open-cast mining (Dinman, 1983; IPCS, 1997). Nepheline and alunite are minerals which have also been used as raw materials for production of aluminium oxide. They are still used at some plants in the Commonweath of Independent States, but are a minor part of world production (Kammer, 1999).
The principal method used in producing aluminium metal involves three main steps (ATSDR, 1999; Browning, 1969; Dinman, 1983; IARC, 1984):
refining of bauxite to produce alumina (Bayer process);
electrolytic reduction of alumina to produce aluminium (Hall-Héroult process); and
casting of aluminium into ingots.
Aluminium is first extracted at 140 - 250°C with caustic soda from the bauxite, precipitated as aluminium hydroxide after the removal of iron and silicon impurities, and subsequently converted to aluminium oxide in a calcination process. These steps encompass the Bayer process (Sleppy, 1992). In the second stage, the aluminium oxide is dissolved in molten cryolite (Na3AlF6) and electrolyzed at temperatures of 920-980°C at carbon electrodes to yield the pure molten metal at the cathode and carbon dioxide at the anode (as well as some carbon monoxide and oxygen). The electrolytic cells are referred to as pots and the work area is the potroom. Because the anodes are consumed, they need to be replaced or generated in situ. In the first instance, pre-bake anodes are employed, while in the second approach (referred to as the Søderberg method) the anode is baked on site and carbon, in the form of a paste of petroleum coke and coal tar, has to be added to the top of the pot (Abramson et al., 1989; Kongerud et al., 1994; Staley & Haupin, 1992). Pre-baked anodes are produced in a separate department by moulding petroleum coke and coal tar pitch binder into blocks and baking at 1000-1200°C. Casting constitutes the final third step and is carried out in the foundry. It involves the pouring of aluminium ingots. Note that aluminium trifluoride (AlF3) is an important additive for the potroom electrolyte. It is prepared from Al2O3 and hydrogen fluoride (Sleppy, 1992; Staley & Haupin, 1992).
Aluminium produced by the Hall-Héroult electrolytic reduction process may be refined to a purity of up to 99.9% by the Badeau low-temperature electrolytic process (ATSDR, 1999; HSDB, 1995). This process is not the only primary refining method. Other approaches have been successfully developed, especially for the production of high purity (99.995%) aluminium (Staley & Haupin, 1992).
Secondary aluminium refining, also referred to as smelting or more commonly recycling, involves recycled aluminium scrap as feed. The scrap is melted in furnaces, fluxes are added, unwanted constituents are removed in the form of dross, and other metals are added if the final products are alloys (Healy et al., 2001). Dross forms on the surface of molten aluminium and consists of aluminium oxide, entrained aluminium, and smaller amounts of aluminium nitride, aluminium carbide and magnesium oxide (Staley & Haupin, 1992). This is further processed to recover the aluminium content.
The use of carbon electrodes in the electrolytic reduction process leads to the generation of volatile by-products including polycyclic aromatic hydrocarbons (PAH) (ATSDR 1999; Dinman, 1983; IARC, 1984).
Aluminium oxide
Aluminium oxide is produced from of bauxite; it is crushed, ground, and then leached with sodium hydroxide to form sodium aluminate from which the aluminium hydrate is precipitated and then calcined to produce aluminium oxide (alumina) (ATSDR, 1999; HSDB, 1995).
Aluminium hydroxide
Aluminium hydroxide is also produced from bauxite; the ore is dissolved in a solution of sodium hydroxide and the aluminium hydroxide precipitated from the resulting sodium aluminate solution by neutralizing with carbon dioxide or by autoprecipitation (ATSDR, 1999; HSDB, 1995; Sax & Lewis, 1987).
Trends in production
Bauxite
In 1992, worldwide production of bauxite was 106 million tonnes; based on a comparison of this quantity with the quarterly average values for 1993 and 1994, production in major producing countries appeared to be fairly constant (IPCS, 1997; World Bureau of Metal Statistics, 1994). However, by 2003 world mine production of bauxite had reached approximately 146 million tons (150 million tonnes) and rose to about 156 million tons (160 million tonnes) in 2004 (USGS, 2005a)1.
Alumina
In 1992, total alumina production worldwide was 33.8 million metric tonnes; this total production figure included 30 million tonnes for metallurgical uses and 3 million tonnes for non-metallurgical uses (IPAI, 1993; IPCS, 1997). By 1998, alumina production reached 47.5 million tonnes (USGS, 2002) and by 2004 was close to 63 million tonnes; Table 4 shows the production figures for different global regions for the period 2000 to 2005 (IAI, 2006a; USGS, 2005b; USGS, 2006a).
Table 4.
World production of alumina 2000-2005a.
| Region | Yearb | |||||
|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | |
| Africa | 0.5 | 0.7 | 0.7 | 0.7 | 0.8 | 0.8 |
| North and South America | 17.0 | 16.3 | 16.7 | 18.6 | 20.0 | 20.1 |
| Asia | 8.7 | 9.1 | 10.5 | 11.6 | 12.8 | 14.4 |
| Europe | 11.3 | 11.4 | 11.7 | 11.9 | 12.3 | 12.6 |
| Oceania | 15.7 | 16.3 | 16.4 | 16.8 | 17.0 | 17.9 |
| Total | 53.2 | 53.8 | 56.0 | 59.6 | 62.9 | 65.8 |
Mega tonnes (Mt)
Data from: IAI (2006a); USGS (2005b); USGS (2006a)
Aluminium hydroxide
Worldwide production of aluminium hydroxide in 2004 was estimated to be 5 million tonnes per annum (E. Nordheim, personal communication, 2006).
Aluminium
It is noted by the International Programme on Chemical Safety (IPCS) (IPAI, 1993; IPCS, 1997) that annual worldwide production of primary aluminium was 14.8 million tonnes in 1992; by 2004 production essentially doubled to 29.6 million tonnes (Table 5). Between 2001 and 2004 production increased by between 6 and 8% per annum. In 2004, primary aluminium was being produced in 41 countries, the largest producers being China (22% of world production) followed by Russia (12%), Canada (9%), the United States (8.5%), Australia (3.8%), Brazil (5%), and Norway (4.5%) (USGS, 2004). China and Russia surpassed the United States as the largest producers of primary aluminium in 2001 (Figure 4).
Table 5.
Primary aluminium production worldwide for 2000-2005a.
| Region | Yearb | ||||||
|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | ||
| Africa | 1.2 | 1.4 | 1.4 | 1.4 | 1.7 | 1.8 | |
| North and South America | 8.2 | 7.2 | 7.6 | 7.8 | 7.5 | 7.8 | |
| Asia | 5.0 | 5.6 | 6.6 | 8.0 | 9.3 | 11.1 | |
| Europe | 7.8 | 8.0 | 8.1 | 8.4 | 8.8 | 9.0 | |
| Oceania | 2.1 | 2.1 | 2.2 | 2.2 | 2.3 | 2.3 | |
| Total | 24.3 | 24.3 | 25.9 | 27.8 | 29.6 | 32.0 | |
Mt
Data from IAI (2006c); USGS (2005c); USGS (2006b)
Figure 4.
Trends in aluminium production by country. Data from USGS (2004).
In addition to primary aluminium production, more than 7 million tonnes is produced per year from post consumer (old) recycled scrap. Almost 100 per cent of all production scrap and over 60 per cent of all old scrap are recycled; it has been noted that the proportion of aluminium produced from scrap (“recycled aluminium”) is rising rapidly (IAI, 2005).
The recycling of aluminium requires much less energy than that used to recover the metal from its ores. The annual amount of aluminium recovered from purchased and tolled (new and old) scrap was about 15 million tonnes in 2004 (IAI, 2005). The automotive industry is the largest consumer of recycled cast aluminium accounting for about 70% of production. It was expected that demand for secondary aluminium would increase significantly as the automotive industry addressed the growing need for lighter vehicles (Wagner, 1999).
In 2004, the total shipments worldwide of aluminium ingots including primary and recycled metal were estimated at 62 million tonnes; shipments of aluminium powder were estimated to be 0.2% (75 000 tonnes per year) of this total (E. Nordheim, personal communication, 2006).
Uses
Industrial and non-industrial uses of aluminium and its compounds are summarized in Tables 6 and 7.
Table 6.
Summary of industrial uses of aluminium and its compounds – industrial compoundsa.
| Compoundb | Common Usesc |
|---|---|
| Aluminium | Metal and alloys; foils; in propellants and pyrotechnics |
| Aluminium alkyls | Catalysts |
| Aluminium alkoxides: ethoxide; tert-butoxide; isopropoxide | Oxidizing agents; catalysts |
| Aluminium antimonide | Semiconductor research |
| Aluminium basic acetate | Dyeing agent; water-/fire-proofing agent |
| Aluminium borate | Polymerization catalyst; component of glass |
| Aluminium borohydride | Reducing agent and jet fuel |
| Aluminium bromide | Acid catalyst |
| Aluminium calcium hydride | Reducing agent |
| Aluminium carbide | Reducing agent; source of methane |
| Aluminium chlorate | Disinfectant; bleaching agent |
| Aluminium chloride | Acid catalyst |
| Aluminium chloride hexahydrate | Preserver; disinfectant; dyeing agent |
| Aluminium fatty-acid salts: oleate, palmitate, stearate, linoleate | Ingredients of high temp lubricants, lacquers, waterproofing materials; protective cover |
| Aluminium fluoride | In ceramics; metallurgical flux; inhibitor of fermentation; catalyst |
| Aluminium hexafluorosilicate | In construction materials; glass manufacture |
| Aluminium hydride | Reducing agent; catalyst |
| Aluminium hydroxide | Adsorbent; emulsifier; dyeing mordant; manufacture of glass; lubricants; detergents; waterproofing fabrics |
| Aluminium hypophosphite | Polymer fibre finishing agent |
| Aluminium iodide | Catalyst |
| Aluminium lactate | Fire extinguishing foam |
| Aluminium lithium hydride | Reducing agent |
| Aluminium magnesium silicate | Thickening agent |
| Aluminium nitrate | Tanning agent; corrosion inhibitor; nitrating agent |
| Aluminium nitride | Semiconductor component |
| Aluminium oxalate | Dyeing mordant |
| Aluminium oxide | Adsorbent; abrasive; in lubricants; water-proofing agent |
| Aluminium phosphate | Cement component; ceramic flux |
| Aluminium phosphide | Fumigant; semiconductor research |
| Aluminium potassium sulphate/ aluminium potassium sulphate dodecahydrate | In manufacture of dyes; specialty cements; tanning agent; staining mordant |
| Aluminium selenide | Semiconductor research |
| Aluminium silicate | In glass; manufacture of ceramics; semiprecious stones; enamels; paint filler |
| Aluminium sodium sulphate/ aluminium sodium sulphate dodecahydrate | As for potassium alum |
| Aluminium sulphate/ aluminium sulphate octadecahydrate | Tanning agent; dyeing mordant; fire/waterproofing agent; decolorizing and clarifying agent |
| Aluminium sulphide | - |
| Aluminium tartrate | Textile dyeing agent |
| Aluminium terachloroaluminate | Catalyst |
| Aluminium thiocyanite | Dyeing mordant |
| Ammonium hexafluoroaluminate | Synthetic agent |
| Ammonium tetrachloroaluminate | Fur processing agent |
| Calcium aluminosilicate | Constituent of cement and refractories |
| Cryolite | Used in aluminium refining |
| Dihydrobis (2-methoxyethanolate- O,O) aluminate (1-) sodium | Reducing agent |
| Fosetyl aluminium | Agricultural fungicide |
| Hydrated magnesium-aluminium-iron silicate | Catalyst; insulation; filler/packing material |
| Indium gallium aluminium phosphide | Use in optical/information processing systems |
| Potassium aluminate | Cement set accelerator |
| Quanidenium aluminium sulphate hexahydrate | - |
| Sodium aluminate | Manufacture of dyes: milk, soap, glass; stone hardener; water softener |
| Tris(8-hydroxyquinoline) aluminium | Active organic light-emitting diode component |
Major sources: Lie et al. (1990/1991); The Merck Index (2001); Office of the Federal Register (2003); ChemFinder.com (http://chemfinder.cambridgesoft.com)
CAS Numbers are listed in Table 1
For some of the compounds listed, uses as pharmaceuticals, cosmetics, and household products are provided in Table 7.
Table 7.
Summary of uses of aluminium and its compounds - pharmaceuticals, food additives, cosmetics and other household productsa.
| Compoundb | Common Usesc |
|---|---|
| Acetylglycerrhetinic acid, aluminium salt | Antiulcerative |
| Aluminium | Aluminium foil; colour ingredient: topical drugs, food packaging |
| Aluminium acetate | Astringent; antiseptic; in food packaging |
| Aluminium ammonium sulphate (anhydrous and dodecahydrate | Astringent; food additive |
| Aluminium basic acetate | Antiseptic; antiperspirant |
| Aluminium benzoate | Antimicrobial |
| Aluminium bis(acetylsalicylate) | Analgesic, antipyretic |
| Aluminium bromohydrate | Antiperspirant |
| Aluminium butyrate | Food packaging ingredient |
| Aluminium carbonate - basic | Antacid; phosphate binder |
| Aluminium chloride hexahydrate | Antiperspirant |
| Aluminium chlorohydrate | Antiperspirant |
| Aluminium chlorohydrex | Topical drug: acne |
| Aluminium citrate | Antiperspirant |
| Aluminium di(2-ethylhexoate) | Food packaging ingredient |
| Aluminium fatty-acid salts | Food packaging ingredient |
| Aluminium glycinate | Antacid |
| Aluminium hexaurea sulphate triiodide | Drinking water antiseptic |
| Aluminium hydroxide | Antacid; antidiarrheal; food colouring agent; topical drugs: diaper rash, anti-fungal, acne; food packaging ingredient; vaccine adjuvant |
| Aluminium hydroxychloride | Astringent; anti-hyperphosphatemic |
| Aluminium lactate | Antiseptic; in dental impression material |
| Aluminium magnesium silicate | Antacid; topical acne |
| Aluminium metal silicates (sodium and/or calcium) | Food Anticaking agent |
| Aluminium methanedisulphonate | Astringent |
| Aluminium nicotinate | Antihyperlipoproteinemic; source of niacin |
| Aluminium nitrate | Antiperspirant |
| Aluminium phenolsulphonate | Antiperspirant; antimicrobial agent |
| Aluminium phosphate | Antacid; vaccine adjuvant; dental cement |
| Aluminium potassium silicate (anhydrous) | Food packaging ingredient |
| Aluminium potassium silicate (hydrated) | Drug colouring agent; cosmetic ingredient |
| Aluminium potassium sulphate/Aluminium potassium sulphate dodecahydrate | Astringent; antiperspirant; water purification agent; baking powder; topical: diaper rash, anti-fungal; food additive; in preparation of vaccine adjuvants |
| Aluminium silicate (anhydrous | Dental cement; food packaging ingredient |
| Aluminium silicate (hydrated) | Food packaging ingredient |
| Aluminium sodium carbonate hexitol complex | Antacid |
| Aluminium sodium sulphate/aluminium sodium sulphate dodecahydrate | As for potassium alum |
| Aluminium sulphate/aluminium sulphate octadecahydrate | Antiperspirant; topical: diaper rash, anti-fungal; food additive |
| Basic aluminium clofibrate | Antilipimic |
| Basic aluminium salicylates | Antidiarrheal |
| Basic aluminium-magnesium carbonate tetrahydrate | Antacid |
| Basic aluminium-magnesium sulphate dihydrate | Antacid |
| Basic sodium aluminium phosphate | Food additive; leavening agent |
| Bismuth aluminate | Antacid |
| Dihydroxy aluminium sodium carbonate | Antacid |
| Dihydroxyaluminium allentoinate | Astringent; antiulcerative |
| Dihydroxyaluminium acetylsalicylate | Analgesic, antipyretic |
| Polyoxyaoluminum acetylsalicylate | Analgesic |
| Sodium aluminium chlorohydroxy lactate | Antiperspirant; cosmetic products |
| Sucrose octakissulphate aluminium salt | Antiulcerative |
Major sources: Lie et al. (1990/1991); The Merck Index (2001); Office of the Federal Register (2003); ChemFinder.com (http://chemfinder.cambridgesoft.com)
CAS Numbers are listed in Table 2
For some of the compounds listed, industrial uses are provided in Table 6.
Aluminium
Aluminium metal is light in weight and is durable because surfaces of products made from it are oxidized to form a thin protective coating of aluminium oxide (alumina). However pure aluminium is extremely soft and therefore is often mixed with other metals and elements (e.g., copper, magnesium, manganese, silicon, lithium and zinc) to form alloys which are stronger and harder and hence of increased versatility (ATSDR, 1999; Wagner, 1999). The tensile strength of some copper-aluminium alloys can exceed that of mild steel by as much as 50% (Wagner, 1999). Reference to the uses of aluminium thus normally relates to those for aluminium as an alloy.
Aluminium metal and its alloys are used extensively in building construction (e.g., siding, roofing, doors, windows), in transportation (in the manufacture of automobiles and aircraft), in packaging (e.g., for beverage cans), and in electrical equipment. Other uses include die-cast motor parts, cooking utensils, decorations, road signs, fencing, beverage cans, coloured kitchenware, food packaging, foil, corrosion-resistant chemical equipment, solid fuel rocket propellants and explosives, dental crowns, jewellery and denture materials. Aluminium is also used for power lines, electrical conductors, insulated cables and wiring (ATSDR, 1999; IAI, 2006b). The largest markets for aluminium are transportation (27%), building and construction (23%), packaging (16%) and electrical equipment (10%). Transportation uses are one of the fastest growing areas for aluminium use showing a growth rate of about 4% per annum in 1999 (Wagner, 1999). Table 8 lists the approximate distribution of the different product segments in which aluminium is used on a global basis (E. Nordheim, personal communication, 2005)
Table 8.
Global markets for aluminium productsa.
| Market Sector | Market Share (%) |
|---|---|
| Building/Construction | 23 |
| Transport-Auto and light truck | 18 |
| Transport-Truck, bus, train, ship | 9 |
| Packaging-cans | 10 |
| Packaging-foil and container | 6 |
| Electrical-cable | 9 |
| Electrical-other | 1 |
| Machinery and Equipment | 7 |
| Consumer Durables | 7 |
| Aerospace | 1 |
| Other | 9 |
Data from E. Nordheim, personal communication (2005)
The major markets for aluminium products in the United States in 1997 are summarized in Table 9 (ATSDR, 1999; USGS, 1997).
Table 9.
Major (U.S.) markets for aluminium products in 1997a.
| Market Sector | Market Share (%) |
|---|---|
| Transportation | 34 |
| Containers/Packaging | 25 |
| Building/Construction | 15 |
| Electrical | 8 |
| Consumer Products | 8 |
| Other (e.g., machinery/equipment) | 10 |
From USGS (1997) in ATSDR (1999)
Over 95% of beer and carbonated drinks are packaged in aluminium cans. Other food-related applications of the metal are as sheet and foil for pie plates, frozen food trays and other food packaging, cooking utensils. Consumer product and medical uses include toys, jewellery, paints and protective coatings, and dental alloys for crowns and dentures (ATSDR, 1999; USGS, 1997).
Aluminium powders
Aluminium powders have been used for over a century in the production of pigments (Dinman, 1987) (see also Table 7). Today, aluminium flake powders constitute an important component of automobile paints (Hong & Kim, 2002). They are produced by milling of gas-atomized powder or foil scrap (Hong & Kim, 2002; James et al., 1991; Lawley, 1986). Flake particles have unique dimensions, with the length and width (measured in micrometres, μm) up to several hundred times its thickness (measured in nanometers, nm). By contrast, gas-atomized particles are smooth and generally spherical or ovoid, with the length, width and thickness being of the same order of magnitude. Sizes are in the nm or μm range corresponding respectively to ultrafine or fine particles and microparticles (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation). Gas-atomization involves aspirating a fine stream of molten metal into a high velocity air or gas jet to generate tiny droplets which, on cooling, solidify (Dinman, 1987; Lawley, 1986; Liddiard, 1984). Aluminium particulate powders are employed as fuel additives in propellants, explosives and pyrotechnics (Dinman, 1987; Meda et al., 2004; Trunov et al., 2005) (see also Table 6).
Other aluminium compounds
Aluminium sulphate is used in water treatment and as an accelerator for concrete solidification (high alumina cements) (ATSDR, 1992; Helmbolt et al., 1985; IPCS, 1997). Aluminium oxide is used in the production of aluminium; more than 95% of alumina produced is used for this purpose (E. Nordheim, personal communication, 2005). Aluminium oxide is also used in the manufacture of abrasives, refractories, ceramics, electrical insulators and resistors, catalysts, paper, spark plugs, and laboratory works, light bulbs, artificial gems, alloys, glass, heat resistant fibres, food additives (as a dispersing agent), in hollow-fibre membrane units for water desalination and in haemodialysis (ATSDR, 1992; 1999; Bradberry et al., 1997; Helmbolt et al., 1985; HSDB, 1995; IPCS, 1997).
Aluminium hydroxide is used widely in non-prescription stomach antacids, in buffered analgesics and other pharmaceuticals, and in antiperspirants and dentifrices, as a filler in cosmetics, plastics, rubber and paper and as a soft abrasive for brass and plastics (ATSDR, 1999; HSDB, 1995); it is also used pharmaceutically to lower the plasma phosphorus levels in patients with renal failure (ATSDR, 1999; Budavari et al., 1989). Aluminium hydroxide is also the basis for producing fire retardant materials.
Food-related uses of aluminium compounds include preservatives, fillers, colouring agents, anti-caking, agents, emulsifiers and baking powders; soy-based infant formula can contain high amounts of aluminium. Natural aluminium minerals especially bentonite and zeolite are used in water purification, sugar refining, brewing and paper industries (ATSDR, 1992; IPCS, 1997).
Legislative controls
Classification and labelling
The carcinogenic risk from aluminium and its compounds has not been evaluated by IARC. However, IARC has deemed that that there is sufficient evidence to show that certain exposures occurring during the production of aluminium cause cancer in humans; therefore “aluminium production” has been classified as carcinogenic to humans (Group I) (IARC, 1987). The U.S. Environmental Protection Agency (EPA) has not classified aluminium for human carcinogenicity (ATSDR, 1999; IRIS, 1999) and the American Conference of Governmental Industrial Hygienists (ACGIH) has designated aluminium as a group A4 substance (“not classifiable as to human carcinogenicity”) (ACGIH, 1996; ATSDR, 1999).
Classification and labelling requirements in the European Union (EU) are based on inherent hazardous properties of a substance, and are laid down in Directive 67/548 (EEC, 1967) and later amendments and adaptations. The requirement covers physico-chemical properties, human health, and environmental toxicity. The classification is based on the results of specific prescribed tests, generally test guidelines developed by the Organization for Economic Cooperation and Development. Discussions regarding the classifications with respect to considerations for either health or environment are conducted in EU expert groups which evaluate the test data and propose the classification. This proposed classification is set out in Directives from the Commission. The classification of aluminium compounds is summarized in Identity, Physical and Chemical Properties, Analytical Methods, Classification.
Due to hepato-, skeletal and neurotoxicity seen in premature infants that received aluminium as a contaminant in total parenteral nutrition solutions (see Effects on Humans, Effects from Non-Occupational Exposure, Bone and Effects on Humans, Subpopulations at Special Risk, Infants and Children) the U.S. Food and Drug Administration enacted a labeling requirement, that went into effect July 26, 2004, which permits no more than 25 μg Al/L in large volume parenterals and a statement of the exact amount of Al present in small volume parenterals (US FDA, 2000).
Occupational exposures
The U.S. Occupational Safety and Health Administration (OSHA) requires employers to reduce exposures to aluminium to or below an 8-hr time-weighted average (TWA) of 15 mg/m3 for total aluminium dust or 5 mg/m3 for the respirable fractions (NIOSH, 2005). Limits have also been set for aluminium in the workplace by the ACGIH (1996) (ATSDR, 1999) and the National Institute for Occupational Safety and Health (NIOSH) (2005) in the United States; these values are listed in Table 10. In general, in the absence of occupational limits, countries in Europe also use those established by the ACGIH (E. Nordheim, personal communication, 2005).
Table 10.
U.S. occupational exposure limits for aluminium.
| Organization | Substance | Value | Reference |
|---|---|---|---|
| OSHA-PELb | Total dust | 15 mg/m3 – 8 hr TWA | NIOSH (2005) |
| OSHA-PEL | Respirable fraction | 5 mg /m3 - 8h hr TWA | NIOSH (2005) |
| ACGIH TLVd | Metal dust | 10 mg /m3 TWA | ACGIH (2005) |
| ACGIH TLV | Welding fume | 5 mg /m3 TWA | ACGIH (1996)a |
| NIOSH RELc | Total dust | 10 mg /m3 - TWA | NIOSH (2005) |
| NIOSH REL | Respirable fraction | 5 mg /m3 - TWA | NIOSH (2005) |
| NIOSH REL | Welding fumes | 5 mg /m3 - TWA | NIOSH (1992)a |
Occupational exposure limits for aluminium oxide (Al2O3) compiled by the Registry of Toxic Effects of Chemical Substances (2006) are shown in Table 11. The values given in this table for the U.S. were established by OSHA; the ACGIH has also set a TLV of 10 mg/m3 (particulate)-TWA (RTECS, 2006).
Table 11.
Limits for occupational exposure to aluminium oxide in Europe, Australia and the U.S.a
| Country | Occupational Exposure Limit | Date |
|---|---|---|
| Austria | MAKb 0.25 fibers/cm3 | Jan, 2006 |
| Belgium | 10 mg/m3 – TWAc | Jan, 1993 |
| Denmark | 10 mg(Al)/m3 – TWA | Oct, 2002 |
| France | VMEd 10 mg/m3 | Jan, 1999 |
| Germany | MAK 1.5 mg/m3, respirable fume | 2005 |
| Netherlands | MAC-TGGe 10 mg/m3 | 2003 |
| Norway | 2 mg(Al)/m3 – TWA MAC 2 mg/m3 – TWA |
Jan, 1999 |
| Poland | MAC 16 mg/m3 – STELf | Jan, 1999 |
| Russia | 6 mg/m3 - TWA | Jun, 2003 |
| Sweden | 4 mg(Al)/m3, respirable dust – TWA | Jan, 1999 |
| Sweden | 10 mg(Al)/m3, total dust – TWA | Jan, 1999 |
| Switzerland | MAK- week 2 mg(Al)/m3 | Jan, 1999 |
| United Kingdom | 10 mg/m3, total inhalable dust – TWA | 2005 |
| United Kingdom | 4 mg/m3, respirable dust – TWA | 2005 |
| Australia | 10 mg/m3 TWA | Jan, 1993 |
| U.S. | PELg 15 mg/m3, total dust-TWA | 1994 |
| U.S. | PEL 5 mg/m3, respirable fraction - TWA | 1994 |
From RTECS (2006)
Maximum workplace concentration
Time weighted average
Limit values for average exposure
Maximum allowable concentration averaged over working life
Short term exposure limit
Permissable exposure limit
Exposures in the general environment
The Joint Food and Agriculture Organization (FAO) / World Health Organization (WHO) Expert Committee on Food Additives and Food Contaminants recommended a provisional tolerable weekly intake (PTWI) of 7.0 mg/kg b.w.; this value includes the intake of aluminium from its use as a food additive (FAO/WHO, 1989; IPCS, 1997). In 2006, the PTWI was lowered to 1.0 mg/kg b.w. citing that aluminium compounds may exert effects on reproductive and developing nervous systems at lower doses than were used in setting the previous guideline (FAO/WHO, 2006). In the United States, an intermediate duration oral exposure Minimal Risk Level (MRL) of 2.0 mg/kg/day has been developed (ATSDR, 1999; IRIS, 1999) based on neurotoxicity in mice (ATSDR, 1999; Golub et al., 1989). No MRLs for any duration of inhalation exposure have been set for aluminium and the EPA has not designated aluminium or its compounds as hazardous air pollutants under the Clean Air Act; however, the EPA has regulated aluminium and certain aluminium compounds under this Act (ATSDR, 1999).
The WHO has not proposed a health-based guideline for aluminium in drinking water because of the limitations in the animal data; however, it has derived “practicable levels” of ≤0.1 and ≤0.2 mg/L for large and small facilities, respectively, based on optimization of the coagulation process in water treatment plants that use aluminium-based coagulants (WHO, 2004). A health based maximum contaminant level (MCL) has also not been promulgated for aluminium under the U.S. Safe Drinking Water Act; a secondary non-enforceable MCL has been set at a concentration range of 0.05 - 0.2 mg/L (ATSDR, 1999; EPA, 1979; IRIS, 1997). The EU directive for aluminium in drinking water is 0.2 mg/L as an “Indicator Parameter” (Lenntech, 2004). Australia has also recommended a drinking water guideline value of 0.2 mg/L based on aesthetic considerations (Queensland Government, 2002). Canada has not set a health-based guideline. However, it is recommended in the Canadian Guidelines for Drinking Water Quality, “as a precautionary measure”, that water treatment plants using aluminium-based coagulants optimize their operations to reduce residual aluminium levels in the treated water to the lowest extent possible. Where aluminium-based coagulants are used in conventional treatment plants, the operational guidance level is <0.1mg/L (based on a 12 month running average of monthly samples); for other types of treatment systems using such coagulants, the operational guidance value is <0.2 mg/L (Federal-Provincial-Territorial Committee on Drinking Water, 2006).
The U.S. Association for the Advancement of Medical Instrumentation has issued a standard in which it is recommended that water used in the preparation of dialysate solution contain less than 10 μg/L Al in order to limit the unintentional administration of aluminium to dialysis patients (AAMI, 1998; ATSDR, 1999). However, there is concern that unless long-term exposure is limited to lower aluminium concentrations, there will be slow, but permanent, exposure of a greater percentage of dialysis patients to aluminium (Fernandez-Martin et al., 2000). These authors suggested the recommended level should be 2 μg/L.
The Kidney Disease Outcomes Quality Initiative suggested Al-based antacids could be used in patients with chronic kidney disease who have serum phosphorus levels >7.0 mg/dL (2.26 mmol/L) as a short-term (4 weeks) therapy, and for one course only, to be replaced thereafter by other phosphate binders and that more frequent dialysis should also be considered in these patients (National Kidney Foundation, 2003).
In the absence of a consensus on the acceptable “safe” concentration of aluminium in plasma to guide therapy, it was suggested that 0.5 to 1.9 μM (13.5 to 51 μg/L) be considered as reflecting increased exposure, 2.0 to 2.9 μM (54 to 78 μg/L) excessive exposure, and > 3 μM (81 μg/L) a toxic concentration, that perhaps warrants mobilization (Fenwick et al., 2005).
Regulations and/or guidelines that have been established for aluminium with respect to the general environment are summarized in Table 12.
Table 12.
Limits for aluminium in general environments.
| Country/Organization | Nature of Limit | Value |
|---|---|---|
| FAO / WHO | Provisional Tolerable Weekly Intake | 1 mg/kg b.w. |
| U.S. | MRL (intermediate duration-oral) | 2 mg/kg/day |
| WHO | Drinking Water Guideline | a |
| EU | Drinking Water Directive | 0.2 mg/L |
| United States | Secondary Drinking Water Regulation | 0.05-0.2 mg/L |
| Australia | Drinking Water Guideline (aesthetic) | 0.2 mg/L |
| Canada | Drinking Water Guideline | b |
| U.S. Association for the Advancement of Medical Instrumentation | Water for dialysate solution | <10 μg/L |
“Practicable levels” of ≤0.1 and ≤0.2 mg/L for large and small facilities, respectively, based on optimization of the coagulation process.
Where aluminium-based coagulants are used in conventional treatment plants, the operational guidance level is <0.1mg/L (based on a 12 month running average of monthly samples); for other types of treatment systems using such coagulants, the operational guidance value is <0.2 mg/L.
HUMAN EXPOSURE
General Discussion
Aluminium is the third most abundant element in the Earth’s crust with oxygen and silicon being the first and second. The respective percentages are 50, 26 and 7.5 (Williams & Fraústo da Silva, 1996). It is therefore not surprising that soils and weathered rocks constitute the major sources of aluminium in environmental media (Bowen, 1979). The mobilization of these natural sources far exceeds the anthropogenic releases into air, in waste-water effluents and industrial waste (ATSDR, 1999). The environmental mobilization, transport and distribution of aluminium, as well as the levels observed in air, water, soils, sediments and food items have been extensively summarized in previous monographs (ATSDR, 1992; IPCS, 1997) and in topic-specific reviews. Rather than reproducing these efforts, only the seminal features of these issues will be highlighted in the present chapter. However, all recent developments and topics pertinent to human risk assessment are reviewed and discussed in detail.
Environmental Levels
Air
Prior to 1987, it was the practice to measure particulate air pollution levels as total suspended particles (TSP) (Samet et al., 2000). TSP constitutes air-borne particles with dae <30 μm (Cyrys et al., 2005). In 1987, annual and 24-hr standards were promulgated by the U.S. EPA for the PM10 aerosol fraction (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation) of 50 and 150 μg/m3, respectively. In 1997, this agency added standards for PM2.5, namely 15 (annual) and 65 μg/m3 (24-hr) (EPA, 1997). Both the PM10 and PM2.5 aerosol fractions have been associated with adverse health effects as reported in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation. Reported ambient aluminium concentrations in these fractions are summarized in Table 13. A perusal of these data indicates mean PM2.5 aluminium levels were in the range 0.035 to 1.82 μg/m3 (excluding the arctic and Antarctica sites) and 0.58-6.97 μg/m3 for PM10. By comparison, the mean total particulate mass reported in the PM2.5 and PM10 fractions for non-remote sites were near or often exceeded the annual standards indicated. Detailed analyses of these data outlined in the original publications have identified the trends and interpretations summarized in the next paragraph.
Table 13.
Concentrations of aluminium in ambient aerosol fractions.
| Area | Year sampled | Particle size (daein μm) | Concentration (ng/m3) | Reference | |
|---|---|---|---|---|---|
| Arith. mean ± sd | Range | ||||
| U.S. cities & industrial areas | 1975-1977 | < 3.5 | - | 48-1983 | Stevens et al. (1978) |
| 1975-1977 | > 3.5 | - | 331-8678 | ||
| Charleston, West Virginia | 1976 | < 3.5 | 74 | - | Lewis & Macias (1980) |
| 1976 | > 3.5 | 1110 | - | ||
| United Kingdom | < 3.5 | ||||
| Chilton, rural | 1971-1989 | < 3.5 | 222±110 | 46-574 | Lee et al. (1994) |
| Windermere, rural | 1970-1989 | < 3.5 | 142±93 | 3-525 | Lee et al. (1994) |
| Lambeth, residential | 1976-1982 | < 3.5 | 636±292 | 282-1214 | Lee et al. (1994) |
| Brent, residential | 1975-1989 | < 3.5 | 489±303 | 232-2415 | Lee et al. (1994) |
| Manchester-1, residential | 1975-1989 | < 3.5 | 545±230 | 232-1530 | Lee et al. (1994) |
| Trafford-1, residential | 1978-1989 | < 3.5 | 563±233 | 177-2289 | Lee et al. (1994) |
| Trafford-2, residential | 1975-1989 | < 3.5 | 694±280 | 238-1402 | Lee et al. (1994) |
| Manchester-2, residential/industrial | 1975-1988 | < 3.5 | 616±220 | 340-1163 | Lee et al. (1994) |
| Walsall, industrial | 1976-1989 | < 3.5 | 838±282 | 246-1481 | Lee et al. (1994) |
| Metropolitan areas of U.S. | 1979-1988 | PM2.5 | - | Laden et al. (2000) | |
| Boston | 1979-1988 | PM2.5 | 66±89 | - | Laden et al. (2000) |
| St. Louis | 1979-1988 | PM2.5 | 161±212 | - | Laden et al. (2000) |
| Knoxville | 1979-1988 | PM2.5 | 153±150 | - | Laden et al. (2000) |
| Madison | 1979-1988 | PM2.5 | 71±127 | - | Laden et al. (2000) |
| Steubenville | 1979-1988 | PM2.5 | 187±238 | - | Laden et al. (2000) |
| Topeka | 1979-1988 | PM2.5 | 130±205 | - | Laden et al. (2000) |
| Central California, U.S. | |||||
| Altamont Pass (costal air mass into San Joaquin Valley) | 1990 | PM2.5 | 76 | 236 (max) | Chow et al. (1996) |
| PM10 | 1147 | 2495 (max) | |||
| Crows Landing (agricultural) | 1990 | PM2.5 | 1390 | 4823 (max) | Chow et al. (1996) |
| PM10 | 6972 | 17316 (max) | |||
| Buttonwillow (agricultural) | 1990 | PM2.5 | 469 | 1956 (max) | Chow et al. (1996) |
| PM10 | 3697 | 14971 (max) | |||
| Acadamy (downwind from City of Fresno) | 1990 | PM2.5 | 631 | 1024 (max) | Chow et al. (1996) |
| PM10 | 2218 | 3083 (max) | |||
| Edison (downwind of City of Bakersfield) | 1990 | PM2.5 | 1824 | 2969 (max) | Chow et al. (1996) |
| PM10 | 2684 | 5395 (max) | |||
| Sequoia NationalPark | 1990 | PM2.5 | 121 | 396 (max) | Chow et al. (1996) |
| PM10 | 1135 | 1767 (max) | |||
| Yosemite National Park | 1990 | PM2.5 | 159 | 242 (max) | Chow et al. (1996) |
| PM10 | 1145 | 1821 (max) | |||
| High arctic | |||||
| Ocean | 1991 | < 2 | 1.50 (median) | - | Maenhaut et al. (1996) |
| < 10 | 7.8 (median) | - | |||
| Ice pack | 1991 | < 2 | 0.5 (median) | - | |
| Brisbane, Australia | 1993/4 | PM10 | 585 | - | Chan et al. (1997) |
| PM2.5 | 160 | - | |||
| Czech Republic, City of Teplice (residential/industrial) | 1993 (summer) | 2.5-8 | 790 | - | Pinto et al. (1998) |
| 1993 | PM2.5 | 510 | - | ||
| 2.5-8 | 1900 | - | |||
| Antarctica, site 1 | 1995/7 | PM10 | 181 | - | Mazzera et al. (2001) |
| site 2 | 1995/7 | PM10 | 254 | - | |
| Singapore | 1996/7 | PM2.5 | 231±313 | - | Orlic et al. (1999) |
| PM10 | 686±423 | - | |||
| Toronto, ON, Canada | 2000/2001 | PM2.5 | 35 | 5.3-3000 | Lee et al. (2003) |
| Buenos Aires, Brazil | 2001 | PM10 | 936 | 346-2541 | Smichowski et al. (2004) |
Aluminium, like silica, may be designated as crustal in origin (Laden et al., 2000; Lee et al., 1994). Calculations of the enrichment factor (the ratio of the concentration of an element of interest and that of a signature element in the air sample divided by the same concentration ratio in the Earth’s crust) support this, namely values below 7.0 (Lee et al., 1994). Further corroboration comes from principal component analysis and the general characteristic that anthropogenic elements (e.g., sulphur and lead) accumulate predominantly in the PM2.5 fraction, while crustal elements (e.g., silicon, titanium, iron, and aluminium) occur frequently in coarser fractions such as such as PM10 (Orlic et al., 1999). The latter was observed for the central California data in Table 13, for which aluminium concentrations for the two agricultural sampling stations were considerably higher in the PM10 fraction (Chow et al., 1996). Road dust, industry and urbanization are also recognized factors in generating higher ambient aluminium levels (Chow et al., 1996; Lee et al., 1994; 2003; Pinto et al., 1998). Finally, remote sites well away from towns and cities exhibit the lowest aluminium air concentrations. Examples are (see Table 13) the Sequoia and Yosemite National Parks in California (Chow et al., 1996), the high arctic (Maenhaut et al., 1996) and Antarctica (Mazzera et al., 2001).
Precipitation
As documented in the ATSDR (1999) and IPCS (1997) monographs, aluminium is present in measurable amounts in precipitation. This is not surprising, based on the observed air levels summarized in Table 13. Clearly, this source constitutes a chronic input for surface water and soil.
Water
In terms of human health risk, dermal contact with and consumption of water are pertinent pathways of exposure. Consequently, aluminium concentrations in surface and drinking water are of primary interest. Further, as documented elsewhere (ATSDR, 1999; IPCS, 1997), aluminium concentrations in marine waters tend to be considerably lower than in fresh water. The latter, therefore, will be the focus in this section.
Environmental acidification is known to mobilize aluminium from land to aquatic environments, and this process has been demonstrated to vary with the seasons or major storm events (ATSDR, 1999). Anthropogenic point sources can add to the aquatic aluminium burden. It is clear from Fig 2.1 in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation that aluminium becomes markedly more soluble below a pH of about 5.
A study by Schintu et al. (2000) provides insight about the levels and speciation (i.e., its chemical and physical forms) of aluminium in raw water and in water after its treatment in the production of drinking water. It corroborates the wide variability of aluminium concentrations and speciation in surface water and that the corresponding parameters for drinking water are more uniform. In characterizing aluminium exposure, some recent epidemiological studies of AD have considered aluminium speciation in their statistical analyses (e.g., Gauthier et al., 2000). Consequently, it is relevant to explore this aspect in some detail here for raw water and in Human Exposure, General Population Exposures, Drinking Water for drinking water.
Fractionation of water samples permits an assessment of a number of forms in which aluminium occurs in addition to the measurement of total aluminium (operationally defined as that dissolved at pH 1 or 2 without filtration), namely total dissolved (that dissolved at pH 1 or 2 after filtration); dissolved organic monomeric; dissolved inorganic monomeric; polymeric/colloidal; and particulate (Driscoll & Letterman, 1995; Schintu et al., 2000). In terms of raw water, the particulate form often dominates, with reported proportions when employing a 0.4 μm polycarbonate filter in the first fractionation step in the 54 to 74% range (e.g. Driscoll & Letterman, 1995) and may exceed 80% for a 0.22 μm filter (Schintu et al., 2000). Average concentrations of total dissolved aluminium after filtration; i.e., inorganic and organic monomeric, plus colloidal and strongly-bound organic typically are 1.9-5.2 μmol/L (50-140 μg/L). These levels corresponded to 3 to 17% of the total aluminium for assessments involving a 0.22 μm filter (Schintu et al., 2000) and 71 to 90% when employing the 0.40 μm filter (Driscoll & Letterman, 1995). Both organic and inorganic forms appear to be present in significant amounts in the dissolved aluminium fraction (Driscoll & Letterman, 1995; Driscoll & Schecher, 1989; Gauthier et al., 2000; Guibaud & Gauthier, 2005; Schintu et al., 2000).
Recent studies have confirmed that, in forest soil extracts or raw water, organic ligands that bind aluminium include citrate, oxalate and humic substances (Drabek et al., 2005; Guibaud & Gauthier, 2005; Mitrovic & Milacic, 2000). Humic substances constitute the predominant component of natural organic substances. They are operationally divided into three fractions: fulvic acids, which are soluble in both acidic and alkaline solutions; humic acids, which are soluble in alkaline solutions, but are precipitated at pH 2; and humin, which is insoluble in water independent of pH (Turner, 1995). Carboxylic acid, alcohol and phenolic functional groups constitute the metal-binding sites in these ligands (Stumm & Morgan, 1996; Turner, 1995). Fulvic acids provide more of these donor sites than humic acids. Respectively, the molecular masses of the corresponding aluminium complexes have been reported for acidic forest soil extracts to be in the 2000-4500 Da and 2700-6000 Da ranges (Mitrovic & Milacic, 2000). Further, 80 to 95% of total water-soluble aluminium was found to be in monomeric form (0.45 μm filter) in the forest soil extracts, with complexes of oxalate, citrate and fluoride contributing 45 to 55%, while 30 to 40% was associated with the molecular mass fractions of the humic substances mentioned (Mitrovic & Milacic, 2000). Drabek et al. (2005) reported that water extracted 32.3 mg/kg of aluminium from acid forest soils as singly-charged species [Al(OH)2+, Al(SO4)+, AlF2+, Al(oxalate)+, and Al(H-citrate)+, among others], 3.1 mg/kg as doubly-charged species [Al(OH)2+, Al(F)2+, etc] and 3.8 mg/kg as Al3+ [Al3+ and hydroxyl aluminium polymers].
Soil and sediment
Dermal contact with and ingestion of sediments and soils is not expected to constitute significant exposure routes, even though the aluminium contents of these media are substantial as mentioned in Human Exposure, General Discussion. Typical aluminium concentrations for major sediments are 9,000 – 94,000 μg/g and 700-300,000 (median 71,000) μg/g in soils (ATSDR, 1999; Bowen, 1979; IPCS,1997; Sanei et al., 2001). As described in the previous section, soils are a source of soluble aluminium species for surface water and, of course, of sediment particles as well (Sanei et al., 2001). Further, aluminium partitions from water to sediment and particulates. Consequently, soils and sediments are determinants for the level and forms of aluminium in surface water and thus raw water as a source for drinking water.
Terrestrial and aquatic organisms
Bioconcentration of aluminium in aquatic plants and plants grown on low pH soils is known (Gallon et al., 2004; IPCS, 1997). Aerial deposition appears to contribute to plant surface levels of aluminium, as illustrated for spruce needles. Because of its high concentrations in sediments, it is difficult to interpret the aluminium concentrations reported (IPCS, 1997) for crustaceans such as crayfish, and bottom feeders such as carp. In other fish, little aluminium seems to be present in edible tissue, but with the gills showing preferential accumulation (IPCS, 1997; Reid et al., 1991; Wilkinson et al., 1993). As reviewed in Human Exposure, General Population Exposures, Food and Beverages, grains, vegetables, legumes, and especially herbs and spices, exhibit significant tissue concentrations of aluminium.
Occupational Exposure
Aluminium production
Exposures experienced by workers employed in aluminium production are influenced by the industrial processes involved; an overview of the primary refining steps and a brief introduction to secondary refining of this metal are presented in Sources of Human Exposure, Anthropogenic Sources, Production Levels and Processes, Aluminium.
Aluminium refinery workers can be exposed to aluminium hydroxide in the Bayer process, and “to airborne particles of variable composition (e.g., aluminium oxides, cryolite, soot), a number of gases (e.g., carbon oxides, sulphur dioxide (SO2), hydrogen fluoride (HF)) and PAHs are generated” (Höflich et al., 2005) in the Hall-Héroult process (also Benke et al., 1998). The emission of dust, including PAHs is more extensive for the Søderberg pots and anode plants. As already pointed out (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation), adsorption of HF and SO2, and presumably also PAHs, onto aluminium oxide and oxyhydroxides is an issue of concern; beryllium emissions pose an additional health risk (Benke et al., 1998). Ultrafine particles in the 10-300 nm diameter range have also been identified in potrooms (Höflich et al., 2005; L’vov et al., 2005; Thomassen et al., 2006). By comparison, for secondary aluminium smelting and relative to accepted occupational exposure limits, inhalable dust, particulate fluorides, HF, lead and aluminium appear to constitute a health risk (Healy et al., 2001).
Although personal measurements of exposure to dust containing aluminium oxide and other aluminium compounds have been made in the aluminium production industry, they are not extensively documented in the published literature. The emphasis has rather been on PAHs, fluorides and HF measurements (Benke et al., 1998). However, the reports by Pierre et al. (1995; 1998) provide a helpful overview. They surveyed 234 workers employed in primary aluminium refining, 88 in secondary aluminium refining and 13 in aluminium powder production. A summary of their findings is given in Table 14. Aluminium air concentrations were measured as the “total” aerosol fraction. Relative to the inhalable aerosol fraction defined in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation, “total” personal samplers undersample particles larger than 15 μm (Vincent, 1994). Unfortunately, for most of the worker groups surveyed, only mean values are available.
Table 14.
Individual exposures to aluminium and its compounds: average daily concentrations in mg/m3 (adapted from Pierre et al., 1995; 1998).
| Primary Aluminium Compound(s) | Plant | Duration of Measurement (days) | Process (Task) | Mean Insolublea Aluminium (range) | Mean Soluble Aluminiumb |
|---|---|---|---|---|---|
| Bauxite | 1,2 | 30 | Mining (unloading) | 1.45 | ≤ 0.005 |
| Alumina | 2,3 | 17 | Bayer process (multiple) | 0.98 | 0.001 |
| Alumina | 2,3 | 15 | Bayer process (pelletising) | 9.88 | 0.008 |
| AlF3, Al2O3 | 3 | 5 | Aluminium fluoride production | 0.33 | 0.03 |
| AlF3, Al2O3 | 3 | 5 | Al-fluoride prod. (bagging) | 4.78 | 0.11 |
| Al-oxides, AlF3, cryolite, metal | 4A | 20 | Potroom, Søderberg (multiple) | 0.30 | 0.28 |
| Al-oxides, AlF3, cryolite, metal | 4B | 27 | Potroom, pre-baked (multiple) | 0.39 (0.29-0.62) | 0.19 |
| Al-oxides, metal | 5 | 24 | Secondary smelting (multiple) | 0.15 (0.19-0.76) | ≤ 0.005 |
| Al-oxides, metal | 5 | 6 | Secondary smelting (slag treatment) | 4.34 | 0.10 |
| Metal | 5 | 6 | Secondary smelting (cutting, rolling) | 0.20 | 0.001 |
| Metal (shot powder) | 6 | 10 | Powder production (blasting, casking) | 15.96 | 0.005 |
| Metal (flake powder) | 6 | 20 | Powder production (multiple) | 0.88 (0.2-2.3) | 0.003 |
Measured as the ‘total’ aerosol fraction, and after removal of the water-soluble subfraction while in the sampler holder (Pierre et al., 1998)
Amount dissolved in 10 mL of distilled water (see footnote a).
Several noteworthy trends are evident from the data in Table 14. Exposure to bauxite, aluminium and the metal (as powder or sheets) is associated with low concentrations of water-soluble aluminium, by contrast to exposures in the potrooms, secondary smelting, and AlF3 production. Consequently, one might expect to observe higher urinary aluminium concentrations among workers in the latter group, which was indeed the case. Surprisingly, the aluminium powder workers exhibited substantially higher before-shift concentrations in urine than other workers. This observation suggests that particulates that deposited and accumulated in the respiratory tract serve as sinks. Further, Gitelman (1995) and Gitelman et al. (1995) conducted a survey involving 40 control subjects and 235 workers employed in 15 plants in the USA engaged in primary and secondary aluminium refining and in the manufacture of products (e.g., powder technologies, cables, rolling mills). Personal exposures ranged from 0.01-1.20 mg/m3, with a median of 0.025 mg/m3 for respirable fractions (<10 μm), and 0.001-3.0 mg/m3 with a median of 0.10 mg/m3 for the “total” aerosol fractions. The maxima in their survey likely pertained to primary refining and work with powders.
The reports by Röllin et al. (1996; 2001) suggest that newer potroom technologies can substantially reduce exposure to aluminium. In a new primary smelter, the median “total” aluminium levels were 0.03 to 0.084 mg/m3, while in a plant using the more standard potroom smelting approach exposures were: mean (range) in mg/m3 of 1.47 (1.25-1.66), potroom 1; 0.35 (0.20-0.57), potroom 2; and other operator, 0.036 (0.02-0.13).
An extensive survey of 7 secondary aluminium smelters in the UK found inhalable aluminium air concentrations of 0.04 to 0.90 mg/m3, with a mean value of 0.31 mg/m3. These measurements are comparable in magnitude to the concentrations reported in Table 14 for jobs involving slag treatment. Healy et al. (2001) did indeed identify the slagging out of rotary furnaces as a dusty operation. Comparable concentrations have been reported for two foundries in South Africa: 0.17 mg/m3 for smelters; 0.027 mg/m3 for operators; and 0.58 mg/m3 for fettlers (i.e., those lining furnaces) (Rollin et al., 1991a).
User industries
Occupational exposures in workplaces using non-powdered aluminium metal well below its melting point (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Compounds, Table 3) may be expected to be considerably lower than those in primary and secondary aluminium refineries. By contrast, exposures in the manufacture of products involving aluminium powder (see Tables 1 and 2 and Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Metal and Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation) are likely to be comparable to those experienced in powder production. Similarly, workers involved in alloy production are likely to have aluminium exposures like those associated with comparable operations described for secondary refining and casting in primary refining. By contrast, exposures for aluminium welders have been reported extensively. Exposures depend on the type of welding: metal inert-gas (MIG), tungsten inert-gas (TIG), or manual metal (MMA) welding, as well as the type of welding electrodes (with or without flux). The following “total” aerosol fractions have been reported: 5-10 mg/m3 (Apostoli et al., 1992); 0.3-10.2 mg/m3 with a mean of 2.4 mg/m3 (Sjögren et al., 1985; MIG); 0.2-5.3 mg/m3 with a mean of 1.5 mg/m3 (Sjögren et al., 1988; mostly MIG); 0.2-6.1 mg/m3 with a mean of 1.4 mg/m3 ((Nielsen et al., 1993; MIG and TIG); 0.17 mg/m3 (electrodes without flux) and 0.81 mg/m3 (flux-coated electrodes) (Vandenplas et al., 1998; MMA)). The particles generated in MIG welding have a mass median diameter of about 0.4 μm, with those for TIG welding being somewhat smaller (Sjögren et al., 1985).
Physico-chemical properties (explosivity, flammability, oxidizing potential)
The explosivity, flammability, oxidizing potential and related reactivities of aluminium and its compounds have been noted in Table 3 and reviewed in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Compounds / Chemical and Morphological Speciation and Identity, Physical and Chemical Properties, Analytical Methods, Classification.
General Population Exposures
Air
In Table 13, aluminium concentrations in the PM2.5 and PM10 aerosol fractions are compiled. In the corresponding text (Human Exposure, Environmental Levels, Air), it was pointed out that the mean total particulate mass found for the PM2.5 and PM10 fraction measurements were near or exceeded the U.S. EPA’s annual standards in most instances for non-remote sites. A perusal of the aluminium-specific data in Table 13 indicates that mean PM2.5 concentrations were in the range 0.035-1.82 μg/m3 and 0.58-6.97 μg/m3 in the PM10 fractions. For agricultural communities, the maximum aluminium concentrations were 4.8 μg/m3 (PM2.5) and 17.3 μg/m3 (PM10), and 2.7 μg/m3 (PM2.5) and 5.4 μg/m3 (PM10) downwind of large urban centres. As pointed out in Human Exposure, Environmental Levels, Air, the natural crustal origin of aluminium accounts for the fact that the highest concentrations are being observed in agricultural areas.
For systemic effects of a toxicant, uptake into the blood stream is required. Consequently, from this perspective the PM10 aerosol fraction is more pertinent than the PM2.5 fraction. Particles are swallowed quickly when deposited in the nasopharynx region of the respiratory tract (inhalation through the nose) and when deposited in the oropharynx region (mouth breathing) (NRC, 1979). Similarly, deposited particles cleared by way of mucous from the ciliated nasal passages and the tracheobronchial tree are either expectorated or swallowed. Because deposition in the entire respiratory system (i.e., upper and lower) is included in the inhalable fraction (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation), it is for this reason that the inhalable aerosol fraction (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation) is recommended in the workplace when assessing potential systemic toxic outcomes (Nieboer et al., 1999; Vincent, 1993). Relative to this, the “total” aerosol fraction discussed in Human Exposure, Occupational Exposure would be second best, followed in order by the PM10 and PM2.5 fractions.
Food and beverages
It is clear that, in the absence of aluminium containing additives, frequently eaten foods contain relatively low concentrations of aluminium. Mean fresh-weight concentrations (in μg/g) typically increase in the order: beverages (1.5); fruit (2.7); fish (fresh or tinned, 3.2); milk, dairy products (4.5); meat, sausage, offal (5.4); vegetables (5.7); sugar, sugar-rich products (6.7); bread, cake and pastries (7.4); edible seeds (beans, peas, etc., 9.3); and meal, flour (9.5) (Müller et al., 1998). Items with higher mean concentrations (in μg/g fresh weight) of which smaller amounts are ingested include: herbs (19); cocoa and cocoa products (33); spices (145) and tea (900) (Müller et al., 1998). Steeped teas often contain 2-5 mg/L of aluminium (Baxter et al., 1989; Jackson & Huang, 1983; Müller et al., 1998; Pennington & Jones, 1989). Rajwanshi et al. (1997) reported concentrations double these values. Pennington & Jones (1989) and Pennington & Schoen (1995) clearly illustrated that food items containing aluminium additives (see Table 7) can appreciatively add to the dietary aluminium intake. They identified the following items as contributing significantly to the estimated daily intake of 11.5 mg/day calculated for a 14-16 year old male: cornbread (36.6% of total intake); American processed cheese (17.2%); pancakes (9.0%); yellow cake with icing (8%); taco/tostada (3.5%); cheeseburger (2.7%); tea (2.0%); hamburger (1.8%); fish sticks (1.5%). It was suspected that aluminium additives in processed cheese and baking ingredients were responsible. Saiyed & Yokel (2005) have confirmed that, due to the presence of aluminium additives (e.g., baking soda; leavening, anticaking, or emulsifying agents (Nieboer et al., 1995); also see Table 7), certain food ingredients and items constitute major sources of dietary aluminium. Examples are: processed cheese; pizzas; flour mixes for cakes and pancakes, and thus many baked goods and crusts; table salt; hot cocoa mix; pickle relish; and non-dairy creamers. Although the details are addressed in Human Exposure, General Population Exposures, Medical, aluminium intake from antacids, buffered aspirins, and antidiarrheal agents would increase by many fold the daily intake of this metal.
In the IPCS (1997) monograph, estimates of the average adult dietary daily intake of aluminium are tabulated for 8 countries. These data and other reported values for adults are depicted in Figure 5. It is clear that, since the mid 1980s, the estimates fall below 15 mg/day. As suggested in the IPCS (1997) review, the lower values in this group likely reflect reduced use of aluminium food additives in some of the countries. The intakes in Figure 5 estimated for or reported in 1985 or before of 20-25 mg/day may be due to the inclusion of larger portions of food items with additives, although analytical limitations cannot be excluded.
Figure 5. Estimates of aluminium consumption (by year).
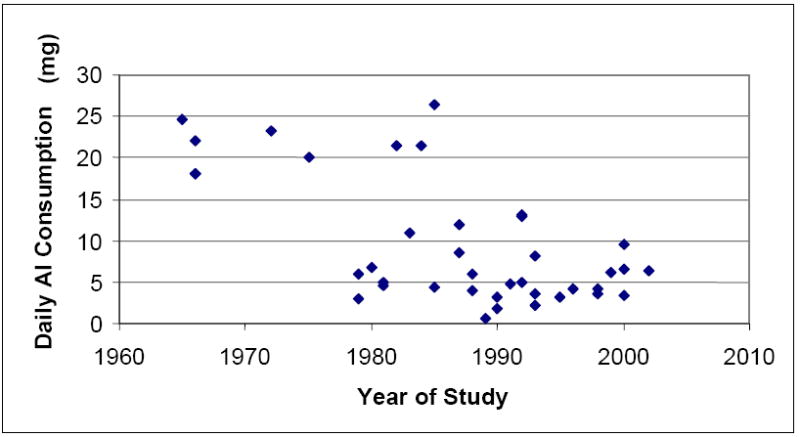
a) Based on daily consumption reported for adults in the period 1965-2001: Biego et al. (1998); CFASD (1984); Delves et al. (1993); Ellen et al. (1990); FASEB (1975); Greger (1985); Greger & Baier (1983); Gramiccioni et al. (1996); Gorsky et al. (1979); Hamilton & Minski (1972/1973); Jorhem & Haegglund (1992); Knutti & Zimmerli (1985); Liu et al. (1992); Liu & Chung (1992); MAFF (1985; MAFF 1993); Matsuda et al. (2001); Müller et al (1997); Neelam et al. (2000); NFA (1993); Pennington (1988); Pennington & Jones (1989); Pennington & Schoen (1995); Santaroni (1990); Soliman & Zikovsky (1999); Teraoka et al. (1981); Tipton et al. (1966); Tripathi et al. (2002); Varo & Koivistoinen (1980); Ysart et al. (2000); Zook & Lehmann (1965).
Intakes for infants appear considerably lower; for example, a 4-month old infant consuming cow’s milk-based formula was estimated to take in 0.03-0.05 mg/day, compared to 0.27-0.53 mg/day when fed soya-based formula (MAFF, 1993; also see Dabeka & McKenzie, 1992; and Pennington & Schoen, 1995). Navarro-Blasco & Alvarez-Galindo (2003) have reported comparable findings (based on the previous PTWI of 7 mg/kg b.w., rather than the current value of 1 mg/kg b.w.): “Standard formulae gave lower intakes amounting to about 4% PTWI; specialized and preterm formulae resulted in moderate intake (11 to 12 and 8 to 10% PTWI, respectively); and soya formulae contributed the highest intake (15% PTWI)”.
The daily intakes in Figure 5 derived from the Pennington & Jones (1989) and Pennington & Schoen (1995) studies include foods that have been in contact with aluminium foil or aluminium containers or that have been cooked in aluminium utensils. It is clear that these storage and preparation steps contribute aluminium to daily intake, especially for acidic foods (e.g., ATSDR, 1999; Fimreite et al., 1997; IPCS, 1997; Neelam et al., 2000; Ranau & Oehlenchälger, 2001). However, relative to the aluminium food additives, such contributions appear not to be appreciable, representing only a small fraction of the total dietary intake.
Drinking water
A perusal of recent reports of total and total dissolved aluminium (for definitions see Human Exposure, Environmental Levels, Water and Table 15) in drinking water after municipal treatment indicated that levels are generally below 7.4 μmol/L (200 μg/L). Recently reported concentrations of dissolved aluminium and the year collected are: 1.36±1.28 μmol/L (38±35 μg/L; 1995/6; (Gauthier et al., 2000); and 2.0-3.6 μmol/L (53-96 μg/L; 1995/6; see Table 15). Total aluminium levels are of comparable magnitude: 0.85-3.9 μmol/L (23-105 μg/L; 1993/7; (Zhao et al., 2001); 0.2-6.1 μmol/L (4-165 μg/L; c.2000; López et al., 2002); and a mean of 2.2 μmol/L (60 μg/L; 1999/2000; Gillette-Guyonnet et al., 2005). Compared to the compilation for the 1980s reported by Nieboer et al. (1995) there appears to be some moderate downward trend in the reported total aluminium concentrations.
Table 15.
Mean concentrations of total aluminium (μg/L) and proportion of constituent fractions % in drinking water (after purification treatment).
| Aluminium Fraction | Driscoll & Letterman (1995) | Schintu et al. (2000) |
|---|---|---|
| Number of plants (country) | 3 (U.S.) | 3 (Italy) |
| Collection period | Dec 1990 – Jan 1992 | Oct 1995 – Feb 1996 |
| Total (unfiltered, acidified), μmol/L (μg/L) | 4.7-6.3 (128-169) | 3.7-6.7 (100-180) |
| Total dissolved (after filtrationa; organic, inorganic and colloidal), % of total | - | 53-96 |
| Total monomeric, % of total | 21-82 | 40-62 |
| Organic monomeric, % of total monomeric | 15-51 | - |
| Organic (monomeric, tightly bound and colloidal), % of total dissolved | - | 12-59 |
| Inorganic monomeric, % of total monomeric | 49-85 | - |
| Inorganic (monomeric, tightly bound and colloidal), % of total dissolved | - | 41-88 |
| Particulate (suspended plus colloidal)b, % of total | 19-83c | 19 (mean) |
0.4 μm filter (Driscoll & Letterman, 1995) and 0.22 μm filter (Schintu et al., 2000)
Particulate aluminium is defined as the total (that available on acidifying unfiltered water) minus total dissolved (that available on acidifying after filtration)
Individual values were 19, 59 and 83 %.
The speciation summary provided in Table 15 illustrates that, after purification treatment, dissolved aluminium levels are not very different from those observed for surface (raw) water (see Human Exposure, Environmental Levels, Water). As in raw water, both inorganic and organic forms are present. However, operational filtering processes in treatment plants using aluminium coagulation can remove or add to the residual dissolved forms in drinking water (Berubé, 2004; Cech & Montera, 2000; Flaten, 2001). Other operational parameters are also known or suspected to influence the amount of dissolved aluminium in drinking water. As the pH decreases or increases from 6, the water-solubility of aluminium increases (Driscoll & Letterman, 1995; Driscoll & Schecher, 1989; Nieboer et al., 1995). Mean pH values of treated drinking water typically are around 8.0, with minimum values as low as 5.3 and maxima just above 9.0 (e.g., Nieboer et al., 1995). Further, addition of filter aids such as sulphate and non-ionic polymers appear effective in repressing residual dissolved aluminium; and solubility increases with water temperature (Driscoll & Letterman, 1995; Kvech & Edwards, 2002). Interestingly, the water quality data reported by Gauthier et al. (2000) suggest that the source of the raw water (e.g., lake, river or ground) may have some bearing. And finally, the type of water purification technology employed is an important determinant. Consequently, and as observed, dissolved aluminium concentrations in household water may be expected to be quite variable.
Many of the organic and inorganic water-soluble species discussed in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation and those shown to exist in surface waters and soil extracts (see Human Exposure, Environmental Levels, Water) may be expected to be stable at the pH values of drinking water.
Aluminium concentrations in bottled commercial drinking water have been reported in a small number of instances. Rosborg et al. (2005) report a median level of total aluminium of 1.33 μmol/L (36 μg/L). Interestingly, when they compared carbonated samples from a single brand, one contained in a plastic bottle and the other in an aluminium can, the respective concentrations were 0.63 μmol/L (17 μg/L) and 2.7 μmol/L (72 μg/L) respectively. Gillette-Guyonnet et al. (2005) reported that aluminium concentrations in 3 of 8 commercial mineral waters obtained in 1992 were below the detection limit of 0.1 μmol/L (3 μg/L), while the remainder had values between 0.2 μmol/L (5 μg/L) and 1.2 μmol/L (32 μg/L). By comparison, levels for 6 city water supplies sampled in the same time period ranged from 0.4 μmol/L (10 μg/L) to 2.3 μmol/L (63 μg/L), and another was 2.2 μmol/L (60 μg/L) in 1999-2000. Finally, López et al. (2002) found a mean ± SD of recoverable aluminium (that found on digestion at 120°C for 90 minutes) of 2.1±1.6 μmol/L (58±43 μg/L) in both 15 regional samples of tap water and 11 samples of glass bottled water bought in supermarkets. By contrast, mineral water purchased in plastic bottles had considerable higher concentrations, namely 4.5±1.2 μmol/L (121±32 μg/L). Leaching from the plastic storage bottles was suspected in this case.
Medical
Based on the aluminium compounds added to non-prescription drugs and anti-ulcerative drugs, the following daily doses (in mg) have been estimated: 840-5000 (antacids); 130-730 (buffered aspirins) and 830 (anti-ulcerative) (ATSDR, 1999; IPCS, 1997; Soni et al., 2001). These intakes are massive compared to the dietary intakes discussed in Human Exposure, General Population Exposures, Food and Beverages / Drinking Water. Antidiarrheal agents also contain considerable levels of aluminium additives, as much as 1450 mg per dose (ATSDR, 1999). Of course, other medical uses of aluminium compounds mentioned in Table 7 and in other sections of this document provide exposure opportunities, namely as astringents, antiseptics, analgesics, antimicrobial agents, vaccine adjuvants, topical drugs, and compounds of dental materials and prosthetics, among others.
In the past, iatrogenic aluminium poisoning has been a serious issue (Savory, 1994). This was due to the use of aluminium contaminated dialysis solutions and aluminium-based phosphate binders in patients with chronic renal failure and, similarly, contaminated human serum albumin and other biological products employed in i.v. therapy (see Nieboer et al. (1995) for a detailed summary; also see Effects on Humans, Subpopulations at Special Risk). The aluminium contamination of total parenteral nutrition solutions (see Sources of Human Exposure, Anthropogenic Sources, Legislative Controls, Classification and Labelling) has been mainly introduced in calcium gluconate and phosphates obtained from small volume parenteral vials (Mouser et al., 1998).
Miscellaneous exposures
Because of the multiple uses of aluminium and its compounds (see Tables 6 and 7), employment in workplaces where they are manufactured or used can potentially lead to exposures not systematically documented in the scientific literature.
Physico-chemical properties (explosivity, flammability, oxidizing potential)
As described in Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Compounds / Chemical and Morphological Speciation, and Identity, Physical and Chemical Properties, Analytical Methods, Classification, explosivity, flammability, oxidizing potential and related reactivities are not considered to be of primary interest with respect to assessing the health impacts of exposure to aluminium and its compounds.
Total Human Intake From All Environmental Pathways (Combined Exposure)
Estimates of daily aluminium intakes are provided in Table 16. For an individual who is not occupationally exposed and does not use antacids, buffered aspirin, or antiulcerative or antidiarrheal preparations (see Human Exposure, General Population Exposures, Medical), food is the major intake source of aluminium. If antacids, buffered aspirin, and other medicinal preparations are used, the food contribution will be relatively insignificant. The same is true for workers with occupational exposures, although in this case inhalation could be relatively more important than food as a source. The potential for anti-perspirants to contribute significant aluminium absorption through the skin has been suggested, but not well demonstrated (see Toxicokinetics, Absorption, Studies in Humans, Dermal Exposure).
Table 16.
Estimates of daily aluminium intakea.
| Source | Aluminium Concentration | Daily Intake (mg) | Bioavailabilityh (uptake) (%) |
|---|---|---|---|
| Foodb | 0.001 – 40 mg/100 g | 7.2 ± 0.3 (females, 14 and older) 8.6 ± 0.7 (males, 14 and older) |
0.05 - 0.1 |
| Drinking Water | ≤ 200 μg/L | 0.16 (mean)c | 0.3 |
| Ambient Aira | 0.6-7.0 μg/m3 (PM10) [occupational: 1-6 mg/m3 ‘total’]g | <0.06f [3.5-21.0]g | 1.0 - 2.0 (depends on water- solubility) |
| Antacidsa,d | 60-160 mg/5 mL or 110-174 mg/tablet | 120-7200 | 0.3 |
| Buffered Aspirina,d | --- | 200-1000 | 0.3 |
| Anti-perspirantse | As powder or solution (25% by w.t.) | ? | ? |
Based on: Van Oostdam et al. (1990).
Source: Pennington & Schoen (1995). It includes contributions from contact with aluminium foil or cookware; values for younger children are lower. Recent determinations of aluminium in foods have confirmed that the highest concentration reported here is typical of some processed foods (Saiyed & Yokel, 2005).
Mean value in drinking water of 107μg/L and a daily intake of 1.5L (Nieboer et al., 1995 and Human Exposure, General Population Exposures, Drinking Water).
From Ott (1990) and ATSDR (1999). Based on the daily recommended dose of 400 milliequivalents neutralizing capacity, typical intake of aluminium ranges 3500-5200 mg/day (Brunton, 1996).
From Hem & White (1989). The active ingredient is aluminium chlorohydrate, Al(OH)5Cl or aluminium chloride hexahydrate, AlCl3•6H2O. Other formulations have been reported (Yokel & McNamara, 2001). Based on the published literature, it remains uncertain whether there is absorption of Al through the skin. Therefore, a value for bioavailability could not be defined.
Intake 23 m3/day of air and 35% particle retention (Van Oostdam et al., 1990).
Intake of 10m3 per 8-hr shift and particle retention of 35% (Van Oostdam et al., 1990; ATSDR, 1999); the occupational air levels shown are typical for a work area with significant exposures to both water-insoluble and soluble aluminium such as those experienced in primary and secondary refining (see Table 14), or for exposures to small-sized particles such as for aluminium welders (see Human Exposure, Occupational Exposure, User Industries). Typical occupational ‘total’ exposure limits are: 10 mg/m3 (metal dust and aluminium oxide); 5 mg/m3 (pyro powders); and 2 mg/m3 (soluble salts) (ACGIH, 2005).
Based on estimates reported in Nieboer et al. (1995) and in Toxicokinetics, Absorption, Studies in Humans.
Uptake
Estimates of the bioavailability of aluminium for the various sources and pathways are summarized in the right-hand column of Table 16. They are based on comparing aluminium intakes by inhalation and ingestion with its urinary excretion (output), as well as toxicokinetic studies. Details are provided in Toxicokinetics, Absorption, Studies in Humans. Food is the primary source for uptake for individuals not occupationally exposed. However, chronic use of antacids, buffered aspirins and other medicinal preparations would likely constitute the major uptake source, even when exposed at work. In the absence of such medicinal usages, occupational exposure would be expected to contribute more to the body burden than food and drinking water.
TOXICOKINETICS
The different chemical forms in which aluminium can exist have a great impact on its toxicokinetics and toxicodynamics. For a discussion of aluminium speciation, complexation, solubility, and reaction rates see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties.
Absorption
Animal studies
Inhalation exposure
Inhalation exposure to aluminium is known to result from cosmetic use and from occupational and environmental sources.
The size of the inhaled particles is expected to have a profound effect on the deposition and absorption of aluminium in the lung. Dust comprises particles from < 1 to > 100 μm in diameter; inhalable particles are those with diameters up to 10 μm. Particles having diameters up to 10 and 2.5 μm are now classified as PM10 and PM2.5 respectively (for more details on these aerosol fractions see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Chemical and Morphological Speciation). Ultrafine particles have diameters < 0.1 μm. Particles can be removed from the respiratory tract by mucociliary clearance, the movement of mucous that covers the respiratory epithelium by cilia projecting from cells lining the respiratory tract. The mucous is moved up and out of the respiratory tract into the upper GI tract. This process has the potential to contribute to the oral route of exposure for substances initially deposited in the respiratory tract. Experimental studies have not isolated the pulmonary from other absorption sites (Rollin et al., 1991b).
The only data from which one can estimate the percentage of aluminium absorbed from inhalation exposure is from exposures in the occupational environment (see 5.1.2.1 below). As the percentage of aluminium estimated to be absorbed during inhalation exposure is greater than from oral aluminium intake (see 5.1.2.2 below), it seems unlikely that absorption from the GI tract accounts for the absorption of all inhaled aluminium. There has been no estimate of the percentage of aluminium absorbed via the intra-nasal route, so its role in relation to pulmonary absorption cannot be delineated.
Aluminium chlorohydrate is present in many aerosol anti-perspirants. Rats and guinea pigs were exposed to aerosolized aluminium chlorohydrate, 0.25, 2.5, or 25 mg/m3, 6 hr/day, 5 days/week, for up to 21 months (guinea pigs) or 24 months (rats). Neither animal species showed appreciable aluminium accumulation in the brain, heart, spleen, kidney, liver or serum, whereas significant increases in aluminium concentrations were seen in the lung of both species, adrenal glands of rats, and peri-bronchial lymph nodes of the guinea pigs (Stone et al., 1979).
Deposition of ~ 2 to 12% of fly ash into the lungs of rats was observed in three studies. Lung and pulmonary deposition of aluminium were 9.8 and 7.9% for spherical monodisperse aluminosilicate particles having a diameter of 2.2 μm (Raabe et al., 1977). Aluminium deposition into rat lung after 7 days of exposure to power plant fly ash with a mass median aerodynamic diameter (MMAD) of ~ 2 μm was 11.8% (Raabe et al., 1982). In another study, the percentage of aluminium in fly ash that deposited in the lung was calculated from the amount of aluminium in the lung of rats after exposure to 73 mg fly ash/m3 for 23 hr/day, 5 days/week for 1 month (Tanaka et al., 1983). The deposition fraction was 1.8%. Similar exposure of rats to a fly ash that was 9.7% aluminium and had a MMAD of 3 μm at 10.4 mg/m3 for 7 hr/day, 5 days/week for 1 month resulted in a deposition fraction of 5.1% (Matsuno et al., 1986). The authors attributed the differences to particle diameter, noting a smaller apparent deposition fraction with larger particles. Rabbits exposed to a mean concentration of 0.56 mg aluminium oxide/m3 for 8 hr/day, 5 days/week for 5 months showed significant increases in aluminium concentrations in the brain, lung, and bone that were, respectively, 247, 15,800 and 122% of the values for the controls, whereas aluminium levels in the heart were significantly lower (70% those of controls) (Rollin et al., 1991b). Serum aluminium levels increased during this study, although not consistently over time.
Oral administration
There has been considerable research on aluminium pharmacokinetics, including its oral absorption. However, most of this research, including all of that carried out prior to 1990, has been conducted using 27Al. To determine oral aluminium bioavailability, which is very low, and to see a significant increase of aluminium in blood, urine or tissue above the endogenous aluminium concentration, it was necessary to give very large doses of 27Al. More recently, studies have been conducted to estimate oral aluminium bioavailability using 26Al.
Bioavailability (fractional absorption) is the amount of a substance absorbed compared to the amount administered. With respect to the toxicokinetics of aluminium, systemic bioavailability, the fraction that ultimately reaches systemic circulation from where it has access to the brain and bone, the target organs for its toxicity, is most relevant. Oral aluminium bioavailability has been determined using several methods. Each of the methods has strengths and weaknesses. One of the first used was the balance study in which absorption was estimated based on the difference between intake and faecal, or urinary-plus- faecal, excretion. Estimating aluminium absorption based on the difference between intake and faecal excretion is not accurate for aluminium, for which oral bioavailability is very low. This approach assumes the difference between aluminium intake and faecal excretion is that which is absorbed and retained or excreted in the urine, which is the major route of elimination of absorbed aluminium. Small errors in determination of aluminium in faeces can significantly influence the estimate of bioavailability. As oral aluminium absorption is 1% or less under most conditions, with the balance passing through the GI tract unabsorbed, it would be very difficult to accurately determine the 1% loss of aluminium due to absorption based on the difference between oral intake and faecal excretion. This was acknowledged by Cam et al. (1976). Therefore, the results of studies that utilized this method (Allen & Fontenot, 1984; Cam et al., 1976; Clarkson et al., 1972) are not considered to be reliable estimates of oral aluminium absorption. Balance studies that estimate retention based on the difference between intake and urinary-plus-faecal excretion tend to overestimate the bioavailability of aluminium because aluminium can be retained on the gut wall and then be eventually excreted in faeces, but not absorbed. Therefore, the results of a study that utilized this method (Gorsky et al., 1979) are not considered a reliable estimate of oral aluminium absorption.
Estimating aluminium bioavailability based on urinary excretion compared to intake has been the method most commonly used to determine aluminium bioavailability. This method has many advantages. Collection of urine is less invasive than the sample collection requirements for most other methods that estimate oral bioavailability, which typically include blood and/or tissue. However, in the single dose, non-steady-state study, collection of all urine, or at least urine collection for a sufficient duration to ensure that nearly all of the urinary aluminium output from the dose has been obtained, is required. This is difficult when 27Al has been used as the aluminium dose because it is necessary to distinguish urinary excretion of the aluminium administered in the test dose from that ingested prior to the study or from other sources during the study. The requirement for total urine collection is also a compliance issue for the human subject. In the steady-state study, a sample collection period representing normal urinary aluminium output should be sufficient to reduce the compliance issue. Calculation of bioavailability assumes that all absorbed aluminium is excreted in the urine. This method may underestimate bioavailability due to the aluminium eliminated in bile, although this is only ~ 1%, (see 5.3.1.2 below), and the aluminium retained during the duration of the study. In a human study, Stauber et al. (1999) utilized two correction factors to estimate oral aluminium bioavailability. They collected urine for 24 hr and multiplied the amount of aluminium excreted by 2.2 to correct for the percentage of i.v. injected aluminium found in the urine after 7 days (72%), and the percentage of total aluminium excreted in 7 days that was excreted in the first day (62%).
Estimation of absorption from a single serum sample and the calculated volume of distribution would be expected to underestimate bioavailability because this approach does not account for aluminium that has not yet been absorbed, has distributed out of the vascular compartment, or has been excreted. It does not assure that peak serum aluminium was sampled unless independently determined. Underestimation of bioavailability by this method was shown by Hohl et al. (1994) who found that peak serum 26Al suggested 0.01% bioavailability, whereas cumulative urine 26Al estimated it to be 0.1%. Similarly, an approximate 10-fold greater estimate of bioavailability was obtained based on urinary aluminium excretion compared with that derived from a single (1, 4 or 24 hr) blood sample (Priest et al., 1996; 1998). The authors concluded: “…bioavailability cannot be accurately determined from blood 26Al or 27Al levels at a single time after administration” (Priest et al., 1996). Furthermore, an accurate estimate of volume of distribution assumes an accurate estimate of the t½ of elimination.
The use of the product of “tissue aluminium concentrations × tissue weights” to determine aluminium bioavailability assumes no aluminium elimination from the sampled tissues. If all tissues are not sampled, this method results in an underestimate of bioavailability. This method was employed by Wilhelm et al. (1992) who used bone and Zafar et al. (1997) who used liver, kidney, spleen, femur, brain, pancreas and blood. Some other studies estimated oral bioavailability from the sum of urinary aluminium excretion and levels of aluminium in bone (and liver and brain) tissue, thereby partially overcoming one of the limitations of using urinary aluminium excretion only to estimate bioavailability (Drüeke et al., 1997; Jouhanneau et al., 1993; 1997a).
Comparison of areas under the “plasma aluminium concentration (AUC) × time curve” after oral vs. i.v. dosing is the method generally accepted for determining the oral bioavailability of most substances (Rowland & Tozer, 1995). This method requires repeated blood sampling, which is a disadvantage. The use of 26Al and this method were employed by Zafar et al. (1997) who compared the AUC after oral, to i.p., not i.v., systemic injection, and by Yokel (2001) who compared the AUCs, or their equivalent, after oral administration of 26Al and i.v. administration of 27Al.
It is clear that aluminium can be orally absorbed. This has been shown by studies in which neurobehavioural changes and elevations of serum, urine and tissue aluminium following oral aluminium dosing of animals and humans have been reported. This corrects misinformation in 1965 stating: “… Large doses of soluble [Al] compounds taken orally will produce … no systemic effects” and “No entity ‘chronic aluminium poisoning’ has been identified in human beings” (Maynard, 1965).
Numerous studies have shown increased serum aluminium and/or urinary aluminium excretion after oral administration of various 27Al-containing products; the increases have often been seen to be influenced by the chemical form of the aluminium. These results suggest aluminium absorption from the GI tract (Beynon & Cassidy, 1990; Gorsky et al., 1979; Haram et al., 1987; Ittel et al., 1991a; 1991b; Kaehny et al., 1977; Knoll et al., 1984; Rauch et al., 1989; Recker et al., 1977; Robertson et al., 1989; Tsou et al., 1991; Winterberg et al., 1987a). However, most of the studies do not permit estimation of oral aluminium bioavailability.
In addition to elevations of serum or urine aluminium levels, numerous studies have shown increased levels of aluminium in the brain after oral 27Al exposure, demonstrating oral aluminium absorption. Some examples follow. Rats and dogs were fed 300 or 3000 mg aluminium hydroxide daily in their food for 5 months. These animals exhibited a significant elevation of brain aluminium concentrations, compared to those not receiving the aluminium hydroxide (Arieff et al., 1979). A significant elevation of brain aluminium was claimed after a single administration of oral aluminium hydroxide to mice (Cutrufo et al., 1984), although these authors did not report their actual results. Rat bone, but not brain, aluminium was significantly elevated after consumption for 8 weeks of a diet containing 570 mg sucralfate/kg. Sucralfate is a sucrose aluminium sulphate complex. The consumption of aluminium was approximately 4 mg/kg/day (Burnatowska-Hledin & Mayor, 1984). Consumption, by rabbits, of distilled water containing 0, 100 or 500 mg Al/L, introduced as aluminium chloride, for 12 weeks produced a positive correlation between aluminium exposure and aluminium in bone, stomach, intestine and kidney, but not in brain (Fulton & Jeffery, 1990). Brain aluminium was significantly increased in rats after 90 daily oral doses of 30 or 100 mg/kg of aluminium chloride (Bilkei-Gorzó, 1993). Consumption by mice of drinking water containing 400 mg Al/L (as aluminium lactate) for 6 months increased aluminium in brain and other tissues (Anghileri et al., 1994). Mice consuming ~20 μg Al/day as aluminium hydroxide gel in their drinking water for 105 days were reported to have 30, 60 and 340% increases in kidney, liver and brain aluminium concentrations, respectively (Sahin et al., 1994).
On the other hand, in many studies, increased blood or tissue aluminium levels were not found after oral 27Al administration. For example, rats consuming approximately 0, 0.01, 0.2 or 5.5 mg Al/kg/day in their drinking water, as aluminium chloride, in the absence or presence of acetate or citrate, failed to show an increase of aluminium in bone or brain (Fulton et al., 1989). Consumption of 0.112 mg Al/day for 20 days by one baboon did not result in increased serum aluminium compared to a 10-day period of 0.06 mg Al/day consumption in the same animal (Turnquest & Hallenbeck, 1991). This exposure level and duration were much smaller than in most other studies, so the negative result from this one animal is not informative. Prior to their report of 0.03% oral aluminium absorption from Zeolite A, Cefali et al. (1995; 1996) did not find significant aluminium absorption from Zeolite A, sodium aluminosilicate or aluminium hydroxide, containing 3.36, 0.90 and 27.8 mg Al/kg, respectively. Rats consuming drinking water containing 3.8 mg Al/L (as the chloride) for 10 weeks, and a diet containing 4 to 5 mg Al/kg, had no elevation of brain, bone or liver aluminium compared to those drinking water containing 0.05 mg Al/L (Glynn et al., 1995). Tissue aluminium concentrations significantly decreased from 0 to 10 weeks after consumption of this diet, perhaps due to consumption of a diet containing more aluminium prior to the study, which might have masked any changes due to the consumption of aluminium in drinking water during the study. At the pHs of these drinking waters, 4.3 to 4.6, speciation calculations suggested 99% of the aluminium should be labile species, and therefore available for absorption (Glynn et al., 1995).
In light of the evidence for oral absorption of aluminium presented in the paragraph immediately above, it must be assumed that there was insufficient aluminium absorbed in the studies described in the previous paragraph. Contributing to the lack of a significant increase of aluminium could be endogenous aluminium in the organism, contamination, and variability in the aluminium assay. The negative studies cannot be taken as proof of lack of oral absorption or as support for the null hypothesis that aluminium is not absorbed after oral administration.
Application of accelerator mass spectrometry to quantify 26Al has enabled study of aluminium toxicokinetics under physiological conditions. This method has been used since ~ 1990. For example, in one study, 2 rats were given a single gastric administration of 26Al in the absence of ligand during access to a normal diet; 2 rats did not receive 26Al. Seven days later the 26Al-treated rats had higher brain 26Al levels than the controls (Fink et al., 1994). In a second study by this group, using conditions that simulate drinking water, 8 rats were given a single gastric administration of 26Al in the absence of ligand after 30 hr of no access to food; 2 rats did not receive 26Al. Two weeks later, brain 26Al concentrations of 2 of the 26Al-dosed rats were comparable to those of controls (3 × 10-9 of the 26Al dose), whereas brain 26Al in the other 6 26Al-dosed rats ranged up to 100 times higher (Walton et al., 1995). The large variability is disconcerting. These results were frequently cited as evidence supporting the hypothesis that aluminium in drinking water contributes to brain aluminium accumulation. Assuming that a comparable fraction of orally consumed aluminium reaches and is permanently retained by the human brain, Walton et al. (1995) concluded that humans drinking alum-treated water (ATW) over seven to eight decades would have ~ 1 mg Al/kg wet brain. This is greater than normal levels of aluminium in the brain. On the other hand, these results could support the hypothesis that aluminium in drinking water does not contribute to brain aluminium accumulation if one uses the lowest brain aluminium level obtained after 26Al dosing, or if aluminium is not permanently retained by the brain. Subsequent studies utilizing 26Al, reviewed below, have clarified this issue.
Studies in animals utilizing 26Al, also reviewed below, have shown the ability of aluminium to enter bone after oral administration.
No published information was found on buccal aluminium absorption.
In summary, there are sufficient studies in which 27Al was utilized by many different research groups to investigate neurobehavioural endpoints or in which blood, urine and/or tissue aluminium levels were studied following controlled administration of 27Al or 26Al, to show that aluminium can be absorbed after oral administration. These studies also show that this route of exposure has the potential to produce toxicity. However, many of the studies with 27Al were conducted utilizing supra-physiological exposures or doses of aluminium. This has led to the controversy of whether or not aluminium exposure, under normal conditions, has the potential to produce toxicity.
Drinking water
The average daily intake of aluminium from drinking water for a 70 kg person is approximately 160 μg, or about 2.3 μg/kg b.w./day. Studies that best model oral aluminium bioavailability from drinking water meet two criteria. They use aluminium doses ~ 2 μg Al/kg b.w./day, which reasonably compares with daily oral aluminium intake from water by adult humans of ~ 2.3 μg Al/kg b.w./day, (see Human Exposure, Total Human Uptake from All Environmental Pathways (Combined Exposure) and Evaluation of Human Health Risks, Health Effects, Exposure Characterization) and they introduce the aluminium either as a chemical species that might be found in drinking water, that is, as the chloride, sulphate or hydroxide, which have the ability to release the free aluminium ion in solution, or they use water from municipal water supplies. As noted above, by necessity, all studies with 27Al use aluminium doses much greater than 2 μg Al/kg. The use of 26Al enables determination of oral aluminium bioavailability after administration of ≤ 2 μg Al/kg.
Estimates of the oral bioavailability of aluminium, based on administration of 27Al chloride in the rat, were 0.06 to 0.2% (Ittel et al., 1987) and 0.04% (Froment et al., 1989a). Administration of 27Al as the chloride, nitrate, lactate or citrate resulted in absorption of 0.57, 1.16, 0.7 to 1.9, and 2.18% in the rabbit (Yokel & McNamara, 1985; 1988). The difference in results obtained in these studies may relate to the difference in physiology of the stomach of these two animal species and/or gastric pH. The rat has a single compartment stomach whereas the rabbit’s stomach has more than one compartment. The pH of suckling rabbit gastric juice is ~ 4 to 6.5 dropping to ~ 1.5 to 2.5 post-weaning (Beauville et al., 1966; Kaplan & Timmons, 1979; Yu & Tsen, 1993) whereas the pH of rat gastric juice is ~ 3.2 (Cunha-Melo et al., 1983; Lysiak, 1963). The human stomach pH is typically 1 to 3. The lower pH may result in conversion of more aluminium to monomeric species. However, as aluminium is not significantly absorbed from the stomach (see Toxicokinetics, Absorption, Animal Studies, Oral Administration, The Site and Mechanisms of Oral Aluminium Absorption), its chemical species would be expected to change as it enters the upper GI tract. Estimates based on studies utilizing 27Al suggest the oral bioavailability of the aluminium ion is < 1%.
Several studies utilized the tracer 26Al and total aluminium doses of 0.1 to 2 μg Al/kg. However, many of these studies had very small sample sizes and some were not well controlled. The amount of 26Al, after administration in the absence of ligand in distilled water that was estimated to be in the plasma of two rats 8, 24 and 48 hr after dosing was 0.0001 to 0.001% (Jouhanneau et al., 1993). These rats had free access to food and water. When plasma aluminium concentrations obtained by Jouhanneau et al. (1993) are compared to those obtained at similar times from one rat after i.v. 26Al injection, in pH 5 saline solution (Meirav et al., 1991), oral 26Al bioavailability is estimated to be 0.033% (8 hrs), 0.087% (24 hrs) or 0.2% (48 hrs). In a study in which 26Al was given to 9 rats/group, the fraction of the dose that was in plasma 0.5 to 5 hr later was estimated (Schönholzer et al., 1997). They pooled the plasma samples at each time point for a single 26Al analysis. The plasma 26Al area under the curve suggested greater 26Al bioavailability in the presence of 27Al citrate + Na citrate than 27Al citrate or 27Al hydroxide. Estimates of bioavailability based on urinary aluminium excretion were 5.1, 0.7 and 0.1%, respectively (Schönholzer et al., 1997). Studies with aluminium hydroxide and aluminium chloride, which may release the free aluminium ion at the low total aluminium concentrations tested, suggested an oral aluminium bioavailability of about 0.06 to 0.1% (Drüeke et al., 1997; Schönholzer et al., 1997). Schönholzer et al. (1997) may have underestimated aluminium bioavailability because they only collected the eliminated urine for 300 minutes after dosing and the urine in the bladder at 300 minutes. Although Jouhanneau et al. (1997a) studied only 2 rats at each euthanasia time point, there were 8 rats/group in the study by Drüeke et al. (1997). Jouhanneau et al. (1997a) collected total urine output for up to 720 hr. Of the total urinary aluminium output, 85% was in the first day, about 4% in the second, an additional 5% in days 3 to 5, and 6% in days 6 to 29. They based their estimate of oral aluminium bioavailability on the sum of the aluminium excreted in urine and that present in bone, liver and brain. Drüeke et al. (1997) based their estimate on cumulative 48 hr urinary aluminium excretion and on aluminium found in the skeleton at 48 hr. These studies were conducted under conditions in which it was likely that the subjects had food in their stomachs (Drüeke et al., 1997; Jouhanneau et al., 1997a) or, possibly, had some stomach contents. Food and/or faeces have been found in the stomachs of rats after a 24-hr (Walton et al., 1995) and 36-hr fast (Yokel et al., unpublished results). This is partly because rats recycle faeces (the practice of coprophagia). Therefore, it is probable that there were stomach contents in the rats studied by Schönholzer et al. (1997) after the 16 hr overnight fast. Zafar et al. (1997) gave 26Al (in the presence of 27Al chloride) by the oral route to 3 subjects and by i.p. injection to 3 others and concluded that oral aluminium bioavailability was 1 to 1.6%. Based on 26Al recovered in urine, liver, spleen and bone after an oral administration of 20 ng 26Al and 200 μg 27Al, as the chloride, oral bioavailability was estimated to be 0.133 % in control rats (Ittel et al., 1997). Oral aluminium bioavailability was determined by concurrent administration of oral aluminium as one isotope, 26Al (in the absence of a ligand), and i.v. aluminium (as Al potassium sulphate) as another, 27Al (Yokel et al., 2001a). Oral aluminium bioavailability was ~ 0.28% in rats that had no stomach contents. Using the rat in situ intestinal perfusion technique, absorption of aluminium from aluminium chloride and equimolar trisodium citrate, based on aluminium in blood and a number of tissues, was estimated to be 0.2% (Arnich et al., 2004).
There is some evidence that fractional absorption of aluminium is dose dependent in the fasted animal. Oral aluminium bioavailability in the rabbit dosed with 108 or 540 mg Al/kg, as aluminium lactate, was 0.7 and 1.9% (Yokel & McNamara, 1985). Although not significantly different, these results suggest a positive correlation between aluminium dose and bioavailability. On the other hand, absorption of aluminium into rat blood and tissues after perfusion of the gut with 48 or 64 mM aluminium chloride at pH 3 was not concentration dependent (Arnich et al., 2004).
Overall, these results suggest that oral aluminium bioavailability from water in the rat and rabbit is in the range of 0.05 to 0.4% and most likely ~ 0.3%.
Beverages and foods
Although food comprises the primary source (> 90%) of aluminium for the typical human (see Human Exposure, General Population Exposures, Food and Beverages), there are very few data on oral aluminium bioavailability from foods, or beverages other than water. The difficulties in estimating aluminium bioavailability from water using 27Al, discussed above, apply to food. It has generally been assumed that oral aluminium bioavailability from food is less than that from water due to the aluminium being incorporated in high molecular weight, relatively insoluble, complexes (Glynn et al., 1995).
It has been suggested that the aluminium in tea leaves has low oral bioavailability (Glynn, 1995). In tea, 91 to 100% of aluminium is in organic complexes with a Mr > 20,000. Even at pH 2, ~ 83% remained bound to the organic matter (French et al., 1989; Gardner & Gunn, 1991). For example, a much lower percentage (15%) of the aluminium in tea was found to be in chemically labile species, compared with that in drinking water (61 to 75%) (Stauber et al., 1999). Approximately 50% of aluminium in tea infusions was as soluble species, ~ 50% as non-labile monomeric aluminium species and a small fraction as labile monomeric aluminium, whereas > 90% of aluminium in tap waters was labile monomeric aluminium (Chen et al., 2004). A large fraction of the aluminium in tea infusions was very strongly bound to unidentified ligands (Alberti et al., 2003). Kralj et al. (2005) found that ~ 10 to 35% of the aluminium in tea was negatively charged aluminium citrate. They thought the remainder was bound to phenolic compounds. Addition of citrate increased the negatively charged aluminium citrate species by up to 40% of total aluminium, whereas milk complexed most of the aluminium that was not associated with citrate to protein, mainly casein. The authors suggested that addition of citrate and milk protein would enhance aluminium absorption. Reiber et al. (1995) suggested that a substantial portion of aluminium, regardless of the form consumed, will be solubilized to monomeric aluminium in the stomach and subsequently converted to poorly soluble aluminium species in the near neutral pH of the upper intestine. As the stomach is not an important site of aluminium absorption, this implies that oral aluminium bioavailability should be aluminium species independent. Citrate and other ligands influence aluminium absorption, suggesting that this hypothesis is an oversimplification.
Weanling rats whose liquid source for three weeks was a diet soft drink from aluminium cans containing 0.47 mg Al/L, had significantly higher liver and bone aluminium concentrations than rats which drank a soft drink from glass bottles containing 0.38 mg Al/L (Kandiah & Kies, 1994). Bone aluminium was 69% higher in rats that drank the soft drink from the aluminium cans than in rats that drank distilled water containing 0.023 mg Al/L. However, liver aluminium concentration was significantly lower, and the concentration of aluminium in bone was non-significantly lower, in rats that drank bottled soft drink than in those that consumed distilled drinking water containing 0.23 mg Al/L, thereby casting doubt on the results of this study. It is surprising that a 24% increase in aluminium intake from beverage would result in a 62, 127 and 84% increase in brain, bone and liver aluminium levels. The authors reported that the rat ration contained 110 mg Al/kg, which is much greater than the beverages. In other studies, rat diet has been found to contain 100, 5, and 51 mg Al/kg (Glynn et al., 1995; Gupta et al., 1986; Yokel et al., unpublished results), mouse diet to contain 131 and 64.5 mg Al/kg (Dlugaszek et al., 2000; Fosmire et al., 1993), guinea pig diet to contain 47 and 60 mg Al/kg (Golub et al., 1996a; Owen et al., 1994), and rabbit diet to contain 297, 1215 and 335 mg Al/kg (Fulton & Jeffery, 1990; Yokel & McNamara, 1985; Yokel et al., unpublished results).
No increases in blood or liver aluminium concentrations were seen in rats which consumed tea as the only source of fluid for 28 days (Fairweather-Tait et al., 1991). In one study, it was reported that increased tissue aluminium concentrations were attributed to the intake of aluminium in food. Guinea pigs that ate a test diet of sponge cake three times weekly for 3 weeks providing a total of 40 mg of aluminium, as acidic sodium aluminium phosphate (SALP) showed a significant elevation in bone aluminium concentrations compared with those that ate only guinea pig chow, which provided a total of 3 mg aluminium (Owen et al., 1994).
In an ambitious but not definitive study, Walton et al. (1994) orally administered various beverages and foods to anaesthetized rats, in the absence of aluminium-treated water. Tail blood and urine, withdrawn by needle aspiration of the bladder, were obtained prior to, and 1, 2, 3 and 4 hr after, dosing. Considering the short period of observation (4 hr) serum aluminium is probably a better indicator of absorbed aluminium than is urinary excretion. Margarine and 8 mg of aluminium, as aluminium sulphate, increased serum aluminium levels. The aluminium content of the margarine was not determined. The other beverages and foods tested, beer, Coca Cola®, coffee, orange juice, tea, wine, apple, broccoli, butter, meat and Vita Wheat®, did not appreciably increase serum aluminium levels when administered alone. This is not surprising as the aluminium dose provided by these beverages and foods, which ranged from 0.005 to 8.6 μg, was ≤ 0.1% of the 8 mg of aluminium in the 1 mL of water which produced an elevation of serum aluminium of 4 to 5-fold. Determination of relative oral bioavailability of aluminium from food and water was not the objective of this study. Little can be learned from these results concerning the oral bioavailability of aluminium from foods.
Although rigorous pharmacokinetic determination of oral aluminium bioavailability from milk could not be determined, it was estimated to be < 1% in rabbits (Yokel & McNamara, 1985).
Weanling rats fed 1 to 2.7 gm of Al/kg diet, added as aluminium hydroxide in the absence and presence of added sodium citrate dehydrate, were estimated to absorb 0.01 to 0.04% of the aluminium (Greger & Powers, 1992). The percentage of aluminium absorbed was lower with the higher dietary concentration of aluminium as the citrate, in contrast to above studies, thereby showing a positive correlation between aluminium dose and fraction absorbed.
There are sufficient studies with reasonably similar results to estimate the oral bioavailability of aluminium from water. Those who suggest water may be a significant contributor of the aluminium body burden assume that aluminium in water is more bioavailable than the aluminium in food. Some recent data support this assumption, as presented in the next paragraph (Yokel & Florence, 2006). Diet provides most of the human’s daily aluminium intake. Those who wish to allay any concern about drinking water as a source of aluminium suggest that the oral bioavailability of aluminium from food and water are similar (Reiber et al., 1995) and have provided some data to support this conclusion (Stauber et al., 1999).
The bioavailability of aluminium from selected foods has been estimated in the rat. 26Al was incorporated into the synthesis of acidic SALP, used as a leavening agent in baked goods, and then incorporated into a biscuit. Basic SALP, used as an emulsifier in cheese, was incorporated into a processed cheese (Yokel et al., 2005). When rats, that had no stomach contents, ate the biscuit containing acidic SALP, it was estimated that oral aluminium bioavailability was ~ 0.1%, significantly less than from water (Yokel & Florence, 2006). Oral Al bioavailability from the cheese containing basic SALP was ~0.1 to 0.3% (Yokel et al., unpublished results). Comparison of oral Al bioavailability from these representative foods to that obtained from water (~0.3%) times the contributions of food and drinking water to the typical human’s daily Al intake (~5 to 10 mg from food and 0.1 mg from water, respectively) suggests food provides ~25-fold more Al to systemic circulation, and potential Al body burden, than does drinking water.
The very limited data available, above, suggest that oral aluminium bioavailability from food is less than from water.
Drugs
Relevant published data on the oral bioavailability of aluminium species from drugs include the use of aluminium hydroxide as an antacid and phosphate binder; sucralfate as an antacid; aluminium lactate as a component in dental products for sensitive teeth; an aluminium silicate product, Zeolite A, which is an inducer of osteoblast proliferation; and aluminium in the presence of citrate. Some of the earlier work, conducted with 27Al, was reviewed by Wilhelm & Ohnesorge (1990). Because of its poor absorption, use of large doses of aluminium was necessitated in studies of bolus 27Al dosing to determine oral aluminium bioavailability. The chemical species of the aluminium, when given in large doses, particularly when the aluminium species has good buffering capacity, such as aluminium hydroxide, would not be expected to be totally affected by the normal chemical reactions in the gut. It is less likely that the series of chemical reactions in the gut that might produce comparable aluminium species irrespective of aluminium species ingested, as proposed by Reiber et al. (1995), would be relevant. Therefore, these studies might more closely reflect the absorption of the aluminium species introduced, albeit after very high doses, assuming there is no saturation of uptake processes.
The bioavailability of aluminium from ingested aluminium hydroxide appears to be less than from the aluminium ion, based on studies using 27Al. In the rabbit, 0.45% of aluminium as the hydroxide was absorbed compared with 0.57 to 1.16% from aluminium chloride and nitrate (Yokel & McNamara, 1988). In the rat, 0.01% of aluminium from the hydroxide was absorbed compared with 0.037% from the chloride (Froment et al., 1989a). Wilhelm et al. (1992) could not detect aluminium absorption after an oral dose of 1 mg Al/kg, as aluminium lactate, illustrating the necessity of using large doses in bolus 27Al dosing studies. Their estimate of 1% oral bioavailability, based on bone aluminium 17 days after oral aluminium dosing, would be an underestimate if any bone aluminium were eliminated within that time, although there may not have been significant bone aluminium elimination (see Toxicokinetics, Elimination and Excretion, Animal Studies, Elimination Rate).
The absorption of aluminium from aluminium citrate is greater than from aluminium hydroxide: 2.18 vs. 0.45% in the rabbit (Yokel & McNamara, 1988), 1.49 vs. 0.015% and 1 vs. 0.1% in the rat (Froment et al., 1989a; Schönholzer et al., 1997). Sucralfate, which, like aluminium hydroxide, is insoluble in water but soluble in acid and base, exhibits oral bioavailability comparable to that of aluminium hydroxide and lower than that of soluble aluminium species. Aluminium bioavailability from sucralfate in the rabbit was 0.6%, and was 0.63% from aluminium lactate, compared with 0.57 to 1.16% from aluminium chloride and nitrate (Yokel & McNamara, 1988). In the rat, oral aluminium bioavailability was 0.015% from aluminium hydroxide and sucralfate, 0.037% from aluminium lactate and aluminium chloride, and 1.49% from aluminium citrate (Froment et al., 1989a).
Cefali et al. (1996) studied aluminium absorption from Zeolite A®, an aluminium silicate product, in dogs; they observed highly variable plasma aluminium levels during the control phase and a small increase of plasma aluminium as a result of the treatments. They did not use baseline-corrected data in their determination of aluminium pharmacokinetics. Therefore, one cannot have much confidence in these results.
Factors influencing oral aluminium absorption
Oral aluminium absorption is dependent on many factors. A summary of the reported factors affecting oral aluminium absorption in animals is shown in Table 17. The following sections describe these effects in much more detail.
Table 17.
Summary of the influence of factors that affect oral aluminium absorption from studies in animals.
| Factor | Influence on absorption | Reference(s) |
|---|---|---|
| Solubility | Greater solubility, increased absorption. | Froment et al. (1989a); Yokel & McNamara (1988) |
| pH | Greater absorption at pH 4 than 7. | Van der Voet & De Wolff (1986-1987) |
| Carboxylic acids (citrate) | Increased | Deng et al. (1998; 2000); Drüeke et al. (1997); Froment et al. (1989a); Partridge et al. (1989; 1992); Schönholzer et al. (1997); Sutherland & Greger (1998); Van der Voet et al. (1989); Yokel & McNamara (1988) |
| Carboxylic acids (acetate, oxalate, lactate, malate, tartrate, gluconate, ascorbate, or carbonate) | Increased, but less than produced by citrate. | Colomina et al. (1994); Domingo et al. (1991a; 1993; 1994); Gómez et al. (1994); Nestel et al. (1994) |
| Silicon-containing compounds | May reduce absorption. | Bellés et al. (1998); Quartley et al. (1993) |
| Fluoride | May enhance. | Allain et al. (1996); Xiao et al. (1992) |
| Iron | Inverse correlation between dietary iron, and iron status, and aluminium absorption. | Brown & Schwartz (1992); Cannata et al. (1991); Fernández et al. (1989); Fernandez Menendez et al. (1991); Florence et al. (1994); Winklhofer et al. (2000) |
| Calcium | Inverse correlation between dietary calcium, and calcium status, and aluminium absorption. | Feinroth et al. (1982); Provan & Yokel (1990); Taneda (1984) |
| Sodium | Reduction increased aluminium uptake. | Van der Voet & de Wolff (1987a) |
| Ethanol | May increase aluminium absorption or decrease elimination. | Flora et al. (1991) |
| Uraemia | Enhanced absorption. | Ittel et al. (1987; 1988; 1991b; 1997); Olaizola et al. (1989); Yokel & McNamara (1988) |
| Foods and dietary Components | Results suggest both that food does and does not inhibit aluminium absorption. | Drüeke et al. (1997); Jouhanneau et al. (1997a); Schönholzer et al. (1997); Yokel et al. (2001b) |
| Aluminium dose | Increased dose increased aluminium bioavailability from soluble aluminium species. | Ittel et al. (1993b); Yokel & McNamara (1985) |
Solubility
There is evidence of greater aluminium absorption from more soluble aluminium species. Aluminium borate, glycinate, hydroxide and sucralfate are much less soluble in water than aluminium chloride, lactate, nitrate and citrate and were generally less well absorbed (0.27, 0.39, 0.45 and 0.60 vs. 0.57, 0.63, 1.16 and 2.18%, respectively (Yokel & McNamara, 1988). Similarly, aluminium hydroxide and sucralfate are less soluble at pH 3, 6 and 7 than aluminium lactate and chloride and were also less well absorbed (0.015 vs. 0.037%), based on urinary aluminium excretion (Froment et al., 1989a).
pH
The chemical speciation of aluminium is known to be greatly influenced by pH (Harris et al., 1996). The bioavailability of aluminium, to some extent, is influenced by the aluminium species. However, Reiber et al. (1995) suggested that the human GI tract acts as a series of reactors. They predicted that, in the stomach, all aluminium would be converted to small molecular weight soluble species due to the predominance of the hydrated aluminium ion at pH 1 to 2. They suggested that, when the stomach contents enter the duodenum/jejunum where the pH increases to 4.5 to 6.8, all aluminium would be converted to hydroxides. They predicted uniform oral bioavailability of aluminium, independent of the chemical species of the aluminium consumed. However, this ignores the presence of ligands to compete with hydroxide to associate with aluminium at the site of absorption. Using the in situ rat intestine preparation with systemic and portal blood sampling, Van der Voet & de Wolff (1986-1987) found aluminium absorption to be higher at pH 4 than at pH 7, presumably due to generation of more soluble aluminium species. However, the predicted aluminium species at acidic pHs, in the absence of binding ligands, are Al(H2O)63+ and Al(OH)2+ (Harris et al., 1996), which would not be expected to permeate the gut wall by diffusion. This is consistent with the assumed primary site of GI aluminium absorption, the upper intestine, discussed below (see Toxicokinetics, Absorption, Animal Studies, Oral Administration, The Site and Mechanisms of Oral Aluminium Absorption).
Carboxylic acids
Citrate and other carboxylic acids form coordination complexes with aluminium. The co-administration of citrate with aluminium-containing drugs is relevant to iatrogenic (medical) aluminium exposure.
The chemistry of aluminium interaction with citrate has been quite extensively studied (Harris et al., 1996) (see also Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Compounds). Citrate can complex aluminium, forming an electrically neutral 1:1 citrate:aluminium complex at pH 2 to 5 in the absence of great excess of citrate. At higher pHs, this complex deprotonates, with a pKa of ~ 4, to a complex with one negative charge held by a carboxylate group that is not involved in the aluminium citrate:complex (Lakatos et al., 2001). More complex chemical species slowly form at higher concentrations (Harris et al., 1996).
Citrate may enhance oral aluminium absorption if there is sufficient citrate present to compete with other binding ligands for aluminium in the GI tract. Speciation calculations indicated that citrate would solubilize ~ 97% of aluminium in the stomach (Glynn et al., 2001). Citrate is the major small molecular weight ligand for aluminium in plasma. Formation of aluminium citrate also enables the distribution of aluminium out of plasma, and may enhance aluminium elimination by the kidney in the presence of renal function. However, in the absence of renal function, citrate can significantly increase aluminium-induced toxicity, presumably by enhancing aluminium distribution out of the blood and the resultant tissue aluminium accumulation. However, the enhanced solubility of aluminium by citrate did not completely account for enhanced aluminium absorption (Froment et al., 1989b). It has been suggested that aluminium citrate is sufficiently lipid soluble from pH 2.5 to 8 to be absorbed by diffusion (Partridge et al., 1992). Taylor et al. (1998) argued that the alkaline environment of the upper intestine could produce insoluble aluminium species, as the aluminium citrate species would not predominate at neutral and higher pHs. However, the typical pH of the human duodenum/jejunum ranges from 4.5 to 6.8, which is not alkaline. Furthermore, in the presence of equimolar aluminium and citrate, aluminium citrate-hydroxide complexes would predominate (W.R. Harris, personal communication, 2004). Alternatively, enhanced aluminium absorption may require sufficient citrate to activate another mechanism of aluminium absorption. Other hypotheses to explain the enhancement of aluminium absorption by citrate are the transport of aluminium citrate into mucosal cells, and citrate opening the tight junctions of the intestinal cells, as discussed in Toxicokinetics, Absorption, Animal Studies, Oral Administration, The Site and Mechanisms of Oral Aluminium Absorption.
Numerous animal studies have shown that aluminium is more bioavailable when administered as the citrate rather than as other chemical species (Cunat et al., 2000; Deng et al., 1998; 2000; Drüeke et al., 1997; Froment et al., 1989b; Partridge et al., 1989; 1992; Schönholzer et al., 1997; Sutherland & Greger, 1998; Van der Voet et al.,1989; Yokel & McNamara, 1988). It was estimated that 3.4 to 4.2% of aluminium was absorbed by rats, from aluminium citrate; however, no direct comparison was made with aluminium dosing in the absence of citrate (Sutherland & Greger, 1998). Numerous studies in humans have also shown enhanced aluminium absorption in the presence of citric and other carboxylic acids (see Toxicokinetics, Absorption, Studies in Humans, Oral Administration, Factors Influencing Oral Aluminium Absorption, Carboxylic Acids). However, a few studies failed to find an increase of aluminium absorption in the presence of citrate. These were conducted in subjects who received aluminium during free food access (Jouhanneau et al., 1993; 1997a).
An increase in the citrate:26Al ratio increased oral aluminium bioavailability in one study (Schönholzer et al., 1997). However, the increase of citrate was accompanied by an increase of the 26Al dose and pH of the delivered solution, confounding the interpretation of the variable that increased oral aluminium bioavailability 5-fold in this study. A citrate-induced increase of brain and bone aluminium has been reported in many studies (Bilkei-Gorzó, 1993; Deng et al., 1998; Domingo et al., 1991a; 1993; Ecelbarger et al., 1994a; Ecelbarger & Greger, 1991; Fulton et al., 1989; Fulton & Jeffery, 1990; Greger & Powers, 1992; Owen et al., 1994; Radunovic et al., 1998; Slanina et al., 1984; 1985; Testolin et al., 1996) though not in others (Jouhanneau et al., 1997a; Van Ginkel et al., 1993). Moreover, Maitani et al. (1994) found that citrate addition decreased tissue aluminium concentrations. It was concluded that the chronic consumption of aluminium and citrate by gavage resulted in greater aluminium retention than when aluminium was delivered in the diet which, in turn, was greater than when delivered in drinking water (Greger & Sutherland, 1997). The authors suggested that the presence of other substances in the GI tract from the diet, which would not be present when aluminium is administered by gavage, may have modified the effect of citrate on aluminium absorption.
Concurrent acute administration of 2 mmole aluminium with 2 mmole citric, gallic, chlorogenic, caffeic or protocatechuic acids resulted in a significant increase in rat blood aluminium 2 hr later only with citric acid. There was no effect on liver aluminium from any ligand, an increase of kidney aluminium with citric and chlorogenic acid, and an increase in tibial aluminium with all but chlorogenic acid (Deng et al., 2000). Consumption by rats of a diet containing 16 mmole/kg of aluminium chloride and carboxylic acid resulted in no significant differences of aluminium in blood, liver, kidney or tibia; but an increase of aluminium in brain after citric and chlorogenic acids (Deng et al., 2000). The very high aluminium concentrations reported in blood and tissues in the control subjects reduce confidence in these results.
Citrate may enhance oral absorption and may also enhance distribution into and out of tissues as well as from the organism by renal elimination, in the presence of renal function, as suggested by (Maitani et al., 1994). Citrate inhibited or produced only a small increase of aluminium uptake into neuroblastoma and human erythroleukemia cells (Guy et al., 1990; McGregor et al., 1991; Shi & Haug, 1990).
Ultrafilterable species may redistribute within the organism, particularly in the presence of reduced renal function. Quartley et al. (1993) gave rats a single oral dose of aluminium citrate; 2, 4 and 24 hr later they found elevated aluminium in all tissues but the brain. The aluminium concentration decreased in most tissues from 2 to 24 hr, except for the bone, in which it increased. Citrate may have enabled redistribution of the aluminium into bone.
Other short chain carboxylic acids, including acetate, oxalate, lactate, malate, tartrate, gluconate, ascorbate, and carbonate have been similarly shown to increase aluminium absorption or tissue aluminium accumulation in some, but not all, animal studies. However, these substances were generally less effective than citrate (Colomina et al., 1994; Domingo et al., 1991a; 1993; 1994; Gómez et al., 1994; Nestel et al., 1994). This may be due to the formation of a more stable complex between aluminium and citrate than these other ligands.
Aluminium can form a complex with the dietary long chain mono-unsaturated FA, oleic acid, but the stability of this complex has apparently not been described (Krasnukhina et al., 1971; Lesnikovich et al., 1993). It appears aluminium does not complex with the saturated long chain FA stearate (Ross & Takacs, 1983). No information was found on aluminium complexation with the dietary saturated long chain FA, palmitic acid, the long chain poly-unsaturated FAs, α-linolenic and arachidonic acids, and the long chain branched chlorophyll degradation products, phytonic and pristonic acids. Aluminium can form a 1:1 complex with phytic acid but the reaction is slower and less favoured thermodynamically than phytic acid complexation with divalent cations (Evans & Martin, 1988). Insoluble aluminium-phytate complexes form at higher aluminium:phytate mole ratios (Evans & Martin,1988).
Silicon-containing compounds
There is evidence suggesting that increased dietary intake of silicon (Si)-containing compounds may reduce aluminium absorption and facilitate aluminium excretion. There are also studies that did not find an effect of silicon-containing compounds on aluminium absorption. Silicon is absorbed in the animal GI tract as monomeric silicic acid. It can react with aluminium to form hydroxyaluminosilicate species and slowly, but eventually, amorphous solids (Birchall et al., 1996). This was thought to reduce aluminium bioavailability, as shown by reduced systemic aluminium absorption in fish (Birchall & Chappell, 1989) and reduced oral aluminium absorption in humans (see Toxicokinetics, Absorption, Studies in Humans, Oral Administration, Factors Influencing Oral Aluminium Absorption, Silicon-Containing Compounds). Addition of sodium metasilicate to the diet was reported to reduce brain hippocampal aluminium accumulation in 28, but not 23, month-old rats (Carlisle & Curran, 1987). The basis for the age difference is unknown. These results have not been independently replicated and are not considered reliable. Addition of 0.5 mM silicic acid to water from 5 days before to 4 hr after aluminium citrate dosing by oral gavage reduced aluminium in most tissues of rats (Quartley et al., 1993). Rats drinking water containing 59 or 118 mg Si/L (as sodium silicate with 27% SiO2) and receiving 450 mg aluminium nitrate nonahydrate injections 5 days weekly had less brain, liver, bone, spleen and kidney aluminium than rats not consuming silicon, suggesting that silicon-containing compounds may have reduced aluminium absorption and/or enhanced its elimination (Bellés et al., 1998). However, as the molar dose of silicon consumed was considerably less than the dose of aluminium administered, the mechanism does not seem to be simply aluminium complexation by silicon. Drüeke et al. (1997) did not find an effect of co-administered silicon dioxide and 26Al on absorption of 26Al given with citrate to rats after eating. It is possible that there was insufficient silicon in the presence of the food to compete with the citrate and interact with the aluminium to influence its bioavailability.
It has been suggested, based on the very low concentrations of silicon in biological fluids, that it is very doubtful that a monomeric aluminium silicate species plays any significant role in the biological chemistry of aluminium, after the aluminium has been absorbed (Harris et al., 1996).
Fluoride
Fluoride appears to be able to increase aluminium absorption. Fluoride forms numerous complexes with aluminium (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Compounds). Speciation calculations indicated fluoride would solubilize > 60% of aluminium in the stomach (Glynn et al., 2001). Aluminium reduced fluoride absorption (Allain et al., 1996; Hobbs et al., 1954; Li et al., 1991; Xiao et al., 1992). The influence of fluoride on aluminium absorption is less clear. Rats consuming a diet containing fluoride and aluminium (chemical species not reported) had decreased levels of aluminium in liver, brain, heart, testes and femur compared to rats consuming aluminium alone, suggesting that fluoride decreased aluminium absorption or enhanced its clearance (Ondreicka et al., 1971). Increasing the aluminium (as the chloride) concentration from 0 to 100 to 500 ppm decreased plasma, bone, liver and urine fluoride concentrations in rabbits concurrently consuming fluoride in their drinking water (Ahn et al., 1995). Increasing the drinking water fluoride concentration from 0 to 1 to 4 to 50 ppm had no significant effect on aluminium levels in plasma, urine, or liver, although the rabbits that drank water containing 50 ppm fluoride and no added aluminium had increased bone aluminium concentrations (Ahn et al., 1995). Sterna, obtained from another study in which rats drank water containing 79 ppm fluoride for 2 years with no added aluminium, showed a significant increase of aluminium (Ahn et al., 1995). Co-administration of aluminium and fluoride to mice increased plasma aluminium 3 hr later when compared to when aluminium chloride or aluminium phosphate was dosed. The increase was similar to that seen with aluminium citrate. Administration of aluminium fluoride to rats increased plasma aluminium more than did administration of aluminium chloride. The rats were given 1.87 mmole Al/kg orally by cannula as aluminium fluoride or aluminium chloride. The time course (sampling at 20, 40, 60 and 90 min) showed aluminium from the aluminium fluoride peaked at ~ 8 μM at ~ 40 min; aluminium from aluminium chloride peaked at ~ 3 μM at ~ 40 min (Allain et al., 1996). In rats fed 130 or 300 mg F/kg, 300 or 1200 mg Al/kg, 130 mg Al + 130 mg F/kg, 300 mg Al + 300 mg F/kg or 1200 mg Al + 300 mg F/kg in their diet for 12 weeks, the lower fluoride concentrations reduced, and the higher concentrations increased, aluminium absorption (Xiao et al., 1992).
Overall, these results suggest that the presence of fluoride can enhance aluminium absorption.
Iron
Iron (Fe) status impacts on the absorption of aluminium and its accumulation in the brain; thus aluminium absorption was generally found to increase in the presence of low amounts of iron. Rats maintained on an iron-deficient diet had greater (0.0065%), and rats maintained on an iron-supplemented diet had lower (0.0028%) oral aluminium bioavailability than controls (0.0040%) (Winklhofer et al., 2000). Oral exposure to aluminium hydroxide produced a greater increase in aluminium excretion and brain aluminium levels in iron-deficient than in normal and iron-overloaded rats, whereas serum aluminium did not show consistent changes (Cannata et al., 1991; Fernández et al., 1989). Similarly, oral exposure to aluminium chloride produced non-significant increases in serum, spleen and liver aluminium concentrations in iron-deficient rats (Brown & Schwartz, 1992). Iron deficiency significantly increased tissue aluminium levels in rats co-administered aluminium and citrate (Brown & Schwartz, 1992). Iron depletion increased aluminium uptake, introduced as the hydroxide, by an intestinal cell line (Cannata et al., 1991; Fernandez Menendez et al., 1991). This is perhaps due to a similar mechanism of GI aluminium and iron uptake, which was suggested to involve an active process mediated by Tf, because aluminium transport was 3-fold greater as the Tf than the citrate (Cannata et al., 1991; Fernandez Menendez et al.,1991). Divalent iron, Fe(II), but not trivalent iron, Fe(III), increased the disappearance of aluminium, introduced as the hydroxide form, from the intestinal lumen in the in situ rat gut preparation and decreased the appearance of aluminium in portal and systemic blood (Van der Voet & de Wolff, 1987b). The authors suggested that Fe(II) may enhance Tf-mediated aluminium uptake, followed by the binding of aluminium in mucosal cells by ferritin. However, although there was a negative correlation between the iron status of Caco-2 cells and their aluminium uptake, both iron-depleted and iron-overloaded Caco-2 cells showed less transport of aluminium citrate, lactate and nitrilotriacetate across the cells than did cells with normal iron content (Alvarez-Hernandez et al., 1994), which is in contrast to the above results. Addition of Tf failed to facilitate aluminium uptake into Caco-2 cells when the aluminium was introduced as aluminium citrate (Alvarez-Hernandez et al., 1994). On the other hand, concurrent administration of 48 or 480 mg/kg Ferromia (sodium ferrous citrate, tetrasodium biscitrato iron (II), E0708) with 50 mg Al/kg as aluminium hydroxide daily 5 times weekly for 16 weeks to 5/6 nephrectomized rats did not affect serum or tissue aluminium concentrations compared to administration of aluminium hydroxide or aluminium citrate alone (Yamazaki et al., 1995). Similarly, iron deficient and iron overloaded rats did not show significantly different urinary aluminium excretion following i.v. aluminium chloride injection than controls, with the exception of less urinary aluminium excretion within the 5 days after aluminium treatment in iron-overloaded rats (Ittel et al. 1996). Brain, bone, liver and muscle aluminium concentrations were not different in iron-depleted vs. iron overloaded rats after 41 days of aluminium supplementation in the drinking water and diet, whereas spleen aluminium concentration was higher in the iron-supplemented rats (Ittel et al., 1996). Surprisingly, urinary aluminium output was greater when aluminium chloride was given orally with iron than saline (Ittel et al., 1996).
Nearly all of the interactions between iron and aluminium are consistent with an enhanced aluminium uptake and retention in the presence of iron deficiency (anaemia). In contrast to iron, zinc (Zn) deficiency did not produce measurable increases of tissue aluminium in rats after 28 days (McNall & Fosmire, 1996).
Calcium and sodium
Like iron, calcium (Ca) status impacts on aluminium absorption and accumulation. Dietary calcium deficiency increased the rate and extent of aluminium absorption, when introduced as the chloride, tissue aluminium accumulation, and aluminium-induced neuropathology in rats (Provan & Yokel, 1990; Taneda, 1984). Increased calcium decreased aluminium uptake and its appearance in plasma in studies that used the rat everted gut sac and in situ rat gut technique, suggesting a common uptake mechanism for aluminium, introduced as the chloride, and calcium (Cunat et al., 2000; Feinroth et al., 1982). Based on ionic radii, it is more likely that aluminium would compete with magnesium than with calcium. Although there is some evidence for aluminium-magnesium competition in vivo, this has not been well investigated.
A reduction of sodium (Na) has been reported to increase aluminium uptake. Replacement of sodium in the perfusate of the in situ gut perfusion study with equimolar choline chloride at pH 3 significantly (~ 50-fold) increased blood aluminium levels, suggesting a negative interaction between sodium and aluminium on aluminium absorption, introduced as the chloride, from the GI tract (Van der Voet & de Wolff, 1987a).
Ethanol
The results of one of two studies suggest that ethanol can affect the toxicokinetics of aluminium, but the mechanism is not known. Rats were given saline, 10% ethanol in drinking water, 25 mg Al/kg, as aluminium nitrate, by gastric intubation (i.g.) or 10% ethanol in drinking water and 25 mg Al/kg i.g. for 6 days/week for 6 weeks (Flora et al., 1991). After the 6 weeks, aluminium concentrations were significantly higher in blood and liver (24 and 76%, respectively), and non-significantly higher in kidney and brain (28 and 31%), in the ethanol-and-aluminium-treated group compared to the group treated only with aluminium. The amount of ethanol consumed was not reported. If fluid consumption was typical for the rat, it would have been 10 to 12 mL/100 g/day. The ethanol-and- aluminium-treated group also showed significant differences from the aluminium-only-treated group with respect to δ-aminolevulinic acid dehydratase, zinc protoporphyrin, and glutamic oxaloacetic transaminase in blood; glutamic pyruvic transaminase in liver; δ-aminolevulinic acid in urine; and dopamine and homovanillic acid in brain. These effects were generally an accentuation of the aluminium effects. However, it is not known if the elevation of blood and liver aluminium levels was due to ethanol alteration of aluminium absorption, distribution or excretion. It is also not known if ethanol increased aluminium toxicity or if these effects were the result of the combined toxicity of these two agents. It has been noted that chronic ethanol ingestion compromises GI tract integrity (Davis, 1993). The author speculated that it may increase aluminium absorption. Results of the combined administration of ethanol and aluminium in rats suggested that ethanol enhances the effects of aluminium, introduced as the chloride, but no information was provided to indicate if ethanol affected aluminium toxicokinetics (Rajasekaran, 2000). However, daily gavage with a 30% v/v ethanol solution delivering 3 g/kg and 91.8 mg aluminium lactate/kg for 90 days did not significantly affect serum aluminium, compared to aluminium alone (Kohila et al., 2004). There are no reports that assess directly the influence of ethanol on aluminium absorption, distribution or elimination.
Uraemia
There is evidence that uraemia enhances aluminium absorption. The primary documented problems with aluminium as a toxicant to bone and the brain have occurred in persons with uraemia (see Toxicokinetics, Absorption, Studies in Humans, Oral Administration, Factors Influencing Oral Aluminium Absorption, Uraemia). Aluminium levels were higher in the liver, but not in other organs, of chronically uraemic rats given oral aluminium hydroxide supplementation than in pair-fed controls (Drüeke et al., 1985). However, this could be due to a difference in absorption, distribution and/or elimination. Blood aluminium and/or urinary aluminium excretion were higher in uraemic, compared to normal, subjects after oral aluminium chloride, hydroxide or lactate administration (Ittel et al., 1987; 1988; 1991a; 1991b; 1996; 1997; Olaizola et al., 1989). The lack of increase of urinary aluminium after i.v. aluminium chloride or lactate administration to uraemic rats suggests the increased urinary aluminium in uraemic rats is due to enhanced absorption (Ittel et al., 1991b). The oral bioavailability of aluminium introduced as 26Al plus 27Al chloride was estimated to be 0.133% in controls and 0.175% in uraemic (5/6 nephrectomized) rats (Ittel et al., 1997). Reduction of renal function in rabbits, to ~ 23% of controls, increased the percentage of aluminium absorbed from aluminium chloride, citrate and lactate by ~ 50 to 100% (Yokel & McNamara, 1988). Systemic clearance was reduced to ~ 74% of that of controls. Ultrafilterable serum aluminium increased, perhaps accounting for the smaller reduction in systemic aluminium clearance than might be expected with a renal function that is 23% that of controls. Serum aluminium concentration was lower in 5/6 nephrectomized rats but urinary aluminium clearance was not significantly different from that of controls, suggesting a greater free fraction of aluminium in uraemia (Ittel et al., 1997). Uraemia may increase aluminium uptake through the paracellular pathway, indicated by increased serum and urinary aluminium in the presence of chemically-induced intestinal mucosal atrophy and by reduced serum aluminium when kinetin, a paracellular pathway blocker, was added to oral but not i.v. aluminium chloride administration (Ittel et al., 1992a; 1996).
Age
It is unclear from animal studies whether age influences aluminium absorption. Comparison of blood and urine aluminium in weanling and growing rats after oral aluminium hydroxide administration led Olaizola et al. (1989) to conclude that there is an inverse relationship between aluminium absorption and age. Rat brain aluminium concentration inversely correlated with age in control rats that were 21 days, 8 months and 16 months old (Domingo et al., 1996), in contrast to most observations in the human (see Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Tissue Aluminium Concentrations, Brain). After consuming aluminium and citrate in drinking water for 6.5 months, elderly rats generally had higher concentrations of aluminium in liver, kidney, spleen, bone and testis than the young rats (Gómez et al., 1997). In contrast, the brain aluminium concentrations were significantly lower in old than young rats (Domingo et al., 1996). The results of these studies are not consistent. Furthermore, none of these studies directly addressed absorption. The differences seen could be due to distribution or elimination.
Foods and dietary components
It has been assumed that the presence of food in the stomach inhibits aluminium absorption, due to aluminium association with organic ligands in food. Only a few studies have directly addressed this hypothesis. The results do not consistently show an influence of food on aluminium absorption. Some of the studies of oral aluminium bioavailability that model aluminium exposure from drinking water restricted food access, whereas others did not. Rodents and rabbits recycle their faeces to increase essential nutrient absorption and usually have stomach contents 24 to 36 hr after food removal. Therefore, simply depriving the animal of food does not guarantee that there will be no stomach contents. In some studies, rats were dosed after a 16 or 24 hr fast (Drüeke et al., 1997; Schönholzer et al., 1997), whereas in others free access to food was allowed (Drüeke et al., 1997). Oral aluminium bioavailability in these studies ranged from 0.06 to 0.36%. The oral bioavailability of 26Al, given in the absence of ligand, in rats that were deprived of food for 24 hr was 0.94% (Drüeke et al., 1997). As stomach contents were found in rats deprived of food for ≥ 24 hr, as noted above, it cannot be assured that there were no stomach contents in the rats in this study. In contrast, Yokel et al. (2001b) did not find a difference in oral 26Al bioavailability, when the Al was given in the absence of ligands, in rats exposed to a procedure that resulted in no stomach contents compared to rats that did have chow in their stomachs. Similarly, the addition of calcium and magnesium carbonates, designed to simulate hard water, did not affect oral aluminium bioavailability (Yokel et al., 2001b). Walton et al. (1994) conducted an ambitious study to assess the influence of beverages and foods on the oral absorption of aluminium given as the sulphate, to produce alum in solution. Their results show increased peak serum aluminium concentrations and urinary aluminium excretion after co-administration of orange juice and, to a much smaller extent, coffee and wine. Meat and carbohydrate/cereal products decreased aluminium absorption. However, neither the blood nor urine samples obtained hourly for 4 hr after dosing enable determination of oral aluminium bioavailability.
Dietary components such as phytate and polyphenols, that chemically associate with aluminium and reduce oral absorption of other minerals, may affect aluminium absorption (Powell & Thompson, 1993; Powell et al., 1993). However, no published studies were found that directly assessed this.
It was speculated that the species of aluminium in the GI tract, which may be present as insoluble or soluble forms and associated with various ligands and, perhaps more importantly, the form of mucus and interaction of aluminium with mucus, vary as a function of time since food consumption and, perhaps, composition of the diet. This may result in variable amounts and composition of absorbable aluminium species, contributing to the variable results in relation to the effect of stomach contents on oral aluminium absorption.
Overall, results suggesting that food composition of the presence of food in the stomach significantly affects oral aluminium bioavailability have been obtained in a few studies (Walton et al., 1994; Drüeke et al., 1997; Yokel & Florence, 2006) whereas another study did not find a significant effect of water quality or the presence of food in the stomach on oral aluminium bioavailability (Yokel et al., 2001b).
Chemical speciation
The chemical form in which aluminium is administered can affect its absorption (Cunat et al., 2000; Deng et al., 2000; Froment et al., 1989b; Schönholzer et al., 1997; Yokel & McNamara, 1988). As noted above in Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption, Carboxylic Acids, addition of citrate, which is believed to solubilize aluminium and perhaps open the paracellular pathway, increased oral aluminium bioavailability in many studies. Consumption by rats of diets for 18 days containing ~ 200 to 260 mg Al/kg diet as aluminium hydroxide, palmatate, lactate or phosphate, or aluminium hydroxide as a reagent grade chemical, or 2 different sources of desiccated gel, resulted in some significant differences in brain, bone and kidney aluminium concentrations (Greger, 1985). For example, aluminium hydroxide raised brain aluminium more than aluminium palmitate and one form of aluminium hydroxide gel raised kidney aluminium levels more than another form but resulted in lower aluminium levels in the tibia. Using the in situ rat gut technique, significantly more aluminium appeared in the plasma after perfusion with the citrate, lactate, tartrate and gluconate forms of aluminium, whereas plasma aluminium was not elevated after perfusion with the chloride, nitrate, sulphate and glutamate forms (Cunat et al., 2000).
It has been suggested that the chemical species of aluminium in drinking water in the absence of fluoride would be primarily labile, monomolecular Al(H2O)6 below pH 5 and Al(OH)1 to 4+2 to -1 at higher pHs (Lazerte et al., 1997). In the presence of fluoride (1 ppm; 53 μM), AlF2+ and AlF3 would be expected at pH < 6.5. At higher, pHs mixed Al(OH)xFy complexes or Al(OH)4- would be expected (Nieboer et al., 1995). It is thought that these species would favour aluminium absorption from drinking water compared with that from food (Martyn et al., 1989) in which aluminium is presumed to be bound to phosphorus-rich compounds such as phytates and casein, a phosphoprotein (Glynn et al., 1995). Glynn et al. (1995) determined soluble and quickly reacting aluminium species (presumably species that might be absorbed) in the presence of 4 mg Al/L, the chloride, added to simulated gastric contents containing 40% rat feed. Less than 50% of the added aluminium was in the soluble fraction. As nearly 100% would be expected to be labile species (Al+3, monomeric hydroxo and sulphato complexes) under these conditions, the authors concluded that the labile aluminium rapidly complexed to components of food. They hypothesized that foods may alter aluminium absorption. The presence of food may change the pH. It has been suggested that food may bind aluminium, hence reducing its bioavailability. Therefore, it has been suggested that enhanced aluminium absorption may occur in the presence of an empty stomach, especially during periods of fasting. This has been discussed in Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption, Foods and Dietary Components. There is no strong evidence to support this notion.
In a subsequent study, rats drank water containing 0, 10, 50 or 500 mg Al/L, introduced as the chloride, for 9 weeks while consuming a diet containing 5 mg Al/kg (Glynn et al., 1999). Bone aluminium increased above control concentrations in the rats drinking the waters containing the higher two aluminium concentrations. In vivo fractionation of the stomach contents of these rats showed an increase of dissolved aluminium as water aluminium concentration increased, up to 50 mg Al/L, and the appearance of measurable quickly reacting aluminium species in those exposed to 500 mg Al/L. The authors interpreted their results as supportive of their hypothesis that aluminium absorption will increase when the aluminium-binding capacity of food in the stomach has been saturated. When compared to typical drinking water aluminium concentrations of 50 to 100 μg Al/L, these results suggest that aluminium would not be absorbed when stomach contents are present, as the aluminium binding capacity would not be saturated. This is not supported by several studies utilizing 26Al in much lower aluminium doses than employed by Glynn et al. (1999). These studies found oral aluminium absorption in the presence of stomach contents (Drüeke et al., 1997; Jouhanneau et al., 1997a; Yokel et al., 2001b).
In contrast to the above hypothesis, Reiber et al. (1995) suggested that a substantial portion of aluminium, regardless of the form consumed, will be solubilized to monomeric aluminium in the stomach. They based this on studies showing solubilization of aluminium from particulate and colloidal sources within 2 hr at pH 1 to 2 and the average residence time in the human stomach, which has a pH of 1.5 to 2, of 1 to 4 hr. They concluded: “…the often raised issue of the relative bioavailability of the aluminium source would seem to be a moot point”, because “Regardless of the consumptive form, the bulk of aluminium will be converted to monomolecular species in the stomach” As they claimed that the stomach is impervious to charged species, they concluded: “In a healthy stomach, however, it is unlikely that any free or complexed aluminium can be absorbed across the stomach wall.” They claimed that the pH of stomach contents rapidly rises to 6.2 when in the duodenum, and then to pH 7.3 after leaving the duodenum. Because the nadir of aluminium solubility, due to aluminium hydroxide formation, is at pH 6.2 (Harris et al., 1996), they suggested: “…more than 99.9 percent of the consumed aluminium should ultimately be excreted in the stool as an aluminium hydroxide precipitate, leaving less than 0.1 percent available for uptake”, explaining why only a small percentage of oral aluminium is absorbed (Reiber et al., 1995). They recognized that this scenario is simplistic. However, at the pH of the human intestine, ~ 6.8, the major aluminium hydroxide species which would be formed in the absence of other ligands to associate with aluminium would be the soluble Al(OH)4- (Harris et al., 1996), suggesting a lower percentage of precipitated aluminium than stated by Reiber et al. (1995). If studies utilizing large doses of 27Al are not considered, there is very little data relevant to comparative oral aluminium bioavailability for different aluminium forms to test the suggestion of Reiber et al. (1995).
The most ambitious study of the influence of beverages and foods on oral aluminium absorption was conducted by Walton et al. (1994). This is a non-peer reviewed report of a study in which large sample sizes were not used. However, an issue that has not been well investigated was addressed. They exposed anesthetized rats to 2 mL of water containing no aluminium, or 8 mg aluminium as aluminium sulphate and/or various beverages and foods by gastric administration. Blood was obtained from the tail, prior to and 1, 2, 3 and 4 hr after, dosing. Urine was obtained, at the same times after dosing, by needle aspiration from the bladder. Considering the short period of observation (4 hr), serum aluminium is probably a better indicator of absorbed aluminium than is aluminium excreted in the urine. Administration of 8 mg aluminium in water increased peak serum aluminium about 4.5-fold and produced a urinary aluminium concentration of 2.6 μg/L. Co-administration of orange juice, coffee and wine with the aluminium significantly increased peak serum aluminium levels, compared to administration of aluminium alone, approximately 17-, 2.5-, and 1.9-fold, respectively. Serum aluminium peaked at 1 hr when given alone. The time of peak serum aluminium in the presence of co-administered foods was not reported. Therefore, it is not known if the rates of aluminium absorption are similar and, as a result, if the 4 blood samples obtained represent similar time profiles of the aluminium absorbed. Urinary aluminium concentration increased approximately 10.5-, 1.3- and 1.8-fold when these three beverages were co-administered with aluminium. Meat and Vita Wheat® biscuits (a carbohydrate/cereal product) significantly attenuated the elevation in serum aluminium caused by aluminium dosing to 62 and 65%, when compared to administration of aluminium alone. Urinary aluminium was attenuated by these two foods to 45 and 57%. These foods were available for 36 hr prior to their co-administration with the aluminium, to “load the gastrointestinal tract with the single food and its breakdown products.” In contrast, no food was available for 36 hr prior to aluminium alone or beverage (plus aluminium) dosing. In preliminary studies, the investigators found food in the stomach 24 hr after removal of food access, so they went to 36-hr food deprivation. They did not verify the lack of stomach contents after 36-hr food deprivation. As noted above, this does not assure the lack of stomach contents. Co-administration of beer, Coca-Cola®, tea, apple, broccoli, butter and margarine produced non-significant effects. Co-administration of both orange juice and Vita Wheat® with aluminium resulted in an increase of serum aluminium levels of 9.1-fold, an attenuation of the result seen with orange juice alone, but urine aluminium concentration was not attenuated by the addition of Vita Wheat® to the aluminium plus orange juice administration. No definitive conclusions can be made from this study. The results from co-administration of coffee and wine, which slightly increased serum and urine aluminium levels, and meat and the cereal product, which decreased serum and urine aluminium levels, suggest that co-administration of certain foods with drugs or foods that contain significant amounts of aluminium warrants further study.
The site and mechanisms of oral aluminium absorption
Aluminium, like most substances, is better absorbed from the upper intestine than from the stomach. The stomach is lined by a thick, mucus-covered membrane. It has a much smaller surface area than the intestine. The primary function of the stomach is digestive, whereas that of the intestine in absorptive. Ionized molecules are usually unable to penetrate the lipid bilayer of cell membranes due to their low lipid solubility (Benet et al., 1996). As aluminium would be expected to be present primarily as the free ion, with associated waters of hydration, at the low pH of the stomach, non-carrier-mediated absorption would not be predicted from the stomach. Studies with in vivo isolated duodenal segments suggested aluminium uptake at pH 2 was due to both an aluminium-concentration-dependent non-saturable process and a saturable process that is at least partially vitamin D dependent, suggesting that aluminium may compete with calcium (Adler & Berlyne, 1985). Uptake of aluminium from the citrate into stomach sacs was much less than into small bowel and colon (Whitehead et al., 1997). Plasma aluminium concentrations peaked 45 minutes after oral citrate intake by rats (Froment et al., 1989a; 1989b). They observed a simultaneous peak of plasma aluminium and glucose and concluded that the site of aluminium absorption is probably the proximal small intestine (Froment et al., 1989b). The lower pH of the proximal duodenum would be expected to result in greater aluminium absorption than more distal intestinal segments (Greger & Sutherland, 1997).
GI aluminium absorption appears to be a two-step process, an initial mucosal cell uptake, followed by much slower release into the blood. This was suggested by Feinroth et al. (1982), based on results obtained with everted rat jejunal sacs and aluminium chloride. Others reached a similar conclusion, based on results with in vivo isolated gut segments and the in situ intestinal perfusion technique with portal and systemic blood sampling after aluminium chloride (Adler & Berlyne, 1985; Van der Voet & de Wolff, 1986-1987) and aluminium chloride, lactate and nitrate addition (Jäger et al., 1991), as did Cochran et al. (1990), after aluminium chloride addition, using a duodenal perfusion preparation. It was shown that aluminium binds to mucus glycoproteins in the GI tract (Crowther & Marriott, 1984). A much greater loss of aluminium from the intestine than appeared in the blood was typically found in the above studies. This loss was attributed to mucosal cell or mucous uptake of aluminium. Support for this suggestion was provided by the finding that nearly all of the aluminium not recovered in the perfusate effluent from the rat gut could be recovered in intestinal mucus (Powell et al., 1994; 1999). This group found tissue aluminium uptake to be up to 1000-fold that of aluminium transport across the gut tissue (Whitehead et al., 1997). Powell et al. (1999) found that the association of aluminium with gelatinous mucus prevents formation of aluminium-hydroxy precipitates. They interpreted their results to show that aluminium is primarily associated with insoluble mucus, which, due to its slow reaction kinetics, reduces aluminium absorption, enabling its faecal elimination (Powell et al., 1999). The finding that ~ 60% of aluminium, introduced as the chloride, taken up from the in situ perfused rat intestine was in the small intestine is consistent with this interpretation (Arnich et al., 2004).
The mechanisms mediating GI aluminium absorption have been suggested to include passive (diffusion) and active (carrier- and vesicular-mediated) transport across intestinal cells as well as paracellular diffusion between these cells. Support has been provided for an energy-dependent uptake process. Dinitrophenol (DNP), cyanide, vanadate, the absence of glucose and low temperature inhibited the uptake of aluminium, introduced as the chloride, in studies utilizing the rat everted gut sac, isolated jejunal slice, and a duodenal perfusion technique (Cochran et al., 1990; Feinroth et al., 1982; Provan & Yokel, 1988b). Reduced glucose in the in situ rat gut technique perfusate decreased aluminium in plasma (Cunat et al., 2000). In contrast, Farrar et al. (1987) and Provan & Yokel (1988a) did not find an effect of DNP using rat duodenal, jejunal or ileum preparations and the in situ rat gut preparation. Whitehead et al. (1997) found no effect of ouabain, suggesting the lack of dependence on Na/K-ATPase. A plateau of aluminium uptake, introduced as the chloride, was observed by Feinroth et al. (1982) and Provan & Yokel (1988b), suggesting a carrier-mediated mechanism. Deleting phosphorus from the in situ rat gut technique perfusate reduced aluminium uptake into plasma which, the authors suggested, could be due to the role of phosphorus in cellular respiration (Cunat et al., 2000). However, the presence of phosphorus, which causes aluminium phosphate precipitation, has been associated with decreased aluminium absorption (Kaehny et al., 1977).
Evidence for an interaction with calcium uptake has been obtained. Increasing calcium perfusate concentration decreased aluminium, introduced as the chloride, uptake (Feinroth et al., 1982) and addition of aluminium chloride to the in situ perfused duodenum decreased calcium uptake (Adler & Berlyne, 1985). Calcium channel blockers decreased, and calcium channel activators increased, uptake into rat jejunal slices (Provan & Yokel, 1988b) and duodenum (Cochran et al., 1990) when introduced as aluminium chloride; however, Provan & Yokel (1988a) did not find an effect of calcium channel blockers or a facilitator in the in situ rat gut preparation.
It has been suggested that citrate facilitates aluminium absorption by opening the tight junctions between GI mucosal cells, by chelating calcium, which is required for tight junction integrity, and that the aluminium is absorbed as aluminium citrate (Froment et al., 1989b). This mechanism of enhanced aluminium absorption was favoured by Taylor et al. (1998). However, they found peak serum citrate concentrations 31 to 32 minutes after aluminium citrate consumption, whereas aluminium concentrations peaked at 77 to 108 minutes, suggesting that aluminium was not absorbed as aluminium citrate due to the faster absorption of citrate and was not released into blood as aluminium citrate (Taylor et al., 1998).
Some evidence suggests a role for sodium transport processes. Low sodium and amiloride, a sodium uptake blocker, increased the uptake t½ of aluminium (Provan & Yokel, 1988a). In contrast, Van der Voet & de Wolff (1987a) found a negative interaction between sodium and aluminium uptake. Further study by Van der Voet & De Wolff (1998) showed that the presence of calcium, but not sodium, reduced aluminium uptake. The presence of both calcium and sodium attenuated the calcium effect, leading the authors to speculate: “… aluminium attempts to mimic calcium in its Na-related intestinal passage”.
Paracellular pathway blockers increased the t½ of aluminium uptake in the in situ gut preparation (Provan & Yokel, 1988a). More support for the hypothesis that the paracellular pathway is involved in aluminium absorption was provided by further studies which used the in situ rat gut preparation and aluminium chloride or citrate (Froment et al., 1989a; Partridge et al., 1992; Provan & Yokel, 1988a), inverted intestinal segments and aluminium citrate (Farrar et al., 1987; Whitehead et al., 1997), and Caco-2 cells exposed to aluminium in the absence of ligand or as the citrate, fluoride, hydroxide or maltolate (Zhou & Yokel, 2005). However, paracellular pathway blockers did not affect aluminium uptake by the jejunal slice (Provan & Yokel, 1988b), which might be expected when the endpoint is uptake into, rather than flux across, tissue.
Tf addition to the medium vascularly perfusing an intestinal preparation increased aluminium uptake, suggesting it facilitated entry of aluminium, introduced as the chloride, into blood, perhaps from intestinal cells (Jäger et al., 1991). It has been suggested that Tf mediates aluminium release from mucosal cells into blood (Greger & Sutherland, 1997). There is no single unifying explanation for these results. It is probable that there is more than one mechanism of aluminium uptake, which might be aluminium species-, pH- and intestinal region-dependent. Multiple mechanisms would account for the lack of ability of manipulations to totally block aluminium uptake, and perhaps the significant results obtained by some, but not other, investigators.
Lower serum aluminium concentrations resulted from portal vein than from i.v. injections of the same aluminium dose, as the sulfate, to rats, indicating significant first-pass pre-systemic clearance of aluminium by the liver (Xu et al., 1992a). These results suggest that determination of aluminium absorption based on area-under-the-curve calculations from multiple blood/serum aluminium determinations over time might underestimate absorbed aluminium, although this method estimates the aluminium that reaches systemic circulation and is available for distribution to organs such as the brain.
The chemical speciation of aluminium, consumed in beer, as it passes through the upper part of the GI tract was modelled by (Sharpe et al., 1995). The citrate concentration in beer exceeded the aluminium concentration, resulting in the predominant aluminium species in lager, the mouth and stomach being aluminium citrate. In the duodenum and jejunum, where the pH is ~ 6.5, the predicted predominant species was aluminium phosphate. This is the primary site of aluminium absorption. At pH 7, neutral aluminium species exceeded charged and “solid” (insoluble) aluminium species at total aluminium concentrations below 7600 μg Al/L, which is well above the median aluminium concentration in the beers studied (100 μg Al/L).
Dermal
Aluminium salts precipitate proteins and have astringent properties. This has led to their use to treat urinary bladder haemorrhage, diaper rash and prickly heat, insect stings and bites and athlete’s foot, and use in styptic pencils and products for dermatitis (Tinea pedis); in anti-diarrheal products and vaginal douches and as a keratolytic in anorectal preparations (Allen et al., 2000; Knodel et al., 1996) (see also Sources of Human Exposure, Anthropogenic Sources, Uses and Table 6).
Aluminium salts are extensively used in antiperspirants because they suppress eccrine sweating more effectively than other metal salts (Hostynek et al., 1993). They are thought to be effective because either they become neutralized in the sweat duct to form a gelatinous or flocculant hydroxide precipitate, or because they denature keratin in the cornified layer that surrounds the opening of the sweat duct. Both mechanisms predict that little aluminium would be absorbed through the sweat duct (Hostynek et al., 1993).
Aluminium poorly penetrates the skin. In a study claimed to be the only report to that time on penetration of aluminium salts through excised skin, it was concluded that minimal aluminium reached the dermis following topical exposure. Abdominal and axillary human skin, 3 cm2, was exposed for up to 23 hr to 5 mL of 20% aluminium chlorohydrate, which would have contained ~ 280 mg aluminium. A 10 mm disk of the exposed skin contained ≤ 7 μg aluminium. Removal of the stratum corneum (the outer layer of the skin that is 25 to 35 μm thick) by stripping with adhesive tape resulted in no greater aluminium penetration into the dermis. The authors noted that very little aluminium reached the dermis, the region of the sweat glands. They attributed the low penetration to binding of unknown aluminium complexes to the outer stratum corneum layers (Blank et al., 1958). Stripping the stratum corneum with tape restored 50% of the function of sweat glands that had been inhibited by aluminium chlorohydrate, suggesting its site of action is quite shallow (Quatrale et al., 1981a). More, ~ 2/3, of sweat gland function inhibited by aluminium zirconium chlorohydrate glycine complex was restored by stripping whereas removal of the stratum corneum did little to reverse the effects of aluminium chloride, suggesting it may have penetrated deeper into the sweat duct (Quatrale et al., 1981a). TEM and morin stain techniques suggested that aluminium chlorohydrate collected in sweat ducts at the layer of the stratum corneum to completely fill the duct as an amorphous mass (Quatrale et al., 1981b; Quatrale, 1985). Sorption, defined to include both adsorption (onto) and absorption (into) the stratum corneum, of unbuffered aqueous solutions of 50% aluminium chlorohydrate at pH 5 to 5.2, or aluminium chloride atomic absorption standard reference solution at pH 3.45 to 4.16, was rapid to guinea pig stratum corneum in vitro (Putterman et al., 1981). Desorption of aluminium chloride was more rapid than aluminium chlorohydrate. The latter was essentially irreversibly bound to the stratum corneum, suggesting that aluminium might not be able to be absorbed through the skin. Hostynek et al. (1993) concluded that the avid formation of aluminium complexes with skin proteins precludes all but very shallow penetration of the epidermis.
A few studies were conducted to assess aluminium absorption through the skin of mice. However, a number of concerns about these studies reduces confidence in the authors’ interpretations. Anane et al. (1995) applied 20 μL of a 0.025 or 0.1 μg aluminium chloride/mL solution to 4 cm2 of skin (0.1 and 0.4 μg/day) on the dorsal shaved surface of mice for 130 days. The total aluminium applied during this time (0.5 to 2 mg/kg) is comparable to a one day aluminium exposure of humans using topical antiperspirants. Twenty-four hr urine samples were obtained starting 1 day after completion of aluminium dosing. Blood and brain samples were also obtained. A statistically significant increase was reported in urinary and serum aluminium concentrations after both aluminium exposures, compared to those for non-exposed mice of the same age. This suggests that a small fraction of the typical human use of a topical antiperspirant might produce a measurable increase in urine and tissue aluminium levels. Increases in brain aluminium levels were 19 to 124% over controls. The aluminium concentration in the hippocampus was reported to be 2 to 3 times the rest of the brain in controls and aluminium-exposed mice. This could be an artifact of aluminium contamination, which would be more pronounced in a smaller sample (hippocampus) than in the rest of the brain. Dermal exposure of mice pups from 2 to 22 days of age to 0.025, 0.05 or 0.1 μg aluminium chloride/cm2 increased their brain aluminium levels by 5 to 24%. Using X-ray energy scanning electron microscopy, the authors reported 11 to 120-fold more aluminium in the hippocampus of treated mice than in those of controls, whereas atomic absorption spectrometric analysis of aluminium showed a 1.6 to 2.2-fold increase. Absorption of aluminium, applied as aluminium chloride, to mouse skin in vitro, was determined in a “static” culture system. Increased aluminium was observed in the compartment that modelled sub-dermal fluid. In a similar study, pregnant mice received dermal application of 0.4 μg aluminium chloride daily for 20 days (Anane et al., 1997). Aluminium levels in maternal serum, amniotic fluid and foetal brain, kidney, and liver were all reported to be statistically increased, by 63, 21, 4.5, 5.0 and 15%, respectively, compared to controls. There is no mention in either report of methods to prevent absorption by non-transcutaneous routes. The aluminium solution was applied over a 4 cm2 area on the back, which represents about 12% of the total body surface area of the mouse. It is quite possible that grooming produced oral aluminium exposure. A 20 g mouse receiving a total of 2 mg Al/kg would receive 0.04 mg of aluminium. Retention of 5 × 10-5 of the administered aluminium by each gram of brain, as has been reported after i.v. aluminium injection (see Toxicokinetics, Distribution (Including Compartmentalization), Animal Studies, Central Nervous System), which corresponds to 100% bioavailability, would raise brain aluminium levels by 2 × 10-3 mg Al/kg (2 ppm). The reported increase of brain aluminium was 18 × 10-3 mg Al/kg, suggesting > 100 % bioavailability, and therefore casting further doubt on the validity of these findings. There is a low degree of confidence in the results and interpretation of this work.
Intranasal
The potential for delivery of compounds to the CNS from the nasal cavity via the olfactory neuron has been proposed. The strongest supporting data have been obtained with manganese (Mn) (Tjälve et al., 1996). There is little evidence supporting similar transport of other metals, such as cadmium, nickel, and mercury. For a review see Tjälve & Henriksson (1999). Anatomically, the olfactory system as a route of delivery to the brain is intriguing as well as problematic for interpretation. The olfactory receptor neurons are the first-order neurons located within the nasal cavity in the olfactory epithelium. Their cell bodies lie in the basal two thirds of the epithelium. Several cilia extend from each cell into the mucous layer of the epithelium. These cells are separated and partially ensheathed by supporting cells of the olfactory epithelium. The axons of these neurons project via the olfactory nerve (the first cranial nerve) to the olfactory bulb and synaptically terminate on the mitral and tufted cells. These cells then relay via neurotransmitter synapses to higher order olfactory structures and to other brain systems. Such neurotransmitter target sites include the olfactory peduncle, the piriform cortex, the olfactory tubercle, the entorhinal cortex, and some amygdaloid nuclei. Projections are made from these primary olfactory cortical structures to other brain regions. While this distinct anatomical pathway exists, to serve as a direct route of exposure to the brain would require the axonal transport of the material of concern as well as the trans-synaptic transport from one neuron to the next within the pathway.
An alternative route from the nasal passage has been proposed, that is exposure via the CSF. In this case, delivery would be from diffusion of the compound through the perineural fluid around the olfactory nerve through the cribriform plate. However, it is not clear if this pathway can mediate distribution from the nasal cavity into the brain as it has been primarily described as a route of drainage from the CSF compartment to the nasal lymphatics (Jackson et al.,1979; Kida et al., 1993). If a compound could diffuse by this route from the nasal cavity into the CSF, it would be expected to initially distribute through the subarachnoid space and over the cortical surface.
The third route of absorption from the nasal cavity is into systemic circulation by the vasculature of the nasal cavity (Landau et al., 1994).
In the initial study to assess whether aluminium can enter the brain from the nasal cavity, Perl & Good (1987) implanted Gelfoam® containing 0.5 mL of 15% aluminium lactate, 5% aluminium chloride, or 15% sodium lactate into the nasal recess of rabbits. The Gelfoam® remained in place for 1 month. Neuropathological changes and elevated aluminium were seen in the olfactory bulb, piriform cortex, hippocampus and cerebral cortex, but not in cerebellum, brainstem or spinal cord. However, this exposure protocol is not realistic with respect to potential human exposure. There is concern that Gelfoam® implants containing such a high aluminium concentration in the nasal cavity for 1 month could damage the nasal epithelia (Lewis et al., 1994). Perl & Good (1987) suggested that a defect in the normally effective olfactory mucosa/olfactory bulb barriers may lead to excessive aluminium exposure. Their experimental conditions may have produced such a defect, which may not be present or as prevalent in the normal condition. Although this study has been frequently cited, it has not been replicated.
In a second study designed to address the possibility that aluminium can enter the brain from the nasal cavity, rats were exposed by inhalation to 20.6 μg aluminium chlorohydrate/m3 via nose-only exposure 6 hr/day for 12 days. The aluminium chlorohydrate was from a commercial source (Pfaltz & Bauer) and was delivered as aerosols generated by a venturi powder dispenser. Although particle size distribution was determined several times during the study, the results were not reported. The rats had a significantly greater aluminium concentration in the olfactory bulb, determined by PIXE, than in non-olfactory brain regions. Aluminium was not seen in the brain of rats that received similar non-aluminium exposures (Divine et al., 1999). Four tissue samples from olfactory bulbs, with 8, 7, 13 and 7 aluminium sites, had average aluminium concentrations of 3.0, 3.8, 7.0, and 2.1 mg Al/kg, respectively. Four brain regions considered non-olfactory bulb associated, i.e., brain stem nuclei in the region of the substantia nigra, had 2, 1, 3 and 2 aluminium sites and average aluminium concentrations of 0.7, 0.5, 1.0, and 0.6 mg Al/kg, respectively. The average aluminium concentration and number of aluminium sites were shown to be significantly different by Student’s t test.
Rats exposed to lipophilic aluminium acetylacetonate under conditions designed to maximize inhalation via the nasal-olfactory system had elevated levels of aluminium in the brain (Zatta et al., 1993). Aluminium was seen in the olfactory bulb, cortex, hippocampus, and entorhinal area, and also in the cerebellum, which is not within the olfactory pathway. Exposure to aluminium via deposition into the nasal cavity from which it might be absorbed may be relevant to environmental exposures, although such exposures are most likely to be from aluminium silicates in airborne dust, which contains very little aluminium in the exchangeable metal ion fraction, as noted below (Lum et al., 1982). This exposure route may also be relevant to occupational settings, such as those of potroom workers, welders and of workers exposed to suspended aluminium particles. Although the study of aluminium chlorohydrate inhalation may involve a route of exposure and chemical species of aluminium relevant for the human, these studies do not model human exposure to typical aluminium species in the environment or workplace. Furthermore, these studies provide no information on the percentage of aluminium introduced into the nasal cavity that might, in turn, be taken up into the brain, which may be very small based on studies with manganese and cocaine (Chow et al., 1999; Dorman et al., 2002).
Intramuscular
There is one reported study that quantified aluminium absorption following i.m. injection. Flarend et al. (1997) prepared 26Al hydroxide and 26Al phosphate adjuvants (which did not contain allergens) and 26Al citrate. Two rabbits were given i.m. 26Al hydroxide, 2 rabbits were given 26Al phosphate adjuvant and 1 rabbit was given i.v. 26Al citrate. In the first 2 days, 40% more 26Al was absorbed from 26Al hydroxide than from 26Al phosphate. Within the first 28 days, 17% of the 26Al from aluminium hydroxide and 51% of the 26Al from aluminium phosphate was absorbed, when compared to the area-under-the-curve for the aluminium citrate. This is based on the assumption that the much greater (300-fold) peak blood aluminium seen after aluminium citrate injection than after adjuvant injections does not confound this interpretation. The authors suggested that all of the injected aluminium may eventually be absorbed. Therefore, 100% of aluminium may eventually be absorbed from muscle, even from insoluble aluminium species. Peak aluminium concentrations following absorption from the i.m. route are lower than from oral and inhalation of small particles due to the prolonged time course of i.m. aluminium absorption.
Studies in humans
Inhalation exposure
Occupational exposure to aluminium fumes, dusts and flakes has been shown to produce elevated levels of urine aluminium and, less frequently, elevated levels of serum and bone aluminium. Workers involved in the electrolytic production of aluminium for an average of 3.8 years, the production of aluminium powder for 10.2 years, the production of aluminium sulphate for 7.4 years, and in aluminium welding for 10.7 years, and a group of patients with renal failure who were receiving dialysis, were compared with a referent group (Sjögren et al., 1983). The dialysis patients had the highest plasma aluminium concentrations. All of the aluminium workers, except those involved in electrolytic aluminium production, had significantly higher serum aluminium levels than the referents. All aluminium workers had significantly higher urine aluminium than their referents. Serum and urine aluminium concentrations were positively correlated. Workers’ plasma and urine aluminium concentrations were higher after a work shift compared to those before the shift and higher on a Friday than on a Monday (Mussi et al., 1984). Plasma and urine aluminium levels increased more after exposure to comparable concentrations of fume than dust (Mussi et al., 1984). Although the particle sizes were not reported, it is likely that those of the dust were larger than those of fume, the mass median diameter of which was ~ 0.4 μm (Sjögren et al., 1988). End-of-shift urinary aluminium concentration correlated well with environmental aluminium concentration. The increase in urinary aluminium level was greater from inhalation of aluminium fumes than from the slightly lower concentrations of aluminium in dust. It is not known if inhalation exposure results in absorption across the lungs, from the GI tract after mucociliary clearance of material from lung to stomach, and/or via the olfactory tract. It is suggested above (see Toxicokinetics, Absorption, Animal Studies, Inhalation Exposure / Intranasal) that absorption from the GI or olfactory tracts is unlikely to account for aluminium absorption after inhalation exposure. Three previously unexposed volunteers and six welders were exposed to welding fumes containing ~ 39% aluminium, as aluminium oxide (Sjögren et al., 1985). The industrial exposures varied from 0.3 to 10.2 mg Al/m3 with a mean 8 hr TWA value of 2.4 mg Al/m3. The urinary aluminium level in one volunteer, who had no previous aluminium exposure and who was exposed to an 8 hr TWA of 7 mg Al/m3, showed an increase to ~ 50 μg Al/L, and then 375 μg Al/L, 12 and 24 hr, respectively, after initiation of inhalation. Urinary aluminium levels in the 3 volunteers increased from < 3 μg Al/L before, to > 100 μg Al/L after, exposure (Sjögren & Elinder, 1992). The t½ of the first phase of elimination was estimated to be ~ 8 hrs. One welder, who had been exposed for one month, had a urinary aluminium concentration of ~ 40 μg/L during a week of exposure to ~ 1.5 mg Al/m3 ~ 7 hr daily. Another welder who had been exposed for 19 years had a urinary aluminium concentration of ~ 300 μg/L during a week of exposure to an 8 hr TWA of 0.5 mg Al/m3 (Sjögren & Elinder, 1992). These results suggested pulmonary absorption. The authors estimated that ~ 0.1 to 0.3% of the inhaled aluminium appeared in urine within a few days (Sjögren et al.,1985). Twenty-five MIG welders exposed to a median of 1.1 mg Al/m3 over periods of 0.3 to 21 years had a median urine aluminium concentration of 82 μg/L (54 mg/kg creatinine). After a period of 16 to 37 days of non-occupational aluminium exposure, the urine aluminium level was 29 μg/L (29 mg/kg creatinine) (Sjögren et al., 1988). Comparison of urinary aluminium concentration in 23 of the welders before and after the exposure-free interval showed that it correlated with the aluminium exposure level before the exposure-free interval whereas, after the exposure-free interval, it related to duration of occupational exposure (Sjögren et al., 1988). The bone aluminium concentration was 18 to 29 mg/kg in two welders after 20 and 21 years of exposure compared to 0.6 to 5 mg/kg in referents, illustrating retention of absorbed aluminium (Elinder et al., 1991). The mean urine aluminium level of 15 workers in an aluminium fluoride plant exposed to a mean of 0.12 mg Al/m3 was 12 μg/L, of 12 potroom workers in an aluminium smelter exposed to a mean of 0.49 mg Al/m3 was 54 μg/L and of 7 foundry workers in the aluminium smelter exposed to a mean of 0.06 mg Al/m3 was 32 μg/L; that for the 230 controls was 5 μg/L (Drabløs et al., 1992). Due to inter-individual variability, these were not significant differences. However, there was a significant correlation between weekly mean aluminium concentration in air and weekly average urine aluminium excretion. Aluminium exposure in the aluminium fluoride plant was mainly from aluminium hydroxide and aluminium fluoride. Exposure in the smelter potroom was mainly from aluminium oxide and partly from aluminium fluoride and, in the foundry, from aluminium oxide and partly from oxidized aluminium metal fume. Although urine aluminium concentrations were poorly correlated with changes in serum aluminium, urinary aluminium and fluoride concentrations were significantly correlated in 8 cryolite workers (Grandjean et al., 1990). Thirty-three foundry workers who worked as smelters, die casting operators, fettlers and sand casters were exposed for 1 to 17 (median 7) years to aluminium in dust and fumes. The exposure concentrations for the smelters, die casting operators and fettlers averaged 0.17, 0.027, and 0.58 mg/m3, respectively. These workers had a significantly higher mean serum aluminium than controls (16 compared to 11.3 μg/L) and non-significantly higher urine aluminium concentrations (18.93 vs.12.90 μg/L) (Rollin et al., 1991a). However, the serum aluminium concentration in the control group was considerably greater than the concentration now considered to be normal, 1.6 μg/L (Nieboer et al., 1995; Roider & Drasch, 1999).
One hundred and fifteen newly employed potroom workers, who had no previous history of work in an aluminium industry, were followed for 36 months. Monthly determinations of airborne aluminium in the first 18 months ranged from 0 to 2.145 mg/m3, with monthly medians ranging from 0.001 to 0.173 mg/m3. Forty-four percent of the total inhalable aluminium was in the respirable fraction, compared to previous determinations by this group of 52 and 87% in two potrooms of an established plant (Rollin et al., 1996). Prior to employment in the potroom, workers’ mean serum aluminium concentration was 3.37 μg/L. The serum aluminium level increased steadily during the first 12 months to a mean of 6.37 μg/L and did not appreciably increase further during the next 24 months. Smokers had higher serum aluminium levels than non-smokers. The mean urine aluminium level before employment was 24.2 μg/L; after 36 months of employment it was 49.1 μg/L.
Based on studies in 8 plants involving refining, casting and pressure moulding that included 119 workers, it was concluded that environmental aluminium concentration, particle size, allotropic state, solubility and the pattern of exposure affect lung aluminium absorption (Apostoli et al., 1992).
The absorption of aluminium from the lung can be estimated from a few studies of occupational aluminium exposure. Daily urinary aluminium excretion by 12 aluminium welders, whose lung aluminium burden may have been approaching a steady state, averaged 0.1 mg. Daily aluminium deposition into their lungs was estimated to be 4.2 mg. This would suggest absorption of ~ 2.4% of the aluminium (Sjögren et al., 1997). The site of absorption cannot be ascertained from such data. Results from workers exposed to ~ 0.2 to 0.5 mg soluble Al/m3 in the air (particle size not described) suggest ~ 2% absorption (Pierre et al., 1995). Fractional absorption was similar in the workers in a second study (Gitelman et al., 1995) who were exposed to a similar air aluminium concentration containing 25% respirable (< 10 μm diameter) aliminium. The urinary aluminium/creatinine ratio correlated better with respirable than total aluminium. However, fractional absorption was inversely related to air aluminium concentration (H.J. Gitelman, personal communication, 1995). These workers showed a better correlation between urinary aluminium excretion (which reflects absorbed aluminium) and respirable aluminium, than total aluminium (Gitelman et al., 1995). These results suggest that the smallest aluminium particles that can distribute to the deepest part of the lung are best absorbed and suggest that absorption is from the pulmonary rather than GI route. The estimate of 26Al absorption from inhalation of 26Al oxide particles which had a MMAD of 1.2 μm by two subjects was 1.9% (Priest, 2004).
Significantly elevated urinary Al levels were seen in 10 volunteers who were exposed for 20 minutes downwind of fumaroles, suggesting respiratory Al absorption from inhalation of the gas (Durand et al., 2004).
There are no good experimental data from which one can estimate aluminium bioavailability from atmospheric sources. A Standard Reference Material containing urban particulate material collected over more than 12 months near St. Louis, MO, that was thought to be representative of an atmospheric sample obtained from an industrialized urban area, was fractionated. Approximately 0.6% of the aluminium was in the exchangeable metal ion fraction (Lum et al., 1982). About half was bound to iron-manganese oxides and half was organically-bound metal ions. Absorption of 3% of the aluminium in the lung to blood was adopted (ICRP, 1981) according to Jones & Bennett (1986), but they note: “there is as yet no firm basis for this estimate”.
Oral administration
Drinking water
The first human studies attempting to estimate oral aluminium bioavailability utilized 27Al. These were balance studies, in which aluminium absorption was estimated from the difference between intake and faecal excretion. Aluminium retention was estimated from the difference between intake and faecal-plus-urinary excretion. However, it has become apparent that the percentage of aluminium that is orally absorbed is quite low (as noted in the animal studies above and more recent human studies, below). Therefore, studies using these methods are not considered reliable, as discussed above.
One of the few human studies of oral aluminium absorption that models drinking water is that reported by Stauber et al. (1999). The 21 subjects consumed a diet that provided a total intake of about 3 mg Al/day. They drank either 1.6 L daily of an ATW from a municipal treatment plant that they found contained 140 μg Al/L or reconstituted soft water (RSW) that had < 1 μg Al/L, with and without sodium citrate. The ATW provided 208 to 233 (in the absence of added sodium citrate) or 253 μg Al/day (when sodium citrate was added). The RSW provided < 1 μg Al/day (when no sodium citrate was added) or 46 μg Al/day (when sodium citrate was added). Oral bioavailability was estimated from the 24 hr urinary aluminium output times 2.2. The value 2.2 was used to correct for the estimated fraction of total urinary aluminium excretion that occurs within the first 24 hr and the fraction of absorbed aluminium that is retained (e.g., does not appear in the urine). This is based on the fraction of aluminium excreted in the urine over 24 hr compared to that excreted over 7 days, which was estimated to be 0.62 by (Priest, 1993). It is also based on the fraction of aluminium administered intravenously that was found in the urine within 7 days by Talbot et al. (1995), 0.72, suggesting the balance of the aluminium was retained. They concluded that the oral bioavailability of aluminium from water was 0.39% in the absence, and 0.36% in the presence, of citrate. This was based on the increase in 24 hr urinary aluminium excretion when the subjects consumed ATW and a controlled diet compared to RSW and the same diet, and the increased aluminium in ATW compared to RSW. The controlled diet was given to all subjects in the same amounts for three meals, three snacks, tea and a banana daily, delivering 1777 calories and 2.9 mg aluminium. It contained standard amounts of specific food components, including meat, cereal, milk, tea, and cookies, each of which was analyzed to determine its aluminium concentration. Aluminium in ATW represented 6 to 7% of total aluminium intake. Its consumption raised urinary aluminium output approximately 9%. Therefore, the response seen was < 10% of background urinary aluminium excretion. A concern about this study is that one might expect the variability in the excretion of aluminium in the urine from food to mask any ability to see an increase in urinary aluminium output from the aluminium in water. The range of 24 hr urinary aluminium output in these subjects was 5-fold (1.8 to 9.3 μg). However, within-subject variability must have been considerably less for the results to produce a statistically significant difference in urinary aluminium output after consuming the ATW compared to RSW. This is indicated by the 95% confidence intervals (CI) that suggest considerably less variability than the 5-fold range of their subjects. The design of this study favoured the study objectives, to measure the amount of aluminium absorbed from water, above that contributed by food. Their study included within-subject comparisons and a defined diet containing a low amount of aluminium for the day before and the day of the study (3 mg vs. normal intake of 3 to 7 mg/day in Australia, according to the authors). Glynn et al. (1995) stated that the concentration of aluminium in drinking water has to be extremely high in relation to food to ensure that aluminium from the water is a significant part of the total oral aluminium intake. Similar concern expressed was: “…biokinetic studies of injected or ingested aluminium in man and animals is complicated by the investigator’s inability to distinguish between aluminium from a test dose and that already present in the body. Most studies of stable aluminium are, thus, of poor sensitivity.” (Priest, 1993). Apparently Stauber et al. (1999) were able to overcome this reservation. The correction factors they used to estimate urinary aluminium output were based on two human studies conducted by other investigators in which 26Al was used. These estimates may not accurately predict the fraction of aluminium excreted in urine in 1 day compared to that excreted to time infinity, and may not accurately predict the percentage retained, when that estimate is also based on 1 vs. 7 days of observations. Although these estimates may influence the absolute estimate of oral bioavailability, they are less likely to influence the relative estimates of aluminium bioavailability from water compared to that from food; this is discussed below.
A second study relevant to consumption of aluminium from drinking water involved 4 subjects who consumed a soft mineral water containing < 1 μg Al/L, the same water with 300 μg Al/L as Al2(SO4)3, and deionized water containing 300 μg Al/L as Al2(SO4)3 (Gardner & Gunn, 1995). Unfortunately, urine was collected only 7 hr after aluminium administration; urinary aluminium excretion rate would not have yet returned to the pre-aluminium-treatment rate in many cases. Therefore, the estimate of oral aluminium bioavailability, which was < 0.1%, is below the true value.
A study of 2 humans who consumed 26Al as aluminium chloride, Hohl et al. (1994) produced an estimate of oral aluminium bioavailability of 0.1%. The authors collected urine for 4 days (although missing the collection of part of the sample from one of the two subjects on day 1). Their estimate of 0.1% oral aluminium bioavailability was not corrected by the aluminium that was retained by the subjects or excreted after the period of sample collection. The authors suggest this would not result in a large error.
Another study that modelled aluminium consumption from drinking water employed two subjects who consumed 26Al added to water from a public supply (Priest et al., 1998). Faeces and urine were collected for 7 days after 26Al administration; these contained 97.6 and 100.4% of the 26Al administered to the two subjects. These results suggest that little aluminium was absorbed. The results also illustrate the inadequacy of the balance method to determine oral aluminium bioavailability. The 26Al in blood obtained 1, 4 and 24 hr after oral 26Al dosing was multiplied by the estimated blood volume of each subject. The results suggested oral aluminium bioavailability of 0.027, 0.034 and 0.012%, based on these three sampling times. Cumulative urine excreted suggested oral aluminium bioavailability was 0.20 and 0.14%, for these two subjects. The authors corrected these values for the percentage of aluminium voided in the urine in the same time period after i.v. injection (72%) (Talbot et al., 1995), as conducted by Stauber et al. (1999). This correction resulted in an estimate of 0.22% oral aluminium bioavailability. This is an order of magnitude greater than the results they obtained by estimating absorption from a single serum sample and the calculated volume of distribution, suggesting this latter method underestimates oral aluminium bioavailability.
Overall, the above results suggest that oral aluminium bioavailability from drinking water is in the range of 0.1 to 0.5%; and most likely approximately 0.3%.
An error resulted in the introduction of 20 tons of 8% aluminium sulphate into a municipal water supply near Camelford, England. Some of the consumers reported that the water had an unpleasant metallic taste; others reported various symptoms (Eastwood et al., 1990). Excessive aluminium, copper, lead and zinc were found in the water. Two of the affected individuals showed elevated urinary aluminium output. Bone biopsies showed the presence of elevated aluminium (Eastwood et al., 1990) that was still elevated 6 and 7, but not 18, months later (McMillan et al., 1993). These results suggest the possibility of elevated bone aluminium in humans with normal renal function after oral consumption of excessive amounts of aluminium. However, only two reports were found of elevated bone aluminium in humans with normal renal function after massive oral consumption of aluminium. One was a 49 year old male with a 25 year history of consumption of aluminium-containing antacids whose bone aluminium concentration, 24 mg/kg dry weight, was between that of three non-dialysis subjects at autopsy, which averaged 6.4 mg Al/kg, and that of 3 dialyzed subjects at autopsy, which averaged 125 mg Al/kg (Recker et al., 1977). The other was a 39 year old female who consumed antacids containing a total of ~ 18 kg of elemental aluminium over 8 years. She had stainable aluminium on 27.6% of the bone surface (Woodson, 1998).
Beverages and foods
Oral aluminium bioavailability from the diet was estimated to be 0.1 to 0.3% based on normal urinary aluminium excretion of 20 to 50 μg/day and a daily aluminium intake of 20 mg (Ganrot, 1986). Daily aluminium intake is now believed to be less. Priest (1993) estimated oral aluminium bioavailability from food to be ~ 0.1% based on a daily aluminium intake of 15 mg, a daily urinary excretion of 0.025 mg and 5% aluminium retention in the body. The same estimate was obtained by comparing an average daily urinary aluminium excretion of 0.004 to 0.012 mg to average daily aluminium intake from food of 5 to 10 mg (Nieboer et al., 1995). Based on daily dietary aluminium intake of 10 mg, a terminal t½ of aluminium of 50 years, and aluminium body burdens of 5 and 60 mg, oral aluminium bioavailability was estimated to be 0.14 and 1.6%, respectively (Priest, 2004).
The bioavailability of aluminium from a low aluminium diet was estimated to be 0.78%, compared to 0.09% when aluminium, as aluminium lactate, was added to the diet, (Greger & Baier, 1983). These results are in the range of values obtained for aluminium from drinking water (see Toxicokinetics, Absorption, Studies in Humans, Oral Administration, Drinking Water). The results suggest an inverse relationship between aluminium dose and oral absorption. However different aluminium species were consumed under these two conditions.
Aluminium bioavailability was estimated based on 24 hr urinary aluminium excretion in subjects consuming a controlled diet that included tea and RSW water (which provided essentially no aluminium) (Stauber et al., 1999). The ~ 3 mg Al/day provided by this diet is below typical dietary intake. Absorption of aluminium from food consumed prior to the study, which likely provided > 3 mg Al/day, may have contributed to the urinary aluminium excretion during the study. This would produce an over-estimation of aluminium bioavailability from the controlled diet. The estimate of oral aluminium bioavailability from food-plus-tea in this study was ~ 0.53%, assuming that 10% of the aluminium in the tea was available for absorption. Based on the assumption that 100% of the aluminium in the tea was available for absorption, oral aluminium bioavailability from food-plus-tea was estimated to be 0.28%. Therefore, the authors concluded that oral aluminium bioavailability from water and food is comparable. They claimed “… recent research suggesting that aluminium in food and aluminium in water have similar bioavailabilities”, citing Priest (1993). However, Priest does not make this statement or provide data to support, or refute, it.
Some studies found increased aluminium in the urine after tea consumption, suggesting absorption of aluminium from the tea. Urinary aluminium concentration increased in the 12 hr after tea consumption (Koch et al., 1988). Equal volumes of coffee or water did not increase the urinary aluminium concentration. They did not report urine volume, or urinary aluminium output, so it is unknown if aluminium excretion increased. However, if urine volume was greater after consuming tea than water, as reported by Powell et al. (1993), urinary aluminium output would have been greater following tea consumption. In the 24 hr after consumption of 2 litres of tea, presumably containing a total of 218 μmoles of aluminium, by one subject, urinary aluminium output was 0.725 μmoles, compared to 0.14 μmoles after consumption of water by the same subject (Powell et al., 1993). This would suggest 0.3% aluminium bioavailability. In contrast, tea, with or without lemon juice or milk, or mineral water was consumed one day with a defined diet, in a cross-over study. The tea contained 2.3 to 2.8 mg aluminium and comprised about 31% of the total daily dietary aluminium intake. Blood was obtained 11 times, from immediately before to 0.5 to 24 hr after dosing. No elevation of serum aluminium, above the pre-treatment mean concentration of 4.2 μg/L, was seen (Drewitt et al., 1993). Four subjects who consumed 2 litres of tea containing ~ 4 mg Al/L eliminated an average of 0.003 mg aluminium within the subsequent 7 hr in their urine, or ~ 0.04% of the aluminium in the tea (Gardner & Gunn, 1995). However, this is clearly an underestimate of the oral aluminium bioavailability because urinary aluminium excretion had not yet returned to the pre-treatment rate in most subjects, suggesting insufficient time to follow aluminium absorption and/or incomplete excretion of the absorbed aluminium.
It was suggested that polyphenols in tea bind most of the aluminium, thus greatly reducing its oral bioavailability (Flaten & Odegard, 1988). Collection of ileostomy effluent from a subject who had not consumed food orally for two weeks but had consumed tea suggested there was no breakdown of the polyphenols from the tea (Powell et al., 1993). These results suggest that digestion does not change the major ligand that binds aluminium in tea.
Chewing gums contain a significant amount of aluminium (0.2 to 4 mg/stick for 3 brands) (Lione & Smith, 1982). Chewing released 2 to 21% of the aluminium, 0.01 to 0.44 mg. The authors concluded that moderate use of chewing gums would not contribute substantially to daily aluminium intake. However, chewing gum may exceed drinking water as a daily source of aluminium.
Drugs
Oral aluminium bioavailability was estimated to be 0.006 and 0.007% from two studies of a commercial aluminium hydroxide product (Linc®); estimates were based on the increase above baseline of urinary aluminium excretion after consumption of 1, 4 or 8 tablets (Haram et al., 1987; Weberg & Berstad, 1986). Weberg & Berstad (1986) observed an inverse relationship between dose and oral aluminium bioavailability. They suggested that this was due to the ability of more tablets to produce a greater increase in the pH of the intragastric milieu, resulting in decreased solubility. However, if aluminium is primarily absorbed from the upper intestine, where the pH is about 6 or 7, this may not be the explanation. It is unknown if diet influenced these results, as this was not controlled or documented. Oral aluminium bioavailability from aluminium hydroxide, and aluminium glycinate taken with aspirin, was estimated to be 0.003 and 0.22%, respectively (Meshitsuka & Inoue, 1998; Meshitsuka et al., 1999).
Oral aluminium bioavailability was estimated from 6-day urinary 26Al output in 2 subjects (Priest et al., 1996). Both subjects received intragastric dosing of 26Al incorporated into aluminium hydroxide, 26Al hydroxide in the presence of citrate, and 26Al citrate. Oral aluminium bioavailability was estimated from urinary aluminium output, corrected for the fraction of aluminium administered intravenously that was found in the urine within 7 days, 0.72, as demonstrated by Talbot et al. (1995), and as used by Stauber et al. (1999) above. Estimates of the percentage of 26Al absorbed from 26Al hydroxide in the absence and presence of citrate averaged 0.01 and 0.14%, respectively; aluminium bioavailability from 26Al citrate was 0.52%. These limited results mirror many reports showing very low aluminium bioavailability from aluminium hydroxide (and sucralfate) and enhancement by citrate.
Oral aluminium bioavailability from aluminium hydroxide appears to be less than from food or water.
Factors influencing oral aluminium absorption
Studies in humans have also shown that oral aluminium absorption is dependent on many factors, although this has been less well studied than in animals. A summary of the reported factors affecting oral aluminium absorption in humans is shown in Table 18. The following sections describe these effects in much more detail.
Table 18.
Summary of the influence of factors that affect oral aluminium absorption from studies in humans.
| Factor | Influence on absorption | Reference(s) |
|---|---|---|
| Solubility | Aluminium absorption is greater from water than aluminium hydroxide or sucralfate, and from sucralfate suspension than tablet. | Haram et al. (1987); Schönholzer et al. (1992); Weberg & Berstad (1986) whereas from drinking water was ~ 0.22 and 0.35% (Conway et al., 1994; Priest et al., 1998; Stauber et al., 1999) |
| pH | Greater absorption at lower gastric pH. | Olaizola Ottonello et al. (1991); Rodger et al. (1991) |
| Carboxylic acids (citrate) | Increased | Coburn et al. (1991); Fairweather- Tait et al. (1994); Lindberg et al. (1993); Mauro et al. (2001); Nestel et al. (1994); Nolan et al. (1990); Nordal et al. (1988a); Priest et al. (1996); Rudy et al. (1991); Slanina et al. (1986); Walker et al. (1990); Weberg & Berstad (1986) |
| Silicon-containing compounds | May reduce absorption. | Edwardson et al. (1993) |
| Iron | Inverse correlation between iron status and aluminium absorption and serum aluminium. | Cannata et al. (1993); Huang et al. (1992) |
| Uraemia | Increased | Ittel et al. (1991a) |
| Dementia | Increased absorption has been suggested in AD and Down’s subjects, but the reported results could be due to differences in aluminium distribution or elimination. | Moore et al. (1997; 2000); Roberts et al. (1998); Taylor et al. (1992) |
| Aluminium dose | Increased dose; decreased aluminium bioavailability from aluminium hydroxide. | Weberg & Berstad (1986) |
Solubility
Oral bioavailability of aluminium from quite insoluble forms, such as aluminium hydroxide and sucralfate, was generally reported to be quite low, e.g. ~ 0.001 to 0.007% (Haram et al., 1987; Weberg & Berstad, 1986), whereas from drinking it water was ~ 0.22 and 0.35% (Priest et al., 1998; Stauber et al., 1999). Higher plasma levels were observed after oral administration of sucralfate in suspension than in tablet form (Conway et al., 1994). This is not consistent with the notion that bioavailability is independent of the chemical species of the aluminium (Reiber et al., 1995), a notion which does not appear to be valid.
pH
Administration of ranitidine, which increased gastric pH, reduced urinary aluminium excretion (Rodger et al., 1991), consistent with an enhancement of aluminium absorption at lower gastric pH. Serum and urine aluminium levels increased in pre-surgery ulcer patients with normal renal function who had a mean gastric pH of 2.09 following administration of 30 mg/kg aluminium hydroxide, whereas the opposite was seen in post-surgery patients whose gastric pH averaged 5.78 (Olaizola Ottonello et al., 1991). In contrast, limited observations in patients with chronic renal failure failed to show a correlation between gastric acid secretion and elevation of serum aluminium after oral aluminium consumption, suggesting gastric acid secretion may not play a critical role in aluminium absorption (Beynon & Cassidy, 1990). There are insufficient reported results to draw a firm conclusion about any effect of gastric pH on oral aluminium absorption.
Carboxylic acids
Numerous studies in humans have shown enhanced aluminium absorption from aluminium hydroxide in the presence of citrate, other carboxylic acids and orange juice (Coburn et al., 1991; Fairweather-Tait et al., 1994; Lindberg et al., 1993; Mauro et al., 2001; Nestel et al., 1994; Nolan et al., 1990; Nordal et al., 1988a; Priest et al., 1996; Rudy et al., 1991; Slanina et al., 1986; Walker et al., 1990; Weberg & Berstad, 1986). For example, Weberg & Berstad (1986) reported that 0.004% of 1 g of aluminium, given as an antacid, was absorbed in the absence of citrate, compared with 0.03% when consumed with orange juice and 0.2% when consumed with citric acid solution that delivered 0.7:1 citrate:aluminium. Similar results have been observed in animal studies (see Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption, Carboxylic Acids). In contrast, co-administered sodium bicarbonate and calcium acetate were not found to increase aluminium absorption (Mauro et al., 2001; Walker et al., 1990).
The first study of oral aluminium absorption using 26Al was conducted in one human subject who received aluminium citrate. The results suggested ≥ 1% oral absorption (Day et al., 1991), whereas two other studies involving 5 and 2 subjects led, respectively, to estimates of 0.015 (Edwardson et al., 1993) and 0.52% (Priest et al., 1996). However, there is a human study that failed to find an increase of aluminium absorption in the presence of citrate. This was conducted in subjects who received aluminium with their diet (Stauber et al., 1999). Generally, increasing the amount of citrate consumed with aluminium hydroxide increased blood aluminium concentrations (Taylor et al., 1992).
Although citrate can increase aluminium absorption, it has not always been reported to increase tissue or cellular aluminium retention. Some animal studies have reported a citrate-induced increase of brain and aluminium (as described above in Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption, Carboxylic Acids), whereas other animal studies and one human study have not (Sakhaee et al., 1993). Therefore, citrate may enhance oral aluminium absorption but may also enhance its distribution into and out of tissues as well as from the organism by renal elimination, in the presence of renal function, as suggested by Maitani et al. (1994), and discussed below (see Toxicokinetics, Elimination and Excretion, Animal Studies, Urinary Excretion).
The primary humic substances in water and soil are humic and fulvic acids. One healthy person who took 1.75 g of aluminium hydroxide, adjusted to pH 2, and 1.25 gm of concentrated humic substances, excreted more aluminium in the urine over the subsequent 50 hr than when the aluminium was taken alone (1.4 v.s. 0.6% of the aluminium dose), suggesting humic substances can increase aluminium absorption (Alexander et al., 1990).
Silicon-containing compounds
Sodium silicate (100 μM) reduced the GI absorption of 26Al (consumed in orange juice, a source of citrate) by 85% in fasted humans (Edwardson et al., 1993). Taylor et al. (1995) cited previous studies showing an inverse relationship between aluminium and silicon concentrations in drinking water. An earlier study showed an inverse relationship between renal function and plasma silicon but no correlation between serum aluminium and silicon in patients with chronic renal failure on regular haemodialysis (Roberts & Williams, 1990). In a later study there appeared to be an inverse relationship between serum aluminium and silicon levels in a subset of haemodialysis patients (Parry et al., 1998). Studies of the aluminium and silicon dioxide concentrations in drinking water suggested that moderate silicon concentration has a slight protective effect against aluminium-associated impaired mental function (Forbes et al., 1995; Forbes & Gentleman, 1998). These results suggest that increasing dietary silicon may reduce aluminium absorption and facilitate excretion.
Fluoride
Fluoride forms numerous complexes with aluminium (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Compounds). Aluminium appears to inhibit fluoride absorption (Brudevold et al., 1973; Hobbs et al., 1954; Li et al., 1991; Spencer et al., 1980a; 1980b; 1981; 1985; Spencer & Kramer, 1985; Spencer & Lender, 1979).
In fluoridated drinking water, decreased pH would favour formation of aluminium-fluoride complexes. In one survey, 19% of aluminium in treated water was complexed with fluoride, when 58 μM (~ 1 mg/L, 1 ppm) fluoride was added (Driscoll & Letterman, 1988). Moderate concentrations of fluoride in drinking water were found to have a slight protective effect against aluminium-associated impaired mental function (Forbes & Agwani, 1994a; 1994b; Forbes & Gentleman, 1998). However, there was no obvious benefit of 40 mg of fluoride given daily for 1 year to patients in the early stages of AD (D. Shore, personal communication, 1992). Although complexation of aluminium with fluoride can be important at the more acidic pHs found occasionally in water, there is not sufficient fluoride in plasma to form any significant amount of binary aluminium fluoride complexes (W.R. Harris, personal communication, 2000). It is not clear if fluoride significantly influences aluminium toxicokinetics.
Iron
Haemodialysis patients who had low or normal serum ferritin levels and were given aluminium hydroxide for 7 days had increased serum aluminium, whereas patients with high serum ferritin did not show an increase of serum aluminium concentration (Cannata et al., 1984). Serum aluminium negatively correlated with serum iron, serum ferritin and Tf saturation in chronic haemodialysis patients (Cannata et al., 1993; Huang et al., 1992). Similarly, there was a negative correlation between serum iron and aluminium absorption in dialysis patients (Cannata et al., 1993). These results are consistent with those of animal studies showing enhanced aluminium absorption in the presence of iron deficiency (see Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption, Iron).
Calcium
Addition of calcium to aluminium hydroxide did not affect aluminium absorption in humans with normal renal function who presumably had normal calcium status (Nolan et al., 1990). Based on ionic radii, it is more likely that aluminium would compete with magnesium than calcium. Although there is some evidence for aluminium-magnesium competition in vivo, this has not been well investigated.
Uraemia
The primary documented problems with aluminium as a toxicant to bone and the brain have occurred in persons with uraemia, who accumulate aluminium, due to their inability to excrete it, and may develop aluminium-induced toxicity (Alfrey, 1980). There is evidence that uraemia may also enhance aluminium absorption. Ittel et al. (1991a) found greater serum aluminium levels in acute and chronic renal failure patients than those with normal renal function receiving the same daily dose of aluminium (1.18 g) as hydroxy-magnesium aluminate, greater daily urinary aluminium excretion in the chronic renal failure patients, and a significant negative correlation between renal aluminium excretion and creatinine clearance. They concluded that there was an enhancement of GI aluminium absorption in the presence of chronic renal failure. However, calculations using the biokinetic model, described in Toxicokinetics, Pharmacokinetic Modelling, failed to find evidence for increased oral aluminium absorption by humans with chronic renal failure (Steinhausen et al., 2004).
Age
The information related to the affect of age on aluminium absorption is from adult and geriatric subjects. No published studies of children were found. A greater increase in blood aluminium was seen in subjects aged > 77 than in those aged < 77 (serum aluminium 101 vs. 38 μg/L at 1 hr), who consumed ~ 4.5 mg/kg aluminium hydroxide and 3.3 to 6.5 g citrate (citrate:aluminium, 1.6:1 to 3.2:1) after an overnight fast (Taylor et al., 1992). Comparing oral aluminium bioavailability in the subjects < 59 with those > 59 years of age failed to reveal a difference (Stauber et al., 1999). Taking these observations together with the results from animal studies, (see Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption, Urinary Excretion) there is a lack of consistent data to be able to conclude that there is a significant effect of age on aluminium absorption, distribution or retention.
Dementia
In a study of 20 AD subjects aged 65 to 76 (n=10) and 77 to 89 (n=10) blood aluminium levels were compared to those of 20 controls. Subjects and controls consumed ~ 4.5 mg/kg aluminium hydroxide and 3.3 to 6.5 g citrate (citrate:aluminium, 1.6:1 to 3.2:1) after an overnight fast (Taylor et al., 1992). In the younger AD subjects, the blood aluminium was significantly greater at 60 minutes than in control subjects (104 vs. 38 μg/L). In the older subjects, the increase in blood aluminium levels was greater, but not statistically different, in the controls than in patients, in contrast to the younger subjects. Aluminium absorption was studied in AD subjects and compared to age-matched controls after consumption of a fruit drink containing 26Al (Moore et al., 2000). The percent of aluminium absorbed in subjects, estimated from single plasma samples obtained 1 hr after the oral aluminium consumption, was 164% of that seen in controls. The authors attributed these differences to absorption, although reduced renal aluminium clearance in the aged could also contribute to this difference. The lack of consistent overall differences as a function of age or dementia status makes it difficult to draw any general conclusion from this study. Zapatero et al. (1995) found significantly higher serum aluminium concentrations in 17 AD subjects, compared to age-matched controls, but no difference between 15 subjects with senile dementias and controls. Based on greater serum and urine aluminium levels in 8 patients with dementia including the Alzheimer’s type who were 65 to 86 years old compared to 144 controls who were 30 to 65 years old (18 and 6 μg/L and 77 and 26 μg/L, respectively), Roberts et al. (1998) claimed to have confirmed earlier findings that patients with dementia appear to absorb more aluminium from the diet than healthy subjects. However, the difference in the serum and urine aluminium levels could be due to factors other than dementia, such as the significant difference in the age of the subjects. AD appears to be associated with a higher serum aluminium concentration than seen in controls. Nothing can be concluded from these observations to elucidate the mechanism of this difference.
Utilizing both 27Al and 26Al in separate studies, greater aluminium absorption was seen in subjects with Down’s syndrome than in controls, 0.14 vs. 0.030% for 27Al administered with citrate and 0.14 vs. 0.022% for 26Al administered with orange juice (Moore et al., 1997). However, these results are based on a single blood sample drawn 60 minutes after aluminium administration, a method noted above in Toxicokinetics, Absorption, Animal Studies, Oral Administration that may not very reliably estimate oral bioavailability. Down’s syndrome is associated with changes in neurodegeneration, particularly increased β-amyloid deposition, that resemble AD.
Aluminium dose
Aluminium bioavailability following consumption of a commercial aluminium hydroxide product was found to be 0.007, 0.004 and 0.001% from aluminium doses of ~ 3.5, 14 and 28 mg/kg, respectively (Weberg & Berstad, 1986). This inverse relationship between dose and oral bioavailablity is not consistent with results from animal studies suggesting increased aluminium absorption with increased dose, as discussed in Toxicokinetics, Absorption, Animal Studies, Oral Administration, Drinking Water.
Site of oral absorption
A significant increase in serum aluminium concentration was seen 30 minutes after consumption of aluminium and citrate, suggesting aluminium absorption from an upper intestinal site (Nordal et al., 1988a). Peak serum aluminium was seen 30 minutes after consumption of an aluminium antacid (Nagy & Jobst, 1994). Oral dosing of human subjects with antacids and citrate produced a peak blood aluminium level ~ 4 hr later (Weberg & Berstad, 1986) that was interpreted as support for intestinal aluminium absorption (Powell & Thompson, 1993). Blood aluminium peaked in four subjects ~ 30 to 45 minutes after they had consumed aluminium citrate (Nordal et al., 1988a; Taylor et al., 1992) and at 30 minutes in one subject after oral ingestion of an aluminium-containing antacid (Nagy & Jobst, 1994). Serum glucose also peaked 30 minutes after oral administration of 50 g of glucose (Nagy & Jobst, 1994). Hohl et al. (1994) saw maximal serum aluminium approximately 2 hr after oral aluminium intake, although the peak was not much higher than seen at 30 minutes, suggesting quite rapid absorption. As concluded from studies in animals (see Toxicokinetics, Absorption, Animal Studies, Oral Administration, The Site and Mechanisms of Oral Aluminium Absorption), aluminium seems to be primarily absorbed from the upper intestine.
Dermal exposure
A study of 26Al chlorohydrate absorption, a primary component of antiperspirants, which was applied once to both underarms of one male and one female subject, revealed an average excretion of 0.012% of the applied 26Al in the urine over the subsequent 53 days (Flarend et al., 2001). Daily application of tape to the underarm area to strip away dead skin and surface aluminium chlorohydrate followed by gentle wiping with a towelette for 6 days after its application removed 39% of the applied material. One might assume that the aluminium chlorohydrate that was not removed by the tape and washing represents the maximum amount of aluminium available for absorption. Based on this assumption and assuming that 85% of the aluminium that would eventually be excreted in the urine was excreted during the time course of this study, the results suggest that a maximum of 0.02% of the aluminium could eventually be absorbed. Given that 50 to 75 mg of Al might be applied daily in antiperspirants, even this very small absorption might be relevant. However, it cannot be assured that this absorption was not from inhalation of Al that flaked off of the application site and it is unknown if comparable aluminium absorption would occur following daily application of aluminium chlorohydrate. This might not occur because of formation of aluminium precipitate in the sweat gland that might impact on the potential for subsequently applied antiperspirant to be absorbed. This study provides the best estimate to date of percutaneous bioavailability of aluminium from antiperspirants.
A woman who applied ~ 1 g of aluminium chlorohydrate-containing antiperspirant to each regularly-shaved underarm daily for 4 years was reported to have experienced bone pain and fatigue (Guillard et al., 2004). Serum aluminium was 3.88 μM (105 μg/L) and 24 hr urinary aluminium excretion was ~ 1.75 μmoles (47 μg)/day, which is elevated, but not as high as would be expected with this serum aluminium concentration in the presence of normal renal function. Aluminium concentrations in serum and urine before antiperspirant use were not reported. After discontinuation of antiperspirant use, urine and serum aluminium concentrations decreased over 7 months, her bone pain nearly disappeared and her fatigue was less severe. Given the extensive world-wide use of aluminium-containing antiperspirants, the lack of other similar reports suggests that this patient was atypical, as speculated by Exley (2004), or that the samples were contaminated.
Distribution (Including Compartmentalization)
Animal studies
Aluminium levels generally decreased in guinea pigs from gestation day 30 to post-natal day 12 (Golub et al., 1996a). Muscle aluminium was reported to increase with age from 2 to 6 to 12 months in rats, but to decrease at 24 months (Kukhtina, 1972). Lung aluminium increased from a mean of 1.7 and 10 mg/kg (wet tissue) in guinea pigs and rats at 6 months of age to 32 and 52 mg/kg at 21 to 24 months, respectively (Stone et al., 1979). Aluminium levels increased with age in liver and kidney of mice, did not change in the brain and heart, and decreased in femur and lung (Massie et al., 1988). Bone and kidney aluminium increased with age in rats (Greger & Radzanowski, 1995). The limited data available suggest that brain and blood aluminium concentrations increase with age. The age-related increase may be due to reduced aluminium clearance with age, as discussed below (see Toxicokinetics, Elimination and Excretion, Animal Studies, Elimination Rate), the large amount of aluminium in the diet of laboratory animals, as noted above (see Toxicokinetics, Absorption, Animal Studies, Oral Administration, Beverages and Foods) and the long t½ of aluminium (as discussed below in Toxicokinetics, Elimination and Excretion, Animal Studies, Elimination Rate).
It has been suggested that citrate can promote the redistribution and elimination of aluminium from plasma. This is dependent on the presence of a significant fraction of aluminium as aluminium citrate. In the presence of normal aluminium concentrations, Tf binds most of the aluminium in plasma (see Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Transport in Blood). Citrate promotion of aluminium distribution and excretion would be favoured in the presence of aluminium concentrations that exceed the Tf metal binding capacity. This very seldom occurs in the human, but has been produced in some of the experimental animal studies. Alternatively, citrate may promote aluminium distribution and excretion prior to association of the aluminium with Tf. The time course of aluminium complexation by Tf in vivo has not been determined; however, when aluminium lactate was incubated with Tf, the association of the aluminium with Tf was complete within 1 minute in vitro at 37 °C (Yokel et al., 1991a). Exchange of aluminium from citrate to transferrrin at pH 7.2 to 8.9 and 25 °C occurred in three kinetic processes. The first, which might be formation of a ternary complex of Tf, aluminium and citrate, was nearly complete within 2 minutes (Hemadi et al., 2003). A transition time for the change of plasma aluminium association from citrate to Tf of 4.2 minutes was tentatively used in a model of aluminium biokinetics (Steinhausen et al., 2004) (see description in Toxicokinetics, Pharmacokinetic Modelling).
In contrast to aluminium citrate, administration of aluminium, as the lactate, chloride and perhaps some other species, may result in clearance of the aluminium by tissues, particularly by the reticuloendothelial organs, and sequestration, reducing renal clearance and increasing the body burden. Tissue aluminium concentrations in rabbits 1 week after completion of a series of 20 i.v. injections of aluminium citrate were considerably lower than after the same molar dose of aluminium lactate (Yokel et al., 1996a). Similarly, addition of equimolar citrate to i.p. injections of aluminium chloride significantly reduced serum aluminium compared to the absence of citrate, whereas equimolar maltol significantly increased both serum and brain levels of aluminium in the rat (Ogasawara et al., 2002).
Administration to dogs of aluminium, as alum, in a mixture of hashed lean beef, lard, cracker meal, bone ash and water or biscuit prepared from flour resulted in a measurable level of aluminium in the blood hours later, leading the author to conclude that the aluminium was absorbed and promptly entered the blood (Steel, 1911). However, no results were reported for dogs that had not been treated with aluminium. Aluminium appeared in bile, lymph and urine within minutes following an i.v. injection (Underhill et al., 1929). Most of the results of animal studies suggest an initial Vd of aluminium consistent with blood volume. When samples were collected over longer periods, greater Vds became evident, although much less than seen with many xenobiotics. The Vd after i.v. and oral administration of 8.1 mg Al/kg in rats and followed for 10 hr was 38 and 46 mL/kg (Gupta et al., 1986). Following 0.1 mg aluminium (as the sulphate)/kg i.v. injection in the rat, Vds of 78, 47, and 42 mL/kg were seen (Pai & Melethil, 1989; Xu et al., 1991; 1992a). After i.v. injection of aluminium lactate, the initial (central compartment) and steady-state Vds in the rabbit were reported to be 54 and 109 mL/kg after 40 μmoles Al/kg (1.1 mg/kg) and 44 and 100 mL/kg after 80 μmoles Al/kg (2.2 mg/kg) (Yokel & McNamara, 1985). When sampling time was increased to 48 hr, initial and steady-state Vds in the normal rabbit after i.v. injection of 100 μmoles (2.7 mg) Al/kg as the lactate were reported to be 159 and 1175 mL/kg and 168 and 516 mL/kg in renally-impaired rabbits (Yokel & McNamara, 1988). When blood aluminium samples were collected over 72 hr, the steady-state Vd was found to be 1091 mL/kg (Yokel & McNamara, 1989-1990). In the dog, the initial Vd was estimated to be 50 mL/kg after 1 mg Al/kg as the chloride given i.v., when studied over a period of 2.5 hr (Henry et al., 1984).
The above results suggest that the initial t½ of aluminium elimination might be aluminium-dose/concentration dependent. The t½ doubled with a 10-fold increase in the aluminium dose in the rat (Pai & Melethil, 1989) and increased 1.8-fold with a doubling of the dose in the rabbit (Yokel & McNamara, 1985). This could be due to greater formation of non-ultrafilterable aluminium species (see Toxicokinetics, Elimination and Excretion, Animal Studies, Elimination Rate).
The steady state serum to whole blood aluminium concentration ratios were ~ 0.9 to 1.1 in rats consuming aluminium in their diet (Mayor et al., 1977). The aluminium concentration ratio in rabbit serum and plasma compared to whole blood was ~ 1.15 to 1.2, showing nearly equal distribution between plasma, serum and the formed elements of blood (Yokel & McNamara, 1985). Over the range of 1 to 20 μg Al/mL, blood to plasma aluminium ratios were 0.8 to 1, also showing near equilibrium of aluminium between blood cells and plasma (Pai & Melethil, 1989).
The percentage of aluminium bound to plasma proteins was reported to be 92 to 98% in the rat, at a serum aluminium concentration of 2000 to 10,000 μg Al/L following aluminium chloride injection (Burnatowska-Hledin et al., 1985). Similarly, 98% of aluminium was found to be protein bound when rat serum aluminium concentrations were 110,000 to 440,000 μg Al/L (Gupta et al., 1986). These aluminium concentrations greatly exceed those seen in humans and the capacity of Tf to bind aluminium. When aluminium, as the chloride, was added to rabbit serum to give a final aluminium concentration of 100 to 32,000 μg/L, the ultrafilterable percentage decreased as aluminium concentration increased in normal, but not renal-impaired, rabbits (Yokel & McNamara, 1988).
Studies of the sub-cellular localization of aluminium in rat liver cells showed considerably more in the nuclear fraction than in the mitochondrial or sub-microscopic fractions when aluminium was introduced as the ion at pH 7, suggesting selective nuclear aluminium uptake (Kushelevsky et al., 1976). In contrast, rat hepatocytes took aluminium into the mitochondrial (~ 45 to 50%) and post-mitochondrial fraction (~ 40 to 45%) but only 6 to 7% into the nucleus. There were no differences when aluminium was introduced as the chloride, nitrate or lactate (Muller & Wilhelm, 1987). In another study, daily i.v. injection for 50 days of 1.5 mg Al/kg, as the chloride, in piglets greatly increased serum and hepatic aluminium levels. Microanalysis with an energy dispersive x-ray spectrometer showed aluminium and phosphate in hepatocyte lysosomes, but aluminium was not seen in all lysosomes or in all hepatocytes (Klein et al., 1987). Aluminium was seen in the lysosomes of kidney cells of rats given i.p. aluminium chloride (Linss et al., 1991; 1992). The nucleus of Caco-2 cells appeared to selectively take up 26Al, irrespective of the chemical species of 26Al to which the cells were exposed (Zhou & Yokel, 2005).
After repeated s.c. aluminium injections to rabbits that produced NFTs, the aluminium in the neurons was seen in the nucleus but not the cytoplasm (Uemura, 1984). Similarly, aluminium was seen in the nucleus (nucleolus, interchromatin granules, euchromatin, and the heterochromatin) and cytoplasmic area (rough endoplasmic reticulum and free ribosomes) of rabbit neurons following administration of aluminium as the powder or chloride into the cisterna magnum or following i.v. injections of aluminium lactate (Wen & Wisniewski, 1985). The intracellular distribution of aluminium in murine neuroblastoma cells that took up aluminium, introduced as aluminium chloride at pH 6, showed 20% to be associated with the nuclear pellet and cell debris, 70% in the supernatant fraction from endoplasmic reticulum, lyosomes and cytosol, and 11% with mitochondria (Shi & Haug, 1990). Aluminium- ethylenediaminetetraacetic acid (EDTA) resulted in equal aluminium distribution in the cytosolic and crude nuclear fractions of human neuroblastoma cells. More aluminium was associated with protein than DNA in the nuclear fraction (Dobson et al., 1998). In contrast, aluminium taken up by rat cerebral organotypic cultures exposed to aluminium chloride was only seen in lysosomes (Schuurmans Stekhoven et al., 1990). A study of 26Al uptake by human neuroblastoma cells exposed to aluminium-EDTA showed ~ 55% of the 26Al in the nuclear fraction and the remainder in the cytoplasm. Within the nuclear fraction, ~ 81% was in the nuclear sap, 17% associated with nuclear protein, 1.2% with DNA and 0.5% with RNA (King et al., 1994). In neuron- and astrocyte-like cells, aluminium maltolate exposure resulted in intranuclear and cytoplasmic vesicular aluminium accumulation, respectively. Aluminium accumulation in astrocyte-like cells after aluminium chloride, aluminium lactate and aluminium fluoride exposure was in small vesicles throughout the cytoplasm and nucleus (Lévesque et al., 2000). When brain (cerebrum) was fractionated to obtain cell nuclei, ~ 27% of the 26Al was seen in the nuclei of suckling, and 47% in those of weaned, rats (Yumoto et al., 2003). Isolation of a nuclear fraction, and then chromatin and supernatant fractions from the nuclear fraction, showed that ~ 89% of the nuclear 26Al was in the chromatin fraction (Yumoto et al., 1997). Aluminium subcellular localization in many of these studies was similar to that seen in human brain (see Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Tissue Aluminium Concentrations, Brain), but some results are different, suggesting that in vitro exposure of cells to some aluminium chemical forms results in different cellular localization than occurs in vivo.
Iron status negatively correlated with tissue aluminium accumulation. It was suggested that this may be due to competition between these two chemically-similar trivalent cations, enabling greater Tf-mediated extravascular distribution and ferritin storage of aluminium in the presence of low iron concentrations (Greger & Sutherland, 1997).
Ferritin isolated from the brain of rats that had received aluminium in their drinking water for 1 year had more aluminium than normal rats (115 vs. 42 mole of Al/mole of ferritin) whereas the molar ratio of aluminium to ferritin in the liver did not increase (4.3 vs. 4.6) (Fleming & Joshi, 1987). Horse spleen ferritin bound up to 98 mole Al/mole ferritin after incubation exposure to aluminium citrate (30 mM) (Cochran & Chawtur, 1988). Further procedures suggested that the aluminium was firmly bound to the ferritin, probably to the core. Horse spleen ferritin that was reconstituted in the presence of aluminium citrate contained an average of 120 mole Al/mole ferritin, whereas apoferritin and reconstituted ferrritin bound only 7.6 and 9.5 mole Al/mole ferritin, in an equilibrium dialysis study (Dedman et al., 1992a). The authors concluded that aluminium can be incorporated into a growing iron core in ferritin, that this can be explained by non-specific binding and that, once the aluminium is bound, it is trapped and cannot dissociate freely, but that in the presence of aluminium-complexing ligands, such as citrate, ferritin will not sequester large amounts of aluminium. Ferritin isolated from the brain of rats that received repeated aluminium injections, where blood and brain aluminium were significantly increased, had 136 mole Al/mole ferritin compared to 3.6 in controls (Sakamoto et al., 2004). Although the authors concluded that ferritin may act as an aluminium detoxicant, they found that the ferritin-associated aluminium corresponded to 5.9% of the total brain aluminium, suggesting it does not sequester a very large percentage of intracellular aluminium.
Repeated aluminium administration to animals results in greater aluminium accumulation in bone of uraemic, than renally-intact, subjects (Alfrey et al., 1985; Chan et al., 1983; Ecelbarger & Greger, 1991; Hirschberg et al., 1985; Walker et al., 1994; Yokel & McNamara, 1988). The bone of uraemic rats contained significantly more 26Al after a single oral administration than did that of control rats, whereas tissues that are thought to receive aluminium by TfR-ME, the liver and spleen, had less 26Al in uraemic, than control rats (Ittel et al., 1997). Brain aluminium concentration was significantly elevated in only one of these studies (Alfrey et al., 1985). Spleen and liver aluminium levels were lower in several of these studies, but liver aluminium increased and muscle aluminium decreased in one (Hirschberg et al., 1985). Uraemia appears to change aluminium in circulation to smaller chemical species (Yokel & McNamara, 1988), possibly enhancing its distribution into some tissue.
After 24 hr, retention of aluminium, administered as aluminium citrate, was greater in the liver, but less in the bone, kidney and spleen, of iron-deficient, than in those of control, rats (Greger et al., 1994). In pregnant rats given a s.c. injection of 26Al at day 15 or 16 of gestation, 0.09% of the dose was seen per gram liver in the dams and 0.0006% in foetal liver at gestational day 20 or 21 (Yumoto et al., 2000; 2001). There was ~ 100-fold more aluminium in the maternal liver than in the brain of these rats, as well as in rats which were similarly injected in other studies (see below). Placental 26Al concentrations were ~ 80 to 90% of liver concentrations. Of the injected dose, ~ 0.2% had transferred to the foetus and a comparable mass amount had been retained by the placenta. The liver contained ~ 5-fold more 26Al than the placenta or foetus. Mice which drank water containing aluminium as aluminium chloride, dihydroxyaluminium sodium carbonate or aluminium hydroxide until they had consumed 700 mg aluminium, which took 159, 182 and 239 days, respectively, had significantly greater aluminium concentrations 48 hr after the last dose in the stomach, kidney, liver and tibia after consuming the aluminium chloride; but only in the stomach after consuming dihydroxyaluminium sodium carbonate and only in the tibia after consuming aluminium hydroxide (Dlugaszek et al., 2000). These results show the influence of speciation on aluminium kinetics.
Some studies suggest that fluoride influences aluminium distribution. Addition of fluoride to s.c. injections of aluminium resulted in significantly higher aluminium levels in liver, spleen and adrenals than those from aluminium-alone injections and increased aluminium-induced behavioural toxicity, suggesting fluoride may alter aluminium distribution or some other aspect of the fate of aluminium (Stevens et al., 1987). Addition of fluoride to the aluminium in the drinking water of rats reduced bone aluminium levels but appeared to exacerbate the osteomalacic lesion of aluminium-associated bone disease (Ittel et al., 1992b). Pre-treating rabbits with 3 mg F/kg/day in their water for 9 days before intrathecal aluminium injection tended to lower brain aluminium levels, but only significantly in the cerebellum. On the other hand, intrathecal aluminium injection in rabbits followed by 3 mg F/kg/day in the drinking water produced no difference in brain aluminium levels compared to those in the controls (Shore et al., 1985).
Although it has been suggested that AlF3 may play a role in aluminium toxicity, the fraction of intracellular aluminium as AlF3 is < 1%, making this unlikely. Intracellular aluminium is more likely associated with phosphate, ATP and phosphorylated proteins. Concurrent gavage administration of aluminium, as 200 mg Al/kg, and folic acid in some rats, as 20 mg/kg, 5 days weekly for 8 weeks, resulted in significantly less aluminium in femur, brain and kidney, but not serum, in the rats that received the folic acid (Bayder et al., 2005). It is not clear if folic acid reduced aluminium absorption or enhanced its elimination. Although the stability constant of the aluminium-folate complex is quite high, (log K = 15.15 (Nayan & Dey, 1970)), the low molar ratio of folate to aluminium of 0.006:1 suggests the results are not due to formation of an aluminium folate complex. Dietary supplementation with vitamin E (5, 15 or 29 mg/g chow) with i.p. injections of aluminium in the rat reduced plasma and brain aluminium compared to the absence of vitamin E supplements, although the effect was most pronounced with the lowest vitamin E concentration (Abubakar et al., 2004). The authors speculated that the vitamin E effect could be due to preservation of cell membrane ion transport and membrane fluidity. However, an effect on aluminium excretion cannot be ruled out.
To determine if there might be genetic influences on aluminium accumulation, mice from 5 inbred strains were maintained on a control diet or one supplemented with 260 mg Al/kg diet for 28 days (Fosmire et al., 1993). C3H mice were the only strain that showed significantly higher tibial aluminium due to aluminium in the diet, DBA the only strain that showed lower aluminium due to aluminium in the diet and the A/J the only strain showing significantly higher aluminium in the absence of aluminium in the diet. DBA strain mice were the only ones showing significantly more brain aluminium due to aluminium in the diet. The bases of the observed differences may be variation in the permeability of various barriers, such as the BBB, as speculated by the authors, but could also be due to other factors affecting aluminium distribution, such as absorption and elimination. No other studies were found that assessed the potential influence of genetics on aluminium kinetics.
The α-hydroxy carboxylic acids (citric, lactic and malic) of interstitial fluids dissolve aluminium from aluminium-containing adjuvants. Citrate solubilizes aluminium phosphate adjuvant faster than aluminium hydroxide adjuvant (Hem, 2002).
Early studies showed a PTH-induced increase of serum and brain aluminium in rats (Mayor et al., 1977). An increase in muscle and liver aluminium was reported in uraemic rats following PTH and aluminium administration, whereas administration of both PTH and vitamin D to nephrectomized rats raised aluminium in heart and muscle but decreased it in brain, bone and liver (Hirschberg et al., 1985). An increase of aluminium in brain, bone and muscle, but not heart, kidney, liver, spleen or serum, was reported following PTH and aluminium administration (Costantini et al., 1989; Hirschberg et al., 1985). These results are consistent with the effects obtained with low calcium, and a low-calcium-induced hyperparathyroidism. In rats maintained on a calcium-deficient diet that developed hyperthyroidism but not given supplemental aluminium, liver and bone aluminium levels were below normal, whereas they were normal in rats given supplemental aluminium (Drüeke et al., 1985). Plasma aluminium was above normal. Liver aluminium content was increased in rats made hyperparathyroid by parathyroid extract treatments. However, subsequent studies failed to show an effect on aluminium absorption following parathyroidectomy (PTX) or PTH administration (Feinroth et al., 1982), an appreciable correlation between PTH status and aluminium absorption (Nordal et al., 1988a), or an effect of PTX on urinary aluminium output in rats that received oral aluminium (Ittel et al., 1987). Because PTH stimulates 1,25-(OH)2-vitamin D, a PTH effect could be mediated by vitamin D. There is evidence suggesting vitamin D enhances oral aluminium absorption in the rat and rabbit, as increased serum and urinary aluminium were seen after oral but not i.v. aluminium administration (Adler & Berlyne, 1985; Ittel et al., 1988; Long et al., 1991; 1994). Supplementation with 1,25-(OH)2-vitamin D resulted in decreased levels of aluminium in the rat (Drüeke et al., 1985). Repletion of 1,25-(OH)2-vitamin D reduced bone aluminium accumulation in 5/6 nephrectomized, thyroparathyroidectomized (TPTX) dogs; however, PTH did not produce the same effect (Malluche et al., 1987). Similarly, 1,25-(OH)2-vitamin D injections decreased bone aluminium deposition in rats treated with i.p. aluminium injections and fluoride in their drinking water (Ittel et al., 1993a). Vitamin D has been shown to increase aluminium levels in serum and in some peripheral tissues, such as bone, heart, liver and muscle (Anthony et al., 1986; Burnatowska-Hledin et al., 1986; Hirschberg et al., 1985; Ittel et al., 1990). The lack of vitamin D-induced increase of serum aluminium when the aluminium was given i.v. suggested the vitamin D effect was on oral absorption (Ittel et al., 1990). However, it usually decreased brain aluminium levels (Anthony et al., 1986; Hirschberg et al., 1985). As the aluminium was given parenterally, the effect of vitamin D was presumably on aluminium distribution or elimination.
Rabbits that consumed a cholesterol rich (1.5% cholesterol) diet had a significant elevation of aluminium in the frontal cortex, spinal cord and kidney after 3 months (Yasui et al., 1990a). A free radical scavenger, vinpocetine, 10 mg/day, significantly reduced the aluminium concentrations to, or below, those of controls.
To address the hypothesis that low levels of calcium and magnesium in the environment contributed to brain aluminium accumulation in the amyotrophic lateral sclerosis (ALS) and parkinsonism dementia disorders (see Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Tissue Aluminium Concentrations, Brain), cynomolgus monkeys were maintained for 41 to 46 months on a normal diet containing 1% calcium, a low calcium diet (0.32%), or a low calcium diet plus 150 mg aluminium and 50 mg manganese daily (Garruto et al., 1989). Preliminary analysis of the spinal cord of one of the animals that received the aluminium-containing diet showed aluminium, but not manganese, accumulation. In another study, mice were fed a standard diet; a diet low in calcium and magnesium; a diet low in calcium and magnesium and high in aluminium; or a diet low in calcium and magnesium, high in aluminium and containing 1,25-(OH)2D3. Addition of aluminium to the diet increased the level of brain, kidney, liver and muscle aluminium. Addition of 1,25-(OH)2D3 enhanced aluminium accumulation in these organs (Yasui et al., 1990b). Rats fed a calcium- and magnesium-deficient diet had a non-significant increase of CNS aluminium, whereas rabbits fed a calcium- and magnesium-deficient diet with added aluminium, as the lactate, did not show an elevation of CNS aluminium compared to rabbits consuming the deficient diet without aluminium (Yase, 1980). However, few details were reported for these two studies. In a study in rats, low amounts of calcium and magnesium significantly increased lumbar spinal and femoral bone aluminium levels. Addition of aluminium increased the aluminium concentration more, but the lack of a group given standard diet with aluminium for comparison does not allow determination whether or not calcium and magnesium deficiency increased nervous system aluminium accumulation in the presence of elevated aluminium in the diet (Yasui et al., 1991a). Mice that consumed a low calcium, low magnesium diet or the same diet plus aluminium, as 15.6 g aluminium hydroxide/kg diet, for 11 to 31 months, had aluminium and calcium deposition in cortical and hippocampal neurons, shown by morin stain (Kihira et al., 2002).
Aluminium uptake into dimethylsulphoxide-induced Friend erythroleukemia cells, which are a model system for erythroid differentiation, was linear for 96 hr when exposed to aluminium Tf (Abreo et al., 1990). Cellular aluminium concentration did not reach the medium aluminium Tf concentration. McGregor et al. (1991) showed that aluminium uptake by erythroleukemia cells was aluminium Tf-concentration dependent. Aluminium uptake was not seen in these cells when exposed to aluminium citrate (Abreo et al., 1990; McGregor et al., 1991). Similarly, there was more aluminium in reticulocytes and an osteoblast-like cell line after exposure to aluminium Tf than to aluminium citrate (Abreo et al., 1990; McGregor et al., 1994).
Aluminium was taken up by hepatocytes to a greater extent when introduced as aluminium Tf than as aluminium citrate (Abreo et al., 1991; 1997). p-Cresol, a compound that accumulates in uraemia, increased aluminium uptake from aluminium Tf, but not from aluminium citrate (Abreo et al., 1997), whereas the anti-oxidants N-acetylcysteine, tetramethylpiperidine 1-oxyl, SOD, and catalase did not (Abreo et al., 2004). Aluminium appears to enter hepatocytes by a Tf-receptor mediated process but can also enter more slowly by another mechanism(s).
Central nervous system
Oral administration of 26Al resulted in higher brain 26Al concentrations than seen in control rats (Drüeke, 2002; Fink et al., 1994; Jouhanneau et al., 1997b; Walton et al., 1995), demonstrating the ability of aluminium to be orally absorbed and to distribute into the brain. There are 2 routes by which aluminium might enter the brain from blood, through the BBB, and through the choroid plexuses into the CSF of the ventricles within the brain and then into the brain. Aluminium can rapidly enter brain extracellular fluid (ECF) and CSF, although its concentrations in these two fluids are less than in blood (Allen & Yokel, 1992; Allen et al., 1995; Xu et al., 1992b; Yokel et al., 1991b). Studies in which rats were injected i.v. with aluminium as aluminium citrate, chloride or aluminium Tf or i.p. in an acidic acetate buffer, using either 27Al or 26Al, resulted in ~ 0.0008 to 0.009% of the aluminium dose/g brain (Allen & Yokel, 1992; Kobayashi et al., 1990; Walker et al., 1994; Yokel, 2001); in pregnant rats injected at day 15 or 16 of gestation, the percentage was 0.00065% and in the foetuses at day 20 it was 0.0002% (Yumoto et al., 2000; 2001).
There appears to be more than one mechanism of aluminium distribution across the BBB into the brain though one has not been directly demonstrated. Evidence has been provided that Tf can mediate aluminium transport across the BBB by TfR-ME of the aluminium Tf complex (Roskams & Connor, 1990). It is also assumed that TfR-ME mediates aluminium uptake into some peripheral tissues (see Toxicokinetics, Pharmacokinetic Modelling). The aluminium Tf complex is the predominant aluminium species in plasma. This process would presumably release free aluminium in brain ECF. Morris et al. (1989) reported a positive correlation between aluminium concentration in neurons in the cortex and hippocampus and the density of Tf receptors. I.v. injection of 26Al Tf resulted in brain 26Al concentrations (~ 0.003 % of the injected dose/g brain) within 4 hr (Yokel et al., 2001a). TfR-ME could account for this appearance of aluminium in the brain if the rate of aluminium transport is similar to that reported for iron. Results of interaction between the TfR and Tf bound to two aluminium atoms suggest that the affinity of the TfR for aluminium-saturated Tf is much lower than for iron-saturated Tf (Hemadi et al., 2003).
Aluminium citrate was given i.v. at a rate that produced plasma concentrations in excess of the ability of Tf to bind the aluminium (Allen et al., 1995). Under this condition aluminium citrate was presumably the predominant aluminium species in plasma. The appearance of aluminium in brain ECF was too rapid to be mediated by TfR-ME. This suggests a second mechanism, independent of Tf, which can transport aluminium citrate into the brain. Brain aluminium uptake, infused as aluminium citrate, was not influenced by a Tf receptor antibody in mice, nor was brain aluminium uptake in hypotransferrinemic mice different from that of controls (Radunovic et al., 1997), providing further evidence of a Tf independent mechanism of brain aluminium entry. Decreased plasma protein binding, produced by uraemia or perhaps by decreased Tf metal binding capacity, should favour formation of aluminium citrate and other small molecular weight aluminium species. This was observed in partially nephrectomized rabbits (Yokel & McNamara, 1988). This may increase brain aluminium distribution of non-protein bound aluminium species, such as aluminium citrate.
S.c. injection of aluminium L-glutamate has been shown to result in elevated levels of aluminium in the brain of the rat (Deloncle et al., 1995; 1999; 2001). This does not prove that the aluminium entered the brain as aluminium glutamate, as claimed by the authors. Speciation calculations suggest only ~ 0.5% of aluminium would be associated with glutamate in the human under extremely high aluminium concentrations (Daydé et al., 2003). I.p. injection of magnesium D-aspartate with or without aluminium L-glutamate significantly decreased brain aluminium levels compared to the absence of magnesium D-aspartate (Deloncle et al., 2002). Although the authors suggested that D-aspartate was acting as a chelator to reduce brain aluminium it is not clear why D-aspartate should produce an effect that is different from L-glutamate, as they have similar complexation constants (Charlet et al., 1984), unless the isomers of the amino acid have differential effects.
Opening of the BBB by i.v. injection of metrazole increased brain aluminium after i.p. injection of potassium aluminium sulphate hydroxide, suggesting that aluminium entered the brain through a paracellular pathway that might have been created by the metrazol (Wu et al., 1999).
There appears to be a mechanism to transport aluminium out of the brain. It has been suggested that citrate may enable aluminium transport out of the brain by a carrier-mediated process. When aluminium citrate was infused i.v. to produce constant brain and blood ECF aluminium concentrations, the brain ECF aluminium concentration was below that in blood ECF (Allen et al., 1995). This suggests a mechanism at the BBB to reduce ECF brain aluminium by transporting it into blood. The concentration of Tf in CSF, and presumably ECF, is very low. It has been suggested that all Tf in brain ECF is iron saturated (Bradbury, 1997) providing no ability of Tf to mediate neuronal aluminium uptake. However, the citrate concentration in brain ECF is higher than in plasma, suggesting that 90, 5, 4 and 1% of aluminium in CSF, and presumably brain ECF, is associated with citrate, hydroxide, Tf and phosphate, respectively, according to calculations conducted by Harris (Yokel, 2001). It is therefore likely that aluminium citrate is the aluminium species transported out of the brain. Aluminium citrate transport across the BBB was assessed using microdialysis under conditions of aluminium equilibrium between blood and brain ECF. The brain to blood aluminium concentration ratio increased after addition to the dialysate of a metabolic inhibitor (2,4-dinitrophenol), pyruvate, a proton ionophore (p- (trifluoromethoxy)phenylhydrazone) or mersalyl acid and when proton availability was decreased by increasing dialysate pH. These results suggested the monocarboxylate-1 (MCT-1) transporter mediated aluminium citrate brain efflux (Ackley & Yokel, 1997; 1998). However, the red blood cell, which expresses MCT-1 and the anion exchanger, did not take up aluminium citrate well, suggesting aluminium citrate is not a substrate for MCT-1 (Yokel, 2002). Aluminium citrate uptake by an immortalized murine BBB endothelial cell line, in the presence of various inhibitors, was suggested to be ATP- but not Na/K-ATPase-dependent and not a substrate for a dicarboxylate carrier, but a substrate for an organic anion transporter (Yokel et al., 2002). Using an immortalized rat BBB endothelial cell line, it was found, in a study focusing on the glutamate transporter, that aluminium citrate uptake was concentrative, temperature- and concentration-dependent, not sodium-dependent, and inhibited by ligands for the sodium-independent L-glutamate/L-cystine exchanger system Xc-. Loading the cells with these ligands enhanced aluminium citrate uptake, interpreted as a trans-stimulatory effect, leading the authors to conclude that system Xc- is a potential candidate for aluminium citrate uptake into the brain across the BBB (Nagasawa et al., 2005).
Brain aluminium concentration in mice consuming a commercial diet increased several fold from 1 week to 4 weeks of age, then remained fairly constant until declining several fold from 52 to 104 weeks of age (Takahashi et al., 2001). The aluminium content of the diet was not described. In contrast, rat brain aluminium showed no consistent changes over the same time period (Takahashi et al., 2001), suggesting no age-related changes in brain aluminium concentration.
Brain aluminium concentrations in pregnant rats, given 0, 200 or 400 mg Al/kg/day during gestation days 1 to 20, were significantly lower than in non-pregnant rats, whereas those in liver, bone (only in non-aluminium treated rats), and kidney (only in rats that received 400 mg Al/kg/day) were significantly higher in the pregnant rats (Bellés et al., 2001).
Neuroblastoma cells, which model human neurons, have been used to study aluminium uptake. A human neuroblastoma cell line showed aluminium uptake with an equilibrium constant of 2.88 nM vs. 1.66 for iron and a similar t½, 3.8 min, for 50% cell internalization of the metal (Morris et al., 1987). Mouse neuroblastoma cells took up aluminium when introduced as aluminium Tf, at pH 7 to 8. Addition of citrate or EDTA inhibited aluminium uptake (Shi & Haug, 1990). Aluminium uptake from a medium containing 25 μM aluminium was saturated, achieving 5 nmole/mg cell protein. Although EDTA, citrate, tartrate, maltolate, fluoride and 8-hydroxyquinoline appeared to inhibit aluminium uptake at pH 6, they did not at pH 7.4. Conversely, although metabolic inhibitors had no effect on aluminium uptake at pH 6, they appeared to reduce uptake at pH 7.4. However, statistical significance was not shown (Shi & Haug, 1990). Human neuroblastoma cells took up more aluminium when introduced as aluminium EDTA than as aluminium citrate or aluminium maltolate. Uptake was concentrative (Guy et al., 1990). Tf enhanced aluminium uptake into neuroblastoma cells 2-fold more than citrate (Abreo et al., 1999).
Primary foetal rat hippocampal and human cortical neurons in culture took up aluminium introduced as the chloride (200 μM) in the presence of 1 mM EGTA. Exposure to a divalent cation ionophore (A23187) increased rat hippocampal intraneuronal aluminium concentration from ~ 3 to 20 mg/kg, and aluminium influx and accumulation in human cortical neurons in culture as well (Mattson et al., 1993; Xie et al., 1996). Explants of rat cortical neurons took up significantly more aluminium, introduced as 340 μM aluminium lactate, aluminium lactate and 328 μM citric acid or aluminium lactate and 2 μM L-glutamic acid, than controls containing no aluminium (Jones & Oorschot, 1998). There was no significant difference in intracellular aluminium amount from the aluminium forms. The aluminium was in the cytoplasm and/or cell nucleus.
Rat cerebellar cells in culture were exposed for a few minutes to aluminium, introduced as 5 mM aluminium citrate or as aluminium fluoride. Aluminium uptake was not seen in granule cells but was observed in GABAergic neurons, that were thought to be Purkinje cells, and flat glial cells, but not star-like (type 2) astrocytes, using synchrotron photoelectronic spectromicroscopy (De Stasio et al., 1993; 1994).
When exposed to aluminium sulphate, rat glioma cells took up significantly more aluminium than did those of controls, whereas murine neuroblastoma cells did not (Campbell et al., 1999). Oligodendrocytes, the glial-forming cells in the CNS, and neurons took up aluminium when introduced at 2.26 μM, as aluminium Tf, but not as aluminium chloride or citrate (Golub et al., 1996b; Golub et al., 1999), consistent with the enhancement of aluminium uptake by Tf in neuroblastoma cells. Aluminium uptake into oligodendrocytes was much greater than into neurons.
Astrocytes, another glial cell, did not take up aluminium (Golub et al., 1999). In contrast, when 1 mM aluminium was added as the chloride, in the presence of 1 mM citrate and 1 mM calcium, the aluminium concentration in astrocytes was greater than in granule neurons isolated from the cerebellum and was even greater in astrocytes that had been co-cultured with neurons. Neuronal aluminium uptake was not affected by co-culture with astrocytes (Suarez-Fernandez et al., 1999). However, the aluminium concentration in these cells was 30 to 50 times that seen in human brain. Aluminium accumulation in neuron- and astrocyte-like cells was significantly increased when introduced as aluminium fluoride and in astrocyte-like cells when introduced as aluminium chloride. Aluminium maltolate and aluminium lactate did not significantly increase aluminium in either cell type nor did aluminium chloride in neuron-like cells. Exposure to up to 1 mM aluminium maltolate resulted in concentration-dependent aluminium uptake that was generally greater in neuron- than astrocyte-like cells (Lévesque et al., 2000). Uptake of aluminium into astrocytes from 0.1 mM aluminium associated with serine, glycine and glutamine was significantly increased after 24 hr, but not from aluminium glutamate or aluminium citrate exposure (Aremu & Meshitsuka, 2005). Inhibition of glutathione (GSH) synthesis increased aluminium uptake from aluminium glutamate and from aluminium glycine but only after 8 hr. However, it did not increase aluminium from aluminium glutamine or serine. A non-specific inhibitor of glycine transporters (doxepin) and a selective blocker of the glycine transporter GlyT1 (sarcosine) increased aluminium uptake from aluminium glycine. A selective blocker (dihydrokainic acid) of the glutamine transporter GLT-1, also called EAAT2, increased aluminium uptake from aluminium glutamine. Neither a non-specific inhibitor of glutamate transporters (L-trans-pyrrolidine-2,4-dicarboxylic acid) nor an inhibitor of Na+/K+-ATPase (ouabain) affected aluminium uptake. The authors concluded that neither amino acid transporters nor Na+/K+-ATPase mediated aluminium uptake, which they suggest might be from diffusion and one or more other mechanisms.
It is difficult to directly compare the results of these studies that had major differences in their methods, including:
the use of different cell types;
the use of similar cells that were obtained from different organisms at different stages of development; and
greatly different, and sometimes physiologically irrelevant, aluminium exposure conditions.
It does appear that Tf enhances uptake into neurons and that many different chemical species of aluminium can enter neurons and glial cells. However, if there are no available binding sites for aluminium on Tf in brain ECF, as suggested by Bradbury (1997), this mechanism may not be very important in vivo. The mechanisms of aluminium uptake by brain cells appear to include diffusion, TfR-ME and other, un-identified, carrier-mediated processes.
Based on brain aluminium concentration in victims of Creutzfeld-Jakob disease, which is associated with widespread neuronal and glial pathology, that were not different from controls, it was concluded that brain damage alone does not result in elevated brain aluminium (Traub et al., 1981). Brain aluminium was not elevated in 20 patients who died from liver disease or other complications of chronic alcoholism compared to 20 patients without a history of alcoholism (Zumkley et al., 1986). Subsequent relevant studies included the intracerebroventricular injection of 0.2 mg aluminium gluconate which resulted in significantly more intraneuronal aluminium accumulation in the hippocampus and parietal and frontal cortex of rats than in those of controls (Szerdahelyi & Kasa, 1988). The increase was greater in the hippocampus and parietal than frontal cortex. Injection of the cholinotoxin AF64A six days before intracerebroventricular aluminium gluconate injection resulted in significantly greater intraneuronal aluminium accumulation in the hippocampus compared to injection of aluminium gluconate alone, suggesting that neuronal toxicity enhanced aluminium uptake (Szerdahelyi & Kasa, 1988). Aluminium uptake was enhanced in neurons exposed in culture to glutamate and calcium, suggesting that aluminium entered during cell degeneration (Mattson et al., 1993). Synaptosomes prepared from rat brain cortex exposed to 11 μM aluminium, from aluminium chloride, took up ~ 2-fold more aluminium when exposed to increased lipid peroxidation induced by 0.8 mM ascorbic acid and 2.5 μM Fe2+ (Amador et al., 1999). Infection of rats with Japanese encephalitis virus resulted in an increased accumulation of aluminium in the brain (Seko et al., 1986). These studies suggest stress (increased lipid peroxidation) and insult to the brain (induced by AF64A, glutamate and calcium and viral infection) can increase brain aluminium accumulation. Although the mechanism is unknown, it appears to be at the cellular level, since this effect was seen in cells in culture.
Bone
Repeated treatment of rats and rabbits with aluminium resulted in ~ 5-fold greater elevation of aluminium levels in bone than brain (DuVal et al., 1986; Fiejka et al., 1996; Garbossa et al., 1998b; Henry et al., 1984; Yokel, 1983). Rats consuming 50 or 500 mg Al/L in their drinking water for 9 weeks had approximately 0.2 and 0.1 × 10-3 % of the total aluminium intake in each gram of bone (Glynn et al., 1999). Three weeks after the i.v. injection of 26Al in one normal rat, the percentage of the 26Al dose in each gram of bone and brain was 9 × 10-1 and 2 × 10-3, respectively, a 37-fold difference (Walker et al., 1994). The 26Al in the bone of two mice two hr after i.v. infusion of 26Al plus citrate was 580-fold that seen in brain (Radunovic et al., 1997). After oral ingestion of 26Al, ~ 0.25 to 0.3% of the administered 26Al was in the skeleton after 2 (Jouhanneau et al., 1997b) and 48 hr (Drüeke et al., 1997). Consistently more 26Al was in the skeleton and urine when it was administered with, than without, citrate, whereas citrate inconsistently increased 26Al in brain and liver (Jouhanneau et al., 1997b). Forty-eight hr after oral 26Al dosing of rats, 1 × 10-3 and 1 × 10-6 % of the dose was in each gram of bone and brain, respectively (Drüeke et al., 1997). In rats orally-dosed with 26Al, the 26Al rapidly entered the bone, peaking within hours, with no significant decrease over the subsequent 720 hr (Jouhanneau et al., 1997b). The percentages of the dose of 26Al in bone and brain were 2 × 10-3 and 2 × 10-6 %, respectively. Multiplying the percentage of the 26Al dose in bone after oral 26Al dosing by 330 (to model 0.3% oral bioavailability) suggests 0.3 to 0.7% of the aluminium that reaches systemic circulation enters each gram of bone. The result of this calculation is comparable to the one obtained by Walker et al. (1994). Comparing this percentage of a systemic dose of 26Al that reaches each gram of bone (~ 0.5 %) to the results summarized above for the percentage that reaches each gram of brain (~ 0.005 %) suggests that about 100-fold more aluminium enters bone than brain after a single exposure. Yet the steady state concentration of aluminium in bone is not 100-fold greater than in brain of human controls (see Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Tissue Aluminium Concentrations) and animal controls, as noted earlier in this section. This suggests that clearance of aluminium from bone is more rapid than from brain. This is reasonable in light of the constant turnover of bone as it remodels, whereas at least some of the cells in the brain, the neurons, undergo very little or no turnover during the animal’s lifetime. Based on the understanding of the cycle of heavy metals in bone, it was suggested that aluminium is transferred to osteoclasts during bone resorption and that some may be released from bone (Priest, 2004). Bone aluminium concentrates at the mineralization front. Potential mechanisms of bone aluminium deposition have been suggested to be heterionic exchange with calcium, co-precipitation with calcium and complexation with organic components of the bone matrix (Priest, 2004).
Fluoride in drinking water (40 mg/L) markedly reduced aluminium accumulation in the bones of uraemic rats given i.p. aluminium injections (Ittel et al., 1993a).
Other tissues and fluids
Aluminium distributes into the placenta and foetus. Injecting aluminium into rabbits during gestation resulted in higher aluminium concentrations in their placenta than in the tissues of 0 to 2 day old rabbits exposed in utero, which were elevated above non-aluminium-exposed offspring (Yokel, 1985). Placental aluminium levels in mice not treated with aluminium were non-significantly higher than in maternal tissues. Injections (i.p.) and oral aluminium administration during gestation significantly increased placental aluminium concentrations above those seen in placenta of saline-control animals as well as the foetuses exposed to the aluminium (Cranmer et al., 1986). Concentrations of aluminium in the placenta of guinea pigs that consumed a diet containing 47 mg Al/kg and in the brain, spinal cord and liver of their newborns were similar, ~ 0.005 mmole/kg (0.135 mg/kg) (Golub et al., 1996a). Brain and spinal cord aluminium generally decreased from gestation day 30 to post-natal day 12 in the offspring.
Aluminium distributes into growing hair. Injection of aluminium lactate (s.c.) into the back of rabbits resulted in a considerable, dose-dependent, increase of the aluminium concentration above the pre-treatment average of ~ 1 mg Al/kg hair in the hair grown over and near the region of the injections (Yokel, 1982). Due to the very large amount of aluminium injected (0.7 to 10.8 mg Al/kg 5 days/week for 4 weeks) and the route of administration, these results cannot be related to the human.
Aluminium distributes into milk. Milk aluminium concentration increased from 0.46 to 0.81 mg/L in cows receiving 114 mg Al/day as alum (Archibald, 1955). Aluminium concentrations in the milk of rabbits receiving 0.4 or 0.8 mmole (10.8 or 21.6 mg) Al/kg s.c. injections 5 days weekly for 4 weeks increased ~ 2 and 5-fold (Yokel, 1984; 1985). The increase of aluminium in milk peaked about 8 hr after i.v. and 12 to 24 hr after oral and s.c. administration in rabbits (Yokel & McNamara, 1985). Prior to aluminium administration, the milk/serum aluminium ratio was 4.9. Approximately 2.4% of an i.v. dose of aluminium and 3.3% of the absorbed dose of aluminium following a s.c. injection were found in the milk (Yokel & McNamara, 1985). Rats were given 10 mg aluminium, as the chloride, daily i.p. from postnatal days 1 to 12 (Muller et al., 1992). Twenty-four hr after the last injection, the aluminium level in the milk of the aluminium-treated rats was 72-fold higher than that in rats not injected with aluminium, 2.02 compared to 0.03 mg/L wet weight. AUC in aluminium-treated rats was 0.3 mg/L wet weight. The results from both rabbit and rat showed a milk/blood ratio considerably > 1 suggesting that a process other than diffusion mediates the distribution of aluminium from blood to milk. These studies with 27Al did not demonstrate an increase of aluminium in the tissue of suckling offspring.
Lactating rats were given daily s.c. injections of 26Al from day 1 to day 20 postpartum. The concentration of 26Al measured in kidney was higher than that in liver which, in turn, was higher than that in brain and blood of suckling offspring euthanized on days 9 and 15, demonstrating the transfer of aluminium to milk followed by its oral absorption and distribution in the suckling offspring (Yumoto et al., 2000). Offspring of rats that were similarly treated were weaned on day 20 and sacrificed 40, 80, 160, 320 and 730 days postpartum. Blood, brain (cerebrum, cerebellum, and hippocampus), spinal cord, parietal bone, kidney and liver were obtained (Yumoto et al., 2003). The amount of 26Al/g tissue in the offspring as a % of the amount injected, on post-partum day 20, was ~ 0.02 in bone, 0.006 in liver, 0.004 in kidney, 3 × 10-5 in brain cerebrum and 3 × 10-6 in blood. The spinal cord had more 26Al/g tissue than the 4 brain regions at postpartum day 20.
The concentration of 26Al in the milk of rats that received daily s.c. injections of 26Al from days 1 to 20 postpartum was greater than that in any of the tissues simultaneously collected from suckling rats (Yumoto et al., 2003). There was about 0.2 to 0.4% of the injected 26Al/g milk. The milk was obtained from the stomachs of the sacrificed suckling rats. As the authors did not report the amount of milk consumed or produced, one cannot determine the percentage of injected 26Al that appeared in the milk.
Human studies
The human whole body aluminium burden has been estimated to be 80 mg (Tipton & Shafer, 1964), probably 35 to 40 mg (Alfrey, 1989) and, more recently, 30 to 50 mg (ATSDR, 1999). Slight age-related increases in blood, bone, brain, and other soft tissues have been reported. Aluminium concentrations increased from ~ 160 mg/kg (in ash) in the lung of 0 to 3 month olds, to ~ 625 in 1 to 12 year olds and to > 2000 in 19 to 89 year old adults, in liver from ~ 100 in 0 to 3 month olds, to ~ 150 in 1 to 12 year olds and to ~ 550 in adults, and in kidney from ~ 150 in 0 to 3 month olds, to ~ 300 in 1 to 12 year olds and to ~ 350 in adults (Stitch, 1957). Similarly, median lung aluminium concentrations increased from ~ 150 mg/kg (wet tissue) in 0 to 12 year olds to ~ 2500 in ≥ 60 year olds (Tipton & Shafer, 1964), and from ~ 2 mg Al/kg (wet tissue) in 21 to 30 year olds to ~ 40 in > 81 year olds (Roider & Drasch, 1999). Brain and bone aluminium increases with age, as discussed in Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Tissue Aluminium Concentrations, Brain / Bone. Kidney, liver and spleen showed a similar trend to lung and brain, an increase with age up to ~ 40 years old, a plateau or slight decrease to age 70, then an increase later in life. Ten 32 to 46 year olds had mean hippocampal and frontal cortex aluminium concentrations of 0.014 and 0.020 mg/kg (wet tissue) whereas those of fifteen 75 to 101 year olds were 0.402 mg/kg and 0.373 mg/kg, respectively (Shimizu et al., 1994). Serum aluminium levels in 356 healthy 20 to 80 year olds increased with age with a significant linear regression of r2 = 0.067 and a mean of 7.3 μg Al/L (Zapatero et al., 1995). In contrast, tissue aluminium levels were not found to increase with age in 36 subjects from Denver, Colorado, U.S. or in 21 from Brisbane, Australia. However, the ages of the subjects were not reported (Alfrey et al., 1980).
In some studies increased levels of aluminium have been found in the brain of persons who had suffered from AD; in other studies no such increases were observed (see Table 19). More details of some of these studies are presented in Speziali & Orvini (2003). It has been noted that aluminium was reported to be higher in the cortex and hippocampus than in other brain structures in normal and AD brains (Gupta et al., 2005). A review was conducted of reports of aluminium in the brain of Parkinson’s disease subjects compared to controls. It revealed some studies that found a significant increase in the former group. However, all of the studies were conducted by the same research team (Speziali & Orvini, 2003).
Table 19.
Comparison of aluminium in the bulk brain samples from AD subjects with those of controls. Adopted and modified from Yokel (2000).
| Referencea | Methodb | # of Subjects | Results | |
|---|---|---|---|---|
| AD | Control | |||
| Crapper et al. (1973) | EAAS | 5 | 3 | Heterogeneous distribution within subjects. Some overlap of AD & control values. Grand mean AD 4 times controls. |
| Crapper et al. (1976) | EAAS | 10 | 7 | Continuation of above 28% of AD values > 3 SD above control value mean. |
| Trapp et al. (1978) | EAAS | 4 | 4 | Grand mean AD 1.4 times controlsc. |
| McDermott et al. (1979) | EAAS | 10 | 9 | No difference AD vs. control. |
| Crapper et al. (1980) | EAAS | Aluminium in nuclear and heterochromatin fractions of AD 2 times control values. | ||
| Yase (1980) | NAA | 3 | 3 | Non-significant, 1.4 times, elevation in AD. |
| Traub et al. (1981) | EAAS | 7 | 4 | 4 of 7 AD considered in normal range. Mean of normal 1.375, of AD 3.07 μg/g, significantly different. |
| Markesbery et al. (1981) | NAA | 12 | 28 | Mean and median of control 1.3 times AD, maximum AD value > max. control value. |
| Yoshimasu et al. (1985) | NAA | 4 | 6 | Significant elevation in AD (average = 33.5 vs. 19.4 μg/g dry weight) |
| Ward & Mason (1987) | NAA | 28 | 30 | Canadian samples: mean AD 8-10 times controlsc. |
| 30 | 30 | UK samples: mean AD 3 times controlsc. | ||
| Jacobs et al. (1989) | EAAS | 6 | 4 | Control mean 2 times AD mean. |
| Lukiw et al. (1991) | EAAS | Aluminium in a chromatin subcompartment, the dinucleosome fraction, 3 times control values. | ||
| Xu et al. (1992c) | EAAS | 10 | 10 | Approx. 2 times as much in hippocampus, inferior parietal lobule and temporal gyri of AD than controlc. |
| Edwardson et al. (1992) | EAAS | 8 | 8 | Control mean 1.2 times AD mean. |
| Yoshida et al. (1993); Yoshida & Yoshimasu (1996) | NAA | 10 | 8 | Aluminium concentrations reported to be above age-matched controls. |
| Andrasi et al. (1995) | ICP-AES & NAA | 9 | 20 | 10 brain regions: AD 2.4-8.1 times control, overall AD 3.8 times controlc. |
| Bjertness et al. (1996) | EAAS | 16 | 14 | No difference. |
| Srivastava & Jain (2002) | ICP-MS | 4 | 4 | Aluminium significantly higher in parietal cortex but not cerebellum. |
| Andrási et al. (2005) | ICP-AES & INAA | 3 | 3 | AD brain had significantly higher Al concentrations. Five brain regions were studied. |
in chronological order of publication
inductively coupled plasma atomic emission spectrometry
statistically significant
NFT bearing neurons from AD brain showed several-fold more aluminium in the tangle than in the nucleus which, in turn, had 1.5 to 2-fold more aluminium than cytoplasm and neuropil. Aluminium distribution in the nucleus, cytoplasm and neuropil of tangle-free neurons was similar (Good et al., 1992). Similarly, another group found more aluminium in the nuclei than cytoplasm of tangle- and non-tangle-bearing neurons of Alzheimer and non-Alzheimer brains (Lovell et al., 1993).
Iron status negatively correlates with tissue aluminium accumulation. Ferritin isolated from the brains of 2 subjects with AD had more aluminium than that from 2 normal human subjects (18.9 vs. 3.4 mole Al/mole ferritin) (Fleming & Joshi, 1987). After equilibrium dialysis of ferritin isolated from human brain and liver against 20 μM aluminium, ferritin from liver had more aluminium than from brain (Fleming & Joshi, 1987). In contrast, aluminium levels were not found to be different in the brain cortex from normal, AD and chronic renal dialysis patients (6.2, 8.9 and 7.2 aluminium atoms/ferritin molecule) (Dedman et al., 1992b).
Transport in blood
The concentration of aluminium in erythrocytes was found to be 110% of that seen in healthy human plasma (Chernov et al., 1977). The aluminium concentration in plasma from haemodialysis patients showed little difference from that in blood cells (Van der Voet & de Wolff, 1985). These results are similar to those from animals, above, showing similar aluminium concentrations in plasma and erythrocytes at equilibrium. In contrast, the whole blood, serum and calculated erythrocyte Al concentrations were 15 & 17, 3 & 4 and therefore 34 & 30 μl/L in two healthy subjects, resulting in serum to erythrocyte ratios of 0.08 and 0.15 (Tamada, 2004). In a study in 15 long-term hemodialysis patients, of which 7 were taking Al hydroxide and 8 were not, the overall serum Al to erythrocyte ratio was 0.06. Mean serum Al was lower in those taking Al hydroxide, and averaged 32 μl/l for all patients (Sharif et al., 2004). After ingestion of 26Al by a healthy subject, plasma 26Al concentration peaked at ~ 1.2 hr and decreased to 5% of the peak value by 24 hr, whereas erythrocyte 26Al peaked at ~ 1.2 days, showing a 1.1 day lag. At 1.2 days, the plasma to erythrocyte concentration was 0.03 (Fifield et al., 1997). However, this is not a peer-reviewed report.
Mean AUC was 92% of that seen in whole blood of 4 renal dialysis patients whose blood aluminium concentration was > 100 μg/L (Sjögren et al., 1983). One hr after ingestion of 26Al citrate by one volunteer, 99% of the aluminium was in plasma (80% with Tf and 4% in a low molecular weight fraction) and the remaining 1% was in erythrocytes. The distribution of aluminium in blood taken 880 days after 26Al citrate injection was 86% in plasma and 14% associated with erythrocytes (Day et al., 1994).
When plasma protein aluminium binding was determined after addition of aluminium to serum at final a aluminium concentrations of 14 to 700 μg/L, the percentage bound increased as aluminium concentration increased from 57 to 89% (Gidden et al., 1980). In uraemic serum, the maximum percentage bound was found to be only 18% (Gidden et al, 1980). The aluminium in uraemic serum containing 36 to 152 μg Al/L was reported to be 70 to 92% protein bound (Graf et al., 1981). Aluminium in serum from normal humans, containing 8 μg Al/L was found to be 86% protein-bound whereas aluminium in serum from uraemic humans, containing 140 μg Al/L was found to be 80% protein-bound (Leung et al., 1985). However, aluminium in serum from normal humans, containing 8 μg Al/L was reported to be 54% protein bound whereas aluminium in serum from uraemic humans, containing 99 μg Al/L was reported to be 67% protein-bound (Rahman et al., 1985). Perez Parajon et al. (1989) found ~ 95% of the aluminium in serum from 8 normal subjects to be plasma-protein-bound, using conventional ultrafiltration, and 92% in 10 normal subjects with ultramicrofiltration, the latter procedure being more reliable. Serum from 10 uraemic patients was 87% bound to plasma proteins before these patients received the chelator desferrioxamine (DFO), and 26% 48 hr later. Based on binding constants, it was estimated that ~ 11% of aluminium is bound to citrate in serum (Ohman & Martin, 1994). Using high pressure liquid chromatograpy (HPLC)-EAAS, it was concluded that ~ 90% of the aluminium was bound to Tf (Wrobel et al., 1995). This percentage did not change as a function of the aluminium concentration or the presence of uraemia, or as a result of kidney transplantation. The remaining 12% was associated with a low molecular mass entity that was thought to be citrate. DFO partially displaced the aluminium from Tf. Using gel filtration and HPLC size exclusion (gel permeation) chromatography, serum aluminium was studied in 3 control and 3 aluminium smelter potroom workers (Rollin & Nogueira, 1997). The workers had a higher percentage bound as a high molecular mass complex (91 vs. 79%), which was thought to contain Tf. Addition of aluminium to the serum samples, to ~ 400 μg Al/L, resulted in 98 to 99% aluminium being bound in the high molecular mass complex. Calculations suggested ~ 91% of plasma aluminium is associated with Tf, 7 to 8% with citrate, < 1% with phosphate and hydroxide and essentially none with fluoride, glutamate or aspartate (Martin, 1996) and as determined by Dr. Wesley R. Harris (in Harris & Messori, 2002; Yokel & McNamara, 2001). Aluminium complexation with citrate in plasma has been demonstrated (Bell et al., 1993). Results of calculation of the ultrafilterable fraction of aluminium in healthy humans and patients with chronic renal failure using the biokinetic model described in Toxicokinetics, Pharmacokinetic Modelling suggested it was 0.06 and 0.11, respectively (Steinhausen et al., 2004). This is relevant to all data reported in this review, with the exception of some 27Al studies where plasma aluminium levels exceeded the aluminium binding capacity of Tf (about 1200 μg/L). Analytical speciation studies suggest that the ultrafilterable species are citrate, phosphate, and citrate-phosphate aluminium complexes (Sanz-Medel et al., 2002). It does not appear that albumin binding of aluminium is physiologically important. Most recent studies concluded that Tf is the sole plasma protein which binds aluminium. Non-protein bound aluminium in plasma is believed to be associated with citrate and, to a lesser extent, phosphate. Therefore, aluminium distribution from blood to tissues probably involves aluminium Tf or aluminium citrate. Aluminium citrate readily distributes out of blood. After i.v. injection of aluminium as the citrate to one subject, > 50% of the aluminium had distributed out of the blood within 15 minutes (Priest, 1994). The effects of citrate on brain and/or bone aluminium concentrations and uptake of aluminium into cells have been inconsistent. Citrate forms a small molecular weight complex with aluminium that appears to enhance aluminium distribution and elimination compared to aluminium Tf (Maitani et al., 1994). In the presence of renal function, citrate may enhance aluminium clearance; in the absence it may enhance the accumulation of aluminium in tissues.
Calculations show that insignificant amounts of aluminium fluoride species will form in the presence of normal plasma fluoride (~ 100 μg/L) and normal or elevated plasma aluminium. This suggests fluoride is unlikely to affect aluminium distribution or elimination, unless it is involved in mixed ligand complexes containing aluminium and other ligands (W.R. Harris, personal communication, 2000). Similarly, silicon concentrations in biological fluids are very low. It was suggested that monomeric aluminium silicate species are quite unlikely to play any significant role in the biological chemistry of aluminium after the aluminium is absorbed (Harris et al., 1996). An inverse relationship was noted between serum aluminium and silicon in patients in one haemodialysis centre, raising the possibility that silicon influences aluminium distribution and/or elimination (Parry et al., 1998).
Plasma aluminium concentrations
Many older studies report aluminium concentrations that were subsequently believed to be elevated due to contamination (Versieck & Cornelis, 1980). A review of the aluminium concentration in normal human plasma or serum by these authors from 12 reports published in 1980 to 1985 showed mean values ranging from 0.07 to 0.38 μM (1.9 to 10.3 μg/L), and an overall median of 0.23 μM (6.2 μg/L) (Versieck & Cornelis, 1989). Eleven reports of blood, serum or plasma aluminium in healthy humans, published from 1986 to 1992 showed a range of 0 to 0.22 μM with an overall median of ~ 0.12 μM (3.2 μg/L). The authors concluded that the true value of aluminium in healthy control human serum/plasma is 0.04 to 0.12 μM (Nieboer et al., 1995). A literature review of studies published the previous 30 years suggested the reference value was 0.5 to 8 μg/L in plasma and serum and 2 to 8 μg/L in whole blood (Caroli et al., 1994). House (1992), summarized 24 previous reports of plasma and serum aluminium concentrations in normal subjects, determined by EAAS, from 1974 to 1991. The results showed a reduction over time to a mean of ~ 27 μg/L around 1990, which is well above the currently accepted normal value. It was suggested that healthy human serum contains 1.6 μg Al/L (Valkonen & Aitio, 1997). Plasma aluminium in 71 office employees did not show a normal distribution. Most values were ≤ 2.7 μg/L, with a mean of 2.67 μg/L (House, 1992). Variables that affected the aluminium concentration were the batch in which the sample was analyzed (thought to reflect sample contamination), sex (males > females), antacid use and cola consumption. It has been suggested that the upper limit of the normal range is 6 μg/L (Daniela et al., 2002). Median blood aluminium was 3.2 μg/L in 67 office workers who had not been exposed to aluminium (Liao et al., 2004). Serum aluminium in a control group was 0.99 ± 0.97 μg/L (range 0.03 to 3.12 μg/L), whereas the values were 4.75 ± 9.23 μg/L (range 0.5 to 45.1 μg/L) in dialyzed patients (Razniewska, 2005). Serum aluminium in normal newborns was reported to average 5.17 μg/L (Sedman et al., 1985) and 8 to 12 μg/L (Litov et al., 1989). In premature infants who received i.v. therapy, the plasma aluminium concentration averaged 37 μg/L (Sedman et al., 1985). Mean plasma aluminium concentrations in pre-term infants born at gestational age 28 to 32 weeks, pre-term infants born at gestational age 33 to 36 weeks, and full-term (mean 39 weeks) were 0.49, 0.39 and 0.29 μM (13.2, 10.5 and 7.8 μg/L), respectively (Bougle et al., 1992). Plasma aluminium averaged 0.59 μM (15.9 μg/L) in those that received parenteral nutrition compared to 0.33 μM (8.9 μg/L) in those that did not (Bougle et al., 1992).
The mean AUC in 44 haemodialysis patients who were receiving aluminium therapy was 32 μg/L compared to 10.8 μg/L in 32 not receiving aluminium (Fenwick et al., 2005). Serum Al concentration was reported to be significantly higher in patients with spontaneous pneumothorax (184 μg/L) than controls (27 μg/L) (Han et al., 2004). However, these values from the healthy individuals are an order of magnitude above what is accepted as the true value of serum aluminium, reducing confidence in this study.
A seasonal variation of aluminium in serum was seen, with higher levels in the autumn, which was speculated to be due to a water-borne factor (Nordal et al., 1988b).
Tissue aluminium concentrations
Aluminium is unequally distributed throughout the body in normal and aluminium-intoxicated humans (Alfrey et al., 1980; Di Paolo et al., 1997). Tissue aluminium concentrations in normal adults at steady state (in mg/kg wet weight unless otherwise stated), were 20 in lung, 1 to 3 in bone (based on dry weight), 1 in liver and spleen, 0.5 in the kidney, 0.45 in the heart, 0.4 in muscle, 0.35 in brain and ~ 0.002 in blood (Nieboer et al., 1995). Similar reference values, of 2.21 to 15.3 mg/kg in the lung, 1.0 to 2.45 mg/kg in the liver and 0.55 to 1.31 mg/kg in the kidneys were obtained from a review of literature published in the prior 30 years (Caroli et al., 1994). With increased age and accumulation of aluminium, late life aluminium body burden has been estimated to be 25 to 50 mg in bone, 20 mg in lung, and 9 to 24 mg in soft tissue (Keith et al., 2002). Based on typical organ weights for a 70 kg adult, ~ 58, 26, 11, 3, 0.95, 0.3, 0.25 and 0.2% of the body burden of aluminium would be in the bone, lung, muscle, liver, brain, heart, kidney and spleen, respectively. The higher concentration in lung of normal humans may reflect entrapment of airborne aluminium particles whereas the higher concentrations in bone, liver and spleen may reflect aluminium sequestration. Skin, taken from the back of 11 chronic haemodialysis patients, had greater aluminium concentration than from 9 controls (1.02 vs. 0.26 mg/kg) (Subra et al., 1991). Skin and serum aluminium concentrations were greater in the patients who had received haemodialysis for a longer time (averages of 156 vs. 49 months) (Subra et al., 1991). It has been suggested that up to 14% of the body burden of aluminium is in the skin, but the potential contribution of contamination to this value has been raised (Priest, 2004). Because of its very low bioavailability by most routes of exposure and the effective urinary clearance of aluminium from blood, human tissue and fluid, aluminium concentrations are low compared to aluminium concentrations in most exposure sources.
Victims of the dialysis encephalopathy syndrome showed elevated aluminium exposure in all tissues (Alfrey et al., 1980). The respective mean aluminium concentrations, in mg/kg dry defatted tissue, in controls, non-dialyzed uraemic patients, dialyzed uraemic patients and dialyzed uraemic patients with encephalopathy were 4.1, 25.5, 161 and 301 in liver; 3.0, 35.3, 243 and 493 in spleen; 4.5, 27.4, 116 and 281 in bone; 1.1, 6.9, 22.5 and 42.7 in heart; 1.2, 2.6, 9.1 and 14.9 in muscle; 55, 75, 90 and 215 in lung; and 2.4, 4.1, 8.5 and 24 in brain grey matter. In a more recent episode of aluminium intoxication in dialysis patients, 10 of 17 patients exposed to dialysate prepared from tap water containing 650 μg Al/L died. The serum aluminium concentrations of those that died and those that did not averaged 808 and 255 μg Al/L, respectively. Tissue aluminium concentrations in 4 of the victims were above reference values: liver 4.7 to 51.7 (reference < 2), bone 7.5 to 88.7 (reference < 2) and cerebral cortex 1.09 to 1.78 (reference 0.14 to 0.22) mg Al/kg.
Two male twins, 32 weeks at birth, received 2500 and 5350 mg total aluminium orally as antacids and 2.96 and 3.2 mg in total parenteral nutrition (TPN) solutions over 5 months. When they died at age 149 and 157 days, their bone, brain, liver, and lung, and in one of the two, kidney, aluminium concentrations were greater than the average values from infants that had not received i.v. fluids or TPN solution for more than 21 days (Bozynski et al., 1989). Considering oral aluminium bioavailability of 0.1%, ~ ½ to ⅔of the aluminium that reached systemic circulation was from the antacids. They received comparable amounts of aluminium from the TPN solution but the twin that received ~ 2-fold more from antacids had higher aluminium concentrations in all organs but the brain.
Aluminium, as the phosphate, was shown to be elevated ~ 10-fold in the synovial fluid, synovial membrane and articular cartilage of 28 chronic haemodialysis patients, some of whom took 5 to 15 g of aluminium hydroxide daily, compared to patients who had not taken aluminium (Netter et al., 1984; 1988). Speciation calculations indicate that a major aluminium species in synovial fluid is the citrate (Silwood & Grootveld, 2005).
Lung, liver and brain aluminium concentrations were greatly elevated (~ 20-, 120- and 20-fold) in a worker who developed fibrosis and encephalopathy after exposure to aluminium powder for 13.5 years (McLaughlin et al., 1962). Aluminium levels were elevated in the lung, liver, spleen and hilar lymph node of a stone mason (Teraoka, 1981).
Gastric mucosal membrane aluminium concentration was elevated in aluminium-consuming patients with normal renal function and those with renal insufficiency who were predialysis and those receiving dialysis, compared to patients with normal renal function who were not consuming aluminium-containing antacids (Zumkley et al., 1984a). The increases paralleled increases in plasma aluminium, and were attributed to consumption of aluminium antacids.
In a case of Wilson disease, increased urinary clearance of aluminium as well as copper and increased aluminium content of the liver was observed (Yasui et al., 1979).
The observations of increased serum aluminium during acute infection and concurrent neurological dysfunction and some deaths suggest infection in humans who have a significant body burden of aluminium causes aluminium release from its storage sites (Davenport et al., 1988; Fenwick et al., 2005).
Brain
A review of the literature on the reports of aluminium concentrations in various brain regions led the authors to conclude that aluminium is generally higher in grey than white matter (Speziali & Orvini, 2003). Human brain aluminium concentration correlated positively with age in several studies. Brain aluminium concentrations in 3 subjects aged 25, 43 and 65 averaged 1.59 mg/kg (dry tissue) and 2.74 mg/kg in 6 subjects aged from 75 to 99 years (McDermott et al., 1979). Brain aluminium levels in 7 subjects from premature to 6 months old were ~ 0.3 mg/kg (wet tissue), increasing as the age of subjects increased, to ~ 0.7 mg/kg in 4 subjects 80 to 99 years old (Markesbery et al., 1984). Similarly, brain aluminium levels increased from ~ 0.2 mg Al/kg (wet tissue) in 21 to 30 year olds to ~ 0.55 mg/kg in > 81 year olds (Roider & Drasch, 1999). Ten adults 32 to 46 years old were compared to fifteen adults 75 to 101 year old. The aluminium concentrations in the hippocampus and frontal lobe were 0.014 and 0.020 vs. 0.40 and 0.37 mg Al/kg, wet weight, respectively (Shimizu et al., 1994). The increase in brain aluminium concentrations with age could be due to increased exposure with age, a decreased ability to remove aluminium from the brain with age, or very slow, or no, elimination of aluminium from the brain. Patients who had elevated aluminium intake and who received haemodialysis before successful renal transplantation up to 8 years prior to death had elevated post-mortem aluminium levels in the brain (McDermott et al., 1978; Reusche et al., 1996), suggesting accumulation of aluminium during haemodialysis that was slowly, or not, cleared after establishment of renal function. Using morin, aluminium was visualized in the disintegrating NFT and senile plaque amyloid core of non-demented elderly subjects. In normal brain tissue, aluminium was seen in the wall of the capillary vessels of the BBB, perivascular glial supporting tissues, nuclei of astrocytes, and nuclei and nucleoli of neurons (Shimizu et al., 1994). However a study of only 4 subjects who died between 58 to 74 years of age and who had a mean brain aluminium concentration of 0.54 mg/kg wet weight failed to find an association between age and brain aluminium concentration (Jacobs et al., 1989). The aluminium concentration in 12 brain regions obtained from 8 neurologically normal subjects was reported to range from a mean of 58 mg Al/kg, wet weight, in the pons to 196 mg Al/kg in temporal cerebrum (Rajan et al., 1997). These very much higher values do not appear to be correct, perhaps due to contamination or incorrect reporting of the units.
Neuromelanin from human brain was reported to contain 220 mg Al/kg, dry weight (Zecca et al., 1994).
Patients who received brain surgery for a tumour after consuming an aluminium-rich antacid for 10 days had 2-fold higher brain aluminium concentration than patients consuming an antacid low in aluminium (Winterberg et al., 1987a), demonstrating aluminium absorption and distribution into the human brain in the presence of normal renal function.
A case of generalized amyloidosis showed greater brain aluminium accumulation than seen in the control or parkinsonism groups, 89.5 mg/kg vs. 17.7 mg/kg and 31.8 mg/kg, respectively (Yasui et al., 1980).
The CNS shows lower aluminium concentrations than many other tissues, even in the presence of overt neurotoxicity. Increased brain aluminium concentrations of ~ 4- to 6-fold in rabbits and somewhat higher increases in victims of dialysis encephalopathy syndrome were associated with neurotoxicity (Alfrey, 1980; Crapper et al., 1976; Yokel, 1983). Results of the many studies of aluminium concentration in bulk brain samples and sub-cellular sites of AD victims inconsistently show elevated aluminium (see Tables 19, 20 and 21). The magnitude of elevation of bulk brain aluminium, when reported, is generally a few-fold or less in AD victims than in controls. The inconsistent findings of a small increase in brain aluminium in AD in relation to the small increase in bulk brain aluminium sufficient to produce neurotoxicity has hindered the resolution of the role of aluminium in AD. If aluminium is elevated in AD brain, it is not reflected in CSF aluminium, which has generally not been found to be elevated (Jagannatha Rao et al., 1999; Kapaki et al., 1993). CSF aluminium concentration was reported to be higher in patients with virus nephro infections, residual manifestations from brain and spinal cord trauma, and pain syndromes, than in controls. Blood serum aluminium concentrations were also reported to be elevated in those with residual effects of brain and spinal cord trauma (0.014 and 0.012% aluminium in the ash vs. 0.0088% in controls) (Del’va, 1962).
Table 20.
Comparison of aluminium in NFTs from AD subjects with those in controls, determined by microprobe analysis or staining. Adopted and modified from Yokel (2000).
| Referencea | Methodb | # of subjects | Results | |
|---|---|---|---|---|
| AD | CONTROL | |||
| Terry & Pena (1965) | EDX | 1 | Aluminium not detected. | |
| Perl & Brody (1980) | EDX | 3 | 3 | Aluminium seen in nucleus & cytoplasm of NFT-positive cells in AD (91 & 29%) and controls (90 & 11%), but not in non-NFT-bearing neurons (2 - 6%). |
| Masters et al. (1985) | EDX | Excessive Aluminium seen. | ||
| Candy et al. (1986) | EDX | Aluminium and silicon were co-localized in plaque cores in AD and mentally-normal subjects. | ||
| Kobayashi et al. (1987) | WDX | 1 | Aluminium not detected. | |
| Jacobs et al. (1989) | EDX | 7 | Aluminium not detected. | |
| Schuurmans Stekhoven et al. (1990) | EDX n=5 LMMS n=3 | 5 | Aluminium not detected. | |
| Moretz et al. (1990) | EDX | 3 | Unable to demonstrate significant aluminium. | |
| Chafi et al. (1991) | WDX SIMS | 7 | Aluminium not detected. | |
| Good et al. (1992) | LMMS | 10 | 4 | In neurons with NFTs, Aluminium in NFTs > cytoplasm, nucleus & neuropil. |
| Sparkman (1993) | EDX | Aluminium detected in NFTs, but not consistently in paired helical filaments. | ||
| Lovell et al. (1993) | LMMS | 7 | 5 | Grand mean [Al] AD cytoplasm of NFT-bearing neurons (2.9 μg/gm), non-NFT-bearing neurons (2.3); control neuron cytoplasm (1.85) [Al] > 3 σ above control means: AD neurons 9.6-14.3%, control 1.3-1.5%. |
| Bouras et al. (1997) | LMMS | 4 | 3 | Aluminium in nuclei of NFT-free neurons and in the neuropil in hippocampus and inferior temporal cortex was 2.2 to 3.4 fold higher than in controls |
| Reusche (1997) | LMMS | Aluminium not detected. | ||
| Makjanic et al. (1998) | Nuclear microscopy | Aluminium seen in neurons and neuropil of fixed, osmicated tissue. Aluminium not see in unstained, untreated tissue. |
||
| Kasa et al. (1995) | solachrone azurine | 10 | 5 | Weak staining of cortical and hippocampal NFTs. |
in chronological order of publication
EDX = Energy dispersive (electron probe) X-ray microanalysis; WDX = wavelength dispersive X-ray microanalysis; SIMS Secondary ion mass spectrometry; LMMS = Laser microprobe mass spectroscopy
Table 21.
Determination of aluminium in plaques by microprobe methods or staining. Adopted and modified from Yokel (2000).
| Referencea | Method | # of Subjects | Results | |
|---|---|---|---|---|
| AD | Control | |||
| Nikaido et al. (1972) | EDX | Aluminium not detected. | ||
| Duckett & Galle (1980) | EDX | 18 | 3 | Highest aluminium seen in plaques & lipofuscin granules in degenerating cells of all brains. |
| Masters et al. (1985) | EDX | Aluminium & silicon in isolated, intact plaque cores. | ||
| Candy et al. (1986) | EDX | Aluminium & silicon in plaques of AD and mentally normal – aluminium 4-19% of plaque core. | ||
| Candy et al. (1986) | SIMS | 3 | Aluminium in in situ plaque cores. | |
| Stern et al. (1986) | LMMS | 3 | Unable to see aluminium in purified plaque cores. | |
| Mori et al. (1988) | EDX | Modest increase in some plaque cores & rims. | ||
| Jacobs et al. (1989) | EDX | 7 | Aluminium not detected. | |
| Larsson et al. (1990) | Proton microprobe | Aluminium not detected. | ||
| Moretz et al. (1990) | EDX | No significant difference in AD brain. | ||
| Chafi et al. (1991) | WDX | 7 | Aluminium not detected. | |
| Landsberg et al. (1992) | Proton (nuclear) microscopy techniques | 5/4 | 2/2 | Studied stained and unstained tissue. <10% of plaque cores had aluminium. Aluminium detected in background and control tissue. Aluminium not detected in plaque cores of unstained tissue, at stated sensitivity of 15 ppm. |
| Smith et al. (1992; 1994) | EDX, SIMS | 4 | 3 | No difference between AD and control brain. No evidence for significant, > 2-fold, aluminium in AD pyramidal neurons. |
| Landsberg et al. (1993) | Proton (nuclear) microscopy techniques | 6 | 2 | Aluminium occurred at ≥ 50 ppm in the cores of 20% of the senile plaques of stained tissue |
| Senitz & Bluthner (1990) | Morin | 3 | Aluminium detected in dense core of plaque. | |
| Favarato et al. (1992) | Morin | 5 | Staining of plaque core. | |
| Kasa et al. (1995) | Solachrome azurine | 10 | 5 | Moderate-intense staining of core and/or rim of some, not all, plaques. |
in chronological order of publication
The indigenous people of several western Pacific foci, the Chamorro on Guam, Japanese on the Kii peninsula of Honshu Island, Japan and the Auyu and Jakai of southern West New Guinea, developed two syndromes having features of ALS and a parkinsonism-dementia (PD). From the time this variant of ALS was first identified in 1945 until 1960, its incidence was 50 to 150 times higher than elsewhere in the world (Kihira et al., 2004). It was suggested that a high aluminium and low calcium and magnesium concentration in the environment contributed to these syndromes (Yase, 1972). Although the manganese concentrations in the soil, river and drinking water were greater in ALS than control areas, the manganese content of plants, crops, livestock and fish was not (Iwata et al., 1976). A specific localization of manganese, aluminium and calcium was observed in the spinal cord of ALS patients (Iwata et al., 1976). Food was not found to be high in aluminium and low in calcium, but the soil was high in aluminium (McLachlan et al., 1989). The aluminium, and calcium, concentration in the brain of victims of ALS has been reported to be greater than in controls, whereas magnesium was not elevated, using neutron activation analysis (Yoshimasu et al., 1976; 1980). Brain aluminium concentrations averaged 33.1 mg/kg in 6 ALS cases and 36.8 mg/kg in 4 PD cases compared to 17.7 mg/kg in controls, determined by neutron activation analysis. Aluminium in the ALS and PD groups was statistically greater than in the controls (Yase, 1980). Calcium was also elevated in the ALS and PD subjects. X-ray microanalysis showed similar calcium, aluminium and manganese distribution in spinal cord of ALS patients (Yase, 1980). Using EAAS, Traub et al. (1981) found elevated brain aluminium levels in two Guamian ALS cases to be 1.7 and 8.9 and in two Guamian PD cases to be 2.0 and 3.9 mg/kg, compared to an average of 1.38 mg/kg in 4 normal subjects. Aluminium, silicon, calcium, vanadium, iron and zinc were reported to be elevated in the frontal cortex of humans with ALS compared to normal subjects and patients with parkinsonism (Mizumoto et al., 1983).
Aluminium was significantly increased in 26 CNS regions in 2 of 6 patients with ALS compared to those in 5 patients without neurological abnormalities. Mean concentrations were 88 and 136 in the two cases vs. 26 and 23 mg Al/kg dry weight in the other 4 cases and controls, respectively (Yasui et al., 1991b; 1991c).
Using SEM with energy dispersive spectrometry, NFT-bearing neurons from ALS-PD and non-afflicted patients were found to have a high aluminium concentration (Perl et al., 1982). Aluminium and calcium were co-localized in the NFT-bearing neurons. Using wavelength dispersive spectrometry coupled with electron beam X-ray microprobe analysis, aluminium and calcium were found to be co-localized in the NFTs of two Guamian PD patients but not in the non-NFT-containing regions of either the PD patients or two control lifelong Guamian residents (Garruto et al., 1984). Semi-quantitative estimates of the highest concentrations were 7200 and 500 mg/kg calcium and aluminium, dry weight, respectively. Garruto & Yase (1986) compared silicon distribution in 5 Guamian Chamorros who had PD and in 2 who had ALS with 2 Guamian and 2 Caucasian normal controls and found a similar distribution, estimated to be up to 3000 mg/kg, with no detectable silicon in the controls. The average brain aluminium concentration was higher in 6 Guam PD cases than 7 Chamorro controls (179 vs. 57 mg/kg dry weight) (Yoshimasu et al., 1985). Aluminium and calcium were seen in the cytoplasm of hippocampal neurons bearing NFTs, using laser microprobe mass spectroscopy (Perl & Pendlebury, 1986). Aluminium and calcium were found to be associated with NFT-bearing hippocampal neurons of PD patients, using secondary ion mass spectrometry (Linton et al., 1987). Using histochemical staining, aluminium was visualized in the hippocampus, spinal cord and frontal cortex in most of 3 Guamian patients with ALS and 5 with PD who had NFTs but not in the 5 neurologically and neuropathologically normal Guamian or Caucasian patients (Piccardo et al., 1988). Staining was observed in the cytoplasm, nucleoli, neuropil, white matter and some endothelial cells and walls of cerebral vessels. X-ray microanalysis confirmed the presence of aluminium.
Using neutron activation analysis, aluminium levels were reported to be higher in 3 Guamian cases than in 4 non-demented controls. The Guamian cases also had high calcium levels in grey and white matter and low zinc levels in grey matter (Yoshida et al., 1993). Using PIXE, extremely high aluminium concentrations were reported in lumbar spinal cord and hippocampus of patients with ALS from Guam and the Kii peninsula of Japan, compared with those in cases with sporadic ALS and in controls (Yoshida et al., 1997; Yoshida, 1999). Aluminium concentrations negatively correlated with calcium and magnesium contents in the birthplace area’s rivers (Yoshida et al., 1997) and positively correlated with iron and copper, and negatively correlated with zinc, in the neural tissue (Yoshida, 1999).
Toenail aluminium concentrations, measured as an indicator of metal exposure, were not different between 22 patients with ALS and 40 controls. Median values were 34.5 and 37.5 mg Al/kg, respectively (Bergomi et al., 2002).
Aluminium levels in 4 cases of Parkinson’s disease were compared to those in the 5 patients without neurological abnormalities in the above cited studies and were found to be significantly higher in the hippocampal gyrus, caudate nucleus, globus pallidus and substantia nigra as well as in the liver, kidney and spleen (Yasui et al., 1992). Magnesium, but not calcium, levels were significantly decreased in these same brain regions, and others as well. However, aluminium was not significantly different in the frontal cortex, caudate nucleus, substantia nigra, and cerebellum of 9 Parkinson’s disease patients or 15 patients with other chronic neurological diseases compared to 12 controls (Uitti et al., 1989).
Bone
Aluminium concentrations, on a dry weight basis, in the bone of normal humans were a few-fold higher than those in the brain (Alfrey et al., 1980; Di Paolo et al., 1997), ~ 1 to 3 mg/kg (Nieboer et al., 1995). Human bone aluminium concentration significantly increased with age in a study of one hundred seventy-two 16-98 year old subjects. It was <0.4 μg/gm dry bone weight in quartile 1 compared to >1.7 in quartile 4 (Hellström et al., 2005). Aluminium contamination in parenteral nutrition solutions in patients with normal renal function led to stainable bone aluminium and osteomalacia (Ott et al., 1983). Oral consumption of aluminium in drinking water and aluminium carbonate by people with normal renal function led to detectible bone levels of aluminium (Eastwood et al., 1990; Recker et al., 1977). Bone aluminium concentrations averaged 6.4 mg/kg dry bone weight in 3 non-dialysis and 125 mg/kg in 3 dialysis patients (Recker et al., 1977) and 5.6 and 102 mg/kg fresh weight in 2 controls and 4 patients with dialysis osteomalacia (Zumkley et al., 1984b). Consumption of a total of ~ 18 kg of elemental aluminium in antacids by a 39 year old female with normal renal function over 8 years led to stainable aluminium on 27.6% of the bone surface, elevated serum and urinary aluminium, and phosphate-depletion-induced osteomalacia that was attributed to aluminium (Woodson, 1998).
Dialysis patients had an average increase of brain and bone aluminium concentrations of 5 and 10-fold, respectively (Alfrey et al., 1980; Di Paolo et al., 1997). Those with dialysis encephalopathy had brain and bone aluminium concentrations about 10- and 85-fold higher than those of controls, respectively (Alfrey et al., 1980). Considerably more aluminium enters, and resides in, the human skeletal system than in the CNS, due to the larger mass of the former. Aluminium was found to be localized at the mineralization front and osteoid of bone (Boyce et al., 1981; Cournot-Witmer et al., 1981; Ott et al., 1982; Schmidt et al., 1984). The aluminium was within 2μm of the calcified bone/osteoid interface at a concentration 20 to 40-fold greater than the whole bone aluminium concentration (Boyce et al., 1981).
Six patients who received aluminium-contaminated TPN solutions for 6 to 72 months had bone aluminium concentrations of 14 to 265 mg/kg, well above the normal range, as well as elevated plasma aluminium (98 to 214 μg/l) and urinary aluminium outputs (Klein et al., 1982). Bone aluminium concentration averaged 2 mg/kg dry weight in infants who received limited i.v. therapy compared to 20 in infants who received ≥ three weeks of i.v. therapy (Sedman et al., 1985).
As bone is a major site of aluminium storage, prolonged urinary aluminium excretion may reflect a prolonged t½ of aluminium in bone. A t½ of 7 years was estimated in one human who had received an i.v. injection of 26Al citrate 3.2 years earlier (Priest et al., 1995). An updated estimate in this individual, based on whole-body monitoring collected up to 3000 days after the injection, suggests the t½ is ~ 50 years (Priest, 2004). This prolonged whole-body t½ may largely reflect the t½ of aluminium in bone.
Clinical evidence suggested that PTH had a protective effect against aluminium-induced bone disease, and encephalopathy (Cannata et al., 1988). Bone aluminium did not correlate with PTH levels in uraemic patients (Alfrey et al., 1979). Subtotal PTX of 10 dialysis patients who had refractory secondary hyperparathyroidism resulted in a significant increase of bone aluminium in 6 of the 7 consuming aluminium (De Vernejoul et al., 1985). These results are not consistent with observations in animals (see Toxicokinetics, Distribution (Including Compartmentalization), Animal Studies).
Aluminium-containing implants have been implicated as a cause of encephalopathy, associated with elevated CSF, serum and urine aluminium concentrations (Hantson et al., 1994; Renard et a., 1994). Using PIXE, it was shown that aluminium uniformly leaked from the site of an implanted aluminium-containing alloy into surrounding bone (Passi et al., 2002).
It has been suggested that the accumulation of aluminium in bone and liver protects patients from the toxic effect of aluminium in other organs (Berend et al., 2001).
Hair
Hair has been used to estimate the body burden and to indicate excessive exposure to metals since the 1960s (Villain et al., 2004). For example, hair has been shown to reflect methylmercury exposure (Johnsson et al., 2005) and to have a 1:270 ratio with blood mercury (IPCS, 1990). The validity of hair to predict the aluminium body burden has not been well established. There can be additional problems with the analysis of hair for metals; procedures for collection have not been standardized. Although hair has been shown in some studies to correlate with other indicators of body burden, it is seldom the preferred tissue for this purpose; commercial panels that test hair for multiple metals have questionable validity (Kales & Goldman, 2002; Villain et al., 2004).
Aluminium was shown to be taken up into human hair from aqueous solution (Wilhelm et al., 1989a). Normal hair aluminium concentration was reported to be < 0.24 to 67 mg/kg in 194 people (Imahori et al., 1979); 6.5 mg/kg in English samples (Alder et al., 1976); 3.7 for US, 8.8 mg/kg for rural Japan, 11.9 mg/kg for Hong Kong and 13.6 mg/kg for samples from Tokyo (IAEA, 1978) and 15 to 18 mg/kg in samples obtained in Kentucky, US, depending on the method used to wash the samples to remove surface contamination (Yokel, 1982). A reference value for aluminium in hair of 0.1 to 36 mg/kg was derived from a literature review of studies published in the prior 30 years (Caroli et al., 1994).There are a few cases reporting elevated hair aluminium in children with emotional problems (Rees, 1979), but the results could be due to contamination. Unexpectedly high aluminium concentrations were observed in the hair of patients with neurological and other disorders, which was thought to be due to dolomite in many of the cases (Roberts, 1981).
The aluminium concentration in hair of 6 control subjects was reported to be 97 ± 25 mg/kg, 118 ± 46 mg/kg in 11 haemodialysis patients and 370 ± 266 in 8 non-dialyzed chronic renal failure patients (Tsukamoto et al., 1979). Hair aluminium concentration positively correlated with AUC and duration of dialysis. Similarly, hair aluminium concentrations were higher in non-dialyzed and dialyzed chronic renal failure patients than in controls, whereas haemofiltered chronic renal failure patients did not have higher concentrations of hair aluminium (Marumo et al., 1984). The elevated aluminium concentrations were attributed to the use of aluminium-contaminated dialysate. Hair aluminium was 10.5 mg/kg in 22 male and 10.1 in 29 female long-term haemodialysis patients who had elevated plasma and bone aluminium concentrations (Winterberg et al., 1987b). There was a significant correlation between hair and bone aluminium in the males, but not the females. Hair aluminium in 18 chronic haemodialysis patients who received an average of 6.3 kg of an aluminium-containing phosphate binder that released aluminium averaged 15.4 mg/kg compared to a hair concentration of 10.5 mg Al/kg in a group of 18 chronic haemodialysis patients who received an average of 6.6 kg of an aluminium-containing phosphate binder that released less aluminium (Winterberg et al., 1987b). The latter group of patients had lower aluminium in their plasma (34.6 vs.101.4 μg/L) and bone (11.6 vs. 26.2 mg/kg). Higher hair aluminium concentration was seen in 39 haemodialyzed patients (6.1 ± 2.8 mg/kg), than in 49 control subjects (3.4 ± 1.6) mg/kg (Chappuis et al., 1988). Hair aluminium did not correlate with serum or bone aluminium, leading the authors to conclude that hair aluminium levels do not predict aluminium-induced osteomalacia (Chappuis et al., 1989). The aluminium hair concentration of 12 home haemodialysis patients tested before introduction of water treatment by reverse osmosis was above that in controls, whereas it was not elevated in 16 patients undergoing continuous ambulatory peritoneal dialysis (Wilhelm et al., 1989b). Because hair aluminium concentration did not relate to daily or cumulative aluminium intake or to bone or plasma aluminium concentrations, the authors concluded that hair aluminium is of very limited value for diagnosis of aluminium exposure.
The concentration of aluminium in the hair of 10 AD patients averaged 7.5 mg/kg compared to 6.2 in 10 age-matched controls (Shore & Wyatt, 1983). In another study, hair aluminium concentration in 35 cases of AD was significantly lower than in 71 comparably-aged controls (Kobayashi et al., 1989). There was no significant effect of age on hair aluminium levels in either group (Kobayashi et al., 1989).
Milk
A literature review of studies published the previous 30 years suggested the reference value was 39 to 250 μg/L (Caroli et al., 1994). Another review concluded the value was 4 to 65 μg Al/L (American Academy of Pediatrics, 1996). Other studies reported means of 30 μg/L (Weintraub et al., 1986), < 5 to 45 (median 14) μg/L (Koo et al., 1988), 49 μg/L (Simmer et al., 1990), 3 to 79 μg/L (Baxter et al., 1991), a median of 161 μg/kg (Coni et al., 1990), a mean of 40 μg/L (Bougle et al., 1992), 9.2 μg/L (Hawkins et al., 1994), a mean of 86 μg/L (Vinas et al., 1997) and a median of 67 μg/L (Krachler et al., 2000). One report found a mean of 380 μg Al/L (Mandic et al., 1995) and another a mean of 350 μg Al/L (Bergerioux & Boivert, 1979); these two values are suspect compared to the others. Speciation calculations suggest ~ 88% of aluminium in human (and bovine) milk at pH 6.8 is Al(citrate)(OH)2-2 and 11% is Al(citrate)(OH)-1, suggesting to the authors that little would be absorbed due to the net charge (Findlow et al., 1990). However, this assumes no change in speciation in the GI tract.
Elimination and Excretion
Animal studies
Urinary excretion
The primary organ for aluminium elimination is the kidney, which is believed to eliminate > 95% of excreted aluminium. Dietary intakes of 3.5 to 11.5 mg Al/day result in a daily excretion of 4 to 12 μg (Nieboer et al., 1995). Many of the reported rates of aluminium clearance are consistent with the glomerular filtration rate (GFR) when the free fraction is considered. Kovalchik et al. (1978) found renal aluminium clearance to be 50% of inulin clearance (GFR) in the dog, over a range of plasma aluminium concentrations of 80 to 600 μg /L. As this range is below the saturation of Tf by aluminium, the aluminium should be ≥ 90% bound to Tf. An aluminium clearance of 116 mL/hr was reported in 18 to 24 kg dogs, or ~ 5.5 mL/kg/hr, after an i.v. aluminium injection that produced blood aluminium concentrations decreasing from ~ 19,000 to ~ 14,000 μg /L (Henry et al., 1984). These are well above the saturation of Tf. The authors found that the renal contribution to plasma aluminium clearance correlated well with GFR in the dog. Systemic clearance in the rabbit was found to be 53 and 72 mL/kg/hr, consistent with GFR, based on the GFR and the free fraction of aluminium in plasma (Yokel & McNamara, 1985; 1988). A renal aluminium clearance of 36 to 60 mL/hr was seen in ~ 0.2 kg rats after an aluminium chloride infusion that produced a serum aluminium concentration of 2000 to 10,000 μg/L (Burnatowska-Hledin et al., 1985). Renal aluminium clearances of 49.6, 44.4 and 41.8, and of 18.4, 18.4 and 17.2 mL/kg/hr were reported after 0.1 or 1 mg Al/kg injections in the rat, respectively, that resulted in serum aluminium concentrations of several thousand decreasing to several hundred μg /L and ~ 20,000 to 30,000 decreasing to ~ 8000 μg /L (Pai & Melethil, 1989; Xu et al., 1991; 1992a). The lower clearance with the greater aluminium concentration may be due to formation of non-ultrafilterable aluminium species.
Several animal studies suggested that aluminium clearance decreases and t½ increases with increased aluminium concentration. For example, renal aluminium clearance in the rat after an i.v. injection of aluminium (8.1 mg/kg) that produced serum aluminium concentrations of 110,000 to 400,000 μg Al/L, was reported to be 4.3 mL/kg/hr (Gupta et al., 1986). Similarly, renal aluminium clearance was reported to decrease from 78 to 3.6 mL/hr as aluminium plasma concentration increased from 40 to 12,400 μg/L (up to 460 μM Al) (Hohr et al., 1989). This may be due to the formation of non-filterable aluminium complexes or aggregates at the higher aluminium concentrations, reducing the plasma filterable aluminium fraction (Lote et al., 1992; Xu et al., 1991; Yokel & McNamara, 1988). The very high aluminium concentrations achieved by Gupta et al. (1986) of 4075 to 16,300 μM far exceed the Tf and citrate aluminium binding capacities of ~ 45 and 100 μM aluminium. It is likely that the aluminium was no longer in solution due to binding by phosphate, which is 1100 μM in serum, or that the aluminium was present as aluminium hydroxides.
The mechanisms of renal aluminium handling are not well understood. Burnatowska-Hledin et al. (1985) interpreted results of their micropuncture studies to suggest that most of the filtered aluminium was reabsorbed. In contrast, Monteagudo et al. (1988) interpreted their results to suggest aluminium excretion in the distal nephron. Micropuncture of Bowman’s space, the late proximal convoluted tubule, and early distal tubule was used to determine aluminium concentration vs. arterial blood and urine in the rat. Aluminium was given in a 3% sodium citrate solution i.v. as doses that produced plasma concentrations of 2.9 to 10 mg/L, a supraphysiological concentration that would exceed the ability of Tf to bind the aluminium, which presumably was present in plasma as the citrate. The results suggested aluminium citrate is filtered at the glomerulus and ~ 25% reabsorbed in the loop of Henle (Shirley et al., 2004). Evidence has been provided that citrate may enhance renal aluminium elimination (Van Ginkel et al., 1990). Lote et al. (1992) found that citrate increased the percentage of aluminium that was ultrafilterable, probably due to formation of aluminium citrate, and enhanced excretion. Others have reported that citrate increased urinary aluminium excretion (Cochran et al., 1994; Maitani et al., 1994), although Cochran et al. (1994) did not find an increase in the ultrafilterable fraction. Urinary silicon and aluminium excretion correlated well, suggesting they may be cleared by a common pathway or as a complex, such as a hydroxyaluminosilicate (Bellia et al., 1994).
Biliary excretion
Biliary aluminium excretion has been reported to account for 0.2% of total aluminium elimination in the dog (Kovalchik et al., 1978), 1.5% in the rabbit (Yokel et al., 1996b), < 0.5 and 1.3% in the rat in the first 12 hr (Xu et al., 1991) and 0.7% in the rat (Yokel et al., unpublished results). These values are in agreement with some of the results from the human (see Toxicokinetics, Elimination and Excretion, Human Studies, Biliary Excretion). Bile aluminium concentration did not increase with increasing oral aluminium doses of 0, 0.2, 0.4 and 0.8 mmole as the lactate given to rats with an average weight of 191 g, suggesting that the higher doses exceeded the ability to excrete aluminium in the bile (Sutherland et al., 1996). Enterohepatic recirculation of aluminium has not been investigated. Considering the very low percentage of aluminium absorbed from the GI tract, it is anticipated that enterohepatic recirculation would not be great. A significant increase of biliary excretion of aluminium and Tf was seen in rats that received 5 mg Al/kg i.v. for 14 days (Klein et al., 1993). These results suggest aluminium bound to Tf may be taken up by hepatocytes and, if excreted as a complex, might be well absorbed because Tf may facilitate aluminium absorption (Jäger et al., 1991).
Elimination rate
Clearance of aluminium from the lung of rats that inhaled fly ash was very slow. The lung concentration decreased from a mean of 53 mg Al/kg immediately after a one-month aluminium exposure, to 27 mg Al/kg six months later, and to 25 mg Al/kg ten months later (Matsuno et al., 1986). The slow clearance was attributed to the solubility of the aluminium in the fly ash. Similarly, after 20 weekly intratracheal installations of 1 mg Al/kg as 1.2 μm MMAD aluminium oxide, only 9% was cleared from the lungs in the subsequent 19 weeks (Schlesinger et al., 2000). A more rapid clearance of 1 to 5 μm diameter coal fly ash particles from mouse lungs after intratracheal administration was described. The aluminium concentration decreased from 980 mg/kg at 1 wk to 519 mg/kg 15 weeks later (Ogugbuaja et al., 2004).
Following inhalation of particles of Montmorillonite (a complex aluminium magnesium silicate clay) by dogs, rats and mice, initial clearance was primarily by the GI tract. Long-term clearance of particles in dogs was predominantly to lung-associated lymph nodes in rats and, in mice, by mechanical clearance by the GI tract (Snipes et al., 1983). The t½ for the clearance of particles that went to the lung-associated lymph nodes of dogs was 3500 days and, from the GI tract, was 6900 days. For mice and rats, the long-term t½s were 490 and 690 days, respectively.
The aluminium concentration was determined in the reactive zone of muscle, which contained macrophage aggregation and lymphoid infiltration, 3 and 6 months after a single i.m. vaccine injection to Cynomolgus monkeys (Macaca fasciculata). The vaccines contained aluminium oxyhydroxide-adjuvated or aluminium phosphate-adjuvated diphtheria and tetanus. At 3 months, the aluminium concentration was 14,280 and 2860 mg Al/kg and at 6 months 11,000 and < 150 mg Al/kg, following the aluminium oxyhydroxide-adjuvated or aluminium phosphate-adjuvated vaccine injections, suggesting more rapid dissolution of aluminium from aluminium phosphate than from aluminium oxyhydroxide adjuvant (Verdier et al., 2005). These results are consistent with the more rapid absorption of aluminium from aluminium phosphate than from aluminium hydroxide reported in rabbits (see Toxicokinetics, Absorption, Animal Studies, Intramuscular).
The rate of aluminium elimination from the entire organism has been determined in animals and humans. The apparent t½ increased with increased duration of sampling after acute aluminium loading of rabbits, suggesting the presence of one or more compartments with very long t½ s. The t½ of aluminium elimination, based on studies in which samples for aluminium determination were collected for ≤ 24 hr, were initially reported to be 1.35 hr in the rat after i.v. injection of aluminium chloride (Wachi & Aikawa, 1975); 5.3 hr after i.v. injection of 8.1 mg Al/kg to the rat, based on sampling to 10 hr (Gupta et al., 1986); 1.2, 1.3, and 1.08 and 2.4, 2.1 and 4 hr after i.v. injection of 0.1 or 1 mg aluminium (as the sulphate)/kg in the rat, based on sampling to 24 hr (Pai & Melethil, 1989; Xu et al., 1991; 1992b); ~ 2 hr from blood after oral aluminium (0.25 to 1 mmole/kg) and citrate (~ 3.4 mmole/kg) and 4.5 to 7.5 hr from liver based on sampling up to 6 hr in the rat (Sutherland & Greger, 1998). Similar values were obtained in the mouse; 1.5 hr after i.p. injection of 54 mg Al/kg as aluminium gluconate or lactate with sampling up to 1.5 hr (Leblondel & Allain, 1980). Similar results were also obtained in rabbits: 2.1 and 3.8 hr after injection of 40 and 80 μmoles Al/kg (1.1 and 2.2 mg Al/kg) as the lactate in lactating rabbits and 8.6 hr after injection of 40 μmoles Al/kg to 17 to 21 day old suckling offspring with sampling up to 24 hr (Yokel & McNamara, 1985). Studies in the dog also provided similar results: ~ 1.5 to 2 hr after 1 or 2 mg aluminium, as the chloride given i.v., when studied up to 2 hr (Kovalchik et al., 1978) and 4.6 hr after i.v. injection of 1 mg aluminium (as the chloride)/kg and sampling to 2.5 hr (Henry et al., 1984). When sampling time increased, longer t½ were observed. When blood was obtained to 48 hr, t½ of 27 and 14 hr were seen in normal and renal-impaired rabbits, respectively, that received 100 μmoles Al/kg (2.7 mg Al/kg) as the lactate (Yokel & McNamara, 1988). A similar study, in which blood was obtained to 72 hr, resulted in a t½ of 43 hr in normal rabbits (Yokel & McNamara, 1989-1990). An initial t½ of 102 to 119 minutes was reported in rats after an oral aluminium dose, but samples were only obtained up to 360 minutes later (Sutherland & Greger, 1998), precluding the ability to observe longer t½.
To determine the t½ of aluminium elimination from organs, adult rabbits were given a single i.v. infusion of 200 μmoles Al/kg (as the lactate) over 6 hr and then terminated up to 128 days later. The t½ of aluminium was estimated to be 113, 74, 44, 42, 4.2 and 2.3 days in spleen, liver, lung, serum, kidney cortex, and kidney medulla, respectively. Another t½ in the kidney greatly exceeded 100 days (Yokel & McNamara, 1989). The whole organism elimination t½ was estimated to be 8 to 24 days in serum, kidney, muscle, liver, tibia and spleen of rats (Greger et al., 1994). The brain aluminium t½ was not determined in either of these studies; this has been done using 26Al (see below). The aluminium concentration in rat tibia, kidney and brain, above that in controls, produced by 30 days of aluminium oral administration, decreased by 88, 85 and 66% respectively (Rahnema & Jennings, 1999), suggesting corresponding elimination t½ of ~ 10, 11 and 18 days. Liver aluminium concentration increased during the 30 days after completion of aluminium administration.
The t½ of aluminium elimination significantly increased following an i.v. injection of 1 mg Al/kg compared to injection of 0.1 mg/kg (Xu et al., 1991). This can be explained by the much lower percentage (5%) of the 10,000 μg Al/L in the plasma that was ultrafilterable after the 1 mg Al/kg injection compared to a greater percentage of ultrafilterable aluminium (22%) of the 1000 μg Al/L in the plasma achieved after the injection of 0.1 mg Al/kg (Xu et al., 1991).
Aluminium persists for a very long time in rat brain following systemic injection of very small doses of 26Al. Rat brain 26Al increased slightly from days 5 to 35 after an i.p. 26Al injection (Kobayashi et al., 1990), suggesting a lack of brain aluminium elimination. However, the possibility of 26Al precipitation and delayed absorption from the peritoneal cavity, the small number of subjects (single rats 10, 15, 25 and 35 days and 2 rats 5 days after dosing) and the lack of a non-26Al dosed group to control for cross-contamination, are of concern. A subsequent study found no decrease in brain 26Al concentration up to 270 days after 26Al injection (Yumoto et al., 1997). When 26Al was given i.v. to rats that were euthanatized 0.17 to 256 days later, the t½ of brain aluminium was estimated to be ~ 150 days (Yokel et al., 2001a). As brain samples were not obtained for at least 3 t½, this estimated terminal t½ of aluminium in the brain is not expected to have a high degree of accuracy. Offspring of rats that were given 26Al injections daily from day 1 to 20 postpartum were weaned on day 20 and sacrificed on days 40, 80, 160, 320 or 730 postpartum. Aluminium concentrations decreased over the 730 days in all tissues (Yumoto et al., 2003). At postpartum day 730, brain 26Al had decreased to ~ 20% of that seen at weaning (day 20 postpartum). The authors did not determine the t½ of aluminium elimination. Calculations conducted for the current review using RSTRIP (Fox & Lamson, 1989) suggest the elimination t½s were ~ 13 and 1635 days in brain. There is little published information on allometric scaling of metal elimination rates that could be used to extrapolate these results from the rat to the human. 150 days is ~ 20% of, and 1365 days exceeds, the rat’s normal life span. For comparison, the whole-body t½ of aluminium in the human was estimated to be 50 years (Priest, 2004) (see Toxicokinetics, Elimination and Excretion, Human Studies, Elimination Rate).
After weanling rats were given a single oral dose of 0.8 mmole aluminium, as lactate, with 0.75 mmole citrate, the aluminium t½ was found to be 16 to 24 days in bone, liver, muscle, and spleen and 8 days in kidney (Greger et al., 1994). The t½ of aluminium elimination from tibia and kidneys positively correlated with the age of rats that received a single gavage of 0.8 mmole aluminium given with 0.75 mmole citrate and were sacrificed 1 to 44 days later (Greger & Radzanowski, 1995). The half lives of aluminium in rats that were 2, 8 and 19 months at dosing were 38, 58 and 173 days in tibia and 9, 12, and 16 days in kidneys. The estimate of the tibial aluminium t½ cannot be considered very definitive because results up to ~ 3 t½ are required for good t½ determination. These results suggest bone aluminium levels should increase with age due to continuous exposure. The t½ of aluminium was significantly greater in liver, muscle and serum of anaemic rats (Greger et al., 1994).
Following a single i.p. injection of 26Al and euthanasia up to 270 days later, liver 26Al was found to decrease considerably from day 5 to 25, then to remain rather constant before beginning to increase from day 75 to 270 (Yumoto et al., 1997). Blood 26Al decreased dramatically from ~ day 35 to 75 then remained fairly constant to day 270.
No decrease of bone aluminium concentration was seen over 6 weeks following aluminium loading in rats that had undergone 5/6 nephrectomy prior to aluminium exposure (Elorriaga et al., 1992). Nor was there an observable decrease of aluminium in bone, liver or brain in rats up to 30 days after oral 26Al administration (Jouhanneau et al., 1997b). Calculations conducted for the current review using RSTRIP, of the t½ of aluminium elimination in the bone of offspring of rats that were given 26Al injections daily from day 1 to 20 postpartum from the results of Yumoto et al. (2003) suggest t½ of ~ 7 and 520 days in parietal bone. After 730 days, the amount of 26Al remaining in the liver and kidneys was ~ 2% of that seen at weaning. For liver and kidney, the t½ were ~ 5 and 430 days and ~ 5 and 400 days, respectively. In blood the values were ~ 16 and 980 days.
Aluminium has been found at the site of s.c. aluminium adjuvant injection to mice and guinea pigs up to 1 year later (Gupta, 1998).
The t½s of aluminium elimination from liver, bone and kidney after a single oral dose of 0, 0.25, 0.5 or 1 mmole Al/kg in rats did not provide any evidence that aluminium elimination is aluminium-concentration dependent (Sutherland & Greger, 1998).
Chelators can increase aluminium clearance into urine, bile and dialysate (Yokel et al., 1996a; 2002). Citrate and perhaps silicon appear to form small molecular weight species with aluminium that can be excreted in the presence of adequate renal function, potentially protecting against the accumulation and toxicity of absorbed aluminium.
Human studies
The primary route of aluminium elimination is via the kidneys, and secondarily via bile. Sweat collected during exercise from 15 normal healthy subjects had a mean aluminium concentration of 15 μg/L, which was similar to their laboratory adult reference values for UK residents of 11 μg/L (Omokhodion & Howard, 1994). Saliva aluminium concentrations in 6 children aged ~ 10 from North Italy averaged 54 μg/L, with a median of 43 μg/L (Sighinolfi et al., 1989). Speciation calculations suggested 94% of aluminium in saliva would be associated with phosphate (Duffield et al., 1991). There are no reports addressing whether aluminium in sweat or saliva reflects aluminium exposure or body burden.
Seminal fluid aluminium concentrations have been reported to average 3.3 mg/kg in 50 subjects (Yamamoto et al., 1959), 0.54 mg/kg in 27 refinery and polyolefin factory employees and 0.87 mg/kg in 45 sperm donor candidates (Hovatta et al., 1998). Although there was no significant difference between the controls and industrially-exposed subjects for seminal plasma aluminium concentration, spermatozoa aluminium was significantly higher in the controls than the industrially-exposed subjects (2.52 vs. 0.93 mg/kg) (Hovatta et al., 1998). The seminal AUC in 64 apparently healthy 21 to 35 year old men was significantly higher in those with low sperm viability, averaging 1.01, 0.59 and 0.18 mg/L in the 18, 26 and 20 subjects with low, medium and high sperm viability, respectively (Dawson et al., 1998). Seminal plasma aluminium concentrations averaged 0.46, 2.0, 1.53 and 0.27 mg/L in 50 adults working in a medical centre, metal ore smelter, petroleum refinery and chemical plant, respectively (Dawson et al., 2000). The authors did not report aluminium in other biological fluids or tissues or the work environment to show if seminal plasma reflects body burden or occupational exposure.
Urinary excretion
The kidneys excrete > 95% of eliminated aluminium, presumably as the citrate. The urinary excretion time of aluminium, as the citrate, into the urine was calculated using the biokinetic model described in Toxicokinetics, Pharmacokinetic Modelling to be 0.4 hr in healthy, and 1.7 hr in patients with chronic renal failure, respectively (Steinhausen et al., 2004).
It has been concluded that humans who consume a normal diet, take no medications containing aluminium, and who have normal renal function excrete 0.15 to 0.45 μmole (4 to 12 μg) (Nieboer et al., 1995), < 20 μg (Wilhelm et al., 1990) and < 50 μg Al/day in urine (Greger & Sutherland, 1997). Other studies reported 24 hr urinary Al excretion of ~ 0.2 μmoles in 21 to 34 year olds (Reffitt et al., 1999) and 0.7, 1.7 and 2 subjects averaging 22, 48 and 69 years old (Morie et al., 1996). Based on published studies during the preceding 30 years, a reference value of 2.3 to 110 μg/L was established (Caroli et al., 1994). Mean serum and urine aluminium levels in 44 non-exposed persons who did not use antacids were 0.06 and 0.33 μM (1.6 and 8.9 μg/L) (Valkonen & Aitio, 1997). Median urine aluminium concentration was 3.3 μg/L in 67 office workers who had not been exposed to aluminium (Liao et al., 2004). Mean urinary aluminium excretion/mmole of creatinine was 0.80, 0.78 and 0.77 μmole in pre-term infants born at gestational age 28 to 32 weeks, pre-term infants born at gestational age 33 to 36 weeks, and full-term infants (mean 39 weeks), respectively (Bougle et al., 1992). Urinary aluminium excretion averaged 0.76 μmole/mmole creatinine in those that received parenteral nutrition compared to 0.47 μmole/mmole creatinine in those that did not (Bougle et al., 1992).
Following oral consumption of 27Al-containing antacids, urinary aluminium levels increased to a greater extent than did those of serum aluminium, suggesting urine is a better indicator of current or very recent aluminium exposure. For example, after consumption of 2.2 g aluminium in antacids, serum aluminium concentration increased 1.3 to 2.8-fold and urine aluminium concentration increased 3 to 34-fold (Kaehny et al., 1977). The increases in both serum and urinary aluminium were less with an aluminium phosphate product than with aluminium hydroxide-, aluminium carbonate- and dihydroxyaluminium aminoacetate-containing products. Similarly, after aluminium-containing antacid consumption, serum aluminium concentration increased ~ 2.4-fold and urine aluminium concentration increased ~4.5-fold (Gorsky et al., 1979). Urine concentration increased to a greater extent and remained elevated for a longer time than did serum aluminium concentration after oral administration of aluminium (Williams et al., 1986). Increasing aluminium antacid dose increased urinary, but not serum, aluminium levels (Nagy & Jobst, 1994).
Nine patients who received aluminium contaminated TPN solutions for 6 to 72 months had daily urinary aluminium outputs of 90 to 3830 μg, well above the normal range, as well as elevated plasma aluminium (23 to 214 μg/L) and bone aluminium concentrations (Klein et al., 1982).
Recent occupational aluminium exposure has been reported to cause increased urinary, and sometimes serum, aluminium levels. Occupational exposure to aluminium fumes and dusts produced a marked increase in urinary, but very little increase in serum, aluminium levels (Mussi et al., 1984). Occupational exposure of 235 workers to median respirable and total aluminium at concentrations of 25 and 100 μg/m3, respectively, was associated with only borderline changes in serum aluminium but significantly higher pre- and post-shift urine aluminium levels than seen in those of 44 controls (Gitelman et al., 1995). Mean plasma aluminium concentrations were 4.6 to 25.1 μg/L in potroom workers from 1981 to 1989 compared to 1.8 μg/L in 24 controls in 1988 (Schlatter et al., 1990; Schlatter & Steinegger, 1992). Although serum aluminium levels did not change in response to occupational aluminium inhalation, there was a significant correlation between mean weekly aluminium concentrations in air and excretion in urine (Drabløs et al., 1992). Median plasma and urine aluminium concentrations were 4.3 and 7.3 μg/L, respectively, in 30 non-exposed controls, whereas workers exposed an average of 151 months to aluminium powder with a mean ambient air concentration of 12.1 mg/m3 had values of 8.6 and 110 μg Al/L (Schmid et al., 1995), showing a greater elevation in urine than blood from the exposure. Similarly, median serum and urine aluminium concentrations were 0.18 μM (4.9 μg/L) and 2.4 μM (65 μg/L), respectively, suggesting greater urine than serum elevation above accepted levels in healthy humans (Hanninen et al., 1994). Blood and urine aluminium positively correlated in 103 exposed workers, but exposure did not significantly increase either (Liao et al., 2004). Workers exposed to mean concentrations of 0.036, 0.35 or 1.47 mg Al/m3 showed significant, and exposure-dependent, increases of urine aluminium (33, 67 and 133 μg/L, respectively, compared to controls which had 24 μg Al/L), but only the group exposed to the highest concentrations of aluminium showed significantly elevated serum aluminium levels (4.1, 4.8 and 7.2, respectively, compared to the control of 4.8 μg Al/L). These results suggested to the authors that only urinary aluminium is a practical index of exposure to aluminium above 0.35 mg Al/m3 (Rollin et al., 1996). By studying aluminium potroom workers before and after occupational exposure, Rollin et al. (1996) found an increase in serum aluminium concentration from 3.3 μg/L before exposure to ~ 6 μg/L after 1 year when it reached a plateau, and a continual increase of urinary aluminium from 24 μg/L before to ~ 48 μg/L after 2.5 years. In this study, the average of the median airborne aluminium concentrations was only 0.04 mg Al/m3, and the respirable fraction only 44% (Rollin et al., 2001). Similarly, three workers were exposed for 6 months to dust containing 3.3 Al/m3 in the air or for 4 months to fumes containing 3.9 mg Al/m3. They showed no consistent elevation of plasma aluminium above that of 10 non-occupationally exposed healthy subjects (mean 6.6 μg/L), whereas urine aluminium was consistently elevated after a work shift, averaging 97 μg Al/L, compared to the beginning of the shift, which averaged 46 μg Al/L. Urine aluminium was much above that of the controls, which averaged 4.6 μg Al/L (Mussi et al., 1984). Forty-four aluminium welders who had an average of 11.4 years of exposure, which averaged 5.6 and 4.5 mg Al/m3 in 1999 and 2001, had median urinary and plasma aluminium concentrations of 130 to 153 and 9.6 to 14.3 μg/L, respectively (Buchta et al., 2005). Plasma aluminium concentrations increased on average ~ 20 to 35% after, compared to before, a shift, but urinary aluminium concentrations did not increase.
There are a number of studies in which urinary, but not serum, aluminium was measured. The median urinary aluminium concentration in workers exposed to aluminium fumes and dusts in Finland from 1992 to 1997 was ~ 0.8 μM (~ 22 μg/L), compared to the upper reference limit for controls of 0.6 μM (16 μg/L) (Kallio et al., 1999). Sixty-seven workers with 2 to 34 years work history exposed to a mean of 0.35 to 0.4 mg aluminium oxide/m3 had a mean urinary aluminium concentration of 42.9 μg/L compared to 20.3 μg/L in 57 controls (Sinczuk-Walczak et al., 2003). Twenty male electrolyzers in the electrolysis department of an aluminium foundry who were exposed to aluminium oxide had a mean urinary aluminium concentration of 56.8 μg/L whereas 55 others in the same department who were crane operators, metallic chargers, locksmiths, and wireman had mean values of 25 to 35 μg/L (Trzcinka-Ochocka et al., 2005). Urinary aluminium concentrations in the control group of 57 wood-shop workers in the same foundry averaged 20 μg/L. A positive relationship was found between urinary aluminium concentrations after a work shift, air aluminium concentrations and duration of exposure (Elinder et al., 1991; Sjögren et al., 1988).
Most urine aluminium outputs have been reported as concentration or daily output, and have not been normalized to creatinine. Schlatter & Steinegger (1992) noted that urinary aluminium clearance is dependent on urine production whereas creatinine clearance is quite constant, making normalization of urine aluminium output to creatinine clearance not appropriate.
Two, five and 11 months after discontinuation of consumption of 6 g aluminium taken as an antacid over 8 years by a 39-year-old female, urinary aluminium levels were 270, 93 and 49 μg/L, respectively (Woodson, 1998). Serum aluminium 11 months after discontinuation of aluminium consumption was 68 μg/L, which is also considerably above normal. Two aluminium welders with 20 and 21 years of exposure had urinary aluminium levels of 107 and 351 μg Al/L (Elinder et al., 1991). Urinary aluminium was also elevated for years after termination of this occupational aluminium exposure.
Median urine aluminium was 2.4 μM (65 μg/L) in MIG welders exposed for 4 years (Hanninen et al., 1994), 22 μg/L in workers exposed > 10 years (Sjögren et al., 1996a), 40 μg/L in aluminium welders exposed for 8 years (Bast-Pettersen et al., 2000), 22 μg/L in aluminium welders with 15 years exposure (Iregren et al., 2001), and 58 μg/L in 1999 and 52 μg/L in 2001 in aluminium welders with > 6 years exposure (Buchta et al., 2003). Akila et al. (1999) studied two groups of aluminium welders who had mean urinary aluminium concentrations of 2.25 and 9.98 μM (61 and 270 μg/L). The mean serum aluminium concentration in 55 workers in an aluminium factory was reported to be 72.7 μg/L compared to 31.1 μg/L in 30 controls (San et al., 1997). Although significantly elevated in the aluminium workers, the control values are well above the normal range, suggesting these values may not be accurate.
Citrate did not increase urinary aluminium levels in the absence of concurrent aluminium administration (Nordal et al., 1988a). Humans consuming 5 mg of fluoride showed increased fluoride and aluminium in urine. Aluminium may have come from endogenous stores (Chiba et al., 2002).
Renal aluminium and silicon excretion following renal transplantation in 15 patients generally correlated, suggesting clearance by the kidney by a similar mechanism or as a complex, such as a hydroxyaluminosilicate species (Bellia et al., 1994). Renal excretion of aluminium, following consumption of 600 μmole silicic acid and 2.67 μmole of aluminium in beer, peaked at the same time as silicon (Bellia et al., 1996). Administration of 600 μmole silicon in water following 26Al administration accelerated the decline in serum 26Al (Bellia et al., 1996); an effect similar to that produced by citrate (Birchall et al., 1996). It was suggested that this resulted from complexation with silicate in urine to form hydroxyaluminosilicate species, which restricted aluminium reabsorption (Birchall et al., 1996). Two subjects were given oral 26Al together with 30 or 540 μM silicon (as a high-silicate mineral water). One was also given 4 and the other 50 mM citrate. They seemed to show more rapid renal aluminium clearance in the first day after they received the higher silicon dose (King et al., 1997). The appearance of 26Al increased in the urine of the subject that received the higher silicon dose and the lower citrate dose whereas less 26Al was eliminated in urine in the subject who received the higher silicon dose and the higher citrate dose. It is unknown if these effects would be seen in more than the one subject tested and if they are due to an effect of silicon on aluminium absorption and/or elimination. In a cross-over study, 3 humans consumed 26Al citrate, 26Al citrate with monomeric silica (orthosilicic acid), which is ~ 50% absorbed and excreted in the urine, or 26Al citrate with oligomeric silica, a polymer of silicic acid that has a much higher binding constant with aluminium, that is not detectably absorbed or excreted. Serum 26Al was lower following oligomeric silica and 26Al ingestion compared to the other two conditions (Jugdaohsingh et al., 2000). These results may explain the differences in the studies of silicon-containing compounds on aluminium absorption and elimination.
In dialysis patients a DFO test (5 mg/kg body weight given i.v.) was recommended for assessing the aluminium burden. If plasma aluminium increased by more than 50 μg/L it indicated that there is a high probability of aluminium-related bone disease. Bone biopsy is however needed for definitive diagnosis (Cronin & Henrich, 2004).
Biliary excretion
The concentration of aluminium in bile was greater than in urine before oral aluminium consumption (mean ~ 63 and 24 μg Al/L). Both increased comparably (~ 4 to 6-fold) suggesting to the authors that bile is an important route of aluminium elimination (Williams & Fraústo da Silva, 1996). It can be noted that the daily output of bile and urine are comparable, ~ 1 to 2 litres, supporting the notion that bile might be a significant route of aluminium elimination (Nieboer et al., 1995). Based on faecal 26Al after an i.v. 26Al citrate injection, biliary aluminium excretion was reported to account for 2.1% of the eliminated aluminium in one human (Priest et al., 1995). During the first 5 days after i.v. 26Al citrate injection in seven humans ~ 1.5% of the injected dose appeared in the faeces and ~ 70% in urine (Priest, 2004). Approximately 9 years after injection, only 1% of the 26Al being excreted in urine and faeces was in faeces (Priest, 2004). The aluminium concentration in bile of dialysis patients was ~ 30-fold higher than in controls (Di Paolo et al., 1997). The discrepancy between the reports of Williams et al. and Priest et al. could be due to enterohepatic recirculation. However, reports from animal studies have not shown a large percentage of aluminium in the bile. If bile was an effective route of aluminium elimination, it would be expected to reduce aluminium in severely renal impaired and anephric patients, which does not appear to be the case.
Elimination rate
Multiple aluminium t½ have been seen, suggesting that there is more than one compartment of aluminium storage from which aluminium is eliminated. Typically, as the duration of sampling after exposure was increased, longer t½ were observed.
The t½ of aluminium elimination positively correlated with the duration of exposure (Ljunggren et al., 1991). Tissue aluminium concentrations were elevated in non-dialyzed uraemic patients (Alfrey et al., 1980), suggesting uraemia facilitated tissue aluminium retention. Based on an estimated human body burden of 60 mg aluminium, a daily dietary intake of 20 mg and absorption of 1%, Jones et al. (1988) calculated a mean retention time of aluminium in the human of 300 days and a t½ of 210 days. This calculation assumed steady state conditions and was based on a single compartment or one compartment that is responsible for a majority of the aluminium body burden. Elimination t½ s of hours, weeks and years were seen after termination of short-term inhalation exposure, < 1 year exposure and upon retirement, respectively (Ljunggren et al., 1991). The aluminium elimination t½ positively correlated with exposure time (Ljunggren et al., 1991). These results are consistent with more than one compartment of aluminium storage. This kinetic behaviour might result from retention of aluminium in a depot from which it is slowly eliminated. This depot is probably bone which stores ~ 58% of the human aluminium body burden. Slow aluminium elimination coupled with continued exposure would be predicted to produce an increasing body burden with age, as noted above.
Multiple phases of elimination were seen in a study in which one human received i.v.26Al citrate suggesting multiple compartments of aluminium distribution. About 85 to 90% of the aluminium was eliminated in < 24 hr. Four percent of the injected 26Al remained after 3.2 years (Priest et al., 1995) and ~ 2% after 9.2 years (Priest, 2004). Calculations based on results up to 14 years after the injection suggested at least three components of the aluminium elimination with t½s of 1.4, 40 and 1727 days, and a retention t½ of ~ 50 years (Priest, 2004). This unusual kinetic behaviour might result from retention of an aluminium species other than that administered, creating a depot, probably in bone, from which the aluminium is slowly eliminated. Slow aluminium elimination coupled with continued exposure predicts an increasing body burden with age.
Following the consumption of aluminium-contaminated drinking water in Camelford, Cornwall, England, stainable bone aluminium was seen in 2 people six and seven months later whereas no stainable aluminium was seen 19 months later (Eastwood et al., 1990). Aluminium was not elevated, as determined by EAAS, at either time (McMillan et al., 1993).
The t½ of the first phase of urinary aluminium elimination in 3 previously non-exposed volunteers exposed to aluminium welding fumes for 1 day was 8 hr (Sjögren et al., 1985). In 5 aluminium welders exposed < 1 year to aluminium fumes, the t½ was estimated to be 8 to 9 days, whereas in workers exposed > 10 years it was ≥ 6 months (Sjögren et al., 1988). The t½ of aluminium elimination in two welders after 20 and 21 years exposure was ~ 3 years, based on daily urinary aluminium excretion compared to the estimated body burden of aluminium which, in turn, was based on bone aluminium concentration (Elinder et al., 1991). This group calculated a t½ of 9.8 years for aluminium elimination based on urine aluminium concentrations of 1 welder (Sjögren et al., 1996b). Aluminium t½ were estimated from urinary aluminium after termination of occupational aluminium exposure of 9 to 50 years duration. Elimination t½ of 0.7 to 7.9 years were estimated, based on a few samples per subject (Ljunggren et al., 1991). These t½s might be underestimates.
The t½ of aluminium elimination in dialysis patients receiving aluminium hydroxide was found to be 85 days, when studied to 900 days (Schulz et al., 1984). In patients undergoing plasmapheresis therapy who received 32 μg Al/kg, the t½ was determined to be 14 hr, when studied to 5 days (Wilhelm et al., 1987).
Priest et al. (1995) provided a formula to predict the aluminium body content (Bτ) as a function of time with repeated daily aluminium exposure, Bτ = 0.52(τ0.68-1), where τ = time in days after the start of the exposure, and Bτ is expressed as a multiple of the daily systemic aluminium intake. Modification, using more recent data, suggests the aluminium body burden after long term (years) of fairly constant aluminium intake would be ~ 400 times the daily aluminium intake (Priest, 2004).
As discussed in Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Tissue Aluminium Concentrations, Brain, brain aluminium levels have been reported to increase with age. Slow, or no, brain aluminium elimination and continued aluminium exposure would produce an increasing aluminium burden with age, as has been seen in the human. Assuming the t½ of brain aluminium in the human to be 20 to 50 years, the amount of aluminium that would accumulate in the brain after 60 years of daily consumption of 300 μg aluminium, assuming that 5 × 10-3 % of each dose enters each gram of brain and resides therein with a t½ of ≥ 20 years, would equal or exceed the amount seen in the normal 60 year old human. One might then conclude that normal concentrations of aluminium in drinking water would significantly contribute to elevated brain aluminium concentrations, and therefore could pose a potential health hazard.
Zapatero et al. (1995) found that serum aluminium concentration positively correlated with age in 356 healthy adults. This could not be attributed to the age-related decrease of renal function. It is unknown if it relates to the long t½ of aluminium in one or more compartments in the human so that steady state is not reached in a lifetime, to age-related increased absorption, or to other factors. In a previous study, Naylor et al. (1990) failed to find a correlation between age and serum, whole blood, urine or hair aluminium concentrations, in 76, 42, 42, and 42 subjects, respecively.
As noted above, injection of 26Al in animals increased 26Al in bone ~ 100-fold more than in brain, yet steady state bone aluminium concentration is < 100-fold greater than that in the brain. This suggests aluminium clearance from bone is more rapid than from brain, which is reasonable considering bone turnover and lack of neuron turnover. The elimination t½ of aluminium from human brain is predicted to be very long.
There are no reported determinations of retention time in specific tissues in humans. No publications of studies of bone aluminium concentration in normal humans as a function of age were found.
Biological Indices of Exposure, Body Burden and Organ Concentration
Human tissue and fluid aluminium concentrations are low compared to those in most exposure sources due to its very low bioavailability by most routes and effective urinary clearance.
Biological monitoring of human exposure to aluminium has been conducted with urine, which has been thought to indicate recent exposure, or serum, which has been thought to better reflect the aluminium body burden and long-term exposure (Alessio et al., 1989; Apostoli et al., 1992; Nieboer et al., 1995). However, neither is a very good predictor of the aluminium body burden.
As noted in Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Plasma Aluminium Concentrations, the serum aluminium level in normal humans has been reported to be ~ 1 to 2 μg/L. In dialysis patients, it was noted that > 30 μg Al/L serum has been associated with osteomalacia and related disorders (Nieboer et al., 1995). This conclusion was based on serum or plasma aluminium levels of 30 to 575 μg Al/L in 11 patients with osteomalacia and 27 to 160 μg Al/L in 11 patients with osteitis fibrosa (Cournot-Witmer et al., 1981), 65 to 360 μg Al/L in 18 patients with osteomalacia (Parkinson et al., 1981), 22 ± 12 μg Al/L in 18 patients with renal osteodystrophy (Gokal et al., 1983), 224 ± 22 μg Al/L in 40 patients with aluminium-related bone disease and 75 ± 13 μg Al/L in 21 patients with osteitis fibrosa (Norris et al., 1985) and 138 to 497 μg Al/L in 7 patients with moderately severe osteomalacia (Smith et al., 1987). It was noted in dialysis patients that concentrations > 80 μg Al/L serum have been associated with encephalopathy (Nieboer et al., 1995). This conclusion was based on serum aluminium concentrations of 89 to 486 μg Al/L in 17 patients (Parkinson et al., 1981) and 175 to 700 μg Al/L in 10 patients (McKinney et al., 1982). Ten dialysis patients who died with acute encephalopathy associated with aluminium contamination of the dialysate had a mean serum aluminium concentration of 808 μg/L. Seventeen patients who were similarly exposed but did not develop encephalopathy or die had a mean of 225 μg Al/L (Berend et al., 2001).
It has been suggested that the serum aluminium concentration should be kept below 30 μg/L (Razniewska, 2005). Psychomotor function in long-term dialysis patients whose mean serum aluminium was 59 μg Al/L was significantly impaired compared to that in controls (Altmann et al., 1989). Others suggested that a level of 40 to 50 μg Al/L warrants discontinuation of aluminium gels, 60 μg Al/L might indicate increased body burden, and > 100 μg Al/L indicates potential encephalopathy and the need for increased monitoring in dialysis patients. An AUC > 100 or 150 μg/L could present a risk of aluminium toxicity in these patients (Alfrey, 1986) and overt aluminium toxicity has been seen when the serum aluminium concentration was > 200 μg/L (Berend et al., 2002; Spencer, 2000). Mild neurophysiological and neuropsychological adverse effects were seen in MIG welders. The body burden threshold associated with these detrimental effects was estimated to be ~ 4 to 6 μM (108 to 162 μg/L) in urine and 0.25 to 0.35 μM (7 to 9 μg/L) in serum (Riihimaki et al., 2000).
Although the serum aluminium level increases with increased aluminium body burden, it does not directly reflect the aluminium body burden from long-term exposure. This is better measured by bone aluminium, the DFO challenge test, which has a high rate of false negative results, or the combined measurements of serum iPTH and the DFO test (Cannata-Andia & Fernadez-Martin, 2002; Mazzaferro et al., 1992; Pei et al., 1992). The peak and increment in serum aluminium after the DFO test in 28 chronic dialysis patients correlated significantly with bone aluminium (De Vernejoul et al., 1989) and in 11 other chronic dialysis patients correlated significantly with skin aluminium (Subra et al., 1991), suggesting utility of this test to indicate the aluminium body burden. Five mg/kg of DFO or less once or twice weekly has been shown to be safe and effective for long-term treatment of aluminium overload, and is as effective when given 1 hr before as after dialysis (Barata et al., 1996; Berend et al., 2002; Cannata-Andia & Fernadez-Martin, 2002). The serum aluminium increase in response to mobilization of aluminium from storage sites, such as produced by DFO, is used to test for aluminium body burden. A positive correlation was found between the oral dose of aluminium in a phosphate binder and bone aluminium content, but not with serum aluminium during the 6 months before the determination of aluminium in bone (Channon et al., 1988).
Conventional x-rays have not been shown useful to reveal aluminium-induced pneumoconiosis (Kraus et al., 2000; Letzel et al., 1996; Saia et al., 1981). High-resolution computed tomography was shown to reveal aluminium-induced lung fibrosis changes (Kraus et al., 2000).
A method using in vivo neutron activation analysis to measure aluminium in the bones of the hand has been developed and is ready for pilot studies in human subjects (Pejovi-Mili et al., 2005).
Pharmacokinetic Modelling
There are no published reports of physiologically based pharmacokinetic (PBPK) modelling of aluminium. The International Commission on Radiological Protection concluded that the t½ would be 100 days, based on 61 mg of aluminium in the human body (21 mg in the skeleton), daily intake of 45 mg in food and fluids, fractional absorption of 0.01 from the GI tract and from inhalation, and distribution of 30% of the aluminium that leaves systemic circulation to bone and 70% to all other organs and tissues (ICRP, 1975). Much of the data obtained during the past 40 years are different from the assumptions used in this prediction. A model with a plasma and two tissue compartments was developed based on t½s of 10.5 and 105 hr, that fit plasma and urine 26Al after 26Al citrate ingestion reasonably well (Fifield et al., 1997; Priest, 2004). Similarly, an 8 compartment model was based on the results obtained during 10 years after 26Al citrate ingestion by one human subject. The model assumes distribution of 60, 24, 7.5, 5.25, 2.2 and 1.25% of the aluminium from blood and ECFs into urine, soft tissues including liver, a rapidly exchangeable bone surface pool, a slowly exchangeable bone surface pool, cortical bone mineral and trabecular bone mineral, respectively. The compartmental t½s were calculated to be 1.43, 6, 45, 10500 and 500 days for the five non-urine compartments, respectively (Priest, 2004). Based on 26Al in blood and urine samples after a single oral 26Al administration to 3 healthy and 2 subjects with renal failure and i.v. in 3 healthy human subjects, Kislinger et al. (1997) developed a tentative open compartment model to describe aluminium biokinetics. The model has a central compartment that incorporates separate plasma and interstitial fluid compartments, each with sub-compartments for aluminium Tf and aluminium citrate. It has three peripheral compartments. One is for bone connected to the interstitial citrate compartment since the authors stated that studies in rats have shown that bones are supplied with aluminium by citrate. A second compartment represents muscles and organs including liver, kidneys and spleen connected to both central Tf compartments (plasma and interstitial fluid) as those organs receive aluminium from Tf. The third peripheral compartment is of unknown identity and was arbitrarily connected to the two central Tf compartments. Aluminium input was represented from a duodenal compartment into both the blood plasma Tf and citrate compartments. Output is described from plasma citrate into urine and a minor pathway from plasma Tf into the residual (beyond duodenal) intestinal tract into the stool. Compartment volumes and transport rates between compartments were determined based on the results of 8 subjects. In another report from the same group, Nolte et al. (2001) presented this model again, modifying the percentage of aluminium bound to citrate in plasma from 20% in the previous model to 6% and assigning the two peripheral non-bone compartments to liver and spleen that receive aluminium from plasma Tf and muscles, heart and the kidneys that receive aluminium from interstitial fluid Tf. Incorporating values reported from studies of aluminium in healthy individuals and renal failure patients, and from healthy and nephrectomized rats as well as rats in iron deficiency and iron overload, they calculated compartment volumes and transport rates between compartments. The same group reported the addition of one subject who received i.v. aluminium citrate, bringing the total to 6 healthy and 2 chronic renal-failure subjects who received 26Al either orally or i.v. Urine samples were obtained for up to 9, and blood for up to, 512 days. Again drawing on much of the published literature, the model was tested (Steinhausen et al., 2004) and the results were found to agree well with model predictions.
EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Single/Acute Exposure
The acute toxicity of aluminium metal and aluminium-containing compounds is relatively low (Appendix A). The reported oral LD50 values of aluminium compounds from toxicological animal studies are between 162 and 980 mg/kg b.w. (IPCS, 1997). The acute toxicity is dependent upon factors such as the solubility and bioavailability of the aluminium compounds, the route of administration, and the physiological status (renal function) of treated animals. Because aluminium hydroxide and aluminium oxide are poorly absorbed after GI, respiratory, dermal or i.v. administration (Flarend et al., 1997; Hem, 2002; Priest, 2004; Priest et al., 1996; Schönholzer et al., 1997) (see also Toxicokinetics, Absorption), it is expected that these compounds also produce low acute toxicity when administered by these routes. The i.p. LD50 (mouse) for aluminium oxide is >3600 mg/kg body weight (Filov, 1988) which suggests that aluminium oxide produces low acute toxicity. Aluminium hydroxide toxicity is predominantly seen in uraemic animals after i.p. injection and is manifested by symptoms of lethargy, periorbital bleeding, anorexia, and subsequently death (Berlyne et al., 1972a). Increased aluminium plasma levels and excessive aluminium deposition in the brain, liver, heart and muscle have also been documented following aluminium hydroxide overload (Berlyne et al., 1972a; Thurston et al., 1972).
Intratracheal/intrapleural exposure
Intratracheal instillation of aluminium compounds in laboratory animals has been used as a simple and relatively inexpensive method for screening aluminium for fibrogenicity or other types of pulmonary toxicity, including carcinogenesis. Experimental studies to evaluate fibrogenic potential in rats i.e., the ability to induce pulmonary fibrosis of 4 different types of aluminium fibres including alpha (uncalcined form) and gamma alumina (calcined form) following single intratracheal injections, were performed by Dalbey & Pulkowski (2000). Six months after dosing, pulmonary function tests (functional residual capacity, deflation pressure-volume curves, maximal forced deflation, single breath carbon monoxide diffusion capacity, and pulmonary resistance) and histopathological evaluation were performed. Rales were noted during the first week of instillation in aluminium treated groups, but not in the groups given glass beads or quartz. Standard lung volume and maximal forced exhalation parameters were decreased at 6 months after instillation in aluminium treated groups as compared to animals injected with saline and glass beads (controls). Single-breath carbon monoxide diffusing capacity was significantly decreased in aluminium treated animals compared to both types of controls which indicated the presence of a physical barrier between the air in the alveoli and the blood. The weight of the postcaval lung lobe was significantly increased for all groups administered aluminas, and the most marked increase was seen in the quartz group. The histopathological changes were similar in all treated groups and consisted of areas of granulomatous inflammation with early collagenization (fibrosis). The presence of multinuclear giant cells and the infiltration of macrophages were suggestive of a foreign body type reaction. Interstitial fibrosis was also apparent and was characterized by a thickening of alveolar walls with collagen. Both groups treated with uncalcined aluminas tended to have a higher incidence and severity of granulomas with fibrosis. Although minor pulmonary changes were noted in the aluminium treated groups, these effects were significantly less pronounced than the changes induced by the instillation of the positive control (quartz). Results are consistent with previously published work (Ess et al., 1993; King et al., 1955; Stacy et al., 1959) and point to the variation in responses to material within the class of alumina compounds. In interpreting these results, it must be considered that large doses were instilled with the intent of overloading normal clearance mechanisms in the lung to exaggerate any reaction that might occur. The dose of 50 mg is equivalent to about 30 mg/g lung, well above the 1 mg/g generally associated with the onset of overloading during long-term studies (Oberdorster et al., 1992). Influx of alveolar macrophages (AM), accumulation of particles, inflammation, and fibrosis are changes which would be expected following the administration of a large dose of relatively insoluble particles producing low toxicity to rats. The main goal of these instillations was to rank several alumina samples for their general potential to induce pulmonary fibrosis.
Lindenschimdt et al. (1990) examined the effects of aluminium on the development of pulmonary fibrosis and histological changes/inflammatory responses in the lungs of rats instilled with 1 or 5 mg Al2O3/100 g body weight. A dose-dependent minimal and generally transient increase in inflammatory responses was measured in the bronchoalveolar lavage fluid (BALF) including; activity of lactate dehydrogenase (LDH) an index of cell membrane damage; beta-glucurnidase and N-acetylglucosaminidase, markers of macrophage/polymorphonuclear membrane damage; and levels of total protein, an index of potential fibrotic activity and/or vascular damage. Increase in total cells at this dose was primarily due to elevation in neutrophils and lymphocytes. At low dose, the only significant change was an increase of neutrophils on day 1 which returned to the control level by day 7. The changes observed at high doses returned slowly to normal values during the 2-month study period. Although intratracheal instillation is not the normal route of exposure, the minimal and generally transient changes induced by Al203 are consistent with the lack of significant lung toxicity found in both humans and animals. Significant pathologic response at high doses might be due to the overload phenomenon of aluminium oxide dust (~9.1 mg/g lung tissue). Morrow (1996) showed that deposition of large amounts of inert dust in the lungs (> 1-2 mg/g lung tissue) resulted in inhibition of phagocytic removal of dust, leading to a delayed clearance from the lung.
Tornling et al. (1993) administered intratracheal instillations of aluminium oxide (primary alumina), aluminium oxide with adsorbed fluorides (secondary alumina), and saline to three different groups of rats. The alumina dust (40 mg) was suspended in saline. BALF was obtained and histological examination of the lungs was performed 1, 4, and 12 months after exposure. No signs of fibrosis were found in any of the animals. No significant changes in alveolar cell concentrations were noted for the group treated with primary aluminium; however the secondary aluminium group exhibited increased concentrations of macrophages and neutrophils one month and one year after exposure. This suggests that fluoride plays an important role in early changes to alveolar cell populations. One year after exposure both the aluminium treated groups exhibited significantly raised concentrations of fibronectin, which indicates that alumina, not fluoride, is essential for this observed effect. The biochemical properties of fibronectin support the formation of an extracellular matrix network, and therefore fibronectin may be an early marker of fibrosis. Due to the administration of aluminium by intratracheal instillation, which is not a physiological route, the results observed in this study need to be confirmed by further investigations in which inhalation is used as a route of exposure. Instillation may have led to pulmonary overload which could have contributed to the development of the observed effects.
Pigott & Ishmael (1992) assessed the effects of a single intrapleural injection (0.2 mL suspension/20 mg suspended solids) of refractory alumina fibres (Saffil fibres) obtained immediately after manufacturing or, later, after extensive thermal ageing. The potential for these fibres, which had different diameters, to result in the development of mesotheliomas in groups of rats was examined. No mesothelioma was detected in any of the rats dosed with the Saffil fibres, or in the negative controls. Malignant mesothelioma was diagnosed in 7 rats in the asbestos group (positive control) and in 3 rats in one of the aluminosilicate groups. However, it must be considered that intra-cavity injections result in a high deposition of the test material directly on the target tissue. This does not reflect inhalation exposures in which the fibres must first be deposited in the alveolar region of the lung and penetrate lung tissue before it reaches the pleural space. The increased mesothelioma proliferation and malignant mesotheliomas detected in the aluminosilicate B group as compared to the aluminosilicate A group is likely a reflection of the size of the fibres. Coarse fibres were found to be more irritating than fine fibres. The results of this study suggest that Saffil alumina fibres are inert and are not associated with mesothelioma induction. This study supports the results from a previously conducted inhalation study (Pigott et al., 1981).
After a single intrapleural instillation of Al(OH)3 at a dose of 0.3 g/mL saline to rats there was an increase in chest wall elastic properties and viscoelastic pressure accompanied by pleural inflammation after 7 days (Albuquerque et al., 2002). The pleural adherence was associated with a marked increase in the type I/type III collagen ratio after 30 days. Histological examination demonstrated no significant differences in lung parenchyma in the aluminium hydroxide treated and control groups.
In vitro studies
Gusev et al. (1993) and Warshawsky et al. (1994) conducted in vitro studies to examine the effects of aluminium on lung cell related functions. Gusev et al. (1993) showed that phagocytosis of alumina dust by rabbit AM did not produce exogenous generation of superoxide radicals and hydrogen peroxide as measured by nitroblue tetrazolium reduction in resting and stimulated cells when compared to quartz dust. Alumina dust exerted no effect on hydrogen peroxide generation and substantially decreased the level of superoxide radical generation by human granulocytes. Warshawsky et al. (1994) also conducted a study to assess the role of AM after exposure to aluminium oxide. The cytotoxicity of aluminium oxide particles (median size was equal or less than 0.36 μm and surface area 198.4 m2/g) to hamster and rat AM in vitro was measured at 0.1-0.5 mg/L ×106 cells at 24 and 48 hr using trypan blue exclusion procedures. The viability of the hamster AM in the presence of aluminium oxide up to the highest concentration was similar to control. After 24 and 48 hr, the viability of the AM was approximately 80 and 70%, respectively. Results demonstrated that aluminium oxide showed no changes in AM viability under in vitro conditions.
Oral exposure
As discussed in Toxicokinetics, Absorption, Animal Studies, Oral Administration, the oral bioavailability of silicon and aluminium from Zeolite A (30 mg/kg), sodium aluminosilicate (16 mg/kg), magnesium trisilicate (20 mg/kg), and aluminium hydroxide (675 mg) in dogs was examined by Cefali et al. (1995). Twelve female dogs received a single oral dose of each compound at one-week intervals. One of the 12 dogs receiving aluminium hydroxide displayed frothy emesis, and two dogs excreted soft stool.
Intracellular binding of aluminium was examined in the mucosa of the stomach, duodenum, jejunum and ileum of adult rats following a single oral administration (300 mg/kg) of aluminium hydroxide (More et al., 1992). A second group of rats received a daily oral administration of Al(OH)3 (300 mg/kg) for 5 days. No marked differences in body weight and no microscopic lesions in the GI tract (body and antrum of the stomach, duodenum, jejunum and ileum) were observed in either group of animals. Six hr after single Al(OH)3 administration, aluminium deposits were observed in the gastric lumen, in the duodenum, and in the lumen of both the jejunum and ileum. After repeated administration, the presence of aluminium-reactive deposits was noted only in the lumen of the stomach (at the bottom of the antral glands) and in the lumen of the intestine from day 3 to day 7. Other data (discussed in Toxicokinetics, Absorption, Animal Studies, Oral Administration, The Site and Mechanisms of Oral Aluminium Absorption) have demonstrated that aluminium absorption occurs in the small intestine by a paracellular pathway process via the tight junctions (Garbossa et al., 1998b; Provan & Yokel 1988a). The results also suggest that after repeated administration of large oral doses, aluminium accumulates in the antral mucosa of the stomach and is released slowly in the digestive tract.
Injection
Aluminium hydroxide is one of the adjuvants most commonly used in routine human vaccines against hepatitis B virus (HBV), hepatitis A virus, and tetanus toxoid (TT) and in veterinary vaccines (see also Effects on Humans, Effects from Non-Occupational Exposure, Irritation, Irritation after Injection of Aluminium-Adsorbed Proteins (Vaccines and Hyposensitization Regimens)). Although it has been investigated since 1926, the mechanisms of action of aluminium adjuvants are not yet fully understood. It is likely that aluminium adjuvants induce immune activation which includes interleukin (IL) -1 production by monocytes, induction of eosinophilia, compliment activation and increased specific and non-specific immunoglobulin (Ig) G1 and IgE antibody responses (Gupta & Siber, 1995; HogenEsch, 2002; Jensen & Koch, 1988; Larsen et al., 2002; Norimatsu et al., 1995; Shi et al., 2001). Limitations of aluminium adjuvants for human vaccination include local reactions, augmentation of IgE antibody responses, ineffectiveness against some antigens and inability to augment cell-mediated immune responses, especially cytotoxic T-cell responses (Gupta, 1998).
Gherardi et al. (2001) administered a single i.m. injection of an aluminium hydroxide-containing HBV vaccine (GenHevac, 250 μL) to rats in an attempt to reproduce lesions characteristic of MMF (also see Effects on Humans, Case Reports). The aluminium hydroxide-containing vaccine induced a large necrotic area containing damaged muscle fibres and neutrophils, surrounded by abundant lymphocytes and macrophages (days 7-15), that progressed to a mature lesion (21 and 28 days). The focal infiltration of densely packed PAS-positive macrophages, without giant cell formation or muscle fibre damage, was similar to the macrophage infiltrate seen in MMF. Crystalline inclusions similar to those of MMF were detected by electron microscopy. It was proposed that aluminium hydroxide forms a deposit which damages the injected tissue, subsequently eliciting a signal from stressed cells. This signal attracts inflammatory and antigen presenting cells and the aluminium hydroxide deposit is then subject to phagocytosis (Balouet et al., 1997; IPCS, 1997; Schijns, 2000). Phagocytized aluminium hydroxide increases survival of macrophages and enhances the effects of granulocyte/monocyte stimulating factor (Hamilton et al., 2000). A number of aluminium loaded macrophages accumulate locally, resulting in the characteristic granuloma formation, while others migrate to the regional lymph nodes (IPCS, 1997). A recent study in monkeys showed that macrophage accumulation persisted more that 1 year after injection (Verdier et al., 2005). A residence time longer than 6 months was observed in rats (Gherardi et al., 2001).
Verdier et al. (2005) evaluated the local reaction and aluminium concentration following i.m. injection of aluminium adjuvant vaccines in Cynomolgus monkeys. Two groups of 12 male monkeys received a single i.m. injection of either aluminium phosphate adjuvant diphtheria-tetanus vaccine or aluminium hydroxide adjuvant diphtheria-tetanus vaccine. Four monkeys from each of the two groups were sacrificed 85, 169, or 366 days after the single i.m. injection, and macroscopic examination of the injected site was performed to detect any sign of local intolerance. Macrophage aggregation was graded as moderate to marked and was accompanied by a lymphoid infiltration in all cases following the initial sacrifice. Analysis of the injection site revealed high aluminium content for both aluminium treated vaccine groups; however, the aluminium concentration of the reactive zones of animals treated with aluminium hydroxide was 4 times higher than in those treated with aluminium phosphate. The size of the inflammatory lesion was greater in the monkeys given the aluminium hydroxide adjuvant. Six months after the vaccine injection 3 out of 4 monkeys exhibited appreciable lesions composed primarily of macrophages. One of the lesions had an extensive cyst-like structure which contained degenerate macrophages. Two of 4 monkeys in the aluminium hydroxide group had persistent macrophages aggregations with associated minor lymphocytic infiltrations one year following the injection. The histological appearance and persistence of the lesion observed at the injection site is similar to the lesions observed in human cases of MMF. Therefore these results suggest that this type of lesion is a usual reaction following the injection of an aluminium adjuvant vaccine by the i.m. route, and can occur in normal healthy animals following the administration of both aluminium phosphate and aluminium hydroxide containing vaccines.
A field trial involving 45 pigs was conducted to validate the hypothesis of aluminium-induced granulomas (Valtulini et al., 2005). The animals were randomly allocated to receive the same aluminium hydroxide adjuvant vaccine which induced the formation of nodules in the muscles of pigs from one particular farm; the adjuvant alone, distilled water, or the adjuvant and distilled water. The pigs were injected twice i.m. and slaughtered at about 165 kg weight. Granulomas located within muscular tissue were observed for all the aluminium containing vaccine groups; granulomas were not detected in any of the pigs who received only water. Granulomas were characterized by aggregates of macrophage-derived epithelioid cells, with some containing oval nucleus, a pale pink cytoplasm, and indistinct cell borders. These cells were surrounded by an infiltrate of mixed inflammatory cells, including large multinucleated giant cells, macrophages, lymphocytes, plasma cells and eosinophilic elements. In most samples, multiple granulomas were joined by a unique fibrous shell. X-ray microanalysis and atomic absorption revealed the presence of considerable amounts of Al, both within and outside the cells. These results indicate that high amounts of aluminium hydroxide have the potential to produce granuloma formulation.
Administration of endotoxin and aluminium hydroxide adjuvant (0.85 mg) s.c. to Sprague-Dawley rats demonstrated that aluminium hydroxide was able to protect animals from the adverse effects of a 15 μg/kg dose of endotoxin. Shi et al. (2001) suggested that the detoxification of endotoxin by Al-containing adjuvants occurs due to the irreversible binding of endotoxin to the surface of Al. These results suggest that all of the surface aluminium in the aluminium hydroxide adjuvant was able to covalently bind to the phosphate groups of endotoxin, and absorption of endotoxin was subsequently inhibited due to phosphate binding. The pro-inflammatory cytokines tumor necrosis factor (TNF)-α and IL-6 were not detected in the serum of animals receiving endotoxin with the aluminium hydroxide adjuvant (Shi et al., 2001).
Dermal exposure
The comparative irritancy of several aluminium salts was assessed by Lansdown (1973) in three different species. Groups of 5 mice, 3 rabbits and 2 pigs were treated daily for 5 consecutive days with applications of 10 % w/v aluminium chloride, aluminium nitrate, aluminium chlorhydrate, aluminium sulphate, aluminium hydroxide (the pH of the solution was highest at 7.2 among these chemical species of Al tested) or basic aluminium acetate. Twenty-four hr after the final treatment with aluminium hydroxide, signs of erythema, thickening, scaling hyperkeratosis, acanthosis, microabsecesses and the presence of aluminium in keratin were not observed. After single dermal application of aluminium hydroxide (10%) on mouse, rabbit and pig skin no signs of dermal irritation or inflammation were found (Lansdown, 1973).
Repeated Exposure
Inhalation/intratrachael exposure
The fibrogenic potential of very fine metallic aluminium powder was investigated by Gross et al. (1973). Three different types of aluminium powder were tested. Pyro powder and flaked powder were composed of flake-like particles, and the atomized powder consisted of atomized spherical particles. Aluminium oxide dust was used as a negative control. Two chambers, containing 30 rats and 30 hamsters each, were held at dust concentrations of 100 mg/m3 of the pyro powder and the atomized metal powder respectively, two additional chambers were held 50 mg/m3 of the respective powders. Six chambers, each containing 30 rats and 15 guinea pigs, were maintained at dust concentrations of 15 and 30 mg/m3 respectively, for each of the three types of metallic aluminium powders. The animals were exposed for 6 hr daily, 5 days each week, for 6 months for the 50 and 100 mg/m3 groups, and for 12 months for all other animals. An additional group of 30 rats and 30 hamsters was exposed to aluminium oxide dust at an average concentration of 75 mg/m3 for 6 months, and 30 rats and 12 guinea pigs were exposed to aluminium oxide at a concentration of 30 mg/m3 for one year. Intratracheal injection of the aluminium powders at different dose levels was also conducted. Pulmonary fibrosis was not apparent following inhalation of the aluminium powders in hamsters and guinea pigs; however scattered small scars resulted from foci of lipid pneumonitis in rats. All three species of animals developed alveolar proteinosis, the severity and extent of which were not consistently or clearly related either to the type of aluminium powder or to the severity of the dust exposure. The alveolar proteinosis resolved spontaneously and the accumulated dust deposits cleared rapidly from the lungs after cessation of exposure. Intratracheal injection of large doses of aluminium powders into rats produced focal pulmonary fibrosis; no fibrosis occurred in the lungs of hamsters following intratracheal injection. The results of this experiment indicate that inhalation of fine metallic aluminium powders does not produce fibrogenic effects, and that intratracheal injection of these powders is likely an artefact of the injection itself.
Christie et al. (1963) examined the pulmonary effects of aluminium in rats and hamsters (see also Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, Inhalation Exposure). Inhalation exposure to 100 mg/hr aluminium, in the form of powder, or 92 mg Al/per 2 hr, as a fume, each day for 9-13 months showed a significant retention of aluminium in the lungs of both groups of animals. The aluminium retention in the lungs in rats and hamsters exposed to fume was much greater than when exposed to powder. Following exposure to fresh air, aluminium oxide was cleared rapidly from the lungs of the both powder and fume groups. Weight of wet lung, ash and aluminium oxide content of lungs in exposed animals increased. The initial pulmonary tissue response was proliferation of macrophages within alveolar spaces as well as lipoid pneumonia. The focal aggregates of macrophages were located around the small bronchioles and small pulmonary arterioles; lymphoid hyperplasia was observed. After chronic exposure to aluminium powder, rats showed focal deposits of hyaline in alveolar walls, and focal areas of lipoid pneumonia developed in hamsters.
The pulmonary reaction to inhalation exposure of refractory alumina fibre (Saffil fibres), either as manufactured or in a thermally aged form, was assessed in rats (Pigott et al., 1981). Animals were exposed to the fibres 5 days a week, for a 6 hr period, for a duration of 86 weeks. Pulmonary reaction to both forms of alumina fibre was minimal. Focal necrosis and regeneration of olfactory epithelium was seen in the nasal cavity in 2 Saffil fibre treated animals, and the appearance of aluminium fibres in the mediastinal lymph nodes indicated that fibres and particles may also have been transported via macrophages into the lymphatic system. Benign and malignant pulmonary tumours were confined to the rats in the positive control group which were dosed with asbestos. The results of this study indicate that inhalation of refractory alumina fibres is not associated with an increase in pulmonary or other tumours.
Ess et al. (1993) studied the fibrogenic effect of intratracheal instillation of 7 alumina samples in rats. Five of the samples were used for aluminium production, one sample was a chemical grade form of alumina characterized by small particle diameter and high chemical purity, and the last sample was a laboratory-produced alumina. Quartz was used as a positive control because of its well-known fibrogenic activity. The alumina samples were administered at a total dose of 50 mg by 5 injections given over a period of 2 weeks. Groups of 5 animals were sacrificed at 60, 90, 180, or 360 days after exposure. Histopathological examinations were carried out on all animals and bronchoalveolar lavage was performed to assess inflammatory reactions. Fibrogenic potential was not detected for any of the 5 aluminas used for primary aluminium production, while it was reported that the other 2 samples induced fibrotic lesions. A correlation between cytological and biochemical parameters studied in BALF and the fibrosis determined by histology was not noted for the alumina-treated animals. A persistent inflammatory alveolar reaction was seen in the animals instilled with the alumina samples, which was less severe than the reaction produced by the instillation of quartz. The route of administration needs to be considered in interpreting these results. Intratracheal instillation may have overloaded clearance mechanisms; however this cannot account for differences of intensity between samples which were administered at the same dose.
There are a number of limitations in these studies. First, most studies do not demonstrate a dose-response relationship. Few data are available concerning exposure conditions and the size of the ambient aerosol. Some studies were of relatively short duration compared with the life-span of the animals employed; consequently, although no adverse effects were reported in nearly all cases, it is not possible to assess how much, if any, of the compound was deposited in the lungs and whether the time-span of the experiment may have been too short to demonstrate delayed effects.
Oral exposure
Several repeated dose toxicity studies have been conducted in order to assess the effects of oral exposure to aluminium hydroxide on clinical signs, food and water consumption, growth, haematology and serum chemistries, tissue and plasma concentrations of aluminium, and histopathology.
In a study conducted by Hicks et al. (1987) there were no treatment-related effects in rats fed up to 288 mg Al/kg b.w./day as aluminium hydroxide in the diet for 28 days.
Berlyne et al. (1972a) investigated the effects of repeated oral, s.c., and i.p. aluminium hydroxide administration in normal and uraemic rats. Groups of nephrectomised rats were administered 1 or 2% AlCl3 or Al2(SO4)3 in the drinking-water or oral Al(OH)3 (150 mg of elemental aluminium/kg/day) by gavage. Groups of non-nephroctomised rats received the same treatments. The duration of the treatment was not indicated. Groups of nephrectomized and normal rats also received i.p. and s.c. injections of Al(OH)3. The clinical signs of intoxication in nephrectomized animals observed following i.p. administration (90 mg/kg b.w.) included periorbital bleeding, lethargy, anorexia and death. Plasma, liver, muscle, heart, brain, and bone levels of aluminium were markedly elevated in the i.p.-treated group. S.c. injection was apparently less toxic, resulting in no mortality, but periorbital bleeding occurred in nephrectomized animals. Aluminium levels were elevated in all tissues, the highest concentration being in the brain. Administration of high doses of aluminium chloride (180 mg/kg b.w.) and aluminium sulphate (300 mg/kg b.w.) in drinking water to nephroectomized rats produced periorbital bleeding and 100% death in treated animals. Periorbital bleeding was noted for the rats which received drinking water supplemented with Al(OH)3. In normal rats only aluminium sulphate produced periorbital bleeding in 3 of 5 rats, but no mortality.
Thurston et al. (1972) examined aluminium deposition in the tissues of rats following dietary aluminium hydroxide exposure in order to assess whether the toxicity of this compound was modified when hypophosphatemia was prevented. Weanling rats (6 per group) were assigned to either a whole meal diet; a whole meal diet with aluminium hydroxide (3.2 g/kg) added; or a whole meal diet with added aluminium hydroxide (3.2 g/kg) plus 10 g/kg disodium hydrogen phosphate. An additional group underwent partial nephrectomy and was assigned to the whole meal diet with added aluminium hydroxide.
The duration of the experiment was 4 weeks; after the treatment the animals were sacrificed, blood samples were taken and a complete post-mortem examination was conducted. Animals in the aluminium hydroxide group exhibited a significant impairment of growth, while animals receiving both aluminium hydroxide and the phosphate supplement showed a normal rate of growth. The adverse effects on growth were more severe in uraemic rats but the pattern was the same for aluminium hydroxide treated and untreated animals. Skeletal aluminium content was raised in the normal animals given aluminium hydroxide or aluminium hydroxide and phosphate; however, the uraemic animals showed the most marked increase in skeletal aluminium levels. These results suggested that some aluminium accumulation is seen following oral exposure but that adverse effects are not exhibited if hypophosphatemia is avoided.
The accumulation of aluminium in bone and various regions of the CNS in rats treated with aluminium hydroxide (100 mg/kg b.w./day) or aluminium citrate (100 mg/kg b.w./day) i.g. for either 4 or 9 weeks (6 times a week) was studied by Slanina et al. (1984). However, a decrease in weight gain was observed after 4 weeks of aluminium hydroxide treatment indicating the presence of subacute adverse effect.
Subchronic oral administration (18 days) of aluminium hydroxide (271.3 μg Al/g diet) resulted in significantly increased tibia weight compared to rats fed aluminium phosphate (272 μg/g), aluminium lactate (262 μg/g), or aluminium palmitate (268 μg/g) (Greger et al., 1985).
Body weight of weanling and adult rats was not affected after repeated oral exposure to high doses of aluminium hydroxide mixed with sucrose in the diet (2000 ppm for 67 days) (Sugawara et al., 1988). Rats were fed test diets that had been supplemented with aluminium hydroxide at levels of 989 and 1070 μg Al/g diet. An additional group of rats was fed a control diet containing 26 μg Al/g. No aluminium-induced anaemia or hypophosphatemia was observed in young or adult rats and serum aluminium did not exceed the normal level. Aluminium concentration in the intestinal tract mucosal membrane increased significantly but no effect on inflammatory infiltration or necrosis was noted in the intestine. Serum and hepatic triglyceride levels and adipose weight were decreased significantly in young rats, but neither serum cholesterol nor phospholipid levels was affected by aluminium ingestion. In the adult group, aluminium hydroxide produced a decrease in only hepatic glycogen content (Sugawara et al., 1988).
The body burden of aluminium in weanling rats fed one of 4 diets for 29 days was assessed by Greger & Powers (1992). Rats were assigned to receive a diet containing 40 μmol Al/g diet with or without citrate, a diet containing 100 μmol Al/g diet with citrate, or a control diet containing 0.39 μmol Al/g diet. Rats were injected with DFO or buffer 24 hr prior to sacrifice. Rats fed Al-supplemented diets accumulated significantly more metal in their tissues than rats fed the basal diet, the accumulation was greatest in the rats fed aluminium with citrate. Haematocrit levels following oral aluminium exposure were inversely correlated to tissue aluminium concentrations. It was expected that DFO might mobilize aluminium from tissues subsequently increasing serum and urinary aluminium levels proportionately to bone aluminium concentrations. However, the changes induced by DFO were small and the elevated serum and urine aluminium concentrations were not more correlated to the body load of Al, as indicated by tibia aluminium concentrations. It was estimated that approximately 0.01 to 0.04% dietary aluminium was absorbed. Aluminium hydroxide added to the diet (0.05%) of rats during the 30 days did not affect vitamin A bioavailability (Favaro et al., 1994). Hicks et al. (1987) found no treatment related effects in rats fed up to 302 mg Al/kg bw as aluminium hydroxide for 28 days.
Oral administration of high doses of aluminium hydroxide (1513, 2697 or 3617 mg/kg) in rats for 30 days did not produce any clinical signs or gross symptoms of intoxication, or any significant differences in body weight and food intake. However, in treated animals, behavioural changes (memory and learning ability disturbances) associated with elevated brain aluminium content were observed (Thorne et al., 1986).
Dlugaszek et al. (2000) examined the effects of long term exposure to aluminium in drinking water, including the distribution of the ingested aluminium and changes in the tissue levels of essential elements. Aluminium was administered in drinking water as aluminium chloride, dihydroxy aluminium sodium carbonate, or aluminium hydroxide. Animals in the Al(OH)3-treated group exhibited an increase in Mg concentration in bones, a decreased Fe concentration in the stomach, and a decline of copper in the kidneys and liver. The group which received AlCl3 exhibited the highest elevation of aluminium in the tissues following oral exposure.
Bilkei-Gorzo (1993) investigated neurotoxic effects following daily oral administration (90 days) of insoluble aluminium hydroxide (300 mg/kg Al(OH)3), water soluble AlCl3 (30 or 100 mg/kg) and chelated aluminium hydroxide (100 mg Al(OH)3/kg + 30 mg citric acid/kg) in rats. The ability to learn (determined by the number of runs necessary to learn the labyrinth) was affected in all aluminium treated groups; the learned performance was altered to a greater extent in the Al(OH)3 and AlCl3 treatment groups. The aluminium content of the brain was elevated in each treatment group; however, the elevation was highest in the groups treated with soluble aluminium compounds. Similarly, all treatments resulted in elevated acetylcholinesterase activity, with significant increases in the AlCl3 group, and in Al(OH)3 chelated to citric acid. No relevant differences in body weights, general conditions, or water and food intake were noted between control and treated groups. These results suggested that, although water-soluble aluminium compounds exhibit greater neurotoxicity, the highly insoluble aluminium hydroxide compound appeared to be absorbed subsequently producing some effect on nervous system functions.
Ecelbarger et al. (1994b) conducted a study to assess the impact of chronic exposure to dietary aluminium on aging rats. Male rats were fed diets containing 0.4 or 36.8 μmol Al/g diet in the form of aluminium hydroxide for 8 months until they reached 23 months of age. One day prior to sacrifice, one-half of the rats in both treatments were i.p. injected with DFO, and the remaining rats were injected with saline in order to investigate the usefulness of DFO for estimating body burden of Al. The rats exhibited little evidence of aluminium toxicity as body weight, feed intake, or changes in the relative size of tissues did not appear to be affected by the treatments.
The possible relation between aluminium intake, levels of aluminium in the brain, and dementia was investigated in rats and dogs following chronic oral aluminium hydroxide exposure (Arieff et al., 1979). Clinical signs of intoxication were not apparent in rats with normal renal function (n=10) or rats with chronic renal failure (n=14) exposed to an oral daily dose of 300 mg aluminium hydroxide, for 5 months. Brain Al3+ was significantly greater than normal for both groups of rats, the most marked increase being in the group with renal failure. The effects of aluminium were also investigated in two groups of mongrel dogs. One group of dogs received a diet which included 3 g of added aluminium hydroxide daily for 5 months, while the other group received the same diet without Al. In the aluminium loaded dogs the content of Al3+ in the cerebral cortex was significantly greater than in that of the controls. Electroencephalograms (EEG) were conducted in the exposed dogs and the results for the aluminium treated dogs were within the normal range. It must be considered that the number of animals in each treatment group was not clearly reported.
A significant increase in tubular phosphate reabsorption with an increase in the apparent velocity of maximal tubular transport was reported in rats following aluminium i.v. administration (Mahieu et al., 1998). Proximal tubule damage was reported in rats (Ebina et al., 1984) and rabbits (Bertholf et al., 1989) following i.v. administration of aluminium. Rats consuming a high aluminium diet (36.8 μmol Al/g diet) for 8 months excreted significantly more protein in urine which is indicative of renal damage (Ecelbarger et al., 1994b).
Studies on the effects of oral administration of aluminium on pregnant animals and their offspring are presented in Effects on Laboratory Mammals and In Vitro Test Systems, Reproductive and Developmental Toxicity.
Injection
Repeated s.c. injection of aluminium hydroxide for 5 or 10 months to rabbits and guinea-pigs did not induce any apparent symptoms or visceral alterations. Limited local lesions characterized by the presence of monocytes and macrophages were observed (Levatidi et al., 1968).
S.c. implants of aluminium foil induced subcutaneous tumours in 8 of 18 rats (O’Gara & Brown, 1967). Histologically, the tumours were fibrosarcomas, rhabdomyosarcomas, or combination of these types. One fibrosarcoma had metastasized to the lungs. The significance of aluminium in tumour induction is unknown as the smooth surface of the aluminium implant may be responsible for the induction of the observed lesions.
Mahieu et al. (2000) examined the effects of chronic i.p. administration of aluminium hydroxide on haematological parameters in rats. Male rats received 80 mg/kg body weight aluminium hydroxide three times a week for 6 months. Control rats were injected with saline solution for the same period. Animals in the aluminium treatment group developed a progressive microcyotosis which became more prominent with time. Significant decreases in mean corpuscular haemoglobin and mean corpuscular volume were noted throughout the duration of the experiment. The haematocrit levels were significantly reduced during months 1, 3, or 4 for the treated animals. The haemoglobin levels of aluminium-treated animals decreased in months 3 and 4 and then began to increase in months 5 and 6. These results suggest that chronic i.p. aluminium administration may interfere with different stages of red blood cell synthesis. Persistent exposure appears to trigger a compensating mechanism leading to restored haematocrit and haemoglobin concentrations, with persistence of microcytosis and depressed mean corpuscular haemoglobin levels.
Bazzoni et al. (2005) studied the effect of repeated i.p. injection of aluminium hydroxide on red blood cell parameters in rats. Male rats were injected with 80 mg/kg body weight of aluminium hydroxide 3 times a week, for a duration of 3 months. A control group of rats was injected with saline at the same frequency and volume. Significant decreases in haematocrit and haemoglobin concentrations were noted in the treated rats as compared to controls. The treated animals showed a high quantity of abnormally shaped (stomatocytes) erythrocytes which became more resistant to haemolysis, and the rigidity index was also substantially higher in the aluminium treated rats. It was reported that the observed effects may have been due to aluminium induced alterations in the mechanical properties of red blood cells resulting in disorganization of the erythrocyte membrane. These alterations likely result in a reduction of the viability of the circulating red blood cells which may lead to anaemia in aluminium intoxicated animals.
Effects of chronic parenteral aluminium administration on parameters of renal function, P and Ca movements at the tubular level, and the possible proximal cell mechanisms were studied by Mahieu et al. (1998). Rats were treated with an 80 mg/kg body weight i.p. dose of aluminium hydroxide (three times per week for 6 months). Control rats received saline for the same duration and frequency. Treated rats showed a significant decrease in asymptotic weight gain and in the initial efficiency of food conversion compared to the controls. Treated rats also exhibited a significant decrease in the haemoglobin level, haematocrit index, and serum Fe concentrations in the peripheral blood compared to control rats. The Ca balance in treated rats was significantly less than in the control group and was accompanied by a significant increase in Ca excreted in faeces, correlated with less intestinal absorption. Accumulation of aluminium on the surface of the trabecular bone and a reduction in the skeletal Ca mass (without any changes in the bone Ca resorption rate), were observed in all treated rats; however, there were no differences when expressed as per 100 g body weight. The reduction in bone turnover was accompanied by a lower recovery velocity from calcemia in the aluminium treated group. The fractional reabsorption of P and sodium was significantly lower in treated animals compared to controls. There did not appear to be any significant differences in the acid-base balance nor in the Ca and P concentrations in the plasma between treated and control animals. It was postulated that aluminium exposure interferes with P excretion by decreasing PTH or by diminishing the affinity for PTH receptors at the level of the renal tubule. These results are in agreement with other studies which demonstrated that aluminium is a potent inhibitor of PTH secretion (Morrisey et al., 1983; Morrissey & Slatopolsky, 1986). Smans et al. (2000) reported that aluminium inhibited PTH secretion in vivo, implying potential for lowered Ca release from the bone to blood.
Cointry et al. (2005) examined aluminium effects on the diaphyseal structure and biomechanics of rat bones. After subchronic i.p. injection of 27 mg Al(OH)3 in 20% glycerol/water solution for a duration of 26 weeks, blood aluminium levels were significantly higher in treated rats. A significant difference was observed between tibial aluminium concentrations in control vs. Al-treated rats. A significant delay in femur length growth was noted in treated animals. The volumetric bone mineral density and cortical bone material properties (elastic modules and the stress at the yield point) were significantly reduced in treated animals compared to controls. The bone geometric and structural properties were unaffected by treatment. The serum Ca and P levels were not affected by aluminium treatment. No changes were observed in urine volume (diuresis) and urinary excretion of Ca and Pi. No significant differences were noted in the body weights of treated and control groups at the end of the study (Cointry et al., 2005).
An aluminium-induced inhibition of bone mineralization (Cointry et al., 2005) is consistent with other studies showing similar aluminium effects on bone mineralization in vitro and in vivo (Ballanti et al., 1989; Bellows et al., 1995; Blumenthal & Posner, 1984; Chan et al., 1987; Mjoberg et al., 1997; Posner & Blumenthal, 1985). Potential mechanisms proposed include; changes in the biosynthesis of collagen, increased collagen cross-links, altered osteocalcin levels (Blahos et al., 1991; Chan et al., 1987; Mjoberg et al., 1997), and physicochemical dissolution of mineral crystals (Bushinsky et al., 1995). Different combinations of these effects were described as aplastic bone disease (Ballanti et al., 1989; Malluche, 2002) and osteomalacia (Galceran et al., 1987; Lieuallen & Weisbrode, 1991; Malluche, 2002; Mjoberg et al., 1997; Quarles et al., 1985). Chronic oral or parenteral aluminium hydroxide administration was found to produce aluminium accumulation in bone and on the bone surface accompanied by a decrease of P and Zn concentrations in tibias (Berlyne et al., 1972a; Ecelbarger et al., 1994b; Greger et al., 1985; Greger & Donnabauer, 1986; Greger & Powers, 1992; Mahieu et al., 1998; Thurston et al., 1972).
Fiejka et al. (1996) conducted a study to determine the compartmentalization of aluminium in mice following i.p. injection of aluminium hydroxide. One group of mice (n=30) received aluminium hydroxide containing 1 mg of elemental aluminium every two weeks; another group (n=30) received an injection of aluminium hydroxide which contained 0.1 mg elemental aluminium 5 days a week. Controls were treated with saline. Ten animals from each group were sacrificed 48 hr after a cumulative dose of 2, 4 or 6 mg of elemental Al. In the liver the development of aluminium containing granulomas represented by swollen macrophages and multinucleated giant cells were observed; the number of granulomas increased with the aluminium dose. These results suggest that the physiochemical nature of the Al(OH)3 is an important factor in the development of this type of foreign-body response (Fiejka et al., 1996).
Histochemical and immunochemical studies were carried out after repeated intracerebroventricular aluminium injections (5.4 or 0.68 μg/day for 5 days) in the adult rat brain in animals allowed to survive for a period of either 1 or 6 weeks. Platt et al. (2001) demonstrated that aluminium concentrated in the white matter of the medial striatum, corpus callosum, and singulate bundle. Inflammatory responses and damage in the singulate bundle was noted in Al-treated animals which led to a severe anterograde degeneration of cholinergic terminals in the cortex and hippocampus. These findings suggested that the enhancement of inflammation and interference with cholinergic signalling may be the modes of action through which aluminium results in learning and memory deficits in mammals (Platt et al., 2001).
The injection of 0.3 ml of a 1% suspension of aluminium (metallic) powder into the CSF (cisterna magna) of adult rabbits induced a slowly progressing encephalopathy characterized by alterations of posture, myoclonic jerks and muscle weakness (Bugiani & Ghetti, 1982). The presence of neurons with neurofibrillary degeneration (NFD) and proximal axon swelling was observed between 1 and 81 days after injection. In some animals, large axons with thin or no myelin sheath were also observed. In treated animals pathological changes in the peripheral nerves and muscles were also found. The neuropathological investigation confirmed that the vulnerability of the brain to aluminium is a time-related event. Within the observed time interval (1-81 days), NFD developed in every nuclear complex of the CNS other than the striatum and the amygdala. The factors regulating the time related degeneration of adult rabbit neurons resulting from exposure to aluminium were not clarified by this study. No relation was found between the chronology or the topography of the NFD and the size of the cell bodies, the site of the injection, and the distance from the subarachnoid space and the ventricles (Bugiani & Ghetti, 1982). These data are consistent with other studies which have demonstrated that aluminium salts induce an encephalopathy with NFD after subarachnoid injection in receptive animals (rabbits, cat, ferret) (Crapper et al., 1973; Klatzo et al., 1965; Wisniewski & Kozlowski, 1982). Although the mechanism by which aluminium induces the accumulation of neurofilaments is unknown, it has been demonstrated that these neurofilaments are morphologically identical to filaments that accumulate following the administration of tubulin-binding agents. These compounds bind to tubulin, induce a disruption of microtubules (Ghetti & Ohcs, 1978; Remillard et al., 1975) that is followed by the accumulation of filaments in the cell pericarion, in dendrites and proximal axonal segments.
Reproductive and Developmental Toxicity
Developmental toxicity
It is noteworthy that the reproductive consequences of aluminium occur only at excessively high non-environmentally amounts. The high amount of aluminium plays a key role in chemical-induced alterations in reproductive function and consequent mammalian development. Various investigators have demonstrated an adverse effect on reproductive capacity due to the chemical species of aluminium ingested (Domingo, 1995; Golub & Domingo, 1996). Embryotoxic and adverse developmental effects were found in rats and mice following administration of aluminium nitrate (Albina et al., 2000; Paternain et al., 1988), aluminium chloride (Colomina et al., 1999; Cranmer et al., 1986; Misawa & Shigeta, 1993) or aluminium lactate (Golub et al., 1987; Gonda et al., 1996; Poulos et al., 1996), although these effects may be directly related to aluminium exposure or the result of a secondary consequence (e.g., maternal toxicity, systemic toxicity). These forms of aluminium are water soluble, and are therefore absorbed to a greater extent than non-soluble aluminium forms, such as Al(OH)3 (Colomina et al., 1994; Domingo, 1995).
The embryotoxic and teratogenic potential of Al(OH)3 administered orally to pregnant mice was investigated by Domingo et al. (1989). Mated female mice were administered 0, 66.5, 133 or 266 mg/kg b.w. of Al(OH)3 daily from gestation days 6 through 15. No signs of maternal toxicity were observed in any group as evidenced by changes in maternal weight gain or gross signs of abnormalities. The number of implantations, number of resorptions, number of live and dead foetuses, and body weights of foetuses were not significantly affected by any dose of aluminium hydroxide administered. The foetuses of Al-treated dams did not exhibit any significant differences in the number and type of external malformations, internal soft-tissue defects or skeletal abnormalities as compared to controls. Domingo et al. (1989) proposed that the lack of any apparent demonstrated embryo/foetal toxicity of Al(OH)3 in mice was likely due to the low GI absorption of this compound as compared to other forms of aluminium. A similar study was also conducted by Gómez et al. (1990). Higher doses of Al(OH)3 were used in order to evaluate the potential of Al(OH)3 to induce adverse developmental effects in rats. Al(OH)3 was administered by gavage at dose levels of 0, 192, 384, and 768 mg/kg b.w./day to pregnant rats from day 6 through 15 of gestation. No significant maternal or developmental toxicity was observed at any Al(OH)3 dose level administered.
Although based on the studies by Domingo et al. (1989) and Gómez et al. (1990) Al(OH)3 alone did not produce adverse reproductive effects, other studies showed that concurrent ingestion of Al(OH)3 with other dietary constituents such as ascorbic acid results in an increase in the GI absorption of aluminium (Colomina et al., 1994; Domingo et al., 1991b) (see also Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption). Gómez et al. (1991) assessed in rats the influence of citric acid on the embryonic and/or teratogenic effects potentially induced by high doses of Al(OH)3. Three groups of pregnant rats were administered daily doses of Al(OH)3 (384 mg/kg b.w.), aluminium citrate (1064 mg/kg b.w.), or Al(OH)3 (384 mg/kg b.w.) concurrently with citric acid (62 mg/kg b.w.) on gestational days 6-15. A fourth group received distilled water and served as a control group (n=17). Maternal and foetal body weights were significantly reduced in the group treated with Al(OH)3 and citric acid. There were no significant treatment-related differences on pre- or post-implantation sites, number of live foetuses per litter, or gender ratio. Although no significant differences in the number of malformations were detected between any of the groups (data not shown), delayed sternabrae and occipital ossification was observed in the foetuses treated with Al(OH)3 and citric acid as compared to the control group (p < 0.05).
The potential influence of lactate on developmental toxicity attributed to high doses of Al(OH)3 was also evaluated in mice (Colomina et al., 1992). Oral daily doses of Al(OH)3 (166 mg/kg b.w.), aluminium lactate (627 mg/kg b), or Al(OH)3 (166 mg/kg b.w.) concurrent with lactic acid (570 mg/kg b.w.) were administered to pregnant mice from gestational day 6 to15. An additional group of mice received lactic acid alone (570 mg/kg b.w.). A control group received distilled water during the same period. Concurrent administration of Al(OH)3 with lactic acid resulted in significant reductions in maternal weight compared to the control group of mice who received distilled water without the addition of aluminium lactate or lactic acid. In the group given lactate only, a quantitative rise in the concentration of aluminium was detected in whole foetuses; however this was not statistically different from the mean levels found in the control group. Aluminium lactate administration resulted in significant decreases in foetal body weight accompanied by increases in the incidence of cleft palate and delayed ossification. Although not statistically significant, the incidence of skeletal variations was higher in the concurrent Al(OH)3 and lactic acid group compared to the control group. No other signs of developmental toxicity were detected in this group.
In a similar experiment, Colomina et al. (1994) assessed the concurrent ingestion of high doses of Al(OH)3 and ascorbic acid on maternal and developmental toxicity in mice. Three groups of pregnant mice were given daily doses of Al(OH)3 (300 mg/kg b.w.), ascorbic acid (85 mg/kg b.w.), or Al(OH)3 concurrent with ascorbic acid (85 mg/kg b.w.) from gestational day 6 to 15. A fourth group of animals received distilled water and served as the control group. No embryotoxic or foetotoxic effects were detected in any group. Gross, internal, or skeletal malformations did not vary according to treatment group. The number of resorptions, dead and live foetuses, percent implantation loss, and foetal body weight did not differ between among the control and treated groups (Colomina et al., 1994). Placenta and kidney concentrations of aluminium were significantly higher in mice receiving Al(OH)3 and Al(OH)3 plus ascorbic acid compared to controls. In contrast, Gómez et al. (1990) found no significant differences in placental concentrations of aluminium in rats administered higher doses of Al(OH)3 alone.
Competition between aluminium and other essential trace elements was proposed as one of the possible mechanisms to explain adverse reproductive outcomes related to aluminium toxicity including delayed ossification, foetal malformations and reduced weight gain (Bellés et al., 2001). Bellés et al. (2001) examined in rats the effect of oral Al(OH)3 on the accumulation and urinary excretion of Ca, Mg, Mn, Cu, Zn and Fe. Three groups of rats were given either 0, 200 or 400mg/kg b.w./day Al(OH)3 from gestational day 1 to 20. Three groups of non-pregnant female rats also received the same doses of Al(OH)3 for 20 consecutive days. Urinary concentrations, as well as samples of liver, bone, spleen, kidney and brain removed post-sacrifice, were analyzed for Al concentrations, as well as levels of Ca, Mg, Zn, Cu, and Fe. Treatment with oral doses of Al(OH)3 did not produce any overt signs of toxicity in pregnant or non-pregnant rats; however, there were differences in the pattern of metal tissue distribution. In pregnant rats, the highest aluminium concentration was found in kidneys while, in non-pregnant animals, the brain had the highest level of aluminium. In the non-pregnant control group, the highest tissue accumulation of aluminium was also in brain. The hepatic and renal concentrations of several essential elements, as well as the levels of calcium in bone and copper in brain, were significantly higher (p < 0.05) in the treatment groups as compared to the control group for the pregnant rats. In contrast, fewer differences between hepatic, renal and bone concentrations of the elements examined were found between the treatment and control groups of the non-pregnant animals. These results suggest that pregnancy may be a period of enhanced susceptibility for aluminium accumulation and subsequent toxic outcome.
Donald et al. (1989) and Golub et al. (1991) evaluated developmental neurobehavioural toxicity. Donald et al. (1989) conducted a study which demonstrated that elevated dietary exposure of mouse dams to aluminium during gestation and lactation resulted in persistent neurological defects during the post weaning period in offspring. Pregnant mice were assigned to receive a diet containing 25, 500, or 1000 μg aluminium lactate/g diet. The experimental diet commenced on day 0 of gestation and continued throughout pregnancy and lactation. Four pups from each litter were selected for further neurobehavioural assessment after weaning. These pups were fed a control diet containing 25 μg aluminium lactate/g. Neither maternal nor reproductive toxicity was detected. Neurobehavioural maturation of the pups was tested on days 8 through 18 with the Wahlsten test battery which included: forelimb and hindlimb grasps, fore- and hindpaw placement on sticks of two widths, vibrissae placing, visual placing, auditory and air puff startle, eye opening, screen grasp, screen cling, and screen climb. The only difference among the aluminium groups in terms of pup toxicity prior to weaning was poor performance in a climbing test in the 1000 μg Al/g diet group. Several significant differences in neurological signs were exhibited between weanlings whose dams were fed control or high aluminium diets; some of these manifestations persisted after a 2 week recovery period from the diet. Foot splay, forelimb and hindlimb grip strengths, and thermal sensitivity (tail removal latency) were associated with higher maternal dietary aluminium levels. These results suggested that maternal dietary exposure to excess aluminium during gestation and lactation resulted in neurobehavioural toxicity in weanling mice even in the absence of maternal toxicity.
In a similar experiment, Golub et al. (1991) fed pregnant mice a diet of either 25 (control) or 1000 (high) μg aluminium lactate/g from conception through lactation. Litters were fostered either within or between groups at birth to create the following 4 groups related to the aluminium concentration of the maternal diet: (1) control during gestation and lactation, (2) high aluminium during gestation, control during lactation, (3) control during gestation, high aluminium during lactation, (4) high aluminium during both gestation and lactation. Forelimb grasp strength was influenced by high aluminium exposure during gestation, negative geotaxis was influenced by exposure during lactation, and hindlimb grasp and temperature sensitivity were affected by exposure during both gestation and lactation. Although aluminium exposure during gestation or lactation did not influence brain and liver aluminium concentrations, exposure during lactation resulted in significantly lower manganese and iron concentrations in the liver, and significantly less manganese concentrations in the brain of the pups at weaning. These results demonstrated that high maternal dietary aluminium intake, during both gestation and lactation, might result in neurodevelopmental adverse effects and altered essential trace element metabolism in offspring.
It has been suggested that maternal stress during pregnancy could enhance aluminium-induced developmental toxicity in mouse and rat offspring (Colomina et al., 1998; 1999; 2005; Roig et al., 2006). Colomina et al. (1998) administered i.p. injections of AlCl3 at 37.5 and 75 mg/kg/day to two groups of pregnant mice on days 6-15 of gestation who were also subjected to restraint for 2 hours/day. AlCl3 was also administered at the same frequency and doses to two groups of mice who were not restrained. Foetal weight was significantly lower (p < 0.05) in the groups whose dams were concurrently exposed to aluminium (37.5mg/kg and 75mg/kg) plus restraint, and in the group exposed to 75 mg/kg without restraint, as compared to those in the group subjected to restraint only. A significant increase (p < 0.05) in the number of litters with skeletal anomalies, as well as the total number of litters with internal and skeletal defects, was observed in the group exposed to 75 mg AlCl3/kg/day plus maternal restraint as compared to any of the other groups.
In a more recent study, Colomina et al. (2005) did not detect a significant influence of maternal restraint on the postnatal developmental and behavioural effects in the offspring of rats exposed parentally to aluminium nitrate nonahydrate in drinking water. In this study, female rats were exposed to 0 (control group), 50, or 100 mg/kg/day of aluminium (as aluminium nitrate nonahydrate) in drinking water with citric acid (355 or 710 mg/kg/day) for a duration of 15 days. The female rats were then mated with untreated males and aluminium exposure was maintained throughout the gestational, lactational, and post-weaning periods. Half of the animals in each group were restrained for 2 hours/day on days 6-20 of gestation. No significant differences were noted in the activity of the offspring (postnatal day 30) measured in an open field test between animals with prenatal aluminium exposure, alone or plus restraint, as compared to the control group. Rats exposed to 100 mg/kg/day all through their life following prenatal restraint stress showed improved performance in a passive avoidance task. A significantly improved performance in a water maze test was also noted for rats exposed to 50mg/kg/day of aluminium as compared to the non-aluminium exposed groups. Maternal restraint did not appear to affect the water maze performance for rats also exposed to aluminium.
Roig et al. (2006) investigated the long-lasting neurobehavioural effects of prenatal restraint stress and oral aluminium exposure in rats. Pregnant females were orally exposed to 0, 50, and 100 mg/kg/day of Al. Each Al exposed group was divided into two subgroups, one of these groups was subjected to restraint stress for 2 hours/day on gestation days 6-20. The offspring of the treated females received the same Al treatment until the time of sacrifice at 1 or 2 years of age. An open field test and a water maze test were conducted to assess behavioural performance of the offspring one or two years after birth. Prenatal restraint did not appear to modify behavioural performance in the rats. In addition, brain aluminium accumulation was significantly higher (p < 0.05) in rats exposed to 100mg/kg/day aluminium, without prenatal restraint, in all brain structures analyzed (cortex: 31.7 ug/g, olfactory bulb: 28.1 ug/g, cerebellum: 36.3 ug/g, striatum: 51.1 ug/g, hippocampus: 79.7 ug/g, and brain stem: 26.7 ug/g), as compared to rats who experienced prenatal restraint and were exposed to the same dose of aluminium (cortex: 0.8 ug/g, olfactory bulb: 9.1 ug/g, cerebellum: 2.6 ug/g, striatum: 7.1 ug/g, hippocampus: 4.2 ug/g, and brain stem: 3.1 ug/g). Therefore, prenatal restraint stress appeared to prevent aluminium accumulation.
Until the actual delivered dose to the foetus, the suckling pup, and to the target organs is well characterized, any determination of a vulnerable window of effect or comparison of animal to human data will remain limited.
Reproductive toxicity
To our knowledge no studies have been conducted to assess the reproductive toxicity of Al(OH)3 or Al2O3 in male mice; however, adverse reproductive effects were documented following i.p. injections of aluminium nitrate to male mice. Llobet et al. (1995) administered i.p. aluminium nitrate injections to adult male mice at doses of 0, 50, 100 or 200 mg/kg b.w./day for 4 weeks before mating with untreated females. The pregnancy rate was significantly reduced for the 100 and 200 mg/kg b.w./day dose groups compared to controls. Testicular and epididymal sperm counts were significantly decreased in the group administered 200 mg/kg b.w./day aluminium nitrate and the count of spermatids was significantly reduced at 100 mg/kg b.w./day. In the 100 and 200 mg/kg b.w./day groups histological changes, including necrosis of spermatocytes/spermatids, were noted in 5 and 6 mice, respectively. Histological lesions were not detected in the control mice or in the group that received 50 mg/kg b.w./day aluminium nitrate.
In another study, focal necrosis of the testes and destruction of spermatozoa was observed following a single intratesticular injection on 4.3 mg Al/kg (as aluminium sulphate) to rats (Kamboj & Kar, 1964). A proliferation of interstitial cells and a reduction in the number and motility of spermatozoa was observed following chronic exposure (6 months) of an oral dose of 2.5 mg/kg (as aluminium chloride) in rats (Krasovskii et al., 1979).
Protective treatments on aluminium-induced developmental effects
Albina et al. (2000) conducted a study to determine if a chelating agent, deferiprone, could protect against aluminium-induced maternal and developmental toxicity in mice. Pregnant mice were randomly divided into 5 groups. One group was administered 1,327 mg/kg b.w. of aluminium nitrate nonahydrate by gavage on gestation day 12, a second group was given 24 mg/kg b.w./day of deferiprone on days 12-15 of gestation, and a third group was given 1,327 mg/kg b.w. of aluminium nitrate nonahydrate on gestation day 12 followed by deferiprone (24 mg/kg b.w. at 2, 24, 48 and 72 hr following aluminium exposure. The controls received sodium nitrate or deionised water. Administration of deferiprone alone did not produce any apparent signs of developmental toxicity. Aluminium-induced maternal toxicity included significant reductions in body weight gain, absolute liver weight and food consumption compared to controls. Administration of deferiprone did not offer protection against these aluminium induced maternal effects. In contrast, deferiprone administration following aluminium exposure resulted in a more pronounced decrease in maternal weight gain and corrected body weight change. Developmental toxicity was manifested by delayed ossification of a number of bones in the aluminium treated groups compared to controls. The group treated with aluminium nitrate and deferiprone exhibited a higher number of litters with foetuses showing skeletal deficiencies. These results suggested that deferiprone is not an effective agent to protect against aluminium-induced developmental toxicity and might increase the severity of aluminium-induced maternal and developmental adverse effects in mice.
Silicon-containing compounds were shown to limit absorption of ingested aluminium (Edwardson et al., 1993). Therefore, it was proposed as exerting a protective effect against aluminium-induced toxicity (see also Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption, Silicon-Containing Compounds). Bellés et al. (1999) conducted a study to test this hypothesis. Aluminium nitrate monohydrate was administered to three groups of pregnant mice by gavage (398 mg/kg b.w./day) on gestation days 6-15. These animals received silicon in drinking water at concentrations of 0, 118 or 236 g/L on days 7-18 of gestation. Three additional groups of pregnant mice received 270.6 mg/kg of sodium nitrate and the same concentrations of silicon in drinking water as the aluminium-treated groups. The percentage of aluminium-induced deaths, abortions and early deliveries was significantly reduced in the group administered 236 mg/L silicon. However, no significant differences were noted at 118 or 236 mg/L silicon on aluminium induced foetotoxicity.
Neurotoxicity
The scientific literature is replete with reports linking (or dissociating) various chemical forms of aluminium with neurotoxicity and neurodegeneration. Interested readers can gain a grasp of the literature and the differing views as to the potential mechanisms of aluminium toxicity, through a number of existing reviews and hypotheses papers (Atchison, 2003; Becaria et al., 2002; Campbell, 2002; 2004; Campbell & Bondy, 2000; Domingo, 1996; Elmore et al., 2003; Emmett, 2004; Exley, 1999; 2005; Flaten, 2001; Gupta et al., 2005; Kagan et al., 2002; Kumar, 1999; Oteiza et al., 2004; Priest, 2004; Rao et al., 1998; Reinke et al., 2003; Rob et al., 2001; Shin et al., 1995; Savory et al., 2003; Sim & Benke, 2003; Solfrizzi et al., 2003; Soni et al., 2001; Szutowicz, 2001; Tanaka, 2004; Van Landegham et al., 1998; Yokel, 2000). Here, we will focus on some of the literature from the last 10 years that addresses the potential for aluminium to cause neurotoxicity and/or provides insight into the mechanisms of toxicity.
In examining the literature on in vivo studies, it is useful to consider several factors in determining whether a given study may provide significant insight. For studies in animals, the over-riding considerations are dose, mode of administration, speciation of the metal, and measures of outcome. Human Exposure, Total Human Uptake from All Environmental Pathways (Combined Exposure) summarizes the most common sources of human exposure to aluminium. For the majority of individuals, these various sources of exposure result serum concentrations of 1-2 μg of aluminium per Litre of plasma. It is notable that daily doses can be much higher in individuals who use aluminium-based antacids (Lione, 1983). Typically, toxicological studies in rodents utilize doses that are 10-20 times the anticipated human dose. This calculation is based on increased metabolic rates for rodents and is meant to account for differences in the metabolism of the toxin. Therefore, one potential approach to relate experimental studies in rodents, which are most commonly used, to humans is to assume that studies in rodents that achieve serum levels of >10-20 μg/L would mimic normal environmental exposure levels of humans. Studies that achieve higher concentrations of aluminium in serum could be viewed as challenging the animal to determine the toxic potential of the metal.
Investigators have used a variety of routes of exposure, including oral, i.v. injection, and i.p. injection. A variety of aluminium salts have been used in these studies. As discussed in Toxicokinetics, soluble aluminium salts that are introduced into the body are subject to re-speciation with most aluminium in serum being bound to Tf, a minority found as aluminium citrate, and smaller amounts bound to other molecules. Aluminium citrate is usually excreted by the kidney rather quickly, and thus one could expect that Tf would be the major carrier of aluminium into the brain. In CSF, which has higher concentrations of citrate, aluminium dissociates from Tf with the majority bound to citrate. In studies where soluble aluminium salts have been given orally or by injection (i.p. or i.v.), the most likely species of aluminium in brain would be bound to Tf and citrate. In studies where aluminium has been injected directly into the brain there would be also be re-speciation but there would be opportunity for exposure to novel aluminium salts. For the purposes of review, studies involving oral and i.p, or i.v., injection methods of exposure are probably most informative regarding potential effects in humans. Studies in which aluminium salts have been injected directly into the brain have a greater probability of producing non-physiologic effects.
Alzheimer’s disease
Studies in both cell culture models and in vivo have clearly established the potential for aluminium to cause significant neurotoxicity. Likewise, well documented cases of encephalopathy associated with long-term dialysis establish a connection between aluminium and neurotoxicity in humans. One of the first proposed consequences of aluminium exposure in humans was an elevated risk of developing AD. AD is defined by both clinical and pathologic symptoms. Clinically, the symptoms include loss of cognitive ability, psychiatric symptoms, and emotional changes, progressing to profound motor dysfunction and death. Pathologically, AD is defined by the presence of senile plaques, composed of fibrillar extracellular deposits of amyloid β peptide, and NFTs, composed of fibrillar intracellular accumulations of hyperphosphorylated tau protein. Several disorders can produce clinical symptoms that overlap with, or duplicate, the clinical symptoms of AD, including frontotemporal dementia and vascular dementia.
The scientific literature contains numerous reviews that discuss the extant literature regarding AD and aluminium exposure. The initial lines of evidence linking AD to aluminium exposure were numerous reports of elevated levels of aluminium in the brains of AD patients and an association of aluminium with disease-specific lesions (amyloid senile plaques and NFTs). However, subsequent studies utilizing more sophisticated technology demonstrated that aluminium levels are not particularly elevated in the brains of AD patients, and aluminium is not disproportionately distributed to senile plaques or NFT (Lovell et al. 1993; Xu et al., 1992c) (see Tables 19-21). A well controlled study by Makjanic et al. (1998), demonstrated that the detection of aluminium in tissues from AD patients may partially result from methods of tissue preparation as measures of aluminium in frozen-untreated tissue preparations failed to detect aluminium (<20 ppm) (Makjanic et al., 1998). Studies of a very limited number of autopsied brains suggested that aluminium may preferentially accumulate in lipofuscin granules (Tokutake et al., 1995), which are a common pathology in the aged, and thought to represent remnants of lysosomes that contain undigestable-material.
In regard to animal studies designed to directly test the potential role of aluminium in the development of AD-related pathologies, Pratico et al. (2002) utilized a transgenic mouse model of Alzheimer amyloid pathology. Animals were fed diets enriched in aluminium (dose and chemical form not clarified in the publication) to determine whether aluminium induced changes in the rate or severity of amyloid deposition in this model. The authors reported significant increases (~ 2-fold) in brain loads of soluble and insoluble amyloid peptide and in the levels of isoprostane 8,12-iso-iPF2α-VI, which was examined as a marker of lipid peroxidation. Huang et al. (1997) also reported increases in the expression of the precursor to amyloid peptide, termed amyloid precursor protein (APP), in cervical spinal cords of rabbits given intracisternal injections of aluminium maltolate (2.5 mmole). Intracellular accumulations of APP immunostaining in neurons of the medulla have also been reported by Zhang et al. (2004), who described increased numbers of neurons immunoreactive for APP in the cortex and hippocampus of rats given aluminium chloride in drinking water (3 mg/mL) for 90 days (Zhang et al., 2003). Together, these data suggest that aluminium may modulate the expression and, or, processing of APP. If a life-time of constant exposure results in sustained elevations in APP expression or production of amyloid peptide, then increases in amyloid peptide could accelerate the rate of senile plaque formation and hasten the onset of AD (Jankowsky et al., 2004).
In a study in which rats were given large doses of aluminium sulphate by oral gavage, El-Rahman (2003) reported histological changes that resemble those found in AD patients. Animals were given daily doses of aluminium ranging from 4.59 to 17 mg/100 g b.w./day for 35 days. The brains of animals receiving the highest doses were reported to have developed both classic amyloid deposits and NFTs in the cortex. The report, however, did not quantify the prevalence of either pathology and relied on histological stains, rather than antibody immunostaining, to define pathology. The highest dose in this study would equate to a daily exposure for humans of 170 mg/kg b.w./day. Unfortunately, serum levels of aluminium were not reported, making if more difficult to assess exposure to tissue. Although this dose is far and above normal exposure levels, humans who take aluminium-containing antacids may be exposed to between 15 and 50 mg/kg b.w./day (Lione, 1983). Therefore the dose used in this study was within the 10 to 20-fold excess that is routinely used in rodent toxicological studies. If replicated, and examined with more precise methods, this study could be construed as evidence that very high exposure to aluminium could lead to AD-related pathologic changes in the brain.
Another potential mechanistic connection between aluminium and AD comes from work of Silva et al. (2002) who examined cholesterol levels in the brains of rats fed high doses of aluminium (1 g per day for 10 days or 0.03 g per day for 4 months). Under both conditions, there were significant and robust changes in the ratio of cholesterol to total phospholipids. There were also robust diminutions in membrane fluidity, attributed to the loss of cholesterol. Recent studies from a number of laboratories (Refolo et al., 2000; Smith et al., 2001) demonstrated that the proteolytic generation of amyloid peptide (the principal component of senile plaques in AD patients) is elevated by hypercholesterolemia. The findings by Silva noted above would suggest that aluminium exposure might indirectly reduce amyloid production. However, such beneficial effects could be offset by the reported ability of aluminium to increase the rate at which amyloid peptides (Kawahara et al., 1994; 2001; Mantyh et al., 1993), and other pathogenic proteins such as α-synuclein (Uversky et al., 2001), to assemble into more stable and pathologic fibrillar structures. Additionally, regardless of whether there is a direct action of aluminium on CNS membranes or an indirect metabolic consequence of aluminium exposure, changes in membrane fluidity and cholesterol content could account for the numerous reports of deleterious effects of aluminium on neurotransmitter systems and synaptic function (Csoti et al., 2001; Dave et al., 2002; El-Rahman, 2003; Nayak & Chatterjee, 2001; Wang et al., 2002; Zatta, 1997; Zhang et al., 2004).
The most convincing data to dissociate AD from aluminium exposure are detailed histological analyses of patients who have undergone long-term haemodialysis and succumbed to encephalopathy. To control serum phosphorus levels, patients take high doses of hydroxyl-aluminium gel over long periods, leading to elevated levels of aluminium in many tissues at autopsy. The brains of these patients have characteristic accumulations of aluminium in sub-cellular compartments of both neurons and glia (Reusche et al., 2001a). Morphologically, these structures are most commonly ovid in nature, intracellular, and appear to be lysosome-derived. In rare cases, where pathologic changes that meet criteria for diagnosis of AD in patients with dialysis-encephalopathy (one case out of 127 analyzed), the pathology associated with each disorder remains morphologically distinct (Reusche, 1997). Importantly, in the study by Reusche et al. (2001a), there was no association between AD-like pathology and long-term ingestion of aluminium. The most informative group of patients included 10 who received high doses of aluminium (> 500 g total intake over at least 5 years), for whom the frequency of AD-related pathology was no greater than controls. Moreover, in patients over 60 years of age the degree of AD-related pathology (subclinical levels) in dialysis patients was similar to that in controls. Collectively, these studies in humans support the view that exposure to aluminium poses no direct risk for development of AD. However, caution in over-stating the importance of the data from DAE patients is warranted given the rather small number of highly definitive cases. The animal studies cited above suggest that a life-time of exposure to low doses of aluminium, or exposure to a single-high-dose, might pose a risk for developing AD or contribute to the aetiology of a subset of cases.
Motor neuron disease
It has been known since 1965 that the rabbit is uniquely vulnerable to aluminium toxicity and produces both clinical and pathologic features of motor neuron disease (Klatzo et al., 1965). Intracisternal (directly into CSF) injection of aluminium salts (chloride, phosphate, maltolate) can be used to cause both acute and chronic neurotoxicity. Single high doses of aluminium (1 mg) cause rapidly progressing motor neuron dysfunction with hindlimb paralysis, whereas repeated low dose injections (100 μg) can induce a chronic motor dysfunction (for review see Savory et al., 2001). Detailed neuropathological studies of the chronic rabbit model have defined the similarities and differences between aluminium intoxication in the rabbit and human disease. First and foremost, aluminium intoxication in the rabbit is reversible. Symptoms and pathology associated with chronic low dose aluminium intoxication can be ameliorated if aluminium exposure is reduced at the first signs of symptoms. In humans, motor neuron disease is progressive and almost uniformly fatal. Pathologically, the major resemblance between aluminium intoxication in the rabbit and human disease is the appearance of intracellular inclusions in motor neurons of the spine and brain stem. These inclusions are formed by filamentous aggregations of neurofilament protein (He & Strong, 2000a; Wakayama et al., 1996). Absent from the rabbit model are significant numbers of inclusions that are immunoreactive with cystatin C, ubiquitin, and tau (Wakayama et al., 1996). Also conspicuously absent is evidence of astrocytic gliosis and activation of microglia, both of which are hallmarks of human ALS (He & Strong, 2000a; 2000b; Wakayama et al., 1996). In a chronic aluminium intoxication model, Ghribi et al. (2002) found evidence of caspase-3 activation, an important step in programmed cell death; however, others failed to detect the definitive marker of apoptosis, DNA fragmentation (He & Strong, 2000a), despite ~50% losses in the numbers of lumbar spinal motor neurons. Relevant to AD, Muma & Singer (1996) reported alterations in tau protein phosphorylation and accumulation, the principal component of NFT pathology of AD, in the spinal motor neurons of rabbits chronically given moderate intracisternal doses of aluminium (~ 200 μg of elemental aluminium). Notably, DAE patients do not show increased levels of neurofibrillary pathology (see below).
In vivo models
Neuropathology
The pathology of DAE patients provides insight into potential mechanisms of aluminium neurotoxicity and its transport into the nervous system. As discussed above, Reusche et al. (1997) noted an abundance of intracellular argentophyllic (silver binding) granules in the brains of DAE patients, which are most commonly ovid, intracellular, and lysosomal in appearance. LAMMA revealed high concentrations of aluminium in the cytoplasm of cells exhibiting these structures. One of the major carriers of aluminium in the serum and interstitial fluids is Tf (see Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Transport in Blood). At physiological pH, aluminium in serum is bound to transferrrin, although less tightly than Fe would be bound. In CSF, higher concentrations of citrate result in significant re-speciation to aluminium citrate (Yokel, 2001). Uptake of Tf by neural cells involves receptor-mediated endocytosis via the Tf receptor. Indeed, in the CNS, Tf-mediated uptake has recently been used as a molecular means of introducing novel compounds or proteins into the brain. Importantly, receptor-mediated endocytosis would be expected to deliver material to the endosomal and then lysosomal compartments. Hence, in DAE patient, the subcellular distribution of aluminium in neural cells (neurons and cells of the choroid plexus) is consistent with a mode of exposure that involves Tf-mediated delivery to the nervous system. The extent to which aluminium citrate is taken up by neural cells is unclear.
Rodent models of aluminium toxicity by direct injection
To bypass limitations of absorption, some investigators have directly i.p. injected aluminium-salts. Esparza et al. (2003) injected rats with aluminium lactate at concentrations of 5 mg/kg/day and 10 mg/kg/day for 8 weeks (5 injections per week) and then assessed markers of oxidative stress and cognitive function (see below). The estimated exposure would be >1000 times normal. Levels of aluminium in cortex, hippocampus, cerebellum and liver were measured, with only cerebellum and liver showing significant accumulations of aluminium. Levels of aluminium in serum were not reported. Significant reductions in the levels of manganese and copper were found in brain, with no change in Fe in any organ. In this model, the hippocampus showed the largest number of changes indicative of oxidative stress, including increased GSH, increased measures of lipid peroxidation (thiobarbituric acid reactive substances (TBARS)), increased levels of oxidized glutathione (GSSG), and increased SOD levels. In the liver, the levels of GSH were similarly increased, however, paradoxically, the levels of lipid peroxidation were lower than control. Importantly, the magnitude of the changes in these markers never approached the level of 2-fold and most were less then 50%.
A follow up study by these investigators (Gómez et al., 2005) utilized a similar paradigm of 7 mg/kg/day i.p. injections of aluminium lactate into rats for 11 weeks (5 injections per week), focusing on oxidative markers in the hippocampus. The most robust evidence of general toxicity included 30% reductions in body weight. The levels of aluminium in hippocampus increased ~5-fold, to 22 μg/g. While this study reported a similar elevation in TBARS (~ 2-fold), increases in GSSG were not detected. Slight elevations (~50%) in mitochondrial superoxide dismutase (SOD) mRNA, an important antioxidant enzyme that is induced by oxidative stress, were noted.
Platt et al. (2001) examined the impact of aluminium on CNS integrity by direct intracerebroventricular injection, via cannula, of 5.4 μg of aluminium chloride for 5 consecutive days; followed by either 7 days or 6 weeks of no treatment before sacrifice. Interpretation of the study is somewhat confounded by damage to the brain resulting from instillation of the cannula and from disruption of the BBB, however several findings were revealing. Aluminium was found to distribute readily along white matter tracts and resulted in activation of both astrocytes and microglia at sites distal to the injection site. Although not a mimic of chronic exposure from drinking water or food sources, these data suggest that acute exposure of neural tissues to aluminium salts elicits responses indicative of neurotoxicity. Overall, however, the reported increases in markers of oxidative stress were of a relatively low magnitude.
In another example of acute toxicity, Sreekumaran et al. (2003) examined several parameters of neuronal morphology following a one-time injection of 8 mg/kg aluminium chloride into CSF, via the cisterna magna of rats. Following a 30 day survival, animals were sacrificed and tissues were prepared for Golgi impregnation to reveal dendritic and axonal structure. Significant reductions (averaging 30 to 40%) in axonal length and dendritic branching were noted in the treated group.
Yang et al. (2004) reported evidence of apoptotic cell death in the CNS of rats given a single injection of aluminium maltolate (100 μl of 500 μM solution). In a small number of animals (n = 4), 5 days after injection, TUNEL positive cells were detected in hippocampus along with biochemical evidence of DNA fragmentation. Biochemical evidence of caspase activation (caspase 3 and 12) was also reported. The study did not identify which type of cell(s) in the hippocampus were affected.
Dramatic evidence of aluminium toxicity in retina of rats was reported by Lu et al. (2002). Chronic i.p. injection of 12 mg of aluminium chloride for 16 weeks resulted in severe atrophy of the retina with losses of photoreceptors. Interestingly, in this model, the location of accumulated aluminium mimicked that seen in DAE patients. Aluminium was concentrated in cytoplasmic granules that resemble lysosomes. There was no report of neurofibrillary pathology or discussion of mechanisms of cell death.
Miu et al. (2003) studied rats given i.p. injections of aluminium gluconate 85 ug/100 g body weight 3 times a week for 6 months. At sacrifice, the authors showed evidence of reductions in neuronal density in the hippocampus, intracellular accumulations of aluminium in dense granules, and thickening of meningeal blood vessels. Serum levels of aluminium were measured at 3 intervals, recording levels of 86.8, 38.9, and 69.7 μg/100 mL of serum (mean values for n=12). These levels were roughly 5 to 40-fold higher than controls. The average level of aluminium in human serum ranges from 1-2 μg/L. Hence the levels in these rats were about 3 to 8-fold the normal human level.
In a parallel study, using the same method of dosing and concentration of aluminium gluconate for 12 weeks, Miu et al. (2004) reported finding similar lesions in addition to changes in myelin structure and evidence of mitochondrial swelling in hippocampal neurons. None of these pathologies was quantified however.
A key issue in the foregoing studies, however, relates to the speciation of aluminium and mechanisms of uptake by neural cells. The pathologic distribution of aluminium in DAE patients, and in some of the animal studies described above, are consistent with Tf-receptor-mediated endocytosis. Free-flow endocytosis of aluminium citrate could produce a similar pattern. Indeed, the pattern of aluminium accumulation in patients suffering from DAE, membrane delineated lysosomal-like structures, is consistent with an endocytic mechanism of uptake. It is unclear as to whether acute exposure to high levels of aluminium salts by direct injection into CNS reasonably simulates the exposures which result from long-term low level intakes via the oral route. In some of the acute exposure studies, aspects of human neurogenerative disease are produced. However, as described above, pathologic analyses of DAE patients do not reveal the pathologies found in AD and other neurodegenerative diseases, including neurofibrillary pathology, symptoms of motor neuron disease (as occurs in rabbits), or senile plaques. Overall, the connection between aluminium exposure and neuropathologic features of human disease is not particularly strong, though some resports of positive associations continue to foster debate.
Rodent models of aluminium toxicity by oral exposure
Aluminium has been implicated in the aetiology of ALS in Kii peninsula of Japan and the islands of Guam. In these environments, the levels of aluminium and manganese in drinking water are high while the levels of calcium and magnesium are low (for review see Garruto, 1991). Kihira et al. (2002) reported that mice feed diets high in aluminium (1.56 g/100 g) and low in Ca/Mg (50% reduction from control diet) developed pathologic features of human disease (Kihira et al., 2002), including neuronal accumulation of tau immunoreactivity in a pattern resembling pre-tangles of AD. Reductions in the density of cortical neurons were also noted in mice on low calcium/magnesium diets and in mice on the aluminium + low Ca/Mg diet. Mice given high doses of aluminium alone showed no evidence of neuronal loss. No symptoms of motor neuron disease were noted and animals exhibited near normal lifespans. Lowering Ca and Mg in diet induced a greater number of abnormalities in general health and appearance than Al laced diets (with or without manipulation of calcium/magnesium levels). Intracellular accumulations of aluminium were noted. Although it is possible that the levels of other minerals in the diet could influence the toxicity of aluminium, the most informative outcome of this study was that chronically high doses of aluminium did not result in obvious neurodegeneration, profound neuropathology, or clinical symptoms relevant to motor neuron disease. The average 25g mouse consumes about 5 g of food per day (formulated to contain 15.60 mg/g of aluminium). Therefore, the estimated consumption of aluminium by these animals would be about 3 g/kg/day, a dose nearly unattainable in humans. Despite this extremely high dose for a prolonged period, these animals developed relatively few phenotypes related to human neurological disease.
In a study of chronic exposure to aluminium in diet (rats fed 32 mg aluminium sulphate per day for 5 weeks), no evidence of apoptotic cells (TUNEL positive) was noted in cerebral cortex (Rodella et al., 2001). Similarly, the relative density of neurons in cortex was not obviously diminished. The authors did report that the density of NADPH-diaphorase positive neurons in cortex was diminished by 50%, but a better validation of such a reduction could be made by unbiased stereological assessments of these neurons (Gundersen et al., 1988).
Swegert et al. (1999) examined the effects of aluminium exposure on oxidative metabolism in rats given diets supplemented with aluminium chloride at an estimated dose of 20 mg/kg/day, which is approximately 20 times higher than the maximum amount normally consumed by humans from food. Animals were treated for 90-120 days at which time tissues were harvested and mitochondria were isolated for further study. Thirty to forty percent reductions in mitochondrial respiration rates were noted in brain mitochondria, with a paradoxical increase (~2-fold) in respiration rates in heart.
Flora et al. (2003) reported similar changes in oxidative markers in rats given aluminium nitrate in water at a concentration of 0.2% (2 g/l) for 8 months. Levels of aluminium in blood increased from ~3 μg/dL to >20 μg/dL. In brain, the levels increased from ~8 μg/dL to ~16 μg/dL. Indices of lipid peroxidation (TBARS) and the levels of GSSG were increased in brain. Again, however, the magnitude of changes in these measures was less than 2-fold with only very modest increases in the levels of GSSG.
Golub et al. (2000) fed mice defined diets containing 1000 μg/g of aluminium lactate from conception to sacrifice at 24 months of age (~ dose 100 mg/kg/day). Several parameters were analyzed (see below for discussion of cognitive behaviour), but relevant to this section was an absence of evidence for oxidative stress (no increase in TBARS). Surprisingly, however, despite the high dose of aluminium in the diet, the levels of accumulated aluminium in the brain were not significantly greater than that of controls.
Collectively, these studies establish that dietary aluminium intake can lead to accumulation of aluminium (speciation uncertain) in the brain of rats and mice. Modest increases in measures of oxidative stress have been noted, but evidence of significant neuropathology related to aluminium intake was not consistently noted. The level of sustained oxidative injury that is required to produce neuropsychological abnormalities is unknown, few of the oxidative markers measured in the foregoing studies increased as much as 2-fold.
Behavioural studies of laboratory animals exposed to aluminium
Before reviewing the literature concerning the cognitive behaviour of laboratory animals exposed to aluminium, it is worth noting that there are detailed longitudinal studies of humans exposed to elevated levels of aluminium in the workplace. Buchta et al. (2003) examined a battery of neuropsychological and motor skills in a large cohort of auto manufacturing workers. They recorded average urine levels of aluminium of 70 μg/L, which compares with 1-2 μg/L in most individuals (Buchta et al., 2003). Individuals who had experienced at least 6 years of exposure were selected for analysis and their results were compared with those of co-workers of similar age, gender, and education who worked in other areas of manufacturing. The only measure by which the workers exposed to aluminium could be distinguished was a small reduction in reaction time (speed to respond to a question or perform a motor task). In all other cognitive measures including intelligence quotient (IQ), verbal intelligence, and the European Neurobehavioral Evaluation System, workers exposed to aluminium were no different from control populations (Buchta et al., 2003). Thus in humans, exposure to significant levels of aluminium does not lead to robust changes in cognitive function. However, the above study did not assess the levels of aluminium in blood, which would indicate absorption. Though there are clearly too few human studies, much of the data from studies in adult animals also suggests aluminium exposure does not lead to significant reductions in cognitive function.
As mentioned above, a lifelong exposure of Swiss Webster and C57BL/6J mice to high doses of aluminium (~100 mg/kg/day in feed) was utilized as a paradigm by Golub et al. (2000). With the caveat that brain levels of aluminium were not elevated in the treated mice, suggesting poor absorption, there were little or no deleterious effects of the aluminium-laced diet on several behavioural measures, including grip strength (slight reduction ~10%), temperature sensitivity (slight increase), and spatial reference memory. In the latter task, data from the C57Bl/6J mice are most informative where no adverse impact on acquisition or retention of memory was noted. A prior study by Golub et al. (1995) fed Swiss Webster mice food supplemented with either 500 μg or 1000 μg/g aluminium lactate (calculated dose 200 mg/kg/day) from conception to sacrifice at 150-170 days of age. In several measures of cognitive function, mice fed the aluminium laced diet performed as well as controls. However, 10 to 15% reductions in fore and hindlimb grip strength were noted.
Similarly, although Esparza et al. (2003) reported increased levels of several oxidative markers in the brains of rats given high doses of aluminium (5 and 10 mg/kg/day by i.p. injection), no changes in performance in a passive avoidance task were noted in animals treated with either dose. The classic passive avoidance task involves an electric shock deterrent in which rats are required to remember that the more preferred location (a dark enclosure next to the lighted open space) is associated with shock. Retention of the memory is usually tested 24 hr after conditioning. Hence the task is a measure of memory function.
In contrast to the results of the study by Esparza et al. (2003), Zhang et al. (2003) reported dramatic reductions in performance in passive avoidance in rats exposed to aluminium through drinking water. In the latter paradigm, aluminium chloride was provided through drinking water at a concentration of 3 mg/mL for 90 days. Serum levels of aluminium were not reported. Interestingly, in this study, an extract of Dispsacus asper (a herbal medicine) and vitamin E were shown to alleviate the memory deficits. The authors suggested that the anti-inflammatory and/or anti-oxidant properties of these drugs contributed to the improvements.
Domingo et al. (1996) examined passive avoidance in rats provided drinking water containing aluminium nitrate at concentrations that would equate to doses predicted to be 50 and 100 mg/kg/day for 6.5 months. No effects of the high aluminium exposure on measures of spontaneous motor activity or learning in passive avoidance tasks were noted.
Struys-Ponsor et al. (1997) studied rats given i.p. injections of 667 μg of aluminium gluconate 3 times per week for 60 days prior to assessment of spatial memory in a radial water maze task. Although a slowing of reaction time was noted, there were no statistically significant deficits in the ability of the aluminium-injected animals to perform the task.
Two studies by Miu et al. (2003; 2004), the pathological findings of which are described above, also assessed neuropsychiatric parameters. The 2003 work reported slight reductions in performance in a passive-avoidance memory task and in a spatial reference memory task. The latter work (2004) reported changes in behaviour in open fields which were interpreted as reductions in spontaneous activity and emotional responses.
Overall, the data on neuropsychological measures in rodents given high doses of aluminium by oral routes are not suggestive of profound toxicity. However, none of these animal studies is able to reproduce life-time exposures that could occur over the life-span of humans. It is clear that humans with compromised renal function develop neuropsychological symptoms upon exposure to elevated levels of aluminium. However, from the study of individuals exposed occupationally to aluminium fumes, humans with normal kidney function seem to tolerate high levels of exposure relatively well (see above). The degree to which the chemical form of aluminium, the route of exposure, and the age/health of the individual could modulate the neurotoxicity of aluminium is uncertain and has not been extensively modeled in animals.
Effects on Bone
The bone constitutes a primary site for the deposition of aluminium (Mahieu et al., 2004) (see also Toxicokinetics, Distribution (Including Compartmentalization), Animal Studies, Bone). Elevated aluminium levels in humans, primarily in individuals with impaired renal function, have been associated with several bone disorders including osteomalacia (excess unmineralized osteoid) and aplastic bone disease which is characterized by normal or decreased osteoid (Firling et al., 1999). The mechanism by which aluminium exerts its effects on bone tissue has not been fully elucidated (Cointry et al., 2005). Experimental evidence in a number of different animal models has led to a variety of proposed ways in which aluminium might influence new bone development. It has been suggested that aluminium may directly interfere with osteoblast activity thereby influencing the production or mineralization of osteoid (Firling et al., 1999). Bone formation may be impaired due to aluminium induced reductions in the total number of osteoblasts (Sedman et al., 1987). Direct physiochemical inhibition of mineralization sites has also been proposed as a potential mechanism (Firling et al., 1999). Aluminium-induced alterations in the PTH-calcium axis have also been extensively investigated with respect to aluminium-induced bone toxicity (Mahieu et al., 2004). One of the functions of the PTH is to stimulate bone resorption by increasing osteoblast activity (Quarles, 1990). It has been proposed that aluminium impairs the secretion of this hormone from parathyroid glands (Morrisey et al., 1983). Numerous studies, using a variety of animal models, have been conducted to investigate the effects of aluminium on bone. In interpreting the results of these studies, it is important to consider the difference in bone remodelling physiology between species. It is thought that larger animals such as the dog and pig approximate the bone physiology of humans more closely than rats and mice (Quarles, 1990). The route of aluminium administration and the duration of the study period may also have had significant impacts on the overall results of these studies. It should also be noted that the use of large doses of aluminium in some of these experimental studies may have resulted in a generalized toxicity to the animals, which could complicate the interpretation of aluminium-induced bone toxicity endpoints (Quarles et al., 1988). Some of the in vitro and animal studies investigating the effects of aluminium and bone have been reviewed and are summarized below.
PTH is considered to enhance osteoblast-directed osteoclast activity, and it has been proposed that aluminium may inhibit the production or secretion of this hormone (Jeffery et al., 1996). Morrissey et al. (1983) used dispersed bovine parathyroid cells to determine if aluminium directly affects PTH secretion. Digested bovine parathyroid glands were placed in media containing varying concentrations of aluminium, ranging from 0.5 to 2.0 mM. The incubations were terminated after 2 hr and the amount of hormone secreted into the medium was determined by radioimmunoassay. The secretion of PTH decreased with increasing amounts of aluminium. Hormone secretion decreased by an average of 68% in cells incubated with 2.0 mM aluminium compared to the cells incubated in the absence of aluminium. To examine if there was an irreversible toxic effect of aluminium with respect to PTH secretion, the cells were incubated for 1 or 6 hr with 2.0 mM aluminium, washed with low calcium buffer, and re-incubated in media without aluminium. Hormone secretion appeared to be restored and was comparable to that of cells which had not been incubated with aluminium. Cells were also incubated with radio-labelled leucine to examine the effect of aluminium on the biosynthesis of proparathyroid hormone, PTH, and parathyroid secretory protein. Examination of the incorporation of this radio-labelled amino acid into these proteins revealed that the biosynthesis of these compounds was not affected by aluminium incubation. Therefore, the results of this study suggest that aluminium directly affects the secretion of protein from parathyroid cells.
Ellis et al. (1979) investigated the effects of aluminium on bone toxicity in a group of 20 rats given daily i.p. injections of aluminium chloride for periods of up to three months. Sixteen rats received daily i.p. injections of 0.27 mg Al/day (as aluminium chloride), increasing gradually to a dose of up to 2.7 mg Al/day. The periods for the injections ranged from 48 to 85 days and, in 5 animals, no further injections were given after 63 or 84 days of treatment until sacrifice, 27 or 49 days later. The total dose of aluminium ranged from 38 mg to 109 mg. Four controls were i.p. injected with saline. The whole femur bone aluminium content was higher in the 16 rats given aluminium chloride (176 ± 8.2 ppm/ash compared with the controls (15.4 ± 4.7 appm). A mineralization defect of bone was detected in the rats after 53 days of aluminium treatment, and this increased in severity as the injections were continued. This was marked by an excess of osteoid on the surface of normally mineralized cartilage at the usual site of endochondral ossification. The excess osteoid was typical of osteomalacia with abnormally wide seams and no calcification front. Endochondral ossification was restored to normal, but osteomalacia persisted for up to 49 days after the cessation of treatment.
Chan et al. (1983) investigated the effects of i.p. aluminium chloride (1.5 mg/kg/day) for a duration of 9 weeks in normal (n=16) and uraemic (n=23) rats. Eight rats in the nonuraemic group and 10 rats in the uraemic group received the aluminium treatment, while no injections were given to the remaining control animals. At the end of the treatment period, the rats were euthanized and tissue aluminium, serum vitamin D metabolites, and quantitative bone histology were measured. Bone aluminium concentrations were higher in uraemic rats (121 ± 27 mg/kg) than in normal rats (47 ± 4 mg/kg), and liver aluminium values were higher in the normal group (175 ± 47 mg/kg) than in the uraemic rats (100 ± 36 mg/kg). Aluminium did not appear to have an effect on the levels of any vitamin D metabolites; however, serum concentrations of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D were reduced as a direct result of uraemia. The nonuraemic aluminium treated animals did not exhibit any significant skeletal changes as compared to the controls. Marrow fibrosis and osteomalacia developed in some of the uraemic, non-aluminium treated animals. However, osteomalacia as defined by (1) an increase in osteoid area (29 ± 13% uraemia + aluminium vs. 3 ± 6% uraemia, no aluminium), (2) an increase in osteoid surface (42 ± 16% vs. 7 ± 11%), and (3) an abnormal pattern of tetracycline uptake at the calcification front, was more severe in uraemic animals treated with aluminium than in untreated uraemic animals.
Robertson et al. (1983) conducted a study to investigate the effects of aluminium on bone histology and PTH levels, and to determine if chronic renal failure accentuates aluminium toxicity. Male Wistar rats were divided into 5 groups. The first group (n=5) received a low dose of aluminium (i.p. injection 0.1 mg aluminium as aluminium chloride 5days/week), the second group (n=5) received a high dose of aluminium (i.p. injection of 1.0 mg aluminium as aluminium chloride 5 days/week); the control group (n=4) was administered an i.p injection of an equal volume of diluent over the same injection schedule. One group (n=6) of rats underwent partial nephrectomy and received an i.p injection of diluent, and another group (n=5) underwent partial nephrectomy and received an i.p. injection of 1.0 mg aluminium as aluminium chloride 5 days/week. The treatment lasted for 120 days for the control and low dose aluminium group, but for a shorter period (between 90-100 days) for the other three groups due to the need for early sacrifice as a result of high attrition in these groups. The trabecular bone of the ischium and the iliac wing was obtained from each animal and examined histologically; the bone mineralization process was evaluated by double tetracycline labelling. There were no differences in bone parameters between the low dose aluminium group and the controls. In the other three groups, the relative osteoid volume (p < 0.02) and the osteoid seam width (p < 0.001) were significantly increased as compared to the controls. These parameters are indicators of osteomalacia. The number of osteoclasts/mm2 increased in nephrectomized rats not exposed to aluminium (p < 0.02) and decreased (p < 0.05) in rats with normal renal function exposed to high doses of aluminium. The number of osteoclasts/mm2 was not significantly different from that of the control level for the aluminium treated nephrectomized rats. Animals with normal renal function given the high dose were the only group to exhibit a decrease in PTH level, which may explain the reduced osteoclast numbers in this group. However, the increase in osteoclasts/mm2, as compared to controls (8.13 ± 2.92 vs. 3.93 ± 100 osteoclasts/mm2), in the non-exposed renal failure group, compared to the reduction of osteoclasts in the exposed renal failure group (2.34 ± 1.38 osteoclasts/mm2 vs. 3.93 ± 100 osteoclasts/mm2), indicates that aluminium may have a direct toxic effect on osteoclastic activity.
Cortical bone growth was measured in rats given aluminium to study the early effects of aluminium on bone (Goodman, 1984). Thirty weanling male rats were assigned to one of three groups: control (n=10), experimental (n=10), or basal (n=10). Rats in the experimental group were given i.p. injections of aluminium chloride (2 mg aluminium, 5 days per week); animals in the control group received an injection of saline vehicle at the same frequency, and the basal animals did not receive any treatment. The treatment period lasted for 44 days. Bone growth was assessed over two consecutive periods of 28 and 16 days in the control and experimental rats, using tetracycline labelling of bone. Rats in the basal group were sacrificed on the first day of the experimental period. Histological measurements in sections of bone obtained from the basal group were interpreted to represent the status of the bone at the beginning of the bone-labelling period in rats from the control and experimental groups. Bone (0.017 ± 0.004 mm3/day) and matrix formation (0.017 ± 0.004mm3/day) in the experimental group remained at control levels (bone formation: 0.020 ± 0.004, matrix formation 0.02 ± 0.004 mm3/day) during the first period of assessment (28 days after treatment initiation); however, both these measurements were significantly lower (p <0.01) than control values at the end of the entire 44 day study (bone formation: 0.014 ± 0.003 mm3/day vs. 0.022 ± 0.003mm3/day, matrix formation:0.014 ± 0.003mm3/day vs. 0.022 ± 0.003mm3/day). Bone and matrix apposition at the periosteum in aluminium treated animals was significantly reduced (p < 0.0001) from control levels at the end of the 44 day treatment period, but was not significantly different at the assessment following 28 days of treatment. Aluminium treatment did not induce a state of osteomalacia as no significant effect on the osteoid width or the mineralization front width was apparent. The results of this study indicate that aluminium reduces bone and matrix formation early in the course of aluminium exposure, prior to the development of histologically apparent osteomalacia. Aluminium may affect matrix synthesis by reducing the total number of active osteoblasts or diminishing the cellular activity of individual osteoblasts.
Goodman et al. (1984a) examined the effect of short-term (4 week exposure) aluminium administration on bone growth and histology, and evaluated the role of renal insufficiency in mediating the skeletal effects of aluminium. Thirty rats underwent partial nephrectomy and were assigned to one of three groups: control, aluminium-treated, or basal control. Thirty additional rats with intact renal function were divided into the same treatment assignments. Aluminium treated rats received i.p. injections of aluminium chloride (AlCl3) in saline 5 days/wk for 4 weeks; the aluminium dose was 2 mg/day. Control rats received injections of saline only, according to the same treatment schedule. Bone growth, bone formation, mineralization, and resorption were measured using double tetracycline labelling of bone. Total bone and matrix formation and periosteal bone and matrix formation were reduced in both nephrectomized and normal renal function rats treated with aluminium as compared to the respective controls. Periosteal bone and matrix formation were similarly reduced in both groups. There was no difference in bone parameters between the control rats of the nephrectomized group as compared to the normal renal function control rats; however, total bone, total matrix, periosteal bone, and periosteal matrix formations were all less in the nephrectomized aluminium treated rats as compared to the non-nephrectomized aluminium treated rats. Resorption surface was greater in both aluminium- (1.70 ± 0.41mm vs. 1.33 ± 0.34 mm) and nephrectomized-aluminium-treated (1.87 ± 0.6 mm vs. 165 ± 0.43 mm) rats compared to the respective controls, and resorptive activity at the endosteum was greater (p < 0.05) in the nephrectomized aluminium treated group (12.2 ± 6.3 μm/d) than in the controls (7.9 ± 4.9 μm/d). Serum calcium and phosphorus concentrations were similar in aluminium-treated and control animals, suggesting that PTH secretion was not substantially affected by aluminium administration. Osteomalacia was not detected, but it should be considered that the duration of this study may not have been of sufficient length for this condition to develop. The results of this study suggest that aluminium exerts is toxic effects on bone by acting directly to reduce new bone and matrix synthesis. In addition, it appears that aluminium may act to increase bone resorption. The enhanced effect of aluminium in nephrectomized rats may be a result of increased aluminium accumulation due to an impaired ability to excrete the metal; however, aluminium in bone was not quantified in this study.
In order to investigate the effects of aluminium on the vitamin D-dependent mineralization process, aluminium chloride (1 mg/kg) was administered i.v. 3 times per week for 3 weeks to normal (n=5) and vitamin D-deficient (n=5) beagle puppies (Quarles et al., 1985). Vitamin deficiency was induced in the 5 dogs by providing a diet deficient in vitamin D and calcium for a period of 15 weeks before treatment initiation. Bone biopsies and plasma were obtained before and after the 3 week treatment period in each group. In the next phase of this study, aluminium chloride administration was continued at a lower dose (1 mg/kg twice a week) and both groups received the diet fortified with calcium and vitamin D. This phase of the study lasted for 11 weeks. The vitamin D deficient dogs displayed biochemical and bone biopsy evidence of osteomalacia before administration of aluminium. Plasma phosphorus, PTH, and 25-hydroxyvitamin D concentrations did not appear to be affected by the 3 weeks of aluminium administration in either group. However, bone aluminium content increased to a greater extent in the vitamin D-deficient dogs (390 ± 24.3 μg/g) than in the normal dogs (73.6 ± 10.6 μg/g). After 3 weeks of aluminium treatment, the bone histology in both groups revealed changes consistent only with aging. Provision of a calcium/vitamin D replete diet to the deficient dogs, and reduction of aluminium dosage for 11 weeks resulted normalization of their plasma biochemistry and healing of the osteomalacia. The bone aluminium content of the non-vitamin D deficient dogs increased during the additional 11 weeks of aluminium exposure to 151.0 ± 11.3 μg/g as compared to a bone aluminium content of 73.6 ± 10.6 μg/g after 3weeks of exposure. In contrast, the bone aluminium content in the vitamin D deficient dogs decreased from 390 ± 24.3 μg/g to 173.5 ± 5.6 μg/g at the end of the additional 11 weeks of exposure. Histochemical staining for bone aluminium revealed no apparent aluminium in the normal group and, in the vitamin-D deficient group, there was identifiable aluminium at the mineralization fronts after the 3 weeks of treatment. At the end of the 11 weeks of additional exposure staining for aluminium was detectable only in the cement lines, indicating that mineralization occurred over the previous sites of aluminium deposition. The results of this investigation indicate that aluminium accumulates preferentially in pre-existent osteomalacic bone and localizes at the calcification front (osteoid-bone interface). The presence of aluminium at the calcification front did not impair vitamin D-dependent mineralization as remineralization occurred in the bones of the osteomalacic pups following vitamin-D repletion. Osteomalacia did not occur in the normal pups; however, this does not eliminate the possibility that aluminium administration at higher doses or for prolonged periods might cause bone toxicity in these animals. Therefore, although aluminium may have the potential to cause osteomalacia, its presence at mineralization fronts does not appear to be the mechanism through which this occurs.
Alfrey et al. (1985) studied aluminium compartmentalization in rats with an induced state of uraemia or hypoparathyroidism. Seventy four male rats were divided into two main study groups. The first group consisted of 10 animals that underwent selective PTX, 10 animals were rendered uraemic by uninephrectomy, 11 animals underwent both nephroctomy and PTX, and 8 animals served as controls. 1,25(OH)2D3 was administered to normalize serum calcium levels. All animals received 1.5 mg/kg aluminium (as AlCl3) by i.p. injection 5 days/wk for 79 days. There was a significantly greater (p < 0.001) trabecular bone osteoid area (percent total bone area) in the PTX uraemic group than in the uraemic group (45 ± 9.7% compared to 13.4 ± 10.6%). Plasma calcium levels were significantly higher in the uraemic PTX group (10.7 ± 0.9 mg/dL) and lower in the control PTX group (9.0 ± 0.5 mg/L) than in their respective controls (9.9 ± 0.6 g/L: uraemic group; 9.8 ± 0.3 mg/L: control group), and phosphorus levels were significantly higher in both the PTX uraemic group (9.3 ± 1.5 mg/L) and PTX control group (8.1 ± 0.6mg/L) as compared to the controls (5.9 ± 0.3mg/L). The second study group was comprised of 35 uraemic rats; 8 of these rats had previously undergone selective PTX and were given 1,25(OH)2D3. The uraemic PTX animals and 8 of the uaremic animals received 1.5 mg AlCl3/kg by i.p. injection 5 days/week for 35 days, and were killed at the end of this treatment period. Aluminium injections were discontinued in the remaining animals, and nine underwent selective PTX at this time. These animals were followed for an additional 30 days. Bone aluminium levels in the animals killed after 35 days were lower (p < 0.05) in the PTX uraemic group than in the uraemic control group (37 ± 7.6 and 47 ± 7.6 mg/kg, respectively). The bone aluminium levels in the uraemic group that underwent PTX after aluminium loading (53 ± 8 mg/kg) and in the uraemic group (48 ± 5.7 mg/kg) 30 days after aluminium discontinuation were not significantly different. The results of this study suggest that PTX affects the compartmentalization of aluminium in bone, especially in animals in a uraemic state. It also appears that PTX may intensify aluminium-induced osteomalacia as the PTX group had significantly greater bone osteoid area than the uraemic group of rats.
Galceran et al. (1987) conducted a study to further characterize the mechanism of aluminium induced osteomalacia. One group of dogs (n=7) was treated i.v. with 0.75 mg aluminium, 5 days a week for 3 months; 7 additional dogs served as controls. At the end of the treatment period, the dogs were killed, and the tibiae were obtained and perfused in vitro. PTH and methylxanthine (an inhibitor of phosphodiesterase) were added to the perfusate. The serum aluminium level was 20.4 ± 2.3 μg/L before treatment, and rose significantly to 206.2 ± 28.3 (p < 0.01) after aluminium administration. No significant difference in PTH levels was detected before and after aluminium treatment. Because PTH acts to stimulate cAMP release from bone, cAMP levels were measured in both groups before and after PTH administration to examine the effect of aluminium on this process. Although the basal cAMP secretion was the same in both groups of dogs, cAMP increased to a peak of 188.2 ± 30.6 pmol/min in the normal dogs vs. 113 ± 8.15 pmol/min in the aluminium treated dogs after PTH was added to the perfusate (p < 0.05). Examination of bone biopsies taken before and after aluminium administration revealed that the number of osteoblasts had decreased 8-fold (p < 0.01) following aluminium treatment. Aluminium treatment led to an increase in the percent total osteoid surface (44.3 ± 4.7% vs. 22.1 ± 4.1%), decreased mineral apposition rate (0.5 ± 0.4 μm/day vs. 1.3 ± 0.2 μm/day), and aluminium deposition at the mineralization front. Despite the decrease in osteoblast number, the histological features of the post aluminium treatment biopsies indicated that aluminium stimulated osteoblastic activity at some point during its administration. This was apparent from the presentation of new woven bone formation in two dogs, and a layer of newly deposited lamellar bone covering all trabecular surfaces in another. Aluminium also appeared to stimulate the activity of fibroblasts as indicated by the presence of extensive marrow fibrosis in five of the treated dogs. These data support the possibility that aluminium is capable of both stimulating and suppressing matrix synthesis at different times throughout the exposure period. Although the levels of PTH were similar in the aluminium exposed and control animals, the decreased generation of cAMP following the addition of PTH to the perfused bones of the aluminium treated group suggests that aluminium may cause bones to be resistant to the effects of PTH.
Sedman et al. (1987) examined the effects of i.v. aluminium injections for a duration of 8 weeks on various bone parameters in growing piglets. Four piglets were administered 1.5 mg AlCl3/kg/day parenterally, and 4 control piglets received daily injections of deionised water for the same period. Quantitative bone histology and measurements of bone formation were assessed at three skeletal sites (two in the proximal tibia and one in the distal femur) in both the experimental and control groups. Bone aluminium was significantly higher (p < 0.001) in experimental animals (241 ± 40 mg/kg) as compared to the controls (1.6 ± 0.9 mg/kg). Osteomalacia, as defined by histological criteria, was documented in all aluminium-treated animals. The rate of mineralized bone formation was also lower in the aluminium-treated group compared to that in the controls at all three sites. However, it was found that, at sites of continued osteoblastic activity, total osteoid production did not differ between the two groups. These results suggest that bone mineralization is inhibited by aluminium via a decrease in the number of active osteoblasts rather than by an inhibition of the calcification of osteoid.
Ott et al. (1987) conducted a study to investigate the development and reversibility of aluminium-induced bone toxicity in weanling and adult rats. Four groups of weanling rats and one group of adult rats were used in this study. Each group consisted of 18 rats. Half the rats in each group received daily i.p. injections 10mg Al/kg as aluminium chloride. The other half of the group was given normal saline solution. Weanling rats were sacrificed after 3, 6, or 9 weeks of treatment. The remaining group of weanling rats received injections for 9 weeks, and was allowed to recover for 3 weeks. The adult group received aluminium treatment for 9 weeks. The effects of aluminium on blood serum levels of various compounds, and on aluminium bone content, and rate of bone formation were assessed after intervals of 3, 6, and 9 weeks. The calcium, phosphate, creatinine, and PTH levels were similar in aluminium-treated rats and controls. Aluminium was detectable by histochemical stain after 6 weeks in the aluminium treated animals; however, other bone parameters did not differ significantly at this time between the treated animals and the controls. A decrease in bone formation (measured by tetracycline labelling) on trabecular and endosteal surfaces was apparent by 9 weeks in the aluminium exposed groups. The weanling rats had a bone formation rate of (0.15 ± 0.2 mm2/100 days vs. 0.46 ± 0.14 mm2/100 days) which was significantly lower (p < 0.02) than the rate in the controls, while the adult rats had a rate of (0.04 ± 0.06 mm2/100 days) vs. (0.23 ± 0.12 mm2/100 days) in the controls. One group of rats was allowed to recover for 3 weeks without any aluminium administration. The bone formation rate in the younger group was similar to that of the controls after 3 weeks of recovery. Adult rats showed signs of early osteomalacia as evidenced by an increase in the length (4.75 ± 2.3 vs. 2.15 ± 1.5% surface) and width (18.4 ± 9.7 vs. 4.8 ± 1.3 μm) of the trabecular osteoid as compared to the controls. In this study it appeared that aluminium administration led to decreased rates of bone formation in rats despite normal calcium and parathyroid levels, and normal renal function. It is possible that aluminium induced decreased bone formation by inhibiting osteoblast formation or activity.
Quarles et al. (1988) conducted a study to define the primary effects of aluminium on bone in the mammalian species, and to examine the dose/time-dependent actions of aluminium on bone. Two year-old beagles were assigned to one of three treatment regimens. The first group (n=6) received 0.75 mg Al/kg as aluminium chloride i.v. three times per week, the second group (n=6) received 1.20 mg Al/kg at the same dosing schedule, and the third group (n=6) received sodium chloride i.v. and served as controls. The treatment period lasted for 16 weeks. Transcortical bone biopsies were taken from each group after 8 weeks and 16 weeks. In both the low and high dose aluminium groups, serum aluminium levels were significantly elevated compared with those of controls, but calcium, or PTH levels were not altered by treatment. Bone biopsies taken at 8 weeks in the low dose group displayed characteristics of a low turnover state, as marked by a reduction of bone resorption (2.6 ± 0.63% vs. 4.5 ± 0.39%) and osteoblast–covered bone surfaces (2.02 ± 0.51% vs. 7.64 ± 1.86%) as compared to the controls. The mineralized bone formation rate was also found to be significantly decreased. Biopsies taken at week 16 of aluminium administration in the low dose group displayed evidence of de novo bone formation, as well as an increase of bone volume (38.9% ± 1.35 vs. 25.2% ± 2.56) and trabecular number (3.56/mm ± 0.23 vs. 2.88 ± 0.11) compared to the controls. The 16 week biopsies displayed a persistence of inactive osteoid marked by a diminished mineralization front in the low dose group compared to the controls (46.0 ± 4.2% vs. 71.9 ± 2.92%). The bone biopsies obtained from the high dose aluminium group at 8 weeks displayed changes similar to those exhibited after 16 weeks of the low dose treatment. De novo bone formation was evidenced by an increase in trabecular number as compared to the controls (3.41 ± 0.18/mm vs. 2.88 ± 0.11/mm) and increased bone volume (36.5 ± 2.38% vs. 25.2 ± 2.56%). Poorly mineralized woven bone accounted for a large proportion of the newly synthesized tissue, comprising 11.5 ± 4.6% of the bone volume. High dose treatment for 16 weeks further enhanced bone volume (50.4 ± 4.61%) and trabecular number (3.90 ± 0.5/mm). The woven osteoid volume at 16 weeks decreased to 2.43 ± 0.96% of the total bone volume, indicating that heterogeneous calcification of this tissue was more complete. The observation of histological changes similar to those observed in disorders characterized by low bone turnover in the low-dose aluminium group after 8 weeks, combined with the observation of new bone formation and stimulation of cellular activity after longer treatment, suggests that aluminium may exert both inhibitory and stimulatory effects on osteoblasts.
To further examine the influence of osteoblast function on aluminium-induced of de novo bone formation, Quarles et al. (1989) compared the effects of aluminium in TPTX beagles (n=4) with beagles which underwent thyroidectomy but had intact parathyroid glands (n=4). The animals underwent TPTX as a means to reduce osteoblast number and activity. The treatment procedure began 2 months after surgery, when sufficient time had elapsed to achieve a new steady state of bone remodelling activity. 1.25 mg AlCl3/kg was administered to both groups of animals by i.v. three times per week for 8 weeks. The TPTX animals received supplements of calcium carbonate and calcitrol in order to maintain normal plasma 1,25-dihydroxyvitamin D levels as well as normocalcemia. Both groups were administered thyroxine to sustain normal free thyroxine concentrations. Although both groups of animals received the same aluminium treatment, TPTX beagles exhibited a significantly higher (p < 0.05) serum aluminium level (2386.1 μg/L) as compared to the controls (1087.0 μg/L). Aluminium administration did not alter the plasma calcium, creatinine, or PTH from baseline levels in either group of animals. Bone biopsies taken from the control animals after 8 weeks of treatment displayed evidence of de novo bone formation as compared to baseline bone parameters. This was evidenced by an increased bone volume (47.0 ± 1.0 vs. 30.4 ± 09%) and trabucular number (4.1 ± 0.2 vs. 3.2 ± 0.2/mm). Deposition of poorly mineralized woven bone accounted for much of the enhanced bone volume (9.9 ± 2.7%). TPTX animals demonstrated significantly less evidence of bone formation. Bone volume (35.5 ± 1.7% vs. 27.7 ± 1.9% at baseline) and woven tissue volume (1.4 ± 0.8% vs. 9.85 ± 2.66%), as well trabecular number (3.3 ± 0.1/mm vs. 4.2 ± 0.2/mm) were significantly less than those of the aluminium treated non-TPTX controls. It appears that the diminished functional osteoblast pool in the TPTX beagles limited the ability of aluminium to stimulate neo-osteogenesis.
Bellows et al. (1999) examined the effects of aluminium on osteoprogenitor proliferation and differentiation, cell survival, and bone formation in long-term rat calvaria cell cultures. The cells obtained from foetal rats were incubated in medium with or without aluminium added. The aluminium treated cells were incubated at various concentrations ranging from 1 uM to 1 mM of aluminium for up to 19 days. The numbers of mineralized and unmineralized bone or osteoid nodules in each culture dish were quantified by in situ staining. Alkaline phosphatase activity, cell viability, and cytotoxicity were also determined. Nodule formation was significantly increased (p < 0.001) by 30-1000 μM aluminium incubation, in a dose dependent manner, at 11 days but not at 17 days. Control and aluminium-treated cultures appeared similar with respect to nodules and cell layers at day 13 of culture. However, at day 17 of culture, aluminium concentrations of 30 μM and above resulted in reduced cellularity and an increased fibrilar appearance of the matrix that had formed outside of, or adjacent to, nodules. Aluminium also increased alkaline phosphatase activity at all time points in a dose-dependent manner. Significantly fewer viable cells were present in the 300 μM aluminium-containing cultures after 13 and 17 days. The results of this experiment indicate that aluminium has a stimulatory effect upon existing osteoprogenitor cells leading to an accelerated rate of osteoblastic differentiation and nodule formation, while inhibiting nodule mineralization. The concentration of aluminium at which this effect occurred resulted in decreased cell viability and enhanced cytotoxicity.
The effect of aluminium administration on bone, in a model of osteopoenia induced by chronic acid overload in rats with normal renal function, was examined by Gomez-Alonso et al. (1999). Thirteen male rats with induced osteopoenia were divided into two groups. The first group (n=8) received 10 mg/kg of AlCl3 i.p. 5 times per week for 4 months, the second group (n=5) did not receive any aluminium treatment. At the end of the experiment, both tibias from each animal were extracted for the determination of aluminium content, in vitro bone densitometry, and histological analysis. Bone mineral density, measured at the proximal end of the tibia, was found to be significantly higher (<0.05) in the aluminium-treated group (0.292 ± 0.01 g/cm2) as compared to the controls (0.267 ± 0.02 g/cm2). Histomorphometric analysis showed a significant increase (p < 0.01) in bone volume (18.59 ± 5.66% vs. 7.69 ± 3.08%), cortical thickness (0.52 ± 0.06 mm vs. 0.36 ± 0.07 mm), osteoid thickness (14.05 ± 4.72 μm vs. 5.25 ± 090 μm), and osteoclast number (2.44 ± 0.52 N Oc/mm2 vs. 1.30 ± 0.01 N Oc/mm2) in the aluminium-treated group as compared to the controls. No significant differences in serum calcium, phosphorus, creatinine, hydroxyproline, or PTH were apparent between the aluminium-treated and control animals. There was no evidence of osteomalacia in the aluminium-treated rats. These findings indicate that aluminium is able to induce bone formation in rats with normal renal function even when osteopoenia is present.
Firling et al. (1999) examined the influence of aluminium citrate administration on tibia formation and calcification in the developing chick embryo. Tibia formation and mineralization were assessed by radiology, total bone calcium content, calcium incorporation rate, collagen synthesis rate, bone alkaline phosphatase activity, and serum levels of osteocalcin, procollagen carboxy-terminal propeptide, and PTH. The chick embryos derived from White Leghorn strain eggs were divided into three treatment groups (aluminium citrate, sodium citrate, sodium chloride), and were treated acutely or chronically. Acutely treated embryos received 100 μL of 60 mM aluminium citrate, 60 mM sodium citrate or 0.7% sodium chloride via injections into the air sac of the egg on day 8 of incubation. Chronically treated embryos received a daily 25 μL dose of the solutions beginning on day 8. The embryos were incubated for an additional 2 to 8 days following treatment. Radiographic analysis of the tibias and femurs of the embryos revealed that the mineralization of the aluminium treated animals was less dense and restricted to a shorter length of the mid-diaphysis as compared to the other two groups. The bone calcium content of embryos acutely or chronically administered aluminium and incubated for 10 to 12 days was significantly lower (p < 0.05) compared to that of the other treatment groups. The calcium content of tibias from embryos chronically treated with aluminium remained lower than the controls for 12 day and 16 day embryos while, by day 14, there were no significant differences in the total calcium content from acutely treated embryos compared to the controls. Significantly higher (p < 0.05) levels of alkaline phosphatase activity were found in the tibias collected from embryos chronically treated with aluminium incubated from 12 (2.16 units/tibia/hr vs. 1.32 units/tibia/hr) to 16 days (10.38 units/tibia/hr vs. 6.78 units/tibia/hr) as compared to the sodium chloride control group. Aluminium did not have a significant effect on the rate of tibia collagen, non-collagenous protein synthesis or serum levels of procollagen carboxy terminal propeptide, osteoclacin or PTH. The lack of change in these parameters suggests that embryonic osteoblast number and activity is not markedly diminished by aluminium exposure at these doses. The authors suggested that the observed under-mineralization of the tibias in the aluminium treated embryos may be a manifestation of the production of defective osteoid, an inhibited terminal maturation of osteoblasts, or physiochemical inhibition of mineralization nucleation sites.
Zafar et al. (2004) investigated the effect of chronic exposure to dietary aluminium on calcium absorption and calbindin concentrations in male weanling rats fed various levels of calcium and aluminium for 3 and 6 week periods. One group of rats (n=40) was fed a calcium adequate diet, three other groups of 40 animals each were fed a calcium-deficient diet with 0, 0.05 or 0.1% aluminium, as aluminium chloride. After 3 weeks, 20 rats per group were fasted overnight and 10 rats per group were given an oral dose of 25 mg calcium labelled with 6 μCi 45Ca by gavage. The other 10 rats in each group were given 6 μCi 45Ca as an interperitoneal injection. Rats were anaesthetized the following day, blood was collected and the femurs were obtained. The remaining animals (20 per group) were switched to a calcium adequate diet containing the same level of aluminium they had been fed previously, and were maintained on these new diets for another 3 weeks. No difference in 45Ca absorption was observed among the 4 groups at either 3 or 6 weeks. Aluminium supplementation at 0.05 and 0.1% of the diet reduced calbindin concentrations (compared to the group receiving a calcium deficient diet without aluminium). Total bone calcium decreased with aluminium supplementation. The bone calcium content was significantly different (p < 0.05) in the calcium deficient-no aluminium group, and in the calcium deficient groups supplemented with 0.05 and 0.1% aluminium, as compared to the calcium adequate group at both 3 and 6 weeks of treatment. In addition, the bone calcium content was significantly lower (p < 0.05) in the calcium deficient, 0.1% aluminium group as compared to all the other groups. Aluminium treatment reduced the breaking strength parameters of the bones from rats on the calcium-deficient diets. When the animals were switched to a calcium adequate diet for 3 weeks, there were no differences in the resistance to breaking due to aluminium intake. The results of this study suggest that dietary aluminium has detrimental effects on bone quality when calcium is deficient.
Cointry et al. (2005) analyzed the effects of aluminium accumulation on whole-bone behaviour in rats. Rats (n=14) received i.p. doses of 27mgAl/day, as aluminium hydroxide (Al(OH)3), for a period of 26 weeks. Fourteen control rats received a 20% glycerol/water solution at the same dosing schedule. At the end of the experimental period, the left tibiae was obtained from each animal for bone ash determination. Both femurs from each animal were also dissected and examined for the volumetric mineral density of the cortical bone region and for cross-sectional properties of the cortical bone region. Mechanical testing of the femurs was also conducted. The Young’s modulus of elasticity (a measure of stiffness) was calculated, as well as the stress of the cortical tissue at the yield point, which is an indicator of the tissue’s ability to support loads before any crack initiation. Aluminium concentration was significantly higher (p < 0.001) in the tibias of treated animals (103 ±18 μg/g) as compared to those of control animals (6 ±1 μg/g). The volumetric bone mineral density was significantly reduced in the treated animals with respect to the controls. Up to the yield point, the structural stiffness and strength of the bones did not differ between groups; however, an aluminium-induced impairment of the ability to resist loads beyond the yield point was observed. Treatment had a negative impact on the bending stiffness (Young’s elastic modulus) and the yield stress of cortical bone. These parameters were decreased by 18 and 13% respectively in treated animals as compared to those of controls (p< 0.05). The cortical second moment of inertia, which is a measure of the architectural efficiency of the cortical bone, was significantly improved in aluminium-treated rats (+10% change, p <0.01) as compared to that of the control group. This suggests that an adaptive response to aluminium treatment may have caused an improvement of the spatial distribution of the available cortical tissue resulting in an enhanced ability of the bone to resist anterior-posterior bending. The results of this study suggest that the apparent adaptive response of the bone to aluminium may have maintained normal stiffness and strength, but aluminium may have reduced the ability of the bone to resist loads beyond the yield point, or the ultimate strength of the bone.
Mineral Metabolism
The effects of aluminium on metabolic parameters in humans are not well understood. There have been relatively few studies of the effects of occupational exposure to aluminium on mineral metabolism. Ulfvarson & Wold (1977) examined the levels of trace metals in the blood of welders of aluminium and stainless steel. Although the authors did not report the levels of aluminium in blood, which would have been useful in gauging exposure, other studies of aluminium welders have noted relatively high levels of exposure (Buchta et al., 2003). In the study by Ulfvarson & Wold (1977), the levels of lead, strontium, rubidium, bromine, gallium, zinc, copper, cobalt, iron, manganese, chromium, calcium, potassium, sulphur, phosphorus, silicon, and magnesium in the blood of aluminium welders were not statistically different from the levels of controls. However, significant variability in the data was noted. Little is known regarding the effects of aluminium on other metabolic parameters in humans.
Most of the data on the effects of aluminium on trace metal, and general, metabolism originates from studies on animals given aluminium by various routes. There is clear evidence that aluminium can influence iron metabolism in the context of haematopoiesis (see Effects on Laboratory Mammals and In Vitro Test Systems, Effects on Haematopoieses). However, as described below, alterations in iron levels in solid organs have also been noted, but with inconsistent outcomes. Studies of the metabolism of trace metals have yielded similarly conflicting outcomes. There have also been reports of alterations in a variety of metabolic pathways in response to aluminium exposure.
Non-haem iron and trace metals
Golub et al. (1995) reported that mice fed diets containing as much as 1 mg Al/g for 150 days had small, but statistically significant, reductions in the levels of iron in spinal cord and liver. The calculated dose of aluminium exposure in this experiment was 200 mg/kg b.w./day, which is about 100-fold greater than dietary exposure in humans. Animals exposed to these levels of aluminium had no significant differences in body weight.
Ward et al. (2001) injected rats (i.p.) with aluminium gluconate (2 mg/kg b.w.) 3 times a week for 8 weeks and then examined iron levels in liver, kidney, heart, spleen, and brain. Two to three-fold increases in tissue iron levels were noted in liver, spleen, and brain. Blood levels of aluminium were not reported, but the levels of aluminium in liver and spleen were recorded as 100 ng/mg protein and 150 ng/mg protein, respectively, indicating substantial exposure.
Han et al. (2000) examined non-haem iron levels in kidney, liver, and intestine of chicks fed diets containing 0.15 and 0.3% (by weight) aluminium for 3 weeks. Chicks receiving the higher dose of aluminium were reported to have gained 28% less weight. Levels of iron in liver were reduced by 40%, with 30% reductions in iron levels in intestine.
Esparza et al. (2003) examined the levels of manganese, iron, and copper in the brain and liver of rats given i.p. injections of aluminium lactate (5 mg/kg b.w.) 5 days per week for 8 weeks. Rats exposed to this level of aluminium showed 5-fold increases in aluminium levels in liver (8.45 μg/g compared to 48.35 μg/g) with a 2-fold increase in cerebellum (5.49 μg/g compared to 12.51 μg/g). The cortex and hippocampus of brain showed trends towards increased aluminium accumulation, but were not statistically different from those of controls. In liver, manganese levels were reduced by 40% with no alterations in iron or copper levels. In cerebellum, there were no statistically significant changes in the levels of any of these metals while, in cortex and hippocampus, the levels of copper were reduced 25 to 40%. Cortex also showed 25% reductions in manganese. Animals injected with aluminium lactate gained 25% less weight than control animals injected with vehicle.
The levels of copper, zinc, and manganese have been measured in the serum of rabbits exposed to aluminium by s.c. injection of aluminium sulphate (600 μmol aluminium/kg b.w./day) five times per week for 3 weeks (Liu et al., 2005). The total aluminium exposure per rabbit for the entire study was 243 mg/kg b.w. Aluminium levels in serum increased from 0.25 μg/mL to 1.3 μg/mL after 21 days of treatment before levelling to 1.2 μg/mL by day 42 of treatment. There were no statistically significant changes in the levels of zinc, copper, or manganese in serum after either 21 days or 42 days of treatment. The authors reported weight loss in the aluminium-treated group but did not specify the degree of loss.
Fattoretti et al. (2004) examined the levels of copper, zinc, and manganese in the brains of rats exposed to aluminium through drinking water (2 g AlCl3 /L) for 6 months beginning at the age of 22 months. Serum levels of aluminium were not reported, making it difficult to assess the level of exposure. The authors sampled 3 domains of the brain; fore- and mid-brain together, pons and medulla together, and cerebellum. In cerebellum, there was no significant change in the levels of any metal. The authors reported accumulations of aluminium in fore- and mid-brain (94% increase) with increases in copper, zinc, and manganese (32, 41, and 50%, respectively). Pons and medulla showed 53% increases in aluminium accumulation with 46, 46, and 41% increases in copper, zinc, and manganese, respectively.
Sanchez et al. (1997) described an analysis of calcium, magnesium, manganese, zinc, copper, and iron levels in rats of 3 age groups exposed to aluminium in drinking water. The doses used were 50 and 100 mg/kg b.w./day of aluminium nitrate with added citrate at 355 and 710 mg/kg/day, respectively, to increase absorption of the metal. The ages at which studies were initiated were at 21 days, 8 months, and 16 months. Studies were terminated after 6.5 months of exposure and the levels of each trace element were measured in liver, bone, testes, spleen, kidney, and brain. The authors did not report the levels of aluminium in serum or bone, so it is difficult to assess the relative exposures of the animals. The amounts of aluminium in the water were roughly 10,000 times the average exposure level of humans and the addition of citrate would likely have increased absorption. Under these conditions, for each trace element, there were statistically significant effects of aluminium for multiple tissues; however, there were not always dose-dependent responses and the response of young vs. older animals sometimes differed. Among the most striking changes were the 25 to 50% reductions in calcium levels in kidney and brain of the mid and old age groups given the highest dose of aluminium. In young animals given the highest dose, however, the levels of calcium in these organs were ~2-fold higher than in those of controls. The testes of older animals also showed 2 to 3-fold increases in calcium levels. The data on copper levels showed significant fluctuations among age and treatment groups. The most robust finding was that, in young animals exposed to both doses of aluminium, the level of copper was reduced 30 to 50% in both kidney and brain. However, in older animals there were no changes in these organs. Magnesium, manganese, iron, and zinc levels fluctuated less among age and treatment groups in the various tissues, and there was less definitive evidence that aluminium caused consistent changes the amount of these elements in the tissues examined. There was evidence of a robust and consistent effect on levels of manganese in spleen, where, in all age groups, the highest dose of aluminium correlated with the highest tissue levels of manganese. There was also evidence of an effect on iron levels in spleen of young animals given the highest dose (30% increase in iron) and in kidney of older animals (25% reduction).
The authors also examined urinary excretion of trace metals at 2 time points within the treatment (at 3 and 6.5 months). The most robust and consistent changes in excretion were noted for zinc with a 3-fold reduction in excreted zinc at both doses in middle-aged group. In all other groups and for all other elements, the changes were either far less robust or inconsistent across age and treatment groups.
Yasui & Ota (1998) examined the levels of magnesium and calcium in serum, spinal cord, and bone in rats fed diets low in calcium (3 mg/100g diet) and high in aluminium (194 mg/100g diet) as aluminium lactate. Control diets contained 1250 mg/100g diet calcium and 10 mg/100g diet aluminium. After 60 days of exposure, the levels of aluminium in serum increased from 0.25 μg/dL to 1.25 μg/dL. Control diet low in calcium had no effect on aluminium levels and only slightly lowered serum levels of calcium. By contrast, there was a 2-fold reduction in serum calcium levels in animals exposed to both low calcium and high aluminium. None of the diets affected serum levels of magnesium. In spinal cord, the combined low calcium/high aluminium diet led to very modest reductions in the levels of magnesium (~10% reduction). However, in lumbar vertebra, magnesium levels were reduced by 25% on the combined diet. Diets low in calcium had no effect in either tissue. The authors did not comment on whether these diets affected weight gain.
In a study of much shorter duration (18 days) in which lower doses of aluminium were used (~270 μg/g of food), Greger et al. (1985) examined the levels of phosphorus, calcium, magnesium, iron, manganese, zinc, and copper in bone, kidney, and liver of rats exposed to aluminium in diet. Two food formulations provided trace metals at levels that were at the minimum requirement and at 2-3 times the minimum requirement. The chemical form of aluminium was varied, using aluminium palmitate, aluminium lactate, aluminium phosphate, and aluminium hydroxide. No significant differences in levels of aluminium accumulation in bone were noted among the different chemical forms of exposure (control 1.9 μg/g; treated 13-15 μg/g). The authors found no significant changes in the levels of any of the studied minerals in the tissues examined in animals exposed to aluminium by any of the methods.
Boudey et al. (1997) examined the effects of low doses of aluminium on growth rates and calcium metabolism of young weanling rats; an additional variable in the study included reductions in calcium levels. Animals were given control diets (8.4 mg Al/kg b.w. or supplemented diet (10.6 mg Al /kg b.w.), with or without altering calcium levels (7.6 g Ca /kg b.w. vs. 0.4 g Ca/kg b.w). Animals exposed to the higher dose of aluminium in calcium deficient diets weighed 40% less at the end of the 90 day study. Aluminium levels in brain, liver, and bone were approximately 3-fold higher than those of animals given diets containing normal levels of calcium and the lower dose of aluminium. The authors also noted that, in the presence of normal levels of calcium, the higher dose of aluminium caused 25 to 40% reductions in the levels of calcium in bone, liver, and brain. One conclusion of the study was that young animals may be more sensitive to the effects of aluminium if diets are deficient in calcium.
In comparison to these outcomes, Julka & Gill (1996) reported that young (100-150 g) rats exposed to very high doses of aluminium by i.p. injection (10 mg/kg b.w. per day) for 4 weeks had elevated levels of calcium (2 to 3-fold) in cortex and hippocampus of brain. Other physiological consequences related to calcium included decreased (~40%) calcium influx in isolated synaptic preparations from the aluminium-treated animals and reduced ability of calmodulin to stimulate cAMP phosphodiesterase (~25% reduction in activity).
Mahieu & Calvo (1998) examined renal function in rats exposed to aluminium by i.p. injection of aluminium hydroxide (80 mg/kg b.w., 3 times per week, for 6 months). After 6 months of exposure, aluminium levels in serum reached 800 μg/L as compared to controls (10 μg/L). These exposure levels are 400 to 800-fold higher than typically found in human serum (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity). Deposition of aluminium in trabecular bone was also observed, indicating significant exposure levels. Similar to results of other studies, the authors reported that treated animals showed reductions in weight (25%) and reduced efficiency of nutrient utilization. No obvious loss of renal function was noted; there were significant reductions in the excretion of phosphorus (40% less) with significant increases in calcium excretion. The suggested mechanism for the effects on renal function involved loss of PTH response, either by lower release of the hormone or reduced receptor sensitivity.
In another study of renal function, Braunlich et al. (1986) reported that aluminium exposure (i.p. injection of 0.5 mg/kg b.w., 5 times weekly for 12 weeks) led to increased urine output (45%) and increased sodium excretion (57%). Notably, the dose used in these studies was more in line with what would be used to achieve 10-fold increases in aluminium loads in rodents.
Mahieu et al. (2004) examined the intestinal absorption of phosphorus and its subsequent deposition in bone in rats exposed to aluminium. Animals were injected i.p. with aluminium lactate (5.75 mg/kg b.w.) 3 times per week for 3 months. By the end of the study, serum levels of aluminium were reported to be 600 μg/dL as compared to 10 μg/dL in controls. Slight reductions in body weight (10%) at 3 weeks of treatment were noted. In serum, there were no differences in calcium or phosphorus levels between control and treated groups at any age tested. At 1, 2, and 3 months of exposure, 25 to 30% reductions in the level of phosphorus excreted in urine were noted. Small, but statistically significant, reductions in the levels of phosphorus absorbed by the intestine were also noted. Similar reductions, small but statistically significant, in calcium absorption were also reported. Slight increases in calcium urinary excretion were detected along with 10% reductions in calcium levels in bone. A significant increase in the bone accretion of phosphorous (32P deposited/32P absorbed) was also noted in treated animals as compared to controls (27% increase). Bone calcium was significantly (p < 0.03) reduced in treated rats (10% decrease). These findings indicate that phosphorous metabolism may be modified by aluminium through direct action on the intestine, kidney, and bone.
General metabolic effects
There are several reports that provide evidence that aluminium may have effects on multiple metabolic pathways. There have been two studies on the effects of aluminium on metabolic enzymes. Rats exposed to 100 μM aluminium chloride in drinking water (2.6 μg Al/mL) for 12 months showed 2-fold elevations in aluminium in brain with reductions in the activities of hexokinase and glucose-6-phosphate dehydrogenase (G6PDH) (73 and 80% of normal) (Cho & Joshi, 1989). Reductions in erythrocyte activities of G6PDH and glutathione reductase (82% of normal for each) were reported in rats injected i.v. with 5 mg AlCl3/kg for 3 consecutive days and then harvested at 4 weeks post treatment (Zaman et al., 1993).
Another study examined nutritional effects of aluminium in drinking water (administered as aluminium nitrate at doses of 360, 720, 3600 mg/kg b.w./day) for 100 days (Domingo et al., 1987). Young female rats exposed to the highest dose gained 50% less weight than animals on lower doses or the controls. The largest difference in weight gain occurred in the first weeks of the study. Animals on the highest dose drank less water, consumed less food, and showed less urine and faecal output. No changes in blood uric acid, cholesterol, glucose, creatine, or urea levels were detected in any treatment group.
Gonzalez et al. (2004) reported that rats given aluminium hydroxide by i.p. injection (27 mg/kg b.w.) 3 times per week for 3 months show 25% reductions in bile flow, 39% reductions in bile salt output, 43% reductions in bile cholesterol output, and 38% reductions in total bile protein output. These effects were correlated with 40% reductions in the expression of multidrug-resistance-associated protein 2, which is the main multi-specific organic anion transporter of the bile duct. Plasma concentration of aluminium in these animals reached 750 μg /L (controls 9 μg/L), indicating very significant exposure. No change in weight was detected in the treated animals.
Effects on Haematopoiesis
One of the most common abnormalities associated with renal failure and haemodialysis is anaemia. Many patients with this disease receive high doses of hydroxyl aluminium gel over long periods to control serum phosphorus levels. As described above in Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, the majority of aluminium found in serum is bound to Tf, which is responsible for the transport of iron. As described below, Tf and iron are crucial regulators of erythropoiesis; thus an immediate suggestion of a mechanism behind anaemia in patients afflicted with renal failure is evident that implicates aluminium.
However, in the majority of these patients, treatment with erythropoietin, a hormonal stimulator of haematopoiesis, is effective in restoring haematocrits (percentage of cells in whole blood that are red blood cells), at least partially (Eschbach et al., 2002; Sakiewicz & Paganini, 1998). In most patients, one physiological basis of anaemia stems from compromised production of erythropoietin by diseased kidneys, leading to chronic anaemia. The condition is often exacerbated by iron deficiency, which can arise from decreased red blood cell t½, chronic loss of blood, decreased uptake, and other nutritional deficiencies (Drüeke, 2001; Sakiewicz & Paganini, 1998; Winearls, 1998). In the majority of haemodialysis patients, treatments with erythropoietin and iron supplements are sufficient to raise haematocrits to levels >30% of normal, a level that alleviates most symptoms of anaemia. Although some patients are hyporesponsive to erythropoietin, the levels of iron-saturated Tf and aluminium do not provide a correlative explanation (Eschbach et al., 2002); unresponsive patients do not have higher serum levels of aluminium or lower levels of iron saturated Tf. Thus, in patients who are exposed to high levels of aluminium, the physiological basis for anaemia is complex and involves, at least in part, a loss of hormonal stimulation. However, as outlined below, there are both cell culture and animal model data to indicate that aluminium has the capacity of perturb haematopoiesis and thus effects on this system should be considered in assessing aluminium toxicity.
In vivo animal studies
The effects of aluminium on haematopoiesis have been investigated in various animal models. In these studies, aluminium exposure has been accomplished by both oral and injection routes. Data from selected studies involving direct injection of aluminium salts will be summarized first. As a point of reference, the average human exposure to aluminium in drinking water, is ~ 2.3 μg/kg b.w./day, with steady-state serum levels of aluminium averaging ~2 μg/L in most individuals (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity).
Chmielnicka et al. (1996) exposed rats to high doses of aluminium chloride (4 mg/kg b.w./day) for 21 days by i.p. injection. Although the levels of aluminium in serum were not reported, this exposure level equates to 800 μg/kg b.w./day of elemental aluminium. At 3, 7, 14, and 21 days of exposure, the authors measured platelet counts, red blood cell counts, serum levels of iron, total haemoglobin levels, and haematocrits. No significant changes in platelet or red blood cell counts were noted at any age. By 21 days of exposure, small, but statistically significant, decreases in total haemoglobin and haematocrit were reported. The most robust effect identified was a 25 to 30% reduction in the level of iron in serum.
Farina et al. (2002) exposed rats to aluminium sulphate (50 μmol/kg b.w. = 2.6 mg of aluminium/kg b.w.) through i.p. injections 5 times a week for 3 months. The levels of aluminium in serum were not reported. At the end of the exposure period, the authors reported finding several indications of toxicity to the haematopoietic system, including 32% reductions in total haemoglobin levels, 24% reductions in haematocrit, and 30% reductions in serum levels of iron. However, total iron-binding capacity of serum from exposed animals was not statistically different from controls.
I.p. injections of aluminium hydroxide (80 mg/kg b.w. – 3 times per week) have also been used as an experimental model of chronic exposure to aluminium. Mahieu et al. (2000) studied animals exposed for up to 28 weeks, while Bazzoni et al. (2005) examined animals treated for 12 weeks. Serum levels of aluminium were not reported in these studies and thus the sustained body burden is not known. The elemental aluminium content of this exposure was calculated to be ~27 mg/kg b.w. at each injection. Several haematological factors were measured in each study. Mahieu et al. (2000) noted progressive decreases in mean corpuscular volume (microcytosis) to a maximum of 28% reductions. However, minimal reductions in red blood cell counts and haematocrit were reported. Modest reductions in total haemoglobin (20% reduction) and small increases in red blood cell fragility were reported. Bazzoni et al. (2005) reported 20% reductions in total haemoglobin with 7% reductions in haematocrit. This latter study reported significant increases in red blood cells with deformed morphology (not quantified) with slight increases in fragility. Bazzoni et al. (2005) also reported that red blood cells in the treated animals were 3 times more rigid and less prone to aggregate.
Farina et al. (2005) conducted a second study of rats exposed to aluminium citrate (30 mM aluminium sulphate with 35 mM sodium citrate) in water for a total of 18 months. The daily exposure to aluminium was estimated to be 54.7 mg/kg b.w. Citrate would be expected to increase absorbance; however serum levels of aluminium were not reported. The authors reported that chronic exposure at this level led to reductions of 20% in red blood cell counts, 13% in haematocrit, 15% in total serum haemoglobin, and 40% in total levels of iron in serum. However there was no change in total iron binding capacity and no increase in red blood cell fragility. Turgut et al. (2004) exposed mice to aluminium for 3 months through drinking water containing aluminium sulphate. The estimated dose was 877 μmol/kg b.w./day = 47 mg of aluminium/kg b.w./day. Serum levels of aluminium were not reported. Reductions in serum haemoglobin (14%) and haematocrit (13%) were described. The levels of iron in serum were elevated by 59% with a small increase in the levels of Tf.
Garbossa et al. (1998a) have reported evidence that aluminium exposure may have direct effects on erythroid differentiation, which may account for the reductions in red blood cell counts and haematocrit in animals exposed to aluminium. Garbossa et al. (1998b) conducted a study in which rats were exposed to aluminium citrate at two levels by two routes for 15 weeks; 1 μmol/g b.w./day by oral gavage 5 days/week and by drinking water containing a 100 mM solution (estimated exposure 14-17 μmol/g b.w. day). Serum levels of aluminium were measured in this study with the dose given by oral gavage leading to serum aluminium levels of 2.9 μmol/L (78 μg/L), as compared to controls with 0.8 μmol/L (21.5 μg/L). The serum levels of aluminium in animals exposed through drinking water were 3.6 μmol/L (97 μg/L). The authors reported that animals given aluminium in drinking water had reductions in haematocrit (11%), increased osmotic fragility of red blood cells (20% more fragile), and 24% reductions in red blood cell t½. At both the lower and higher dose, there were reductions in the ability of isolated bone marrow stem cells to differentiate into erythrocytes after exposure to erythropoietin (colony-forming units – erythroid). In a later study by the same group, Vittori et al. (1999) exposed rats to aluminium via drinking water containing 80 mM aluminium citrate for 8 months. At the end of the study, the serum level of aluminium in the treated rats was 205 μg/L (range 120-790 μg/L) whereas the level in control animals was 15 μg/L (range 5 to 90 μg/L). These levels are between 10 (control) and 200 (treated) times the average level of aluminium in human serum (1-2 μg/L - see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity). At this high exposure level, very significant reductions in erythrogenesis were found ~60% reduction in erythroid colony forming units (CFU-E) and decreased uptake of iron (~30% reduction). The authors also reported significant increases in abnormal red blood cell morphology.
In vitro studies
Studies of the effects of aluminium on erythroid cell differentiation have suggested potential mechanisms by which aluminium could impact haematopoiesis. In a study by Vittori et al. (1999), direct evidence that aluminium inhibits erythroid differentiation was reported. Erythroid progenitor cells were concentrated from human blood and exposed to 100 μmol aluminium citrate (8 mg/L elemental aluminium) for 10 days and induced to differentiate with erythropoietin. The authors reported 30% reductions in CFU-E activity. However, in a similar study, Mladenovic (1988) reported that aluminium at levels of 1,035 ng/mL (1.035 mg/L) in medium did not significantly diminish CFU-E. However, if Tf was added to the medium along with aluminium, then CFU-E was diminished by 90%. If Tf was first saturated with iron, aluminium had no effect. The negative effects of Tf-aluminium were not overcome by adding excess levels of erythropoietin. One conclusion that could be drawn from this study was that a primary effect of aluminium on erythroid differentiation was mediated by competition for the Tf receptor. If excess Tf saturated with aluminium is present, then erythroid differentiation is inhibited by the binding of aluminium-metallated-Tf (aluminoxamine) to its receptor. However, the affinity of Tf for iron is 5 orders of magnitude greater than its affinity for aluminium. Hence excess aluminium alone cannot displace the iron from Tf-iron complexes that pre-exist in culture medium. Adding demetallated Tf to the medium allows aluminium to bind and thus creates the opportunity for Tf-aluminium-complexes to compete for binding to the Tf receptor. The extent to which a similar scenario may occur in vivo depends upon the levels of free Tf in serum at the time of aluminium exposure and on the level of iron in the serum. If sufficient iron is present, then aluminium binding to Tf would be less favoured. Individuals with iron deficiency could therefore be at greater risk for developing haematopoietic abnormalities upon exposure to aluminium.
Direct effects of aluminium salts on the morphology of red blood cells have been reported (Suwalsky et al., 2004; Vittori et al., 2002; Zatta et al., 1997). However, as outlined above in Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, most studies of aluminium metabolism indicate that most of the aluminium, absorbed from drinking water or food, present in plasma would be bound to Tf. Thus, the amount of soluble aluminium salts that red blood cells would be exposed to is limited. However, Tf-mediated delivery of aluminium to erythroid progenitors does provide a means to expose these cells directly to the metal. Vittori et al. (2002) noted that red blood cells exposed to aluminium citrate in vitro acquired abnormal morphology and showed increased degradation of a protein involved in maintenance of cell shape (band 3 protein). Suwalsky et al. (2004) similarly reported that red blood cells exposed to aluminium fluoride in vitro developed abnormal morphology.
Irritation
Oral exposure
There are reports of animal studies in which aluminium was given in large amounts orally for a considerable period of time that do not mention GI irritation, corrosion or sensitivity. These GI effects were not specifically examined in many of these studies and it is not known whether such effects were considered at all in these studies. Generally, the only adverse effect observed in most of the studies was a decrease in body weight gain. Some examples follow. Gross necropsy of male Sprague-Dawley rats fed diets containing 7000 to 30,000 mg/kg basic SALP, which is used as emulsifying agent, equivalent to 67 to 288 mg Al/kg b.w./day, for 28 days, showed no evidence of GI irritation (Hicks et al., 1987). Beagle dogs fed 80 mg Al/kg b.w./day as basic SALP for 26 weeks demonstrated histopathological changes in the liver and kidney that were attributed to reduced food intake, but no adverse effects on the stomach were noted (Pettersen et al., 1990). Male and female beagle dogs fed acidic SALP, a leavening agent, in a diet containing 0.3, 1.0 or 3.0% SALP for 6 months, showed no evidence of GI irritation on gross autopsy and histopathological examination (Katz et al., 1984). These SALPs are not very soluble, except in dilute HCl, which might be achieved in the stomach. Male Weizman Institute strain rats given 1% aluminium chloride or sulphate in their drinking water demonstrated periorbital bleeding after an unspecified period of exposure, but were not noted to demonstrate GI irritation. However, histology may not have been conducted on the GI tract (Berlyne et al., 1972b). Female Sprague-Dawley rats were given drinking water containing aluminium nitrate (375, 750 or 1500 mg/kg b.w./day, equivalent to 27, 54 and 108 mg Al/kg b.w./day, respectively) for 28 days; mild histological changes occurred in the liver and spleen but no histological changes in the presence of elevated aluminium were seen in the stomach or small or large intestine (Gómez et al., 1986).
In contrast to the above reports that were negative for aluminium-induced GI irritation, male albino rats given a single daily gavage dose of aluminium sulphate (17 to 172 mg/kg b.w./day) or potassium aluminium sulphate (29 or 43 mg Al/kg b.w./day) for 21 days demonstrated gastric mucosal layer thickening, hyperplasia and ulceration after exposure to 86 and 172 mg/kg b.w./day aluminium sulphate, but not to lower doses (Roy et al., 1991). It therefore appears that aluminium has the potential to produce irritation in rats after oral administration when high concentrations of soluble aluminium salts are administered (see Effects on Laboratory Mammals and In Vitro Test Systems, Single/Acute Exposure and Effects on Laboratory Mammals and In Vitro Test Systems, Repeated Exposure for more information on the effects of oral exposure to aluminium in mammals).
Inhalation exposure
The pulmonary response to various species of aluminium has been studied in animals after inhalation exposure and after intratracheal instillation (see Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, Intratrachael Exposure). Many of these studies were conducted to better understand the adverse effects observed in humans exposed to very high levels of air-borne aluminium, and other materials, in industrial settings during the Second World War, and the postulated protective effect of inhaled aluminium against quartz dust-induced pulmonary fibrosis; this practice started in the mid-1940’s and continued for at least 10 years (see Effects on Humans, Effects from Occupational Exposure, Irritation, Inhalation Exposure). Various species of aluminium caused responses described as typical of foreign body reaction, alveolar proteinosis and wall thickening, some nodule formation, but not the extent of fibrosis caused by quartz dust.
Rabbits exposed to aluminium dust 1 to 2 hr daily for 20 to 40 days showed increased connective tissue in their lungs (Goralewski, 1939). In studies by Jötten & Eickhoff (1942), inhalation of finely powdered aluminium dust by rabbits caused few lung changes and no fibrosis. However, addition of a pneumococcal infection resulted in a diffuse sclerosis, collagen formation and rapid death, suggesting that aluminium somehow enhanced the toxicity of the infectious agent.
Exposure of rabbits to aluminium dusts resulted in three phases of pulmonary change, an initial exudation of leukocytes for a few hr or days, several months of an intermediate monocytic phase, and a final foreign body granulomatous phase that developed after several months of exposure (De Marchi, 1947). These are changes associated with a foreign body reaction (Chen et al., 1978; De Vuyst et al., 1987). Rats and guinea pigs were exposed to arc-produced fumes of pure aluminium oxide (alumina), silica, or “stack dust” (a mixture of alumina, silica and other oxides) for 18.3 hr daily for 6 months. Six months after completion of exposure, their lungs contained 1 to 11% of fume material. The alumina-exposed animals exhibited nodule-like collections of endothelial cells, fibroblasts and mononuclear leucocytes, similar to silicotic nodules (MacFarland & Hornstein, 1949). Inhalation of aluminium hydrate by guinea pigs led to hypertrophic reticular pneumonia and a histiolymphoid reaction as a general response to this insult and formation of nodules in the alveoli, demonstrating the harmfulness of this aluminium species (Jullien et al., 1952).
To assess the role of paraffin, which was used to coat aluminium dust during its production, in the toxicity associated with aluminium production, rabbits were exposed to paraffin-coated aluminium dust. They developed an interstitial fibrosis that began to appear about 75 days later, which was less frequent and severe than seen in rabbits exposed to uncoated dust (Van Marwyck & Eickhoff, 1950). These results did not support the hypothesis of Perry (1947) (see Effects on Humans, Effects from Occupational Exposure, Irritation, Inhalation Exposure) that paraffin substances coating the aluminium are the cause of aluminium-induced fibrosis, but are consistent with the hypothesis of Corrin (1963a) (see below).
Inhalation of 40 to 120 mg/m3 of boehmite, a gelatinous aluminium oxide, or 21 to 33 mg/m3 aluminium oxide, considered to be gibbsite, by guinea pigs 8 hr daily, 6 days weekly for 14 months apparently did not produce adverse pulmonary alterations (Gardner et al., 1944). The authors did note that this inhalation unfavourably influenced the resistance to tuberculosis, consistent with the response to concurrent exposure to finely powdered aluminium dust and pneumococci (see above).
Aluminium oxide exists in many chemical species (see Identity, Physical and Chemical Properties, Analytical Methods, Physical and Chemical Properties, Properties of Aluminium Compounds). The γ transitional form was believed to be the most biologically active (Klosterkotter, 1960). Mice and rats were exposed to a relatively well-defined γ transitional form of aluminium oxide having an average particle size of 0.005 to 0.04 μm and a surface area of 95 m2/g, although these particles aggregated during the experiment. Exposure was to 33 g/m3 by inhalation 5 hr daily for 285 days or by intratracheal instillation of a single dose of 35 mg of γ-Al2O3 suspended in 1 mL of tap-water. It was noted by the experimenters that this very high level inhalational exposure could not be tolerated and would be avoided by industrial workers. Inhalation exposure resulted in some deaths, some connective tissue thickening in the alveolar walls and bronchioles with mild collagenous fibrosis, but not the collagenous nodules seen in silicosis. These effects have been interpreted as alveolar proteinosis, a non-specific response to pulmonary dust exposure (Dinman, 1988).
Wistar rats and hamsters inhaled a powder composed of 20% aluminium and 80% aluminium oxide, 0.05 to 7 μm particle width, which was introduced hourly during an 8 hr day for 3 to 19 months (Christie et al., 1963). Increased exposure duration produced enlarged lungs covered by slightly thickened pleura with sub-pleural plaques. This progressed to consolidated areas surrounding the small bronchioles. Cystic spaces, usually sub-pleural, were seen after 9 months. They were surrounded by firm, fibrous plural walls and filled with pulpy material. Microscopic examination revealed diffuse alveolar catarrh, large macrophages, eosinophilic foamy vacuolated cytoplasm, some thickening of the alveolar walls which were lined by proliferation macrophages, foreign body giant cells, granulomas formed by histiocytes (tissue macrophages), and foreign body giant cells with large lipoid clefts. This constellation of effects was considered to be a lipid pneumonia. Acute inhalation of aluminium oxide particles, produced by a Wright dust constant feed generator, with a mean count size of 0.2 μm, and maximum size of 1 μm, as 0.38 or 0.58 mg/L (380 or 580 mg/m3), for 30 minutes by guinea pigs caused a constriction of pulmonary air flow (Robillard & Alarie, 1963a). In contrast, inhalation of aluminium oxide at a concentration of 0.38 mg/L (380 mg/m3) by rats for 3, 7 or 15 minutes produced a time-dependent lung dilatory effect, the opposite to that seen in the human, guinea pig, dog and cat (Robillard & Alarie, 1963b).
Three metallic aluminium powders were introduced at dust concentrations of 15, 30, 50 or 100 mg/m3 into chambers containing rats, guinea pigs or hamsters 6 hr daily, 5 days weekly for 6 months. The powders were; pyro coated, flake-like particles (4% < 1 μm, 87% 1 to 4 μm and 8.5 % > 4 μm; mean 2.5 μm diameter), flake-like particles (19% 1 to 4 μm and 71 % > 4 μm; mean 4.8 μm diameter), and atomized spherical particles (1.5% < 1-4 μm, 96% 1 to 4 μm and 3 % > 4 μm; mean 2.2 μm diameter) (Gross et al., 1973). The response among all 3 animal species was alveolar proteinosis, characterized by generalized alveolar epithelial reactions, the appearance of pneumocytes, and occasional macrophages, but no fibrosis.
Male Syrian golden hamsters were exposed by inhalation to a propylene glycol complex of aluminium chloride hydroxide, which was used in some antiperspirants. Exposure was to 164 mg/m3 of the complex, for 6 hr on the first day and for 4 hr on the second and third days. The animals demonstrated acute bronchopneumonia and moderate thickening of the alveolar walls. Rabbits exposed to 212 mg/m3 of the complex for 5 days showed similar effects. Exposure of hamsters to 52 mg/m3 of the complex 6 hr daily 5 days weekly for 30 exposures demonstrated persistent numerous foci of macrophages and heterophils, especially at the bronchioalveolar junction (Drew et al., 1974). Fisher 344 rats and Hartley strain guinea pigs received inhalation exposure to large (2.45 to 3.09 μm mean mass equivalent aerodynamic diameter) aluminium chlorhydrate particles, 0.25, 2.5 or 25 mg/m3 6 hr daily, 5 days weekly for 6 months. This resulted in multi-focal granulomatous pneumonia characterized by proliferation and infiltration of mononuclear inflammatory cells and foci of giant macrophages in the alveoli, often containing vacuoles of fibrillar basophilic material, and granulomatous lesions in peribronchial lymph nodes, evidence of active phagocytosis and necrosis (Steinhagen et al., 1978; Stone et al., 1979). Rats exposed to aluminium chloride and aluminium fluoride dusts by inhalation, 1.3 to 1.8 mg/m3 for over 5 months, showed that aluminium affected AM integrity and increased lysozyme, alkaline phosphatase, and initial protein levels in lung lavage fluid, suggesting effects on Type II alveolar cells. This was considered to be an adaptive response to the dusts (Finelli et al., 1981). Inhalation exposure of male Fischer 344 rats for 4 hr to aluminium flakes, at aluminium concentrations of 10, 50, 100, 200 or 1000 mg /m3, demonstrated an influx of polymorphonuclear neutrophils into bronchopulmonary lavage fluid that persisted for 6 months post-exposure, an indication of a continued irritant response. Multifocal microgranulomas in lungs and hilar lymph nodes were also seen (Thomson et al., 1986).
Clinical observations of uncontrolled studies suggested inhalation of aluminium dust was beneficial in the treatment of silicosis and did not produce apparent adverse effects (see Effects on Humans, Effects from Occupational Exposure, Irritation, Inhalation Exposure). This treatment was based on the theory that silica exerted its toxicity following its dissolution to silicic acid. Aluminium was found to coat the silica particles with a thin film of hydrated gelatinous aluminium oxide, reducing particle solubility (Crombie et al., 1944). Denny et al. (1937;1939) found that exposure to 1% metallic aluminium powder and quartz dust for 6 months protected against quartz-induced silicosis in the rabbit. They then exposed rabbits to freshly ground, fine particulate metallic aluminium dust particles, (the majority with diameter < 3 μm), at an average airborne particle concentration of 7 × 109 /m3 for 12 hr daily for 14 months. This resulted in a dried lung aluminium concentration of 2,700 to 12,000 mg/kg. The authors observed no harmful effects other than occasional thickening of the alveolar walls that consisted of alveolar epithelial proliferation. Concurrent exposure to admixtures of quartz and 0.5 to 3% aluminium dusts resulted in fibrosis in some animals that had < 1% aluminium in their lungs, but no fibrosis was seen in rabbits whose lungs had hydrated aluminium oxide. Aluminium was visualized by aurine. Similarly, exposure of rabbits for 40 minutes to aluminium dust containing 7 × 109 particles/m3 before or after being exposed to quartz dust (20 × 109 particles/m3) for 12 hr daily protected against quartz-dust induced fibrosis. Exposure of guinea pigs to 2 types of hydrated aluminium oxide (aluminium hydroxide) for ≥ 1 year produced no adverse effects on the lungs, but did have a protective effect against silicosis (Gardner et al., 1944). Dworski (1955) described a series of studies, begun by Gardner and continued by others, on the effects of colloidal aluminium hydroxide, powdered aluminium hydrate and metallic aluminium powder on normal animals, on the prevention of quartz dust-induced-silicotic reaction and on the treatment of pre-established silicotic lesions. A beneficial effect was attributed to aluminium when given before, or with, quartz, irrespective of the route of quartz or aluminium administration, when the quartz and aluminium distributed to the same location. Some retardation of not-yet-mature silicotic lesions was observed, but aluminium did not affect fibrotic lesions. Metallic aluminium powder produced less effect and lower tissue aluminium concentrations. One observation related by the author of unintended aluminium exposure producing less than expected toxicity from quartz exposure during the course of their studies undermines confidence in the experimental control of these studies. Addition of 2% metallic aluminium powder, as used by Denny et al. (1937;1939), to quartz dust delayed the development of nodular retilulinosis and collagenous silicosis, which occurred by ~ the 200th and 300th days in the absence of aluminium, and by ~ the 300th and 400th days in the presence of aluminium, respectively (King & Wright, 1950). Addition of aluminium at a concentration of 4 mg/m3 as McIntyre powder to an inhaled dust mixture of 75% finely ground lean coal and 25% Dörentrup quartz powder, ~ 45 mg/m3 with a particle count of ~ 2. 5 × 109/m3, did not reduce the development of fibrosis or have any other beneficial effects (Weller et al., 1966). When the aluminium dust was inhaled after the coal-quartz dusting, further cellular granulomas reactions were seen. The authors concluded from studies of inhalation of the aluminium dust alone that it cannot be considered totally inert. Aluminium salts were shown to be beneficial in rats exposed to pure quartz or coal-quartz. The aluminium aerosols showed no toxicity (Le Bouffant et al., 1975). Aluminium lactate alone produced no significant effects. Treating quartz with aluminium lactate reduced the inflammatory response and attenuated oxidation and proteolysis of fibronectin (Brown et al., 1989). Exposure of sheep with silica-induced silicosis to 100 mg aluminium lactate aerosol in saline monthly for the first year, and weekly for the next 2 years, starting 1 year after radiographic evidence of silicosis, produced no benefit (Bégin et al., 1995).
Many, but not all, of the studies aimed at assessing the potential protective effect of aluminium against fibrosis induced by quartz dust reported beneficial effects, as reviewed above. It was often noted that the aluminium alone had little toxicity.
Zeolite (hydrated alkali aluminium silicate) inhalation produced respiratory disease in rats (Gloxhuber et al., 1983).
Inhalation of power plant fly ash, which had a MMAD of ~ 2 μm, at concentrations of up to 4.2 mg/m3, 8 hr/day for 180 consecutive days, produced no significant adverse effects in rats (Raabe et al., 1982). However, the increased number of macrophages found in the lung was interpreted as a natural response to inhaled particles.
Aluminium starch octenylsuccinate is used in cosmetic formulations as an anti-caking and viscosity-increasing agent. Rats exposed to 200 g/m3 for 1 hr showed no gross changes from 1 hr to 14 days later (Nair & Yamarik, 2002).
Intratracheal exposure
Implantation of a fine, pure aluminium wire in the lungs of Swiss mice produced interstitial infiltration of lymphocytes, macrophages, and diffusely spreading fibrosis (Greenberg, 1977). The author thought the effects were more extensive than those produced by powdered aluminium and suggested that metallic aluminium might act as an antigen in a hypersensitivity reaction and that aluminium-induced pulmonary fibrosis may be an expression of tissue immunity.
Intratracheal administration, rather than inhalation, of aluminium has been employed in many studies. Intratracheal injection of a rather coarse form of metallic aluminium powder produced an extensive foreign-body granuloma in rats (Belt & King, 1943). Intratracheal injection of stack fume dust, a causative agent in Shaver’s disease, to guinea pigs produced a rather abruptly developing fibrosis 8 to 12 months later (Pratt, 1950).
Intratracheal instillation of 100 mg of the same gelatinous, hydrated aluminium oxide (laths of 200 to 400 Å length and 50 to 100 Å width, or aggregates, 300 m2/g surface area) studied by Gardner et al. (1944) resulted in extensive confluent nodular and diffuse confluent grade 5 fibrosis after 270 days (King et al., 1955). Similar intratracheal application of fumes, comprising largely amorphous spherical particles of 0.02 to 0.5 μm with a surface area of 10 m2/g, from a corundum furnace produced mild, grade 2 to 3 fibrosis. Introduction of 50 mg of aluminium phosphate, in the form of thin tablets of 0.5 to 5 μm diameter and having a surface area of 1 m2/g, produced moderate, grade 3, fibrosis after 390 days (King et al., 1955). Further studies were conducted by this group in hooded rats given intratracheal instillation of various aluminium forms. Fourteen mg of aluminium hydroxide resulted in the development of firm, discrete, rarely confluent lung lesions and collagenous fibrosis. Fifty mg of aluminium oxide, aluminium phosphate or the equivalent dose as aluminium hydroxide were similarly injected. The aluminium oxide studied was the same as used by others (Gardner et al., 1944). Intratracheal instillation produced numerous white patches, often confluent, as early as 60 days after exposure. After 180 days there were firm fibrotic areas and, at day 210, almost the entire lung was a dense mass of fibrous tissue that progressed to a mass of confluent collagenous tissue. Aluminium hydroxide produced firm, confluent, fibrotic patches in the lungs after 150 or more days. Aluminium phosphate produced similar effects, although less severe than seen with aluminium oxide, whereas catalytically inert α-aluminium oxide did not produce adverse lung effects (Stacy et al., 1959). Similar experiments with powdered metallic aluminium (amorphous, 0.05 to 1 μm diameter) resulted in nodular fibrosis in hooded rats, similar to changes produced by quartz dust (King et al., 1958). The intratracheal administration of 35 mg of aluminium oxide produced slight (grade 1 to 2) fibrosis 150 days later, and grade 2 to 3 fibrosis 180 days later (Klosterkotter, 1960).
An intratracheal injection of 100 mg of stamped, thin, flake-like, aluminium particles (50% < 1 μm, 47% 1 to 5 μm and 3% > 5 μm) in 1 mL saline to Wistar rats produced nodules, enlarged hilar lymph nodes, macrophage infiltration, the development of collagen fibres and fibrosis over months. In contrast, instillation of granular aluminium (11% 1 to 5 μm, 17% 5 to 10 μm and 72% > 10 μm) resulted in a small number of macrophages and occasional collagen fibres after many months, but very little fibrosis (Corrin, 1963a).
Intratracheal installation of 2 to 100 mg of coated, flake-like particles or atomized spherical particles or 2 to 24 mg of the flaked powder to rats and hamsters resulted in dose-dependent collagenous fibrosis which was not seen below a dose of 24 mg (Gross et al., 1973).
Rats were given a single intratracheal instillation of a saline suspension of potroom dust from an aluminium reduction plant. Seven days after instillation of 0.5 mg dust, changes were seen at the pulmonary surface. After a 5 mg dose, marked changes were seen at the pulmonary surface and in lung tissue. There was a 16 to 20-fold increase in the number of polymorphonuclear leukocytes in the lung. By contrast, 5 mg virginal (primary) aluminium oxide caused an 8-fold increase which was interpreted by the authors as a standard response to a nuisance dust. Twenty-two weeks after a single intratracheal instillation of 5 mg of potroom dust or virginal aluminium oxide, all parameters measured had returned to control levels. The acute irritation and inflammation produced in rat lung suggested that this type of dust may have produced similar effects in human lung, thus contributing to the acute respiratory symptoms experienced by some potroom workers (White et al., 1987).
Dinman (1988) reviewed the experimental studies of the toxicity of aluminium oxide in the lung and concluded that the catalytically-active low temperature forms of aluminium oxide and the forms that are not catalytically-active and defined as γ can produce irreversible fibronodular changes, but only after intratracheal instillation. He termed this “alumina-related pulmonary disease”. He noted a positive correlation between the surface area of the aluminium oxide particles and the fibronodular response.
The ability of 7 aluminium oxide samples to produce lung cytotoxicity, measured by LDH and polymorphonuclear neutrophils in BALF, was tested in rats by comparing the intratracheal instillation of a total of 50 mg of aluminium oxide, with that of 25 mg of quartz, each given in 5 instillations over 2 weeks (Ess et al., 1993). The samples were smelter grade aluminas obtained from industries involved in aluminium production and a chemical grade and a laboratory produced aluminium oxide. They had median particle diameters of 1.3 to 12 μm except for the chemical grade that had a median diameter of 0.008 μm. Increased lung cytotoxicity was seen as surface area increased and α-content decreased. I.p. injection of 5 mg of the smelter grade aluminas in mice produced nodules, but no fibrosis. The BALF of rats given a single intratracheal instillation of 40 mg of potroom aluminium oxide was studied 1, 4 and 12 months later (Tornling et al., 1993). Compared to controls, there was a significant decrease of lymphocytes at 1 month, a significant decrease of albumin at 4 months and a significant increase of fibronectin at 12 months. The authors suggested that the increase of fibronectin might contribute to the inflammatory reaction and build up of an extrcellular matrix network. Rats were given a single intratracheal instillation of 20 mg of either condensed aerosols/dusts collected from a foundry or pure chemical grade α-alumina. They were suspended in 0.5 mL of saline. Before installation, samples were passed through a 36 μm diameter strainer (Halatek et al., 2005). Multiple endpoints were determined in the BALF 3, 6 and 9 months later. Clara cell protein 16 (CC16) was significantly lower in rats that received α-alumina 3 months after instillation and in rats that received foundry aluminium 6 months after instillation, compared to controls. Hyaluronic acid was significantly increased only in the rats terminated 6 months after α-alumina instillation. Nine months after treatment, neutrophils increased ~ 3-fold in rats given foundry aluminium and ~ 5-fold in those given α-alumina. Lung weights increased in the α-alumina-treated rats but not those that received foundry aluminium. Three months after α-alumina instillation macrophage accumulation was seen; after 6 and 9 months granuloma-like structures were observed. Six months after foundry aluminium instillation, young forms of lymphocytes, macrophages and fibroblasts were seen; after 9 months interstitial fibrosis was observed. The authors concluded that foundry aluminium can cause irritation and inflammation in the rat lung and that a lowering of CC16 was the most sensitive biomarker for this damage.
Some of the studies of the potential for aluminium to protect against quartz dust-induced pulmonary fibrosis employed intratracheal aluminium administration. Intratracheal insufflation in rats of 150 mg of quartz dust mixed with 3 mg of powdered aluminium in 1.5 mL milk-saline as a vehicle resulted in silicotic nodules in the presence of 1% aluminium in the lung in nearly all subjects, showing that aluminium failed to prevent experimentally-induced silicosis (Dworski, 1955). Similarly, aluminium alone (20 to 50 mg) produced an extensive foreign body reaction seen at ~ 16 months (Belt & King, 1943). Administration of 400 mg of a mixture of 98% quartz and 2% of the aluminium dust used by Denny et al. (1937; 1939) in 4 mL saline into the lungs of rabbits reduced lesions and collagen formation up to 1 year later and to a greater extent than when only quartz was administered (Belt & King, 1943; King et al., 1945). King et al. (1958) conducted further studies in hooded rats given intratracheal instillation of various aluminium forms. Powdered metallic aluminium given intratracheally in large amounts relative to quartz, which was given similarly, did not reduce quartz-induced fibrosis (King et al., 1958). Intratracheal administration in sheep of 100 mg quartz produced sustained increases in bronchoalveolar lavage cells, an alveolitis at 60 days, and early nodular silicotic lesions at 10 months. This was attenuated by treating the quartz with 11 or 100 mg aluminium lactate, which was believed to mask some of the active sites on the silica (Bégin et al., 1986; 1987; Dubois et al., 1988). Monthly inhalation of 100 mg aluminium lactate after intratracheal instillation of 100 mg quartz or instillation of 100 mg aluminium lactate-treated quartz enhanced pulmonary quartz clearance compared to when only quartz was inhaled (Dufresne et al., 1994).
I.v. and i.p. administration of an aluminium dextran complex, Mr = 150,000, protected against the development of silicotic nodules in the liver of mice injected i.v. with silica dust (James et al., 1960).
Aluminium species used in pharmaceuticals and cosmetics have been tested for pulmonary irritation and toxicity. Intratracheal installation of kaolin (hydrated aluminium silicate) in rats produced pulmonary toxicity manifested as fibrosis (Martin et al., 1975). Bentonite (hydrated colloidal aluminium silicate) given intratracheally to rats produced an increase in polymorphonuclear lymphocytes in the lung (Sykes et al., 1982), inflammation, and bronchopneumonia progressing to necrosis and storage-focal tissue reaction (Tatrai et al., 1983), elevated macrophages and acid phosphatase activity (Tatrai et al., 1985) and increased phospholipid (Adamis et al., 1986). Intratracheal montmorillonite (an aluminium magnesium silicate clay) produced dose-dependent interstitial fibrosis (Schreider et al., 1985). Intratracheal zeolite administration produced pneumoconiosis in rats (Kruglikov et al., 1990) and a storage type reaction that progressed to mild pulmonary fibrosis (Tatrai et al., 1991).
Single intratracheal administration of attapulgite (hydrated magnesium aluminium silicate) or Fiberfrax (an aluminium silicate) in rats produced granulomas accompanied by multinucleated giant macrophages and enhanced IL-1-like activity. Additionally, early fibrosis was seen after Fiberfrax exposure, and irreversible fibrosis 8 months following attapulgite administration (Lemaire, 1991; Lemaire et al., 1989).
Rats that inhaled refractory aluminium oxide fibre as manufactured, or after thermal aging for 86 weeks, showed minimal pulmonary reaction (Pigott et al., 1981). When 20 mg of these refractory aluminium oxide fibres were given by an intrapleural injection, they did not produce the malignant mesothelioma produced by aluminosillicate and asbestos fibres (Pigott & Ishmael, 1992).
Dermal application
Aluminium was applied in 0.5 mL solution as 2.5, 5, 10 and 25% aluminium chloride, or 10 or 25% aluminium chlorhydrate to 2 cm2 of shaved skin on the back of TF1 strain mice for 5 consecutive days. Aluminium was similarly applied for 5 days to mice, New Zealand rabbits and white strain pigs (1 mL of solution to 4 cm2) as 10% aluminium chloride, nitrate, chlorhydrate, sulphate, hydroxide (in suspension) and basic acetate (in suspension), and as 25% aluminium chlorhydrate. The 10% solutions of aluminium chloride and nitrate produced epidermal changes that included slight to severe hyperplasia with focal ulceration, epidermal damage, dermal inflammatory cell infiltration, hyperkeratosis, acanthosis, microabscesses, aluminium deposition and abnormal keratin (Lansdown, 1973). The other 4 aluminium forms did not produce these changes. The author suggested that the aluminium ion interacted with keratin to denature it, making the stratum corneum more permeable, thereby allowing aluminium penetration through the stratum corneum to cause toxicity to the epidermal cells. Magnesium aluminium silicate was a weak primary skin irritant in rabbits (CFTA, 1970). Therefore, aluminium can produce dermal irritation that is aluminium species-dependent.
Injection
The i.p. injection of ground aluminium phosphate in rats produced extensive fibrosis in the peritoneal cavity (Evans & Zeit, 1949). Zeolite injection produced a peritoneal fibrosis (Suzuki & Kohyama, 1984). Sub-plantar injection of bentonite produced granulomas in rats (Marek & Blaha, 1985).
Aluminium is used as an adjuvant in vaccines and hyposensitization treatments for allergies. Precipitation of toxins and toxoids by alum was found to enhance their antigenic properties and reduce the rate of absorption and elimination of the antigen (Glenny et al., 1926; 1931). However, an in vitro study demonstrated that proteins present in interstitial fluid that have a larger adsorption coefficient can displace aluminium-adsorbed proteins with a smaller coefficient, with > 50% protein displacement within 15 minutes. It has been suggested that this result does not support the proposed mechanism of aluminium-enhanced antigenicity by producing a persistent depot of antigen (Heimlich et al., 1999). In addition to delaying release, aluminium strengthens the immunological properties of weak antigens to improve antibody response, as shown by addition of aluminium hydroxide to triple vaccine, 2.5 mg in the 0.5 mL injection. This reduced toxicity and increased potency in laboratory tests, and reduced reactions in children. However it produced s.c. nodules (Butler et al., 1969) (see also Effects on Humans, Effects from Non-Occupational Exposure, Irritation, Irritation after Injection of Aluminium-Adsorbed Proteins (Vaccines and Hyposensitization Regimens)). Aluminium adjuvants may also increase antigenicity by increasing the production of a local granuloma which contains antibody-producing plasma cells (White et al., 1955); by increasing the immunogenicity of the aluminium-antigen complex; by increasing antigen specific as well as total IgE antibodies (Gupta, 1998); and by stimulating the body’s immune competent cells through activation of complement, induction of eosinophilia, and activation of macrophages, lymphocytes and lymph nodes (Gupta, 1998; Hunter, 2002). Aluminium hydroxide-stimulated macrophages contain aluminium and differentiate into mature, specialized antigen-producing cells that express surface molecules similar to those seen in cultured dendritic cells, including HLA-DRhigh, CD-86high, CD14-, and CD83 (Rimaniol et al., 2004). These effects seem to be aluminium-dependent, because aluminium, phosphate-adsorbed TT produced a prolonged synthesis of specific IgE, whereas calcium phosphate adsorbed TT did not (Vassilev, 1978). This is discussed further in Effects on Humans, Effects from Non-Occupational Exposure, Irritation, Irritation after Injection of Aluminium-Adsorbed Proteins (Vaccines and Hyposensitization Regimens).
There is other evidence for aluminium induction of the immune system. I.p. administered ovalbumin-adsorbed-aluminium silicate enhanced IgE antibody production (Fujimaki et al., 1984). Aluminium silicate is a component of fly ash. IgE and IgG production were increased by intratracheal instillation of ovalbumin adsorbed onto 0.02 or 0.2 mg of aluminium silicate, or onto alum or kaolin (Fujimaki et al., 1986).
Aluminium phosphate-precipitated toxoid prolonged the response to the toxoid in rabbits and guinea pigs, producing granulomas and antibody-containing cells 4 to 7 weeks after injection (White et al., 1955). The intradermal injection of alum-precipitated antigens produced lymph node infiltration of histiocytes (Turk & Heather, 1965). Intradermal injection of aluminium chlorhydrate into guinea pigs produced granulomas consisting of aggregations of undifferentiated macrophages that followed lymph drainage to the regional lymph nodes where they collected (Gaafar & Turk, 1970). Intradermal injection of 0.1 mL of alum-precipitated protein or aluminium hydroxide emulsion into Hartley guinea pig ears produced histiocyte infiltration within 4 days, persisting up to 28 days, that was mainly localized in close proximity to the entrance to the afferent lymphatics (Gaafar & Turk, 1970). I.m. injection of aluminium hydroxide or aluminium-adsorbed TT (0.1 mL containing 0.05 mg aluminium) in mice produced interstitial oedema and degenerative/necrotic changes and polymorphonuclear leukocyte infiltration at 24 hr. This acute reaction decreased over 72 hr when macrophage infiltration began, leading to a chronic granulomatous reaction beginning 1 to 2 weeks later that peaked at 8 and persisted for 16 to 20 weeks (Goto & Akama, 1982). Intradermal injections of 0.05, 0.5 or 5 mg aluminium chlorhydrate or zirconium aluminium glycinate (ZAG) or 0.065, 0.65 or 6.5 mg of aluminium hydroxide were given to Hartley strain guinea pigs. The aluminium hydroxide injections produced granulomas after the higher 2 doses. Those produced by 6.5 mg aluminium hydroxide persisted for longer than 28 days. These granulomas demonstrated undifferentiated macrophages and occasional giant cells around the injection. There was little evidence of infiltration and none of fibrosis. Aluminium chlorhydrate and ZAG injections produced skin thickness increases that reached a maximum at 28 days, and granulomas consisting of shredded bundles of basophilic collagen, giant cells and histiocytes which were pleomorphic, strongly hyperchromatic and sometimes phagocytic, followed by intense fibrosis (Turk & Parker, 1977). The authors suggested aluminium induced persistent nodule formation by a nonallergic direct toxic effect (foreign body reaction). I.m. injection of aluminium hydroxide-adsorbed TT into mice produced muscle fibre necrosis and eosinophil infiltration 4 days later (Walls, 1977). When aluminium chlorhydrate or ZAG were injected into the toe pad of New Zealand rabbits twice weekly for 6 weeks for a total dose of 1.4 mg, foreign-body granulomas, but not positive skin reactivity, were induced (Kang et al., 1977). Intradermal injection of 0.25 mL of vaccine containing ~ 0.3 mg aluminium into the backs and sides of rabbits produced s.c. nodules at all sites 8 days later, which persisted in most rabbits for 56 days and in 1 for 72 days (Pineau et al., 1992). The nodules had greater aluminium concentration than did normal skin. The infiltrate intensity and aluminium concentration in the nodules positively correlated. However, this is not the typical route for such injections. Aluminium hydroxide gel and suspension (aluminium concentration, 1 or 3 mg/mL) were injected i.m. (0.5 mL) or s.c. (1 mL) as 3 mg aluminium with ovalbumin into the hind legs of Hartley strain guinea pigs. The aluminium hydroxide gel produced granulomatous inflammatory reactions characterized by macrophages with foamy cytoplasm, small lymphocytes and giant cells at the injection sites. These effects persisted at least 8 weeks (Goto et al., 1997).
Aluminium compounds are the only adjuvants widely used in routine human vaccines and are the most commonly used adjuvants in veterinary vaccines. Two production methods have been used. One is the addition of alum to the antigen to form a precipitate of protein aluminate, termed alum-precipitated vaccines, which are similar in composition and physicochemical characteristics to aluminium phosphate adjuvants. The second is the addition of the antigen solution to preformed aluminium hydroxide, aluminium phosphate, mixed aluminium hydroxide and phosphate, or gamma aluminium oxide to produce aluminium-adsorbed vaccines (Clements & Griffiths, 2002; HogenEsch, 2002). Aluminium hydroxide (chemically: crystalline aluminium oxyhydroxide) and aluminium phosphate (chemically: aluminium hydroxyphosphate) are most commonly used (Hem, 2002). The former has an isoelectric point of 11.4 and is positively charged in interstitial fluid, at pH 7.4, thereby adsorbing negatively charged antigens by electrostatic attractive forces. Aluminium phosphate has an isoelectric point between 4.5 and 6, is negatively charged at neutral pH and this adsorbs positively charged antigens. Rational selection of the aluminium form is based on the charge of the protein to be adsorbed.
When aluminium starch octenylsuccinate was prepared in suspension and injected intracutaneously into a depilated site on the back of guinea pigs and rabbits, thrice weekly the first week and weekly for 7 more weeks, no abnormal skin reactions were observed (Nair & Yamarik, 2002). Subarachnoid (cisternal magna) injection of kaolin into foetal lambs and monkeys produced a fibrotic reaction and inflammatory cell response of the meninges and infiltration of kaolin-containing macrophages into the subarachnoid space (Edwards et al., 1984).
Ophthalmic exposure
With respect to studies relevant to industrial aluminium exposure, instillation of aluminium sulphate, potash alum, and ammonium alum into the eye resulted in conjunctivitis and purulent ophthalmitis (Grekhova et al., 1994).
Of relevance to the potential effects of human exposure to aluminium in cosmetics, instillation of aluminium starch octenylsuccinate, 70 mg in 0.1 mL, into the conjunctival sac of rabbits produced a slight reddening of the conjunctiva that was seen from 1 to 24 hr later; this was considered “unlikely” to be an ocular irritant in humans (Nair & Yamarik, 2002). When placed in the eye of rabbits as an eye shadow containing 15% of this aluminium form, the irritation potential was considered mild by the Draize classification system (Nair & Yamarik, 2002). Formulations containing 1 and 2.5% of this aluminium form were considering non-irritating in the chorioallantoic membrane vesicular assay (Nair & Yamarik, 2002). Magnesium aluminium silicate caused minimal eye irritation in a Draize eye irritation test (Hazelton Laboratories, 1968). Bentonite caused severe iritis after injection into the anterior chamber of the eyes of rabbits. When injected intralamellarly, widespread corneal infiltrates and retrocorneal membranes were recorded (Austin & Doughman, 1980).
Implantation exposure
Discs of synthetic auditory ossicle composed of aluminium oxide were implanted s.c. in the interscapular region of 16 rats and removed 1, 3, 7 and 14 days later. After 1 day, this resulted in an acute inflammatory reaction in which macrophages and neutrophils predominated and that almost disappeared after 7 days. Fibrosis began to be observed at 3 days (Ye et al., 1998).
In vitro test systems
Many reports are cited in Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, Inhalation Exposure / Intratrachael Exposure, and Effects on Humans, Effects from Occupational Exposure, Irritation, Inhalation Exposure of the ability of aluminium to cause pneumoconiosis, an inflammation of the lung that can progress to fibrosis, which is typically caused by inhalation of dust. There are also many reports of negative findings. The discrepancy may be due to the chemical form (species) of the inhaled aluminium, granular vs. flake-like particles. In this section, studies addressing the mechanism(s) of action of these irritant effects are discussed.
Corrin (1963a;1963b) noted that aluminium reacts with water but is not able to do so when coated with inert aluminium oxide. Granular aluminium coated with aluminium oxide is produced without use of lubricating agents, such as spindle oil and stearine. The author attributed many of the reports of negative effects to exposure to aluminium oxide or aluminium oxide-coated aluminium. The author also found that spindle oil- or stearine-coated aluminium powder reacted with water, presumably forming reactive aluminium hydroxide, whereas stearine-coated aluminium powder did not, and noted that the substitution of mineral product for stearine coincided with the onset of aluminium-induced pneumoconiosis. The explanation offered was that respirable aluminium particles that can react with water in the lung can be toxic.
Aluminium oxide was shown to release histamine from rat peritoneal mast cells (Casarett et al., 1968). The greatest effect, seen with 4.4 μm maximal median diameter particles at pH 6.8, was comparable to that see with iron oxide but less than seen with chromium oxide. This may contribute to bronchoconstriction caused by inhaled aluminium particles. Four clays containing aluminium silicate (montmorillonite, bentonite, kaolinite and erionite), caused lysis of human umbilical vein endothelia, N1E-115 neuroblastoma and ROC-1 oligodendroglial cells (Murphy et al., 1993). The authors suggested these clays might disrupt the BBB, allowing their entry into the brain. Exposure of mouse peritoneal macrophages, human type II alveolar tumour (A549) cells and Chinese hamster V79-4 lung cells to 11 minerals, including short and long fibres of attapulgite (a hydrated magnesium aluminium silicate) revealed some dusts that were non-toxic to all three cell types, some that were toxic toward mouse peritoneal macrophages, as shown by LDH release, and some that were toxic to all 3 cell types, additionally causing increased diameter of the A549 cells and reduced survival of 79-4 cells (Chamberlain et al., 1982). The results suggest differential sensitivity of cells to toxicity produced by these dusts and that long-fibred dusts are more toxic than short-fibred dusts. Exposure of rabbit AM to hydrated aluminium silicate resulted in toxicity, as evidenced by reduced viability and ATP (Hatch et al., 1985). Hydrated aluminium silicate caused concentration-dependent haemolysis of erythrocytes (Woodworth et al., 1982). Murine neuroblastoma cells exposed to hydrated aluminium silicate showed an increase in membrane electrical conductance and loss of excitable activity, as evidence of toxicity (Banin & Meiri, 1990).
Immunotoxicity/Immunosuppression
The immune system and its reactions involve interactions between various cell types and soluble mediators. These responses can be clustered into innate (natural and non-specific) and acquired (adaptive) responses for which the reaction is directed to an antigenic determinant or epitope. Non-specific responses involve effector cells such as macrophages, natural killer cells, granulocytes, and mediator systems such as the complement system. Biologically, components of the immune system are present throughout the body and interactions between the immune system and other organ systems are a normal component of immunoregulation. While a number of metals have been demonstrated to have immunotoxic properties, little has been reported for aluminium (IPCS/WHO, 1996). In addition, aluminium hydroxide has been used as an adjuvant in many human vaccines (Roit et al., 1998) (see Table 7). Vaccine efficiency is enhanced by aluminium’s capacity to absorb antigen particles forming granulomas in the point of injection. Early studies suggested altered immune responses following excess aluminium exposure. Pregnant Swiss-Webster mice exposed to aluminium (500 or 1,000 μg Al/g diet; as aluminium lactate) showed a lower resistance to bacterial Listeria monocytogene infection while non-pregnant mice showed the reverse (Yoshida et al., 1989). Acute injection of aluminium (1-10 mg/kg body weight to non-pregnant mice) resulted in a lower mortality rate to L. monocytogenes as compared to controls (Yoshida et al., 1989). In these studies, the offspring showed no differences in mortality rates. However, Golub et al. (1993) suggested that excess aluminium exposure (1000 μg Al/g diet; as aluminium lactate) in Swiss Webster mice from conception to 6 months of age resulted in alterations in immune effector cell function. Splenic lymphocytes showed a depressed response to concanavalin A. When the spleen weight was measured in mice fed 1000 ppm in the food from weaning to adulthood (4 week and 8 week exposures) no changes were detected relative to controls (Golub & Keen, 1999). Based upon these studies, Tsunoda & Sharma (1999) examined pro-inflammatory cytokine mRNA levels in the brain and immune organs of mice following a 1-month exposure to 125 ppm aluminium ammonium sulphate in the drinking water. Isolated splenic macrophages and lymphocytes showed no aluminium-related changes in the basal mRNA levels of TNFα, IL-1β, or IFNγ. However, the authors suggested that, while low and somewhat variable, basal mRNA levels for TNFα were increased in the brain of aluminium exposed mice (Tsunoda & Sharma, 1999).
Effects on the Endocrine System
With the exception of experimental studies in which the effect of aluminium exposure on the reproductive system has been examined (Effects on Laboratory Mammals and In Vitro Test Systems, Reproductive and Developmental Toxicity, Reproductive Toxicity), those designed to examine adverse effects on the endocrine system have been limited to the parathyroid response given that aluminium overload leads to PTH suppression.
Many of the PTH receptors of interest for aluminium toxicity are present in both the bone and kidney; thus, much of the data with regard to the effect of aluminium exposure on the bone discussed in Effects on Laboratory Mammals and In Vitro Test Systems, Effects On Bone is related to the alterations in serum PTH levels and calcium homeostasis. For example, Pun et al. (1990) demonstrated that lower concentrations of aluminium (4μm and 40 μM) inhibited the cyclic AMP response to PTH challenge via a decrease in PTH receptor binding in both clonal osteoblastic UMR-106 cells and in dog renal cortical membrane. Bourdeau et al. (1987) examined the endocrine response of porcine parathyroid gland tissue slices to aluminium at concentrations of 20 to 500 ng/mL. High concentrations of aluminium inhibited induced PTH release in a calcium-dependent manner. Gonzalez-Suarez et al. (2003) reported that 8 weeks of exposure to aluminium chloride (AlCl3) (i.p. daily) reduced serum PTH levels and cell proliferation in the parathyroid glands, yet did not alter serum phosphorus levels, cell apoptosis or the calcium sensing receptor expression in young adult male Wistar rats surgically nephrectomized (7/8th tissue excised). In a similar study, Diaz-Corte et al. (2001), surgically nephrectomized adult male Wistar rats maintained on a high dietary phosphorus intake received 2 daily ip. injections of AlCl3 five weeks after surgery and examined 2 weeks post-injection. While significant decreases in serum PTH levels and mRNA levels for PTH in the parathyroid gland were seen in the aluminium-injected group, no differences were seen in serum calcium and phosphorus levels, renal function or body weight. Similar decreases in plasma PTH concentrations have been reported in cats with stable chronic renal failure when maintained on a diet restricted in phosphorus and protein with aluminium hydroxide included as an intestinal phosphate binding agent (Barber et al., 1999).
Genotoxicity and Mutagenicity
Aluminium compounds have produced negative results in most short-term mutagenic assays. As early as 1976, aluminium (Al2(SO4)3) had been shown to decrease DNA synthesis without affecting replication fidelity (Sirover & Loeb, 1976). At concentrations from 20 μM to 150 mM, the accuracy of DNA synthesis in vitro was maintained. In a rat osteoblast cell line, UMR 106, DNA synthesis as determined by 3H-thimidine incorporation was shown to be decreased in the absence of an increase of protein synthesis as determined by 3H-leucine incorporation by exposure to 30 μM aluminium (Blair et al., 1989). Aluminium concentrations of 0.01 mM to 0.1 M, as AlCl3, showed no potential to induce depurination of DNA as measured by the release of adenine or guanine in calf-thymus DNA (Schaaper et al., 1987). Calf-thymus DNA was used by Ahmad et al. (1996) to examine alterations in DNA binding by AlCl3 (0.6-25 mM). These authors reported that aluminium was bound to the backbone PO2 group and the guanine N-7 site of the G-C base pairs by the process of chelation.
In bacteria, aluminium compounds have been considered, in general, to be non-mutagenic. Aluminium showed no mutagenic activity as measured by the Rec-assay using Bacillus subtilis (Nishioka, 1975). At concentrations of 1 to 10 mM, Al2O3, AlCl3, and Al2(SO4)3 were also negative in the Rec-assay with the Bacillus subtilis H17 rec+ and M45 rec- strains (Kada et al., 1980; Kanematsu et al., 1980). Both aluminium and hydrated aluminium chloride (AlCl3-6H2O) at concentrations ranging from 10 to 100 nM per plate failed to induce reverse mutations in the Salmonella typhimurium TA102 strain as identified by his gene mutations (Marzin & Phi, 1985). Positive results have been obtained in studies using dye-alumina complexes; however these (positive) results have been attributed to impurities in the complexes rather than an effect of aluminium (Brown et al., 1979). The absence of mutagenic effects of aluminium compounds on various bacterial strains including Salmonella typhimurium and Escherichia coli demonstrated in these early studies has been supported by the findings of more recent studies (Ahn & Jeffery, 1994; Gava et al., 1989; Marzin & Phi, 1985; Olivier & Marzin, 1987; Prival et al., 1991; Seo & Lee, 1993; Shimizu et al., 1985; Venier et al., 1985; Zeiger et al., 1987). In their assessment of mutagenicity, Ahn & Jeffrey (1994) failed to find positive findings with aluminium chloride (0.3 and 3.0 ppm) using the biological endpoint of his mutation induction in the absence of S9 metabolic activation in the TA98 strain. No induction of his mutations were seen by Marzin & Phi (1985) in the 102 strain exposed to aluminium chloride hexahydrate (10-100 nmol/plate), and by Gava et al. (1989) in the TA104, TA92, TA98, TA1000 strains exposed to aluminium acetylacetonate (1.9-48 μmol/plate), aluminium lactate (1.8 - 5.5 μmol/plate), or aluminium maltolate (0.5-3.7 μmol/plate). The Salmonella typhimurium strain, TA104, showed a his gene mutation to aluminium acetylacetonate (1.8 – 48 μmol/plate); however, negative responses were reported when comparisons were made between the absence and presence of S9 metabolic activation. Shimizu et al. (1985) showed no positive response to aluminium fluoride (0.02-119 μmol/plate) in TA98, TA100, TA1535, TA1537, and TA1538 strains. Zeiger et al. (1987) showed similar results in the TA98, TA100, TA1535, and TA1537 strains following exposure to sodium aluminium silicate (0.96 - 38.5 μmol/plate). Prival et al. (1991) showed no positive responses to sodium aluminium silicate (0.36 - 108.1 μmol/plate) or calcium aluminosilicate (0.033 - 10 mg/plate) in TA98, TA100, TA1535, TA1537, and TA1538 strains. Studies with Escherichia coli (WP2 strain) have shown negative responses in trp mutations with aluminium chloride (0.8 mol) (Seo & Lee, 1993); aluminium fluoride (12 nmol-1.6 mmol/plate) (Shimizu et al., 1985); calcium aluminosilicate (0.33-10 mg/plate), and sodium aluminium silicate (0.96.1 μmol/plate) (Prival et al., 1991). Using the SOS chromotest, Raabe et al. (1993) evaluated the genotoxicity potential of 36 characteristic waste products resulting from aluminium plasma etching. While a majority of the products show some genotoxic activity, a correlation between the organic constituents and biological effect could not be established. Using the common soil bacterium Rhizobium, Octive et al. (1991) reported preliminary findings showing no change in cell survival following an 18 hr exposure to 50 mM aluminium in both RDG 2002 and NZP2037 strains. In each strain, exposure to rafampicin for 3-14 days decreased cell growth; the data suggested a slight resistance in the RDG 2002 strain exposed to aluminium.
Studies have shown that aluminium compounds can inhibit cell division and produce chromosomal aberrations in plants. The relevance of data derived from plant studies to assess the carcinogenic potential in mammalian systems has come under question given that metal salts of various carcinogenic potential can give similar results in short-term plant assays (Léonard & Gerber, 1988). An early study by Gelfant (1963) showed that mammalian cell division can be inhibited by aluminium salts; however, later studies showed that this did not translate to morphological transformation of Syrian hamster embryo cells. In these cells, AlCl3 and Al2(SO4) at concentrations up to 20 μg/mL medium neither caused morphological transformation nor enhanced their transformation induced by a simian adenovirus SA7 (Casto et al., 1979; Di Paolo & Casto, 1979). In the L5178Y mouse lymphoma assay, AlCl3, 500-620 μg/mL, did not induce forward mutations at the thymidine kinase locus (Oberly et al., 1982). Chromosome aberrations have been reported in spermatocytes of grasshoppers (Phloeoba antennata) 48 – 60 hr post aluminium chloride (10 mg/0.21 g b.w.; Manna & Parida, (1965)) and in mammalian peritoneal cells (Nashed, 1975). Aberrations in the bone marrow cells of mice injected with aluminium chloride (0.1M aluminium chloride 1 mL/30 g b.w., acute ip. dose) have also been reported in a single study (Manna & Das, 1972).
Aluminium is known to act as a cross-linking agent for various cellular filaments. Using ascites hepatoma cells from Sprague-Dawley rats, Wedrychowski et al. (1986a; 1986b) reported that AlCl3 could serve as a stimulator for the crosslinking of chromosomal proteins. However, in an Epstein-Barr virus-transformed Burkitts human lymphoma cell line, AlCl3 showed no ability to crosslink DNA protein at either cytotoxic or non-cytotoxic concentration levels (Costa et al., 1996). Human blood lymphocytes showed positive responses for both micronuclei formation (Migliore et al., 1999; Roy et al., 1990) and sister chromatin exchange (Roy et al., 1990) at AlCl3 levels from 11.6 μmol/mL (Roy et al., 1990) to 500 - 4000 μM (Migliore et al., 1999). Roy et al. (1990) reported that the increase in micronuclei formation was significant only in cells obtained from adult donors, and sister chromatin exchange was significant only in cells from females. The chromatid aberrations reported in the Roy et al. (1990) study were limited to an increase in gaps and breaks and the study was done in phosphate-free media. One additional study (Alfaro Moreno et al., 1997) conducted using BALB/c mouse 3T3 cells reported anaphasic alterations as a possible step in the process of chromosomal dysfunction following exposure to Mexicali dust (98% potassium aluminium silicates and 2% sodium dioxide- 0.67 mMl/L). The work of Karlik et al. (1980a; 1980b) suggested that interactions of aluminium with DNA would be dependent upon pH.
During the 1990s, a number of investigators conducted experiments to determine the genotoxic potential of aluminium compounds administered to the whole animal. Short-term studies examining changes occurring within the first 24 - 48hr of systemic dosing with aluminium compounds showed contradictory results. Isolated DNA from the liver of male Wistar rats showed no changes in the formation of 8-hydroxydeoxyguanosine (Umemura et al., 1990) following a high dose of aluminium nitrilotriacetate complex (7 mg Al/kg [259 μmol/kg] i.p.). Takagi et al. (1990) exposed F-344 rats to 1.2% aluminium clofibrate in the diet; within 1-12 months of exposure the rats showed an elevation in hepatic peroxisomal beta-oxidation enzyme activity. One early preliminary study using high levels of aluminium chloride (0.01 - 0.1 mol/mouse, i.p.) reported the induction of chromosomal aberrations (Manna & Das, 1972). Two later published studies used high dose levels of aluminium sulphate (100 - 500 mg/kg bw (0.3 - 1.5 mmol Al/kg b.w.)) as a known inducer of either micronucleated polychromatic peripheral erythrocyte (mnPCE) formation (Roy et al., 1992) or SCEs (Dhir et al., 1993) in murine bone marrow cells. As expected, a significant increase in mnPCEs was induced 24 hrs after a second aluminium dose of 500mg/kg b.w. in Swiss albino mice. No changes were seen at the 250 mg/kg b.w. dose level (Roy et al., 1992). The work of Dhir et al. (1993) showed an induction of SCEs in bone marrow from male Swiss albino mice in a dose-related fashion 24 hr after a single dose of aluminium sulphate. The induction of SCEs was detected by bromodeoxyuridine (BrdU) pre-labelling (50 mg BrdU paraffin-coated tablet implanted subcutaenously) followed by a single dose of aluminium sulphate (100, 200, or 400 mg/kg b.w.) and a single i.p. injection of colchicine (4 mg/kg b.w.) 22 hrs later.
Studies in which in either male rats (Rattus norvegicus) or sheep were exposed to complex mixtures containing aluminium were indicative of positive changes. Bauer et al. (1995) reported mnPCE formation in male and female Wistar rats that received vacuum pump oils contaminated by waste products from a BC13/C12 aluminium plasma etching process (dose of 1000 mg/kg/day in a gavage dosing volume of 0.54 mL/kg/day). Numerous compounds and elements were identified in the waste; however, the aluminium contribution to the waste was at approximately 2000 ppb. MnPCE formation in bone marrow was not detected until the animals had been exposed for 28 days. In another mixture study, the emissions of aluminium and other ionic forms of metals from an aluminium refining plant were administered orally in distilled water for one year to sheep (Sivikova & Dianovsky, 1995). The total calculated concentration of aluminium delivered was 1.1 or 2.4 mmol Al/animal/day. A significant increase in SCEs was found in the cultured lymphocytes of the high dose group only. A mitotic delay in both dose groups was reported by the authors based upon differences in cell metaphases relative to that seen in the controls. Spotheim-Maurizot et al. (1992) reported on the antigenotoxicity of aluminium. The in vitro frequency of single- and double-stranded breaks in plasmid DNA, as induced by radiation, was significantly inhibited by low concentrations of aluminium chloride. A 50% reduction in double-stranded breaks occurred with 0.2 mM aluminium chloride. A similar reduction was seen in single-stranded breaks with 0.04 mM concentration levels. The authors speculated that the reduction was due to structural changes occurring in the DNA that prevented the access of OH+ radicals.
Carcinogenicity
Overall, experimental animal studies have failed to demonstrate carcinogenicity attributable solely to aluminium compounds (for reviews, see ATSDR, 1999; Furst, 1971; Furst & Haro, 1969; Haddow & Hornig, 1960; IPCS, 1997; Shubik & Hartwell, 1969). The data that exist for aluminium compounds support the relevance of the physical characteristics of aluminium in relation to the adverse endpoint under study as was suggested by Krueger et al. (1984). As an example, in a very early study, O’Gara & Brown (1967) reported an increase in sarcomas (8 out of 18 rats) in NIH black rats implanted s.c. with 0.05 mm thick aluminium foil. However, intrapleural or i.p. administration of 3.5 μm diameter aluminium fibres to rats showed no indication of carcinogenicity (Pigott & Ishmael, 1981; 1992; Stanton, 1974). It has been proposed that the results of these studies support the hypothesis put forth by Bischoff & Gyson (1964) that the dimension of the implant rather than chemical composition is related to carcinogenicity (for review, see Krueger et al. (1984)). A similar pattern is evident in inhalation studies. Steinhagen et al. (1978) reported a dose-related increase in lung lesions in both rats (Fischer 344 males and females) and guinea pigs (Hartley) following inhalation of aluminium chlorhydrate for six months. Fifty-percent of the animals showed lesions following exposure to 2.5 mg/Al/m3 increasing to 100% of animals that had been exposed to 25 mg Al/m3. In guinea pigs, approximately 10% showed an increase in AM at a low dose of 0.25 mg Al/m3. The pathology was characterized by an increase in mononuclear inflammatory cells and large macrophages in alveoli around the termination of air passageways. These data suggest a progression in the inflammatory response of the lungs with aluminium chlorohydrate exposure that may be related to the tissue response to foreign bodies and the associated irritant response. A later study showed no increased tumour incidence in rats (male and female Wistar) following inhalation of alumina fibres (2.18 or 2.45 mg/m3; Al2O3 and approx. 4% silica) of a similar diameter for 86 weeks (Pigott & Ishmael, 1981). When Al2O3 dust was intratracheally instilled in hamsters, Stenback et al. (1973) concluded that Al2O3 was not carcinogenic for the respiratory system. Of two additional early studies Schroeder & Mitchner (1975a) reported an increased incidence in gross tumours of approximately 52% as compared to 16% in control male Long-Evans rats exposed to aluminium (KAl(SO4)2) at a concentration of 5 ppm in drinking water for approximately 2-2.5 yr. This was observed in males only. In the second study, no increase in the incidence of neoplasms in either male or female Wistar rats exposed to aluminium phosphide/ammonium carbamate in the diet for 2 years was reported (Hackenberg,1972).
A limited number of studies have been conducted in mice. Two studies examined the effects of oral exposure to aluminium potassium sulphate in male and female mice. Schroeder & Mitchener (1975b) reported an increase in the incidence of gross tumours in female Swiss Webster mice (46 vs. 30%) following exposure to 5 ppm aluminium in drinking water (1.2 mg/kg b.w./day for 2-2.5 years). A more recent study by Oneda et al. (1994) showed no increase in the incidence of gross tumours, neoplastic lesions, or other proliferative lesions in B6C3F1 mice following dietary exposure to up to 979 mg Al/kg/day for 20-24 months. Interestingly, in all groups, the incidence of spontaneous hepatocellular carcinoma was significantly decreased in the females. This was also significantly decreased in the high-dose group males (5.5 vs. 20.5% in controls) and the incidence of myocardial eosinophilic cytoplasm showed a dose-dependent decrease with aluminium exposure. When the route of delivery was changed and an i.p. injection of Al2O3 was given in male and female mice at 2 and 3 months of age, examination at the end of normal lifespan showed an increase in mesothelioma in the peritoneum (Frash et al., 1992).
Aluminium compounds have been proposed as possible chemotherapeutic agents. Experimental animal studies showed that aluminium nitrate (50 - 400 mg/kg; optimal dose: 150mg/kg b.w.) could significantly reduce the growth of i.p. transplanted Walker 256 carcinosarcomas in female Sprague-Dawley rats (Adamson et al., 1975; Hart & Adamson, 1971). A similar effect was not evident against P388 leukaemia cells, L1210 leukaemia, K1964 leukaemia, YPC-1 plasma cells, or Ehrlich ascites carcinoma cells. These effects may be related to differential uptake of aluminium by these cells (Adamson et al., 1975) or, alternatively, by direct alterations of the immune response (Pauwels et al., 1979). Pretreatment with a s.c. injection of 0.1 mL 0.2% aluminium chloride (AlCl3 - 6H2O) reduced the number of developing nodules induced by dimethyl nitrosamine (Yamane & Ohtawa, 1979).
Interactions Between Aluminium and Other Agents
With additional investigation, the roles of social, environmental, and biological factors as either modifiers of potential risk or susceptibility factors for adverse effects from chemical exposure are becoming more evident. With regards to genetic factors, Fosmire et al. (1993) examined the level of aluminium in the brains of 5 strains of inbred mice following dietary exposure to 260 mg Al/kg b.w. for 28 days. While no difference could be detected between the A/J, BALB/c, and C57BL/6 strains, higher levels of aluminium were seen in the brains of DBA/2 and C3H/2 strains. Interestingly, the C3H/HeJ and the C57BL/6 strain differ in bone density (Beamer et al., 1996) and calcium metabolism which is thought to occur partially via the vitamin D and PTH endocrine systems’ regulatory influence on extracellular calcium (Chen & Kalu, 1999), both of which can be influenced by aluminium. Tf plays a significant role in the biological availability of aluminium to organ systems. The ability to saturate Tf with iron was reported to be approximately 10 times greater in C57BL/6 and BALB/c mice than in DBA/2 and AKR mice; however, serum Tf levels were equivalent across these strains (Leboeuf et al., 1995). For a discussion of other potentially modifying factors such as age, and interactions with other chemical species, see Toxicokinetics.
EFFECTS ON HUMANS
Case Reports
Although the majority of human cases of aluminium toxicity have been reported in either preterm infants or patients with renal failure, several case reports of aluminium toxicity have been documented in other populations (Nieboer et al., 1995). Reports specifically related to infants and renal impaired patients are included in the Effects on Humans, Subpopulations at Special Risk section. Case reports allow for potentially important adverse consequences to be identified; however, this information is not collected in a controlled, systematic manner and, in general, includes data obtained from small sample sizes. Therefore case reports can not be generalized to support causal associations and are not subject to statistical verification for biological relevance.
A case of osteomalacia was reported subsequent to chronic antacid use in a patient with normal renal function (Woodson, 1998). The 39 year old female patient consumed approximately 70g of a commercial antacid daily for approximately 8 years, which is equivalent to consumption of over 18 kg of elemental aluminium. The daily dose ingested by this individual exceeded the manufacturer=s recommended maximum daily dose for this antacid. The patient developed severe osteomalacia due to phosphate depletion. However, the patient=s condition improved after cessation of antacid use and supplementation with calcium, phosphate and vitamin D.
Severe osteomalacia secondary to ingestion of large amounts of an aluminium- containing antacid was also found in a 75-year old woman (Kassem et al., 1991). This patient presented with symptoms of severe osteopoenia, muscle pain and elevated levels of serum alkaline phosphatase activity. Medical records revealed that she consumed 70-85 mL of antacid daily, which is equivalent to 8-9 g of aluminium hydroxide. The patient=s condition improved after cessation of antacid use and treatment with vitamin D2, calcium, phosphate and sodium fluoride.
Gilbert-Barness et al. (1998) reported the case of a 9-year-old female who was found not to be progressing developmentally at the age of 2 months. At the age of 4 months the child was diagnosed with a neurodegenerative disorder with severe mental retardation. Congenital metabolic disorders were considered as a manifestation; however, all enzyme levels related to these disorders were within the normal range. The infant had a high Apgar score at birth and there was no recorded neonatal distress. The patient’s condition progressively worsened, resulting in death at the age of 9 years. Autopsy revealed CNS cortical atrophy, small basal ganglia, and hypomyelination of the spinal cord, cerebral cortex, subcortex and cerebellar white matter. Following autopsy it was discovered that the mother had taken an average of 75 Maalox® tablets (containing 200 mg of aluminium hydroxide per tablet) each day during the pregnancy. These results suggested that the high levels of aluminium intake by the mother, during critical periods of the foetus’ brain development, resulted in neurological damage to the infant. It should also be considered that concurrent ingestion of aluminium-containing antacids with acid-containing foodstuffs can results in enhanced GI absorption of aluminium (Tytgat et al., 2003). Pregnant women may be more likely to consume these types of foods (e.g. orange juice, yogurt), further adding to the concern of aluminium-containing antacid use during pregnancy.
Reusche et al. (2001b) reported a case of fatal aluminium encephalopathy following reconstructive otoneurosurgery in which bone reconstruction was performed with aluminium-containing cement. Six weeks after the surgery the 52 year old female patient experienced disturbances of consciousness, subacute coma, and grand-mal seizures. The patient died 6 weeks after presentation of these symptoms, due to septic complications. The role played by aluminium in this fatality remains unclear. Two similar cases of encephalopathy following the surgical use of aluminium-containing bone cement were reported by Hantson et al. (1994). A 29 year old man died from brain dysfunction following surgery for Meniere’s disease in which aluminium containing bone cement was used to bridge bone defects. The same operation was conducted on a 54 year old female. This patient developed confusion, alterations of consciousness, and grand-mal seizures and the patient died 80 days after surgery. In both cases the bone cement came in contact with CSF (Hantson et al., 1994).
Several cases of adverse reactions to aluminium in vaccines were reported. MMF is a type of inflammatory myopathy characterized by muscular infiltrate of macrophages containing aluminium inclusions (Di Muzio et al., 2004). Several cases of macrophagic myofaciitis were observed following i.m. injection of aluminium containing vaccines in both adult (Authier et al., 2001; Gherardi et al., 2001) and paediatric populations (Di Muzio et al., 2004; Gherardi et al., 2001; Lacson et al., 2002; Nevo et al., 2004). Clinical features of this condition include diffuse muscle pain, muscle weakness, fever, and in some cases overt CNS manifestations are apparent (Authier et al., 2001; Di Muzio et al, 2004). Authier et al. (2001) reported 7 cases of MMF accompanied by demyelinating CNS symptoms. All of these patients received an aluminium-containing vaccine 3-78 months before the presentation of symptoms. Gheradi et al. (2001) recorded information regarding immunizations for 50 patients presenting with macrophagic myofaciitis. All patients received aluminium hydroxide-containing vaccines 3-96 months before biopsy. The low prevalence of this disorder in relation to the number of individuals who were vaccinated with aluminium-containing adjuvants suggests that there is inter-subject variability in the elimination of aluminium. This would account for differences in the occurrence of MMF in only a predisposed subset of individuals with impaired ability to eliminate aluminium from muscle (Nevo et al., 2004). MMF has been linked to aluminium hydroxide-containing vaccines due to the detection of aluminium hydroxide crystals within MMF lesions, a temporal relationship between vaccination and occurrence of MMF, and location of the lesions at the same site where aluminium hydroxide-containing vaccine was administered (Gherardi et al., 2001). However, due to the invasiveness of the biopsy procedure, cases of MMF have not been compared with asymptomatic controls (Netterlid et al., 2004) and the pathogenic mechanisms through which aluminium induces this disorder is currently unknown (Authier et al., 2001).
Contact allergic reactions to aluminium following injection of aluminium-adsorbed vaccines have also been documented (Akyol et al., 2004; Bergfors et al., 2003; Cox et al., 1988a; 1988b, Netterlid et al., 2004) (see also Effects on Humans, Effects from Non-Occupational Exposure, Irritation, Irritation after Injection of Aluminium-Adsorbed Proteins (Vaccines and Hyposensitization Regimens)). This reaction is usually manifested by symptoms including itching nodules or granulomas at the injection site (Bergfors et al., 2003). Hypersensitivity to aluminium is considered to be a rare occurrence (Akyol et al., 2004; Bergfors et al., 2003). Netterlid et al. (2004) conducted a prospective cluster randomized surveillance study to estimate the extent of late local reactions and contact allergic reactions to aluminium in 22,365 Swedish 10-year olds following vaccination with an aluminium-containing diphtheria-tetanus vaccine. The presence of local itching nodules persisting for at least 2 months was detected in 3-6 per 10,000 children (Netterlid et al., 2004).
Effects from Occupational Exposure
Respiratory tract effects
Aluminium smelter (potroom) workers
There have been numerous reports of the effects of working in the aluminium industry on the respiratory tract, particularly in aluminium potroom (or smelter) workers. Asthma-like symptoms, known as “potroom asthma” have been reported in several studies. The exact cause of potroom asthma has not been conclusively established, but hydrogen fluoride and particulate fluoride have been implicated (Barnard et al., 2004; Soyseth & Kongerud, 1992). Radon et al. (1999) further suggested that lung function impairment may be only partly due to fluoride exposure and that working in aluminium carbon plants (with exposure to carbon dusts) may cause acute lung function changes. A detailed review of studies (in chronological order) follows.
A cross-sectional respiratory survey of white males conducted from October 1979 to May 1980 at an aluminium smelter in British Columbia (Chan-Yeung et al., 1983) compared the results in 797 potroom workers, 495 with high exposure (who spent more than 50% of their working time in the potroom) and 302 with medium exposure (who spent less than 50% of their working time in the potroom), with 713 men with no significant exposure to air contaminants (who worked in the office and casting departments). There were two components to the study, a health study and an industrial hygiene survey. For the health study, trained interviewers administered a questionnaire using a standardized technique; data on smoking habits and detailed occupational history were collected. A limited physical examination was conducted, including respiratory symptoms, and tests for common skin allergens. Chest roentgenograms were obtained (from 60% of workers), and pulmonary function tests were conducted. To determine if work place exposure to contaminants had acute effects on the airways, pre-shift and post-shift spirometry was conducted on a subset of the medium and high exposure groups. In addition, pre-shift, post-shift and end of the work week urine samples were collected to measure urinary fluoride levels. The industrial hygiene survey involved personal sampling on representative workers of different job categories for levels of air contaminants, including total airborne particulates, gaseous and particulate fluoride, carbon monoxide, sulphur dioxide and benzo-alpha-pyrene. Highly exposed potroom workers were significantly younger, were employed for significantly shorter periods of time, and were more likely to smoke than the control group. Potroom workers had a greater prevalence of cough, sputum, and wheeze compared with the control group; these results were statistically significant for the high exposure group but not for the medium exposure group. Less than 10% of the workers who had a chest radiograph exhibited abnormal findings; most of these changes were determined to have resulted from previous pulmonary tuberculosis. Chest radiographs revealed the presence of diffuse reticulonodular shadows in 28 workers with no association with any particular group. When pulmonary function was adjusted for age, height, duration of employment and smoking, potroom workers in the high exposure category were found to have significantly lower mean FEV1 and lower maximal mid-expiratory flow rate. The same result was observed in the medium exposure group, but was not statistically significant. Pulmonary function was lower when measured post-shift as compared to the pre-shift measurement in both potroom and control workers. Urinary fluoride levels increased in potroom workers after one shift, while the control group exhibited a slight decrease in urinary fluoride levels after one shift. Results from personal samplers revealed that potroom workers were exposed to higher levels of air contaminants (total particulate, fluoride, carbon monoxide, sulphur dioxide, and benzo-alpha-pyrene) than the controls. These results suggest that the increased frequency of adverse pulmonary effects seen in the potroom workers, compared with the controls, may be a result of the irritant properties of the air contaminants on the potline. Although no cases of asthma were detected in the potroom workers, five workers in the control group indicated that they left the potroom as a result of asthma. In interpreting the results of this study, it must be considered that only 60% of the sample received chest radiographs. In addition, the article states that many of the workers had previously been miners which could have had an impact on the workers= current pulmonary status.
The possible role of atopy in the occurrence of acute bronchoconstrictive impairment was investigated in a cross-sectional study of 227 workers using the Alu-Swiss process for the electrolytic extraction of aluminium (Saric et al., 1986). Subjects underwent an examination which consisted of an interview regarding respiratory symptoms and history of atopy, measurement of FEV1, and intradermal skin testing with common allergens. Bronchial hyperreactivity was assessed using non-specific bronchoprovocative tests with histamine or methacholine. The fraction of workers diagnosed with atopy was within the expected range. Seven workers exhibited signs of paroxysmal wheezing and dyspnoea. Three of these workers had a positive skin test, but only one worker had increased IgE levels. Bronchoprovocative tests with histamine or methacholine indicated the presence of bronchial hyperreactivity in five of these workers. These results suggest that bronchial hyperreactivity is an important mechanism in the occurrence of acute respiratory impairment. Atopy did not appear to be associated with the asthma-like symptoms.
O’Donnell et al. (1989) reported on 57 cases of potroom asthma at an aluminium smelter from early 1971 to the end of 1986. Beginning in 1982, subjects’ symptoms were diagnosed at biennial medical examinations. When asthma was suspected, the subject underwent skin tests to assess background atopy, blood eosinophil count, serum IgE concentration, a progressive incremental exercise test, and the measurement of nonspecific bronchial reactivity to methacholine. All employees had regular chest X-rays. Thirty-one subjects had been diagnosed with asthma prior to the study commencement in 1982, when these employees were assessed in detail for the first time. All subjects were transferred from potrooms to other areas of the smelter after diagnosis. From 1982, subjects underwent annual physical examination, spirometry and assessment of non-specific bronchial reactivity. Subjects were followed until the end of 1986. Seventeen of the 57 subjects tested positive for atopy, and 34 subjects exhibited nonspecific bronchial hyperreactivity to methacholine. The majority of workers showed improvement of symptoms during the first 2 years after diagnosis and transfer from the potrooms. However, deterioration of symptoms, as assessed by return to abnormal nonspecific bronchial reactivity, was noted in several subjects. Forty-three subjects were available for review in 1986. Twelve reported frequent or persistent symptoms of asthma, and a further 11 were taking some regular medication for intermittent symptoms of asthma. Persistence of symptoms may be a reflection of the severity of the asthma. It is possible that workers affected more severely also experienced persistent epithelial damage or continuing inflammatory changes due to irritant particulate in the airways. It should be noted that 16 of the 31 subjects diagnosed with asthma had been transferred from potroom work prior to the detailed functional assessment in 1982. These patients had time to recover from the potroom exposure, and the baseline symptom measurements may not reflect their symptoms at the time of diagnosis. This was essentially a monitoring of the respiratory health of potroom workers with a view to moving those who were adversely affected to different jobs, rather than an epidemiological study.
Mackay et al. (1990) conducted a case control study to test the hypothesis that immunological determinants and certain genetic markers are related to the risk of developing asthma in aluminium smelter employees. Data were collected from 33 asthmatic (23 prevalent cases, 10 incident cases) and 127 non-asthmatic potroom workers stratified by age and length of service. Subjects were tested for atopy (positive wheal and flare response to environmental antigens), serum Ig levels, autoantibodies, and antinuclear antibodies. The genetic markers assessed included typing for ABO blood groups, and standard microcytotoxicity for A and B locus human leucocyte antigens for histocompatibility testing. Sixty-four percent of the asthmatic workers had a background of atopy while the remaining 36% of the group did not. Of the asthmatics who were atopic, 48% had elevated IgE levels; however, the difference in frequency of IgE levels greater than 100 IU/mL was not significant between the asthmatic and the nonasthmatic group. No other major differences in Ig levels, genetic markers, frequency of antinuclear or other autoantibodies were noted between the case and control groups. The results of this study suggest that the immune parameters and genetic markers assessed did not have a significant impact on the risk of developing aluminium smelter-related asthma.
Respiratory symptoms and lung function were examined in 1,679 male and 126 female potroom workers from seven Norwegian aluminium reduction plants (Kongerud et al., 1990; 1994; Kongerud & Samuelsen, 1991). This longitudinal study began in 1986 and the active work force was examined each year for a period of four years. All subjects leaving their occupation and all new employees were recorded until the end of 1989. Self-administered questionnaires were employed to record workers= symptoms of dyspnoea, wheezing and cough during the previous year. Information about allergy, smoking habits, former work exposure, length of employment in the potrooms, duration of symptoms and use of airway protection was also recorded. FEV1 and FVC was measured by spirometry. Personal samplers were used to obtain measurements of total airborne dust and total fluorides. A total of 9 exposure categories was established within each plant. Subjects within the same exposure category were assigned identical exposures based on the average level of total airborne dust and total fluorides for the previous year.
Kongerud et al. (1990) reported the results of a cross-sectional survey from this Norwegian cohort covering the period from the beginning of September to the end of October, 1986 in the 7 aluminium reduction plants. This analysis, which covered 1,760 members of the active work force during this period, demonstrated a significant association between length of potroom employment and the presence of work-related asthma. Work-related asthmatic symptoms occurred in 15% of the workers with an exposure period of 10 years or more, and in 8% of the workers who had been employed for less than 5 years. The odds ratio (OR) for asthmatic symptoms was 3.44 (95% CI: 2.06-5.76) for workers employed for 10 years or more compared to workers exposed for less than five years after controlling for sex, age, familial disposition for asthma, allergy, smoking, and use of airway protection. Airflow limitation was also significantly higher for workers employed for over 10 years as compared to those employed for less than 5 years (OR= 2.6, 95% CI: 1.68-3.85).
A prospective study of the respiratory health in Norwegian aluminium potroom workers was also conducted of newly enrolled workers who were examined on at least 2 occasions from January 1, 1986 to December 31, 1989 (Kongerud & Samuelsen, 1991). Dyspnoea and wheezing were reported by 105 of the 1301 workers during the follow-up. Symptomatic and asymptomatic workers did not differ in FEV1 or FVC measurements. The probability of developing symptoms was 7% for people who had never smoked, and 23% for those who currently smoked, during the first 2 years of employment. Smoking and total fluoride exposure were determined to be the most important predictors of development of dyspnoea and wheezing, based on proportional hazards regression analysis. The incidence of respiratory symptoms was highest in the first year of work and appeared to decrease after two years of exposure. The estimated probability for the development of symptoms was approximately 20% after 4 years of exposure. Proportional hazard ratios by smoking status compared to those who never smoked were: ex-smokers 4.39 (95% CI: 1.30-14.82), low 2.19 (1.23-3.91), and high 3.29 (1.72-6.29). Compared to the lowest level of fluoride exposure of 0-0.4 mg/m3, the hazard ratio for 0.41-0.8 mg/m3 was 3.35 (1.51-7.41), and for >0.8 mg/m3 was 5.20 (2.02-13.34). Some of these CIs are broad, indicating small numbers which give a less stable estimate of the risk.
To investigate whether chronic exposures in potrooms decrease pulmonary function, Kilburn & Warshaw (1992) compared the pulmonary function status of aluminium refinery workers to that of regional controls who worked as pipefitters. Selection criteria included having had initial occupational exposure to asbestos at least 15 years previously, and a duration of exposure of 5 years. The controls were volunteers who may not have been representative of their occupation, but their age, height, weight and smoking status were well matched with the aluminium refinery workers. Both groups were exposed to asbestos. The referent group was also exposed to ferrous metal welding and copper brazing, but not aluminium. Six hundred and seventy aluminium workers and 659 controls were included in this analysis. Subjects completed health questionnaires, received a physical chest examination, and chest radiograph. Spirometry was performed and alveolar carbon monoxide was measured. The prevalence of reported asthma was 13.5% in the aluminium exposed group as compared to 8.7% in the comparison group (p < 0.05). Wheezing heard at the physical examination was also significantly higher in the aluminium workers (32%) as compared to the pipefitters (11.8%). The prevalence of chronic bronchitis was equal between the two groups. The occurrence of irregular opacities in the lung was higher in the aluminium workers, and the pattern of these irregularities differed from that of the regional controls. The prevalence of asthma in non-smoking workers was 7.6% for the aluminium group and 4.0% for the controls; wheezing was detected in 21.4% of non-smoking aluminium workers compared to 2.4% of non-smoking controls. Asthma prevalence was 16.4% for aluminium workers who were current smokers vs. 9.4% in the referent group, and wheezing was heard in 43.7% of aluminium workers who were current smokers compared with 21.1% in the controls. These results suggest that aluminium workers had significantly more asthma, wheezing, airway obstruction and pulmonary parenchymal abnormalities than did a control group of a similar socio-economic status and type of employment not involving exposure to aluminium. Due to the lack of industrial hygiene measurements of exposure to gases, fumes, and other particulates, it is difficult to speculate on whether a specific agent was responsible for the higher occurrence of asthma in the aluminium workers. The differences in mean pulmonary function values were not attributed to asbestos because both groups had similar rates of exposure and prevalence of asbestosis. It is possible that systematic differences in terms of lifestyle and other previous exposures between the two groups of workers might have been responsible for the differences in respiratory symptoms.
Sorgdrager et al. (1995) conducted a nested case control study in two Dutch aluminium production plants in order to identify workers with an increased risk of developing potroom asthma. The cases and controls from this study were drawn from two aluminium smelter plants. The first group contained workers employed in plant A between 1978 and 1988; the second consisted of workers from plant B employed between 1971 and 1991. Cases were defined as potroom workers who were unable to work because of respiratory symptoms, and who met clinical criteria for potroom asthma. These cases were identified through worker consultation with the occupational health officer for respiratory complaints, or through periodic medical examinations. Cases were matched to controls (co-workers who did not have potroom asthma) based on age and year of employment commencement. In Plant A, 57 out of 300 workers were diagnosed with potroom asthma, while 174 of 1,208 employees were diagnosed with potroom asthma in plant B. After the matching process, 182 case-control pairs were identified from the two plants combined and included in the analysis. Subjects underwent pre-employment examinations which included spirometry, bronchial responsiveness, and blood eosinophil count. Leucocyte count was also obtained from workers in plant B. Lung function did not differ between the cases and controls in plant A. In plant B only, FEV1/FVC was significantly lower in cases than in controls at pre-employment examination. Pre-employment eosinophil counts were significantly higher in cases than in controls in the workers of both plants (OR = 1.28, p = 0.0002 controlling for childhood respiratory problems, positive family history, smoking and lung function). These results suggest that the development of potroom asthma is not related to lung function or bronchial responsiveness before employment. Elevated blood eosinophil count may be a risk factor for potroom asthma for workers without clinical signs of respiratory disease prior to employment. The authors suggested that the combined effects of fluoride exposure with elevated eosinophil counts might induce an immunological or cytotoxic process.
Three hundred and seventy aluminium potroom workers in a Norwegian plant participated in a cross-sectional survey to assess bronchial responsiveness, lung function, and respiratory symptoms in relation to occupational exposure to air contaminants in the potroom (Soyseth & Kongerud, 1992). Three hundred and thirty-seven subjects completed a questionnaire regarding respiratory symptoms within the previous year, familial asthma, smoking habits, and years of employment in the potrooms. Work related asthmatic symptoms were defined as dyspnoea and wheezing which improved during absence from work. Lung function tests and methacholine challenge for bronchial responsiveness were conducted. An estimate of exposure to dust and fluorides was made for each worker based on job specific exposure measurements and personal sampling equipment. Adjusted ORs were statistically significantly elevated for subjects exposed to total fluorides above 0.5 mg/m3 compared to workers exposed to total fluorides at concentrations less than 0.5 mg/m3, for dyspnoea only, dyspnoea and wheezing, cough and work-related asthmatic symptoms. The equivalent ORs for dust exposure were not elevated. These findings suggest that exposure to fluorides may be an important determinant of work related asthmatic symptoms in potroom workers. However, it should be considered that bronchial responsiveness and spirometry, which are more objective measures of respiratory function, were not associated with exposure to fluorides or dust. Therefore, it is possible that the positive association between exposure to fluoride and respiratory symptoms is a spurious result because of over-reporting of symptoms in the high exposure group, misclassification of exposure, or selection bias.
Søyseth et al. (1994) examined the relationship between plasma fluoride levels and bronchial responsiveness in the same 26 aluminium potroom workers described above. Subjects were tested for bronchial responsiveness and blood samples were obtained and analysed for fluoride levels every third month for two years. A positive association was found between bronchial responsiveness and plasma fluoride levels. This result contrasts with the finding of a negative association between fluoride exposure and bronchial responsiveness from the previous cross-sectional survey in the same plant (Soyseth & Kongerud, 1992). The differences between these two studies may be a reflection of their study designs. Selection bias is a concern in cross-sectional studies; in a longitudinal design subjects serve as their own controls which allows selection bias to be minimized. However, the small size of the longitudinal study may limit its value; for example, such a small subset of aluminium potroom workers may not be representative of potroom workers in general. It is also possible that fluoride levels serve as a marker for another non-fluoride containing agent that is present in the potroom and correlated with fluoride levels.
The bronchial responsiveness of the 26 members of the index group was also compared to that of 12 aluminium potroom workers who were relocated due to their work-related asthmatic symptoms (Soyseth et al., 1995). A decrease in bronchial responsiveness during the follow-up visits occurred in both groups; however, the decline appeared to be greater for the relocated workers. Subjects who were most reactive at inclusion experienced the largest improvement in bronchial responsiveness during the follow-up. The results of this study indicate that removal of potroom workers from the exposure causes a decrease in bronchial responsiveness. However, the subjects who were relocated were more likely to have more severe symptoms than the non-relocated workers. Thus the observed decrease in bronchial responsiveness may be, in part, due to regression to the mean. This phenomenon refers to the tendency of severe symptoms to naturally decline towards normal levels. Again, the small number of participants in this study may mean that the results cannot be generalised to other potroom workers.
Søyseth et al. (1996) expanded their cross-sectional survey into a longitudinal study to compare the variation in bronchial responsiveness over time between potroom workers reporting work-related asthmatic symptoms and asymptomatic workers. Of the 379 potroom workers who completed a respiratory questionnaire in the cross-sectional survey, 26 men met the inclusion criteria for the study which was age under 60 years and normal lung function during symptom free periods. A reference group was comprised of new male employees who reported no respiratory symptoms on the questionnaire. The subjects were examined at regular intervals over a period of two years. At each interval, subjects were asked to record the frequency and severity of dyspnoea, wheezing and coughing. FEV1 and bronchial responsiveness was also measured. Eight subjects in the index group shifted between bronchial hyperresponsiveness and normal bronchial responsiveness, while the degree of bronchial response did not vary for any of the subjects in the reference group. In addition, it was found that the variability of bronchial responsiveness was positively associated with mean symptom scores. One possible limitation of this study is the difference between the index group and the reference group in age (mean of 38.1 in the index group vs. 25.3 in the reference group), duration of employment, and smoking habits. However, the authors stated that neither age nor duration of employment were determinants of bronchial responsiveness in a previous experiment. The observations from this small longitudinal study indicate that severity of symptoms in aluminium potroom workers with work related asthma reflects the variability in bronchial responsiveness.
The effect of exposure to potroom pollutants on the development of lung function in potroom workers was investigated in an additional longitudinal study by Søyseth et al. (1997). All employees working in potrooms at the Hydro Aluminium Plant in Årdal, Norway, in September 1986 or later were invited to participate in this study. Employees starting work in the potrooms during the follow up were also recruited to the cohort. The workers were examined annually during the period 1986-1992; 265 were included in the final analyses. Pulmonary function was measured by spirometry Measurement of exposure was conducted by dividing the potrooms into several job categories. Each year a randomly selected number of workers were asked to wear samplers for 8 hr shifts to calculate annual mean exposures in each job category. Exposure to particulates increased the decline in FEV1. It was found that the decline in FEV1 accelerated with age and smoking. This study suggests that potroom workers may be at increased risk of developing chronic obstructive lung disease due to the decline in FEV1 as a result of particulate exposure. This contrasts with the previous findings of Søyseth & Kongerud (1992) and Søyseth et al. (1994) which demonstrated that respiratory symptoms and bronchial responsiveness were associated with fluoride levels but not with exposure to particulates. This may be explained by differences in exposure classification and participant loss to follow-up between the different studies, as well as to the small number of participants in some of the analyses.
Sorgdrager et al. (1998) conducted a study to investigate whether reduction of exposure and the introduction of the histamine provocation test (HPT) as a worker selection instrument resulted in a lower incidence of potroom asthma. The data used for this analysis originated from employment history, clinical history and some exposure measurements for 179 potroom workers (contributing 2,845 person years) who developed work-related respiratory disease between 1970 and 1990. This period was divided into three segments. No exposure data were available during period 1, (1970-1975). For period 2, (1976-1981), exposure data based on determination of fluoride in urine samples were available. In the third period (1982-1990) the HPT was incorporated in the pre-employment medical examination. After the introduction of the HPT as a selection tool in pre-employment screening, the incidence density of potroom asthma decreased from 11.6 (period 2) to 2.5 (period 3). However, potroom asthma was also shown to occur in employees with normal bronchial responsiveness. In addition, there was no difference in time lag for the cases occurring before and after introduction of the HPT. This suggests that potroom exposure acts as an inducer of hyper-responsiveness. The decrease in fluoride levels in the aluminium plant between period 2 and period 3 may have contributed to the decrease of potroom asthma observed.
Fritschi et al. (1999) conducted a cross-sectional study of 1,529 male participants in an attempt to compare the prevalence of work-related respiratory symptoms and levels of lung function in different departments within two different aluminium smelters. Employees of two Australian aluminium smelters were interviewed to obtain information regarding demographics, respiratory symptoms, and occupational history. Information was collected about symptoms of wheeze, dyspnoea, chest tightness, and rhinitis. Subjects who indicated that symptoms were improved at the beginning of the working week, on days when away from work, or when on holidays, were considered to have a work-related symptoms. Spirometry was performed on all subjects; FEV1 and FVC were recorded. Skin prick tests to common allergens were also performed to determine atopic status. Employees were divided into groups according to the type and area of work at the time of the survey. Employees in administrative type jobs were considered to be unexposed. Mean time-weighted averages were available for some job titles from the two different plants. Multiple logistic regression analysis was performed with each of the four work-related symptoms (wheeze, chest tightness, shortness of breath, and rhinitis) as outcomes. The participants from the two smelters completed an interview. Workers in the ingot process group in Smelter A had about 5 times greater odds (after adjusting for smoking and age) of reporting each of the work-related symptoms compared to the administration group. The odds of reporting work-related wheeze and chest tightness was approximately three times greater in the anode and potroom groups than in the administration group. The ingot workers in Smelter B had approximately 3 times greater odds of work-related wheeze, and potroom employees had 3.5 times the odds of work-related rhinitis, compared to the unexposed group. Rhinitis was the only symptom reported more commonly by the potroom employees than by the administration employees in smelter B. The likelihood of reporting work-related symptoms increased with duration of work in the potrooms, while ingot mill workers and anode process workers were more likely to report symptoms within the first year of work. The differences in results between the potrooms in the two different smelters may be a reflection of the age of the smelter, the type of respiratory equipment used, or the selection process for employees at the two different plants. Another major difference between the two smelters was that Smelter A produced alloy while Smelter B did not. The results of cross-sectional studies must be interpreted cautiously as selection and survival bias is often inherent in this type of design. Subjects who left the company as a result of their symptoms would not have participated in this study.
Radon et al. (1999) conducted a cross-sectional survey in a German aluminium smelter to investigate the combined influence on respiratory health of smoking and exposure in an aluminium potroom. Seventy-eight smelter employees participated in this survey (23 never smokers, 38 current smokers, and 17 ex-smokers). Fifty-seven laboratory, workshop and gate workers (18 non-smokers, 22 current smokers, and 17 ex-smokers) from within the plant served as controls. Information regarding respiratory symptoms during the preceding year, smoking habits, medical and occupational history was collected in standardized interviews. Spirometry tests were preformed for all subjects. Potroom workers who were current smokers at the time had the lowest prevalence of respiratory symptoms, while, respiratory symptoms in controls tended to be highest in those who were smokers. No effects of potroom work on the prevalence of respiratory symptoms could be detected. The highest spirometric values in the potroom workers were found in ex-smokers. In contrast, the spirometric results were lower in ex-smokers than in never smokers and lowest in smokers of the control group. Therefore, it appears that the effect of workplace exposure was greatest in subjects who did not smoke, and the consequence of smoking was only found in non-exposed subjects. This indicates the lack of a combined effect of smoking and occupational exposure. Due to the cross-sectional design of this study, workers who left the job due to airway symptoms or disease and other factors related to respiratory function could not be captured. Therefore, it is important to recognize the impact of selection processes in cross-sectional studies of occupational exposures; ignoring these effects can lead to underestimation of the health effects of the hazard. For example, in this study, comparison of only the workers who were current smokers in both the exposed and control group revealed an inverse effect of potroom work on respiratory symptoms. The results likely reflect the increased probability for least susceptible subjects to continue to smoke and work in an atmosphere with respiratory irritants.
An Australian nested case-control study assessed employee risk factors for occupational asthma in primary aluminium smelting (Barnard et al., 2004). The study included 545 workers employed in areas with moderate to high levels of smelting dust and fume. Workers were included in the study if they had had their first employment medical examination between 1982 and 1995; the cohort was followed until December 31, 2000. Cases of potroom asthma were diagnosed by a set of standard criteria during regular medical surveillance of the cohort; some cases were self-referred. Subjects with chronic obstructive pulmonary disease due to welding or metal fume were excluded. Forty-five subjects were diagnosed with occupational asthma of aluminium smelting, or potroom asthma. Four controls per case were matched for the same year of employment-commencement and age within 5 years. The pre-employment medical questionnaires were examined and information regarding demographics, details of allergic symptoms, respiratory symptoms, and spirometric data were extracted. Follow-up data were collected to obtain information regarding hay fever and family history of asthma, full work history while at the smelter, and tobacco smoking history at termination, diagnosis or at cessation of the study. The investigators were blinded to the status (case or control) of the subject. Occupational hygiene monitoring was used to obtain the mean concentrations of respirable dust, fluorides, and sulphur dioxide in all areas. This allowed for the creation of five different exposure categories ranging from grade 4 (potrooms and potroom services) to grade 0 (areas of low air contamination). A cumulative exposure index for each individual was calculated based on the years worked in a specific area. The mean exposure index for cases was 21.21 and, for controls, was 17.84. Forty three of the 45 cases were working in potroom areas at the time of diagnosis. Subjects who were diagnosed with hay fever at the initial medical examination or during the follow-up period had an increased risk of developing potroom asthma (adjusted OR = 3.58, 95% CI: 1.57-8.21). Higher forced expiratory ratio at employment reduced the risk of developing potroom asthma (adjusted OR = 0.93, 95% CI: 0.88-0.99). These results suggest that individuals with hay fever may be more susceptible to developing asthma upon exposure to airborne irritants found in aluminium smelting potrooms. The authors proposed that this may have been a result of reduced nasal filtration and increased bronchial hyper-responsiveness in these individuals.
Welders working with aluminium (n=64), stainless steel (n=46) and railroad track workers (n=149) were studied by Sjögren & Ulfvarson (1985) in relation to an age matched referent group of nonwelding industrial workers and railroad workers. Welding fume samples were measured by filters placed inside the welding helmets of the subjects. Participants were interviewed based on a questionnaire regarding respiratory symptoms. FVC and FEV1 were measured at lest three times for each subject. All three groups of welders reported higher frequencies of chronic bronchitis and other respiratory symptoms than their respective referents. Ozone appeared to be more highly correlated with the observed respiratory symptoms than aluminium particles among the aluminium welders. Pulmonary function, as assessed by FVC and FEV1, did not differ between the three groups of welders, or between the welders and the control group. Subjects with long exposure periods did not exhibit decreased pulmonary function variables.
Nielsen et al. (1993) studied 25 welders exposed to aluminium. The exposed workers were matched for age and smoking habits to 25 males employed in a warehouse for wines. Subjects were interviewed by a physician to obtain information regarding symptoms of conjunctivitis, rhinitis, pharyngitis, and asthma. The subjects were asked if these symptoms were related to work (worsened during the work week). History of chronic bronchitis and bronchial hyperreactivity was also obtained. A physical examination was performed, including pulmonary auscultation. Allergic status was inferred from information obtained about the subjects= histories of atopic symptoms, and the administration of a skin prick test to common allergens. Air sampling analysis was conducted in the breathing zones of 19 welders and a urine sample was collected from each welder on Friday after the workshift and on the following Monday morning before work. Lung function tests were conducted to measure vital capacity, FEV1, and the maximum expiratory flow. The air sampling analysis indicated that the exposure to welding fumes was lower than PELs. However, the welders showed an increase in urinary aluminium, indicating a significant uptake. A higher prevalence of work-related eye and airways symptoms was noted in the welders than in the controls. There did not appear to be an association between allergic status and the development of symptoms. Welders who had been employed for less than 2.5 years experienced more symptoms than did long-term welders. This may reflect a selection for long term workers who are not susceptible to the work-related effects. Long-term welders exhibited a steeper slope of the alveolar plateau and a slight increase in volume of trapped gas as compared to the controls, following a methacholine inhalation test. These observations indicate that welding fumes may cause increased reactivity of the small airways. The welders in this study were exposed to both aluminium and stainless steel, so it is not known to which welding fume the effects were attributable, or if the effects were a result of a combination of these fumes, or other air contaminants in the plant. However, the authors stated that aluminium welding was far more frequent than stainless steel welding in this group of workers.
Occupational exposure to aluminium dust
Pulmonary function of workers exposed to different types of aluminium dust has not been extensively studied epidemiologically. A small case-control study was conducted in Turkey (55 aluminium workers, 30 controls) to investigate the effects of aluminium dust on pulmonary function and to look for the existence of a dose-response relationship (San et al., 1997). The authors found that the mean serum aluminium levels of workers (72.7 ± 9.9 ng/mL) were significantly higher than those of controls, and reflected a range of aluminium exposures considered toxic. Spirometric parameters correlated negatively with both exposure time and serum aluminium levels. The authors stated that protective masks were not consistently worn by the aluminium workers; use of masks and working fewer hr would reduce exposure levels (San et al., 1997). Case reports presented by Mitchell et al. (1961) implicated very fine aluminium powder (finely divided aluminium) used for fireworks as a cause of pulmonary fibrosis (also discussed in Effects on Humans, Effects from Occupational Exposure, Irritation, Inhalation Exposure). The authors concluded that duration of exposure, particle size, density of the dust, and presence or absence of stearin (thought to be protective) as well as individual characteristics were all relevant to the development of pulmonary fibrosis due to aluminium exposure.
It seems that most respiratory problems experienced by aluminium workers – potroom workers, foundry workers, welders – were not due to exposure to aluminium compounds, but rather to other substances in the work place. The exception appears to be workers who were exposed to very fine aluminium dust, who developed pulmonary fibrosis; based on reports in the literature, this seems to have occurred relatively rarely – precise figures are not available.
Neurotoxicity
Sjögren et al. (1990) studied neuropsychiatric symptoms among welders exposed to potentially neurotoxic metals including aluminium, chromium, lead, manganese and nickel. They found that welders (n=78) exposed to aluminium welding fumes for at least 10 years had significantly more neuropsychiatric symptoms than the referent group which was defined as welders aged 20-29 (n=23). The composition of the referent group could have affected the authors’ conclusions, since some neuropsychiatric measures differ by age, e.g., memory problems.
Hosovski et al. (1990) compared psychomotor and intellectual abilities of 87 aluminium foundry workers with non-exposed workers matched on age, job seniority and social status. Exposure to aluminium was assessed by measuring levels in whole blood and urine. Total body burden of aluminium was assessed following the administration of desferroxamine. They found slower psychomotor reaction, dissociation of oculomotor coordination, reduced memory ability and disturbed emotional balance in the exposed workers. They concluded that these differences could be due to long-term toxic effects of aluminium.
Neurological symptoms of 25 patients, who had worked in an aluminium smelting plant for more than a decade, were described by White et al. (1992). Fifteen of the patients were working at the time of examination, and 10 had taken early retirement or medical leave due to their symptoms. The patients received a standardized evaluation which included the completion of a health questionnaire, a neurological examination, and neuropsychological evaluation. An exposure index based on level and duration of exposure in the potroom was calculated for each worker. The most common symptoms reported by the patients were frequent loss of balance (88%) and memory loss (84%). Signs of incoordination in 84% were revealed by neurological examination, and neuropsychological tests showed a substantial impairment of memory function for 70-75% of the subjects. The degree of exposure in the potroom was graded for different job categories, level 1 represented the lowest exposure to dust and fumes from the potline, and level 4 represented the highest degree of exposure. The exposure index was significantly associated with signs and symptoms of incoordination. Workers were exposed to several contaminants in the potroom including; alumina, aluminium fibres, fluorides, calcium and cryolite. The neurotoxic properties of aluminium suggest that this particular potroom exposure may be responsible for the neurological symptoms described in these workers. It should be noted that this was not an epidemiological study, but rather a case report of a fairly large number of cases. There was no referent group which could have demonstrated the differences between the cases and controls. However, this study has been frequently quoted as the basis for conducting further studies of the possible neurological effects of working in an aluminium smelting plant.
Salib & Hillier (1996) conducted a case-control study to examine the association between AD and occupational aluminium exposure. Patients with a clinical diagnosis of probable or possible AD (n=198) were compared to two control groups with respect to their occupational history. The first control group was comprised of patients with dementias other than AD (n=164); the second control group consisted of individuals with no dementia (n= 176). Informants of both cases and controls were interviewed using a structured questionnaire. The subjects= occupational histories and other personal variables were obtained. Twenty-two of the 198 cases with AD had been exposed to aluminium at some point in their occupational history, compared to 39 of the 340 controls. The OR obtained for Alzheimer=s cases compared to all controls was 0.98 (95% CI: 0.53-1.75). The OR was 0.95 (95% CI: 0.5-1.8) when the cases were compared to only the other dementias group, and similarly an OR of 0.95 (95% CI: 0.5-1.9) was observed when the non-demented subjects were used as the comparison group. These results suggest that there is no association between previous occupational aluminium exposure and the risk of AD. A limitation of this study is the lack of detail of the occupational exposure information. The questionnaire contained a broad classification of occupation by industry type and it is likely that the variation of the extent and type of aluminium exposure in specific occupational settings was not adequately captured in this study.
Another case control study examining the occupational risk factors for AD was conducted by Gun et al. (1997). A wide range of physical and chemical agents was assessed including hydrocarbon solvents, lead, mercury, organophosphates, aluminium, asbestos, vibrations, physical inactivity, and aluminium. One hundred and seventy cases with possible or probable AD from two Sydney hospitals were compared to an equal number of age and sex matched controls recruited from general practices. Informants were interviewed about the subjects= occupational histories and about other suspected risk factors. The industry and occupation titles of every job for each participant were entered into the Job-Exposure Matrix (JEM) database which is maintained by NIOSH. This database provided an estimate of the probability of exposure to specific chemicals in each occupation for each subject. Occupational hygienists used the JEM information and their own knowledge of likely exposures to assess the probability of exposure of each subject to each of the substances of interest in the study. This information was used to generate a lifetime cumulative exposure record for each subject. The lifetime exposure status, total time of any exposure, average daily duration of exposures, and the average intensity of exposure were determined for each specific exposure of interest. The estimated OR associated with possible or probable exposure to aluminium was 0.33 (95% CI: 0.01-4.16). No statistically significant associations were found among any of the exposures and the occurrence of AD. Although the exposure estimate was more detailed than the case-control study conducted by Salib & Hillier (1996) exposure sampling measurements were not available. This study is also limited by the small number of patients with significant exposure to the agents of interest.
Two studies of miners exposed to McIntyre powder (a finely ground aluminium powder), formerly used as a prophylactic measure against the development of silicosis, have been conducted to examine a possible association with cognitive impairment or AD. Rifat et al. (1990) initially reported poorer performance on a battery of cognitive tests by workers exposed to McIntyre powder than by workers who were not exposed. In a second report which examined a number of possible confounders, Rifat (1992) found interaction between mother tongue and exposure, and some effect of education and place of residence, but the observed effect still held. Nieboer et al. (1995) noted that a weakness of the study was the absence of information on exposure to other underground substances. A further report did not confirm the earlier findings (Occupational Disease Panel (Industrial Disease Standards Panel), 1998) and concluded that the results did not allow any definitive conclusion to be reached regarding the safety of historical exposure to McIntyre powder. A study of Cornish tin miners compared mortality of workers in two tin mines, one of which used McIntyre powder as described above (McDonald et al., 1996). The study did not support a link between inhalation of aluminium in powder form and AD.
In two studies designed to test specific cognitive functions, differences between workers exposed and unexposed to aluminium were seen (Akila et al., 1999; Polizzi et al., 2002; Riihimäki et al., 2000). In the earlier analysis of cognitive performance in Finnish metal inert gas welders exposed to aluminium, Akila et al. (1999) compared 28 welders with low aluminium exposure and 24 with high exposure (based on urinary aluminium concentrations) to a reference group of 28 mild steel workers. The mean urinary aluminium concentrations were 0.46 μmol/l in the reference group, 2.25 μmol/l in the low exposure group and 9.98 in the high exposure group. Blood lead levels were measured to exclude one possible confounder and were all in the normal range (0.1 – 0.4 μmol/l). Information on age, education, smoking and alcohol consumption was taken into account. The interview also included occupational history, past and present exposure to neurotoxic agents, past and present diseases, injuries, clinical symptoms, medication (including antacids containing aluminium) and questions about general health. A comprehensive neuropsychological examination was administered to assess psychomotor function, simple visual reaction time, attention related taskes, verbal and visual, or visuospatial abilities, as well as verbal and visual learning and mamory. No differences were seen on the finger tapping, Santa Ana dexterity, simple visual reaction times, verbal memory tasks, the similarities subtest of the Wechsler adult intelligence scale, or the Stroop task. However, the low exposed group performed more poorly on the memory for designs and on more difficult block design items. An exposure-response relationship was seen for the time limited synonym task, embedded figures, digit symbol speed, and the backward counting component of the divided attention task. In summary, the performance difficulties were mainly in tasks demanding complex attention, requiring working memory, and particularly in time limited tasks involving visually presented material. The more recent analysis included essentially the same study population (Riihimäki et al., 2000) (65 metal inert-gas welders vs. 52 in the earlier analysis, and 25 mild steel workers vs. 28) with referents, low-exposure and high-exposure groups’ mean serum aluminium levels of 0.08, 0.14 and 0.46 μmol/l respectively and the corresponding values for urine-aluminium being 0.4, 1.8 and 7.1 μmol/l. They expanded their findings, showing subjective symptoms including exposure-related increases in fatigue, mild depression, and memory and concentration problems. In addition to the findings based on neuropsychological testing, visual EEG analysis showed pathological findings only for aluminium welders. Mild diffuse abnormalities were seen in 17 percent of the low exposure group and 27 percent of the high exposure group; mild to moderate epileptiform abnormalities were found at a frequency of 7 percent and 17 percent respectively. Riihimäki et al. (2000) concluded that both objective neurophysiological and neuropsychological testing as well as subjective symptomatology indicated mild but unequivocal findings with a dose-response relationship with the level of aluminium body burden. The strength of this study was its detailed neuropsychological assessment of very specific functions; a weakness is the lack of measurement in blood or urine of possible additional exposures (other than lead) that could have affected the results. However, the authors conducted site visits to rule out confounding exposures such as solvents, and collected occupational histories of the workers, including past and present exposure to neurotoxic agents.
Letzel et al. (2000) conducted a cross-sectional study with a follow-up five years later at a German aluminium powder plant to evaluate possible cognitive decline. In the first analysis, 53 exposed workers were compared with 30 controls who were comparable in age, education and smoking habits. Some members of the exposed group had higher levels of alcohol consumption and were also less likely to participate in the follow-up. Therefore comparisons were made separately with the exposed group that was available for follow-up at both phases of the study (n = 21); this was the exposed group on which the comparisons noted below were based. The investigation included a standardized medical history, biological monitoring of aluminium concentration in urine, in creatinine and in plasma as well as measurement of post prandial blood glucose levels and serum gamma-glutathione levels. The neuropsychological tests included a multiple choice vocabulary test, three subtests of the Wechsler Adult Intelligence Survey, German version, (digit span (verbal short-term memory), digit symbol (visuomotor speed), and block design (constructive abilities)); the trail making test (visuomotor speed); syndrome short test (SKT-D, visuoverbal short- and long-term memory, attention), and a visual discriminative reaction task (sustained attention). Statistically significant differences were found in the levels of aluminium in urine (median value of 98.8ug/L (range 5.0 – 336.6) in the exposed group that was followed up vs. 7.6 (2.6 – 73.8) in controls), creatinine (77.1 ug/g (4.6 – 321.4) vs. 9.0 (1.9 – 51.8)) and in plasma (8.5 ug/L (5.4 – 25.0) vs. 4.3 (1.6 – 7.1)) of the exposed in relation to the controls at the first phase of the study, and at the second phase, in urine (24.1 ug/L (3.4 – 218.9) vs. 6.5 (2 – 25.4)) and creatinine (19.8 ug/g (3 – 202.7) vs. 4.5 (2.2 – 15.9)), but not in the plasma. Differences due to consumption of medication containing aluminium were ruled out. No significant differences were seen in the results of the neuropsychological tests. There was no dose-effect relationship for the length of exposure of internal aluminium concentrations in plasma or urine and any of the primary neurologic variables. The study was small and the participants were generally younger than those at higher risk of cognitive problems.
Pollizi et al. (2002) conducted a cross-sectional case-control study of 64 workers formerly exposed to aluminium dust and 32 demographically similar controls. Participants were required to have retired at least 10 years before the beginning of the study (no rationale was given). They excluded people on continuous medication containing aluminium, with renal failure, past head trauma, on centrally acting drugs, or diagnosed with other psychiatric, somatic and neurological disorders, as well as with profound hearing loss or blindness. The controls were matched for age, professional training, economic status, educational and clinical features. They administered the Mini-Mental State Exam (MMSE), the Clock Drawing Test (CDT), and the auditory evoked Event-Related Potential (ERP-P300). To detect mild cognitive impairment, the time taken to conduct the MMSE and the CDT was also recorded. The former aluminium workers had significantly higher levels of serum aluminium and blood iron. There was a negative relationship between serum aluminium levels and MMSE score and CDT score; there was a positive relationship between serum aluminium levels and MMSE time and CDT time. There was a significant difference in the latency of P300, MMSE score, MMSE time, CDT score and CDT time between the exposed and the control population. The authors controlled for potential confounders including age, height, weight, blood pressure, schooling, alcohol and consumption, and smoking. The neurological testing in this study was not as comprehensive as in the Finnish study (Akila et al., 1999), but is consistent with its results. This study measured blood levels of a greater number of other potential neurotoxins; specifically blood levels of Mn, Pb and Fe, and serum levels of Cu and Zn. These studies provide evidence of very specific forms of mild cognitive impairment in workers exposed to aluminium as measured in their serum.
Doll (1993), in his review of AD and environmental aluminium, concluded that, while there was evidence that aluminium is neurotoxic and defects of coordination were pronounced, cognitive impairment was less evident, and there was no evidence of occupational aluminium exposure leading to AD.
Irritation
One mineral form of aluminium oxide, a hexagonally, very closely packed form, corundum, is extensively used as an abrasive and polisher in sandpaper, emery, etc. This can cause abrasion of the skin, eyes or any other tissue against which it is rubbed.
Skin and eye
Foreign body entry into the eye of material containing bentonite resulted in anterior segment inflammation. Based on a similar response to bentonite, introduction into animal eyes, it was concluded that bentonite was the causative agent (Austin & Doughman, 1980).
Inhalation exposure
Several lung diseases have been associated with work in the aluminium industry: pulmonary fibrosis, alveolar proteinosis, granulomatosis, chronic obstructive lung disease, asthma, and lung cancer. There have been numerous reports of diffuse pulmonary fibrosis and interstitial emphysema, but without nodule formation. Some cases were fatal, attributed to inhalation of metallic aluminium dust in workers in aluminium processing or manufacturing. Most of the exposures that resulted in the more severe outcomes occurred in Germany during World War II, in industrial environments that were heavily contaminated with airborne aluminium flake powder (used in explosives), and where there was unsatisfactory ventilation due to blackout. It is estimated that the highest air levels of aluminium occurred during production of pyro powders in the 1940s when concentrations reached 50 to 100 mg/m3. This lead to a fibrotic lung disease, aluminosis (Sjögren, 2000). The case reports and studies are summarized in Table 22.
Table 22.
Inhalation exposures to aluminium in the workplace – case studies.
| Exposure conditions | Subjects described in the report | Outcomes | Comments | References |
|---|---|---|---|---|
| Cases of severe lung fibrosis due to aluminium exposure in Germany in the 1930s & 1940s | Cited by others as reporting the first case of pulmonary fibrosis in an aluminium worker. | Doese (1938) | ||
| Aluminium powder factory: ~ 4 billion particles/g dust (an explosive concentration of aluminium dust). | 700 workers making aluminium powder (98% metallic aluminium) by a stamping process just before and during World War II. | Dry cough, dyspnoea, lymphocytosis X-rays similar to those seen in silicosis, rapid spread of diffuse hyaline degeneration, spontaneous pneumothorax was common, loss of elastic tissue. 26% of 628 workers judged to be uncomplicated cases of pulmonary aluminosis. 4 men with 1.5 to 16 years exposure had spontaneous pneumothorax. One fatal case had collagenous fibres enclosing phagocytes which contained particles. | Goralewski (1941) stated: “ aluminium lung is a specific disease entity.” Paraffin-like substances were used due to a shortage of stearine in war-time Germany. The author believed the increase of serious cases of aluminium dust disease was due to omission of stearine. | Goralewski (1939; 1940; 1941; 1943; 1948) (most of the information was obtained from Perry (1947)) |
| Cases of severe lung fibrosis due to aluminium exposure in Germany in the 1930s & 1940s. | Koelsch (1942) | |||
| 28 cases of aluminium dust poisoning | Severe fibrosis of the lung due to aluminium exposure. | The action of the dust is partly mechanical, partly chemical. | Meyer & Kasper (1942a; 1942b) | |
| Aluminum Company of America - Pittsburg plant - making aluminium powder. | 125 with 6 to 23, an average of 12, years of exposure. | Health better than 3,000 workers in other parts of the plant in New Kensington. | Concluded inhalation of finely particulate aluminium powder is not harmful to human lung. | Crombie et al. (1944) |
| Grinding duralumin (95% aluminium, 3.5 to 4.5% copper, traces of other metals, ≤0.7 % silicon) and in a factory making aluminium powder. | 92 of 97 workers exposed to aluminium dust. Grinding process: Average aluminium concentration 3 to 5 mg l/m3, 1/3 was 2 to 7 μm diameter, 2/3 was ≥7 μm. Alumina particles mostly < 1 μm diameter. Polishing process: Average aluminium concentration 50 to 100 mg /m3, 95 mg/ m3 of 7 to 40 μm, 1.4 mg/ m3 of 2 to 7 μm, 2.7 mg/ m3 of < 0.4 to 2 μm diameter. | X-rays in 7 showed shadows in peripheral parts of their lungs. 27 claimed to have some cough, 10 were somewhat short of breath. | X-ray changes and reported symptoms were not thought to be significant because they worked without obvious effects on their health. There was no evidence that the dust caused any disease of the trachea, bronchi or lungs. It was noted by Mitchell et al. (1961) that nearly all respirable particles were alundum abrasive and that the aluminium particles were generally > 7 μm diameter. | Hunter et al. (1944) |
| Mostly furnace feeding, with some crane operations, in the manufacture of an abrasive, corundum, an aluminium oxide, from bauxite. Most fume particles were amorphous and < 0.5 μm in diameter. | 23 cases 23 months to 15 years employment | X-rays showed shadows accompanied by pneumothorax, dyspnoea, and sudden attacks of extreme breathlessness. Some cases showed diffuse non-nodular, interstitial fibrosis. Some were fatal. | Has features resembling the condition attributed to dust inhalation by Goralewski (1943). The hypothesis is that intense exposure to amorphous aluminium dust may play a dominant role. Stated by McLaughlin et al. (1962) that they were also exposed to fumes of amorphous silica. |
Riddell (1948); Shaver & Riddell (1947); Wyatt & Riddell (1949) |
| Aluminium smelting | 97 workers were studied. | Heavy damage to the lungs of 18 operators in smelting and alloying departments who had been employed an average of 6 years. | Aluminosis in 8, 1 with fibrosis, and other changes caused by aluminium compounds in 4. | Mödder and Schmitt (1951) |
| Aluminium pulverizing | A single case report | Died 3 years after terminating 3 years employment. | Death attributed to aluminium dust | Ueda et al.(1958) |
| Stamping of aluminium powder. Average dust concentration 4 to 50 mg/m3, most < 4 μm diameter. | 5 cases among a group of 35 workers. | Breathlessness after 2 to 4 years, and 13 years exposure in one worker. Exposure was terminated; 3 recovered, 2 did not, 1 died, showing non-specific lung inflammation, fibrosis and emphysema. | Edling (1961) | |
| Flash filler (filling fireworks with aluminium powder [many particles < 5 μm] and potassium perchlorate by hand). | A case report of a 26 year old female who had 5 years exposure. | Dyspnoea and gross pulmonary fibrosis were seen. She appeared to have had tuberculosis. |
Similar to cases reported by Goralewski (1947), Shaver (1948) and Mitchell (1959) | Jordan (1961) |
| Pyro stamping. Total dust concentration (means of 5 & 7 samples): 615 & 685 mg/m3. Respirable dust: 51 & 52 mg/m3. Stamping, screening and weighing room mean respirable dust concentration: 19 to 114 mg/m3; mean daily exposure time to dusty work: 3.5 hr. 70% of particles < 5 μm diameter. 81% free aluminium, 17% aluminium oxides and hydroxides, 0.5% stearine (½ palmitic, ½ stearic acid), 0.5% silicon. | Examined 27 of 30 workers at risk. 12 were exposed to fine aluminium powder (2 died, 2 were affected). 15 worked with coarser powder (2 had radiological symptoms). | 6 had pulmonary fibrosis. There were 2 fatal cases where pulmonary fibrosis was conclusive, in 3 cases it was sufficient and in 1 suggestive. | A follow-up of Mitchell (1959), case report. Concluded that finely divided aluminium was responsible for the lung damage. |
Mitchell et al. (1961) |
| A ball mill operator in an aluminium flake powder factory that incorporated 1.5 to 5 % stearic acid. Average concentrations from 2 sites: total dust 0.94 and 1.46 mg/m3 (60 & 71% aluminium) Respirable dust concentration: 0.24 and 0.38 mg/m3 (42 & 48% aluminium) | A case report of 49 year old male with 13.5 years exposure. | Lung shadows, adhesions in pleural cavities, diffuse fibrosis of upper lobes. 20 μm aluminium flakes were seen in his lungs, which had 340 & 430 mg Al/kg lung (wet weight). | Histological picture similar to that described by Mitchell et al. (1961) | McLaughlin et al. (1962) |
| Aluminium welder | Case report | Diffuse interstitial infiltrate and mild dyspnoea. Microscopic examination of material from lung biopsy showed extensive interstitial granulomas composed of macrophages, foreign body giant cells and crystals containing Al. | Chen et al. (1978) | |
| Aluminium welding | Case report | Desquamative interstitial pneumonia Lung biopsy showed large amounts of particles containing Al, particularly in alveolar macrophages. |
A nonspecific response to inhaled particles. | Herbert et al. (1982) |
| Welding with exposure exclusively to aerosols of oxidized aluminium | A case report of a 35 year old male with 17 years exposure who was a 60 pack per year smoker. | Shortness of breath. X-ray showed ill-defined nodular, infiltrative lesions, especially in the upper airway. Fibrous pleural adhesions were present. |
Vallyathan et al. (1982) | |
| Aluminium rail grinding, often in an extremely dusty work environment | Case report of a 44 year old with 6 years exposure. | Shortness of breath X-ray showed diffuse infiltrates and restrictive lung disease. Lung tissue contained > 342,000,000 particles/g dry weight, of which 94.8% were pure aluminium. | Concluded the inhaled aluminium particles caused this pulmonary alveolar proteinosis. | Miller et al. (1984) |
| Exposure to aluminium dust | A case report. Developed first symptoms of cough, sputum after 3 years exposure, diagnosis of pneumoconiosis made 13 years after initiation of exposure at age 33. | Pneumoconiosis, characterized by intensive lung fibrosis with an hyperelastogenic reaction X-ray showed extensive, defacing fibrosis in both lungs and pseudo-tumoral shadows. Dyspnoea, which was attributed to fibrosis, which led to fatal chronic cor pulmonale. |
Aluminium concentration 50 to 70 mg/kg in mediastinal lymph nodes and 0.4 to 2.2 mg/kg in lung parenchyma | Freour et al. (1966) |
| Metal polishing | A case report of a 61 year old male with 20 years exposure who was a 45 pack per year smoker. | Shortness of breath, dry cough, and dyspnoea. X-ray showed dense, diffuse interstitial infiltrate, which was denser in upper lung. Bronchoalveolar lavage, lung & lymph node particles ~ 1/3 aluminium, ~ ¼ silicon. Large amount of 0.5 to 5 μm particles in lung. |
Diagnosis of pneumoconiosis based on severe, diffuse, fibrosis; massive dust deposits and no specific sign of other lung disease. No silicotic nodules were seen. |
De Vuyst et al. (1986) |
| Bauxite refining for aluminium oxide production (8 cases), cold grinding of aluminium (3 cases), other occupations (2 cases) | 13 males, mean 53 years old. Awarded compensation by Turin branch of INAIL (National Institute for Insurance against Occupational Accidents) | X-ray showed irregular opacities and nodular fibrosis. | Relatively benign character of modern aluminium lung. | Avolio et al. (1989) |
| 19 males, mean 54.7 years old, mean 16 year dust inhalation. Awarded compensation by Venice branch of INAIL. | X-ray showed 10 cases had signs of fibrosis, 9 cases of chronic obstructive pulmonary disease. | Confirmation of aluminium pneumoconiosis with moderate functional alterations. | Lorusso et al. (1992) | |
| Aluminium production | A case report of a 62 year old male industrial engineer with 23 years exposure. | Exertional dyspnoea. X-ray showed bilateral interstitial infiltrates. Pulmonary function tests were consistent with severe restrictive ventilatory defect. | Al-Masalkhi & Walton (1994) |
These observations led to the conduct of studies in animals, reviewed above, to further understand the causes and conditions of the aluminium-associated pulmonary diseases. The apparently contradictory findings in some of the studies may be explained by the different types of aluminium, as noted by Corrin (1963b) (see also Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, In Vitro Test Systems).
A review and historical perspective of the outcomes of these exposures to high concentrations of airborne aluminium follows. Four men who worked for 1.5 to 16 years in an aluminium powder factory demonstrated dyspnoea, lymphocytosis and lung x-rays similar to those seen in silicosis (Goralewski, 1941). Goralewski (1939; 1940; 1941; 1943) studied 700 workers who made a stamped aluminium powder that comprised 98% aluminium coated with a thin film of paraffin-like substance. Some workers had a dry cough and radiographs with focal shadows in the apical region, 4 had spontaneous pneumothorax and 1, on autopsy, had collagenous fibres enclosing phagocytes containing granular particles. Similar effects were produced in animals (Jötten & Eickhoff, 1942). It was suggested that aluminium dissolved in the lung and then co-precipitated with protein to cause this problem (Goralewski, 1947; 1948; Jäger & Jäger, 1941). It has also been suggested that the paraffinic substances were the causal agents (Perry, 1947), although Van Marwyck & Eickhoff (1950) found paraffin-coated aluminium dust produced less diffuse interstitial fibrosis than uncoated dust (see Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, Inhalation Exposure).
Shaver & Riddell (1947) described a lung disease seen in 23 furnace feeders and ore bin operators, who had worked for 23 months to 15 years in a facility manufacturing the aluminium oxide abrasive, corundum. These workers were exposed to fumes containing considerable amounts of very fine aluminium oxide as well as silica and smaller quantities of many other substances. The disease was characterized by dyspnoea, sudden attacks of extreme breathlessness, pneumothorax, and diffuse, irregular, lace-like, granular shadows rapidly progressing to a non-nodular fibrosis. This became known as Shaver’s disease, a pulmonary fibrosis seen in workers in bauxite refining or exposed to finely divided aluminium powders, especially flake (pyro) powders. The fumes were shown to contain 30% silicon dioxide and 55% aluminium oxide; the silicon particle size (0.02 to 05 μm) was smaller than that associated with classical silicosis (Anon, 1956). More, similar, cases were described by Riddell (1948) who was not able to identify the cause and Wyatt & Riddell (1949) who found the majority of the fume particles to be amorphous and ≤ 0.5 μm in diameter. They believed the initial lesion was intracellular oedema with fibroblastic proliferation, followed by inflammatory cell infiltration within the thickened alveolar walls and then by collagen deposition. They hypothesized that intense exposure to amorphous aluminium dust may play a role in this disorder.
A fatal case described by Mitchell (1959) concerned exposure for 2.75 years to finely divided aluminium powder having a wide range of particle sizes, with ~ 10 mg/m3 being < 5 μm diameter (pyro powder). The powder was stearine coated and produced by a stamping machine. Mitchell et al. (1961) also described another fatal case of a worker who performed the same job for 3.5 years after the first case. These two workers were exposed to 615 and 685 mg/m3 of pyro powder and ~ 50 mg/m3 of respirable (< 10 μm) dust. Four additional cases identified among the 27 workmen of this small factory were investigated (Mitchell et al., 1961). The case described by Jordan (1961) worked for 5 years as a “flash filler”, hand filling fireworks with pyro powder, then frequently sweeping the floor, raising a considerable amount of dust. Pneumoconiosis was seen in 5 workers exposed to aluminium pyrotechnic flake powder in an aluminium powder mill (Swensson et al., 1962). One of these men died 6 years after exposure due to pulmonary oedema caused by cor pulmonale and aluminosis, another died 20 years after exposure due to respiratory insufficiency due to aluminosis, and another died 34 years later with pulmonary fibrosis. When the remaining 2 survivors were studied 40 years later they had no respiratory symptoms and had no further vital lung capacity deterioration (Sjögren et al., 1996b). McLaughlin et al. (1962) provided a frequently cited report of a 49 year old who worked for 13.5 years in the ball-mill room of an aluminium powder factory and who died with interstitial and nodular fibrosis and dementia. Two samples of dust in the workroom contained 0.94 and 1.46 mg/m3 total dust, of 71 and 60% aluminium, and 0.24 and 0.38/m3 respirable dust, of 48 and 42% aluminium. Chen et al. (1978) described an aluminium welder who they claimed to be the first reported case having pulmonary granulomatosis associated with aluminium inhalation, but not associated with aluminium processing. The case presented as a diffuse interstitial infiltrate and mild dyspnoea. Microscopic examination of material from an open lung biopsy showed extensive interstitial granulomas composed of macrophages, foreign body giant cells, and crystals containing aluminium.
Pneumoconiosis has been attributed to kaolin and montmorillonite exposure (Gough et al., 1956; Lynch & McIver, 1954; Sakula, 1961). A case of multiple kaolin-induced granulomas was reported (Herman et al., 1982) in which large amounts of kaolinite were found in a pneumoconiotic lesion of a worker exposed to kaolin. The presence of silica could not be detected (Lapenas & Gale, 1983). Pulmonary interstitial inflammation, fibrosis and granulomatosis have also been reported in those exposed to aluminium silicates (Musk et al., 1980; Sherwin et al., 1979); this was later concluded not to be caused by the aluminium silicates (Musk et al., 1988). The prevalence of simple and complicated pneumoconiosis in kaolin workers was 3.2 and 0.63%, respectively (Morgan et al., 1988). China clay workers who had a high quartz content of the dust recovered from the lung demonstrated nodular fibrosis whereas those who had a high content of kaolinite dust in the lungs had interstitial fibrosis (Wagner et al., 1986).
Desquamative interstitial pneumonia, a non-specific response to inhaled particles, was observed in an aluminium welder. Lung biopsy showed large amounts of particles containing aluminium, particularly in AM (Herbert et al., 1982).
Aluminium oxide refinery workers exposed to some low temperature transitional aluminas demonstrated little evidence of pulmonary fibrosis and pneumoconiosis. The noted adverse effects were consistent with non-specific chronic industrial bronchitis associated with excessive, prolonged exposures to nuisance dust (Townsend et al., 1985). The aluminium lung concentrations in 4 workers exposed to aluminium oxide for 10 to 37 years were 400 to 1080 mg/kg, respectively and correlated well with the extent of fibrosis (Gaffuri et al., 1985). About 1/3 of 197 workers engaged in electrolytic reduction of aluminium oxide, exposed > 3 years to dust ranging in size from 0.5 to 5 μm and in concentration from 0.4 to 3.1 mg/m3, and to gaseous fluoride at concentrations of 0.13 to 0.96 mg/m3 (below the TLV-TWA for fluorides of 2.5 mg/m3 (ACGIH, 2005), demonstrated bronchitis. X-rays indicated a slowly evolving pneumoconiosis (Clonfero et al., 1978). Similarly, the incidence of chronic bronchitis increased with duration of occupational exposure in aluminium foundry workers, to 38% in those with > 20 years exposure. Dust, fluoride and sulphate levels were half of their TLVs (Clonfero et al., 1980a). This group provided a case report of aluminium smelter workers who developed lung fibrosis or fibronodular changes, a pneumoconiosis (Clonfero et al., 1980b). An aluminium arc welder for 17 years presented with fibrotic nodules with seemingly necrotic centres in his lungs (Vallyathan et al., 1982). A 44 year old who had worked for 6 years as an aluminium rail grinder, often in an extremely dusty work environment, presented with shortness of breath, diffuse x-rays infiltrates and restrictive lung disease. Lung tissue was found to contain > 342,000,000 particles/g dry weight, of which 94.8% were pure aluminium. It was concluded that the inhaled aluminium particles caused the pulmonary alveolar proteinosis (Miller et al., 1984). Similarly, a 50 year old who had worked for 14 years in a potroom and died 3 years later of respiratory insufficiency had ~ 1,300,000,000 fibrous particles containing aluminium oxide/g dry lung and 15,000,000,000 non-fibrous Al-containing particles/g dry lung, respectively. The Al present was α Al oxide, not γ Al oxide or Al metal. This is ~ 1000-fold more aluminium in the lung than seen in the general population. The authors speculated that the fibres themselves may have played a role in aluminium-induced fibrosis/pneumoconiosis in cases of massive aluminium exposure (Gilks & Churg, 1987). Avolio et al. (1989) reported 13 cases of lung disease in workers in bauxite refining, preparation of aluminium oxide abrasives, cold grinding of aluminium and other jobs which exposed them to airborne dust. They felt their cases supported the possibility that exposure to aluminium can cause interstitial fibrosis but noted that this was associated with a relatively benign lung condition.
Population studies of aluminium smelter operators suggested minimal (Saia et al., 1981) or no fibronodular disease (Chan et al., 1983; Discher & Breitenstein, 1976) or increased mortality associated with pneumoconiosis (Gibbs, 1985). Doese et al. (1938) found no effects in 70 workers in an aluminium foundry or in 16 in a fabricating plant. However, it was noted that aluminium and aluminium bronze dusts produced upper respiratory tract lesions manifested as a head cold, irritation and atrophy of the nasal mucosa and septal perforation. Crombie et al. (1944) studied 125 workers in an aluminium stamp mill who had been exposed for 6 to 23 years and found no chest radiographic evidence of abnormalities attributable to dust inhalation. The authors concluded that grinders of aluminium airplane propellers, exposed to a mixture of 3 mg/m3 aluminium dust (1/3 between 2 and 7 μm and 2/3 > 7 μm diameter) and aluminium oxide (mostly < 7 μm diameter) and workers in a factory making aluminium powder showed no evidence of adverse effects (Hunter et al., 1944). In a study of 9 workers who had abnormal chest x-rays and a mean exposure of 25 years to aluminium oxide in the production of aluminium oxide abrasives, biopsies from 3 indicated interstitial fibrosis, an absence of silicotic nodules and aluminium levels several orders of magnitude above background. Aluminium constituted > 90% of the metals in the lung. This suggested that aluminium oxide was the cause of the pulmonary fibrosis (Jederlinic et al., 1990).
In reviewing the experimental studies and clinical reports on aluminium oxide, Dinman (1988) suggested that increased thermodynamic instability and enhanced bioreactivity occurs as particle surface area increases, contributing to pulmonary fibrogenicity, and that the thermodynamically active catalytic activity of the low temperature transitional forms also contributes to pulmonary bioreactivity. In their review of evidence for a relationship between work in the aluminium industry and lung disease, Abramson et al. (1989) concluded that pulmonary fibrosis had not been shown to be a significant problem. The difficulty of determining whether the pulmonary fibrosis and pleural abnormalities associated with working with aluminium are due to aluminium and/or asbestos was discussed (Kilburn & Warshaw, 1992).
Nemery presented a case of aluminium exposure causing a sarcoid-like lung granulomatosis and suggested that this might be evidence that aluminium causes a cell-mediated immune response (Nemery, 1990). A 32 year old aluminium polisher who was extensively exposed to metallic aluminium, aluminium oxide powders and other metals for 8 years developed severe lung fibrosis with T-lymphocyte alveolitis and sarcoid-like epitheloid granulomas which contained aluminium. Aluminium comprised ~ 1/3 and silicon ~ ¼ of the mineral species in his lung. The aluminium particles were 0.5 to 5 μm in diameter (De Vuyst et al., 1986). A case of pneumoconiosis in a stoneworker was presented with a high lymph node aluminium content that was attributed to aluminium oxide inhalation (Sunami et al., 1997). Assessment of bronchial and dermal responsiveness was conducted in 127 aluminium smelter workers. Hypersensitive reactions to pure aluminium and aluminium smelter dust were seen in some workers, but bronchial hyperresponsiveness to aluminium aerosol was not seen. The authors suggested that these changes might be have been due to an immunological disturbance in the aluminium smelter workers who had experienced long exposures to toxic compounds, mainly those of aluminium (Hosovski et al., 1998).
Observations in guinea pigs exposed to aluminium hydroxide (Gardner et al., 1944) led to issuing a warning that large amounts of aluminium inhalation may lower the resistance of patients to tuberculosis (Council on Industrial Health, 1946).
In reviewing metal-induced pulmonary toxicity, Nemery (1990) suggested that the rarely-observed pulmonary granulomatous fibrosis seen following aluminium exposure (aluminosis), is a form of induced pneumoconiosis, similar to that from exposure to beryllium and its compounds; it was also suggested that this may also be a cell-mediated immune response. This was also suggested by De Vuyst et al. (1986).
Observations that aluminium inhalation reduced the ability of quartz to produce lung fibrosis in some experimental animals lead to its application in humans. Initially a number of uncontrolled studies were conducted. Crombie et al. (1944) treated 34 silicotic coal miners with 200 to 300 inhalations of a fine aluminium powder, initially for 5 minutes, then increasing to 30 minutes. Clinical improvement was reported in 19 cases, evidenced by lessening or disappearance of shortness of breath, cough, chest pain and fatigue, whereas 6 of 9 control cases that did not receive aluminium reported progression of their disease. There were no apparent adverse effects, leading the investigators to conclude that inhalation of fine particulate aluminium powder is not harmful to human lung. Aluminium treatment was reported to produce symptomatic improvement in 135 of 143 industrial workers who had developed silicosis from exposure to siliceous dust (Hannon, 1944). Symptomatic improvement was reported in 49 non-ferrous metal workers treated with metallic aluminium. Forty-three percent of those treated with hydrated aluminium oxide reported improvement compared to 30% of those treated with metallic aluminium oxide. There was no objective measure of improvement in these cases (Bamberger, 1945). Improvement was reported in 56% of 75 silicosis patients treated with metallic aluminium powder (Johns & Petronella, 1945). In 1946, E. J. King & C. L. Sutherland (as noted in Berry, 1948) reviewed the evidence for the use of aluminium to prevent or treat silicosis and concluded that there was no definitive evidence that aluminium prevented further development or caused reversal of established disease. It was concluded, after reviewing the reports to that time, that the improvements reported by many silicotics were subjective and that there had been no adequately controlled studies (Berry, 1948). Twenty-six silicotic patients inhaled a dust containing 300,000,000 particles of hydrated aluminium oxide per cubic foot or air, equivalent to 35 mg/m3, for 20 minutes daily, 5 days weekly for 4 week periods alternating with 4 week periods of no inhalation, for up to 1 year; comparison was made with 16 controls who inhaled room air. All subjects were blinded. Subjective improvement was seen in both groups. No objective improvement was seen in those inhaling aluminium, suggesting the beneficial effects were psychological (Berry, 1948). It was noted that the observation of improved function coincided with improved dust control in these industries (Anon, 1956). Carefully planned and properly controlled studies were recommended (Kennedy, 1956). This led to an investigation that was conducted in pottery workers and coal miners of the efficacy of inhalation of 50 mg pure metallic aluminium dust, of which > 90% was respirable, for 15 minutes, 3 times weekly, for up to 3.5 years; comparison was made with inhalation of 5 mg of carbon black containing 1 mg/kg aluminium powder (Kennedy, 1956). No regression of the x-ray picture or improvement of functional capacity was seen. It was claimed that no nodulation was seen in 11 workers in a refractory plant who inhaled aluminium for 11 years, whereas this was seen in 11 of 12 controls (Hannon, 1953). Five percent of 164 workers who inhaled aluminium for 5 minutes a week in a foundry manufacturing large castings showed a reduction of respiratory function after 6 years; 9% of 125 who originally had functional incapacity deteriorated while 90% improved (Osmond, 1955).
Asthma was seen in workers in the aluminium industry, but this has been largely attributed to the presence of fluorine and substances other than aluminium. However, there are a number of reports suggesting that aluminium can cause asthma and related symptoms.
An airway obstruction called potroom asthma was seen in workers in the aluminium industry (Chan-Yeung et al., 1983; Frostad, 1936; Kongerud et al., 1994). Exposure to emissions from aluminium industry pots was thought to be the cause of bronchoconstriction among susceptible workers (Field, 1984). As the pot emissions contained aluminium oxide, carbon dusts, particulate polycyclic organics, gaseous and particulate fluorides, carbon monoxide, carbon dioxide, sulphur dioxide and nitrogen oxides, it has so far not been possible to identify the causative agent(s) of potroom asthma; however, fluoride compounds have been implicated (Söyseth et al., 1994). Determination of the contribution of fluoride, inspirable dust, sulphur dioxide, oil mist and the benzene-soluble fraction of coal tar pitch volatiles to respiratory symptoms in aluminium smelter workers suggested the first two were causative agents (Fritschi et al., 2003). Respirable sodium aluminium tetrafluoride fibres were found in the work environment of the primary aluminium industry (Gylseth et al., 1984). Fibres found in the lungs of previous workers in the aluminium industry who had pulmonary problems were similar and evidently persisted in the lung for years (Voisin et al., 1996).
Occupational airway hyperreactivity and nocturnal asthma were seen in 16 young male workers, representing 20% of workers, exposed to aluminium fluoride and in 3 exposed to aluminium sulphate in another plant. Although inhalation challenge tests were negative in 3 workers, intracutaneous injections produced positive responses in 3 of 8 workers. The authors concluded that the aluminium salts were the most likely cause of the occupational nocturnal asthma (Simonsson et al., 1985). Increased bronchial hyperreactivity in aluminium welders was attributed to the aluminium (Nielsen et al., 1993). Park et al. (1996) described a worker who had been exposed for 5 years to aluminium powder and who developed dyspnoea, cough and sputum production which began 1 hr after beginning work. These symptoms and a decreased FEV1 were seen to a challenge with 10 mg aluminium powder and 10 mg/mL of aluminium chloride solution. The worker’s neutrophil chemotactic activity increased during the challenge. Skin tests to aluminium chloride produced no immediate reaction. The authors suggested that aluminium powder might have produced bronchoconstriction mediated by a non-immunological mechanism that could have involved neutrophils. A welder who had intermittently welded aluminium for 4 years developed symptoms of asthma 1 to 4 hr after ceasing welding (Vandenplas et al., 1998). Although skin tests with aluminium sulphate and nitrate did not show an immediate reactivity, the worker exhibited a large decrease in FEV1 following aluminium welding. As positive skin tests to aluminium usually appear after 24 to 48 hr (see Effects on Humans, Effects from Occupational Exposure, Irritation, Dermal Exposure) the absence of immediate reactivity may not rule out hypersensitivity to aluminium. Asthmatic symptoms in this subject appeared after aluminium welding in the absence of detectable fluorides. A positive response was seen to a bronchial provocation test with aluminium chloride in a foundry worker who had 19 years exposure to molten aluminium and who experienced potroom asthma (Burge et al., 2000). An aluminium soldering flux containing potassium aluminium tetrafluoride has been shown to cause a potroom asthma reaction (Hjortsberg et al., 1994).
Dermal exposure
Four cases of contact dermatitis to dural, an alloy of 95 to 95.5% aluminium, 3.5 to 4% copper, 0.5% manganese and 0.5% magnesium, and 2 cases of contact dermatitis to aluminium were seen in 202 cases of contact dermatitis among aircraft workers (Hall, 1944). Itching from an irritant dermatitis that has been reported among potroom workers in factories using recycled aluminium was probably due to aluminium dust mixed with sulphur dioxide and traces of other metals (Johannessen & Bergan-Skar, 1980).
The use of a compressed air pistol to accidently impel newly milled narrow aluminium particles into the hand for 1.5 years of a worker resulted in erythema, hyperkeratosis, fissuring and partial desquamation of the hand. Patch testing was positive for aluminium filings in petroleum lubricant. Testing was not conducted with aluminium alone, so it is not known if this reaction was due to aluminium (Peters et al., 1998).
Corrosivity
Skin, eye and upper respiratory tract contact with trimethyl and triethyl aluminium can produce thermal burns, redness and swelling (Roychowdhury, 1993).
Organo-aluminium compounds have an aluminium-carbon bond in which the carbon atom forms part of an organic radical. Most aluminium alkyls are liquids and react with oxygen and oxygen-containing compounds. This is particularly true for the lower alkylaluminiums such as the alkylaluminium chlorides and hydrides that are violently reactive with oxygen, spontaneously flammable in air, and explosive on contact with water, or when mixed with strong oxidizing agents or halogenated hydrocarbons. They are extremely destructive to living tissue producing an immediate reaction that results in deep, painful burns with subsequent scarring if not immediately treated. Contacted areas should be immediately flushed with much water and covered with a burn ointment (Anderson, 1963). Skin, eye and upper respiratory tract contact with trimethyl and triethyl aluminium can produce thermal burns, redness and swelling (Roychowdhury, 1993).
Effects on the endocrine system
In a report on several biological monitoring studies of aluminium workers in Italy, Alessio et al. (1989) compared 22 workers exposed to aluminium with 12 non-exposed workers. They noted that thyroid-stimulating hormone and prolactin levels were depressed 8 months after starting exposure to aluminium, but subsequently returned to normal. No other epidemiological studies have been published on this topic.
Immunotoxicity/immunosuppression
No epidemiological studies investigating the effects of occupational aluminium exposure on immune parameters in workers have been published on this topic. There is some evidence describing a potential immunosuppressive effect of aluminium in renal transplant patients (see Effects on Humans, Effects from Non-Occupational Exposure, Immunotoxicity/Immunosuppression).
Renal
No epidemiological studies have been published on occupational aluminium exposure and its effects on the renal system. There is extensive literature describing the role of renal insufficiency in the development of aluminium induced toxicity (see Effects on Humans, Case Reports and Effects on Humans, Subpopulations at Special Risk).
Bone
No information was found specifically describing occupational aluminium exposure and adverse effects of the skeletal system. For a description of non-occupational aluminium exposure and bone toxicity see Effects on Humans, Effects from Non-Occupational Exposure, Bone.
Anaemia
No information was found specifically describing aluminium induced anaemia as a result of an occupational exposure. For a description of non-occupational related aluminium exposure and anaemia see Effects on Humans, Effects from Non-Occupational Exposure, Anaemia.
Cancer
The potential for carcinogenic effects from exposure specifically to aluminium or its compounds in the workplace has not been studied. However, according to IARC, processes used in the production of aluminium are carcinogenic in humans (IARC 1984; 1985; 1987; Straif et al., 2005). See below for further discussion on research papers relevant to the IARC ranking.
A synthesis of different epidemiological studies from 5 countries on the risk of cancer from occupational to aluminium is presented in Table 23. These studies show excess cancers at multiple sites, especially the bladder and the lung. In the occupational environment, certain PAHs, such as tar-pitch volatiles, which are emitted when organic matter is burned, are established carcinogens. However, uncertainty exists regarding the exposure-response relationship with respect to PAH. Two review articles have been published on epidemiological studies in which the relationship between exposure to PAH and lung and bladder cancers in occupationally exposed subjects was investigated (Armstrong et al., 2004; Mastrangelo et al., 1996).
Table 23.
Epidemiological studies of occupational exposure to aluminium and cancer.
| Reference | Study Location | Design | Exposure | Outcome | Association | Significance | Comments |
|---|---|---|---|---|---|---|---|
| Armstrong et al. (2004) | 4 countries | Meta-analysis: 36 cohorts – 3 case-controls samples – 1 case cohort | PAH | Lung cancer | +/- | ||
| Moulin et al. (2000) | France, 1950- 1994 | Cohort of male workers from an aluminium reduction plant (n=2,133) 335 died. | Proxy for PAH | Bladder cancer (7) | SMR = 1.77 (0.71-3.64) | - | - few events |
| Lung cancer (19) | SMR = 0.63 (0.38-0.98) | + | - a possible negative confounding by smoking (for lung cancer) | ||||
| Romundstad et al. (2000b) | Norway, 1953- 1996 | Cohort of male workers from an aluminium plant (n=11,103). | Job exposure matrix for PAH | Bladder cancer (130) | SIR = 1.3 (1.1-1.5) | + | - adjusted for smoking habits |
| Lung cancer (189) | SIR = 1. (0.9-1.2) | - | - 6 study plants | ||||
| Kidney cancer (55) | SIR = 1.1 (0.8-1.4) | - | |||||
| Pancreatic cancer (46) | SIR = 0.9 (0.7-1.2) | - | |||||
| Romundstad et al. (2000a) | Norway, 1953- 1995 | Cohort of male workers from an aluminium plant (n=1,790). | Job exposure matrix for PAH | Bladder cancer (23) | SIR = 1.3 (0.8-1.9) | - | - adjusted for smoking habits |
| Lung cancer (27) | SIR = 0.9 (0.6-1.3) | - | - only one study plant (limited study size) | ||||
| Ronneberg et al.. (1999) | Norway, 1953- 1993 | Cohort of male workers from an aluminium smelter (n=5,908). | PAH | Bladder cancer (36) (production subcohort) | SIR = 1.52 for ≥ 1200μg/m3 (p =0.03 for trend) | + | - bladder cancer and PAH exposure 30 years or more before observation |
| Lung cancer (93) | SIR = 0.96 (0.69-1.29) | - | - significant excess of lung cancers for short-term workers (<4 years) | ||||
| Selden et al. (1997) | Sweden, 1958-1992 | Cohort of workers from aluminium foundries and secondary aluminium smelters (n=6,454). | Proxy for PAH | Bladder cancer (19) | SIR = 0.87 (0.52-1.36) | - | - lung cancer risks higher in short duration of employment (< 5 years) |
| Lung cancer (51) | SIR = 1.49 (1.11-1.96) | + | - anorectal cancers not etiologically linked to occupational risk factors | ||||
| Anorectal cancer (33) | SIR = 2.13 (1.47-2.99) | + | |||||
| Sinonasal cancer (4) | SIR = 4.70 (1.28-12.01) | + | |||||
| Schroeder et al. (1997) | U.S., 1941-1985 | Case-controls study of automotive workers (667 cases, 3,041 matched controls) | Aluminium machining | Lung cancer | OR = 2.85 (1.43-5.68) (for 0.9-1.7 years of exposure v.s. 0, for 20 years lagged exposure) | + | All workers exposed to aluminium were also exposed to soluble oils |
| Armstrong et al. (1994) | Canada, 1950-1988 | Case-controls (338 deaths) and a random sub-cohort (1,138 subjects) of workers from an aluminium production plant | Benzo-a-pyrene/ Benzene- soluble matter | Lung cancer | OR = 2.25 (1.50-3.38) (for 10-19 mg/m3-years benzene soluble) | + | -adjusted for smoking habits |
| Spinelli et al. (1991) | Canada, 1950- 1985 | Cohort of workers from an aluminium production plant (n=4,213). | Proxy for PAH | Bladder cancer (incident=16, death=3) | SIR = 1.69 (1.06-2.57) | + | - limited study size |
| SMR = 1.37 (0.37-3.54) | - | adjusted for smoking habits | |||||
| Lung cancer (incident=37, death=32) | SIR = 0.97 (0.73-1.28) | - | |||||
| SMR = 0.93 (0.68-1.25) | + | ||||||
| Brain cancer (incident=8, death=10) | SIR = 1.94 (0.97-3.50) | - | |||||
| SMR = 2.17 (1.18-3.68) | + | ||||||
| Mur et al. (1987) | France, 1950- 1976 | Cohort of workers from an aluminium production plant (n=6,455). | None | Bladder cancer (7) | SMR = 2.09 (0.96-3.68) | - | - limited study size |
| Lung cancer (37) | SMR = 1.14 (0.85-1.48) | - | - lack of information on the cause of death for 29% of the deaths | ||||
| Edling et al. (1987) | Sweden, 1958- 1983 | Cohort of workers manufacturing abrasive materials (n=521) | Total dust | All cancers (17) | SMR = 0.93 (0.5-1.5) | - | - limited study size |
| Lung cancer (2) | SIR = 0.57 (0.1-2.1) | - | |||||
| Gibbs (1985) | Canada, 1950- 1977 | Cohorts of aluminium smelters (n=5,406) | Proxy for PAH | Malignant neoplasm: | (ever exposed) | ||
| Bladder (12) | SMR = 1.61 | - | - limited study size | ||||
| Lung (101) | SMR = 1.43 (p< .05) | + | - exposure on or before 1950-1951 | ||||
| Rockette & Arena1 (1983) | U.S. | Cohorts of aluminium reduction workers (n= 21,829) | None | (more than 20 years of expsure) | - | A factor common to the plants would be related to the excess of pancreatic cancers | |
| Lung cancer (27) | SMR = 1.01 | - | |||||
| Pancreatic (16) | SMR = 1.98 (p<0.05) | + |
In Canada, a significant excess of mortality or morbidity from lung or bladder cancers has been observed in three studies (Armstrong et al., 1994; Gibbs, 1985; Spinelli et al., 1991). Gibbs (1985), in a study of 5,406 Canadian aluminium smelter workers, found that mortality from malignant neoplasms of the bladder and the lung was meaningfully related to the number of tar-years (an exposure index calculated for each year 1950-1977, which corresponds to a weighted sum of the total number of years in the tar-exposed occupation) and number of years of exposure (Gibbs, 1985).
Edling et al. (1987) in Sweden found no significant increase in cancer morbidity or mortality in 521 workers exposed to aluminium oxide in an abrasive-manufacturing plant and the cohort was followed up between 1958 and 1983. Selden et al. (1997) in Sweden investigated the risk of developing cancers at different sites in workers employed in secondary aluminium (scrap) smelters. They obtained significantly increased risk estimates for specific sites or organ-systems such as the rectum and anus (standardized incidence ratio (SIR) =2.13, 95% CI: 1.47-2.99), the nose and paranasal sinuses in a small number of cases (SIR= 4.70, 95% CI: 1.28-12.01), and the lung and pleura (SIR=1.49, 95% CI: 1.11-1.96). They also observed an increase in anorectal cancer, but this was not etiologically related to occupational determinants of risk. In contrast to previous findings in primary aluminium smelters, there was no overall increase in urinary bladder cancer in this study including workers from secondary aluminium smelters.
Two mortality studies on this topic have been conducted in France. In the first, Mur et al. (1987) investigated workers at 11 plants of the Aluminium Pechiney Company; the follow-up period was from 1950 to 1976. A slight but non-significant lung cancer excess of 1.14 was found based on 37 observed deaths. The second study conducted by the French National Institute for Research and Safety by request of the Aluminium Pechiney Company was an update of the mortality study of workers in the largest plant from the previous study (Moulin et al., 2000). An improvement in the study design was made by collecting the causes of death from death certificates over the period 1968-1994. The statistical analysis, based on 7 deaths, showed a non-significant excess of mortality from bladder cancer (standardized mortality ratio (SMR) =1.7, 95% CI: 0.71-3.64) and the observed mortality from lung cancer (19 deaths) was significantly lower than expected (SMR=0.63, 95% CI: 0.38-0.98).
The most recent Norwegian studies showed no elevated risk of lung cancer even among the most heavily PAH-exposed workers (Romundstad et al., 2000a; Ronneberg et al., 1999). However, these investigators obtained inconsistent results regarding bladder cancer. Limitations in study size have been problematic in interpreting the results of these studies and the latter one (Romundstad et al., 2000b) was initiated to clarify possible associations between PAH exposure and the incidence of lung, bladder, kidney and pancreatic cancers in a combined update of 6 Norwegian aluminium cohorts, i.e., 11,103 workers including 130 with bladder and 189 with lung cancers. This study showed an association between bladder cancer and exposure to PAH, but gave no support to an association with lung, kidney or pancreatic cancers.
Higher than expected mortality from other cancers, including lymphoreticular and genitourinary malignancies (Rockette & Arena, 1983) or brain cancer (Spinelli et al., 1991) have also been reported in aluminium reduction plants but again concomitant exposure to PAHs was likely to have been involved in these plants.
Occupational exposure to aluminium occurs not only in the primary refining of this metal but also in user industries such as automobile manufacturing. For example, inhalation exposure can occur during metalworking. In a nested case-control study of 667 cases (members of the cohort (n=45,000) who died before January 1, 1985) and 3,041 matched controls, Schroeder et al. (1997) analyzed the effect of aluminium machining on the risk of lung cancer. Semiquantitative estimates, based on years of exposure, were available for additives including sulphur and biocides, for the metals, steel, iron and aluminium, and for nitrosamines. A positive association between exposure to aluminium and lung cancer mortality was observed, particularly after an exposure period of 20 years. There was no evidence of a dose-response relationship between years of exposure and the outcome. However, ORs from these models indicate there are risks from exposure to aluminium together with soluble oils (a class of machining fluid), rather than from exposure specifically to aluminium. The likelihood that Al exposure is causally related to lung cancer in this cohort is small.
Armstrong et al. (2004) has reviewed the risk of lung cancer from inhalation exposure to PAHs in different aluminium-related industries. Meta-analysis of the data from eight of these industries showed the risk of lung cancer to be equivalent to 1.16 (95% CI: 1.05-1.28) for exposure to 100 μg/m3 benzo(a)pyrene.
Several epidemiological studies have shown that workers in the aluminium industry have an increased risk of developing lung cancer or bladder cancer; however, in all these studies this risk has been attributed to exposure to the PAHs generated during aluminium production, rather than to exposures to aluminium compounds. Benke et al. (1998) recommended that more attention be given to exposures to other contaminants and agents such as aromatic amines and nitro compounds, asbestos, heat stress and magnetic fields in future studies of cancer in aluminium smelter workers.
Genotoxicity
A case control study was conducted by Arnaiz et al. (2003) to examine whether genetic polymorphisms associated with asthma in the general population were also associated with the development of the asthma-like condition among aluminium potroom workers. The genotype at beta2-adrenoreceptor 16 and 27 and the high affinity IgE receptor 181 and 183 amino acid positions were studied. Only 52 cases agreed to participate; 13 were identified as new cases. The study did not show any association between genotype and the development of asthma for workers employed in a potroom. However this study might not have been of sufficient power to detect an association because of the limited number of subjects.
Reproductive toxicity
Effects on fertility
Epidemiological investigations of the effects of aluminium on fertility have not been published. A demographic analysis of fertility in French aluminium industry workers was conducted, but the aim of this study was to evaluate the potential effects of occupational exposure to heat and static magnetic fields (Mur et al., 1998). A comparison was made between the number of live births in the group of male workers (n=692) exposed to heat and magnetic fields from 11 aluminium producing factories with that in workers in the same factories who were not exposed to these factors (n=588); the workers were mainly employed as maintenance operators and had never worked in potrooms. In the passageways where potroom workers mainly stay, the maximum level of the magnetic fields to which exposures occurred was 20 mT2. The index taken for estimating the fertility of these workers was the number of live births since marriage, after the start of occupational exposure. They observed a significantly greater birthrate in the “exposed” group (3.11 ± 1.74) than in the “control” group (2.63 ± 1.34) 30 years after marriage, for instance. However, in this study, factors which could have influenced the results had not been taken into account, that is the contraceptive practices of couples and non-occupational factors influencing the fertility of the man and/or woman (e.g., smoking, which can alter male fertility, was more frequent in the “exposed” than in the “non-exposed” group; this could have been a bias). Comparison with a national reference (the average number of live births in the French population is 2.28) also reveals that the fertility of (“exposed” and “control”) subjects from these aluminium industries is greater than that of the population as a whole. However other factors can explain the disparities in fertility rates, especially the fertility of the socio-professional category of the subjects studied here (blue collar workers) which is higher than that of other socio-professional categories included in the national reference. This study has too many drawbacks to reach any conclusion on an association between occupational exposure to aluminium and fertility.
Developmental effects
No published epidemiological studies of the effects of occupational aluminium on developmental effects were found. There is at least one case report of severe developmental retardation in a child whose mother had taken high doses of aluminium containing antacids during pregnancy (see Effects on Humans, Case Reports).
Modifying factors
Gene-environment interactions
As described above in Effects on Humans, Effects from Occupational Exposure, Genotoxicity, Arnaiz et al. (2003) examined whether genetic polymorphisms associated with asthma in the general population are also associated with the development of an asthma-like condition among aluminium potroom workers. No associations between genotype and the development of asthma in workers in a potroom were found.
Confounding factors
The major weakness of studies of occupational exposures to Al is the lack of information required to rule out exposure to other toxic substances as the cause of the observed effect. Most of these studies involved a cohort of workers with a small number of cases which resulted in inconsistent results. Also confounding factors such as smoking histories, or other occupational exposure to dust or substances such as asbestos have not always been controlled for. Armstrong et al. (1994), in a case-cohort study, adjusted for smoking histories from the medical records of the company. They obtained a clear excess of lung cancer risk in men who had worked in the Søderberg potrooms and in jobs in which there were generally high exposures to coal tar-pitch volatiles. Linear modelling predicted a rate ratio of 1.25 for lung cancer after 40 years at the TLV for coal tar pitch volatiles of 0.2 mg/m3 (ACGIH, 2005) matter, implying a lifetime absolute excess risk of 2.2 percent. Furthermore, they showed that the risk was not due to confounding by smoking. In only 4 of the 12 studies reported in Table 23 has an adjustment been made in the analyses for smoking habits. However, Armstrong et al. (2004) have noted that confounding is unlikely, where comparisons are being generally made between groups with only moderately differing smoking habits (particularly different groups of manual workers).
Producing aluminium leads to the release of a chemically complex mixture including both gases and particulates of varying chemical composition. Most attention has been focused on the effects of the particulate phase of the coal-tar-pitch volatiles (CTPV), principally because CTPV contain carcinogenic PAHs and other carcinogenic constituents (e.g., arylamines and nitro-PAH). Differences in duration, level and chemical composition of exposure may in part explain the conflicting results. Earlier studies (Rockette & Arena, 1983; Spinelli et al., 1991) were not as detailed in their exposure assessments as those of Armstrong et al. (1994) and Ronneberg et al. (1999).
Explosivity, flammability, oxidizing potential
Aluminium is a reactive chemical element with a natural affinity for oxygen. Metallic aluminium is produced commercially from its ores by the breakage of the aluminium-oxygen bonds (see Sources of Human Exposure, Anthropogenic Sources, Production Levels and Processes). This requires energy and if it recombines with oxygen in water or air this energy will in turn be released. Within the industrial environment, explosions continue to be reported as a result of the combination of molten aluminium and water when water turns to steam as it is trapped between the molten metal and a solid. Such a rapid expansion and rise in temperature has lead to a number of different types of steam-related explosions, producing a significant amount of damage. In the mid-1980’s the Aluminium Association initiated a reporting program for incidents of three different types of explosions. Defined as level of Force 1, 2, or 3 they cover steam explosions (less than 10 pounds of metal dispersed less than 15 feet), violent steam explosions (metal dispersed 15 to 50 feet); and catastrophic explosions resulting from reactions with air or water and the metal is dispersed more than 50 feet with little remains of the metal, respectively. A recent review outlines these types of industrial accidents and provides incident data by type of operation over the last 20 years (Epstein, 2005). As expected, the majority of the incidents are within the Force 1 level however, there has been relatively consistent number of Force 2 incidents over the years. While Force 1 incidents are associated with burn related injuries, Force 2 incidents represent the majority of fatalities and serious injuries.
When assessed, the explosibility of metallic dusts dispersed in air showed that minimum explosive concentrations of aluminium dusts ranged from 70 to 90 g/m3 when ignited by a 1000 J system up to a maximum of 350 g/m3 at 5000 J. The maximum adiabatic flame temperature is 3550 °K and the limit flame temperature is 2150 °K. It has been suggested that, in such cases, aluminium combustion depends on metal evaporation in order to support flame propagation (Hertzberg et al., 1991).
Mixtures of aluminium and halogenated organic compounds in which the aluminium concentration can have high reactivity and present an explosion hazard depend on the reactivity limits (RL). Most mixtures with iodinated compounds are not very reactive and many mixtures of aluminium with fluorinated hydrocarbons do not have a lower RL. In the presence of heptane, ternary mixtures of aluminium with fluoro-, chloro-, or bromo-substituted compounds have a decreased range of reactivity (Cardillo & Nebuloni, 1992).
Effects from Non-Occupational Exposure
Neurotoxicity
It has been shown that aluminium is neurotoxic, based on the literature on dialysis encephalopathy (see Effects on Humans, Subpopulations at Special Risk, Individuals with Impaired Renal Function). This section reviews studies, in chronological order, of aluminium exposure and cognitive impairment, and dementia, including AD.
Aluminium in drinking water
A number of studies have examined the effects of aluminium in drinking water on cognitive impairment and dementia including AD, as well as other neurological conditions such as Parkinson=s disease and amyotrophic lateral sclerosis. These studies are listed in Table 24.
Table 24.
Summary of epidemiological studies of aluminium in drinking water and cognitive impairment, dementia and AD.
| Study | Design | Subjects | Age range | Outcome measures | Case ascertainment | Association with aluminium |
|---|---|---|---|---|---|---|
| Martyn et al. (1989), UK | Ecological study | 445 probable AD, 221 possible AD, 519 other dementia | 40-69 years | Probable AD, possible AD, other dementia | Computerized tomographic scanning | Positive for probable AD (statistically significant) |
| Flaten (1990), Norway | Ecological study | 14,727 subjects, 586 demented | Dementia | Death certificates | Positive (statistically significant) | |
| Frecker (1991), Canada | Ecological study | 568,345 total deaths, 379 demented | Dementia | Death certificates | Positive (statistically significant) | |
| Neri & Hewitt (1991), Canada | Ecological study, case-control analysis | 2,232 AD or presenile dementia, 2,232 controls | 55 years and over | AD, presenile dementia | Hospitalization records | Positive (statistically significant) |
| Forbes et al. (1991; 1992; 1994), Canada | Prospective cohort | 782 subjects, 400 with some symptoms of mental impairment | 45 years at baseline | Impaired mental functioning | Questionnaire completed by the subjects and verified by family members | Positive if aluminium high and fluoride low or high (statistically significant) |
| Wettstein et al. (1991), Switzerland | Population survey | 805 subjects, 99 demented | 82-85 years | Mnestic and naming skills | Mnestic and naming subtests of the MMSE. | No statistically significant association |
| Jacqmin et al. (1994), Jacqmin- Gadda et al. (1996), France | Prospective cohort | 3,777 subjects | 65 years and over | Cognitive impairment | Score <24 on Mini- Mental State Exam | Positive for aluminium only if silica and pH low (statistically significant) |
| Forster et al. (1995), Taylor et al. (1995), UK | Case-control | 109 PDAT, 109 controls | <65 years | Presenile dementia of the Alzheimer type | Clinical diagnosis | No statistically significant association |
| McLachlan et al. (1996), Canada | Case-control | 119 AD, 51 controls | AD | Autopsy | Positive (statistically significant) | |
| Sohn et al. (1996), Korea | Cross-sectional study | 558 subjects, 45 cognitively impaired | 60 years and over | Cognitive impairment | Mini Mental State test, Korean version | No statistically significant association |
| Martyn et al. (1997), UK | Case-control | Males only; 106 AD, 441 controls | Born from 1916 to 1945 | AD | Computerized tomographic (CT) records, review of hospital case notes | No statistically significant association |
| Gauthier et al. (2000), Canada | Case-control | 68 AD, 68 controls | 70 years and over | AD | Clinical diagnosis | Positive (statistically significant) association with organic monomeric Al.; negative for all forms of Al. |
| Rondeau et al. (2000), France | Prospective cohort | 253 demented, 182 AD | 65 years and over | AD and dementia | Clinical diagnosis | Positive (statistically significant) |
Martyn et al. (1989) conducted an ecological study of the incidence of AD in men and women aged 40-69 in 88 county districts from the records of 7 CT scanning units to which patients had been referred for diagnosis, in relation to levels of aluminium in drinking water over the previous 10 years. CT scans covered a 3-year period, either 1983-85 or 1984-86. There was a wide range of water aluminium concentrations. Rates were adjusted to compensate for different rates of referral according to the distance from the nearest CT scanning unit. Dementia was classified as probable AD (242 men, 203 women), possible AD (112 men, 109 women), cerebrovascular dementia (162 men, 111 women) and other dementia (154 men, 92 women). To examine differences in referral practices in different areas, a group of 2,936 epilepsy patients was also included. The risk of probable AD was 1.5 times higher in districts where the mean aluminium concentration exceeded 0.11 mg/L than in districts with concentrations of <0.01 mg/L. There was no evidence of a dose-response relationship where there were intermediate levels of aluminium in water. There was no evidence of a relationship between other types of dementia or epilepsy and aluminium concentrations in water. It should be noted that this study included an unusually young population in relation to risk of AD (rates increase dramatically from ages 65-69 - the oldest people in this study – to those aged 85 and over). The authors justified exclusion of older age groups in which AD is more common on the basis that younger people presenting with clinical symptoms of dementia would more likely be more aggressively assessed and more likely to have a CT scan. The study was not population-based, i.e. not all people developing AD had an equal probability of being included in the analysis, only those under age 70 receiving a CT scan. It is not known how this sample differed from all AD cases in the area studied, but it was not representative.
Flaten (1990) examined geographical associations in Norway between aluminium in drinking water and death rates (underlying or contributing cause) from dementia including AD, as well as Parkinson=s disease and amyotrophic lateral sclerosis. Water samples were collected seasonally from October 1982 to August 1983 from 384 Norwegian waterworks supplying 70.9% of the Norwegian population, covering 193 municipalities. Death rates were standardized to the 1979 Norwegian population, for 3 5-year periods (1969-73, 1974-78, 1979-1983) and for the 10-year period, 1974-83. Municipalities were grouped into low, medium and high levels of aluminium (<0.05 mg/L, 0.05-0.2 mg/L and >0.2mg/L respectively), and correlation and regression analyses were conducted. Age-adjusted rates per 100,000 population (1974-1983) grouped by aluminium concentration in water showed relative risks (RR) for dementia in males of 1.00, 1.15 and 1.32 for low, medium and high levels of aluminium, respectively; for women, the corresponding values were 1.00, 1.19 and 1.42. This relationship did not hold true for mortality from Parkinson=s disease or amyotrophic lateral sclerosis. Flaten appropriately cautioned that these associations were ecological, serving to generate hypotheses for further study. The study has the strength of being population-based. The association could be stronger than reported if there was under-reporting of dementia on death certificates; however, since both underlying and contributing causes were used, this problem would be reduced. Typically, report of dementia on death certificates is specific but not as highly sensitive; that is, where dementia is recorded, it is highly likely that the decedent had dementia, but where it is not recorded, it will have been missed in some proportion of the death records.
Frecker (1991) examined the 1985-86 dementia mortality in Newfoundland to determine if there were unusual geographic distributions of disease. Data included age, sex, birth place, place of death, cause of death and surname of parents, as well as aluminium concentration, pH and colour of water. Two areas of high aluminium levels in drinking water had different rates of dementia mortality. No conclusive evidence of family clustering was found.
Neri & Hewitt (1991) conducted a case-control analysis, using hospital discharge records of 2,344 people aged 55 or over, hospitalized for AD or presenile dementia, and 2,232 people with non-psychiatric diagnoses, matched by age and sex to the cases. Drinking water data included the level of aluminium in treated municipal water. They found an OR of 1.46 for aluminium concentrations of ≥ 0.200 mg/L compared to <0.01 mg/L. While the analysis was conducted as a case-control study, the study was really ecological in that it did not have any data on potential confounders or effect modifiers.
In the Ontario Longitudinal Study of Aging (Forbes et al., 1991), 2,000 men were followed for about 30 years and, of the 782 who remained in the study in 1991, 400 demonstrated mental impairment at age 76. Aluminium and fluoride levels in drinking water were obtained from provincial sources and allocated by place of residence of the study participants. In their first publication, Forbes et al. (1991) noted only that ‘low’ and ‘high’ referred to below and above the 50th percentile; there was no mention of what concentrations of aluminium or fluoride were considered to be ‘low’ and ‘high’. They found that men living in areas with high fluoride were more likely to be cognitively normal; men in areas with high aluminium were less likely to be cognitively normal. The combination of high aluminium and low fluoride gave the lowest likelihood of being cognitively normal (OR=0.37); conversely, the OR for low aluminium and high fluoride was 1.5. No CIs or levels of statistical significance were given. Two further papers in 1992 and 1994 expanded on the first report (Forbes & Agwani, 1994a; Forbes et al., 1992). The median concentration of aluminium in drinking water was 0.0847 mg/L, and, for fluoride, 0.88 mg/L in treated drinking water. Drinking water levels were classified as “high” if the concentration aluminium or fluoride was greater than, or equal to, the median value, and “low” if it was less than the median value. The OR was 2.72 (p < 0.01) for subjects in areas with high concentrations of aluminium and low levels of fluoride in the drinking water, compared to those exposed to low aluminium and high fluoride concentrations. An analysis of the risk of impaired mental functioning according to pH, in addition to aluminium and fluoride, levels resulted in small numbers, which makes interpretation difficult (Forbes et al., 1994). There was a lower risk of impaired mental functioning with a neutral pH, between 7.85 and 8.05. Additional caveats include that the sample was not representative of the general population (a sample of males aged 45 in 1959), the possibility that those with mental impairment were more likely to drop out of the study, and the difficulty of accurately identifying impaired mental functioning based on a brief screening test and subjective impressions of the responses to the overall interview.
Wettstein et al. (1991) conducted a well-designed population-based study of 805 residents, aged 81 to 85, living for more than 15 years in districts with high (98 μg/L) or low (4 μg/L) aluminium concentrations in their drinking water. They measured memory skills using the Mini Mental Status test. Mnestic and naming skills did not differ between the high and low exposure groups. Urinary aluminium and serum aluminium levels were measured in 10 clinically diagnosed Alzheimer=s patients and 10 controls in both areas; no significant difference was found, adding strength to the negative findings. However, the highest concentration of aluminium in drinking water was below 100 μg/L. Perhaps it would be more accurate to say that no association was seen between memory loss (or cognitive impairment) and low and medium levels of exposure.
There have been reports of adverse neurological effects resulting from the accidental contamination of drinking water in the Camelford area in Cornwall, England in 1988 (Altmann, 1999; McMillan et al., 1993). The water aluminium concentrations recorded at the time of the contamination event ranged from 30 to 620 mg/L (Eastwood et al., 1990). McMillan et al. (1993) reported evidence of memory impairment in 10 individuals; this was not an epidemiological study, but a description of 10 cases referred by their general practitioners. Altmann et al. (1999) reported on a self-selected group of 55 adults who were considering litigation due to the alleged effects of the water contamination three years after the incident. A control group consisted of 15 siblings (all who were available) who had not lived in the Camelford area since before the water contamination incident. An additional control group of 42 similarly aged adults underwent the same psychological tests. Subjects completed the national adult reading test as a method of assessing IQ, and the Bexley Maudsley automated psychological screening to measure cognitive impairment. All affected participants complained of short term memory loss and impaired concentration. The mean pre-morbid IQ as assessed by the national adult reading test was above average. The IQ measure of the affected subjects was similar to their unaffected siblings. Affected subjects performed poorly on the symbol digit coding test as compared to the 42 unrelated controls. The symbol digit coding test is thought to be a measure of organic brain disease. The 15 unexposed siblings had significantly better symbol digit coding test results and flash-pattern differences than did their affected siblings. Many methodological issues must be considered in interpreting the results of this study. The cases were self selected and were pursuing litigation for cerebral damage claimed to be a result of the Camelford incident. It is possible that these cases may have already had unexplained symptoms and cognitive problems, and the incident served to highlight a potential cause (David, 2000). In addition, individuals who believed that they had suffered damage as a result of the incident may have been more likely to have shown errors in psychological tests which they knew were designed to detect abnormalities (Esmonde, 2000). The level of aluminium in tap water in the period following the incident and the current levels of aluminium in the tap water were not reported in the study. There was also no indication of aluminium concentrations in the drinking water of the controls (Esmonde, 2000).
Jacqmin et al. (1994) investigated the relationship between aluminium, fluorine, calcium, and pH in drinking water and the risk of cognitive impairment. This investigation used data collected from the population-based Paquid study. The study sample included 3,777 individuals 65 years of age and older. To assess their cognitive status, subjects completed the MMSE. Seventy-eight areas of distribution of drinking water were defined and aluminium, calcium, fluorine, and pH were measured in two separate surveys. A weighted mean of exposure was calculated for each water supply. Of the 3,697 subjects who competed the MMSE, 24.5% were defined as cognitively impaired. A mixed effects logistical regression was performed to estimate the association between the exposure variables and cognitive impairment while adjusting for age, sex, educational level, and occupation of the participants. Higher calcium levels in drinking water appeared to have a protective effect for cognitive impairment; the OR was 0.8 (p = 0.015, CI not provided) for a calcium concentration of 75 mg/L or greater compared to less than 75mg/L. The association between aluminium levels and cognitive impairment was not significant when pH was ignored. A weak interaction was detected between aluminium and pH levels. The association between aluminium and cognitive impairment was reported to be positive up to a pH of 7.0, and negative above that pH level. For example an OR of 1.35 was calculated for a concentration of aluminium of 100 ug/L compared to 5 ug/L at a pH of 7.0, while an OR of 0.5 was obtained at the same concentration level with a pH of 8.5. The CIs for these measures of association were not reported; thus the stability and significance of these results is not known.
In a subsequent analysis, the effect of silica in water on cognitive impairment was assessed in 3,450 participants in 71 parishes (Jacqmin-Gadda et al., 1996). A weighted mean of the measures obtained from two different surveys of each component of drinking water (pH, and concentrations of aluminium, calcium, and fluorine) was computed for each of the 71 areas. Three categories of silica levels were used (low, medium and high), defined by the first and last quartiles of the distribution. The prevalence of cognitive impairment for each of the categories was 24.0 (n=775), 21.8 (n=1,828), and 28.3% (n=847), respectively. In the regression model, in which first quartiles of exposure for the different water components were used as cut points, a significant relationship between silica, aluminium, and cognitive impairment was detected. In this model, when the level of silica and the pH were both low, subjects exposed to an aluminium concentration of 3.5 ug/L or more were more likely to have cognitive impairment compared to subjects in the lowest quartile of aluminium exposure (OR=3.94, 95% CI: 1.39-11.2). This CI is wide, indicating that the results were based on small numbers. ORs were calculated for eight different categories defined by the distributions of aluminium, pH, and silica. Low aluminium, high silica, and high pH was chosen as the reference category. An OR for cognitive impairment of 1.30 (95% CI: 0.75-2.24) was obtained for water districts with high aluminium, but low silica levels, and low pH. A lower OR (0.74, 95% CI: 0.53-1.02) was obtained for subjects living in areas with high aluminium levels, low pH, and high silica. These results support the hypothesis of a protective effect of silica in the development of cognitive impairment.
Forster et al. (1995) conducted a case control study to investigate the relationship between presenile dementia of the Alzheimer type (PDAT), family history, medical history, cigarette smoking, and exposure to aluminium. Cases had been diagnosed between 1981 and 1989 as having dementia before the age of 65 years, and were referred for specialist hospital services. Subjects were required to meet the DSM-III-R criteria for dementia (American Psychiatric Association, 1987), and the NINCDS-ADRDA criteria for AD (McKhann et al., 1984). Subjects completed the MMSE (Folstein et al., 1975); those scoring from 17 to 24 underwent another test (Geriatric Mental State Examination) to exclude any possible non-organic psychiatric conditions. Each case was paired with a control randomly selected from the population matched for age and sex. Informants of both cases and controls answered questionnaires designed to obtain information on general health, demographic factors, possible risk factors for AD, and family history of dementia. Historical data on aluminium levels in the drinking water were related to the place of residence at which the individual had lived the longest in the 10 years before the onset of symptoms of dementia. A total of 109 case-control pairs, for which there were available data on aluminium concentrations, were included in the analysis. The levels of aluminium in drinking water examined were <50 ug/L, 50-99 ug/L, 100-149 ug/L and 150 ug/L and over. No significant relationship was detected between the mean aluminium concentration in drinking water at place of residence and the onset of dementia. This study did not examine the effects of other water constituents, such as silica, fluoride, calcium or pH on the risk estimate.
Silicon-containing compounds in drinking water are thought to reduce the bioavailability of aluminium, and is therefore considered to be protective against the development of AD. Taylor et al. (1995) conducted an analysis of the same 109 case-control pairs included in the study described above in order to determine if an inverse relationship exists between dissolved silicon and aluminium in the drinking water. Water samples were collected from the tap water in the residences of the 109 cases and 109 controls. Historical data of the silicon levels in water were not available; therefore the case-control pairs were compared based on the current levels of silicon at their residences. An inverse relationship between aluminium and dissolved silicon was found. Silicon values at the residences of the cases were not significantly different than in the controls (p = 0.65). The aluminium levels in the current water samples were also not significantly different (p = 0.60). Using a threshold value for soluble silicon of 3 mg/L or above, the OR in the case-control pairs was calculated as 0.8 (95% CI: 0.34-1.83). It must be considered that the potential protective effect of silicon was measured after the onset of presenile dementia of the Alzheimer=s type, however, silicon levels in the same water source are thought to be relatively stable over time.
McLachlan et al. (1996) found an elevated risk of AD where levels of aluminium in drinking water were ≥100 μg/L, based on 296 neuropathologically confirmed cases of AD and two series of controls (n = 125 with no pathology and 170 with other neurodegenerative diseases). Municipal drinking water data were obtained from the Ontario Ministry of Environment and Energy=s Drinking Water Surveillance Program. Residential histories were collected from next of kin; one analysis was based on drinking water at the last place of residence before death (OR = 1.7, p < 0.05), and a second analysis, on the weighted residential histories (OR = 2.5, p < 0.05). This is the only study based on neuropathologically confirmed cases of AD, which is a strength. However, there were two weaknesses: only aluminium levels (not fluoride, silica or pH) in drinking water were obtained, and the analysis did not control for possible confounders or effect modifiers.
Sohn et al. (1996) conducted a cross-sectional study to investigate the relationship between aluminium, calcium and magnesium and cognitive impairment in a sample of elderly individuals living in rural communities in Korea. Two hundred and forty-nine men and 309 women aged 60 years and older participated in this study. The MMSE was used to measure cognitive status. The geographic region from which the participants were selected was divided into 66 areas, and the levels of aluminium, calcium, and magnesium were measured in two separate surveys. The mean concentrations of the 3 drinking water components, as well as the pH, were determined for each area. No significant correlation was detected between cognitive impairment and the concentrations of the drinking water components or the pH level of the drinking water. The cross-sectional design of this study is a weakness; no information was available on residential history or change in the levels of the drinking water components over time.
Martyn et al. (1997) conducted a case-control study to investigate the effects of aluminium and silicon in drinking water on the risk of AD. Subjects were identified from the computerized tomographic records of eight neuroradiology centres in England and Wales. Male patients with a possible diagnosis of dementia or primary brain cancer were selected from the files. For each possible case of dementia the next male patient in the file who was born during the same 5-year period and had a diagnosis other than dementia (brain tumour, epilepsy, or chronic disabling disease) was also selected. One hundred and six men with AD were identified for inclusion in the study. Ninety-nine men with other dementing illnesses, 226 men with brain cancer, and 441 men with other diseases of the nervous system were included in the analysis as controls. Subjects or informants completed a questionnaire which was designed to obtain information regarding residential history. The response rate was 58% due to failure to obtain permission from the general practitioner to approach the patient or next of kin (303 subjects), difficulty in tracing the patient or next of kin (112 subjects), and refusal of the patient or next of kin (209 subjects). The average aluminium and silica concentration for each address and period of residence was calculated by obtaining data from the water plants supplying each area. Aluminium levels in the water supplies ranged from 0.004 to 0.481 mg/L, with a median level of 0.043 mg/L. Cases were compared to each of the control groups to examine the relationship between aluminium and silica levels and AD. The risk of AD did not appear to increase with increasing aluminium concentration. The average levels of silicon in the water ranged from 1.2 to 23.0 mg/L and were inversely related to the average aluminium concentrations. No significant association for AD risk was found for subjects living in areas where the concentration of silica was above 6 mg/L as compared to men exposed to silica concentrations below this threshold. In summary, this study did not find an association between the risk of AD and aluminium in drinking water (at an average concentration of up to about 0.2 mg/L), and the concentration of silica in drinking water did not appear to exert a protective effect. The low response rate was a weakness of the study; its effect on the results is unknown.
Gauthier et al. (2000) conducted a study (68 cases, 68 matched controls) in the Saguenay-Lac-Saint-Jean region of Quebec to examine aluminium speciation in drinking water and genetic characteristics in relation to AD. AD diagnosis was established through a three shage process. Subjects were first screened for cognitive impairment, those screening positive underwent a standardized clinical interview by a nurse, and finally the diagnosis of dementia was established according to DSM IV criteria by two external evaluators. Municipal water data collected in 1995-1996 was used to estimate long term exposure to aluminium species in the drinking water. On average, the subjects had lived 44 years in the residence they occupied at the time of disease onset. They found that only exposure to organic monomeric aluminium at the onset of disease was associated with AD (OR = 2.678, 95% CI: 1.04-6.90) after adjusting for education, family history of AD and the apoliprotein E gene (ApoE) ε4. They did not see any effect of silica or fluoride, as has been hypothesized (Jacqmin-Gadda et al., 1996; Kraus & Forbes, 1992; Taylor et al., 1995).
Rondeau et al. (2000) conducted an analysis of the Paquid data to examine the effect of aluminium and silica in drinking water on the risk of dementia and AD. The analysis included 2,698 participants aged 65 and over living in the community in 75 civil parishes in Gironde and Dordogne in south-western France, for whom drinking water data and covariates were available. The mean length of residence in the same parish was 41 years. Over an 8-year follow-up, 253 incident cases of dementia, including 182 with AD, were identified. The effect of consumption of bottled water was examined separately (data collected at the 3-year follow-up). The RR of dementia adjusted for age, gender, educational level, place of residence and wine consumption was 1.99 (95% CI: 1.20 - 3.26) for those exposed to an aluminium concentration >0.1 mg/L. For AD the adjusted RR was 2.14 (95% CI: 1.21 - 3.80). There was an inverse association with silica (RR = 0.74, p = 0.021) (see also Effects on Humans, Effects from Non-Occupational Exposure, Modifying Factors). There was no statistically significant interaction between aluminium and silica concentrations or between aluminium and mineral water consumption.
Overall, many of the studies of aluminium in drinking water and risk of cognitive impairment, dementia and/or AD have had methodological weaknesses. Early studies were ecological, taking into account only the level of cognitive impairment, dementia, or AD in relation to aluminium in drinking water. Most of the case-control and cohort studies took few or no possible confounders or effect modifiers into account in their analyses. The exception to this is the analysis by Rondeau et al. (2000), which is by far the strongest methodologically of the epidemiological studies conducted to date. However, one limitation of this study is a lack of an intermediate exposure category, and therefore the inability to examine the possibility of a dose-response relationship.
Aluminium in antacids and antiperspirants
Graves et al. (1990) conducted a case-control study to investigate the association between exposure to aluminium, from the lifetime use of antiperspirants and antacids, and the risk of AD. Subjects diagnosed with AD were selected from one of two clinics. Selection of cases was limited to those who were married at the time of diagnosis since the spouse served as an informant. Friends of the case and/or informant were selected as controls; when this was not possible, a non-blood relative of the case was identified. The controls were within 10 years of age of the cases and did not suffer from memory loss. Case and control informants were interviewed about the subjects’ antiperspirant/deodorant use and antacid use. Subjects were defined as an antiperspirant/deodorant user if they were reported to have used these products at least once a month for one year prior to the reference year. Frequency, duration, and the name of the most common brands used were also obtained. A subject was considered to have been exposed to antacids if he or she had used antacids daily or almost daily for at least one year prior to the reference year. One hundred and thirty case-control pairs were included in this analysis. The age-adjusted OR for use of aluminium-containing antiperspirants vs. no use of antiperspirant was 1.7 (95% CI: 1.1-2.4). This component of the analysis included only 63 matched pairs as a result of missing information. There was a statistically significant dose-response relationship between the lifetime use of aluminium-containing antiperspirants and the risk of AD, adjusted for age (p = 0.01). The age-adjusted OR for antacid use vs. no use was 3.1 (95% CI: 1.3-7.5). A dose-response gradient was found for any antacid use (p = 0.009), and the adjusted OR for the highest level of exposure was 11.66 (95% CI: 1.28-106.4). However, no association was found when only aluminium-containing antacids were considered (OR=0.8, 95% CI: 0.3-2.1). There are some methodological issues which should be considered in interpreting the results of this study. The necessary use of surrogate informants may have resulted in misclassification of exposure data and a high frequency of missing information. Also the small numbers of subjects in the exposure categories, especially in the subanalyses, may have led to unstable estimates of the ORs.
Flaten et al. (1991) compared the rate of mortality from dementia (as an underlying or contributory cause) of a cohort of 4,179 patients who had surgery for gastro-duodenal ulcer disease between 1911 and 1987 to national rates. It was assumed that the majority of the patients operated on after 1963 were heavy users of antacids containing aluminium, although no precise exposure information was available. No statistically significant results were seen. The SMR for dementia was 1.10 (95% CI: 0.85 – 1.40, n=64) for all patients, and for those operated on in the period 1967-78 it was 1.25 (95% CI: 0.66 – 2.13, n=13). Limitations of the study include misclassification both in diagnosis and exposure; in addition, the observation period may have been too short to detect an effect. Thus the possibility of an effect cannot be ruled out based on this study.
The Canadian Study of Health and Aging included a prospective analysis of risk factors for AD (Lindsay et al., 2002). The baseline population of this study was comprised of 6,434 subjects aged 65 years or older in 1991. Of these, 4,615 subjects participated in the follow-up study in 1996. The analysis included 194 AD cases, and 3,894 cognitively normal controls. Detailed information regarding a variety of risk factors was obtained through a self-administered questionnaire completed by the subjects at baseline. No association was found between the regular use of antiperspirants OR=0.77 (95% CI: 0.55-1.08), or antacid use OR=0.91 (95% CI: 0.65-1.26) and the risk of AD.
In addition to investigating the relationship between PDAT and exposure to aluminium in drinking water, Forster et al. (1995) also investigated the effects of aluminium exposure from medicinal sources. In an analysis of 109 case-control pairs matched for age and sex, there was no significant association between use of antacids for 6 months or more and PDAT (OR=1.6, 95% CI: 0.77-3.51).
Exley (1998) suggested that little is known about absorption of aluminium from antiperspirants, and that more study is required. There have been no epidemiological studies showing an association between antacid consumption and AD (Doll, 1993).
Aluminium in food
Although the main source of exposure to aluminium for most people probably is food, it is believed that very little is absorbed (Marcus et al., 1992) (see also Toxicokinetics, Absorption, Studies in Humans, Oral Administration). There have only been a few epidemiological studies of the effects of aluminium in food in the general population. A small study (23 cases, 23 matched controls) reported positive results (Rogers & Simon, 1999). While they reported on some aspects of medical history, there is no indication that they considered possible differences between cases and controls in renal function or vitamin deficiencies relevant to cognitive decline. A comprehensive review of the role of diet in cognitive decline (Solfrizzi et al., 2003) referred to the possibility of a link between aluminium in the diet and AD as “controversial” (see Effects on Humans, Subpopulations at Special Risk).
Aluminium in vaccines
Verreault et al. (2001) examined the association between past exposure to conventional vaccines and the risk of AD in order to determine if changes to the immune system could be a factor in the development of this condition. The study population in this investigation consisted of subjects 65 years of age or older who participated in the Canadian Study of Health and Aging, which is a prospective cohort study of dementia. Subjects were screened for dementia at baseline, and cognitively normal subjects completed a risk factor questionnaire which included information about exposure to vaccines. Respondents were asked whether they had ever received vaccinations for tetanus, diphtheria, poliomyelitis, or influeneza. Subjects were screened again for dementia 5 years later. Multivariate logistic regression models were used to determine the association between vaccine exposure and the risk of AD, and to adjust for a variety of other suspected risk factors. One hundred and eighty three subjects, with information about past exposure to vaccines, were diagnosed with probable or possible AD during the 5 year follow up period; 3,682 subjects remained cognitively normal at follow-up. A statistically significant lower risk of AD was demonstrated for subjects who reported at least one vaccination against diptheria/tetanus (OR=0.40, 95% CI: 0.25-0.65), and at least one vaccination against poliomyelitis (OR=0.54, 95% CI: 0.30-0.97). Exposure to influenza vaccine was also associated with a lower risk of AD but did not reach statistical significance (OR= 0.81, 95% CI: 0.55-1.19). These results suggest that past exposure to vaccines may protect against the development of AD.
Irritation
Corundum, one of the mineral forms of aluminium oxide, a hexagonally, very closely packed form, is extensively used as an abrasive and polisher in sandpaper, emery, etc. This can obviously cause abrasion of the skin, eyes or any other tissue against which it is rubbed.
Oral exposure
Oral exposure to aluminium hydroxide gel has been implicated as a contributing factor in porphyria cutanea tarda, a vesiculobullous disorder, seen in chronic renal failure patients on haemodialysis (Fisher, 1984). This suggestion has been supported by the clinical observations of Gafter et al. (1996), King et al. (1983), McColl et al. (1986), Peserico (1979), Suga & Ikezawa (1995) but not by the findings of Tercedor et al. (1997).
The accidental introduction of 20 tons of concentrated aluminium sulphate into a treated water reservoir in the Camelford area of Cornwall, England in 1988 resulted in a finished water serving ~ 20,000 people that had low pH and high aluminium concentrations for 3 days. Some people experienced GI disturbances and oral ulceration. In addition to the low pH and elevated aluminium in the water, copper, zinc and lead, that were dissolved from domestic plumbing, were also elevated, making it difficult to attribute the irritation solely to aluminium (Coggon, 1991; Altmann, 1999).
Dermal application
Contact sensitivity to aluminium is very rare. Sensitization has been reported during continuous application of aluminium-containing antiperspirants, after injection of aluminium-adjuvant containing vaccines and pollen extracts, topical aluminium application, and occupational exposure to aluminium dust and filings (see below).
Two types of reaction patterns have been described: recurrent eczema from topical application of antiperspirants, and persistent granuloma at the injection site. Sensitization is more common after injection of aluminium-adsorbed vaccines and hyposensitization extracts than from topical application of antiperspirants and medications (Peters et al., 1998). Fifty reported cases of aluminium contact sensitivity were reviewed by Böhler-Sommeregger & Lindemayr (1986), showing that granulomas, eczema and nodules had been reported in 26, 15 and 5 of the cases, respectively. Only one case that was positive to follow-up tests with aluminium was discovered among 853 people who were working in a hard metal manufacturing plant (Fischer & Rystedt, 1982). During the 4.5 years of seeing patients, Veien et al. (1986) encountered 13 children and 1 adult who had positive aluminium patch tests. Moderate positive aluminium patch test reactions were seen in 4 of 1922, primarily adult, patients (Hemmer et al., 1996). Other studies found no positive cases among 25 children with ectopic dermatitis and 251 adults with suspected contact dermatitis (Frost et al., 1985).
Aluminium applied as a nearly saturated solution of aluminium chloride in absolute alcohol to the axillary region to treat hyperhidrosis produced irritation and soreness, particularly at the beginning of a regimen of nightly applications for 1 week, alternate nightly applications for 1 week, then twice weekly for 2 weeks (Ellis & Scurr, 1979). Similarly, daily application of 20% aluminium chloride hexahydrate in ethanol to the palm produced skin irritation in 4 of 12 patients, which disappeared in 3 of these patients after 1 week (Goh, 1990).
Contact sensitivity has been attributed to the use of aluminium-containing antiperspirants. A patient who experienced itchy axillary dermatitis while using an antiperspirant demonstrated contact sensitivity to the aluminium disc used in routine patch testing (Finn Chamber®). He showed a 2+ positive reaction to 2 and 5% aluminium chloride, a positive response to intracutaneous 0.1% aluminium hydroxide, but not a positive reaction to aluminium foil, or to 1% aluminium acetoacetate (Fischer & Rystedt, 1982). Positive patch tests to aluminium were seen in two adults who had used topical aluminium acetotartrate (Meding et al., 1984). Positive reactions were seen to aluminium metal and aluminium chloride, but not to aluminium subacetate (Kotovirta et al., 1984). Two brothers who had suffered from a scaling eruption on their faces and limbs since infancy similarly showed a reaction to the aluminium Finn Chamber and a subsequent aluminium chloride patch test (Dwyer & Kerr, 1993). Cox et al. (1988b) reported four cases of sensitivity to the aluminium Finn Chamber, and one to aluminium salts as well, which they believe was induced by diphtheria toxoid, TT and pertussis (DTP) vaccination. Similar cases of aluminium contact sensitivity demonstrated by positive reactions to aluminium chambers and aluminium salts were reported in children and adults by (Akyol et al., 2004; Bajaj et al., 1997; Castelain et al., 1988; Clemmensen & Knudsen, 1980; Cosnes et al., 1990; Dwyer & Kerr, 1993; Fawcett et al., 1985; Fischer & Rystedt, 1982; Hyry & Hook-Nikanne, 2004; Kalveram et al., 1980; Kotovirta et al., 1984; O’Driscoll et al., 1991; Stables et al., 1996; Tosti et al., 1990; Veien et al., 1986). Hemmer et al. (1996) further described the use of patch testing with aluminium chloride, aluminium hydroxide and potassium aluminium sulphate in patients suspected of having a cutaneous hypersensitivity to aluminium chloride hexahydrate. An acutely inflamed axillary mass of ~ 1 year duration developed following use of an aluminium zirconium tetrachloridehydrex glycerine antiperspirant for several years. The authors attributed this foreign body-hypersensitivity reaction to the aluminium zirconium complex (Skelton et al., 1993).
Not all cases of hypersensitivity to aluminium result in clinical problems. In presenting a case of aluminium sensitivity revealed by the use of the aluminium-containing Finn Chambers, Kotovirta et al. (1984) suggested that a patient’s sensitivity to aluminium, as well as those in some previous cases (Meding et al., 1984), had no clinical relevance, and was only discovered as an accidental finding of the testing procedure.
Use of 5% aluminium acetate ear drops resulted in a 2 year history of eczema (contact sensitivity) to aluminium in a 9 year old which resolved when use of the eardrops was discontinued (O’Driscoll et al., 1991). The aluminium allergy was believed to have been caused by DTP vaccination. Similarly, three 2 to 3 year olds experienced pruritic infiltrates in response to toothpaste containing aluminium oxide that resolved 4 to 6 weeks after discontinuation of use of this toothpaste. Two of the 3 showed return of symptoms when challenged with the toothpaste. All showed positive patch tests to aluminium (Veien et al., 1993).
Application of lotions containing 1 or 3% aluminium starch octenylsuccinate resulted in “slight” irritation potential and no evidence for potential to induce allergic sensitization (Nair & Yamarik, 2002).
Irritation after injection of aluminium-adsorbed proteins (vaccines and hyposensitization regimens)
Aluminium adjuvants are used in vaccines which are given i.m. and s.c., and in allergen immunotherapy injections which are given s.c. Reports of a workshop on this topic appear in a 2002 issue of Vaccine (20:S1-S64). Aluminium hydroxide and/or phosphate is used as an adjuvant in all DTP (including acellular pertussis; DTaP), some Haemophilus influenza type B, and in most hepatitis A and B, meningococcal, pneumococcal, tick-borne encephalitis, some rabies, and anthrax vaccines. On the other hand, inactivated poliovirus vaccines, measles, mumps, rubella, some rabies, yellow fever, Japanese encephalitis, adenovirus, typhoid, plague, cholera, bacillus calmette-guerin (BCG), meningococcal, and some Haemophilus influenza type B do not contain aluminium (Baylor et al., 2002; Bergfors, 2005; Eickhoff & Myers, 2002). Erythema is occasionally associated with aluminium adsorbed vaccines (Gupta, 1998). Booster injections containing aluminium-adsorbed vaccines produced more local reactions, including redness, swelling and itching, in children who had primary immunization with aluminium-adsorbed vaccine than in children who received un-adsorbed primary vaccines (Gupta, 1998). Immediate, inflammatory reactions that produce palpable s.c. nodules are commonly seen with subsequent injections in hyposensitized patients; these reactions usually heal without treatment after a few weeks (Garcia-Patos et al., 1995; Lopez et al., 1994). About 20 to 30% of patients develop s.c. nodules at the injection site after the acute local reaction dissipates, whereas the nodules persist in ~ 4% of patients (Lopez et al., 1994). This occurs more commonly after s.c. than i.m. injection (Hutteroth & Quast, 1990). The most common reactions are a necrotizing granulamatoma with associated fibrosis and chronic inflammation, seen in ~ 60% of cases, and a mixed inflammation with fibrous and histocytic or lymphocytic proliferation, seen in ~ 40% of cases (Miliauskas et al., 1993).
Aluminium adjuvants occasionally produce sterile abscesses, s.c. nodules and dermal granulomatous inflammation at the injection site (Böhler-Sommeregger & Lindemayr, 1986). The incidence of transient nodules lasting weeks has been estimated to be 5 to 10% (Fiejka & Aleksandrowicz, 1993), 2 to 19% (Orfan et al., 1995) and 20 to 30% (Vogelbruch et al., 2000) whereas the incidence of persistent nodules that may last for years is less common (estimated to be 0.1% or lower (Bernier et al., 1981) and 0.5 to 6% (Garcia-Patos et al., 1995)).
Erythema and swelling were seen in the first 24 to 48 hr after s.c. injection of vaccine containing aluminium phosphate at a concentration of 10 mg/mL but not in those who received the vaccine without aluminium. Induration at the injection site was seen in 33% of those receiving the aluminium-containing vaccine vs. 10 to 14% of those receiving vaccine without aluminium (Medical Research Council, 1955). Voss & Tolki (1960) reported a granuloma removed ~ 1 year after vaccination with aluminium oxide adsorbed vaccine showed the presence of aluminium in the granuloma, using a stain. Orell (1962) reported 15 s.c. lesions after influenza vaccination. By injecting adult guinea pigs with each component of the vaccine he found that fine aluminium oxide, as used in the vaccine, produced a granulomatous nodule, whereas injection of commercial aluminium oxide did not, leading him to conclude that particle size was important. Persisting nodules, histologically characterized as a nonspecific chronic perivascular inflammatory infiltrate and degenerated collagen, were seen at the site of tetanus vaccination, and attributed to the aluminium in the vaccine (Lenz, 1966). Deep s.c. immunization of children with triple vaccine containing aluminium hydroxide at a concentration of 2.5 mg/0.5 mL resulted in s.c. nodules in 17 to 21% of the children, and 1 sterile abscess; none occurred in the absence of aluminium (Butler et al., 1969). Erdohazi & Newman (1971) reported 2 cases of post-immunization granuloma that persisted for months after injection of aluminium hydroxide vaccine. Aluminium was identified in the lump by stain and x-ray crystallography in the one case in which it was looked for. Three children developed painful s.c. nodules 1 month to 2 years after injection of aluminium hydroxide-adsorbed DTP vaccine (Slater et al., 1982). Another case of aluminium hydroxide-induced granuloma that persisted for 8 years was reported (Savage, 1973). A foreign body granuloma, which developed in the injection area of a 16-year old after hyposensitization therapy, was found to contain aluminium hydroxide (Linse et al., 1979).
Aluminium was found in histiocytes in a granulomatous inflammatory infiltrate of an 8-month old child. The lesion was seen 2 weeks after i.m. vaccine injection in rats (Mrak, 1982). Three children developed itching, eczema and circumscribed hypertrichosis over nodules following immunization with aluminium hydroxide-adsorbed vaccines (Pembroke & Marten, 1979). Cases in which two similar patients also developed hyperpigmentation and itching, that lasted for 2 years in one, were described (Orlans & Verbov, 1982). Standard patch testing of a patient who developed eczema during hyposensitization injections with an aluminium-adsorbed allergen, and who developed a contact dermatitis to aluminium-containing antiperspirants, revealed positive reactions to the aluminium Finn Chamber discs used for testing. The patch tests were negative in 53 controls and 9 patients who had received hyposensitization injections with aluminium-precipitated allergens. The patient showed a positive response to patch tests with aluminium salts in plastic disks and prick and intradermal testing with aluminium hydroxide. The eczema disappeared when hyposensitization was discontinued (Clemmensen & Knudsen, 1980). Three more cases of injection site granulomas associated with pruritus or tenderness which developed 2 to 9 months after injection of aluminium-adsorbed vaccine were reported by Fawcett & Smith (1984) who demonstrated the presence of aluminium in the nodules by stain. In another case, a positive test to the Finn Chambers and aluminium chloride hexahydrate and a moderate response to aluminium acetate and aluminium powder were shown (Fawcett et al., 1985). These authors produced a similar response in rats by injecting aluminium-adsorbed vaccine. A case of a patient with a positive patch test reaction to aluminium hydroxide who had been sensitized by aluminium-adsorbed vaccine was reported (Böhler-Sommeregger & Lindemayr, 1986). Thirteen children and one adult, who had received hyposensitization therapy with aluminium-bound pollen extracts or childhood immunizations with aluminium-bound vaccine and who had pruritic, excoriated papules, had positive patch tests to 2% aluminium chloride. One child developed axillary eczema after antiperspirant use (Veien et al., 1986). Two additional cases of aluminium sensitivity were seen in adults which was attributed to inoculation of aluminium-precipitated pollen or dust extracts for hyposensitization (Castelain et al., 1988). Persistent nodular lesions were noted by Fernandez et al. (1990) and Hutteroth & Quast (1990) who described a case of palpable, ~ 3 cm diameter, itchy nodules 3 years after inoculation. Cosnes et al. (1990) reported 2 adult cases of pruritic nodules and contact allergy to aluminium attributed to aluminium-adsorbed vaccine. A follow-up study of 202 children who received hyposensitization with aluminium-containing allergens showed that 13 had severe itching for 1 to 3 years, and persisting, s.c. nodules. Excised nodules from 6 showed infiltration with lymphocytes, macrophages, plasma cells, mast cells and some eosinophils. Aluminium was found in nodules from 5 children. Four showed positive patch tests to aluminium (Frost et al., 1985). Twenty one children had cutaneous granulomas following aluminium hydroxide-vaccine immunization and positive tests to aluminium chloride and/or an aluminium Finn Chamber. Clearing of symptoms was experienced by 5, improvement by 11 and no change by 5 over a period of 1 to 8 years (Kaaber et al., 1992). Two of 4 children patch-tested again 4 years later showed negative responses.
All children who were referred to 2 private dermatological practices over 6 years and who had pruritus and s.c. infiltrates in the areas of immunization with an aluminium hydroxide-containing DTP vaccine were patch tested with an aluminium Finn Chamber or with 2% aqueous aluminium chloride. Contact allergy to aluminium was demonstrated in 32 children. Patch testing with 2% aluminium chloride occluded with an aluminium Finn Chamber proved to be the most sensitive (Nielsen et al., 1992). Aluminium was demonstrated by x-ray microanalysis in necrotic foci of 4 patients who developed s.c. nodules 4 to 22 months after vaccine injection (Miliauskas et al., 1993), of 2 patients who developed localized nodular reactions at the site of prior TT injections (Cominos et al., 1993), and of 3 children who developed s.c. nodules with necrotizing granulomatous reactions at the site of DTPT (DTP + poliovirus) injections (Bordet et al., 2001). Similarly it was demonstrated in the macrophages of itching nodules that developed following immunization with aluminium hydroxide-adsorbed vaccine in 2 patients (Nagore et al., 2001). Garcia-Patos et al. (1995) concluded that 60 to 80% of patients with delayed hypersensitivity reaction to aluminium show a positive response to patch tests or aluminium Finn Chambers. Positive responses to patch tests with aluminium salts in plastic van der Bend chambers were demonstrated in 4 patients who developed pruritic, painful nodules and papules after vaccination (Skowron et al., 1998). Fiejka & Aleksandrowicz (1993) suggested that nodules that persist > 6 weeks after injection of aluminium-adsorbed proteins may indicate aluminium hypersensitivity. The prevalence of aluminium sensitivity was studied in 40 patients who had received aluminium-containing antigens, 20 of whom had persistent s.c. nodules of > 2 months duration, and in 20 controls. The only positive aluminium chloride patch tests were in 4 patients who had persistent nodules (Lopez et al., 1994). Ten patients who developed persistent s.c. nodules 1 month to 6.5 years following immunotherapy were studied. Patch tests with 2% aluminium chloride were positive in 5. Histopathological examination revealed 2 different patterns. Some lesions of < 9 months duration showed a pure foreign body histiocytic reaction. Other lesions showed a delayed hypersensitivity granulomatous reaction in association with an histiocytic foreign body response, suggesting that 2 characteristic histopathological patterns may be produced by injection of aluminium-adsorbed proteins (Garcia-Patos et al. 1995). A case was reported of apparently permanent local fibrosis and disfigurement due to alum-precipitated hyposensitization injections in a woman who showed a positive patch test to 2% aluminium chloride and itchy eczematous axillary rash to aluminium-containing antiperspirants (Orfan et al., 1995). Persistent intra-dermal granulomas developed after inappropriate intradermal injections of aluminium-containing hyposensitization solutions (Vogelbruch et al., 2000). An unexpectedly high frequency of persistent itching nodules at the vaccination site was observed from aluminium hydroxide-adsorbed DTP vaccines, leading to a longitudinal observational study of the incidence of aluminium sensitization (Bergfors et al., 2003). The authors estimated that only ~ 100 cases had been described in the previous 40 years. Itching nodules were found in 645 children following 76,000 s.c. and i.m. vaccinations (0.8%); these appeared in 28, 117, 494, 1 and 6 children after the 1st, 2nd and 3rd, 4th and 6th vaccination, respectively. By 2005, 740 cases had been observed (Bergfors, 2005). The onset averaged 3 months, ranging from 2 weeks to 5 years. Hypertrichosis, hyperpigmentation and/or local eczematous reactions were seen in 32%. Although the symptoms decreased and the nodules resolved over time, 75% of the children had symptoms after a mean of 4 years.
Similarly, large injection site reactions were more frequent after the fifth DTaP immunization than after the previous four (Pichichero et al., 2000). Seventy-seven per cent of the children with itching nodules had positive patch tests to 2% aluminium chloride using a plastic IQ chamber and the metallic aluminium of the aluminium Finn Chamber as compared with 8% of their symptom-less siblings (Bergfors et al., 2003), consistent with the conclusion of Garcia-Patos et al. (1995) that a delayed hypersensitivity granulomatous reaction occurs in 0.5 to 6% of cases. In contrast to the Swedish experience described by Bergfors et al. (2003), itching nodules were reported in only 30 children in Denmark during 1997 to 2003 when 3,600,000 doses of vaccines containing the same aluminium hydroxide-adsorbant were administered (Thierry-Carstensen & Stellfeld, 2004). It was suggested that the use of i.m. injections in Denmark, which are claimed to produce this reaction less frequently than s.c., may explain the difference (Thierry-Carstensen & Stellfeld, 2004) but the Swedish group doubts this (Trollfors et al., 2005), leaving the cause of this difference unresolved.
Epicutaneous tests demonstrated hypersensitivity to aluminium in 77% (393 of 512) of children who had persistent itching s.c. nodules at the injection site of the vaccine, aluminium hydroxide-adsorbed DTP (Bergfors, 2005). The aluminium hypersensitivity was manifest in local dermatitis after application of aluminium-containing antiperspirants and sun protection lotions. Eight percent (17 of 221) of children who had received the same vaccinations but who did not experience any local reaction also demonstrated positive aluminium tests, whereas a positive reaction was not seen in any of 54 children who earlier had received an aluminium phosphate-adsorbed vaccine. In contrast to the experience with aluminium hydroxide-adsorbed vaccines, no cases of persistent nodules were reported among 1.7 million Swedish children who received aluminium phosphate-adsorbed DT vaccine from 1979 to 1996 (Bergfors, 2005).
A follow-up study was conducted of 22,365 pupils who received i.m. aluminium hydroxide- or aluminium phosphate-adsorbed diphtheria and tetanus vaccines. The results revealed 405 reports of itching and nodules from the parents. Seventy-five reports were positive for both itching and nodules, sixty-five had immediate symptoms with a duration of < 3-4 weeks, and ten had a persistent itching nodule persisting for > 2 months involving both aluminium adjuvants (Netterlid et al., 2004).
The above reviewed reports suggest persistent adverse effects associated with aluminium adjuvants are much more frequent following use of aluminium hydroxide than aluminium phosphate adjuvants. The s.c. injection site seems to cause these problems more frequently than that of i.m. injection.
An apparently new medical condition, termed MMF, characterized by myalgia, arthralgia, muscle weakness, asthenia and fever has been described (Gherardi & Cherin, 1998). A study of 50 patients revealed all had received aluminium hydroxide-containing vaccines, 3 to 96 (median 36) months prior to muscle biopsy with an onset of mylagia 11 months (median) after vaccine injection. Muscle biopsy showed macrophage infiltration. X-ray microanalysis of inclusions showed aluminium in each of the 8 cases tested; nuclear microanalysis showed elevated levels of aluminium in macrophages and EAAS showed elevated aluminium levels. A similar syndrome was produced in rats after aluminium hydroxide-containing vaccine injection (Gherardi et al., 2001). Although aluminium can persist after i.m. injection as a vaccine adjuvant, there does not appear to be strong evidence that it causes a diffuse inflammatory muscle disease (Siegrist, 2005).
The only vaccine licensed in the U.S. containing aluminium hydroxide that is administered s.c., rather than i.m., is anthrax vaccine, adsorbed. However, when given i.m. it produced less erythema and induration than when injected s.c. (Pittman, 2002).
It has been suggested that subsequent injection of aluminium-containing vaccine in a patient who developed an aluminium-induced granuloma from previous injection runs the risk of a similar outcome (Hutteroth & Quast, 1990). This risk needs to be weighed against the benefit to be derived from the subsequent injection.
Microscopic examination of 4 cases of aluminium-induced injection site reactions suggested manifestations in addition to the commonly seen histiocyte sheets and necrotizing granulomatous reaction with associated fibrosis and chronic inflammation or mixed inflammatory reaction with fibrosis and histiocyte or lymphocyte proliferation (Culora et al., 1996).
In Denmark, vaccinations are sometimes given into the upper part of the breast region because the s.c. tissue there is thought to reduce the discomfort of the injection. Twenty-two cases of vaccination granulomas in the breast region caused by injection of aluminium-adsorbed vaccines have been described (Al-Suliman et al., 1999; Nielsen et al., 1991).
A tattoo resulted in a delayed, localized, intermittently pruritic granuloma that developed 4 weeks later. It was shown to contain aluminium and titanium particles but no other inorganics (McFadden et al., 1989). The patient did not show a positive response to epicutaneous testing with aluminium Finn Chambers and patch tests with aluminium chlorhydrate, aluminium chloride or aluminium sulphate but did show an erythematous nodular reaction 2 weeks after intradermal injection of a 10% aluminium chloride suspension. Schwarze et al. (2000) reported a case in which a woman developed erythematosquamous lesions that appeared 6 days after receiving blepharopigmentation (tattooing to create a permanent line along the eyelid margin) with an aluminium silicate compound. This delayed hypersensitivity granulomatous reaction was attributed to the aluminium salt.
The mechanism of aluminium adjuvant-induced nodules has been thought to be due to a type-I immediate hypersensitivity reaction or a specific type IV cell-mediated delayed hypersensitivity response to aluminium (Frost et al., 1985; Garcia-Patos et al., 1995; Lopez et al., 1994; Orfan et al., 1995) and to a foreign-body reaction to aluminium (Garcia-Patos et al., 1995; Turk & Parker, 1977). Aluminium adjuvants attract eosinophils to the injection site (Walls, 1977) and may induce IgE production (Nagel et al., 1977). As noted in Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, Injection above, the aluminium-adsorbed protein may increase IgE production. IgE antibodies were higher in adults who received aluminium hydroxide-adsorbed TT than in those who received the plain TT (Cogne et al., 1985). Following primary immunization with aluminium-adsorbed pertussis toxin, the specific IgE response, and local side effects, were greater in children who received aluminium-adsorbed than those who received non-aluminium-adsorbed booster immunization (Odelram et al., 1994). IgE responses, and local side effects, increased much more in children given a booster who had received primary vaccine containing aluminium-phosphate-adsorbed diphtheria, tetanus and/or pertussis than in those whose primary vaccine was not aluminium-adsorbed (Mark et al., 1995). An investigation of the in vitro effects of alum on peripheral blood mononuclear cells from atopic human donors revealed that it down-regulates the allergen-driven Th2 cytokine response, providing more support for its use as an adjuvant in allergen immunotherapy vaccines (Wilcock et al., 2004).
Contact hypersensitivity is initiated by activation of CD4+ T lymphocytes that are activated by an antigen bound to a relevant major histocompatibility complex class II protein on the surface of antigen-presenting cells. Metals, such as chromium, cobalt, nickel and platinum may combine with serum proteins to act as haptens and become antigens (Park et al., 1996; Sinigaglia, 1994). It does not appear that the ability of aluminium to similarly serve as a hapten has been evaluated, nor has it been shown that there is a specific IgE antibody to aluminium (Park et al., 1996). Park et al. (1996) suggested that aluminium-induced occupational bronchoconstriction/asthma is mediated by a non-immunologic mechanism. Aluminium has been shown to increase the delayed-type of hypersensitivity to an infection in mice, suggesting it damages T system and B system-specific immunity (Simonyte et al., 2004).
Although the British Ministry of Health recommended aluminium-free vaccines in 1957, the Committee on Control of Infectious Diseases of the American Academy of Pediatrics advised in 1964 that alum-precipitated DTP or aluminium hydroxide- or aluminium phosphate-adsorbed vaccines should be used (Baylor et al., 2002). Aluminium compounds are the only adjuvants used in vaccines in the U.S. The U.S. Food and Drug Administration allows ≤0.85 mg Al/dose in vaccines if the level is assayed, ≤1.14 mg Al/dose if determined by calculation based on the amount of the aluminium compound added, and ≤1.25 mg Al/dose in biological products, the last to be consistent with WHO standards (Baylor et al., 2002). In the U.S., currently used vaccines that contain aluminium do so as aluminium hydroxide, aluminium phosphate, or a combination of these. In spite of the above problems, it has been suggested that the benefits and safety profile of aluminium salts as adjuvants in vaccines will result in their continued use for some time (Baylor et al., 2002; Clements & Griffiths, 2002). Precipitation of toxins and toxoids by alum (and cerium nitrate, zinc sulfate, calcium chloride dialyzed iron and tungstic acid) was found to enhance their antigenic properties and reduce the rate of antigen absorption and elimination in animals (Glenny et al., 1926; 1931). Addition of alum to diphtheria toxoid increased the percentage of infants and children showing detectable antitoxin (Volk & Bunney, 1942; Barr et al., 1952) and aluminium phosphate addition increased the antitoxin titer to diphtheria and tetanus when given with DPT (Greenberg & Benoit, 1956). However, some studies produced negative results (Barr et al., 1955; Feldman, 1957). The rate of adverse reactions to aluminium in vaccines is very small (Clements & Griffiths, 2002).
Implantation exposure
Aluminium oxide ceramic implants did not produce any allergic skin reactions (cutaneous hypersensitivity) in 250 patients (Thomas et al., 2003).
Effects on the endocrine system
PTH function may be affected by exposure to aluminium which may, in turn, affect bone metabolism (see Effects on Humans, Effects from Non-Occupational Exposure, Bone) (Cannata Andía, 2000). No epidemiological studies have been published on this topic.
Immunotoxicity/immunosuppression
There is little information available regarding the effects of aluminium exposure on immunological parameters in humans.
There is some evidence of a possible link between aluminium overload and immunosuppression in renal transplant recipients (Davenport et al., 1989; Garrett, 1989; Nordal et al., 1988c). Nordal et al. (1988c) followed 94 recipients of kidney allografts for 3 years. Subclinical aluminium toxicity was found in 66 of these patients. Factors potentially influencing the rejection rate in these patients was analyzed by multivariate analysis. Only the source of kidney (cadavers v.s. living donors) and aluminium accumulation were found to influence the rejection rate (Nordal et al., 1988c). In a similar study, Davenport et al. (1989) followed 58 patients after successful renal allograft transplantation. The patients were divided into two groups based on the number of rejection episodes following the transplant. The median daily urinary excretion of aluminium was greater in the group with one or no rejection episodes than in the group with two or more. These results suggest that aluminium accumulation in renal transplant patients exerts an immunosuppressive effect.
Graske et al. (2000) conducted a small experimental study on volunteers to examine the effects of oral aluminium exposure on the immune system. The study group was comprised of 18 healthy volunteers aged 28-57 years. The 18 subjects were randomly assigned into two groups. Individuals in the treatment group (n=13) ingested 3 daily doses of 10 mL antacid mixture (Al content 588 mg/10mL) for 6 weeks, subjects in the control group (n=5) did not ingest any antacids. Blood samples were taken for analysis of lymphocyte subpopulations, nitrogen induced lymphocyte proliferation, and in vitro production and circulating plasma concentration of various immunoglobins. A slightly smaller primed cytotoxic T-cell population in the exposed individuals was the only noted difference in the immune parameters investigated. CD8+ cells are thought to play a role in the down-regulation of the immune response. In contrast to the immunosuppressive effects of aluminium observed in renal transplant patients, these finding suggest that aluminium exposure may have slight stimulatory effect on the immune system in healthy subjects. This finding has not been confirmed in a larger study.
Renal
There is extensive literature on the effects of renal insufficiency and renal failure on the absorption of aluminium (see Effects on Humans, Subpopulations at Special Risk, Individuals with Impaired Renal Function), but no literature to suggest that aluminium exposure causes renal damage.
Bone
Renal failure results in inability to eliminate aluminium, so that the absorbed aluminium, or aluminium introduced directly into blood, e.g. during dialysis, cannot be eliminated. Absorbtion of this aluminium by bones can result in bone disease (Cannata Andia, 1996; Cannata Andia, 2000; Jeffery et al., 1996). Cannata Andía (2000) described renal osteodystrophy as representing a wide spectrum of bone derangements, ranging from high bone turnover to low bone turnover, with two main forms: aluminium-induced and non-aluminium-induced adynamic bone disease. Aluminium-induced osteomalacia involves low bone turnover, possibly by indirectly inhibiting PTH function.
Jeffery et al. (1996) reviewed the systemic toxic effects of aluminium on bone in patients receiving renal dialysis. They noted that some renal dialysis patients developed aluminium-dependent bone disease, while others did not; the reason for this was not clear. They speculated that it could have been due to differences in handling and accumulation of aluminium leading to differences in the bone aluminium load, or to more complex interactions between aluminium accumulation and factors such as the presence, absence, or degree of, hyperparathyroidism.
Recker et al. (1977) studied aluminium absorption in six subjects with normal renal function, and examined aluminium content in bones from autopsies of both dialysis and non-dialysis patients. They concluded that the gut barrier is permeable to heavy aluminium load and they suggested that bone aluminium occurs in humans with normal renal function.
Eastwood et al. (1990) reported the cases of a 41 year-old woman and 49 year old man who had acute symptoms of mouth and nasal ulcers following the Camelford contamination incident. The water aluminium concentrations recorded at the time of the contamination event ranged from 30 to 620 mg/L. These patients were examined approximately 7 months following the incident and bone biopsies were obtained. Subjective and objective static and dynamic bone measurements appeared to be within the normal range. Infrequent lines of positive staining for aluminium by solochrome azurine were detected within the trabecular and cortical bone of these patients; however, in a quantitative analysis, aluminium could only be identified in a single trabecula in one patient. Aluminium concentrations in the bone specimens were within the normal range. The authors reported that the local areas of aluminium staining and the incorporation of these lines into the lamellar structure indicated that these deposits were formed by a single event of short duration. It was concluded: “..under certain conditions normal individuals can absorb aluminium via the gut, and that such aluminium can be deposited in bone.” This is a case report only, involving two patients and no controls; the findings may not be generalizable. In addition, the amount of aluminium that these individuals were exposed to during the period of contamination is unknown.
McMillan et al. (1993) presented follow up data on the two patients described previously by Eastwood et al. one year after their initial bone biopsies. In addition, data from seven additional individuals who underwent bone biopsies 12-17 months after the accident were presented. These patients developed musculoskeletal symptoms as well as indications of impaired memory following the contamination incident. In the two patients previously described, there was no residual aluminium staining and all quantitative and qualitative measurements were normal. Three of the new patients exhibited borderline osteopoenia, as detected by cancellous bone measurements which were reported to be 2-3 standard deviations below that of a control group. However, the control group was not described, and the data were not presented in the article. The aluminium content of the patients was normal.
The elevated bone Al seen in premature infants who received Al-contaminated total parenteral nutrition (Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Tissue Aluminium Concentrations, Bone) has been implicated in a metabolic bone disease characterized by pain and patchy osteomalacia and associated with reduced serum 1,25-dihydroxyvitamin D, reduced or low-normal serum immunoreactive parathyroid hormone, hypercalciuria, mild hyperphosphatemia, and mild hypercalcemia (Klein et al., 1982; McCullough & Hsu, 1987).
There have been no epidemiological studies of the effects of aluminium on bone.
Anaemia
Microcytic anaemia is a feature of aluminium intoxication seen in dialysis patients, which is reversible with deferoxamine treatment (Swartz et al., 1987; Tielemans et al., 1985). Severity of anaemia in these patients has been shown to be correlated with erythrocyte aluminium levels (Yuan et al., 1989), which suggests that there is a dose response relationship. The physiological basis for aluminium induced anaemia is complex, a discussion of the mechanisms involved and issues surrounding the treatment of this disorder is provided in Effects on Laboratory Mammals and In Vitro Test Systems, Effects on Haematopoiesis. No epidemiological studies have been published on this topic.
Cancer
Aluminium salts are the major constituents of many widely used antiperspirant products. Aluminium chloride was the first compound to be used for this type of application; currently, however, the most widely used antiperspirant compound is aluminium chlorohydrate. However, only a few epidemiological studies have been undertaken of the possible carcinogenic risks of antiperspirants.
In a case-control study, Mirick et al. (2002) investigated a possible relationship between use of products applied for underarm perspiration and the risk for breast cancer in Western Washington State. Case patients (n=810) were women aged 20-74 years who had first been diagnosed with breast cancer between November 1992 and March 1995. Control subjects (n=793) were women without breast cancer, identified by random-digit dialling from the same population as the case patients, who were frequency-matched to the case patients by 5-year age groups. In-person interviews were used to gather the information. The findings did not support the hypothesis that antiperspirant use increases the risk of breast cancer with any of the following activities:
antiperspirant (OR=0.9; p=0.23) or deodorant use (OR=1.2; p=0.19);
product use among subjects who shaved with a blade razor; or;
application of products within 1 hr of shaving (for antiperspirant, OR=0.9 and p=0.40; for deodorant, OR=1.2 and p=0.16).
No information pertaining to the time of exposure was collected.
McGrath et al. (2003) used a cohort of 437 women to study the association between antiperspirant and deodorant usage and the risk of breast cancer. In this retrospective study, a written questionnaire was sent (1st January, 1993 to 31st December, 2001) to surviving female breast cancer patients whose mean age was 65.7 years (range: 31 to 94 years). The 437 members of the cohort comprised those of 1,344 women who had returned their questionnaire. This cohort was subdivided into usage groups identified by the authors as: “maximum”, “middle”, “minimum”, “none”, and “all” levels of use. The mean age at which breast cancer had been diagnosed was also compared between subjects who started using these products before the age of 16 and those who started at or after the age of 16. The frequency and an earlier onset of antiperspirant/deodorant usage with underarm shaving were associated with an earlier age of breast cancer diagnosis. A dose-response relationship was observed with the mean age of breast cancer diagnosis being progressively lower from the no to the high usage groups. Aluminium salts from antiperspirants could be absorbed through the dermal barrier, however toxicokinetics studies have shown that aluminium poorly penetrates the skin (see Toxicokinetics, Absorption, Animal Studies, Dermal). We do not really know which of the components are responsible for the observed relationship and we should not exclude the fact that other components of the products or the characteristics of the women could be confounding factors. This small study cannot conclusively link a woman’s use of underarm products with breast cancer.
Genotoxicity
The aetiology of AD is complex and both environmental and genetic causes are involved. Moreover, although highly controversial, it has been suggested that aluminium could play a role in AD (see Effects on Humans, Effects from Non-Occupational Exposure, Neurotoxicity). Tf is a major transport protein for both iron and aluminium. The Tf protein has many variants, but the C1 and C2 variants account for the majority of the population of all races. Farrar et al. (1990) have suggested that deficiency of aluminium binding to Tf may increase the amount of unbound aluminium which could cross the BBB thereby increasing the risk of neurotoxic effects. Defective binding of iron and aluminium to the Tf variant C2 could be present in AD and individuals carrying the C2 allele could therefore be at a greater risk of developing AD. Contradictory results have been obtained in case controls studies. Namekata et al. (1997) obtained a significantly higher C2 allele frequency in AD patients than in age-matched controls. More recently, however, Hussain et al. (2002) did not find a significant association between the C2 allele and AD. They obtained a significant excess of the C2 allele only in AD cases without ε4 allele. In contrast, Namateka et al. (1997) suggested that the C2 allele increases the risk of late onset AD and the C2 allele frequency in AD patients homozygous for the ApoE ε4 allele was increased compared to patients carrying zero or one copy of the ε4 allele. Lleo et al. (2002) did not find any association with Tf or with any other genetic factor.
In a recent cohort study, Rondeau et al. (2006) investigated whether the Tf C2 allele (Tf C2) might be responsible for susceptibility to AD in a sample of 292 subjects (55 of whom had AD) aged 75 years and over from south-west France; some of these subjects (n=181) had been exposed to high levels of aluminium in tap water, (i.e., levels higher than 100 μg/L), and others (n=111 subjects) to low levels of aluminium. The combined genetic effects of the Tf C2 variant and the ε4 allele of ApoE was also examined. Logistic regression analysis showed that neither Tf C2 itself nor its interaction with aluminium or with the ε4 allele of the ApoE was significantly associated with the risk of AD. These results could be attributed to a lack of power in the statistical analyses due to small sample size. This appears to be the only epidemiological study which addresses the issue of the interaction between aluminium in tap water and the influence of Tf C2 allele in AD.
Developmental effects
Various developmental effects of aluminium in animals have been described, particularly in rats and mice (Domingo, 1995) (see also Effects on Laboratory Mammals and In Vitro Test Systems, Reproductive and Developmental Toxicity, Developmental Toxicity); however, few studies have been conducted in human populations. Studies of the effects of aluminium on human pregnancy have been located and a recent review article describes some results on this topic (Reinke et al., 2003). The report of Weberg et al. (1986) described women with dyspepsia who could have ingested large amounts of aluminium from antacids at various times during their pregnancies. They analyzed the concentrations of aluminium in serum from the mother and her newborn child, in consecutive cases where the mothers had used antacids during the pregnancy (antacid-use group comprised 9 mothers and their newborns), and in randomly selected cases where the mother had not used antacids (control group = 32). The median aluminium concentration in serum was 0.19 (0.04-0.70) in the control group and 0.26 (0.04-1.59) in their newborns, compared to 0.19 (0.04-0.52) and 0.26 (0.07-0.52) in the antacid-use group. This small study did not show significant differences in aluminium concentration in serum between those who had consumed antacids and the controls, or between mothers and newborns within any of the two groups.
Severe developmental retardation has been documented in a child whose mother had taken high doses of aluminium-containing antacids during pregnancy (Gilbert-Barness et al., 1998) (see Effects on Humans, Case Reports). In most clinical situations, antacids that contain either calcium or aluminium are safe when taken at the recommended doses. However caution should be exercised if using aluminium-containing antacids when large doses are taken for long periods of time. As a result, a panel of experts has developed algorithms that present healthcare professionals with treatment options (Tytgat et al., 2003). The Panel recommended, for instance antacids “on-demand” as the first-line over-the-counter treatment in reflux, and as rescue medication for immediate relief when reflux breaks through with proton pump inhibitors. Calcium/magnesium-based antacids would be the treatment of choice for pregnant women because of their good safety profile.
Toxicokinetics, Distribution (Including Compartmentalization), Human Studies, Tissue Aluminium Concentrations, Milk, presents reported values of Al in human breast milk. This would suggest that aluminium may generally reach breast-fed newborns, but toxicity data are lacking.
Bishop et al. (1997) investigated the effects of perinatal exposure to i.v. aluminium on the neurological development of 227 infants born prematurely. One group of premature infants received standard feeding solutions and the other group received aluminium-depleted i.v. feeding solutions. They were evaluated using the Bayley scales of infant development at 18 months of age. The Bayley index was significantly lower in the 39 infants that received more than 10 days of intravenous feeding of the standard solution than in the 41 infants that received more than 10 days of intravenous feeding of the Al-depleted solution (92 ± 20 vs. 102 ± 17 respectively, p = 0.02). The result implies an increased risk of subsequent learning problems in the former group. The standard and aluminium-depleted solutions delivered median daily aluminium intakes of 187 and 28 μg respectively. None of these infants were judged to have neuromotor impairment, which interferes with performance of the Bayley scales. A further analysis suggested that infants given intravenous feeding that delivered 45 μg Al/kg/day would experience a one point reduction of the Bayley index for each day of feeding.
Modifying factors
Increasingly, studies are showing that analyses of possible relationships between aluminium in drinking water and the risk of AD or cognitive impairment must be made after adjusting for other water constituents that could play a confounding role by masking the neurotoxicity of ingested aluminium. Other water components or other characteristics of the geographical area may influence this possible association. It is thus important to include diverse geographical areas in epidemiological studies.
In particular, the confounding effect of silicon in tap water has been studied. Silicic acid could protect against aluminium toxicity by the likely formation of hydroxylaluminosilicate compounds (Birchall & Chappell, 1989). Edwardson et al. (1993) confirmed this conclusion in a study of five healthy men which showed that the GI absorption of aluminium administered in orange juice is reduced if sodium silicate is added to the beverage (see also Toxicokinetics, Absorption, Studies in Humans, Oral Administration, Factors Influencing Oral Aluminium Absorption, Silicon-Containing Compounds). From these studies, two hypotheses have been proposed. The first was suggested by Birchall & Chappell (1989), who pointed out that, generally, aluminium and silicon concentrations in drinking water are negatively correlated, confirming that silicon may be a confounding factor in the reported statistical association between aluminium in drinking water and AD. Taylor et al. (1995) showed a significant inverse correlation between soluble silicon and soluble aluminium (r = −0.43, p < 0.001). Moreover, drinking water could be an important source of the daily intake of silcon in a form that is particularly available for association with aluminium. As a second hypothesis, Birchall & Chapell (1989) suggested that the low association between aluminium levels in drinking water and AD might be a consequence of the protective effect of silicon in the water. Silicon in water might protect populations not only from aluminium in water but also from the effect of intake of aluminium in the diet. More specifically, Exley & Birchall (1993) suggested that a concentration of silicic acid, produced when sodium silicate solution is acidified, of >100 μmol/L might prevent the absorption of aluminium from the GI tract and facilitate its excretion by the kidney.
The results of the Paquid cohort study also showed that high silica levels (≥ 11.25 mg/L) are associated with a lower risk of dementia (adjusted RR = 0.74, 95% CI: 0.58–0.96) and AD (adjusted RR = 0.73, 95% CI: 0.55–0.99) (Rondeau et al., 2000) (see also Effects on Humans, Effects from Non-Occupational Exposure, Neurotoxicity). The same relationship was observed with cognitive decline (Rondeau et al., 2001). The results are concordant with the hypothesis of Birchall & Chapell (1989) that failure to detect an association between aluminium levels in drinking water and AD might be due to a protective effect of silicon. If this assumption is true, then the exact risk attributable to aluminium is probably underestimated in the Paquid cohort, which does not consider total daily aluminium intake (which is difficult to measure). On the other hand, the results obtained by Martyn et al. (1997) in a case-control study on younger subjects (43-75 years) do not support a protective role of silicon.
Several investigators have described how the pH of drinking water may affect the solubility of aluminium components. It is plausible that the biological availability of aluminium is higher for low than for high pH, which would lead to an interaction between pH and aluminium. These results were not confirmed recently (Rondeau et al., 2000). Another interaction has been described in the literature by Forbes et al. (1994); they found that a neutral pH, a relatively low aluminium concentration and relatively high fluoride concentration decreased the odds of cognitive impairment by a factor of about 5, compared with other type of drinking water.
Subpopulations at Special Risk
Individuals with impaired renal function
Historically, patients with impaired renal function were exposed to aluminium via trace amounts of aluminium in the water used to prepare dialysis solutions, as well as from orally administered aluminium compounds prescribed as GI phosphate binders (Alfrey, 1993). Patients receiving dialysis treatment are at higher risk of aluminium toxicity from contaminated dialysate because GI absorption is bypassed, resulting in direct entry of metal into the circulatory system (Hawkins et al., 1994). In addition, patients with sub-optimal renal function have decreased ability to eliminate aluminium from the body as the renal pathway is the main route for the excretion of metals (Alfrey, 1993).
Encephalopathy in dialysis patients has been well documented (Alfrey, 1993; Foley et al, 1981). The main symptoms of this disorder include alterations in behaviour and memory, speech disorders, convulsions and myoclonus (Foley et al., 1981). Many outbreaks of encephalopathy were reported in association with the use of dialysis fluids containing aluminium; however, aluminium induced encephalopathy was also found in patients with renal failure, who did not undergo dialysis treatment (Foley et al., 1981; Moreno et al., 1991; Sedman et al., 1984). Foley et al. (1981) reported the onset of encephalopathy in 5 paediatric patients who had not undergone dialysis therapy but were receiving chronic administration of aluminium hydroxide gel. Encephalopathy developed in an 8-year old female after 6 years of orally administered aluminium therapy before end stage renal failure occurred (Sedman et al., 1984). A fatal case of encephalopathy was reported in a 59 year old male with severe chronic renal failure (Zatta et al., 2004). This patient was not undergoing dialysis but had consumed large doses of aluminium-containing antacids for at least 3 years.
Encephalopathy was also found in patients with renal failure who received DFO therapy to treat bone aluminium toxicity with chelation therapy (Sherrard et al., 1988). It was proposed that DFO-aluminium complexes may cross the BBB more readily than the naturally circulating aluminium which is predominantly bound to serum Tf and albumin (Sherrard et al., 1988).
Aluminium toxicity was also implicated in a variety of haematological disorders (Mahieu et al., 2000). Yuan et al. (1989) assessed the prevalence of aluminium associated anemia in an outpatient dialysis population. Anaemia was determined to be a significant problem in approximately 18% of the haemodialysis population studied. Patients with the highest quartile of red blood cell aluminium values had significantly lower haematocrit levels compared to those in the lowest quartile (Yuan et al., 1989). Aluminium-induced microcytosis was documented following a failure in the water treatment system of a dialysis facility (Caramelo et al., 1995). Twenty-three haemodialysis patients were exposed to high aluminium concentrations. The patients exhibited a decrease in the erythrocyte: plasma ferritin ratio which suggested the existence of a decreased rate of iron uptake, although the plasma ferritin levels indicated normal stores of iron.
Bia et al. (1989) examined the relationship between haemoglobin concentration and aluminium overload in dialysis patients, as well as the haematopoietic response to DFO treatment. Haemoglobin levels significantly decreased as the degree of aluminium burden rose, as assessed by bone surface aluminium staining. Haemoglobin concentrations increased significantly in 8 patients following DFO treatment, but did not change for the remaining 11 patients. The lack of response to DFO was not correlated to a greater degree of aluminium overload; however, the erythropoietin levels were significantly higher in responders than in non-responders. These results suggest that therapy with DFO significantly reduces anaemia in patients with sufficient levels of erythropoietin such that there may be stimulation in the development of mature red blood cells (Bia et al., 1989).
Aluminium induced microcytosis was reported in a child who had suffered moderate renal failure when given high doses of aluminium compounds for the treatment of hyperphosphatemia. The microcytosis reversed in 2 months following the discontinuation of aluminium treatment (Shah et al., 1990).
Bullous dermatosis is another condition which has been linked to aluminium toxicity in end-stage renal patients (Gafter et al., 1996). It was suggested that high serum aluminium concentrations in these patients may disrupt certain enzymes in the haeme biosynthetic pathway resulting in an overproduction and accumulation of porphyrins. This condition is characterized by skin lesions which can be very painful and are susceptible to secondary infections (Topi et al., 1981).
Aluminium was also associated with skeletal toxicity in this susceptible population (Jeffery et al., 1996; Mathias et al., 1993). Aluminium was found to disrupt PTH secretions, and impair the conversion of 25-hydroxyvitamin D to its biological active hormone, 1, 25-dihydroxyvitamin D (Klein, 2003). Aluminium bone toxicity results in either increased bone remodelling, which produces conditions such as osteitis fibrosa, while some patients exhibit low bone turnover resulting in osteomalacia (Ng et al., 2004). Mixed osteodystrophy also occurs as some patients exhibit features of both osteomalacia and osteitis fibrosis (Jeffery et al., 1996). Aluminium in patients with osteomalacia accumulates in the area between calcified and non-calcified bone (Alfrey, 1993), preventing further mineralization (Bougle et al., 1997).
The incidence of aluminium intoxication in dialysis patients has decreased considerably in recent years due to more thorough treatment of water used in all dialysis solutions, and replacement of orally administered aluminium hydroxide with calcium containing phosphate binding agents (Hdez-Jaras et al., 1998). However there have been reports of aluminium toxicity due to failures in treating the water used in dialysis solutions or due to contamination of the solutions (Berend et al., 2001; Burwen et al., 1995; Caramelo et al., 1995). Hdez-Jaras et al. (1998) reported aluminium intoxication in 8 patients undergoing acetate-free biofiltration with bicarbonate solutions contaminated with aluminium. Serum ferritin and aluminium concentrations were markedly increased in these patients; however there were no indications of neurological or skeletal abnormalities. Aluminium intoxication was reported following contamination of the water system supplying a dialysis centre on the island of Curacao in 1996 (Berend et al., 2001). Aluminium had leached from the cement mortar lining a water distribution pipe exposing 27 dialysis patients to high levels of aluminium. Ten patients died from acute aluminium encephalopathy while the remaining 17 patients exhibited only minor symptoms. The serum aluminium concentrations available for 7 of the non-survivors were significantly higher than in the survivors.
Infants and children
Infants, especially at the pre-term stage, are at particular risk for aluminium toxicity due to immaturity of the GI wall, the BBB, and renal system (Hawkins et al. 1994). The limited capacity of infants to excrete aluminium can result in accumulation of aluminium to levels which may be toxic (Koo et al., 1986). Pre-term infants receiving parenteral nutrition are exposed to aluminium as the solutions used have intrinsic aluminium content and may also become contaminated with aluminium during processing and storage (Advenier et al., 2003). Cognitive deficits have been reported among premature infants that received standard feeding solutions in comparison to infants receiving aluminium-depleted i.v. feeding (total parenteral nutrition) solutions (Bishop et al., 1997 (see also Effects on Humans, Effects from Non-Occupational Exposure, Developmental Effects)). Elevated bone Al seen in premature infants who received aluminium-contaminated total parenteral nutrition solutions has been implicated in a metabolic bone disease (see Effects on Humans, Effects from Non-Occupational Exposure, Bone). Klein (2003) found that hepatic aluminium concentrations in 5 children receiving parenteral nutrition were 5 to 27 times higher than normal concentrations. Therefore, aluminium was implicated in the development of parenteral nutrition-associated cholestasis in infants; however this association has not been fully elucidated (Arnold et al., 2003). Some highly modified infant formula and soy formula were reported to contain significantly more aluminium than breast milk (Goyens & Brasseur, 1990; Hawkins et al., 1994). Von Stockhausen et al. (1990) determined serum aluminium levels in premature infants receiving long-term parenteral nutrition. There was a significantly negative correlation between serum aluminium levels and gestational ages among the 22 premature infants examined (von Stockhausen et al., 1990). Tsou et al. (1991) investigated plasma aluminium levels in infants with normal renal function during prolonged use of aluminium-containing antacids (Tsou et al., 1991). Ten infants who had been receiving antacids for at least one week were compared with 16 controls. The plasma aluminium level of the treated patients (37.2 ± 7.13 μg/L) was significantly higher than that of the control group (4.13 ± 0.66 μg/L).
The growing bone is a particular target for aluminium in infants and young children (Koo & Kaplan, 1988). A 3-month old premature infant developed rickets after 6 weeks of therapy with an aluminium containing antacid (Pattaragarn & Alon, 2001). Bougle et al. (1997) examined the relationship between aluminium and bone mineralization in healthy premature infants. Serum aluminium levels appeared to be a more significant variable in determining bone-mineral density variations than the usual biomarkers of bone metabolism such as calcium and phosphorus levels (Bougle et al., 1997).
EVALUATION OF HUMAN HEALTH RISKS
General Aspects
This section provides a synthesis of data from the salient studies on potential health effects of exposure to aluminium for the purpose of quantifying health risks. The discussion follows the following four-step process specified by the National Research Council (1983).
Hazard identification (the qualitative identification of adverse effects by route of exposure, regardless of level of exposure, and the determination of whether those effects are likely in humans);
Exposure assessment (quantification of human exposure by route);
Dose-response assessment (quantification of the relationship between the level of exposure and the probability of effects identified as part of hazard identification); and
Risk characterization (the combination of exposure assessment and dose-response assessment information to develop an estimate of the probability of adverse outcomes in actual populations).
Health Effects
Hazard identification
Both animal and human data are used to identify potential human health hazards. Where plentiful, hazard identification is based primarily on epidemiological data derived from exposed human populations. However, to the extent that it is useful, hazard identification may also make use of animal data. The epidemiological information base for aluminium includes many occupational studies describing a range of health effects at high levels of inhalation exposure, and non-occupational studies of populations exposed to aluminium in food and drinking water. In addition, vaccine safety studies document the impact of exposure to aluminium via injection.
The detailed review of the available literature presented earlier in this report permits the identification of health effects for which there is sufficient evidence of an adverse effect at some level of exposure to warrant investigation of the dose-response relationship (see Evaluation of Human Health Risks, Health Effects, Exposure Characterization). As described in Effects on Humans, Case Reports, case studies have documented reported cases of a range of effects following exposure to aluminium. These include:
osteomalacia following chronic use of high doses of antacids (Kassem et al., 1991; Woodson, 1998) and exposure to aluminium-contaminated dialysis solutions;
mental retardation and death (at nine years of age) following in-utero exposure to aluminium associated with maternal use of antacids at extremely high doses during pregnancy (Gilbert-Barness et al., 1998);
fatal aluminium encephalopathy following the chronic use of high doses of antacids and exposure to aluminium-contaminated dialysis solutions in patients with reduced or absent renal function (dialysis encephalopathy/dementia), following short term exposure to large amounts of alum instilled into the urinary bladder in patients with reduced renal function (Nakamura et al., 2000; Phelps et al., 1999), and following reconstructive otoneurosurgery using bone cement containing aluminium (Hantson et al., 1994; Reusche et al., 2001b), although the role of aluminium in these cases was not clear;
contact reactivity to aluminium (see Effects on Humans, Effects from Non-Occupational Exposure, Irritation, Dermal Application);
adverse reactions to vaccines containing aluminium (Authier et al., 2001; Di Muzio et al., 2004; Gherardi et al., 2001; Lacson et al., 2002; Nevo et al., 2004); and
contact allergic reactions to aluminium following injection of vaccines containing aluminium (Akyol et al., 2004; Bergfors, 2005; Cox et al.,1988b; Netterlid et al., 2004).
Although these case reports provide information suggestive of adverse health effects in humans, they do not involve the collection of data in a controlled and systematic manner from large samples of the general population. The results of such studies, therefore, cannot be generalized.
The remainder of this section describes results from more detailed studies of aluminium exposure in human populations. The discussion is organized by route of exposure for two reasons. First, information on dose-response and exposure is also organized by route of exposure. Second, the route of exposure can strongly influence a substance’s toxicity. This influence stems from differences in terms of the organs exposed and from differences in the degree of absorption (see Toxicokinetics). Where relevant, we also note the influence of chemical speciation on toxicity. However, the chemical species of aluminium in vivo is often unknown. Methods to separate in vivo aluminium species for identification and quantification are not generally employed, because there is great potential for changes in speciation during separation of metal complexes from each other due to perturbation of chemical equilibria during the process. An alternative approach is to use computational models that include the stability constants of metal-ligand complexes to calculate in vivo metal species concentrations. As there is not good agreement for the aluminium binding constants for many important ligands of aluminium, there is often not good agreement among the results of speciation modelling (Caruso et al., 2005).
Within each exposure route, we consider the following health endpoints: 1) acute toxicity, 2) irritation, 3) corrosivity, 4) sensitization, 5) repeated dose toxicity, 6) mutagenicity, 7) carcinogenicity, 8) reproductive toxicity, and 9) other forms of toxicity, as described by the European Commission (2003). For each outcome and route of exposure, the weight of evidence for its potential to occur in humans, as evaluated based on a systematic narrative review (Weed, 2005) and consensus among the papers’ authors, is judged to be strong, modest, limited, or having no clear evidence. Evaluation of Human Health Risks, Health Effects, Exposure Characterization seeks to quantitatively evaluate the dose-response relationship information for outcomes having either strong or modest supporting evidence. For evidence of an effect to be strong, the preponderance of epidemiological data must be positive, with at least some coming from multiple studies with modest or large sample size and reasonably sound design. A study is judged to have a sound design if 1) its sample size is large enough to detect an effect of moderate magnitude; 2) it controls potential confounding effects sufficiently to ensure that their introduction of a spurious association is at most modest; and 3) it quantifies exposure to aluminium among subjects or groups of study subjects. Epidemiological evidence that is mixed or from studies of limited size or poor design can result in, at most, a modest finding of support for the existence of an effect. If there are only a small number of positive studies of decent design and size, evidence can be rated as limited. If there is minimal or no evidence of an effect, we rate support for it as having no clear evidence. Animal evidence on its own is insufficient to result in classification of an association as “strong.” Animal evidence can, however, serve to support epidemiological evidence. Strong animal evidence for an effect can also serve as the basis for classifying the weight of evidence for an effect as modest.
It is important to keep in mind that with the exception of the injection pathway, this section confines discussion to members of the population that are not believed to be especially susceptible to the effects of aluminium exposure. The section on individuals with impaired renal function (Evaluation of Human Health Risks, Health Effects, Hazard Identification, Sensitive Subpopulations) considers these individuals. Nor does this section in general place substantial weight on associations observed at extremely high levels of exposure (e.g., following chronic exposure to dialysis fluids containing aluminium), as such extreme exposures are not informative for the purpose of characterizing risks at exposure levels more typical of other pathways, which may be orders of magnitude smaller.
Finally, we note risks associated with aluminium’s physico-chemical properties.
Inhalation exposure
Studies investigating the impact of inhalation exposure to aluminium have considered the following endpoints listed in the introduction to Evaluation of Human Health Risks, Health Effects, Hazard Identification: (2) irritation; (6) mutagenicity; (7) carcinogenicity; (8) reproductive/developmental effects; and (9) other effects, including neurological toxicity.
Endpoint (2) – irritation
The association between respiratory symptoms and occupational exposure to aluminium among aluminium smelter workers has been investigated in a number of studies (see Effects on Humans, Effects from Occupational Exposure, Respiratory Tract Effects). Those studies have reported compromised pulmonary function in exposed subjects. However, in many cases, the subjects were exposed to other agents, such as particulate fluoride or carbon dusts, that may have been responsible for this effect.
On the other hand, a series of studies have documented physiological changes attributable to aluminium powder exposure. These changes have included fibrosis, and have been associated with severe symptoms, such as dry cough, dyspnoea, and shortness of breath (see Effects on Humans, Effects from Occupational Exposure, Irritation, Inhalation Exposure). These studies were generally conducted at least 50 years ago, although the consistency of the findings in multiple populations lends support to the hypothesis that these exposures are capable of causing this type of irritation. There have been no studies of these physiological effects in non-occupationally exposed populations. However, animal data support the existence of this effect (see Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, Inhalation Exposure). On the strength of the occupational cohort studies showing evidence of physiological changes to the lung and severe associated symptoms, and the supporting animal data, this evidence is rated as strong.
Endpoint (6) - mutagenicity
Evidence of genotoxicity has not been compelling (see Effects on Laboratory Mammals and In Vitro Test Systems, Carcinogenicity; Effects on Humans, Effects from Occupational Exposure, Genotoxicity; and Effects on Humans, Effects from Non-Occupational Exposure, Genotoxicity), and is judged to be limited.
Endpoint (7) – carcinogenicity
Evidence of an association between aluminium exposure and cancer in humans derives largely from studies of occupationally exposed populations. Interpretation of these studies, which are summarized in Table 22, is complicated by the fact that, in most of them, aluminium exposure is confounded by exposure to PAHs, substances that have themselves been implicated in causing cancer. Other potential confounders include aromatic amines, nitro compounds, asbestos, heat stress, and magnetic fields. The International Agency for Research on Cancer (IARC, 1984; Straif et al., 2005) has designated processes associated with aluminium production as “carcinogenic in humans”, but did not implicate aluminium itself as a human carcinogen. Neither non-occupational studies (see Effects on Humans, Effects from Non-Occupational Exposure, Cancer) nor studies in experimental animal studies have provided evidence that aluminium is carcinogenic (see Effects on Laboratory Mammals and In Vitro Test Systems, Interactions between Aluminium and Other Agents). We therefore conclude that there is no clear evidence of cancer due to inhalation of aluminium.
Endpoint (8) – reproductive/developmental effects
A study of French aluminium industry workers identified an increase in fertility among those with a higher exposure index (Mur et al., 1998). However, this study is inadequate for the purpose of assessing the impact of aluminium exposure on this endpoint. First, the study was designed to evaluate the influence of exposure to heat and static magnetic fields. Second, the study did not control for other factors that may explain the results, especially differences in socio-economic status. We therefore conclude evidence for this effect is limited.
Endpoint (9) – other effects
Sufficient data exist to evaluate the weight of evidence for neurotoxicity following inhalation exposure to aluminium.
Studies of an association between inhalation exposure to aluminium and neurological effects have been limited to occupationally exposed populations. Results among studies of occupationally exposed populations have been mixed (see Effects on Humans, Effects from Occupational Exposure, Neurotoxicity). Akila et al. (1999) and Riihimäki et al. (2000) reported that workers exposed to either low or high levels of aluminium (serum aluminium levels of 0.14 and 0.46 μmol/L, respectively) had lower neuropsychological test scores compared to referants (serum aluminium level of 0.08 μmol/L). EEG abnormalities were most common in the high exposure group and second most common in the low exposure group. The study measured lead exposure in order to rule out its potential confounding influence but did not assess other possible neurotoxic agents. The most recent publication based on this cohort had a modest sample size that included 65 metal inert-gas welders and 25 mild steel workers. The earlier study was smaller.
Pollizi et al. (2002) compared cognitive function in 64 aluminium workers and 32 controls. They reported an inverse relationship between aluminium exposure and performance on MMSE and the CDT. This study controlled for a greater range of possible neurotoxins. Nonetheless, its sample size is modest.
While Sjögren et al. (1996a) observed an elevated risk of neuropsychiatric symptoms among welders, those workers were also exposed to chromium, lead, manganese, and nickel. Hosovski et al. (1990) identified differences in psychomotor and intellectual abilities in aluminium foundry workers and attributed the differences to aluminium metal exposure. White et al. (1992) identified a ‘neurological syndrome’ among aluminium smelter workers (exposed to alumina, aluminium fibres, fluorides, calcium and cryolite), although the sample size was small (n = 25).
Evidence of an association between occupational exposure to aluminium and risk of AD has generally been negative, although the strength of the conclusions from these studies has been tempered by sample size considerations and inadequate characterization of exposure levels. A review by Doll (1993) concluded there was no evidence that occupational exposure causes AD. A subsequent case control study conducted by Salib & Hiller (1996) also found no association between AD and occupational exposure to aluminium. More recent studies (Akila et al., 1999; Riihimäki et al., 2000; Pollizi et al., 2002) reported cognitive effects. These studies were all small, however, and the first study (Akila et al., 1999 and Riihimäki et al., 2000) did not control for agents that might have a neurotoxic effect.
Overall, evidence of an association between inhalation exposure and neurological effects is limited.
Oral ingestion
Studies of the impact of oral exposure to aluminium compounds have considered the following endpoints listed in the introduction to Evaluation of Human Health Risks, Health Effects, Hazard Identification: (2) irritation; (6) mutagenicity; (7) carcinogenicity; (8) reproductive/developmental effects; and (9) other effects, including neurological impacts, bone toxicity, and metabolism. For this pathway, no occupational exposure data were available.
Note that because internal exposure following oral exposure depends on absorption and retention of aluminium, effects described here depend on health status. For the purpose of this discussion, we confine our attention to the general population. As noted in the introduction to this section, Evaluation of Human Health Risks, Health Effects, Hazard Identification, Sensitive Subpopulations addresses individuals, including infants and children, and individuals with impaired renal function, with specific factors that increase their susceptibility to aluminium exposure.
Endpoint (2) – irritation
In 1988, a drinking water treatment error lead to 20,000 individuals in Cornwall, England being exposed to elevated levels of aluminium sulphate in their drinking water over a three day period. Although members of the population reported GI disturbances and oral ulcerations, the role of aluminium could not be isolated because of other associated changes in water chemistry, including a decrease in pH and elevation in the concentrations of copper, zinc, and lead (dissolved from domestic plumbing fixtures). Animal data do not support the potential for oral and GI effects (see Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, Oral Exposure). We therefore conclude that evidence for these endpoints is limited.
Endpoint (6) - mutagenicity
See discussion in Evaluation of Human Health Effects, Health Effects, Hazard Identification, Inhalation Exposure, Endpoint (6) - mutagenicity.
Endpoint (7) – carcinogenicity
There are no human data for this endpoint. Animal data (see Effects on Laboratory Mammals and In Vitro Test Systems, Interactions between Aluminium and Other Agents) are negative. We therefore conclude that there is no clear evidence for the carcinogenicity of aluminium by oral ingestion.
Endpoint (8) – reproductive/developmental effects
There are no epidemiological data on reproductive or developmental outcomes following oral exposure to aluminium. However, Effects on Laboratory Mammals and In Vitro Test Systems, Reproductive and Developmental Toxicity, describes a substantial number of rodent bioassays investigating the impact of aluminium exposure via oral administration. The results from the animal studies indicate that the effects depend strongly on the bioavailability of the aluminium species. Species that are water soluble have been shown to have adverse effects, including aluminium nitrate (Albina et al., 2000; Paternain et al., 1988), aluminium chloride (Colomina et al., 1999; Cranmer et al., 1986; Misawa & Shigeta, 1993), or aluminium lactate (Golub et al., 1987; Gonda et al., 1996; Poulos et al., 1996), although these effects may be directly related to aluminium exposure or the result of a secondary consequence.
On the other hand, administration of aluminium hydroxide (Al(OH)3), which is absorbed to a more limited extent (Colomina et al., 1994; Domingo et al., 1991a), has been reported to have a more limited impact on reproductive outcomes (Gómez et al., 1991), or no adverse impact (Domingo et al., 1989). Co-administration of ascorbic acid appears to increase the impact of aluminium hydroxide on reproductive toxicity (see e.g., (Colomina et al., 1994). Donald et al. (1989) reported neurological defects following exposure of dams to aluminium lactate during lactation. Administration of aluminium caused significant reductions in maternal weight.
Based on the animal data, we conclude that evidence for this endpoint is modest, although we emphasize that it is highly dependent on aluminium species, most likely because of differences in bioavailability.
Endpoint (9a) – other effects - neurological
Effects on Humans, Effects from Non-Occupational Exposure, Neurotoxicity of this report describes 15 studies of the association between aluminium in drinking water and the risk of AD. Nine of these studies report an elevated risk, although methodological limitations have been identified for most of these investigations. That of Martyn et al. (1989) was not population based; those of Flaten (1990) and Neri & Hewitt (1991) were ecological in nature and hence did not control for relevant confounders. Forbes et al. (1991; 1992) reported that men living in areas with elevated aluminium concentrations in drinking water were less likely to be cognitively normal. However, interpretation of this study’s results is complicated by the fact that it evaluated the joint impact of aluminium and fluoride exposure. Other potential problems with this study included sample size limitations and lack of representativeness. McMillan et al. (1993) and Altmann et al. (1999) reported on the impact of a short-term spike in exposure to aluminium in drinking water following operational problems in a local drinking water treatment facility in the UK. However, because cases in this study were self-selected and may have been motivated by the potential for compensatory rewards, the results may be biased. Jacqmin et al. (1994) and Jacqmin-Gadda et al. (1996) reported the association between cognitive impairment and aluminium and silica drinking water concentrations considered jointly. (These results support the hypothesis that silica protects against this condition. Because silica limits exposure to aluminium, this finding may be supportive of a link between aluminium exposure and cognitive impairment.) McLachlan et al. (1996) reported an elevated risk of AD among individuals exposed to higher levels of aluminium in drinking water. However, the study did not control for fluoride, silica, or pH; nor did it control for other potential confounders. Gauthier et al. (2000) reported an association between risk of AD and exposure to organic monomeric aluminium at onset. Finally, in a relatively large study involving 2,698 participants aged 65 and older, Rondeau et al. (2000) reported an association between exposure to aluminium in drinking water and risk of AD. However, because this study lacked an intermediate exposure group, it is not possible to determine if there is a dose-response relationship. Six other studies described in Effects on Humans, Effects from Non-Occupational Exposure, Neurotoxicity, Aluminium in Drinking Water reported no association between aluminium exposure and development of AD or cognitive impairment.
In addition to the studies of neurological effects following exposure to aluminium in drinking water, there are two other studies that consider this endpoint and its association with oral aluminium exposure. Graves et al. (1990) reported a positive association between lifetime antacid use and risk of AD. However, this association vanished when consideration was limited to antacids containing aluminium. A small study of exposure to aluminium in food (Rogers & Simon, 1999), which followed only 23 case control pairs, reported positive findings.
Animal models lend some support to the biological plausibility of an association between aluminium exposure and the development of AD. Several studies report that intake of aluminium (chemical species not specified) (Pratico et al., 2002), aluminium maltolate (Huang et al., 1997), aluminium chloride (Zhang et al., 2003), or aluminium sulphate (El-Rahman, 2003) increases expression of amyloid protein in rodent tissues, a step that may be critical to the development of AD. Kihira et al. (2002) reported that administering aluminium in drinking water to rats lead to the accumulation of tau immunoreactivity in a pattern resembling AD pre-tangles. Increased oxidative stress in the brains of rodents exposed to aluminium may have been noted, but this evidence falls short of demonstrating neuropathology (see Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, In Vivo Models, Rodent Models of Aluminium Toxicity by Oral Exposure). Other studies (e.g., Silva et al., 2002) suggest that aluminium may decrease amyloid plaque formation. However, there may be other effects on neurotransmitter systems and synaptic function.
Although many of the epidemiological studies reporting a positive association between aluminium exposure and the development of AD or other forms of cognitive impairment have limitations, they provide credibility for the potential for such an association. The autopsy data cast doubt on the association. In addition, animal study data are mixed. In light of that negative evidence and only limited epidemiological support, we conclude the strength of the evidence for neurological effects is modest.
Endpoint (9b) – other effects - bone toxicity
Only a small number of animal studies have investigated the impact of aluminium exposure via oral intake (or gastric intubation) on bone toxicity and none has demonstrated an adverse effect. Slanina et al. (1984) reported that administration of aluminium citrate to rats substantially elevated the concentration of aluminium in bone compared to controls (by a factor of 40), and that administration of citric acid increased the concentration of aluminium in the bone of animals receiving diet spiked with aluminium. Animals receiving aluminium hydroxide did not have elevated concentrations of aluminium in bone. These findings suggest that the potential for aluminium to affect bone may be limited by the extent to which it is absorbed. Yasui & Ota (1998) reported that rats consuming a diet containing 194 mg Al /100 g feed (equivalent to 1.94 g/kg feed) as aluminium lactate exhibited decreased spinal cord magnesium concentration. Because magnesium is important to bone metabolism, the authors concluded that their findings suggest the potential for aluminium to cause bone toxicity.
With the exception of a single case study involving a chronic consumer of antacids, there is no evidence of bone toxicity in people with normal renal function (see Evaluation of Human Health Risks, Health Effects, Hazard Identification, Exposure via Injection, Endpoint (9b) – other – bone toxicity for individuals on dialysis). Among members of the population with normal kidney function, there is limited evidence that exposures to high levels of aluminium can result in deposits of aluminium in bone tissue (see Effects on Humans, Effects from Non-Occupational Exposure, Bone). However, these studies have been limited in size and have not shown adverse effects. We therefore conclude that there is no clear evidence for this endpoint.
Endpoint (9c) – other effects - metabolism
As concluded in Effects on Laboratory Mammals and In Vitro Test Systems, Mineral Metabolism, studies of an association between aluminium exposure and metabolic impacts are inconsistent in terms of their results, with the possibility that differences across studies may be due, at least in part, to factors such as the level of exposure, route of exposure, and age of the animals tested. In addition, other than body weight, the only effects observed were changes in tissue trace mineral concentrations. Because none of these changes is indicative of an adverse toxic effect, we conclude the evidence for a toxic metabolism effect is limited.
Dermal and other external exposure
Studies investigating the impact of dermal and other external exposure to aluminium have considered endpoint (2) - irritation.
In occupational settings, instillation of aluminium sulphate, potash alum, and ammonium alum have resulted in conjunctivitis and purulent ophthalmitis (Grekhova et al., 1994). Other forms of aluminium relevant to cosmetics (aluminium starch octenylsuccinate) have also been shown to cause mild eye irritation in rabbits, although the authors concluded that this exposure was unlikely to cause irritation in humans (Nair & Yamarik, 2002). Magnesium aluminium silicate caused minimal eye irritation in a Draize eye irritation test (Hazelton Laboratories, 1968). When injected intralamellarly, widespread corneal infiltrates and retrocorneal membranes were recorded (Austin & Doughman, 1980).
Reports of skin irritation due to contact with aluminium have been limited in extent (e.g., 2 of 202 cases of contact dermatitis among aircraft workers reported by Hall (1944), or not definitively associated with aluminium (Johannessen & Bergan-Skar, 1980; Peters et al., 1998). Non-occupational evidence of contact sensitivity to aluminium is generally limited to case reports (see Effects on Humans, Effects from Non-Occupational Exposure, Irritation, Dermal Application). We therefore conclude that evidence for this endpoint is limited.
Exposure via injection
Studies investigating the impact of exposure to aluminium via injection have considered the following endpoints listed in the introduction to Evaluation of Human Health Risks, Health Effects, Hazard Identification: (2) irritation; (8) reproductive/developmental effects; and (9) other effects, including neurological effects, bone toxicity, and metabolism.
Endpoint (2) – irritation
The fact that some vaccines contain aluminium hydroxide and/or aluminium phosphate adjuvant (all DTP, some Haemophilus influenza type B, most hepatitis A and B, meningococcal, pneumococcal, tick-born encephalitis, some rabies, and anthrax vaccines) while others do not (inactivated poliovirus, measles, mumps, rubella, some rabies, yellow fever, Japanese encephalitis, adenovirus, typohoid, plague, cholera, BCG, meningococcal, and some Haemophilus influenza type B) affords an opportunity to study the extent to which aluminium might cause irritation. Effects on Humans, Effects from Non-Occupational Exposure, Irritation, Irritation after Injection of Aluminium-Adsorbed Proteins (Vaccines and Hyposensitization Regimens) summarizes the numerous studies that have reported a range of dermal irritation effects following administration of vaccines containing aluminium. Animal studies support the existence of this effect (see Effects on Laboratory Mammals and In Vitro Test Systems, Irritation, Injection). We conclude that evidence for this evidence is strong.
Endpoint (8) – reproductive/developmental effects
Llobet et al. (1995) reported that injecting male mice with aluminium nitrate for four weeks prior to mating decreased pregnancy rates. Because there is only a single animal study and no epidemiological data, we conclude that there is no clear evidence for this endpoint.
Endpoint (9a) – other – neurological effects
As documented in Effects on Laboratory Mammals and In Vitro Test Systems, Neurotoxicity, In Vivo Models, Rodent Modesl of Aluminium Toxicity by Direct Injection, a number of animal studies have shown that rodents suffer a range of adverse nervous system effects following direct injection of aluminium. Effects include signs of oxidative stress as indicated by increased glutathione (GSH), increased measures of lipid peroxidation (TBARS), increased levels of oxidized glutathione (GSSG), and increased superoxide dismutase levels (Esparza et al., 2003); changes to neuronal morphology (reductions in axonal length (Sreekumaran et al., 2003)); CNS cell death (Yang et al., 2004); retinal toxicity (Lu et al., 2002); and reductions in neuronal density (Miu et al., 2003).
Autopsy evidence from individuals who died of encephalopathy following long-term dialysis treatment argues against a role for aluminium in the development of one particular neurological effect, namely AD. Those patients are especially susceptible to aluminium exposure (see Evaluation of Human Health Risks, Health Effects, Hazard Identification, Sensitive Subpopulations). Nonetheless, those studies show no association between aluminium exposure and the development of Alzheimer’s-like lesions.
In any case, there are no human data to support the existence of neurological effects following injection. On the basis of the animal evidence, we rate the evidence for neurological effects as modest.
Endpoint (9b) – other - bone toxicity
As detailed in Effects on Laboratory Mammals and In Vitro Test Systems, Effects on Bone, the vast majority of animal studies investigating the potential for aluminium to cause bone toxicity have used injection as the exposure pathway. Studies have reported that aluminium exposure decreases bone formation in rats (Goodman, 1984; Goodman et al., 1984a; Ott et al., 1987) and has an adverse impact on the functional properties of bone (Cointry et al., 2005; Zafar et al., 2004). Effects on various aspects of bone formation or metabolism have also been observed in other species, including pigs (Sedman et al., 1987) and dogs (Goodman et al., 1984b; Quarles et al., 1985; 1988; 1989). Experiments in these larger animals may have greater relevance to the prediction of impacts in humans for this endpoint than do the ones in rats. On the other hand, in dialysis patients, contaminated solution (administered either i.v. or via i.p. injection) is responsible for observed bone toxicity. Based on this evidence, we judge the evidence for bone toxicity via the injection pathway to be modest.
Endpoint (9c) – other - metabolism
Our comments on the oral exposure pathway for this endpoint are applicable to the injection pathway, as well. We conclude that evidence for a toxic metabolism effect is limited.
Sensitive subpopulations
Individuals with impaired renal function do not clear aluminium as effectively as healthy individuals. This population can also be exposed to higher levels of aluminium that are administered inadvertently via their i.v. feeds. This route of exposure may be particularly significant because it bypasses the barrier imposed by GI absorption characteristics.
Researchers have reported cases of encephalopathy among members of this population (Alfrey, 1993; Foley et al., 1981), resulting in alterations to memory and behaviour, speech disorders, convulsions, and myoclonus. Yuan et al. (1989) noted that, in dialysis patients, haematocrit levels were lowest in patients with the highest RBC aluminium values. Bia et al. (1989) reported that haemoglobin levels were significantly lower among individuals with higher aluminium burdens. Gafter et al. (1996) reported a link between bullous dermatosis and aluminium toxicity in end-stage renal patients.
Infants, especially those born pre-term, are especially vulnerable to aluminium exposure due to immaturity of the GI wall, the BBB, and the renal system (Hawkins et al., 1994). One study (Bishop, 1997) reported an association between exposure to aluminium (following administration of i.v. feeding solutions just after birth) and cognitive development measured using the Bayley Mental Development Index at 18 months. The study used a randomized design. On the other hand, it is limited in size. Nor has the study been replicated. Other evidence of a neurological effect in this population is limited to case studies.
We therefore designate evidence for susceptibility to toxicity among renal patients to be strong. However, among infants and premature infants, we designate the evidence as limited.
Physico-chemical properties
As noted in Effects on Humans, Effects from Occupational Exposure, Explosivity, Flammability, Oxidizing Potential, aluminium is a reactive agent that can pose a risk of physical injury in industrial settings. Airborne aluminium powder can pose an ignition danger, although only at very high concentrations. Finally, mixtures of aluminium and halogenated organic compounds can pose an explosion hazard under certain conditions.
Summary
Table 25 summarizes our findings for the strength of the evidence for each pathway and health effect. There is strong evidence that aluminium can cause irritation following exposure via either inhalation or injection. Modest evidence of an effect exists for reproductive toxicity following oral exposure, for neurological toxicity following either oral or injection exposure, and for bone toxicity following injection exposure. All other effects were judged to be supported by either limited evidence or no clear evidence at all.
Dose response
This section identifies quantitative benchmarks of toxicity for the health effects for which there is modest or strong supporting evidence (see Evaluation of Human Health Risks, Health Effects, Hazard Identification). The discussion is organized by exposure pathway.
Inhalation exposure
For the inhalation pathway, irritation is the only health effect for which there is strong or modest supporting evidence. Epidemiological findings quantifying exposures associated with this effect are limited to occupationally exposed cohorts, and this information draws heavily on the experience of German workers from the World War II era (see Effects on Humans, Effects from Occupational Exposure, Irritation, Inhalation Exposure). Those workers are thought to have been exposed to airborne aluminium powder concentrations as high as 50 to 100 mg/m3. Sjögren (2000) reports that these exposures lead to aluminosis, a fibrotic disease of the lung.
Animal bioassays confirm the potential for airborne aluminium to cause frank effects at exposures in the order of 100 mg/m3 or more. Effects included constriction of pulmonary air flow following exposure of rats to airborne aluminium concentrations of 380 mg/m3 or of guinea pigs to airborne aluminium concentrations of 580 mg/m3 (Robillard & Alarie, 1963a); acute bronchopneumonia and moderate thickening of the alveolar walls following exposure of Syrian Golden hamsters to airborne aluminium chloride hydroxide concentrations of 164 mg/m3 (Drew et al., 1974); and similar effects in rabbits following their exposure to airborne aluminium chloride hydroxide concentrations of 212 mg/m3 (Drew et al., 1974).
At exposures approximately one to two orders of magnitude lower, frank effects are not often reported. On the other hand, physiological changes have been observed, including development of granulomatous pneumonia in rats following exposure to airborne aluminium concentrations of either 2.5 or 25 mg/m3 (Steinhagen et al., 1978); Type II alveolar cell effects in rats following exposure to airborne aluminium concentrations of 1.3 or 1.8 mg/m3 (Finelli et al., 1981), and an influx of polymorphonuclear neutrophils into bronchopulmonary lavage fluid following exposure of rats to airborne aluminium concentrations as low as 10 mg/m3 (Thomson et al., 1986). While some of these effects persisted after cessation of exposure (e.g., Thomson et al. (1986) reported effects 6 months after exposure ended), there are no reports indicating that these responses were more than temporary, or that they lead to any long term risk of disease.
On the strength of the occupational exposure studies and the supporting findings from the animal studies, we identify an airborne aluminium powder concentration of 50 mg/m3 as a level at which adverse effects have been observed. Although physiological effects have been observed in animal studies at lower exposure levels, there is no evidence of effects at these levels in humans. Nor is there any evidence that these effects lead to a permanent decline in functional status.
Oral exposure
Neurological effects
If there is an association between aluminium exposure and risk of either AD or other forms of dementia, the results of positive studies of exposure to aluminium in drinking water and these health effects would serve to characterize the nature of the dose response relationship. Martyn (1989) reported that individuals consuming drinking water with aluminium concentrations exceeding 0.11 mg/L had a 1.5-fold greater risk for AD than those who consumed water with aluminium concentrations less than 0.01 mg/L. Flaten (1990) reported RRs for subjects consuming drinking water with aluminium concentrations > 0.2 mg/L and those consuming drinking water with aluminium concentrations from 0.05 to 0.2 mg/L. The comparator group included individuals consuming water with aluminium concentrations < 0.05 mg/L. For the high exposure group, the RRs were 1.32 (male) and 1.42 (female). For the mid-exposure group, RRs were 1.15 (male) and 1.19 (female). Neri & Hewitt (1991) reported a RR of 1.46 for individuals consuming drinking water with aluminium concentrations > 0.2 mg/L, when compared to individuals consuming drinking water with concentrations below 0.01 mg/L. Rondeau et al. (2000) reported an elevated risk of AD among individuals consuming water with aluminium concentrations exceeding 100 μg/L. Recall that these studies have a number of limitations, as described in Evaluation of Human Health Risks, Health Effects, Hazard Identification, Oral Ingestion, Endpoint (9a) – other effects - neurological. Interpretation of other drinking water studies for this health endpoint is complicated by the fact that other water chemistry characteristics were considered in conjunction with aluminium exposure.
For the purpose of dose-response analysis, we limit our attention to the studies described in the preceding paragraph. Those studies collectively suggest that relative to very low levels of aluminium exposure, drinking water concentrations amounting to 0.2 mg/L elevate AD risk by approximately a factor of 1.3 to 1.5 (see Flaten (1990) and Neri & Hewitt (1991)). The study by Martyn (1989) suggests the risk may be somewhat higher, while the mid-exposure group results from the Flaten (1990) study are consistent with this estimate. The Rondeau et al. (2000) study finding of a risk associated with aluminium concentrations exceeding 100 μg/L serves as the basis for the level of concern identified here.
Reproductive toxicity effects
Human data for this endpoint are limited to extremely high exposures (due to chronic high doses of antacids) studied in a very small number of individuals (see Effects on Humans, Effects from Occupational Exposure, Reproductive Toxicity, Developmental Effects). Effects on Laboratory Mammals and In Vitro Test Systems, Reproductive and Developmental Toxicity, Reproductive Toxicity describes seven animal bioassays that studied the association between reproductive toxicity and the impact of oral exposure to aluminium. Two of these studies investigated administration of Al(OH)3 alone (Domingo et al., 1989; Gómez et al., 1990); neither reported any effects. The Domingo et al. (1989) study evaluated the impact of exposures to Al(OH)3 on gestation days 6-15 as high as 266 mg/kg b.w/day in mice. Gómez et al. (1990) evaluated the impact of exposures as high as 768 mg/kg b.w./day on gestation days 6-15.
On the other hand, studies of the concurrent administration of Al(OH)3 and citric acid (Gómez et al., 1991), lactate (Colomina et al., 1992), or ascorbic acid (Colomina et al., 1994) did report reproductive toxicity effects. Gómez et al. (1991) reported delayed ossification following administration of 384 mg/kg b.w./day in dams along with 62 mg/kg b.w./day citric acid, with no effects when either of these substances was administered alone. On the other hand, there was no treatment related impact on pre- or post-implantation sites, number of live foetuses per litter, or gender ratio. Colomina et al. (1992) reported that administration of 627 mg aluminium lactate/kg b.w./day and 570 mg lactic acid/kg b.w./day to dams on gestation days 6-15 decreased maternal body weight and foetal body weight, increased the incidence of cleft palate, and delayed occification. Colomina et al. (1994) found that concurrent administration of ascorbic acid and aluminium (300 mg/kg b.w./day) had no impact on reproductive toxicity.
The available results suggest that, when consumed alone, even high oral doses of aluminium (in particular, Al(OH)3) are insufficient to cause reproductive toxicity. However, when consumed concurrently with acid, which increases absorption (see Toxicokinetics, Absorption, Animal Studies, Oral Administration, Factors Influencing Oral Aluminium Absorption, Carboxylic Acids), there is sufficient internal exposure to result in toxicity. Even with concurrent administration of acid, these effects have been documented only at maternal exposures of approximately 400 mg/kg body weight/day or higher.
Dermal exposure
The available evidence for this exposure route’s health effects did not achieve a modest or strong level of support.
Exposure via injection
Irritation
As described in Effects on Humans, Effects from Non-Occupational Exposure, Irritation, Irritation after Injection of Aluminium-Adsorbed Proteins (Vaccines and Hyposensitization Regimens), the quantities of aluminium in vaccine adjuvents are sufficient to trigger localized irritation in humans.
Neurological effects
Doses were reported in terms of mg/kg body weight/day in three of the studies that investigated the association between neurological effects and exposure to aluminium via injection. Esparza et al. (2003) reported that administration of 5 or 10 mg aluminium lactate/kg body weight/day via injection for 8 weeks (5 times per week) resulted in signs of oxidative stress in the hippocampus. Changes in these markers remained well below two-fold despite the fact that this exposure was 1,000 times normal. Gómez et al. (2005) administered 7 mg/kg body weight/day aluminium lactate via injection for 11 weeks (5 times per week). Exposed animals experienced 30% reductions in body weight. Some signs of oxidative stress in the hippocampus (elevations of approximately 50% in mitochondrial SOD mRNA) were also reported. Finally, Miu et al. (2003) injected rats with 0.85 mg aluminium gluconate/kg b.w./day for 6 months (3 times per week). The study reported evidence of reductions in neuronal density in the hippocampus, intracellular accumulations of aluminium in dense granules, and thickening of the meningeal blood vessels.
Exposure characterization
Human Exposure comprehensively reviews the available data quantifying human exposure to aluminium in the environment.
Ambient respirable exposures for the general population average as much as nearly 2 μg/m3 (PM2.5 fraction) to 7 μg/m3 (PM10 fraction) of aluminium in ambient dust fractions (see Table 13). Occupational exposures for this route average as much as 1 to 6 mg/m3 (see Table 15).
Table 16 summarizes estimated intake rates for food, drinking water, ambient air (followed by swallowing), antacid use, buffered aspirin use, and from antiperspirants. The exposures associated with each of the exposure routes listed span approximately five orders of magnitude. The largest exposures are associated with the use of antacids (7,200 mg/day) and aspirin (1,000 mg/day). The smallest exposures are associated with inhalation of ambient air and consumption of drinking water. Using that information, Table 26 estimates body weight normalized aluminium uptake. Differences in bioavailability for the various routes of exposure change the ranking of the exposure routes, but only to a limited degree.
The values in this table indicate that intake of aluminium from food dominates that from drinking water. Even inhalation contributes more aluminium than does consumption of drinking water. As expected, occupational exposures exceed general population exposures by more than two orders of magnitude. However, consumption of medications containing aluminium makes the greatest potential contribution to internal aluminium exposure. Contributions from these two sources exceed the contribution from food by a factor of about 100 (aspirin) or 1,000 (antacid). The contribution of anti-perspirant use to aluminium uptake could potentially be of the same order of magnitude as the contribution of diet, although the degree to which aluminium in anti-perspirants is absorbed has not been well demonstrated (see Toxicokinetics, Absorption, Studies in Humans, Dermal Exposure).
Risk characterization
Table 27 summarizes the level of concern values identified in Evaluation of Human Health Risks, Health Effects, Dose Response and the exposure levels listed in Evaluation of Human Health Risks, Health Effects, Exposure Characterization. The table also reports “margin of exposure” values, representing the ratio of each level of concern and the corresponding exposure level. Separate values are reported for occupational and general populations, where appropriate. For the general population, the margins of exposure are generally large, with the exception of the estimated margin of exposure associated with drinking water consumption (margin of exposure equal to approximately unity), and exposure via injection (margin of exposure less than unity). In particular, aluminium exposures accompanying a single injection may result in irritation, a relatively minor health effect. Neurological health effects are also possible, although for typical injections (e.g., for vaccination), only following daily injections over a period of many months. In the past, dialysis patients were exposed to very high levels of aluminium due to contamination of dialysis fluid and/or use of aluminium phosphate binders. Such contamination is possible now, but occurs only rarely. For the general population, health effects associated with injections containing aluminium are not likely. For the occupational population, only one margin of exposure value could be estimated. For inhalation exposure in occupational settings, we estimated a margin of exposure value of approximately eight for the irritation endpoint.
Although we judge the available toxicity information for aluminium as inadequate to develop dose response relationships that could be used to estimate the probability of adverse outcomes, it is sufficient to identify those exposure pathways and health outcomes that are of greatest concern. Comparing the exposure levels of concern to established exposure limits (summarized in Table 28) further informs this characterization.
Table 28.
Established exposure limits for aluminiuma.
| Pathway | Exposure Limit | Source of standard |
|---|---|---|
| Occupational Inhalation | 2 mg/m3 | Aluminium oxide – Poland, TWA MAC Aluminium oxide – Switzerland, 2 week TWA |
| 4 mg/m3 | Respirable dust – Sweden, TWA Respirable dust – UK, TWA |
|
| 5 mg/m3 | Respirable fraction – OSHA-PEL, 8 hr TWA Respirable fraction – NIOSH REL, 10 hr TWA |
|
| 6 mg/m3 | Aluminium oxide – Russia, TWA | |
| 10 mg/m3 | Metal dust – ACGIH TLV, 8 hr TWA Welding fume – ACGIH, TWA Aluminium oxide – ACGIH, TWA Total dust – NIOSH REL – 10 hr TWA Aluminium oxide – Belgium – TWA Aluminium oxide – Denmark – TWA Aluminium oxide – France – VME Total dust – Sweden – TWA Total inhalable dust – UK – TWA |
|
| 15 mg/m3 | Total dust – OSHA-PEL – 8 hr TWA | |
| 16 mg/m3 | Aluminium oxide – Poland, STEL MAC | |
| General Population Oral Intake | 1 mg/kg-week | FAO/WHO PTWI |
| 2 mg/kg-day | U.S. MRL (intermediate duration) | |
| Population Drinking Water | 0.05 to 0.2 mg/L | U.S. Secondary Drinking Water Regulation |
| 0.2 mg/L | WHO – Drinking Water Guideline European Union – Drinking Water Directive Australia – Drinking Water Guideline |
|
| Injection Fluids | 10 μg/L | U.S. Association for the Advancement of Medical Instrumentation |
For the general population, the evidence suggests that exposure levels to airborne aluminium are well below plausible levels of concern. The identified exposure level for the general population (0.007 mg/m3) is approximately a factor of 300 below the lowest of the occupational exposure limits for airborne exposure listed in Table 28. Although these limits are not established for the general population, the 0.007 mg/m3 concentration is also nearly four orders of magnitude below the concern level identified for aluminium powder in this assessment (see Table 27). Occupational exposure is below the level of concern we identified (by approximately a factor of 8), although the exposure level we identified does exceed some of the exposure limits listed in Table 28. In any case, it appears that airborne exposure to aluminium is generally below levels where health effects have been identified, and in the case of the general population, there is a large margin of exposure.
For oral exposure, we identified two health effects. For reproductive toxicity, there is a margin of exposure of nearly 3000. On the other hand, for neurological effects (in particular, an increased risk for the development of AD), exposure to aluminium in drinking water (0.1 mg/L) may be similar to the level of concern we identified. This level of 0.1 mg/L is less than the recommended limits established by the WHO, the European Union, and Australia (see Table 28). Secondary drinking water regulations in the U.S. establish a limit of 0.05 to 0.2 mg/L for aluminium (Table 28).
The implication of the comparisons outlined here is that health effects associated with oral exposure to aluminium in general, and the neurological (AD) health endpoint in particular, deserve the greatest scrutiny. Limitations to the epidemiological evidence (confounding, uncertain exposure levels, and so forth) complicate its use for establishing toxicity benchmarks (such as a reference dose).
As pointed out by Nieboer et al. (1995), the epidemiological evidence for a possible association between aluminium in drinking water and AD must be viewed in the context that this disease has a complex genetic trait. It is considered multifactorial, involving multiple genes that interact with multiple environmental (including life-style) factors. Early onset AD is strongly linked to mutations in 3 genes, with autosomal-dominant inheritance patterns (Bertram & Tanzi, 2004; 2005). By contrast, the more frequent late onset (at or older than age 65) AD, only one genetic risk factor has been consistently established and involves polymorphism of the ApoE gene; its epsilon-4 allele constitutes a risk factor (Bertram & Tanzi, 2004; 2005). Many other genetic loci have been implicated, but “no other genetic risk factor has been found to consistently confer susceptibility to ‘late onset’ AD” (Bertram & Tanzi, 2004). Identification of specific environmental factors has been more elusive, but age, low-level education, head trauma, and vascular risk factors (including cardiovascular genetic susceptibility factors) are plausible candidates (Bertram & Tanzi, 2005; De la Torre, 2002; Kivipelto et al., 2005; Lindsay et al., 2002). In a recent study of twins, heritability for AD was estimated to be between 58-79% (Gatz et al., 2006). This evidence for a genetic contribution to the aetiology of AD does not preclude the possibility of an environmental contribution. However, positive findings in epidemiological studies completed to date need to be interpreted carefully in light of possible confounding and inherent study weaknesses.
A chronic exposure animal bioassay might help to provide information on the neurological impact of oral exposure to aluminium. If such a study is conducted, it should expose animals via the relevant exposure route (oral exposure), rather than by other routes (e.g., injection). Before committing the time and resources to such a study, however, the limitations inherent to such a study need to be considered. First, while a laboratory animal study can identify alterations in the nervous system and neurobehavioural function, clinical relevance to neurological effects in humans, especially those related to a cognitive disease, are not clear. Second, such a study would likely necessitate use of high doses, making downward extrapolation to environmentally relevant doses and maintained neurobehavioural dysfunction necessary. While standard algorithms can be used to develop a point of departure (e.g., a benchmark dose) and identify appropriate adjustment factors (for calculation of a reference dose, for example), the implications of the resulting exposure value for human risk may not be clear. It should be noted that the exposure level of concern estimated here for aluminium in drinking water (100 μg/L) suggests that absorption of 6.9 × 10-6 mg/kg-day aluminium is of concern. If that is the case, then exposures associated with food intake (uptake of 1 × 10-4 mg/kg-day) or inhalation (1.7 × 10-5 mg/kg-day) would also be of concern (see Table 26). This possibility underlines the need to better understand the robustness of the causal association between neurological effects and exposure to aluminium, i.e., the effect associated with exposure via drinking water intake.
RESEARCH NEEDS
Research Needs for Risk Assessment
The following research needs were identified as important research requirements to further improve risk assessments on aluminium:
Studies should be conducted to quantify peak and cumulative air-borne aluminium exposure of workers in the aluminium industry and to characterize aluminium-containing aerosols in terms of particle composition and size. Concomitant assessments of the bioavailability of the inhaled aerosols are crucial.
In many occupational studies of aluminium workers, it was not known whether respiratory tract illness was due to exposure to aluminium or other substances. There have been very few studies of neurological effects of occupational exposure via inhalation to aluminium and aluminium compounds (as measured in serum), and it is not known if the very specific neurological deficits observed lead to more severe illness such as AD. Therefore, large-scale, longitudinal, studies of occupational exposure to aluminium and aluminium compounds via inhalation, with precise methods of exposure measurement, are needed to assess the risks of respiratory tract disease and neurological effects due to aluminium and aluminium compounds.
Further studies are needed to settle the debate over the link between aluminium in drinking water and neurological disorders and cognitive impairment. Ideally, individual level data on drinking water exposure as well as other relevant risk factors would be obtained; in the absence of this, replication of the Rondeau et al. (2000) analysis in other study populations, with the ability to control for important confounders and effect modifiers, is needed to assess this potential risk.
Scientific Data Gaps
The following data gaps represent areas of scientific interest to further understanding on the human health implications of aluminium exposure:
At present, there is no long-term animal bioassay focusing on the neurotoxic effects of aluminium. Although this represents a major scientific data gap, the Panel questioned whether the availability of such a study would greatly improve our ability to establish human exposure guidelines for aluminium, given the availability of direct epidemiological evidence in this regard. If such a study were to be conducted for the purpose of informing human health risk assessment, careful consideration should be given to the neurological endpoints that would be of most relevance to humans, the most appropriate animal species and route of exposure, and the detailed experimental design, including the number of animals tested, and number and selection of doses. The Panel also noted the lack of data on neurodevelopmental effects in animals, but was unclear on the need for additional data in this regard for risk assessment purposes.
Quantify aluminium absorption through the skin in humans after repeated application of an aluminium-containing antiperspirant.
Given the high degree of inhalation exposure to aluminium, further examination of aluminium uptake from the nasal cavity and distribution directly to the brain, rather than secondary via the circulatory system in the rat or non-human primate, would provide information on the potential for inhalation exposure to contribute to aluminium neurotoxicity by this route.
Develop a better understanding of the significance of ligands associated with aluminium on its absorption, distribution, biotransformation and elimination, and aluminium-induced effects. This would include determination if aluminium complexes with fluoride or natural organic ligands such as phytates, humic acids and phenols in water, infant formula or food are stable in vivo, and if not, the rate they biotransform and the aluminium species formed. The experimental approach will probably include computational modelling. This work would also address whether the aluminium species significantly affects aluminium pharmacokinetics, such as oral bioavailability, and aluminium-induced effects, such as the association between aluminium consumption and cognitive loss. The pharmacokinetic studies are probably best conducted using 26Al as a tracer.
Develop a physiologically-based pharmacokinetic (PBPK) model in the human for aluminium that would reduce the need for further pharmacokinetic studies necessary to predict the risk from various aluminium exposures.
One of the most difficult tasks is to relate the level of exposure of the animal to the human. For rodent studies, the general rule is that the metabolic rate of rodents is about 10 times that of humans. Therefore, in testing the efficacy or toxicity of a given compound, doses that are at least 10 times the normal human dose are usually used. However, in gauging the relative exposure of the rodent to aluminium, it would be preferable to document the steady-state level of aluminium in serum as a marker of exposure. This measure would provide information on absorption, which would be one variable to consider in assessing differences in metabolism between species. Internal biomarkers of exposure and target tissue body burdens are important for assessing neurotoxicity data on any chemical. Unfortunately, relatively few studies in the existing literature provide this important piece of information. As described in Toxicokinetics, the steady-state level of aluminium in serum of the average adult human is 1-2 μg/L. Most of this aluminium is bound to transferrin. Therefore, a study of rodents in which levels of aluminium in serum were maintained at 10-20 μg/L would be viewed as a reasonable approximation of normal human exposure. Studies that achieved higher steady-state levels (5 and 10 times normal serum levels or 50-100 μg/L to 100-200 μg/L) could be viewed as more rigorous tests of toxicity and would provide very useful information regarding the potential long-term effects of Al on various organ systems. From the data reviewed here, there is evidence that high levels of aluminium are toxic to the nervous system, can affect bone, and may affect hematopoiesis. There remains however, an incomplete understanding of the threshold of aluminium exposure required to induce toxicity in vulnerable tissues.
It should be determined if aluminium can serve as a hapten to produce contact hypersensitivity and if there is a specific IgE antibody to aluminium.
As the results from some, but not all, epidemiological and dietary studies suggest silica species can reduce aluminium oral absorption and/or enhance aluminium excretion and protect against aluminium-induced adverse effects, further studies are needed to investigate this, focusing on the identity of the silica species, their molar ratio to aluminium, and the magnitude of the observed effects.
There is a need for further epidemiological studies that look at all aluminium components of ingestion. The regular ingestion of aluminium-containing antacids represents a major source of exposure to aluminium. However, very few studies have related their use to the development of AD. Epidemiological studies on aluminium exposure from food or on the consumption of food-containing aluminium additives are also uncommon, although food is a more significant source of aluminium ingestion than water.
Acknowledgments
In 2004, the McLaughlin Centre for Population Health Risk Assessment at the University of Ottawa was asked by the International Aluminium Institute to conduct a comprehensive review of the potential human health risks associated with aluminium, aluminium oxide, and aluminium hydroxide. The U.S. Environmental Protection Agency agreed to co-sponsor the risk assessment. An international scientific advisory committee was formed to provide independent oversight the risk assessment. The scientific advisory committee was comprised of: Jose L Domingo, Rovira i Virgili University, Spain, Anders Glynn, Swedish National Food Administration, Vesa Riihimaki, Finnish Institute of Occupational Health, and Thomas Wisniewski, New York University School of Medicine.
The authors wish to acknowledge Ian Arnold, Eirik Nordheim, Chris Bayliss and Ed O’Hanian for providing helpful scientific background information on aluminium and Mari Golub and Wesley Harris for providing comments on the manuscript. The assistance of Nicole Boom and Nataliya Karyakina who served as research assistants, contributing to the development of background material on toxicological and epidemiological aspects is also gratefully acknowledged. Finally, Fan Mo helped to create a database of reference material, Nagarajkumar Yenugadhati provided editorial assistance, and Robert Clarke helped with organizational aspects of the work.
D. Krewski is the NSERC/SSHRC/McLaughlin Chair in Population Health Risk Assessment at the University of Ottawa.
ABBREVIATIONS
- dae
aerodynamic diameters
- ATW
alum-treated water
- AM
alveolar macrophages
- AD
Alzheimer’s disease
- ACGIH
American Conference of Governmental Industrial Hygienists
- APP
amyloid precursor protein
- ALS
amyotrophic lateral sclerosis
- ApoE
apoliprotein E gene
- AA
atomic absorption
- BCG
bacillus calmette-guerin
- BBB
blood-brain barrier
- BrdU
bromodeoxyuridine
- BALF
bronchoalveolar lavage fluid
- Bτ
body content
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CAS
Chemical Abstracts Service
- CC16
clara cell protein 16
- CDT
clock drawing test
- CTPV
coal-tar-pitch volatiles
- CT
computerized tomographic
- CI
confidence interval
- DFO
desferrioxamine
- DAE
dialysis associated encephalopathy
- DNP
dinitrophenol
- DTP
diphtheria toxoid, tetanus toxoid, and pertussis
- EEG
electroencephalogram
- EAAS
electrothermal atomic absorption spectrometry
- EDX
energy dispersive (electron probe) x-ray microanalysis
- EDXS
energy dispersive x-ray spectrometry
- CFU-E
erythroid colony forming units
- EDTA
ethylenediaminetetraacetic acid
- EEC
European Economic Union Council
- EINECS
European Inventory of Existing Commercial Substances
- ERP-P300
event-related potential
- ECF
extracellular fluid
- FA
fatty acid
- FAO
Food and Agriculture Organization
- FEV1
forced expiratory volume
- FVC
forced vital capacity
- i.g.
gastric intubation
- GI
gastrointestinal
- GFR
glomerular filtration rate
- G6PDH
glucose-6-phosphate dehydrogenase
- GSH
glutathione
- t½
half life
- HBV
hepatitis B virus
- HPT
histamine provocation test
- Ig
immunoglobulin
- ICP-MS
inductively-coupled plasma mass spectrometry
- IQ
intelligence quotient
- IL
interleukin
- IARC
International Agency for Research on Cancer
- IPCS
International Programme on Chemical Safety
- i.m.
intramuscular
- i.p.
intraperitoneal
- i.v.
intravenous
- JEM
job-exposure matrix
- LDH
lactate dehydrogenase
- LAMMA
laser microprobe mass analysis
- LMMS
laser microprobe mass spectroscopy
- VME
limit values for average exposure
- MMF
macrophagic myofasciitis
- MMA
manual metal
- MOE
margin of exposure
- MMAD
mass median aerodynamic diameter
- MCL
maximum contaminant level
- MAK
maximum workplace concentration
- Mt
mega tonnes
- MIG
metal inert-gas
- PIXE μbeam
micro-beam proton-induced X-ray emission
- mnPCE
micronucleated polychromatic peripheral erythrocyte
- MMSE
mini-mental state exam
- MRL
minimal risk level
- MCT-1
monocarboxylate-1
- INAIL
National Institute for Insurance Against Occupational Accidents
- NIOSH
National Institute for Occupational Safety and Health
- NFD
neurofibrillary degeneration
- NFT
neurofibrillary tangle
- NAA
neutron activation analysis
- OR
odds ratio
- GSSG
oxidized glutathione
- PTH
parathyroid hormone
- PTX
parathyroidectomy
- PD
Parkinsonism-dementia
- PM
particulate matter
- PEL
permissible exposure limit
- PBPK
physiologically based pharmacokinetic
- AUC
plasma aluminium concentration
- PAH
polycyclic aromatic hydrocarbons
- PDAT
presenile dementia of the Alzheimer type
- PTWI
provisional tolerable weekly intake
- RL
reactivity limit
- REL
recommended exposure limit
- RSW
reconstituted soft water
- RR
relative risk
- R
risk phrase
- S
safety phrase
- SEM
scanning electron microscopy
- SIMS
secondary ion mass spectrometry
- STEL
short term exposure limit
- SALP
sodium aluminium phosphate
- SIR
standardized incidence ratio
- SMR
standardized mortality ratio
- s.c.
subcutaneous
- TT
tetanus toxoid
- TBARS
thiobarbituric acid reactive substances
- TLV
threshold limit value
- TPTX
thyroparathyroidectomized
- TWA
time-weighted average
- TPN
total parenteral nutrition
- TSP
total suspended particles
- Tf
transferrin
- TfR-ME
transferrin-receptor mediated endocytosis
- TEM
transmission electron microscopy
- TIG
tungsten inert-gas
- TNF
tumor necrosis factor
- EPA
U.S. Environmental Protection Agency
- OSHA
U.S. Occupational Safety and Health Administration
- Vd
volume of distribution
- WDX
wavelength dispersive X-ray microanalysis
- WHO
World Health Organization
- ZAG
zirconium aluminium glycinate.
APPENDIX A
Summary of the toxic effects of aluminium on laboratory animals and in vitro.
| Reference | Compound, dose, duration | Species | End-points examined | Results |
|---|---|---|---|---|
| SINGLE/ACUTE EXPOSURE | ||||
| Intratracheal/intrapleural exposure | ||||
| Lindenschmidt et al. (1990) | Animals were instilled with silica (0.2, 1.0, or 5.0 mg/100 g b.w.), titanium dioxide (1.0 or 5 mg/100 g b.w.), aluminium oxide (1.0 or 5.0 mg/100g b.w.) or saline. | Male Fischer- 344 rats | Endpoints were assessed following termination at 1, 7, 14, 28, and 63 days following instillation. BAL performed on 5 rats/group. Levels of lactate dehydrogenase, B-glucuronidase, N-acetyglucosaminidase, and total protein were measured. The lungs of 3 rats from each group were examined for increased collagen at the end of the study period. | Biochemical and cellular parameters increased in a dose-related fashion at the earlier time points for all groups, the magnitude of change was greatest for silica. The response for silica dust steadily increased, while the levels for the other two groups decreased toward normal values. Minimal histological changes were seen for the aluminium oxide or titanium dioxide groups. |
| Pigott & Ishmael (1992) | Groups of 24 male and 24 female rats were dosed by single intrapleural injection of refractory alumina fibres (Saffil) as manufactured or after extensive thermal ageing or of one of two types of aluminosilicate fibres with different diameters. Asbestos and saline were used as positive and negative controls, respectively | Male and female albino rats of the Alpk: AP (Wistar derived) strain | Rats were maintained to 85% mortality and were closely examined for the presence of mesothelioma. | Malignant mesothelioma was diagnosed in 7 rats dosed with asbestos, and 3 dosed with aluminosilicate fibre B. Mesothelioma was not detected in any rat dosed with Saffil or in negative controls. |
| Tornling et al. (1993) | Single intratracheal instillation of aluminium oxide (n=21) (40 mg), or aluminium oxide with adsorbed fluorides (n=21) (40 mg). | Sprague Dawley rats | BAL performed 1.4 and 12 months after exposure. | Cells in BAL fluid were increased significantly by secondary alumina (adsorbed fluorides) but not with primary alumina. Fibronectin concentrations were increased one year after exposure in both groups. |
| Dalbey & Pulkowski (2000) | Groups of 15 rats were administered a single intratracheal instillation (50 mg) of Bayer pseudoboehmite, Bayer gamma-Al2O3, Ziegler pseudoboehmite, or Ziegler gamma-Al203. Positive controls were instilled with quartz, and negative controls with glass beads. | Male Sprague Dawley rats | 6 months after administration animals were evaluated for lung volumes, pulmonary pressure- volume curves, pulmonary hydroxyproline content, lung weights and histopathology. | Rales were noted in the rats in the four groups receiving aluminas but not in the groups given glass beads or quartz. Lung lobe weight significantly increased with quartz and all groups given aluminas. Areas of granulomatous inflammation and early collagenization were noted in the alumina treatment groups. None of the aluminas caused a reaction as severe as quartz. |
| Alberquerque et al. (2002) | Male rats were instilled once intrapleurally with saline or Al(OH)3 (0.15 g/mL). Animals were studied 7 or 30 days after the instillation. | Male Wistar rats | Respiratory system, lung, and chest wall elastic, resistive, and viscelastic/inhomogeneous pressures were measured. Inflammation and collagen deposition was assessed. | Chest wall elastic and viscoelastic pressures increased after Al(OH)3 instillation. An increase in the type I/type III collagen ratio was present 30 days after instillation. No differences in lung parenchyma among the groups were noted. |
| In Vitro | ||||
| Gusev et al. (1993) | Rabbit alveolar macrophages and monocytes, and human monocytes and granulocytes were incubated with different amounts of quartz, silica, and alumina dust. | Rabbit alveolar macrophages and moncytes, human granulocytes and monocytes | Active oxygen total generation by cells was measured. The intensity of generation of superoxide radicals by cells was conducted. | Cells incubated with quartz dust exhibited a significant increase in total generation of active oxygen. Silica dust and alumina dust at the same concentrations caused much less of an increase in active oxygen production. |
| Warshawsky et al. (1994) | The cytotoxicity of various particles, including aluminium oxide, to alveolar macrophages obtained from hamsters and rats was assessed. | Hamster and rat alveolar macrophage | Cytotoxicity was measured at 24 and 48 h using dye exclusion procedures. | The cells treated with aluminium oxide showed minimal to no changes in viability. |
| Oral exposure | ||||
| More et al. (1992) | One group of 42 rats was sacrificed 1, 3, 6, 12, 24, 48, and 96 hr after a single (300 mg/kg b.w.) oral administration of aluminium hydroxide. A second group (n=30) received daily the same dose of aluminium hydroxide and were sacrificed after 3, 4, 5, 6, and 7 days of treatment. | Male Sprague Dawley rats | Intracellular binding of aluminium was examined in the mucosa of the stomach, duodenum, jejunum, and ileum of adult rats. | Aluminium deposits occurred only in the antral gland of the stomach and in rats treated for at least 3 days. |
| Injection | ||||
| Gherardi et al. (2001) | 4 rats received an i.m. injection of an aluminium hydroxide containing vaccine. | Sprague Dawley rats | Animals were sacrificed at 7, 14, 21, and 28 days post-injection and muscles close to, and remote from, the site of injection were examined by electron microscopy. | The vaccine induced a large necrotic area containing damaged muscle fibres and neutrophils surrounded by abundant lymphocytes and macrophages. Crystalline inclusions similar to those of MMF were discovered. |
| Shi et al. (2001) | 42 rats received s.c. injections of 1 of 4 treatments: (1) 0.9% NaCl, (2) Endotoxin solution, (3) Endotoxin and aluminium phosphate adjuvant, (4) Endotoxin and aluminium hydroxide adjuvant. The dose of endotoxin was 1 μg, and the dose of aluminium was 0.85 mg. | Female Sprague Dawley rats | To measure systemic response, the levels of TNFα, and IL-6 were measured in the serum. | TNFα and IL-6 were observed in the group which received an endotoxin solution or endotoxin and aluminium phosphate adjuvant. No TNFα or IL-6 was detected n the group that received endotoxin and Al(OH)3 adjuvant. |
| Valtulini et al. (2005) | Investigation followed repeated reports of up to 65% of pigs from one particular farm having one or more nodules in the muscles of the neck. In total, 45 pigs were assigned to: the original vaccine, the adjuvant alone, distilled water, or with the adjuvant and distilled water | Pigs (cross between Italian Landrace and Italian Large White) | Areas of muscular-cutaneous tissue were sampled from the neck of the slaughtered pigs. Granulomas underwent histological and histochemical analysis. | All groups administered the vaccine, adjuvant or adjuvant/water developed granulomas. No granulomas were found in the group administered water alone. |
| Verdier et al. (2005) | 2 groups of 12 monkeys received an i.m. injection of vaccines which were adjuvanted with either aluminium hydroxide or aluminium phosphate. | Cynomolgus monkeys | 4 monkeys from each group were sacrificed 3, 6, or 12 months after vaccination. Histopathological examination and aluminium assays were performed on the quadriceps muscle sections. | Histopathological lesions similar to the MMF described in humans were observed and persisted 3 months after aluminium phosphate and 12 months after aluminium hydroxide administration. Increased aluminium concentration in the area of the lesion was also observed. |
| Dermal Exposure | ||||
| Lansdown (1973) | Topically applied solutions of six aluminium salts were administered to the skin of mice, rabbits, and pigs. The animals were treated for 5 consecutive days at various concentrations. | Female albino mice, New Zealand white rabbits, and pigs (large white strain). | Animals were killed 24 hr after the final treatment and the skin was examined for erythema, thickening, and scaling. | Epidermal changes consisting of hyperplasia, microabscess formation, and dermal inflammatory cell infiltration were evident in all three species treated with aluminium chloride (10%) or nitrate (10%), but not with aluminium sulphate (10%), aluminium hydroxide (10%), acetate (10%), or chlorhydrate (10% and 25%) |
| REPEATED EXPOSURE | ||||
| Inhalation/intratracheal instillation exposure | ||||
| Christie et al. (1963) | Rats and hamsters were exposed to powder or fume produced from aluminium. Powder was blown into the experimental chambers hourly and fume every two hr throughout an 8 hr day. The quantity recovered in one period was 100 mg from the powder chamber and 92 mg from the fume chamber. | Male hamsters and male Wistar rats | Lungs were examined for gross abnormalities and microscopic examination was also conducted. Aluminium content of the lung was also measured. | Weights of wet lung, ash and aluminium oxide content of lungs were greater in the lungs of exposed rats than controls. In rats and hamsters exposed to aluminium powder and alumina fume the initial response was a proliferation of macrophages within alveolar spaces. After long exposure to aluminium powder, rats showed focal deposits of hyaline material in alveolar walls. |
| Gross et al. (1973) | Rats, guinea pigs, and hamsters were exposed to 3 different types of aluminium powder (British pyro powder- flake like particles, American powder with flake like particles, and powder comprised of atomized spherical particles) in inhalation chambers at varying concentrations. Animals were exposed for 6 hr/day, 5 days/week, for 6 months; some animals were exposed for 1 year. | Rats, hamsters, and guinea pigs | Histological examination of the lungs | Scattered small scars resulted from foci of lipid pneumonitis in rats in the treatment group. Alveolar proteinosis developed in all three species that inhaled aluminium dust, and was most pronounced in the rats. These symptoms resolved spontaneously and the accumulated dust deposits cleared rapidly from the lungs after cessation of the exposure. |
| Pigott et al. (1981) | Rats were exposed to refractory alumina fibre (Saffil) as manufactured or aged; asbestos was used as a positive control. Rats were placed in inhalation chambers and were exposed to fibres 5 days/week for 6 hr period. Exposures were terminated after 86 weeks for the Saffil group and 77 weeks for the asbestos group. Animals were maintained until a mortality rate of 85% was reached. | Wistar Albino rats | Histopathological examination of all grossly abnormal tissue and of the major organs was conducted. | Pulmonary reaction to both forms of alumina fibre was minimal. The asbestos group exhibited the most severe fibrosis. Pulmonary tumours were confined to rats dosed with asbestos. |
| Ess et al. (1993) | Fibrogenicity of 7 alumina samples was tested in rats by intratracheal instillation. Quartz was used as a positive control. The total dose (50 mg for each of the alumina samples and 25 mg for quartz) was administered by 5 instillations over a period of 2 weeks. | Female Sprague Dawley rats | Groups of 5 animals were sacrificed 60, 90, 180, and 360 days after exposure. Histological examinations were carried out and BAL was performed on the rats. | Fibrogenic potential was not noted for any of the 5 aluminas used for primary aluminium production; a chemical grade and laboratory produced sample induced fibrotic lesions. |
| Oral exposure | ||||
| Berlyne et al. (1972a) | Groups of nephrectomised rats were administered 1% or 2% AlCl3 or Al2(SO4)3 in drinking-water or oral Al(OH)3 (150 mg of elemental aluminium/kg b.w./day) by gavage. Groups of non-nephrectomised rats received the same treatments. The duration of the treatment was not indicated. | Male rats Weizmann Institute strain | Plasma and tissue levels of aluminium were assessed. Histological examination of the cornea, lungs, liver, and other major organs was conducted. | Periorbital bleeding was noted in all of the oral treatment groups in the nephrectomised rats, except for the Al(OH)3 group. |
| Thurston et al. (1972) | Groups of 6 rats were assigned to (a) a control diet, (b) diet with Al(OH)3 3.2 g/kg, (c) diet with Al(OH)3 + 10 g/kg disodium hydrogen phosphate. An additional group of rats underwent partial nephrectomy and received diet (b). | Rats | Line test for rachitic change was carried out on the skeleton of each animal. Histological examination was conducted and the skeletal aluminium content assessed. | Al(OH)3 impaired the growth rate of normal rats and produced rachitic bone changes; this effect could have been corrected by phosphate supplements. |
| Arieff et al. (1979) | Normal rats and rats with renal failure were given oral Al(OH)3 (300 mg daily for 5 months). A group of mongrel dogs also received the same dose and frequency of treatment. An additional group of rats and dogs served as controls. | Sprague Dawley rats, and mongrel dogs | Aluminium levels were measured in the liver, skeletal muscle and cerebral cortex of the rats. In the dogs, aluminium was measured in the cerebral cortex and subcortical white matter. EEGs were conducted on the dogs. | EEGs were normal in the aluminium treated dogs. |
| Thorne et al. (1986) | Rats were fed diets with no added aluminium, or with at doses of added Al(OH)3 1500, 2500 or 3500 mg/kg b.w./day. These diets were maintained for 30 days, after the treatment period animals were fed a basal diet. | Male Long Evans rats | A variety of behavioural tests was conducted. Brain aluminium content was measured. | Brain aluminium content correlated with the amount of aluminium in the diet. Elevated brain aluminium correlated with relatively poor performance on two of the behavioural tests. |
| Sugawara et al. (1988) | 22 weanling rats, and 19 adult rats were fed either a control diet, a diet with 2000 ppm Al(OH)3 or 2000 ppm AlK(SO4)2 for 67 days. | Male Wistar rats | Blood samples were taken to determine haematocrit and haemoglobin. Serum, liver, epididymal adipose tissue, femur, and intestine were analyzed for aluminium and lipids. | No aluminium induced anaemia or hyophosphatemia was observed. Serum triglyceride was decreased by aluminium. Neither serum cholesterol nor phospholipids was affected by aluminium ingestion. |
| Bilkei-Gorzo (1993) | Rats were treated orally once a day with NaCl + citric acid, AlCl3 (30 or 100 mg/kg), Al(OH)3 (100 mg/kg) + citric acid, or Al(OH)3 (300 mg/kg). The treatment was maintained for 90 days. | Long Evans rats | Learning ability was determined by the labyrinth test at day 90. Choline-acetyl transferase, acetylcholinesterase activity and aluminium content of the brains were measured. | Learning ability was impaired in the groups which received AlCl3 or Al(OH)3 + citric acid. Aluminium content of the brain was elevated in all treatment groups, most significantly in the AlCl3 (100 mg/kg) group. Choline-acetyltransferase activity decreased in all groups except the Al(OH)3 treated group. |
| Ecelbarger et al. (1994b) | Rats were fed diets containing 0.4 or 36.8 μmol Al/g diet as aluminium hydroxide for 8 months until 23 months of age. | Sprague Dawley rats | Urine and tissue samples were collected for aluminium analysis. | The rats exhibited little evidence of aluminium toxicity as body weight, feed intake, or changes in the relative size of tissues. |
| Dlugazek et al. (2000) | Aluminium was administered in drinking water as aluminium chloride, dihydroxyaluminium sodium carbonate or aluminium hydroxide. Animals were exposed to a total dose of 700 mg aluminium for each compound. The treatment period was 159 days for Al(OH)3. | Female mice (Pzh:SFIS) | Concentrations of Al, Ca, Mg, Zn, Cu, and Fe in the stomach, kidneys, bone and liver of the animals were analyzed. | In the Al(OH)3 treated group an increase in Mg concentration in bones, a decline in the Fe concentration in the stomach, and a decline in Cu in liver and kidney was observed. |
| Injection | ||||
| O’Gara & Brown (1967) | Aluminium foil was implanted s.c. into rats. | NIH black male rats | Rats were examined grossly and microscopically for lesions | 8 of 18 rats developed sarcomas following implantation. The smooth surface of the implant is likely responsible for the induction of sarcomas. |
| Levaditi et al. (1968) | Injections of Al(OH)3 s.c. were given weekly to rabbits and guinea pigs for a duration of 5 or 10 months. | Rabbits and guinea pigs | Animals were examined for visceral alterations. | Limited local inflammatory lesions were observed. |
| Bugiani & Ghetti (1982) | The cisterna magna of 31 rabbits were injected with 0.3 mL of a 1% suspension of (metallic) aluminium powder. 11 additional rabbits served as controls. | Male New Zealand Albino rabbits | Neurological examinations were conducted. Sections of the spinal cord were examined for the number of nerve cells, and neurons with neurofibrillary degeneration. | The injection induced a slowly progressing encephalomyelopathy. The large neurons of the anterior horns showed the most severe neurofibrillary degeneration and axonal swellings. Neurogenic muscular atrophy appeared in animals killed in the 2nd and 3rd month after injection. |
| Fiejka et al. (1996) | Mice received 1 mg Al, as Al(OH)3, i.p. every two weeks; another group received an i.p. injection of 0.1 mg elemental aluminium in the form of Al(OH)3 5 days/week. 10 animals from each group were killed after treatment with 2, 4 and 6 mg of elemental aluminium. | Mice (strain Pzh:SFIS) | Al concentrations in liver, bone and brain were evaluated. Liver tissue was examined histologically. | Development of resorption granulomas was observed in the liver. |
| Mahieu et al. (1998) | Rats were given Al(OH)3 (80 mg/kg b.w.) i.p. 3 time per week for a duration of 6 months. The diet was supplemented with calcium and phosphorus. Control rats received i.p. injections of saline. | Male Wistar rats | Phosphate tubular transport capacity was evaluated and parathyroid gland function was assessed. | The fractional excretion of P and Na were significantly lower in treated animals than in control animals. Calcemia recovery following a hypocacaemic stimulus and the nephrogenic excretion of cAMP were diminished. |
| Mahieu et al. (2000) | Rats were treated 3 times a wk with an i.p. injection of Al(OH)3 (80 g/kg b.w.) for a period of 6 months. | Male Wistar rats | Haematological parameters, and the osmotic resistance of red cells were determined in blood. aluminium and Fe concentrations as well as Tf saturation rate were determined in serum. Urea and creatine plasma levels were also measured. | Animals in the aluminium treatment group developed progressive microcytosis which became more prominent with time. The haematocrit levels were significantly reduced during months 1, 3, and 4 for the treated animals. The haemoglobin levels decreased on months 3 and 4, and then began to increase in months 5 and 6. |
| Platt et al. (2001) | Rats received intracerebroventricular injections of aluminium (0.68 μg or 5.4 μg over a period of 5 days). Animals were left for a period of either 7 days or 6 weeks. | Male Wistar rats | The brain of the animals was histologically examined. | A greater inflammatory response was noted in Al-injected animals compared with controls. Damage of the cingulated bundle in Al-treated animals led to a severe anterograde degeneration of cholinergic terminals in the cortex and hippocampus. |
| Bazzoni et al. (2005) | 7 rats were injected i.p. with Al(OH)3 (80 mg/kg b.w) 3 times a week for three months. A control group (n=7) was injected with saline at the same frequency and duration. | Male Wistar rats | Blood samples were collected to examine various haematological parameters. | Significant decreased in haematocrit and blood haemoglobin concentrations were found in the treatment group. Erythrocytes became more resistant to hypotonic haemolysis. Erythrocyte aggregation rate decreased in the treatment group. |
| Cointry et al. (2005) | 14 rats received i.p. doses of 27 mg/day aluminium as Al(OH)3 for 26 weeks. | Female Wistar rats | Femur diaphyses were studied tomographically and mechanically tested in bending. | Treatment reduced the cortical bone mineralization, and had a negative impact on the bending stiffness and the yield stress of cortical bone. |
Footnotes
DISCLAIMER: Although the present report is based primarily on peer-reviewed scientific literature, several abstracts of work in-progress have been cited along with some personal communications that were considered by the authors to be of relevance to their task. The authors included all relevant peer-reviewed scientific literature as of September 1, 2006 in their work. However, the conclusions drawn and the assessment of the health risks of aluminium are restricted to information appearing in the scientific peer-reviewed literature. All doses cited in the report are the doses as the Al form administered according to the original study.
No conflict of interest was declared.
Reference states that data were not available for the United States.
milliTesla: the strength of the magnetic field.
Publisher's Disclaimer: The manuscript has been reviewed and approved for publication by internal review at the US Environmental Protection Agency. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- AAMI (Association for the Advancement of Medical Instrumentation) Water Quality for Dialysis. 3. Arlington: AAMI WQD; 1998. [Google Scholar]
- Abramson MJ, Wlodarczyk JH, Saunders NA, Hensley MJ. Does aluminum smelting cause lung disease? Am Rev Respir Dis. 1989;139:1042–1057. doi: 10.1164/ajrccm/139.4.1042. [DOI] [PubMed] [Google Scholar]
- Abreo K, Glass J, Sella M. Aluminum inhibits hemoglobin synthesis but enhances iron uptake in friend erythroleukemia cells. Kidney Int. 1990;37:677–681. doi: 10.1038/ki.1990.33. [DOI] [PubMed] [Google Scholar]
- Abreo K, Jangula J, Jain S, Sella M, Glass J. Aluminum uptake and toxicity in cultured mouse hepatocytes. J Am Soc Nephrol. 1991;1:1299–1304. doi: 10.1681/ASN.V1121299. [DOI] [PubMed] [Google Scholar]
- Abreo K, Sella M, Gautreaux S, De Samet R, Vogeleere P, Ringoir S, Vanholder R. P-cresol, a uremic compound, enhanced the uptake of aluminum in hepatocytes. J Am Soc Nephrol. 1997;8:935–942. doi: 10.1681/ASN.V86935. [DOI] [PubMed] [Google Scholar]
- Abreo K, Abreo F, Sella M, Jain S. Aluminum enhances iron uptake and expression of neurofibrillary tangle protein in neuroblastoma cells. J Neurochem. 1999;72:2059–2064. doi: 10.1046/j.1471-4159.1999.0722059.x. [DOI] [PubMed] [Google Scholar]
- Abreo K, Sella M, Alvarez-Hernandez X, Jain S. Antioxidants prevent aluminum-induced toxicity in cultured hepatocytes. J Inorg Biochem. 2004;98:1129–1134. doi: 10.1016/j.jinorgbio.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Abubakar MG, Taylor A, Ferns GA. Regional accumulation of aluminium in the rat brain is affected by dietary vitamin. Eur J Trace Elem Med Biol. 2004;18:53–59. doi: 10.1016/j.jtemb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- ACGIH (American Conference of Governmental Industrial Hygienists) 1995-1996 Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 1996. [Google Scholar]
- ACGIH (American Conference of Governmental Industrial Hygienists) Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 2005. [Google Scholar]
- Ackley DC, Yokel RA. Aluminum citrate is transported from brain into blood via the monocarboxylic acid transporter located at the blood-brain barrier. Toxicology. 1997;120:89–97. doi: 10.1016/s0300-483x(97)03640-8. [DOI] [PubMed] [Google Scholar]
- Ackley DC, Yokel RA. Aluminum transport out of brain extracellular fluid is proton dependent and inhibited by mersalyl acid, suggesting mediation by the monocarboxylase transporter (MCTI) Toxicology. 1998;127:59–67. doi: 10.1016/s0300-483x(98)00037-7. [DOI] [PubMed] [Google Scholar]
- Adamis Z, Timar M, Kofler L, Tatrai E, Ungvary G. Biological effects of the respirable dusts from ore mines. Environ Res. 1986;41:319–326. doi: 10.1016/s0013-9351(86)80193-1. [DOI] [PubMed] [Google Scholar]
- Adamson RH, Canellos GP, Sieber SM. Studies on the antitumor activity of gallium nitrate (NSC-15200) and other group IIIa metal salts. Cancer Chemother Rep. 1975;59:599–610. [PubMed] [Google Scholar]
- Adler AJ, Berlyne GM. Duodenal aluminum absorption in the rat: effect of vitamin D. Am J Physiol. 1985;249:209–213. doi: 10.1152/ajpgi.1985.249.2.G209. [DOI] [PubMed] [Google Scholar]
- Advenier E, Landry C, Colomb V, Cognon C, Pradeau D, Florent M, Goulet O, Ricour C, Corriol O. Aluminum contamination of parenteral nutrition and aluminum loading in children on long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 2003;36:448–453. doi: 10.1097/00005176-200304000-00005. [DOI] [PubMed] [Google Scholar]
- Ahmad R, Naoui M, Neault JF, Diamontoglou S, Tajmir-Riahi HA. An FTIR spectroscopic study of calf-thymus DNA complexation with Al(III) and Ga(III) cations. J Biomol Struc Dyn. 1996;13:795–802. doi: 10.1080/07391102.1996.10508892. [DOI] [PubMed] [Google Scholar]
- Ahn HW, Jeffery EH. Effect of aluminum on fluoride uptake by Salmonella typhimurium TA98; implications for the Ames mutagenicity assay. J Toxicol Environ Health. 1994;4:357–368. doi: 10.1080/15287399409531849. [DOI] [PubMed] [Google Scholar]
- Ahn HW, Fulton B, Moxon D, Jeffery DH. Interactive effects of fluoride and aluminum uptake and accumulation in bones of rabbits administered both agents in their drinking water. J Toxicol Environ Health. 1995;44:337–350. doi: 10.1080/15287399509531963. [DOI] [PubMed] [Google Scholar]
- Akila R, Stollery BT, Riihimäki V. Decrements in cognitive performance in metal inert gas welders exposed to aluminum. Occup Environ Med. 1999;56:632–639. doi: 10.1136/oem.56.9.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akyol A, Boyvat A, Kundakci N. Contact sensitivity to aluminum. Dermatology. 2004;43:942–943. doi: 10.1111/j.1365-4632.2004.01983.x. [DOI] [PubMed] [Google Scholar]
- Al-Suliman NN, Grabau DA, Kiaer H, Rasmussen M, Bak M. A tumour in the breast: vaccination granuloma as a differential diagnosis. Eur J Surg Oncol. 1999;25:34–37. doi: 10.1053/ejso.1998.0596. [DOI] [PubMed] [Google Scholar]
- Alberti G, Biesuz R, Profumo A, Pesavento M. Determination of the total concentration and speciation of Al(III) in tea infusions. J Inorg Biochem. 2003;97:79–88. doi: 10.1016/s0162-0134(03)00247-2. [DOI] [PubMed] [Google Scholar]
- Albina ML, Bellés M, Sanchez DJ, Domingo JL. Evaluation of the protective activity of deferiprone, an aluminum chelator, on aluminum-induced developmental toxicity in mice. Teratology. 2000;6:86–92. doi: 10.1002/1096-9926(200008)62:2<86::AID-TERA4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Albuquerque D, Seidl V, Santos V, Oliveria-Neto J, Capelloi V, Rocco P, Zin W. The effect of experimental pleurodesis caused by aluminium hydroxide on lung and chest wall mechanics. Lung. 2002;179:293–303. doi: 10.1007/s004080000069. [DOI] [PubMed] [Google Scholar]
- Alder JF, Samuel AJ, West TS. The single element determination of trace metals in hair by carbon-furnace atomic absorption spectrometry. Anal Chim Acta. 1976;87:313–321. doi: 10.1016/S0003-2670(01)82260-1. [DOI] [PubMed] [Google Scholar]
- Alessio L, Apostoli P, Ferioli A, Sipio Di I, Mussi I, Rigosa C, Albertini A. Behaviour of biological indicators of internal dose and some neuro-endocrine tests in aluminium workers. Med Lav. 1989;80:290–300. [PubMed] [Google Scholar]
- Alexander J, Gjessing E, Nordal KP, Dahl E, Halse J, Thomsassen Y. A preliminary study of aluminium in serum and other human materials in subjects from different areas of Norway. Environ Geochem Health. 1990;12:83–86. doi: 10.1007/BF01734054. [DOI] [PubMed] [Google Scholar]
- Alfaro Moreno E, Flores Rojas G, Orozco de la Huerta A, Quintana Belmares R, Osornio Vargas AR. In vitro induction of abnormal anaphases by contaminating atmospheric dust from the city of Mexicali, Baja California, and Mexico. Arch Med Res. 1997;28:549–553. [PubMed] [Google Scholar]
- Alfrey AC. Aluminum metabolism in uremia. Neurotoxicology. 1980;1:43–53. [Google Scholar]
- Alfrey AC. Aluminum metabolism. Kidney Int. 1986;29(Suppl 18):S8–S11. [PubMed] [Google Scholar]
- Alfrey AC. Physiology of aluminum in man. In: Gitelman HJ, editor. Aluminum and Health A Critical Review. New York: Marcel Dekker, Inc; 1989. pp. 101–124. [Google Scholar]
- Alfrey AC. Aluminum toxicity in patients with chronic renal failure. Ther Drug Monit. 1993;15:593–597. doi: 10.1097/00007691-199312000-00025. [DOI] [PubMed] [Google Scholar]
- Alfrey AC, Hegg A, Miller N, Berl T, Berns A. Interrelationship between calcium and aluminum metabolism in dialyzed uremic patients. Miner Electrol Metab. 1979;2:81–87. [Google Scholar]
- Alfrey AC, Hegg A, Craswell P. Metabolism and toxicity of aluminum in renal failure. Am J Clin Nutr. 1980;33:1509–1516. doi: 10.1093/ajcn/33.7.1509. [DOI] [PubMed] [Google Scholar]
- Alfrey AC, Sedman A, Chan YL. The compartmentalization and metabolism of aluminum in uremic rats. J Lab Clin Med. 1985;105:227–233. [PubMed] [Google Scholar]
- Allain P, Gauchard F, Krari N. Enhancement of aluminum digestive absorption by fluoride in rats. Res Commun Mol Pathol Pharmacol. 1996;91:225–231. [PubMed] [Google Scholar]
- Allen DD, Yokel RA. Dissimilar aluminum and gallium permeation of the blood-brain barrier demonstrated by in vivo microdialysis. J Neurochem. 1992;58:903–908. doi: 10.1111/j.1471-4159.1992.tb09342.x. [DOI] [PubMed] [Google Scholar]
- Allen DD, Orvig C, Yokel RA. Evidence for energy-dependent transport of aluminum out of brain extracellular fluid. Toxicology. 1995;98:31–39. doi: 10.1016/0300-483x(94)02953-r. [DOI] [PubMed] [Google Scholar]
- Allen LV, Jr, Berardi RR, Desimone EM, Engle JP, Popovich NG, Rosenthal WM, Tietze KJ. Handbook of Nonprescription Drugs. Washington: American Pharmaceutical Association; 2000. [Google Scholar]
- Allen VG, Fontenot JP. Influence of aluminium as sulfate, chloride and citrate on magnesium and calcium metabolism in sheep. J Anim Sci. 1984;59:798–804. doi: 10.2527/jas1984.593798x. [DOI] [PubMed] [Google Scholar]
- Al-Masalkhi A, Walton SP. Pulmonary fibrosis and occupational exposure to aluminum. J Ky Med Assoc. 1994;92:59–61. [PubMed] [Google Scholar]
- Altmann P, Dhanesha U, Hamon C, Cunningham J, Blair J, Marsh F. Disturbance of cerebral function by aluminium in haemodialysis patients without overt aluminium toxicity. Lancet. 1989;2:7–12. doi: 10.1016/s0140-6736(89)90254-7. [DOI] [PubMed] [Google Scholar]
- Altmann P, Cunningham J, Dhanesha U, Ballard M, Thompson J, Marsh F. Disturbances of cerebral function in people exposed to drinking water contaminated with aluminum sulphate: retrospective study of the Camelford water incident. Br Med J. 1999;319:807–811. doi: 10.1136/bmj.319.7213.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Hernandez X, Madigosky SR, Stewart B, Glass J. Iron status affects aluminum uptake and transport by Caco-2 cells. J Nutr. 1994;124:1574–1580. doi: 10.1093/jn/124.9.1574. [DOI] [PubMed] [Google Scholar]
- Amador FC, Santos MS, Oliveira CR. Lipid peroxidation facilitates aluminum accumulation in rat brain synaptosomes. J Toxicol Environ Health A. 1999;58:427–435. doi: 10.1080/009841099157151. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics, Committee on Nutrition. Aluminum toxicity in infants and children. Pediatrics. 1996;97:413–416. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: American Psychiatric Association; 1987. [Google Scholar]
- Anane R, Bonini M, Grafeille J, Creppy E. Skin absorption and hippocampal bioaccumulation of water soluble aluminum chloride in mice (abstract) Hum Exp Toxicol. 1995;14:531. [Google Scholar]
- Anane R, Bonini M, Creppy EE. Transplacental passage of aluminum from pregnant mice to fetus organs after maternal transcutaneous exposure. Hum Exp Toxicol. 1997;16:501–504. doi: 10.1177/096032719701600904. [DOI] [PubMed] [Google Scholar]
- Anderson AR. Aluminum compounds: aluminum organometallic compounds. In: Mark HF, McKette JJ Jr, Othmer DF, editors. Kirk-Othmer Encyclopedia of Chemical Technology. 2. Vol. 2. New York: Interscience Publishers; 1963. pp. 226–41. [Google Scholar]
- Andrási E, Farkas E, Scheibler H, Reffy A, Bezur L. Al, Zn, Cu, Mn and Fe levels in brain in Alzheimer’s disease. Arch Gerontol Geriatr. 1995;21:89–97. doi: 10.1016/0167-4943(95)00643-y. [DOI] [PubMed] [Google Scholar]
- Andrási E, Páli N, Molnár Z, Kösel S. Brain aluminum, magnesium and phosphorus contents of control and Alzheimer-diseased patients. J Alzheimers Dis. 2005;7:273–284. doi: 10.3233/jad-2005-7402. [DOI] [PubMed] [Google Scholar]
- Anghileri LJ, Maincent P, Thouvenot P. Long-term oral administration of aluminum in mice. Aluminum distribution in tissues and effects on calcium metabolism. Ann Clin Lab Sci. 1994;24:22–26. [PubMed] [Google Scholar]
- Anon. Aluminium and silicosis. Lancet. 1956;271:500–501. [PubMed] [Google Scholar]
- Anthony JS, Fadl S, Mason C, Davison A, Berry J. Absorption, deposition and distribution of dietary aluminium in immature rats: effects of dietary vitamin D3 and food-borne chelating agent. J Environ Sci Health B. 1986;21:191–205. doi: 10.1080/03601238609372518. [DOI] [PubMed] [Google Scholar]
- Apostoli P, Lucchini R, Maccarrone R, Alessio L. Biological monitoring of occupational exposure to aluminum. Med Lav. 1992;83:475–483. [PubMed] [Google Scholar]
- Archibald JD. Aluminum in cow’s milk. J Dairy Sci. 1955;38:159–162. [Google Scholar]
- Aremu DA, Meshitsuka S. Accumulation of aluminum by primary cultured astrocytes from aluminum amino acid complex and its apoptotic effect. Brain Res. 2005;1031:284–296. doi: 10.1016/j.brainres.2004.06.090. [DOI] [PubMed] [Google Scholar]
- Arieff AI, Cooper JD, Armstrong D, Lazarowitz VC. Dementia, renal failure, and brain aluminum. Ann Int Med. 1979;90:741–747. doi: 10.7326/0003-4819-90-5-741. [DOI] [PubMed] [Google Scholar]
- Armstrong BC, Tremblay D, Baris, Theriault G. Lung cancer mortality and polynuclear aromatic hydrocarbons: a case-cohort study of aluminum production workers in Arvida, Quebec, Canada. Am J Epidemiol. 1994;139:250–262. doi: 10.1093/oxfordjournals.aje.a116992. [DOI] [PubMed] [Google Scholar]
- Armstrong BE, Hutchinson E, Unwin J, Fletcher T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta-analysis. Environ Health Perspect. 2004;112:970–978. doi: 10.1289/ehp.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz NO, Kaufman JD, Daroowala FM, Quiqley S, Farin F, Checkoway H. Genetic factors and asthma in aluminum smelter workers. Arch Environ Health. 2003;58:197–200. doi: 10.3200/AEOH.58.4.197-200. [DOI] [PubMed] [Google Scholar]
- Arnich N, Cunat L, Lanhers MC, Burnel D. Comparative in situ study of the intestinal absorption of aluminum, manganese, nickel, and lead in rats. Biol Trace Elem Res. 2004;99:157–171. doi: 10.1385/BTER:99:1-3:157. [DOI] [PubMed] [Google Scholar]
- Arnold CJ, Miller GG, Zello GA. Parenteral nutrition-associated cholestasis in neonates: the role of aluminum. Nutr Rev. 2003;61:306–310. doi: 10.1301/nr.2003.sept.306-310. [DOI] [PubMed] [Google Scholar]
- Atchison WD. Effects of toxic environmental contaminants on voltage-gated calcium channel function: from past to present. J Bioeng Biomembr. 2003;35:507–532. doi: 10.1023/b:jobb.0000008023.11211.13. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Aluminum and Compounds. Atlanta, GA.: U.S Department of Health and Human Services, Public Health Service; 1992. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) A Toxicological Profile for Aluminum. Atlanta, GA.: U.S Department of Health and Human Services, Public Health Service; 1999. [Google Scholar]
- Austin PS, Doughman DJ. Reaction to intraocular penetration of bentonite. Am J Ophthamol. 1980;89:719–723. doi: 10.1016/0002-9394(80)90294-9. [DOI] [PubMed] [Google Scholar]
- Authier FJ, Cherin P, Creange A, Bonnotte B, Ferrer X, Abdelmoumni A, Ranoux D, Pelletier J, Figarella-Branger D, Granel B, Maisonobe T, Coquet M, Degos JD, Gherardi RK. Central nervous system disease in patients with macrophagic myofasciitis. Brain. 2001;124:974–983. doi: 10.1093/brain/124.5.974. [DOI] [PubMed] [Google Scholar]
- Avolio G, Galietti F, Iorio M, Oliaro A. Aluminum lung as an occupational disease. Case reports. Minerva Med. 1989;80:411–414. [PubMed] [Google Scholar]
- Baes CF, Mesmer RE. The Hydrolysis of Cations. New York: John Wiley & Sons, Inc; 1976. [Google Scholar]
- Bajaj AK, Gupta SC, Pandey RK, Misra K, Rastogi S, Chatterji AK. Aluminium contact sensitivity. Contact Dermatitis. 1997;37:307–308. doi: 10.1111/j.1600-0536.1997.tb02479.x. [DOI] [PubMed] [Google Scholar]
- Ballanti P, Mocetti P, Della Rocca C, Bonucci E, Costantinit S, Giordano R, Ioppolo A, Mantovani A. Experimental aluminium intoxication and parathormone: effects on the mineralization process. Mineral Electrolyte Metab. 1989;15:233–240. [PubMed] [Google Scholar]
- Balouet G, Baret M, Relyveld E, Ravisse P, Levaditi J. Role of antigens and adjuvant substances in the histological response in experimental granulomas (immunogenic granuloma) Ann Anat Pathol. 1997;22:159–170. [PubMed] [Google Scholar]
- Bamberger PJ. Aluminum therapy in silicosis-a clinical study of the comparative effects of the metallic powder and hydrated aluminum. Ind Med Surg. 1945;14:477–479. [Google Scholar]
- Banin E, Meiri H. Toxic effects of alumino-silicates on nerve cells. Neurosci. 1990;39:171–178. doi: 10.1016/0306-4522(90)90230-2. [DOI] [PubMed] [Google Scholar]
- Barata JD, D’Haese PC, Pires C, Lamberts LV, Simoes J, De Broe ME. Low-dose (5 mg/kg) desferrioxamine treatment in acutely aluminium-intoxicated haemodialysis patients using two drug administration schedules. Nephrol Dial Transplant. 1996;11:125–132. [PubMed] [Google Scholar]
- Barber PJ, Rawlings JM, Markwell PJ, Elliott J. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract. 1999;40:62–70. doi: 10.1111/j.1748-5827.1999.tb03039.x. [DOI] [PubMed] [Google Scholar]
- Barker J, Day JP, Aitken TW, Charlesworth TR, Cunningham RC, Durum PV, Lilley JS, Newton GW, Smithson MJ. Development of Al-26 accelerator mass-spectrometry for biological and toxicological applications. Nucl Instrum Methods Phys Res B. 1990;52:540–543. [Google Scholar]
- Barnard CG, McBride DI, Firth HM, Herbison GP. Assessing individual employee risk factors for occupational asthma in primary aluminium smelting. Occup Environ Med. 2004;61:604–608. doi: 10.1136/oem.2003.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M, Hignett S, Glenny AT, Randall KJ. Antigenic efficiency of fluid and precipitated diphtheria prophylactics in very young babies and lambs. Lancet. 1952;2:803–805. doi: 10.1016/s0140-6736(52)92333-7. [DOI] [PubMed] [Google Scholar]
- Barr M, Glenny AT, Butler NR. Immunization of babies with diphtheriatetanus-pertusis prophylactic. Br Med J. 1955;2:635–639. doi: 10.1136/bmj.2.4940.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast-Pettersen R, Skaug V, Ellingsen D, Thomassen Y. Neurobehavioral performance in aluminum welders. Am J Ind Med. 2000;37:184–192. doi: 10.1002/(sici)1097-0274(200002)37:2<184::aid-ajim4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bauer S, Wolff I, Werner N, Schmidt R, Blume R, Pelzing M. Toxicological investigations in the semiconductor industry: IV. Studies on the subchronic oral toxicity and genotoxicity of vacuum pump oils contaminated by waste products from aluminum plasma etching processes. Toxicol Ind Health. 1995;11:523–541. doi: 10.1177/074823379501100506. [DOI] [PubMed] [Google Scholar]
- Baxter MJ, Burrell JA, Crews HM, Massey RC. Aluminium in infant formulae and tea and leaching during cooking. In: Massey R, Taylor D, editors. Aluminium in Food and the Environment. Cambridge, UK: The Royal Society of Chemistry; 1989. pp. 77–87. [Google Scholar]
- Baxter MJ, Burrell JA, Crews H, Massey RC. Aluminum levels in milk and infant formulae. Food Addit Contam. 1991;8:653–660. doi: 10.1080/02652039109374019. [DOI] [PubMed] [Google Scholar]
- Bayder T, Nagymatényi L, Isimer A, Sahin G. Effect of folic acid supplementation on aluminum accumulation in rats. Nutrition. 2005;21:406–410. doi: 10.1016/j.nut.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Baylor NW, Egan W, Richman P. Aluminum salts in vaccines-US perspective. Vaccine. 2002;20(Suppl 3):S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Bollini A, Hernandez G, del Carmen Contini M, Chiarotto M, Rasia M. In vivo effect of aluminium upon the physical properties of the erythrocyte membrane. J Inorg Biochem. 2005;99:822–827. doi: 10.1016/j.jinorgbio.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- Beauville M, Pradal G, Raynaud P. Influence of fasting on the components of the gastric juice in rabbits. C R Seances Soc Biol Fil. 1966;160:404–408. [PubMed] [Google Scholar]
- Becaria A, Campbell A, Bondy SC. Aluminum as a toxicant. Toxicol Ind Health. 2002;18:309–320. doi: 10.1191/0748233702th157oa. [DOI] [PubMed] [Google Scholar]
- Bégin R, Massé S, Rola-Pleszczynski M, Martel M, Desmarais Y, Geoffroy M, LeBouffant L, Daniel H, Martin J. Aluminum lactate treatment alters the lung biological activity of quartz. Exp Lung Res. 1986;10:385–399. doi: 10.3109/01902148609058289. [DOI] [PubMed] [Google Scholar]
- Bégin R, Massé S, Sébastien P, Martel M, Bosse J, Dubois F, Geoffrey M, Labbe J. Sustained efficacy of aluminum to reduce quartz toxicity in the lung. Exp Lung Res. 1987;13:205–222. doi: 10.3109/01902148709064319. [DOI] [PubMed] [Google Scholar]
- Bégin R, Massé S, Dufresne A. Further information on aluminum inhalation in silicosis. Occup Environ Med. 1995;52:778–780. doi: 10.1136/oem.52.11.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JD, Kubal G, Radulovic S, Sadler PJ, Tucker A. Detection of aluminium(III) binding to citrate in human blood plasma by proton nuclear magnetic resonance spectroscopy. Analyst. 1993;118:241–244. doi: 10.1039/an9931800241. [DOI] [PubMed] [Google Scholar]
- Bellés M, Sanchez DJ, Gómez M, Corbella J, Domingo JL. Silicon reduces aluminum accumulation in rats: relevance to the aluminum hypothesis of Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:83–87. doi: 10.1097/00002093-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Bellés M, Albina ML, Sanchez DJ, Domingo JL. Lack of protective effects of dietary silicon on aluminium-induced maternal and developmental toxicity in mice. Pharmacol Toxicol. 1999;85:1–6. doi: 10.1111/j.1600-0773.1999.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Bellés M, Albina ML, Sanchez DJ, Corbella J, Domingo JL. Effects of oral aluminum on essential trace elements metabolism during pregnancy. Biol Trace Elem Res. 2001;79:67–81. doi: 10.1385/BTER:79:1:67. [DOI] [PubMed] [Google Scholar]
- Bellia JP, Newton K, Davenport A, Birchall JD, Roberts NB. Silicon and aluminium and their inter-relationship in serum and urine after renal transplantation. Eur J Clin Invest. 1994;24:703–710. doi: 10.1111/j.1365-2362.1994.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Bellia JP, Birchall JD, Roberts NB. The role of silicic acid in the renal excretion of aluminum. Ann Clin Lab Sci. 1996;26:227–233. [PubMed] [Google Scholar]
- Bellows CG, Aubin JE, Heersche JNM. Aluminium inhibits both initiation and progression of mineralization of osteoid nodules formed in differentiating rat calvaria cell cultures. J Bone Mineral Res. 1995;10:2011–2016. doi: 10.1002/jbmr.5650101222. [DOI] [PubMed] [Google Scholar]
- Bellows CG, Heersche JN, Aubin JE. Aluminum accelerates osteoblastic differentiation but is cytotoxic in long-term rat calvaria cell cultures. Calcif Tissue Int. 1999;65:59–65. doi: 10.1007/s002239900658. [DOI] [PubMed] [Google Scholar]
- Belt TF, King EJ. Failure of aluminium to prevent experimental silicosis. J Path Bact. 1943;55:69–73. [Google Scholar]
- Benet LZ, Kroetz DL, Sheiner LB. Pharmacokinetics. The dynamics of drug absorption, distribution, and elimination. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. pp. 3–28. [Google Scholar]
- Benke G, Abramson M, Sim M. Exposures in the alumina and primary aluminium industry: a historical review. Ann Occup Hyg. 1998;42:173–189. doi: 10.1016/s0003-4878(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Berend K, van der Voet G, Boer WH. Acute aluminum encephalopathy in a dialysis center caused by a cement mortar water distribution pipe. Kidney Int. 2001;59:746–753. doi: 10.1046/j.1523-1755.2001.059002746.x. [DOI] [PubMed] [Google Scholar]
- Berend K, van der Voet G, de Wolff A. Acute aluminum intoxication. In: Roesky H, Atwood D, editors. Structure and Bonding. Berlin: Springer; 2002. pp. 1–58. [Google Scholar]
- Bergerioux G, Boivert J. Rapid neutron activation method for the determination of minerals in milk. Int J Nucl Med Biol. 1979;6:128–131. doi: 10.1016/0047-0740(79)90011-1. [DOI] [PubMed] [Google Scholar]
- Bergfors E. 406 children with contact allergy to aluminium after the use of adsorbed vaccines. Sixth Keele Meeting on Aluminium; February 26-March 2, 2005; Bussaco, Portugal. 2005. [Google Scholar]
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64–69. doi: 10.1016/s0264-410x(03)00531-0. [DOI] [PubMed] [Google Scholar]
- Bergomi M, Vinceti M, Nacci G, Pietrini V, Bratter P, Alber D, Ferrari A, Vescovi L, Guidetti D, Sola P, Malagu S, Aramini C, Vivoli G. Environmental exposure to trace elements and risk of amyotrophic lateral sclerosis: a population-based case-control study. Environ Res. 2002;89:116–123. doi: 10.1006/enrs.2002.4361. [DOI] [PubMed] [Google Scholar]
- Berlyne GM, Yagil R, Ari JB, Weinberger G, Knopf E, Danovitch GM. Aluminium toxicity in rats. Lancet. 1972a;1:564–568. doi: 10.1016/s0140-6736(72)90357-1. [DOI] [PubMed] [Google Scholar]
- Berlyne GM, Yagil RJ, Ben Ari J, Danovitch GM. Aluminium toxicity. Lancet. 1972b;1:1070–1071. doi: 10.1016/s0140-6736(72)91252-4. [DOI] [PubMed] [Google Scholar]
- Bernier RH, Frank JA, Nolan TF. Abcesses complicating DTP vacccination. Am J Dis Child. 1981;135:826–828. doi: 10.1001/archpedi.1981.02130330036011. [DOI] [PubMed] [Google Scholar]
- Berry JW. Aluminum therapy in advanced silicosis. Amer Rev Tuberc. 1948;57:557–573. doi: 10.1164/art.1948.57.6.557. [DOI] [PubMed] [Google Scholar]
- Bertholf RL, Herman MM, Savory J, Carpenter RM, Sturgill B, Katsetos CD, Vandenberg SR, Wills MR. A long-term intravenous model of aluminum maltol toxicity in rabbits: tissue distribution, hepatic, renal, and neuronal cytoskeletal changes associated with systemic exposure. Toxicol Appl Pharmacol. 1989;98:58–74. doi: 10.1016/0041-008x(89)90134-8. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. The current status of Alzheimer’s disease genetics: what do we tell the patients? Pharmacol Res. 2004;50:385–396. doi: 10.1016/j.phrs.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005;115:1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berubé D. Speciation analysis and the occurrence of aluminium turbidity. J Toxicol Environ Health A. 2004;67:1655–1666. doi: 10.1080/15287390490493457. [DOI] [PubMed] [Google Scholar]
- Beynon H, Cassidy MJ. Gastrointestinal absorption of aluminum. Nephron. 1990;55:235–236. doi: 10.1159/000185966. [DOI] [PubMed] [Google Scholar]
- Bia MJ, Cooper K, Schnall S, Duffy T, Hendler E, Malluche H, Solomon L. Aluminum induced anemia: pathogenesis and treatment in patients on chronic hemodialysis. Kidney Int. 1989;36:852–858. doi: 10.1038/ki.1989.271. [DOI] [PubMed] [Google Scholar]
- Biego GH, Joyeux M, Hartemann P, Debry G. Daily intake of essential minerals and metallic micropollutants from foods in France. Sci Total Environ. 1998;217:27–36. doi: 10.1016/s0048-9697(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzó A. Neurotoxic effect of enteral aluminum. Food Chem Toxicol. 1993;31:357–361. doi: 10.1016/0278-6915(93)90191-z. [DOI] [PubMed] [Google Scholar]
- Birchall JD, Chappell JS. Aluminum water chemistry, and Alzheimer’s disease. Lancet. 1989;1:953. doi: 10.1016/s0140-6736(89)92523-3. [DOI] [PubMed] [Google Scholar]
- Birchall JD, Bellia JP, Roberts NB. On the mechanisms underying the essentiality of silicon-interactions with aluminum and copper. Coor Chem Rev. 1996;149:231–240. [Google Scholar]
- Bischoff R, Gyson G. Carcinogenesis through solid state surfaces. Proc Exp Tumor Res. 1964;5:85–133. doi: 10.1159/000385997. [DOI] [PubMed] [Google Scholar]
- Bishop NJ, Morley R, Day JP, Lucas A. Aluminum neurotoxicity in preterm infants receiving intravenous-feeding solutions. N Eng J Med. 1997;336:1557–1561. doi: 10.1056/NEJM199705293362203. [DOI] [PubMed] [Google Scholar]
- Bjertness E, Candy JM, Torvik A, Ince P, McArthur F, Taylor GA, Johansen SW, Alexander J, Grønnesby JK, Bakketeig LS, Edwardson JA. Content of brain aluminum is not elevated in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:171–174. doi: 10.1097/00002093-199601030-00006. [DOI] [PubMed] [Google Scholar]
- Blahos J, Care AD, Abbas M, Corlett SC. Auminium induced decrease in osteocalcin levels in the chick. Horm Metab Res. 1991;23:50–51. doi: 10.1055/s-2007-1003611. [DOI] [PubMed] [Google Scholar]
- Blair HC, Finch JL, Avioli R, Crouch LL, Slatopolisky L, Teitelbaum SL. Micromolar aluminum levels reduce 3H-thymidine incorporation by cell line UMR 106-01. Kidney Int. 1989;35:1119–1125. doi: 10.1038/ki.1989.99. [DOI] [PubMed] [Google Scholar]
- Blank IH, Jones JL, Gould E. A study of the penetration of aluminum salts into excised skin. Proc Sci Sect Toilet Goods Assoc. 1958;29:32–35. [Google Scholar]
- Blumenthal NC, Posner AS. In vitro model of aluminium induced osteomalacia: inhibition of hydroxyapatite formation and growth. Calcif Tissue Int. 1984;36:439–441. doi: 10.1007/BF02405357. [DOI] [PubMed] [Google Scholar]
- Böhler-Sommeregger K, Lindemayr H. Contact sensitivity to aluminium. Contact Dermatitis. 1986;15:278–281. doi: 10.1111/j.1600-0536.1986.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Bordet AL, Michenet P, Cohen C, Arbion F, Ekindi N, Bonneau C, Kerdraon R, Coville M. Post-vaccination granuloma due to aluminium hydroxide. Ann Pathol. 2001;21:149–152. [PubMed] [Google Scholar]
- Boudey MF, Bureau C, Place D, Neuville M, Drosdowsky, Arhan P, Bougle D. Effect of small variations of aluminum intake on calcium metabolism in young rats. J Pediatr Gastroenterol Nutr. 1997;24:124–127. doi: 10.1097/00005176-199702000-00002. [DOI] [PubMed] [Google Scholar]
- Bougle D, Bureau F, Voirin J, Neuville D, Duhamel JF. A cross-sectional study of plasma and urinary aluminum levels in term and preterm infants. J Parenter Enteral Nutr. 1992;16:157–159. doi: 10.1177/0148607192016002157. [DOI] [PubMed] [Google Scholar]
- Bougle D, Bureau F, Morello R, Guillow B, Sabatier JP. Aluminum in the premature infant. Trace Elem Electrolytes. 1997;14:24–26. [Google Scholar]
- Bouras C, Giannakopoulos P, Good PF, Hsu A, Hof PR, Perl DP. A laser microprobe mass analysis of brain aluminum and iron in dementia pugilistica: comparison with Alzheimer’s disease. Eur Neurol. 1997;38:53–58. doi: 10.1159/000112903. [DOI] [PubMed] [Google Scholar]
- Bourdeau AM, Plachot JJ, Cournet-Witmer G, Pointillart A, Balsan S, Sachs C. Parathyroid response to aluminum in vitro: ultrastructural changes and PTH release. Kidney Int. 1987;31:15–24. doi: 10.1038/ki.1987.3. [DOI] [PubMed] [Google Scholar]
- Bowen HJM. Environmental Chemistry of the Elements. London: Academic Press; 1979. [Google Scholar]
- Boyce BF, Eider HY, Fell SG, Nicholson WA, Smith GD, Dempster DW, Gray CC, Boyle IT. Quantitation and localisation of aluminum in human cancellous bone in renal osteodystrophy. Scan Electron Microsc. 1981;3:329–337. [PubMed] [Google Scholar]
- Bozynski ME, Sedman AB, Naglie RA, Wright EJ. Serial plasma and urinary aluminum levels and tissue loading in preterm twins. J Parenter Enteral Nutr. 1989;13:428–431. doi: 10.1177/0148607189013004428. [DOI] [PubMed] [Google Scholar]
- Bradberry SM, Beer ST, Vale JA. UKPID monograph Aluminium oxide. 1997 [online] Cited 24 January 2007 www.intox.org/databank/documents/chemical/alumoxde/ukpid33.htm.
- Bradbury MW. Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem. 1997;69:443–454. doi: 10.1046/j.1471-4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- Bradley C, Leung FY. Aluminum determined in plasma and urine by atomic absorption spectroscopy with a transversely heated graphite atomizer furnace. Clin Chem. 1994;40:431–434. [PubMed] [Google Scholar]
- Bräunlich H, Fleck C, Kersten L, Stein G, Laske V, Müller A, Keil E. Renal effects of aluminium in uraemic rats and rats with intact kidney function. J Appl Toxicol. 1986;6:55–59. doi: 10.1002/jat.2550060112. [DOI] [PubMed] [Google Scholar]
- Brown GM, Donaldson K, Brown DM. Bronchoalveolar leukocyte response in experimental silicosis: modulation by a soluble aluminum compound. Toxicol Appl Pharmacol. 1989;101:95–105. doi: 10.1016/0041-008x(89)90215-9. [DOI] [PubMed] [Google Scholar]
- Brown JP, Dietrich PS, Bakner CM. Mutagenicity testing of some drug and cosmetic dye lakes with the Salmonella/mammalian microsome assay. Mutat Res. 1979;66:181–185. doi: 10.1016/0165-1218(79)90064-8. [DOI] [PubMed] [Google Scholar]
- Brown TS, Schwartz R. Aluminum accumulation in serum, liver and spleen of Fe-depleted and Fe-adequate rats. Biol Trace Elem Res. 1992;34:1–10. doi: 10.1007/BF02783892. [DOI] [PubMed] [Google Scholar]
- Browning E. Aluminum. In: Browning E, editor. Toxicity of Industrial Metals. New York: Appleton-Century-Crofts; 1969. pp. 3–22. [Google Scholar]
- Brudevold F, Bakhos Y, Gron P. Fluoride in human saliva after ingestion of aluminium chloride and sodium fluoride or sodium monofluorophosphate. Arch Oral Biol. 1973;18:699–706. doi: 10.1016/0003-9969(73)90005-8. [DOI] [PubMed] [Google Scholar]
- Brunton LL. Agents for control of gastric acidity and treatment of peptic ulcers. In: Hardman JG, Limbird LE, editors. The Pharmacological Basis of Therapeutics. 9. New York: McGraw-Hill; 1996. pp. 901–915. [Google Scholar]
- Brusewitz S. Aluminum vol 203. Stockholm, Sweden: University of Stockholm Institute of Theoretical Physics; 1984. [Google Scholar]
- Buchanan MRC, Ihle BU, Dunn CM. Haemodialysis related osteomalacia: a staining method to demonstrate aluminum. J Clin Pathol. 1981;34:1352–1354. doi: 10.1136/jcp.34.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta M, Kiesswetter E, Otto A, Schaller KH, Seeber A, Hilla W, Windorfer K, Stork J, Kuhlmann A, Gefeller O, Letzel S. Longitudinal study examining the neurotoxicity of occupational exposure to aluminium-containing welding fumes. Int Arch Occup Environ Health. 2003;76:539–548. doi: 10.1007/s00420-003-0450-9. [DOI] [PubMed] [Google Scholar]
- Buchta M, Kiesswetter E, Schaper M, Zchiesche W, Schaller KH, Kuhlmann A, Letzel S. Neurotoxicity of exposures to aluminium welding fumes in the truck trailer construction industry. Environ Toxicol Pharmacol. 2005;19:677–685. doi: 10.1016/j.etap.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Budavari S, O’Neil MJ, Smith A, Heckelman PE. The Merck Index. 11. Rathway, N.J.: Merck & Co. Inc; 1989. [Google Scholar]
- Bugiani O, Ghetti B. Progressing encephalomyelopathy with muscular athropy, induced by aluminum powder. Neurobiol Aging. 1982;3:209–222. doi: 10.1016/0197-4580(82)90041-0. [DOI] [PubMed] [Google Scholar]
- Burge PS, Scott JA, McCoach J. Occupational asthma caused by aluminum. Allergy. 2000;55:779–780. doi: 10.1034/j.1398-9995.2000.00641.x. [DOI] [PubMed] [Google Scholar]
- Burnatowska-Hledin MA, Mayor GH. The effects of aluminum loading on selected tissue calcium and magnesium concentrations in rats. Biol Trace Elem Res. 1984;6:531–535. doi: 10.1007/BF02987206. [DOI] [PubMed] [Google Scholar]
- Burnatowska-Hledin MA, Mayor GH, Lau K. Renal handling of aluminum in the rat: clearance and micropuncture studies. Am J Physiol. 1985;249:F192–F197. doi: 10.1152/ajprenal.1985.249.2.F192. [DOI] [PubMed] [Google Scholar]
- Burnatowska-Hledin MA, Doyle TM, Eadie MJ, Mayor GH. 1,25 Dihydroxy-vitamin D increases serum and tissue accumulation of aluminum in rats. J Lab Clin Med. 1986;108:96–102. [PubMed] [Google Scholar]
- Burwen DR, Olsen SM, Bland LA, Arduino MJ, Reid MH, Jarvis WR. Epidemic aluminum intoxication in hemodialysis patients traced to use of an aluminum pump. Kidney Int. 1995;48:469–474. doi: 10.1038/ki.1995.315. [DOI] [PubMed] [Google Scholar]
- Bushinsky DA, Sprague SM, Hallegot P, Girod C, Chabala JM, Levisetti R. Effects of aluminium on bone surface ion composition. J Bone Mineral Res. 1995;10:1988–1997. doi: 10.1002/jbmr.5650101219. [DOI] [PubMed] [Google Scholar]
- Butler NR, Voyce MA, Burland WL, Hilton ML. Advantages of aluminium hydroxide adsorbed combined diptheria, tetanus, and pertussis vaccines for the immunization of infants. Br Med J. 1969;1:663–666. doi: 10.1136/bmj.1.5645.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cam JM, Luck VA, Eastwood JB, De Wardener HE. The effect of aluminium hydroxide orally on calcium, phosphorus and aluminium in normal subjects. Clin Sci Mol Med. 1976;51:407–414. doi: 10.1042/cs0510407. [DOI] [PubMed] [Google Scholar]
- Campbell A. The potential role of aluminum in Alzheimer’s disease. Nephrol Dial Transplant. 2002;17:17–20. doi: 10.1093/ndt/17.suppl_2.17. [DOI] [PubMed] [Google Scholar]
- Campbell A. Inflammation, neurodegnerative diseases, and environmental exposures. Ann N Y Acad Sci. 2004;1035:117–132. doi: 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- Campbell A, Bondy SC. Aluminum induced oxidative events and its relation to inflammation: a role for the metal in Alzheimer’s disease. Cell Mol Biol. 2000;46:721–730. [PubMed] [Google Scholar]
- Campbell A, Prasad KN, Bondy SC. Aluminum-induced oxidative events in cell lines: glioma are more responsive than neuroblastoma. Free Radic Biol Med. 1999;26:1166–1171. doi: 10.1016/s0891-5849(98)00308-6. [DOI] [PubMed] [Google Scholar]
- Candy J, Klinowski J, Perry R, Perry E, Fairbain A, Oakley A, Carpenter T, Atack J, Blessed G, Edwardson J. Aluminosilicates and senile plaque formation in Alzheimer’s disease. Lancet. 1986;1:354–357. doi: 10.1016/s0140-6736(86)92319-6. [DOI] [PubMed] [Google Scholar]
- Cannata Andía JB. Aluminium toxicity: its relationship with bone and iron metabolism. Nephrol Dial Transplant. 1996;11(Suppl 3):69–73. doi: 10.1093/ndt/11.supp3.69. [DOI] [PubMed] [Google Scholar]
- Cannata Andía JB. Adynamic bone and chronic renal failure: an overview. Am J Med Sci. 2000;320:81–84. doi: 10.1097/00000441-200008000-00003. [DOI] [PubMed] [Google Scholar]
- Cannata JB, Suarez C, Cuesta V, Roza RR, Allende MT, Herrera J, Llanderal JP. Gastrointestinal aluminum absorption: is it modulated by the iron-absorptive mechanism? Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1984;21:354–359. [PubMed] [Google Scholar]
- Cannata JB, Diaz Lopez JB, Fernandez Menendez MJ, Virgos MJ. The parathyroid gland and aluminum overload: an overview. Contrib Nephrol. 1988;64:113–119. [PubMed] [Google Scholar]
- Cannata JB, Fernandez-Soto I, Fernandez-Menendez MJ, Fernandez-Martin JL, McGregor SJ, Brock JH, Halls D. Role of iron metabolism in absorption and cellular uptake of aluminum. Kidney Int. 1991;39:799–803. doi: 10.1038/ki.1991.98. [DOI] [PubMed] [Google Scholar]
- Cannata JB, Olaizola IR, Gomez-Alonso C, Menendez-Fraga P, Alonso-Suarez M, Diaz-Lopez JB. Serum aluminum transport and aluminum uptake in chronic renal failure: role of iron and aluminum metabolism. Nephron. 1993;65:141–146. doi: 10.1159/000187456. [DOI] [PubMed] [Google Scholar]
- Cannata-Andía JB, Fernadez-Martin JL. The clinical impact of aluminum overload in renal failure. Nephrol Dial Transplant. 2002;17(Suppl 2):9–12. doi: 10.1093/ndt/17.suppl_2.9. [DOI] [PubMed] [Google Scholar]
- Caramelo CA, Cannata JB, Rodeles MR, Fernandez Martin JL, Mosquera JR, Monzu B, Outeirino J, Blum J, Andrea C, Lopez Farre AJ, Acuna J, Casado S, Hernando L. Mechanisms of aluminum-induced microcytosis: lessons from accidental aluminum intoxication. Kidney Int. 1995;47:164–168. doi: 10.1038/ki.1995.19. [DOI] [PubMed] [Google Scholar]
- Cardillo P, Nebuloni M. Reactivity limits of aluminium and halohydrocarbon mixtures: determination by the ASTM CHETAH program. J Loss Prev Process Ind. 1992;5:81–88. [Google Scholar]
- Carlisle EM, Curran MJ. Effect of dietary silicon and aluminum on silicon and aluminum levels in rat brain. Alzheimer Dis Assoc Disord. 1987;1:83–89. doi: 10.1097/00002093-198701020-00003. [DOI] [PubMed] [Google Scholar]
- Caroli S, Alimonti A, Coni E, Petrucci F, Senofonte O, Violante N. The assessment of reference values for elements in human biological tissues and fluids: a systematic review. Crit Rev Anal Chem. 1994;24:363–398. [Google Scholar]
- Caruso JA, Klaue B, Michalke B, Rocke DM. Group assessment: elemental speciation. Ecotoxicol Environ Saf. 2003;56:32–44. doi: 10.1016/s0147-6513(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Caruso JA, Wuilloud RG, Altamirano JC, Harris WR. Modeling and separation-detection methods to evaluate the speciation of metals for toxicity assessment. J Toxicol Environ Health B Crit Rev. 2005;9:41–61. doi: 10.1080/15287390500196172. [DOI] [PubMed] [Google Scholar]
- Casarett MG, Casarett LJ, Hodge HC. An in vitro study of mast cell response to praticulate materials. Pharmacology. 1968;1:271–282. doi: 10.1159/000135975. [DOI] [PubMed] [Google Scholar]
- Castelain PY, Castelain M, Vervloet D, Garbe L, Mallet B. Sensitization to aluminium by aluminium precipitated dust and pollen extracts. Contact Dermatitis. 1988;19:58–60. doi: 10.1111/j.1600-0536.1988.tb02869.x. [DOI] [PubMed] [Google Scholar]
- Casto BC, Meyers J, DiPaolo JA. Enhancement of viral transformation for evaluation of the carcinogenic or mutagenic potential of inorganic metal salts. Cancer Res. 1979;39:193–198. [PubMed] [Google Scholar]
- Cech I, Montera J. Spatial variations in total aluminum concentrations in drinking water supplies studied by geographic information system (GIS) methods. Wat Res. 2000;34:2703–2712. [Google Scholar]
- Cefali EA, Nolan JC, McConnell WR, Walters DL. Pharmacokinetic study of zeolite A, sodium aluminosilicate, magnesium silicate, and aluminum hydroxide in dogs. Pharm Res. 1995;12:270–274. doi: 10.1023/a:1016291228957. [DOI] [PubMed] [Google Scholar]
- Cefali EA, Nolan JC, McConnell WR, Walters DL. Bioavailability of silicon and aluminum from Zeolite A in dogs. Int J Pharm. 1996;127:147–154. [Google Scholar]
- CFTA. Safety data of magnesium aluminum silicate. 1970 Unpublished data. [Google Scholar]
- Chafi AH, Hauw JJ, Rancurel G, Berry J-P, Galle C. Absence of aluminium in Alzheimer’s disease brain tissue: electron microprobe and ion microprobe studies. Neurosci Lett. 1991;123:61–64. doi: 10.1016/0304-3940(91)90158-p. [DOI] [PubMed] [Google Scholar]
- Chamberlain M, Davies R, Brown RC, Griffiths DM. In vitro tests for the pathogenicity of mineral dusts. Ann Occup Hyg. 1982;26:583–592. [PubMed] [Google Scholar]
- Chan WS, Marshall JF, Hart IR. Photodynamic therapy of a murine tumor following sensitisation with chloro aluminum sulfonated phthalocyanine. Photochem Photobiol. 1987;46:867–871. doi: 10.1111/j.1751-1097.1987.tb04861.x. [DOI] [PubMed] [Google Scholar]
- Chan YC, Simpson RW, Mctainsh GH, Vowles PD, Cohen DD, Bailey GM. Characterisation of chemical species in PM2.5 and PM10 aerosols in Brisbane, Australia. Atmos Environ. 1997;31:3773–3785. doi: 10.1016/s0048-9697(00)00571-4. [DOI] [PubMed] [Google Scholar]
- Chan YL, Alfrey AC, Posen S, Lissner D, Hills E, Dunstan CR, Evans RA. Effect of aluminum on normal and uremic rats: tissue distribution, vitamin D metabolites, and quantitative bone histology. Calcif Tissue Int. 1983;35:344–351. doi: 10.1007/BF02405056. [DOI] [PubMed] [Google Scholar]
- Chan-Yeung M, Wong R, MacLean L, Tan F, Dorken E, Schulzer M, Dennis R, Grzybowski S. Epidemiologic health study of workers in an aluminum smelter in British Columbia, Canada: Effects on respiratory system. Am Rev Respir Dis. 1983;127:465–469. doi: 10.1164/arrd.1983.127.4.465. [DOI] [PubMed] [Google Scholar]
- Channon SM, Arfeen S, Ward MK. Long-term accumulation of aluminium in patients with renal failure. Trace Elem Med. 1988;5:154–157. [Google Scholar]
- Chappuis P, Duhaux L, Paolaggi F, De Vernejoul MC, Rousselet F. Analytical problems encountered in determining aluminum status from hair in controls and hemodialyzed patients. Clin Chem. 1988;34:2253–2255. [PubMed] [Google Scholar]
- Chappuis P, de Vernejoul MC, Paolaggi F, Rousselet F. Relationship between hair, serum and bone aluminium in hemodialyzed patients. Clin Chim Acta. 1989;179:271–278. doi: 10.1016/0009-8981(89)90089-2. [DOI] [PubMed] [Google Scholar]
- Charlet P, Deloume JP, Duc G, Thomas-David G. Chelation of aluminum(3+) ions by succinic, aspartic, and glutamic acids and 1-histidine. Potentiometric study. Bull Soc Chimique France. 1984;N7:222–226. [Google Scholar]
- Chen C, Kalu DN. Strain differences in bone density and calcium metabolism between C3H/HeJ and C57BL/6J mice. Bone. 1999;25:413–420. doi: 10.1016/s8756-3282(99)00185-4. [DOI] [PubMed] [Google Scholar]
- Chen J, Huang C, Hu B, Jiang Z. Speciation of aluminum in drink samples by 8-hydroxyquinoline loaded silylanization silica gel microcolumn separation with off-line ICP-MS detection. J Agric Food Chem. 2004;52:6843–6847. doi: 10.1021/jf049576o. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Monnat RJ, Chen M, Mottet NK. Aluminum induced pulmonary granulomatosis. Hum Pathol. 1978;9:705–712. doi: 10.1016/s0046-8177(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Chernov VK, Lazareva V, Asmolov AI, Markova SB. Content of some trace elements in plasma and erythrocytes. Zdravookhranenie Kazakhstana. 1977;4:80–81. [Google Scholar]
- Chiba J, Kusumoto M, Shirai S, Ikawa K, Sakamoto S. The influence of fluoride ingestion on urinary aluminum excretion in humans. Tohoku J Exp Med. 2002;196:139–149. doi: 10.1620/tjem.196.139. [DOI] [PubMed] [Google Scholar]
- Chmielnicka J, Nasiadek M, Lewandowska-zyndul E, Pinkowski R. Effect of aluminum on hematopoiesis after intraperitoneal exposure in rats. Ecotoxicol Environ Saf. 1996;33:201–206. doi: 10.1006/eesa.1996.0026. [DOI] [PubMed] [Google Scholar]
- Cho SW, Joshi JG. Inactivation of glucose-6-phosphate dehydrogenase isozymes from human and pig brain by aluminum. J Neurochem. 1989;53:616–621. doi: 10.1111/j.1471-4159.1989.tb07378.x. [DOI] [PubMed] [Google Scholar]
- Chow HS, Chen Z, Matsuura GT. Direct transport of cocaine from the nasal cavity to the brain following intranasal cocaine administration in rats. J Pharm Sci. 1999;88:754–758. doi: 10.1021/js9900295. [DOI] [PubMed] [Google Scholar]
- Chow JC, Watson JG, Lu Z, Lowenthal DH, Frazier CA, Solomon PA, Thuillier RH, Magliano K. Descriptive analysis of PM(2.5) and PM(10) at regionally representative locations during SJVAQS/AUSPEX. Atmos Envrion. 1996;30:2079–2112. [Google Scholar]
- Christie H, Mackay RJ, Fisher AM. Pulmonary effects of inhalation of aluminum by rats and hamsters. Ind Hyg J. 1963;24:47–56. doi: 10.1080/00028896309342923. [DOI] [PubMed] [Google Scholar]
- Clark RA, Krueger GL. Aluminon: its limited application as a reagent for the detection of aluminum species. J Histochem Cytochem. 1985;33:729–732. doi: 10.1177/33.7.3891845. [DOI] [PubMed] [Google Scholar]
- Clarkson EM, Luck VA, Hynson WV, Bailey RR, Eastwood JR, Woodhead JS, Clemets VR, O’Riordan JL, De Wardener HE. The effect of aluminum hydroxide on calcium, phosphorus and aluminum balances, the serum parathyroid hormone concentration and the aluminum content of bone in patients with chronic renal failure. Clin Sci. 1972;43:519–531. doi: 10.1042/cs0430519. [DOI] [PubMed] [Google Scholar]
- Clements CJ, Griffiths E. The global impact of vaccines containing aluminium adjuvants. Vaccine. 2002;20(Suppl 3):S24–S33. doi: 10.1016/s0264-410x(02)00168-8. [DOI] [PubMed] [Google Scholar]
- Clemmensen O, Knudsen HE. Contact sensitivity to aluminium in a patient hyposensitized with aluminium precipitated grass pollen. Contact Dermatitis. 1980;6:305–308. doi: 10.1111/j.1600-0536.1980.tb04953.x. [DOI] [PubMed] [Google Scholar]
- Clonfero E, Cortese S, Saia B, Marcer G, Crepet M. Pulmonary pathology in a plant of electrolytic reduction of aluminum. Med Lav. 1978;69:613–619. [PubMed] [Google Scholar]
- Clonfero E, Caroldi S, Cortese M, Zambon P. Health problems in aluminum foundry workers of Porto Marghera [Italy]. Part II. Chronic bronchitis. Securitas. 1980a;64:60–62. [Google Scholar]
- Clonfero E, Caroldi S, Cortese M, Zambon P. Health problems in aluminum foundry workers of Porto Marghera [Italy]. Part III. Alumina-induced pneumoconiosis. Securitas. 1980b;64:63–66. [Google Scholar]
- Coburn JW, Mischel MG, Goodman WG, Salusky IB. Calcium citrate markedly enhances aluminum absoprtion from aluminum hydroxide. Am J Kidney Dis. 1991;17:708–711. doi: 10.1016/s0272-6386(12)80356-8. [DOI] [PubMed] [Google Scholar]
- Cochran M, Chawtur V. Interaction of horse-spleen ferritin with aluminium citrate. Clin Chim Acta. 1988;178:79–84. doi: 10.1016/0009-8981(88)90271-9. [DOI] [PubMed] [Google Scholar]
- Cochran M, Goddard G, Ludwigson N. Aluminum absorption by rat duodenum: further evidence of energy-dependent uptake. Toxicol Lett. 1990;51:287–294. doi: 10.1016/0378-4274(90)90071-s. [DOI] [PubMed] [Google Scholar]
- Cochran M, Chawtur V, Phillips JW, Dilena B. Effect of citrate infusion on urinary aluminium excretion in the rat. Clin Sci. 1994;86:223–226. doi: 10.1042/cs0860223. [DOI] [PubMed] [Google Scholar]
- Coggon D. Camelford revisited. Br Med J. 1991;303:1280–1281. doi: 10.1136/bmj.303.6813.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogne M, Ballet JJ, Schmitt C, Bizzini B. Total and IgE antibody levels following booster immunization with aluminium absorbed and nonabsorbed tetanus toxoid in humans. Ann Allergy. 1985;54:148–151. [PubMed] [Google Scholar]
- Cointry GR, Capozza RF, Negri AL, Ferretti JL. Biomechanical impact of aluminium accumulation on the pre and post yield behavior of rat cortical bone. J Bone Miner Metab. 2005;23:15–23. doi: 10.1007/s00774-004-0535-x. [DOI] [PubMed] [Google Scholar]
- Colomina M, Gomez TM, Domingo JL, Llobet M, Corbella J. Concurrent ingestion of lactate and aluminum can result in developmental toxicity in mice. Res Commun Chem Pathol Pharmacol. 1992;77:95–106. [PubMed] [Google Scholar]
- Colomina MT, Gómez M, Domingo JL, Llobet JM, Corbella J. Lack of maternal and developmental toxicity in mice given high doses of aluminium hydroxide and ascorbic acid during gestation. Pharmacol Toxicol. 1994;74:236–239. doi: 10.1111/j.1600-0773.1994.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Colomina MT, Esparza JL, Corbella J, Domingo JL. The effect of maternal stress on developmental toxicity of aluminum in mice. Neurotoxicol Teratol. 1998;20:651–656. doi: 10.1016/s0892-0362(98)00025-7. [DOI] [PubMed] [Google Scholar]
- Colomina MT, Sanchez DJ, Domingo JL, Sanchez-Turet M. Exposure of pregnant mice to aluminum and restraint stress: Effects on postnatal development and behaviour of the offspring. Psychobiol. 1999;27:521–529. [Google Scholar]
- Colomina MT, Roig JL, Torrente M, Vicens P, Domingo JL. Concurrent exposure to aluminum and stress during pregnancy in rats: Effects on postnatal development and behavior of the offspring. Neurotoxicol Teratol. 2005;27:565–574. doi: 10.1016/j.ntt.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Cominos D, Strutton G, Busmanis I. Granulomas associated with tetanus toxoid immunization. Am J Dermatopathol. 1993;15:114–117. doi: 10.1097/00000372-199304000-00003. [DOI] [PubMed] [Google Scholar]
- CFASD (Committee on Food Additives Survey Data) Poundage Update of Food Chemicals, 1982. Washington D.C.: National Academy Press; 1984. [Google Scholar]
- Comsa CD, Prestwich WV, McNeil FE, Byun SH. Application of spectral decomposition analysis to in vivo quantification of aluminum by neutron activation analysis. Appl Radiat Isot. 2004;61:1353–1360. doi: 10.1016/j.apradiso.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Coni E, Stacchini A, Caroli S, Falconieri P. Analytical approach to obtaining reference values for minor and trace elements in human milk. J Anal At Spectrom. 1990;5:581–586. [Google Scholar]
- Conway EL, O’Callaghan C, Drummer OH, Howes LG, Louis WG. A single-dose comparison of the bioavailability of aluminum from two formulations of sucralphate in normal volunteers. Biopharm Drug Dispos. 1994;15:253–261. doi: 10.1002/bdd.2510150307. [DOI] [PubMed] [Google Scholar]
- Corrin B. Aluminium pneumoconiosis. II. Effect on the rat lung of intratracheal injections of stamped aluminium powders containing different lubricating agents and of a granular aluminium powder. Br J Ind Med. 1963a;20:268–276. doi: 10.1136/oem.20.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrin B. Aluminium pneumoconiosis. I. In vitro comparison of stamped aluminium powders containing different lubricating agents and a granular aluminium powder. Br J Ind Med. 1963b;20:264–267. doi: 10.1136/oem.20.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosnes A, Flechet M-L, Revuz J. Inflammatory nodular reactions after hepatitis B vaccination due to aluminium sensitization. Contact Dermatitis. 1990;23:65–67. doi: 10.1111/j.1600-0536.1990.tb03224.x. [DOI] [PubMed] [Google Scholar]
- Costa M, Zhitkovich A, Gargas M, Paustenbach D, Finley B, Kuykendall J, Billings R, Carlson TJ, Wetterhahn K, Xu J, Pateirno S, Bogdanffy M. Interlaboratory validation of a new assay for DNA-protein crosslinks. Mutat Res. 1996;369:13–21. doi: 10.1016/s0165-1218(96)90043-9. [DOI] [PubMed] [Google Scholar]
- Costantini S, Giordano R, Ioppolo A, Mantovani A, Ballanti P, Mocetti P, Bonucci E. Distribution of aluminium following intraperitoneal injection of aluminium lactate in the rat. Pharmacol Toxicol. 1989;64:47–50. doi: 10.1111/j.1600-0773.1989.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. 4. New York: John Wiley & Sons, Inc; 1980. [Google Scholar]
- Council on Industrial Health. JAMA. 1946;130:1223. [Google Scholar]
- Cournot-Witmer G, Zinngraff J, Plachot JJ, Escaig F, Lefevre R, Boumati P, Bourdeau A, Garadedian M, Galle P, Bourdon R, Drüeke T, Balsan S. Aluminium localization in bone from hemodialyzed patients: Relationship to matrix mineralization. Kidney Int. 1981;20:375–378. doi: 10.1038/ki.1981.149. [DOI] [PubMed] [Google Scholar]
- Cox NH, Moss C, Forsyth A. Allergy to non-toxoid constituents of vaccines and implications for patch testing. Contact Dermatitis. 1988a;18:143–146. doi: 10.1111/j.1600-0536.1988.tb04500.x. [DOI] [PubMed] [Google Scholar]
- Cox NH, Moss C, Forsyth A. Cutaneous reactions to aluminium in vaccines: an avoidable problem. Lancet. 1988b;2:43. doi: 10.1016/s0140-6736(88)92971-6. [DOI] [PubMed] [Google Scholar]
- Cranmer JM, Wilkins JD, Cannon DJ, Smith L. Fetal-placental-maternal uptake of aluminum in mice following gestational exposure: Effect of dose and route of administration. Neurotoxicology. 1986;7:601–608. [PubMed] [Google Scholar]
- Crapper DR, Krishnan SS, Dalton AJ. Brain aluminum distribution in Alzheimer’s disease and experimental neurofibrillary degeneration. Science. 1973;180:511–513. doi: 10.1126/science.180.4085.511. [DOI] [PubMed] [Google Scholar]
- Crapper DR, Krishnan SS, Quittkat S. Aluminum, neurofibrillary degeneration and Alzheimer’s disease. Brain. 1976;99:67–80. doi: 10.1093/brain/99.1.67. [DOI] [PubMed] [Google Scholar]
- Crapper D, Quittkat S, Krishnan S, Dalton A, De Boni U. Intranuclear aluminum content in Alzheimer’s disease, dialysis encephalopathy and experimental aluminum encephalopathy. Acta Neuropathol. 1980;50:19–24. doi: 10.1007/BF00688530. [DOI] [PubMed] [Google Scholar]
- Crombie DW, Blaisdell JL, MacPherson G. The treatment of silicosis by aluminum powder. Can Med Assoc J. 1944;50:318–328. [PMC free article] [PubMed] [Google Scholar]
- Cronin RE, Henrich WL. UpToDate®. 2004 [online] https://store.utdol.com/app/index.asp.
- Crowther RS, Marriott C. Counter-ion binding to mucus glycoproteins. J Pharm Pharmacol. 1984;36:21–26. doi: 10.1111/j.2042-7158.1984.tb02980.x. [DOI] [PubMed] [Google Scholar]
- Csoti T, Gyori J, Salanki J, Erdelyi L. pH-dependent actions of aluminum on voltage-activated sodium currents in snail neurons. Neurotoxicology. 2001;22:109–116. doi: 10.1016/s0161-813x(00)00006-1. [DOI] [PubMed] [Google Scholar]
- Culora GA, Ramsay AD, Theaker JM. Aluminum and injection site reactions. J Clin Pathol. 1996;49:844–847. doi: 10.1136/jcp.49.10.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunat L, Lanhers MC, Joyeux M, Burnel D. Bioavailability and intestinal absorption of aluminum in rats: effects of aluminum compounds and some dietary constituents. Biol Trace Elem Res. 2000;76:31–55. doi: 10.1385/BTER:76:1:31. [DOI] [PubMed] [Google Scholar]
- Cunha-Melo JR, Gonzaga HM, Alzamora F, Freire-Maia L. Effects of purified scorpion toxin (tityustoxin) on gastric secretion in the rat. Toxicology. 1983;21:843–848. doi: 10.1016/0041-0101(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Cutrufo C, Caroli S, Delle FP, Ortolani E, Palazzesi S, Violante N, Zapponi GA, Loizzo A. Experimental aluminium encephalopathy: quantitative EEG analysis of aluminium bioavailability. J Neurol Neurosurg Psych. 1984;47:204–206. doi: 10.1136/jnnp.47.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrys J, Hochadel M, Gehring U, Hoek G, Diegmann V, Brunekreef B, Heinrich J. GIS-based estimation of exposure to particulate matter and NO2 in an urban area: stochastic versus dispersion modeling. Environ Health Perspect. 2005;113:987–992. doi: 10.1289/ehp.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabeka RW, McKenzie AD. Graphite-furnace atomic absorption spectrometric determination and survey of total aluminum, copper, manganese, molybdenum, and tin in infant formulas and evaporated milks. J Assoc Off Anal Chem Int. 1992;75:954–963. [Google Scholar]
- Dalbey W, Pulkowski C. Comparison of synthetic zeolite catalysts and alumina binders administered intratracheally to rats. J Toxicol Environ Health A. 2000;60:355–374. doi: 10.1080/00984100050030136. [DOI] [PubMed] [Google Scholar]
- Daniela B, Antonella D, Antonela L. Aluminum contamination in home parenteral nutrition patients. J Parenter Enteral Nutr. 2002;26:S30–S31. [Google Scholar]
- DaSilva JJ, Williams RJ. The Biological Chemistry of the Elements. Oxford: Clarenden Press; 1991. [Google Scholar]
- Dave KR, Syal AR, Katyare SS. Effect of long-term aluminum feeding on kinetics attributes of tissue cholinesterases. Brain Res Bull. 2002;58:225–233. doi: 10.1016/s0361-9230(02)00786-4. [DOI] [PubMed] [Google Scholar]
- Davenport A, Williams PS, Roberts NB, Bone JM. Sepsis: a cause of aluminum release from tissue stores associated with acute neurological dysfunction and mortality. Clin Nephrol. 1988;30:48–51. [PubMed] [Google Scholar]
- Davenport A, Davison AM, Will EJ, Toothill C, Newton KE, Giles GR. Aluminium accumulation and immunosuppression. BMJ. 1989;298:458–459. doi: 10.1136/bmj.298.6671.458-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. Cerebral dysfunction after water pollution incident in Camelford: Results were biased by self selection of cases. Br Med J. 2000;320:1337. [PMC free article] [PubMed] [Google Scholar]
- Davis WM. Is aluminum an etiologic contributor to alcoholic amnesia and dementia? Med Hypotheses. 1993;41:341–343. doi: 10.1016/0306-9877(93)90079-6. [DOI] [PubMed] [Google Scholar]
- Dawson EB, Ritter S, Harris WA, Evans DR, Powell LC. Comparison of sperm viability with seminal plasma metal levels. Biol Trace Elem Res. 1998;64:215–219. doi: 10.1007/BF02783337. [DOI] [PubMed] [Google Scholar]
- Dawson EB, Evans DR, Harris WA, Powell LC. Seminal plasma trace metal levels in industrial workers. Biol Trace Elem Res. 2000;74:97–105. doi: 10.1385/BTER:74:2:97. [DOI] [PubMed] [Google Scholar]
- Day JP, Barker J, Evans LJ, Perks J, Seabright PJ, Ackrill P, Lilley JS, Drumm PV, Newton GW. Aluminium absorption studied by 26Al tracer. Lancet. 1991;337:1345. doi: 10.1016/0140-6736(91)93016-3. [DOI] [PubMed] [Google Scholar]
- Day JP, Barker J, King SJ, Miller RV, Templar J, Lilley JS, Drumm PV, Newton GWA, Fifield LK, Stone JOH, Allan GL, Edwardson JA, Moore PB, Ferrier IN, Priest ND, Newton D, Talbot RJ, Brock JH, Sanchez L, Dobson CB, Itzhaki RF, Radunovic A, Bradbury MWB. Biological chemistry of aluminium studied using 26Al and accelerator mass spectrometry. Nucl Instrum Methods Phys Res. 1994;B29:463–468. [Google Scholar]
- Daydé S, Brumas V, Champmartin D, Rubini P, Berthon G. Aluminum speciation studies in biological fluids. Part 9. A quantitative investigation of aluminum(III)-glutamate complex equilibria and their potential implications for aluminum metabolism and toxicity. J Inorg Biochem. 2003;97:104–117. doi: 10.1016/s0162-0134(03)00244-7. [DOI] [PubMed] [Google Scholar]
- De la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- De Marchi J. Uber experimentelle aluminium-staubungen. Grenzgeb Einzeldarst. 1947;4:28. [Google Scholar]
- De Stasio G, Dunham D, Tonner BP, Mercanti D, Ciotti MT, Angelini A, Coluzza C, Perfetti P, Margaritondo G. Aluminum in rat cerebellar neural cultures. Neuroreport. 1993;4:1175–1178. [PubMed] [Google Scholar]
- De Stasio G, Mercanti D, Ciotti MT, Dunham D, Droubay TC, Tonner BP, Perfetti P, Margaritondo G. Aluminium in rat cerebellar primary cultures: glial cells and GABAergic neurones. Neuroreport. 1994;5:1973–1976. doi: 10.1097/00001756-199410000-00034. [DOI] [PubMed] [Google Scholar]
- De Vernejoul MC, Marchais S, London G, Morieux C, Bielakoff J, Miravet L. Increased bone aluminum deposition after subtotal parathyroidectomy in dialyzed patients. Kidney Int. 1985;27:785–791. doi: 10.1038/ki.1985.81. [DOI] [PubMed] [Google Scholar]
- De Vernejoul MC, Marchais S, London G, Bielakoff J, Chappuis P, Morieux C, Llach F. Deferoxamine test and bone disease in dialysis patients with mild aluminum accumulation. Am J Kidney Dis. 1989;14:124–130. doi: 10.1016/s0272-6386(89)80188-x. [DOI] [PubMed] [Google Scholar]
- De Vuyst P, Dumortier P, Rickaert F, Van De Weyer R, Lenclud C, Yernault JC. Occupational lung fibrosis in an aluminium polisher. Eur J Respir Dis. 1986;68:131–140. [PubMed] [Google Scholar]
- De Vuyst P, Dumortier P, Schandené L, Estenne M, Verhest A, Yernault JC. Sarcoid-like lung granulomatosis induced by aluminum dust. Am Rev Respir Dis. 1987;135:493–497. doi: 10.1164/arrd.1987.135.2.493. [DOI] [PubMed] [Google Scholar]
- Dedman DJ, Treffry A, Harrison PM. Interaction of aluminium citrate with horse spleen ferritin. Biochem J. 1992a;287:515–520. doi: 10.1042/bj2870515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedman DJ, Treffry A, Candy JM, Taylor GA, Morris CM, Bloxham CA, Perry MH, Edwardson JA. Iron and aluminum in relation to brain ferritin in normal individuals and Alzheimer’s-disease and chronic renal-dialysis patients. Biochem J. 1992b;287:509–514. doi: 10.1042/bj2870509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del’va VA. Changes in the concentration of aluminum of the blood and cerebrospinal fluid in some pathologic processes. Ref Zh Khim Biol Khim. 1962;19:193–195. [Google Scholar]
- Deloncle R, Guillard O, Huguet F, Clanet F. Modification of the blood-brain barrier through chronic intoxication by aluminum glutamate. Possible role in the etiology of Alzheimer’s disease. Biol Trace Elem Res. 1995;47:227–233. doi: 10.1007/BF02790121. [DOI] [PubMed] [Google Scholar]
- Deloncle R, Huguet F, Babin P, Fernandez B, Quellard N, Guillard O. Chronic administration of aluminum L-glutamate in young mature rats: effects on iron levels and lipid peroxidation in selected brain areas. Toxicol Lett. 1999;104:65–73. doi: 10.1016/s0378-4274(98)00345-2. [DOI] [PubMed] [Google Scholar]
- Deloncle R, Huguet F, Fernandez B, Quellard N, Babin P, Guillard O. Ultrastructural study of rat hippocampus after chronic administration of aluminum L-glutamate: an acceleration of the aging process. Exp Gerontol. 2001;36:231–244. doi: 10.1016/s0531-5565(00)00214-x. [DOI] [PubMed] [Google Scholar]
- Deloncle R, Fauconneau B, Piriou A, Huguet F, Guillard O. Aluminum L-glutamate complex in rat brain cortex: in vivo prevention of aluminum deposit by magnesium D-aspartate. Brain Res. 2002;946:247–252. doi: 10.1016/s0006-8993(02)02891-3. [DOI] [PubMed] [Google Scholar]
- Delves HT, Sieniawaska CE, Suchak B. Total and bioavailable aluminium in foods and beverages. Anal Proc (London) 1993;30:358–360. [Google Scholar]
- Deng ZY, Coudray C, Gouzoux L, Mazur A, Rayssiguier Y, Pepin D. Effect of oral aluminum citrate on blood level and short-term tissue distribution of aluminum in the rat. Biol Trace Elem Res. 1998;63:139–147. doi: 10.1007/BF02778873. [DOI] [PubMed] [Google Scholar]
- Deng Z, Coudray C, Gouzoux L, Mazur A, Rayssiguier Y, Pepin D. Effects of acute and chronic coingestion of AlCl3 with citrate or polyphenolic acids on tissue retention and distribution of aluminum in rats. Biol Trace Elem Res. 2000;76:245–256. doi: 10.1385/BTER:76:3:245. [DOI] [PubMed] [Google Scholar]
- Denny JJ, Robson WD, Irwin DA. The prevention of silicosis by metallic aluminum. Can Med Assoc J. 1937;37:1–11. [PMC free article] [PubMed] [Google Scholar]
- Denny JJ, Robson WD, Irwin DA. The prevention of silicosis by metallic aluminum II. Can Med Assoc J. 1939;40:213–228. [PMC free article] [PubMed] [Google Scholar]
- Dhir H, Roy AK, Sharma A. Relative efficiency of Phyllanthus emblica fruit extract and ascorbic acid in modifying lead and aluminum-induced sister-chromatid exchanges in mouse bone marrow. Environ Mol Mutagen. 1993;21:229–236. doi: 10.1002/em.2850210305. [DOI] [PubMed] [Google Scholar]
- Di Muzio A, Capasso M, Verrotti A, Trotta D, Lupo S, Pappalepore N, Manzoli C, Chiarelli F, Uncini A. Macrophagic myofasciitis: an infantile Italian case. Neuromuscul Disord. 2004;14:175–177. doi: 10.1016/j.nmd.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Di Paolo N, Masti A, Comparini IB, Garosi G, Di Paolo M, Centini F, Brardi S, Monaci G, Finato V. Uremia, dialysis and aluminium. Int J Artif Organs. 1997;20:547–552. [PubMed] [Google Scholar]
- Di Paolo JA, Casto BC. Quantitative studies of in vitro morphological transformation of Syrian hamster cells by inorganic metal salts. Cancer Res. 1979;39:1008–1013. [PubMed] [Google Scholar]
- Díaz-Corte C, Fernández-Martin JL, Barreto S, Gómez C, Fernández-Coto T, Braga S, Cannata JB. Effect of aluminium load on parathyroid hormone synthesis. Nephrol Dial Transplant. 2001;16:742–745. doi: 10.1093/ndt/16.4.742. [DOI] [PubMed] [Google Scholar]
- Dinman BD. Aluminium, alloys and compounds. In: Parmeggiani L, editor. Encyclopedia of Occupational Health and Safety. Geneva: International Labour Organisation; 1983. pp. 131–135. [Google Scholar]
- Dinman BD. Aluminum in the lung: the pyropowder conundrum. J Occup Med. 1987;29:869–876. [PubMed] [Google Scholar]
- Dinman BD. Alumina-related pulmonary disease. J Occup Med. 1988;30:328–335. [PubMed] [Google Scholar]
- Discher DP, Breitenstein BD. Prevalence of chronic pulmonary disease in aluminum potroom workers. J Occup Med. 1976;18:379–386. [Google Scholar]
- Divine KK, Lewis JL, Grant PG, Bench G. Quantitative particle-induced x-ray emission imaging of rat olfactory epithelium applied to the permeability of rat epithelium to inhaled aluminum. Chem Res Toxicol. 1999;12:575–581. doi: 10.1021/tx9900268. [DOI] [PubMed] [Google Scholar]
- Dlugaszek M, Fiejka MA, Graczyk A, Aleksandrowicz JC. Effects of various aluminium compounds given orally to mice on Al tissue distribution and tissue concentrations of essential elements. Pharmacol Toxicol. 2000;86:135–139. doi: 10.1034/j.1600-0773.2000.d01-25.x. [DOI] [PubMed] [Google Scholar]
- Dobson CB, Day JP, King SJ, Itzhaki RF. Location of aluminium and gallium in human neuroblastoma cells treated with metal-chelating agent complexes. Toxicol Appl Pharmacol. 1998;152:145–152. doi: 10.1006/taap.1998.8489. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An association between air pollution and motality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Doese M. Industrial medicine studies on injury to health caused by aluminium, in particular lung disease from aluminium dust. Arch Gewerbepathol. 1938;8:501–531. [Google Scholar]
- Doll R. Review: Alzheimer’s disease and environmental aluminium. Age Ageing. 1993;22:138–153. doi: 10.1093/ageing/22.2.138. [DOI] [PubMed] [Google Scholar]
- Domingo JL. Reproductive and developmental toxicity of aluminum: a review. Neurotoxicol Teratol. 1995;17:515–521. doi: 10.1016/0892-0362(95)00002-9. [DOI] [PubMed] [Google Scholar]
- Domingo JL. Adverse effects of aluminum-chelating compounds for clinical use. Adv Drug React Toxicol Rev. 1996;15:145–165. [PubMed] [Google Scholar]
- Domingo JL, Llobet JM, Gómez M, Tomás JM, Corbella J. Nutritional and toxicological effects of short-term ingestion of aluminum by the rat. Res Commun Chem Pathol Pharmacol. 1987;56:409–419. [PubMed] [Google Scholar]
- Domingo JL, Gómez M, Bosque MA, Corbella J. Lack of teratogenicity of aluminum hydroxide in rats. Life Sci. 1989;45:243–247. doi: 10.1016/0024-3205(89)90256-7. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Gómez M, Llobet JM, Corbella J. Influence of some dietary constituents on aluminum absorption and retention in rats. Kidney Intl. 1991a;39:598–601. doi: 10.1038/ki.1991.70. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Gómez M, Llobet JM, Richart C. Effect of ascorbic acid on gastrointestinal aluminium absorption. Lancet. 1991b;338:1467. doi: 10.1016/0140-6736(91)92776-x. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Gómez M, Sanchez DJ, Llobet JM, Corbella J. Effect of various dietary constituents on gastrointestinal absorption of aluminum from drinking water and diet. Res Commun Chem Pathol Pharmacol. 1993;79:377–380. [PubMed] [Google Scholar]
- Domingo JL, Gómez M, Llobet JM, del Castillo D, Corbella J. Influence of citric, ascorbic and lactic acids on the gastrointestinal absorption of aluminum in uremic rats. Nephron. 1994;66:108–109. doi: 10.1159/000187777. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Llorens J, Sanchez DJ, Gómez M, Llobet JM, Corbella J. Age-related effects of aluminum ingestion on brain aluminum accumulation and behavior in rats. Life Sci. 1996;58:1387–1395. doi: 10.1016/0024-3205(96)00108-7. [DOI] [PubMed] [Google Scholar]
- Donald JM, Golub MS, Gershwin ME, Keen CL. Neurobehavioral effects in offspring of mice given excess aluminum in diet during gestation and lactation. Neurotoxicol Teratol. 1989;11:345–351. doi: 10.1016/0892-0362(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. Olfactory transport: a direct route of delivery of inhaled manganese phosphate to the rat brain. J Toxicol Environ Health A. 2002;65:1493–1511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- Drabek O, Mladkova L, Boruvka L, Szakova J, Nikodem A, Nemecek K. Comparison of water-soluble and exchangeable forms of Al in acid forest soils. J Inorg Biochem. 2005;99:1788–1795. doi: 10.1016/j.jinorgbio.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Drabløs PA, Hetland S, Schmidt F, Thomassen Y. Uptake and Excretion of Aluminum in Workers Exposed to Aluminum Fluoride and Aluminum Oxide. Aluminum Association. Aluminum and Health, 2nd International Conference; Tampa, Florida. 1992. pp. 157–160. [Google Scholar]
- Drew RT, Gupta BN, Bend JR, Hook GE. Inhalation studies with a glycol complex of aluminum-chloride-hydroxide. Arch Environ Health. 1974;28:321–326. doi: 10.1080/00039896.1974.10666500. [DOI] [PubMed] [Google Scholar]
- Drewitt PN, Butterworth KR, Springall CD, Moorhouse SR. Plasma levels of aluminium after tea ingestion in healthy volunteers. Food Chem Toxicol. 1993;31:19–23. doi: 10.1016/0278-6915(93)90173-v. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Letterman RD. Chemistry and fate of aluminum (III) in treated drinking water. J Environ Eng. 1988;114:21–37. [Google Scholar]
- Driscoll CT, Letterman RD. Factors regulating residual aluminium concentrations in treated waters. Environmetrics. 1995;6:287–309. [Google Scholar]
- Driscoll CT, Schecher WD. Aqueous chemistry of aluminum. In: Gitelman HJ, editor. Aluminum and Health a Critical Review. New York: Marcel Dekker; 1989. pp. 27–65. [Google Scholar]
- Drüeke T. Hyporesponsiveness to recombinant human erythropoietin. Nephrol Dial Transplant. 2001;16:25–28. doi: 10.1093/ndt/16.suppl_7.25. [DOI] [PubMed] [Google Scholar]
- Drüeke TB. Intestinal absorption of aluminium in renal failure. Nephrol Dial Transplant. 2002;17(Suppl 2):13–16. doi: 10.1093/ndt/17.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- Drüeke T, Lacour B, Touam M, Basile C, Bourdon R. Oral aluminum administration to uremic, hyperparathyroid, or vitamin D-supplemented rats. Nephron. 1985;39:10–17. doi: 10.1159/000183329. [DOI] [PubMed] [Google Scholar]
- Drüeke TB, Jouhanneau P, Banide H, Lacour B, Yiou F, Raisbeck G. Effects of silicon, citrate and the fasting state on the intestinal absorption of aluminium in rats. Clin Sci. 1997;92:63–67. doi: 10.1042/cs0920063. [DOI] [PubMed] [Google Scholar]
- Dubois F, Bégin R, Cantin A, Massé S, Martel M, Bilodeau G, Dufresne A, Perreault G, Sébastien P. Aluminum inhalation reduces silicosis in a sheep model. Am Rev Respir Dis. 1988;137:1172–1179. doi: 10.1164/ajrccm/137.5.1172. [DOI] [PubMed] [Google Scholar]
- Duckett S, Galle P. Electron microscope-microprobe studies of aluminum in the brains of cases of Alzheimer’s disease and ageing patients. J Neuropathol Exp Neurol. 1980;39:350. [Google Scholar]
- Duffield JR, Edwards K, Evans DA, Morrish DM, Vobe A, Williams D. Low molecular mass aluminum complex speciation in biofluids. J Coord Chem. 1991;23:277–290. [Google Scholar]
- Dufresne A, Sébastien P, Michaud D, Perrault G, Bégin R. Influence of aluminum treatments on pulmonary retention of quartz in sheep silicosis. Exp Lung Res. 1994;20:157–168. doi: 10.3109/01902149409064380. [DOI] [PubMed] [Google Scholar]
- Durand M, Florkowski C, George P, Walmsley T, Weinstein P, Cole J. Elevated trace element output in urine following acute volcanic gas exposure. J Volcanol Geotherm Res. 2004;134:139–148. [Google Scholar]
- DuVal G, Brubb B, Bentley P. Aluminum accumulation in the crystalline lens of humans and domestic animals. Trace Elem Med. 1986;3:100–104. [Google Scholar]
- Dworski M. Prophylaxis and treatment of experimental silicosis by means of aluminum; an experimental study. Arch Ind Health. 1955;12:229–246. [PubMed] [Google Scholar]
- Dwyer CM, Kerr RE. Contact allergy to aluminum in 2 brothers. Contact Dermatitis. 1993;29:36–38. doi: 10.1111/j.1600-0536.1993.tb04534.x. [DOI] [PubMed] [Google Scholar]
- Eastwood JB, Levin GE, Pazianas M, Taylor AP, Denton J, Freemont AJ. Aluminum deposition in bone after contamination of drinking water supply. Lancet. 1990;336:462–464. doi: 10.1016/0140-6736(90)92012-7. [DOI] [PubMed] [Google Scholar]
- Ebina Y, Okada S, Hamazaki S, Midorikawa O. Liver, kidney, and central nervous system toxicity of aluminum given intraperitoneally to rats: a multiple-dose subchronic study using aluminum nitrilotriacetate. Toxicol Appl Pharmacol. 1984;72:211–218. doi: 10.1016/0041-008x(84)90203-5. [DOI] [PubMed] [Google Scholar]
- Ecelbarger CA, Greger JL. Dietary citrate and kidney function affect aluminum, zinc, and iron utilization in rats. J Nutr. 1991;121:1755–1762. doi: 10.1093/jn/121.11.1755. [DOI] [PubMed] [Google Scholar]
- Ecelbarger CA, MacNeil GG, Greger JL. Tissue aluminum accumulation and toxic consequences in rats chronically fed aluminum with and without citrate. J Agric Food Chem. 1994a;42:2220–2224. [Google Scholar]
- Ecelbarger CA, MacNeil GG, Greger JL. Aluminum retention by aged rats fed aluminum and treated with desferrioxamine. Toxicol Lett. 1994b;73:249–257. doi: 10.1016/0378-4274(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Edling NPG. Aluminum pneumoconiosis: A roentgendiagnostic study of five cases. Acta Radiol. 1961;56:170–178. [PubMed] [Google Scholar]
- Edling C, Jarvholm B, Andersson L, Axelson O. Mortality and cancer incidence among workers in an abrasive manufacturing industry. Br J Ind Med. 1987;44:57–59. doi: 10.1136/oem.44.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MS, Harrison MR, Halks-Miller M, Nakayama DK, Berger MS, Glick PL, Chinn DH. Kaolin-induced congenital hydrocephalus in utero in fetal lambs and rhesus monkeys. J Neurosurg. 1984;60:115–122. doi: 10.3171/jns.1984.60.1.0115. [DOI] [PubMed] [Google Scholar]
- Edwardson JA, Candy JM, Ince PG, McArthur FK, Morris CM, Oakley AE, Taylor GA, Bjertness E. Aluminium accumulation, beta-amyloid deposition and neurofibrillary changes in the central nervous system. In: Chadwick DJ, Whelan J, editors. Aluminium in Biology and Medicine. New York: John Wiley; 1992. pp. 165–179. discussion 179-185. [DOI] [PubMed] [Google Scholar]
- Edwardson JA, Moore PB, Ferrier IN, Lilley JS, Newton GW, Barker J, Templar J, Day JP. Effect of silicon on gastrointestinal absorption of aluminium. Lancet. 1993;342:211–212. doi: 10.1016/0140-6736(93)92301-9. [DOI] [PubMed] [Google Scholar]
- EEC (European Economic Union Council) EEC directive 67/548/EEC of 27 June 1967 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. 1967:1–98. [Google Scholar]
- Eickhoff TC, Myers M. Aluminum in vaccines. Vaccine. 2002;20:S1–S4. doi: 10.1016/s0264-410x(02)00163-9. [DOI] [PubMed] [Google Scholar]
- El-Rahman SS. Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment) Pharmacol Res. 2003;47:189–194. doi: 10.1016/s1043-6618(02)00336-5. [DOI] [PubMed] [Google Scholar]
- Elinder CG, Ahrengart L, Lidums V, Pettersson E, Sjögren B. Evidence of aluminium accumulation in aluminium welders. Br J Ind Med. 1991;48:735–738. doi: 10.1136/oem.48.11.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen G, Egmond E, Van Loon JW, Sahertian ET, Tolsma K. Dietary intakes of some essential and non-essential trace elements, nitrate, nitrite and N-nitrosamines, by Dutch adults: estimated via a 24-hour duplicate portion study. Food Addit Contam. 1990;7:207–221. doi: 10.1080/02652039009373885. [DOI] [PubMed] [Google Scholar]
- Ellis H, Scurr JH. Axillary hyperhidrosis - topical treatment with aluminum chloride hexahydrate. Postgrad Med J. 1979;55:868–869. doi: 10.1136/pgmj.55.650.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HA, McCarthy JH, Herrington J. Bone aluminium in haemodialysed patients and in rats injected with aluminium chloride: relationship to impaired bone mineralisation. J Clin Pathol. 1979;32:832–844. doi: 10.1136/jcp.32.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KJ, Keleher S, Raciti A, Savory J, Wills M. In vivo monitoring of skeletal aluminum burden in patients with renal failure. J Radioanal Nucl Chem. 1988;124:85–95. [Google Scholar]
- Elmore AR. Cosmetic ingredient review expert panel. Final report on the safety assessment of aluminum silicate, calcium silicate, magnesium aluminum silicate, magnesium silicate, magnesium trisilicate, sodium magnesium aluminum silicate, zirconium silicate, attapulgite, bentonite, Fuller’s earth, hectorite, kaolin, lithium magnesium silicate, lithium magnesium sodium silicate, montmorillonite, pyrophyllite, and zeolite. Int J Toxicol. 2003;22(Suppl 1):S37–S102. [PubMed] [Google Scholar]
- Elorriaga R, Fernández Martin JL, Menéndez Fraga PM, Naves ML, Braga S, Cannata JB. Aluminium removal: short and long-term preliminary results with L1 in rats. Drugs Today. 1992;28:177–182. [Google Scholar]
- Emmett M. A comparison of clinically useful phosphorus binders for patients with chronic kidney failure. Kidney Int Suppl. 2004;90:S25–S32. doi: 10.1111/j.1523-1755.2004.09005.x. [DOI] [PubMed] [Google Scholar]
- Eng PJ, Trainor TP, Brown GE, Waychunas GA, Newville M, Sutton SR. Structure of the hydrated α–Al2O3 (0001) surface. Science. 2000;288:1029–1033. doi: 10.1126/science.288.5468.1029. [DOI] [PubMed] [Google Scholar]
- Englert N. Fine particles and human health-a review of epidemiological studies. Toxicol Lett. 2004;149:235–242. doi: 10.1016/j.toxlet.2003.12.035. [DOI] [PubMed] [Google Scholar]
- EPA (Environmental Protection Agency) U.S. Environmental Protection Agency. Code of Federal Regulations. 1979 40 CFR 143.3. [Google Scholar]
- EPA (Environmental Protection Agency) National ambient air quality standards for particulate matter; final rule. Fed Reg. 1997;62:38651. [Google Scholar]
- Epstein SG. A summary of findings from twenty years of molten metal incident reporting. Light Metals 2005 2005 [Google Scholar]
- Erdohazi M, Newman RL. Aluminium hydroxide granuloma. Br Med J. 1971;3:621–623. doi: 10.1136/bmj.3.5775.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschbach JW, Varma A, Stivelman JC. Is it time for a paradigm shift? Is erythropoietin deficiency still the main cause of renal anaemia? Nephrol Dial Transplant. 2002;17(Suppl 5):2–7. doi: 10.1093/ndt/17.suppl_5.2. [DOI] [PubMed] [Google Scholar]
- ESIS (European Chemical Substances Information System) 2007 [online]. Cited 24 January 2007. http://ecb.jrc.it/esis.
- Esmonde TF. Cerebral dysfunction after a water pollution incident in Camelford: Study has several methodological errors. Br Med J. 2000;320:1337–1338. [PubMed] [Google Scholar]
- Esparza JL, Gómez M, Romeu M, Mulero M, Sanchez DJ, Mallol J, Domingo JL. Aluminum-induced pro-oxidant effects in rats: protective role of exogenous melatonin. J Pineal Res. 2003;35:32–39. doi: 10.1034/j.1600-079x.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Ess S, Steinegger A, Ess H, Schlatter C. Experimental study on the fibrogenic properties of different types of alumina. Am Ind Hyg Assoc J. 1993;45:360–370. doi: 10.1080/15298669391354810. [DOI] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst WJ. Localization of aluminum in the maize root apex: can morin detect cell wall-bound aluminum? J Exp Bot. 2005;56:1351–1357. doi: 10.1093/jxb/eri136. [DOI] [PubMed] [Google Scholar]
- European Commission. Technical guidance document on risk assessment. 2003 [online] Cited 24 January 2007 http://ecb.jrc.it/tgd/
- Evans SM, Zeit W. Tissue responses to physical forces. II. The response of connective tissue to piezoelectrically active crystals. J Lab Clin Med. 1949;34:592–609. [PubMed] [Google Scholar]
- Evans WJ, Martin CJ. Heat of complex formation of Al(III) and Cd(II) with phytic acid. IX. J Inorg Biochem. 1988;34:11–18. doi: 10.1016/0162-0134(88)85013-x. [DOI] [PubMed] [Google Scholar]
- Exley C. Does antiperspirant use increase the risk of aluminium-related disease, including Alzheimer’s disease? Molec Med Today. 1998;4:107–109. doi: 10.1016/s1357-4310(98)01209-x. [DOI] [PubMed] [Google Scholar]
- Exley C. A molecular mechanism of aluminum-induced Alzheimer’s disease? J Inorg Biochem. 1999;76:133–140. doi: 10.1016/s0162-0134(99)00125-7. [DOI] [PubMed] [Google Scholar]
- Exley C. Aluminum in antiperspirants: more than just skin deep. Am J Med. 2004;117:969–970. doi: 10.1016/j.amjmed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Exley C. The aluminium-amyloid cascade hypothesis and Alzheimer’s disease. Subcell Biochem. 2005;38:225–234. doi: 10.1007/0-387-23226-5_11. [DOI] [PubMed] [Google Scholar]
- Exley C, Birchall JD. Aluminium and Alzheimer’s disease. Age Ageing. 1993;22:391–392. doi: 10.1093/ageing/22.5.391-a. [DOI] [PubMed] [Google Scholar]
- Fairweather-Tait SJ, Piper Z, Fatemi SJ, Moore GR. The effect of tea on iron and aluminium metabolism in the rat. Br J Nutr. 1991;65:61–68. doi: 10.1079/bjn19910066. [DOI] [PubMed] [Google Scholar]
- Fairweather-Tait S, Hickson K, McGaw B, Reid M. Orange juice enhances aluminum absorption from antacid preparations. Eur J Clin Nutr. 1994;48:71–73. [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization/World Health Organization) Aluminium. Evaluation of Certain Food Additives and Contaminants; Thirty-third report of the Joint FAO/WHO Expert Committee on Food Additives; Geneva: World Health Organization; 1989. pp. 28–31. WHO Technical Report Series No. 776. [Google Scholar]
- FAO/WHO (Food and Agriculture Organization/World Health Organization) Summary and conclusions of the sixty-seventh meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) 2006 [online] Cited 24 January 2007 http://www.who.int/ipcs/food/jecfa/summaries/summary67.pdf.
- Farina M, Lara FS, Brandao R, Jacques R, Rocha JB. Effects of aluminum sulfate on erythropoiesis in rats. Toxicol Lett. 2002;132:131–139. doi: 10.1016/s0378-4274(02)00077-2. [DOI] [PubMed] [Google Scholar]
- Farina M, Rotta LN, Soares FA, Jardim F, Jacques R, Souza DO, Rocha JB. Hematological changes in rats chronically exposed to oral aluminum. Toxicology. 2005;209:29–37. doi: 10.1016/j.tox.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Farrar G, Morton AP, Blair JA. The intestinal absorption and tissue distribution of aluminium, gallium and scandium: a comparative study. Biochem Soc Trans. 1987;15:1164–1165. [Google Scholar]
- Farrar G, Altmann P, Welch S, Wychrij O, Ghose B, Lejeune J, Corbett J, Prasher V, Blair JA. Defective gallium-transferrin binding in Alzheimer’s disease and Down’s syndrome: possible mechanism for accumulation of aluminum in brain. Lancet. 1990;335:747–750. doi: 10.1016/0140-6736(90)90868-6. [DOI] [PubMed] [Google Scholar]
- FASEB (Federation of American Societies for Experimental Biology) Evaluation of the Health Aspects of Aluminum Compounds as Food Ingredients. NTIS Report No-262 665. Springfield VA: 1975. p. 26. National Technical Information Service Sponsored by the US Food and Drug Administration. US FDA Report FDA/BF-77/24. [Google Scholar]
- Fattoretti P, Bertoni-Freddari C, Balietti M, Giorgetti B, Solazzi M, Zatta P. Chronic aluminum administration to old rats results in increased levels of brain metal ions and enlarged hippocampal mossy fibers. Ann N Y Acad Sci. 2004;1019:44–47. doi: 10.1196/annals.1297.010. [DOI] [PubMed] [Google Scholar]
- Favarato M, Zanoni S, Zatta PF. Unambiguous aluminum(III) localization in senile plaque core from Alzheimer’s disease brains. Neurobiol Aging. 1992;13(Suppl 1):S40. [Google Scholar]
- Favaro RM, Silva HC, Vannucchi H. Bioavailability of vitamin A in the rat following ingestion of neomycin sulfate or aluminium hydroxide. Int J Vit Nutr Res. 1994;64:98–103. [PubMed] [Google Scholar]
- Fawcett HA, Smith NP. Injection-site granuloma due to aluminum. Arch Dermatol. 1984;120:1318–1322. [PubMed] [Google Scholar]
- Fawcett HA, McGibbon D, Cronin E. Persistent vaccination granuloma due to aluminium hypersensitivity. Br J Dermatol. 1985;113(Suppl 29):101–102. [Google Scholar]
- Federal-Provincial-Territorial Committee on Drinking Water. Summary of guidelines for Canadian drinking water quality. 2006 [online] Cited 24 January 2007 www.healthcanada.gc.ca/waterquality.
- Feinroth M, Feinroth MV, Berlyne GM. Aluminum absorption in the rat everted gut sac. Miner Electrolyte Metab. 1982;8:29–35. [PubMed] [Google Scholar]
- Feldman GV. Pertussin antibody response after triple antigen. Arch Dis Child. 1957;32:111–113. doi: 10.1136/adc.32.162.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng TL, Gurian PL, Healy MD, Barron AR. Aluminum citrate: isolation and structural characterization of a stable trinuclear complex. Inorg Chem. 1990;29:408–411. [Google Scholar]
- Fenwick S, Roberts EA, Mahesh BS, Roberts NB. In end-stage renal failure, does infection lead to elevated plasma aluminium and neurotoxicity? Implications for monitoring. Ann Clin Biochem. 2005;42:149–152. doi: 10.1258/0004563053492757. [DOI] [PubMed] [Google Scholar]
- Fernández F, Bernaola G, Muñoz DG, Fernandez de Corres L. Nodulos subcutáneous por sensibilización a aluminio en pacientes tratados mediante inmunoterapia con extratos semidepot. Dermititis Contacto. 1990;16:57–60. [Google Scholar]
- Fernández I, Fernández JL, Rodriquez R, Sanz-Medel A, Cannata JB. The influence of iron stores on aluminum gastrointestinal absorption. Rev Esp Fisiol. 1989;45:33–39. [PubMed] [Google Scholar]
- Fernández-Martin JL, Canteros A, Alles A, Massari P, Cannata-Andia J. Aluminum exposure in chronic renal failure in iberoamerica at the end of the 1990s: overview and perspectives. Am J Med Sci. 2000;320:96–99. doi: 10.1097/00000441-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Fernandez Menendez MJ, Fell GS, Brock JH, Cannata JB. Aluminium uptake by intestinal cells: effect of iron status and precomplexation. Nephrol Dial Transplant. 1991;6:672–674. doi: 10.1093/ndt/6.9.672. [DOI] [PubMed] [Google Scholar]
- Fiejka M, Aleksandrowicz J. Aluminum as an adjuvant in vaccines and post-vaccine reactions. Rocz Panstw Zakl Hig. 1993;44:73–80. [PubMed] [Google Scholar]
- Fiejka M, Fiejka E, Dlugaszek M. Effect of aluminum hydroxide administration on normal mice: tissue distribution and ultrastructural localization of aluminum in liver. Pharmacol Toxicol. 1996;78:123–128. doi: 10.1111/j.1600-0773.1996.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Field GB. Pulmonary function in aluminum smelters. Thorax. 1984;39:743–751. doi: 10.1136/thx.39.10.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifield LK, Day JP, Oldham C, Carling RS. Department of Nuclear Physics, Annual Report. Canberra: Australian National University; 1997. Study of the kinetics of aluminium absorption and excretion in humans; pp. 97–101. [Google Scholar]
- Filov VA. Vrednie Chemichescie Veshestva Neorganicheskie Soedinenia Elementov I-IV Groopp [Hazardous Substances Inorganic Substances Containing I-IV Group Elements] Leningrad: Chemistry; 1988. [Google Scholar]
- Fimreite N, Hansen OO, Pettersen HC. Aluminum concentrations in selected foods prepared in aluminum cookware, and its implications for human health. Bull Environ Contam Toxicol. 1997;58:1–7. doi: 10.1007/s001289900292. [DOI] [PubMed] [Google Scholar]
- Findlow JA, Duffield JR, Williams DR. The chemical speciation of aluminum in milk. Chem Speciat Bioavail. 1990;2:3–32. [Google Scholar]
- Finelli VN, Que Hee SS, Niemeier RW. Influence of exposure to aluminum chloride and fluoride dusts on some biochemical and physiological parameters in rats. In: Brown SS, Davies DS, editors. Organ-Directed Toxicity Chemical Indices and Mechanisms. New York: Pergamon Press; 1981. pp. 291–295. [Google Scholar]
- Fink D, Walton J, Hotchkis MA, Jacobsen GE, Lawson EM, Smith AM, Tuniz C, Wilcox D. First 26Al analyses at the ANATARES AMS Centre: uptake via oral ingestion of 26Al in rats. Nucl Instr Meth Phys Res Sect B. 1994;92:473–477. [Google Scholar]
- Firling CE, Hill TA, Severson AR. Aluminum toxicity perturbs long bone calcification in the embryonic chick. Arch Toxicol. 1999;73:359–366. doi: 10.1007/s002040050674. [DOI] [PubMed] [Google Scholar]
- Fischer T, Rystedt I. A case of contact sensitivity to aluminium. Contact Dermatitis. 1982;8:343. doi: 10.1111/j.1600-0536.1982.tb04247.x. [DOI] [PubMed] [Google Scholar]
- Fisher AA. Reactions to aluminum and its salts. Cutis. 1984;33:154–159. [PubMed] [Google Scholar]
- Flarend RE, Hem SL, White JL, Elmore D, Suckow MA, Rudy AC, Dandashli EA. In vivo absorption of aluminum-containing vaccine adjuvants using 26Al. Vaccine. 1997;15:1314–1318. doi: 10.1016/s0264-410x(97)00041-8. [DOI] [PubMed] [Google Scholar]
- Flarend R, Bin T, Elmore D, Hem SL. A preliminary study of the dermal absorption of aluminum from antiperspirants using aluminum-26. Food Chem Toxicol. 2001;39:163–168. doi: 10.1016/s0278-6915(00)00118-6. [DOI] [PubMed] [Google Scholar]
- Flaten TP. Geographical associations between aluminium in drinking water and death rates with dementia (including Alzheimer’s disease), Parkinson’s disease and amyotrophic lateral sclerosis in Norway. Environ Geochem Health. 1990;12:152–167. doi: 10.1007/BF01734064. [DOI] [PubMed] [Google Scholar]
- Flaten TP. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull. 2001;55:187–196. doi: 10.1016/s0361-9230(01)00459-2. [DOI] [PubMed] [Google Scholar]
- Flaten TP, Odegard M. Tea, aluminium and Alzheimer’s disease. Food Chem Toxicol. 1988;26:959–960. doi: 10.1016/0278-6915(88)90095-6. [DOI] [PubMed] [Google Scholar]
- Flaten TP, Glattre E, Viste A, Sooreide O. Mortality from dementia among gastroduodenal ulcer patients. J Epidemiol Community Health. 1991;45:203–206. doi: 10.1136/jech.45.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J, Joshi JG. Ferritin: Isolation of aluminum-ferritin complex from brain. Proc Natl Acad Sci. 1987;84:7866–7870. doi: 10.1073/pnas.84.22.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora SJ, Dhawan M, Tandon SK. Effects of combined exposure to aluminium and ethanol on aluminium body burden and some neuronal, hepatic and haematopoietic biochemical variables in the rat. Hum Exp Toxicol. 1991;10:45–48. doi: 10.1177/096032719101000108. [DOI] [PubMed] [Google Scholar]
- Flora SJ, Mehta A, Satsangi K, Kannan GM, Gupta M. Aluminum-induced oxidative stress in rat brain: response to combined administration of citric acid and HEDTA. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:319–328. doi: 10.1016/s1532-0456(02)00269-7. [DOI] [PubMed] [Google Scholar]
- Florence AL, Gauthier A, Ponsar C, Van den Bosch de Aguilar P, Crichton RR. An experimental animal model of aluminium overload. Neurodegeneration. 1994;3:315–323. [PubMed] [Google Scholar]
- Foley CM, Polinsky MS, Gruskin AB, Baluarte HJ, Grover WD. Encephalopathy in infants and children with chronic renal disease. Arch Neurol. 1981;38:656–658. doi: 10.1001/archneur.1981.00510100084016. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forbes WF, Agwani N. Geochemical risk factors for mental functioning, based on the Ontario, Longitudinal study of aging (LSA). III. The effects of different aluminum-containing compounds. Can J Aging. 1994a;13:488–498. [Google Scholar]
- Forbes WF, Agwani N. A suggested mechanism for aluminum biotoxicity. J Theor Biol. 1994b;171:207–214. doi: 10.1006/jtbi.1994.1224. [DOI] [PubMed] [Google Scholar]
- Forbes WF, Gentleman JF. Risk factors, causality, and policy initiatives: the case of aluminum and mental impairment. Exp Gerontol. 1998;33:141–154. doi: 10.1016/s0531-5565(97)00061-2. [DOI] [PubMed] [Google Scholar]
- Forbes WF, Hayward LM, Agwani N. Dementia, aluminum, and fluoride. Lancet. 1991;338:1592–1593. doi: 10.1016/0140-6736(91)92411-t. [DOI] [PubMed] [Google Scholar]
- Forbes WF, Hayward LM, Agwani N. Geochemical risk factors for mental functioning, based on the Ontario Longitudial Study of Aging (LAS). I. Results from a preliminary investigation. Can J Aging. 1992;11:269–280. [Google Scholar]
- Forbes WF, McAiney CA, Hayward LM, Agwani N. Geochemical risk factors for mental functioning, based on the Ontario longitudinal study of aging (LSA): II. The role of pH. Can J Aging. 1994;13:249–267. [Google Scholar]
- Forbes WF, Agwani N, Lachmaniuk P. Geochemical risk factors for mental functioning, based on the Ontario Longitudinal Study of Aging (LAS) IV. The role of silicon-containing compounds. Can J Aging. 1995;14:630–641. [Google Scholar]
- Forster DP, Newens AJ, Kay DW, Edwardson JA. Risk factors in clinically diagnosed presenile dementia of the Alzheimer type: a case-control study in northern England. J Epidemiol Commun Health. 1995;49:253–258. doi: 10.1136/jech.49.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosmire GJ, Focht SJ, McClearn GE. Genetic influences on tissue deposition of aluminum in mice. Biol Trace Elem Res. 1993;37:115–121. doi: 10.1007/BF02783787. [DOI] [PubMed] [Google Scholar]
- Fox JL, Lamson ML. RSTRIP: Pharmacokinetic Data Stripping/Least Squares Parameter Optimization. Salt Lake City: MicroMath, Inc; 1989. [Google Scholar]
- Frash VN, Vanchugova NN, Rukoleeva SN, Zykova VA, Grebennikov SA, Shcherbakov SV. Carcinogenic effects of some nonfibrous mineral dusts. Byull Eksp Biol Med. 1992;114:648–651. [PubMed] [Google Scholar]
- Frecker MF. Dementia in Newfoundland: Identification of a geographical isolate? J Epidemiol Comm Health. 1991;45:307–311. doi: 10.1136/jech.45.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French P, Gardner MJ, Gunn AM. Dietary aluminium and Alzheimer’s disease. Food Chem Toxicol. 1989;27:495–498. doi: 10.1016/0278-6915(89)90040-9. [DOI] [PubMed] [Google Scholar]
- Freour P, Germouty J, Jault D, Plancel F, Warin JF. A case of pneumoconiosis due to aluminum dust. La Presse Medicale. 1966;74:2547–2549. [PubMed] [Google Scholar]
- Fritschi L, Beach J, Sim M, Abramson M, Benke G, Musk AW, de Klerk N, McNeil J. Respiratory symptoms and lung function in two prebake aluminum smelters. Am J Ind Med. 1999;35:491–498. doi: 10.1002/(sici)1097-0274(199905)35:5<491::aid-ajim6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Fritschi L, Sim MR, Forbes A, Abramson MJ, Benke G, Musk AW, de Klerk NH. Respiratory symptoms and lung-function changes with exposure to five substances in aluminium smelters. Int Arch Occup Environ Health. 2003;76:103–110. doi: 10.1007/s00420-002-0398-1. [DOI] [PubMed] [Google Scholar]
- Froment DH, Buddington B, Miller NL, Alfrey AC. Effect of solubility on the gastrointestinal absorption of aluminum from various aluminum compounds in the rat. J Lab Clin Med. 1989a;114:237–242. [PubMed] [Google Scholar]
- Froment DP, Molitoris BA, Buddington B, Miller N, Alfrey AC. Site and mechanism of enhanced gastrointestinal absorption of aluminum by citrate. Kidney Int. 1989b;36:978–984. doi: 10.1038/ki.1989.290. [DOI] [PubMed] [Google Scholar]
- Frost L, Johansen P, Pedersen S, Veien N, Aabel Östergaard P, Nielsen MH. Persistent subcutaneous nodules in children hyposensitized with aluminium-containing allergen extracts. Allergy. 1985;40:368–372. doi: 10.1111/j.1398-9995.1985.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Frostad AW. Fluorine intoxication in Norwegian aluminium plant workers. Tidskr Nor Laegenforen. 1936;56:179–182. [Google Scholar]
- Fujimaki H, Ozawa MT, Kubota K, Watanabe N. Adjuvant effects of aluminum silicate on IgE and IgG1 antibody production in mice. Int Arch Allergy Appl Immunol. 1984;75:351–356. doi: 10.1159/000233646. [DOI] [PubMed] [Google Scholar]
- Fujimaki H, Ozawa M, Ashikawa T, Kubota K, Watanabe N. Induction of IgE antibody production to aerosolized ovalbumin in mice treated intratracheally with aluminum silicate. Int Arch Allergy Appl Immunol. 1986;79:206–210. doi: 10.1159/000233972. [DOI] [PubMed] [Google Scholar]
- Fulton B, Jeffery EH. Absorption and retention of aluminum from drinking water.1. Effect of citric and ascorbic acids on aluminum tissue levels in rabbits. Fundam Appl Toxicol. 1990;14:788–796. doi: 10.1016/0272-0590(90)90303-2. [DOI] [PubMed] [Google Scholar]
- Fulton B, Jaw S, Jeffery EH. Bioavailability of aluminum from drinking water. Fundam Appl Toxicol. 1989;12:144–150. doi: 10.1016/0272-0590(89)90069-9. [DOI] [PubMed] [Google Scholar]
- Furst A. Trace elements related to specific chronic diseases. Cancer Geol Soc Am Mem. 1971;123:109–114. [Google Scholar]
- Furst A, Haro RT. A survey of metal carcinogenesis. Prog Exp Tumor Res. 1969;12:102–133. [PubMed] [Google Scholar]
- Gaafar SM, Turk JL. Granuloma formation in lymph-nodes. J Pathol. 1970;100:9–20. doi: 10.1002/path.1711000103. [DOI] [PubMed] [Google Scholar]
- Gaffuri E, Donna A, Pietra R, Sabbioni E. Pulmonary changes and aluminum levels following inhalation of alumina dust: A study on four exposed workers. Med Lav. 1985;76:222–227. [PubMed] [Google Scholar]
- Gafter U, Mamet R, Korzets A, Malachi T, Schoenfeld N. Bullous dermatosis of end-stage renal disease: a possible association between abnormal porphyrin metabolism and aluminium. Nephrol Dial Transplant. 1996;11:1787–1791. [PubMed] [Google Scholar]
- Galceran T, Finch J, Bergfeld M, Coburn JW, Martin K, Teitelbaum S, Slatopolsky E. Biological effects of aluminum on normal dogs: studies on the isolated perfused bone. Endocrinology. 1987;121:406–413. doi: 10.1210/endo-121-1-406. [DOI] [PubMed] [Google Scholar]
- Gallon C, Munger C, Permont S, Campbell PG. Hydroponic study of aluminum accumulation by aquatic plans: effects of fluoride and pH. Water Air Soil Poll. 2004;153:135–155. [Google Scholar]
- Ganrot PO. Metabolism and possible health effects of aluminum. Environ Health Perspect. 1986;65:363–441. doi: 10.1289/ehp.8665363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbossa G, Galvez G, Castro ME, Nesse A. Oral aluminum administration to rats with normal renal function. 1. Impairment of erythropoiesis. Hum Exp Toxicol. 1998a;17:312–317. doi: 10.1177/096032719801700605. [DOI] [PubMed] [Google Scholar]
- Garbossa G, Galvez G, Perez G, Stripeikis J, Tudino M, Nesse A. Oral aluminum administration to rats with normal renal function. 2. Body distribution. Hum Exp Toxicol. 1998b;17:318–322. doi: 10.1177/096032719801700606. [DOI] [PubMed] [Google Scholar]
- Garcia-Patos V, Pujol RM, Alomar A, Cistero A, Curell R, Fernandez-Figueras MT, de Moragas JM. Persistent subcutaneous nodules in patients hyposensitized with aluminum-containing allergen extracts. Arch Dermatol. 1995;131:1421–1424. [PubMed] [Google Scholar]
- Gardner LU, Dworski M, Delahant AB. Aluminum therapy in silicosis: An experimental study. J Ind Hyg Toxicol. 1944;26:211–223. [Google Scholar]
- Gardner MJ, Gunn AM. Bioavailability of aluminium from food and drinking water. Alzheimer’s Disease and the Environment; Proceedings of the conference, Royal Society of Medicine, Round Table Series No 26, ed Lord Walton of Detchant; London UK. 1991. pp. 78–86. [Google Scholar]
- Gardner MJ, Gunn AM. Speciation and bioavailability of aluminum in drinking waer. Chem Speciat Bioavail. 1995;7:9–16. [Google Scholar]
- Garrett P. Aluminium accumulation and immunosuppression. BMJ. 1989;298:755. doi: 10.1136/bmj.298.6675.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garruto RM. Pacific paradigms of environmentally-induced neurological disorders: clinical, epidemiological and molecular perspectives. Neurotoxicology. 1991;12:347–377. [PubMed] [Google Scholar]
- Garruto RM, Yase Y. Neurodegenerative disorders of the Western Pacific: the search for mechanisms of pathogenesis. Trends Neurosci. 1986;9:368–374. [Google Scholar]
- Garruto RM, Fukatsu R, Yanagihara R, Gajdusek DC, Hook G, Fiori CE. Imaging of calcium and aluminum in neurofibrillary tangle-bearing neurons in parkinsonism-dementia of Guam. Proc Natl Acad Sci. 1984;81:1875–1879. doi: 10.1073/pnas.81.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garruto RM, Shankar SK, Yanagihara R, Salazar AM, Amyx HL, Gajdusek DC. Low-calcium high-aluminum diet-induced motor neuron pathology in cynomolgus monkeys. Acta Neuropathol. 1989;78:210–219. doi: 10.1007/BF00688211. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D. Aluminium forms in drinking water and risk of Alzheimer’s disease. Environ Res A. 2000;84:234–246. doi: 10.1006/enrs.2000.4101. [DOI] [PubMed] [Google Scholar]
- Gava C, Perazzolo M, Zentilin L, Levis AG, Corain B, Bombi GG, Palumbo M, Zatta P. Genotoxic potentiality and DNA binding properties of acetylacetone, maltol, and their aluminum (III) and chromium (III) neutral complexes. Toxicol Environ Chem. 1989;22:149–157. [Google Scholar]
- Gelfant S. Inhibition of cell division: a critical and experimental analysis. In: Bourne GH, Danielli JF, editors. International Review of Cytology. New York: Academic Press; 1963. pp. 1–39. [DOI] [PubMed] [Google Scholar]
- Gherardi RK, Cherin P. Macropagic fascitis: a new entity. Groupe d’etudes et recherche surles maladies musculaires acquises et disimmunitaires (GERMMAD) de l’association francaise contre les myopathies (AFM) Rev Med Interne. 1998;19:617–618. doi: 10.1016/s0248-8663(99)80039-3. [DOI] [PubMed] [Google Scholar]
- Gherardi RK, Coquet M, Cherin P, Belec L, Moretto P, Dreyfus PA, Pellissier JF, Chariot P, Authier FJ. Macrophagic myofasciitis lesions assess long-term persistence of vaccine-derived aluminium hydroxide in muscle. Brain. 2001;124:1821–1831. doi: 10.1093/brain/124.9.1821. [DOI] [PubMed] [Google Scholar]
- Ghetti B, Ohcs S. On the relation between microtubule denisty and axoplasmic transport in nerves treated with maytansine in vitro. In: Canal N, Pozza G, editors. Periferal Neuropathies. Amsterdam: Elsevier/North Holland Biomedical Press; 1978. pp. 177–186. [Google Scholar]
- Ghribi O, Herman MM, Savory J. The endoplasmic reticulum is the main site for caspase-3 activation following aluminum-induced neurotoxicity in rabbit hippocampus. Neurosci Lett. 2002;324:217–221. doi: 10.1016/s0304-3940(02)00147-7. [DOI] [PubMed] [Google Scholar]
- Gibbs GW. Mortality of aluminum reduction plant workers, 1950 through 1977. J Occup Med. 1985;27:761–770. [PubMed] [Google Scholar]
- Gidden H, Holland FF, Klein E. Trace metal protein binding in normal and dialyzed uremic serum. Trans Am Soc Artif Intern Organs. 1980;26:133–138. [PubMed] [Google Scholar]
- Gilbert-Barness E, Barness LA, Wolff J, Harding C. Aluminum toxicity. Arch Pediatr Adolesc Med. 1998;152:511–512. doi: 10.1001/archpedi.152.5.511. [DOI] [PubMed] [Google Scholar]
- Gilks B, Churg A. Aluminum-induced pulmonary fibrosis: do fibers play a role? Am Rev Respir Dis. 1987;136:176–179. doi: 10.1164/ajrccm/136.1.176. [DOI] [PubMed] [Google Scholar]
- Gillette-Guyonnet S, Andrieu S, Nourhashemi F, de La Guéronnierè VH, Grandjean H, Vellas B. Cognitive impairment and composition of drinking water in women: findings of the EPIDOS study. Am J Clin Nutr. 2005;81:897–902. doi: 10.1093/ajcn/81.4.897. [DOI] [PubMed] [Google Scholar]
- Gitelman HJ. Aluminum exposure and excretion. Sci Total Environ. 1995;163:129–135. doi: 10.1016/0048-9697(95)04483-h. [DOI] [PubMed] [Google Scholar]
- Gitelman HJ, Alderman FR, Kurs-Lasky M, Rockette HE. Serum and urinary aluminium levels of workers in the aluminium industry. Ann Occup Hyg. 1995;39:181–191. doi: 10.1016/0003-4878(94)00113-f. [DOI] [PubMed] [Google Scholar]
- Glenny AT, Popk CG, Waddington H, Wallace U. Immunological notes. XXIII. The antigenic value of toxoid precipitated by potassium alum. J Pathol Bacteriol. 1926;29:38–39. [Google Scholar]
- Glenny AT, Buttle GA, Stevens MF. Rate of disappearance of diphtheria toxoid injected into rabbits and guinea pigs: toxoid precipitated with alum. J Pathol Bacteriol. 1931;34:267–275. [Google Scholar]
- Gloxhuber C, Potokar M, Pittermann W, Wallat S, Bartnik F, Reuter H, Braig S. Zeolithe A--A phosphate substitute for detergents: toxicological investigation. Food Chem Toxicol. 1983;21:209–220. doi: 10.1016/0278-6915(83)90238-7. [DOI] [PubMed] [Google Scholar]
- Glynn AW, Sparen A, Danielsson LG, Haegglund G, Jorhem L. Bioavailability of labile aluminum in acidic drinking water: A study in the rat. Food Chem Toxicol. 1995;33:403–408. doi: 10.1016/0278-6915(95)00002-j. [DOI] [PubMed] [Google Scholar]
- Glynn AW, Sparen A, Danielsson LG, Sundstrom B, Jorhem L. Concentration-dependent absorption of aluminum in rats exposed to labile aluminum in drinking water. J Toxicol Environ Health A. 1999;56:501–512. doi: 10.1080/009841099157944. [DOI] [PubMed] [Google Scholar]
- Glynn AW, Sparen A, Danielsson LG, Sundstrom B, Jorhem L. The influence of complexing agents on the solubility and absorption of aluminium in rats exposed to aluminium in water. Food Addit Contam. 2001;18:515–523. doi: 10.1080/02652030118639. [DOI] [PubMed] [Google Scholar]
- Goh CL. Aluminum choride hexahydrate versus palmar hyperhidrosis. Evaporimeter assessment. Int J Dermatol. 1990;29:368–370. doi: 10.1111/j.1365-4362.1990.tb04766.x. [DOI] [PubMed] [Google Scholar]
- Gokal R, Ramos JM, Ellis HA, Parkinson I, Sweetman V, Dewar J, Ward MK, Kerr DN. Histological renal osteodystrophy, and 25 hydroxycholecalciferol and aluminum levels in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 1983;23:15–21. doi: 10.1038/ki.1983.4. [DOI] [PubMed] [Google Scholar]
- Golub MS, Domingo JL. What we know and what we need to know about developmental aluminum toxicity. J Toxicol Environ Health. 1996;48:585–597. doi: 10.1080/009841096161087. [DOI] [PubMed] [Google Scholar]
- Golub MS, Keen CL. Effects of dietary aluminum on pubertal mice. Neurotoxicol Teratol. 1999;21:595–602. doi: 10.1016/s0892-0362(99)00016-1. [DOI] [PubMed] [Google Scholar]
- Golub MS, Gershwin ME, Donald JM, Negri S, Keen CL. Maternal and developmental toxicity of chronic aluminum exposure in mice. Fundam Appl Toxicol. 1987;8:346–357. doi: 10.1016/0272-0590(87)90084-4. [DOI] [PubMed] [Google Scholar]
- Golub MS, Donald JM, Gershwin ME, Keen CL. Effects of aluminum ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol. 1989;11:231–235. doi: 10.1016/0892-0362(89)90064-0. [DOI] [PubMed] [Google Scholar]
- Golub MS, Han B, Keen CL. Al and Mn: Interactions in adult and developing mice. Teratology. 1991;43:490. [Google Scholar]
- Golub MS, Takeuchi PE, Gershwin ME, Yoshida SH. Influence of dietary aluminum on cytokine production by mitogen-stimulated spleen cells, from Swiss Webster mice. Immunopharmacol Immunotoxicol. 1993;15:605–619. doi: 10.3109/08923979309019733. [DOI] [PubMed] [Google Scholar]
- Golub MS, Han B, Keen CL, Gershwin ME, Tarara RP. Behavioral performance of Swiss Webster mice exposed to excess dietary aluminum during development or during development and as adults. Toxicol Appl Pharmacol. 1995;133:64–72. doi: 10.1006/taap.1995.1127. [DOI] [PubMed] [Google Scholar]
- Golub MS, Han B, Keen CL. Developmental patterns of aluminum and five essential mineral elements in the central nervous system of the fetal and infant guinea pig. Biol Trace Elem Res. 1996a;55:241–251. doi: 10.1007/BF02785283. [DOI] [PubMed] [Google Scholar]
- Golub MS, Han B, Keen CL. Aluminum alters iron and manganese uptake and regulation of surface transferrin receptors in primary rat oligodendrocyte cultures. Brain Res. 1996b;719:72–77. doi: 10.1016/0006-8993(96)00087-x. [DOI] [PubMed] [Google Scholar]
- Golub MS, Han B, Keen CL. Aluminum uptake and effects on transferrin mediated iron uptake in primaary cultures of rat neurons, astrocytes and oligodendrocytes. Neurotoxicology. 1999;20:961–970. [PubMed] [Google Scholar]
- Golub MS, Germann SL, Han B, Keen CL. Lifelong feeding of a high aluminum diet to mice. Toxicology. 2000;150:107–117. doi: 10.1016/s0300-483x(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Gomez-Alonso C, Mendez-Rodriquez P, Virgos-Soriano MJ, Fernandez-Martin JL, Fernandez-Coto MT, Cannata-Andia JB. Aluminum-induced osteogenesis in ostopenic rats with normal renal function. Calcif Tissue Int. 1999;64:534–541. doi: 10.1007/s002239900645. [DOI] [PubMed] [Google Scholar]
- Gómez M, Domingo JL, Llobet JM, Tomas JM, Corbella J. Short-term oral toxicity study of aluminium in rats. Arch Farmacol Toxicol. 1986;12:145–151. [PubMed] [Google Scholar]
- Gómez M, Bosque MA, Domingo JL, Llobet JM, Corbella J. Evaluation of the maternal and developmental toxicity of aluminum from high doses of aluminum hydroxide in rats. Vet Hum Toxicol. 1990;32:545–548. [PubMed] [Google Scholar]
- Gómez M, Domingo JL, Llobet JM. Developmental toxicity evaluation of oral aluminum in rats: Influence of citrate. Neurotoxicol Teratol. 1991;13:323–328. doi: 10.1016/0892-0362(91)90078-b. [DOI] [PubMed] [Google Scholar]
- Gómez M, Domingo JL, Llobet JM, Richart C, Corbella J. Effect of frequent dietary organic constituents on the gastrointestinal absorption of aluminum. In: Collery P, Poirier LA, Littlefield NA, Etienne JC, editors. Metal Ions in Biology and Medicine. Paris: John Libbey Eurotext; 1994. pp. 91–95. [Google Scholar]
- Gómez M, Sanchez DJ, Llobet JM, Corbella J, Domingo JL. The effect of age on aluminum retention in rats. Toxicology. 1997;116:1–8. doi: 10.1016/s0300-483x(96)03512-3. [DOI] [PubMed] [Google Scholar]
- Gómez M, Esparza JL, Nogues MR, Giralt M, Cabre M, Domingo JL. Pro-oxidant activity of aluminum in the rat hippocampus: gene expression of antioxidant enzymes after melatonin administration. Free Radic Biol Med. 2005;38:104–111. doi: 10.1016/j.freeradbiomed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Gonda Z, Lehotzky K, Miklosi A. Neurotoxicity induced by prenatal aluminum exposure in rats. Neurotoxicology. 1996;17:459–469. [PubMed] [Google Scholar]
- Gonzalez MA, Roma MG, Bernal CA, Alvarez L, Carrillo MC. Biliary secretory function in rats chronically intoxicated with aluminum. Toxicol Sci. 2004;79:189–195. doi: 10.1093/toxsci/kfh085. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez I, Naves M, Diaz-Corte C, Fernandez-Martin JL, Menendez-Rodriguez P, Cannata-Andia JB. Effect of aluminium on calcium-sensing receptor expression, proliferation, and apoptosis of parathyroid glands from rats with chronic renal failure. Kidney Int. 2003;85:S39–S43. doi: 10.1046/j.1523-1755.63.s85.10.x. [DOI] [PubMed] [Google Scholar]
- Good PF, Perl DP, Bierer LM, Schmeidler J. Selective accumulation of aluminium and iron in the neurofibrillary tangles of Alzheimer’s disease: a laser microprobe (LAMMA) study. Ann Neurol. 1992;31:286–292. doi: 10.1002/ana.410310310. [DOI] [PubMed] [Google Scholar]
- Goodman WG. Short-term aluminum administration in the rat: reductions in formation without osteomalacia. J Lab Clin Med. 1984;103:749–757. [PubMed] [Google Scholar]
- Goodman WG, Gilligan J, Horst R. Short-term aluminum administration in the rat: effects on bone formation and relationship with renal osteomalacia. J Clin Invest. 1984a;73:171–181. doi: 10.1172/JCI111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WG, Henry DA, Horst R, Nudelman RK, Alfrey AC, Coburn JW. Parenteral aluminum administration in the dog: II. Induction of osteomalacia and effect on vitamin D metabolism. Kidney Int. 1984b;25:370–375. doi: 10.1038/ki.1984.26. [DOI] [PubMed] [Google Scholar]
- Goralewski G. Klinische und tierexperimentelle studien sur frage der aluminum-staublunge. [Clinical and animal experimental studies and the question of the aluminum-dusty lung] Arch Gewerbepathol Gewerbehyg. 1939;9:676–688. [Google Scholar]
- Goralewski G. Zur symptomalogie der aluminum-staublunge. [The symptomatology of the aluminum dust lung] Arch Gewerbepathol Gewerbehyg. 1940;10:384–408. [Google Scholar]
- Goralewski G. Zur klinik der aluminumlunge [Clinical examination of the aluminum lung] Arch Gewerbepathol Gewerbehyg. 1941;11:108–116. [Google Scholar]
- Goralewski G. Weitere erfahrungen zum krankheitsbild der aluminumlunge. Dtsch Tuberk Bl. 1943;17:2–10. [Google Scholar]
- Goralewski G. Die aluminiumlunge-eine neue gewerbeerkrankung. [The aluminum lung: a new industrial disease] Z Gesamte Inn Med. 1947;2:665–673. [Google Scholar]
- Goralewski G. The aluminium lung: A new industrial disease. Br J Ind Med. 1948;6:53–54. Abstract of Goralewski, 1947. [Google Scholar]
- Gorsky JE, Dietz AA, Spencer H, Osis D. Metabolic balance of aluminum studied in six men. Clin Chem. 1979;25:1739–1743. [PubMed] [Google Scholar]
- Goto N, Kato H, Maeyama J, Shibano M, Saito T, Yamaguchi J, Yoshihara S. Local tissue irritating effects and adjuvant activities of calcium phosphate and aluminium hydroxide with different physical properties. Vaccine. 1997;15:1364–1371. doi: 10.1016/s0264-410x(97)00054-6. [DOI] [PubMed] [Google Scholar]
- Goto N, Akama K. Histopathological studies of reactions in mice injected with aluminum-adsorbed tetanus toxoid. Microbiol Immunol. 1982;26:1121–1132. doi: 10.1111/j.1348-0421.1982.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Gough J, Hale LW, King EJ, Nagelschmidt G. Pneumoconiosis of kaolin workers. Br J Ind Med. 1956;13:251–259. doi: 10.1136/oem.13.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyens P, Brasseur D. Aluminum and infants. Pediatrics. 1990;86:650–652. [PubMed] [Google Scholar]
- Graf H, Stummvoll HK, Meisinger V, Kovarik J, Wolf A, Pinggera WF. Aluminum removal by hemodialysis. Kidney Int. 1981;19:587–592. doi: 10.1038/ki.1981.56. [DOI] [PubMed] [Google Scholar]
- Gramiccioni L, Ingrao G, Milana MR, Santaroni P, Tomassi G. Aluminium levels in Italian diets and in selected foods from aluminium utensils. Food Addit Contam. 1996;13:767–774. doi: 10.1080/02652039609374464. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Hörder M, Thomassen Y. Fluoride, aluminum, and phosphate kinetics in cryolite workers. J Occup Med. 1990;32:58–63. doi: 10.1097/00043764-199001000-00015. [DOI] [PubMed] [Google Scholar]
- Graske A, Thuvander A, Johannisson A, Gadhasson I, Schutz A, Festin R, Wicklund Glynn A. Influence of aluminium on the immune system--an experimental study on volunteers. Biometals. 2000;13:123–133. doi: 10.1023/a:1009215924071. [DOI] [PubMed] [Google Scholar]
- Graves AB, White E, Koepsell TD, Reifler BV, Van Belle G, Larson EB. The association between aluminum-containing products and Alzheimer’s disease. J Clin Epidemiol. 1990;43:35–44. doi: 10.1016/0895-4356(90)90053-r. [DOI] [PubMed] [Google Scholar]
- Greenberg L, Benoit R. Control of potency and the dosage of diphtheria and tetanus toxoids. J Am Med Assoc. 1956;160:108–113. doi: 10.1001/jama.1956.02960370018005. [DOI] [PubMed] [Google Scholar]
- Greenberg S. The pulmonary effects of pure aluminum in the Swiss mouse. Lab Invest. 1977;36:339. [Google Scholar]
- Greger JL. Aluminum content of the American diet. Food Technol. 1985;39:73–80. [Google Scholar]
- Greger JL, Baier MJ. Excretion and retention of low or moderate levels of aluminium by human subjects. Food Chem Toxicol. 1983;21:473–477. doi: 10.1016/0278-6915(83)90105-9. [DOI] [PubMed] [Google Scholar]
- Greger JL, Donnabauer S. Retention of aluminium in the tissue of rats after the discontinuation of oral exposure to aluminium. Food Chem Toxicol. 1986;24:1331–1334. doi: 10.1016/0278-6915(86)90067-0. [DOI] [PubMed] [Google Scholar]
- Greger JL, Powers CF. Assessment of exposure to parenteral and oral aluminum with and without citrate using a desferrioxamine test in rats. Toxicology. 1992;76:119–132. doi: 10.1016/0300-483x(92)90159-c. [DOI] [PubMed] [Google Scholar]
- Greger JL, Radzanowski GM. Tissue aluminium distribution in growing, mature and ageing rats: relationship to changes in gut, kidney and bone metabolism. Food Chem Toxicol. 1995;33:867–875. doi: 10.1016/0278-6915(95)00059-b. [DOI] [PubMed] [Google Scholar]
- Greger JL, Sutherland JE. Aluminum exposure and metabolism. Crit Rev Clin Lab Sci. 1997;34:439–474. doi: 10.3109/10408369709006422. [DOI] [PubMed] [Google Scholar]
- Greger JL, Bula EN, Gum ET. Mineral metabolism of rats fed moderate levels of various aluminum compounds for short periods of time. J Nutr. 1985;115:1708–1716. doi: 10.1093/jn/115.12.1708. [DOI] [PubMed] [Google Scholar]
- Greger JL, Chang MM, MacNeil GG. Tissue turnover of aluminum and Ga-67: effect of iron status. Proc Soc Exp Biol Med. 1994;207:89–96. doi: 10.3181/00379727-207-43796. [DOI] [PubMed] [Google Scholar]
- Grekhova TD, Neizvestnova EM, Konstantinova LI, Dobroliubova LP, Babakova OM, Petelina EV, Fomina AS. Rationale for maximum allowable exposure level of aluminum sulfate and its coagulants in the air of the workplace. Med Tr Prom Ekol. 1994;1:26–28. [PubMed] [Google Scholar]
- Gross P, Harley RA, Detreville RT. Pulmonary reaction to metallic aluminum powders. Arch Environ Health. 1973;26:227–236. doi: 10.1080/00039896.1973.10666264. [DOI] [PubMed] [Google Scholar]
- Guibaud G, Gauthier C. Aluminium speciation in the Vienne river on its upstream catchment (Limousin Region, France) J Inorg Biochem. 2005;99:1817–1821. doi: 10.1016/j.jinorgbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Guillard O, Fauconneau B, Olichon D, Dedieu G, Deloncle R. Hyperaluminemia in a woman using an aluminum-containing antiperspirant for 4 years. Am J Med. 2004;117:956–959. doi: 10.1016/j.amjmed.2004.07.047. [DOI] [PubMed] [Google Scholar]
- Gun RT, Korten AE, Jorm AF, Hendersen AS, Broe GA, Creasy H, McCusker E, Mylvaganam A. Occupational risk factors for Alzheimer disease: A case control study. Alzheimer Dis Assoc Disord. 1997;11:21–27. doi: 10.1097/00002093-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32:155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Siber GR. Adjuvants for human vaccines-current status, problems and future prospects. Vaccine. 1995;13:1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Waters DH, Gwilt PR. Absorption and disposition of aluminium in the rat. J Pharm Sci. 1986;75:586–589. doi: 10.1002/jps.2600750613. [DOI] [PubMed] [Google Scholar]
- Gupta VB, Anitha S, Hegde ML, Zecca L, Garruto RM, Ravid R, Shankar SK, Stein R, Shanmugavelu P, Jagannatha Rao KS. Aluminium in Alzheimer’s disease: are we still at a crossroad? Cell Mol Life Sci. 2005;62:143–158. doi: 10.1007/s00018-004-4317-3. [DOI] [PubMed] [Google Scholar]
- Gusev VA, Danilovskaja YV, Vatolkina OY, Lomonosova OS, Velichkovsky BT. Effects of quartz and alumina dust on generation of superoxide radicals and hydrogen peroxide by alveolar macrophages, granulocytes, and monocytes. Br J Ind Med. 1993;50:732–735. doi: 10.1136/oem.50.8.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy SP, Seabright PJ, Day JP, Itzhaki RF. Uptake of aluminium by human neuroblastoma cells. Biochem Soc Transactions. 1990;18:392–393. doi: 10.1042/bst0180392. [DOI] [PubMed] [Google Scholar]
- Gylseth B, Bjoerseth O, Dugstad O, Gjoennes J. Occurrence of fibrous sodium aluminum tetrafluoride particles in potrooms of the primary aluminum industry. Scand J Work Environ Health. 1984;10:189–195. doi: 10.5271/sjweh.2341. [DOI] [PubMed] [Google Scholar]
- Hackenberg U. Chronic ingestion by rats of standard diet treated with aluminum phosphide. Toxicol Appl Pharmacol. 1972;23:147–158. doi: 10.1016/0041-008x(72)90214-1. [DOI] [PubMed] [Google Scholar]
- Haddow A, Hornig ES. On the carcinogenicity of an iron-dextran complex. J Natl Cancer Inst. 1960;24:109–147. [PubMed] [Google Scholar]
- Halatek T, Opalska B, Lao I, Stetkiewicz J, Rydzynski K. Pneumotoxicity of dust from aluminum foundry and pure alumina: a comparative study of morphology and biomarkers in rats. Int J Occup Med Environ Health. 2005;18:59–70. [PubMed] [Google Scholar]
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179–185. [Google Scholar]
- Hamilton E, Minski M. Abundance of the chemical elements in man’s diet and possible relations with environmental factors. Sci Total Environ. 19721973;1:375–394. [Google Scholar]
- Hamilton JA, Byrne R, Whitty G. Particulate adjuvants can induce macrophage survival, DNA synthesis, and a synergistic proliferative response to GM-CSF and CSF-1. J Leukoc Biol. 2000;67:226–232. [PubMed] [Google Scholar]
- Han J, Han J, Dunn MA. Effect of dietary aluminum on tissue nonheme iron and ferritin levels in the chick. Toxicology. 2000;142:97–109. doi: 10.1016/s0300-483x(99)00119-5. [DOI] [PubMed] [Google Scholar]
- Han SH, Sakinci U, Kose SK, Yazkan R. The relationship between aluminum and spontaneous pneumothorax: treatment, prognosis, follow-up? Interact Cardiovas Thorac Surg. 2004;3:79–82. doi: 10.1016/S1569-9293(03)00226-3. [DOI] [PubMed] [Google Scholar]
- Hanninen H, Matikainen E, Kovala T, Valkonen S, Riihmahki V. Internal load of aluminum and the central nervous system function of aluminum welders. Scand J Work Environ Health. 1994;20:279–285. doi: 10.5271/sjweh.1397. [DOI] [PubMed] [Google Scholar]
- Hannon JW. Aluminum therapy in silicosis. Trans Canad Min. 1944;47:180. [Google Scholar]
- Hannon JW. Proceedings of the 5th Conference of McIntyre Research Foundation on silicosis.1953. [Google Scholar]
- Hantson P, Mahieu P, Gersdorff M, Sindic SJ, Lauwerys R. Encephalopathy with seizures after use of aluminum containing bone cement. Lancet. 1994;344:1647. doi: 10.1016/s0140-6736(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Haram EM, Weberg R, Berstad A. Urinary excretion of aluminum after ingestion of sucralfate and an aluminum-containing antacid in man. Scand J Gastroenterol. 1987;22:615–618. doi: 10.3109/00365528708991908. [DOI] [PubMed] [Google Scholar]
- Harris WR. Equilibrium model for speciation of aluminum in serum. Clin Chem. 1992;38:1809–1818. [PubMed] [Google Scholar]
- Harris WR, Messori L. A comparative study of aluminum (III), gallium (III), indium (III), and thallium (III) binding to human serum transferrin. Coord Chem Rev. 2002;228:237–262. [Google Scholar]
- Harris WR, Berthon G, Day JP, Exley C, Flaten TP, Forbes WF, Kiss T, Orvig C, Zatta PF. Speciation of aluminum in biological systems. J Toxicol Environ Health. 1996;48:543–568. [PubMed] [Google Scholar]
- Hart MM, Adamson RH. Antitumor activity and toxicity of salts of inorganic group IIIa metals: aluminum, gallium, indium, and thallium. Proc Natl Acad Sci. 1971;68:1623–1626. doi: 10.1073/pnas.68.7.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GE, Boykin E, Graham JA, Lewtas J, Pott F, Loud K, Mumford JL. Inhalable particles and pulmonary host defense: in vivo and in vitro effects of ambient air and combustion particles. Environ Res. 1985;36:67–80. doi: 10.1016/0013-9351(85)90008-8. [DOI] [PubMed] [Google Scholar]
- Hawkins NM, Coffey S, Lawson MS, Delves HT. Potential aluminum toxicity in infants fed special infant formula. J Pediatr Gastroenterol Nutr. 1994;19:377–381. doi: 10.1097/00005176-199411000-00002. [DOI] [PubMed] [Google Scholar]
- Hazelton Laboratories. Acute ocular and dermal testing with magnesium aluminum silicate. 1968 Unpublished data. [Google Scholar]
- Hdez-Jaras J, Galan A, Sanchez P. Accidental aluminum intoxication in patients undergoing acetate-free biofiltration. Nephron. 1998;78:274–277. doi: 10.1159/000044935. [DOI] [PubMed] [Google Scholar]
- He BP, Strong MJ. Motor neuronal death in sporadic amyotrophic lateral sclerosis (ALS) is not apoptotic. A comparative study of ALS and chronic aluminium chloride neurotoxicity in New Zealand white rabbits. Neuropathol Appl Neurobiol. 2000a;26:150–160. doi: 10.1046/j.1365-2990.2000.026002150.x. [DOI] [PubMed] [Google Scholar]
- He BP, Strong MJ. A morphological analysis of the motor neuron degeneration and microglial reaction in acute and chronic in vivo aluminum chloride neurotoxicity. J Chem Neuroanat. 2000b;17:207–215. doi: 10.1016/s0891-0618(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Healy J, Bradley SD, Northage C, Scobbie E. Inhalation exposure in secondary aluminum smelting. Ann Occup Hyg. 2001;45:217–225. [PubMed] [Google Scholar]
- Heimlich JM, Regnier FE, White JL, Hem SL. The in vitro displacement of adsorbed model antigens from aluminium-containing adjuvants by interstitial proteins. Vaccine. 1999;17:2873–2881. doi: 10.1016/s0264-410x(99)00126-7. [DOI] [PubMed] [Google Scholar]
- Hellström HO, Mjöberg B, Mallmin H, Michaëlsson K. The aluminum content of bone increases with age, but is not higher in hip fracture cases with and without dementia compared to controls. Osteoporos Int. 2005;16:1982–1988. doi: 10.1007/s00198-005-1981-6. [DOI] [PubMed] [Google Scholar]
- Helmboldt O, Hudson LK, Misra C, Wefers K, Stark H, Danner M. Aluminum compounds, inorganic. In: Gerhartz W, Yamamoto YS, Campbell FT, Pfefferkorn R, Rounsaville JF, editors. Ullmann’s Encyclopedia of Industrial Chemistry - Volume A1: Abrasives to Aluminum Oxide. Weinheim: Verlag Chemie; 1985. pp. 527–541. [Google Scholar]
- Hem SL. Elimination of aluminium adjuvants. Vaccine. 2002;20(Suppl 3):S40–S43. doi: 10.1016/s0264-410x(02)00170-6. [DOI] [PubMed] [Google Scholar]
- Hem SL, White JL. Pharmaceutical uses of aluminum. In: Gitelman HJ, editor. Aluminum and Health A Critical Review. New York: Marcel Dekker; 1989. pp. 257–282. [Google Scholar]
- Hemadi M, Miquel G, Kahn PH, Hage Chahine JM. Aluminum exchange between citrate and human serum transferrin and interaction with transferrin receptor 1. Biochem. 2003;42:3120–3130. doi: 10.1021/bi020627p. [DOI] [PubMed] [Google Scholar]
- Hemmer W, Wantke F, Focke M, Gotz M, Jarisch R. Evaluation of cutaneous hypersenstivity to aluminum by routine path testing with AlCl. Contact Dermatitis. 1996;34:217–218. doi: 10.1111/j.1600-0536.1996.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Henry DA, Goodman WG, Nudelman RK, Didomenico NC, Alfrey AC, Slatopolsky E, Stanley TM, Coburn JW. Parenteral aluminum administration in the dog: I. Plasma kinetics, tissue levels, calcium metabolism, and para-thyroid hormone. Kidney Int. 1984;25:362–369. doi: 10.1038/ki.1984.25. [DOI] [PubMed] [Google Scholar]
- Herbert A, Sterling G, Abraham J, Corrin B. Desquamative interstitial pneumonia in an aluminum welder. Hum Pathol. 1982;13:694–699. doi: 10.1016/s0046-8177(82)80291-8. [DOI] [PubMed] [Google Scholar]
- Herman SJ, Olscamp GC, Weisbrod GL. Pulmonary kaolin granulomas. J Can Assoc Radiol. 1982;33:279–280. [PubMed] [Google Scholar]
- Hertzberg M, Zlochower IA, Cashdollar KL. Explosibility of metal dusts. Comb Sci Tech. 1991;75:161–165. [Google Scholar]
- Hicks JS, Hackett DS, Sprague GL. Toxicity and aluminium concentration in bone following dietary administration of two sodium aluminium phosphate formulations in rats. Food Chem Toxicol. 1987;25:533–538. doi: 10.1016/0278-6915(87)90205-5. [DOI] [PubMed] [Google Scholar]
- Hirschberg R, von Herrath D, Voss R, Bossaller W, Mauelshagen U, Pauls A, Schaefer K. Organ distribution of aluminium in uremic rats: influence of parathyroid hormone and 1,25-dihydroxyvitamin D3. Miner Electrolyte Metab. 1985;11:106–110. [PubMed] [Google Scholar]
- Hjortsberg U, Ørbæk P, Arborelius M, Karlsson JE. Upper airway irritation and small airways hyperreactivity due to exposure to potassium aluminium tetrafluoride flux: an extended case report. Occup Environ Med. 1994;51:706–709. doi: 10.1136/oem.51.10.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs CS, Moorman RP, Griffith JM, West JL, Merriman GM, Hansard SL, Chamberlain CC. The University of Tennessee Agricultural Exeriment Station Knoxville, Report No 235. Knoxville, Tennessee: University of Tennessee; 1954. Fluorosis in cattle and sheep. [Google Scholar]
- Höflich BL, Wentzel M, Ortner HM, Weinbruch S, Skogstad A, Hetland S, Thomassen Y, Chaschin VP, Nieboer E. Chemical composition of individual aerosol particles from working areas in a nickel refinery. J Environ Monit. 2000;2:213–217. doi: 10.1039/b001146k. [DOI] [PubMed] [Google Scholar]
- Höflich BL, Weinbruch S, Theissmann R, Gorzawski H, Ebert M, Ortner HM, Skogstad A, Ellingsen DG, Drablos PA, Thomassen Y. Characterization of individual aerosol particles in workroom air of aluminum smelter potrooms. J Environ Monit. 2005;7:419–424. doi: 10.1039/b418275h. [DOI] [PubMed] [Google Scholar]
- HogenEsch H. Mechanisms of stimulation of the immune response by aluminium adjuvants. Vaccine. 2002;20(Suppl 3):S34–S39. doi: 10.1016/s0264-410x(02)00169-x. [DOI] [PubMed] [Google Scholar]
- Hohl C, Gerisch P, Korschinek G, Nolte E, Ittel TH. Medical application of 26Al. Nucl Instr Meth Phys Res B. 1994;92:478–482. [Google Scholar]
- Höhr D, Abel J, Wilhelm M. Renal clearance of aluminium: studies in the isolated perfused rat kidney. Toxicol Lett. 1989;45:165–174. doi: 10.1016/0378-4274(89)90006-4. [DOI] [PubMed] [Google Scholar]
- Hong SH, Kim BK. Effects of lifter bars on the ball motion and aluminum foil milling in tumbler ball mill. Mater Lett. 2002;57:275–279. [Google Scholar]
- Hosovski E, Mastelica Z, Sunderic D, Radulovic D. Mental abilities of workers exposed to aluminium. Med Lav. 1990;81:119–123. [PubMed] [Google Scholar]
- Hosovski E, Vidakovic A, Hosovski M. Dermal and bronchial responsiveness of aluminum smelter workers. J Occup Health. 1998;40:44–49. [Google Scholar]
- Hostynek JJ, Hinz RS, Lorence CR, Price M, Guy RH. Metals and the skin. Crit Rev Toxicol. 1993;23:171–235. doi: 10.3109/10408449309117116. [DOI] [PubMed] [Google Scholar]
- House RA. Factors affecting plasma aluminum concentrations in nonexposed workers. J Occup Med. 1992;34:1013–1017. [PubMed] [Google Scholar]
- Hovatta O, Venalainen ER, Kuusimaki L, Heikkila J, Hirvi T, Reima I. Aluminium, lead and cadmium concentrations in seminal plasma and spermatozoa, and semen quality in Finnish men. Hum Reprod. 1998;13:115–119. doi: 10.1093/humrep/13.1.115. [DOI] [PubMed] [Google Scholar]
- HSDB (Hazardous Substances Data Bank) National Library of Medicine, National Toxicology Program. Bethesda MD: 1995. [Google Scholar]
- Huang JY, Huang CC, Lim PS, Wu MS, Leu ML. Effect of body iron stores on serum aluminum level in hemodialysis patients. Nephron. 1992;61:158–162. doi: 10.1159/000186864. [DOI] [PubMed] [Google Scholar]
- Huang Y, Herman MM, Liu J, Katsetos CD, Wills MR, Savory J. Neurofibrillary lesions in experimental aluminum-induced encephalopathy and Alzheimer’s disease share immunoreactivity for amyloid precursor protein, A beta, alpha1-antichymotrypsin and ubiquitin-protein conjugates. Brain Res. 1997;771:213–220. doi: 10.1016/s0006-8993(97)00780-4. [DOI] [PubMed] [Google Scholar]
- Hunter D, Milton R, Perry KMA, Thompson DR. Effects of aluminum and alumina on the lung in grinders of duralumin aeroplane propellers. Br J Ind Med. 1944;1:159–164. [Google Scholar]
- Hunter RL. Overview of vaccine adjuvants: present and future. Vaccine. 2002;20(Suppl 3):S7–S12. doi: 10.1016/s0264-410x(02)00164-0. [DOI] [PubMed] [Google Scholar]
- Hussain RI, Ballard CG, Edwardson JA, Morris CM. Transferrin gene polymorphism in Alzheimer’s disease and dementia with Lewy bodies in humans. Neurosci Lett. 2002;317:13–16. doi: 10.1016/s0304-3940(01)02403-x. [DOI] [PubMed] [Google Scholar]
- Hütteroth TH, Quast U. Aluminum hydroxide granuloma following hepatitis B vaccination. Dtsch Med Wochenschr. 1990;115:476. [PubMed] [Google Scholar]
- Hyry H, Hook-Nikanne J. Aluminium and milk allergies in an adult. Contact Dermatitis. 2004;50:107. doi: 10.1111/j.0105-1873.2004.0295i.x. [DOI] [PubMed] [Google Scholar]
- IAEA (International Atomic Energy Agency) Activation analysis of hair as an indicator of contamination of man by environmental trace element pollutants. Vienna. 1978 Report No IAEA/RL/50. [Google Scholar]
- IAI (International Aluminium Institute) Alternative source data, Aluminium recovered from purchased or tolled scrap. 2005 [online] Cited 27 November 2005 Available from www.world-aluminium.org/iai/stats/
- IAI (International Aluminium Institute) IAI statistics. 2006a [online] Cited 24 January 2007 http://world-aluminium.org/iai/stats/index.asp.
- IAI (International Aluminium Institute) Aluminium applications. 2006b [online] Cited 24 January 2007 http://www.world-aluminium.org/applications/index.html.
- IAI (International Aluminium Institute) Consolidated IAI primary aluminium production reports, 20 December, 2006. 2006c [online] Cited 24 January 2007 http://www.world-aluminium.org/iai/stats/formServer.asp?form=16.
- IARC (International Agency for Research on Cancer) Polynuclear Aromatic Compounds, Part 3, Industrial Exposures in Aluminum Production, Coal Gasification, Coke Production, and Iron and Steel Founding. Lyon, France: World Health Organization, International Agency for Research on Cancer; 1984. [Google Scholar]
- IARC (International Agency for Research on Cancer) Polynuclear aromatic compounds. Part 4: Bitumens, coal-tars and derived products, shale oils and soots. IARC Monogr Eval Carcinog Risks Hum. 1985;35:1–271. [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs (Volumes 1 to 42) Lyon, France: World Health Organization, International Agency for Research on Cancer; 1987. [Google Scholar]
- ICRP (International Commission of Radiological Protection) Limits on Intakes of Radionuclides by Workers. Oxford: Pergamon; 1975. Metabolic data for aluminium; pp. 12–13. [Google Scholar]
- ICRP (International Commission of Radiological Protection) Limits for Intakes of Radionuclides by Workers. Oxford: Pergamon Press; 1981. [Google Scholar]
- Ilyin A, Gromov A, An F, Faubert V, de Izarra C, Espagnscq A, Brunet L. Characterization of aluminum powders I. Parameters of reactivity of aluminum powders. Propell Explos Pyrot. 2002;27:361–364. [Google Scholar]
- Imahori A, Fukushima I, Shiobara S, Yanagida Y, Tomura K. Multielement neutron activation analysis of human scalp hair. A local population survey in the Tokyo metropolitan area. J Radioanal Chem. 1979;52:167–180. [Google Scholar]
- IPAI (International Primary Aluminium Institute) Statistical Summary - Volume 5. London: International Primary Aluminium Institute; 1993. [Google Scholar]
- IPCS (International Programme on Chemical Safety) Methylmercury Environmental Health Criteria 144. Geneva: World Health Organization; 1990. [Google Scholar]
- IPCS (International Programme on Chemical Safety) Aluminum Environmental Health Criteria 194. Geneva: World Health Organization; 1997. [Google Scholar]
- IPCS/WHO (International Programme on Chemical Safety/World Health Organization) Principles and Methods for Assessing Direct Immunotoxicity Associated with Exposure to Chemicals Environmental Health Criteria 180. Geneva: World Health Organization; 1996. [Google Scholar]
- Iregren A, Sjögren B, Gustafsson K, Hagman M, Nylen L, Frech W, Andersson M, Ljunggren KG, Wennberg A. Effects on the nervous system in different groups of workers exposed to aluminum. Occup Environ Med. 2001;58:453–460. doi: 10.1136/oem.58.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRIS (Integrated Risk Information System) Cincinnati, OH: U.S. Environmental Protection Agency; 1997. [Google Scholar]
- IRIS (Integrated Risk Information System) Cincinnati, OH: U.S. Environmental Protection Agency; 1999. [Google Scholar]
- Ittel TH, Buddington B, Miller NL, Alfrey AC. Enhanced gastrointestinal absorption of aluminum in uremic rats. Kidney Int. 1987;32:821–826. doi: 10.1038/ki.1987.282. [DOI] [PubMed] [Google Scholar]
- Ittel TH, Kluge R, Sieberth HG. Enhanced gastrointestinal absorption of aluminium in uraemia: time course and effect of vitamin D. Nephrol Dial Transplant. 1988;3:617–623. doi: 10.1093/oxfordjournals.ndt.a091716. [DOI] [PubMed] [Google Scholar]
- Ittel TH, Koppe BK, Sieberth HG. Differential effect of steroids and chloroquine on the intestinal absorption of aluminium and calcium. Nephrol Dial Transplant. 1990;5:860–867. doi: 10.1093/ndt/5.10.860. [DOI] [PubMed] [Google Scholar]
- Ittel TH, Gladziwa U, Mück W, Sieberth HG. Hyperaluminaemia in critically ill patients: role of antacid therapy and impaired renal function. Eur J Clin Invest. 1991a;21:96–102. doi: 10.1111/j.1365-2362.1991.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Ittel TH, Griessner A, Sieberth HG. Effect of lactate on the absorption and retention of aluminum in the remnant kidney rat model. Nephron. 1991b;57:332–339. doi: 10.1159/000186284. [DOI] [PubMed] [Google Scholar]
- Ittel TH, Paulus CP, Handt S, Hofstadter F, Sieberth HG. Induction of intestinal mucosal atrophy by difluoromethylornithine: a nonuremic model of enhanced aluminum absorption. Miner Electrolyte Metab. 1992a;18:15–23. [PubMed] [Google Scholar]
- Ittel TH, Gruber E, Heinrichs A, Handt S, Hofstädter F, Sieberth HG. Effect of fluoride on aluminum-induced bone disease in rats with renal failure. Kidney Int. 1992b;41:1340–1348. doi: 10.1038/ki.1992.198. [DOI] [PubMed] [Google Scholar]
- Ittel TH, Gladziwa U, Sieberth HG. Synergistic effect of 1,25-vitamin D3 and fluoride on bone aluminum accumulation. Bone. 1993a;14:427–432. doi: 10.1016/8756-3282(93)90175-a. [DOI] [PubMed] [Google Scholar]
- Ittel TH, Gerisch P, Nolte E, Sieberth HG. Fractional absorption of aluminium is dose-dependent: A 26Al tracer study. Nephrol Dial Transplant. 1993b;8:993. [Google Scholar]
- Ittel TH, Kinzel S, Ortmanns A, Sieberth HG. Effect of iron status on the intestinal absorption of aluminum: a reappraisal. Kidney Int. 1996;50:1879–1888. doi: 10.1038/ki.1996.509. [DOI] [PubMed] [Google Scholar]
- Ittel TH, Steinhausen C, Kislinger G, Kinzel S, Nolte E, Sieberth HG. Ultrasensitive analysis of the intestinal absorption and compartmentalization of aluminium in uraemic rats: a 26Al tracer study employing accelerator mass spectrometry. Nephrol Dial Transplant. 1997;12:1369–1375. doi: 10.1093/ndt/12.7.1369. [DOI] [PubMed] [Google Scholar]
- Iwata S, Kasajima K, Yase Y, Yoshimasu F, Kamiayashi Y. Radioactivation analysis of amyotrophic lateral sclerosis. Kyoto Daigaku Genshiro Jikkensho Gakujutsu Koenkai Koen Yoshishu. 1976;10:33–38. [Google Scholar]
- Jackson ML, Huang PM. Aluminum of acid soils in the food chain and senility. Sci Total Environ. 1983;28:269–276. doi: 10.1016/s0048-9697(83)80024-2. [DOI] [PubMed] [Google Scholar]
- Jackson RTJ, Tigges J, Arnold W. Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch Otolaryngol. 1979;105:180–184. doi: 10.1001/archotol.1979.00790160014003. [DOI] [PubMed] [Google Scholar]
- Jacobs RW, Duong T, Jones RE, Trapp GA, Scheibel AB. A re-examination of aluminium in Alzheimer’s disease: Analysis by energy dispersive x-ray microprobe and flameless atomic absorption spectrophotometry. Can J Neurol Sci. 1989;16:498–503. doi: 10.1017/s0317167100029838. [DOI] [PubMed] [Google Scholar]
- Jacqmin H, Commenges D, Letenneur L, Barberger-Gateau P, Dartigues JF. Components of drinking water and risk of cognitive impairment in the elderly. Am J Epidemiol. 1994;139:48–57. doi: 10.1093/oxfordjournals.aje.a116934. [DOI] [PubMed] [Google Scholar]
- Jacqmin-Gadda H, Commenges D, Letenneur L, Dartigues JF. Silica and aluminum in drinking water and cognitive impairment in the elderly. Epidemiology. 1996;7:281–285. doi: 10.1097/00001648-199605000-00011. [DOI] [PubMed] [Google Scholar]
- Jagannatha Rao K, Rao R, Shanmgaveu P, Menon R. Trace elements in the cerebrospinal fluid in Alzheier’s disease. Alz Rep. 1999;2:333–338. [Google Scholar]
- Jäger DE, Wilhelm M, Witte G, Ohnesorge FK. Intestinal absorption of aluminium: studies in the isolated perfused rat intestinal preparation. J Trace Elem Electrolytes Health Dis. 1991;5:81–85. [PubMed] [Google Scholar]
- Jäger R, Jäger F. Colloid chemistry and histochemistry in the problem of pulmonary disease caused by aluminum dust. Archiv fuer Gewerbepathologie und Gewerbehygiene. 1941;11:117–130. [Google Scholar]
- James DM, Morris TG, Marks J. Drug treatment of experimental silicosis. Br J Ind Med. 1960;17:36–40. doi: 10.1136/oem.17.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JD, Pounds CA, Wilshire B. Production and characterization of flake metal powders for fingerprint detection. Powder Metall. 1991;34:39–43. [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jederlinic PJ, Abraham JL, Churg A, Himmelstein JS, Epler GR, Gaensler EA. Pulmonary fibrosis in aluminum oxide workers. Investigation of nine workers, with pathologic examination and microanalysis in three of them. Am Rev Respir Dis. 1990;142:1179–1184. doi: 10.1164/ajrccm/142.5.1179. [DOI] [PubMed] [Google Scholar]
- Jeffery EH, Abreo K, Burgess E, Cannata J, Greger JL. Systemic aluminum toxicity: effects on bone, hematopoietic tissue, and kidney. J Toxicol Environ Health. 1996;48:649–665. doi: 10.1080/009841096161122. [DOI] [PubMed] [Google Scholar]
- Jensen OM, Koch C. On the effect of Al(OH)3 as an immunological adjuvant. APMIS. 1988;96:257–264. doi: 10.1111/j.1699-0463.1988.tb05299.x. [DOI] [PubMed] [Google Scholar]
- Johannessen H, Bergan-Skar B. Itching problems among potroom workers in factories using recycled alumina. Contact Dermatitis. 1980;6:42. doi: 10.1111/j.1600-0536.1980.tb03892.x. [DOI] [PubMed] [Google Scholar]
- Johns DR, Petronella SJ. Aluminum therapy for silicosis. Monthly Bull Indiana State Board Health. 1945;43:203. [Google Scholar]
- Johnson KE, Treble RG. Determination of aluminum in biological fluids by furnace atomic absorption spectrophotometry. J Clin Lab Anal. 1992;6:264–268. doi: 10.1002/jcla.1860060504. [DOI] [PubMed] [Google Scholar]
- Johnsson C, Schutz A, Sallsten G. Impact of consumption of freshwater fish on mercury levels in hair, blood, urine, and alveolar air. J Toxicol Environ Health A. 2005;68:129–140. doi: 10.1080/15287390590885992. [DOI] [PubMed] [Google Scholar]
- Jones KC, Bennett BG. Exposure of man to environmental aluminum-An exposure commitment assessment. Sci Total Environ. 1986;52:65–82. doi: 10.1016/0048-9697(86)90105-1. [DOI] [PubMed] [Google Scholar]
- Jones KR, Oorschot DE. Do aluminium and/or glutamate induce Alz-50 reactivity? A light microscopic immunohistochemical study. J Neurocytol. 1998;27:45–57. doi: 10.1023/a:1006986903687. [DOI] [PubMed] [Google Scholar]
- Jones P, Ebdon L, Williams T. Determination of trace amounts of aluminum by ion chromatography with fluorescence detection. Analyst. 1988;113:641–644. [Google Scholar]
- Jordan JW. Pulmonary fibrosis in a worker using an aluminum powder. Br J Ind Med. 1961;18:21–23. [Google Scholar]
- Jorhem L, Haegglund G. Aluminium in foodstuffs and diets in Sweden. Zeitschrift für Lebensmittel-Untersuchung und-Forschung. 1992;194:38–42. doi: 10.1007/BF01191038. [DOI] [PubMed] [Google Scholar]
- Jötten KW, Eickhoff W. Risk of lung damage by Al dust. I. Literature and discussion of hygienic technique. Archiv Fuer Hygiene und Backteriologie. 1942;127:344–367. [Google Scholar]
- Jouhanneau P, Lacour B, Raisbeck G, Yiou F, Banide H, Brown E, Drüeke T. Gastrointestinal absorption of aluminum in rats using 26Al and accelerator mass spectrometry. Clin Nephrol. 1993;40:244–248. [PubMed] [Google Scholar]
- Jouhanneau P, Raisbeck GM, Yiou F, Lacour B, Banide H, Drüeke TB. Gastrointestinal absorption, tissue retention, and urinary excretion of dietary aluminum in rats determined by using 26Al. Clin Chem. 1997a;43:1023–1028. [PubMed] [Google Scholar]
- Jouhanneau P, Raisbeck GM, Yiou F, Lacour B, Banide H, Drüeke TB. Gastrointestinal absorption, tissue retention, and urinary excretion of dietary aluminum in rats determined by using 26Al. Clin Chem. 1997b;43:1023–1028. [PubMed] [Google Scholar]
- Jugdaohsingh R, Reffitt DM, Oldham C, Day JP, Fifield LK, Thompson RP, Powell JJ. Oligomeric but not monomeric silica prevents aluminum absorption in humans. Am J Clin Nutr. 2000;71:944–949. doi: 10.1093/ajcn/71.4.944. [DOI] [PubMed] [Google Scholar]
- Julka D, Gill KD. Altered calcium homeostasis: a possible mechanisms of aluminium-induced neurotoxicity. Biochimica et Biophysica Acta. 1996;1315:47–54. doi: 10.1016/0925-4439(95)00100-x. [DOI] [PubMed] [Google Scholar]
- Jullien G, Vallecalle E, Leandri M. Harmful effect of aluminium hydroxide in inhalation and its effect on the pulmonary tissue. Arch Mal Prof. 1952;13:31–38. [PubMed] [Google Scholar]
- Kaaber K, Nielsen AO, Veien NK. Vaccination granulomas and aluminum allergy: course and prognostic factors. Contact Dermatitis. 1992;26:304–306. doi: 10.1111/j.1600-0536.1992.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Kada T, Hirano K, Shirasu Y. Screening of environmental chemical mutagens by the rec(-) assay system with Bacillus subtilis. In: Hollaender A, Serres FJ, editors. Chemical Mutagens: Principles and Methods for their Detection. New York: Plenum Press; 1980. pp. 149–173. [Google Scholar]
- Kaehny WD, Hegg AP, Alfrey AC. Gastrointestinal absorption of aluminum from aluminum-containing antacids. N Engl J Med. 1977;296:1389–1390. doi: 10.1056/NEJM197706162962407. [DOI] [PubMed] [Google Scholar]
- Kagan BL, Hirakura Y, Azimov R, Azimova R, Lin MC. The channel hypothesis of Alzheimer’s disease: current status. Peptides. 2002;23:1311–1315. doi: 10.1016/s0196-9781(02)00067-0. [DOI] [PubMed] [Google Scholar]
- Kales SN, Goldman RH. Mercury exposure: current concepts, controversies, and a clinic’s experience. J Occup Environ Med. 2002;44:143–154. doi: 10.1097/00043764-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Kallio A, Kiilunen M, Kivisto H, Pekari K, Valkonen S. Results of biomonitoring analyses in Biomonitoring Laboratory, Helsinki, Finland in 1997. Toxicol Lett. 1999;108:249–257. doi: 10.1016/s0378-4274(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Kalveram KJ, Rapp-Frick C, Sorck G. Misleading patch test results with aluminium Finn chambers and mercury salts. Contact Dermatitis. 1980;6:507–508. doi: 10.1111/j.1600-0536.1980.tb05587.x. [DOI] [PubMed] [Google Scholar]
- Kamboj VP, Kar AB. Antitesticular effect of metallic and rare earth salts. J Reprod Fertil. 1964;7:21–28. doi: 10.1530/jrf.0.0070021. [DOI] [PubMed] [Google Scholar]
- Kammer C. Aluminum Handbook. Dusseldorf: Aluminium Verlag; 1999. [Google Scholar]
- Kandiah J, Kies C. Aluminum concentrations in tissues of rats: effect of soft drink packaging. Biometals. 1994;7:57–60. doi: 10.1007/BF00205195. [DOI] [PubMed] [Google Scholar]
- Kanematsu N, Hara M, Kada T. Rec assay and mutagenicity studies on metal compounds. Mutat Res. 1980;77:109–116. doi: 10.1016/0165-1218(80)90127-5. [DOI] [PubMed] [Google Scholar]
- Kang KY, Bice D, Hoffman E, D’Amato R, Salvaggio J. Experimental studies of sensitization to beryllium, zirconium, and aluminum compounds in the rabbit. J Allergy Clin Immunol. 1977;59:425–436. doi: 10.1016/0091-6749(77)90005-7. [DOI] [PubMed] [Google Scholar]
- Kapaki EN, Zourmas CP, Segdistsa IT, Xenos DS, Papageorgiou CT. Cerebrospinal fluid aluminum levels in Alzheimer’s disease. Biol Psych. 1993;33:679–681. doi: 10.1016/0006-3223(93)90114-s. [DOI] [PubMed] [Google Scholar]
- Kaplan HM, Timmons EH. The Rabbit A Model for the Principles of Mammalian Physiology and Surgery. New York: Academic Press; 1979. [Google Scholar]
- Karlik SJ, Eichhorn GL, Crapper DR. Molecular interactions of aluminum with DNA. Neurotoxicology. 1980a;1:83–88. [Google Scholar]
- Karlik SJ, Eichhorn GL, Levis PN, Crapper DR. Interaction of aluminum species with deoxyribonucleic acid. Biochemistry. 1980b;19:5991–5998. doi: 10.1021/bi00567a008. [DOI] [PubMed] [Google Scholar]
- Kasa P, Szerdahelyi P, Wisniewski HM. Lack of topographical relationship between sites of aluminum deposition and senile plaques in the Alzheimer’s disease brain. Acta Neuropathol. 1995;90:526–531. doi: 10.1007/BF00294815. [DOI] [PubMed] [Google Scholar]
- Kassem M, Eriksen EF, Melsen F, Mosekilde L. Antacid-induced osteomalacia: a case report with a histomorphometric analysis. J Intern Med. 1991;229:275–279. doi: 10.1111/j.1365-2796.1991.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Katz AC, Frank DW, Sauerhoff MW, Zwicker GM, Freudenthal RI. A 6-month dietary toxicity study of acidic sodium aluminium phosphate in beagle dogs. Food Chem Toxicol. 1984;22:7–9. doi: 10.1016/0278-6915(84)90045-0. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Muramoto K, Kobayashi K, Mori H, Kuroda Y. Aluminum promotes the aggregation of Alzheimer’s amyloid beta-protein in vitro. Biochem Biophys Res Commun. 1994;198:531–535. doi: 10.1006/bbrc.1994.1078. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Kato M, Kuroda Y. Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of beta-amyloid protein. Brain Res Bull. 2001;55:211–217. doi: 10.1016/s0361-9230(01)00475-0. [DOI] [PubMed] [Google Scholar]
- Keith LS, Jones DE, Chou CH. Aluminum toxicokinetics regarding infant diet and vaccinations. Vaccine. 2002;20(Suppl 3):S13–S17. doi: 10.1016/s0264-410x(02)00165-2. [DOI] [PubMed] [Google Scholar]
- Kennedy MC. Aluminium powder inhalations in the treatment of silicosis of pottery workers and pneumoconiosis of coal-miners. Br J Ind Med. 1956;13:85–101. doi: 10.1136/oem.13.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Kihira T, Yoshida S, Yase Y, Ono S, Kondo T. Chronic low-Ca/Mg high-Al diet induces neuronal loss. Neuropathology. 2002;22:171–179. doi: 10.1046/j.1440-1789.2002.00441.x. [DOI] [PubMed] [Google Scholar]
- Kihira T, Yoshida S, Kondo T, Yase Y, Ono S. ALS-like skin changes in mice on a chronic low-Ca/Mg high-Al diet. J Neurol Sci. 2004;219:7–14. doi: 10.1016/j.jns.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Warshaw RH. Irregular opacities in the lung, occupational asthma, and airways dysfunction in aluminum workers. Am J Ind Med. 1992;21:845–853. doi: 10.1002/ajim.4700210607. [DOI] [PubMed] [Google Scholar]
- King EJ, Wright BM. Effect of aluminium on the silicosis-producing action of inhaled quartz. Br J Ind Med. 1950;7:27–36. doi: 10.1136/oem.7.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EJ, Rogers N, Gilchrist M. Attempts to prevent silicosis with aluminium. J Pathol Bacteriol. 1945;57:281. [Google Scholar]
- King EJ, Harrison C, Mohanty G, Nagelschmidt G. The effect of various forms of alumina on the lungs of rats. J Pathol Bacteriol. 1955;69:81–93. doi: 10.1002/path.1700690113. [DOI] [PubMed] [Google Scholar]
- King EJ, Harrison CV, Mohanty GP, Yoganathan M. The effect of aluminum and of aluminum containing 5 per cent. of quartz in the lungs of rats. J Pathol Bacteriol. 1958;75:429–434. doi: 10.1002/path.1700750222. [DOI] [PubMed] [Google Scholar]
- King J, Day RS, Milne FJ, Bezwoda WR, Viljoen JD, Kramer S. Delayed onset of overt porphyria cutanea tarda in a patient on long-term haemodialysis. A case report. S Afr Med J. 1983;63:743–746. [PubMed] [Google Scholar]
- King SJ, Templar J, Miller RV, Day JP, Itzhaki RF, Fifield LK, Allan G. Aluminium and Alzheimer’s disease: sites of aluminium binding in human neuroblastoma cells determined using 26Al and accelerator mass spectrometry. Nuc Instr Meth Phys Res B. 1994;92:469–472. [Google Scholar]
- King SJ, Day JP, Oldham C, Popplewell JF, Ackrill P, Moore P, Taylor G, Edwardson JA, Fifield LK, Liu K, Cresswell RG. The influence of dissolved silicate on the physiological chemistry of aluminium, studies in humans using tracer 26Al and accelerator mass spectrometry. Nuc Instr Meth Phys Res. 1997;123:254–258. [Google Scholar]
- Kislinger G, Steinhausen C, Alvarez-Bruckmann M, Winklhofer C, Ittel T, Nolte E. Investigations of the human aluminium biokinetics with 26Al and AMS. Nuc Instr Meth Phys Res B. 1997;123:259–265. [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Klatzo I, Wisniewski H, Streicher E. Experimental production of neurofibrillary degeneration: 1. Light microscopic observation. J Neuropathol Exp Neurol. 1965;24:187–199. doi: 10.1097/00005072-196504000-00002. [DOI] [PubMed] [Google Scholar]
- Klein GL. Aluminum contamination of parenteral nutrition solutions and its impact on the pediatric patient. Nutr Clin Pract. 2003;18:302–307. doi: 10.1177/0115426503018004302. [DOI] [PubMed] [Google Scholar]
- Klein GL, Ott SM, Alfrey AC, Sherrard DJ, Hazlet TK, Miller NL, Maloney NA, Berquist WE, Ament ME, Coburn JW. Aluminum as a factor in the bone disease of long-term parenteral nutrition. Trans Assoc Am Physicians. 1982;95:155–164. [PubMed] [Google Scholar]
- Klein GL, Sedman AB, Heyman MB, Marathe G, Battifora HA, Worrall JL, Horst RL, Brewer GJ, Miller NL, Alfrey AC. Hepatic abnormalities associated with aluminum loading in piglets. J Parenter Enteral Nutr. 1987;11:293–297. doi: 10.1177/0148607187011003293. [DOI] [PubMed] [Google Scholar]
- Klein GL, Goldblum RM, Moslen MT, Pyron DL, Mann PA, Lee TC, Alfrey AC. Increased biliary transferrin excretion following parenteral aluminum administration to rats. Pharmacol Toxicol. 1993;72:373–376. doi: 10.1111/j.1600-0773.1993.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Klosterkötter W. Effects of ultramicroscopic gamma-aluminum oxide on rats and mice. AMA Arch Ind Health. 1960;21:458–472. [PubMed] [Google Scholar]
- Knodel L, Kendall S, Young L, Hickey M. Nonprescription Products: Formulations and Features ’96-97. Washington: American Pharmaceutical Association; 1996. [Google Scholar]
- Knoll O, Kellinghaus H, Bertram HP, Zumkley H, Graefe U. Gastrointestinal absorption of aluminum in chronic renal insufficiency. Contrib Nephrol. 1984;38:24–31. doi: 10.1159/000408063. [DOI] [PubMed] [Google Scholar]
- Knutti R, Zimmerli B. Investigation of the daily portions of Swiss food establishments: III. Lead, cadmium, mercury, nickel and aluminium. Mitt Geb Lebensm Hyg. 1985;76:206–232. [Google Scholar]
- Kobayashi S, Hirota N, Saito K, Utsuyama M. Aluminum accumulation in tangle-bearing neurons of Alzheimer’s disease with Balint’s syndrome in a long-term aluminum refiner. Acta Neuropathol. 1987;74:47–52. doi: 10.1007/BF00688337. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Fujiwara S, Arimoto S, Koide H, Fukuda J, Shimode K, Yamaguchi S, Okada K, Tsunematsu T. Hair aluminium in normal aged and senile dementia of Alzheimer type. Prog Clin Biol Res. 1989;317:1095–1109. [PubMed] [Google Scholar]
- Kobayashi K, Yumoto S, Nagai H, Hosoyama Y, Imamura M, Masuzawa S, Koizumi Y, Yamashita H. 26Al tracer experiment by accelerator mass spectrometry and its application to the studies for amyotrophic lateral sclerosis and Alzheimer’s disease. I Proc Jpn Acad B. 1990;66:189–192. [Google Scholar]
- Koch KR, Pougnet MA, de Villiers S, Monteagudo F. Increased urinary excretion of Al after drinking tea. Nature. 1988;333:122. doi: 10.1038/333122a0. [DOI] [PubMed] [Google Scholar]
- Koelsch F. Pulmonary disease caused by aluminum dust. Beitr Klin Tuberk. 1942;97:688–693. [Google Scholar]
- Kohila T, Parkkonen E, Tahti H. Evaluation of the effects of aluminium, ethanol and their combination on rat brain synaptosomal integral proteins in vitro and after 90-day oral exposure. Arch Toxicol. 2004;78:276–282. doi: 10.1007/s00204-003-0530-3. [DOI] [PubMed] [Google Scholar]
- Kongerud J, Samuelsen SO. A longitudinal study of respiratory symptoms in aluminium potroom workers. Am Rev Respir Dis. 1991;144:10–16. doi: 10.1164/ajrccm/144.1.10. [DOI] [PubMed] [Google Scholar]
- Kongerud J, Gronnesby JK, Magnus P. Respiratory symptoms and lung function of aluminium potroom workers. Scand J Work Environ Health. 1990;16:270–277. doi: 10.5271/sjweh.1785. [DOI] [PubMed] [Google Scholar]
- Kongerud J, Boe J, Soyseth V, Naalsund A, Magnus P. Aluminum potroom asthma: The Norwegian experience. Eur Resp J. 1994;7:165–172. doi: 10.1183/09031936.94.07010165. [DOI] [PubMed] [Google Scholar]
- Koo WW, Kaplan LA. Aluminum and bone disorders with specific reference to aluminum contamination of infant nutrients. J Am Coll Nutr. 1988;7:199–214. doi: 10.1080/07315724.1988.10720237. [DOI] [PubMed] [Google Scholar]
- Koo WW, Kaplan LA, Bendon R, Succop T, Tsang RC, Horn J, Steichen JJ. Response to aluminum in parenteral nutrition during infancy. J Pediatr. 1986;109:877–883. doi: 10.1016/s0022-3476(86)80718-1. [DOI] [PubMed] [Google Scholar]
- Koo WW, Kaplan LA, Krug-Wispe SK. Aluminum contamination of infant formulas. J Parenter Enteral Nutr. 1988;12:170–173. doi: 10.1177/0148607188012002170. [DOI] [PubMed] [Google Scholar]
- Kotovirta ML, Salo OP, Visa-Tolvanen K. Contact sensitivity to aluminium. Contact Dermatitis. 1984;11:135. doi: 10.1111/j.1600-0536.1984.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Kovalchik MT, Kaehny WD, Hegg AP, Jackson JT, Alfrey AC. Aluminum kinetics during hemodialysis. J Lab Clin Med. 1978;92:712–720. [PubMed] [Google Scholar]
- Krachler M, Prohaska T, Koellensperger G, Rossipal E, Stingeder G. Concentrations of selected trace elements in human milk and in infant formulas determined by magnetic sector field inductively coupled plasma-mass spectrometry. Biol Trace Elem Res. 2000;76:97–112. doi: 10.1385/BTER:76:2:97. [DOI] [PubMed] [Google Scholar]
- Kralj B, Krizaj I, Bukovec P, Slejko S, Milacic R. Speciation of aluminium in tea infusions by use of SEC and FPLC with ICP-OES and ES-MS-MS detection. Anal Bioanal Chem. 2005;383:467–475. doi: 10.1007/s00216-005-3312-3. [DOI] [PubMed] [Google Scholar]
- Krasnukhina AV, Laptev SF, Vakhramova SP. Reactions of some metal cations with oleic acid in water. Sb Tr Tsent Nauch Issled-Inst Olovyan Prom. 1971;1:69–71. [Google Scholar]
- Krasovskii GN, Vasukovich LY, Chariev OG. Experimental study of biological effects of lead and aluminum following oral administration. Environ Health Perspect. 1979;30:47–51. doi: 10.1289/ehp.30-1637724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus AS, Forbes WF. Aluminum, fluoride and the prevention of Alzheimer’s disease. Can J Pub Health. 1992;83:97–100. [PubMed] [Google Scholar]
- Kraus T, Schaller KH, Angerer J, Letzel S. Aluminium dust-induced lung disease in the pyro-powder-producing industry: detection by high-resolution computed tomography. Int Arch Occup Environ Health. 2000;73:61–64. doi: 10.1007/pl00007939. [DOI] [PubMed] [Google Scholar]
- Krueger GL, Morris TK, Suskind RR, Widner EM. The health effects of aluminum compounds in mammals. Crit Rev Toxicol. 1984;13:1–24. doi: 10.3109/10408448409029320. [DOI] [PubMed] [Google Scholar]
- Kruglikov GG, Velichkovskii BT, Garmash TI. Morphology of pneumoconiosis induced by natural zeolite. Gig Tr Prof Zabol. 1990;5:14–17. [PubMed] [Google Scholar]
- Kukhtina AP. Effect of age and the functional condition of the central nervous system on the level of some trace elements in the skeletal muscles and blood of white rats. Mikroelementy v Meditsine. 1972;3:123–126. [Google Scholar]
- Kumar S. Aluminum-induced biphasic effect. Med Hypotheses. 1999;52:557–559. doi: 10.1054/mehy.1997.0693. [DOI] [PubMed] [Google Scholar]
- Kushelevsky A, Yagil R, Alfasi Z, Berlyne GM. Uptake of aluminium ion by the liver. Biomed. 1976;25:59–60. [PubMed] [Google Scholar]
- Kvech S, Edwards M. Solubility controls on aluminium in drinking water at relatively low and high pH. Water Res. 2002;36:4356–4368. doi: 10.1016/s0043-1354(02)00137-9. [DOI] [PubMed] [Google Scholar]
- L’vov BV, Polzik LK, Weinbruch S, Ellingsen DG, Thomassen Y. Theoretical aspects of fluoride air contaminant formation in aluminium smelter potrooms. J Environ Monit. 2005;7:425–430. doi: 10.1039/b501302j. [DOI] [PubMed] [Google Scholar]
- Lacson AG, D’Cruz CA, Gilbert-Barness E, Sharer L, Jacinto S, Cuenca R. Aluminum phagocytosis in quadriceps muscle following vaccination in children: relationship to macrophagic myofasciitis. Pediatr Dev Pathol. 2002;5:151–158. doi: 10.1007/s10024001-0137-8. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos A, Banyai I, Decock P, Kiss T. Time-dependent solution speciation of the AlIII-citrate system: potentiometric and NMR studies. Eur J Inorg Chem. 2001;2001:461–469. [Google Scholar]
- Landau AJ, Eberhardt RT, Frishman WH. Intranasal delivery of cardiovascular agents: an innovative approach to cardiovascular pharmacotherapy. Am Heart J. 1994;127:1594–1599. doi: 10.1016/0002-8703(94)90391-3. [DOI] [PubMed] [Google Scholar]
- Landsberg JP, McDonald B, Watt F. Absence of aluminium in neuritic plaque cores in Alzheimer’s disease. Nature. 1992;360:65–68. doi: 10.1038/360065a0. [DOI] [PubMed] [Google Scholar]
- Landsberg J, McDonald B, Grime G, Watt F. Microanalysis of senile plaques using nuclear microscopy. J Geriatr Psychiatry Neurol. 1993;6:97–104. doi: 10.1177/089198879300600206. [DOI] [PubMed] [Google Scholar]
- Lansdown AB. Production of epidermal damage in mammalian skins by some simple aluminum compounds. Br J Dermatol. 1973;89:67–76. doi: 10.1111/j.1365-2133.1973.tb01919.x. [DOI] [PubMed] [Google Scholar]
- Lapenas DJ, Gale PN. Kaolin pneumoconiosis. A case report. Arch Pathol Lab Med. 1983;107:650–653. [PubMed] [Google Scholar]
- Larsen S, Lund R, Nielsen D, Thygesen P, Poulsen O. Adjuvant effect of di-n-butyl-, di-n-octyl-, di-iso-nonyl and di-iso-decyl phtalate in a subcutaneous injection model using BALB/c mice. Pharmacol Toxicol. 2002;91:264–272. doi: 10.1034/j.1600-0773.2002.910508.x. [DOI] [PubMed] [Google Scholar]
- Larsson NPO, Tapper UAS, Sturesson K, Odselius R, Brun A. Technical aspects of nuclear microprobe analysis of senile plaques from Alzheimer patients. Nuc Inst Meth Phys Res B. 1990;49:472–475. [Google Scholar]
- Lawley A. Modern powder metallurgy science and technology. JOM. 1986;38:15–26. [Google Scholar]
- Lazerte BD, Van Loon G, Anderson B. Aluminum in water. In: Yokel RA, Golub M, editors. Research Issues in Aluminum Toxicity. Washington DC: Taylor & Francis; 1997. pp. 17–45. [Google Scholar]
- Le Bouffant L, Daniel H, Martin JC. The therapeutic action of aluminium compounds on the development of experimental lesions produced by pure quartz or mixed dust. Inhaled Part. 1975;4:389–401. [PubMed] [Google Scholar]
- Leblondel G, Allain P. Blood and brain aluminium concentrations in mice after intraperitoneal injection of different aluminium compounds. Res Commun Chem Pathol Pharmacol. 1980;27:579–586. [PubMed] [Google Scholar]
- Leboeuf RC, Tolson D, Heinecke JW. Dissociation between tissue iron concentrations and transferrin saturation among inbred mouse strains. J Lab Clin Med. 1995;126:128–136. [PubMed] [Google Scholar]
- Lee DS, Garland JA, Fox AA. Atmospheric concentrations of trace elements in urban areas of the United Kingdom. Atmos Environ. 1994;28:2691–2713. [Google Scholar]
- Lee PK, Brook JR, Dabek-Zlotorzynska E, Mabury SA. Identification of the major sources contributing to PM2.5 observed in Toronto. Environ Sci Technol. 2003;37:4381–4840. doi: 10.1021/es026473i. [DOI] [PubMed] [Google Scholar]
- Lemaire I. Selective differences in macrophage populations and monokine production in resolving pulmonary granuloma and fibrosis. Am J Pathol. 1991;138:487–495. [PMC free article] [PubMed] [Google Scholar]
- Lemaire I, Dionne PG, Nadeau D, Dunnigan J. Rat lung reactivity to natural and man-made fibrous silicates following short-term exposure. Environ Res. 1989;48:193–210. doi: 10.1016/s0013-9351(89)80034-9. [DOI] [PubMed] [Google Scholar]
- Lenntech. EU’s drinking water standards. 2004 [online] Cited 24 January 2007 http://www.lenntech.com/EU’s-drinking-water-standards.htm.
- Lenz TR. Foreign body granuloma caused by jet injection of tetanus toxoid. Rocky Mt Med J. 1966;63:48. [PubMed] [Google Scholar]
- Léonard A, Gerber GB. Mutagenicity, carcinogenicity and teratogenicity of aluminium. Mutat Res. 1988;196:247–257. doi: 10.1016/0165-1110(88)90009-7. [DOI] [PubMed] [Google Scholar]
- Lesnikovich AI, Shunkevich TM, Vorob’eva JA, Maumenko VN. Interaction of highly dispersed aluminum with oleic acid. Vestnik Belorusskogo Gosudarstvennogo Universiteta, Seriya 2: Khimiya Biologiya Geografiya. 1993;1:7–9. [Google Scholar]
- Letzel S, Schaller KH, Angerer J, Drexler H, Weber A, Schmid K, Weltle D. Biological monitoring of occupational aluminum powder exposure. Occup Hyg. 1996;3:271–280. [Google Scholar]
- Letzel S, Lang C, Schaller KH, Angerer J, Fuchs S, Neudörfer B, Lehnert G. Longitudinal study of neurotoxicity with occupational exposure to aluminium dust. Neurology. 2000;54:997–1000. doi: 10.1212/wnl.54.4.997. [DOI] [PubMed] [Google Scholar]
- Leung FY, Hodsman AB, Muirhead N, Henderson AR. Ultrafiltration studies in vitro of serum aluminum in dialysis patients after deferoxamine chelation therapy. Clin Chem. 1985;31:20–23. [PubMed] [Google Scholar]
- Levatidi J, Guibert L, Wolfromm R. Experimental study of tissue reactions following repeated injections of aluminium hydroxide. Ann Inst Pasteur. 1968;114:511–517. [PubMed] [Google Scholar]
- Lévesque L, Mizzen CA, McLachlan DR, Fraser PE. Ligand specific effects on aluminum incorporation and toxicity in neurons and astrocytes. Brain Res. 2000;877:191–202. doi: 10.1016/s0006-8993(00)02637-8. [DOI] [PubMed] [Google Scholar]
- Lewis CW, Macias ES. Composition of size-fractioned aerosols in Charleston, WV. Atmos Environ. 1980;14:185–194. [Google Scholar]
- Lewis JL, Hahn FF, Dahl AR. Transport of inhaled toxicants to the central nervous system. Characteristics of a nose-brain barrier. In: Isaacson RL, Jensen KF, editors. The Vulnerable Brain and Environmental Risks, Vol 3: Toxins in Air and Water. New York: Plenum Press; 1994. pp. 77–103. [Google Scholar]
- Li D, Xiao B, Dong Q, Li S, Zhan C. Aluminum and fluorine absorption in a perfusion system of rat small intestine in vivo. J West China Univ Med Sci. 1991;22:189–191. [PubMed] [Google Scholar]
- Liang L, D’Haese PC, Lamberts LV, De Broe ME. Direct calibration for determining aluminum in bone and soft tissues by graphite furnace atomic absorption spectrometry. Clin Chem. 1991;37:461–466. [PubMed] [Google Scholar]
- Liao YH, Yu HS, Ho CK, Wu MT, Yang CY, Chen JR, Chang CC. Biological monitoring of exposures to aluminium, gallium, indium, arsenic, and antimony in optoelectronic industry workers. J Occup Environ Med. 2004;46:931–936. doi: 10.1097/01.jom.0000137718.93558.b8. [DOI] [PubMed] [Google Scholar]
- Liddiard PD. Aluminum powder metallurgy in perspective. Powder Metall. 1984;27:193–200. [Google Scholar]
- Lie DR, editor. CRC Handbook of Chemistry and Physics. 71. Boca Raton, FL: The Chemical Rubber Publishing Company; 19901991. [Google Scholar]
- Lieuallen WG, Weisbrode SE. Effects of systemic aluminium on the resolution of a uremic and dieteary phosphorus-dependent model of uremic osteomalacia in rats. J Bone Mineral Res. 1991;6:751–757. doi: 10.1002/jbmr.5650060713. [DOI] [PubMed] [Google Scholar]
- Lindberg JS, Copley JB, Koenig KG, Cushner HM. Effect of citrate on serum aluminum concentrations in hemodialysis patients: a prospective study. South Med J. 1993;86:1385–1388. doi: 10.1097/00007611-199312000-00013. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt RC, Driscoll KE, Perkins MA, Higgins JM, Maurer JK, Belfiore KA. The comparison of a fibrogenic and two nonfibrogenic dusts by bronchoalveoar lavage. Toxicol Appl Pharmacol. 1990;102:268–281. doi: 10.1016/0041-008x(90)90026-q. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Linse R, Hadlich J, Kirsten D. Cutaneous foreign body granulomas casued by aluminium hydroxide after desensitization with Mischpollen-Depotallergen. Dermatol Monatsschr. 1979;165:653–657. [PubMed] [Google Scholar]
- Linss VW, Martin R, Stein G, Bräunlich H, Fleck C. Electron microscopic evidence of aluminium in lysosomes of kidney cells by electron energy loss spectroscopy. Acta Histochem. 1991;90:65–73. [PubMed] [Google Scholar]
- Linss W, Martin R, Stein G, Bräunlich H, Fleck C. The occurrence of aluminum-containing lysosomes in the kidney of experimentally treated rats. Acta Histochem Suppl. 1992;42:273–276. [PubMed] [Google Scholar]
- Linton RW, Bryan SR, Griffis DP, Shelburne JD, Fiori CE, Garruto RM. Digital imaging studies of aluminum and calcium in neurofibrillary tangle-bearing neurons using secondary ion mass spectrometry. Trace Elem Med. 1987;4:99–104. [Google Scholar]
- Lione A. The prophylactic reduction of aluminium intake. Food Chem Toxicol. 1983;21:103–109. doi: 10.1016/0278-6915(83)90277-6. [DOI] [PubMed] [Google Scholar]
- Lione A, Smith JC. The mobilization of aluminum from three brands of chewing gum. Food Chem Toxicol. 1982;20:945–946. doi: 10.1016/s0015-6264(82)80233-2. [DOI] [PubMed] [Google Scholar]
- Litov RE, Sickles VS, Chan GM, Springer MA, Cordano A. Plasma aluminum measurements in term infants fed human milk or a soy-based infant formula. Pediatrics. 1989;84:1105–1107. [PubMed] [Google Scholar]
- Liu P, Yao YN, Wu SD, Dong HJ, Feng GC, Yuan XY. The efficacy of deferiprone on tissues aluminum removal and copper, zinc, manganese level in rabbits. J Inorg Biochem. 2005;99:1733–1737. doi: 10.1016/j.jinorgbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Liu SM, Chung C. Trace elements in Taiwanese diet in different seasons. J Radioanal Nucl Chem. 1992;161:27–38. [Google Scholar]
- Liu SM, Chung C, Chan CC. Daily dietary intake of Pratas islanders in the South China Sea. J Radioanal Nucl Chem. 1992;162:363–370. [Google Scholar]
- Ljunggren KG, Lidums V, Sjögren B. Blood and urine concentrations of aluminum among workers exposed to aluminum flake powders. Br J Ind Med. 1991;48:106–109. doi: 10.1136/oem.48.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A, Blesa R, Angelopoulos C, Pastor-Rubio P, Villa M, Oliva R, Bufill E. Transferrin C2 allele, haemochromatosis gene mutations, and risk for Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72:820–821. doi: 10.1136/jnnp.72.6.820-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet JM, Colomina MT, Sirvent JJ, Domingo JL, Corbella J. Reproductive toxicology of aluminum in male mice. Fundam Appl Toxicol. 1995;25:45–51. doi: 10.1006/faat.1995.1038. [DOI] [PubMed] [Google Scholar]
- Long JF, Renkes G, Steinmeyer CL, Nagode LA. Effect of calcitriol infusions on serum aluminum in vitamin D-depleted rabbits fed an aluminum-supplemented ration. Res Commun Chem Pathol Pharmacol. 1991;74:89–104. [PubMed] [Google Scholar]
- Long JF, Nagode LA, Stemeyer CL, Renkes G. Comparative effects of calcitriol and parathyroid hormone on serum aluminum in vitamin D-depleted rabbits fed an aluminum-supplemented diet. Res Commun Chem Pathol Pharmacol. 1994;83:3–14. [PubMed] [Google Scholar]
- López FF, Cabrera C, Lorenzo ML, López M. Aluminium content of drinking waters, fruit juices and soft drinks: contribution to dietary intake. Sci Total Environ. 2002;292:205–213. doi: 10.1016/s0048-9697(01)01122-6. [DOI] [PubMed] [Google Scholar]
- Lopez S, Pelaez A, Navarro LA, Montesinos E, Morales C, Carda C. Aluminum allergy in patients hyposensitized with aluminum-precipitated antigen extracts. Contact Dermatitis. 1994;31:37–40. doi: 10.1111/j.1600-0536.1994.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Lorusso A, Sama B, Giacomazzi G, Magarotto G. A retrospective assessment of 19 subjects compensated for the inhalation of aluminum powders (item 48, D.P.R. 482/75) Med Lav. 1992;83:451–455. [PubMed] [Google Scholar]
- Lote CJ, Wood JA, Saunders HC. Renal filtration, reabsorption and excretion of aluminium in the rat. Clin Sci. 1992;82:13–18. doi: 10.1042/cs0820013. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Ehmann WD, Markesbery WR. Laser microprobe analysis of brain aluminium in Alzheimer’s disease. Ann Neurol. 1993;33:36–42. doi: 10.1002/ana.410330107. [DOI] [PubMed] [Google Scholar]
- Lu ZY, Gong H, Amemiya T. Aluminum chloride induces retinal changes in the rat. Toxicol Sci. 2002;66:253–260. doi: 10.1093/toxsci/66.2.253. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Krishnan B, Wong L, Kruck TP, Bergeron C, Crapper McLachlan DR. Nuclear compartmentalization of aluminum in Alzheimer’s disease (AD) Neurobiol Aging. 1991;13:115–121. doi: 10.1016/0197-4580(92)90018-s. [DOI] [PubMed] [Google Scholar]
- Lum KR, Betteridge JS, Macdonald RR. The potential availability of P, Al, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn in urban particulate matter. Env Technol Lett. 1982;3:57–62. [Google Scholar]
- Lynch KM, McIver FA. Pneumoconiosis from exposure to kaolin dust kaolinosis. Am J Pathol. 1954;30:1117–1127. [PMC free article] [PubMed] [Google Scholar]
- Lysiak M. Somatotropic hormone and pH of the gastric juice. Patol Pol. 1963;14:477–478. [PubMed] [Google Scholar]
- MacFarland HN, Hornstein N. An experimental investigation of the effects of the inhalation of alumina and other fumes. Can Chem Process Industries. 1949;33:145. [Google Scholar]
- Mackay IR, Oliphant RC, Laby B, Smith MM, Fisher JN, Mitchell RJ, Propert PN, Tait BD. An immunologic and genetic study of asthma in workers in an aluminum smelter. J Occup Med. 1990;32:1022–1026. [PubMed] [Google Scholar]
- Maenhaut W, Ducastel G, Leck C, Nilsson ED, Heintzenberg J. Multi-elemental composition and sources of the high arctic atmospheric aerosol during summer and autumn. Tellus B. 1996;48:300–321. [Google Scholar]
- Mahieu S, Calvo ML. Effect of chronic poisoning with aluminum on the renal handling of phosphate in the rat. Toxicol Lett. 1998;94:47–56. doi: 10.1016/s0378-4274(97)00101-x. [DOI] [PubMed] [Google Scholar]
- Mahieu S, Calvo ML, Millen N, Gonzalez M, Contini MC. Growth and metabolism of calcium in rats chronically poisoned with aluminium hydroxide. Acta Physiol Pharmacol Ther Latinoam. 1998;48:32–40. [PubMed] [Google Scholar]
- Mahieu S, del Carmen Contini M, Gonzalez M, Millen N, Elias MM. Aluminum toxicity. Hematological effects. Toxicol Lett. 2000;111:235–242. doi: 10.1016/s0378-4274(99)00184-8. [DOI] [PubMed] [Google Scholar]
- Mahieu ST, Navoni J, Millen N, del Carmen CM, Gonzalez M, Elias MM. Effects of aluminum on phosphate metabolism in rats: a possible interaction with vitamin D3 renal production. Arch Toxicol. 2004;78:609–616. doi: 10.1007/s00204-004-0579-7. [DOI] [PubMed] [Google Scholar]
- Maitani T, Kubota H, Hori N, Yoshihira K, Takeda M. Distribution and urinary excretion of aluminum injected with several organic acids into mice: Relationship with chemical state in serum studied by the HPLC-ICP method. J Appl Toxicol. 1994;14:257–261. doi: 10.1002/jat.2550140403. [DOI] [PubMed] [Google Scholar]
- Makjanic J, McDonald B, Li-Hsian Chen CP, Watt F. Absence of aluminum in neurofibrillary tangles in Alzheimer’s disease. Neurosci Lett. 1998;240:123–126. doi: 10.1016/s0304-3940(97)00940-3. [DOI] [PubMed] [Google Scholar]
- Malluche HH. Aluminum and bone disease in chronic renal failure. Nephrol Dial Tranplant. 2002;172(Suppl 2):21–24. doi: 10.1093/ndt/17.suppl_2.21. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Faugere MC, Friedler RM, Matthews C, Fanti P. Calcitriol, parathyroid hormone, and accumulation of aluminum in bone in dogs with renal failure. J Clin Invest. 1987;79:754–761. doi: 10.1172/JCI112881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandic ML, Grgic J, Grgic Z, Seruga M, Hasenay D. Aluminum levels in human milk. Sci Total Environ. 1995;170:165–170. doi: 10.1016/0048-9697(95)04702-4. [DOI] [PubMed] [Google Scholar]
- Manna GK, Das RK. Chromosome aberration in mice induced by aluminum chloride. Nucleus. 1972;15:180–186. [Google Scholar]
- Manna GK, Parida BB. Aluminum chloride induced meiotic chromosome aberrations in the grasshopper. Phloeoba antennata Naturwissenschaften. 1965;52:647–648. [Google Scholar]
- Mantyh PW, Ghilardi JR, Rogers S, Demaster E, Allen CJ, Stimson ER, Maggio JE. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J Neurochem. 1993;61:1171–1174. doi: 10.1111/j.1471-4159.1993.tb03639.x. [DOI] [PubMed] [Google Scholar]
- Marchante-Gayon JM, Muniz CS, Alonson JI, Sanz-Medel A. Multielemental trace analysis of biological materials using double focusing inductively coupled plasma mass spectrometry detection. Analyt Chim Acta. 1999;400:307–320. [Google Scholar]
- Marcus DL, Wong S, Freedman ML. Dietary aluminum and Alzheimer’s disease. J Nutr Elder. 1992;12:55–61. doi: 10.1300/j052v12n02_04. [DOI] [PubMed] [Google Scholar]
- Marek J, Blaha V. Some methodical and morphological aspects of the bentonite-induced inflammatory reaction in rat. Acta Univ Palacki Olomuc Fac Med. 1985;108:151–170. [PubMed] [Google Scholar]
- Mark A, Björkstén B, Granström M. Immunoglobulin E responses to diptheria and tetanus toxoids after booster with aluminium-adsorbed and fluid DT-vaccines. Vaccine. 1995;13:669–673. doi: 10.1016/0264-410x(94)00017-h. [DOI] [PubMed] [Google Scholar]
- Markesbery W, Ehmann W, Hossain T, Alauddin M, Goodin D. Instrumental neutron activation analysis of brain aluminum in Alzheimer disease and aging. Ann Neurol. 1981;10:511–516. doi: 10.1002/ana.410100604. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Ehmann WD, Alauddin M, Hossain TIM. Brain trace element concentrations in aging. Neurobiol Aging. 1984;5:19–28. doi: 10.1016/0197-4580(84)90081-2. [DOI] [PubMed] [Google Scholar]
- Martin JC, Daniel H, Le Bouffant L. Short-and long-term experimental study of the toxicity of coal-mine dust and of some of its constituents. Inhaled Part. 1975;4(Pt 1):361–371. [PubMed] [Google Scholar]
- Martin RB. The chemistry of aluminum as related to biology and medicine. Clin Chem. 1986;32:1797–1806. [PubMed] [Google Scholar]
- Martin RB. Aluminum: a neurotoxic product of acid rain. Acc Chem Res. 1994;27:204–210. [Google Scholar]
- Martin RB. Ternary complexes of Al3+ and F with a third ligand. Coord Chem Rev. 1996;141:23–32. [Google Scholar]
- Martyn CN, Barker DJ, Osmond O, Harris EC, Edwardson JA, Lacey RF. Geographical relation between Alzheimer’s disease and aluminium in drinking water. Lancet. 1989;1:59–62. [PubMed] [Google Scholar]
- Martyn CN, Coggon DN, Inskip H, Lacey RF, Young WF. Aluminum concentrations in drinking water and risk of Alzheimer’s disease. Epidemiology. 1997;8:281–286. doi: 10.1097/00001648-199705000-00009. [DOI] [PubMed] [Google Scholar]
- Marumo F, Tsukamoto Y, Iwanami S, Kishimoto T, Yamagami S. Trace element concentrations in hair, fingernails and plasma of patients with chronic renal failure on hemodialysis and hemofiltration. Nephron. 1984;38:267–272. doi: 10.1159/000183321. [DOI] [PubMed] [Google Scholar]
- Marzin DR, Phi HV. Study of the mutagenicity of metal derivatives with Salmonella typhimurium TA102. Mutat Res. 1985;155:49–51. doi: 10.1016/0165-1218(85)90024-2. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Tuttle RS. Aluminum in the organs and diet of ageing C57BL/6J mice. Mech Ageing Dev. 1988;45:145–156. doi: 10.1016/0047-6374(88)90104-2. [DOI] [PubMed] [Google Scholar]
- Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer’s disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985;4:2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo G, Fadda E, Marzia V. Polycyclic aromatic hydrocarbons and cancer in man. Environ Health Perspect. 1996;104:1166–1170. doi: 10.1289/ehp.961041166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R, Salusky I, Harman W, Paredes A, Emans J, Segre G, Goodman W. Renal bone disease in pediatric and young adult patients on hemodialysis in a children’s hospital. J Am Soc Nephrol. 1993;3:1938–1946. doi: 10.1681/ASN.V3121938. [DOI] [PubMed] [Google Scholar]
- Matsuda R, Sasaki K, Sakai H, Aoyagi Y, Saeki M, Hasegawa Y, Hidaka T, Ishii K, Mochizuki E, Yamamoto T, Miyabe M, Tamura Y, Hori S, Ikebe K, Tsuji M, Kojima M, Saeki K, Matsuoka S, Nishioka C, Fujita H, Shiroma H, Oshiro Z, Toyoda M. Estimation of daily dietary intake of aluminum. Shokuhin Eiseigaku Zasshi. 2001;42:18–23. doi: 10.3358/shokueishi.42.18. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Tanaka I, Kodama Y. Pulmonary deposition and clearance of a coal fly ash aerosol by inhalation. Environ Res. 1986;41:195–200. doi: 10.1016/s0013-9351(86)80181-5. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Ehmann WD, Markesbery WR. Comparison of the effects of elevated intracellular aluminum and calcium levels on neuronal survival and tau immunoreactivity. Brain Res. 1993;602:21–31. doi: 10.1016/0006-8993(93)90236-g. [DOI] [PubMed] [Google Scholar]
- Mauro LS, Kuhl DA, Kirchhoff JR, Mauro VF, Hamilton RW. Impact of oral bases on aluminum absorption. Am J Therap. 2001;8:21–25. doi: 10.1097/00045391-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Maynard EA. Gold, silver, manganese, and other minor metals. In: DiPalma JR, editor. Drill’s Pharacology in Medicine. 3. New York: McGraw-Hill Book Company; 1965. pp. 872–887. [Google Scholar]
- Mayor GH, Keiser JA, Makdani D, Ku PK. Aluminum absorption and distribution: Effect of parathyroid hormone. Science. 1977;197:1187–1189. doi: 10.1126/science.897661. [DOI] [PubMed] [Google Scholar]
- Mazzaferro S, Coen G, Ballanti P, Costantini S, Bondatti F, Giordano R, Manni M, Pasquali M, Perruzza I, Sardella A. Deferoxamine test and PTH serum levels are useful not to recognize but to exclude aluminum-related bone disease. Nephron. 1992;61:151–157. doi: 10.1159/000186863. [DOI] [PubMed] [Google Scholar]
- Mazzera DM, Lowenthal DH, Chow JC, Watson JG, Grubisic V. PM10 measurements at McMurdo station, Antarctica. Atmos Environ. 2001;35:1891–1902. [Google Scholar]
- McColl KE, Simpson K, Laiwah AY, Thompson GG, McDougall A, Moore MR. Hemodialysis-related porphyria cutanea tarda-treatment failure with charcoal hemoperfusion. Photdermatol. 1986;3:169–173. [PubMed] [Google Scholar]
- McCullough ML, Hsu N. Metabolic bone disease in home total parenteral nutrition. J Am Diet Assoc. 1987;87:915–920. [PubMed] [Google Scholar]
- McDermott JR, Smith A, Ward MK, Parkinson IS, Kerr DN. Brain-aluminium concentration in dialysis encephalopathy. Lancet. 1978;1:901–904. doi: 10.1016/s0140-6736(78)90681-5. [DOI] [PubMed] [Google Scholar]
- McDermott JR, Smith I, Iqbal K, Wisniewski HM. Brain aluminum in aging and Alzheimer disease. Neurology. 1979;27:809–814. doi: 10.1212/wnl.29.6.809. [DOI] [PubMed] [Google Scholar]
- McDonald B, Haszard R, Spence A, Osbourne K. A mortality study of Alzheimer’s disease and aluminum exposure through inhalation of McIntyre powder in Cornish tin miners. Neurobiol Aging. 1996;17:S122–S123. [Google Scholar]
- McFadden N, Lyberg T, Hensten-Pettersen A. Aluminum-induced granulomas in a tattoo. J Am Acad Dermatol. 1989;20:903–908. doi: 10.1016/s0190-9622(89)70104-3. [DOI] [PubMed] [Google Scholar]
- McGrath KG. An earlier age of breast cancer diagnosis related to more frequent use of antiperspirants/deodorants and underarm shaving. Eur J Cancer Prev. 2003;12:479–485. doi: 10.1097/00008469-200312000-00006. [DOI] [PubMed] [Google Scholar]
- McGregor SJ, Brock JH, Halls D. The role of transferrin and citrate in cellular uptake of aluminium. Biol Metals. 1991;4:173–175. doi: 10.1007/BF01141310. [DOI] [PubMed] [Google Scholar]
- McGregor SJ, Fernandez Menendez MJ, Naves ML, Elloriaga R, Brock JH, Cannata JB. The uptake of aluminum and its effect on iron metabolism in the osteoblast like cell line MG-63. Trace Elem Electrolytes. 1994;11:187–191. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKinney TD, Basinger, Dawson E, Jones MM. Serum aluminum levels in dialysis dementia. Nephron. 1982;32:53–56. doi: 10.1159/000182802. [DOI] [PubMed] [Google Scholar]
- McLachlan DR, Crapper McLachlan CD, Krishnan B, Krishnan SS, Dalton AJ, Steele JC. Aluminum and calcium in soil and food from Guam, Palau and Jamaica: implications for amyotrophic lateral sclerosis and parkinsonism-dementia syndromes of Guam. Environ Geochem Health. 1989;11:45–53. doi: 10.1007/BF01782992. [DOI] [PubMed] [Google Scholar]
- McLachlan DR, Bergeron C, Smith JE, Boomer D, Rifat SL. Risk for neuropathologically confirmed Alzheimer’s disease and residual aluminum in municipal drinking water employing weighted residential histories. Neurology. 1996;46:401–405. doi: 10.1212/wnl.46.2.401. [DOI] [PubMed] [Google Scholar]
- McLaughlin AIG, Kazantzis G, King E, Teare D, Porter RJ, Owen R. Pulmonary fibrosis and encephalopathy associated with the inhalation of aluminium dust. Br J Ind Med. 1962;19:253–263. doi: 10.1136/oem.19.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan TM, Freemont AJ, Herxheimer A, Denton J, Taylor AP, Pazianas M, Cummin AR, Eastwood JB. Camelford water poisoning accident: serial neuropsychological assessments and further observations on bone aluminium. Hum Exp Toxicol. 1993;12:37–42. doi: 10.1177/096032719301200108. [DOI] [PubMed] [Google Scholar]
- McNall AD, Fosmire GJ. Zinc status does not affect aluminum deposition in tissues of rats. Biol Trace Elem Res. 1996;53:7–18. doi: 10.1007/BF02784540. [DOI] [PubMed] [Google Scholar]
- Meda L, Marra G, Galfetti L, Inchingalo S, Severini F, De Luca L. Nano-composites for rocket and solid propellants. Compos Sci Technol. 2004;65:769–773. [Google Scholar]
- Medical Research Council. Medical Research Council’s Committee on Clinical Trials of Influenza, V., Second progress report by the 1955 Antibody responses and clinical reactions with saline and oil adjuvant influenza virus vaccines. Br Med J. 1955;2:1229–1232. [PMC free article] [PubMed] [Google Scholar]
- Meding B, Augustsson A, Hansson C. Short communications. Patch test reactions to aluminium. Contact Dermatitis. 1984;10:107. doi: 10.1111/j.1600-0536.1984.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Meirav O, Sutton RA, Fink D, Middleton R, Klein J, Walker VR, Halabe A, Vetterli D, Johnson RR. Accelerator mass spectrometry: application to study of aluminum kinetics in the rat. Am J Physiol. 1991;260:F466–469. doi: 10.1152/ajprenal.1991.260.3.F466. [DOI] [PubMed] [Google Scholar]
- Meshitsuka S, Inoue M. Urinary excretion of aluminum from antacid ingestion and estimation of its apparent biological half-time. Trace Elem Electrolytes. 1998;15:132–135. [Google Scholar]
- Meshitsuka S, Matsushima F, Nose T. Absorption of aluminum from aspirin preparations with aluminum glycinate. Trace Elem Electrolytes. 1999;16:175–176. [Google Scholar]
- Meyer FA, Kasper W. Examination of the effects of aluminium on the lung. Dtsch Arch Klin Med. 1942a;189:471–495. [Google Scholar]
- Meyer FA, Kasper W. Results of examination of workers in an aluminum-stamping works. Sitzber physik -med Sozietat Erlangen. 1942b;73:71–75. [Google Scholar]
- Migliore L, Cocchi L, Nesti C, Sabbioni E. Micronuclei assay and FISH analysis in human lymphocytes treated with six metal salts. Environ Mol Mutagen. 1999;34:279–284. doi: 10.1002/(sici)1098-2280(1999)34:4<279::aid-em8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Miliauskas JR, Mukherjee T, Dixon B. Postimmunization (vaccination) injection-site reactions. A report of four cases and review of the literature. Am J Surg Pathol. 1993;17:516–524. [PubMed] [Google Scholar]
- Miller RR, Churg AM, Hutcheon M, Lom S. Pulmonary alveolar proteinosis and aluminum dust exposure. Am Rev Respir Dis. 1984;130:312–315. doi: 10.1164/arrd.1984.130.2.312. [DOI] [PubMed] [Google Scholar]
- Mineralogy Database. 2006 [online] Cited 24 January 2007. http://webmineral.com.
- MAFF (Ministry of Agriculture Fisheries and Food) Fifteenth report of the Steering Group on Food Surveillance, the working party on the monitoring of foodstuffs for heavy metals Food Surveillance Paper No 15. London, UK: The Ministry; 1985. Survey of Aluminium, Antimony, Chromium, Cobalt, Indium, Nickel, Thallium and Tin in Food. [Google Scholar]
- Ministry of Agriculture, Fisheries and Food (MAFF) The thirty-ninth report of the Steering Group on Chemical Aspects of Food Surveillance (MAFF) Food Surveillance Paper No 39. London, UK: The Ministry; 1993. Aluminium in Food. [Google Scholar]
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578–1580. doi: 10.1093/jnci/94.20.1578. [DOI] [PubMed] [Google Scholar]
- Misawa T, Shigeta S. Effects of prenatal aluminum treatment on development and behavior in the rat. J Toxicol Sci. 1993;18:43–48. doi: 10.2131/jts.18.43. [DOI] [PubMed] [Google Scholar]
- Mitchell J. Pulmonary fibrosis in an aluminium worker. Br J Ind Med. 1959;16:123–125. doi: 10.1136/oem.16.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Manning GB, Molyneux M, Lane RE. Pulmonary fibrosis in workers exposed to finely powdered aluminium. Br J Ind Med. 1961;18:10–23. doi: 10.1136/oem.18.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic B, Milacic R. Speciation of aluminum in forest soil extracts by size exclusion chromatography with UV and ICP-AES detection and cation exchange fast protein liquid chromatography with ETAAS detection. Sci Total Environ. 2000;258:183–194. doi: 10.1016/s0048-9697(00)00569-6. [DOI] [PubMed] [Google Scholar]
- Miu AC, Andreescu CE, Vasiu R, Olteanu AI. A behavioral and histological study of the effects of long-term exposure of adult rats to aluminum. Int J Neurosci. 2003;113:1197–1211. doi: 10.1080/00207450390232292. [DOI] [PubMed] [Google Scholar]
- Miu AC, Olteanu AI, Miclea M. A behavioral and ultrastructural dissection of the interference of aluminum with aging. J Alzheimers Dis. 2004;6:315–328. doi: 10.3233/jad-2004-6312. [DOI] [PubMed] [Google Scholar]
- Mizumoto Y, Iwata S, Sasajima K, Yoshimasu F, Yase Y. X-ray florescence analysis for trace elements in frontal cortex in amytrophic lateral sclerosis. Kinki Daigaku Genshiryoku Kenkyusho Nenpo. 1983;20:1–4. [Google Scholar]
- Mjoberg B, Hellquist E, Mallmin H, Lindh U. Aluminum, Alzheimer’s disease and bone fragility. Acta Orthop Stand. 1997;68:511–514. doi: 10.3109/17453679708999016. [DOI] [PubMed] [Google Scholar]
- Mladenovic J. Aluminum inhibits erythropoiesis in vitro. J Clin Invest. 1988;81:1661–1665. doi: 10.1172/JCI113502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mödder H, Schmitt T. Spreading of aluminosis. Dtsch Med Wochenschr. 1951;76:84–87. doi: 10.1055/s-0028-1116591. [DOI] [PubMed] [Google Scholar]
- Monteagudo FS, Isaacson LC, Wilson G, Hickman R, Folb PI. Aluminum excretion by the distal tubule of the pig kidney. Nephron. 1988;49:245–250. doi: 10.1159/000185064. [DOI] [PubMed] [Google Scholar]
- Moore PB, Edwardson JA, Ferrier IN, Taylor GA, Lett D, Tyrer SP, Day JP, King SJ, Lilley JS. Gastrointestinal absorption of aluminum is increased in Down’s syndrome. Biol Psychiatry. 1997;41:488–492. doi: 10.1016/S0006-3223(96)00045-5. [DOI] [PubMed] [Google Scholar]
- Moore PB, Day JP, Taylor GA, Ferrier IN, Fifield LK, Edwardson JA. Absorption of aluminium-26 in Alzheimer’s disease, measured using accelerator mass spectrometry. Dem Geriatr Cognitive Dis. 2000;11:66–69. doi: 10.1159/000017216. [DOI] [PubMed] [Google Scholar]
- More J, Fioramonti J, Bueno L. Aluminium hydroxide uptake in the stomach and in the intestine of the rat: a histochemical study. Acta Anatom. 1992;145:50–54. [PubMed] [Google Scholar]
- Moreno A, Dominguez P, Dominguez C, Ballabriga A. High serum aluminum levels and acute reversible encephalopathy in a 4-year-old boy with acute renal failure. Eur J Pediatr. 1991;150:513–514. doi: 10.1007/BF01958436. [DOI] [PubMed] [Google Scholar]
- Moretz RC, Iqbal K, Wisniewski HM. Microanalysis of Alzheimer disease NFT and plaques. Environ Geochem Health. 1990;12:15–16. doi: 10.1007/BF01734044. [DOI] [PubMed] [Google Scholar]
- Morgan WK, Donner A, Higgins IT, Pearson MG, Rawlings W. The effects of kaolin on the lung. Am Rev Respir Dis. 1988;138:813–820. doi: 10.1164/ajrccm/138.4.813. [DOI] [PubMed] [Google Scholar]
- Mori H, Swyt C, Smith QR, Atack JR, Rapoport SI. In situ x-ray microanalysis of senile plaques in Alzheimer’s disease. Neurology. 1988;38(Suppl 1):230. [Google Scholar]
- Morie T, Iwamoto M, Harada N, Masumoto S, Yamada M, Kusunose Y. Urinary excretion of aluminium: effects of aging and diurnal variation. Arch Gerontol Geriatr. 1996;22:287–295. doi: 10.1016/0167-4943(96)00701-7. [DOI] [PubMed] [Google Scholar]
- Morrissey J, Slatopolsky E. The effect of aluminum on parathyroid hormone secretion. Kidney Int Suppl. 1986;29(Suppl 18):S41–S44. [PubMed] [Google Scholar]
- Morrissey J, Rothstein M, Mayor GH, Slatopolsky E. Suppression of parathyroid hormone secretion by aluminum. Kidney Int. 1983;2:699–704. doi: 10.1038/ki.1983.81. [DOI] [PubMed] [Google Scholar]
- Morris CM, Candy JM, Court JA, Whitford CA, Edwardson JA. The role of transferrin in the uptake of aluminum and manganese by the IMR 32 neuroblastoma cell line. Biochem Soc Trans. 1987;15:498. [Google Scholar]
- Morris CM, Candy JM, Oakley AE, Taylor GA, Mountfort S, Bishop H, Ward MK, Bloxham CA, Edwardson J. Comparison of the regional distribution of transferrin receptors and aluminium in the forebrain of chronic renal dialysis patients. J Neurol Sci. 1989;94:295–306. doi: 10.1016/0022-510x(89)90238-4. [DOI] [PubMed] [Google Scholar]
- Morrow PE, Haseman KJ, Hobbs CH, Driscoll KW, Vu V, Oberdorster G. The maximum tolerated dose for inhalation bioassays: toxicity vs overload. Fundam Appl Toxicol. 1996;29:155–167. doi: 10.1006/faat.1996.0017. [DOI] [PubMed] [Google Scholar]
- Moulin JJ, Clavel T, Buclez B, Laffitte-Rigaud G. A mortality study among workers in a french aluminum reduction plant. Int Arch Occup Environ Health. 2000;73:323–330. doi: 10.1007/s004200000124. [DOI] [PubMed] [Google Scholar]
- Mouser JF, Wu AH, Herson VC. Aluminum contamination of neonatal parenteral nutrient solutions and additives. Am J Health Syst Pharm. 1998;55:1071–1072. doi: 10.1093/ajhp/55.10.1071. [DOI] [PubMed] [Google Scholar]
- Mrak RE. Muscle granulomas following intramuscular injection. Muscle Nerve. 1982;5:637–639. doi: 10.1002/mus.880050808. [DOI] [PubMed] [Google Scholar]
- Muller G, Hutin MF, Burnel D, Lehr PR. Aluminum transfer through milk in female rats intoxicated by aluminum chloride. Biol Trace Elem Res. 1992;34:79–87. doi: 10.1007/BF02783900. [DOI] [PubMed] [Google Scholar]
- Muller L, Wilhelm M. Uptake and distribution of aluminium in rat hepatocytes and its effect on enzyme leakage and lactate formation. Toxicology. 1987;44:203–212. doi: 10.1016/0300-483x(87)90150-8. [DOI] [PubMed] [Google Scholar]
- Müller M, Anke M, Illing-Günther H. Oral aluminium exposure of adults in Germany - A long-term survey. In: Fischer P, L’Abbé M, Cockell K, Gibson R, editors. Trace Elements in Man and Animals -9: Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals. Ottawa, Canada: NRC Research Press; 1997. pp. 177–178. [Google Scholar]
- Müller M, Anke M, Illing-Günther H. Aluminium in foodstuffs. Food Chem. 1998;61:419–428. [Google Scholar]
- Muma NA, Singer SM. Aluminum-induced neuropathology: transient changes in microtubule-associated proteins. Neurotoxicol Teratol. 1996;18:679–690. doi: 10.1016/s0892-0362(96)00126-2. [DOI] [PubMed] [Google Scholar]
- Mur JM, Moulin JJ, Meyer-Bisch C, Massin N, Couloun JP, Loulergue J. Mortality of aluminum reduction plant workers in France. Int J Epidemiol. 1987;16:257–264. doi: 10.1093/ije/16.2.257. [DOI] [PubMed] [Google Scholar]
- Mur JM, Wild P, Rapp R, Vautrin JP, Coulon JP. Demographic evaluation of the fertility of aluminium industry workers: influence of exposure to heat and static magnetic fields. Hum Reprod. 1998;13:2016–2019. doi: 10.1093/humrep/13.7.2016. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Roberts E, Horrocks LA. Aluminum silicate toxicity in cell cultures. Neuroscience. 1993;55:597–605. doi: 10.1016/0306-4522(93)90527-m. [DOI] [PubMed] [Google Scholar]
- Musk AW, Greville HW, Tribe AE. Pulmonary disease from occupational exposure to an artificial aluminum silicate used for cat litter. Br J Ind Med. 1980;37:367–372. doi: 10.1136/oem.37.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musk AW, Beck BD, Greville HW, Brain JD, Bohannon DE. Pulmonary disease from exposure to an artificial aluminium silicate: further observations. Br J Ind Med. 1988;45:246–250. doi: 10.1136/oem.45.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussi I, Calzaferri G, Buratti M, Alessio L. Behaviour of plasma and urinary aluminum levels in occupationally exposed subjects. Int Arch Occup Environ Health. 1984;54:155–161. doi: 10.1007/BF00378518. [DOI] [PubMed] [Google Scholar]
- Nagasawa K, Ito S, Kakuda T, Nagai K, Tamai T, Tsuji A, Fujimoto S. Transport mechanism for aluminum citrate at the blood-brain barrier: kinetic evidence implies involvement of system Xc- in immortalized rat brain endothelial cells. Toxicol Lett. 2005;155:289–296. doi: 10.1016/j.toxlet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Nagel J, Svec D, Waters T, Fireman P. IgE synthesis in man-I. Development of specific IgE antibodies after immunization with tetanus-diphtheria (Td) toxoids. J Immunol. 1977;118:334–341. [PubMed] [Google Scholar]
- Nagore E, Martinez-Escribano JA, Tato A, Sabater V, Vilata JJ. Subcutaneous nodules following treatment with aluminium-containing allergen extracts. Eur J Dermatol. 2001;11:138–140. [PubMed] [Google Scholar]
- Nagy E, Jobst K. The kinetics of aluminum-containing antacid absorption in man. Eur J Clin Chem Clin Biochem. 1994;32:119–121. doi: 10.1515/cclm.1994.32.3.119. [DOI] [PubMed] [Google Scholar]
- Nair B, Yamarik TA. Final report on the safety assessment of aluminum starch octenylsuccinate. Int J Toxicol. 2002;21(Suppl 1):1–7. doi: 10.1080/10915810290096379. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Rose PG, Blumer JL, Reed MD. Acute encephalopathy due to aluminum toxicity successfully treated by combined intravenous deferoxamine and hemodialysis. J Clin Pharmacol. 2000;40:296–300. doi: 10.1177/00912700022008847. [DOI] [PubMed] [Google Scholar]
- Namekata KM, Imagawa M, Terashi A, Ohta S, Oyama F, Ihara Y. Association of transferrin C2 allele with late-onset Alzheimer’s disease. Hum Genet. 1997;101:126–129. doi: 10.1007/s004390050600. [DOI] [PubMed] [Google Scholar]
- Nashed N. Preparation of peritoneal cell metaphases of rats, mice and Chinese hamsters after mitogenic stimulation with magnesium sulfate and/or aluminium hydroxide. Mutat Res. 1975;30:407–416. [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1–S201. [PubMed] [Google Scholar]
- Navarro-Blasco I, Alvarez-Galindo JI. Aluminium content of Spanish infant formula. Food Addit Contam. 2003;20:470–481. doi: 10.1080/0265203031000098704. [DOI] [PubMed] [Google Scholar]
- Nayak P, Chatterjee AK. Effects of aluminum exposure on brain glutamate and GABA systems: an experimental study in rats. Food Chem Toxicol. 2001;39:1285–1289. doi: 10.1016/s0278-6915(01)00077-1. [DOI] [PubMed] [Google Scholar]
- Nayan R, Dey AK. Avidity of folic acid for carcinogenic metal ions aluminium(3), chromium(3), beryllium(II), lead(II) and uranium(VI) Z Naturforsch B. 1970;25:1453–1457. doi: 10.1515/znb-1970-1229. [DOI] [PubMed] [Google Scholar]
- Naylor GJ, Sheperd B, Treliving L, McHarg A, Smith A, Ward N, Harper M. Tissue aluminum concentrations stability over time, relationship to age, and dietary intake. Biol Psychiatry. 1990;27:884–890. doi: 10.1016/0006-3223(90)90469-i. [DOI] [PubMed] [Google Scholar]
- Neelam M, Bamji S, Kaladhar M. Risk of increased aluminium burden in the Indian population: contribution of aluminium from cookware. Food Chem. 2000;70:57–61. [Google Scholar]
- Nemery B. Metal toxicity and the respiratory tract. Eur Respir J. 1990;3:202–219. [PubMed] [Google Scholar]
- Neri LC, Hewitt D. Aluminum, Alzheimer’s disease, and drinking water. Lancet. 1991;338:390. doi: 10.1016/0140-6736(91)90531-s. [DOI] [PubMed] [Google Scholar]
- Nestel AW, Meyers AM, Paiker J, Rollin HB. Effect of calcium supplement preparation containing small amounts of citrate on the absorption of aluminium in normal subjects and in renal failure patients. Nephron. 1994;68:197–201. doi: 10.1159/000188256. [DOI] [PubMed] [Google Scholar]
- Netter P, Kessler M, Burnel D, Hutin MF, Delones S, Benoit J, Gaucher A. Aluminum in the joint tissues of chronic renal failure patients treated with regular hemodialysis and aluminum compounds. J Rheumatol. 1984;11:66–70. [PubMed] [Google Scholar]
- Netter P, Kessler M, Gaucher A, Gillet P, Delons S, Burnel D, Benoit J, Got C. Aluminium and dialysis arthropathy. Lancet. 1988;1:886–887. doi: 10.1016/s0140-6736(88)91635-2. [DOI] [PubMed] [Google Scholar]
- Netterlid E, Bruze M, Hindsén M, Isaksson M, Olin P. Persistent itching nodules after the fourth dose of diphtheria-tetanus toxoid vaccines without evidence of delayed hypersensitivity to aluminium. Vaccine. 2004;22:3698–3706. doi: 10.1016/j.vaccine.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Nevo Y, Kutai M, Jossiphov J, Livne A, Neeman A, Arad T, Popovitz-Biro R, Atsmon J, Shapira Y, Soffer D. Childhood macrophagic myofasciitis-consanguinity and clinicopathological features. Neuromuscul Disord. 2004;14:246–252. doi: 10.1016/j.nmd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- NFA (National Food Authority) The 1992 Australian Market Basket Survey - A Total Diet Survey of Pesticides and Contaminants. Canberra: The Authority; 1993. [Google Scholar]
- Ng AH, Hercz G, Kandel R, Grynpas MD. Association between fluoride, magnesium, aluminum and bone quality in renal osteodystrophy. Bone. 2004;34:216–224. doi: 10.1016/j.bone.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Nieboer E, Fletcher GG. Determinants of reactivity in metal toxicology. In: Chang LW, editor. Toxicology of Metals. Boca Raton, FL: CRC Publishing; 1996. pp. 113–132. [Google Scholar]
- Nieboer E, Richardson DHS. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Env Pollut (Series B) 1980;1:3–26. [Google Scholar]
- Nieboer E, Gibson BL, Oxman AD, Kramer JR. Health effects of aluminum: A critical review with emphasis on aluminum in drinking water. Environ Rev. 1995;3:29–81. [Google Scholar]
- Nieboer E, Fletcher GG, Thomassen Y. Relevance of reactivity determinants to exposure assesssment and biological monitoring of the elements. J Environ Monit. 1999;1:1–14. doi: 10.1039/a808849g. [DOI] [PubMed] [Google Scholar]
- Nieboer E, Thomassen Y, Chashchin V, Odland JO. Occupational exposure assessment. J Environ Monit. 2005;7:411–415. [PubMed] [Google Scholar]
- Nielsen AO, Kaaber K, Veien NK. Aluminum allergy caused by DTP vaccine. Ugeskr Laeger. 1992;154:1900–1901. [PubMed] [Google Scholar]
- Nielsen J, Dahlqvist M, Welinder H, Thomassen Y, Alexandersson R, Skerfving S. Small airways function in aluminum and stainless steel welders. Int Arch Occup Environ Health. 1993;65:101–105. doi: 10.1007/BF00405727. [DOI] [PubMed] [Google Scholar]
- Nielsen VT, Hjorth L, Moller JC. Vaccination granuloma in the breast region-differential diagnosis. Ugesker Laeger. 1991;153:2180–2182. [PubMed] [Google Scholar]
- Nikaido T, Austin J, Trueb L, Rinehart R. Studies in ageing of the brain. II. Microchemical analyses of the nervous system in Alzheimer patients. Arch Neurol. 1972;27:549–554. doi: 10.1001/archneur.1972.00490180085017. [DOI] [PubMed] [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health) Recommendations for Occupational Safety and Health -- Copendium of Policy Documents and Statements. Cincinnati, OH: Department of Health and Human Services, National Institute for Occupational Safety and Health; 1992. [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health) Pocket guide to chemical hazards, aluminum. 2005 [online] Cited 24 January 2007 http://www.cdc.gov/niosh/npg/npgd0022.html.
- Nishioka H. Mutagenic activities of metal compounds in bacteria. Mutat Res. 1975;31:185–189. doi: 10.1016/0165-1161(75)90088-6. [DOI] [PubMed] [Google Scholar]
- Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int. 1990;38:937–941. doi: 10.1038/ki.1990.294. [DOI] [PubMed] [Google Scholar]
- Nolte E, Beck E, Winklhofer C, Steinhausen C. Compartmental model for aluminium biokinetics. Hum Exp Toxicol. 2001;20:111–117. doi: 10.1191/096032701673730925. [DOI] [PubMed] [Google Scholar]
- Nordal KP, Dahl E, Sorhus K, Berg KJ, Thomassen Y, Kofstad J, Halse J. Gastrointestinal absorption and urinary excretion of aluminium in patients with predialysis chronic renal failure. Pharmacol Toxicol. 1988a;63:351–354. doi: 10.1111/j.1600-0773.1988.tb00967.x. [DOI] [PubMed] [Google Scholar]
- Nordal KP, Dahl E, Thomassen Y, Brodwall EK, Halse J. Seasonal variations in serum aluminium concentrations. Pharmacol Toxicol. 1988b;62:80–83. doi: 10.1111/j.1600-0773.1988.tb01850.x. [DOI] [PubMed] [Google Scholar]
- Nordal KP, Dahl E, Albrechtsen D, Halse J, Leivestad T, Tretli S, Flatmark A. Aluminium accumulation and immunosuppressive effect in recipients of kidney transplants. BMJ. 1988c;297:1581–1582. doi: 10.1136/bmj.297.6663.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norimatsu M, Ogikubo Y, Aoki A, Takahasi T, Watanabe G, Taya K, Sasamoto S, Tsuchiya M, Tamura Y. Effects of aluminium adjuvant on systemic reactions of lipopolysaccharides in swine. Vaccine. 1995;13:1325–1329. doi: 10.1016/0264-410x(95)00023-t. [DOI] [PubMed] [Google Scholar]
- Norris KC, Crooks PW, Nebeker HG, Hercz G, Milliner DS, Gerszi K, Slatopolsky E, Andress DL, Sherrard DJ, Coburn JW. Clinical and laboratory features of aluminum-related bone disease: differences between sporadic and “epidemic” forms of the syndrome. Am J Kidney Dis. 1985;6:342–347. doi: 10.1016/s0272-6386(85)80091-3. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) Airborne Particles. Baltimore: University Park Press; 1979. [Google Scholar]
- NRC (National Research Council) Risk Assessment in the Federal Government: Managing the Process. Washingon D.C.: National Academy Press; 1983. [PubMed] [Google Scholar]
- O’Donnell TV, Welford B, Coleman ED. Potroom asthma: New Zealand experience and follow-up. Am J Ind Med. 1989;15:43–49. doi: 10.1002/ajim.4700150106. [DOI] [PubMed] [Google Scholar]
- O’Driscoll JB, Beck M, Kesseler E, Ford G. Contact sensitivity to aluminium acetate eardrops. Contact Dermatitis. 1991;24:156–157. doi: 10.1111/j.1600-0536.1991.tb01686.x. [DOI] [PubMed] [Google Scholar]
- O’Gara R, Brown J. Comparison of the carcinogenic actions of subcutaneous implants of iron and aluminium in rodents. J Nat Cancer Inst. 1967;38:947–957. [PubMed] [Google Scholar]
- Oberdorster G, Ferin J, Morrow PE. Volumetric loading of alveolar macrophages (AM): A possible basis for diminished AM-mediated particle clearance. Exp Lung Res. 1992;18:87–104. doi: 10.3109/01902149209020653. [DOI] [PubMed] [Google Scholar]
- Oberly TJ, Piper CE, McDonald DS. Mutagenicity of metal salts in the L5178Y mouse lymphoma assay. J Toxicol Environ Health. 1982;9:367–376. doi: 10.1080/15287398209530170. [DOI] [PubMed] [Google Scholar]
- Occupational Disease Panel (Industrial Disease Standards Panel) Annual Report 1997/98. 1998 [online] Cited 24 January 2007 http://www.canoshweb.org/odp/html/an98.htm.
- Octive JC, Wood M, Johnson AC. Mutagenic effects of aluminium. Mutat Res. 1991;264:135–137. doi: 10.1016/0165-7992(91)90130-v. [DOI] [PubMed] [Google Scholar]
- Odelram H, Granström M, Hedenskog S, Duchen K, Björkstén B. Immunoglobulin E and G responses to pertussis toxin after booster immunization in relation to atopy, local reactions and aluminium content of the vaccines. Allergy Immunol. 1994;5:118–123. doi: 10.1111/j.1399-3038.1994.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Office of the Federal Register, National Archives and Records Administration. Code of Federal Regulations, Title 21- Food and Drugs. College Park, MD: Center for Food Safety and Applied Nutrition, U.S Food and Drug Administration; 2003. [Google Scholar]
- Ogasawara Y, Sakamoto T, Ishii K, Takanasi H, Tanabe S. Effects of the administration routes and chemical form of aluminium on aluminium accumulation in the rat brain. Biol Trace Element Res. 2002;86:269–278. doi: 10.1385/BTER:86:3:269. [DOI] [PubMed] [Google Scholar]
- Ogugbuaja VO, Onyeyili PA, Gadam AA, William A. The bioaccumulation of aluminium from coal ash on the tissue of rabbits: an environmental toxicology study. J Chem Soc Nigeria. 2004;29:1–4. [Google Scholar]
- Ohman LO, Martin RB. Citrate as the main small molecule binding Al3+ in serum. Clin Chem. 1994;40:598–601. [PubMed] [Google Scholar]
- Olaizola I, Fernández Martin JL, Fernández Menéndez MJ, Vizoso Piñeiro FJ, Virgós MJ, Fernández Soto I, Roza Suárez M, Cannata JB. Aluminium hydroxide absorption: Effect of age, renal failure and aluminium overload. Kidney Int. 1989;36:147. [Google Scholar]
- Olaizola Ottonello I, Serrano AM, Vizoso PF, Rodrigo SL, Garcia MM, Cannata Andia JB. Aluminum absorption in the presence of normal kidney function: the effect of the pH. Revista Clinica Espanola. 1991;188:442–445. [PubMed] [Google Scholar]
- Olivier P, Marzin D. Study of the genotoxic potential of 48 inorganic derivatives with the SOS chromotest. Mutat Res. 1987;189:263–269. doi: 10.1016/0165-1218(87)90057-7. [DOI] [PubMed] [Google Scholar]
- Oller A, Bates H. Metals in perspective. J Environ Monit. 2005;6:411–412. doi: 10.1039/b406573p. [DOI] [PubMed] [Google Scholar]
- Omokhodion FO, Howard JM. Trace elements in the sweat of acclimatized persons. Clin Chim Acta. 1994;231:23–28. doi: 10.1016/0009-8981(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Ondreicka R, Kortus J, Ginter E. Aluminum, its aborption, distribution and effects on phosphous metabolism. In: Skoryna SC, Waldron-Edward D, editors. Intestinal Absorption of Metal Ions, Trace Elements and Radionuclides. Oxford: Pergamon; 1971. pp. 293–305. [Google Scholar]
- Oneda S, Takasaki T, Kuriwaki K, Ohi Y, Umekita Y, Hatanaka S, Fujiyoshi T, Yoshida A, Yoshida H. Chronic toxicity and tumorigenicity study of aluminum potassium sulfate in B6C3F1 mice. In Vivo. 1994;8:271–278. [PubMed] [Google Scholar]
- Orell SR. Subcutaneous granulomata following inoculation of influenza vaccine. Acta Pathol Microbiol Scand. 1962;56:127–134. doi: 10.1111/j.1699-0463.1962.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Orfan NA, Dykewicz MS, Barnowsky L. Extensive subcutaneous fibrosis in a patient treated with alum precipitated allergenic extract. Ann Allergy Asthma Immunol. 1995;75:453–456. [PubMed] [Google Scholar]
- Orlans D, Verbov J. Skin reactions after triple vaccine. Practit. 1982;226:1295–1296. [PubMed] [Google Scholar]
- Orlic I, Wen X, Ng TH, Tang SM. Two years of aerosol pollution monitoring in Singapore. A review. Nucl Instrum Meth B. 1999;150:457–464. [Google Scholar]
- Osmond LH. Experiences with the control of silicosis in a foundry. A M A Arch Ind Health. 1955;12:221–225. [PubMed] [Google Scholar]
- Oteiza PI, Mackenzie GG, Verstraeten SV. Metals in neurodegeneration: involvement of oxidants and oxidant-sensitive transcription factors. Mol Aspects Med. 2004;25:103–115. doi: 10.1016/j.mam.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Ortner HM, Hoffman P, Stadermann FJ, Weinbruch S, Wentzel M. Chemical characterization of environmental and industrial particulate samples. Analyst. 1998;123:833–842. [Google Scholar]
- Ott SM, Maloney NA, Coburn JW, Alfrey AC, Sherrard DJ. The prevalence of bone aluminum deposition in renal osteodystrophy and its relation to the response to calcitriol therapy. N Engl J Med. 1982;307:709–713. doi: 10.1056/NEJM198209163071202. [DOI] [PubMed] [Google Scholar]
- Ott SM, Maloney NA, Klein GL, Alfrey AC, Ament ME, Coburn JW, Sherrard DJ. Aluminum is associated with low bone formation in patients receiving chronic parenteral nutrition. Ann Intern Med. 1983;98:910–914. doi: 10.7326/0003-4819-98-6-910. [DOI] [PubMed] [Google Scholar]
- Ott SM, Feist E, Andress DL, Liu CC, Sherrard DJ, Alfrey AC, Slatopolsky E, Howard GA. Development and reversibility of aluminum-induced bone lesions in the rat. J Lab Clin Med. 1987;109:40–47. [PubMed] [Google Scholar]
- Ott SM. Sources of aluminium. In: de Broe ME, Coburn JW, editors. Aluminum and Renal Failure. Dordrecht, Netherlands: Kluwer Academic; 1990. pp. 189–201. [Google Scholar]
- Owen LM, Crews HM, Bishop NJ, Massey RC. Aluminum uptake from some foods by guinea pigs and the characterization of aluminum in in vivo intestinal digesta by SEC-ICP-MS. Food Chem Toxicol. 1994;32:697–705. doi: 10.1016/s0278-6915(09)80002-1. [DOI] [PubMed] [Google Scholar]
- Pai SM, Melethil S. Kinetics of aluminum in rats I: Dose-dependent elimination from blood after intravenous administration. J Pharmac Sci. 1989;78:200–202. doi: 10.1002/jps.2600780305. [DOI] [PubMed] [Google Scholar]
- Park HS, Uh ST, Park CS. Increased neutrophil chemotactic activity is noted in aluminum induced occupational asthma. Kor J Int Med. 1996;11:69–73. doi: 10.3904/kjim.1996.11.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson IS, Ward MK, Kerr DN. Dialysis encephalopathy, bone disease and anaemia: the aluminum intoxication syndrome during regular haemodialysis. J Clin Pathol. 1981;34:1285–1294. doi: 10.1136/jcp.34.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry R, Plowman D, Delves HT, Roberts NB, Birchall JD, Bellia JP, Davenport A, Ahmad R, Fahal I, Altmann P. Silicon and aluminium interactions in haemodialysis patients. Nephrol Dial Transplant. 1998;13:1759–1762. doi: 10.1093/ndt/13.7.1759. [DOI] [PubMed] [Google Scholar]
- Partridge NA, Regnier FE, White JL, Hem SL. Influence of dietary constituents on intestinal absorption of aluminum. Kidney Int. 1989;35:1413–1417. doi: 10.1038/ki.1989.142. [DOI] [PubMed] [Google Scholar]
- Partridge NA, Regnier FE, Reed WM, White JL, Hem SL. Contribution of soluble aluminium species to absorption of aluminium from the rat gut in situ. Clin Sci. 1992;83:425–30. doi: 10.1042/cs0830425. [DOI] [PubMed] [Google Scholar]
- Passi P, Zadro A, Galassini S, Rossi P, Moschini G. PIXE micro-beam maping of materials in human peri-implant tissues. J Mat Sci Mater Med. 2002;13:1083–1089. doi: 10.1023/a:1020309108950. [DOI] [PubMed] [Google Scholar]
- Paternain JL, Domingo JL, Llobet JM, Corbella J. Embryotoxic and teratogenic effects of aluminum nitrate in rats upon oral administration. Teratology. 1988;38:253–257. doi: 10.1002/tera.1420380309. [DOI] [PubMed] [Google Scholar]
- Pattaragarn A, Alon US. Antacid-induced rickets in infancy. Clin Pediatr. 2001;40:389–393. doi: 10.1177/000992280104000705. [DOI] [PubMed] [Google Scholar]
- Pauwels R, Bazin H, Platteau B, Van der Straeten M. The influence of different adjuvants on the production of IgE and IgE antibodies. Ann Immunol. 1979;130C:49–58. [PubMed] [Google Scholar]
- Pearson A. Aluminum oxide (alumina) In: Kroschwitz JI, Howe-Grant M, editors. Othmer Encyclopedia of Chemical Technology. New York: John Wiley & Sons Inc; 1992. [Google Scholar]
- Pei Y, Hercz G, Greenwood C, Sherrard D, Segre G, Manuel A, Saiphoo C, Fenton S. Non-invasive prediction of aluminum bone disease in hemo- and peritoneal dialysis patients. Kidney Int. 1992;41:1374–1382. doi: 10.1038/ki.1992.202. [DOI] [PubMed] [Google Scholar]
- Pejovi-Mili A, Byun SH, Comsa DC, McNeill FE, Prestwich WV. In vivo measurement of bone aluminium: recent developments. Sixth Keele Meeting on Aluminium; February 26-March 2, 2005; Bussaco, Portugal. 2005. [DOI] [PubMed] [Google Scholar]
- Pembroke AC, Marten RH. Unusual cutaneous reactions following diphtheria and tetanus immunization. Clin Exp Dermatol. 1979;4:345–348. doi: 10.1111/j.1365-2230.1979.tb02649.x. [DOI] [PubMed] [Google Scholar]
- Pennington JA. Aluminium content of foods and diets. Food Addit Contam. 1988;5:161–232. doi: 10.1080/02652038809373696. [DOI] [PubMed] [Google Scholar]
- Pennington JA, Jones J. Aluminum in american diets. In: Gitelman HJ, editor. Aluminum in Health, A Critical Review. New York: Marcel Dekker; 1989. pp. 67–100. [Google Scholar]
- Pennington JA, Schoen SA. Estimates of dietary exposure to aluminium. Food Addit Contam. 1995;12:119–128. doi: 10.1080/02652039509374286. [DOI] [PubMed] [Google Scholar]
- Perez Parajon J, Gonzalez FB, Cannata JB, Sanz Medel A. A critical appraisal of the speciation of aluminum in serum b ultrafiltration. Trace Elem Med. 1989;6:41–46. [Google Scholar]
- Perl DP, Brody AR. Alzheimer’s disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science. 1980;208:297–299. doi: 10.1126/science.7367858. [DOI] [PubMed] [Google Scholar]
- Perl DP, Good PF. Uptake of aluminum into central nervous system along nasal-olfactory pathways. Lancet. 1987;1:1028. doi: 10.1016/s0140-6736(87)92288-4. [DOI] [PubMed] [Google Scholar]
- Perl DP, Pendlebury WW. Aluminum neurotoxicity--potential role in the pathogenesis of neurofibrillary tangle formation. Can J Neurol Sci. 1986;13:S441–S445. doi: 10.1017/s0317167100037082. [DOI] [PubMed] [Google Scholar]
- Perl DP, Gajdusek DC, Garruto RM, Yanagihara RT, Gibbs CJ. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Science. 1982;217:1053–1055. doi: 10.1126/science.7112111. [DOI] [PubMed] [Google Scholar]
- Perry KM. Diseases of the lung resulting from occupational dusts other than silica. Thorax. 1947;2:91–120. doi: 10.1136/thx.2.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peserico A. Pathogenesis of Porphyria cutanea tarda-like syndrome. Nephron. 1979;24:51. doi: 10.1159/000181683. [DOI] [PubMed] [Google Scholar]
- Peters T, Hani N, Kirchberg K, Gold H, Hunzelmann N, Scharffetter-Kochanek K. Occupational contact sensitivity to aluminium in a machine construction plant worker. Contact Dermatitis. 1998;39:322–323. doi: 10.1111/j.1600-0536.1998.tb05958.x. [DOI] [PubMed] [Google Scholar]
- Pettersen JC, Hackett DS, Zwicker GM. Twenty-six week toxicity study with kasal (basic sodium aluminum phosphate) in beagle dogs. Environ Geochem Health. 1990;12:121–123. doi: 10.1007/BF01734061. [DOI] [PubMed] [Google Scholar]
- Phelps KR, Naylor K, Brien TP, Wilbur H, Haqqie SS. Encephalopathy after bladder irrigation with alum: case report and literature review. Am J Med. 1999;318:181–185. doi: 10.1097/00000441-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Piccardo P, Yanagihara R, Garruto RM, Gibbs CJ, Gajdusek CD. Histochemical and X-ray microanalytical localization of aluminum in amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Acta Neuropathol. 1988;77:1–4. doi: 10.1007/BF00688235. [DOI] [PubMed] [Google Scholar]
- Pichichero ME, Edwards KM, Anderson EL, Rennels MB, Englund JA, Yerg DE, Blackwelder WC, Jansen DL, Meade BD. Safety and imunogenicity of six acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fifth dose in four to six year old children. Pediatrics. 2000;105:e11. doi: 10.1542/peds.105.1.e11. [DOI] [PubMed] [Google Scholar]
- Pierre F, Baruthio F, Diebold F, Biette P. Effect of different exposure compounds on urinary kinetics of aluminum and fluoride in industrially exposed workers. Occup Environ Med. 1995;52:396–403. doi: 10.1136/oem.52.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre F, Diebold F, Baruthio F. Biomonitoring of aluminium in production workers. In: Priest ND, O’Donnell TV, editors. Health in the Aluminium Industry. London, UK: Middlesex University Press; 1998. pp. 68–69. [Google Scholar]
- Pigott GH, Ishmael J. An assessment of the fibrogenic potential of two refractory fibres by intraperitoneal injection in rats. Toxicol Lett. 1981;8:153–163. doi: 10.1016/0378-4274(81)90044-8. [DOI] [PubMed] [Google Scholar]
- Pigott GH, Ishmael J. The effects of intrapleural injections of alumina and aluminoosilicate (ceramic) fibres. Int J Exp Pathol. 1992;73:137–146. [PMC free article] [PubMed] [Google Scholar]
- Pigott GH, Gaskell BA, Ishmael J. Effects of long term inhalation of alumina fibres in rats. Br J Exp Pathol. 1981;62:323–331. [PMC free article] [PubMed] [Google Scholar]
- Pineau A, Chappuis P, Arnaud J, Baruthio F, Zawislak R, Jaudon MC. Interlaboratory tests: completion of a method of aluminum assay in serum by electrothermal atomic absorption spectrometry. Annales de Biologie Clinique. 1992;50:577–585. [PubMed] [Google Scholar]
- Pinto JP, Stevens RK, Willis RD, Kellogg R, Mamane Y, Novak J, Santroch J, Benes I, Bures V, Lenicek J. Czech air quality monitoring and receptor modeling study. Environ Sci Technol. 1998;32:843–854. [Google Scholar]
- Pittman PR. Aluminum-containing vaccine associated adverse events: role of route of administration and gender. Vaccine. 2002;20(Suppl 3):S48–S50. doi: 10.1016/s0264-410x(02)00172-x. [DOI] [PubMed] [Google Scholar]
- Platt B, Fiddler G, Riedel G, Henderson Z. Aluminum toxicity in the rat brain: histochemical and immunocytochemical evidence. Brain Res Bull. 2001;55:257–267. doi: 10.1016/s0361-9230(01)00511-1. [DOI] [PubMed] [Google Scholar]
- Polizzi S, Pira E, Ferrara M, Bugiani M, Papaleo A, Albera R, Palmi S. Neurotoxic effects of aluminium among foundry workers and Alzheimer’s disease. Neurotoxicology. 2002;23:761–774. doi: 10.1016/S0161-813X(02)00097-9. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J A M A. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner AS, Blumenthal NC. Model of aluminium-induced osteomalacia. Inhibition of apatite formation and growth. In: Ornoy A, Harell A, Sela J, editors. Current Advances in Skeletogenesis. Amsterdam: Exerpta Medica; 1985. pp. 299–304. [Google Scholar]
- Poulos BK, Perazzolo M, Lee MY, Rudelli R, Wisniewski HM, Soifer D. Oral aluminum administration during pregnancy and lactation produces gastric and renal lesions in rat mothers and delay in CNS development in their pups. Mol Chem Neuropathol. 1996;29:15–25. doi: 10.1007/BF02815190. [DOI] [PubMed] [Google Scholar]
- Powell JJ, Thompson RP. The chemistry of aluminium in the gastrointestinal lumen and its uptake and absorption. Proc Nutr Soc. 1993;52:241–253. doi: 10.1079/pns19930056. [DOI] [PubMed] [Google Scholar]
- Powell JJ, Greenfield SM, Parkes HG, Nicholson JK, Thompson RP. Gastro-intestinal availability of aluminum from tea. Food Chem Toxicol. 1993;31:449–454. doi: 10.1016/0278-6915(93)90162-r. [DOI] [PubMed] [Google Scholar]
- Powell JJ, Ainley CC, Evans R, Thompson RP. Intestinal perfusion of dietary levels of aluminium: association with the mucosa. Gut. 1994;35:1053–1057. doi: 10.1136/gut.35.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JJ, Whitehead MW, Ainley CC, Kendall MD, Nicholson JK, Thompson RP. Dietary minerals in the gastrointestinal tract: hydroxypolymerization of aluminum is regulated by luminal mucins. J Inorg Biochem. 1999;75:167–180. doi: 10.1016/s0162-0134(99)00094-x. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J. 2002;16:1138–1140. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- Pratt PC. 6th Saranac Laboratory Symposium. New York: 1950. Pneumoconiosis. [Google Scholar]
- Priest ND. The bioavailability and metabolism of aluminium compounds in man. Proc Nutr Soc. 1993;52:231–240. doi: 10.1079/pns19930055. [DOI] [PubMed] [Google Scholar]
- Priest ND. A Human Volunteer Feeding Study using Aluminium-26 Labelled Aluminium Citrate, Aluminium Hydroxide and Aluminium Hydroxide in the Presence of Citrate. Harwell, Oxfordshire: AEA Technology; 1994. Report No AEA-TPD-268. [Google Scholar]
- Priest ND. The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. J Environ Monitor. 2004;6:375–403. doi: 10.1039/b314329p. [DOI] [PubMed] [Google Scholar]
- Priest ND, Newton D, Day JP, Talbot RJ, Warner AJ. Human metabolism of aluminium-26 and gallium-67 injected as citrates. Hum Exp Toxicol. 1995;14:287–293. doi: 10.1177/096032719501400309. [DOI] [PubMed] [Google Scholar]
- Priest ND, Talbot RJ, Austin JG, Day JP, King SJ, Fifield K, Cresswell RG. The bioavailability of 26Al-labelled aluminium citrate and aluminium hydroxide in volunteers. BioMetals. 1996;9:221–228. doi: 10.1007/BF00817919. [DOI] [PubMed] [Google Scholar]
- Priest ND, Talbot RJ, Newton D, Day JP, King SJ, Fifield LK. Uptake by man of aluminium in a public water supply. Hum Exp Toxicol. 1998;17:296–301. doi: 10.1177/096032719801700602. [DOI] [PubMed] [Google Scholar]
- Prival MJ, Simmon VF, Mortelmans KE. Bacterial mutagenicity testing of 49 food ingredients gives very few positive results. Mutat Res. 1991;260:321–329. doi: 10.1016/0165-1218(91)90017-g. [DOI] [PubMed] [Google Scholar]
- Provan SD, Yokel RA. Aluminum uptake by the in situ rat gut preparation. J Pharmacol Exp Therap. 1988a;245:928–931. [PubMed] [Google Scholar]
- Provan SD, Yokel RA. Influence of calcium on aluminum accumulation by the rat jejunal slice. Res Commun Chem Pathol Pharmacol. 1988b;59:79–92. [PubMed] [Google Scholar]
- Provan SD, Yokel RA. Reduced intestinal calcium and dietary calcium intake increase aluminum absorption and tissue concentrations in the rat. Biol Trace Elem Res. 1990;23:119–132. doi: 10.1007/BF02917183. [DOI] [PubMed] [Google Scholar]
- Pun KK, Ho PW, Lau P. Effects of aluminum on the parathyroid hormone receptors of bone and kidney. Kidney Int. 1990;37:72–78. doi: 10.1038/ki.1990.10. [DOI] [PubMed] [Google Scholar]
- Putterman GJ, Strassburger J, Fitzgerald JJ. In vitro sorption of aluminum complex to guinea pig stratum corneum. J Invest Dermatol. 1981;77:319–324. doi: 10.1111/1523-1747.ep12482522. [DOI] [PubMed] [Google Scholar]
- Quarles LD. Attenuated bone aluminum deposition in nonuremic beagles with reduced bone remodeling. Am J Physiol. 1990;258:E576–E581. doi: 10.1152/ajpendo.1990.258.4.E576. [DOI] [PubMed] [Google Scholar]
- Quarles LD, Dennis VW, Gitelman HJ, Harrelson JM, Drezner MK. Aluminum deposition at the osteoid-bone interface. J Clin Invest. 1985;75:1441–1447. doi: 10.1172/JCI111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles LD, Gitelman HJ, Drezner MK. Induction of bone formation in the beagle: a novel effect of aluminum. J Clin Invest. 1988;81:1056–1066. doi: 10.1172/JCI113417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles LD, Gitelman HJ, Drezner MK. Aluminium-induced de novo bone formation in the beagle: a parathyroid hormone-dependent event. J Clin Invest. 1989;83:1644–1650. doi: 10.1172/JCI114063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartley B, Essehnont G, Taylor A, Dobrota M. Effect of oral aluminum citrate on short-term tissue distribution of aluminum. Food Chem Toxicol. 1993;31:543–548. doi: 10.1016/0278-6915(93)90203-b. [DOI] [PubMed] [Google Scholar]
- Quatrale RP. Mechanism of antiperspirant action. Cosmet Toiletries. 1985;100:23–26. [Google Scholar]
- Quatrale R, Waldman A, Rogers J, Felger C. The mechanism of antiperspirant action by aluminum salts. I. The effect of cellophane tape stripping on aluminum salt-inhibited eccrine sweat glands. J Soc Cos Chem. 1981a;32:67–73. [Google Scholar]
- Quatrale R, Coble D, Stoner K, Felger C. The mechanism of antiperspirant action by aluminum salts. II. Histological observation of human eccrine sweat glands inhibited by aluminum chlorohydrate. J Soc Cos Chem. 1981b;32:107–136. [Google Scholar]
- Queensland Government. Public health guidance note Aluminum. 2002 [online] Cited 24 January 2007 http://www.health.qld.gov.au/phs/Documents/ehu/4177.pdf.
- Raabe OG, Yeh H, Newton GJ, Phalen RF, Velasquez DJ. Deposition of inhaled monodisperse aerosols in small rodents. In: Walton WH, editor. Inhaled Particles. IV. New York: Pergamon Press; 1977. pp. 3–21. [PubMed] [Google Scholar]
- Raabe OG, Tyler WS, Last JA, Schwartz LW, Lollini LO, Fisher GL, Wilson FD, Dungworth DL. Studies of the chronic inhalation of coal fly ash by rats. Ann Occup Hyg. 1982;26:189–211. [PubMed] [Google Scholar]
- Raabe R, Janz S, Wolff G, Merten H, Landrock A, Birkenfeld T, Herzschuh R. Genotoxicity assessment of waste products of aluminum plasma etching wih SOS chromotest. Mutat Res. 1993;300:99–109. doi: 10.1016/0165-1218(93)90127-y. [DOI] [PubMed] [Google Scholar]
- Radon K, Nowak D, Heinrich-Ramm R, Szadkowski D. Respiratory health and fluoride exposure in different parts of the modern primary aluminum industry. Int Arch Occup Environ Health. 1999;72:297–303. doi: 10.1007/s004200050378. [DOI] [PubMed] [Google Scholar]
- Radunovic A, Ueda F, Raja KB, Simpson RJ, Templar J, King SJ, Lilley JS, Day JP, Bradbury MW. Uptake of 26-Al and 67-Ga into brain and other tissues of normal and hypotransferrinaemic mice. BioMetals. 1997;10:185–191. doi: 10.1023/a:1018399611243. [DOI] [PubMed] [Google Scholar]
- Radunovic A, Delves HT, Bradbury MW. Uptake of aluminum and gallium into tissues of the rat: influence of antibody against the transferrin receptor. Biol Trace Elem Res. 1998;62:51–64. doi: 10.1007/BF02820021. [DOI] [PubMed] [Google Scholar]
- Rahman H, Skillen AW, Channon SM, Ward MK, Kerr DN. Methods for studying the binding of aluminum by serumprotein. Clin Chem. 1985;31:1969–1973. [PubMed] [Google Scholar]
- Rahnema S, Jennings F. Accumulation and depletion of aluminum from various tissues of rats following aluminum citrate ingestion. Ohio J Sci. 1999;99:98–101. [Google Scholar]
- Rajan MT, Jagannatha Rao KS, Mamatha BM, Rao RV, Shanmugavelu P, Menon RB, Pavithran MV. Quantification of trace elements in normal human brain by inductively coupled plasma atomic emission spectrometry. J Neurol Sci. 1997;146:153–166. doi: 10.1016/s0022-510x(96)00300-0. [DOI] [PubMed] [Google Scholar]
- Rajasekaran K. Effects of combined exposure to aluminium and ethanol on food intake, motor behaviour and a few biochemical parameters in pubertal rats. Environ Toxicol Pharmacol. 2000;9:25–30. doi: 10.1016/s1382-6689(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Rajwanshi P, Singh V, Gupta MK, Kumari V, Shrivastav R, Ramanamurthy M, Dass S. Studies on aluminum leaching from cookware in tea and coffee and estimation of aluminum content in toothpaste, baking powder, and paan masala. Sci Total Environ. 1997;193:243–249. doi: 10.1016/s0048-9697(96)05347-8. [DOI] [PubMed] [Google Scholar]
- Ranau R, Oehlenchälger SH. Aluminium levels of fish fillets backed and grilled in aluminium foil. Food Chem. 2001;73:1–6. [Google Scholar]
- Rao JK, Katsetos CD, Herman MM, Savory J. Experimental aluminum encephalomyelopathy. Relationship to human neurodegenerative disease. Clin Lab Med. 1998;18:687–698. [PubMed] [Google Scholar]
- Rauch H, Fleischer F, Bohrer H, Jurs G, Wilhelm M, Krier C. Serum aluminium levels of intensive care patients treated with two different antacids for prevention of stress ulceration. Intensive Care Med. 1989;15:84–86. doi: 10.1007/BF00295982. [DOI] [PubMed] [Google Scholar]
- Razniewska G, Trzcinka-Ochocka M, Gazewski A. Serum aluminum concentration in dialyzed patients. Sixth Keele Meeting on Aluminium; February 26-March 2, 2005; Bussaco, Portugal. 2005. [Google Scholar]
- Recker RR, Blotcky AJ, Leffler JA, Rack EP. Evidence of aluminum absorption from the gastrointestinal tract and bone deposition by aluminum carbonate ingestion with normal renal function. J Lab Clin Med. 1977;90:810–815. [PubMed] [Google Scholar]
- Rees EL. Aluminum toxicity as indicated by hair analysis. J Orthomol Psych. 1979;8:37–43. [Google Scholar]
- Reffitt DM, Jugdaohsingh R, Thompson RP, Powell JJ. Silicic acid: its gastrointestinal uptake and urinary excretion in man and effects on aluminium excretion. J Inorg Biochem. 1999;76:141–147. doi: 10.1016/s0162-0134(99)00126-9. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Reiber S, Kukull W, Standish-Lee P. Drinking water aluminum and bioavailability. J Am Water Works Assoc. 1995;87:86–100. [Google Scholar]
- Reid SD, McDonald DG, Rhem RR. Acclimation of sublethal aluminum; modifications of metal-gill surface interactins of juvenile rainbow trout (Oncorhynchus mykiss) Can J Fish Aquat Sci. 1991;48:1996–2005. [Google Scholar]
- Reinke CM, Breitkreutz J, Leuenberger H. Aluminium in over-the-counter drugs: risks outweigh benefits? Drug Saf. 2003;26:1011–1025. doi: 10.2165/00002018-200326140-00003. [DOI] [PubMed] [Google Scholar]
- Remillard S, Rebhun LI, Howie GA, Kupchan SM. Antimitotic activity of the potent tumor inhibitor maytanisine. Science. 1975;189:1002–1005. doi: 10.1126/science.1241159. [DOI] [PubMed] [Google Scholar]
- Renard JL, Felten D, Bequet D. Post-otoneurosurgery aluminum encephalopathy. Lancet. 1994;344:63–64. doi: 10.1016/s0140-6736(94)91089-8. [DOI] [PubMed] [Google Scholar]
- Reusche E. Argyrophilic inclusions distinct from Alzheimer neurofibrillary changes in one case of dialysis-associated encephalopathy. Acta Neuropathol. 1997;94:612–616. doi: 10.1007/s004010050757. [DOI] [PubMed] [Google Scholar]
- Reusche E, Koch V, Friedrich HJ, Nunninghoff D, Stein P, Rob PM. Correlation of drug-related aluminum intake and dialysis treatment with deposition of argyrophilic aluminum-containing inclusions in CNS and in organ systems of patients with dialysis-associated encephalopathy. Clin Neuropathol. 1996;15:342–347. [PubMed] [Google Scholar]
- Reusche E, Koch V, Lindner B, Harrison AP, Friedrich HJ. Alzheimer morphology is not increased in dialysis-associated encephalopathy and long-term hemodialysis. Acta Neuropathol. 2001a;101:211–216. doi: 10.1007/s004010000253. [DOI] [PubMed] [Google Scholar]
- Reusche E, Pilz P, Oberascher G, Lindner B, Egensperger R, Gloeckner K, Trinka E, Iglseder B. Subacute fatal aluminum encephalopathy after reconstructive otoneurosurgery: a case report. Hum Pathol. 2001b;32:1136–1140. doi: 10.1053/hupa.2001.28251. [DOI] [PubMed] [Google Scholar]
- Riddell AR. Pulmonary changes encountered in employees engaged in the manufacture of alumina abrasives. Occup Med. 1948;5:710–717. [PubMed] [Google Scholar]
- Rifat SL. Cognitive deficit after exposure to McIntyre powder: exposure effect or artifact?. Second international conference on aluminum and health; Tampa, Florida. 1992. [Google Scholar]
- Rifat SL, Eastwood MR, McLachlan DR, Corey PN. Effect of exposure of miners to aluminium powder. Lancet. 1990;336:1162–1165. doi: 10.1016/0140-6736(90)92775-d. [DOI] [PubMed] [Google Scholar]
- Riihimaki V, Hanninen H, Akila R, Kovala T, Kuosma E, Paakkulainen H, Valkonen S, Engstrom B. Body burden of aluminum in relation to central nervous system function among metal inert-gas welders. Scan J Work Environ Health. 2000;26:118–130. doi: 10.5271/sjweh.521. [DOI] [PubMed] [Google Scholar]
- Rimaniol AC, Gras G, Verdier F, Capel F, Grigoriev VB, Porcheray F, Sauzeat E, Fournier JG, Clayette P, Siegrist CA, Dormont D. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. 2004;22:3127–3135. doi: 10.1016/j.vaccine.2004.01.061. [DOI] [PubMed] [Google Scholar]
- Rob PM, Niederstadt C, Reusche E. Dementia in patients undergoing long-term dialysis: aetiology, differential diagnoses, epidemiology and management. CNS Drugs. 2001;15:691–699. doi: 10.2165/00023210-200115090-00003. [DOI] [PubMed] [Google Scholar]
- Roberts NB, Williams P. Silicon measurement in serum and urine by direct current plasma emission spectrometry. Clin Chem. 1990;36:1460–1465. [PubMed] [Google Scholar]
- Roberts NB, Clough A, Bellia JP, Kim JY. Increased absorption of aluminum from a normal dietary intake in dementia. J Inorg Biochem. 1998;69:171–176. doi: 10.1016/s0162-0134(97)10015-0. [DOI] [PubMed] [Google Scholar]
- Roberts RJ. Dolomite as a source of toxic metals. N Engl J Med. 1981;304:423. doi: 10.1056/nejm198102123040712. [DOI] [PubMed] [Google Scholar]
- Robertson JA, Felsenfeld AJ, Haygood CC, Wilson P, Clarke C, Llach F. Animal model of aluminum-induced osteomalacia: role of chronic renal failure. Kidney Int. 1983;23:327–335. doi: 10.1038/ki.1983.23. [DOI] [PubMed] [Google Scholar]
- Robertson JA, Salusky IB, Goodman WG, Norris KC, Coburn JW. Sucralfate, intestinal aluminum absorption, and aluminum toxicity in a patient on dialysis. Ann Intern Med. 1989;111:179–181. doi: 10.7326/0003-4819-111-2-179. [DOI] [PubMed] [Google Scholar]
- Robillard E, Alarie Y. Static volume-pressure relations in guinea pig lungs after inhalation of aluminum or iron oxide particles and bronchodilator aerosols. Can J Biochem Physiol. 1963a;41:461–468. [PubMed] [Google Scholar]
- Robillard E, Alarie Y. Pressure-volume curves in isolated atelectatic rat lungs after aluminum oxide microparticle inhalation. Can J Biochem Physiol. 1963b;41:1257–1265. [PubMed] [Google Scholar]
- Rockette HE, Arena VC. Mortality studies of aluminum reduction plant workers: Potroom and carbon department. J Occup Med. 1983;25:549–557. [PubMed] [Google Scholar]
- Rodella L, Rezzani R, Lanzi R, Bianchi R. Chronic exposure to aluminium decreases NADPH-diaphorase positive neurons in the rat cerebral cortex. Brain Res. 2001;889:229–233. doi: 10.1016/s0006-8993(00)03044-4. [DOI] [PubMed] [Google Scholar]
- Rodger RS, Muralikrishna GS, Halls DJ, Henderson JB, Forrest JA, MacDougall AI, Fell GS. Ranitidine suppresses aluminum absorption in man. Clin Sci. 1991;80:505–508. doi: 10.1042/cs0800505. [DOI] [PubMed] [Google Scholar]
- Rodushkin I, Odman F. Application of ICP-MS for elemental analysis of urine. J Trace Elem Med Biol. 2001;14:241–247. doi: 10.1016/S0946-672X(01)80010-9. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Simon DG. A preliminary study of dietary aluminum intake and risk of Alzheimer’s disease. Age Aging. 1999;28:205–209. doi: 10.1093/ageing/28.2.205. [DOI] [PubMed] [Google Scholar]
- Roider G, Drasch G. Concentration of aluminum in human tissues-investigations on an ocupationally non-exposed population in Southern Bavaria (Germany) Trace Elem Electrolytes. 1999;16:77–86. [Google Scholar]
- Roig JL, Fuentes S, Colomina MT, Vicens P, Domingo JL. Aluminum, restraint stress and aging: behavioral effects in rats after 1 and 2 years of aluminum exposure. Toxicology. 2006;218:112–124. doi: 10.1016/j.tox.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Roit I, Brostoff J, Male D. Vaccination, Immunology. London: Mosby; 1998. [Google Scholar]
- Rollin HB, Nogueira CM. Identification of aluminium fractions in serum using the techniques of high performance liquid chromatography, ultrafiltration and Zeeman atomic absorption spectrometry. Eur J Clin Chem Clin Biochem. 1997;35:215–222. doi: 10.1515/cclm.1997.35.3.215. [DOI] [PubMed] [Google Scholar]
- Rollin HB, Theodorou P, Kilroe-Smith TA. The effect of exposure to aluminium on concentrations of essential metals in serum of foundry workers. Br J Ind Med. 1991a;48:243–246. doi: 10.1136/oem.48.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin HB, Theodorou P, Kilroe-Smith TA. Deposition of aluminum in tissues of rabbits exposed to inhalation of low concentrations of Al203 dust. Br J Ind Med. 1991b;48:389–391. doi: 10.1136/oem.48.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin HB, Theodorou P, Cantrell AC. Biological indicators of exposure to total and respirable aluminum dust fractions in a primary aluminum smelter. Occup Environ Med. 1996;53:417–421. doi: 10.1136/oem.53.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin HB, Theodorou P, Nogueir CM, Levin J. Aluminium uptake and excretion in potroom workers of a new primary aluminium smelter during the construction stage. J Environ Monit. 2001;3:560–564. doi: 10.1039/b105849p. [DOI] [PubMed] [Google Scholar]
- Romundstad P, Haldorsen T, Andersen A. Lung and bladder cancer among workers in a Norwegian aluminum reduction plant. Occup Environ Med. 2000a;57:495–499. doi: 10.1136/oem.57.7.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romundstad P, Andersen A, Haldorsen T. Cancer incidence among workers in six Norwegian aluminum plants. Scand J Work Environ Health. 2000b;26:461–469. doi: 10.5271/sjweh.569. [DOI] [PubMed] [Google Scholar]
- Rondeau V, Commenges D, Jacqmin-Gadda H, Dartigues JF. Relation between aluminum concentrations in drinking water and Alzheimer’s disease: an 8-year follow-up study. Am J Epidemiol. 2000;152:59–66. doi: 10.1093/aje/152.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau V, Jacqmin-Gadda H, Commenges D, Dartiques JF. Aluminum in drinking water and cognitive decline in elderly subjects: the Paquid cohort. Am J Epidemiol. 2001;154:288–290. doi: 10.1093/aje/154.3.288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau V, Iron, Letenneur L, Commenges D, Duchene F, Arveiller B, Dartiques JF. Analysis of the effect of aluminum in drinking water and transferrin C2 allele on Alzheimer’s disease. Eur J Neurol. 2006;13:1022–1025. doi: 10.1111/j.1468-1331.2006.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronneberg A, Haldorsen T, Romundstad P, Andersen A. Occupational exposure and cancer incidence among workers from an aluminum smelter in western Norway. Scand J Work Environ Health. 1999;25:207–214. doi: 10.5271/sjweh.425. [DOI] [PubMed] [Google Scholar]
- Rosborg I, Nihlgard B, Gerhardsson L, Gernersson ML, Ohlin R, Olsson T. Concentrations of inorganic elements in bottled waters on the Swedish market. Environ Geochem Health. 2005;27:217–227. doi: 10.1007/s10653-004-1612-8. [DOI] [PubMed] [Google Scholar]
- Roskams AJ, Connor JR. Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci. 1990;87:9024–9027. doi: 10.1073/pnas.87.22.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Takacs A. Heterogeneous reactions of aluminum and copper surfaces with stearic acid. Ind Eng Chem Prod Res Dev. 1983;22:280–286. [Google Scholar]
- Rowland M, Tozer TM. Clinical Pharmacokinetics Concepts and Applications. 3. Philadelphia: Williams & Wilkins, Media; 1995. [Google Scholar]
- Roy AJ, Talukder G, Sharma A. Effects of aluminium sulphate on human leukocyte chromosomes in vitro. Mutat Res. 1990;244:179–183. doi: 10.1016/0165-7992(90)90069-v. [DOI] [PubMed] [Google Scholar]
- Roy AJ, Talukder G, Sharma A. Similar effects in vivo of two aluminum salts on the liver, kidney, bone, and brain of Rattus norvegicus. Bull Environ Contam Toxicol. 1991;47:288–295. doi: 10.1007/BF01688654. [DOI] [PubMed] [Google Scholar]
- Roy AJ, Dhir H, Sharma A. Modification of metal induced micronuclei formation in mouse bone marrow erythrocytes by Phyllanthus fruit extract and ascorbic acid. Toxicol Lett. 1992;62:9–17. doi: 10.1016/0378-4274(92)90072-r. [DOI] [PubMed] [Google Scholar]
- Roychowdhury M. A review of safety and health hazards of metalorganic compounds. Am Ind Hyg Assoc J. 1993;54:607–614. [Google Scholar]
- RTECS (Registry of Toxic Effects of Chemical Substances) Aluminum oxide. 2006 [online] Cited 24 January 2007 www.cdc.gov/niosh/rtecs/bd124f80.html.
- Rudy D, Sica DA, Comstock T, Davis J, Savory J, Schoolwerth AC. Aluminum-citrate interaction in end-stage renal disease. Int J Artif Organs. 1991;14:625–629. [PubMed] [Google Scholar]
- Ruster M, Abendroth K, Lehmann G, Stein G. Aluminum deposition in the bone of patients with chronic renal failure--detection of aluminum accumulation without signs of aluminum toxicity in bone using acid solochrome azurine. Clin Nephrol. 2002;58:305–312. doi: 10.5414/cnp58305. [DOI] [PubMed] [Google Scholar]
- Sahin G, Varol I, Temizer A, Benli K, Demirdamar R, Duru S. Determination of aluminum levels in the kidney, liver, and brain of mice treated with aluminum hydroxide. Biol Trace Elem Res. 1994;41:129–135. doi: 10.1007/BF02917223. [DOI] [PubMed] [Google Scholar]
- Saia B, Cortese S, Piazza G, Camposampietro A, Clonfero E. Chest x-ray findings among aluminium production plant workers. Med Lav. 1981;72:323–329. [PubMed] [Google Scholar]
- Saiyed SM, Yokel RA. Aluminium content of some foods and food products in the USA, with aluminium food additives. Food Addit Contam. 2005;22:234–244. doi: 10.1080/02652030500073584. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Ogasawara Y, Ishii K, Takahashi H, Tanabe S. Accumulation of aluminum in ferritin isolated from rat brain. Neurosci Lett. 2004;366:264–267. doi: 10.1016/j.neulet.2004.05.045. [DOI] [PubMed] [Google Scholar]
- Sakhaee K, Wabner CL, Zerwekh JE, Copley JB, Pak L, Poindexter JR, Pak CY. Calcium citrate without aluminum antacids does not cause aluminum retention in patients with functioning kidneys. Bone Miner. 1993;20:87–97. doi: 10.1016/s0169-6009(08)80040-2. [DOI] [PubMed] [Google Scholar]
- Sakiewicz P, Paganini E. The use of iron in patients on chronic dialysis: mistake and misconceptions. J Nephrol. 1998;11:5–15. [PubMed] [Google Scholar]
- Sakula A. Pneumoconiosis due to Fuller’s earth. Thorax. 1961;16:176–179. doi: 10.1136/thx.16.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salib E, Hillier V. A case-control study of Alzheimer’s disease and aluminum occupation. Br J Psychiatry. 1996;168:244–249. doi: 10.1192/bjp.168.2.244. [DOI] [PubMed] [Google Scholar]
- Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- San LN, Uysal H, Gokbel H, Bediz LS, Sayal A. Pulmonary function of workers in the aluminum industry. Am J Ind Med. 1997;33:305–307. doi: 10.1002/(sici)1097-0274(199803)33:3<305::aid-ajim13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sanchez DJ, Gómez M, Llobet JM, Corbella J, Domingo JL. Effects of aluminum on the mineral metabolism of rats in relation to age. Pharmacol Toxicol. 1997;80:11–17. doi: 10.1111/j.1600-0773.1997.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Sanei H, Goodarzi F, Flier-Keller EV. Historical variation of elements with respect to different geochemical fractions in recent sediments from Pigeon Lake, Alberta, Canada. J Environ Monit. 2001;3:27–36. doi: 10.1039/b006819p. [DOI] [PubMed] [Google Scholar]
- Santaroni P. Ingestion of aluminum in the diet and evaluation of safety levels in Italy. Rivista di Tossicologia: Sperimentale e Clinica. 1990;20:25–30. [Google Scholar]
- Sanz-Medel A, Cabezuelo ABS, Milacic R, Polak TB. The chemical speciation of auminium in human serum. Coord Chem Rev. 2002;228:373–383. [Google Scholar]
- Saric M, Godnic-Cvar J, Gomzi M, Stillnovic L. The role of atopy in potroom workers asthma. Am J Ind Med. 1986;9:239–242. doi: 10.1002/ajim.4700090306. [DOI] [PubMed] [Google Scholar]
- Savage J. Proceedings: Aluminium hydroxide granuloma. Proc R Soc Med. 1973;66:984–985. [PMC free article] [PubMed] [Google Scholar]
- Savory J. Iatrogenic aluminum poisoning. Clin Chem. 1994;40:1477–1478. [PubMed] [Google Scholar]
- Savory J, Wills MR. Analysis of aluminum in biological materials. In: Siegel H, Siegel A, editors. Metal Ions in Biological Systems, Vol 24: Aluminum and its Role in Biology. New York: Marcel Dekker; 1988. pp. 347–371. [Google Scholar]
- Savory J, Brown S, Bertholf RL, Mendoza N, Wills MR. Aluminum. Methods Enzymol. 1988;158:289–301. doi: 10.1016/0076-6879(88)58061-8. [DOI] [PubMed] [Google Scholar]
- Savory J, Ghribi O, Forbes MS, Herman MM. Aluminium and neuronal cell injury: inter-relationships between neurofilamentous arrays and apoptosis. J Inorg Biochem. 2001;87:15–19. doi: 10.1016/s0162-0134(01)00309-9. [DOI] [PubMed] [Google Scholar]
- Savory J, Herman MM, Ghribi O. Intracellular mechanisms underlying aluminum-induced apoptosis in rabbit brain. J Inorg Biochem. 2003;15:151–154. doi: 10.1016/s0162-0134(03)00258-7. [DOI] [PubMed] [Google Scholar]
- Sax NI, Lewis RJ Sr, editors. Hawley’s Condensed Chemical Dictionary. 11. New York, NY: Van Nostrand Reinhold; 1987. pp. 1248–1249. [Google Scholar]
- Schaaper RM, Koplitz RM, Tkeskelashvili LK, Loeb LA. Metal-induced lethality and mutagenesis: possible role of apurinic intermediates. Mutat Res. 1987;177:179–188. doi: 10.1016/0027-5107(87)90001-7. [DOI] [PubMed] [Google Scholar]
- Schijns VE. Immunological concepts of vaccine adjuvant activity. Curr Opin Immunol. 2000;12:456–463. doi: 10.1016/s0952-7915(00)00120-5. [DOI] [PubMed] [Google Scholar]
- Schintu M, Meloni P, Contu A. Aluminum fractions in drinking water from reservoirs. Ecotoxicol Environ Saf. 2000;46:29–33. doi: 10.1006/eesa.1999.1887. [DOI] [PubMed] [Google Scholar]
- Schlatter C, Steinegger A. Limitations of biological monitoring of aluminium exposure. Second international conference on aluminum and health; Tampa, Florida. 1992. [Google Scholar]
- Schlatter C, Steinegger A, Rickenbacher U, Hans C, Lengyel A. Aluminum levels in the blood plasma of persons working in the aluminum industry. Environ Geochem Health. 1990;12:59–64. doi: 10.1007/BF01734049. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB, Snyder CA, Chen LC, Gorchzynski JE, Menarche M. Clearance and translocation of aluminium oxide (alumina) from the lungs. Inhal Toxicol. 2000;12:927–939. doi: 10.1080/08958370050137996. [DOI] [PubMed] [Google Scholar]
- Schmid K, Angerer J, Letzel S, Sturm G, Lehnert G. Use of bone mineral content determination by x-ray absorptiometry in the evaluation of osteodystrophy among workers exposed to aluminum powders. Sci Total Environ. 1995;163:147–151. doi: 10.1016/0048-9697(95)04493-k. [DOI] [PubMed] [Google Scholar]
- Schmidt PF, Zumkley H, Bertram H, Lison A, Winterberg B, Barckhaus R. Localization of aluminum in bone of patients with dialysis osteomalacia. In: Bratter P, Schramel P, editors. Trace Elements-Analytical Chemistry in Medicine and Biology. Vol. 3. New York: Walter de Gruyter & Co; 1984. pp. 475–482. [Google Scholar]
- Schönholzer K, Sutton R, Walker V, Sossi V, Schulzer M, Venczel E, Johnson R, Vetterli D, Dittrich B, Suter M. Is intestinal aluminum absorption enhanced in the renal failure rat? J Am Soc Nephrol. 1992;3:676. abstract. [Google Scholar]
- Schönholzer KW, Sutton RA, Walker VR, Sossi V, Schulzer M, Orvig C, Venczel E, Johnson RR, Vetterli D, Dittrich-Hannen B, Kubik P, Sutter M. Intestinal absorption of trace amounts of aluminium in rats studied with 26aluminium and accelerator mass spectrometry. Clin Sci. 1997;92:379–383. doi: 10.1042/cs0920379. [DOI] [PubMed] [Google Scholar]
- Schreider JP, Culbertson MR, Raabe OG. Comparative pulmonary fibrogenic potential of selected particles. Environ Res. 1985;38:256–274. doi: 10.1016/0013-9351(85)90090-8. [DOI] [PubMed] [Google Scholar]
- Schroeder HA, Mitchener M. Life-term effects of mercury, methyl mercury, and nine other trace metals on mice. J Nutr. 1975a;105:452–458. doi: 10.1093/jn/105.4.452. [DOI] [PubMed] [Google Scholar]
- Schroeder HA, Mitchener M. Life-term studies in rats: effects of aluminum, barium, beryllium, and tungsten. J Nutr. 1975b;105:421–427. doi: 10.1093/jn/105.4.421. [DOI] [PubMed] [Google Scholar]
- Schroeder JC, Tolbert PE, Eisen EA, Monson RR, Hallock MF, Smith TJ, Woskie SR, Hammond SK, Milton DK. Mortality studies of machining fluid exposure in the automobile industry. IV: A case-control study of lung cancer. Am J Ind Med. 1997;31:525–533. doi: 10.1002/(sici)1097-0274(199705)31:5<525::aid-ajim5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schulz W, Deuber HJ, Popperl G. On the differential diagnosis and therapy of dialysis osteomalacia under special consideration to a therapy with oral phosphate binders. Trace Elem Med. 1984;1:120–127. [Google Scholar]
- Schuurmans Stekhoven JH, Renkawek K, Otte-Holler I, Stols A. Exogenous aluminum accumulates in the lysosomes of cultured rat cortical neurons. Neurosci Lett. 1990;119:71–74. doi: 10.1016/0304-3940(90)90758-2. [DOI] [PubMed] [Google Scholar]
- Schwarze HP, Giordano-Labadie F, Loche F, Gorguet MB, Bazex J. Delayed-hypersensitivity granulomatous reaction induced by blepharopigmentation with aluminum-silicate. J Am Acad Dermatol. 2000;42:888–891. doi: 10.1016/s0190-9622(00)90264-0. [DOI] [PubMed] [Google Scholar]
- Sedman AB, Wilkening GN, Warady BA. Clinical and laboratory observations. Encephalopathy in childhood secondary to aluminum toxicity. J Pediatr. 1984;105:836–838. doi: 10.1016/s0022-3476(84)80318-2. [DOI] [PubMed] [Google Scholar]
- Sedman AB, Klein GL, Merritt RJ, Miller NL, Weber KO, Gill WL, Anand H, Alfrey AC. Evidence of aluminum loading in infants receiving intravenous therapy. N Engl J Med. 1985;312:1337–1343. doi: 10.1056/NEJM198505233122101. [DOI] [PubMed] [Google Scholar]
- Sedman AB, Alfrey AC, Miller NL, Goodman WG. Tissue and cellular basis for impaired bone formation in aluminum-related osteomalacia in the pig. J Clin Invest. 1987;79:86–92. doi: 10.1172/JCI112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko Y, Nakamura I, Sugamata M, Nonaka K, Miura T. A possible increase in the concentration of aluminum in the brain of rats infected with Japanese encephalitis virus; an investigation by neutron activation analysis. Igaku to Seibutsugaku. 1986;113:367–370. [Google Scholar]
- Selden AI, Westberg HB, Axelson O. Cancer morbidity in workers at aluminum foundries and secondary aluminum smelters. Am J Ind Med. 1997;32:467–477. doi: 10.1002/(sici)1097-0274(199711)32:5<467::aid-ajim6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Senitz D, Bluthner K. The presence of aluminum in cerebral vessels in Alzheimer’s disease. Zentralblatt allg Pathology. 1990;136:329–335. [PubMed] [Google Scholar]
- Seo KS, Lee CS. Mutagenicity of metal compounds using Escherichia coli WP2 UVRA. Misaengmul Hakhoechi. 1993;31:527–531. [Google Scholar]
- Shah NR, Oberkircher OR, Lobel JS. Aluminum-induced microcytosis in a child with moderate renal insufficiency. Am J Pediatr Hematol Oncol. 1990;12:77–79. doi: 10.1097/00043426-199021000-00015. [DOI] [PubMed] [Google Scholar]
- Sharif AAM, Ghafourian H, Ahmadiniar A, Waqif Husain S, Saber-Tehrani M, Ghods H. Determination of aluminum levels in serum and red blood cells from long-term haemodialysis patients using instrumental neutron activation analysis. J Radioanal Nucl Chem. 2004;262:473–477. [Google Scholar]
- Sharpe FR, Vobe RA, Williams DR. Chemical speciation of aluminum in beers. Chem Spec Bioavail. 1995;7:49–55. [Google Scholar]
- Shaver CG. Pulmonary changes encountered in employees engaged in the manufacture of alumina abrasives. Occup Med. 1948;5:718–728. [PubMed] [Google Scholar]
- Shaver CG, Riddell AR. Lung changes associated with the manufacture of alumina abrasives. J Ind Hyg Toxicol. 1947;29:145–157. [PubMed] [Google Scholar]
- Sherrard DJ, Walker JV, Boykin JL. Precipitation of dialysis dementia by deferoxamine treatment of aluminum-related bone disease. Am J Kidney Dis. 1988;12:126–130. doi: 10.1016/s0272-6386(88)80007-6. [DOI] [PubMed] [Google Scholar]
- Sherwin RP, Barman ML, Abraham JL. Silicate pneumoconiosis of farm workers. Lab Invest. 1979;40:576–582. [PubMed] [Google Scholar]
- Shi B, Haug A. Aluminum uptake by neuroblastoma cells. J Neurochem. 1990;55:551–558. doi: 10.1111/j.1471-4159.1990.tb04169.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, HogenEsch H, Regnier FE, Hem SL. Detoxification of endotoxin by aluminium hydroxide adjuvant. Vaccine. 2001;19:1747–1752. doi: 10.1016/s0264-410x(00)00394-7. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Suzuki Y, Takemura N, Goto S, Matsushita H. Results of microbial mutation test for forty-three industrial chemicals. Sangyo Igaku. 1985;27:400–419. doi: 10.1539/joh1959.27.400. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Mori T, Koyama M, Sekiya M, Ooami H. A correlative study of the aluminum content and aging changes of the brain in non-demented elderly subjects. Nippon Ronen Igakkai Zasshi. 1994;31:950–960. doi: 10.3143/geriatrics.31.950. [DOI] [PubMed] [Google Scholar]
- Shin RW, Lee VM, Trojanowski JQ. Neurofibrillary pathology and aluminum in Alzheimer’s disease. Histol Histopathol. 1995;10:969–978. [PubMed] [Google Scholar]
- Shirley DG, Walter MF, Walter SJ, Thewles A, Lote CJ. Renal aluminium handling in the rat: a micropuncture assessment. Clin Sci. 2004;107:159–165. doi: 10.1042/CS20040052. [DOI] [PubMed] [Google Scholar]
- Shore D, Wyatt RJ. Aluminum and Alzheimer’s disease. J Nerv Ment Dis. 1983;171:553–558. doi: 10.1097/00005053-198309000-00005. [DOI] [PubMed] [Google Scholar]
- Shore D, Sprague SM, Mayor GH, Moreno EC, Apostoles PS, Wyatt RJ. Aluminum-fluoride complexes: preclinical studies. J Environ Pathol Toxicol Oncol. 1985;6:9–13. [PubMed] [Google Scholar]
- Shubik P, Hartwell JL. Survey of compounds which have been tested for carcinogenic activity. 1969;(Suppl 2) U.S. Public Health Service Publication, No. 149. [Google Scholar]
- Siegrist CA. Vaccine adjuvants and macrophagic myofasciitis. Arch Pediatr. 2005;12:96–101. doi: 10.1016/j.arcped.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Sighinolfi GP, Gorgoni C, Bonori O, Cantoni E, Martelli M, Simonetti L. Comprehensive determination of trace elements in human saliva by ETA-AAS. Microchimica Acta. 1989;97:171–179. [Google Scholar]
- Silva VS, Cordeiro JM, Matos MJ, Oliveira CR, Goncalves PP. Aluminum accumulation and membrane fluidity alteration in synaptosomes isolated from rat brain cortex following aluminum ingestion: effect of cholesterol. Neurosci Res. 2002;44:181–193. doi: 10.1016/s0168-0102(02)00128-1. [DOI] [PubMed] [Google Scholar]
- Silwood CJ, Grootveld M. Evaluation of the speciation status of aluminium(III) ions in isolated osteoarthritic knee-joint synovial fluid. Biochim Biophys Acta. 2005;1725:327–339. doi: 10.1016/j.bbagen.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Sim M, Benke G. World at work: hazards and controls in aluminium potrooms. Occup Environ Med. 2003;60:989–992. doi: 10.1136/oem.60.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer K, Fudge A, Teubner J, James SL. Aluminum concentrations in infant formulae. J Paediatr Child Health. 1990;26:9–11. doi: 10.1111/j.1440-1754.1990.tb02370.x. [DOI] [PubMed] [Google Scholar]
- Simonsson BG, Sjöberg A, Rolf C, Haeger-Aronsen B. Acute and long-term airway hyperreactivity in aluminium-salt exposed workers with nocturnal asthma. Eur J Respir Dis. 1985;66:105–118. [PubMed] [Google Scholar]
- Simonyte S, Cherkashin G, Sadauskiene I, Planciuniene R, Stapulionis R, Ivanov L. Effects of lead and aluminum on the specific immune response of growing mice. Ekologija. 2004;2:16–20. [Google Scholar]
- Sinczuk-Walczak H, Szymczak M, Razniewska G, Matczak W, Szymczak W. Effects of occupational exposure to aluminum on nervous system: clinical and electroencephalographic findings. Int J Occup Med Environ Health. 2003;16:301–310. [PubMed] [Google Scholar]
- Sinigaglia F. The molecular basis of metal recognition by T cells. J Invest Dermatol. 1994;102:398–401. doi: 10.1111/1523-1747.ep12372149. [DOI] [PubMed] [Google Scholar]
- Sirover MA, Loeb LA. Infidelity of DNA synthesis in vitro: screening for potential metal mutagens or carcinogens. Science. 1976;194:1434–1436. doi: 10.1126/science.1006310. [DOI] [PubMed] [Google Scholar]
- Sivikova K, Dianovsky J. Sister-chromatid exchanges after exposure to metal-containing emissions. Mutat Res. 1995;327:17–22. doi: 10.1016/0027-5107(94)00061-9. [DOI] [PubMed] [Google Scholar]
- Sjögren B. Occupational aluminum exposure. Neurotoxicology. 2000;21:871. [Google Scholar]
- Sjögren B, Elinder CG. Proposal of a dose-response relationship between aluminium welding fume exposure and effect on the central nervous system. Med Lav. 1992;83:484–488. [PubMed] [Google Scholar]
- Sjögren B, Ulfvarson U. Respiratory symptoms and pulmonary function among welders working with aluminum, stainless steel and railroad tracks. Ulfnd J Work Environ Health. 1985;11:27–32. doi: 10.5271/sjweh.2257. [DOI] [PubMed] [Google Scholar]
- Sjögren B, Lundberg I, Lidums V. Aluminium in the blood and urine of industrially exposed workers. Br J Ind Med. 1983;40:301–304. doi: 10.1136/oem.40.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren B, Lidums V, Hakansson M, Hedstrom L. Exposure and urinary excretion of aluminum during welding. Scand J Work Environ Health. 1985;11:39–43. doi: 10.5271/sjweh.2255. [DOI] [PubMed] [Google Scholar]
- Sjögren B, Elinder CG, Lidums V, Chang G. Uptake and urinary excretion of aluminum among welders. Int Arch Occup Environ Health. 1988;60:77–79. doi: 10.1007/BF00381484. [DOI] [PubMed] [Google Scholar]
- Sjögren B, Gustavsson P, Hogstedt C. Neuropsychiatric symptoms among welders exposed to neurotoxic metals. Br J Ind Med. 1990;47:704–707. doi: 10.1136/oem.47.10.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren B, Iregren A, Frechn W, Hagman M, Johansson L, Tesarz M, Wennberg A. Effects on the nervous system among welders exposed to aluminum and maganese. Occup Environ Med. 1996a;53:32–40. doi: 10.1136/oem.53.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren B, Ljunggren KG, Almkvist O, Frech W, Basun H. A follow-up study of five cases of aluminosis. Int Arch Occup Environ Health. 1996b;68:161–164. doi: 10.1007/BF00381625. [DOI] [PubMed] [Google Scholar]
- Sjögren B, Elinder CG, Iregren A, McLachlan DRC, Riihimaki V. Occupational aluminum exposure and its health effects. In: Yokel RA, Golub MS, editors. Aluminum Toxicity. Washington DC.: Taylor & Francis; 1997. pp. 165–183. [Google Scholar]
- Skaugset NP, Elingsen DG, Jordbekken L, Notø H, Thomassen Y. Measurement strategies for peak exposure to gases and aerosols. Abstract 0-60, Fifth International Symposium on Modern Principles of Air Monitoring (Including Biomonitoring), Airmon 2005; Loen, Norway. 2005. [Google Scholar]
- Skelton HG, 3rd, Smith KJ, Johnson FB, Cooper CR, Tyler WF, Lupton GP. Zirconium granuloma resulting from an aluminum zirconium complex: a previously unrecognized agent in the development of hypersensitivity granulomas. J Am Acad Dermatol. 1993;28:874–876. doi: 10.1016/0190-9622(93)70122-a. [DOI] [PubMed] [Google Scholar]
- Skowron F, Grezard P, Berard F, Balme B, Perrot H. Persistent nodules at sites of hepatitis B vaccination due to aluminium sensitization. Contact Dermatitis. 1998;39:135–136. doi: 10.1111/j.1600-0536.1998.tb05865.x. [DOI] [PubMed] [Google Scholar]
- Slanina P, Falkeborn Y, Frech W, Cedergren A. Aluminium concentrations in the brain and bone of rats fed citric acid, aluminium citrate or aluminium hydroxide. Food Chem Toxicol. 1984;22:391–397. doi: 10.1016/0278-6915(84)90369-7. [DOI] [PubMed] [Google Scholar]
- Slanina P, Frech W, Bernhardson A, Cedergren A, Mattsson P. Influence of dietary factors on aluminium absorption and retention in the brain and bone of rats. Acta Pharmacol Toxicol. 1985;56:331–336. doi: 10.1111/j.1600-0773.1985.tb01299.x. [DOI] [PubMed] [Google Scholar]
- Slanina P, Frech W, Ekström LG, Lööf L, Slorach S, Cedergren A. Dietary citric acid enhances absorption of aluminium in antacids. Clin Chem. 1986;32:539–541. [PubMed] [Google Scholar]
- Slater DN, Underwood JC, Durrant TE, Gray T, Hopper IP. Aluminium hydroxide granulomas: light and electron microscopic studies and X-ray microanalysis. Br J Dermatol. 1982;107:103–108. doi: 10.1111/j.1365-2133.1982.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Sleppy WC. Aluminum compounds. In: Kroschwitz JI, Howe-Grant M, editors. Kirk-Othmer Encylopedia of Chemical Technology. Vol. 2. New York: John Wiley & Sons; 1992. pp. 252–267. [Google Scholar]
- Smans KA, D’Haese PC, Van Landeghem GF, Andries LJ, Lamberts LV, Hendy GN, De Broe ME. Transferrin-mediated uptake of aluminium by human parathyroid cells results in reduced parathyroid hormone secretion. Nephrol Dial Transplant. 2000;15:1328–1336. doi: 10.1093/ndt/15.9.1328. [DOI] [PubMed] [Google Scholar]
- Smichowski P, Gomez DR, Dawidowski LE, Gine MF, Bellato AC, Reich SL. Monitoring trace metals in urban aerosols from Buenos Aires city. Determination by plasma-based techniques. J Environ Monit. 2004;6:286–294. doi: 10.1039/b312446k. [DOI] [PubMed] [Google Scholar]
- Smith CC, Hyatt PJ, Stanyer L, Betteridge DJ. Platelet secretion of beta-amyloid is increased in hypercholesterolaemia. Brain Res. 2001;896:161–164. doi: 10.1016/s0006-8993(01)02080-7. [DOI] [PubMed] [Google Scholar]
- Smith GD, Winney RJ, McLean A, Robson JS. Aluminium-related osteomalacia: response to reverse osmosis water treatment. Kidney Int. 1987;32:96–101. doi: 10.1038/ki.1987.177. [DOI] [PubMed] [Google Scholar]
- Smith PS, McClure J. Localization of aluminum by histochemical and electron probe x-ray microanalytical techniques in bone tissue of cases of renal osteodystrophy. J Clin Pathol. 1982;35:1283–1293. doi: 10.1136/jcp.35.11.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Q, Mori H, Swyt C, Atack J, Rapoport S. Electron probe microanalysis of aluminum and silicon in Alzheimer’s disease senile palques using unfixed, unstained tissue. Aluminum Association. Aluminum and Health, 2 nd International Conference; Tampa, Florida. 1992. [Google Scholar]
- Smith Q, Deng Q, Brady D, Swyt C, Nguyen J, Gillen G. Aluminum localization and analysis in stained and unstained human brain tissue using X-ray microanalysis and secondary ion mass spectrometry. Aluminum Association. Aluminum and Health, 3 rd International Conference; Miami, Florida. 1994. [Google Scholar]
- Snipes MB, Boecker BB, McClellan RO. Retention of monodisperse or polydisperse aluminosilicate particles inhaled by dogs, rats, and mice. Toxicol Appl Pharmacol. 1983;69:345–362. doi: 10.1016/0041-008x(83)90258-2. [DOI] [PubMed] [Google Scholar]
- Sohn SJ, Shin JH, Park YS, Rhee JA, Choi JS. Components of drinking water and risk of cognitive impairment in the rural elderly. Chonnam J Med Sci. 1996;9:189–193. [Google Scholar]
- Solfrizzi V, Panza F, Capurso A. The role of diet in cognitive decline. J Neural Transm. 2003;110:95–110. doi: 10.1007/s00702-002-0766-8. [DOI] [PubMed] [Google Scholar]
- Soliman K, Zikovsky L. Concentrations of Al in food sold in Montreal, Canada, and its daily dietary intake. J Radioanal Nucl Chem. 1999;242:807–809. [Google Scholar]
- Soni MG, White SM, Flamm WG, Burdock GA. Safety evaluation of dietary aluminum. Regul Toxicol Pharmacol. 2001;33:66–79. doi: 10.1006/rtph.2000.1441. [DOI] [PubMed] [Google Scholar]
- Sorgdrager B, Pal TM, de Looff AJ, Dubois AE, de Monchy JG. Occupational asthma in aluminium potroom workers related to pre-employment eosinophil count. Eur Respir J. 1995;8:1520–1524. [PubMed] [Google Scholar]
- Sorgdrager B, de Looff AJ, de Monchy JG, Pal TM, Dubois AE, Rijcken B. Occurrence of occupational asthma in aluminum potroom workers in relation to preventive measures. Int Arch Occup Environ Health. 1998;71:53–59. doi: 10.1007/s004200050250. [DOI] [PubMed] [Google Scholar]
- Søyseth V, Kongerud J. Prevalence of respiratory disorders among aluminum potroom workers in relation to exposure to fluoride. Br J Ind Med. 1992;49:125–130. doi: 10.1136/oem.49.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søyseth V, Kongerud J, Kjuus H, Boe J. Bronchial responsiveness and decline in FEV in aluminum potroom workers. Eur Resp J. 1994;7:888–894. [PubMed] [Google Scholar]
- Søyseth V, Kongerud J, Aalen OO, Botten G, Boe J. Bronchial responsiveness decreases in relocated aluminum potroom workers compared with workers who continue their potroom exposure. Int Arch Occup Environ Health. 1995;67:53–57. doi: 10.1007/BF00383133. [DOI] [PubMed] [Google Scholar]
- Søyseth V, Kongerud J, Boe J. Increased variability in bronchial responsiveness in aluminum potroom workers with work-related asthma-like symptoms. Occup Environ Med. 1996;38:66–69. doi: 10.1097/00043764-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Søyseth V, Boe J, Kongerud J. Relation between decline in FEV1 and exposure to dust and tobacco smoke in aluminum potroom workers. Occup Environ Med. 1997;54:27–31. doi: 10.1136/oem.54.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman DR. X-ray probe microanalysis of Alzheimer disease soluble and insoluble paired helical filaments. Neurosci Lett. 1993;151:153–157. doi: 10.1016/0304-3940(93)90009-a. [DOI] [PubMed] [Google Scholar]
- Spencer H, Kramer L. Osteoporosis: calcium, fluoride, and aluminum interactions. J Am Coll Nutr. 1985;4:121–128. doi: 10.1080/07315724.1985.10720071. [DOI] [PubMed] [Google Scholar]
- Spencer H, Lender M. Adverse effects of aluminum-containing antacids on mineral metabolism. Gastroenterology. 1979;76:603–606. [PubMed] [Google Scholar]
- Spencer H, Kramer L, Norris C, Wiatrowski E. Effect of aluminum hydroxide on fluoride metabolism. Clin Pharmacol Therap. 1980a;28:529–535. doi: 10.1038/clpt.1980.198. [DOI] [PubMed] [Google Scholar]
- Spencer H, Kramer L, Osis D, Wiatrowski E, Norris C, Lender M. Effect of calcium, phosphorus, magnesium, and aluminum on fluoride metabolism in man. Ann N Y Acad Sci. 1980b;355:181–194. doi: 10.1111/j.1749-6632.1980.tb21337.x. [DOI] [PubMed] [Google Scholar]
- Spencer H, Kramer L, Norris C, Wiatrowski E. Effect of aluminum hydroxide on plasma fluoride and fluoride excretion during a high fluoride intake in man. Toxicol Appl Phamacol. 1981;58:140–144. doi: 10.1016/0041-008x(81)90124-1. [DOI] [PubMed] [Google Scholar]
- Spencer H, Kramer L, Osis D, Wiatrowski E. Effects of aluminum hydroxide on fluoride and calcium metabolism. J Environ Pathol Toxicol Oncol. 1985;6:33–41. [PubMed] [Google Scholar]
- Spencer PS. Aluminum and its compounds. In: Spencer PS, Schaumburg HH, editors. Experimental and Clinical Neurotoxicology. New York: Oxford University Press; 2000. pp. 142–151. [Google Scholar]
- Speziali M, Orvini E. Metals distribution and regionalization in the brain. In: Zatta P, editor. Metal Ions and Neurodegenerative Disorders. Singapore: World Scientific Publishing Co. Pte. Ltd; 2003. pp. 15–65. [Google Scholar]
- Spinelli JJ, Band PR, Svirchev LM, Gallagher RP. Mortality and cancer incidence in aluminum reduction plant workers. J Occup Med. 1991;33:1150–1155. doi: 10.1097/00043764-199111000-00011. [DOI] [PubMed] [Google Scholar]
- Spotheim-Maurizot M, Garnier F, Sabattier R, Charlier M. Metal ions protect DNA against strand breakage induced by fast neutrons. Int J Radiat Biol. 1992;62:659–666. doi: 10.1080/09553009214552601. [DOI] [PubMed] [Google Scholar]
- Sreekumaran E, Ramakrishna T, Madhav TR, Anandh D, Prabhu BM, Sulekha S, Bindu PN, Raju TR. Loss of dendritic connectivity in CA1, CA2, and CA3 neurons in hippocampus in rat under aluminum toxicity: antidotal effect of pyridoxine. Brain Res Bull. 2003;59:421–427. doi: 10.1016/s0361-9230(02)00944-9. [DOI] [PubMed] [Google Scholar]
- Srivastava RA, Jain JC. Scavenger receptor class B type I expression and elemental analysis in cerebellum and parietal cortex regions of the Alzheimer’s disease brain. J Neurol Sci. 2002;196:45–52. doi: 10.1016/s0022-510x(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Stables GI, Forsyth A, Lever RS. Patch testing in children. Contact Dermatitis. 1996;34:341–344. doi: 10.1111/j.1600-0536.1996.tb02219.x. [DOI] [PubMed] [Google Scholar]
- Stacy BD, King EJ, Harrison CV, Nagelschmidt G, Nelson S. Tissue changes in rats’ lungs caused by hydroxides, oxides and phosphates of aluminium and iron. J Pathol Bacteriol. 1959;77:417–426. doi: 10.1002/path.1700770212. [DOI] [PubMed] [Google Scholar]
- Staley JT, Haupin W. Aluminum and aluminum alloys. In: Kroschwitz JI, Howe-Grant M, editors. Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 2. New York: John Wiley & Sons; 1992. [Google Scholar]
- Stanton MF. Fiber carcinogenesis: is asbestos the only hazard? J Natl Cancer Inst. 1974;52:633–634. doi: 10.1093/jnci/52.3.633. [DOI] [PubMed] [Google Scholar]
- Stauber JL, Florence TM, Davies CM, Adams MS, Buchanan SJ. Bioavailability of Al in alum-treated drinking water. J Am Water Works Assoc. 1999;91:84–93. [Google Scholar]
- Steel M. On the absorption of aluminium from aluminized food. Am J Physiol. 1911;28:94–102. [Google Scholar]
- Steinhagen WH, Cavender FL, Cockrell BY. Six month inhalation exposures of rats and guinea pigs to aluminum chlorhydrate. J Environ Pathol Toxicol. 1978;1:267–277. [PubMed] [Google Scholar]
- Steinhausen C, Kislinger G, Winklhofer C, Beck E, Hohl C, Nolte E, Ittel TH, Alvarez-Bruckmann MJ. Investigation of the aluminium biokinetics in humans: a 26Al tracer study. Food Chem Toxicol. 2004;42:363–371. doi: 10.1016/j.fct.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Stenback F, Ferrero A, Shubik P. Synergistic effects of diethylnitrosamine and different dusts on respiratory carcinogenesis in hamsters. Cancer Res. 1973;33:2209–2214. [PubMed] [Google Scholar]
- Stern AJ, Perl DP, Munoz-Garcia D, Good PF, Abraham C, Selkoe DJ. Investigation of silicon and aluminum content in isolated senile plaque cores by laser microprobe mass analysis (LAMMA) J Neuropathol Exp Neurol. 1986;45:361. [Google Scholar]
- Stevens BJ, Willis GL, Humphrey TJ, Atkins RC. Combined toxicity of aluminum and fluoride in the rat: possible implications in hemodialysis. Trace Elem Med. 1987;4:61–66. [Google Scholar]
- Stevens RK, Dzubay TG, Russwurm G, Rickel D. Sampling and analysis of atmospheric sulfates and related species. Atmos Environ. 1978;12:55–68. [Google Scholar]
- Stitch SR. Trace elements in human tissue. I. A semi-quantitative spectrographic survey. Biochem J. 1957;67:97–103. doi: 10.1042/bj0670097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CJ, McLaurin DA, Steinhagen WH, Cavender FL, Haseman JK. Tissue deposition patterns after chronic inhalation exposures of rats and guinea pigs to aluminum chlorhydrate. Toxicol Appl Pharmacol. 1979;49:71–76. doi: 10.1016/0041-008x(79)90278-3. [DOI] [PubMed] [Google Scholar]
- Straif K, Baan R, Gross Y, Secretan B, El Ghissassi F, Cogliano V. on behalf of the WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol. 2005;6:931–932. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- Struys-Ponsar C, Kerkhofs A, Gauthier A, Soffie M, van den Bosch de Aguilar P. Effects of aluminum exposure on behavioral parameters in the rat. Pharmacol Biochem Behav. 1997;56:643–648. doi: 10.1016/s0091-3057(96)00515-1. [DOI] [PubMed] [Google Scholar]
- Stumm W, Morgan JJ. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York: John Wiley & Sons, Inc; 1996. [Google Scholar]
- Suarez-Fernandez MB, Soldado AB, Sanz-Medel A, Vega JA, Novelli A, Fernandez-Sanchez MT. Aluminum-induced degeneration of astrocytes occurs via apoptosis and results in neuronal death. Brain Res. 1999;835:125–136. doi: 10.1016/s0006-8993(99)01536-x. [DOI] [PubMed] [Google Scholar]
- Subra JF, Krari N, Tirot P, Mauras Y, Balit G, Van-Weydevelt FC, Allain P. Aluminum determination in the skin of patients with and without end-stage renal failure. Nephron. 1991;58:170–173. doi: 10.1159/000186409. [DOI] [PubMed] [Google Scholar]
- Suga C, Ikezawa Z. Porphyria cutanea tarda in hemodialyzed patients. Nippon Rinsho. 1995;53:1484–1490. [PubMed] [Google Scholar]
- Sugawara C, Sugawara N, Kiyosawa H, Miyake H. Decrease of serum triglyceride in normal rat fed with 2000 ppm aluminum diet for 67 days. II. Feeding young and adult rats a sucrose diet with addition of aluminum hyroxide and aluminum potassium sulfate. Fundam Appl Toxicol. 1988;10:616–623. doi: 10.1016/0272-0590(88)90188-1. [DOI] [PubMed] [Google Scholar]
- Sunami K, Yamadroi I, Kishimoto T, Ozaki S, Kawabata Y. Unilateral mixed-dust pneumoconiosis with aluminum deposition associated with interstitial pneumonia. Nihon Kyobu Shikkan Gakkai Zasshi. 1997;35:189–195. [PubMed] [Google Scholar]
- Sutherland JE, Greger JL. Kinetics of aluminum disposition after ingestion of low to moderate pharmacological doses of aluminum. Toxicology. 1998;126:115–125. doi: 10.1016/s0300-483x(98)00005-5. [DOI] [PubMed] [Google Scholar]
- Sutherland JE, Radzanowski GM, Greger JL. Bile is an important route of elimination of ingested aluminum by conscious male Sprague-Dawley rats. Toxicology. 1996;109:101–109. doi: 10.1016/0300-483x(96)03310-0. [DOI] [PubMed] [Google Scholar]
- Suwalsky M, Norris B, Villena F, Cuevas F, Sotomayor P, Zatta P. Aluminum fluoride affects the structure and functions of cell membranes. Food Chem Toxicol. 2004;42:925–933. doi: 10.1016/j.fct.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kohyama N. Malignant mesothelioma induced by asbestos and zeolite in the mouse peritoneal cavity. Environ Res. 1984;35:277–292. doi: 10.1016/0013-9351(84)90136-1. [DOI] [PubMed] [Google Scholar]
- Swartz R, Dombrouski J, Burnatowska-Hledin M, Mayor G. Microcytic anemia in dialysis patients: reversible marker of aluminum toxicity. Am J Kidney Dis. 1987;9:217–223. doi: 10.1016/s0272-6386(87)80058-6. [DOI] [PubMed] [Google Scholar]
- Swensson A, Nordenfelt O, Forssman S, Lundgren KD, Öhman H. Aluminum dust pneumoconiosis: A clinical study. Int Arch Gewerbepathol Gewerbehyg. 1962;19:131–148. [Google Scholar]
- Swegert CV, Dave KR, Katyare SS. Effect of aluminum induced Alzheimer’s like condition on oxidative energy metabolism in rat liver, brain and heart mitochondria. Mech Ageing Dev. 1999;112:27–42. doi: 10.1016/s0047-6374(99)00051-2. [DOI] [PubMed] [Google Scholar]
- Sykes SE, Morgan A, Evans JC, Evans N, Holmes A, Moores SR. Use of an in vivo test system to investigate the acute and subacute responses of the rat lung to mineral dusts. Ann Occup Hyg. 1982;26:593–605. doi: 10.1093/annhyg/26.5.593. [DOI] [PubMed] [Google Scholar]
- Szerdahelyi P, Kasa P. Intraventricular administration of the cholinotoxin AF64A increases the accumulation of aluminum in the rat parietal cortex and hippocampus, but not in the frontal cortex. Brain Res. 1988;444:356–360. doi: 10.1016/0006-8993(88)90946-8. [DOI] [PubMed] [Google Scholar]
- Szutowicz A. Aluminum, NO, and nerve growth factor neurotoxicity in cholinergic neurons. J Neurosci Res. 2001;66:1009–1018. doi: 10.1002/jnr.10040. [DOI] [PubMed] [Google Scholar]
- Takagi A, Sai K, Umemura T, Hasegawa R, Kurokawa K. Relationship between hepatic peroxisome proliferation and 8-hydroxydeoxyguanosine formation in liver DNA of rats following long-term exposure to three peroxisome proliferators; di(2-ethylhexyl)phthalate, aluminium clofibrate and simfibrate. Cancer Lett. 1990;53:33–38. doi: 10.1016/0304-3835(90)90007-k. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Takahashi I, Sato H, Kubota Y, Yoshida S, Muramatsu Y. Age-related changes in the concentrations of major and trace elements in the brain of rats and mice. Biol Trace Elem Res. 2001;80:145–158. doi: 10.1385/BTER:80:2:145. [DOI] [PubMed] [Google Scholar]
- Talbot RJ, Newton D, Priest ND, Austin JG, Day JP. Inter-subject variability in the metabolism of aluminium following intravenous injection as citrate. Hum Exp Toxicol. 1995;14:595–599. doi: 10.1177/096032719501400707. [DOI] [PubMed] [Google Scholar]
- Tamada T. Fluorimetric determination of aluminum in human serum and whole blood. Bunseki Kagaku. 2004;53:435–440. [Google Scholar]
- Tanaka A. Toxicity of indium arsenide, gallium arsenide, and aluminium gallium arsenide. Toxicol Appl Pharmacol. 2004;198:405–411. doi: 10.1016/j.taap.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Tanaka I, Matsuno K, Kodama Y, Akiyama T. Pulmonary deposition of a fly ash aerosol by inhalation. J UOEH. 1983;5:423–431. doi: 10.7888/juoeh.5.423. [DOI] [PubMed] [Google Scholar]
- Taneda M. Effect of aluminum on rat brain. Enhancement by calcium deficiency. Hokkaido Igaku Zasshi. 1984;59:312–337. [PubMed] [Google Scholar]
- Tang S, Parsons PJ, Slavin W. Effect of acids, modifiers and chloride on the atomization of aluminum in electrothermal atomic absorption spectrometry. J Anal At Spectrom. 1995;10:521–526. [Google Scholar]
- Tatrai E, Adamis Z, Timar M, Ungvary G. Comparative histopathological and biochemical analysis of early stages of exposure to non-silicogenic aluminium silicate- and strongly silicogenic quartz-dust in rats. Exp Pathol. 1983;23:163–171. doi: 10.1016/s0232-1513(83)80054-1. [DOI] [PubMed] [Google Scholar]
- Tatrai E, Ungvary G, Adamis Z, Timar M. Short term in vivo method for prediction of the fibrogenic effect of different mineral dusts. Exp Pathol. 1985;28:111–118. doi: 10.1016/s0232-1513(85)80022-0. [DOI] [PubMed] [Google Scholar]
- Tatrai E, Wojnarovits I, Ungvary G. Non-fibrous zeolite induced experimental pneumoconiosis in rats. Exp Pathol. 1991;43:41–46. doi: 10.1016/s0232-1513(11)80140-4. [DOI] [PubMed] [Google Scholar]
- Taylor A, Walker AW. Measurement of aluminum in clinical samples. Ann Clin Biochem. 1992;29:377–389. doi: 10.1177/000456329202900402. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Ferrier IN, McLoughlin IJ, Fairbairn AF, McKeith IG, Lett D, Edwardson JA. Gastrointestinal absorption of aluminium in Alzheimer’s disease: Response to aluminium citrate. Age Aging. 1992;21:81–90. doi: 10.1093/ageing/21.2.81. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Newens AJ, Edwardson JA, Kay DW, Forster DP. Alzheimer’s disease and the relationship between silicon and aluminium in water supplies in northern England. J Epidemiol Community Health. 1995;49:323–324. doi: 10.1136/jech.49.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GA, Moore PB, Ferrier IN, Tyrer SP, Edwardson JA. Gastrointestinal absorption of aluminium and citrate in man. J Inorg Biochem. 1998;69:165–169. doi: 10.1016/s0162-0134(97)10014-9. [DOI] [PubMed] [Google Scholar]
- Teagarden DL, Radavich JF, White JL, Hem SL. Aluminum chlorohydrate II: Physicochemical properties. J Pharm Sci. 1981;70:762–764. doi: 10.1002/jps.2600700712. [DOI] [PubMed] [Google Scholar]
- Templeton DM, Ariese F, Cornelis R, Danielsson LG, Muntau H, van Leeuwen HP, Lobinski R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC recommendations 2000) Pure Appl Chem. 2000;72:1453–1470. [Google Scholar]
- Teraoka H. Distribution of 24 elements in the internal organs of normal males and metallic workers in Japan. Arch Environ Health. 1981;36:155–165. doi: 10.1080/00039896.1981.10667620. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Morii F, Kobayashi J. The concentrations of 24 elements in foodstuffs and the estimate of their daily intake. J Jap Soc Nutr Food Sci. 1981;34:221–239. [Google Scholar]
- Tercedor J, Lopez-Hernandez B, Rodenas JM. Bullous dermatosis of end-stage renal disease and aluminium. Nephrol Dial Transplant. 1997;12:1083. doi: 10.1093/ndt/12.5.1083a. [DOI] [PubMed] [Google Scholar]
- Terry RD, Pena C. Experimental production of neurofibrillary degeneration. 2. Electron microscopy, phosphatase histochemistry, and electron probe analysis. J Neuropathol Exp Neurol. 1965;24:200–210. doi: 10.1097/00005072-196504000-00003. [DOI] [PubMed] [Google Scholar]
- Testolin G, Erba D, Ciappellano S, Bermano G. Influence of organic acids on aluminium absorption and storage in rat tissues. Food Addit Contam. 1996;13:21–27. doi: 10.1080/02652039609374378. [DOI] [PubMed] [Google Scholar]
- The Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals. 13. Whitehouse Station, NJ: Merck & Co., Inc; 2001. [Google Scholar]
- Thierry-Carstensen B, Stellfeld M. Itching nodules and hypersensitivity to aluminium after the use of adsorbed vaccines from SSI. Vaccine. 2004;22:1845. doi: 10.1016/j.vaccine.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Thomas P, Barnstorf S, Summer B, Willmann G, Przybilla B. Immuno-allergological properties of aluminium oxide (Al2O3) ceramics and nickel sulfate in humans. Biomaterials. 2003;24:959–966. doi: 10.1016/s0142-9612(02)00432-5. [DOI] [PubMed] [Google Scholar]
- Thomassen Y, Koch W, Dunkhorst W, Ellingsen DG, Skaugset NP, Jordbekken L, Arne Drabløs P, Weinbruch S. Ultrafine particles at workplaces of a primary aluminium smelter. J Environ Monit. 2006;8:127–133. doi: 10.1039/b514939h. [DOI] [PubMed] [Google Scholar]
- Thomson SM, Burnett DC, Bergmann JD, Hixson CJ. Comparative inhalation hazards of aluminum and brass powders using bronchopulmonary lavage as an indicator of lung damage. J Appl Toxicol. 1986;6:197–209. doi: 10.1002/jat.2550060311. [DOI] [PubMed] [Google Scholar]
- Thorne BM, Donohoe T, Lin KN, Lyon S, Mederios DM, Weaver ML. Aluminum ingestion and behavior in the Long-Evans rat. Physiol Behav. 1986;36:63–67. doi: 10.1016/0031-9384(86)90074-0. [DOI] [PubMed] [Google Scholar]
- Thurston H, Gilmore GR, Swales JD. Aluminium retention and toxicity in chronic renal failure. Lancet. 1972;1:881–883. doi: 10.1016/s0140-6736(72)90743-x. [DOI] [PubMed] [Google Scholar]
- Tielemans C, Collart F, Wens R, Smeyers-Verbeeke J, van Hoff I, Dratwa M, Verbeelen D. Improvement of anemia with deferoxamine in hemodialysis patients with aluminum-induced bone disease. Clin Nephrol. 1985;24:237–241. [PubMed] [Google Scholar]
- Tikhov SF, Potapova YV, Sadykov VA, Fenelonov VB, Tsybulya SV, Salanov AN, Ivanov VP, Kolomiichuk VN. Porous Al2O3 /Al metal ceramics prepared by the oxidation of aluminum powder under hydrothermal conditions followed by thermal dehydration: III. The reactivity of aluminum, the reaction mechanism of its oxidation with water vapour, and the microtexture of cermets. Kinet Catal. 2003;44:297–308. [Google Scholar]
- Tipper JL, Hatton A, Nevelos JE, Ingham E, Doyle C, Streicher R, Nevelos AB, Fisher J. Alumina-alumina artificial hip joints. Part II: characterization of the wear debris from in vitro hip joint simulations. Biomaterials. 2002;23:3441–3448. doi: 10.1016/s0142-9612(02)00048-0. [DOI] [PubMed] [Google Scholar]
- Tipton IH, Shafer JJ. Statistical analysis of lung trace element levels. Arch Environ Health. 1964;8:58–67. doi: 10.1080/00039896.1964.10663632. [DOI] [PubMed] [Google Scholar]
- Tipton IH, Stewart PL, Martin PG. Trace elements in diets and excretia. Health Phys. 1966;12:1683–1689. [Google Scholar]
- Tjälve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–195. [PubMed] [Google Scholar]
- Tjälve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol. 1996;79:347–356. doi: 10.1111/j.1600-0773.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Tokutake S, Nagase H, Morisaki S, Oyanagi S. Aluminium detected in senile plaques and neurofibrillary tangles is contained in lipofuscin granules with silicon, probably as aluminosilicate. Neurosci Lett. 1995;185:99–102. doi: 10.1016/0304-3940(94)11234-a. [DOI] [PubMed] [Google Scholar]
- Topi GC, Alessandro GL, Cancarini GC, De Costanza F, Griso D, Ravelli M. Porphyria cutanea tarda in a haemodialysed patient. Br J Dermatol. 1981;104:579–580. doi: 10.1111/j.1365-2133.1981.tb08175.x. [DOI] [PubMed] [Google Scholar]
- Tornling G, Blaschke E, Eklund A. Long term effects of alumina on components of bronchioalveolar lavage fluid from rats. Br J Ind Med. 1993;50:172–175. doi: 10.1136/oem.50.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosti A, Vincenzi C, Peluso AM. Accidental diagnosis of aluminium sensitivity with Finn Chambers. Contact Dermatitis. 1990;23:48–49. doi: 10.1111/j.1600-0536.1990.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Townsend MC, Enterline PE, Sussman NB, Bonney TB, Rippey LL. Pulmonary function in relation to total dust exposure at a bauxite refinery and alumina-based chemical products plant. Am Rev Respir Dis. 1985;132:1174–1180. doi: 10.1164/arrd.1985.132.6.1174. [DOI] [PubMed] [Google Scholar]
- Trapp GA, Miner GD, Zimmermann RL, Mastri AR, Heston LL. Aluminum levels in brain in Alzheimer’s disease. Biol Psychiatry. 1978;13:709–718. [PubMed] [Google Scholar]
- Traub RD, Rains TC, Garruto RM, Gajdusek DC, Gibbs CJ., Jr Brain destruction alone does not elevate brain aluminum. Neurology. 1981;31:986–990. doi: 10.1212/wnl.31.8.986. [DOI] [PubMed] [Google Scholar]
- Trentini PL, Ascanelli M, Zanforlini B, Venturini F, Buci G, Fagioli F. Determination of aluminium by inductively coupled plasma mass spectrometry in serum of patients treated by haemodialysis, dialysis solutions and tap water, and a comparison with atomic absorption spectrometry. J Anal At Spectrom. 1993;8:905–909. [Google Scholar]
- Tripathi RM, Mahapatra S, Raghunath R, Vinod Kumar A, Sadasivan S. Daily intake of aluminium by adult population of Mumbai, India. Sci Total Environ. 2002;299:73–77. doi: 10.1016/s0048-9697(02)00224-3. [DOI] [PubMed] [Google Scholar]
- Trollfors B, Bergfors E, Inerot A. Vaccine related itching nodules and hypersensitivity to aluminium. Vaccine. 2005;23:975–976. doi: 10.1016/j.vaccine.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Trunov MA, Schoenitz M, Zhu X, Dreizin EL. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust Flame. 2005;140:310–318. [Google Scholar]
- Trzcinka-Ochocka M, Razniewska G, Sinczuk-Walczak H, Gazewski A. Urinary aluminum levels of workers in the aluminium foundry. Sixth Keele Meeting on Aluminium; February 26-March 2, 2005; Bussaco, Portugal. 2005. [Google Scholar]
- Tsou VM, Young RM, Hart MH, Vanderhoof JA. Elevated plasma aluminum levels in normal infants receiving antacids containing aluminum. Pediatrics. 1991;87:148–151. [PubMed] [Google Scholar]
- Tsukamoto Y, Iwanami S, Marumo F. Study of trace elements in patients with chronic renal failure by neutron activation analysis. Proc Eur Dial Transplant Assoc. 1979;16:665–667. [PubMed] [Google Scholar]
- Tsunoda M, Sharma RP. Modulation of tumor necrosis factor alpha expression in mouse brain after exposure to aluminum in drinking water. Arch Toxicol. 1999;73:419–426. doi: 10.1007/s002040050630. [DOI] [PubMed] [Google Scholar]
- Turgut G, Kaptanoglu B, Turgut S, Enli Y, Genc O. Effects of chronic aluminum administration on blood and liver iron-related parameters in mice. Yonsei Med J. 2004;45:135–139. doi: 10.3349/ymj.2004.45.1.135. [DOI] [PubMed] [Google Scholar]
- Turk JL, Heather CJ. A histological study of lymph nodes during the development of delayed hypersensitivity to soluble antigens. Int Arch Allergy Appl Immunol. 1965;27:199–212. doi: 10.1159/000229612. [DOI] [PubMed] [Google Scholar]
- Turk JL, Parker D. Granuloma formation in normal guinea pigs injected intradermally with aluminum and zirconium compounds. J Invest Dermatol. 1977;68:336–340. doi: 10.1111/1523-1747.ep12496358. [DOI] [PubMed] [Google Scholar]
- Turner DR. Problems in trace metal speciation modeling. In: Tessier A, Turner DR, editors. Metal Speciation and Bioavailability in Aquatic Systems. Chichester, England: John Wiley & Sons; 1995. pp. 149–203. [Google Scholar]
- Turnquest EM, Hallenbeck WH. Blood aluminum levels as a function of aluminum intake from drinking water. Bull Environ Contam Toxicol. 1991;46:554–560. doi: 10.1007/BF01688199. [DOI] [PubMed] [Google Scholar]
- Tytgat GN, Heading RC, Muller-Lissner S, Kamm MA, Scholmerich J, Berstad A, Fried A, Chaussade S, Jewell D, Briggs A. Contemporary understanding and management of reflux and constipation in the general population and pregnancy: a consensus meeting. Aliment Pharacol Ther. 2003;18:291–301. doi: 10.1046/j.1365-2036.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- Ueda M, Mizoi Y, Maki Z, Maeda R, Takada R. A case of aluminum dust lung: A necropsy report. Kobe J Med Sci. 1958;4:91–99. [Google Scholar]
- Uemura E. Intranuclear aluminum accumulation in chronic animals with experimental neurofibrillary changes. Exp Neurol. 1984;85:10–18. doi: 10.1016/0014-4886(84)90155-9. [DOI] [PubMed] [Google Scholar]
- Uitti RJ, Rajput AH, Rozdilsky B, Bickis M, Wollin T, Yuen WK. Regional metal concentrations in Parkinson’s disease, other chronic neurological diseases, and control brains. Can J Neurol Sci. 1989;16:310–314. doi: 10.1017/s0317167100029140. [DOI] [PubMed] [Google Scholar]
- Ulfvarson U, Wold S. Trace-element concentrations in blood samples from welders of stainless steel or aluminium and a reference group. Scand J Work Environ Health. 1977;3:183–191. doi: 10.5271/sjweh.2774. [DOI] [PubMed] [Google Scholar]
- Umemura T, Sai K, Takagi A, Hasegawa R, Kurokawa Y. Formation of 8-hydroxydeoxyguanosine (8-OH-dG) in rat kidney DNA after intraperitoneal administration of ferric nitrilotriacetate (Fe-NTA) Carcinogenesis. 1990;11:345–347. doi: 10.1093/carcin/11.2.345. [DOI] [PubMed] [Google Scholar]
- Underhill FP, Peterman FI, Steel SL. Studies in the metabolism of aluminium. IV. The fate of intravenously injected aluminium. Am J Physiol. 1929;90:52–61. [Google Scholar]
- US FDA (US Food and Drug Administration) Aluminum in large and small volume parenterals used in total parenteral nutrition. Code of Federal Regulations, Vol 21CFR201.323 [Docket No 90N-056] and Fed Regist 1998. 2000;63:176–185. [PubMed] [Google Scholar]
- USGS (US Geological Survey) US Geological Survey Minerals Yearbook: Aluminum. 1997 [online] Cited 24 January 2007 http://minerals.er.usgs.gov/minerals/pubs/commodity/aluminum/050497 pdf.
- USGS (US Geological Survey) US Geological Survey Minerals Yearbook: Bauxite and Alumina. 2002 [online] Cited 24 January 2007 http://minerals.usgs.gov/minerals/pubs/commodity/bauxite/bauximyb02 pdf.
- USGS (US Geological Survey) US Geological Survey Minerals Yearbook: Aluminum. 2004 [online] Cited 24 January 2007 http://minerals.usgs.gov/minerals/pubs/commodity/aluminum/alumimyb 04.pdf.
- USGS (US Geological Survey) Mineral Commodity Summaries. 2005a [online] Cited 24 January 2007 http://minerals.usgs.gov/minerals/pubs/commodity/bauxite/bauximcs05 pdf.
- USGS (US Geological Survey) US Geological Survey Minerals Yearbook: Bauxite & Alumina. 2005b [online] Cited 24 January 2007 http://minerals.usgs.gov/minerals/pubs/commodity/bauxite/bauximyb04 pdf.
- USGS (US Geological Survey) US Geological Survey Minerals Yearbook: Aluminum. 2005c [online] Cited 24 January 2007 http://minerals.usgs.gov/minerals/pubs/commodity/aluminum/alumimyb 04.pdf.
- USGS (US Geological Survey) US Geological Survey Minerals Yearbook: Bauxite & Alumina. 2006a [online] Cited 24 January 2007 http://minerals.usgs.gov/minerals/pubs/commodity/bauxite/bauximyb05 pdf.
- USGS (US Geological Survey) US Geological Survey Minerals Yearbook: Aluminum. 2006b [online] Cited 24 January 2007 http://minerals.usgs.gov/minerals/pubs/commodity/aluminum/alumimyb 05.pdf.
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkison’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- Valkonen S, Aitio A. Analysis of aluminium in serum and urine for the biomonitoring of occupational exposure. Sci Total Environ. 1997;199:103–110. doi: 10.1016/s0048-9697(97)05485-5. [DOI] [PubMed] [Google Scholar]
- Vallyathan V, Bergeron WN, Robichaux PA, Craighead JE. Pulmonary fibrosis in an aluminum arc welder. Chest. 1982;81:372–374. doi: 10.1378/chest.81.3.372. [DOI] [PubMed] [Google Scholar]
- Valtulini S, Macchi C, Ballanti P, Cherel Y, Laval A, Theaker JM, Bak M, Ferretti E, Morvan H. Aluminium hydroxide induced granulomas in pigs. Vaccine. 2005;23:3999–4004. doi: 10.1016/j.vaccine.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Van der Voet GB, de Wolff FA. Intestinal absorption of aluminium in rats. Arch Toxicol Suppl. 1985;8:316–318. doi: 10.1007/978-3-642-69928-3_60. [DOI] [PubMed] [Google Scholar]
- Van der Voet GB, de Wolff FA. Intestinal absorption of aluminium from antacids: a comparison between hydrotalcite and algeldrate. J Toxicol Clin Toxicol. 19861987;24:545–553. doi: 10.3109/15563658608995393. [DOI] [PubMed] [Google Scholar]
- Van der Voet GB, de Wolff FA. Intestinal absorption of aluminium in rats: effect of sodium. Arch Toxicol Suppl. 1987a;11:231–235. doi: 10.1007/978-3-642-72558-6_39. [DOI] [PubMed] [Google Scholar]
- Van der Voet GB, de Wolff FA. The effect of di- and trivalent iron on the intestinal absorption of aluminum in rats. Toxicol Appl Pharmacol. 1987b;90:190–197. doi: 10.1016/0041-008x(87)90326-7. [DOI] [PubMed] [Google Scholar]
- Van der Voet GB, De Wolff FA. Intestinal absorption of aluminum: effect of sodium and calcium. Arch Toxicol. 1998;72:110–114. doi: 10.1007/s002040050476. [DOI] [PubMed] [Google Scholar]
- Van der Voet GB, van Ginkel MF, de Wolff FA. Intestinal absorption of aluminum in rats: stimulation by citric acid and inhibition by dinitrophenol. Toxicol Appl Pharmacol. 1989;99:90–97. doi: 10.1016/0041-008x(89)90114-2. [DOI] [PubMed] [Google Scholar]
- Van Ginkel MF, van der Voet GB, de Wolff FA. Improved method of analysis for aluminum in brain tissue. Clin Chem. 1990;36:658–661. [PubMed] [Google Scholar]
- Van Ginkel MF, van der Voet GB, D’Haese PC, De Broe ME, de Wolff FA. Effect of citric acid and maltol on the accumulation of aluminum in rat brain and bone. J Lab Clin Med. 1993;121:453–460. [PubMed] [Google Scholar]
- Van Landeghem GF, de Broe ME, D’Haese PC. Al and Si: their speciation, distribution, and toxicity. Clin Biochem. 1998;31:385–397. doi: 10.1016/s0009-9120(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Van Marwyck C, Eickhoff W. Result of further experiments on the pulmonary pathogenicity of aluminum dusts. Arch Hyg Bakteriol. 1950;133:139–157. [PubMed] [Google Scholar]
- Van Oostdam JC, Zwanenburg H, Harrison JR. Canadian perspectives on aluminum. Environ Geochem Health. 1990;12:71–74. doi: 10.1007/BF01734051. [DOI] [PubMed] [Google Scholar]
- Vandenplas O, Delwiche JP, Vanbilsen ML, Joly J, Roosels D. Occupational asthma caused by aluminium welding. Eur Resp J. 1998;11:1182–1184. doi: 10.1183/09031936.98.11051182. [DOI] [PubMed] [Google Scholar]
- Varo P, Koivistoinen P. Mineral element composition of Finnish foods: XII. General discussion and nutritional evaluation. Acta Agric Scand Suppl. 1980;22:165–171. [Google Scholar]
- Vassilev TL. Aluminium phosphate but not calcium phosphate stimulates the specific IgE response in guinea pigs to tetanus toxoid. Allergy. 1978;33:155–159. doi: 10.1111/j.1398-9995.1978.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Veien NK, Hattel T, Justesen O, Norholm A. Aluminium allergy. Contact Dermatitis. 1986;15:295–297. doi: 10.1111/j.1600-0536.1986.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199–200. doi: 10.1111/j.1600-0536.1993.tb03399.x. [DOI] [PubMed] [Google Scholar]
- Venier P, Montaldi A, Busi L, Gava C, Zentilin L, Tecchio G, Bianchi V, Levis AG. Genetic effects of chromium tannins. Carcinogenesis. 1985;6:1327–1335. doi: 10.1093/carcin/6.9.1327. [DOI] [PubMed] [Google Scholar]
- Verbueken AH, van de Vyver FL, Van Grieken RE, De Broe ME. Microanalysis in biology and medicine ultrastructural localization of aluminum. Clin Nephrol. 1985;24:S58–S77. [PubMed] [Google Scholar]
- Verdier F, Burnett R, Michelet-Habchi C, Moretto P, Fievet-Groyne F, Sauzeat E. Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the Cynomolgus monkey. Vaccine. 2005;23:1359–1367. doi: 10.1016/j.vaccine.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Verreault R, Laurin D, Lindsay J, DeSerres G. Past exposure to vaccines and subsequent risk of Alzheimer’s disease. CMAJ. 2001;165:1495–1498. [PMC free article] [PubMed] [Google Scholar]
- Versieck J, Cornelis R. Measuring aluminum levels. N Engl J Med. 1980;302:468–469. doi: 10.1056/NEJM198002213020818. [DOI] [PubMed] [Google Scholar]
- Versieck J, Cornelis R. Trace Elements in Human Plasma or Serum. Boca Raton, Florida: CRC Press; 1989. [Google Scholar]
- Villain M, Cirimele V, Kintz P. Hair analysis in toxicology. Clin Chem Lab Med. 2004;42:1265–1272. doi: 10.1515/CCLM.2004.247. [DOI] [PubMed] [Google Scholar]
- Vinas P, Campillo N, Lopez-Garcia I, Hernandez-Cordoba M. Electrothermal atomic absorption spectrometric determination of molybdenum, aluminium, chromium and manganese in milk. Anal Chim Acta. 1997;356:267–276. [Google Scholar]
- Vincent JH. Perspectives on international standards for health-related sampling of airborne contaminants. Appl Occup Environ Hyg. 1993;8:233–238. [Google Scholar]
- Vincent JH. Measurement of coarse aerosols in workplaces. A review. Analyst. 1994;119:13–18. doi: 10.1039/an9941900013. [DOI] [PubMed] [Google Scholar]
- Vincent JH. Aerosol Science for Industrial Hygienists. Oxford: Elsevier Science Ltd; 1995. [Google Scholar]
- Vincent JH. Health-related aerosol measurement: a review of existing sampling criteria and proposals for new ones. J Env Monit. 2005;7:1037–1053. doi: 10.1039/b509617k. [DOI] [PubMed] [Google Scholar]
- Vittori D, Nesse A, Perez G, Garbossa G. Morphologic and functional alterations of erythroid cells induced by long-term ingestion of aluminium. J Inorg Biochem. 1999;76:113–120. doi: 10.1016/s0162-0134(99)00122-1. [DOI] [PubMed] [Google Scholar]
- Vittori D, Garbossa G, Lafourcade C, Perez G, Nesse A. Human erythroid cells are affected by aluminium. Alteration of membrane band 3 protein. Biochim Biophys Acta. 2002;1558:142–150. doi: 10.1016/s0005-2736(01)00427-8. [DOI] [PubMed] [Google Scholar]
- Vogelbruch M, Nuss B, Korner M, Kapp A, Kiehl P, Bohm W. Aluminium-induced granulomas after inaccurate intradermal hyposensitization injections of aluminium-adsorbed depot preparations. Allergy. 2000;55:883–887. [PubMed] [Google Scholar]
- Voisin C, Fisekci F, Buclez B, Didier A, Couste B, Bastien F, Bouchard P, Pairon JC. Mineralogical analysis of the respiratory tract in aluminium oxide-exposed workers. Eur Resp J. 1996;9:1874–1879. doi: 10.1183/09031936.96.09091874. [DOI] [PubMed] [Google Scholar]
- Volk VK, Bunney WE. Diphtheria immunization with fluid toxoid and alum-precipitated toxoid. Am J Public Health Nations Health. 1942;32:690–699. doi: 10.2105/ajph.32.7.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stockhausen HB, Schrod L, Bratter P, Rosick U. Aluminum loading in premature infants during intensive care as related to clinical aspects. J Trace Elem Electrolytes Health Dis. 1990;4:209–213. [PubMed] [Google Scholar]
- Voss H, Tolki U. On a vaccine granuloma approximately one year old in man. Arch Hyg Bakteriol. 1960;178:291–299. [PubMed] [Google Scholar]
- Wachi M, Aikawa K. Serum aluminum assay by flameless atomic absorption spectroscopic method and oral absorption of some aluminum compounds by rats. Oyo Yakuri. 1975;10:359–363. [Google Scholar]
- Wagner JC, Pooley FD, Gibbs A, Lyons J, Sheers G, Moncrieff CB. Inhalation of china stone and china clay dusts: relationship between the mineralogy of dust retained in the lungs and pathological changes. Thorax. 1986;41:190–196. doi: 10.1136/thx.41.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W. Canadian Minerals Yearbook. Ottawa: Natural Resources Canada; 1999. [Google Scholar]
- Wakayama I, Nerurkar VR, Strong MJ, Garruto RM. Comparative study of chronic aluminum-induced neurofilamentous aggregates with intracytoplasmic inclusions of amyotrophic lateral sclerosis. Acta Neuropathol. 1996;92:545–554. doi: 10.1007/s004010050560. [DOI] [PubMed] [Google Scholar]
- Walker JA, Sherman RA, Cody RP. The effect of oral bases on enteral aluminum absorption. Arch Intern Med. 1990;150:2037–2039. [PubMed] [Google Scholar]
- Walker VR, Sutton RA, Meirav O, Sossi V, Johnson R, Klein J, Fink D, Middleton R. Tissue disposition of 26aluminum in rats measured by accelerator mass spectrometry. Clin Invest Med. 1994;17:420–425. [PubMed] [Google Scholar]
- Walls RS. Eosinophil response to alum adjuvants: involvement of T cells in non-antigen-dependent mechanisms. Proc Soc Exp Biol Med. 1977;156:431–435. doi: 10.3181/00379727-156-39951. [DOI] [PubMed] [Google Scholar]
- Walton J, Hams G, Wilcox D. Bioavailability of Aluminium From Drinking Water: Co-exposure with Foods and Beverages. Melbourne, Australia: Urban Water Research Association of Australia, Melbourne Water Corporation; 1994. Research Report No 83. [Google Scholar]
- Walton J, Tuniz C, Fink D, Jacobsen G, Wilcox D. Uptake of trace amounts of aluminum into the brain from drinking water. Neurotoxicology. 1995;16:187–190. [PubMed] [Google Scholar]
- Wang M, Chen JT, Ruan DY, Xu YZ. The influence of developmental period of aluminum exposure on synaptic plasticity in the adult rat dentate gyrus in vivo. Neuroscience. 2002;113:411–419. doi: 10.1016/s0306-4522(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Ward NI, Mason JA. Neutron activation analysis techniques for identifying elemental status in Alzheimer’s disease. J Radioanal Nucl Chem. 1987;113:515–526. [Google Scholar]
- Ward RJ, Zhang Y, Crichton RR. Aluminium toxicity and iron homeostasis. J Inorg Biochem. 2001;87:9–14. doi: 10.1016/s0162-0134(01)00308-7. [DOI] [PubMed] [Google Scholar]
- Warshawsky D, Reilman R, Cheu J, Radike M, Rice C. Influence of particle dose on the cytotoxicity of hamster and rat pulmonary alveolar macrophage in vitro. J Toxicol Environ Health. 1994;42:407–421. doi: 10.1080/15287399409531891. [DOI] [PubMed] [Google Scholar]
- Weberg R, Berstad A. Gastrointestinal absorption of aluminium from single doses of aluminium containing antacids in man. Eur J Clin Invest. 1986;16:428–432. doi: 10.1111/j.1365-2362.1986.tb01018.x. [DOI] [PubMed] [Google Scholar]
- Weberg R, Berstad A, Ladehaug B, Thomassen Y. Are aluminum containing antacids during pregnancy safe? Acta Pharmacol Toxicol. 1986;59:63–65. doi: 10.1111/j.1600-0773.1986.tb02709.x. [DOI] [PubMed] [Google Scholar]
- Wedrychowski A, Schmidt WN, Hnilica LS. The in vivo cross-linking of proteins and DNA by heavy metals. J Biol Chem. 1986a;261:3370–3376. [PubMed] [Google Scholar]
- Wedrychowski A, Schmidt WN, Hnilica LS. DNA-protein crosslinking by heavy metals in Novikoff hepatoma. Arch Biochem Biophys. 1986b;251:397–402. doi: 10.1016/0003-9861(86)90345-0. [DOI] [PubMed] [Google Scholar]
- Weed DL. Weight of evidence: a review of concept and methods. Risk Anal. 2005;25:1545–1557. doi: 10.1111/j.1539-6924.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- Weinbrüch S, van Aken P, Ebert M, Thomassen Y, Skogstad A, Chashchin VP, Nikonov A. The heterogeneous composition of working place aerosols in a nickel refinery: a transmission and scanning electron microscope study. J Environ Monit. 2002;4:344–350. doi: 10.1039/b110504n. [DOI] [PubMed] [Google Scholar]
- Weintraub R, Hams G, Meerkin M, Rosenberg AR. High aluminum content of infant milk formulas. Arch Dis Child. 1986;61:914–916. doi: 10.1136/adc.61.9.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller W, Reif E, Ulmer WT. Long term inhalation studies on rats on the problem of silicosis prophylaxis with McIntyre aluminum powder. Histology, determination of oxyproline and dust content, examination of respiration and circulation. Int Arch Arbeitsmed. 1966;22:77–94. [PubMed] [Google Scholar]
- Wen GY, Wisniewski HM. Histochemical localization of aluminum in the rabbit CNS. Acta Neuropathol. 1985;68:175–184. doi: 10.1007/BF00690191. [DOI] [PubMed] [Google Scholar]
- Wettstein A, Aeppli J, Gautschi K, Peter M. Failure to find a relationship between mnestic skills of octogenarians and aluminium in drinking water. Int Arch Occup Environ Health. 1991;63:97–103. doi: 10.1007/BF00379071. [DOI] [PubMed] [Google Scholar]
- White DM, Longstreth WT, Jr, Rosenstock L, Claypoole KH, Brodkin CA, Townes BD. Neurologic syndrome in 25 workers from an aluminum smelting plant. Arch Intern Med. 1992;152:1443–1448. [PubMed] [Google Scholar]
- White LR, Steinegger AF, Schlatter C. Pulmonary response following intratracheal instillation of potroom dust from an aluminum reduction plant into rat lung. Environ Res. 1987;42:534–545. doi: 10.1016/s0013-9351(87)80220-7. [DOI] [PubMed] [Google Scholar]
- White RG, Coons AH, Connolly JM. Studies on antibody production. III. The alum granuloma. J Exp Med. 1955;102:73–82. doi: 10.1084/jem.102.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MW, Farrar G, Christie GL, Blair JA, Thompson RP, Powell JJ. Mechanisms of aluminum absorption in rats. Am J Clin Nutr. 1997;65:1446–1452. doi: 10.1093/ajcn/65.5.1446. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Guidelines for Drinking-Water Quality. 3. Vol. 1. Geneva: World Health Organization; 2004. Recommendations. [Google Scholar]
- Wilcock LK, Francis JN, Durham SR. Aluminium hydroxide down-regulates T helper 2 responses by allergen-stimulated human peripheral blood mononuclear cells. Clin Exp Allergy. 2004;34:1373–1378. doi: 10.1111/j.1365-2222.2004.02052.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ohnesorge FK. Influence of storage conditions on aluminum concentrations in serum dialysis fluid, urine, and tap water. J Anal Toxicol. 1990;14:206–210. doi: 10.1093/jat/14.4.206. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Sprenger KB, Vossas U, Ohnesorge FK. Aluminum load in chronic intermittent plasma exchange. J Toxicol Clin Toxicol. 1987;25:209–220. doi: 10.3109/15563658708992625. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Passlick J, Busch T, Szydlik M, Ohnesorge FK. Scalp hair as an indicator of aluminium exposure: comparison to bone and plasma. Hum Toxicol. 1989a;8:5–9. doi: 10.1177/096032718900800102. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ohnesorge FK, Lombeck I, Hafner D. Uptake of aluminum, cadmium, copper, lead, and zinc by human scalp hair and elution of the adsorbed metals. J Anal Toxicol. 1989b;13:17–21. doi: 10.1093/jat/13.1.17. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Jäger DE, Ohnesorge FK. Aluminium toxicokinetics. Pharmacol Toxicol. 1990;66:4–9. doi: 10.1111/j.1600-0773.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Zhang XJ, Hafner D, Ohnesorge FK. Single-dose toxicokinetics of aluminum in the rat. Arch Toxicol. 1992;66:700–705. doi: 10.1007/BF01972620. [DOI] [PubMed] [Google Scholar]
- Wilkinson KJ, Bertsch PM, Jagoe CH, Campbell PGC. Surface complexation of aluminum on isolated fish gill cells. Environ Sci Technol. 1993;27:1132–1138. [Google Scholar]
- Williams JW, Vera SR, Peters TG, Luther RW, Bhattacharya S, Spears H, Graham A, Pitcock JA, Crawford AJ, Palmieri GM. Biliary excretion of aluminum in aluminum osteodystrophy with liver disease. Ann Intern Med. 1986;104:782–786. doi: 10.7326/0003-4819-104-6-782. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Fraústo da Silva JJ. The Natural Selection of the Chemical Elements. New York: Oxford University Press; 1996. [Google Scholar]
- Winearls CG. Recombinant human erythropoietin: 10 years of clinical experience. Nephrol Dial Transplant. 1998;13:S3–S8. doi: 10.1093/ndt/13.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- Winklhofer C, Steinhausen C, Beck E, Alvarez-Bruckmann M, Kinzel S, Ittel TH, Nolte E. Effect of iron status on the absorption, speciation and tissue distribution of aluminium in rats. Nuc Inst Meth Phys Res B. 2000;172:920–924. [Google Scholar]
- Winterberg B, Bertram H, Rolf N, Roedig N, Kisters K, Remmers S, Spieker C, Zumkley H. Differences in plasma and tissue aluminum concentrations due to different aluminum-containing drugs in patients with renal insufficiency and with normal renal function. J Trace Elem Electrolytes Health Dis. 1987a;1:69–72. [PubMed] [Google Scholar]
- Winterberg B, Bertram H, Korte R, Niederlain G, Remmers S, Remmers J, Zumkley H. Hair analysis for aluminum monitoring in patients on long-term hemodialysis. Trace Elem Med. 1987b;4:72–74. [Google Scholar]
- Wisniewski HM, Kozlowski PB. Evidence for blood-brain barrier changes in senile dementia of the Alzheimer type (SDAT) Ann N Y Acad Sci. 1982;396:119–129. doi: 10.1111/j.1749-6632.1982.tb26848.x. [DOI] [PubMed] [Google Scholar]
- Woodson GC. An interesting case of osteomalacia due to antacid use associated with stainable bone aluminum in a patient with normal renal function. Bone. 1998;22:695–698. doi: 10.1016/s8756-3282(98)00060-x. [DOI] [PubMed] [Google Scholar]
- Woodworth CD, Mossman BT, Craighead JE. Comparative effects of fibrous and nonfibrous minerals on cells and liposomes. Environ Res. 1982;27:190–205. doi: 10.1016/0013-9351(82)90070-6. [DOI] [PubMed] [Google Scholar]
- World Bureau of Metal Statistics. World bauxite production. World Met Stat. 1994;47:6. [Google Scholar]
- Wrobel K, Gonzalez EB, Wrobel K, Sanz-Medel A. Aluminum and silicon speciation in human serum by ion-exchange high-performance liquid chromatography-electrothermal atomic absorption spectrometry and gel electrophoresis. Analyst. 1995;120:809–815. doi: 10.1039/an9952000809. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou Z, Xiong Y, Wang Y, Sun J. Distribution of aluminum of alunite in blood and brain of normal mice and mice with high permeability of blood-brain barrier. Zhongguo Zhongyao Zazhi. 1999;24:234–235. [Google Scholar]
- Wyatt JP, Riddell ACR. The morphology of bauxite-fume pneumoconiosis. Am J Pathol. 1949;25:447–465. [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Dong Q, Li S, Li D, Zhan C. Aluminum and fluorine in blood and bone of rats fed on diet mixed with various contents of aluminum, fluoride or their mixture. Hua Xi Yi Ke Da Xue Xue Bao. 1992;23:185–189. [PubMed] [Google Scholar]
- Xie CX, Mattson MP, Lovell MA, Yokel RA. Intraneuronal aluminum potentiates iron-induced oxidative stress in cultured rat hippocampal neurons. Brain Res. 1996;743:271–277. doi: 10.1016/s0006-8993(96)01055-4. [DOI] [PubMed] [Google Scholar]
- Xu N, Majidi V, Markesbery WR, Ehmann WD. Brain aluminum in Alzheimer’s disease using an improved GFAAS method. Neurotoxicology. 1992c;13:735–743. [PubMed] [Google Scholar]
- Xu ZC, Tang JP, Xu ZX, Melethil S. Kinetics of aluminum in rats. IV: Blood and cerebrospinal fluid kinetics. Toxicol Lett. 1992b;63:7–12. doi: 10.1016/0378-4274(92)90102-p. [DOI] [PubMed] [Google Scholar]
- Xu ZX, Pai SM, Melethil S. Kinetics of aluminum in rats. II: Dose-dependent urinary and biliary excretion. J Pharm Sci. 1991;80:946–951. doi: 10.1002/jps.2600801009. [DOI] [PubMed] [Google Scholar]
- Xu ZX, Tang JP, Badr M, Melethil S. Kinetics of aluminum in rats. III: Effect of route of administration. J Pharm Sci. 1992a;81:160–163. doi: 10.1002/jps.2600810212. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Watsuji T, Ishikawa M, Harina Y, Yabuki T. Zinc, copper, and aluminum contents of various human organs, especially of the male genital organs. Kobe Ika Daigaku Kiyo. 1959;16:185–190. [Google Scholar]
- Yamane Y, Ohtawa M. Possible mechanisms of suppressive action of aluminium chloride on lung carcinogenesis in mice induced by 4-nitro-quinoline 1-oxide: II. Effects of aluminium chloride on metabolism of 4-niroquinoline 1-oxide. Gann. 1979;70:147–152. [Google Scholar]
- Yamazaki K, Kajima T, Yoshida Y, Yuzuriha T, Wakabayashi T, Shigematsu T, Yamamoto H, Kawaguchi Y. Sodium ferrous citrate does not cause aluminum retention in rats with experimentally induced renal failure. J Bone Miner Metab. 1995;13:87–92. [Google Scholar]
- Yang SJ, Lee JE, Lee KH, Huh JW, Choi SY, Cho SW. Opposed regulation of aluminum-induced apoptosis by glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor in rat brains. Brain Res Mol Brain Res. 2004;127:146–149. doi: 10.1016/j.molbrainres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Yase Y. The pathogenesis of amyotrophic lateral sclerosis. Lancet. 1972;2:292–296. doi: 10.1016/s0140-6736(72)92903-0. [DOI] [PubMed] [Google Scholar]
- Yase Y. The role of aluminum in CNS degeneration with interaction of calcium. Neurotoxicology. 1980;1:101–109. [Google Scholar]
- Yasui M, Yoshimasu F, Yase Y, Uebayashi Y. Wilson’s disease; increased aluminum in liver. Folia Psychiatr Neurol Jpn. 1979;33:547–552. doi: 10.1111/j.1440-1819.1979.tb03194.x. [DOI] [PubMed] [Google Scholar]
- Yasui MS, Kita SF, Yoshimasu F, Higashi Y, Yase Y, Uebayashi Y, Miyamoto K, Nagasaki Y, Miyoshi K, Hayashi S, Ogura Y. Accumulation of aluminum in central nervous system and other organs in a case of generalized amyloidosis. Nippon Naika Gakkai Zasshi. 1980;69:982–991. doi: 10.2169/naika.69.982. [DOI] [PubMed] [Google Scholar]
- Yasui M, Yano I, Ota K, Oshima A. Calcium, phosphorus and aluminium concentrations in the central nervous system, liver and kidney of rabbits with experimental atherosclerosis: preventive effects of vinpocetine on the deposition of these elements. J Int Med Res. 1990a;18:142–152. doi: 10.1177/030006059001800208. [DOI] [PubMed] [Google Scholar]
- Yasui M, Yano I, Yase Y, Ota K. Aluminum contents in soft tissues of mice fed low Ca-Mg, low Ca-Mg+ high aluminum, and low Ca-Mg+ high aluminum diets with administration of 1,25-(OH)2D3. Igaku no Ayumi. 1990b;153:601–602. [Google Scholar]
- Yasui M, Yase Y, Ota K, Garruto RM. Evaluation of magnesium, calcium and aluminum metabolism in rats and monkeys maintained on calcium-deficient diets. Neurotoxicology. 1991a;12:603–614. [PubMed] [Google Scholar]
- Yasui M, Yase Y, Ota K, Mukoyama M, Adachi K. High aluminum deposition in the central nervous system of patients with amyotrophic lateral sclerosis from the Kii Peninsula, Japan: two case reports. Neurotoxicology. 1991b;12:277–283. [PubMed] [Google Scholar]
- Yasui MS, Yase Y, Ota K, Garruto RM. Aluminum deposition in the central nervous system of patients with amyotrophic lateral sclerosis from the Kii peninsula of Japan. Neurotoxicology. 1991c;12:615–620. [PubMed] [Google Scholar]
- Yasui MS, Kihira T, Ota K. Calcium, magnesium and aluminum concentrations in Parkinson’s disease. Neurotoxicology. 1992;13:593–600. [PubMed] [Google Scholar]
- Yasui M, Ota K. Aluminum decreases the magnesium concentration of spinal cord and trabecular bone in rats fed a low calcium, high aluminum diet. J Neurol Sci. 1998;157:37–41. doi: 10.1016/s0022-510x(98)00075-6. [DOI] [PubMed] [Google Scholar]
- Ye Q, Ohsaki K, Ii K, Li DJ, Matsuoka H, Tenshin S, Yamamoto T. A subcutaneous tissue reaction in the early stage to a synthetic auditory ossicle (Bioceram) in rats. J Med Invest. 1998;44:173–177. [PubMed] [Google Scholar]
- Yokel RA. Hair as an indicator of excessive aluminum exposure. Clin Chem. 1982;28:662–665. [PubMed] [Google Scholar]
- Yokel RA. Persistent aluminum accumulation after prolonged systemic aluminum exposure. Biol Trace Elem Res. 1983;5:467–474. doi: 10.1007/BF02988939. [DOI] [PubMed] [Google Scholar]
- Yokel RA. Toxicity of aluminum exposure during lactation to the maternal and suckling rabbit. Toxicol Appl Pharmacol. 1984;75:35–43. doi: 10.1016/0041-008x(84)90073-5. [DOI] [PubMed] [Google Scholar]
- Yokel RA. Toxicity of gestational aluminum exposure to the maternal rabbit and offspring. Toxicol Appl Pharmacol. 1985;79:121–133. doi: 10.1016/0041-008x(85)90374-6. [DOI] [PubMed] [Google Scholar]
- Yokel RA. The toxicology of aluminum in the brain: a review. Neurotoxicology. 2000;21:813–828. [PubMed] [Google Scholar]
- Yokel RA. Aluminum toxicokinetics at the blood-brain barrier. In: Exley C, editor. Aluminium and Alzheimer’s Disease. New York: Elsevier; 2001. pp. 233–260. [Google Scholar]
- Yokel RA. Aluminum chelation principles and recent advances. Coord Chem Rev. 2002;228:97–113. [Google Scholar]
- Yokel RA, Florence RL. Aluminum bioavailability from the approved food additive leavening agent acidic sodium aluminum phosphate, incorporated into a baked good, is lower than from water. Toxicology. 2006;227:86–93. doi: 10.1016/j.tox.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Yokel RA, McNamara PJ. Aluminum bioavailability and disposition in adult and immature rabbits. Toxicol Appl Pharmacol. 1985;77:344–352. doi: 10.1016/0041-008x(85)90334-5. [DOI] [PubMed] [Google Scholar]
- Yokel RA, McNamara PJ. Influence of renal impairment, chemical form, and serum protein binding on intravenous and oral aluminum kinetics in the rabbit. Toxicol Appl Pharmacol. 1988;95:32–43. doi: 10.1016/s0041-008x(88)80005-x. [DOI] [PubMed] [Google Scholar]
- Yokel RA, McNamara PJ. Elevated aluminium persists in serum and tissues of rabbits after a six-hour infusion. Toxicol Appl Pharmacol. 1989;99:133–138. doi: 10.1016/0041-008x(89)90118-x. [DOI] [PubMed] [Google Scholar]
- Yokel RA, McNamara PJ. The influence of dietary calcium reduction on aluminum absorption and kinetics in the rabbit. Biol Trace Elem Res. 19891990;23:109–117. doi: 10.1007/BF02917182. [DOI] [PubMed] [Google Scholar]
- Yokel RA, McNamara PJ. Aluminum toxicokinetics: an updated minireview. Pharmacol Toxicol. 2001;88:159–167. doi: 10.1034/j.1600-0773.2001.d01-98.x. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Datta AK, Jackson EG. Evaluation of potential aluminum chelators in vitro by aluminum solubilization ability, aluminum mobilization from transferrin and the octanol/aqueous distribution of the chelators and their complexes with aluminum. J Pharmacol Exp Ther. 1991a;257:100–106. [PubMed] [Google Scholar]
- Yokel RA, Lidums V, McNamara PJ, Ungerstedt U. Aluminum distribution into brain and liver of rats and rabbits following intravenous aluminum lactate or citrate: A microdialysis study. Toxicol Appl Pharmacol. 1991b;107:153–163. doi: 10.1016/0041-008x(91)90339-g. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Ackrill P, Burgess E, Day JP, Domingo JL, Flaten TP, Savory J. Prevention and treatment of aluminum toxicity including chelation therapy: status and research needs. J Toxicol Environ Health. 1996a;48:667–683. [PubMed] [Google Scholar]
- Yokel RA, Meurer KA, Skinner TL, Fredenburg AM. The 3-hydroxypyridin-4-ones more effectively chelate aluminum in a rabbit model of aluminum intoxication than does desferrioxamine. Drug Metab Dispos. 1996b;24:105–111. [PubMed] [Google Scholar]
- Yokel RA, Rhineheimer SS, Sharma P, Elmore D, McNamara PJ. Entry, half-life, and desferrioxamine-accelerated clearance of brain aluminum after a single (26)Al exposure. Toxicol Sci. 2001a;64:77–82. doi: 10.1093/toxsci/64.1.77. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Rhineheimer SS, Brauer RD, Sharma P, Elmore D, McNamara PJ. Aluminum bioavailability from drinking water is very low and is not appreciably influenced by stomach contents or water hardness. Toxicology. 2001b;161:93–101. doi: 10.1016/s0300-483x(01)00335-3. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wilson M, Harris WR, Halestrap AP. Aluminum citrate uptake by immortalized brain endothelial cells: implications for its blood-brain barrier transport. Brain Res. 2002;930:101–110. doi: 10.1016/s0006-8993(02)02234-5. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Urbas AA, Lodder RA, Selegue JP, Florence RL. 26Al containing acidic and basic sodium aluminum phosphate preparation and use in studies of oral aluminum bioavailability from foods utilizing 26Al as an aluminum tracer. Nucl Instr Meth Phys Res B. 2005;229:471–478. [Google Scholar]
- Yoshida H, Yoshimasu F. Alzheimer’s disease and trace elements. Nippon Rinsho. 1996;54:111–116. [PubMed] [Google Scholar]
- Yoshida H, Yoshimsu F, Chen KM. The study on trace elements in brains of dementia patients. Wakayama Igaku. 1993;44:529–539. [Google Scholar]
- Yoshida S. Application of PIXE in medical study: environmental minerals and neurodegenerative disorders. Int J PIXE. 1999;9:245–257. [Google Scholar]
- Yoshida S, Gershwin ME, Keen CL, Donald JM, Golub MS. The influence of aluminum on resistance to Listeria monocytogenes in Swiss-Webster mice. Int Arch Allergy Appl Immunol. 1989;89:404–409. doi: 10.1159/000234983. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Wakayama I, Kihira T, Sasajima K, Yoshida K. Environmental minerals in Kii amyotrophic lateral scerosis in Japan: a PIXE analysis featuring aluminum. Int J PIXE. 1997;6:543–554. [Google Scholar]
- Yoshimasu F, Uebayashi Y, Yase Y, Iwata S, Sasajima K. Studies on amyotrophic lateral sclerosis by neutron activation analysis. Folia Psychiatr Neurol Jpn. 1976;30:49–55. doi: 10.1111/j.1440-1819.1976.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Yoshimasu F, Yasui M, Yase Y, Iwata S, Gajdusek DC, Gibbs CJ, Jr, Chen KM. Studies on amyotrophic lateral sclerosis by neutron activation analysis --- 2. Comparative study of analytical results on Guam PD, Japanese ALS and Alzheimer’s disease cases. Folia Psychiatr Neurol Jpn. 1980;34:75–82. doi: 10.1111/j.1440-1819.1980.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Yoshimasu F, Yasui M, Yoshida H, Yoshida S, Uebayashi Y, Yase Y, Gajdusek DC, Chen KM. Aluminum in Alzheimer’s disease in Japan and parkinsonism-dementia in Guam. In 13th world congress of neurology. J Neurol. 1985;232(Suppl):61. [Google Scholar]
- Ysart G, Miller P, Croasdale M, Crews H, Robb P, Baxter M, de L’Argy C, Harrison N. 1997 UK Total Diet Study--dietary exposures to aluminium, arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, tin and zinc. Food Addit Contam. 2000;17:775–786. doi: 10.1080/026520300415327. [DOI] [PubMed] [Google Scholar]
- Yu B, Tsen HY. Lactobacillus cells in the rabbit digestive tract and the factors affecting their distribution. J Appl Bacteriol. 1993;75:269–275. doi: 10.1111/j.1365-2672.1993.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Yuan B, Klein MH, Contiguglia RS, Mishell JL, Seligman PA, Miller NL, Molitoris BA, Alfrey AC, Shapiro JI. The role of aluminum in the pathogenesis of anemia in an outpatient hemodialysis population. Ren Fail. 1989;11:91–96. doi: 10.3109/08860228909066949. [DOI] [PubMed] [Google Scholar]
- Yumoto S, Nagai H, Imamura M, Matsuzaki H, Hayashi K, Masuda A, Kumazawa H, Ohashi H, Kobayashi K. 26Al uptake and accumulation in the rat brain. Nuc Inst Meth Phys Res B. 1997;123:279–282. [Google Scholar]
- Yumoto S, Nagai H, Matsuzaki H, Kobayashi T, Tada W, Ohki Y, Kakimi S, Kobayashi K. Transplacental passage of 26Al from pregnant rats to fetuses and 26Al transfer through maternal milk to suckling rats. Nuc Inst Meth Phys Res B. 2000;172:925–929. [Google Scholar]
- Yumoto S, Nagai H, Matsuzaki H, Matsumura H, Tada W, Nagatsuma E, Kobayashi K. Aluminium incorporation into the brain of rat fetuses and sucklings. Brain Res Bull. 2001;55:229–234. doi: 10.1016/s0361-9230(01)00509-3. [DOI] [PubMed] [Google Scholar]
- Yumoto S, Nagai H, Kobayashi K, Tamate A, Kakimi S, Matsuzaki H. 26Al incorporation into the brain of suckling rats through maternal milk. J Inorg Biochem. 2003;97:155–160. doi: 10.1016/s0162-0134(03)00246-0. [DOI] [PubMed] [Google Scholar]
- Zafar TA, Weaver CM, Martin BR, Flarend R, Elmore D. Aluminum (26AI) metabolism in rats. Proc Soc Exp Biol Med. 1997;216:81–85. doi: 10.3181/00379727-216-44159. [DOI] [PubMed] [Google Scholar]
- Zafar TA, Teegarden D, Ashendel C, Dunn MA, Weaver CM. Aluminium negatively impacts calcium utilization and bone in calcium-deficient rats. Nutr Res. 2004;24:234–259. [Google Scholar]
- Zaman K, Zaman W, Siddique H. Hematological and enzymatic results of aluminum intoxication in rats. Comp Biochem Physiol C. 1993;105:73–76. doi: 10.1016/0742-8413(93)90060-x. [DOI] [PubMed] [Google Scholar]
- Zapatero MD, Garcia de Jalon A, Pascual F, Calvo ML, Escanero J, Marro A. Serum aluminum levels in Alzheimer’s disease and other senile dementias. Biol Trace Elem Res. 1995;47:235–240. doi: 10.1007/BF02790122. [DOI] [PubMed] [Google Scholar]
- Zatta P, Bordin C, Favarato M. The inhibition of trypsin and alpha-chymotrypsin proteolytic activity by aluminum(III) Arch Biochem Biophys. 1993;303:407–411. doi: 10.1006/abbi.1993.1302. [DOI] [PubMed] [Google Scholar]
- Zatta P, Zambenedetti P, Toffoletti A, Corvaja C, Corain B. Aluminum(III) induces alterations on the physical state of the erythrocytic membrane: an ESR evaluation. J Inorg Biochem. 1997;65:109–114. doi: 10.1016/s0162-0134(96)00095-5. [DOI] [PubMed] [Google Scholar]
- Zatta P, Zambenedetti P, Reusche E, Stellmacher F, Cester A, Albanese P, Meneghel G, Nordio M. A fatal case of aluminium encephalopathy in a patient with severe chronic renal failure not on dialysis. Nephrol Dial Transplant. 2004;19:2929–2931. doi: 10.1093/ndt/gfh439. [DOI] [PubMed] [Google Scholar]
- Zecca L, Pietra R, Goj C, Mecacci C, Radice D, Sabbioni E. Iron and other metals in neuromelanin, substantia nigra, and putamen of human brain. J Neurochem. 1994;62:1097–1101. doi: 10.1046/j.1471-4159.1994.62031097.x. [DOI] [PubMed] [Google Scholar]
- Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. Salmonella mutagenicity tests. III. Results from the testing of 255 chemicals. Environ Mol Mutagen. 1987;9:1–109. [PubMed] [Google Scholar]
- Zhang ZJ, Qian YH, Hu HT, Yang J, Yang GD. The herbal medicine Dipsacus asper wall extract reduces the cognitive deficits and overexpression of beta-amyloid protein induced by aluminum exposure. Life Sci. 2003;73:2443–2454. doi: 10.1016/s0024-3205(03)00649-0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Nie A, Bai W, Meng Z. Effects of aluminum chloride on sodium current, transient outward potassium current and delayed rectifier potassium current in acutely isolated rat hippocampal CA1 neurons. Food Chem Toxicol. 2004;42:1453–1462. doi: 10.1016/j.fct.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Zhao HX, Mold MD, Stenhouse EA, Bird SC, Wright DE, Demaine AG, Millward BA. Drinking water composition and childhood-onset Type 1 diabetes mellitus in Devon and Cornwall, England. Diabet Med. 2001;18:709–717. doi: 10.1046/j.1464-5491.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yokel RA. The chemical species of aluminum influences its paracellular flux across and uptake into Caco-2 cells, a model of gastrointestinal absorption. Toxicol Sci. 2005;87:15–26. doi: 10.1093/toxsci/kfi216. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hattori R, Fujimori E, Umemura T, Haraguchi H. Multielemental determination of trace metals in river water (certified reference material JSAC 0301-1) by high efficiency nebulization ICP-MS after 100-fold preconcentration with a chelating resin-packed minicolumn. Anal Sci. 2005;21:199–203. doi: 10.2116/analsci.21.199. [DOI] [PubMed] [Google Scholar]
- Zook E, Lehmann J. Total diet study: Content of ten minerals - aluminum, calcium, phosphorus, sodium, potassium, boron, copper, iron, manganese and magnesium. J Assoc Off Agr Chem. 1965;48:850–855. [Google Scholar]
- Zumkley H, Spieker C, Kisters K, Zidek W, Fromme HG, Losse H, van Husen N, Bertram HP. Aluminum concentrations in gastric mucous membrane in renal insufficiency. Trace Subst Environ Health. 1984a;18:40–46. [Google Scholar]
- Zumkley H, Schmidt PF, Bertram HP, Lison AE, Winterberg B, Spieker K, Losse H, Barckhaus R. Aluminum concentration of bone cells in dialysis osteomalacia. Trace Elem Med. 1984b;1:103–106. [Google Scholar]
- Zumkley H, Bertram HP, Brandt M, Rodig M, Spieker C. Magnesium, aluminum and lead in various brain areas. Trace Subst Environ Health. 1986;20:29–35. [Google Scholar]


