Abstract
Objectives
To investigate the association of carotenoids and retinol (vitamin A) with intestinal barrier function in children in an urban community in Fortaleza, northeastern Brazil.
Methods
Descriptive analysis of serum carotenoids and retinol concentrations with intestinal barrier function in 102 children from an urban community, July 2000 to August 2001.
Results
The weight for height z score (wasting) showed that 19.6% (20/102) had mild malnutrition (–1 to –2 z score). All of the children's serum retinol concentrations were determined and none were severely deficient (≤0.35 μmol/L), 2.9% (3/102) were moderately (0.36–0.70 μmol/L) deficient, 20.6% (21/102) were mildly (0.71–1.05 μmol/L) deficient; 76.5% (78/102) were vitamin A sufficient (>1.05 μmol/L). The lactulose:mannitol (L/M) ratio was elevated (≥0.0864) in 49% (47/97) of children when compared with healthy children with normal L/M ratio (<0.0864) in the same geographic area. Serum carotenoids, lutein, β-cryptoxanthin and β-carotene showed significant inverse correlations with the L/M ratio, but not lutein after adjusting for age. Acute phase proteins (C-reactive protein and β-acid glycoprotein) were significantly inversely correlated with retinol but not with carotenoids. Retinol and retinol-binding protein were not significantly associated with L/M ratio.
Conclusions
These data suggest a disruption of intestinal barrier function in the paracellular pathway with low serum concentrations of carotenoids. Carotenoids may provide a better marker for disrupted intestinal barrier function than retinol-binding protein or retinol.
Keywords: Carotenoids, Intestinal barrier function, Retinol, Retinol-binding protein, Transthyretin
Malnutrition, including vitamin A deficiency (VAD) and infectious diseases, especially enteric and acute respiratory infections, are common health problems in developing countries (1–3). One of the important reasons for prolonged duration of an episode of diarrhea is believed to be a delay in the repair of the intestinal mucosa, which increases the risk for the vicious cycle of diarrhea and malnutrition. Vitamin A may play a role in maintaining the integrity of the epithelial cell lining of the gastrointestinal tract, as well as in immunological function (4–9). Gut integrity, as measured by the lactulose:mannitol ratio, in infants in developing areas is often abnormal and is closely linked to VAD and growth impairment (4,10). It has been shown that biochemical measurements of gut integrity were best at a time of the year when dietary vitamin A is most abundant. This suggests a direct effect of vitamin A on the gut cells, or that increased intake of vitamin A may have systemic effects on many systems that collectively bring about improved gut integrity as measured by the lactulose:mannitol ratio (5,11).
Vitamin A has a critical role in cellular differentiation, which is particularly important in growth and in maintaining epithelial integrity (4,5,10). VAD induces changes on epithelial integrity in numerous epithelial surfaces, including the eye, skin, trachea, salivary gland, vaginal epithelium, and gastrointestinal tract, suggesting a potentially important role for retinol and carotenoids in maintaining the integrity of intestinal barrier function. Thus, retinol and carotenoids also may help prevent bacterial transmigration and consequent complications such as bacteremia and sepsis, especially in children with malnutrition and living in highly endemic areas for malnutrition and diarrheal diseases.
Children with severe malnutrition and VAD have low serum retinol concentrations, and these significantly correlate with disruption of the intestinal barrier function measured by lactulose:mannitol ratio (5,10). Clinical nutritional interventions employing either retinol or carotenoids have reported improvements in abnormal intestinal barrier function in children with mild to moderate malnutrition (10,11). One intervention study carried out in India provided retinol to hospitalized infants (mean age 9 months) and in a community health center. All children who received retinol showed a significant improvement in gut integrity measured by lactulose:mannitol ratio (11). In Brazil, an intervention study using retinol in 2- to 97-month-old children also showed an improvement in gut integrity (ie, decreased lactulose:mannitol ratio) (10). In South Africa, children who become human immunodeficiency virus–infected from their infected mothers who took retinol and β-carotene supplements had less deterioration in gut integrity as measured by the lactulose:mannitol excretion ratio (12). Data on intestinal barrier function in vitro also have shown functional beneficial effects of retinol and carotenoids, suggesting that these nutrients coregulate gut integrity (13–15).
We hypothesize that serum carotenoids and retinol concentrations are associated with intestinal barrier function and that the serum retinol concentration is functionally correlated with serum retinol-binding protein (RBP), transthyretin (TTR), and acute phase response protein concentrations. The purpose of the present study was to assess the association between carotenoids and retinol with intestinal barrier function in children living in an area with a high prevalence of VAD, located in northeastern Brazil. In addition, we explored the relation among RBP, TTR, and the acute phase response proteins C-reactive protein (CRP) and α-acid glycoprotein (AGP).
PATIENTS AND METHODS
Ethics
The study protocol and informed consent were in accordance with the ethical standards of the responsible institutional committees on human investigation: the Federal University of Ceará, the University of Virginia, and the National Institutes of Health.
Geographic Location and Study Population
The study population is located in an urban community called Parque Universitário, in Fortaleza, Ceará, in northeastern Brazil. The urban community Parque Unversitário is about 5 km from the Clinical Research Unit and Institute of Biomedicine/Center for Global Health (www.upcibimed.ufc.br) laboratories in Fortaleza. Fortaleza has approximately 2.6 million inhabitants and the infant mortality rate is 35 deaths per 1000 births. The prevalence of VAD in Fortaleza is estimated to be 40% in children 0.5 to 4.9 years old (16).
The data from the 1998 census at Parque Universitário showed a total population of 3541 inhabitants with 27% (957) being children younger than 9 years old. Parents or guardians of children 2 months to 9 years old were invited to participate in the study protocol after they signed the consent form.
Study Design, Eligibility, and Exclusion Criteria for Enrollment of Subjects
This is a descriptive study in 102 children and the data analyzed in this article were collected from June 2000 to August 2001. The following eligibility criteria were used for enrollment of subjects: children from 2 months to 9 years old with height-for-age score less than median (–0.06) for the Parque Universitário community, residents of this urban community, and parental or guardian consent. Children were excluded if they were exclusively breast-fed, participants in any study in the past 2 years, or ill with fever >38°C at time of enrollment.
Enrollment of Children, Anthropometrics Measurements, and Sample Collection and Handling
A surveillance team (field nurse coordinator and 3 health care workers) visited the mothers for interviews after the responsible parent or guardian signed the consent form. A questionnaire was filled out with demographic data and illness information.
The field team also collected weight and height measurements using calibrated methods and standardized techniques from each child at the household visit to evaluate the nutritional status of each child. Children wearing light clothing were weighed using a calibrated digital scale (Solar Scale, Tanita, Arlington, IL) with a precision of 100 g. Supine (children younger than 24 months old) or standing (children 24 months old or older) height was recorded for all children using an anthropometric board with an accuracy of 0.1 cm.
Fresh stool samples were collected and brought to the laboratory in a cooler within 3 hours after collection, and a small amount of this sample was used for initial microbiology procedures to examine stool characteristics and lactoferrin, and for direct microscopic observation of parasites and stool concentration to identify ova for helminths and protozoa using standard protocols. A fecal lactoferrin measurement was performed using enzyme-linked immunoassay according to the instructions provided in the kit assay (IBD-Scan, Techlab, Blacksburg, VA).
Blood samples from each child participating in the study were collected early in the morning while the children were in the fasted state. The serum retinol (1.2285 ± 0.2769 vs 1.2727 ± 0.2814 μmol/L; P > 0.05), lutein (0.0949 ± 0.1388 vs 0.0380 ± 0.0863 μmol/L; P > 0.05), β-cryptaxanthin (0.0797 ± 0.0841 vs 0.0640 ± 0.1023 μmol/L; P > 0.05), and β-carotene (0.0128 ± 0.0291 vs 0.0117 ± 0.0267 μmol/L; P > 0.05) concentrations were not significantly different when samples collected in the dry versus rainy seasons were compared, even when controlling for acute phase response proteins. The samples were collected in a serum separator tube without anticoagulant and carried to the laboratory with proper precautions to prevent exposure to light. The samples were transported within 3 hours to the laboratories and frozen at –80°C until the time of analysis.
Serum Retinol, Carotenoids, RBP, and TTR Determinations
Plasma/serum levels of retinol and carotenoids were measured using high-performance liquid chromatography (HPLC) methods that allow for the simultaneous determination of plasma/serum retinol, β-carotene, α-carotene, lycopene, β-cryptaxanthin, lutein, and zeaxanthin (17,18). Retinyl acetate and echinenone were used as internal standards for assessing the recovery of the retinol and carotenoids, respectively. Dr H. Bagavahan (Hoffmann-LaRoche, Nutley, NJ) provided authentic standards of retinol, carotenoids and echinenone. The assay variability for assays performed on the same day is between 3% and 6%, and for assays performed on different days the variability is between 5% and 8%. On each day of analysis, a quality control serum standard containing known concentrations of retinol, α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin was processed along with the experimental samples. If any concentration of analyte determined for the quality control standard on a particular day was outside the range of the value ±2 standard deviations, then the entire day's analysis was repeated.
To measure serum/plasma levels of RBP and TTR, we have made use of specific and sensitive radioimmunoassay procedures that we have described previously (19). As standards in the radioimmunoassays, we use either homogeneously purified human serum RBP or homogeneously purified serum TTR. Within assay, coefficients of variation for the 2 radioimmunoassays are approximately 4%, and between-assay coefficients of variation are 5% for TTR and 6% for RBP.
Acute Phase Protein Determinations
CRP and AGP were measured in serum samples by radial immunodiffusion procedure, a technique that is routinely used for measuring the concentrations of various soluble antigens (usually proteins) in biological fluids (20–22) (human CRP “LL” NANORID and AGP BINDARID, obtained from The Binding Site, Birmingham, UK). Reference limits used to indicate a positive acute phase response were those suggested by the manufacturer (ie, > 8 mg/L for CPR and > 1000 mg/L for AGP).
Intestinal Permeability Test
The lactulose:mannitol test was done as we have previously reported (23–25), and it is considered a consistent and sensitive method to measure small intestinal epithelial area, paracellular and transcellular transport, damage, and permeability. Briefly, fasted children ingested a solution containing lactulose (250 mg/mL; Lactulona, Luitpold Produtos Farmacêuticos Ltda Barueri, São Paulo, Spain) and mannitol (50 mg/mL) in a dose of 2 mL/kg of weight or a maximum 20 mL. The children were allowed to return to their regular diet 1 hour after the initiation of the lactulose:mannitol test. The children were asked to empty their bladders whenever possible before ingesting the test sugar solution, followed by the total urine collection for 5 hours in appropriate flasks containing 1 mL of clorhexidine (40 mg/mL; Sigma Chemical, St Louis, MO). Total urine volume was measured and a sample of 5 mL was saved in a –80°C freezer, until used to measure lactulose and mannitol. Lactulose and mannitol concentrations were determined by HPLC using pulsed amperometric detection (25). A total of 50 μL of each urine sample was diluted with 50 μL of mellibiose solution (3.6 mmol/L) and completed with distilled and deionized water up to 2.9 mL. All of the samples were filtered (0.22 μm) and 50 μL from each sample was automatically injected (AS40 Automated Sampler, Dionex, Sunnyvale, CA) into the HPLC column system. The quantification of lactulose, mellibiose (internal standard control), and mannitol was evaluated using the GP40 Gradient Pump and MA1 CarboPac column from Dionex. A pulsed amperometric detector (ED40 Electrochemical Detector) from Dionex was used to determine the amount of each sugar. The integration and quantification of the isolated peaks in the chromatograms were completed using the Peak Net software program (also from Dionex). The calculation of the final percentage of urinary sugar excretion was done using the Excel software program (Microsoft, Redmond, WA). The lactulose:mannitol permeability test was considered abnormal or positive for comparison purposes if the lactulose:mannitol ratio was ≥0.0864. These values are equal to normal mean plus 2 standard deviations, as previously reported (25).
Statistical Analysis
The data were collected and entered twice by different people and were validated using the Access software program (Microsoft). EpiInfo and Nutstat software programs (version 6.0 from Centers for Disease Control and Prevention, Atlanta, GA) were used to calculate the anthropometric parameters. Statistical analysis was performed using Statistical Package for Social Sciences (version 11.5 from SPSS, Chicago, IL). P < 0.05 were considered statistically significant.
The percent of lactulose and mannitol urinary excretion and lactulose:mannitol ratio were calculated using the Excel software program (Microsoft). Lactulose:mannitol ratio data are presented as mean and standard deviation (SD). The best to fit function analyses were used to determine the correlation coefficients of the intestinal permeability test to retinol or carotenoids and the linearity model using logarithm transformed from the best to fit function equation were used for statistical analysis on each correlation.
The retinol-RBP, retinol-TTR, retinol-CRP, and retinol-AGP relations were analyzed using the best to fit function analyses. Serum retinol concentrations between children undergoing an acute phase response or not undergoing an acute phase response were compared by using Student 2-tailed t test.
RESULTS
Table 1 shows the distribution of children from the urban community Parque Universitário in Fortaleza by age, sex, and degree of malnutrition. The median age of the children was 41 months (range 5–110 months); 49% (50/102) of the children were male and 51% (52/102) female. The weight-for-height (wasting), weight-for-age, and height-for-age (stunting) z scores in these children showed that none, 8.8%, and 18.6% were considered malnourished (conventional cutoff < –2 z scores), respectively.
TABLE 1.
Demographic characteristics of children from Parque Universitário in Fortaleza, Ceará, Brazil
| Characteristics | Baseline data | ||
|---|---|---|---|
| Age, mo | |||
| Median (range) | 41 (5–110) | ||
| Sex | |||
| Male (%) | 50 (49) | ||
| Anthropometric parameters* |
|||
| Degree of malnutrition† | Weight-for-height | Weight-for-age | Height-for-age |
| Normal (≥–1) | 82 (80.4) | 51 (50.0) | 35 (34.3) |
| Mild (–2 to –1) | 20 (19.6) | 42 (41.2) | 48 (47.1) |
| Moderate (–3 to –2) | – | 9 (8.8) | 16 (15.7) |
| Severe (<–3) | – | – | 3 (2.9) |
| Degree of VAD, μmol/L‡ | No. of children (%) |
|
|
| Sufficient (>1.05) | 78 (76.5) | ||
| Mild (0.70–1.05) | 21 (20.6) | ||
| Moderate (0.35–0.70) | 3 (2.9) | ||
| Lactulose:mannitol ratio§ | No. of children (%) |
|
|
| Abnormal (≥0.0864) | 47 (48.5) | ||
Stratified by age, sex, nutritional status, vitamin A deficiency, and intestinal permeability test.
Number of children and percentage on each anthropometric parameter stratified by degree of malnutrition.
Degree of malnutrition measured by z score intervals.
Degree of vitamin A deficiency (VAD) by serum retinol concentration (μmol/L) intervals.
The lactulose:mannitol ratio was considered abnormal for comparison purposes if the ratio was ≥0.0864. This value equal to normal mean + 2 SD (26).
The prevalence of vitamin A deficiency on the basis of serum retinol for a total of 102 children is summarized in Table 1. Twenty-four percent (24/102) of children showed insufficient serum vitamin A concentrations (retinol ≤1.05 μmol/L), although only 3% (3/102) were moderately vitamin A deficient and none were severely vitamin A deficient. We analyzed a higher cutoff for retinol (retinol ≤1.05 μmol/L) to indicate mild vitamin A deficiency. At this serum retinol concentration, the children are at increased risk for morbidity and mortality even though they will show no overt clinical evidence for VAD. The intestinal permeability test was abnormal (≥0.0864) in 49% (47/97) of the total children tested (Table 1).
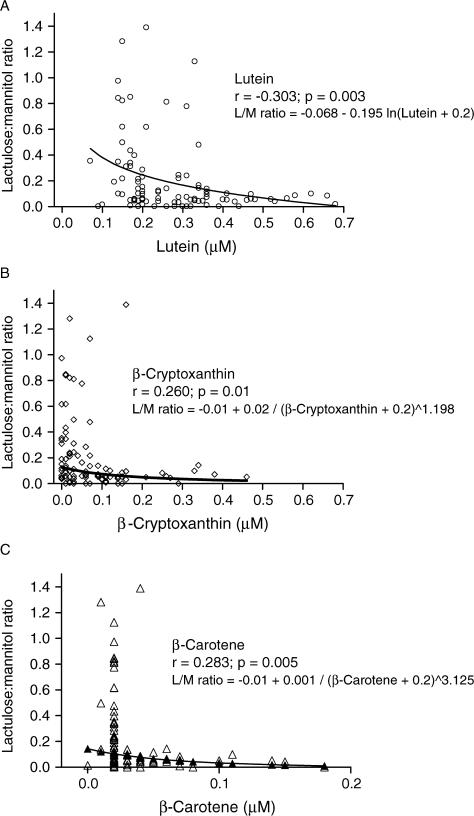
We explored possible relations between serum vitamin A concentrations and gut integrity in children from Parque Universitário in Fortaleza as assessed by the lactulose:mannitol test. We found no significant correlation between retinol concentration with mannitol (r = 0.132, P = 0.196), lactulose (r = 0.035, P = 0.727), or lactulose:mannitol ratio (r = 0.046, P = 0.652). However, when we analyzed the concentrations of common dietary carotenoids (lutein, β-cryptoxanthin, lycopene, β-carotene, and α-carotene) in all serum samples collected from the children of this study by HPLC, we found significant correlations between lactulose:mannitol ratio and lutein (r = –0.303, P = 0.003, n = 97), β-cryptoxanthin (r = 0.260, P = 0.01, n = 97), and β-carotene (r = 0.283, P = 0.005, n = 97), as shown in Figure 1. In addition, we observed significant correlations between lutein (r = –0.210, P = 0.038) and β-cryptoxanthin (r = 0.233, P = 0.022), but not β-carotene (r = 0.173, P = 0.091), with lactulose, but not with mannitol urinary excretion. CRP and AGP did not correlate significantly with lutein (r = 0.071 and 0.0142, P > 0.05), β-cryptoxanthin (r = –0.0958 and –0.0111, P > 0.05), and β-carotene (r = –0.1113 and –0.0304, P > 0.05).
FIG. 1.
Scatter diagram relates (A) serum lutein concentration to urinary lactulose:mannitol (L/M) ratio for Brazilian children; (B) serum β-cryptoxanthin concentration to urinary L/M ratio for Brazilian children; and (C) serum β-carotene concentration to urinary L/M ratio for Brazilian children. Urine L/M ratio reflects percentage of each dose excreted in urine. The line represents the best fit of the data. Negative values on β-carotene serum concentrations means values lower than the level determined by standard values.
To avoid the influence of ill children in the correlations analysis, the children with acute phase reactions—ie, with a recent or presenting infection as defined by CRP >8 mg/L and/or AGP >1000 mg/L—were excluded and we further adjusted for age and seasonal variation in subsequent analyses. The statistical analyses were done using the linearity model using logarithm transformations on the best to fit function equations evaluated for each correlation. β-Cryptoxanthin (r = –0.221, P = 0.0481, n = 82) and β-carotene (r = –0.2325, P = 0.0380, N=82) were significantly inversely correlated with lactulose:mannitol ratio. However, after correcting for age, lutein (r = –0.1676, P = 0.137, n = 82) was not significantly correlated with lactulose:mannitol ratio. When these children were adjusted only for season the correlation of lutein with lactulose:mannitol ratio was significant (r = –0.2779, P = 0.012, n = 82), but it was not significantly different when adjusted only for age (r = –0.1629, P = 0.146, n = 82). The last result suggested an age influence on lutein serum concentrations’ association with intestinal epithelial integrity. In fact, there was a statistically significant correlation of serum lutein concentrations with age in these children (r = 0.4420, P < 0.0001, n = 82), but not with β-cryptoxanthin or β-carotene serum concentrations (r = 0.1352, P = 0.2147, n = 82; and r = 0.0.1744, P = 0.1083, n = 82, respectively).
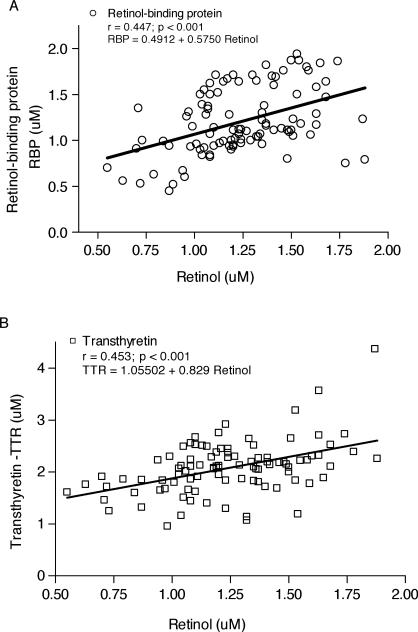
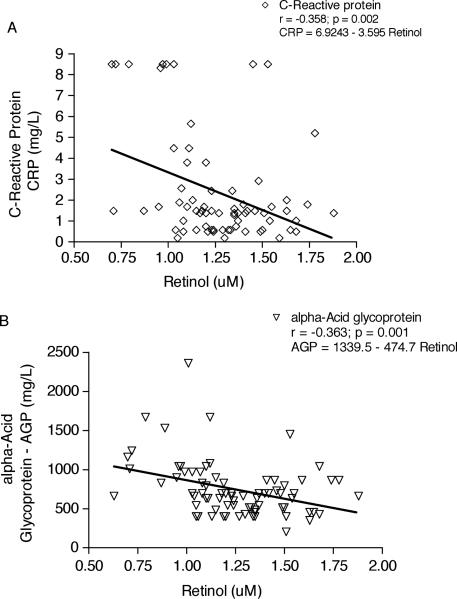
The relation between RBP and retinol in serum of the 99 children from Parque Universitário is illustrated in Figure 2. The line in Figure 2A represents the best fit between retinol and RBP. Retinol and RBP have a linear correlation (r = 0.447). Serum retinol concentrations also correlate with serum TTR (r = 0.4530) as shown in Figure 2B. Moreover, serum retinol showed an inverse correlation with CRP (r = –0.3583) and AGP (r = –0.3627), as can be seen in Figure 3A and 3B, respectively. RBP also was inversely correlated with AGP (P < 0.05; data not shown). RBP did not correlate significantly with TTR (r = 0.1003, P = 0.3375).
FIG. 2.
Regression analysis of serum retinol (A) vs serum retinol binding protein (RBP) and (B) vs serum transthyretin (TTR). The line represents the line of best fit of the data. The root mean square error of the residuals of retinol around the best fit line was 0.447 (P < 0.001, n = 99) and 0.453 (P < 0.001, n = 96) for RBP and TTR, respectively.
FIG. 3.
Regression analysis of serum retinol (A) vs serum C-reactive protein (CRP) and (B) vs serum α1-acid glycoprotein (AGP). The line represents the line of best fit of the data. The root mean square error of the residuals of retinol around the best fit line was –0.358 (P = 0.002, n = 72) and –0.363 (P = 0.001, n = 80) for CRP and AGP, respectively.
Because the concentration of retinol in the circulation is known to be influenced by infection, retinol was compared in children experiencing (acute phase positive) or not experiencing (acute phase negative) infection. CRP was elevated (>8 mg/L) in 9 of the 72 children and AGP was elevated (>1000 mg/L) in 13 of the 80 children studied.
To assess serum RBP concentrations as a surrogate measure for serum retinol, we calculated a cutoff of 1.003 μmol/L RBP from a receiver operating characteristic statistical analysis as described by Blaner (19) that corresponds to 1.05 μmol/L retinol in the present study. On the basis of a cutoff of 1.003 μmol/L RBP and serum retinol concentration ≤1.05 μmol/L, the RBP serum measurement has a 70.8% sensitivity and an 80.0% specificity.
On the basis of a cutoff of 1.931 μmol/L TTR and serum retinol concentration ≤1.05 μmol/L, the TTR serum measurement has a 73.9% sensitivity and 74.0% specificity.
Twelve (13%) out of 93 stool samples were liquid and 40% (37/93) of the samples were positive for lactoferrin. White blood cells were positive in 1% of these samples. Ninety samples were concentrated and examined for parasitic infections. Twenty seven samples (30%) were positive for at least 1 parasite. The most frequent parasitic infections found were as follow: Ascaris lumbricoides, 19 (21%); Trichuris trichiura, 9 (10%); Entamoeba coli, 4 (4%); Giardia lamblia, 3 (3%); Hymenolepis diminuta, 1 (1%); and E nana, 1 (1%).
Parasitic infections were not significantly associated with seasonality, age, acute phase reaction, or lactoferrin (P > 0.05). The lactulose:mannitol ratio or serum carotenoids (lutein, β- cryptoxanthin, or β-carotene) concentrations were not significantly associated with these parasitic infections in the study children. Although acute phase reaction proteins were not associated with parasitic infections, serum retinol concentrations were significantly lower in the group with parasitic infections (1.110 ± 0.2417 vs 1.292 ± 0.2639 μmol/L; N = 90; P = 0.003). Because of this last result, further analyses were done to see whether serum retinol from those children with and without parasitic infections were associated with differences in the lactulose:mannitol findings, and the result showed no significant association (P > 0.05).
DISCUSSION
Nutrition interventions can save the lives of up to 2.5 million children each year worldwide. Vitamin A supplementation can also prevent approximately 500,000 children from irreversible blindness associated with VAD (16,26,27). Although the association of VAD with disrupted intestinal barrier function, reproductive function, and immunological function was poorly recognized, improved methods to evaluate subclinical and prepathological marginal VAD are now possible. This study was conducted primarily to examine the association of carotenoids and retinol with intestinal barrier function in children from an urban community, Parque Universitário, located in northeastern Brazil, where VAD is common.
VAD is an important problem in more than 60 countries, especially in Asia, Africa, and Latin America, where the prevalence rate is considered high (16,28). The World Health Organization lists Brazil as having a high rate of subclinical VAD (15). In northeastern Brazil, the prevalence rate for VAD using serum retinol concentration was 49% (maximum accepted is 20%) for children 2 to 8 years old (16,29). In 1991, the prevalence rate in Fortaleza was 40% for children 0.5 to 4.9 years old (15). In the present study, we found a prevalence of 24% of at least mild VAD in children 0.4 to 9 years old.
To explore possible roles for carotenoids and retinol in maintaining gut integrity of the children from Parque Universitário in Fortaleza, we used the lactulose:mannitol test. This test provides a noninvasive method to assess impaired intestinal barrier function. On the basis of our previous findings, we expected that children from Parque Universitário with mild or moderate vitamin A deficiency would show an inverse correlation of serum retinol concentration with lactulose:mannitol ratio, suggesting an impaired intestinal barrier function (5). However, the effects of vitamin A deficiency on intestinal permeability are likely to be subtle in a population with mild VAD, which may likely explain why there was no significant correlation between retinol and intestinal permeability in the children from Parque Universitário. What is new for us is the inverse correlation of carotenoids with lactulose:mannitol ratio, which suggests that the low concentration of carotenoids (precursors of retinol) may be a more sensitive predictor of impaired intestinal barrier function. These inverse correlations of carotenoids (especially with lutein) were further seen with the urinary excretion of lactulose but not with mannitol. These data suggest that the disruption in the paracellular pathway is the most affected. Because this is a cross-sectional study, additional data will be necessary to better define the causality of carotenoid effects on intestinal barrier function. As shown in the analyses in which age, season, and ill children were adjusted, and controlled for any influence on carotenoids associated with intestinal integrity, lutein seems to be dependent on age. This result deserves further study into the biology behind the mechanism by which lutein is modulated in the intestinal epithelium in children in the developmental age window in this study.
Retinol and carotenoids are essential for normal cell growth, differentiation, and maintenance of epithelial tissues (30,31). Retinoids and retinol regulate expression of cell cycle proteins and intestinal brush border enzymes involved in the digestive process (7,30). In addition, retinol is associated with tight junctions between cells and adherence junction proteins (31). Provitamin A carotenoids, such as α- and β-carotene, are precursors of retinol and all carotenoids are known to function as potent and effective scavengers of free oxygen radicals (31,32). Recent findings have showed that carotenoids such as lutein improved intestinal barrier function induced by a lipid peroxidation substance called tertiary-butylhydroperoxide (33).
Vitamin A and carotenoid deficiencies in children with and without malnutrition can play a major role in intestinal immunity and infection, which can influence gut integrity. Recent reports have shown that retinol plays a key role in gut-associated lymphoid tissue dendritic cell promotion of T regulatory cells in the lamina propria and intestinal epithelium (34,35). Data from an intervention study showed that vitamin A supplementation has been associated with reducing intestinal enteropathogenic Escherichia coli infection (36), and preliminary data from the intervention study with vitamin A that followed this study showed a significant decrease in the prevalence of total intestinal parasitic or Giardia spp infection (unpublished data). These data suggest that gut integrity impairments associated with vitamin A and carotenoid deficiencies also could reflect the indirect effect of a higher prevalence of intestinal infections in children with vitamin A and carotenoid deficiencies. These issues are being addressed in detail in analyses of the vitamin A intervention study that will follow the current descriptive data. The present study agrees with earlier published demonstrations that serum retinol concentration decreases in conjunction with elevations of acute phase serum proteins (37), which are frequently associated with infectious diseases such as respiratory and enteric infections that are highly prevalent in these children in this impoverished community in northeastern Brazil (38,39). This could significantly influence the marginal VAD correlation with the intestinal barrier function as previously shown with severe VAD in children from the same area (5).
In contrast to retinol, carotenoids do not vary with acute phase serum protein elevations, and they are more stable even at serum concentrations with marginal VAD. This likely helps explain the association of carotenoids with intestinal barrier function described in this article. These findings and other reports postulate that carotenoids ameliorate disrupted intestinal barrier function, via scavenging free oxygen radicals or via maintenance of tight junctions and adherence junction proteins.
In conclusion, we find an increased intestinal permeability in 50% of children in a poor urban area (and presumably some degree of malabsorption and an enteropathy), with mild VAD in 25% and an association of impaired intestinal permeability with low serum carotenoids. Although serum retinol concentration did not predict disrupted intestinal barrier function, serum carotenoids concentrations (lutein, β-cryptoxanthin, and β-carotene, which are precursors of retinol) did correlate inversely with disrupted intestinal barrier function. In addition, serum retinol was correlated with RBP and TTR and it was inversely correlated with AGP and CRP. Hence, the retinol precursor carotenoids may provide a better marker for disrupted intestinal barrier function than measurements of RBP or retinol itself.
Acknowledgments
Supported, in part, by Conselho Nacional de Desenvolvimento Científico e Tecnológico and National Institutes of Health International Collaborations in Infectious Disease Research grant 5 U01 AI026512.
M.M.V., J.P., and W.S.B. were responsible for the retinol, carotenoids, retinol-binding protein, transthyretin, and acute phase proteins serum concentration measurements. A.M.S. and R.M.S.M. were responsible for the data management, safety, and analysis. R.L.G. and A.A.M.L. were responsible for the study design, collection and validation of the data, lactulose and mannitol measurements, provision of advice, and consultation. All of the authors participated in the writing of the manuscript. M.M.V. was a postdoctoral fellow at Columbia University under adviser W.S.B. and a doctoral student at Federal University of Ceará. All of the authors had no financial or personal relations with the organization sponsoring the research at the time that the research was conducted.
REFERENCES
- 1.Nalin DR, Russel R. Vitamin A, xerophthalmia, and diarrhea. Lancet. 1980;2:1411. doi: 10.1016/s0140-6736(80)92670-7. [DOI] [PubMed] [Google Scholar]
- 2.Grotto I, Mimouni M, Gdalevich M, et al. Vitamin A supplementation and childhood morbidity from diarrhea and respiratory infections: a meta-analysis. J Pediatr. 2003;142:297–304. doi: 10.1067/mpd.2003.116. [DOI] [PubMed] [Google Scholar]
- 3.Long KZ, Montoya Y, Hertzmark E, et al. A double-blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. Am J Clin Nutr. 2006;83:693–700. doi: 10.1093/ajcn.83.3.693. [DOI] [PubMed] [Google Scholar]
- 4.Dewan V, Patwari AK, Jain M, et al. A randomized controlled trial of vitamin A supplementation in acute diarrhea. Indian Pediatr. 1995;32:21–5. [PubMed] [Google Scholar]
- 5.Quadro L, Gamble MV, Vogel S, et al. Retinol and retinol-binding protein: gut integrity and circulating immunoglobulins. J Infect Dis. 2000;182(Suppl 1):S97–102. doi: 10.1086/315920. [DOI] [PubMed] [Google Scholar]
- 6.Zile M, Bunge C, Deluca HF. Effect of vitamin A deficiency on intestinal cell proliferation in the rat. J Nutr. 1977;107:552–60. doi: 10.1093/jn/107.4.552. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler TR, Evan ME, Fernandez-Estivariz C, et al. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–31. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 8.Long KZ, Estrada-Garcia T, Rosado JL, et al. The effect of vitamin A supplementation on the intestinal immune response in Mexican children is modified by pathogen infections and diarrhea. J Nutr. 2006;136:1365–70. doi: 10.1093/jn/136.5.1365. [DOI] [PubMed] [Google Scholar]
- 9.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J Exp Med. 2007;8:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Soares AM, Lima AA, et al. Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. J Health Popul Nutr. 2003;21:309–15. [PubMed] [Google Scholar]
- 11.Thurnham DI, Northrop-Clewes CA, McCullough FSW, et al. Innate immunity, gut integrity, and vitamin A in Gambian and Indian infants. J Infect Dis. 2000;182(Suppl 1):S23–8. doi: 10.1086/315912. [DOI] [PubMed] [Google Scholar]
- 12.Filteau SM, Rollins NC, Coutsoudis A, et al. The effect of antenatal vitamin A and β-carotene supplementation on gut integrity of infants of HIV-infected South African women. J Pediatr Gastroenterol Nutr. 2001;32:464–70. doi: 10.1097/00005176-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Briviba K, Schnabele K, Schwertle E, et al. Beta-carotene inhibits growth of human colon carcinoma cells in vitro by induction of apoptosis. Biol Chem. 2001;382:1663–8. doi: 10.1515/BC.2001.201. [DOI] [PubMed] [Google Scholar]
- 14.Swartz-Basile DA, Wang L, Tang Y, et al. Vitamin A deficiency inhibits intestinal adaptation by modulating apoptosis, proliferation, and enterocyte migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G424–32. doi: 10.1152/ajpgi.00524.2002. [DOI] [PubMed] [Google Scholar]
- 15.Nagira M, Tomita M, Mizuno S, et al. Ischemia/reperfusion injury in the monolayers of human intestinal epithelial cell line caco-2 and its recovery by antioxidants. Drug Metab Pharmacokinet. 2006;21:230–7. doi: 10.2133/dmpk.21.230. [DOI] [PubMed] [Google Scholar]
- 16.Ramalho RA, Flores H, Saunders C. Hypovitaminosis A in Brazil: a public health problem. Rev Panam Salud Publica. 2002;12:117–22. doi: 10.1590/s1020-49892002000800007. [DOI] [PubMed] [Google Scholar]
- 17.Mills JL, Tuomilehto J, Yu KF, et al. Maternal vitamin levels during pregnancies producing infants with neural tube defects. J Pediatrics. 1992;120:863–71. doi: 10.1016/s0022-3476(05)81951-1. [DOI] [PubMed] [Google Scholar]
- 18.Redlich CA, Grauer JN, van Bennekum AM, et al. Characterization of carotenoid, vitamin A, and α-tocopherol levels in human lung tissue and pulmonary macrophages. Am J Res Crit Care Med. 1996;154:1436–43. doi: 10.1164/ajrccm.154.5.8912761. [DOI] [PubMed] [Google Scholar]
- 19.Blaner WS. Radioimmunoassays for retinol-binding protein, cellular retinol-binding protein, and cellular retinoic acid-binding protein. Meth Enzymol. 1990;189:270–1. doi: 10.1016/0076-6879(90)89298-v. [DOI] [PubMed] [Google Scholar]
- 20.Mancini G, Vaerman JP, Carbonara AO, et al. A single radial diffusion method for the immunological quantitation of proteins. In: Peeters H, editor. Protides of the Biological Fluids (XI Colloquium) Elsevier; Amsterdam: 1964. pp. 370–3. [Google Scholar]
- 21.Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965;2:235–54. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- 22.Fahey JL, McKelvey EM. Quantitative determination of serum immunoglobulins in antibody-agar plates. J Immunol. 1965;94:84–90. [PubMed] [Google Scholar]
- 23.Bao Y, Silva TMJ, Guerrant RL, et al. Direct analysis of mannitol, lactulose, and glucose in urine samples by high-performance anion-exchange chromatography with pulse amperometric detection. Clinical evaluation of intestinal permeability in human immunodeficiency virus infection. J Chromatogr B Biomed Appl. 1996;685:105–12. doi: 10.1016/0378-4347(96)00159-4. [DOI] [PubMed] [Google Scholar]
- 24.Lima AA, Silva TM, Gifoni AM, et al. Mucosal injury and disruption of intestinal barrier function in HIV-infected individuals with and without diarrhea and cryptosporidiosis in northeast Brazil. Am J Gastroenterol. 1997;92:1861–6. [PubMed] [Google Scholar]
- 25.Barboza Junior MS, Silva TM, Guerrant RL, et al. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz J Med Biol Res. 1999;32:1499–504. doi: 10.1590/s0100-879x1999001200008. [DOI] [PubMed] [Google Scholar]
- 26.Organización Mundial de la Salud . Prevención y tratamiento de la carencia de vitamina A y de la xeroftalmia. Organización Mundial de la Salud; Geneva: 1982. (Série Informes Técnicos, 672) [Google Scholar]
- 27.Vitamin A Field Support Project . Tercer taller regional sobre dediciencias de vitamina A y otros micronutrients en América Latina y el Caribe (Recife, Brasil. US Agency for International Development; Arlington, VA: 1993. [Google Scholar]
- 28.Mora JO, Gueri M, Mora OL. Vitamin A deficiency in Latin America and the Caribbean: an overview. Rev Panam Salud Publica. 1998;4:178–86. doi: 10.1590/s1020-49891998000900005. [DOI] [PubMed] [Google Scholar]
- 29.Zaiger G, Nur T, Barshack I, et al. Vitamin A exerts its activity at the transcriptional level in the small intestine. Eur J Nutr. 2004;43:259–66. doi: 10.1007/s00394-004-0466-2. [DOI] [PubMed] [Google Scholar]
- 30.Park CK, Ishimi Y, Ohmura M, et al. Vitamin A and carotenoids stimulate differentiation of mouse osteoblastic cells. J Nutr Sci Vitaminol (Tokyo) 1997;43:281–6. doi: 10.3177/jnsv.43.281. [DOI] [PubMed] [Google Scholar]
- 31.Baltes S, Nau H, Lampen A. All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev Growth Differ. 2004;46:503–4. doi: 10.1111/j.1440-169x.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- 32.Khachik F, Beecher GR, Smith JC., Jr Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem Suppl. 1995;22:236–46. doi: 10.1002/jcb.240590830. [DOI] [PubMed] [Google Scholar]
- 33.Nagira M, Tomita M, Mizuno S, et al. Ischemia/reperfusion injury in the monolayers of human intestinal epithelial cell line caco-2 and its recovery by antioxidants. Drug Metab Pharmacokinet. 2006;21:230–7. doi: 10.2133/dmpk.21.230. [DOI] [PubMed] [Google Scholar]
- 34.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoid acid–dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long KZ, Estrada-Garcia T, Rosado JL, et al. The effect of vitamin A supplementation on the intestinal immune response in Mexican children is modified by pathogen infections and diarrhea. J Nutr. 2006;136:1365–70. doi: 10.1093/jn/136.5.1365. [DOI] [PubMed] [Google Scholar]
- 37.Thurnham DI, McCabe GP, Northrop-Clewes CA, et al. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet. 2003;362:2052–8. doi: 10.1016/s0140-6736(03)15099-4. [DOI] [PubMed] [Google Scholar]
- 38.Lima AA, Moore SR, Barboza MS, Jr, et al. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J Infect Dis. 2000;181:1643–51. doi: 10.1086/315423. [DOI] [PubMed] [Google Scholar]
- 39.Castro MX, Soares AM, Fonseca W, et al. Common infectious diseases and skin test anergy in children from an urban slum in northeast Brazil. Braz J Infect Dis. 2003;7:387–94. doi: 10.1590/s1413-86702003000600006. [DOI] [PubMed] [Google Scholar]