Abstract
A current debate in the HIV-1 vaccine field concerns the ability of an immunodeficiency virus to elicit a protective response. One argument is that HIV-1 superinfections are frequent in healthy individuals, because virus evades conventional immune surveillance, a serious obstacle to vaccine design. The opposing argument is that protection from superinfection is significant, reflecting a robust immune response that might be harnessed by vaccination to prevent disease. In an experiment designed to address the debate, two macaques received an I.V. inoculation with SHIV KU-1-d (a derivative of SHIV KU-1) and were rested for ≥10 months. Infection elicited diverse neutralizing antibody activities in both animals. Animals were then exposed to SHIV 89.6P (I.V.), a virus carrying a heterologous envelope protein relative to the vaccine strain. Infection was monitored by viral load and CD4+ T-cell measurements. All control animals were infected and most succumbed to disease. In contrast, protection from superinfection was statistically significant in test monkeys; one animal showed no evidence of superinfection at any time point and the second showed evidence of virus at only one time point over a 6-month observation period. Neither animal showed signs of disease. Perhaps this protective state may serve as a ‘gold-standard’ for HIV-1 vaccine development, as a similar degree of protection against immunodeficiency virus infections in humans would be much desired.
Keywords: rhesus macaques, protective immunity, SHIV, neutralization, vaccine
INTRODUCTION
Recently, after more than 25 years of research, the HIV-1 field received the disappointing news that a front-runner vaccine candidate had failed in clinical trials [1]. This news has prompted considerable debate among HIV-1 researchers as to when and how a successful HIV-1 vaccine might be designed. Some researchers argue that the immune system is precisely armed to combat HIV-1 due to its sophisticated array of antibodies and T-cell receptors. By somatically rearranging variable (V), diversity (D), joining (J), and constant (C) region genes, the immune system tags virtually every new lymphocyte with a unique receptor, creating a plethora of weapons with which variant HIV-1 may be attacked. Other researchers note that there has never been a clinical success in the HIV-1 vaccine field, and argue that conventional immune responses toward HIV-1 are inadequate [2-7].
Part of this ongoing debate is the question of whether a protective response can be induced by natural infection with an immunodeficiency virus. The question is one of critical importance, because in many vaccine fields, the protection elicited by natural infection defines a gold-standard with which to measure the success of new vaccine candidates. If protection cannot be elicited by natural infection, the task of developing a protective vaccine can be perceived as difficult or perhaps impossible [5;7-9]
The macaque model provides an attractive platform for controlled studies of superinfections, as viral exposures may be deliberate rather than presumed (in clinical research, exposure dates are generally unknown). While a number of experiments have revealed protective immunity following SIV or SHIV infections, results and interpretations of these experiments have been variable [10-20]. Here, we address the debate with a description of macaques that were rested for ≥10 months after infection with a derivative of SHIV-KU-1 and then exposed to the heterologous, pathogenic SHIV-89.6P.
METHODS
Viruses and animal inoculations
SHIV KU-1 (carrying an HIV-1IIIB-derived envelope, [21]) was kindly provided by Dr. O. Narayan and the NIH AIDS Research and Reference Reagent Program (NARRRP). The virus was grown in limiting dilution cultures on MT-2 cells and wells with sensitivity to neutralization by immunoglobulin from HIV-1-infected humans were selected for further expansion (this process was implemented to avoid acquisition of HIV-1-specific antibody resistance, as is sometimes observed following the in vivo passage of SHIVs [21;22]). Further virus expansion was with intermittent positive selections of infected cells on HIV-1-specific-antibody-coated dynabeads. The final virus expansion was on rhesus PBMCs. The resultant virus stock was termed SHIV-KU-1-d. In a pilot experiment, two adult Indian rhesus macaques (Macaca mulatta, CG83 and CA35) were inoculated (I.V.) respectively with 250 and 25 TCID50 virus (TCID50 were measured on rhesus PBMC). The 2 test and 7 naive animals were then exposed to SHIV 89.6P (approximately 50 TCID50 per animal, I.V. [23;24]). One animal from the control group (CE46) and one animal from the test group (CA35) received approximately 40 TCID50 of the challenge virus by I.V. inoculation, and 10 TCID50 virus by the subcutaneous route due to technical difficulties. All study procedures followed IACUC Guidelines.
Antibody assays
Enzyme-linked immunosorbant (ELISA) tests were performed with kits as recommended by manufacturers (HIVABTM HIV-1/HIV-2 (rDNA) EIA, Abbott Laboratories) with sera diluted 10−3 or 10−4. Serum samples (diluted 1:50) were also tested for neutralization of HIV-1 isolates SF2, 30e, 310a, IIIB, and SHIV 89.6P. Human HIV-negative and HIV-positive serum samples were used as controls. Neutralization assays were performed using GHOST cells expressing either CXCR4 (for viruses 30e, 310a, IIIB and SHIV 89.6P) or CCR5 (for virus SF2). Aliquots of viruses were mixed with positive and negative control human serum samples or with test monkey serum samples for overnight incubation at 37°C. Virus/antibody mixtures were then incubated with washed, adherent GHOST cells in a 96-well plate (approximately 10 TCID50 virus per well). Following an overnight incubation, cells were washed several times and then incubated for an additional 24–72 hours. Virus was measured by p24 (for HIV-1) or p27 (for SHIV) antigen assays as described by manufacturers (Coulter or Immunodiagnostics); in some experiments, plates were coated with monoclonal antibody 183-H12-5C (NARRRP) to capture P24 from culture supernatants.
Virus load and CD4+T-cell percentages
To measure SIV virus loads, branched DNA (bDNA) assays were performed on plasma samples by Bayer Reference Testing Laboratory (Berkeley, CA). As confirmation of bDNA assays, SIV RNA levels in plasma samples were also quantified by real time RT-PCR in a Prism 7700 Sequence Detection System using Taqman Two Step Gold Kit (Applied Biosystems (ABI), Foster City, CA) with SIV LTR primers and probe, as defined previously [25]. Briefly, single tube reactions were at 50°C for 2 min followed by 95°C for 10 min. Amplification was with 40 cycles at 95°C for 15 sec and 60°C for 60 sec. Serial dilutions of transcribed SIV LTR RNA ranging from 108 to 100 copies, (plasmid kindly provided by M. Murphey-Corb, University of Pittsburgh) were reacted in parallel with samples to generate a standard curve with a sensitivity threshold of 10 copies per reaction. RNA copy numbers from the unknown plasma samples were calculated from the standard curve and expressed as RNA copies per milliliter of plasma.
To determine CD4+ and CD3+ T-cell counts, blood in EDTA anti-coagulent was stained with anti-CD4 antibodies-APC (Becton Dickinson (BD) Biosciences, San Diego, CA) and anti-CD3 antibodies-FITC (BD). Erythrocytes were lysed and the leukocytes were fixed using the TQ-Prep system and reagents (Beckman-Coulter).
RESULTS
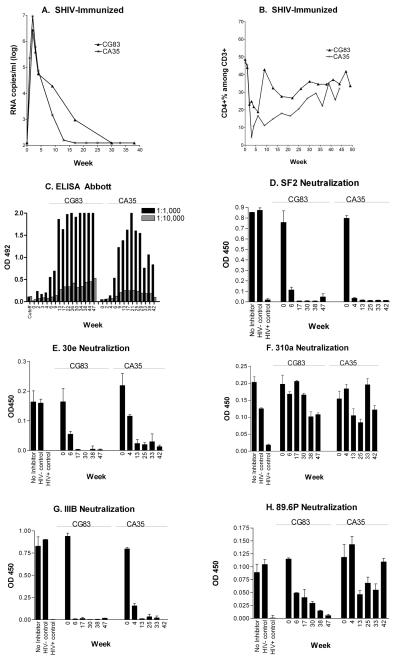
Two adult rhesus macaques were inoculated with SHIV KU-1-d by the intravenous route. Inoculations were with 250 TCID50 and 25 TCID50 for animals CG83 and CA35, respectively. As shown in Figure 1A, both animals were infected and showed peak viral loads of over one million RNA copies/ml by four weeks post-inoculation as determined by bDNA analyses. Animal CG83 required approximately seven months to clear circulating virus (virus dropped below detection, <100 RNA copies/ml, within 30 weeks). In animal CA35, virus dropped below detection within four months (by week 17). As shown in Figure 1B, CD4+ T-cell percentages dropped precipitously in both animals by week four post-infection. Recovery was gradual. Both animals maintained good health throughout the experimental course, demonstrating that the SHIV KU-1-d differed from its pathogenic SHIV KU-1 parent [21].
Figure 1. Infectious SHIV vaccine elicits binding and neutralizing antibodies.
Two macaques were inoculated with the SHIV-KU-1 and monitored for viral load by bDNA analyses (panel A), and for blood CD4+ T-cell percentages within the CD3+ cell population (panel B). Serum antibodies were also monitored for HIV-1-binding antibodies at 1:1,000 (black bar) and 1:10,000 (grey bar) serum dilutions (panel C). Additionally, serum samples (diluted 1:50) were tested for neutralization of HIV-1 isolates SF2 (panel D), 30e (panel E), 310a (panel F), IIIB (panel G), and SHIV 89.6P (panel H).
The Abbott clinical ELISA (based on HIV-1-IIIB-derived antigens) was used to measure antibody responses. Shown in Figure 1C are results of tests with serially diluted (1:1,000 and 1:10,000) sera. Antibodies were of higher magnitude in CG83 compared to CA35. Sera were also tested (1:50 dilution) for neutralization of SF2 (Figure 1D), 30e (Figure 1E), 310a (Figure 1F), IIIB (Figure 1G) and SHIV 89.6P (Figure 1H). Activity was detected against all viruses (but was relatively weak toward virus 310a). CG83 showed the better breadth of neutralizing activity, including robust activity toward 89.6P at week 47 after SHIV KU-1 infection. The neutralization by both animals of viruses SF2, 30e and IIIB prompted the conduct of tests with higher serum dilutions (1:500 and 1:5000). Positive scores (>50% neutralization) were measured in both animals at a 1:500 serum dilution for all three viruses, and at a 1:5,000 serum dilution for 30e and IIIB (data not shown). Virus-specific T-cells were not measured in the current study, but it is likely that these cells were generated, and contributed to experimental outcome.
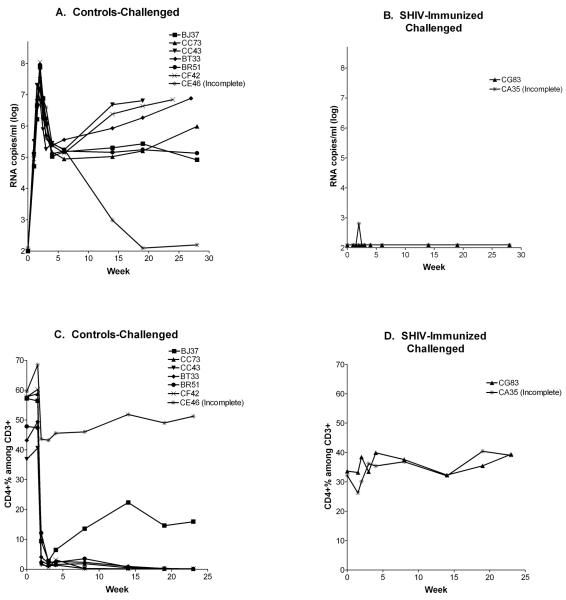
After 49 weeks of infection for CG83 and 44 weeks of infection for CA35, the macaques were challenged with pathogenic SHIV-89.6P (approximately 50 TCID50 by the I.V. route). Seven naive animals were also challenged. One animal from the control group (CE46) and one animal from the test group (CA35) received approximately 40 TCID50 of the challenge virus by I.V. inoculation and 10 TCID50 by the subcutaneous route due to technical difficulties. As demonstrated in Figure 2A, infections occurred and peak viral loads exceeded 107 RNA copies/ml in all controls, as measured by branched DNA (bDNA) analyses. Animal CE46, having received the smaller 89.6P I.V. inoculum, cleared virus from the circulation by week 20, while all other animals maintained levels of greater than 104 RNA copies/ml. Both SHIV KU-1 immunized animals controlled virus following SHIV 89.6P challenge (Figure 2B). Challenge virus was never detected in animal CG83, and there was only one virus-positive score at the two-week time point in CA35. The RT-PCR tests confirmed bDNA results in control animals (data not shown). For test animals, the RT-PCR results were negative at every time point including the 2 week time point of CA35 (data not shown). Differences between test and control animals were statistically significant (p<.05, Students T test).
Figure 2. Infectious SHIV vaccine protects against heterologous pathogenic SHIV challenge.
Seven controls and two test monkeys were challenged with SHIV-89.6P and monitored for viral load by bDNA analyses. Results from control and test animals are shown in panels A and B, respectively. Animals were also monitored for CD4+ T-cell percentages among CD3+ populations after challenge with SHIV-89.6P (Panels C and D).
Figures 2C and D show the CD4+ T-cell percentages among CD3+ cells after SHIV 89.6P challenge. All seven control animals experienced a drop in CD4+ T-cell percentages from pre-challenge levels by at least 20%, while the two test animals did not (Fishers Exact Test, p<.05) . CA35 showed a minor drop in CD4+ T-cell percentages after challenge, followed by rapid recovery whereas there was no identifiable drop in CD4+ T-cell percentages in animal CG83. Five of the seven control animals succumbed to disease (all were sacrificed due to disease, except for animals BJ37 and CE46). There were no signs of disease in either of the test animals.
DISCUSSION
Our study illustrated that two animals infected with SHIV-KU-1-d, a derivative of SHIV-KU-1, developed robust and diverse neutralizing antibody responses. When animals were later challenged with an envelope-heterologous pathogenic SHIV, they were each protected. One animal showed no virus at any time point. The second animal experienced a marginal viral bDNA score at only one time point, two weeks post-challenge. These two animals remained healthy throughout the course of the experiment. In contrast, seven of seven control animals had peak virus titers of ≥107 RNA copies/ml, and 5 of 7 animals died. The experiment clearly demonstrated statistically significant protection conferred by a live SHIV vaccine. This degree of protection in humans would be a much desired ‘gold-standard’ outcome in the HIV-1 vaccine field.
The current results supplement numerous additional studies revealing the potency of immune responses elicited by immunodeficiency virus infections and demonstrating the efficacy of infectious SIV and SHIV vaccines [11;13;15;19]. For example, a recent report by Reynolds et. al. demonstrated that the infection of macaques with SIVmac239Δnef controlled replication (either completely or partially) following challenge with SIVsmE660 [26]. Another recent study by Yankee et. al. demonstrated that the infection of macaques with ΔvpuSHIVPPC (by DNA and then virus inoculation) conferred full protection against challenge with pathogenic viruses SHIVKU2 and SIVmac239 in most animals [27].
What are the precise immune correlates of protection? Answers to this question are many. Yeh et. al. have suggested that there is no correlation between immune response and protection in non-human primate superinfection studies. This conclusion was reached when authors found no significant differences between immune responses prior to challenge in vaccinated/protected animals and vaccinated/partially-protected animals [12]. As an alternative strategy, Yankee et. al. compared immune responses in vaccinated/protected animals and unvaccinated/unprotected animals (following the ΔvpuSHIVPPC study described above [27]). They then found that cellular, but not humoral immune responses correlated with protection (no neutralizing antibodies were identified in vaccinated animals prior to challenge in this case). In still other situations, the humoral response was identified as a protective correlate [28]. For example, researchers showed that the passive transfer of SIVhyperimmune sera to naïve recipients conferred complete protection against SIV challenge [29]. Surprisingly, the same sera scored negatively for neutralization against the challenge virus in vitro, demonstrating a limitation in the in vitro neutralization assay [29-36]. Taken together, these results suggest that despite limitations in current immune assays, both B-cell and T-cell activities can be correlated with protection.
How do the infectious SHIV and SIV vaccines elicit protective immunity? Again, opinions are many. One hypothesis is that the answer lies in a complex interplay between virus and the immune system: Upon first exposure to an immunodeficiency virus, a sero-negative subject cannot efficiently attack virus. The virus may therefore traffic to privileged sites such as the brain. Even though virus-specific B-cell and T-cell responses activate in the periphery, they cannot reach and clear sequestered virus. Escape virus mutants (bearing a cocktail of diverse antigens) are therefore generated from virus sanctuaries, followed by activation of new B-cell and T-cell populations [37]. After numerous cycles of virus escape and immune activation, diverse B-cells and T-cells are primed to function as a composite, to target the diverse viruses of nature as necessary for protection from superinfection.
It is likely that healthy HIV-1-infected humans who experience months of virus-lymphocyte co-evolution without disease also exhibit considerable protection when exposed to a second virus [38-41]. A compelling study is that of Chakraborty et. al., revealing no detectable virus transmission between members of HIV-1 sero-concordant couples [42]. However, superinfections can occur, particularly during early stages of virus infection (before immune cells are triggered toward the virus and its escape mutants) and during late stages of infection (when T-cell responses decline, [38;43-45]). The ever-increasing sensitivity of nucleic acid assays may ultimately reveal ‘new’ HIV-1 sequences in all infected persons [46], although the relationship of these sequences to temporal virus exposures, and to virus-induced pathology, will remain a topic of continued debate.
The ability of a sequestered virus (SIV, SHIV or HIV) to elicit protective immunity against superinfection is not unique to the immunodeficiency viruses. Rather, there are numerous viruses that establish chronic infections while eliciting a protective immune response. These include Epstein-Barr Virus (EBV) and Varicella Zoster Virus (VZV). Each of these viruses, like HIV-1, can pose a threat to the host throughout life due to its sequestration in privileged sites. If the immune system experiences an insult (perhaps due to an unrelated illness), an explosive state of virus growth, morbidity and mortality may occur. As examples, VZV and EBV can be fatal in immunocompromised subjects, causing Zoster and EBV-induced lymphoproliferative disease, respectively. Fortunately, the association of virus with chronic infection does not preclude development of an effective vaccine. The primed immune system is better able to protect against infection at peripheral surfaces than to eradicate infection following virus sequestration at privileged sites, as demonstrated by the recent licensure of VZV and papilloma virus vaccine products.
Debates are certain to continue regarding the correlation of HIV-1-specific immune responses with protection, the value of various primate models (positive results in both SIV [47-49] and SHIV [6;50] experiments have been followed by disappointments in the clinic), and the best design of an HIV-1 vaccine for humans. With reference to the latter point, the live immunodeficiency virus vaccine is currently considered unsafe for testing in humans because of its reversion potential [51]. As an alternative means of presenting diverse antigens to the immune system, HIV-1 vaccine cocktails are now being designed by a number of laboratories [2;52;53]. Cocktails are attractive in that they have been proven safe and efficacious in numerous other vaccine fields (e.g. polio, VZV, papilloma virus, influenza virus). Perhaps a cocktail vaccine designed to harness the ‘gold-standard’ activity typifying SIV/SHIV-infected primates will ultimately protect humans from infections with HIV-1 [2;3;54-57].
ACKNOWLEDGEMENTS
We thank Dr. N. Letvin, Dr. Preston A. Marx, Tessa Williams and the virus stock core at Tulane National Primate Research Center, for the SHIV-89.6P challenge inoculum. We thank Drs. R.V. Srinivas and J. Levy and the NIH AIDS Research and Reference Reagent Program (NARRRP) for viruses IIIB, SF2, 30e and 310a. The SHIV KU-1 (carrying an HIV-1IIIB-derived envelope) was kindly provided by Dr. O. Narayan and the NARRRP. We thank B. Chesebro and H. Chen for the p24 hybridoma 183-H12-5C and V KewalRamani and D Littman for GHOST cells, provided through NARRRP. We thank Harold P. Stamey and the Tennessee Blood Services, Inc. for providing blood donor samples to the study. We finally thank the Tulane National Primate Research Center veterinary and clinical staff for excellent animal care, Tamika Bridges-Malveo for RT-PCR analyses, Calvin Lanclos for flow analyses, and Lynn Fresh for coordination and shipping of samples.
This work was supported in part by grants from the NIH NCI: R01 CA57419 and Cancer Center Support Core Grant P30-CA21765, NIH-NIAID: P01 AI45142 and R21-AI56974, NIH NCRR base grant P51-RR00164 to the Tulane National Primate Research Center, the Federated Department Stores, the Mitchell Fund, the Anderson Foundation, the Pendleton Fund, the Pioneer Fund and the American Lebanese Syrian-Associated Charities (ALSAC).
References
- 1.Robb ML. Failure of the Merck HIV vaccine: an uncertain step forward. Lancet. 2008;372:1857–8. doi: 10.1016/S0140-6736(08)61593-7. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz JL, Zhan X, Brown SA, et al. HIV-1 vaccine development: Tackling virus diversity with a multi-envelope cocktail. Frontiers Bioscience. 2008;13:609–20. doi: 10.2741/2706. [DOI] [PubMed] [Google Scholar]
- 3.Zhan X, Martin LN, Slobod KS, et al. Multi-envelope HIV-1 vaccine devoid of SIV components controls disease in macaques challenged with heterologous pathogenic SHIV. Vaccine. 2005;23:5306–20. doi: 10.1016/j.vaccine.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Nyambi PN, Nadas A, Mbah HA, et al. Immunoreactivity of intact virions of human immunodeficiency virus type 1 (HIV-1) reveals the existence of fewer HIV-1 immunotypes than genotypes. J Virol. 2000;74:10670–80. doi: 10.1128/jvi.74.22.10670-10680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston MI, Fauci AS. An HIV vaccine--challenges and prospects. N Engl J Med. 2008;359:888–90. doi: 10.1056/NEJMp0806162. [DOI] [PubMed] [Google Scholar]
- 6.Fauci AS, Johnston MI, Dieffenbach CW, et al. HIV vaccine research: the way forward. Science. 2008;321:530–2. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- 7.Douek DC, Kwong PD, Nabel GJ. The rational design of an AIDS vaccine. Cell. 2006;124:677–81. doi: 10.1016/j.cell.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: The human immunodeficiency virus (HIV) Vaccine. 2006;24:4062–81. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Jefferys R. Pleas for a back-to-basics approach. IAVI Report, The Publication on International AIDS Vaccine Research. 2008 [Google Scholar]
- 10.Berry N, Stebbings R, Ferguson D, et al. Resistance to superinfection by a vigorously replicating, uncloned stock of simian immunodeficiency virus (SIVmac251) stimulates replication of a live attenuated virus vaccine (SIVmacC8) J Gen Virol. 2008;89:2240–51. doi: 10.1099/vir.0.2008/001693-0. [DOI] [PubMed] [Google Scholar]
- 11.Connor RI, Montefiori DC, Binley JM, et al. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–9. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh WW, Jaru-Ampornpan P, Nevidomskyte D, et al. Partial protection of Simian immunodeficiency virus (SIV)-infected rhesus monkeys against superinfection with a heterologous SIV isolate. J Virol. 2009;83:2686–96. doi: 10.1128/JVI.02237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements JE, Montelaro RC, Zink MC, et al. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–44. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–41. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 15.Stephens EB, Joag SV, Atkinson B, et al. Infected macaques that controlled replication of SIVmac or nonpathogenic SHIV developed sterilizing resistance against pathogenic SHIV(KU-1) Virology. 1997;234:328–39. doi: 10.1006/viro.1997.8662. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen RA, Montefiori DC, Robinson HL, McClure HM, Ruprecht RM. Heterologous neutralizing antibody induction in a simian-human immunodeficiency virus primate model: lack of original antigenic sin. J Infect Dis. 2001;184:1603–7. doi: 10.1086/324582. [DOI] [PubMed] [Google Scholar]
- 17.Kim EY, Busch M, Abel K, et al. Retroviral recombination in vivo: viral replication patterns and genetic structure of simian immunodeficiency virus (SIV) populations in rhesus macaques after simultaneous or sequential intravaginal inoculation with SIVmac239Deltavpx/Deltavpr and SIVmac239Deltanef. J Virol. 2005;79:4886–95. doi: 10.1128/JVI.79.8.4886-4895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay GA, Liu Z, Singh DK, et al. Protection against late-onset AIDS in macaques prophylactically immunized with a live simian HIV vaccine was dependent on persistence of the vaccine virus. J Immunol. 2004;173:4100–7. doi: 10.4049/jimmunol.173.6.4100. [DOI] [PubMed] [Google Scholar]
- 19.Cranage MP, Whatmore AM, Sharpe SA, et al. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology. 1997;229:143–54. doi: 10.1006/viro.1996.8419. [DOI] [PubMed] [Google Scholar]
- 20.Cranage MP, Sharpe SA, Whatmore AM, et al. In vivo resistance to simian immunodeficiency virus superinfection depends on attenuated virus dose. J Gen Virol. 1998;79(Pt 8):1935–44. doi: 10.1099/0022-1317-79-8-1935. [DOI] [PubMed] [Google Scholar]
- 21.Cayabyab M, Karlsson GB, Etemad-Moghadam BA, et al. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2) J Virol. 1999;73:976–84. doi: 10.1128/jvi.73.2.976-984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao HX, Etemad-Moghadam B, Montefiori DC, et al. Induction of antibodies in guinea pigs and rhesus monkeys against the human immunodeficiency virus type 1 envelope: neutralization of nonpathogenic and pathogenic primary isolate simian/human immunodeficiency virus strains. J Virol. 2000;74:254–63. doi: 10.1128/jvi.74.1.254-263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimann KA, Li JT, Voss G, et al. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reimann KA, Li JT, Veazey R, et al. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–8. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amedee AM, Rychert J, Lacour N, Fresh L, Ratterree M. Viral and immunological factors associated with breast milk transmission of SIV in rhesus macaques. Retrovirology. 2004;14:1–17. doi: 10.1186/1742-4690-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds MR, Weiler AM, Weisgrau KL, et al. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J Exp Med. 2008;205:2537–50. doi: 10.1084/jem.20081524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yankee TM, Sheffer D, Liu Z, et al. Longitudinal study to assess the safety and efficacy of a live-attenuated SHIV vaccine in long term immunized rhesus macaques. Virology. 2009;383:103–11. doi: 10.1016/j.virol.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrantelli F, Ruprecht RM. Neutralizing antibodies against HIV -- back in the major leagues? Curr Opin Immunol. 2002;14:495–502. doi: 10.1016/s0952-7915(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 29.Van Rompay KK, Berardi CJ, Dillard-Telm S, et al. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis. 1998;177:1247–59. doi: 10.1086/515270. [DOI] [PubMed] [Google Scholar]
- 30.Baba TW, Liska V, Hofmann-Lehmann R, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 31.Foresman L, Jia F, Li Z, et al. Neutralizing antibodies administered before, but not after, virulent SHIV prevent infection in macaques. AIDS Res Hum Retroviruses. 1998;14:1035–43. doi: 10.1089/aid.1998.14.1035. [DOI] [PubMed] [Google Scholar]
- 32.Glaudin M-C, Parren PWHI, Weir R, Barbas CF, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nature Medicine. 1997;3:1389–93. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 33.Mascola JR, Lewis MG, Stiegler G, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–18. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parren PW, Marx PA, Hessell AJ, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–7. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prince AM, Reesink H, Pascual D, et al. Prevention of HIV infection by passive immunization with HIV immunoglobulin. Aids Res Hum Retroviruses. 1991;7:971–3. doi: 10.1089/aid.1991.7.971. [DOI] [PubMed] [Google Scholar]
- 36.Ferrantelli F, Hofmann-Lehmann R, Rasmussen RA, et al. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS. 2003;17:301–9. doi: 10.1097/00002030-200302140-00003. [DOI] [PubMed] [Google Scholar]
- 37.Wrin T, Crawford L, Sawyer L, Weber P, Sheppard HW, Hanson CV. Neutralizing antibody responses to autologous and heterologous isolates of human immunodeficiency virus. J Acquir Immune Defic Syndr. 1994;7:211–9. [PubMed] [Google Scholar]
- 38.Gottlieb GS, Nickle DC, Jensen MA, et al. Dual HIV-1 infection associated with rapid disease progression. Lancet. 2004;363:619–22. doi: 10.1016/S0140-6736(04)15596-7. [DOI] [PubMed] [Google Scholar]
- 39.Gerhardt M, Mloka D, Tovanabutra S, et al. In-depth, longitudinal analysis of viral quasispecies from an individual triply infected with late-stage human immunodeficiency virus type 1, using a multiple PCR primer approach. J Virol. 2005;79:8249–61. doi: 10.1128/JVI.79.13.8249-8261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coetzer M, Cilliers T, Papathanasopoulos M, et al. Longitudinal analysis of HIV type 1 subtype C envelope sequences from South Africa. Aids Res Hum Retroviruses. 2007;23:316–21. doi: 10.1089/aid.2006.0207. [DOI] [PubMed] [Google Scholar]
- 41.Casado C, Pernas M, Alvaro T, et al. Coinfection and superinfection in patients with long-term, nonprogressive HIV-1 disease. J Infect Dis. 2007;196:895–9. doi: 10.1086/520885. [DOI] [PubMed] [Google Scholar]
- 42.Chakraborty B, Valer L, De MC, Soriano V, Quinones-Mateu ME. Failure to detect human immunodeficiency virus type 1 superinfection in 28 HIV-seroconcordant individuals with high risk of reexposure to the virus. Aids Res Hum Retroviruses. 2004;20:1026–31. doi: 10.1089/aid.2004.20.1026. [DOI] [PubMed] [Google Scholar]
- 43.Smith DM, Strain MC, Frost SD, et al. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology. 2006;355:1–5. doi: 10.1016/j.virol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Grobler J, Gray CM, Rademeyer C, et al. Incidence of HIV-1 dual infection and its association with increased viral load set point in a cohort of HIV-1 subtype C-infected female sex workers. J Infect Dis. 2004;190:1355–9. doi: 10.1086/423940. [DOI] [PubMed] [Google Scholar]
- 45.Gonzales MJ, Delwart E, Rhee SY, et al. Lack of detectable human immunodeficiency virus type 1 superinfection during 1072 person-years of observation. J Infect Dis. 2003;188:397–405. doi: 10.1086/376534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piantadosi A, Chohan B, Chohan V, McClelland RS, Overbaugh J. Chronic HIV-1 infection frequently fails to protect against superinfection. PLoS Pathog. 2007;3:e177. doi: 10.1371/journal.ppat.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berman P, Murthy KK, Wrin T, et al. Protection of MN-rgp120-immunized chimpanzees from heterologous infection with a primary isolate of human immunodeficiency virus type 1. J Infect Dis. 1996;173:52–9. doi: 10.1093/infdis/173.1.52. [DOI] [PubMed] [Google Scholar]
- 48.Hu S-L, Abrams K, Barber GN, et al. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–9. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 49.Berman PW, Gray AM, Wrin T, et al. Genetic and immunologic characterization of viruses infecting MN-rgp120-vaccinated volunteers. J Infect Dis. 1997;176:384–97. doi: 10.1086/514055. [DOI] [PubMed] [Google Scholar]
- 50.Morgan C, Marthas M, Miller C, et al. The use of nonhuman primate models in HIV vaccine development. PLoS Med. 2008 Aug 12;5(8):e173. doi: 10.1371/journal.pmed.0050173. 2008; 5:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–5. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 52.Pal R, Kalyanaraman VS, Nair BC, et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006;348:341–53. doi: 10.1016/j.virol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 53.Catanzaro AT, Roederer M, Koup RA, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–92. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 54.Hurwitz JL, Lockey TD, Jones B, et al. First phase I clinical trial of an HIV-1 subtype D gp140 envelope protein vaccine: immune activity induced in all study participants. AIDS. 2008;22:149–51. doi: 10.1097/QAD.0b013e3282f174ed. [DOI] [PubMed] [Google Scholar]
- 55.Stambas J, Brown SA, Gutierrez A, et al. Long lived multi-isotype anti-HIV antibody responses following a prime-double boost immunization strategy. Vaccine. 2005;23:2454–64. doi: 10.1016/j.vaccine.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 56.Slobod KS, Lockey TD, Howlett N, et al. Subcutaneous administration of a recombinant vaccinia virus vaccine expressing multiple envelopes of HIV-1. Eur J Clin Microbiol Infect Dis. 2004;23:106–10. doi: 10.1007/s10096-003-1075-3. [DOI] [PubMed] [Google Scholar]
- 57.Sealy R, Slobod KS, Flynn P, et al. Preclinical and clinical development of a multi-envelope, DNA-virus-protein (D-V-P) HIV-1 vaccine. Int Rev Immunol. 2009;28:49–68. doi: 10.1080/08830180802495605. [DOI] [PMC free article] [PubMed] [Google Scholar]