Abstract
We aimed to test if stimulation of both adenosine A2A and A2B receptors is required to produce an effective cardioprotection against reperfusion injury. Isolated rat hearts were subjected to 30 min regional ischemia followed by 2 h of reperfusion. The adenosine A1/A2 receptor agonist 5′-(N-ethylcarboxamido) adenosine (NECA) given at reperfusion reduced infarct size, an effect that was reversed by both the adenosine A2A antagonist SCH58261 and the A2B antagonist MRS1706. The A2B agonist BAY 60-6583 but not the selective A2A agonist CGS21680 reduced infarct size. Interestingly, a combination of BAY 60-6583 and CGS21680 further reduced infarct size. These results suggest that both A2A and A2B receptors are involved in NECA’s anti-infarct effect at reperfusion. NECA attenuated mitochondrial swelling upon reperfusion and this was blocked by both SCH58261 and MRS1706, indicating that activation of A2 receptors with NECA can modulate reperfusion-induced mitochondrial permeability transition pore (mPTP) opening. In support, NECA also prevented oxidant-induced loss of mitochondrial membrane potential (ΔΨm) and matrix Ca2+ overload in cardiomyocytes via both the A2 receptors. In addition, NECA increased mitochondrial glycogen synthase kinase 3β (GSK-3β) phosphorylation upon reperfusion and this was again blocked by SCH58261 and MRS1706. In conclusion, A2A and A2B receptors work in concert to prevent reperfusion injury in rat hearts treated with NECA. NECA may protect the heart by modulating the mPTP opening through inactivating mitochondrial GSK-3β. A simultaneous stimulation of A2A and A2B receptors at reperfusion is required to produce a strong cardioprotection against reperfusion injury.
Keywords: NECA, reperfusion injury, adenosine A2 receptors, mPTP, GSK-3β
Introduction
Cardioprotection against myocardial infarction has been successfully acquired experimentally with ischemic preconditioning and numerous chemicals. Because the protective effects of these interventions are established when applied prior to the onset of index ischemia, it is not possible to apply the interventions in patients with acute myocardial infarction (AMI). However, recent studies have proposed that adenosine analogues selectively targeting A2 receptors are promising to protect the heart at reperfusion. There are two adenosine A2 receptor subtypes (A2A and A2B). Activation of A2A receptors at reperfusion with the selective agonist CGS21680 reduced infarct size in rabbit and dog hearts [1, 2]. The protective effect of A2A activation at reperfusion was also demonstrated in other experimental models [3-5]. Recently it has been reported that postconditioning protects the reperfused heart by activating A2A receptors [6]. In contrast to these reports, the effectiveness of CGS21680 at reperfusion was questioned by other studies [7, 8].
Compared to the active investigation on the role of A2A receptor in cardioprotection at reperfusion, the role of A2B receptor had largely been unexplored until recently when Philipp et al. reported that postconditioning protects the heart by activating A2B receptors [9]. It was also reported that BAY 60-6583, a highly selective A2B receptor agonist, limited infarct size in rabbit hearts when applied at reperfusion [10]. A recent study further demonstrated that the infarct-limiting effect of PKG activator at reperfusion is attributed to activation of A2B receptors [11]. Thus, based on these observations it is possible that both A2A and A2B receptors are important to prevent reperfusion injury. A previous study [7] investigating the cardioprotective effects of AMP579, a novel A1/A2 receptor agonist, on reperfusion injury further support the importance of both A2A and A2B receptors in prevention of reperfusion injury. In that study, the protective effect of AMP579 was blocked by the selective A2A antagonist 8-(3-chlorostyryl)caffeine (CSC) but not by the A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), implying that A2A receptors but not A1 receptors were responsible for the action of AMP579 . Interestingly, the protective effect of AMP579 at reperfusion mimicked by another A1/A2 receptor agonist 5′-(N-ethylcarboxamido) adenosine (NECA) but not by the selective A2A receptor agonist CGS21680, indicating that AMP579’s cardioprotection at reperfusion required some other drug effect or other receptor subtype working in concert with A2A receptors. NECA has been consistently shown to protect the heart at reperfusion [10, 12, 13] and Ki values of NECA for A2A and A2B receptors are 20 and 2400 nM, respectively, whereas CGS21680 has Ki values for A2A and A2B receptors of 27 and 88800 nM [14]. Obviously, NECA has a much higher affinity for A2B receptors (37-fold higher) than CGS21680, although their Ki values for A2A are similar. Because no selective agonist for A2B receptors was available, NECA had been the most potent A2B agonist [15] until recently when Bayer Healthcare developed the selective A2B antagonist BAY 60-6583 [10, 16]. Thus, it is highly likely that A2A and A2B receptors work in concert to confer protection in the hearts treated with NECA at reperfusion. Here, we would hypothesize that a simultaneous stimulation of A2A and A2B receptors might confer a strong and consistent protection.
Materials and Methods
This study conforms to the NIH Guide for the Care and Use of Laboratory Animals (NIH publication NO. 85-23, revised 1996).
Chemicals
NECA, SCH58261, MRS1706, CGS21680, and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) were purchased from Tocris Bioscience (Ellisville, MO). BAY 60-6580 was obtained from Bayer Healthcare in Germany. All antibodies were purchased from Cell Signaling (Beverly, CA). Fluorescence dyes were obtained from Molecular Probes (Eugene, OR).
Perfusion of isolated rat hearts
Male Wistar rats (250-350 g) were anesthetized with thiobutabarbital sodium (100 mg/kg i.p.). The hearts were removed rapidly and mounted on a Langendorff apparatus. The hearts were perfused with Krebs-Henseleit buffer containing (in mM) 118.5 NaCl, 4.7 KCl, 1.2 MgSO4, 1.8 CaCl2, 24.8 NaHCO3, 1.2 KH2PO4, and 10 glucose, which was heated to 37°C and gassed with 95 % O2/5 % CO2. A latex balloon connected to a pressure transducer was inserted into the left ventricle through the left atrium. The left ventricular pressure and heart rate were continuously recorded with a PowerLab system (ADInstruments, Mountain View, CA). A 4-0 silk suture was placed around the left coronary artery, and the ends of the suture were passed through a small piece of soft vinyl tubing to form a snare. All hearts were allowed to stabilize for at least 20 min. Ischemia was induced by pulling the snare and then fixing it by clamping the tubing with a small hemostat. Total coronary artery flow was measured by timed collection of the perfusate dripping from the heart into a graduated cylinder.
Measurement of infarct size
At the end of the experiments, the coronary artery was reoccluded, and fluorescent polymer microspheres (2-9 μM diameter, Duke Scientific Corp) were infused to demarcate the risk zone as the tissue without fluorescence. The hearts were weighed, frozen and cut into 1 mm slices. The slices were incubated in 1 % triphenyltetrazolium chloride (TTC) in sodium phosphate buffer at 37°C for 20 min. The slices were immersed in 10 % formalin to enhance the contrast between stained (viable) and unstained (necrotic) tissue and then squeezed between glass plates spaced exactly 1 mm apart. The myocardium at risk was identified by illuminating the slices with U.V. light. The infarcted and risk zone regions were traced on a clear acetate sheet and quantified with ImageTool. The areas were converted into volumes by multiplying the areas by slice thickness. Infarct size is expressed as a percentage of the risk zone.
Isolation of adult rat cardiomyocytes
Rat cardiomyocytes were isolated enzymatically [17]. Male Wistar rats weighing 250-350 g were anesthetized with thiobutabarbital sodium (100mg/kg, i.p.). A midline thoracotomy was performed and the heart was removed and rapidly mounted on a Langendorff apparatus. The heart was perfused in a non-recirculating mode with Krebs-Henseleit buffer (37°C) containing (in mM) NaCl 118, NaHCO3 25, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 1.25, and glucose 10 for 5 min to wash out blood. The buffer was bubbled with 95 % O2/5 % CO2. Then the heart was perfused with a calcium-free buffer that contained all of the above components except CaCl2. After 5 min of perfusion, collagenase (type II) was added to the buffer (0.1 %) and the heart was perfused in a recirculating mode for ~15 min. The heart was removed from the apparatus and the ventricles were placed into a beaker containing the calcium-free buffer. The ventricles were agitated in a shaking bath (37°C) at a rate of 50 cycles/min until individual cells were released. The released cells were suspended in an incubation buffer containing all the components of the calcium-free buffer, 1 % bovine serum albumin, 30 mM HEPES, 60 mM taurine, 20 mM creatine, and amino acid supplements at 37°C. Calcium was gradually added to the buffer containing the cells to a final concentration of 1.2 mM. The cells were filtered through nylon mesh and centrifuged briefly. Finally the cells were suspended in culture medium M199 for 4 h before experiments.
Mitochondrial isolation
Mitochondria and cytosolic fractions were isolated by differential centrifugation as previously described [18]. Cardiac samples (or isolated cardiomyocytes) were homogenized in a buffer containing 250 mM sucrose, 10 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, and protease inhibitor cocktail. The homogenate was centrifuged at 1000g for 10 minutes to remove nuclei and debris. The supernatant was centrifuged at 10000g for 30 minutes. The resultant supernatant was subsequently centrifuged at 10000g for 1 hour to yield the cytosolic fraction. The 10000g pellet, corresponding to the mitochondrial fraction, was resuspended and centrifuged again at 10000g for 30 minutes. Mitochondria were then resuspended in swelling buffer (for swelling experiments) and homogenized (for Western and immunoprecipitation).
Confocal Imaging of ΔΨm
ΔΨm was measured using confocal microscopy as reported previously [19]. Briefly, cardiac cells cultured in a specific temperature-controlled culture dish were incubated with tetramethylrhodamine ethyl ester (TMRE, 100 nM) in standard Tyrode solution containing (mM) NaCl 140, KCl 6, MgCl2 1, CaCl2 1, HEPES 5, and glucose 5.8 (pH 7.4) for 10 min. Cells were then mounted on the stage of an Olympus FV 500 laser scanning confocal microscope. The red fluorescence was excited with a 543 nm line of argon-krypton laser line and imaged through a 560 nm long-path filter. Temperature was maintained at 37°C with Delta T Open Dish Systems (Bioptechs, Butler, PA). The images recorded on a computer were quantified using Image J.
Confocal imaging of mitochondrial Ca2+
Mitochondrial Ca2+ is determined with Rhod-2. This mitochondrial Ca2+ indicator has a net positive charge, a property that promotes its sequestration into mitochondria. Cardiomyocytes were loaded with 2 μM Rhod-2-AM for 1 h at 4°C and then incubated for 30 min at 37°C. This two-step cold loading/warm incubation protocol achieves exclusive loading of Rhod-2 into mitochondria [20]. The red fluorescence was excited with a 543 nm line of argon-krypton laser line and imaged through a 560 nm long-path filter.
Measurement of mitochondrial swelling
Intact mitochondria (0.3 mg/ml) isolated from cardiac samples taken 10 min after the onset of reperfusion were suspended in a buffer containing (in mM) 120 KCl, 10 Tris·HCl, 5 KH2PO4, and 20 MOPS. Mitochondrial swelling was assessed spectrophotometrically as a decrease in absorbance at 520 nm (A520) [21].
Western blotting analysis
Myocardial samples taken from risk zones were homogenized in ice-cold lysis buffer. Equal amounts of protein were loaded and eletrophoresed on SDS-polyacrylamide gel and transferred to a PVDF membrane. Membranes were blocked with nonfat milk, and then incubated with the primary antibodies (1:1000) at 4 °C overnight. The primary antibody bindings were detected with a secondary anti-rabbit antibody (1:2000) and visualized by the ECL method.
Evaluation of adenosine A2B receptor expression by quantitative RT-PCR in rat cardiomyocytes
Total mRNA of rat cardiomyocytes was isolated using Trizol (Invitrogen) reagent. RNA was converted to cDNA using reverse transcriptase. GADPH and A2B mRNA sequences were identified using Rat Genome Resources of NCBI (http://www.ncbi.nlm.nih.gov). Primer pairs for GAPDH (Forward: CAGGTTGTCTCCTGCGACTT, Reverse: ATGTAGGCCATGAGGTCCAC) and A2B (Forward: CCAAGGACAAGCCCAAATG, Reverse: CCGTCTGGCAGAGAACGTAT) were designed using Primer3 program. Gene expressions were assessed by quantitative RT-PCR using QuantiTect SYBR Green PCR Kit (Qiagen) and ABI 7900HT Fast Real-Time PCR Systems.
Experimental protocols
All hearts were subjected to a 30 min regional ischemia followed by 2 h of reperfusion. Infusion of NECA and inhibitors were started 5 min before the onset of reperfusion and continued for 35 min. Biopsies were collected from risk zones at 5 and 10 min after the onset of reperfusion. Infarct size was measured 2 h after reperfusion. In the experiments measuring ΔΨm, isolated rat cardiomyocytes were exposed to 100 μM H2O2 for 20 min. Agonists were given 10 min before exposure to H2O2 and antagonists were applied 5 min before the application of the agonists.
Statistical Analysis
Data are expressed as mean ± SEM and were obtained from 4 to 10 separate experiments. Statistical significance was determined using Student t-test or one-way ANOVA followed by Tukey’s test. A value of P < 0.05 was considered as statistically significant.
Results
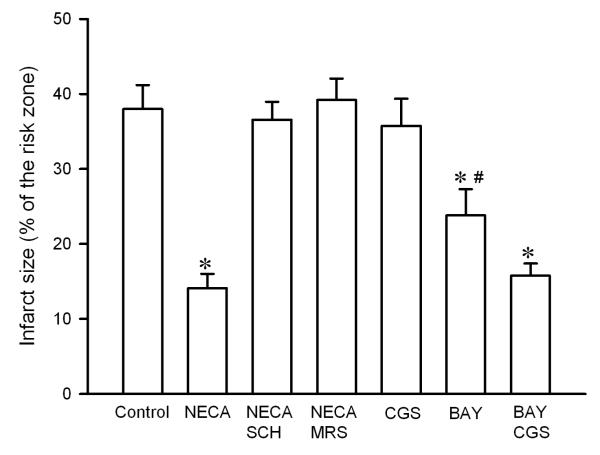
NECA (100 nM) given at reperfusion significantly reduced infarct size (14.1 ± 1.9 % of risk zone) compared to the control (37.9 ± 3.2 % of risk zone) (Fig. 1). The anti-infarct effect of NECA was reversed by both the selective A2A antagonist SCH58261 (15 nM) and the selective A2B antagonist MRS1706 (15 nM) (Fig. 1), indicating that both A2A and A2B receptors are involved in the action of NECA. The selective A2A agonist CGS21680 did not reduce infarct size (35.8 ± 3.6 % of risk zone). In contrast, the selective A2B agonist BAY 60-6580 (300 nM) reduced infarct size to 23.8 ± 3.5 % of risk zone, although the reduction was not as great as NECA did. Interestingly, a combination of BAY 60-6580 and CGS21680 further reduced infarct size to 15.8 ± 1.6 % of risk zone, an effect that was equipotent with NECA, implying that a simultaneous stimulation of A2A and A2B receptors may produce a strong protection.
Fig. 1.
Infarct size in isolated rat hearts. Hearts were subjected to 30 min regional ischemia followed by 2 h of reperfusion. NECA ( n = 7) given at reperfusion reduced infarct size compared the control (n = 6). Both the selective A2A receptor antagonist SCH58261 (SCH, n = 6) and A2B receptor antagonist MRS1706 (MRS, n = 6) reversed the anti-infarct effect of NECA. The selective A2A agonist CGS21680 (CGS, n = 6) failed to reduce infarct size, whereas the selective A2B receptor antagonist BAY 60-6583 (BAY, n = 6) slightly but significantly reduced infarct size. A combination of CGS21680 and BAY 60-6583 (n =7) further reduced infarct size. * p < 0.05 vs. control; # p < 0.05 vs. NECA and BAY+CGS.
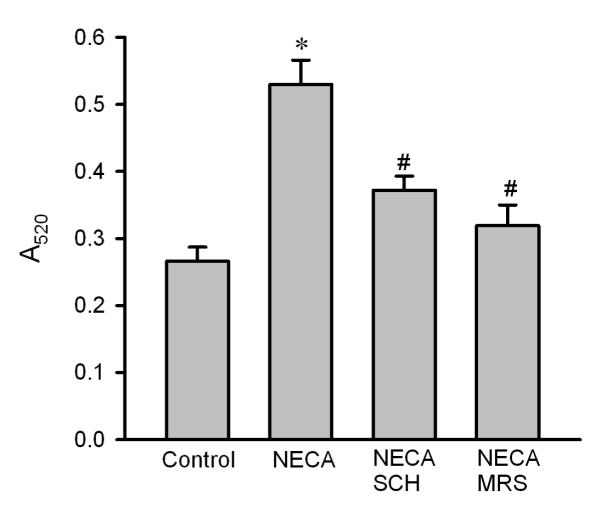
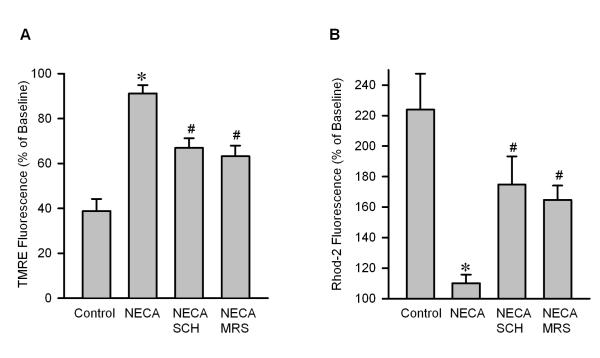
To test if NECA prevents reperfusion injury by targeting the mPTP opening, we evaluated the effect of NECA on mitochondrial swelling upon reperfusion by measuring light scattering at A520. Compared to the control (0.266 ± 0.020), mitochondria isolated from the heart treated with NECA had a higher value of A520 (0.530 ± 0.036), indicating that NECA may modulate the mPTP opening at reperfusion (Fig. 2). This effect of NECA was abrogated by both SCH58261(0.372 ± 0.021) and MRS1706 (0.319 ± 0.031) (Fig. 2), indicating that the preventive effect of NECA on the mPTP opening is mediated by both A2A and A2B receptors. To confirm the effect of NECA on the mPTP opening, we tested the effect of NECA on oxidant-induced loss of ΔΨm by monitoring TMRE fluorescence with confocal microscopy in isolated rat cardiomyocytes. As shown in Fig. 3A, 100 μM H2O2 dramatically decreased TMRE fluorescence in control cells (38.8 ± 5.4 % of baseline), indicating that oxidant stress induces the mPTP opening. In contrast, cells treated with NECA showed a much higher TMRE fluorescence intensity (91.2 ± 3.7 % of baseline), implying that NECA may prevent the mPTP opening. This action of NECA was partially but significantly inhibited by both SCH58261 (69.9 ± 4.3 % of baseline) and MRS1706 (63.2 ± 4.7 % of baseline) (Fig. 3A). To further corroborate the inhibitory action of NECA on the mPTP opening, we then tested the effect of NECA on oxidant induced mitochondrial Ca2+ overload by detecting Rhod-2 fluorescence with confocal microscopy in isolated rat cardiomyocytes. Fig. 3B shows that treatment of cardiomyocytes with 100 μM H2O2 for 20 min markedly increased Rhod-2 fluorescence intensity (223.9 ± 23.5 of baseline), indicating that oxidant stress caused mitochondrial Ca2+ overload, which is the main cause of the mPTP opening. NECA dramatically reduced the red fluorescence intensity to 110.0 ± 5.6 % of baseline (Fig. 3B). The action of NECA was partially but significantly blocked by both SCH58261 (174.8 ± 18.5 % of baseline) and MRS1706 (164.7 ± 9.4 % of baseline).
Fig. 2.
Mitochondrial swelling in isolated rat hearts. Mitochondria were isolated from heart samples collected at 10 min after the onset of reperfusion. Mitochondrial swelling was measured as a decrease in absorbance at 520 nm (A520). NECA (n = 5) prevented mitochondrial swelling at reperfusion, an effect that was blocked by both SCH58261 (SCH, n = 5) and MRS1706 (MRS, n = 5). * p < 0.05 vs. control; # p < 0.05 vs. NECA.
Fig. 3.
A, Summarized data for TMRE fluorescence intensity measured with confocal microscopy 20 min after exposure to 100 μM H2O2 in isolated rat cardiomyocytes. NECA (n =5) prevented oxidant-induced TMRE fluorescence reduction compared to the control (n = 7) and this action was partially but significantly inhibited by both SCH58261 (SCH, n = 5) and MRS1706 (MRS, n = 5). * p < 0.05 vs. control; # p < 0.05 vs. NECA. B, Summarized data for Rhod-2 fluorescence intensity measured with confocal microscopy 20 min after exposure to 100 μM H2O2 isolated rat cardiomyocytes. NECA (n = 5) inhibited oxidant-induced increase in Rhod-2 fluorescence compared to the control (n = 5), indicating that NECA can prevent oxidant-induced matrix Ca2+ overload. This action of NECA was partially but significantly blocked by both SCH58261 (SCH, n = 5) and MRS1706 (MRS, n = 5). * p < 0.05 vs. control; # p < 0.05 vs. NECA.
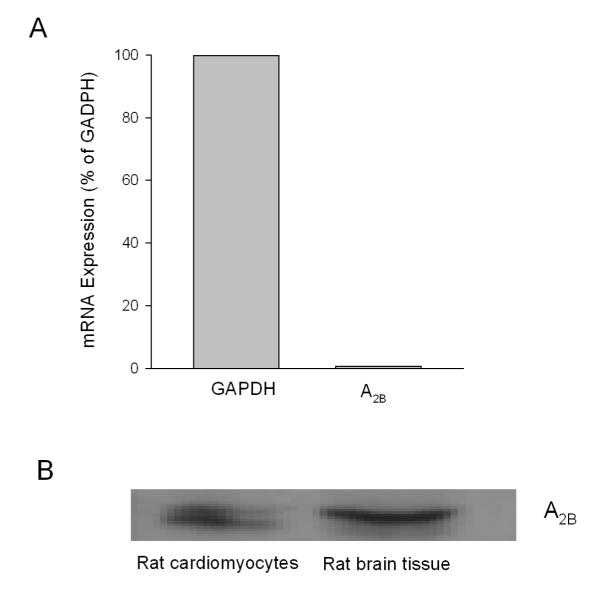
To test if adenosine A2B receptors exist in cardiomyocytes, we determined A2B receptor mRNA expression in isolated rat cardiomyocytes with RT-PCR. As shown in Fig. 4A, A2B receptor mRNA was expressed in rat cardiomyocytes (0.7 % of GAPDH). In support, Western blotting analysis showed that A2B receptor protein was expressed in isolated cardiomyocytes (Fig. 4B).
Fig. 4.
A, A2B receptor mRNA expression in isolated adult rat cardiomyocytes. B, Western blot analysis of A2B receptor expression in isolated adult rat cardiomyocytes (n = 3).
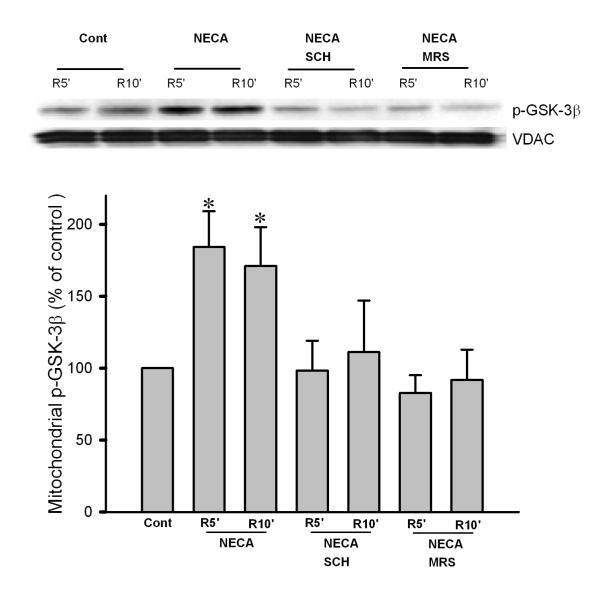
To determine the potential role of mitochondrial GSK-3β in the cardioprotective effect of NECA, we measured phosphorylation levels of mitochondrial GSK-3β at Ser9. NECA significantly enhanced mitochondrial GSK-3β phosphorylation at 5 and 10 min of reperfusion, which was abrogated by both SCH58261 and MRS1706 (Fig. 5). We further found that the mitochondrial total GSK-3β levels were increased upon reperfusion by the treatment with NECA, implying that GSK-3β is translocated to mitochondria by NECA (Fig. 6).
Fig. 5.
Western blot analysis of mitochondrial phospho-GSK-3β (Ser9) in cardiac samples collected at 5 and 10 min after the onset of reperfusion. NECA (n = 6) significantly enhanced mitochondrial GSK-3β phosphorylation, indicating that NECA can inactivate GSK-3β at reperfusion. The effect of NECA was abrogated by both SCH58261 (SCH, n = 5) and MRS1706 (MRS, n = 5). * p < 0.05 vs. control.
Fig. 6.
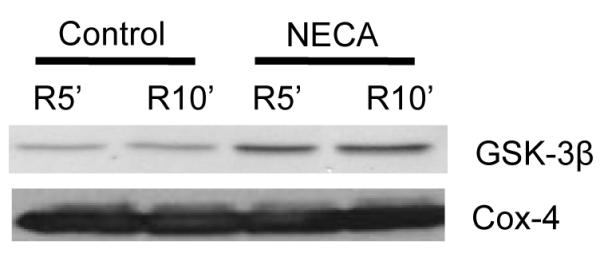
Western blot analysis of mitochondrial total GSK-3β in cardiac samples collected at 5 and 10 min after the onset of reperfusion. NECA (n = 5) increased the mitochondrial GSK-3β protein level, indicating that NECA may translocate GSK-3β to mitochondria.
Discussion
The present study demonstrates that both adenosine A2A and A2B receptors contribute to the cardioprotective effect of NECA at reperfusion and that a simultaneous stimulation of the two receptor subtypes at reperfusion may produce a strong and indubitable cardioprotection against ischemia/reperfusion injury. Adenosine A2 receptor activation at reperfusion protects the heart presumably by modulating the mPTP opening through inactivation of mitochondrial GSK-3β phosphorylation.
Although preconditioning as well as many other interventions applied prior to ischemia are well established to protect the heart from ischemia/reperfusion injury, pretreatment is seldom possible in the clinical setting of AMI. Thus, to save myocardium in patients with AMI, it is required that interventions must be effective when applied after ischemia has begun or at the onset of reperfusion. In this regard, adenosine A2 receptor agonists have been frequently demonstrated to be effective at reperfusion in various experimental settings. There are two A2 receptor subtypes: A2A and A2B. Early studies showed that CGS21680, the selective A2A receptor agonist, given at reperfusion protects the heart in various experimental models [1-4, 22]. Recently it has been reported that activation of A2A receptors at reperfusion with ATL146e, another selective A2A agonist, reduced infarct size in mouse hearts [5, 23]. In addition, the cardioprotective effect of postconditioning was attenuated in A2A adenosine receptor knockout mouse hearts, indicating that A2A receptor activation is involved in the mechanism of postconditioning [24]. In isolated rabbit hearts, AMP579, a novel adenosine A1/A2 receptor agonist, given at reperfusion attenuated contracture and limited infarct size, an effect that was blocked by the selective A2A receptor antagonist CSC but not by the A1 receptor antagonist DPCPX, indicating that A2A but not A1 receptors are involved in the action of AMP579 [7]. Interestingly, the protective effect of AMP579 was mimicked by NECA but not by the selective A2A receptor agonist CGS21680, thus casting doubt on the effectiveness of A2A receptor activation alone at reperfusion [7]. Similarly, Kis et al. demonstrated that the anti-infarct effect of AMP579 given at reperfusion was abrogated by ZM241385, an A2A receptor antagonist, but was not mimicked by CGS21680 [8]. These observations suggest that although A2A receptor activation is crucial for prevention of reperfusion injury, stimulation of some other receptor subtypes may also be required to produce an effective protection. Recently, it has been reported that the selective A2B agonist BAY 60-6583 applied at reperfusion reduced infarct size in rabbit hearts [10], indicating that A2B receptor activation leads to cardioprotection at reperfusion. Further studies demonstrated the importance of A2B receptor activation in cardioprotection induced by both postconditioning and PKG activation [9, 11]. Thus, it seems likely that both A2A and A2B receptors are important to protect the heart at reperfusion. Nevertheless, little has been done to test if a simultaneous activation of A2A and A2B receptors can lead to a strong protection at reperfusion. We assumed that A2A and A2B receptors may work in concert to protect the heart at reperfusion.
In the present study we tested our hypothesis with NECA. NECA has a high affinity for A2A receptors (20 nM) and had been the most potent A2B agonist [15] until recently when the selective A2B agonist BAY 60-6583 was available [16]. Therefore, it is highly likely that infusion of heart with NECA at reperfusion can activate both A2A and A2B receptors. We found that the anti-infarct effect of NECA was reversed by both the potent and selective A2A antagonist SCH58261 (323-, 53-, and 100-fold more selective for A2A receptors than A1, A2B, and A3 receptors, respectively) and the selective A2B antagonist MRS1706 (113-, 81-, and 165-fold more selective for A2B receptors than A1, A2A, and A3 receptors, respectively). However, the selective A2A agonist CGS21680 alone did not mimic the action of NECA. Interestingly, although BAY 60-6583 (EC50 < 10 nM for human A2B receptors and > 10 μM for A1, A2A, and A3 receptors (Table 1) [10]) could reduce infarct size, the protection was not as great as that of NECA and the combination of CGS21680 and BAY 60-6583 further reduced infarct size. Further experiments showed that the effects of NECA on the mPTP opening (mitochondrial swelling, ΔΨm, and mitochondrial Ca2+) and GSK-3β phosphorylation were also blocked by SCH58261 and MRS1706. These results clearly indicate that both A2A and A2B receptors account for the anti-infarct effect of NECA and that simultaneous stimulation of A2A and A2B receptors at reperfusion might confer a strong and consistent protection. However, one may have a question: why both the receptors are needed to induce cardioprotection? Since both of these receptors are coupled to Gs proteins and thus should have similar downstream signals. One potential answer might be that the receptor density in cardiomyocytes is so low that both types need to be occupied in order to launch sufficient signals. In addition, the observation that the selective A2A agonist CGS21680 was not protective but augmented BAY 60-6583 induced cardioprotection may suggest that A2A receptors play a role in both transmission and enhancement of the protective signal from the A2b receptors.
Table 1.
Binding affinity of the agonists and antagonist for adenosine receptor subtypes (Ki/Kd in nM)
The mPTP opening is a critical determinant of myocardial ischemia/reperfusion injury [25] and the mPTP is an important target of cardioprotection [26]. Because the mPTP opens upon reperfusion but not during ischemia [27], interventions that modulate the pore opening may protect the heart. It has been demonstrated that suppression of the mPTP opening by sanglifehrin-A during first few minutes of reperfusion leads to cardioprotection against infarction [28]. Inhibition of the mPTP opening also plays a essential role in postconditioning [29]. Recently, our group has demonstrated that cardioprotectants such as bradykinin, IB-MECA, and morphine applied at reperfusion protects the heart by modulating the mPTP opening [30-32]. Thus, it is reasonable to assume that NECA may also protect the heart at reperfusion by inhibiting the mPTP opening. In this study, NECA attenuated mitochondrial swelling upon reperfusion in perfused rat hearts and prevented oxidant-induced ΔΨm loss and mitochondrial Ca2+ overload in isolated rat cardiomyocytes, suggesting that NECA may protect the heart at reperfusion by modulating the mPTP opening. Moreover, the inhibitory action of NECA on the mPTP opening was suppressed by the antagonists of both A2A and A2B receptors, suggesting that adenosine A2 receptor activation induced cardioprotection is attributed to inhibition of the mitochondrial death pathway upon reperfusion. This result also supports our view that both A2A and A2B receptors contribute to the cardioprotective effect of NECA at reperfusion. However, in this study the preventive effects of NECA on the mPTP opening were partially but not completely blocked by either SCH58261 or MRS1706, implying that the mPTP is not the only target of A2 receptor activation-induced cardioprotective signaling. Since we evaluated the effects of NECA on ΔΨm and mitochondrial matrix Ca2+ by imaging isolated adult rat cardiomyocytes with confocal microscopy, these findings also indicate that both A2A and A2B receptors exist in rat cardiomyocytes. Previous studies have demonstrated the presence of A2A receptors in adult rat cardiomyocytes [33, 34]. In addition, Liang et al. reported that A2B receptors exist in chick embryonic cardiac cells [35]. The current study has also shown that the A2B receptors were detectable in isolated rat cardiomyocytes. Thus, these observations strongly suggest that A2 receptors in cardiomyocytes are responsible for the cardioprotective effect of NECA.
Although the exact signaling mechanism by which various cardioprotective interventions prevent the mPTP opening remains unclear, GSK-3β has been proposed to mediate the convergence of cardioprotective signaling pathways to inhibit mPTP opening [36]. In support, inactivation of GSK-3β is critical for prevention of the mPTP opening by preconditioning [37], postconditioning [38], and bradykinin [30]. However, a recent study failed to demonstrate that NECA given at reperfusion significantly increases GSK-3β phosphorylation at Ser9 in isolated rabbit hearts, although there was a strong trend for increased GSK-3β phosphorylation [13]. In this study, we found that NECA increased the phosphorylation level of GSK-3β in mitochondria, which was reversed by both A2A and A2B receptors. A recent study by Ohori et al. demonstrated that erythropoietin prevents oxidant-induced apoptosis in cardiomyocytes by inactivating mitochondrial GSK-3β [39]. Thus, it is tenable to reason that activation of adenosine A2 receptors may also share the common mechanism with erythropoietin to modulate the mPTP opening through the inactivated form of mitochondrial GSK-3β. However, it remains unknown if NECA-induced mitochondrial GSK-3β phosphorylation took place within mitochondria, because our data also showed that NECA translocated GSK-3β (total) to mitochondria, which may suggest that the increase in mitochondrial GSK-3β phosphorylation might be due in part to the translocation of the phosphorylated form of GSK-3β from cytosol. Although GSK-3β interactions with the mPTP components ANT (adenine nucleotide translocator) and cyclophilin D have been proposed to be the mechanisms by which erythropoietin [37] and resveratrol [40] modulate the mPTP opening, it remains to be determined if NECA modulates the pore opening through the similar mechanism. Obviously, more studies are required to define the precise mechanism by which the mitochondrial phosphorylated form of GSK-3β suppresses the mPTP opening in the heart treated with adenosine A2 receptor agonists.
Study limitations
We investigated the roles of adenosine A2A and A2B receptors in NECA-induced cardioprotection against reperfusion injury applying the antagonists and agonists that target the both receptor subtypes in perfused rat hearts or in isolated rat cardiomyocytes. Although the antagonists and agonists used were potent and selective, use of animal lacking a specific gene that codes an A2 receptor subtype will further help us to precisely determine the specific A2 receptor subtype (s) that is protective when stimulated.
In conclusion, A2A and A2B receptors work in concert to prevent reperfusion injury in rat hearts treated with NECA. NECA may protect the heart by modulating the mPTP opening through inactivating mitochondrial GSK-3β. A simultaneous stimulation of A2A and A2B receptors at reperfusion might be required to produce a strong and consistent cardioprotection against reperfusion injury.
Acknowledgement
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-08336. We are grateful to Bayer HealthCare in Germany for providing the selective A2B receptor agonist BAY 60-6583.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Norton ED, Jackson EK, Turner MB, Virmani R, Forman MB. The effects of intravenous infusions of selective adenosine A1-receptor and A2-receptor agonists on myocardial reperfusion injury. Am Heart J. 1992;123:332–338. doi: 10.1016/0002-8703(92)90643-a. [DOI] [PubMed] [Google Scholar]
- [2].Schlack W, Schäfer M, Uebing A, Schäfer S, Borchard U, Thamer V. Adenosine A2-receptor activation at reperfusion reduces infarct size and improves myocardial wall function in dog heart. J Cardiovasc Pharmacol. 1993;22:89–96. doi: 10.1097/00005344-199307000-00015. [DOI] [PubMed] [Google Scholar]
- [3].Zhao Z-Q, Sato H, Williams MW, Fernandez AZ, Vinten-Johansen J. Adenosine A2-receptor activation inhibits neutrophil-mediated injury to coronary endothelium. Am J Physiol. 1996;271:H1456–H1464. doi: 10.1152/ajpheart.1996.271.4.H1456. [DOI] [PubMed] [Google Scholar]
- [4].Jordan JE, Zhao Z-Q, Sato H, Taft S, Vinten-Johansen J. Adenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherence. J Pharmacol Exp Ther. 1997;280:301–309. [PubMed] [Google Scholar]
- [5].Rork TH, Wallace KL, Kennedy DP, Marshall MA, Lankford AR, Linden J. Adenosine A2A receptor activation reduces infarct size in the isolated, perfused mouse heart by inhibiting resident cardiac mast cell degranulation. Am J Physiol. 2008;295:H1825–1833. doi: 10.1152/ajpheart.495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao Z-Q, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- [7].Xu Z, Downey JM, Cohen MV. AMP 579 reduces contracture and limits infarction in rabbit heart by activating adenosine A2 receptors. J Cardiovasc Pharmacol. 2001;38:474–81. doi: 10.1097/00005344-200109000-00016. [DOI] [PubMed] [Google Scholar]
- [8].Kis A, Baxter GF, Yellon DM. Limitation of myocardial reperfusion injury by AMP579, an adenosine A1/A2A receptor agonist: role of A2A receptor and Erk1/2. Cardiovasc Drugs Ther. 2003;17:415–25. doi: 10.1023/b:card.0000015856.02691.fa. [DOI] [PubMed] [Google Scholar]
- [9].Philipp S, Yang X-M, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- [10].Kuno A, Critz SD, Cui L, Solodushko V, Yang X-M, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–271. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kuno A, Solenkova NV, Solodushko V, Dost T, Liu Y, Yang X-M, Cohen MV, Downey JM. Infarct limitation by a protein kinase G activator at reperfusion in rabbit hearts is dependent on sensitizing the heart to A2b agonists by protein kinase C. Am J Physiol. 2008;295:H1288–1295. doi: 10.1152/ajpheart.00209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang XM, Krieg T, Cui L, Downey JM, Cohen MV. NECA and bradykinin at reperfusion reduce infarction in rabbit hearts by signaling through PI3K, ERK, and NO. J Mol Cell Cardiol. 2004;36:411–21. doi: 10.1016/j.yjmcc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- [13].Förster K, Paul I, Solenkova N, Staudt A, Cohen M, Downey J, Felix S, Krieg T. NECA at reperfusion limits infarction and inhibits formation of the mitochondrial permeability transition pore by activating p70S6 kinase. Basic Res Cardiol. 2006;101:319–326. doi: 10.1007/s00395-006-0593-4. [DOI] [PubMed] [Google Scholar]
- [14].Klotz K-N. Adenosine receptors and their ligands. Naunyn-Schmiedebergs Arch Pharmacol. 2000;362:382–91. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- [15].Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- [16].Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by Ecto-5′-Nucleotidase (CD73) and A2B Adenosine Receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- [17].Xu Z, Park SS, Mueller RA, Bagnell RC, Patterson C, Boysen PG. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc Res. 2005;65:803–12. doi: 10.1016/j.cardiores.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [18].Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- [19].Jang Y, Wang H, Xi J, Mueller RA, Norfleet EA, Xu Z. NO mobilizes intracellular Zn2+ via cGMP/PKG signaling pathway and prevents mitochondrial oxidant damage in cardiomyocytes. Cardiovasc Res. 2007;75:426–433. doi: 10.1016/j.cardiores.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Trollinger DR, Cascio WE, Lemasters JJ. Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca(2+)-indicating fluorophores. Biophys J. 2000;79:39–50. doi: 10.1016/S0006-3495(00)76272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang G, Liem DA, Vondriska TM, Honda HM, Korge P, Pantaleon DM, Qiao X, Wang Y, Weiss JN, Ping P. Nitric Oxide Donors Protect the Murine Myocardium Against Infarction via Modulation of Mitochondrial Permeability Transition. Am J Physiol. 2005;288:H1290–5. doi: 10.1152/ajpheart.00796.2004. [DOI] [PubMed] [Google Scholar]
- [22].Zhao Z-Q, Budde JM, Morris C, Wang N-P, Velez DA, Muraki S, Guyton RA, Vinten-Johansen J. Adenosine attenuates reperfusion-induced apoptotic cell death by modulating expression of Bcl-2 and Bax proteins. J Mol Cell Cardiol. 2001;33:57–68. doi: 10.1006/jmcc.2000.1275. [DOI] [PubMed] [Google Scholar]
- [23].Yang Z, Linden J, Berr SS, Kron IL, Beller GA, French BA. Timing of adenosine 2A receptor stimulation relative to reperfusion has differential effects on infarct size and cardiac function as assessed in mice by MRI. Am J Physiol. 2008;295:H2328–2335. doi: 10.1152/ajpheart.00091.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morrison RR, Tan XL, Ledent C, Mustafa SJ, Hofmann PA. Targeted deletion of A2A adenosine receptors attenuates the protective effects of myocardial postconditioning. Am J Physiol. 2007;293:H2523–2529. doi: 10.1152/ajpheart.00612.2007. [DOI] [PubMed] [Google Scholar]
- [25].Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- [26].Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- [27].Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- [29].Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning Inhibits Mitochondrial Permeability Transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- [30].Park SS, Zhao H, Mueller R, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permiability transition pore through glycogen synthase kinase 3β. J Mol Cell Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- [31].Park S-S, Zhao H, Jang Y, Mueller RA, Xu Z. N6-(3-Iodobenzyl)-adenosine-5′-N-methylcarboxamide confers cardioprotection at reperfusion by inhibiting mitochondrial permeability transition pore opening via glycogen synthase kinase 3β. J Pharmacol Exp Ther. 2006;318:124–131. doi: 10.1124/jpet.106.101477. [DOI] [PubMed] [Google Scholar]
- [32].Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology. 2008;108:243–250. doi: 10.1097/01.anes.0000299437.93898.4a. [DOI] [PubMed] [Google Scholar]
- [33].Xu H, Stein B, Liang B. Characterization of a stimulatory adenosine A2a receptor in adult rat ventricular myocyte. Am J Physiol. 1996;270:H1655–61. doi: 10.1152/ajpheart.1996.270.5.H1655. [DOI] [PubMed] [Google Scholar]
- [34].Dobson JGJ, Fenton RA. Adenosine A2 receptor function in rat ventricular myocytes. Cardiovasc Res. 1997;34:337–347. doi: 10.1016/s0008-6363(97)00023-0. [DOI] [PubMed] [Google Scholar]
- [35].Liang BT, Haltiwanger B. Adenosine A2a and A2b receptors in cultured fetal chick heart cells. High- and low-affinity coupling to stimulation of myocyte contractility and cAMP accumulation. Circ Res. 1995;76:242–51. doi: 10.1161/01.res.76.2.242. [DOI] [PubMed] [Google Scholar]
- [36].Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nishihara M, Miura T, Miki T, Tanno M, Yano T, Naitoh K, Ohori K, Hotta H, Terashima Y, Shimamoto K. Modulation of the mitochondrial permeability transition pore complex in GSK-3β-mediated myocardial protection. J Mol Cell Cardiol. 2007;43:564–570. doi: 10.1016/j.yjmcc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- [38].Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3β by postconditioning Is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- [39].Ohori K, Miura T, Tanno M, Miki T, Sato T, Ishikawa S, Horio Y, Shimamoto K. Ser9 phosphorylation of mitochondrial GSK-3β is a primary mechanism of cardiomyocyte protection by erythropoietin against oxidant-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;295:H2079–2086. doi: 10.1152/ajpheart.00092.2008. [DOI] [PubMed] [Google Scholar]
- [40].Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3[beta] and mitochondrial permeability transition pore. Eur J Pharmacol. 2009;604:111–116. doi: 10.1016/j.ejphar.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]