Introduction
The transfusion of allogeneic red blood cell (RBC) concentrates is common practice in critical care medicine: up to 45% of critically ill patients are transfused during their stay in an intensive care unit (ICU)10,53. Generally, RBC transfusions are performed with the intention to increase arterial oxygen content and thus oxygen delivery to the tissues.
To this end, RBC transfusions have undoubtedly been proven effective in many medical and surgical conditions, thereby particularly improving the survival of patients with critical impairment of tissue oxygenation, i.e. those with profound anaemia or circulatory shock.
Nevertheless, systematic evidence about the clinical effectiveness of blood transfusions is still difficult to obtain. The most relevant clinical studies report inconsistent effects of blood transfusion on morbidity and mortality as primary outcome parameters.
Patients requiring allogeneic blood are commonly reported to have worse outcomes than those who do not10,22,53. However, patients having required blood transfusions were usually more critically ill as documented by higher illness and organ failure scores at baseline. Irrespective of the final outcome, the efficacy of blood transfusions may be higher in patients who immediately need allogeneic blood transfusions, while the benefit of a RBC transfusion may be more difficult to assess in stable patients.
Whether a RBC transfusion may be effective or not remains an individual decision based on a thorough analysis of the patient’s risk/benefit ratio and the identification of an appropriate transfusion trigger.
The present article reviews: (i) physiological principles of oxygen transport, including compensatory mechanisms of acute anaemia and limits of anaemia tolerance, (ii) clinical evidence regarding the effects of RBC transfusion on mortality and morbidity, and (iii) the effects of such transfusions on oxygen transport and tissue oxygenation.
Compensation and limits of acute anaemia
It has been known for a long time, that adequate tissue oxygenation does not depend on a normal haemoglobin concentration – always presumed that normovolaemia is maintained35,46. Since oxygen delivery to the tissues (DO2) is defined as the product of cardiac output and arterial oxygen content (CaO2), compensatory mechanisms of dilutional anaemia include an increase of cardiac output and an increase of arterio-venous oxygen extraction18.
Increase of cardiac output
The anaemia-related decrease of haematocrit results in a proportional decline of blood viscosity. As a consequence, venous return to the heart and left ventricular preload increase, while systemic vascular resistance and thus left ventricular afterload decrease16,17. Both effects increase left ventricular performance and, therefore, cardiac output.
In anaesthetised humans, the compensatory increase of cardiac output is – in the initial phase of acute normovolaemic anaemia – predominantly achieved by an increase of left ventricular stroke volume. In more profound stages of anaemia, this mechanism is accompanied by an increase of heart rate. In awake humans, heart rate increases already in early stages of dilutional anaemia, while an early increase of heart rate in anaesthetised subjects may be indicative of hypovolaemia55,56.
Increase of arterio-venous oxygen extraction
At the microcirculatory level, the decrease of blood viscosity entails a redistribution and homogenisation of regional blood flow, which enables an increase of oxygen extraction rate (O2-ER)18. The increase of O2-ER is reflected by a decrease of mixed-venous (SvO2) and/or central venous oxygen saturation (ScvO2)39–41,47. Which of these parameters is most appropriate for estimating total body oxygen supply is presently a matter of controversy.
“Luxury-DO2”
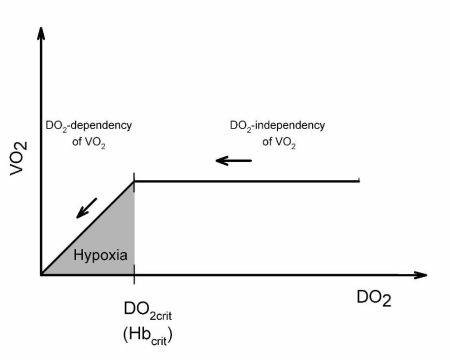
Oxygen delivery to the tissues (DO2) begins to decrease at haematocrit values lower than 25% (corresponding to a haemoglobin concentration of ~8g/dL). At haematocrit values of ~25%, the compensation of dilutional anaemia via an increase in cardiac output becomes exhausted and DO2 starts to decrease. However, since DO2 exceeds oxygen demand under physiological conditions by a factor of three to four, the organism’s oxygen demand (reflected by total body oxygen consumption - VO2 - under quiescent conditions) can be met over a large range despite a decreasing DO2 (oxygen supply independency of VO2, Figure 1).
Figure 1.
Relationship between oxygen consumption (VO2) and oxygen delivery (DO2). Physiologically, DO2 amounts to three- to four-fold the VO2. Over a long period, VO2 remains independent of DO2 despite the anaemia-related decrease of DO2 (oxygen supply-independency of DO2). When a critical haemoglobin concentration (Hbcrit) is reached, DO2 falls short of the actual oxygen demand and VO2 begins to decrease (onset of oxygen supply-dependency of VO2).
Limits of anaemia tolerance
At extreme degrees of dilutional anaemia, the DO2 falls below a critical value (DO2crit). The amount of oxgyen delivered to the tissues becomes insufficient to meet their oxygen demand and VO2 starts to decline (oxygen supply dependency of VO2, cf. figure 1)8. This indirectly indicates the onset of tissue hypoxia. The haemoglobin value that corresponds to the inflection of VO2 is called the “critical haemoglobin” (Hbcrit) and reflects the physiological limit of dilutional anaemia.
In a standardised experimental protocol, it could be demonstrated that the persistence of DO2crit without any treatment leads to death within less than 3 hours33.
Both DO2crit and Hbcrit vary within and between individuals and are influenced by different physiological circumstances (Table I). In previous experimental studies, Hbcrit was found at values between 2 and 3 g/dL. It has been clinically observed in anaesthetised patients that extremely low haemoglobin concentrations (Hb 3.0 ± 0.8g/dL in children undergoing major spine surgery14 and Hb 1.1g/dL in an unexpected massive blood loss57) have been tolerated without meeting the DO2crit. (Table I)
Table I.
Limits of acute anaemia tolerance in different species as reflected by the individual critical Hb-concentration
| Author | Species | Anaesthesia | FiO2 | Plasma substitute | Identification of Hbcrit | Hbcrit[g/dL] |
|---|---|---|---|---|---|---|
| Fontana et al.14 | Man (Child) | Isoflurane | ||||
| Sufentanil | 1.0 | Albumin | Decay of VO2 | 2.1 | ||
| Vecuronium | ||||||
| van Woerkens et al.52 | Man (84 yrs.) | Enflurane | ||||
| Fentanyl | 0.4 | Gelatine | Decay of VO2 | 4 | ||
| Pancuronium | ||||||
| Zollinger et al.57 | Man (58 yrs.) | Propofol | ||||
| Fentanyl | 1.0 | Gelatine | ST-segment depression | ~ 1.1 | ||
| Pancuronium | ||||||
| Cain8 | Dog | Pentobarbital | 0.21 | Dextrane | Decay of VO2 | 3.3 |
| Meier et al.33 | Pig | Propofol | 0.21 | HES | Decay of VO2 | 3.1 ± 0.4 |
| Fentanyl | ||||||
| Pape et al.38 | Pig | Propofol | ||||
| Fentanyl | 0.6 | HES | Decay of VO2 | 1.5 ± 0.4 | ||
| Midazolam Pancuronium | ||||||
| Kemming et al.25 | Pig | Midazolam | ||||
| Morphine | 0.21 | HES | ST-segment depression | 2.6 ± 0.3 | ||
| Pancuronium | ||||||
| Meisner et al.34 | Pig | Diazepam | ||||
| Morphine | 0.21 | Albumine | ST-segment depression | 2.0 ± 0.8 | ||
| Pancuronium | ||||||
| Meier et al.32 | Pig | Propofol | ||||
| Fentanyl | 0.21 | HES | Decay of VO2 | 2.6 ± 0.4 | ||
| Pancuronium |
These data demonstrate that the tolerance of acute normovolaemic anaemia is high in anaesthetised subjects. However, the presented concept of DO2crit refers to a critical limitation of total body oxygen supply. The limiting factor of anaemia tolerance is the oxygenation of the myocardium as the motor of haemodynamic compensation: when the DO2crit is reached, the deteriorated myocardial performance represents imminent breakdown of total body oxygenation.
While DO2crit reflects anaemia tolerance at the level of the whole body, particular organs (brain, kidneys, splanchnic organs) may reach their specific critical DO2 at an earlier stage of dilutional anaemia. This implies that the oxygenation of a single organ may be critically impaired at a higher haemoglobin concentration than the total-body Hbcrit37.
Effects of transfusion on oxygen transport and tissue oxygenation
In clinical routine, a transfusion trigger (based on a pre-defined haemoglobin concentration or on the recognition of a physiological transfusion trigger, see below) is usually met before the individual anaemia tolerance is completely exhausted. In this situation, the transfusion of RBC concentrates is intended to increase DO2 and hence tissue oxygenation.
Global oxygen transport
To what extent DO2 is actually enhanced by a RBC transfusion depends mainly on the degree of anaemia at the initiation of transfusion. Besides increasing CaO2, the augmentation of the haematocrit - and hence blood viscosity - counteracts the effects of acute anaemia on left ventricular pre- and afterload and thereby the major compensatory mechanisms of acute anaemia.
As a consequence, the cardiac output may decrease so that DO2 may not necessarily increase following the transfusion of allogeneic RBC. Inasmuch, it may also be scrutinised whether total body oxygen uptake and consumption (VO2) can be increased by RBC transfusions. Hebert et al. recently reviewed 18 clinical studies investigating the effects of RBC transfusions on the DO2/VO2 relationship in critically ill patients. In all of these studies, the haematocrit and CaO2 could be elevated by RBC transfusions. However, an increase of DO2 was documented in only 14 studies and an increase of VO2 was observed in only five of them21. The inconsistent effects of RBC transfusions on VO2 may also be related to methodological problems including the imprecision of calculating VO2 using the reverse Fick principle24. Moreover, standard methods for determination of VO2 (reverse Fick principle or, more validly, indirect calorimetry) assess total body oxygen consumption, but not regional VO2.
Whether a RBC transfusion may increase VO2 depends on how severely tissue oxygenation is impaired, i.e. on the existence of tissue hypoxia and oxygen debt. Typically, a supply-dependency of VO2 is observed in shock11 and in critical normovolaemic anaemia30. In anaesthetised dogs subjected to cardiopulmonary bypass with critically decreased pump flow, van der Linden et al. demonstrated that supply dependency could be reverted by the transfusion of fresh RBC as effectively as by the establishment of an appropriate pump flow51. In critically ill patients suffering from acute respiratory distress syndrome with oxygen supply dependency and lactic acidosis, Kruse et al. demonstrated that blood transfusions contributed to the augmentation of DO2, thereby providing a significant increase of VO2 28.
Tissue oxygenation
At the level of the microcirculation, oxygen is released to the tissues from microvessels (arterioles and capillaries). Apart from convective oxygen transport, RBC also seem to contribute to tissue oxygenation by maintaining microvascular perfusion. In an experimental study of haemorrhagic shock using the hamster window chamber model, Cabrales et al. demonstrated that functional capillary density could be restored as effectively by non-functional RBC (haemoglobin oxidised to methaemoglobin) as by functional, oxygen-carrying RBC. While both oxygen-carrying and non-functional RBC were superior to acellular fluid resuscitation with HES with regards to functional capillary density, oxygen-carrying RBC provided the highest microvascular and tissue tpO2 values7.
RBC transfusions were also demonstrated to increase functional capillary density in anaemic preterm infants15 and in septic adult ICU patients with impaired microvascular function43. In patients with traumatic brain injury or subarachnoid haemorrhage, RBC transfusion and maintenance of haematocrit values > 30% was found to improve cerebral perfusion and hence the oxygenation of brain tissue29,45.
The impact of the storage process and the so-called storage lesion on oxygen-carrying properties and the efficacy of RBC regarding tissue oxygenation are much debated at present. Alterations of RBC physiology have been comprehensively described ex vivo. Reduced deformability, increased adhesiveness and aggregability of stored RBC impair their rheological properties; anaerobic cellular metabolism with reduced contents of 2,3 disphosphoglycerate and ATP increases oxygen affinity and impairs oxygen release to the tissues23. However, the clinical significance of these findings remains unclear. In healthy volunteers subjected to acute normovolaemic anaemia, Weiskopf et al. found no differences between fresh (< 5 hours) and stored (> 3 weeks) RBC regarding their efficacy in restoring neurocognitive deficits observed during haemodilution55. Likewise, Hebert et al. found no differences regarding morbidity and mortality in ICU patients transfused with RBC stored for 4 or 19 days19.
Contrasting with these findings, some older studies suggest that the transfusion of stored RBC is associated with an increased risk of nosocomial pneumonia50 or with impaired oxygenation of the gastric mucosa31.
Physiological transfusion triggers
The individual decision to give a RBC-transfusion can be based either on pre-defined haemoglobin concentrations (e.g., 6–7 g/dL in young and healthy patients or 8–10 g/dL in patients with pre-existing cardiovascular disease)1 or on the occurrence of ‘physiological transfusion triggers’48.
Parameters indicating a physiological transfusion trigger include increased catecholamine demand, tachycardia, ST-segment alterations, newly occurring arrhythmias, compromised left ventricular contractility (TEE), lactic acidosis, and increased total body oxygen extraction (SvO2<65%). Prerequisites for the correct interpretation of these physiological signs are the maintenance of normovolaemia and appropriate depth of anaesthesia.
These parameters reflect a potentially reversible limitation of oxygen supply to peripheral tissues or to the myocardium as the motor of haemodynamic compensation of acute anaemia. Inasmuch, the reversal of a physiological transfusion trigger by a RBC transfusion can be judged as a direct indicator of transfusion effectiveness.
Effects of transfusion on mortality and morbidity
In the presence of an adequate transfusion trigger, the increase in oxygen transport capacity with RBC-transfusions restores adequate tissue oxygenation and is, therefore, life-saving in situations of critically impaired oxygen supply. However, the benefit of blood transfusions with regards to reduced morbidity and mortality has not been universally documented in large clinical studies.
Mortality
In a meta-analysis of ten randomised controlled trials investigating the outcome of a liberal versus a restrictive transfusion strategy, Carson et al. reported that a restrictive transfusion policy was not associated with an increase in mortality. Indeed, mortality was averaged to be one fifth lower in restrictively transfused patients, although this difference from the liberally transfused cohort was not statistically significant (p=0.07)9.
The largest study included in this meta-analysis was the Transfusion Requirements in Critical Care (TRICC) study by Hebert et al., which contributed 83% of the pooled mortality data. The TRICC trial enrolled 838 patients treated in Canadian ICU between November 1994 and November 1997. The overall finding of this study was that the 30-day mortality did not differ between patients allocated to a liberal versus a restrictive transfusion policy (target haemoglobin concentration 10–12 g/dL versus 7–9 g/dL). Moreover, in certain subgroups (patients younger than 55 years, patients with APACHE scores <20), a restrictive transfusion policy was even associated with a significantly reduced 30-day mortality rate.
In a smaller randomised prospective trial (n=99 patients undergoing elective vascular surgery), Bush et al. consistently reported no differences in mortality rates between deliberately or restrictively transfused patients6. Similar results were reported in premature infants with extremely low birth weight assigned to a transfusion algorithm with low or high transfusion thresholds26.
The large ABC study (Anaemia and Blood Transfusion in Critically Ill Patients), a prospective observational study of 3,534 patients from 145 western European ICU, investigated the impact of transfusion on mortality and morbidity by propensity-matched comparison of transfused and non-transfused patients enrolled in November 1999. This study reported significantly higher mortality rates (overall, ICU and 28-day mortality) in patients who received blood transfusions than in those who did not53.
Similarly, 4,892 patients from 248 ICU in the USA were prospectively enrolled in the observational CRIT study (enrolment period: August 2000 – April 2001) to investigate the impact of anaemia and RBC transfusions on clinical outcomes by propensity-matched comparison of transfused and non-transfused patients. This study identified the following independent predictors of mortality: (i) the number of RBC transfusions a patient received during the ICU stay, and (ii) anaemia with a nadir haemoglobin concentration below 9 g/dL10.
The above mentioned findings of the TRICC, ABC and CRIT studies were recently challenged by the results of the SOAP study (Sepsis Occurrence in Acutely Ill Patients). Similarly to the ABC and CRIT studies, the SOAP study was conducted as a prospective observational study; it was carried out in 198 European ICU and involved 3,147 patients who were enrolled in May 2002. Again, the aim was to investigate the impact of RBC transfusions on mortality and morbidity (propensity case matching). The major finding of this study was that the 30-day survival rate was significantly higher in transfused patients54.
Morbidity
The results of clinical studies investigating the effects of blood transfusion on morbidity are at least as inconsistent as those regarding the effects on mortality. In a small randomised prospective study of patients undergoing major vascular surgery, Bush et al. found no differences in cardiac morbidity between patients assigned to a restrictive or a liberal transfusion regimen6. While the maintenance of high haematocrit levels was demonstrated to reduce neurological morbidity in preterm infants (reduced incidence of intraparenchymal brain haemorrhage and apnoeic episodes3) and adults with traumatic brain injury or subarachnoid haemorrhage45, a retrospective study of 11,963 patients undergoing coronary artery bypass grafting demonstrated that transfusion was independently associated with an increased risk of post-operative morbid events (renal failure, prolonged ventilatory support, infections, cardiac complications, neurological events)27.
However, the large prospective TRICC, ABC, CRIT and SOAP studies concordantly documented an elevated morbidity rate in critically ill patients requiring allogeneic RBC transfusions. In particular, Hebert et al. found that cardiac morbidity (myocardial infarction, pulmonary oedema) was increased in patients assigned to the liberal transfusion strategy22. In their ABC study, Vincent et al. demonstrated that blood transfusion was associated with an increased time spent in the ICU and a higher incidence of organ failure, as assessed by the sequential organ failure assessment (SOFA) score53. The CRIT study by Corwin et al. identified the number of RBC transfusions and a nadir haemoglobin level <9 g/dL as independent predictors of adverse clinical outcome as reflected by increased durations of ICU and hospital stays and a higher incidence of complications10. Despite the reduction of mortality in transfused patients, the SOAP study by Vincent et al. also demonstrated increased durations of ICU and hospital stays among patients who received RBC concentrates. However, in this study it was well documented that transfused patients were more critically ill than non-transfused patients on admission to the ICU, as reflected by higher initial SOFA scores in the former54.
Limitations
Only very few randomised prospective trials have assessed the efficacy of allogeneic RBC transfusions. Most of them included only a small number of patients and the majority of the trials were performed before the implementation of the general leucodepletion programme.
Study design and quality of evidence
The ideal design of a trial investigating the effects of a certain treatment – a prospective, randomised, placebo-controlled and double-blinded study protocol – is most appropriate to balance known and unknown patients’ characteristics that may influence the investigated outcome parameters, since ideally the treatment intervention is the only difference between the study groups. However, for several reasons (including ethical and practical considerations), the transfusion of blood can usually not be subjected to a randomised, placebo-controlled study protocol.
A possible compromise was achieved with the design of the TRICC study, in which patients were randomly assigned to a prospectively defined treatment modality (liberal versus restrictive transfusion strategy, see above). Inasmuch, this trial fulfils the criteria of a large, randomised prospective trial (evidence level Ib).
In contrast, the above-cited ABC, CRIT and SOAP studies were prospective observational studies (evidence level IIa). In these studies, transfused patients were compared with non-transfused patients. As patients requiring blood transfusions are usually supposed to be sicker than non-transfused patients and blood transfusions were not administered on the basis of a prospective randomisation, the study populations were very likely to have differed in many other variables than only the treatment intervention (i.e., transfusion versus non-transfusion). To statistically balance these potential confounders (i.e., variables with potential impact on outcome, so-called covariates), the propensity score method is frequently chosen in observational studies. Basically, this method involves a two-step logistic regression model to predict the probability of exposure (“propensity”) to a treatment condition (so-called treatment model) and secondly, to evaluate the exposure-outcome relationship (outcome model). Usually, all measured confounders are summarised a propensity-score, which allows for case-matching in non-randomised studies42.
A major source of bias is that only observed baseline covariates are included, while unmeasured co-variables may not be considered. Due to the opacity of this statistical process, propensity score analyses are somewhat suggestive of a “black box feeling”36.
Impact of leucoreduction
Among the large studies reporting the impact of RBC transfusions on patients’ outcome, the SOAP study by Vincent et al. was the first to carried out after the implementation of the general leucodepletion programme54.
This finding is relevant, as white blood cells are responsible for transfusion-related morbidity, such as transfusion-related lung injury and transfusion-related immunomodulation, and the transmission of cell-associated viruses (cytomegalovirus, human T-cell lymphotropic virus, Epstein-Barr virus). Moreover, the effects of storage and white blood cell content seem to interact: Anniss and Sparrow observed, in vitro, increased adherence of stored RBC to an endothelial monolayer, which was significantly lower in leucoreduced RBC samples2, indicating that some of the detrimental effects of blood storage may be reduced in leucoreduced blood23.
Consistently, recent meta-analyses by Fergusson et al.13 and Blumberg et al.5 suggest a beneficial effect of leucoreduction through a decrease in post-operative rates of infection. In a retrospective analysis of two cohorts of surgical patients transfused before and after implementation of the universal leucoreduction programme (total n=14,786), Hebert et al. found that the transfusion of leucoreduced blood was associated with fewer febrile reactions and reduced need for antibiotic treatment20. In contrast, randomised controlled trials by Dzik et al.12 and Bilgin et al.4 found no differences regarding morbidity or antibiotic use in patients transfused with leucoreduced or standard buffy-coat depleted RBC concentrates.
In any case, the exclusive use of leucoreduced blood products is nowadays prescribed in many European countries49.
Conclusions
The transfusion of allogeneic RBC concentrates is intended to improve tissue oxygenation and hence patients’ outcome. Whether transfusion actually meets these expectations is difficult to estimate for several reasons. Firstly, the individual organism can substantially compensate for acute anaemia, with the range of anaemia tolerance being particularly dependent on individual cardiovascular health status. The efficacy of a RBC transfusion will, therefore, only become apparent in the presence of an adequate transfusion trigger. Secondly, large clinical trials have yielded inconsistent results. While some studies documented increased morbidity and mortality rates in ICU patients who had received RBC transfusions, a recent large observational study found, for the first time, a reduction of mortality in transfused patients. However, most relevant clinical studies have methodological limitations suggesting that their results should be interpreted with caution. Thirdly, there is still a lack of adequate monitoring of tissue oxygenation in clinical transfusion practice. This is important, as the elevation of haematocrit with increases in arterial oxygen content (CaO2) and blood viscosity counteracts major compensatory mechanisms in acute normovolaemic anaemia (in particular, the increase of cardiac output), so that DO2 may not increase despite elevated CaO2. As the dynamics of the haematocrit may have a distinct effect on microcirculatory function, monitoring of regional tissue oxygenation is of particular importance when assessing the efficacy of a blood transfusion. Finally, the efficacy of RBC transfusions - as reflected by morbidity and mortality - may also be influenced by leucodepletion and storage of blood products. The relevance of these issues required further research.
At present there are some direct and indirect indicators available for evaluating the efficacy of RBC transfusions. The most convincing direct indicators of blood transfusion effectiveness include the reduction of mortality in acute situations with otherwise fatal or life-threatening blood loss and the reversal of a physiological transfusion trigger by restoration of adequate tissue oxygenation. Indirect indicators of efficacy were provided by the CRIT study, which suggested that anaemia in the critically ill patient is also associated with increased morbidity. Moreover, the main result of the TRICC trial (i.e., a restrictive transfusion policy is at least as effective as a liberal strategy) implies that even a moderate quantity of allogeneic RBC may be sufficient to achieve a satisfactory outcome.
In conclusion, whether a RBC transfusion will affect clinical outcome remains dependent on the individual patient's demand (dynamics of blood loss, prevalence of an oxygen debt, choice of transfusion trigger).
The decision to administer a blood transfusion should, therefore, be based on clinical judgement of the individual's risk/benefit ratio including risks associated with transfusion and anaemia, respectively. To obtain systematic evidence about blood transfusion efficacy, we still need large randomised prospective trials investigating appropriate transfusion triggers in various populations of patients.
Presented in part at the “XXXVIII Convegno Nazionale di Studi di Medicina Trasfusionale” (Rimini, Italy, September, 24–27, 2008).
References
- 1.Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Anniss AM, Sparrow RL. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion. 2006;46:1561–7. doi: 10.1111/j.1537-2995.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 3.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilgin YM, van de Watering LM, Eijsman L, et al. Double-blind, randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac valve surgery. Circulation. 2004;109:2755–60. doi: 10.1161/01.CIR.0000130162.11925.21. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg N, Zhao H, Wang H, et al. The intention-to-treat principle in clinical trials and meta-analyses of leukoreduced blood transfusions in surgical patients. Transfusion. 2007;47:573–81. doi: 10.1111/j.1537-2995.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- 6.Bush RL, Pevec WC, Holcroft JW. A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. Am J Surg. 1997;174:143–8. doi: 10.1016/s0002-9610(97)00073-1. [DOI] [PubMed] [Google Scholar]
- 7.Cabrales P, Intaglietta M, Tsai AG. Transfusion restores blood viscosity and reinstates microvascular conditions from hemorrhagic shock independent of oxygen carrying capacity. Resuscitation. 2007;75:124–34. doi: 10.1016/j.resuscitation.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cain SM. Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J Appl Physiol. 1977;42:228–34. doi: 10.1152/jappl.1977.42.2.228. [DOI] [PubMed] [Google Scholar]
- 9.Carson JL, Hill S, Carless P, et al. Transfusion triggers: a systematic review of the literature. Transfus Med Rev. 2002;16:187–99. doi: 10.1053/tmrv.2002.33461. [DOI] [PubMed] [Google Scholar]
- 10.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT study: anemia and blood transfusion in the critically ill - current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 11.Dunham CM, Siegel JH, Weireter L, et al. Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock. Crit Care Med. 1991;19:231–43. doi: 10.1097/00003246-199102000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Dzik WH, Anderson JK, O’Neill EM, et al. A prospective, randomized clinical trial of universal WBC reduction. Transfusion. 2002;42:1114–22. doi: 10.1046/j.1537-2995.2002.00182.x. [DOI] [PubMed] [Google Scholar]
- 13.Fergusson D, Khanna MP, Tinmouth A, Hebert PC. Transfusion of leukoreduced red blood cells may decrease postoperative infections: two meta-analyses of randomized controlled trials. Can J Anaesth. 2004;51:417–24. doi: 10.1007/BF03018302. [DOI] [PubMed] [Google Scholar]
- 14.Fontana JL, Welborn L, Mongan PD, et al. Oxygen consumption and cardiovascular function in children during profound intraoperative normovolemic hemodilution. Anesth Analg. 1995;80:219–25. doi: 10.1097/00000539-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Genzel-Boroviczeny O, Christ F, Glas V. Blood transfusion increases functional capillary density in the skin of anemic preterm infants. Pediatr Res. 2004;56:751–5. doi: 10.1203/01.PDR.0000141982.38959.10. [DOI] [PubMed] [Google Scholar]
- 16.Habler OP, Kleen MS, Hutter JW, et al. Effects of hyperoxic ventilation on hemodilution-induced changes in anesthetized dogs. Transfusion. 1998;38:135–44. doi: 10.1046/j.1537-2995.1998.38298193095.x. [DOI] [PubMed] [Google Scholar]
- 17.Habler OP, Kleen MS, Podtschaske AH, et al. The effect of acute normovolemic hemodilution (ANH) on myocardial contractility in anesthetized dogs. Anesth Analg. 1996;83:451–8. doi: 10.1097/00000539-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Habler OP, Messmer KF. The physiology of oxygen transport. Transfus Sci. 1997;18:425–35. doi: 10.1016/S0955-3886(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 19.Hebert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–8. doi: 10.1213/01.ANE.0000148690.48803.27. [DOI] [PubMed] [Google Scholar]
- 20.Hebert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003;289:1941–9. doi: 10.1001/jama.289.15.1941. [DOI] [PubMed] [Google Scholar]
- 21.Hebert PC, van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin. 2004;20:187–212. doi: 10.1016/j.ccc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Hebert PC, Wells GA, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 23.Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 2003;31:S687–S697. doi: 10.1097/01.CCM.0000099349.17094.A3. [DOI] [PubMed] [Google Scholar]
- 24.Kemming GI, Meisner FG, Kleen M, Habler OP. Calculation is unsuitable for determination of O2-consumption (VO2) in case of O2-supply-dependency. Eur J Med Res. 2002;7:139–48. [PubMed] [Google Scholar]
- 25.Kemming GI, Meisner FG, Kleen MS, et al. Hyperoxic ventilation at the critical haematocrit. Resuscitation. 2003;56:289–97. doi: 10.1016/s0300-9572(02)00408-2. [DOI] [PubMed] [Google Scholar]
- 26.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 28.Kruse JA, Haupt MT, Puri VK, Carlson RW. Lactate levels as predictors of the relationship between oxygen delivery and consumption in ARDS. Chest. 1990;98:959–62. doi: 10.1378/chest.98.4.959. [DOI] [PubMed] [Google Scholar]
- 29.Leal-Noval SR, Rincon-Ferrari MD, Marin-Niebla A, et al. Transfusion of erythrocyte concentrates produces a variable increment on cerebral oxygenation in patients with severe traumatic brain injury: a preliminary study. Intensive Care Med. 2006;32:1733–40. doi: 10.1007/s00134-006-0376-2. [DOI] [PubMed] [Google Scholar]
- 30.Madjdpour C, Spahn DR, Weiskopf RB. Anemia and perioperative red blood cell transfusion: a matter of tolerance. Crit Care Med. 2006;34:S102–S108. doi: 10.1097/01.CCM.0000214317.26717.73. [DOI] [PubMed] [Google Scholar]
- 31.Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–9. [PubMed] [Google Scholar]
- 32.Meier J, Pape A, Loniewska D, et al. Norepinephrine increases tolerance to acute anemia. Crit Care Med. 2007;35:1484–92. doi: 10.1097/01.CCM.0000265740.62130.1C. [DOI] [PubMed] [Google Scholar]
- 33.Meier JM, Kemming GI, Kisch-Wedel H, et al. Hyperoxic ventilation reduces 6-hour mortality at the critical hemoglobin concentration. Anesthesiology. 2004;100:70–6. doi: 10.1097/00000542-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Meisner FG, Kemming GI, Habler OP, et al. Diaspirin crosslinked hemoglobin enables extreme hemodilution beyond the critical hematocrit. Crit Care Med. 2001;29:829–38. doi: 10.1097/00003246-200104000-00030. [DOI] [PubMed] [Google Scholar]
- 35.Messmer KF. Acceptable hematocrit levels in surgical patients. World J Surg. 1987;11:41–6. doi: 10.1007/BF01658458. [DOI] [PubMed] [Google Scholar]
- 36.Nuttall GA, Houle TT. Liars, damn liars, and propensity scores. Anesthesiology. 2008;108:3–4. doi: 10.1097/01.anes.0000296718.35703.20. [DOI] [PubMed] [Google Scholar]
- 37.Pape A, Habler O. Alternatives to allogeneic blood transfusions. Best Pract Res Clin Anaesthesiol. 2007;21:221–39. doi: 10.1016/j.bpa.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Pape A, Meier J, Kertscho H, et al. Hyperoxic ventilation increases the tolerance of acute normovolemic anemia in anesthetized pigs. Crit Care Med. 2006;34:1475–82. doi: 10.1097/01.CCM.0000215826.45839.36. [DOI] [PubMed] [Google Scholar]
- 39.Reinhart K, Bloos F. The value of venous oximetry. Curr Opin Crit Care. 2005;11:259–63. doi: 10.1097/01.ccx.0000158092.64795.cf. [DOI] [PubMed] [Google Scholar]
- 40.Reinhart K, Rudolph T, Bredle DL, et al. Comparison of central-venous to mixed-venous oxygen saturation during changes in oxygen supply/demand. Chest. 1989;95:1216–21. doi: 10.1378/chest.95.6.1216. [DOI] [PubMed] [Google Scholar]
- 41.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 42.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52:249–64. [PubMed] [Google Scholar]
- 43.Sakr Y, Chierego M, Piagnerelli M, et al. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35:1639–44. doi: 10.1097/01.CCM.0000269936.73788.32. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro MJ. To filter blood or universal leukoreduction: what is the answer? Crit Care. 2004;8(Suppl 2):S27–30. doi: 10.1186/cc2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MJ, Stiefel MF, Magge S, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33:1104–8. doi: 10.1097/01.ccm.0000162685.60609.49. [DOI] [PubMed] [Google Scholar]
- 46.Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93:242–55. doi: 10.1097/00000542-200007000-00035. [DOI] [PubMed] [Google Scholar]
- 47.Trouwborst A, Tenbrinck R, van Woerkens EC. Blood gas analysis of mixed venous blood during normoxic acute isovolemic hemodilution in pigs. Anesth Analg. 1990;70:523–9. doi: 10.1213/00000539-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Vallet B, Adamczyk S, Barreau O, Lebuffe G. Physiologic transfusion triggers. Best Pract Res Clin Anaesthesiol. 2007;21:173–81. doi: 10.1016/j.bpa.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Vamvakas EC, Blajchman MA. Universal WBC reduction: the case for and against. Transfusion. 2001;41:691–712. doi: 10.1046/j.1537-2995.2001.41050691.x. [DOI] [PubMed] [Google Scholar]
- 50.Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39:701–10. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 51.van der Linden P, De HS, Belisle S, et al. Comparative effects of red blood cell transfusion and increasing blood flow on tissue oxygenation in oxygen supply-dependent conditions. Am J Respir Crit Care Med. 2001;163:1605–8. doi: 10.1164/ajrccm.163.7.2001003. [DOI] [PubMed] [Google Scholar]
- 52.van Woerkens EC, Trouwborst A, van Lanschot JJ. Profound hemodilution: what is the critical level of hemodilution at which oxygen delivery-dependent oxygen consumption starts in an anesthetized human? Anesth Analg. 1992;75:818–21. doi: 10.1213/00000539-199211000-00029. [DOI] [PubMed] [Google Scholar]
- 53.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 54.Vincent JL, Sakr Y, Sprung C, et al. Are blood transfusions associated with greater mortality rates? Results of the Sepsis Occurrence in Acutely Ill Patients study. Anesthesiology. 2008;108:31–9. doi: 10.1097/01.anes.0000296070.75956.40. [DOI] [PubMed] [Google Scholar]
- 55.Weiskopf RB, Feiner J, Hopf H, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–20. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Weiskopf RB, Feiner J, Hopf HW, et al. Oxygen reverses deficits of cognitive function and memory and increased heart rate induced by acute severe isovolemic anemia. Anesthesiology. 2002;96:871–7. doi: 10.1097/00000542-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Zollinger A, Hager P, Singer T, et al. Extreme hemodilution due to massive blood loss in tumor surgery. Anesthesiology. 1997;87:985–7. doi: 10.1097/00000542-199710000-00036. [DOI] [PubMed] [Google Scholar]