Abstract
Sphingomyelin breakdown product ceramide has recently been found to induce an adaptive response and reduce myocardial ischemia/reperfusion injury. Since activation of MAP kinases plays an essential role in myocardial adaptation to ischemic stress and since ceramide is involved in lipid raft formation where MAP kinases can be translocated in response to stress, we reasoned that preconditioning may potentiate the translocation of MAP kinases into the lipid raft. To test the hypothesis, rats were divided into five groups: i) control, ii) ischemia/reperfusion (I/R), iii) I/R + C2-ceramide, iv) adapted and v) adapted+ desipramine, an inhibitor of ceramide formation. Isolated hearts were preperfused for 15 min with Krebs Henseleit Bicarbonate (KHB) buffer in the absence or presence of 10 μM desipramine followed by adaptation induced by four cyclic episodes of 5 min ischemia and 10 min reperfusion. For myocardial adaptation to ischemia with ceramide, the hearts were perfused with 1μM C-2-ceramide. All hearts were then subjected to 30 min ischemia and 2 h of reperfusion. As expected, both ischemic adaptation and ceramide adaptation made the heart resistant to I/R injury as evidenced by improved ventricular performance and reduced myocardial infarct size and cardiomyocyte apoptosis, which were significantly blocked with desipramine indicating the involvement of ceramide in ischemic adaptation. Ceramide also participated in the formation of lipid raft, and desipramine disrupted the raft formation. In the adapted hearts, there was an increased association of the proapoptotic p38MAPKα with caveolin-1 while there was a reduced association of anti-apoptotic p38MAPKβ with caveolin-3 indicating reduced amount of p38MAPKα and increased amount of p38MAPKβ were available to the adapted hearts thereby generating a survival signal. Desipramine decreased the association of P38MAPKα and C-2 ceramide increased the association of P38MAPKα with the lipid raft. The survival signal was further confirmed by increased phosphorylation of AKT and enhanced induction of expression of Bcl-2 during adaptation and its reversal with desipramine. The results indicated a unique ceramide signaling the ischemic and PC hearts involving lipid rafts, which generated a survival signal by differentially associating the p38MAPKα and p38MAPKβ with the caveolin-1 and caveoli-3, respectively.
Keywords: Ceramide, lipid raft, caveolin, p38MAP kinase, Survival signal, ischemia/reperfusion
INTRODUCTION
The sphingolipid ceramide has been implicated to function as an important intracellular lipid mediator and a second messenger related to a variety of cell functions including differentiation and cell death. In general, ceramide inhibits cell growth and induces apoptosis, while its metabolite, sphingosine-1-phosphate promotes growth and survival. Ceramide can result in transmembrane receptor activation modulating the activities of a variety of kinases and phosphatases that regulate cell death process.
A recent study demonstrated a duel role of ceramide in cardiomyocytes death and survival [1], in which ischemia/reperfusion induced cardiac dysfunction and cell death were partially restored with desipramine, a blocker of sphingomyelinase, and thus to ceramide synthesis. When the ischemic heart was made resistant to cell death by preconditioning (PC) or adaptation, it improved postischemic ventricular recovery and reduced myocardial infarct size and cardiomyocyte apoptotosis[1]. The cardio protective abilities of PC were abolished with desipramine, which also downregulated PC-mediated increase in antiapoptotic protein Bcl-2. The apparent paradoxical role of desipramine was explained by the increase in proapoptotic ceramide content in the ischemic reperfused heart that was blocked with desipramine and an increase in antiapoptotic sphingosine-1-P content in the preconditioned heart.
Another related study showed that ischemia/reperfusion resulted in the breakdown of sphingomyelin with corresponding accumulation of ceramide and sphingosine [2]. Immunoprecipitation with eNOS-specific antibody revealed the association of eNOS with caveolin-1 fraction of the heart. Ischemia/reperfusion caused an increased cell death and myocardial infarct size, which were reduced by preperfusing the hearts with desipramine, which simultaneously prevented ceramide accumulation and eNOS association with caveolin-1[3]. The results indicated that ischemia/reperfusion caused an increase in eNOS, which was unavailable to the ischemic heart because of its association with caveolin-1. Ceramide played a crucial role in this process, because prevention of ceramide formation either by myocardial adaptation to ischemia or with desipramine resulted in an inhibition of eNOS association with caveolin-1 thereby reducing myocardial ischemic reperfusion injury.
Since ceramide appears to precondition the ischemic heart, we reasoned that ceramide might modulate MAP kinase signaling, which plays a crucial role in the preconditioning process. Our results determined that ceramide indeed preconditioned the ischemic heart by differentially regulating p38MAP kinases association to the lipid rafts thereby converting the death signal into a survival signal.
MATERALS AND METHODS
Chemicals
sphingosine-1-phosphate (sphingosine-1-P), C2-ceramide and desipramine were obtained from Sigma, St. Louis, Mo, Antibodies against caveolin-1, caveolin-3, p38MAPKβ, and Bcl-2 were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. P38MAPKα, AKT, and Phospho-AKT were purchased from Cell Signaling technology, Danvers, MA
Experimental Protocol
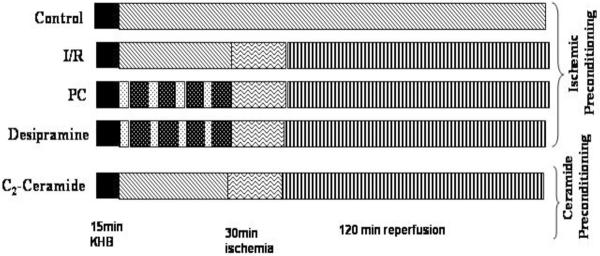
The study used two different protocols using isolated working rat heart model: i) Ischemic preconditioning in the absence and presence of desipramine and ii) preconditioning with C-2 ceramide. For the first, the isolated working rat hearts were preperfused with the Krebs Henseleit bicarbonate (KHB) buffer for 15 min in the absence or presence of desipramine (10 μM) followed by 30 min ischemia and 2 h of reperfusion. Ischemic preconditioning was achieved by subjecting the hearts to four cyclic episodes of 5 min ischemia each followed by another 10 min reperfusion. The preconditioned hearts were then subjected to 30 min ischemia and 2 hour of reperfusion (Figure 1). For the second model, the hearts were perfused for 15 min in the absence or presence of C-2 ceramide (1 μM) followed by 30 min ischemia and 2 hours of reperfusion.
Fig 1.
Schematic representation of perfusion protocol of different groups of heart.
Isolated Working Rat Heart Preparation
Male Sprague Dawley rats of 250 gm body weight were used for this study. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institute of Health (NIH Publication No. 86–23, revised 1985). The rats were anesthetized with sodium pentobarbital (80 mg/kg b/w, ip.) (Abbott Laboratories, North Chicago, IL, USA) and anticoagulated with heparin sodium (500 IU/kg b.w, i.p) (Elkin-Sinn Inc., Cherry Hill, NJ, USA) injection.
After ensuring sufficient depth of anesthesia, the hearts were excised and perfused in retrograde Langendorff mode at constant perfusion pressure of 100 cm of water (10kPa) for stabilization period [4]. Any heart that showed any cardiac disturbance (ventricle arrhythmia, and fibrillation) during the entire experiment was excluded from this study. All hearts were perfused by working mode according to the protocol described above. To ascertain the normal function of the heart, the heart rate, left ventricular developed pressure (the difference between the maximum systolic and diastolic pressure), left ventricular end-diastolic pressure, and the first derivative of developed pressure were recorded by Gould p23XL transducer (Gould Instrument System Inc., Valley View, OH). The signal was amplified by using Gould 6600 series signal conditioner (Gould Instrument System Inc., Valley View, OH) and monitored on Cordat II real-time acquisition system (Triton technologies, San Diego, CA) [5]. The aortic flow was measured by flow meter. The coronary flow was measured by time-collection of the coronary effluent dripping from the heart.
Measurements of the infarct size
After a global ischemic procedure the heart was infused with 10 % solution of the Triphenyl tetrazolium (TTC) in phosphate buffer through the aortic cannula for 20 minutes [6]. The left ventricle was removed and sliced into 1-mm thickness of cross-sectional pieces and weight PCR buffer. Each slice was scanned with computer-assisted scanner (Scanjet). The risk area of the whole myocardium was stained in red by TTC while the infarct zone remained unstained by TTC. These were measured by using of computerized software (Scion Image); areas were multiplied by the weight of the each section, and summed up to obtain the total of the risk zone and an infarct zone. The infarct size was expressed as the ratio of the infarct zone to the risk zone.
TUNEL Assay for assessment of Apoptotic Cell Death
Immunohistochemical detection of apoptotic cells was carried out by TUNEL assay using ApopTag in situ apoptosis detection kit (Intergen Company, Purchase, N.Y.) [7]. Cells were counted at 100X magnification and at least 4 fields per sample. The sections were incubated again with mouse monoclonal antibody recognizing cardiac myosin heavy chain to specifically recognize apoptotic cardiomyocytes. The fluorescence staining was viewed with a confocal laser microscope. The number of apoptotic cells was counted and expressed as a percent of total myocytes population.
Isolation of Caveolin-rich Membrane Fractions
The hearts were homogenized in sodium carbonate buffer containing protease inhibitor cocktail, pH 11.0 using a Polytron homogenizer [three 10s bursts] [Brinkman Instruments, Westbury, N.Y.]. The homogenate was sonicated [three 20s bursts], and adjusted to 45% sucrose by the addition of 2 ml of 90% sucrose prepared in MBS [25 mM Mes, pH 6.5, 0.15 M NaCl] and placed at the bottom of an ultracentrifuge tube as described previously [8]. A 35% discontinuous sucrose gradient was formed above [4 ml of 5% sucrose/4 ml of 35% sucrose – both in MBS containing 250 mM sodium carbonate] and centrifuged at 39,000 rpms for 16–20 h in an SW41 rotor [Beckman Instruments, Palo Alto, CA]. From the top of each gradient, 1 ml gradient fractions were collected to yield a total of 12 fractions as described elsewhere [8]. Caveolin migrates mainly in fractions 5 and 6 of these sucrose density gradients [9].
Immunoprecipitation with Caveolin 1 and Caveolin 3
Only caveolin rich fractions (fraction 5 & 6) were used for immunoprecipitation. Immunoprecipitation was performed with Protein-A sepharose CL-B4 (Pharmacia Biotech Inc) using a polyclonal antibody against caveolin-1 or caveolin -3[Santa Cruz Biotechnology, Santa Cruz, CA] Incubation conditions were maintained as instructed by the supplier. Western blot analysis was then performed with antibodies against P38MAPKα, P38 MAPKβ, AKT and Phospho-AKT and Bcl-2 according to established Method [9].
Estimation of Ceramide and Sphingosine-1-P in Caveolin-rich Membrane Fraction
Ceramide and its breakdown products were analyzed in the caveolin-rich membrane by HPTLC method as described previously (2). The plates were developed with ethyl acetate-methanolacetic acid 85:10:5 (v/v) as mobile phase in a Camag HPTLC twin-trough chamber lined with a saturation pad (Analtech, Newark, DE), and equilibrated with the mobile phase. The samples and standard zones were quantified by linear scanning at 254 nm with a Camag TLC Scanner II with a deuterium source; slit dimension settings of length 4, width 4, and scanning rate of 4 mm s−1.
Statistical Analysis
The values for number of apoptotic cardiomyocytes and infarct sizes as well as the functional parameters were all expressed as the Mean ± Standard Error of Mean (SEM) for at least six animals per group. The Western blot analyses were performed with at least three animals per group. The statistical analysis was performed by analysis of variance followed by Bonferroni's correction for any differences between the mean values of all groups. Differences between data were analyzed for significance by performing a Student's t-test. The results were considered significant if p < 0.05.
RESULTS
Cardioprotection by ischemic adaptation and C2-ceramide adaptation
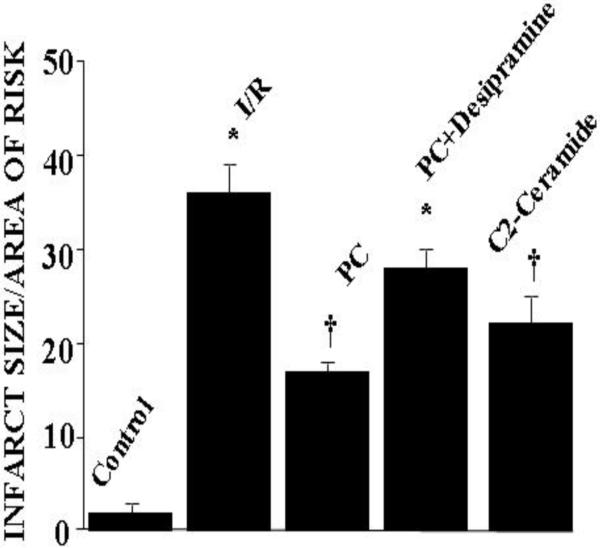
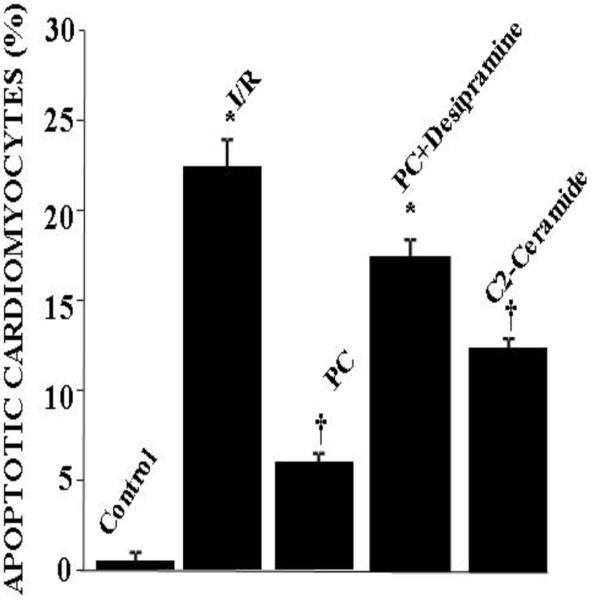
As expected, ischemia/ reperfusion caused significant amount of tissue injury as reflected by increased amount of myocardial infarct size and cardiomyocyte apoptosis [Figure 2]. Ischemic preconditioning mediated by four cyclic episodes of 5 min ischemia each followed by another period of ten minutes of reperfusion reduced the tissue injury as evidenced by decreased amount of infarct size [Figure 2] and apoptosis [Figure 3]. In concert, post-ischemic ventricular function was significantly higher in the preconditioned hearts compared to non-PC hearts [Table I]. LV developed pressure and aortic flow were reduced during the postischemic reperfusion in all groups. However, the reduction in recovery was significantly less for the ischemic PC groups, which reached significance compared to non-PC group at 60 and 120 min of reperfusion. The reduction of ischemia/reperfusion-mediated contractile function was also partially restored with desipramine.
Fig 2.
Effect of ischemia reperfusion, preconditioning, desipramine and ceramide on the infarct size The results are mean ± S.E.M. of six animal per group. * p<0.05 vs control, †p< 0.05 vs I/R.
Fig 3.
Effect of ischemia reperfusion, preconditioning, desipramine and ceramide on cardiomyocyte apoptosis. The results are mean ± S.E.M of six animals per group. *p<0.05 vs control, †p< 0.05 vs I/R.
TABLE 1.
EFFECT OF ISCHEMIC PC AND CERAMIDE PC ON POST-ISCHEMIC VENTRICULAR PERFORMANCE
| Baseline | I/R | PC | PC + Desipramine | C-2 Ceramide | |
|---|---|---|---|---|---|
| Heart Rate [beats/min] | 285± 5.4 | 275 ± 4.8 | 282 ± 3.9 | 279 ± 5.0 | 281 ± 3.2 |
| Aortic Flow [ml/min] | 30.6 ± 2.2 | 9.8 ± 1.9* | 17.6 ± 2.7*† | 9.2 ± 1.4*† | 15.5 ± 2.1*† |
| Coronary Flow [ml/min] | 19.5 ± 0.8 | 17.3 ± 1.3 | 19.2 ± 2.0 | 17.5 ± 1.6 | 19.7 ± 1.6 |
| Left ventricular developed pressure [mm Hg] | 110 ± 5.8 | 42 ± 7.5 | 68 ± 5.1 | 46 ± 3.3 | 60 ± 4.8 |
| Maximum first derivative of developed pressure[m Hg/Sec] | 3825 ± 72 | 1375 ± 67* | 2776 ± 55*† | 1423 ± 48* | 2910 ± 84*† |
Results are shown at the end of each experiments and expressed as Means ± SEM of six animals per group.
p<0.05 vs baseline,
p< 0.05 vs I/R
Similar to ischemic preconditioning, C-2 ceramide also successfully preconditioned the heart as evidenced by the lowering of myocardial infarct size and cardiomyocyte apoptosis [Figure 1 and Figure 2] and improved recovery of contractile function [Table I]. It is important to note that both ischemic preconditioning and ceramide preconditioning achieved cardioprotection in a comparable manner.
Effects of Ischemic adaptation and ceramide adaptation on the accumulation of ceramide in the caveolin fraction
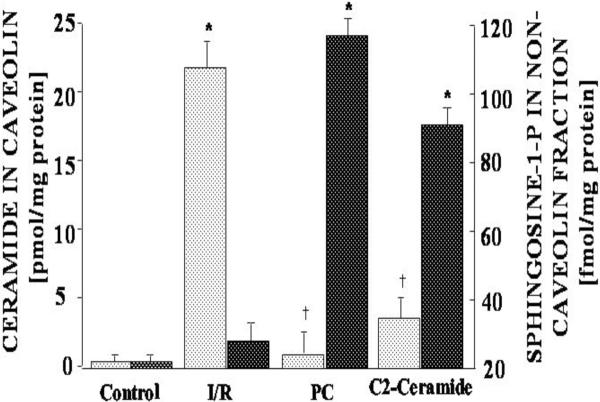
Ceramide and sphingosine-1-P content were estimated in the isolated caveolae fractions. Significant amount of ceramide was associated with caveolae fraction after I/R. But ceramide in caveolae fraction was significantly low when heart was subjected to ischemic adaptation (PC) and C2-ceramide treatment. In the PC (ischemic and ceramide) heart, the reduction of ceramide content was associated with an enhancement in sphingosine-1-P content in caveolae fraction, suggesting that PC trigger the breakdown of ceramide and accumulation of sphingosine-1-P. [Figure 4]
Fig 4.
Generation of ceramide and sphingosine -1-P in caveolae rich fraction of heart during Ischemia/ reperfusion and preconditioning. The results are mean ± S.E.M of six animals per group. *p<0.05 vs control, †p< 0.05 vs I/R.  = Ceramide
= Ceramide  =Sphingosine-1-phosphate
=Sphingosine-1-phosphate
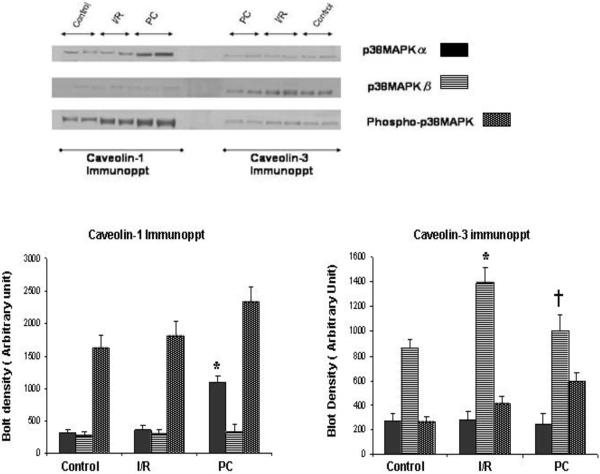
Differential Association of p38MAPKα and p38MAPKβ with caveolin 1 vs. caveolin
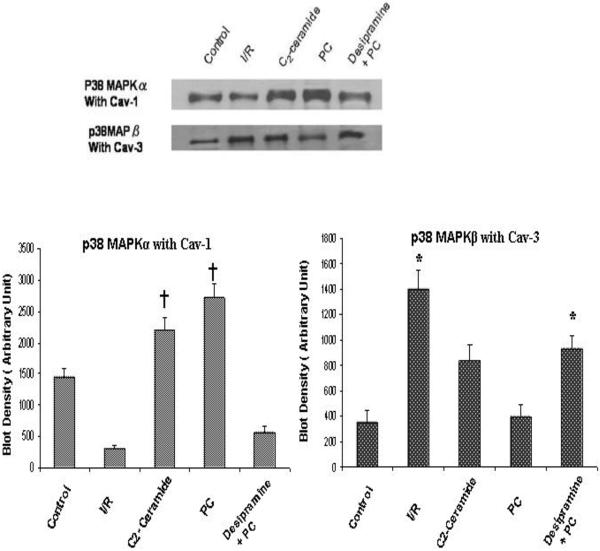
Caveolin rich fractions (5th and 6th fraction of sucrose density gradient) of all five groups of heart were immuno-precipitated with caeolin-1 and caveolin-3. The examination of the existence of p38MAPKα/β in the caveolin immunopreciptated sample by Western blot revealed that high amount of p38MAPKα bind with caveolin-1 whereas interaction of caveolin-3 with p38MAPKα was very negligible. In contrast, high amount of p38MAPKβ bind with Caveolin-3 whereas its binding with caveolin-1 was very negligible. [Figure 5]. In PC hearts, binding of p38MAPKα with caveolin-1 was much higher compared to the I/R hearts and desipramine treated hearts. Similarly in C2 ceramide treated hearts, binding of p38MAPKα with caveolin-1 was greater compared to the I/R and desipramine treated hearts. However, the binding of p38MAPKβ with caveolin-3 in PC hearts was much less compared the I/R heart and desipramine treated heart. [Figure 6]. The binding of p38MAPKβ with caveolin-3 in C2 ceramide treated heart was also lower compared to the I/R hearts.
Fig 5.
Differential interaction of p38MAPKα and p38MAPKβ with caveolin-1 and caveolin-3 during ischemia reperfusion and preconditioning. Bar graph represents the density of the blot. The results are mean ± S.E.M of three animals per group. *p<0.05 vs control, †p< 0.05 vs I/R.
Fig 6.
Effect of ischemia reperfusion, preconditioning, desipramine and ceramide on the binding of p38MAPK with caveolin. Bar graph represents the density of the blot. The results are mean ± S.E.M of three animals per group. *p<0.05 vs control, †p< 0.05 vs I/R.
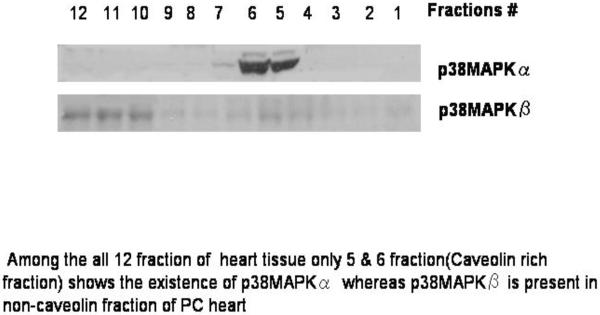
In order to further confirm the association of p38MAPKα and β in caveolin fractions, we examined their presence in the non-caveolin fractions. As shown in Figure 7, p38MAPKα was completely absent in non-caveolin fraction (only present in caveolin rich fraction) p38MAPKβ was present in noncaveolin fractions (mainly fraction 10, 11, 12) further supporting PC-mediated differential translocation of p38MAPKα and p38MAPKβ in caveolin-1 and caveolin-3, respectively. From these results it can be concluded that the hearts received the survival signal during PC when greater amount p38MAPKα and lower amount of p38MAPKβ was bound to caveolin, indicating availability of higher amount of anti-apoptotic p38MAPKβ and lower amount of pro-apoptotic p38MAPKα to the heart.
Fig 7.
Distribution of p38MAPKα/β in different fraction preconditioned heart. Within all the 12 fractions of the heart tissue only 5th & 6th fraction (caveolin rich fraction) shows the existence of p38MAPKα whereas p38MAPKβ is present in the non-caveolin fraction of PC heart (mainly 10th, 11th & 12th fractions)
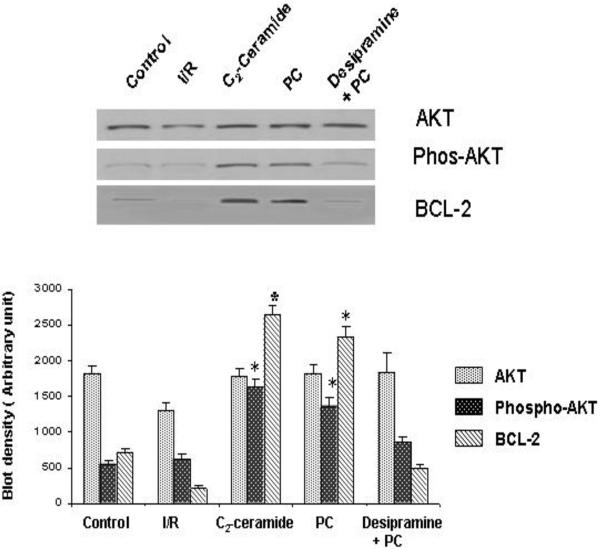
Expression of AKT and Bcl-2 in PC, Desipramine & C2 ceramide treated heart
Bcl-2 and AKT are known to transmit survival signal. We did not find these proteins in the caveolin immunoprecipitated samples. The expression of these proteins was found in the cytosolic and crude fraction in of the heart. PC heart showed the upregulation of Bcl-2 compared to the control and desipramine abolished such increase in Bcl-2 [Figure 8]. Phospho-AKT was only present in the PC and creamide treated heart but not in the control, I/R and desipramine treated hearts.
Fig 8.
Effect of ischemia reperfusion, preconditioning, desipramine and ceramide on the induction of the expression of Bcl-2, AKT. Phospho-AKT. Bar graph represents the density of the blot. The results are mean ± S.E.M of three animals per group. *p<0.05 vs control.
DISCUSSION
The present study demonstrated an important role of ceramide, a sphingomyelin breakdown product, in myocardial ischemia/reperfusion injury and ischemic preconditioning. Earlier investigation of our laboratory showed that cardioprotective ability of preconditioning was blocked by desipramine, an inhibitor of ceramide synthesis[1]. Furthermore, ceramide itself could mimic the effects of preconditioning, as preperfusion of the hearts with a cell permeable ceramide analog, C-2 ceramide was found to be equally cardioprotective. Interestingly enough, p38MAPKα was translocated into caveolin-1 while p38MAPKβ was translocated into caveolin-3. Both ischemic PC and ceramide PC enhanced the translocation of p38MAPKα into the caveolin-1 while they reduced the translocation of p38MAPKβ into caveolin-3 thereby generating an overall survival signal to the preconditioned hearts while desipramine reduced such translocation. The survival signal was reflected by increased phosphorylation of AKT and enhanced induction of the expression of Bcl-2 in both ischemic and ceramide PC hearts.
The MAP kinases play an essential role in mediating intracellular signal transduction during ischemic preconditioning. In response to extracellular stimulation, MAP kinases are rapidly activated and regulate cellular functions by inducing the phosphorylation of proteins, such as an oncogene product c-jun, S6 ribosomal protein kinase, and MAP kinase activated protein kinase 2 [10, 11]. The participation of MAP kinases in the preconditioned hearts involves a tyrosine kinase- phospholipase D- MAP kinases- MAPKAP kinase 2 signaling pathway [12, 13]. It appears that activation of stress-activated protein kinases are obligatory for myocardial adaptation to ischemia, but the activation is only transient, and the activities rapidly come down after subsequent ischemia and reperfusion.
In a previous study, we have shown that preconditioning translocates p38MAPK from the nucleus to the cytosol prior to activation of MK2 and HSP27 phosphorylation [14]. To date, four members of the p38MAPK family have been identified: p38α, p38β, p38γ and p38δ [15]. Although these isoforms share functional similarities, differences exist in their upstream and downstream kinases specificity, suggesting that they may have non-overlapping functions [16]. In general p38MAPKα is linked to the death signal while p38MAPKβ is linked with the survival signal [17]. Mitogen-activated protein kinase 3 (MKK3) preferentially activates p38MAPKα by dual phosphorylation and increases cell death [18]. In case of heart, MK2 is activated specifically by p38MAPKα and p38MAPKβ. In the present study, decreased amount of p38MAPKα was associated with caveolin-1 during I/R and its association was increased during PC indicating that caveolin-1 being instrumental in converting p38MAPK-mediated death signal into a survival signal. Conversely, PC reduced the association of p38MAPKβ into caveolin-3 as compared to I/R thereby generating a further survival signal.
Recent investigation has suggested an emerging paradigm whereby stress-responsive intracellular signaling pathways involve survival gene Bcl-2 through PI-3-kinase pathway [19]. Akt is a critical regulator of PI3-kinase-mediated cell survival [20]. Constitutive activation of Akt signaling is sufficient to block cell death induced by a variety of apoptotic stimuli [21]. Several down stream targets of Akt have been recognized as apoptosis regulatory molecules including Bcl-2-family member BAD [22] procaspase-9, [20] cAMP response element-binding protein (CREB) [23], and forkhead family of transcription factors [24, 25]. Insulin like growth factor-1 (IGF-1) also regulates PI3-kinase and Akt [26], and induces over expression of Bcl-xL and down-regulation of Bax. Akt is activated by PC as a result of PI3-kinase activation leading to the stimulation of PKC and endothelial NO synthase (eNOS) [27]. Because of the suggested role of Akt in cell survival that directly antagonize mitochondrial-directed apoptosis, and because Akt is reported to be regulated by PC, we investigated if p38MAPKα and p38MAPKβ-mediated survival signal is transmitted through Akt. Our results revealed rapid translocation of p38MAPKα into caveolin-1 and p38MAPKβ into caveolin-3, which was blocked by an inhibitor of ceramide formation indicating a crucial role of ceramide in p38MAP kinase signaling in lipid raft.
This was followed by the phosphorylation of AKT and activation of Bcl-2 survival protein. This finding is in consistent with the previous findings in other cell lines, in which Akt acts at the level of mitochondria to release cytochrome- C via Bcl-2 in adult cardiomyocyte. PI-3 kinase and Akt signaling pathways were also found to play a critical role in the prevention of apoptotic cell death by PC [20].
In summary, the results of this study showed for the first time differential interaction of p38MAPKα and p38MAPKβ with caveolin -1 and caveolin -3 respectively. Ischemia/reperfusion cause rapid translocation and activation of p38 MAPKs in the caveolin fraction. In I/R heart less p38MAPKα binds to caveolin-1 and more p38MAPKβ binds to caveolin-3 – making p38MAPKα more abundance in the heart to induce death signal. In contrast, in ischemic PC and ceramide PC heart, more p38MAPKα binds to caveolin-1 while less p38MAPKβ binds with caveolin-3. As a result more p38MAPKβ and less p38MAPKα are available to heart to induce survival signal. It appears that differential translocation and/or binding of p38MAPKα/β to caveolin-1/3 functions as a switch for the conversion of I/R induced death signal into PC induced survival signal. Additionally, PC stimulates the generation of sphingosine-1-P at the expense of ceramide thereby further converting the death signal into survival signal. The survival signal was confirmed by the activation of AKT and induction of the expression of Bcl-2 in PC heart.
ACKNOWLEDGEMENTS
This study was supported by NIH HL 22559, HL33889 and HL 34360.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1).Cui J, Engleman RM, Maulik N, Das DK. Role of ceramide in Ischemia preconditioning. J Am Coll Surg. 2004;198(5):770–777. doi: 10.1016/j.jamcollsurg.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 2).Cordis GA, Yoshida T, Das DK. HPTLC analysis of sphingomyelin, ceramide and sphingosine in ischemia/ reperfusion heart. Journal of Pharmaceutical and Biochemical Analysis. 1998;16:1189–1193. doi: 10.1016/s0731-7085(97)00260-4. [DOI] [PubMed] [Google Scholar]
- 3).Der P, Cui J, Das DK. Role of lipid raft in ceramide and nitric oxide signaling in ischemic and preconditioned hearts. J Mol Cell Cardiol. 2006;40:313–320. doi: 10.1016/j.yjmcc.2005.10.005. (2006) [DOI] [PubMed] [Google Scholar]
- 4).Maulik N, Engleman RM, Rouson JA, Flack JE, Deaton D, Das DK. Ischemia preconditioning reduces apoptosis by regulating anti death gene Bcl-2. Circulation. 1999;100:II369–II 375. doi: 10.1161/01.cir.100.suppl_2.ii-369. [DOI] [PubMed] [Google Scholar]
- 5).Beresewicz A, Maczewski M, Duda M. Effect of classic preconditioning and diazoxide on endothelial function and O2 and NO generation in the post-ischemic guinea-pig heart. Cardiovasc Res. 2004;172:201–10. doi: 10.1016/j.cardiores.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 6).Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgenic overexpression of α B-crystallin confers simultaneous protection against cardiac myocyte apoptosis and necrosis during myocardial ischemia reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 7).Maulik N, Sasakli H, Addya S, Das DK. Regulation of cardiomyocyte apoptosis by redox sensitive transcription factor. FEBS lett. 2000;485:7–12. doi: 10.1016/s0014-5793(00)02174-8. [DOI] [PubMed] [Google Scholar]
- 8).Song KS, Li S, Okamoto T, Qutrilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomain. J. Biol. Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 9).Suzuki YJ, Nagase H, Day RM, Das DK. GATA-4 regulation of myocardial survival in preconditioned heart. J. Mol, Cell. Cardiol. 2004;37:1195–1203. doi: 10.1016/j.yjmcc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 10).Sato M, Cordis GA, Maulik N, Das DK. SAPKs regulation of ischemic preconditioning. Am. J. Physiol. 2000;279:H901–H907. doi: 10.1152/ajpheart.2000.279.3.H901. [DOI] [PubMed] [Google Scholar]
- 11).Das S, Otani H, Maulik N, Das DK. Redox regulation of angiotensin-II preconditioning of the myocardium requires MAP kinase signaling. J. Mol. Cell. Cardiol. 2006 doi: 10.1016/j.yjmcc.2006.03.009. In press. [DOI] [PubMed] [Google Scholar]
- 12).Maulik N, Watanabe M, Zu YL, Huang CK, Cordis GA, Schley JA, Das DK. Ischemic preconditioning triggers the activation of MAP kinases and MAPKAP kinase 2 in rat hearts. FEBS. Lett. 1996;369:233–237. doi: 10.1016/0014-5793(96)01109-x. [DOI] [PubMed] [Google Scholar]
- 13).Zu YL, Ai Y, Gilchrist A, Maulik N, Watras J, Shaafi RI, Das DK, Huang CK. High expression and activation of MAP kinase-activated protein kinase 2 in cardiac muscle cells. J.Mol. Cell. Cardiol. 1997;29:2150–2168. doi: 10.1006/jmcc.1997.0449. [DOI] [PubMed] [Google Scholar]
- 14).Shiroto K, Otani H, Yamamoto F, Huang CK, Maulik N, Das DK. MK-2 gene knock out mouse heart carry anti-apoptotic signal and are resistant to ischemia reperfusion injury. J.Mol. Cell. Cardiol. 2005;38:93–97. doi: 10.1016/j.yjmcc.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Wrobleski ST, Doweyko AM. Structural comparison of p38 inhibitor-protein complexes: a review of recent p38 inhibitors having unique binding interactions. Current Topics in Med Chem. 2005;5:1005–1016. doi: 10.2174/1568026054985894. [DOI] [PubMed] [Google Scholar]
- 16.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 17.Sumbayev VV, Yasinska IM. Regulation of MAP kinase-dependent apoptotic pathway: implication of reactive oxygen and nitrogen species. Arch Biochem Biophys. 2005;436:406–412. doi: 10.1016/j.abb.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Kyosseva SV. Mitogen-activated protein kinase signaling. Int Rev Neurobiol. 2004;59:201–220. doi: 10.1016/S0074-7742(04)59008-6. [DOI] [PubMed] [Google Scholar]
- 19.Maulik N, Sato M, Prince BD, Das DK. An essential role of NFκB in tyrosine kinase signaling of p38MAP kinase regulation of myocardial adaptation to ischemia. FEBS. Lett. 1998;429:365–369. doi: 10.1016/s0014-5793(98)00632-2. [DOI] [PubMed] [Google Scholar]
- 20.Uchiyama T, Engleman RM, Maulik N, Das DK. Role of AKT signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109(24):3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 21.O'Gorman DM, McKenna SL, McGAhon AJm, Konx KA, Cotter TG. Sensitization of HL66 human leukaemic cells to cytotoxic drug induced apoptosis by inhibition of PI-3-kinase survival signal. Leukemia. 2000;14:602–611. doi: 10.1038/sj.leu.2401726. [DOI] [PubMed] [Google Scholar]
- 22.Maulik N, Engleman RM, Rousou JA, Flack JE, Deaton DW, Das DK. Ischemic preconditioning suppress apoptosis by upregulating the anti-death gene Bcl-2. Surg. Forum. 1998;49:209–211. doi: 10.1161/01.cir.100.suppl_2.ii-369. [DOI] [PubMed] [Google Scholar]
- 23.Das S, Cordis GA, Maulik N, Das DK. Pharmacological preconditioning of resveratrol: a role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor. Am. J. Physiol. Heart. Circ. 2005;288(1):H328–H351. doi: 10.1152/ajpheart.00453.2004. [DOI] [PubMed] [Google Scholar]
- 24.Das DK, Maulik N, Engleman RM. Redox regulation of angiotensin II signaling in the heart. J. Cell. Mol. Med. 2004;8(1):144–152. doi: 10.1111/j.1582-4934.2004.tb00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maulik N, Das DK. Preconditioning potentiates redox signaling and converts death signal into survival signal. Archives of Biochemistry and Biophysics. 2003;420:305–311. doi: 10.1016/j.abb.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Takasuga S, Katada T, Ui M, Hazeki O. Enhancement of adenosine and insulin induced activation of PI-3-kinase and protein kinase B in rat adipocyte. J. Biol. Chem. 1999;274:19545–19550. doi: 10.1074/jbc.274.28.19545. [DOI] [PubMed] [Google Scholar]
- 27.Das S, Tosaki A, Bagchi D, Maulik N, Das DK. Rsveratrol- mediated activation of cAMP response: Element binding protein through adenosine A3 receptor by AKT dependent and independent pathway. The Journal of Pharmacology and Experimental Therapeutics. 2005;314:762–769. doi: 10.1124/jpet.105.084285. [DOI] [PubMed] [Google Scholar]