Abstract
Marijuana use is associated with anxiety, particularly among those anxiety conditions in which panic is common. It may therefore be that risk factors for panic increase the likelihood that marijuana users will experience problematic anxiety symptoms. The current study investigated the role of one such risk factor, anxiety sensitivity (AS), or the extent to which an individual is frightened of anxiety symptoms. We examined whether AS interacts with frequency of marijuana use to increase anxious responding (using a three-minute voluntary hyperventilation procedure). The sample consisted of 153 adolescents (46.4% female) age 11-17 (M = 14.92, SD = 1.49). As predicted, AS moderated the link between lifetime marijuana use frequency and both post-challenge physiological anxiety (as indexed by skin conductance) and post-challenge subjective anxiety in female (but not male) adolescents such that those with high AS and more frequent marijuana use demonstrated the highest level of challenge-induced fear response. This effect remained even after controlling for relevant variables (e.g., age, trait anxiety, lifetime alcohol and cigarette use). Present findings suggest AS appears to serve as an important and potentially specific anxiety-related variable that deserves serious attention as a potential vulnerability factor among frequent marijuana-using females.
Keywords: Marijuana, Cannabis, Anxiety, Anxiety Sensitivity, Biological Challenge, Panic
1. Introduction
Empirical evidence indicates a link between anxiety and frequent marijuana use, marijuana-related impairment, and marijuana use disorders (Abel, 1971; Agosti et al., 2002; Buckner et al., 2007; Buckner et al., 2006a; Buckner et al., 2006b; Buckner et al., 2008; Oyefeso, 1991; Patton et al., 2002; Zvolensky et al., 2006a). The association between marijuana and anxiety appears strongest among anxiety conditions in which panic is common (e.g., social anxiety, panic disorder). To illustrate, social anxiety disorder (SAD) in adolescence appears to serve as a risk for marijuana dependence in adulthood (Buckner et al., 2008). Further, more frequent marijuana use is prospectively associated with panic attacks and panic disorder (Zvolensky et al., in press) and panic attacks appear to be one of the most common consequences of marijuana use (Thomas, 1996).
Despite the link between marijuana use and certain anxiety states and disorders, little attention has been paid to individual-difference characteristics that may contribute to these relations. Given that marijuana use tends to co-occur with anxiety conditions in which panic commonly occurs, it may be that risk factors for panic increase the likelihood that marijuana users will experience problematic anxiety. One factor relevant to panic is anxiety sensitivity (AS), or the extent to which an individual fears symptoms of anxiety (Reiss and McNally, 1985). To illustrate, an individual high in AS may fear that heart palpitations signal a heart attack whereas someone low in AS may interpret the same sensations as non-threatening. AS has been found to predict panic attacks and panic disorder above and beyond relevant factors such as trait anxiety (Schmidt et al., 1997; Schmidt et al., 1999; Schmidt et al., 2006).
Given that marijuana use can produce bodily sensations such as acute changes in heart rate (O'Leary et al., 2002; Pillard et al., 1974), blood pressure (O'Leary et al., 2002), and respiratory functioning (Nahas and Latour, 1992), high AS individuals may interpret marijuana-related bodily sensations as threatening, thereby increasing the likelihood that they will experience acute anxiety and/or panic. In support of this conceptualization, marijuana use has been found to interact with AS to predict self-reported panic symptoms among young adult tobacco smokers (Zvolensky et al., 2006b). Specifically, high AS marijuana users reported the highest levels of panic symptoms and panic-related cognitions compared to high AS non-users and low AS individuals regardless of marijuana use status.
Although promising, the extant literature linking marijuana use to panic psychopathology is limited in that research to date has been conducted primarily with adults. Yet rates of marijuana use are particularly high among adolescents (Comeau et al., 2001; Johnston et al., 2004; Substance Abuse and Mental Health Services Administration, 2006). Further, the examination of the relations between marijuana use and anxiety among psychologically healthy adolescents (i.e., free from current or past Axis I psychopathology) limits the extent to which findings may be confounded. For instance, if other types of psychopathology common to marijuana use are present in adult samples, observed relations could be due to the presence of psychopathology rather than the predictor variables of interest. Additionally, the use of a young, healthy sample allows for the examination of the relations between marijuana use, AS, and anxious reactivity prior to the point at which marijuana use problems and clinically meaningful AS and/or anxiety could interact to create a vicious cycle of marijuana use leading to increased anxiety leading to increased marijuana use to medicate anxiety reactions (Buckner et al., 2008).
The primary goal of the current study was to further clarify the role of AS in the association between marijuana use and intensity of challenge-induced anxious responding (cognitive and physical). Biological challenges (e.g., hyperventilation) are widely used procedures for the investigation of psychological and neurobiological factors associated with panic and anxiety responses (Zvolensky and Eifert, 2000). Specifically, we sought to test whether AS moderated the relation between marijuana use and anxious reactivity. We extended prior work in several ways. First, we examined challenge-induced anxiety responses (elicited by voluntary hyperventilation). Second, given data suggesting that frequent marijuana use is associated with greater levels of anxiety and panic compared to individuals who use marijuana less frequently (Oyefeso, 1991; Zvolensky et al., in press), we examined frequency of marijuana use. It was hypothesized that even after controlling for relevant variables (alcohol use, trait anxiety, tobacco use), AS would moderate the association between frequency of marijuana use and challenge response such that individuals with the greatest frequency of marijuana use and highest levels of AS would demonstrate the greatest challenge-induced anxiety relative to frequent marijuana users with low AS and individuals with low levels of marijuana use regardless of AS. Third, in light of data indicating the relations between marijuana and anxiety is specific to females (Buckner et al., 2006a), we examined conducted analyses separately by sex.
2. Method
2.1. Participants
The sample consisted of 153 adolescents (46.4% female) age 11-17 (Mage = 14.92, SD = 1.49) recruited through the general community. Advertising consisted primarily of flyers placed in the community (e.g., arcades, primary care offices, adolescent-oriented community centers). Advertisement booths were also set up in a local well-traveled marketplace on two separate occasions (approximately two months apart). The racial distribution of the sample generally reflected that of the local population: 90.8% Caucasian, 2.0% African American, 0.7% Native Hawaiian, 0.7% Asian, 0.7% American Indian, 2.0% “other,” and 3.3% did not specify race. The study was approved by the University of Vermont Institutional Review Board. Although there are other published reports from this dataset (Leen-Feldner et al., 2005; Leen-Feldner et al., 2006; Leen-Feldner et al., 2007), each focused on a distinct theoretical question.
To determine eligibility, all participants were administered the Anxiety Disorders Interview Schedule for the Diagnostic and Statistical Manual—Fourth Edition [DSM-IV]: Child Version (ADIS-C) (Silverman and Albano, 1996). Research personnel (e.g., doctoral-level graduate students, clinical post-doctoral students) were trained to mastery in the use of the instrument. Training involved intensive didactic sessions, direct observation of administrations, and diagnostic comparison until 100% diagnostic reliability was reached. In addition, ongoing supervision was provided throughout the study. No participant had current or past Axis I psychopathology and no participant met exclusionary criteria for the present study, including: (a) current or past cardiopulmonary (chronic) illness; (b) current acute respiratory illness; (c) current or past psychotropic medication use; (d) suicidality; and (e) limited mental competency and the inability to give informed, written consent/assent. These criteria serve to reduce potential confounds introduced by individual difference factors known to affect responding to the hyperventilation procedure as well as reduce medical complications and risks that might arise from hyperventilation.
2.2. Measures
2.2.1. Pre-challenge assessment
The 18-item Childhood Anxiety Sensitivity Index (CASI) (Silverman et al., 1991) was used to index anxiety sensitivity. Participants rated their perceptions of the aversive nature of anxiety symptoms by endorsing 1 (none), 2 (some), or 3 (a lot) in response to items such as “I don't want other people to know when I feel afraid”. This psychometrically sound scale (α =.83; current sample) was developed for use with youth and evidences satisfactory validity estimates (Silverman et al., 1991). Trait anxiety was measured using the well-validated state-trait anxiety index (STAI) (Spielberger et al., 1983). Substance use behaviors were assessed using items from the Youth Risk Behavior Survey (YRBS) (Division of Adolescent and School Health, 2004). Lifetime frequency of marijuana use was assessed using a 6-point scale ranging from “a” (0 times) to “f” (40 or more times). To evaluate the effects of using other substances, alcohol and tobacco cigarette use were included as covariates. Lifetime alcohol use was assessed on a 7-point scale ranging from “a” (0 days) to “g” (100 or more days). The YRBS assesses lifetime tobacco cigarette use dichotomously (“yes” or “no”). The YRBS substance items have demonstrated excellent test-retest reliability (Brener et al., 2002).
2.2.2. Challenge assessment
The Acute Panic Inventory (API) (Dillon et al., 1987), a 23-item assessment, was used to measure post-challenge panic-relevant responding. Each item on the API represents a DSM-IV defined panic attack symptom (e.g., Do you feel faint?) to which respondents endorse 0 (not at all), 1 (slight), 2 (moderate), or 3 (severe), yielding a total intensity score ranging from 0-69. The API has sound psychometric properties (α =.83; current sample) and has been successfully employed in previous biological challenge research with children and adolescents (Pine et al., 2000). Consistent with empirical precedent (Zvolensky et al., 2005), we examined the cognitive and physical API items separately.
2.2.3. Challenge
Hyperventilation served as the challenge procedure because it can be safely employed with adolescents, its parametric properties are well studied, and it can reliably produce bodily arousals that mimic panic attack symptoms (Hornsveld et al., 1990). The challenge involved a 3-minute hyperventilation with a breathing rate of 30 respiratory cycles/minute. A J&J Engineering I-330-C2 system was used to digitally record physiological data on-line at a sample rate of 1024 samples per second across all channels using J&J Engineering Physiolab Software. Raw electrocardiogram data were collected with disposable Ag/AgCl electrodes placed in a standard bilateral configuration on the palmar side of each wrist using adhesive foam patch and pellet, along with Signal Cream conductive cream. Skin conductance levels, converted to microsiemens, were obtained using an RV-5 skin resistance lead connected to SE-35 electrodes placed on the middle finger. In the present study, average heart-rate and skin conductance levels (sampled every .25 s) were computed across the 5-minute quiet period to index baseline physiological responding and for three minutes post-challenge.
2.3. Procedure
Upon arrival to the laboratory, participants and their guardians were told that the purpose of the study was to learn about “adolescent emotions” and that participants had the right to discontinue participation at any time without penalty (no participant withdrew from the study). In regard to the challenge, participants were informed that they would be asked to participate in an exercise that involved breathing in and out at a standard rate for three minutes. Participants also were advised as to the potential side-effects of the procedure. Following completion of the consent/assent forms, trained research staff conducted the psychiatric interview and eligible participants completed the pre-challenge assessments.
For the challenge procedures, participants were seated and electrodes were attached. Participants were instructed to sit as still as possible during the procedures. The experimenter then left the room and participants sat quietly for a five-minute adaptation period. Audiotaped directions then described the hyperventilation procedure and provided breathing instructions. Psychophysiological data indicated the procedure was effective in increasing psychophysiological reactivity (Leen-Feldner et al., 2006).
API ratings were collected directly following the hyperventilation challenge. At the end of the study, participants and their parental guardians were debriefed and compensated $50.
2.4 Data analytic strategy
Given hypothesized sex differences, analyses were conducted separately for male and female participants. First, zero-order correlations were computed to provide initial examination of the associations between predictor and criterion variables. To control for Type I error, a probability factor of .01 was used to interpret these associations.
To test the hypothesis that AS would moderate the relation between marijuana use frequency and post-challenge anxiety even after controlling for relevant covariates, hierarchical linear regression analyses were performed. This approach ensures that observed effects for the interactions cannot be attributed to shared variance with the variables at lower steps of the model (Cohen and Cohen, 1983). Interaction terms were centered to reduce multicollinearity (Aiken and West, 1991).The criterion variables were post-challenge heart rate, post-challenge skin conductance, and post-challenge API scores (API-physical and API-cognitive). Predictor variables in all regressions were the main effects of AS and marijuana frequency and the interaction of AS × marijuana frequency. Predictor variables were divided into three steps in the hierarchy: (a) the covariates of baseline heart rate or skin conductance (when applicable), age, trait anxiety, and lifetime alcohol and cigarette quantity were entered at step 1, (b) the main effects of each predictor variable in the interaction were entered at step 2, and (c) the interaction term was entered at step 3 (Baron and Kenny, 1986). The forms of significant interactions were inspected (Cohen and Cohen, 1983) and we conducted post-hoc probing of all significant moderator models (Aiken and West, 1991; Holmbeck, 2002). We also examined whether the observed relations occurred for past-month substance use and found that the nature and magnitude of the findings were similar whether past-month or lifetime indices were utilized. Thus, only analyses concerning lifetime indices are reported.
3. Results
3.1. Relations among predictor and criterion variables
Among females, marijuana use frequency was significantly positively correlated with age and alcohol use frequency, although negatively related to tobacco cigarette use. AS was unrelated to substance use variables. Post-challenge API scales (physical and cognitive) were positively correlated with trait anxiety and demonstrated a trending positive association with marijuana use frequency. AS was positively related to API-cognitive and trended toward a significant positive relation with API-physical. See Table 1.
Table 1.
Correlations between age, anxiety, lifetime substance use, and post-challenge measures by sex
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | - | .18 | .12 | .39** | .48** | -.18 | -.19 | -.18 | -.14 | -.18 | 14.80 (1.44) |
| 2. Anxiety sensitivity | .22 | - | .49** | -.02 | .01 | .18 | -.17 | -.02 | .26* | .12 | 23.58 (4.18) |
| 3. Trait anxiety | .47** | .63** | - | .00 | -.00 | .02 | .06 | .09 | .31** | .18 | 29.08 (6.74) |
| 4. Marijuana use frequency | .35** | .06 | .25* | - | .69** | -.59** | -.06 | -.13 | -.02 | -.03 | |
| 5. Alcohol use frequency | .39** | .07 | .32** | .80** | - | -.56** | -.09 | .04 | -.02 | -.15 | |
| 6. Cigarette use | .21* | -.12 | -.29* | -.75** | -.66** | - | -.12 | -.02 | .02 | .08 | |
| 7. Post-challenge heart rate | -.33** | .11 | -.09 | -.01 | .04 | -.05 | - | .06 | -.02 | .10 | 77.79 (10.26) |
| 8. Post-challenge skin conductance | -.09 | .04 | .01 | -.12 | -.03 | -.06 | .18 | - | .12 | .26* | 5.68 (2.30) |
| 9. API: Physical | .13 | .30* | .42** | .26* | .16 | -.29* | -.14 | -.03 | - | .40** | 7.82 (5.49) |
| 10. API: Cognitive | .11 | .50** | .49** | .29* | .16 | -.26* | .02 | .04 | .72** | - | 1.48 (2.95) |
| Mean (SD) | 15.04 (1.56) | 24.69 (5.11) | 31.10 (6.64) | 77.40 (10.30) | 4.97 (1.89) | 8.49 (6.76) | 1.56 (2.47) |
Correlations for male participants are presented above the diagonal. Correlations for female participants are presented below the diagonal. Lifetime tobacco cigarette use was dummy coded (1 = Yes, 2 = No). Given that substance use variables are categorical, means are not reported.
p < .05,
p < .01.
Among males, lifetime marijuana use frequency was also positively correlated with age and alcohol use frequency, and negatively related to cigarette use. AS was unrelated to substance use. The API-physical scale demonstrated a trending positive relation with AS. See Table 1.
3.2. Moderator analyses controlling for relevant variables
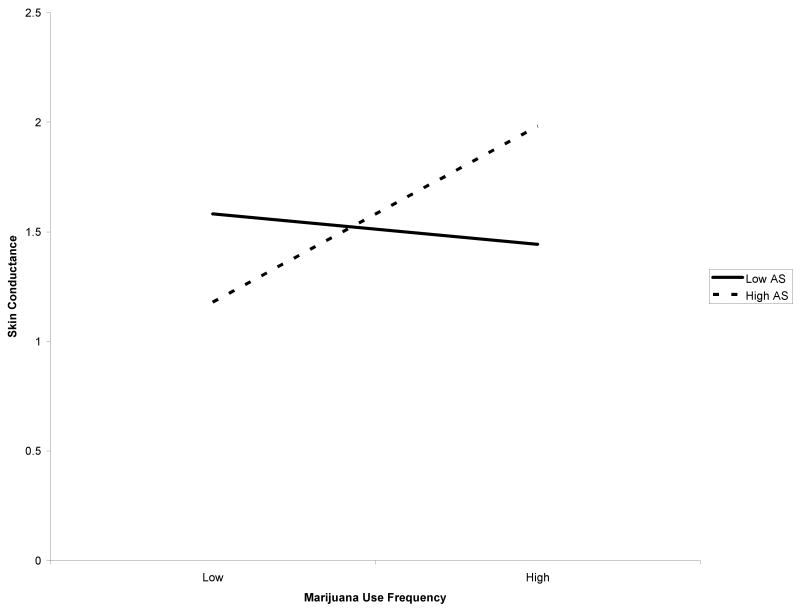
Among females, AS moderated the relation between marijuana use frequency and post-challenge skin conductance even after controlling for relevant variables, Fchange(1, 55) = 8.11, p = .006, with a medium effect size as indexed by f2 (Cohen, 1988) (Table 2). Regression analyses indicated that the predictor variables together predicted 89.6% of the overall variance. After controlling for the covariates, the main effects of AS and marijuana frequency significantly predicted 0.4% of the unique variance in skin conductance. The interaction of AS × marijuana frequency significantly predicted an additional 1.5% of the total variance. The form of the interaction was examined and, as predicted, high AS and greater frequency of marijuana use were associated with the greatest post-challenge skin conductance (Figure 1). Only the simple slope of the high AS moderator variable was significant (t = 2.13, p<.05). In other words, post-challenge skin conductance was significantly greater for frequent versus infrequent marijuana users among individuals with high AS. However, among adolescents with low AS, these relations were not significant (t = -.52, p = .61). AS did not moderate the link between marijuana use frequency and post-challenge heart rate, Fchange (1, 55) = .63, p = .43.
Table 2.
Individual variable contributions predicting post-challenge outcomes (female adolescents)
| ΔR2 | T | β | f2 | p | |
|---|---|---|---|---|---|
| Dependent Variable: Post-Challenge Heart Rate | |||||
| Step 1: Covariates | .772 | <.001 | |||
| Baseline heart rate | 12.32 | .83 | 2.62 | <.001 | |
| Age | -2.36 | -.18 | 0.10 | .02 | |
| Trait anxiety | 1.53 | .11 | 0.04 | .13 | |
| Alcohol frequency | .86 | .08 | 0.01 | .39 | |
| Cigarette use | .42 | .04 | 0.00 | .68 | |
| Step 2: Main Effects | .014 | .18 | |||
| Anxiety sensitivity | 1.70 | .14 | 0.05 | .10 | |
| Marijuana frequency | -.86 | -.11 | 0.01 | .40 | |
| Step 3: Interaction Effect | .002 | .43 | |||
| Anxiety sensitivity × Marijuana frequency | -.79 | -.05 | 0.01 | .43 | |
| Dependent Variable: Post-Challenge Skin Conductance | |||||
| Step 1: Covariates | .877 | <.001 | |||
| Baseline skin conductance | 19.97 | .05 | 6.89 | <.001 | |
| Age | .87 | -.06 | 0.01 | .39 | |
| Trait anxiety | -1.09 | -.05 | 0.02 | .28 | |
| Alcohol frequency | -.70 | .04 | 0.01 | .49 | |
| Cigarette frequency | -1.32 | -.08 | 0.03 | .19 | |
| Step 2: Main Effects | .004 | .36 | |||
| Anxiety sensitivity | .86 | .05 | 0.01 | .39 | |
| Marijuana frequency | 1.11 | .11 | 0.02 | .27 | |
| Step 3: Interaction Effect | .015 | .006 | |||
| Anxiety sensitivity × Marijuana frequency | 2.85 | .13 | 0.15 | .006 | |
| Dependent Variable: Post-Challenge API-Physical | |||||
| Step 1: Covariates | .221 | <.01 | |||
| Age | -.96 | -.13 | 0.02 | .34 | |
| Trait anxiety | 3.23 | .44 | 0.17 | .002 | |
| Alcohol frequency | -.71 | -.11 | 0.01 | .48 | |
| Cigarette frequency | -1.63 | -.25 | 0.04 | .11 | |
| Step 2: Main Effects | .027 | .35 | |||
| Anxiety sensitivity | .35 | .05 | 0.00 | .73 | |
| Marijuana frequency | 1.41 | .32 | 0.03 | .16 | |
| Step 3: Interaction Effect | .000 | .91 | |||
| Anxiety sensitivity × Marijuana frequency | .11 | .01 | 0.00 | .91 | |
| Dependent Variable: Post-Challenge API-Cognitive | |||||
| Step 1: Covariates | .295 | <.001 | |||
| Age | -1.53 | -.20 | 0.04 | .18 | |
| Trait anxiety | 4.30 | .55 | 0.30 | <.001 | |
| Alcohol frequency | -.67 | -.10 | 0.01 | .25 | |
| Cigarette frequency | -1.37 | -.20 | 0.03 | .98 | |
| Step 2: Main Effects | .131 | .002 | |||
| Anxiety sensitivity | 2.40 | .31 | 0.10 | .03 | |
| Marijuana frequency | 2.71 | .54 | 0.12 | .001 | |
| Step 3: Interaction Effect | .083 | .003 | |||
| Anxiety sensitivity × Marijuana frequency | 3.13 | .29 | 0.17 | .003 | |
API=Acute Panic Inventory. Anxiety sensitivity was measured using the Childhood Anxiety Sensitivity Index (CASI), trait anxiety was measured using the Spielberger Trait Anxiety Inventory (STAI). β = standardized beta weight provided for multiple regression.
Figure 1.
Post-challenge skin conductance among high and low marijuana users based on AS (female adolescents).
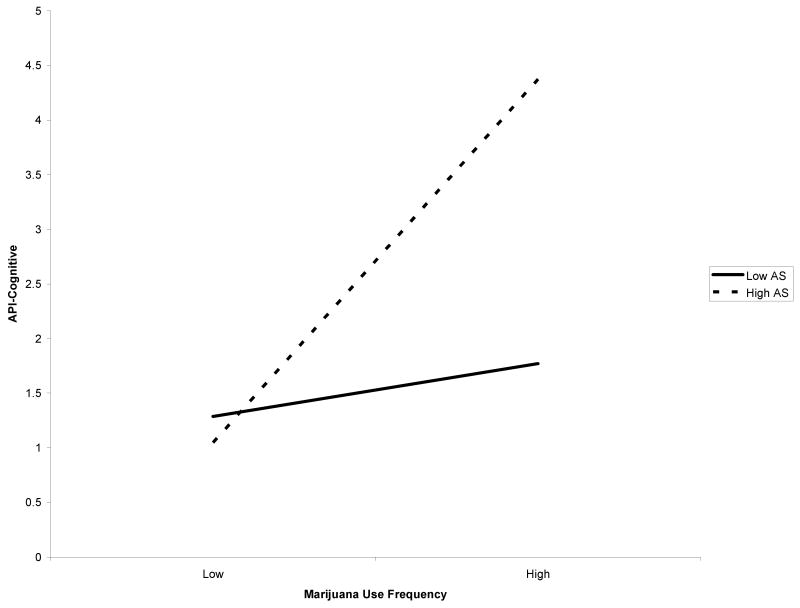
In regard to self-reported intensity of panic symptoms, AS moderated only the association between frequency of lifetime marijuana use and API-cognitive scores after controlling for relevant covariates, Fchange (1, 58) = 9.78, p = .003, with a medium effect size as indexed by f2 (Table 2). Regression analyses suggest that the predictor variables together predicted 50.9% of the overall variance. After controlling for relevant variables, the main effects of AS and marijuana frequency significantly predicted 13.1% of the unique variance in marijuana symptoms. The interaction of AS × marijuana frequency significantly predicted an additional 8.3% of the total variance. The form of the interaction was examined and, as predicted, high AS and greater frequency of marijuana use were associated with the greatest post-challenge API-cognitive scores (Figure 2). Only the simple slope of the high AS moderator variable was significant (t = 3.96, p < .001). In other words, the relationship between frequency of marijuana use and post-challenge API-cognitive scores was significantly different for individuals with high AS but not individuals with low AS (t = .89, p = .38). AS did not moderate the link between marijuana use frequency and post-challenge API-physical scores, Fchange (1, 57) = .01, p = .91.
Figure 2.
Post-challenge API-Cognitive scores among high and low marijuana users based on AS (female adolescents).
Due to limitations of conducting moderator analyses using cross-sectional data, one method of increasing confidence in observed effects is to conduct additional regressions after reversing the proposed moderator with the independent variable. This strategy allows for the examination of the competing hypothesis that high AS adolescents are hyper-reactive and use marijuana to self-medicate. Frequency of marijuana use served as the dependent variable in two additional hierarchical regression models. Predictor variables included the main effects of centered AS and post-challenge reactivity (skin conductance or API-cognitive score) and their interaction. Predictors were divided into three steps in the hierarchy: (a) covariates were entered at step 1, (b) the main effects of each interaction term were entered at step 2, and (c) the interaction term was entered at step 3. Neither model was significant (post-challenge skin conductance Fchange (1, 55) = 2.46, p = .12; API-Cognitive Fchange (1, 55) = .01, p = .92). In other words, these models failed to account for the data thereby increasing confidence in the original models' moderational effects.
Among male participants, AS did not moderate the relation between marijuana use frequency and any post-challenge measure, Fchange's < .44, p's > .51.
To examine the specificity of AS as a moderator, we examined whether trait anxiety demonstrated a similar moderational effect. Trait anxiety did not moderate the link between marijuana use frequency and any post-challenge measure (biological indices and API subscales) for female adolescents, Fchange's < 1.09, p's > .30, or for male adolescents, Fchange < 1.53, p's > .20.
4. Discussion
Overall, findings from the present study suggest that female adolescents who have frequently used marijuana throughout their lives may be particularly vulnerable to increased anxious responding if they possess high levels of the trait-like quality AS. Interestingly, our data suggest that marijuana use may not increase anxious responding among those with low AS. These findings are consistent with prior work suggesting that AS moderates the association between marijuana use and self-reported panic-relevant indices among adults (Zvolensky et al., 2006b). We built upon prior work by examining whether these relations were evident on behavioral measures of anxiety (i.e., response to hyperventilation challenge). Although the additional variance explained by the interaction term was small, given the magnitude of variance accounted for at earlier steps for each model (e.g., 88%), it is noteworthy that the interaction of AS and marijuana use frequency enhanced the model's predictive power at all (Abelson, 1985) and future study is necessary to evaluate the practical utility of this finding. Importantly, the observed effects were not attributable to other factors related to marijuana use and AS (e.g., lifetime alcohol and tobacco cigarette use, trait anxiety, age). It is also of note that trait anxiety did not demonstrate a moderator effect, providing further support that AS may serve a specific role in anxious responding among marijuana users.
An alternative explanation of the current data is that high AS female adolescents are vulnerable to anxious responding and therefore use marijuana to dampen anxious arousal. This interpretation is consistent with the self-medication hypothesis that has received at least partial support in prior reports (e.g., Carrigan and Randall, 2003). Although the cross-sectional nature of our research design does not allow us to speak to temporal ordering of these associations, we attempted to rule out this alternative explanation by evaluating whether AS and anxious responding interacted to predict frequency of lifetime marijuana use. This model was not significant for either psychophysiological or self-reported post-challenge response, strengthening confidence in our contention that frequent marijuana use among high AS individuals may place them at risk for increased anxious arousal. However, prospective work will be a critical next step in demarcating temporal sequencing of these relations.
It is of note that AS moderated the marijuana use-anxious responding link while participants were ostensibly not under the influence of marijuana. This finding indicates there may be long-term effects of marijuana use on high AS female adolescents, such that high AS females who use marijuana are more likely to experience greater anxiety even when not intoxicated.
The present study is the first known investigation of the relations between marijuana use, AS, and anxious responding by sex and it is notable that lifetime frequency of marijuana use was related to some forms of anxiety (trait anxiety, post-challenge API) in female (but not male) adolescents. It is also noteworthy that AS increased post-challenge anxiety response only among female adolescents. These data are in line with emerging literature indicating that marijuana-related problems are related to anxiety among female (but not male) college students (Buckner et al., 2006a) and marijuana use is more strongly related to the development of depression and anxiety among female than male adolescents (Patton et al., 2002).
Yet what accounts for higher rates of anxiety among female marijuana users? Differential motivations for marijuana use may be at least partially responsible. Although females are more likely to use marijuana to cope with negative affect (Simons et al., 1998), male adolescents are more likely to use marijuana to enhance sociability and positive affect than females (Newcomb et al., 1988). It may therefore be that if males use marijuana and experience anxiety, they are less likely to subsequently use marijuana (as its use produced negative, not positive affect).
The present study should be considered in light of limitations that point to further work in this area. First, the cross-sectional nature of the study hinders our ability to draw causal inferences. Second, the present study examined a non-referred group of adolescents rather than a treatment-seeking sample. Although these data are thereby generalizable to our population of interest, 70% of participants were non-users and future study is necessary to determine whether the observed relations generalize to more severe marijuana-using populations. Third, panic symptoms were evaluated by administering the API following the hyperventilation challenge thereby providing information regarding the overall magnitude of panic responding. Future work is thus necessary to evaluate change in panic symptoms before and after challenge engagement. Fourth, given the lack of significant findings for male adolescents, replication using a multi-modal, multi-informant approach would strengthen confidence in the present findings.
Overall, the present investigation represents an important step toward the delineation of mechanisms that may contribute to the high rates of co-occurring marijuana use and anxiety pathology. The results suggest that AS plays an important role in some types of anxious responding among frequent marijuana using female adolescents. Future work is necessary to determine the temporal relations among marijuana use, AS, and anxiety as such work will have important implications for the prevention and treatment of this high-risk population.
Acknowledgments
This research was supported in part by a National Research Service Award from the National Institute of Drug Abuse (F31DA021457) awarded to Julia D. Buckner. This work was also supported by National Institute on Drug Abuse research grants (1 R01 MH076629-01, 1 R01 DA018734-01A1, and R03 DA16307-01) awarded to Michael J. Zvolensky. Data for the present study were collected in the Anxiety and Health Research Laboratory at the University of Vermont.
The authors thank Laura Dixon, Natalie Feldman, Kate Follansbee, Marc Hartigan, Rachel Jones, Justin McCormick, Amanda O'Dell, Stephanie Sinisi, Nick Want, and Lindsay Van Zanten for their assistance with this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Changes in anxiety feelings following marihuana smoking. British Journal of Addiction. 1971;66:185–187. doi: 10.1111/j.1360-0443.1971.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Abelson RP. A variance explanation paradox: When a little is a lot. Psychological Bulletin. 1985;97:129–133. [Google Scholar]
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. American Journal of Drug and Alcohol Abuse. 2002;28:643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interaction. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Brener ND, Kann L, McManus T, Kinchen SA, Sundberg EC, Ross JG. Reliability of the 1999 Youth Risk Behavior Survey questionnaire. Journal of Adolescent Health. 2002;31:336–342. doi: 10.1016/s1054-139x(02)00339-7. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Bonn-Miller MO, Zvolensky MJ, Schmidt NB. Marijuana use motives and social anxiety among marijuana using young adults. Addictive Behaviors. 2007;32:2238–2252. doi: 10.1016/j.addbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Mallott MA, Schmidt NB, Taylor J. Peer influence and gender differences in problematic cannabis use among individuals with social anxiety. Journal of Anxiety Disorders. 2006a;20:1087–1102. doi: 10.1016/j.janxdis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Bobadilla L, Taylor J. Social anxiety and problematic cannabis use: Evaluating the moderating role of stress reactivity and perceived coping. Behaviour Research and Therapy. 2006b;44:1007–1015. doi: 10.1016/j.brat.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Lang AR, Small J, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. Journal of Psychiatric Research. 2008;42:230–239. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan MH, Randall CL. Self-medication in social phobia: A review of the alcohol literature. Addictive Behaviors. 2003;28:269–284. doi: 10.1016/s0306-4603(01)00235-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1983. [Google Scholar]
- Comeau N, Stewart SH, Loba P. The relations of trait anxiety, anxiety sensitivity and sensation seeking to adolescents' motivations for alcohol, cigarette and marijuana use. Addictive Behaviors. 2001;26:803–825. doi: 10.1016/s0306-4603(01)00238-6. [DOI] [PubMed] [Google Scholar]
- Dillon DJ, Gorman JM, Liebowitz MR, Fyer AJ, Klein DF. Measurement of lactate-induced panic and anxiety. Psychiatry Research. 1987;20:97–105. doi: 10.1016/0165-1781(87)90002-3. [DOI] [PubMed] [Google Scholar]
- Division of Adolescent School Health, Centers for Disease Control. Youth Risk Behavior Surveillance-United States, 2003. Morbidity and Mortality Weekly Report. 2004;53:1–29. [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Hornsveld H, Garssen B, Dop MF, Van Spiegel P. Symptom reporting during voluntary hyperventilation and mental load: Implications for diagnosing Hyperventilation Syndrome. Journal of Psychosomatic Research. 1990;34:687–697. doi: 10.1016/0022-3999(90)90113-i. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future: National results on adolescent drug use-Overview of key findings (NIH Publication No 04-5506) National Institute on Drug Abuse; Bethesda, MD: 2004. [Google Scholar]
- Leen-Feldner EW, Feldner MT, Bernstein A, McCormick JT, Zvolensky MJ. Anxiety sensitivity and anxious responding to bodily sensations: A test among adolescents using a voluntary hyperventilation challenge. Cognitive Therapy and Research. 2005;29:593–609. [Google Scholar]
- Leen-Feldner EW, Feldner MT, Tull MT, Roemer L, Zvolensky MJ. An examination of worry in relation to anxious responding to voluntary hyperventilation among adolescents. Behaviour Research and Therapy. 2006;44:1803–1809. doi: 10.1016/j.brat.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Leen-Feldner EW, Reardon LE, Zvolensky MJ. Pubertal status and emotional reactivity to a voluntary hyperventilation challenge predicting panic symptoms and somatic complaints: A laboratory-based multi-informant test. Behavior Modification. 2007;31:8–31. doi: 10.1177/0145445506295058. [DOI] [PubMed] [Google Scholar]
- Nahas G, Latour C. The human toxicity of marijuana. The Medical Journal of Australia. 1992;156:495–497. [PubMed] [Google Scholar]
- Newcomb MD, Chou Cp, Bentler PM, Huba GJ. Cognitive motivations for drug use among adolescents: Longitudinal tests of gender differences and predictors of change in drug use. Journal of Counseling Psychology. 1988;35:426–438. [Google Scholar]
- O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, Boles Ponto LW, Leonard G, Hurtig RR, Hichwa RD. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Oyefeso A. Personality differences among five categories of student cannabis users. Indian Journal of Behaviour. 1991;15:28–35. [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in younger people: Cohort study. British Medical Journal. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillard RC, McNair DM, Fisher S. Does marijuana enhance experimentally induced anxiety? Psychopharmacologia. 1974;40:205–210. doi: 10.1007/BF00429414. [DOI] [PubMed] [Google Scholar]
- Pine DS, Klein RG, Coplan JD, Papp LA, Hoven CW, Martinez J, Kovalenko P, Mandell DJ, Moreau D, Klein DF, Gorman JM. Differential carbon dioxide sensitivity in childhood anxiety disorders and nonill comparison group. Archives of General Psychiatry. 2000;57:690–967. doi: 10.1001/archpsyc.57.10.960. [DOI] [PubMed] [Google Scholar]
- Reiss S, McNally RJ. The expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical issues in behavior therapy. Academic Press; New York: 1985. pp. 107–121. [Google Scholar]
- Schmidt NB, Lerew DR, Jackson RJ. The role of anxiety sensitivity in the pathogenesis of panic: Prospective evaluation of spontaneous panic attacks during acute stress. Journal of Abnormal Psychology. 1997;106:355–364. doi: 10.1037//0021-843x.106.3.355. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Lerew DR, Jackson RJ. Prospective evaluation of anxiety sensitivity in the pathogenesis of panic: Replication and extension. Journal of Abnormal Psychology. 1999;108:532–537. doi: 10.1037//0021-843x.108.3.532. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Zvolensky MJ, Maner JK. Anxiety sensitivity: Prospective prediction of panic attacks and Axis I pathology. Journal of Psychiatric Research. 2006;40:691–699. doi: 10.1016/j.jpsychires.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Silverman WK, Albano AM. Anxiety Disorders Interview Schedule (ADIS-IV): Child and Parent Versions. Physiological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Silverman WK, Fleisig W, Rabian B, Peterson RA. Childhood Anxiety Sensitivity Index. Journal of Clinical Child Psychology. 1991;20:162–168. doi: 10.1207/s15374424jccp2801_9. [DOI] [PubMed] [Google Scholar]
- Simons J, Correia CJ, Carey KB, Borsari BE. Validating a five-factor marijuana motives measure: Relations with use, problems, and alcohol motives. Journal of Counseling Psychology. 1998;45:265–273. [Google Scholar]
- Spielberger CD, Gorusch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Mind Garden; Palo Alto, CA: 1983. [Google Scholar]
- Results from the 2005 National Survey on Drug Use and Health: National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2006. [Google Scholar]
- Thomas H. A community survey of adverse effects of cannabis use. Drug and Alcohol Dependence. 1996;42:201–207. doi: 10.1016/s0376-8716(96)01277-x. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bernstein A, Sachs-Ericsson N, Schmidt NB, Buckner JD, Bonn-Miller MO. Lifetime associations between cannabis, use, abuse, and dependence and panic attacks in a representative sample. Journal of Psychiatric Research. 2006a;40:477–786. doi: 10.1016/j.jpsychires.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bonn-Miller MO, Bernstein A, McLeish AC, Feldner MT, Leen-Feldner EW. Anxiety sensitivity interacts with marijuana use in the prediction of anxiety symptoms and panic-related catastrophic thinking among daily tobacco users. Behaviour Research and Therapy. 2006b;44:907–924. doi: 10.1016/j.brat.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Eifert GH. A review of psychological factors/processes affecting anxious responding during voluntary hyperventilation and inhalations of carbon dioxide-enriched air. Clinical Psychology Review. 2000;21:375–400. doi: 10.1016/s0272-7358(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Leen-Feldner EW, Gibson LE, Abrams K, Gregor K. Acute nicotine withdrawal symptoms and anxious responding to bodily sensations: A test of incremental predictive validity among young adult regular smokers. Behaviour Research and Therapy. 2005;43:1683–1700. doi: 10.1016/j.brat.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lewinsohn PM, Bernstein A, Schmidt NB, Buckner JD, Seeley J, Bonn-Miller MO. Prospective associations between cannabis use, abuse, and dependence and panic attacks and disorder. Journal of Psychiatric Research. doi: 10.1016/j.jpsychires.2007.10.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]