Antithrombin concentrates
Introduction
Antithrombin (AT) concentrates can be therapeutically useful in cases of primary and acquired AT deficiency; their use, to be reserved to clinical conditions in which low levels of functional AT are associated with a thrombotic imbalance in haemostasis, has yet to be supported by clear scientific evidence.
Notions of physiology
AT is a glycoprotein synthesised by the liver. Its molecular weight is 58,000 Da and it circulates in the plasma at a concentration of 150 mg/mL1–3. Belonging to the family of serpins or inhibitors of serine proteases, it inhibits proteases. AT is the most potent naturally occurring inhibitor of coagulation and plays a fundamental role in maintaining haemostatic balance. Furthermore, it has anti-inflammatory and anti-aggregant properties mediated through the release of prostacyclins from endothelial cells3.
Normal values of AT activity in the plasma range from 80% to 120%. In normal conditions its biological half-life is 1.5–2.5 days; in conditions of acquired deficiency and in the presence of heparin, the half-life of AT can be notably shorter, being reduced to even a few hours.
Preparations of AT
AT concentrates, like all other plasma derivates, are prepared from pools of human plasma, made from at least 1,000 different donors4.
Various companies have been licensed to manufacture this product for clinical use. AT preparations undergo microbial inactivation by pasteurisation, sometimes followed by nanofiltration. Vials containing 500, 1,000, 1,500 and 2,000 UI are available.
Mechanism of action
AT is used as replacement therapy in conditions of acquired or inherited deficiency, in particular circumstances. Its anticoagulant activity is mainly due to the inhibition of thrombin, activated factor X (FXa) and, to a lesser degree, also other activated clotting factors (FIXa, FXIa, FXIIa)1. The rate of formation of the thrombin-antithrombin complex is very greatly increased by heparan sulphate, present on the surface of endothelial cells. Subjects lacking AT have an increased risk of thrombosis, particularly in the presence of other thrombophilic conditions.
Congenital AT deficiency
The estimated prevalence is 1/2,000–5,000 in the general population and 2–3% in a selected population of patients with thrombotic events1,5.
There are two different types of congenital deficiency of AT, which are inherited in an autosomal dominant manner:
- TYPE I (quantitative defect), in which there are proportional decreases in the concentration and, therefore, functional activity of the AT.
- TYPE II (qualitative defect), characterised by normal levels of protein, but a reduction in its functional activity.
Acquired AT deficiency
Various clinical conditions are associated with acquired AT deficiency5,6:
- Dilution5:
- - massive transfusion;
- - plasma exchange;
- - extracorporeal circulation.
Indications
The use of AT concentrates, to be reserved to clinical conditions in which low levels of functional AT are associated with a thrombotic imbalance in haemostasis, has yet to be supported by clear scientific evidence.
1. Patients with congenital AT deficiency
In the absence of symptoms or risk factors, congenital AT deficiency is not an indication for replacement therapy with AT concentrates, which should be reserved, on a temporary basis and in association with heparin therapy, to the following circumstances (Grade of Recommendation: 2C)1,6:
- prophylaxis of deep vein thrombosis and thromboembolism in high-risk conditions: major surgery, obstetric procedures (such as delivery or abortion), trauma, immobilisation;
- treatment of ongoing thrombosis, until the indicated level of oral anticoagulation is reached. Patients with congenital AT deficiency and repeated episodes of thromboembolism must receive life-long oral anticoagulant therapy (Grade of Recommendation: 2C+)1,6 (Table I).
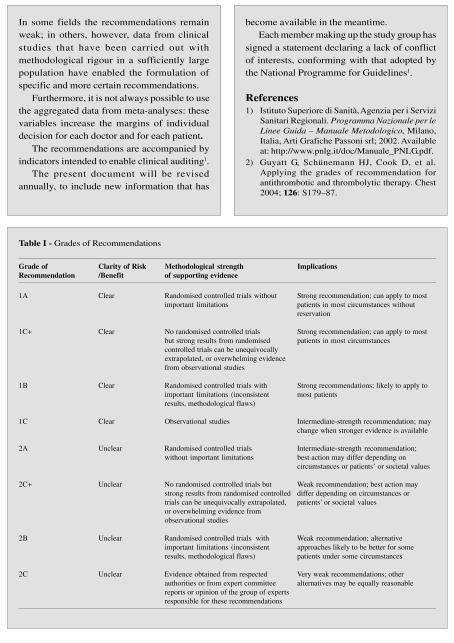
Table I.
Indications for the use of antithrombin
| Clinical conditions | Notes | GoR |
|---|---|---|
| Congenital antithrombin deficiency | ||
| Prophylaxis of deep vein thrombosis and thrombo-embolism in high-risk situations | For the entire time that the high-risk state is present | 2C |
| Treatment of ongoing thrombosis | Until the indicated level of oral anticoagulation is reached | 2C |
| This latter must be maintained life-long in cases of repeated thromboembolism | 2C+ | |
| Acquired antithrombin deficiency | ||
| Increased consumption (in DIC associated with severe sepsis) | Administration of high doses, not associated with heparin, may improve survival | 2C+ |
|
There is little evidence of the efficacy of treatment with AT in these clinical circumstances | |
GoR: Grade of Recommendation
2. Patients with acquired AT deficiency
There is little evidence concerning treatment with AT in conditions of acquired deficiency; replacement therapy with AT may be useful, although the levels of evidence are not high, in DIC associated with severe sepsis, in which the use of high doses, not associated with heparin, could improve the survival of patients (Grade of Recommendation: 2C+)3,5,15–18,20–36.
Further studies are needed on the use of AT concentrates in the case of1,6:
- DIC associated with trauma, burns, pregnancy;
- neonates of mothers with AT deficiency or a family history of severe venous thromboembolism;
- ongoing thrombosis with low levels of AT and resistance to heparin;
- acute thromboembolism during treatment with Lasparaginase9–12;
- extracorporeal circulation;
- thrombosis of the hepatic artery following orthotopic liver transplantation;
- veno-occlusive disease following bone marrow transplantation37,38;
Furthermore, the use of AT is not generally indicated (given the lack of proof of clinical efficacy), even when AT levels are considerably below normal, in the following conditions of chronic, not decompensated deficiency: acute or chronic liver disease, nephrotic syndrome, protein-losing enteropathy, pre-eclampsia, neonatal respiratory distress syndrome, multiple trauma and postoperatively in the absence of DIC (Table I).
Calculation of the dose of AT to administer
There is no evidence that higher than normal levels of AT provide greater protection than physiological levels, just as an overdose does not imply an increased risk of bleeding. Before starting replacement therapy with a specific concentrate, it is advisable to assay AT functional activity. Given that the administration of 1 UI/kg of body weight increases plasma AT activity by 1.5%, the dose to administered is calculated as follows:
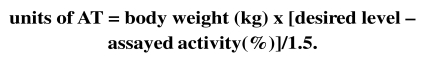 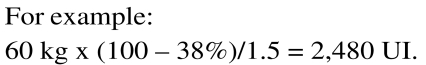
|
The dose and timing of subsequent administrations are based on the results of monitoring plasma AT activity every 12–48 h.
Monitoring indices for clinical auditing
Use of AT treatment in the following conditions:
- congenital AT deficiency in the absence of symptoms or risk factors and/or with AT values > 70%.
Side effects and adverse reactions
AT infusions are generally well tolerated; allergictype reactions are, however, possible.
The use of AT concentrates contemporaneously with heparin increases the risk of bleeding and careful clinical and laboratory monitoring is, therefore, necessary, particularly in patients at high haemorrhagic risk.
Recommendations
It is recommended that all the details of the product infused, including its batch number, are recorded in the clinical records.
References
- 1.Hathaway WE, Goodnight SH., Jr . Malattie dell’Emostasi e Trombosi. Milan, Italy: McGraw-Hill Companies Italia; 1994. [Google Scholar]
- 2.Blondel-Hill E, Mant MJ. The pregnant antithrombin III deficient patient: management without antithrombin III concentrate. Thromb Res. 1992;65:193–8. doi: 10.1016/0049-3848(92)90239-7. [DOI] [PubMed] [Google Scholar]
- 3.Inthorn D, Hoffmann JN, Hartl WH, et al. Effect of antithrombin III supplementation on inflammatory response in patients with severe sepsis. Shock. 1998;2:90–6. doi: 10.1097/00024382-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Burnouf T. Modern plasma fractionation. Transfus Med Rev. 2007;21:101–17. doi: 10.1016/j.tmrv.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prinoth O.Servizio Aziendale di Immunoematologia e Trasfusione - Comprensorio Sanitario di Bolzano. Terapia con emocomponenti e plasmaderivati: linee guida ed aspetti medico-legali January2007. Available at: http://www.asbz.it/portal/it/document/IT/direzione/LINEE%20GUIDA%20ALLA%20TRASFUSIONEITALIANE%20(integrale).pdf
- 6.Lechner K, Kyrle PA. Antithrombin III concentrates: are they clinically useful? Thromb Haemost. 1995;73:340–8. [PubMed] [Google Scholar]
- 7.Ribeiro AA, Lourenco DM, Toledo CF, et al. Antithrombin III concentrate use in patients with cirrhosis with coagulation disorders. Rev Assoc Med Bras. 1997;43:189–94. doi: 10.1590/s0104-42301997000300004. [DOI] [PubMed] [Google Scholar]
- 8.Langley PG, Keays R, Hughes RD, et al. Antithrombin III supplementation reduces heparin requirement and platelet loss during hemodialysis of patients with fulminant hepatic failure. Hepatology. 1991;14:251–6. [PubMed] [Google Scholar]
- 9.Bushman JE, Palmieri D, Whinna HC, Church FC. Insight into the mechanism of asparaginase-induced depletion of antithrombin III in treatment of childhood acute lymphoblastic leukemia. Leuk Res. 2000;24:559–65. doi: 10.1016/s0145-2126(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 10.Beinart G, Damon L. Thrombosis associated with Lasparaginase therapy and low fibrinogen levels in adult acute lymphoblastic leukemia. Am J Hematol. 2004;77:331–5. doi: 10.1002/ajh.20230. [DOI] [PubMed] [Google Scholar]
- 11.Andrew M, Brooker L, Mitchell L. Acquired antithrombin III deficiency secondary to asparaginase therapy in childhood acute lymphoblastic leukaemia. Blood Coagul Fibrinolysis. 1994;5(Suppl 1):S24–36. doi: 10.1097/00001721-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki A, Suminoe A, Hara T. Antithrombin III supplementation in childhood acute lymphoblastic leukemia treated with L-asparaginase. Pediatr Hematol Oncol. 2002;19:601–3. doi: 10.1080/08880010290108744. [DOI] [PubMed] [Google Scholar]
- 13.Vaziri ND, Paule P, Toohey J, et al. Acquired deficiency and urinary excretion of antithrombin III in nephrotic syndrome. Arch Int Med. 1984;144:1802–3. [PubMed] [Google Scholar]
- 14.Citak A, Emre S, Sairin A, et al. Hemostatic problems and thromboembolic complications in nephrotic children. Pediatr Nephrol. 2000;14:138–42. doi: 10.1007/s004670050029. [DOI] [PubMed] [Google Scholar]
- 15.Hauptman JG, Hassouna HI, Bell TG, et al. Efficacy of antithrombin III in endotoxin-induced disseminated intravascular coagulation. Circ Shock. 1988;25:111–22. [PubMed] [Google Scholar]
- 16.Vinazzer H. Therapeutic use of antithrombin III in shock and disseminated intravascular coagulation. Sem Thromb Hemost. 1989;15:347–52. doi: 10.1055/s-2007-1002727. [DOI] [PubMed] [Google Scholar]
- 17.Taylor FB, Jr, Emerson TE, Jr, Jordan R, et al. Antithrombin III prevents the lethal effects of Escherichia coli infusion in baboons. Circ Shock. 1988;26:227–35. [PubMed] [Google Scholar]
- 18.Emerson TE, Jr, Fournel MA, Leach WJ, Redens TB. Protection against disseminated intravascular coagulation and death by antithrombin III in the Escherichia coli endotoxemic rat. Circ Shock. 1987;21:1–13. [PubMed] [Google Scholar]
- 19.Mangione S, Giarratano A. The role of antithrombin III in critical patients in obstetrics. Minerva Anestesiol. 2002;68:449–53. [PubMed] [Google Scholar]
- 20.Fourrier F, Chopin C, Huart JJ, et al. Double-blind placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest. 1993;104:882–8. doi: 10.1378/chest.104.3.882. [DOI] [PubMed] [Google Scholar]
- 21.Inthorn D, Hoffmann JN, Hartl WH, et al. Antithrombin III supplementation in severe sepsis: beneficial effects on organ dysfunction. Shock. 1997;8:328–34. doi: 10.1097/00024382-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Eisele B, Lamy M, Thijs LG, et al. Antithrombin III in patients with severe sepsis. A randomized, placebo-controlled, double-blind multicenter trial plus a metaanalysis on all randomized, placebo-controlled, double-blind trials with antithrombin III in severe sepsis. Intens Care Med. 1998;24:663–72. doi: 10.1007/s001340050642. [DOI] [PubMed] [Google Scholar]
- 23.Baudo F, Caimi TM, de Cataldo F, et al. Antithrombin III (ATIII) replacement therapy in patients with sepsis and/or postsurgical complications: a controlled double-blind, randomized, multicenter study. Intens Care Med. 1998;24:336–42. doi: 10.1007/s001340050576. [DOI] [PubMed] [Google Scholar]
- 24.Warren BL, Eid A, Singer P, et al. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869–78. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 25.Messori A, Vacca F, Vaiani M, et al. Antithrombin III in patients admitted to intensive care units: a multicenter observational study. Crit Care. 2002;6:447–51. doi: 10.1186/cc1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fourrier F, Jourdain M, Tournoys A. Clinical trial results with antithrombin III in sepsis. Crit Care Med. 2000;28(Suppl 9):S38–43. doi: 10.1097/00003246-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann JN, Muhlbayer D, Jochum M, Inthorn D. Effect of long-term and high-dose antithrombin supplementation on coagulation and fibrinolysis in patients with severe sepsis. Crit Care Med. 2004;32:1851–9. doi: 10.1097/01.ccm.0000139691.54108.1f. [DOI] [PubMed] [Google Scholar]
- 28.Levi M. Antithrombin in sepsis revisited. Crit Care. 2005;9:624–5. doi: 10.1186/cc3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kountchev J, Bijuklic K, Bellmann R, et al. Reduction of D-dimer levels after therapeutic administration of antithrombin in acquired antithrombin deficiency of severe sepsis. Crit Care. 2005;9:R596–600. doi: 10.1186/cc3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann JN, Wiedermann CJ, Juers M, et al. Benefit/risk profile of high-dose antithrombin in patients with severe sepsis treated with and without concomitant heparin. Thromb Haemost. 2006;95:850–6. [PubMed] [Google Scholar]
- 31.Wiedermann CJ. Clinical review: molecular mechanisms underlying the role of antithrombin in sepsis. Crit Care. 2006;10:209. doi: 10.1186/cc4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonano C, Sitzwohl C, Meitner E, et al. Four-day antithrombin therapy does not seem to attenuate hypercoagulability in patients suffering from sepsis. Crit Care. 2006;10:R160–5. doi: 10.1186/cc5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kienast J, Juers M, Wiedermann CJ, et al. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J Thromb Haemost. 2006;4:90–7. doi: 10.1111/j.1538-7836.2005.01697.x. [DOI] [PubMed] [Google Scholar]
- 34.Wiedermann CJ, Hoffmann JN, Juers M, et al. High-dose antithrombin III in the treatment of severe sepsis in patients with a high risk of death: efficacy and safety. Crit Care Med. 2006;34:285–92. doi: 10.1097/01.ccm.0000194731.08896.99. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann JN, Schick K. Antithrombin and hypercoagulability in sepsis: insights from thromboelastography? Crit Care. 2007;11:115. doi: 10.1186/cc5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann JN, Fertmann JM, Jauch KW. Microcirculatory disorders in sepsis and transplantation: therapy with natural coagulatory inhibitors antithrombin and activated protein C. Curr Opin Crit Care. 2006;12:426–30. doi: 10.1097/01.ccx.0000244121.54495.f7. [DOI] [PubMed] [Google Scholar]
- 37.Morris JD, Harris RE, Hashmi R, et al. Antihrombin-III for the treatment of chemotherapy-induced organ dysfunction following bone marrow transplantation. Bone Marrow Transplant. 1997;20:871–8. doi: 10.1038/sj.bmt.1700985. [DOI] [PubMed] [Google Scholar]
- 38.Haire WD, Ruby EI, Stephens LC, et al. A prospective randomized double-blind trial of antithrombin III concentrate in the treatment of multiple-organ dysfunction syndrome during hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 1998;4:142–50. doi: 10.1053/bbmt.1998.v4.pm9923412. [DOI] [PubMed] [Google Scholar]
Prothrombin complex concentrates
Introduction
Prothrombin complex concentrate (PCC) may be therapeutically useful for the acute and temporary correction of deficiencies of factors in the prothrombin complex. There are no randomised, controlled clinical trials providing clear evidence on the use of PCC, but only observational or retrospective studies, on the basis of which the following recommendations were formulated. Reference is also made to the guidelines from the Italian Association of Haemophilia Centres (AICE) and the Italian Federation of Anticoagulation Clinics (FCSA)1–3.
Preparations of PCC
PCC contain factor II (FII), factor IX (FIX) and factor X (FX), with procoagulant effects, as well as naturally occurring and physiological inhibitors of coagulation such as protein C, protein S and traces of antithrombin, heparin and vitronectin4. PCC containing unactivated clotting factors and one concentrate of activated factors are available. PCC, like all other plasma derivates, are prepared from pools of human plasma, made from at least 1,000 different donors5.
Various companies have been licensed to manufacture PCC for clinical use. These products are subjected to viral inactivation, both by physical methods (heating or vapour) and chemical methods (use of solvent-detergent). Vials containing 200, 500 and 1,000 UI are available.
Indications
Congenital deficiencies
Unactivated PCC is used only in the case of documented isolated deficiencies of FII and FX, for the prophylaxis or treatment of bleeding (table II); if it is not available, fresh-frozen plasma (FFP) can be used as an alternative. Similarly, in cases of congenital deficiencies of FVII and FIX, PCC should only be used when the specific clotting factor is not available (Grade of Recommendation: 2C)1,6–8.
Table II.
Indications for the use of PCC
| Congenital deficiencies of clotting factors in the prothrombin complex | GoR |
| Deficiencies of factors II and X: for prophylaxis or treatment of bleeding. If PCC are not available, FFP can be used as an alternative. | 2C |
| Haemophilia B and inherited factor VII deficiency: when the specific factor IX and VII concentrates are not available. | |
| Haemophilia A with inhibitors, during bleeding episodes (activated PCC). | |
| Acquired deficiencies of clotting factors in the prothrombin complex (Factors II, IX, X) | GoR |
| Bleeding episodes in the presence of deficiencies of one or more factors of the prothrombin complex. | |
| Limitations to the use of FFP because of the risk of circulatory overload or the need for immediate haemostasis, in the following circumstances: | |
| -severe liver disease with serious bleeding or in preparation for elective surgery carrying the risk of bleeding (liver transplantation); | |
| -vitamin K deficiency in the presence of life-threatening bleeding; | |
| -acquired haemophilia (activated PCC). | 2C |
| -Excessive doses of dicoumarols or the need to suspend them in emergency conditions (acute haemorrhage, urgent surgery). | 2C+ |
GoR: Grade of Recommendation
Activated PCC is a therapeutic option, together with recombinant activated factor VII (rFVIIa), for the treatment of bleeding episodes in patients with haemophilia A with inhibitors (Grade of Recommendation: 2C)1,6,9.
The dose indicated for congenital deficiency of FII or FX is 20–30 UI/kg, depending on the severity, site and extension of the bleeding. Once the first dose has been administered, the level of the individual deficient factor must be monitored in order to be able to decide the subsequent maintenance dose, taking into consideration that the minimum level required for haemostasis is 20–30 UI/dL for FII and 10–15 UI/dL for FX.
Greater detail can be gained from the AICE guidelines or by referring to Centres with expertise in the management of disorders of haemostais1 (Table II).
Acquired deficiencies
In patients with acquired deficiencies of factors in the prothrombin complex (due to severe liver disease, reduction due to loss or dilution) PCC can be administered, as a second choice alternative to FFP, taking into account that the risk of thrombosis is higher with PCC than with plasma10–12.
The administration of PCC is indicated (Table II):
in patients with deficiency of one or more of the factors of the prothrombin complex, in the presence of bleeding (Grade of Recommendation: 2C)7,8,13,14.
- when there are limitations to the use of FFP because of the risk of circulatory overload or the need for immediate haemostasis, in the following circumstances:
-
to correct excessive use of dicoumarols or when suspending oral anticoagulant therapy in emergency circumstances (acute major haemorrhage, urgent surgery) (Grade of Recommendation: 2C+)2,3,15–28.
In the case of oral anticoagulant therapy, PCC may be the treatment of first choice, although depending on the cause, site and extent of the potential or actual bleeding, the use of other therapeutic strategies, such as vitamin K and/or FFP, should be considered.
Although results from adequate clinical trials are not yet available, in life-threatening, extremely urgent situations, an infusion of rFVIIa can be considered as a replacement for PCC, when this latter is not available (as stated by the FCSA) (Grade of Recommendation: 2C)2,3.
in acquired haemophilia, in which PCC containing activated clotting factors can be used (Grade of Recommendation: 2C)9,29–33.
Posology and method of administration
The doses and duration of replacement therapy must be decided on the basis of the haemostatic imbalance, the site and extent of the bleeding, and the clinical situation10,22,28.
Before PCC is administered – compatibly with the urgency of the clinical situation – tests of haemostasis should be performed (PT/INR, aPTT and, if possible, assays of the factors in the prothrombin complex), in order to decide the dose and duration of the treatment.
In the case of severe bleeding or major surgery, the average first dose to administer, as a bolus, is 20–25 UI/kg. The PT and INR must be evaluated 30–60’ after the administration of the PCC, in order to determine whether to continue with this treatment and, if so, at what dose.
Correction of excessive anticoagulation from oral anticoagulant therapy
In the case of major bleeding or surgery that cannot be postponed2,3:
suspend the ongoing oral anticoagulant therapy;
measure the INR.
administer vitamin K intravenously at a dose of 10 mg/100 mL of physiological saline, slowly over about 30’.
- infuse the following doses of PCC slowly, over about 10–15’:
- - for INR < 2 administer 20 UI/kg;
- - for INR between 2 – 4 administer 30 UI/kg;
- - for INR > 4 administer 50 UI/kg.
measure the INR again at the end of the infusion and ensure that is < 1.5; if this is not the case, repeat the administration of PCC, according to the above scheme.
Alternatively, and particularly if PCC is not available, administer FFP at a starting dose of 15–20 mL/kg.
Monitoring indices for clinical auditing
Use of PCC treatment in the following conditions:
- in the absence of major bleeding;
- in patients on oral anticoagulation undergoing elective surgery with an INR < 1.5.
Contraindications, side effects and adverse reactions
DIC is a contraindication to the use of PCC10.
Possible side effects and adverse reactions are10–12:
- thromboembolic complications;
- allergic and anaphylactic reactions;
- fever;
- development of inhibitors of the clotting factors present in the PCC.
As other blood derivatives, PCC can be considered safe from an infectious point of view, although with some query concerning the potential transmission of prions.
Recommendations
It is recommended that all the details of the product infused, including its batch number, are recorded in the clinical records.
References
- 1.Santagostino E.Linee guida per la terapia sostitutiva dell’emofilia e dei difetti ereditari della coagulazione. Associazione Italiana dei Centri Emofilia, 2003. Available at: http://www.aiceonline.it/documenti/LineeGuida/ITALIA_Coagulopatie.pdf
- 2.Federazione Centri per la diagnosi della trombosi e la Sorveglianza delle terapie Antitrombotiche (FCSA). Pazienti in Terapia Anticoagulante Orale – Che Cosa Fare in Caso di: Emorragia Intracranica, Emorragie Maggiori, Emorragie Minori (con o senza Eccessiva Anticoagulazione), Correzione di Eccessiva Anticoagulazione in Assenza di Emorragie FCSA; 2006
- 3.Guida alla Terapia con Anticoagulanti Orali. 7a ed. FCSA; 2008. Federazione Centri per la diagnosi della trombosi e la Sorveglianza delle terapie Antitrombotiche (FCSA) [Google Scholar]
- 4.Brigulla M, Thiele T, Scharf C, et al. Proteomics as a tool for assessment of therapeutics in transfusion medicine: evaluation of prothrombin complex concentrates. Transfusion. 2006;46:377–85. doi: 10.1111/j.1537-2995.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 5.Burnouf T. Modern plasma fractionation. Transfus Med Rev. 2007;21:101–17. doi: 10.1016/j.tmrv.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United Kingdom Haemophilia Centre Doctors’ Organization (UKHCDO) Guidelines on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. Haemophilia. 2003;9:1–23. [Google Scholar]
- 7.Bolton-Maggs PHB, Perry DJ, Chalmers EA, et al. The rare coagulation disorders – review with guidelines for management from the United Kingdom Haemophilia Centre Doctors’ Organization. Haemophilia. 2004;10:593–628. doi: 10.1111/j.1365-2516.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 8.Acharya SS, Coughlin A, Dimichele DM, North American Rare Bleeding Disorder Study Group Rare Bleeding Disorder Registry: deficiencies of factors II, V, VII, X, XIII, fibrinogen and dysfibrinogenemias. J Thromb Haemost. 2004;2:248–56. doi: 10.1111/j.1538-7836.2003.t01-1-00553.x. [DOI] [PubMed] [Google Scholar]
- 9.Hay CR, Brown S, Collins PW, et al. The diagnosis and management of factor VIII and IX inhibitors: a guideline from the United Kingdom Haemophila Centre Doctors Organization. Br J Haematol. 2006;133:591–605. doi: 10.1111/j.1365-2141.2006.06087.x. [DOI] [PubMed] [Google Scholar]
- 10.Hellstern P, Halbmayer WM, Kohler M, et al. Prothrombin complex concentrates: indications, contraindications and risk: a task force summary. Thromb Res. 1999;95(Suppl 1):S3–6. doi: 10.1016/s0049-3848(99)00077-8. [DOI] [PubMed] [Google Scholar]
- 11.Kohler M, Hellstern P, Lechler E, et al. Thromboembolic complications associated with the use of prothrombin complex and factor IX concentrates. Thromb Haemost. 1998;80:399–402. [PubMed] [Google Scholar]
- 12.Kohler M. Thrombogenicity of prothrombin complex concentrates. Thromb Res. 1999;95(Suppl 1):S13–7. doi: 10.1016/s0049-3848(99)00079-1. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz R, Kienast J, Otto U, et al. Efficacy and safety of a prothrombin complex concentrate with two virusinactivation steps in patients with severe liver damage. Eur J Gastroenterol Hepatol. 2003;15:15–20. doi: 10.1097/00042737-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Prinoth O.Servizio Aziendale di Immunoematologia e Trasfusione - Comprensorio Sanitario di Bolzano. Terapia con emocomponenti e plasmaderivati: linee guida ed aspetti medico-legali Gennaio 2007. Available at: http://www.asbz.it/portal/it/document/IT/direzione/LINEE%20GUIDA%20ALLA%20TRASFUSIONEITALIANE%20(integrale).pdf
- 15.Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Hematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- 16.Makris M, Greaves M, Phillips WS, et al. Emergency oral anticoagulant reversal: the relative efficacy of infusion of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost. 1997;77:477–80. [PubMed] [Google Scholar]
- 17.Watson HG, Baglin T, Laidlaw SL, et al. A comparison of the efficacy and rate of response to oral and intravenous vitamin K in reversal of over-anticoagulation with warfarin. Br J Haematol. 2001;115:145–9. doi: 10.1046/j.1365-2141.2001.03070.x. [DOI] [PubMed] [Google Scholar]
- 18.Preston FE, Laidlaw ST, Sampson B, Kitchen S. Rapid reversal of oral anticoagulation with warfarin by a prothrombin complex concentrate (Beriplex): efficacy and safety in 42 patients. Br J Haematol. 2002;116:619–24. doi: 10.1046/j.0007-1048.2001.03295.x. [DOI] [PubMed] [Google Scholar]
- 19.Evans G, Luddington R, Baglin T. Beriplex P/N reverses severe warfarin-induced overanticoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol. 2001;115:998–01. doi: 10.1046/j.1365-2141.2001.03214.x. [DOI] [PubMed] [Google Scholar]
- 20.Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–70. doi: 10.1161/01.STR.0000221786.81354.d6. [DOI] [PubMed] [Google Scholar]
- 21.Yasaka M, Minematsu K, Naritomi H, et al. Predisposing factors for enlargement of intracerebral hemorrhage in patients treated with warfarin. Thromb Haemost. 2003;89:278–83. [PubMed] [Google Scholar]
- 22.Baglin T, Keeling DM, Watson HG, British Committee for Standards in Haematology Guideline on oral anticoagulation (warfarin): third edition-2005 update. Br J Haematol. 2006;132:277–85. doi: 10.1111/j.1365-2141.2005.05856.x. [DOI] [PubMed] [Google Scholar]
- 23.Lankiewicz MW, Hays J, Friedman KD, et al. Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost. 2006;4:967–70. doi: 10.1111/j.1538-7836.2006.01815.x. [DOI] [PubMed] [Google Scholar]
- 24.Kessler CM. Urgent reversal of warfarin with prothrombin concentrate: where are the evidence-based data? J Thromb Haemost. 2006;4:963–6. doi: 10.1111/j.1538-7836.2006.01944.x. [DOI] [PubMed] [Google Scholar]
- 25.Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfus Med Rev. 2007;21:37–48. doi: 10.1016/j.tmrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Viguè B, Ract C, Tremey B, et al. Ultra-rapid management of oral anticoagulant therapy-related surgical intracranial hemorrhage. Intensive Care Med. 2007;33:721–5. doi: 10.1007/s00134-007-0528-z. [DOI] [PubMed] [Google Scholar]
- 27.Riess HB, Meier-Hellmann A, Motsch J, et al. Prothrombin complex concentrate (Octaplex®) in patients requiring immediate reversal of oral anticoagulation. Thromb Res. 2007;121:9–16. doi: 10.1016/j.thromres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Salamat A, Seaton J, Watson HG. Impact of introducing guidelines on anticoagulant reversal. Transfus Med. 2005;15:99–105. doi: 10.1111/j.0958-7578.2005.00558.x. [DOI] [PubMed] [Google Scholar]
- 29.Sallah S. Treatment of acquired haemophilia with factor eight inhibitor bypassing activity. Haemophilia. 2004;10:169–73. doi: 10.1046/j.1365-2516.2003.00856.x. [DOI] [PubMed] [Google Scholar]
- 30.Tjønnfiord GE. Activated prothrombin complex concentrate (FEIBA) treatment during surgery in patients with inhibitors to FVIII/IX: the updated Norwegian experience. Haemophilia. 2004;10(Suppl 2):41–5. doi: 10.1111/j.1365-2516.2004.00939.x. [DOI] [PubMed] [Google Scholar]
- 31.Tjønnfiord GE. Surgery in patients with hemophilia and inhibitors: a review of the Norwegian experience with FEIBA. Semin Hematol. 2006;43(Suppl 4):S18–21. doi: 10.1053/j.seminhematol.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Berntorp E, Gringeri A, Leissinger C, et al. New approaches to using FEIBA in the treatment of inhibitor patients. Semin Thromb Hemost. 2006;32(Suppl 2):22–7. doi: 10.1055/s-2006-946911. [DOI] [PubMed] [Google Scholar]
- 33.Kraut EH, Aledort LM, Arkin S, et al. Surgical interventions in a cohort of patients with haemophilia A and inhibitors: an experiential retrospective chart review. Haemophilia. 2007;13:508–17. doi: 10.1111/j.1365-2516.2007.01523.x. [DOI] [PubMed] [Google Scholar]