Abstract
Background
Blood safety must be maintained throughout the whole transfusion chain to prevent the transfusion of incorrect blood components. The estimated risk of an incorrect transfusion is in the order of 1 per 10,000 units of blood. Although several kinds of errors contribute to “wrong blood” events, 70% of errors occur in clinical areas with the most common being due to failure of the pre-transfusion bedside checking procedure.
Materials and Methods
Several methods are available to reduce such errors. The I-TRAC Plus system by Immucor consists of an identification bracelet which is a bar-coded wristband and a handheld portable computer that identifies patients and blood bags by a scanner and prints the information through a portable printer. The labels attached on the blood order forms and on the sample tubes are read and recorded in the blood bank’s informatics system (EmoNet INSIEL). Labels showing the bar-code of the assigned number, which includes the ID number of the patient, the ID number of the unit and a code identifying the kind of product and use (allogeneic or autologous), are generated and applied to the blood components. The transfusions are administered after checking the unit and the patient’s wristband using the scanner of a portable PC.
Results
In 5 years a total of 71,400 units of blood components were transfused to 15,430 patients using the I-TRAC Plus system. The system prevented 12 cases of mis-identification of patients (5 in 2003, 0 in 2004, 1 in 2005, 1 in 2006 and 5 in 2007).
Conclusions
In 2003 we introduced the use of a bar-code matching system between a patient’s wristband and the blood bag to avoid mistakes at the bedside. In 5 years the system provided benefits by avoiding errors in the identification of patients, thus preventing “wrong blood” transfusions.
Keywords: Recipient identification, transfusion safety, mistransfusion
Introduction
Transfusion safety concerns the delivery of blood components to patients. It includes blood safety but focuses on critical steps related to the medical use of blood components and the outcome of patients1. Blood safety must be maintained throughout the whole transfusion chain to prevent the transfusion of incorrect blood components. The estimated risk of an incorrect transfusion is in the order of 1 per 10,000 units of blood2. The risk of mistransfusion is considerable as shown by the SHOT Annual Report 2007, which contains a large amount of information about failures in bedside transfusion3. Mistransfusion has consistently been the leading cause of death4. Although several kinds of errors contribute to “wrong blood” events, 70% of errors occur in clinical areas with the most common being due to failure of the pre-transfusion bedside checking procedure.
The current set of data probably underestimates the magnitude of the problem because only a third of errors in ABO compatibility have clinical consequences. The contribution of insufficient nursing staff to unsafe conditions among hospitalised patients has been assessed5. Processes to ensure identification of patients, blood samples and blood units need to be considered as an addition to the existing policies and procedures for administration of blood6. Indeed, as far as concerns risk management in healthcare, the second most important goal is to increase the accuracy of patient identification (the first is drug identification)7. One of the best strategies to prevent identification errors is a technological approach to patient identification (Table I).
Table I.
Technological approaches to reduce the risk of mistransfusion
| 1. | Mechanical barriers |
| 2. | Bar-codes |
| 3. | Microchips and RFID |
| 4. | Anthropometric readers |
| 5. | Portable computers |
| 6. | Smart fluid pumps |
| 7. | Automated blood bank refrigerators |
| 8. | Mobile computer wireless network |
The first positive donor-recipient identification system was described in 19738. It is now possible to use active identification systems linked to mechanical devices or palm computers able to read bracelets carrying bar-codes, microchips or radiofrequency identifier devices (RFID) to control transfusions at the patient’s bedside; furthermore, a fingerprint reader has recently been suggested9 (Table II).
Table II.
Devices designed to aid patient identification
Identification bracelets with alphanumeric codes
|
Bar-code bracelets read by handheld scanners and bedside computers
|
Radiofrequency identification systems, handheld scanners and bedside computers
|
Fingerprint sensor and bar-code labels read by handheld scanners and bedside computers
|
In order to tackle medical errors, we must have “a health-care system that makes it easy to do the right thing and difficult to do the wrong thing” 10. The forcing solutions are patterns that make it impossible to forget a step in work instructions11. “As error is the price we pay for having a creative mind”12, we can use uncreative technical devices that can check repetitive and tedious pre-transfusion procedures, preventing possible errors caused by distraction.
Technology-based solutions to prevent transfusion errors are designed to improve the identification of the transfusion recipient: what is needed is an independent control of the identity of the patient who gives the blood sample, and the patient who receives the blood bag. The identification systems used are of three types: bracelets with an alphanumeric code that opens a mechanical barrier system, machine-readable bracelets with bar-codes or RFID (smart tags), and machine-readable anthropometric data. Technologies are used to force operators to self-correct when an error is detected and to monitor the process, providing automatic traceability of all the steps.
Labelling of blood for transfusion was defined many years ago and the bar-code solution is a well accepted standard. Few hospitals in the world have implemented new technologies to reduce mistransfusion and in many cases only limited numbers of transfusions have been involved. Furthermore, there is paucity of reports on these experiences. A review on transfusion recipient identification published in 200613 listed six published cases of errors prevented in bedside administration of blood as a result of the use of technological devices (Table III).
Table III.
Results of the introduction of patient identification technologies
| Year | Author | System | Number of units transfused | Number of errors prevented |
|---|---|---|---|---|
| 1991 | Wenz | BloodLoc | 672 | 3 |
| 1996 | AuBuchon | BloodLoc | 86,000 | 3 |
| 1996 | Mercuriali | BloodLoc | 10,995 | 4 |
| 1996 | Turner | I-TRAC | 51 | 0 |
| 2000 | Marconi | I-TRAC | 621 | 0 |
Materials and methods
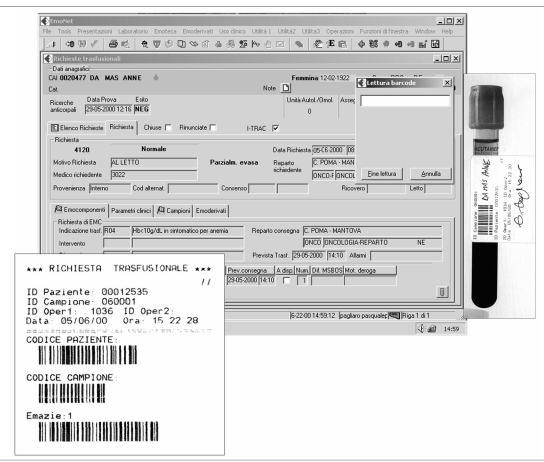
The I-TRAC Plus system by Immucor (http://www.immucor.it/) consists of an identification bracelet that is a bar-coded wristband and a handheld portable computer that identifies patients and blood bags by a scanner and prints information through a portable printer (Figure 1). Three types of bracelet are possible to manage all a patient’s entry in the hospital. The first is a bar-coded wristband (year + medical record #) printed at hospital admission in the admissions’ office with the patient’s name, sex and date of birth. The second is an outpatient wristband printed in the Blood Bank Transfusion Service (unique code sequence) with the patient’s name, sex and date of birth. The third is a preprinted anonymous bar-coded wristband (unique code sequence), for emergency rooms. The nurse identifies him or herself and the patient, and produces labels for the request and for the tube.
Figure 1.
The I-TRAC device
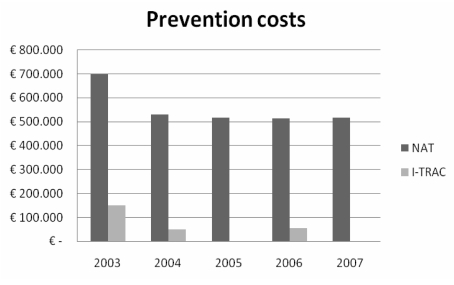
The labels attached on the blood order forms and on the sample tubes are read and recorded in the computer programme (EmoNet INSIEL) of the blood bank at Carlo Poma Hospital transfusion service (Figure 2). Labels showing the bar-code of the assigned number, which includes the ID number of the patient, the ID number of the unit, a code identifying the kind of product, and the use (allogeneic or autologous), are generated and applied to the blood components. The transfusions are administered after the identification of the doctor and the nurse, checking the patient’s wristband and the unit by the scanner of the portable PC. At the end of the transfusion, the nurse closes the operation and prints a haemovigilance report. After the purchase of 48 portable PC and 48 printers for 36 wards in the hospital, the Blood Bank staff and 460 nurses were trained and qualified. Costs compared with those of implementing nucleic acid amplification testing (NAT) for hepatitis B, hepatitis C and human immunodeficiency viruses are summarised in Figure 3. The starting phase, with weekly implementation of homogeneous groups, was completed in December 2002.
Figure 2.
Data input in EmoNet
Figure 3.
Comparative costs of NAT technology and the I-TRAC system
Results
In 5 years (2003–2007), a total of 71,400 units of blood components were transfused to 15,430 patients using the I-TRAC Plus system. Nurses strongly supported the use of the system because it provides accountability and documentation of blood transfusions. The system avoided 12 cases of patient misidentification (5 in 2003, 0 in 2004, 1 in 2005, 1 in 2006, 5 in 2007). In ten cases there was the wrong blood in the tube. In one case a nurse used a wrong bracelet to request a transfusion: the bracelets of two ambulatory patients were in the documentation but not on the patient’s wrist. The system intercepted the error because the wristband was related to another patient in the blood bank software. In one case at the bedside of the wrong patient, the system avoided an ABO-incompatible mistransfusion: the request for the blood used to take the red cells from the blood bank was for another patient. In the same period, for ten times the cost, NAT identified only one donor positive for hepatitis B virus but negative according to serological testing (HBsAg).
Discussion and conclusions
In 1996 AuBuchon and Littenberg concluded that the application of a barrier system to prevent mistransfusion, and related morbidity and mortality, could be cost-effective: other systems that do not involve expensive disposable devices are probably cheaper and more cost-effective14. Regardless of this, strategies to reduce transfusion risks must be promoted, by appealing to the precautionary principle as for other laboratory technologies applied to blood safety.
In 1993 the French parliament passed a law on blood transfusion safety and implemented a mandatory haemovigilance system co-ordinated by a national agency15. The 2007 Report on the data collected stated that in years 2000–2006 there had been an ABO transfusion error every 144,060 units transfused16.
Quality improvement programmes assessing blood administration practices are needed, but results regarding tracing blood units to their recipients are still unsatisfactory17. In 2007 AuBuchon reported that over 1,000 units of red blood cells (1 in every 12,000) are transfused to the wrong patients each year in the United States18.
If descriptive data about the efficacy of identification systems have been published and misidentification of a patient during the transfusion process is the most important cause of incorrect blood component transfused, why has there been such a poor diffusion of patients’ positive identification systems in the last 15 years? It cannot be claimed that financial constraints are the cause per se, since many more resources have been used for the implementation of NAT, which is well-recognised to be not cost-effective19. The need for a specific regulatory mandate may be important, but a legal requirement currently exists in Italy20. The possibility of blaming someone (even a nurse) for a transfusion given to a wrong patient or, conversely, the possibility of disregarding mistransfusions in a complex clinical context diverts attention from the utility of introducing technological devices to improve transfusion safety. If, on the other hand, “near misses” are reported, valuable information can be gained for error management; it is, however, important that the purpose of the specific reporting system is non-punitive21. However, simple technological devices that are not specific for medicine and transfusion, such as bar-codes, have not been applied in hospitals until very recently and mostly to improve efficiency in laboratories rather than safety in blood banks. Bar-code technology, like other commercial distribution technologies, has the potential to increase the safety of blood transfusion, but should be accompanied by comprehensive education, training and continued support22. Great effort must be expended in the implementation of new procedures in the transfusion process in order to obtain the compliance of operators and to change the design of patients’ identification, and particular attention must be given to including nurses in the clinical quality improvement23. Methods of encoding patient identification can be integrated in the informatics system of the Transfusion Service to enable detection of various errors and thereby prevent complications, to increase documentation of the transfusion process and to improve haemovigilance24. Bar-coded identification of all patients is suggested to improve electronic cross-matching processes25,26.
In general, the organisational changes and training necessary to implement a patient identification system in a hospital are notable: the promoter of these changes – who may be the transfusion manager – must carry out a pilot study and then involve clinical governance and the nursing staff to face the changes over a long period, step by step. The motivation to change is strong and the decision to adopt a positive identification device is easier if an important clinical incident occurs. However, if a haemovigilance system is not effective, the perception of any problems remains unclear and the necessary solutions do not become a priority.
Research in blood and patient identification confirms the value of positive technological checks27 and the use of electronic reading of bar-codes or other computerised aids will increase, although even the most sophisticated measures will fail if they are not applied accurately28.
After a long period of underuse, methods to ensure accuracy and precision in identification of transfusion units and recipients at the bedside are ready for widespread introduction, and the coming years are the right time for another important step in transfusion safety.
Presented in part at the “XXXVIII Convegno Nazionale di Studi di Medicina Trasfusionale” (Rimini, Italy, September, 24–27, 2008).
References
- 1.Dzik WH. Emily Cooley Lecture 2002: transfusion safety in the hospital. Transfusion. 2003;43:1190–8. doi: 10.1046/j.1537-2995.2003.00523.x. [DOI] [PubMed] [Google Scholar]
- 2.Linden JV, Wagner K, Voytovich AE, Sheehan J. Transfusion errors in New York State: an analysis of ten years’ experience. Transfusion. 2001;40:1207–13. doi: 10.1046/j.1537-2995.2000.40101207.x. [DOI] [PubMed] [Google Scholar]
- 3.Serious hazard of transfusion: annual report 2007. Available from: http://www.shotuk.org/
- 4.Sazama K. Reports of 355 transfusion associated deaths. 1976 through 1985. Transfusion. 1990;30:583–90. doi: 10.1046/j.1537-2995.1990.30790385515.x. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrook R. Nursing in the crossfire. N Engl J Med. 2002;346:1757–66. doi: 10.1056/NEJM200205303462225. [DOI] [PubMed] [Google Scholar]
- 6.Goodnough LT, Shander A, Brecher ME. Transfusion Medicine: looking to the future. Lancet. 2003;361:161–169. doi: 10.1016/S0140-6736(03)12195-2. [DOI] [PubMed] [Google Scholar]
- 7.WHO Collaborating Centre for Patient Safety Solutions. Nine patient safety solutions. Available from http://www.ccforpatientsafety.org/
- 8.Chambers RW, Rubin MI, Rath CE, et al. A positive donor-recipient identification system for a regional blood transfusion service. Transfusion. 1973;13(1):34–6. doi: 10.1111/j.1537-2995.1973.tb05434.x. [DOI] [PubMed] [Google Scholar]
- 9.Bennardello F, Fidone C, Cabibbo S, et al. Use of an identification system based on biometric data for patients requiring transfusions guarantees transfusion safety and traceability. Blood Transfus. 2009;7:193–203. doi: 10.2450/2009.0067-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohn LT, Corrigan JM, Donaldson MS, editors. To Err Is Human: Building a Safer Health System. National Academy Press; Washington: 2000. [PubMed] [Google Scholar]
- 11.Leape L. Error in Medicine. JAMA. 1994;272:1851. [PubMed] [Google Scholar]
- 12.Reason J. Human Error. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 13.Pagliaro P, Rebulla P. Transfusion recipient identification. Vox Sang. 2006;9:97–101. doi: 10.1111/j.1423-0410.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 14.AuBuchon J, Littemberg B. A cost-effectiveness analysis of the use of a mechanical barrier system to reduce the risk of mistransfusion. Transfusion. 1996;36:222–26. doi: 10.1046/j.1537-2995.1996.36396182139.x. [DOI] [PubMed] [Google Scholar]
- 15.Delbosc A, Weiller J, Dessert P. Blood monitoring at the dawn of the 21st century. Presse Med. 2000;29(19):1066–71. [PubMed] [Google Scholar]
- 16.Agence francaise de sécurité sanitarie des produit de santé. Rapport Annuel 2006 Unité Hémovigilance. Available from: http://www.afssaps.sante.fr/
- 17.Ballard S, Buck J, Llewelyn C, et al. Tracing blood units to their recipient: results of a two-centre study. Transfusion Medicine. 2003;13:127–30. doi: 10.1046/j.1365-3148.2003.00432.x. [DOI] [PubMed] [Google Scholar]
- 18.AuBouchon JP. Process controls to avert mistransfusion. ISBT Science Series. 2007;2:253–56. [Google Scholar]
- 19.Bush MP, Doddy RY. NAT and blood safety: what is the paradigm? Transfusion. 2000;40:1147. doi: 10.1046/j.1537-2995.2000.40101157.x. [DOI] [PubMed] [Google Scholar]
- 20.Ministero della Salute. Raccomandazione N°5 del 31/3/2008. Available from http://www.ministerosalute.it/
- 21.Kaplan HS, Callum JL, Fastman BR, Merkley LL. Medical event reporting system for transfusion medicine: will it help get the right blood to the right patient? Transfus Med Rev. 2002;16:86–102. doi: 10.1053/tmrv.2002.31459. [DOI] [PubMed] [Google Scholar]
- 22.Clark P, Renne I, Rawlinson S. Effect of a formal education programme on safety of transfusion. Br Med J. 2001;323:1118–20. doi: 10.1136/bmj.323.7321.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foss ML, Moore SB. Evolution of quality management: integration of quality assurance function into operations, or “quality is everyone’s responsibility”. Transfusion. 2003;43:1330–36. doi: 10.1046/j.1537-2995.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- 24.Butch SH. Computerization in the Transfusion Service. Vox Sang. 2002;83(1):105–10. doi: 10.1111/j.1423-0410.2002.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 25.Judd WJ. Requirements for the Electronic Crossmatch. Vox Sang. 1998;74(2):409–17. doi: 10.1111/j.1423-0410.1998.tb05450.x. [DOI] [PubMed] [Google Scholar]
- 26.Miyata S, Kawai T, Yamamoto S, et al. Network computer-assisted transfusion-management system for accurate blood component-recipient identification at the bedside. Transfusion. 2004;44:364–72. doi: 10.1111/j.1537-2995.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 27.Sandler SG, Langeberg A, DeBandi L, et al. Radiofrequency identification technology can standardize and document blood collection and transfusions. Transfusion. 2007;47:763–70. doi: 10.1111/j.1537-2995.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 28.International Forum Transfusion safety in the hospital. Vox Sang. 2004;87:48–62. doi: 10.1111/j.0042-9007.2004.00527.x. [DOI] [PubMed] [Google Scholar]