Abstract
Background
Factor VII (FVII) is a plasma glycoprotein that participates in the coagulation process leading to the generation of fibrin. The aim of this study was to construct, express and purify recombinant FVII fused to a polyhistidine (his) tag using Gateway technology.
Methods
To construct the entry clone, blunt-end FVII cDNA and subsequent polymerase chain reaction (PCR) product isolated from a HepG2 cell line was TOPO-cloned into a pENTR TOPO vector. To construct the expression clone, a LR recombination reaction was carried out between the entry clone and destination vector, pDEST26. Chinese hamster ovary (CHO) cells were transfected with 1 μg of DNA of PDEST26–FVII using the FuGENE HD transfection reagent. Two cell lines that permanently expressed recombinant FVII were established. The expression of recombinant FVII was confirmed by reverse transcriptase PCR and enzyme-linked immunosorbent assay. Culture medium containing his-tagged FVII was added to the nickel-nitrilotriacetic acid resin column and bound protein was eluted. The purified protein was detected by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis. The biological activity of the recombinant FVII was determined by a prothrombin time assay using FVII-depleted plasma.
Results
The results showed that human recombinant FVII was successfully cloned and the accuracy of the nucleotide sequence of the gene and its frame in the vector were confirmed by DNA sequencing. Stable clones transfected with the construct expressed FVII mRNA and related protein but no expression was detected in the CHO cells containing an empty vector. A protein of about 52 KDa was detected in SDS-PAGE and was further confirmed by western blot analysis. A three-fold decrease in clotting time was observed using this recombinant FVII.
Conclusion
As far as we are aware, this is the first report of expression of recombinant FVII fused with a his-tag through Gateway technology. The next steps, including large scale expression, purification, activation and stabilisation, are underway.
Keywords: Gateway technology, haemophilia, recombinant FVII, His-tag, purification
Introduction
Factor VII (FVII) is a plasma glycoprotein that participates in the coagulation process leading to the generation of fibrin1,2. It is synthesised in the liver where vitamin K is required for the formation of approximately 10 γ-carboxyglutamic acid residues that are present in the amino-terminal region of the protein2–4. FVII is a single-chain glycoprotein that is secreted into the blood and circulates in a zymogen form5–8. It is converted to activated FVII (FVIIa) by activated factors X7, 9, XII7,10–12, and IX12 or thrombin9 through minor proteolysis. FVIIa initiates the extrinsic coagulation pathway by binding to tissue factor on the surface of cells that have become exposed to circulating blood following injury13,14. Recombinant human FVIIa can counterbalance factor VIII and factor IX deficiencies and could be used for the treatment of bleeding in patients with haemophilia A or B who produce antibodies (inhibitors) against factor VIII or factor IX13,15.
There are currently two different types of FVII preparation: plasma-derived and recombinant. Disadvantages of plasma-derived FVII are the low yield and possible viral contamination. A commercially available recombinant FVIIa (NovoSeven) is produced in baby hamster kidney (BHK) cell line that secretes FVII into the culture medium in its single chain form. The product is purified with murine monoclonal anti-FVII antibodies and subsequent ion exchange chromatography. Because these purification steps are time-consuming and expensive, the aim of this study was to construct, express and purify recombinant FVII fused to a poly histidine (his) tag using Gateway technology.
Materials and methods
Plasmids and bacteria
The plasmids pENTR TOPO/D and pDEST26 (Invitrogen, Carslbad, CA, USA) were used in different stages of the cloning and expression procedure. pENTR TOPO/D was used for initial cloning and sequencing. The E. coli DH5α bacterial strain (Invitrogen, USA) was used as a host for cloning of the constructs.
The pDEST26 expression vector provides the opportunity to clone the desired insert as a fusion protein with an N-terminal poly-his tag. This tag facilitates detection of the expressed protein with anti-his antibody and also purification of the protein using the metal-binding site for affinity purification of the recombinant protein.
Cell culture
A human hepatoma cell line (HepG2) and Chinese hamster ovary cell line (CHO) were obtained from National Cell Bank of Iran. These cell lines were grown in RPMI-1640 medium (Gibco-BRL, Eggenstein, Germany) containing 10% foetal bovine serum (FBS), 1 μg/mL vitamin K1, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco-BRL, Germany).
Isolation of FVII cDNA and plasmid construction
The HepG2 cell line was used as a source of human FVII gene. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The quality of RNA was determined by electrophoresis. Reverse transcription was performed by SuperScript III reverse transcriptase (Invitrogen, USA) with 2 μg of total RNA followed by DNaseI (Invitrogen, USA) treatment and heat inactivation. Full-length human FVII cDNA was isolated using specific primers by reverse transcription polymerase chain reaction (RT-PCR). PCR was performed using Platinum Taq DNA polymerase (Invitrogen, USA) in a GeneAmp PCR system 9600 (PerkinElmer Life And Analytical Sciences, Inc., Wellesley, MA, USA). After initial denaturation (5 min at 94ºC), cDNA was subjected to 30 cycles of PCR. The primer set for amplification of the full-length human FVII gene containing Kozak sequence site included the forward primer: 5’-CACCATGGTC TCC CAG GCCCTC AGG CTCC-3’ and reverse primer: 5’-T AGG GAA ATG GGG CTC GCA G-3’. The PCR annealing temperature was 60ºC. The PCR products were separated in 2% agarose gel. The blunt-end PCR products were then TOPO-cloned into pENTR TOPO/D vector according to the manufacturer’s protocol (Invitrogen, USA). The reaction mixture was incubated for 5 min at room temperature. The reaction was then placed on ice and the pENTR TOPO/D-FVII construct was transformed to competent E. coli according to the manufacturer’s protocol (Invitrogen, USA). Positive clones were selected on LB medium containing 100 μg/mL kanamycin. Plasmid DNA was isolated using a high pure plasmid extraction kit (Roche, Mannheim, Germany). The presence of the insert was confirmed by PCR and, finally, to confirm the fidelity of the sequence, DNA sequencing was performed. This construct is called the entry clone. The LR recombination reaction was then carried out between the entry clone and destination vector, pDEST26, according to the manufacturer’s instructions (Invitrogen, USA). The products of LR recombination were transformed to competent E. coli according to the manufacturer’s protocols. Positive clones were analysed by culturing them on LB medium containing 100 μg/mL ampicillin and 30μg/mL chloramphenicol. Afterwards, the plasmid DNA was isolated using a commercially available plasmid extraction kit and was further analysed by restriction enzyme digestion and PCR. Finally this expression vector was transfected into the CHO cell line.
Transfection and generation of stable FVII-expressing cells
CHO cells (5 ×105) were seeded and upon reaching 70% confluence were transfected with 1 μg of pDEST26–FVII DNA using the FuGENE HD transfection reagent (Roche, Germany) according to the manufacturer’s protocol. pDEST26 DNA was used as a control. CHO cells containing pDEST26-FVII construct were selected in a medium containing 600 μg/mL geneticin (Roche, Germany) for at least 14 days. Several stable clones were generated by dilution of the cells and their culture in 96-well culture plates. The expression of FVII was demonstrated by RT-PCR and enzyme-linked immunosorbent assay (ELISA; Diagnostica Stago, France) according to the manufacturer’s protocol.
Purification of polyhistidine-tagged FVII fusion protein and its characterisation
The FVII encoded by pDEST26 carries six histidine residues at its N-terminus. Polyhistidine has a high affinity for a nickel-nitrilotriacetic acid resin permitting single-step purification of the fusion protein. The nickel-nitrilotriacetic acid resin was washed and culture medium containing FVII was added to the column; the bound protein was eluted according to the manufacturer’s instruction (Invitrogen, USA).
Detection of the purified protein
The protein concentration was quantified using a Bio-Rad protein assay kit according to the supplier’s instructions (Bio-Rad, USA). Purified protein was detected by running the samples heated in 1x sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer at 95°C for 5 min on 12% gels followed by Coomassie blue staining. The proteins were also blotted onto polyvinylidene fluoride (PVDF) membranes (Hi-bond Amersham Biosciences, USA) and blocked with a solution containing 5% skimmed milk and 0.1% Tween 20. The blocked membranes were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and incubated with horseradish peroxidase-conjugated histidine antibody according to the supplier’s instructions (Roche, Germany) at room temperature for 1 h. Afterwards, the membranes were washed four times with PBS containing 0.1 % Tween 20, and finally the membranes were developed with 3,3 diaminobenzidine (DAB) solution (Sigma, Dusseldorf, Germany). Purified protein was also detected using goat anti-human FVII antibody (R&D Systems, Minneapolis, MN, USA) with 0.75 μg/mL peroxidise-conjugated anti-goat IgG (Dako, Denmark) as the secondary antibody. The coagulant activity of the purified recombinant FVII was measured using FVII-depleted human plasma (Diagnostica Stago, France). Thromboplastin preparations utilised in the assay were from humans (Hoechst Canada Inc., Behring Diagnostics, Montreal, Quebec, Canada). Prothrombin time assays were performed using fibrin timer cups and a mechanical fibrometer with a built-in automatic timer device (BBL, Division of Bioquest, Cockeysville, MD, USA).
Results
Isolation and construction of the recombinant vectors
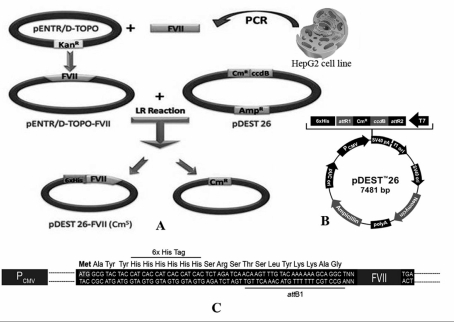
An outline of the procedure is illustrated in figure 1. Specific primers were designed to amplify the full length of the human FVII gene. The expected size of the PCR product was about 1400 bp. The blunt-end FVII PCR product was directly TOPO-cloned into the pENTR TOPO/D vector and the accuracy of the nucleotide sequence of the gene and its frame in the vector were confirmed by DNA sequencing (the gene is accessible in Genbank, accession number EU557239). Next, to construct the expression clone, a LR recombinant reaction was carried out between the pENTR TOPO/D-FVII and pDEST26 vectors. To check the putative expression clones, these were grown in the presence of chloramphenicol and desired clones, which were ampicillin-resistant and chloramphenicol-sensitive, were chosen for further analysis using PCR, which proved the existence of the insert. Finally, to confirm that the FVII gene is in frame with the appropriate tag, DNA sequencing was performed. Figure 1C represents the N-terminal sequence of the construct and its frame in detail.
Figure 1.
A; Diagram of the construction of the entry and expression clones. The HepG2 cell line was used as a source of FVII cDNA. Blunt-end PCR product was TOPO-cloned into the pENTR TOPO/D vector. The construct was used to transform competent E. coli and positive clones were selected on LB medium containing an appropriate antibiotic. This construct is called the entry clone. Next, LR recombinant reaction was carried out between pENTR TOPO/D-FVII and pDEST26 vector to construct the expression clone. The resulting pDEST26-FVII, which is called the expression clone, was transfected into CHO cells. Stable clones expressing recombinant FVII were established in the presence of geneticin. B; pDEST26 vector. C; N-terminal sequence of the fusion protein.
Expression of FVII by the CHO cell line
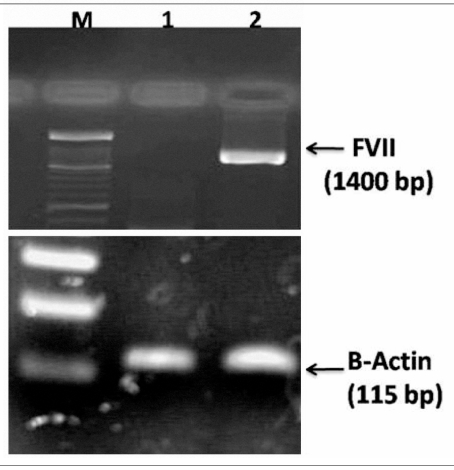
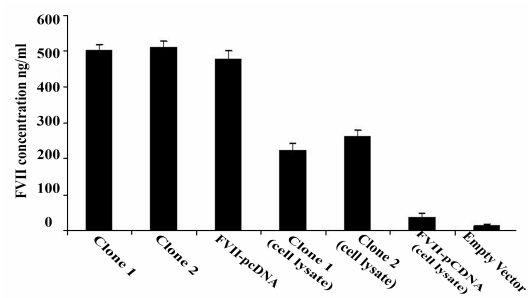
To investigate whether pDEST26-FVII-transfected cells express human FVII, RT-PCR was performed. Stable clones transfected with the construct expressed FVII mRNA, while there was no expression in the CHO cells transfected with pDEST26 vector (Figure 2). The cell culture medium of stably pDEST26-FVII transfected cells was used for detection of FVII protein by ELISA. The cells transfected with pDEST26-FVII expressed FVII protein while cells transfected with pDEST26 vector did not (Figure 3). About 40% of the his-tagged FVII was found to be intracellular and not secreted into the medium. In another study, in which the FVII gene was cloned into pcDNA3.1 expression vector, which lacks a his-tag, 20% of the FVII was intracellular, indicating that fusion of FVII to the his-tag may impair secretion of the protein.
Figure 2.
Expression of the recombinant FVII by stable clones of CHO. RNA was extracted from the CHO cells transfected with the pDEST26-FVII construct and the CHO cells transfected with the empty vector. cDNA was synthesised and RT-PCR was performed. CHO cells transfected with the construct expressed FVII mRNA, an approximately 1400 bp length fragment (Lane 2), while no expression was observed in CHO cells transfected with empty vector (Lane 1). The lower figure shows the expression of β-actin in both stable clones of CHO, i.e. transfected with the pDEST26-FVII or empty vector. M: 100 bp ladder marker.
Figure 3.
Detection of recombinant FVII by ELISA. Cell culture medium and lysate of the stable clones were evaluated. The expression of recombinant FVII was confirmed by ELISA. His-tagged FVII was detected both in cell culture medium and cell lysate. Two stable clones (Clone 1 and 2), CHO transfected with pcDNA 3.1-FVII and empty vector are shown in the figure. No FVII expression was detected in the medium of CHO cells transfected with empty vector (pcDEST26 3.1). Data are presented as mean ± SD.
Purification and characterisation of the expressed recombinant FVII
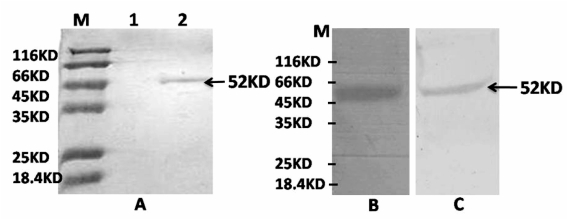
The recombinant fusion protein was purified using a nickel–sepharose column and the eluted protein was analysed by SDS-PAGE and western blotting. A protein of approximately 52 KDa was detected by SDS-PAGE (Figure 4A) and further confirmed by western blot analysis using both anti-his and anti-FVII antibodies (Figure 4B and C).
Figure 4.
SDS-PAGE and western blot analysis of recombinant FVII fusion protein. A; SDS-PAGE analysis. The recombinant fusion protein was purified using a nickel–sepharose column. Af ter purification, a single protein band was detected in 12% SDS-PAGE (Lane 2). The cell culture medium of CHO cells transfected with empty vector was also passed through a nickel–sepharose column, but no band was observed (Lane 1). B; Western blot analysis of eluted protein using anti-his-tag and anti-FVII antibodies (C). Eluted protein reacted with both antibodies, visualised by development with DAB solution.
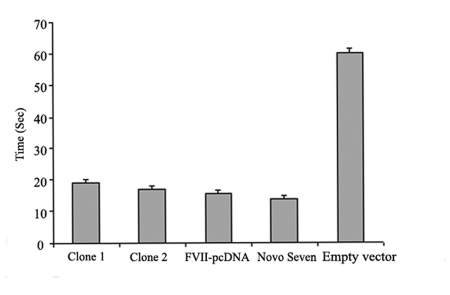
To test whether the purified protein is biologically active, 500 ng/mL of recombinant his-tagged FVII protein was obtained from 10 mL culture medium and a prothrombin time test was carried out. A three-fold decrease in clotting time was observed when human FVII-depleted plasma was used in combination with human thromboplastin and the recombinant FVII produced in this study. Similar results were observed when his-tag-free recombinant FVII, expressed using a pcDNA3.1 expression vector, and also a commercially available recombinant FVII (NovoSeven) were used (Figure 5). Taken together, these results confirm the biological activity of the expressed his-tagged recombinant FVII and the fact that its function is not affected following fusion.
Figure 5.
Prothrombin time assay. The biological activity of the purified recombinant his-tagged FVII was evaluated by the prothrombin time test. A three-fold decrease in clotting time was observed when human FVII-depleted plasma was used in combination with human thromboplastin and the his-tagged recombinant FVII produced in this study. About a four-fold decrease in clotting time was observed when a commercially available preparation of recombinant FVII (NovoSeven), was tested. The experiment was performed in triplicate and the data are presented as mean ± SD.
Discussion
We report, for the first time, the expression and purification of human recombinant FVII fused to a his-tag. Gateway technology was employed for construction of the recombinant vectors. Gateway technology, a universal cloning method based on the site-specific recombination properties of lambda bacteriophage16, provides a rapid and highly efficient way to clone DNA sequences into multiple vector systems for functional analysis and protein expression17. Briefly, two types of vectors are used in this technology. One is an entry vector and the other is a destination or expression vector; accordingly there are two types of clones i.e. entry and expression clones. In the present study, pENTR TOPO/D and pDEST26 were the entry and expression vectors, respectively. The PCR product, FVII cDNA, was directly TOPO-cloned into the pENTR TOPO/D vector in 5 minutes with 100% efficiency, and with no ligase, post-PCR procedures, or restriction enzymes required. The pDEST26 vector was used as the expression vector. During LR recombination between pENTR TOPO/D-FVII and pDEST26, the FVII gene was inserted into the destination vector resulting in construction of pDEST26-FVII which was in frame with the appropriate his-tag, as determined by DNA sequencing. The CHO cell line was used as a host for expression of recombinant the FVII protein.
Kemball-Cook et al. also expressed recombinant FVII in CHO cells. However, the vector they used (pNeoIG502) was different from the one we used (pDEST26). Furthermore, they used electroporation for transfection of the construct into the CHO cells, while we used FuGENE HD transfection reagent18. This reagent has the advantages of requiring simple methodology, minimal optimisation, low cytotoxicity and the ability to provide high transfection efficiency even in the presence of serum. Expression of recombinant rabbit FVII has been reported by Ruiz et al. They utilised pCMV5 for cloning and expression of rabbit recombinant FVII in the human 293 cell line19. The rabbit recombinant FVII was purified using a variation of the barium citrate precipitation technique followed by DEAE sepharose FF and benzamidine agarose column chromatography19,20. A commercially available human recombinant FVIIa (NovoSeven) is produced in BHK cells and is purified by consecutive chromatography steps, including immunoaffinity chromatography using murine monoclonal antibodies. In our study, the recombinant FVII encoded by pDEST26 carries six histidine residues at its N-terminus. Polyhistidine has a high affinity for a nickel-nitrilotriacetic acid resin permitting single-step and fast (in about 1.5 h) purification of the recombinant FVII protein. This recombinant FVII protein was detected as a single band in SDS-PAGE and western blot analysis indicating high purity of the protein, obviating additional purification steps.
Considering the fact that FVII is a secretion glycoprotein and recognition of its signal protein by a signal recognition particle (SRP) is essential for its entry into the lumen of the endoplasmic reticulum (ER), it might be questioned how the his-tag remains in the fusion protein. In other words, is the signal peptide removed? There are several possibilities: (i) the fusion protein enters the ER non-specifically, i.e. without the involvement of a SRP; (ii) the protein is recognised by the SRP and enters the ER but the signal peptide is not removed by signal peptidase; and (iii), some of the protein enters the ER normally in which signal peptide is removed and some enters through either the first or second above-mentioned pathways. Further, complementary studies are required to clarify the precise mechanism of entry into the ER. However, our results clearly confirm the existence of the his-tagged FVII fusion protein in the cell culture medium, as determined following elution using a nickel column, SDS-PAGE and western blot analysis with both anti-his and anti-FVII antibodies. It is worth noting that no recombinant FVII protein was detected in the culture medium of CHO cells producing his-tag-free recombinant FVII by expression of a pcDNA3.1 vector following passage of the medium through a nickel column (data not shown), which proves the specific elution of his-tagged FVII. However, the intracellular accumulation of his-tagged FVII expressed by the pDEST26 vector was much greater than that of his-tag-free recombinant FVII expressed by pcDNA 3.1. This indicates that fusion of FVII to the his-tag might impair secretion of the protein.
Finally, it was observed that the recombinant FVII produced in this study decreased clotting time three-fold, indicating that it was biologically active and that its function was not affected by fusion. However, because the CHO cells were cultured in serum-containing medium, contamination of the purified FVII protein by FBS was possible. The use of a serum-free medium should be considered to overcome this problem in subsequent studies and for clinical purposes.
Conclusion
As far as we are aware, this is the first report of expression of a recombinant FVII fused with a his-tag using Gateway technology. Our results revealed that the recombinant hybrid protein was biologically active. The simple methodology, minimal optimisation, high efficiency, low costs and fast procedure are potential advantages of this technology. The next steps, including large scale expression, purification, activation and stabilisation of the recombinant clotting factor, are under study.
Acknowledgments
We thank Mr. Mehdi Tabrizi for his help in preparing this manuscript.
References
- 1.Davie EW, Fujikawa K, Kurachi K, Kisiel W. The role of serine proteases in the blood coagulation cascade. Adv Enzymol Relat Areas Mol Biol. 1979;48:277–318. doi: 10.1002/9780470122938.ch6. [DOI] [PubMed] [Google Scholar]
- 2.Frederick SH, Charles LG, Patrick O, et al. Characterization of a cDNA coding for human factor VII (blood coagulation/DNA sequence/vitamin K-dependent proteins) Proc Natl Acad Sci USA. 1986;83:2412–6. doi: 10.1073/pnas.83.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiScipio RG, Hermodson MA, Yates SG, Davie EW. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977;16:698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- 4.Rapaport SI, Aas K, Owren PA. The effect of glass upon the activity of the various plasma clotting factors. J Clin Invest. 1955;34:9–19. doi: 10.1172/JCI103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisiel W, Davie EW. Isolation and characterization of bovine factor VII. Biochemistry. 1975;14:4928–34. doi: 10.1021/bi00693a023. [DOI] [PubMed] [Google Scholar]
- 6.Radcliffe R, Nemerson Y. Activation and control of factor VII by activated factor X and thrombin. Isolation and characterization of a single chain form of factor VII. J Biol Chem. 1975;250:388–95. [PubMed] [Google Scholar]
- 7.Miletich JP, Broze GJ, Majerus PW. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Human factor VII. Methods Enzymol. 1980;105:304–10. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj SP, Rapaport SI, Brown SF. Isolation and characterization of human factor VII. Activation of factor VII by factor Xa. J Biol Chem. 1981;256:253–9. [PubMed] [Google Scholar]
- 9.Radcliffe R, Nemerson Y. Mechanism of activation of bovine factor VII. Products of cleavage by factor Xa. J Biol Chem. 1976;251:4749–802. [PubMed] [Google Scholar]
- 10.Kisiel W, Fujikawa K, Davie EW. Activation of bovine factor VII (proconvertin) by factor XIIa (activated Hageman factor) Biochemistry. 1977;19:4189–94. doi: 10.1021/bi00638a009. [DOI] [PubMed] [Google Scholar]
- 11.Radcliffe R, Bagdasarian S, Colman R, Nemerson Y. Activation of bovine factor VII by Hageman factor fragments. Blood. 1977;50:611–7. [PubMed] [Google Scholar]
- 12.Seligsohn U, Osterud B, Brown SF, et al. Activation of human factor VII in plasma and in purified systems: roles of activated factor IX, kallikrein, and activated factor XII. J Clin Invest. 1979;64:1056–65. doi: 10.1172/JCI109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolt G, Kristensen C, Dock TS. Posttranslational N-glycosylation takes place during the normal processing of human coagulation factor VII. Glycobiology. 2005;15:541–7. doi: 10.1093/glycob/cwi032. [DOI] [PubMed] [Google Scholar]
- 14.Rapaport SI, Rao LV. Initiation and regulation of tissue factor-dependent blood coagulation. Arterioscler Throm. 1992;12:1111–21. doi: 10.1161/01.atv.12.10.1111. [DOI] [PubMed] [Google Scholar]
- 15.Astermark J. Treatment of the bleeding inhibitor patient. Semin Thromb Haemost. 2003;29:77–85. doi: 10.1055/s-2003-37972. [DOI] [PubMed] [Google Scholar]
- 16.Landy A. Dynamic, structural, and regulatory aspects of lambda site-specific recombination. Ann Rev Biochem. 1989;58:913–49. doi: 10.1146/annurev.bi.58.070189.004405. [DOI] [PubMed] [Google Scholar]
- 17.Hartley JL, Temple GF, Brasch MA. DNA cloning using in vitro site-specific recombination. Genome Res. 2000;10:1788–95. doi: 10.1101/gr.143000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemball CG, Garner I, Imanaka Y, et al. High-level production of human blood coagulation factors VII and XI using a new mammalian expression vector. Gene. 1994;139:275–9. doi: 10.1016/0378-1119(94)90769-2. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz SM, Sridhara S, Blajchman MA, Clarke BJ. Expression and purification of recombinant rabbit factor VII. Thromb Res. 2000;98:203–11. doi: 10.1016/s0049-3848(99)00227-3. [DOI] [PubMed] [Google Scholar]
- 20.Clarke BJ, Ruiz S, Sridhara S, Blajchman MA. Yolk antibodies against a conserved mammalian protein. FASEB J. 1990;4:2528–32. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]