Abstract
Objective
Few patients take inhaled corticosteroids as recommended. This study aimed to determine the effectiveness of school-based supervised asthma therapy in improving asthma control. The primary hypothesis was that the supervised asthma therapy group would have a lower proportion of children experiencing an episode of poor asthma control (EPAC) each month compared to the usual care group.
Patients and Methods
Children were eligible if they had physician-diagnosed persistent asthma, the need for daily controller medication, and the ability to use a dry-powder inhaler and a PFM. The trial used a two-group randomized longitudinal design with 15 month follow-up. 290 children from 36 schools were randomly assigned to either: school-based supervised asthma therapy or usual care. Ninety-one percent of children were African American and 57% were male. Mean age was 11 years (SD = 2.1). An EPAC was defined as one or more of the following each month: 1) an absence from school due to respiratory illness/asthma; 2) average use of rescue medication more than two times per week (not including pre-exercise treatment); or 3) at least one red or yellow PFM reading.
Results
240 children completed the study. There were no differences in the likelihood of an EPAC between the baseline and follow-up period in the usual care group (p=0.77); however, among those in the supervised therapy group, the odds of experiencing an EPAC during the baseline period were 1.57 times the odds of experiencing an EPAC during the follow-up period (90% CI: 1.20, 2.06, p=0.006). GEE modeling revealed a marginally significant interaction between the intervention and time period (p=0.065) indicating that children in the supervised therapy group showed greater improvement in asthma control.
Conclusions
Supervised asthma therapy improves asthma control. Clinicians who have pediatric asthma patients with poor outcomes that may be due to non-adherence should consider supervised therapy.
Keywords: asthma, child, anti-asthmatic drugs, schools
Introduction
Inhaled corticosteroids reduce pediatric asthma morbidity and decrease hospitalizations and emergency department visits by as much as 50%.1–5 However, few asthma patients take these medications as recommended.6, 7,8 Therefore, pediatric asthma guidelines recommend testing interventions to increase adherence.9
Among children, rates of daily controller medication use range from 18% to 65%,10, 11 with most reports indicating less than 50% adherence. Medication adherence is lower among children in non-white or low-income families.12, 13 Among children enrolled in Medicaid, which covers medication costs, asthma controller medication adherence was 22%.2
Directly observed therapy can ensure adherence14 and help establish the habit of taking a daily medication. This strategy has been used for diseases where treatment adherence is essential due to infectiousness and potential for drug resistance (Tuberculosis)15 or where medication resources are limited (HIV).16
Schools are a logical place to promote adherence among children.17 With an average minimum of 180 school days each year,18 schools provide an opportunity to help children establish good health habits, especially for chronic conditions requiring regular treatment regimens. Furthermore, asthma morbidity is seasonal with the greatest number of exacerbations occurring during the school year.19–21 Three previous studies suggest that supervised therapy at school improves asthma outcomes.22,23,24 Two small studies indicated that school based delivery of steroids improved asthma control;23–24 however, both studies had small sample sizes and short follow-up periods. A larger study of 180 children indicated that school based supervision of inhaled steroids improves asthma symptoms but only among children who were not exposed to secondhand smoke.22 This manuscript describes a randomized trial of the effectiveness of school-based supervised asthma therapy in improving asthma control.
Methods
Participants
Children with physician-diagnosed persistent asthma, need for daily controller medication, enrolled in one of 36 participating schools, who had the ability to use a dry-powder inhaler and a peak flow meter (PFM) were enrolled. Children were prescribed the same inhaled corticosteroid and were excluded if their physician felt it inappropriate to change their prescription to budesonide inhalation powder. Children were recruited through local schools, physician offices, and health departments. Parents provided written informed consent; children provided assent. The University of Alabama at Birmingham’s Institutional Review Board and an independent Data Safety and Monitoring Board approved and monitored the study.
Upon enrollment, children were assessed by a physician (their primary care provider or, if no primary care provider was identified, the study physician) and prescribed budesonide inhalation powder in a single daily dose specific to their need (1–4 puffs).25–31 Inhaled steroids and rescue medication were provided at no cost. Refills were supplied by mail upon telephone request. Children were also provided with an asthma action plan and two PFMs (one for home, one for school). Children could take additional asthma medications if their physician felt it was warranted; these medications were not supervised by study staff.
Study Design
Using a two-group randomized longitudinal design with a 15 month follow-up, children were randomly assigned to: school-based supervised asthma therapy or usual care (parent-supervised asthma therapy). Upon enrollment by study staff, children were assigned an ID number corresponding to their school. A random sequence of treatment codes, stratified by school system, was generated using the SAS System (Version 9.1, Cary, North Carolina) by the statistician.
Intervention
For children randomized to the supervised therapy arm, use of inhaled corticosteroids was supervised by study staff at school each day. A standard daily time was arranged with each school, during which staff observed children taking their medication. If a child arrived later in the day, study staff returned to the school to supervise medication use. If a child was observed using their inhaler incorrectly, staff provided education with the aid of a placebo inhaler. Those randomized to usual care continued their usual parent- or self-supervised use of daily inhaled corticosteroids at home.
Data collection
Baseline data collection occurred from October 2005-December 2005. In January 2006, children were randomized to either supervised therapy or usual care. Randomization occurred at the individual student level within each school system, to account for factors that differ across school systems. Follow-up data were collected from January 2006 – December 2006 in the same manner as the baseline. To avoid differences in the frequency of episodes of poor asthma control (EPAC) due to seasonal variation, the main comparison uses the October through December data in both school years.20, 21
Daily PFM readings, rescue medication use and school absences were collected at school on all children. Previous experience indicated that daily data collection is difficult to achieve without interrupting school schedules.32 To minimize these interruptions, investigators collaborated with Blue Cross and Blue Shield of Alabama to develop a web-based data collection system (Asthma Agents System). A detailed description of this system has been published.33
PFM readings were based on the values representing the child’s “best” peak flow rates during healthy periods at the beginning of each school year. Red readings indicated peak flow rates less than or equal to 50% of the “best” value; yellow readings indicated peak flow rates at 50–80% of the “best” value; green were readings above 80%. School staff were trained to have children log on to the Asthma Agents System at the same time each day to prevent diurnal variation in PFM readings. Designated school staff also logged on to the system to verify PFM readings and report on child absences.
At-school rescue medication use was monitored using a Doser™ (Meditrack, Hudson, MA) attached to the top of the inhaler which activated automatically to record each inhalation taken. Study staff read each child’s Doser™ every two weeks to record usage. No data were collected on rescue medication or inhaled steroid use at home either on weekends or during school breaks. As children spend the majority of their time at school during the time when asthma symptoms are most common,18, 20 the cost and effort of collecting data at home were thought excessive in relation to the return. Data on health care utilization, secondhand smoke exposure, and quality of life (QOL) were collected at the beginning of each school year and at the end of the study through telephone interviews with the parent. Secondhand smoke exposure status was assessed using two questions: (1) Are there smokers in the house where your child lives? and, (2) Are there smokers in other places where your child spends a lot of time, such as a day-care or a friend’s house? Smoke exposure was grouped into three categories (No secondhand smoke exposure; Secondhand smoke exposure outside of the primary household only; Secondhand smoke exposure inside the primary household). QOL was assessed using the Juniper Pediatric Asthma Caregiver Quality of Life Questionnaire.34 Asthma safety events were monitored by the DSMB using daily data from the internet based collection system (asthma symptoms and peak flow meter readings). In addition, teachers and parents were asked to report adverse events throughout the study period. Study staff had weekly contact with teachers and reminded them to report adverse events related to the study drug (e.g., thrush) as well as emergency room visits or hospitalizations due to asthma. Parents were sent information regarding adverse events at the beginning of each school year and asked to call study personnel if these occurred. In addition, parents were interviewed by phone three times during the study period.
Outcome Variables
The primary hypothesis was that the supervised asthma therapy group would have a lower proportion of children experiencing an episode of poor asthma control (EPAC) each month compared to the usual care group. An EPAC was defined as one or more of the following each month: 1) an absence from school due to respiratory illness/asthma; 2) average use of rescue medication more than two times per week (not including pre-exercise treatment); or 3) at least one red or yellow PFM reading.
Sample Size
A sample size of 100 children per group was calculated to have a minimum 80% power to detect a 10% time averaged difference in the proportion of EPACs between the groups (two-sided alpha of 0.05).35 We assumed a correlation no larger than 0.25 among a child’s outcomes and adjusted for seasonality in the rate of EPACs.
Statistical Analysis
The primary hypothesis was tested in two ways. A Chi-Square test was used to examine the difference in the probability of experiencing EPACs between the two groups for each individual month, as well as the probability of experiencing EPACs in each of the baseline and follow-up periods. Generalized estimating equations (GEEs) were fitted, in which the outcome indicated if each individual experienced an EPAC in each month of the study period. We examined the interaction between the intervention and period (baseline, follow-up) in order to assess whether the effect of the intervention differed by period. Primary analyses considered the complete definition of an EPAC, while secondary analyses considered each of the three components of the definition of an EPAC individually.
Results
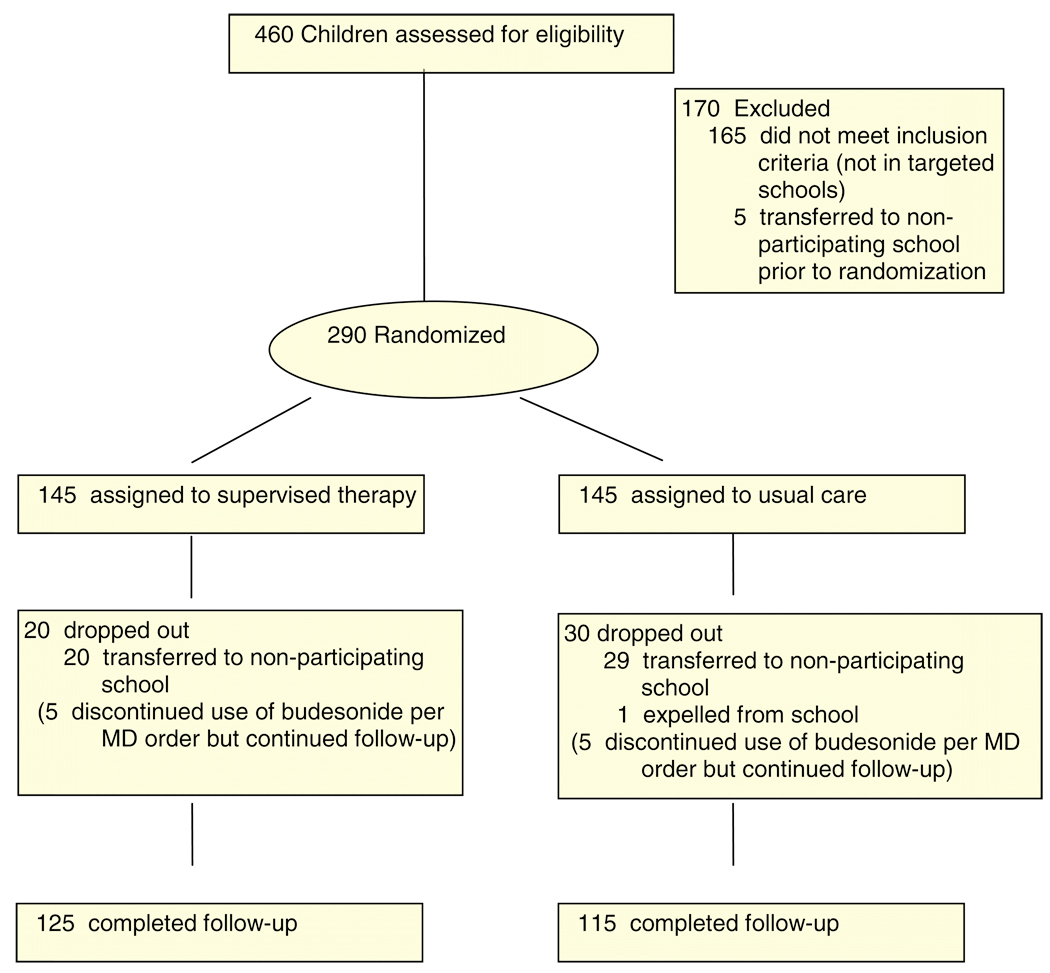
Four hundred sixty children were assessed for eligibility (See Figure 1), 295 were eligible (97% of those ineligible were not enrolled in the targeted schools), and 290 were randomized. Attrition was primarily due to children transferring to a non-participating school (98%). There was no significant difference between treatment groups in attrition rate (p=0.26). After randomization, children who discontinued use of budesonide in accordance with their physician’s order continued providing daily data.
Figure 1.
Flow of participants through the study
The demographic and asthma characteristics of children randomized are shown in Table 1. There were no significant differences between the supervised therapy and usual care groups in any of the demographic or asthma characteristics. Children had an average age of 11 years, were 57% male and primarily black race (91%). Seventy-nine percent of children had moderate persistent asthma yet only 14% had rescue medication at school prior to the study. During the baseline period, there was no difference between the two treatment groups in the percent of expected budesonide refills that were filled (Supervised therapy = 57.3%; Usual Care = 54.2%, p=0.40). Because medication was administered daily by the study staff to the children in the supervised therapy group, we induced a high level of adherence among these children. However, the average adherence over the entire study period in the usual care group was 38% (SD = 25%). Further, 78% of children in the usual care group were ≤50% adherent indicating that the distribution of adherence is both highly variable and highly skewed.
Table 1.
Demographic Characteristics of Randomized Participants according to treatment group.
| Supervised Therapy (n=145) |
Usual Care (n=145) |
Total (n=290) |
p-value¥ | |
|---|---|---|---|---|
| Age | 11.1 (2.0) | 10.8 (2.1) | 11.0 (2.1) | 0.25 |
| Male Gender | 87 (60%) | 78 (54%) | 165 (57%) | 0.29 |
| Black Race | 133 (92%) | 130 (90%) | 263 (91%) | |
| Asthma Severity (2 missing) |
||||
| Mild persistent | 22 (15%) | 24 (17%) | 46 (16%) | 0.54 |
| Moderate persistent | 113 (79%) | 115 (80%) | 228 (79%) | |
| Severe persistent | 9 (6%) | 5 (3%) | 14 (5%) | |
| Rescue Medication at school prior to study |
25 (17%) | 16 (11%) | 41 (14%) | 0.18 |
| Number of daily puffs of budesonide prescribed |
2.7 (1.1) Range 1–4 |
2.7 (1.1) Range 1–4 |
2.7 (1.1) Range 1–4 |
1.0 |
| % of expected budesonide refills that were filled* |
57.3% SD = 28.7% |
54.2% SD = 31.5% |
55.5% SD = 30.1% |
0.40 |
| Secondhand smoke exposure** |
0.42 | |||
| In home | 42 (31%) | 35 (27%) | 77 (29%) | |
| Outside the home | 28 (21%) | 22 (17%) | 50 (19%) | |
| ≥ 1 hospitalization for asthma in past year |
12 (9%) | 15 (12%) | 27 (10%) | 0.54 |
| ≥ 1 emergency department visit for asthma in past year |
53 (40%) | 52 (41%) | 105 (40%) | 0.90 |
| ≥ 1 urgent care visit for asthma in the past year |
90 (67%) | 84 (66%) | 174 (66%) | 0.79 |
| Average days absent | 4.3 (3.9) | 4.1 (3.8) | 4.2 (3.8) | 0.61 |
| Average days absent due to respiratory illness |
1.0 (2.2) | 0.7 (1.5) | 0.9 (1.9) | 0.10 |
Refill data calculated from enrollment through the end of the baseline period; defined as proportion of expected refills that were actually refilled
Exposure data available on 265 children (135 supervised therapy and 130 usual care)
p-values based on t-test or chi-squares, as appropriate
The internet based data collection system allowed us to collect high quality data with very few missing reports.36 During the study period, there were a total of 26,417 daily reports expected from the teachers and children. These daily reports provided information on peak flow meter readings, asthma symptoms, and absences. Of the observed reports, 25,744 (97.5%) provided data regarding asthma control. There were no adverse events related to the study drug reported.
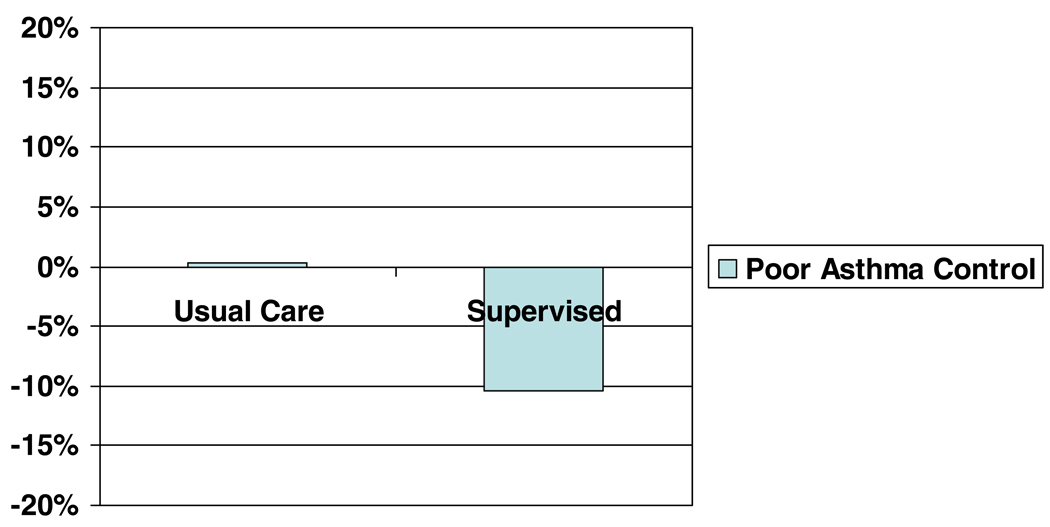
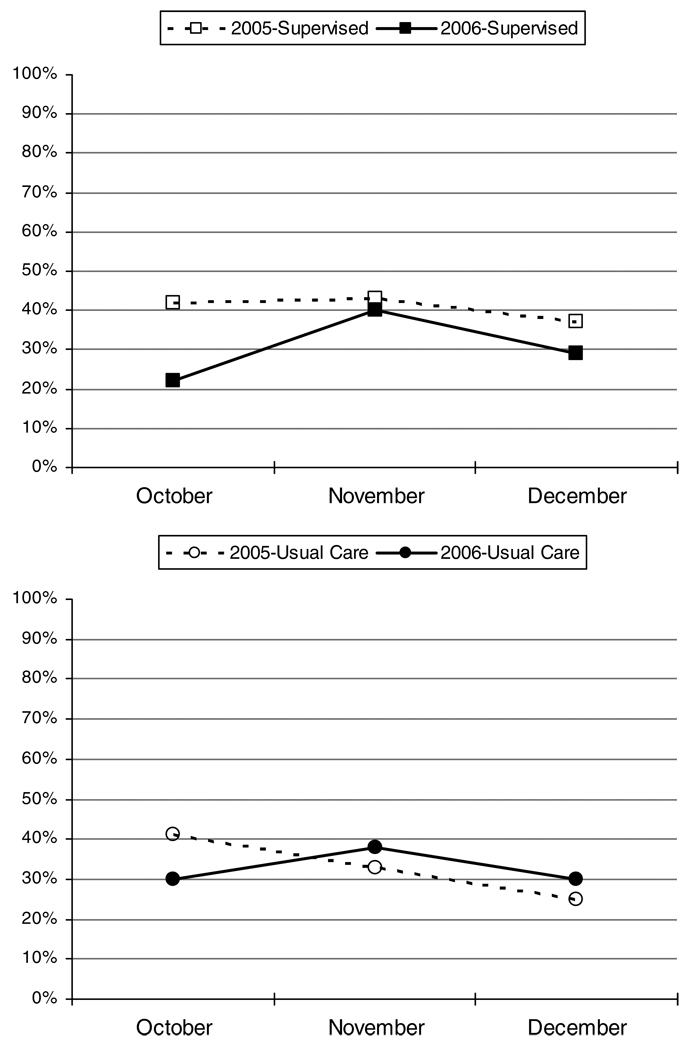
There were no differences in the likelihood of experiencing an EPAC between the baseline and follow-up period in the usual care group (p=0.94); however, among those in the supervised therapy group, the odds of experiencing an EPAC during the baseline period were 1.57 times the odds of experiencing an EPAC during the follow-up period (90% CI: 1.20, 2.06, p=0.006). Figure 2 shows the decrease in percent of children experiencing an EPAC from baseline to follow-up by treatment group and Figure 3 shows the percent of children experiencing an EPAC each month by treatment group. Table 2 shows results of the univariate analysis by month. Higher rates of EPACs were reported for the supervised therapy group than the usual care in November (not statistically significant) and December (p=0.04) of the baseline period.
Figure 2.
Change in percent of children experiencing an episode of poor asthma control from baseline to intervention by treatment group.
P=0.065 for interaction
Figure 3.
Exacerbations by month: supervised therapy1 and usual care2
1P = 0.0064 for Supervised Therapy 2005 to 2006
2P = 0.94 for Usual Care 2005 to 2006
Table 2.
Episodes of poor asthma control by month, according to treatment group.
| Baseline | Intervention | |||||
|---|---|---|---|---|---|---|
| Oct | Nov | Dec | Oct | Nov | Dec | |
| Episodes of poor asthma control | ||||||
| Usual | 59 (41%) | 48 (33%) | 36 (25%) | 36 (30%) | 45 (38%) | 35 (30%) |
| Supervised | 61 (42%) | 62 (43%) | 53 (37%) | 28 (22%) | 50 (40%) | 36 (29%) |
| p-value | 0.91 | 0.12 | 0.04 | 0.19 | 0.79 | 0.89 |
| Individual components of asthma control | ||||||
| Red or yellow peak flow meter readings | ||||||
| Usual | 38 (26%) | 30 (21%) | 16 (11%) | 12 (10%) | 19 (16%) | 22 (19%) |
| Supervised | 49 (34%) | 42 (29%) | 40 (28%) | 16 (13%) | 29 (23%) | 22 (18%) |
| p-value | 0.20 | 0.13 | <0.001 | 0.55 | 0.20 | 0.87 |
| Absence due to respiratory illness | ||||||
| Usual | 27 (19%) | 19 (13%) | 8 (6%) | 12 (10%) | 25 (21%) | 16 (14%) |
| Supervised | 23 (16%) | 26 (18%) | 18 (12%) | 13 (10%) | 29 (23%) | 20 (16%) |
| p-value | 0.64 | 0.33 | 0.06 | >0.99 | 0.76 | 0.72 |
| Rescue medication use | ||||||
| Usual | 10 (7%) | 12 (9%) | 14 (10%) | 15 (13%) | 14 (12%) | 9 (8%) |
| Supervised | 7 (5%) | 10 (7%) | 6 (4%) | 5 (4%) | 4 (3%) | 1 (<1%) |
| p-value | 0.62 | 0.83 | 0.10 | 0.017 | 0.012 | 0.008 |
GEE modeling revealed a marginally significant interaction between the intervention and time period (p=0.065) indicating that children in the supervised therapy group showed greater improvement in asthma control from baseline to follow-up than children in the usual care group. The odds of experiencing an EPAC among those in the supervised group during the baseline period were significantly higher (1.38; 90% CI: 1.03, 1.87) than the odds of those in the usual care group during the same period, while the odds of experiencing an EPAC during the follow-up period among those in the supervised group were lower (0.89; 90% CI: 0.64, 1.22) than those in the usual care group during the baseline period. Looking at the individual components of the EPAC definition, there were no significant differences in either the interaction between or the main effects for treatment or time period (tests of interaction: PFM readings, p=0.20; absence due to respiratory illness, p=0.62; rescue medication, p=0.11).
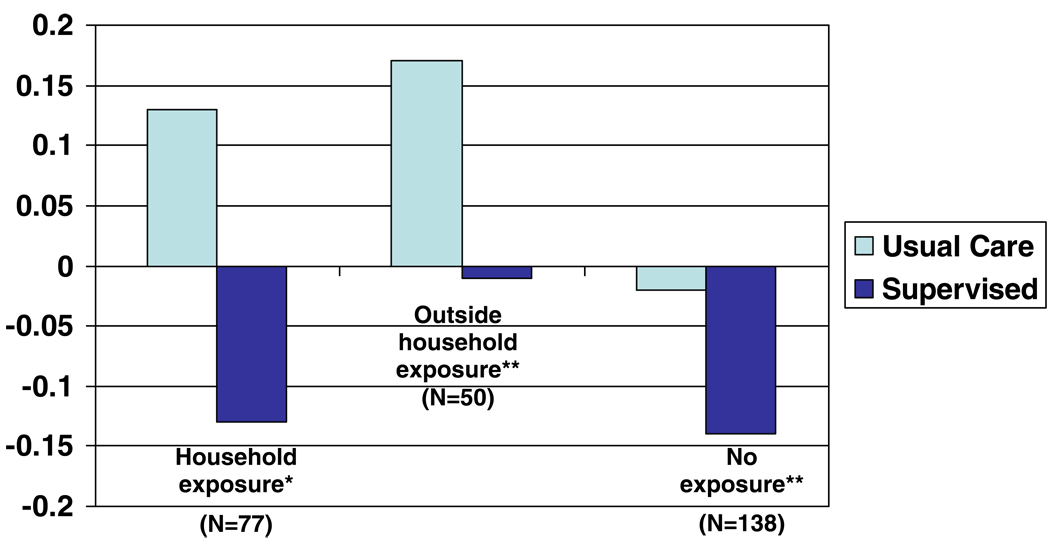
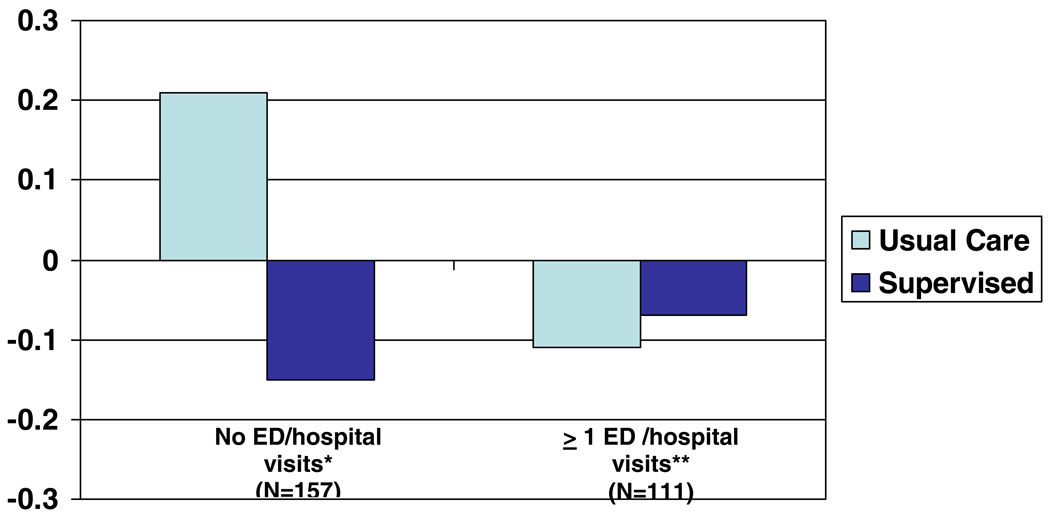
There was no observed relationship between age (p=0.13), gender (p=0.18) or race (p=0.24) and the frequency of EPACs, nor was the relationship between intervention and the frequency of EPACs modified by including any of these covariates in the model. There was a significant relationship between school system (p=0.08) and the frequency with which EPACs occurred; however, these differences did not modify the relationship observed between the intervention and the frequency of EPACs. There was also a significant relationship between treatment and the likelihood of EPAC among specific subgroups of smoke exposure at baseline and emergency room visits and hospitalizations (Figure 4 and Figure 5). For those exposed to smoke in the household, the magnitude of the interaction increases, compared to when the entire cohort is included. The p-value for the interaction in this group is 0.0997, suggesting that within those exposed to smoke in the household there is a difference in the frequency of EPACs between the usual and supervised groups. However, among those children either not exposed to smoke at baseline, or those exposed outside of the household only, there is no evidence of a treatment effect. Among those children who reported, at baseline, no ER visits or overnight hospitalizations during the past 12 months, there was a large difference in the effect of the intervention (p for interaction=0.0057). However, among those reporting at least one ER visit or overnight hospitalization, no differences in treatment were seen, either by year (i.e., no interaction), or overall. In these secondary analyses, subgroups had small samples; thus we lacked power to detect important differences in these groups.
Figure 4.
Change in percent of children experiencing an episode of poor asthma control from baseline to intervention by secondhand smoke exposure and treatment group.
*P=0.0997
**NS
Figure 5.
Change in percent of children experiencing an episode of poor asthma control from baseline to intervention by baseline ED/hospitalizations visits and treatment group.
*P=0.0057
**NS
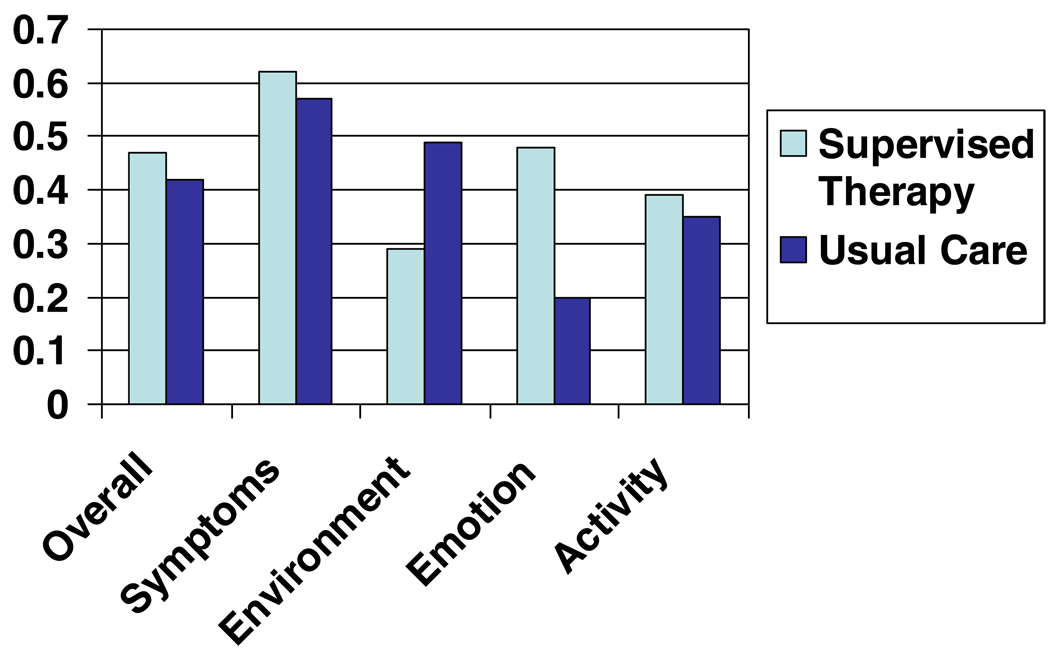
Figure 6 shows the baseline and post-intervention QOL scores for both treatment groups. Significant increases in QOL were seen in overall scores as well as on domains for both treatment groups. There were no significant differences between the groups.
Figure 6.
Mean change in quality of life overall score and subscales by treatment group*.
* Changes greater than 0 indicate improvement. All p-values for within group changes were <0.05; all p-values for between group changes were >0.05.
Discussion
Our findings are consistent with two small studies which indicate that supervised asthma therapy improves asthma control among urban school children. Inhaled corticosteroids offer significant protection against asthma exacerbations1–5; yet adherence with these medications is low.6, 7 Self-management education theories37 suggest that adherence to daily medications must become habit. While few schools have school-based health clinics and many do not have full-time school nurses, approximately 5% of children receive medication at school on a typical day.38 Rescue medications for asthma and those for Attention Deficit Hyperactivity Disorder are most common.38–41 In this research study, we used seven personnel in the 36 schools. These personnel not only supervised daily medication but also conducted quality control on data collection procedures and monitored adverse events. In many of our local school systems, medication supervision responsibilities lie with office personnel. These personnel have been trained and are successfully supervising medication administration. Therefore, it is feasible for that even schools without nurses could implement supervised asthma therapy to ensure adherence to asthma controller medications. The once-a-day dosing of inhaled budesonide is as effective as twice daily27, 29–31 and was used in this trial to facilitate adherence and the implementation of the intervention. This aspect of the intervention increases the feasibility of implementing supervised asthma therapy in a school setting.
The test of interaction for this study was marginally significant. However, in certain contexts, it has been recommended that tests of interaction be evaluated at the 0.10 level.42 Whether the interaction is evaluated at the 0.05 or 0.10 level, the results indicate an intervention effect. There are several possible reasons why the effect observed was not large. To collect quality data for this study, substantial intervention in the usual care group was necessary. Children in both treatment groups received an internet-based asthma education program as well as daily monitoring of symptoms and PFM readings. Other investigators report improvement in asthma symptoms among children who monitor their asthma.43 Recording of behavior sensitizes an individual to that behavior, thereby facilitating behavior change.44 It is likely the monitoring system created a heightened sensitivity to the children’s asthma and may have served a cue to take their rescue medications or engage in behaviors to decrease exposure to asthma triggers.
Furthermore, inhaled steroids were provided to both treatment groups, at no cost to the family, and obtaining refills was simplified thereby removing financial and access barriers to adherence45 in the usual care group. However, adherence was low among both groups during the baseline period (Table 1) and in the usual care group throughout the study. Despite the elimination of financial and access barriers, the average adherence over the entire study period in the usual care group was 38% (SD = 25%) similar to that found in a large CDC funded trial of inner-city children.46 This suggests there are additional barriers to medication adherence in this predominantly black urban population. Recent literature has described such barriers in inner-city populations including beliefs related to medications45, 47–53 and believing that asthma is an episodic disease requiring only episodic medication administration.54 Both groups of children were also provided with rescue medication at no cost in order to ensure their safety, as well as our ability to monitor rescue medication use at school. Prior to the start of this study, only 14% of children had rescue medication at school.
Thus, there was not a true control group as intervention occurred in both groups. This most likely biased our results toward no difference between groups. In a previous study investigating the impact of providing low-dose inhaled corticosteroids at school or at home, Millard et al24 found that provision of inhaled steroids without school supervision improved asthma control compared to usual care but not as much as school-based administration of steroids. In addition to the above mentioned issues, there are other changes in behavior that often occur simply from being involved in a clinical trial that could also have led to positive effects in the usual care group. Not surprisingly, significant improvement in quality of life was seen in both treatment groups but there was no difference between the groups, further indicating that the usual care group may not have been a true control group.
Three additional points should be considered when interpreting these results. First, medication was supervised only on school days. Medication use was not supervised on weekends, school holidays, during the summer, or when the child was absent for the entire school day. Children in the supervised group were provided with inhaled budesonide for use at home, but it is not known how commonly it was used on these days. Yet, improvements in asthma control were seen in this group. This may indicate that children in the supervised therapy group were more likely to take their medication at home because they were acquiring the daily habit at school, or that provision of inhaled steroids at a “less than perfect” adherence rate still improves asthma control.55 Second, at baseline, children in the supervised therapy group experienced a higher rate of EPACs than children in the usual care group. Because randomization occurred after baseline data collection, and children randomized to the supervised therapy group experienced higher rates of EPACs during the baseline period, our analyses were biased toward the null which could account for the small observed effect. Third, it is important to acknowledge that the use of the October--December time period misses the “back to school” outbreaks of asthma symptoms described in the literature.20, 21, 56 However, logistical issues of implementing the Asthma Agents System in 36 schools within 5 school systems at the beginning of the school year prevented us from getting all schools on-line until the end of September.
Secondary analyses suggest that certain subgroups of children benefit more from supervised therapy. The intervention appeared to have a greater effect in children who were exposed to secondhand smoke at home and in those who reported no ED visits or overnight hospitalizations in the past year at baseline. The results concerning secondhand smoke conflict with those reported by Halterman et al22 who found that supervised asthma therapy was only effective in improving symptom free days among children who were not exposed to secondhand smoke. Relatively small numbers in both our study and the Halterman study indicate that more research is needed in this area. Additionally, there is variability in clinical response to inhaled corticosteroids and data suggests that black children who have poor asthma control have increased risk for corticosteroid insensitivity.57, 58 Thus, there may be a biological basis for explaining why the effect did not work as well in kids who had ED visits or hospitalizations. However, sample sizes in these subgroups are small and results must be interpreted with caution.
Conclusion
The justification for supervised therapy is the benefit that can be achieved by decreasing asthma morbidity. Once-a-day supervised asthma therapy is a simple intervention that improves asthma control. Clinicians who have pediatric asthma patients with poor outcomes possibly due to medication non-adherence should consider coordinating supervised therapy with the parent and the child’s school. As medication administration at school is common, this is a reasonable approach for reducing asthma morbidity. There are federal and state guidelines for taking medication in school and each school district may have its own policies and mechanisms.59 Physicians should be familiar with state and local policies and the forms required for medications in school. In the absence of established policies, physicians may need to work with the school or parents to establish a protocol.60 Furthermore, many schools do not have full-time nurses; therefore, physicians may want to work with other school personnel to train them to properly supervise inhaled steroid use among children.
Acknowledgements
We would like to thank our school system partners without whom this study could not have been performed. These partners included Birmingham City Schools, Bessemer City Schools, Jefferson County Schools, Midfield City Schools, and Tarrant City Schools.
Funding. This trial was sponsored by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL075043). Blue Cross and Blue Shield of Alabama provided support for the internet based “Asthma Agents” monitoring system. Pulmicort Turbuhalers® were provided by AstraZeneca Pharmaceuticals.
Trial Registration: www.ClinicalTrials.gov Trial identifier: NCT00110383
Abbreviations
- PFM
Peak flow meter
- EPAC
Episode of poor asthma control
- QOL
Quality of life
Footnotes
Financial Disclosures: None reported.
References
- 1.Adams RJ, Fuhlbrigge A, Finkelstein JA, Lozano P, Livingston JM. Impact of Inhaled Antiinflammatory Therapy on Hospitalization and Emergency Departments Visits for Children With Asthma. [4-2001];Pediatrics. 2001 107(4):706–711. doi: 10.1542/peds.107.4.706. [DOI] [PubMed] [Google Scholar]
- 2.Camargo CA, Jr, Ramachandran S, Ryskina KL, Lewis BE, Legorreta AP. Association between common asthma therapies and recurrent asthma exacerbations in children enrolled in a state Medicaid plan. Am J Health Syst Pharm. 2007 May 15;64(10):1054–1061. doi: 10.2146/ajhp060256. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Man SF. Low-dose Inhaled Corticosteroid Therapy and Risk of Emergency Department Visits for Asthma. [7-22-2002];Arch Intern Med. 2002 162:1591–1595. doi: 10.1001/archinte.162.14.1591. [DOI] [PubMed] [Google Scholar]
- 4.Smith JM, Raxcati KL, McWilliams CB. Inhaled anti-inflammatory pharmacotherapy and subsequent hospitalizations and emergency department visits among patients with asthma in the Texas Medicaid program. [7-13-2003];Ann Allergy Asthma Immunol. 2003 92:40–60. doi: 10.1016/S1081-1206(10)61708-5. [DOI] [PubMed] [Google Scholar]
- 5.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343(15):1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 6.De Smet BD, Erickson SR, Kirking DM. Self-reported adherence in patients with asthma. Ann Pharmacother. 2006;40(3):414–420. doi: 10.1345/aph.1G475. [DOI] [PubMed] [Google Scholar]
- 7.Erickson SR, Coombs JH, Kirking DM, Azimi AR. Compliance from self-reported versus pharmacy claims data with metered-dose inhalers. Ann.Pharmacother. 2001;35:997–1003. doi: 10.1345/aph.10379. [DOI] [PubMed] [Google Scholar]
- 8.NHLBI Expert Panel 3. U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung and Blood Institute; Guidelines for the Diagnosis and Management of Asthma. 2007
- 9.American Academy of Allergy AaI. Pediatric Asthma: Promoting Best Practice: Guide for Managing Asthma in Children. Rochester, NY: American Academy of Allergy, Asthma, and Immunology; 1999. [Google Scholar]
- 10.Boychuk RB, Demesa CJ, Kiyabu KM, et al. Change in approach and delivery of medical care in children with asthma: results from a multicenter emergency department educational asthma management program. Pediatrics. 2006 Apr;117(4 Pt 2):S145–S151. doi: 10.1542/peds.2005-2000L. [DOI] [PubMed] [Google Scholar]
- 11.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. Journal of Allergy and Clinical Immunology. 1996;98:1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 12.Bender B, Wamboldt FS, O'Connor SL, et al. Measurement of children's asthma medication adherence by self report, mother report, canister weight, and doser CT. Ann.Allergy Asthma Immunol. 2000;85:416–421. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 13.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. Journal of Pediatric Psychology. 2003;28(5):323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 14.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005 Aug 4;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 15.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362(9387):887–899. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 16.Myung P, Pugatch D, Brady MF, et al. Directly observed highly active antiretroviral therapy for HIV-infected children in Cambodia. Am J Public Health. 2007 Jun;97(6):974–977. doi: 10.2105/AJPH.2005.083253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kann L, Collins JL, Pateman BC, Small ML, Ross JG, Kolbe LJ. The School Health Policies and Programs Study (SHPPS): rationale for a nationwide status report on school health programs. J Sch Health. 1995 Oct;65(8):291–294. doi: 10.1111/j.1746-1561.1995.tb03376.x. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. [Accessed June 12, 2007];Average length of school year and average length of school day, by selected characteristics: United States, 2001–02. http://nces.ed.gov/surveys/pss/tables/table_15.asp.
- 19.Dales RE, Schweitzer I, Toogood JH, et al. Respiratory infections and the autumn increase in asthma morbidity. Eur Respir J. 1996;9:72–77. doi: 10.1183/09031936.96.09010072. [DOI] [PubMed] [Google Scholar]
- 20.Silverman RA, Ito K, Stevenson L, Hastings HM. The Relationship of Fall School Opening and Emergency Department Asthma Visits in a Large Metropolitan Area. Arch Pediatr Adolesc Med. 2005 September;159:818–823. doi: 10.1001/archpedi.159.9.818. [DOI] [PubMed] [Google Scholar]
- 21.Johnston NW, Johnston SL, Ducan JM, et al. The September epidemic of asthma exacerbations in children: A search for etiology. J Allergy Clin Immunol. 2005;115:132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halterman JS, Szilagyi PG, Yoos HL, et al. Benefits of a School-Based Asthma Treatment Program in the Absence of Secondhand Smoke Exposure: Results of a randomized clinical trial. Arch Pediatr Adolesc Med. 2004;158:460–467. doi: 10.1001/archpedi.158.5.460. [DOI] [PubMed] [Google Scholar]
- 23.McEwen M, Johnson P, Neatherlin J, Millard MW, Lawrence G. School-Based Management of Chronic Asthma Among Inner-City African-American Schoolchildren in Dallas, Texas. Journal of School Health. 1998 May;68(5):196. doi: 10.1111/j.1746-1561.1998.tb01300.x. 196p. [DOI] [PubMed] [Google Scholar]
- 24.Millard MW, Johnson PT, McEwen M, et al. A Randomized Controlled Trial Using the School for Anti-Inflammatory Therapy in Asthma. Journal of Asthma. 2003;40(7):769–776. doi: 10.1081/jas-120023504. [DOI] [PubMed] [Google Scholar]
- 25.McFadden ER, Casale TB, Edwards TB, et al. Administration of budesonide once daily by means of turbuhaler to subjects with stable asthma. J Allergy Clin Immunol. 1999;104(1):46–52. doi: 10.1016/s0091-6749(99)70112-0. [DOI] [PubMed] [Google Scholar]
- 26.de Kluijver J, Evertse CE, Schrumpf JA, et al. Asymptomatic worsening of airway inflammation during low-dose allergen exposure in asthma: protection by inhaled steroids. Am J Respir Crit Care Med. 2002;166(3):294–300. doi: 10.1164/rccm.2112097. [DOI] [PubMed] [Google Scholar]
- 27.Mallol J, Aguirre V. Once versus twice daily budesonide metered-dose inhaler in children with mild to moderate asthma: effect on symptoms and bronchial responsiveness. Allergol Immunopathol (Madr) 2007 Jan–Feb;35(1):25–31. doi: 10.1157/13099092. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro GG, Mendelson LM, Pearlman DS. Once-daily budesonide inhalation powder (Pulmicort Turbuhaler) maintains pulmonary function and symptoms of asthmatic children previously receiving inhaled corticosteroids. Ann Allergy Asthma Immunol. 2001;86(6):633–640. doi: 10.1016/S1081-1206(10)62291-0. [DOI] [PubMed] [Google Scholar]
- 29.Moller C, Stromberg L, Oldaeus G, Arwestrom E, Kjellman M. Efficacy of Once-Daily Versus Twice-Daily Administration of Budesonide by Turbuhaler in Children with Stable Asthma. Pediatric Pulmonology. 1999;28:337–343. doi: 10.1002/(sici)1099-0496(199911)28:5<337::aid-ppul5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Campbell LM, Bodalia B, Gogbashian CA, Gunn SD, Humphreys PJ, Powell JP. Once-daily budesonide: 400 micrograms once daily is as effective as 200 micrograms twice daily in controlling childhood asthma. Int J Clin Pract. 1998;52(4):213–219. [PubMed] [Google Scholar]
- 31.Herjavecz I, Blomqvist P, Serrano A. Efficacy of once- and twice-daily administration of budesonide via Turbuhaler as initial therapy in patients with mild persistent asthma. Respir Med. 1999;93:230–235. doi: 10.1016/s0954-6111(99)90018-5. [DOI] [PubMed] [Google Scholar]
- 32.Gerald LB, Redden D, Wittich AR, et al. Outcomes for a comprehensive school-based asthma management program. J Sch Health. 2006 Aug;76(6):291–296. doi: 10.1111/j.1746-1561.2006.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangan JM, Gerald LB. Asthma Agents: Monitoring Asthma in School. Journal of School Health. 2006;76(6):300–302. doi: 10.1111/j.1746-1561.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 34.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Quality of Life Research. 1996;5(1):27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]
- 35.Diggle P, Liang K, Zeger S. Analysis of Longitudinal Data. New York: Oxford University Press; 1994. [Google Scholar]
- 36.McClure LA, Harrington KF, Graham H, Gerald LB. Internet-based monitoring of asthma symptoms, peak flow meter readings, and absence data in a school-based clinical trial. Clinical Trials. doi: 10.1177/1740774507086647. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creer TL, Boekarts M, Pintrich P, Zeidner M. Handbook of Self-Regulation. San Diego: Academic Press; 2000. Self-management of chronic illness. [Google Scholar]
- 38.McCarthy AM, Kelly MW, Reed D. Medication administration practices of school nurses. Journal of School Health. 2000;70(9):371–376. doi: 10.1111/j.1746-1561.2000.tb07277.x. [DOI] [PubMed] [Google Scholar]
- 39.Johnson PE, Hayes JM. Medication use in schools. Am J Health Syst Pharm. 2006;63(1):1277–1285. doi: 10.2146/ajhp050392. [DOI] [PubMed] [Google Scholar]
- 40.Weller L, Fredrickson DD, Burbach C, Molgaard CA, Ngong L. Chronic disease medication administration rates in a public school system. Journal of School Health. 2004;74(5):161–165. doi: 10.1111/j.1746-1561.2004.tb08214.x. [DOI] [PubMed] [Google Scholar]
- 41.Price JH, Dake JA, Murnan J, Tellijohann SK. Elementary school secretaries' experiences and perceptions of administering prescription medication. Journal of School Health. 2003;73(10):373–379. doi: 10.1111/j.1746-1561.2003.tb04179.x. [DOI] [PubMed] [Google Scholar]
- 42.Grizzle J. The two period change-over design and its use in clinical trials. Biometrics. 1965;21(2):467–480. [PubMed] [Google Scholar]
- 43.Jan RL, Wang JY, Huang MC, Tseng SM, Su HJ, Liu LF. An internet-based interactive telemonitoring system for improving childhood asthma outcomes in taiwan. Telemed J E Health. 2007 Jun;13(3):257–268. doi: 10.1089/tmj.2006.0053. [DOI] [PubMed] [Google Scholar]
- 44.Verbrugge LM. Health diaries. Med Care. 1980 Jan;18(1):73–95. doi: 10.1097/00005650-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol.Allergy Clin.N.Am. 2005;25:107–130. doi: 10.1016/j.iac.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Warman K, Silver EJ, Wood PR. Asthma risk factor assessment: what are the needs of inner-city families? Ann Allergy Asthma Immunol. 2006 Jul;97(1) Suppl 1:S11–S15. doi: 10.1016/s1081-1206(10)60779-x. [DOI] [PubMed] [Google Scholar]
- 47.Bauman LJ, Wright E, Leickly FE, et al. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002 July;110(1):6. doi: 10.1542/peds.110.1.e6. [DOI] [PubMed] [Google Scholar]
- 48.Mansour ME, Lanphear BP, DeWitt TG. Barriers to asthma care in urban children: parent perspectives. Pediatrics. 2000 September;106(3):512–519. doi: 10.1542/peds.106.3.512. [DOI] [PubMed] [Google Scholar]
- 49.Chan PWK, DeBruyne JA. Parental concern towards the use of inhaled therapy in children with chronic asthma. Pediatrics International. 2000;42:547–551. doi: 10.1046/j.1442-200x.2000.01278.x. [DOI] [PubMed] [Google Scholar]
- 50.Yoos HL, Kitzman H, McMullen A. Barriers to anti-inflammatory medication use in childhood asthma. Ambulatory Pediatrics. 2003;3(4):181–190. doi: 10.1367/1539-4409(2003)003<0181:btamui>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.George M, Freedman TG, Norfleet AL, Feldman HI, Apter AJ. Qualitative research-enhanced understanding of patients' beliefs: results of focus groups with low-income, urban, African American adults with asthma. Journal of Allergy and Clinical Immunology. 2003;111:967–973. doi: 10.1067/mai.2003.1459. [DOI] [PubMed] [Google Scholar]
- 52.Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it's not just black and white. Journal of Allergy and Clinical Immunology. 2003;111:1219–1226. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 53.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. Journal of Psychosomatic Research. 1999;47(6):555–567. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 54.Nelson BW, Clark NM, Valerio MA, Houle CR, Brown RW, Brown C. Working with a head start population with asthma: lessons learned. J Sch Health. 2006;76(6):273–275. doi: 10.1111/j.1746-1561.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 55.Boushey HA, Sorkness C, King TS, et al. Daily versus As-Needed Corticosteroids for Mild Persistent Asthma. New England Journal of Medicine. 2005;352:1519–1528. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 56.Blaisdell CJ, Weiss SR, Kimes DS, et al. Using seasonal variations in asthma hospitalizations in children to predict hospitalization frequency. Journal of Asthma. 2002;39(7):567–575. doi: 10.1081/jas-120014921. [DOI] [PubMed] [Google Scholar]
- 57.Chan MT, Leung DY, Szefler SJ, Spahn JD. Difficult-to-control asthma: clinical characteristics of steroid-insensitive asthma. J Allergy Clin Immunol. 1998;101(5):594–601. doi: 10.1016/S0091-6749(98)70165-4. [DOI] [PubMed] [Google Scholar]
- 58.Federico MJ, Covar RA, Brown EE, Leung DY, Spahn JD. Racial differences in T-lymphocyte response to glucocorticoids. CHEST. 2005;127(2):571–578. doi: 10.1378/chest.127.2.571. [DOI] [PubMed] [Google Scholar]
- 59.Guidelines for the administration of medication in school. Committee on School Health. Pediatrics. 2003 Sep;112(3 Pt 1):697–699. [PubMed] [Google Scholar]
- 60.Bannon MJ, Ross EM. Administration of medicines in school: who is responsible? Bmj. 1998 May 23;316(7144):1591–1593. doi: 10.1136/bmj.316.7144.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]