Abstract
Objectives
To investigate age-associated changes in the expression and deposition of collagen and hyaluronan (HA) in aged vocal folds.
Methods
Thirty, Sprague-Dawley rats were involved in this study. For gene expression analyses, fifteen animals were divided into three age groups of young (2 month), adult (9 month), and elderly (18 month) rats. Real-time PCR was used to quantify messenger RNA expression of procollagen type I, -III, matrix metalloproteinase (MMP) -2, -9, and hyaluronan synthase (HAS)-1, -2, -3. The remaining fifteen animals were divided into the same three groups and underwent histological analyses to investigate age-associated changes in the deposition of collagen and HA.
Results
Results revealed downregulated expression of procollagen type I, -III, MMP-2, -9, and HAS-1, -2, -3 in adult and elderly vocal folds, compared to young vocal folds. Histologically, staining of collagen was dense and HA was less dense in the vocal folds of adult and elderly rats, compared to young rats.
Conclusions
A slowdown in the expression of procollagens and MMPs was associated with dense collagen in aged vocal folds, as observed in elderly humans. A similar decrease in the expression of genes coding HAS was consistent with low density of extracellular matrix HA in the vocal folds of elderly rats.
Keywords: age, vocal fold, collagen, hyaluronan, turnover
Introduction
Voice disorders are common in the elderly and have a significant impact on communication and quality of life.1 The vocal folds, which protect the airway and modulate airflow through the glottis during phonation, are composed of an extracellular matrix (ECM) of fibrous proteins, interstitial proteins, and glycosaminoglycans. The density and spatial arrangement of these elements is important, as changes in their deposition can alter the vocal folds biomechanical properties and vibratory function.2 Age-related alterations in the deposition of the ECM have been reported in humans, including excessive accumulation of collagen, dense collagen bundles, reduced elastin, and decreased hyaluronan (HA). 3-6
One hypothesis that has been proposed attributes age-related histologic changes of the vocal fold to a disruption in the normal balance of ECM turnover.7 It has been suggested that the vocal folds undergo an age-dependent slowdown in the synthesis and degradation of collagen. 7 Whether the slowdown in the synthesis of collagen and in the expression of genes coding matrix degrading enzymes results in the overaccumulation of ECM collagen observed in elderly humans is unknown. Also unknown is whether decreased levels of HA are the result of a similar decrease in the synthesis of mRNA coding hyaluronan synthase (HAS). Therefore, the purpose of the current study was threefold: (1) to investigate the senescent expression of genes coding collagen and HAS; (2) to investigate the senescent expression of genes coding matrix metalloproteinases; and (3) to examine histologic changes in ECM levels of collagen and HA in aged vocal folds.
Materials And Methods
Animals
Thirty male Sprague-Dawley rats were involved in this study. Fifteen animals were equally divided into three groups of rats aged 2 months (young), 9 months (adult), and 18 months (elderly) (five per group), and assigned to the PCR study arm. The fifteen remaining rats were divided into three equal groups and assigned to the histological study arm. Whole larynges were harvested from all animals following an overdose intracardiac injection of pentobarbital sodium (150 mg/kg).
Polymerase chain reaction (PCR)
A light microscope was used to remove all layers of the vocal fold above the thyroarytenoid muscle for PCR analysis. Specimens included all mucosal layers of the lamina propria and epithelium. A Mixer Mill MM 301 (Retsch Inc. Pittsburgh, PA, USA) was used for homogenization of vocal fold specimens. Total RNA was isolated from homogenate using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and treated with ribonuclease-free deoxyribonuclease I (QIAGEN, Valencia, CA, USA) to minimize contamination from genomic DNA. The A260/A280 ratio was used to determine the quantity of total RNA. Electrophoresis was used to evaluate the quality of RNA based on the appearance of the 18S and 28S ribosomal RNA bands. A High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used to perform reverse transcription according to the manufacturers recommended reaction protocol. A Biometra TGradient Thermocycler (LABREPCO, Horsham, PA, USA) was used to perform reactions using the following conditions: 25°C for 10 min, 37°C for 120 min, 85°C for 5 sec, and 4°C for 5 min.
Rat-specific primers for procollagen type I, procollagen type III, MMP-2, -9, HAS-1, -2, -3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used.8,9 Primers were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Table 1 displays primer sequences. All primers generated a single PCR band of the expected size and PCR products were verified by DNA sequencing. The iQ™SyBR Green Supermix Kit (BioRad, CA, USA) was used to perform real-time PCR in a 25 μL volume reaction mixture composed of 500 nM primer1, 500 nM primer 2, 12.5 μL iQ SyBR Green Supermix, 1 μL of template complementary DNA, and 9 μL of ribonuclease-free water. Real-time PCR was performed under the following conditions: 1 cycle at 95°C for 3 min, followed by 45 cycles at 95°C for 20 seconds, 58.7°C for 20 seconds, 72°C for 40 seconds, and 1 cycle at 95°C for 2 min, 55°C for 2 min, and then 1 cycle at 55°C to 95°C in 0.2°C increments to make a melting curve. PCR products were detected using an iCycler iQ™ Optical System (Software version 2.0; Bio-Rad, Hercules, CA, USA). Electrophoresis was used to separate PCR products in 1% agarose gels containing 0.5μg/mL ethidium bromide for verification of PCR products according to fragment size.
Table 1.
Primer Sequences
| Procollagen type I | Forward | 5′- AAG GGT GAG ACA GGC GAA CAA -3′ |
| Reverse | 5′- TTG CCA GGA GAA CCA GCA GAG -3′ | |
| Procollagen type III | Forward | 5′- ATG GTG GCT TTC AGT TCA GC -3′ |
| Reverse | 5′- TGT CTT GCT CCA TTC ACC AG -3′ | |
| MMP-2 | Forward | 5′-GTC ACT CCG CTG CGC TTT TCT CG-3′ |
| Reverse | 5′-GAC ACA TGG GGC ACC TTC TGA-3′ | |
| MMP-9 | Forward | 5′-CGG AGC ACG GGG ACG GGT ATC-3′ |
| Reverse | 5′-AAG ACG AAG GGG AAG ACG CAC ATC-3′ | |
| HAS-1 | Forward | 5′-TAG GTG CTG TTG GAG GAG ATG TGA-3′ |
| Reverse | 5′-AAG CTC GCT CCA CAT TGA AGG CTA-3′ | |
| HAS-2 | Forward | 5′-CCA ATG CAG TTT CGG TGA TG-3′ |
| Reverse | 5′-ACT TGG ACC GAG CCG TGT AT-3′ | |
| HAS-3 | Forward | 5′-CCT CAT CGC CAC AGT CAT ACA A-3′ |
| Reverse | 5′-CCA CCA GCT GCA CCG TTA GT-3′ | |
| GAPDH | Forward | 5′-GAG TCA ACG ATT TGG TCG T-3′ |
| Reverse | 5′-GAC AAG CTT CCC GTT CTC AG-3′ |
MMP – Matrix metalloproteinase; HAS – Hyaluronan synthase; GAPDH – Glyceraldehyde-3- phosphate dehydrogenase.
Standard curves were used to determine the relative ratio of gene expression. Gene expression ratios from young rats (2 month) were used for PCR control. Target gene ratios were normalized by comparing the ratios of the internal control gene (GAPDH) with gene expression ratios from PCR control. The normalization consisted of using the differences between the cycle thresholds (ΔCT) and the expression level for GAPDH to calculate the ETΔCT(1-2)/ERΔCT(1-2) ratio, where (ET) corresponds to the expression level of the target gene, (ΔCT(1-2)) corresponds to the differences between the cycle thresholds (ΔCT) for the internal control gene and the control sample, and (ER) corresponds to the expression level for the internal control gene. Separate one-way analysis of variance (ANOVA) tests were used to investigate differences in gene expression ratios across age groups. An adjusted alpha level of .01 was used to control for type I error. For group analyses, vocal folds were averaged for each animal and the expression ratios were log-transformed to better meet the assumptions of the ANOVA. If the F test revealed a significant main effect, pairwise comparisons between age groups were examined using Tukey's HSD. For post-hoc testing, threshold for significance was set to p < .0167 (.05/3) to account for multiple pairwise comparisons. All analyses were performed with the use of 2-tailed p-values. Data were analyzed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Histology
Whole larynges were soaked in embedding medium (OCT compound, Tissue-Tek, Torrance, CA, USA), frozen quickly with a combination of acetone and dry ice, and stored in a deep freezer (-80°C). Ten-micron thick serial sections were prepared in the coronal plane. The sections were made in the same systematic manner from the posterior to the anterior portion of the vocal fold. Verhoeff's elastic stain was performed to identify collagen. The hyaluronidase digestion technique was used to detect HA. For the hyaluronidase digestion procedure, 50 mg of bovine testes hyaluronidase (Sigma, St Louis, MO, USA) were diluted in 100ml PBS solution, and each section was incubated in this solution for 1 hour at 37°C. Next, the sections were stained with Alcian blue (pH 2.5). HA was detected by comparing the sections without digestion to those with digestion.
Results
PCR
Results of ANOVA revealed a significant main effect for procollagen type I, procollagen type III, MMP-2, and MMP-9 among young (2 month), adult (9 month), and elderly vocal folds (18 month) (p <.0001). Pairwise comparisons revealed that the expression of procollagen type I decreased significantly in adult (Figure 1A; p <.0001) and elderly vocal folds (Figure 1A; p <.0001) compared to young vocal folds. The expression of procollagen type I was also significantly lower in elderly vocal folds, compared to adult vocal folds (Figure 1A; p =.001). Procollagen type III expression decreased significantly in adult (Figure 1B; p <.0001) and elderly vocal folds (Figure 1B; p <.0001) compared to young vocal folds. Procollagen type III was also significantly lower in elderly vocal folds, compared to adult vocal folds (Figure 1B; p =.003). The expression of MMP-2 decreased significantly in adult (Figure 1C; p =.001) and elderly vocal folds (Figure 1C; p <.0001) compared to young vocal folds. MMP-9 expression decreased significantly in adult (Figure 1D; p <.0001) and elderly vocal folds (Figure 1D; p <.0001) compared to young vocal folds.
Fig 1.
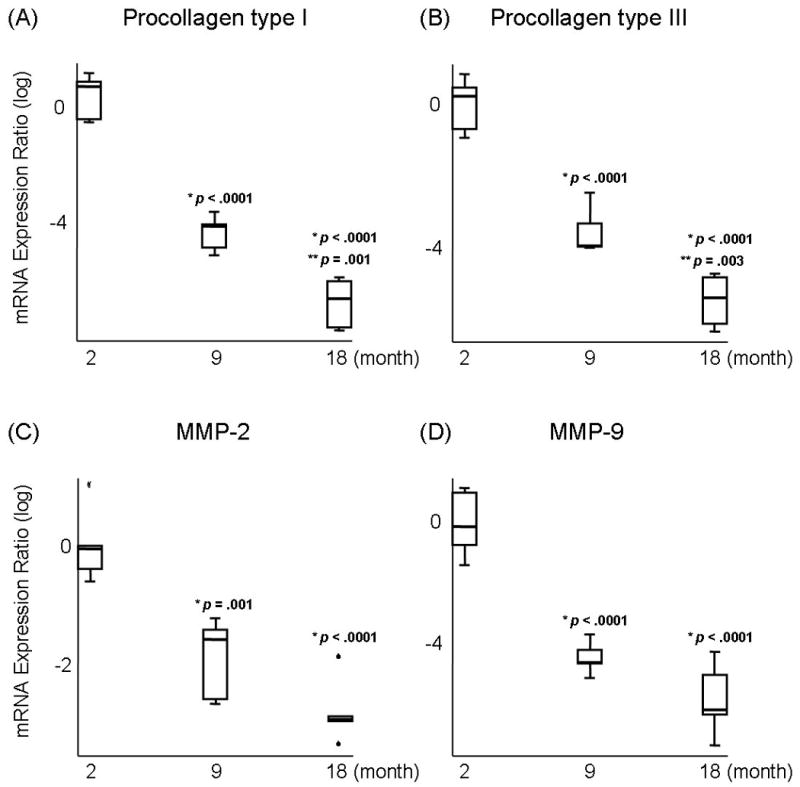
Log-transformed mRNA expression ratios of (A) Procollagen type I, (B) Procollagen type III, (C) Matrix metalloproteinase (MMP)-2, and (D) MMP-9 in young (2 month), adult (9 month), and elderly vocal folds (18 month). Boxes indicate first and third quartiles. Whisker caps indicate smallest and largest non-outlier observations. Outlier indicated by °. Extreme indicated by  . Median observations denoted by thick black line in each box. p values indicate a significant difference from (*) young and (**) adult vocal folds.
. Median observations denoted by thick black line in each box. p values indicate a significant difference from (*) young and (**) adult vocal folds.
Results of ANOVA revealed a significant main effect for HAS-1, HAS-2, and HAS-3 expression across age (p <.0001). Pairwise comparisons revealed that the expression of HAS-1 decreased significantly in adult (Figure 2A; p <.0001) and elderly vocal folds (Figure 2A; p <.0001) compared to young vocal folds. HAS-2 expression decreased significantly in adult (Figure 2B; p =.003) and elderly vocal folds (Figure 2B; p <.0001) compared to young vocal folds. HAS-2 expression was also significantly lower in elderly vocal folds, compared to adult vocal folds (Figure 2B; p =.004). The expression of HAS-3 decreased significantly in adult (Figure 2C; p <.0001) and elderly vocal folds (Figure 2C; p <.0001) compared to young vocal folds. HAS-3 expression was also significantly lower in elderly vocal folds, compared to adult vocal folds (Figure 2C; p <.0001).
Fig 2.
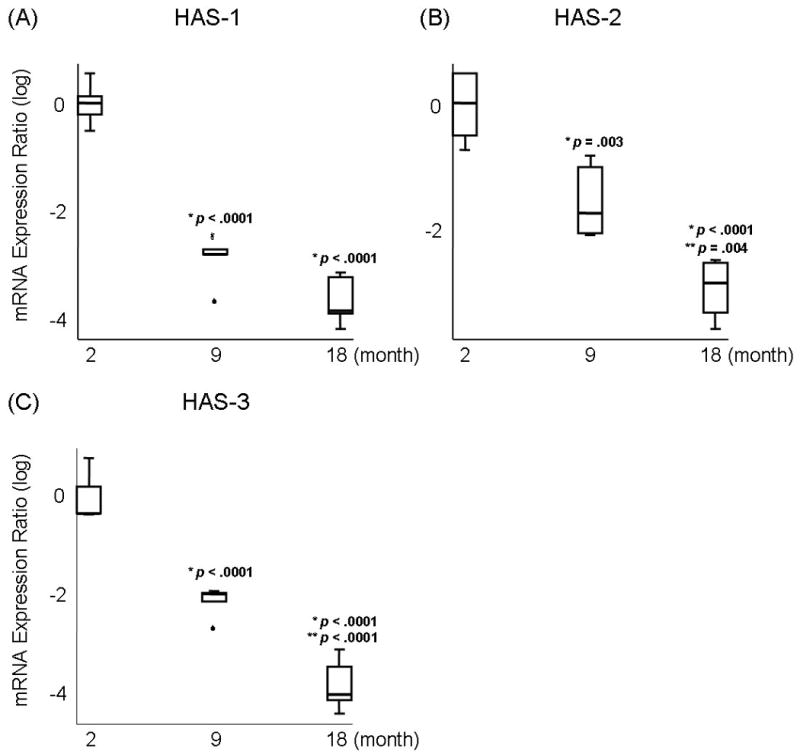
Log-transformed mRNA expression ratios of (A) Hyaluronan synthase (HAS)-1, (B) HAS-2, and (C) HAS-3 in young (2 month), adult (9 month), and elderly vocal folds (18 month). Boxes indicate first and third quartiles. Whisker caps indicate smallest and largest non-outlier observations. Outlier indicated by °. Extreme indicated by  . Median observations denoted by thick black line in each box. p values indicate a significant difference from (*) young and (**) adult vocal folds.
. Median observations denoted by thick black line in each box. p values indicate a significant difference from (*) young and (**) adult vocal folds.
Histology
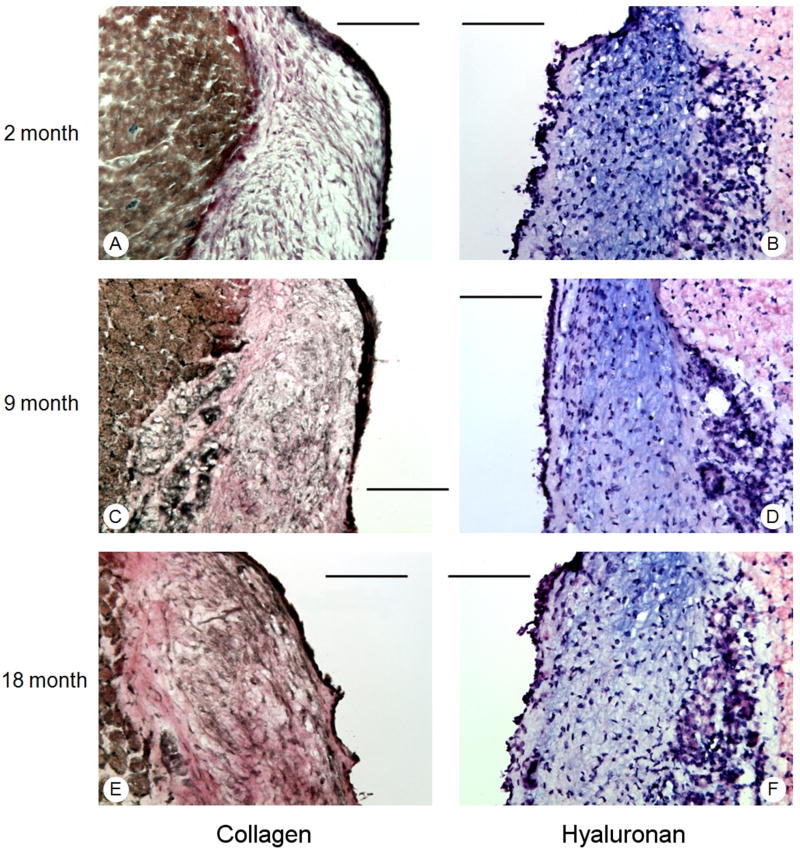
Young vocal folds revealed sparse collagen and dense HA throughout the lamina propria (Figure 3A, 3B). In contrast, dense staining of collagen and sparse HA was present in adult and elderly vocal folds (Figure 3C, 3D, 3E, 3F).
Fig 3.
Coronal sections of young (2 month) (A,B); adult (9 month) (C,D); and elderly (18 month) (E,F) vocal folds stained with Verhoeff's elastic stain (collagen) and Alcian blue stain (hyaluronan). Results revealed dense deposition of collagen (pink staining) in adult and elderly vocal folds, compared to young vocal folds, and less dense staining of hyaluronan (blue stain) in elderly vocal folds, compared to young and adult vocal folds. Bars represent 100μm.
Discussion
Matrix metalloproteinases (MMPs) are a family of zinc-containing endopeptidases involved in the turnover of structural components of the ECM. 10 MMP-2 (gelatinase A) and MMP-9 (gelatinase B) display substrate specificity for collagen. 11,12 MMP-2 encodes an enzyme which degrades the major structural component of the basement membrane (type IV collagen). MMP-9 encodes for an enzyme which displays broad spectrum activity and affinity for denatured collagens, membranous collagens (type IV), interstitial collagens (type V collagen), and elastin.
Results from the current study revealed that the expression of MMP-2 and MMP-9 decreased significantly in adult and elderly vocal folds, compared to young vocal folds. No further decline in MMP expression was observed beyond adulthood. That is, adult and elderly vocal folds revealed similar expression levels of MMP-2 and MMP-9. Findings are similar to Ding et al. who found decreased expression of MMP-2 and MMP-9 in adult and elderly vocal folds, compared to young vocal folds.7 These same investigators found that the expression of MMPs, albeit decreased, did not diminish any further after adulthood.7
In contrast, procollagen type I and procollagen type III expression decreased significantly in adult vocal folds, compared to young vocal folds, and continued to diminish beyond adulthood. Elderly vocal folds displayed significantly decreased procollagen type I and procollagen type III expression levels in comparison to adult vocal folds. Histology revealed that young vocal folds contained sparse collagen throughout the lamina propria, while the deposition of collagen was dense in adult and elderly vocal folds.
The advantages of a rat model for studies involving aging of the vocal folds include the wide availability of extracellular matrix primers. Ding et al. also postulated that the rat model provides an opportunity to investigate the effects of aging without the contamination of biomechanical force effects.7 While it is likely that the biomechanical forces experienced by rats during vocalization is different than humans, rats do produce meaningful ultrasonic vocalizations. 13 The types of vocalizations emitted by rats depend on a number of factors, including the animal's age, its environmental conditions, and its affective state. For example, adult rats produce a 22-kHZ vocalization when faced with an inescapable aversive stimulus, such as exposure to a predator, foot shock, or an alarming sound. 14 These calls are typically between 300 to 4000 ms in duration and are produced at a sound pressure level of 65 to 85 dB. 14 Rats also emit 50-kHZ vocalizations in situations that are rewarding such as sexual activity, playful behavior, and/or arousal over winning a fight.14 Given the likelihood that the rats in this study vocalized less in their shorter lifespan than elderly humans do in the course of an average lifetime, the findings of decreased expression of procollagens and MMPs in adult and elderly vocal folds, in the context of increased ECM collagen, provides support for the hypothesis proposed by Ding et al of an age-dependent slowdown in the synthesis and turnover of vocal fold collagen. 7 These data may also suggest that the increase in collagen deposition observed in elderly humans may not be due to the effects of biomechanical forces alone. This is because, in the absence of human phonatory forces in the rat vocal fold, the balance between synthesis and degradation of collagen appears to result in an increased amount of collagen, as observed in elderly humans. 3,4 In other words, the ECM changes observed in human and rat vocal folds may be related to normal age-related changes in gene expression and not necessarily due to vocal fold use and abuse over a lifetime. Interestingly, and perhaps providing additional evidence for this is the finding that age-related changes in the expression of vocal fold collagen and elastin are comparatively similar to lung and skin, suggesting that these mRNA changes may be indicative of an age-dependent degenerative process across various organ systems in the body. 7,15 The overaccumulation of ECM collagen in humans is likely to have adverse effects on stiffness of the lamina propria, as collagen type I provides tensile strength to the tissue, and collagen type III, a reticular fiber, contributes to structural maintenance of the vocal fold. 16 However, the question of whether increased stiffness of the aged vocal fold is the result of an increased amount of collagen and/or increased collagen cross-linking remains unknown. In actuality, the answer to this question may not be so simple. Theories that attribute stiffness alterations of the vocal fold to structural changes in fibrous proteins alone exclude the possibility that alterations in other key ECM components, such as interstitial proteins and glycosaminoglycans may play a contributing role.
HA is an important component of the ECM and contributes to the viscoelastic properties of the vocal fold lamina propria.17 Butler et al reported decreased HA levels in the vocal fold lamina propria in elderly humans.6 Thus, a second aim of this study was to investigate whether the histologic deposition of HA would be similarly decreased in adult and elderly vocal folds, and to determine whether a slowdown in the synthesis of genes coding HAS is consistent with decreased ECM HA. Results revealed that the expression of HAS-1, -2, and -3 decreased significantly in adult and elderly vocal folds, compared to young vocal folds. HAS-1 expression decreased significantly in adult vocal folds, compared to young vocal folds, but expression levels were similar between adult and elderly vocal folds. In contrast, HAS-2 and HAS-3 expression decreased significantly in adult vocal folds, compared to young vocal folds, and continued to decrease after adulthood. Histologically, ECM HA levels were less dense in elderly vocal folds, compared to young and adult vocal folds.
Findings of decreased expression of genes coding HAS was consistent with decreased density of ECM HA in the aged vocal fold. It would be interesting in the future to also investigate whether the expression of genes coding hyaluronidases (HA degrading enzymes) are similarly decreased in the aged vocal fold, which may provide evidence for a slowdown in the synthesis and turnover of HA during the aging process. An alternative explanation for decreased ECM HA may be that the expression of genes coding hyaluronidases are upregulated, giving way to unchecked turnover of HA in the aged vocal fold. However, in the context of the age-associated slowdown in ECM gene expression mentioned already, this appears unlikely.
Future studies should also consider the effects of cell phenotype and decreased fibroblast density in the observed mRNA changes associated with aging of the vocal fold. For example, the accumulation of dysfunctional senescent cells may contribute to the observed mRNA changes and play a role in the transcription and translation of proteins and the associated histologic manifestations observed in the aged vocal fold. It would also be interesting to determine the individual contributions of various cell populations (fibroblasts, epithelial cells, etc.) on synthesis and maintenance of the aged vocal fold. The isolation of epithelial cells and cells of mesenchymal origin from aged vocal fold specimens will be necessary to address these questions. In the current study, all mucosal layers above the thyroarytenoid muscle, including epithelium were used for PCR. It was not determined how specific cells might have contributed to the mRNA and histologic findings. However, it may be possible in the future to investigate cellular production from individual cell types.
Collectively, these results suggest that the issue of increased stiffness of the aged vocal fold is likely a result of more than just structural changes in fibrous proteins. Furthermore, given the findings of decreased expression of genes coding MMPs, procollagens, HAS and associated histologic changes, these candidate genes may provide logical targets for treatments aimed at stimulating synthesis and turnover of the ECM, and for optimizing tissue viscoelastic properties.
Conclusion
We performed a prospective study to investigate age-associated changes in the expression of genes coding collagens, HA, MMPs, and histologic changes in the deposition of collagen and HA in the aged vocal fold. Results revealed that in the absence of human phonatory forces, a slowdown in the expression of procollagens and MMPs results in an increased amount of collagen in the aged vocal fold, as observed in humans. A similar decrease in the expression of genes coding HAS was observed in elderly vocal folds, which was consistent with decreased density of ECM HA. These data provide a foundation for future studies aimed at investigating strategies for the treatment of age-related vocal fold changes in the rat model and potential targets for treatments aimed at stimulating synthesis and turnover of the extracellular matrix.
Acknowledgments
Research supported by NIH grant R21 DC 009873 from the National Institute of Deafness and Other Communication Disorders (NIDCD).
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center.
References
- 1.Roy N, Stemple J, Merrill RM, Thomas L. Epidemiology of voice disorders in the elderly: preliminary findings. Laryngoscope. 2007;117:628–33. doi: 10.1097/MLG.0b013e3180306da1. [DOI] [PubMed] [Google Scholar]
- 2.Hirano M, Kakita Y. Cover-body theory of vocal fold vibration. In: Daniloff RG, editor. Speech science. San Diego, Calif: College Hill Press; 1985. pp. 1–46. [Google Scholar]
- 3.Hammond TH, Gray SD, Butler JE. Age- and gender-related collagen distribution in human vocal folds. Ann Otol Rhinol Laryngol. 2000;109:913–20. doi: 10.1177/000348940010901004. [DOI] [PubMed] [Google Scholar]
- 4.Sato K, Hirano M, Nakashima T. Age-related changes of collagenous fibers in the human vocal fold mucosa. Ann Otol Rhinol Laryngol. 2002;111:15–20. doi: 10.1177/000348940211100103. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Hirano M. Age-related changes of elastic fibers in the superficial layer of the lamina propria of vocal folds. Ann Otol Rhinol Laryngol. 1997;106:44–48. doi: 10.1177/000348949710600109. [DOI] [PubMed] [Google Scholar]
- 6.Butler JE, Hammond TH, Gray SD. Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope. 2001;111(5):907–11. doi: 10.1097/00005537-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Ding H, Gray SD. Senescent expression of genes coding collagens, collagen-degrading metalloproteinases, and tissue inhibitors of metalloproteinases in rat vocal folds: comparison with skin and lungs. J Gerontol A Biol Sci Med Sci. 2001;56(4):B145–52. doi: 10.1093/gerona/56.4.b145. [DOI] [PubMed] [Google Scholar]
- 8.Ohno T, French LC, Hirano S, Ossoff RH, Rousseau B. Effect of Hepatocyte Growth Factor on Gene Expression of Extracellular Matrix During Wound Healing of the Injured Rat Vocal Fold. Ann Otol Rhinol Laryngol. 2008;117:696–702. doi: 10.1177/000348940811700912. [DOI] [PubMed] [Google Scholar]
- 9.Liuzzi GM, Mastroianni CM, Latronico T, Mengoni F, Fasano A, Lichtner M, Vullo V, Riccio P. Anti-HIV drugs decrease the expression of matrix metalloproteinases in astrocytes and microglia. Brain. 2004;127(Pt 2):398–407. doi: 10.1093/brain/awh049. Epub 2003 Dec 8. [DOI] [PubMed] [Google Scholar]
- 10.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001;276:7549–58. doi: 10.1074/jbc.M010242200. [DOI] [PubMed] [Google Scholar]
- 12.Manuel JA, Gawronska-Kozak B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biol. 2006;25(8):505–14. doi: 10.1016/j.matbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T. Limb use and complex ultrasonic vocalization in a rat model of Parkinson's disease: Deficit-targeted training. Parkinsonism and Related Disorders. 2008;14:S172–S175. doi: 10.1016/j.parkreldis.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of the American Association for Laboratory Animal Science. 2007;46(1):28–34. [PubMed] [Google Scholar]
- 15.Ding H, Gray SD. Senescent expression of genes coding tropoelastin, elastase, lysyl oxidase, and tissue inhibitors of metalloproteinases in rat vocal folds: comparison with skin and lungs. Journal of Speech, Language, and Hearing Research. 2001;44:317–326. doi: 10.1044/1092-4388(2001/026). [DOI] [PubMed] [Google Scholar]
- 16.Gray SD, Hirano M, Sato K. Vocal fold physiology: frontiers of basic science. San Diego: Singulair Publishing Group; 1993. Molecular and cellular structure of vocal fold tissue; pp. 1–34. [Google Scholar]
- 17.Chan RW, Titze IR. Hyaluronic acid (with fibronectin) as a bioimplant for the vocal fold mucosa. Laryngoscope. 1999;109:1142–1149. doi: 10.1097/00005537-199907000-00026. [DOI] [PubMed] [Google Scholar]