Summary
A declining cell-mediated immunity to varicella zoster virus (VZV) with advancing age or immunosuppression results in virus reactivation from latently infected human ganglia anywhere along the neuraxis. Virus reactivation produces zoster, often followed by chronic pain (postherpetic neuralgia or PHN) as well as vasculopathy, myelopathy, retinal necrosis and cerebellitis. VZV reactivation also produces pain without rash (zoster sine herpete). Vaccination after age 60 reduces the incidence of shingles by 51%, PHN by 66% and the burden of illness by 61%. However, even if every healthy adult over age 60 years is vaccinated, there would still be about 500,000 zoster cases annually in the United States alone, about 200,000 of whom will experience PHN. Analyses of viral nucleic acid and gene expression in latently infected human ganglia and in an animal model of varicella latency in primates are serving to determine the mechanism(s) of VZV reactivation with the aim of preventing reactivation and the clinical sequelae.
Keywords: VZV, latency, zoster, postherpetic neuralgia, vaccination, vasculopathy
Introduction
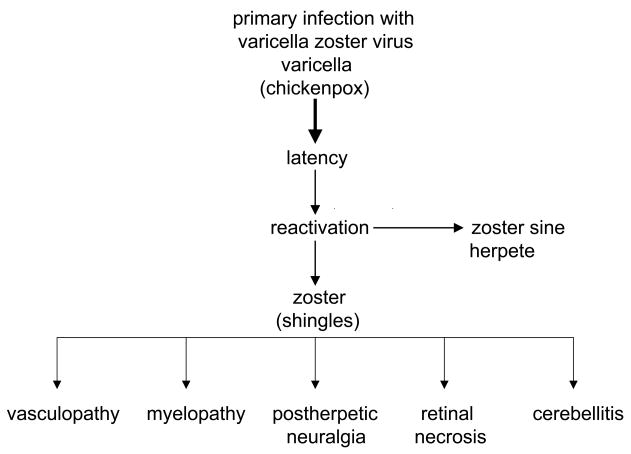
Varicella zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus. Primary infection causes varicella (chickenpox), after which virus becomes latent in cranial nerve ganglia, dorsal root ganglia and autonomic ganglia along the entire neuraxis. Years later, as cell-mediated immunity to VZV declines with age or immunosuppression, such as in cancer transplant or AIDS patients, VZV reactivates to cause zoster (shingles), often followed by chronic pain (postherpetic neuralgia or PHN) as well as vasculopathy, myelopathy, retinal necrosis and cerebellitis (Figure 1). VZV reactivation can also produce pain without rash (zoster sine herpete). In fact, all neurological complications of VZV reactivation can occur without rash.
Figure 1.
Neurologic disease produced by reactivation of varicella zoster virus.
Herpes zoster
Zoster affects approximately 1,000,000 individuals in the U.S. per year. Most patients are over age 60 [1] or immunocompromised [2]. The annual incidence of zoster is 5 to 6.5 per 1,000 individuals at age 60, increasing to 8 to 11 per 1,000 at age 70 [2]. Unlike varicella, which occurs primarily in the spring, there is no seasonal predilection for zoster. The development of zoster may be viewed in the context of a continuum in immunodeficient individuals, ranging from a natural decline in VZV-specific cell-mediated immunity with age, to more serious immune deficits seen in cancer patients and transplant recipients, and ultimately in patients with AIDS [3]. Not surprisingly, zoster in otherwise young, healthy individuals may be the first manifestation of HIV infection [4]. Interestingly, varicella in infancy predisposes to zoster earlier in life [5].
Herpes zoster usually begins with pain, itching, paresthesias, dysesthesias, and/or allodynia in one to three dermatomes followed days later by the appearance of a unilateral maculopapular rash that evolves into vesicles over the affected area. VZV is highly infectious and transmission occurs by direct contact with these skin lesions or by respiratory aerosols. Immunosuppression increases the risk of disseminated zoster [6]. In most patients, the disappearance of skin lesions is accompanied by decreased pain and complete resolution of pain in 4–6 weeks. Magnetic resonance imaging (MRI) may show enhancement of ganglia and affected nerve roots [7].
Because VZV becomes latent in ganglia along the entire neuraxis, zoster can develop anywhere on the body. Zoster can affect all cranial nerves [8–19] and spinal nerves at all levels; zoster can also be associated with lower motor neuron type weakness in the arm or leg [20,21], diaphragm [22] or abdominal muscles [23]. In addition, VZV can reactivate subclinically (without pain or rash) in astronauts [24], including shedding of infectious virus [25], most likely reflecting transient stress-induced depression of cell-mediated immunity to VZV [26]. Asymptomatic shed of human neurotropic alphaherpsesviruses is not restricted to VZV as shown by subclinical reactivation of HSV-1 [27] and HSV-2 [28].
Zoster is presumed to develop by retrograde transport of virus from ganglia to skin in a host partially immune to VZV. VZV has also been isolated from the blood of immunocompromised patients with localized and disseminated zoster [29], suggesting a role for hematogenous spread. Cardinal pathological features of zoster are inflammation and hemorrhagic necrosis with associated neuritis, localized leptomeningitis, unilateral segmental poliomyelitis, and degeneration of related motor and sensory roots [30,31]. Demyelination occurs in areas with mononuclear cell (MNC) infiltration and microglial proliferation. Intranuclear inclusions, viral antigen and herpesvirus particles have been detected in acutely infected ganglia [32–35].
Treatment
In immunocompetent individuals under age 50, analgesics are used to relieve pain. Antivirals (famciclovir, 500 mg orally three times daily or valacyclovir, 1 g three times daily for 7–10 days) are not required but speed healing of rash. In immunocompetent individuals age 50 and older, treatment with both analgesics and antivirals are recommended and are essential in patients with ophthalmic distribution zoster. Similarly, treatment of patients within 7 days of onset of the Ramsay reportedly improves recovery [36–38]. We also use prednisone (60 mg orally for 3–5 days) to reduce the inflammatory response, although double-blind placebo-controlled studies to prove additional efficacy are lacking. In immunocompromised patients, intravenous acyclovir (5–10 mg/kg three times per day for 5–7 days) is recommended.
Postherpetic neuralgia (PHN)
About 40% of zoster patients over age 60 will experience PHN [39,40]. PHN is characterized by constant, severe, stabbing or burning, dysesthetic pain that persists for at least three months and sometimes years after resolution of rash. The cause and pathogenesis of PHN are unknown. Two non-mutually exclusive theories are that: (1) excitability of ganglionic or even spinal cord neurons is altered; and (2) persistent or low-grade productive virus infection exists in ganglia. Support for the concept that PHN is produced by low-level ganglionitis comes from the detection of VZV DNA and proteins in blood MNCs of many patients with PHN [41–43], and from the favorable response of some PHN patients to antiviral treatment [44–46].
While not life-threatening, PHN is difficult to manage. Treatment is supportive with use of neuroleptic drugs and various analgesics, including opiates to alleviate pain, but no universally accepted treatment exists. Pregabalin is given at 75 to 150 mg orally twice daily or 50 to 100 mg orally three times daily (150 to 300 mg/day). If minimal relief is obtained at 300 mg daily for two weeks, the dose can be increased to a maximum of 600 mg/day in two or three divided doses. In addition, oxycodone (controlled-release, 10–40 mg orally every 12 hr) or controlled-release morphine sulfate and tricyclic antidepressants (TCAs) are used [47]. Levorphanol produces morphine-like analgesia, at a dose of 2 mg orally every 6–8 hr as needed with maximum doses of 6–12 mg daily [48,49]. Combination treatment with morphine and gabapentin also decreases pain more than either drug alone or placebo [50]. TCAs, including amitriptyline (10–25 mg orally at bedtime with a maximum dose of 150–200 mg/day), nortriptyline, mapotriline, and despramine lessen the pain of PHN.
Numerous studies indicate that antiviral therapy with oral acyclovir, famcyclovir or valacyclovir may reduce the duration and severity of pain after zoster [51–53]. In a recent prospective, open-label phase I/II clinical trial, 15 patients with moderate to severe PHN were treated with intravenous acyclovir for 2 weeks, followed by oral valacyclovir for 1 month; 8 of 15 (53%) patients reported improvement of pain [46].
VZV vasculopathy
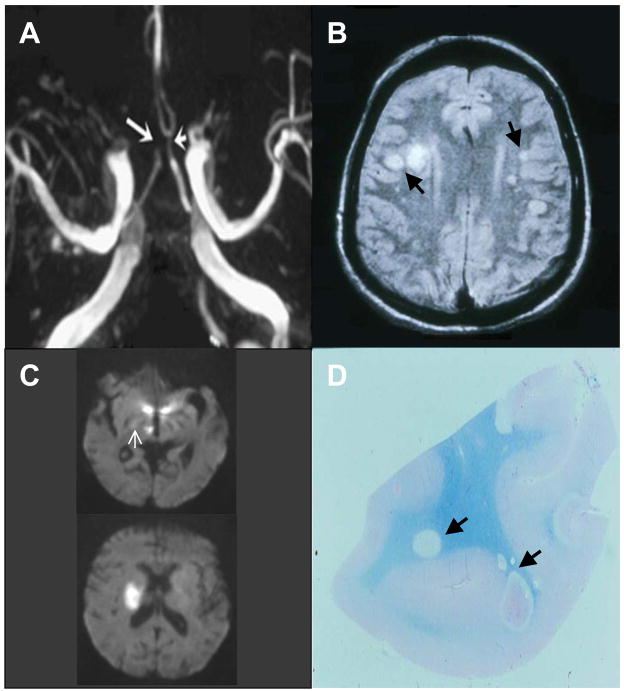
VZV vasculopathy results from productive virus infection in large or small cerebral arteries, or both. Patients present with headache, fever, mental status changes, transient ischemic attacks (TIAs) and/or focal deficit (stroke). The clinical spectrum of VZV vasculopathy is protean and includes TIAs, ischemic [54] and hemorrhagic stroke [55], aneurysms [56] and dissection [57]. VZV vasculopathy often occurs without rash [58]. The cerebrospinal fluid (CSF) usually, but not always, reveals a mononuclear pleocytosis and oligoclonal bands. The oligoclonal IgG is antibody directed against VZV [59]. Brain imaging usually reveals ischemic and/or hemorrhagic infarcts, more deep-seated than cortical lesions and at gray-white matter junctions. Cerebral angiography also reveals areas of focal arterial stenosis or occlusion (Figure 2). Macroscopically, a predominance of gray-white matter junction lesions is seen. Microscopically, virus is present in affected cerebral arteries [60] but not in areas of infarction, although in chronic cases, virus may be seen in brain parenchyma, usually close to arteries and veins. The primary site of VZV infection is in cerebral arteries, which contain multinucleated giant cells, Cowdry A inclusion bodies and herpes virus particles. Postmortem virological analysis has demonstrated both VZV DNA and VZV antigen (Figure 3) in cerebral vessels [60].
Figure 2. Characteristic angiographic, imaging and pathologic abnormalities in varicella zoster virus (VZV) vasculopathy.
(A) Three-dimensional time-of-flight magnetic resonance angiography of the Circle of Willis shows marked narrowing of the left anterior cerebral artery (short arrow) and occlusion of the right anterior cerebral artery (long arrow). (B) Brain magnetic resonance imaging (MRI) scan shows multiple areas of infarction in both hemispheres, primarily involving white matter and grey-white matter junctions (arrows). (C) Diffusion-weighted MRI in a patient with small vessel VZV vasculopathy. Top scan reveals two ischemic lesions in the posterior thalamus, one in the hypothalamus, and a small ischemic lesion (arrow) in the posterior limb of the internal capsule; one week later, the patient became hemiplegic. A new MRI (bottom) showed a discrete infarct in the area of the posterior limb of the internal capsule, although the ischemic thalamic and hypothalamic lesions had resolved. (D) Macroscopic changes in brain from a patient who died of chronic VZV vasculopathy; arrows indicate ovoid areas of ischemia/demyelination of varying size, primarily at grey-white matter junctions.
Reproduced with permission from [58].
Figure 3. Immunohistochemical analysis of cerebral artery from a patient with varicella zoster virus (VZV) vasculopathy.
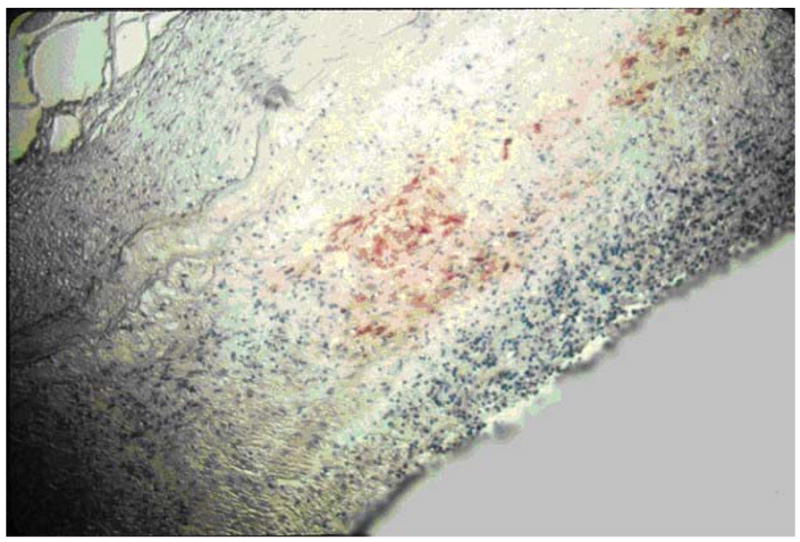
VZV antigen (red staining) is detected after incubation of artery with rabbit antiserum against the VZV open reading frame 63 protein.
Reproduced with permission from [54].
Confirmation of VZV vasculopathy requires virological analysis to detect amplifiable VZV DNA or anti-VZV IgG antibodies or both in the CSF. The CSF does not always contain PCR-amplifiable VZV DNA, but does contain anti-VZV IgG [61]. The detection of anti-VZV IgG, but not VZV DNA, likely reflects the chronic, protracted course of disease. Testing for both VZV DNA and anti-VZV IgG must be done, and only negative findings in both can exclude the diagnosis of VZV vasculopathy. Also, since VZV vasculopathy can occur without rash, all vasculopathies of unknown etiology should be evaluated for VZV. Rapid diagnosis of VZV vasculopathy is important since the mortality without treatment is 25% [62], while treatment with intravenous acyclovir, even after neurologic disease has been present for months, can be curative [63].
VZV myelopathy
There are various presentations of VZV myelopathy. One form is a self-limiting, monophasic spastic paraparesis, with or without sensory features and sphincter problems. This so-called post-infectious myelitis usually occurs in immunocompetent patients, days to weeks after acute varicella or zoster. Its pathogenesis is unknown. The CSF usually reveals a mild mononuclear pleocytosis with a normal or slightly elevated protein. Steroids are used to treat these patients [64], although some improve spontaneously [65]. Rarely, VZV myelitis recurs, even in immunocompetent patients [66].
VZV myelopathy may also present as an insidious, progressive and sometimes fatal myelitis, mostly in immunocompromised individuals. Because AIDS is so prevalent, this has become the most common condition associated with VZV myelitis. CSF examination reveals a mild, predominantly mononuclear pleocytosis with elevated protein. MRI reveals longitudinal serpiginous enhancing lesions. Diagnosis is confirmed by the presence of VZV DNA or anti-VZV IgG or both in CSF [66]. Pathological and virological analyses of the spinal cord from fatal cases have shown frank invasion of VZV in the parenchyma [67], and in some instances, spread of virus to adjacent nerve roots [68]. Not surprisingly, some patients respond favorably to antiviral therapy [69–71]. Importantly, VZV myelitis may develop with rash. Early diagnosis and aggressive treatment with intravenous acyclovir has been helpful, even in immunocompromised patients [69]. The benefit of steroids in addition to antiviral agents is unknown.
VZV can also produce spinal cord infarction identified by diffusion-weighted MRI (DWI) and confirmed virologically [72]. Thus, VZV vasculopathy can cause stroke in the spinal cord as well as the brain.
VZV retinal necrosis
VZV produces both acute retinal necrosis (ARN) and progressive outer retinal necrosis (PORN).
Acute retinal necrosis (ARN)
VZV can produce ARN in both immunocompetent and immunocompromised hosts. Patients present with periorbital pain and floaters with hazy vision and loss of peripheral vision. Treatment is typically intravenous acyclovir, steroids and aspirin followed by oral acyclovir [73]. Intravitreal injections of foscarnet and oral acyclovir have been used in early, milder cases.
Progressive outer retinal necrosis (PORN)
PORN is caused almost exclusively by VZV and occurs primarily in AIDS patients with CD4 counts typically less than 10 cells/mm3 of blood [74], as well as in other immunosuppressed individuals [75]. PORN may be preceded by retrobulbar optic neuritis and aseptic meningitis [76], central retinal artery occlusion or ophthalmic distribution zoster [77], and may occur together with multifocal vasculopathy or myelitis. Patients present with sudden painless loss of vision, floaters and constricted visual fields with resultant retinal detachment. Multifocal, discrete opacified lesions begin in the outer retinal layers peripherally and/or posterior pole; only late in disease are inner retinal layers involved. Diffuse retinal hemorrhages and whitening with macular involvement bilaterally are characteristic findings. Treatment with intravenous acyclovir has given poor or inconsistent results [78], and even when acyclovir helped, VZV retinopathy recurred when drug was tapered or stopped. PORN patients treated with a combination of ganciclovir and foscarnet or with ganciclovir alone had a better final visual acuity than those treated with acyclovir or foscarnet [79]. The best treatment for PORN in AIDS patients may be prevention with HAART, which appears to decrease the incidence of this syndrome [80].
Zoster sine herpete
Zoster sine herpete (pain without rash) is due to reactivation of VZV [81], a concept first supported by the description of dermatomal distribution radicular pain in areas distinct from pain with rash in zoster patients [82]. Currently, most clinicians regard zoster sine herpete exclusively as the rare occurrence of chronic radicular pain without rash with virological confirmation of VZV reactivation. In recent years, the detection of VZV DNA and anti-VZV antibody in patients with meningoencephalitis, vasculopathy, myelitis, cerebellar ataxia and polyneuritis cranialis, all without rash, has expanded the spectrum of zoster sine herpete. Prevalence estimates of VZV-induced pathology without rash await virological analysis of additional patients with prolonged radicular pain or other neurological symptoms and signs. Analyses should include tests for both anti-VZV IgG and PCR-amplifiable VZV DNA in CSF, as well as examination of blood MNCs for VZV DNA.
Vaccination
Widespread, aggressive VZV vaccination has reduced the total number of varicella cases by ~85% and the number of moderate-to-severe cases by 95–100% [83]. Now, like the live varicella vaccine for children, there is a live zoster vaccine that appears to be safe and effective clinically. The results of a prospective, double-blind, placebo-controlled trial of attenuated VZV vaccine designed to prevent zoster and PHN in men and women over age 60 were recently reported [84]. Otherwise healthy adults age 60 years or older (median 69 years) were vaccinated with placebo or an attenuated Oka/Merck-VZV vaccine containing 18,700 to 60,000 plaque-forming units (PFUs) of virus, considerably greater than the ~1350 PFUs in the Oka/Merck-VZV vaccine administered to American children since 1995. More than 38,000 recipients of the “zoster vaccine” were followed closely for 3 years. The incidence of zoster in the placebo group was 11.1 per 1000 person-years, approximating the results of an epidemiological survey performed a decade ago, which revealed zoster exceeding 10 cases per 1000 person-years in individuals older than 75 years [2]. The effect of zoster vaccine was impressive; compared to placebo, vaccination reduced the incidence of shingles by 51%, the incidence of PHN by 66% and the burden of illness by 61%.
Serious adverse effects and deaths occurred in 1.4% of both vaccine and placebo recipients. In more than 6000 subjects who kept daily diaries of minor adverse effects for 42 days, 48% of vaccine recipients reported injection site erythema, pain or tenderness, swelling and pruritis, as compared to 16% of placebo recipients. In the same 6000 subjects, serious adverse effects were significantly more frequent (p = 0.03) in vaccine recipients (1.9%) compared to placebo recipients (1.3%), although no specific serious effects emerged. The relative impact of these side effects on the elderly (age ≥ 70) compared to younger patients was not examined but might be important in future analyses, since the at-risk population over age 70 years is projected to increase substantially in the coming decades. Although the Oka/Merck VZV vaccine on rare occasions unmasks a childhood immunodeficiency disorder, no cases of disseminated zoster that might have been attributed to zoster vaccine in a person with undiagnosed lymphoma, leukemia or similar disorders were reported.
In 2006, zoster vaccine received FDA approval for healthy VZV-seropositive adults over age 60. Zoster vaccine increases cell-mediated immunity to VZV in such individuals, and the boost is likely to last for decades. Since zoster and its attendant neurological complication of PHN are common and serious, it seems prudent to recommend zoster vaccine. The Census Bureau projects that by the year 2050, there will be more than 21 million Americans 85 years of age or older [201].
Despite the development of a vaccine to prevent zoster, even if every healthy adult in the United States over age 60 years is vaccinated, there would still be ~500,000 zoster cases annually, about 200,000 of whom will experience PHN, as well as stroke, blindness and myelopathy caused by VZV reactivation. Furthermore, because zoster vaccine is not approved for immunocompromised individuals, neurologic disease produced by VZV reactivation in this population will be a continuing problem.
Molecular aspects of VZV latency
Primary VZV infection results in virus replication in the nasopharyngeal region where infected T-cells transfer virus to the skin, leading to varicella [85]. Nerve fibers innervating sites of active virus replication can transport virus anterograde to neurons in ganglia. When virus DNA has gained access the neuronal nucleus, the incoming VZV genome either orchestrates expression of genes leading to production and release of newly formed infectious virus, or limits its own gene transcription such that no infectious virus is released. During productive virus infection in culture, transcripts from all predicted VZV open reading frames (ORFs) are detected [86,87], whereas during latent infection, less than 10% of the VZV ORFs are transcribed.
VZV was the first herpesvirus for which the sequence of the entire genome was determined [88]. A search of the National Center for Biotechnology Information database shows the complete DNA sequence of 22 VZV isolates [202]. The VZV genome is stable [89], with a genetic variation of 0.00063 differences per nucleotide [90]. The stability and uniformity of the VZV genome imply that virus from various geographic regions does not undergo significant genetic mutation when propagated in the laboratory. While analysis of the VZV genome is unencumbered by mutations induced by in vitro propagation, the study of VZV latency is strictly dependent upon in vivo-derived samples.
VZV is an exclusively human pathogen. Unlike herpes simplex virus type 1 (HSV-1) for which mouse and rabbit models are available to analyze latency and either spontaneous [91,92] or induced [93–95] virus reactivation, there are no animal models of VZV latency and reactivation. Consequently, much of the information on VZV latency has been obtained through analysis of human ganglia removed at autopsy. VZV DNA is detected in all cranial nerve, dorsal root or autonomic ganglia along the entire neuraxis. Within ganglia, VZV is detected predominately, if not exclusively, in 1–7% of individual neurons [96–102]. Unlike VZV DNA extracted from virus particles where termini of the viral DNA molecule are mostly free [103], the virus genome during latency forms a circle or multiple concatemers [104]. In addition, latent VZV DNA is associated with cellular histones [105].
In latently infected human ganglia, the VZV DNA copy number does not differ significantly in the left and right trigeminal ganglia from the same individual; however, there is considerable variation (37-3500 VZV DNA copies per 100 ng trigeminal ganglia DNA) in the virus burden among individuals [106,107]. Although less than 20% of the ~70 VZV genes have been studied, transcription of VZV ORF 63 appears to be a hallmark of virus latency. Analysis of latently infected human trigeminal ganglia by in situ hybridization with gene-specific antisense oligonucleotide probes detected transcripts mapping to VZV gene 4 in 2 of 12 (17%) subjects, VZV gene 18 in 4 of 15 (27%) subjects, VZV gene 21 in 7 of 11 (64%) subjects, VZV gene 28 in 0 of 7 (0%) subjects, VZV gene 29 in 5 of 13 (38%) subjects, VZV 40 in 1 of 5 (20%) subjects, VZV gene 61 in 0 of 3 (0%) subjects, VZV gene 62 in 4 of 10 (40%) subjects and VZV gene 63 in 8 of 17 (47%) subjects [108]. However, in situ hybridization is a capricious technique subject to investigator interpretation [109]. For example, one in situ hybridization study located latent VZV exclusively in non-neuronal satellite cells [110], a finding not substantiated by subsequent in situ hybridization [99], in situ PCR [97], whole ganglia dissociation [100,101] or laser-capture microdissection [102].
To avoid the problems inherent with in situ techniques, latently infected human ganglia have been analyzed by Northern blot analysis. VZV genes 29 and 62, but not 28 or 61 transcripts were detected by Northern blots; however, to obtain sufficient quantities of polyadenylated RNA, ganglia from hundreds of individuals were pooled [111]. PCR analysis of human ganglia provides the sensitivity needed to detect latent VZV gene transcripts in individual ganglia. When coupled with DNA sequencing, this confirms that the PCR product was derived from the VZV gene transcript. Overall DNA sequence analysis of RT-PCR products generated from latently infected human ganglia showed that VZV genes 21, 29, 62, 63 and 66, but not VZV genes 4, 10, 40, 51, and 61 are transcribed [112,113]. Subsequently, the prevalence and abundance of VZV gene transcripts identified by DNA sequence analysis in latently infected ganglia was determined by real-time (quantitative) RT-PCR [114]. Analysis of 28 trigeminal ganglia from 14 humans indicated that VZV gene 63 transcripts were detected most often (63%), followed by gene 66 (43%), gene 62 (36%), and gene 29 (21%). No gene 21 transcripts were detected in any of the ganglia. Quantitative analysis also showed that VZV gene 63 RNA was also the most abundant (3,710 – 6,895 copies per ug mRNA), followed by VZV gene 29 (491–594, VZV gene 66 (117–85), and VZV gene 62 (64–38). While VZV gene 63 transcripts are the most prevalent and abundant in latently infected human ganglia, expression VZV ORFs 4, 18, 21, 29, 40, 62, and 66 may occur when small numbers of neurons undergo limited reactivation.
While VZV gene 63 transcripts are consistently detected in during latency, detection of immediate early (IE) 63 protein, the product of VZV ORF 63, is difficult. In situ immunohistochemistry using rabbit polyclonal antibody applied to latently infected human ganglia detected VZV IE63 in 2 of 9 subjects [98]. In one subject, IE63 was detected in all sections from four of five thoracic ganglia, while in the second subject; IE63 was detected in all sections from one trigeminal ganglion. Approximately 6% neurons stained positive for IE63 by in situ immunohistochemistry in dorsal root ganglia from three subjects [115]. In both studies, IE63 was found predominantly in the cytoplasm of latently infected ganglionic neurons [98,115]. In situ immunohistochemistry has also detected proteins encoded by VZV ORFs 4, 21, 29, 62, 63 in latently infected ganglia from three subjects [115]. Further In situ immunohistochemistry analysis of latently infected human ganglia detected proteins encoded by VZV ORFs 21, 29, 62 and 63 [116], VZV ORF 62 [117–119] and VZV ORF 66 [113]. While multiple reports have detected VZV proteins in latently infected ganglia, all used the same technique of in situ immunohistochemistry and require confirmation by independent technology.
Many viruses, including VZV, can induce apoptotsis upon infection of cells. VZV induces apoptotic pathways in infected fibroblasts [120] and in peripheral blood MNCs [121]. A study by Hood et al. [122] to compare infection of human foreskin fibroblasts with that of human dorsal root ganglionic cells showed that apoptosis was induced only in the fibroblasts, a finding of particular importance since VZV establishes and maintains latency in human neurons for the lifetime of the host.
Additional studies suggest that VZV IE63, a gene duplicated in the viral genome and expressed during lytic and latent infection, may play a role in inhibition of apoptosis in VZV-infected neurons [123]. This is consistent with the findings in HSV-1 that some viral genes have an anti-apoptotic function [124]. In cultured human dorsal root ganglionic cells infected with VZV deleted in one copy of ORF 63, apoptosis was increased as compared to that after wild-type VZV infection, suggesting VZV IE63 can modulate this process in a dose-dependent manner.
Apoptosis proceeds primarily through two distinct pathways: 1) an extrinsic pathway initiated by ligand interaction with death receptors, leading to activation of caspase-8 [125]; and 2) an intrinsic pathway activated by an imbalance between pro-apoptotic (e.g., Bax and Bak) and anti-apoptotic (e.g., Bcl-2 and Bcl-xL) proteins in mitochondria, resulting in the release of cytochrome c from mitochondria which activates caspase-9 [126]. Both pathways converge through activation of caspase-3 which along with other effector caspases, cleaves the cellular proteins and results in cell death. Our studies of the animal model of simian varicella virus (SVV)-infected cultured monkey kidney cells revealed SVV-induced apoptosis, as evidenced by nuclear condensation and positive TUNEL staining [Pugazhenthi, Personal Communication]. Western blotting analysis to further characterize the apoptotic mechanisms indicated a significant increase in levels of the cleaved active form of caspase-3 and elevated levels of active caspase-9 in infected cells, whereas active caspase 8 was not detected; expression of anti-apoptotic Bcl-2 decreased significantly at the mRNA and protein levels in virus-infected cells (Figure 4). Immunofluorescence labeling of cells infected with green fluorescent protein (GFP) tagged SVV confirmed the colocalization of active forms of caspase-3 and -9 with GFP. Finally, release of cytochrome c, an activator of caspase-9, from mitochondria into the cytoplasm was also detected. Together, these findings indicate that SVV induces apoptosis in cultured cells through the intrinsic pathway. Information on VZV-induced apoptosis in infected cells remains limited. Further studies are needed to identify the mechanisms that induce apoptosis in one cell type but prevent it in another during VZV infection, and to elucidate how the virus modulates cellular mechanisms to enable maintenance of a lifelong latent infection.
Figure 4. Intrinsic pathway of apoptosis in SVV-infected cells.
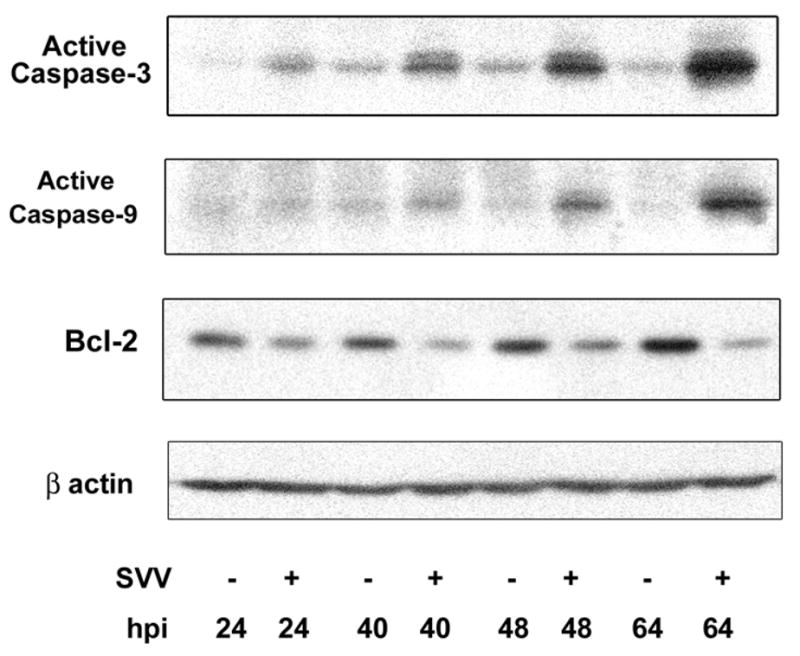
Uninfected (−) and SVV-infected (+) Vero cells were harvested at multiple times. Cell lysates were analyzed by Western blot analysis for the active forms of caspase-3 and caspase-9 and Bcl-2. Blots were reprobed for β-actin. Representative blots from four experiments are shown. Note the increased levels of caspases-3 and -9 and the decreased Bcl-2 expression in infected cells at 64 hr post-infection (hpi).
Development of animal models
Since VZV infects only humans, there is a need for a reliable animal model in which the pattern of disease seen in humans can be reproduced. Any animal model of VZV infection that can be used to study latency must fulfill the following criteria: (i) presence of virus nucleic acids in ganglia, but not in non-ganglionic tissues such as lung and liver; (ii) restricted transcription of the virus genome; (iii) presence of virus exclusively in neurons of ganglia; and (iv) ability to reactivate.
Inoculation of guinea pigs, rabbits, mice and rats with VZV by different routes leads to seroconversion in the absence of clinical symptoms [127,128]. Intramuscular inoculation of guinea pigs with VZV produces a papular exanthem, but not vesicles [129]. VZV spreads to guinea pig ganglia after subcutaneous inoculation [130]; however, because non-ganglionic tissues from virus-infected guinea pigs have not been analyzed for the presence of virus nucleic acids, it is difficult to evaluate this model to study VZV latency. Finally, VZV reactivation has not been demonstrated in guinea pigs.
Subcutaneous inoculation of VZV into adult rats along the spine does not produce chickenpox. However, VZV DNA, RNA and proteins can be detected in neurons from ganglia removed at different time points up to 9 months in these rats. Since ganglia were cultured for 3–12 days in these studies, it is difficult to rule out VZV reactivation in culture [131]. Transcripts associated with VZV latency (gene 21) but not with active replication (gene 40) have been detected in ganglia of newborn rats 5–6 weeks after intraperitoneal injection [132]. Neurons as well as non-neuronal cells of dorsal root ganglia were shown to harbor VZV DNA in rats at 1 and 3 months [133], although the latentcy-associated VZV gene 63 protein was detected in neurons of dorsal root ganglia rats 12–18 months after foot-pad inoculation [134,135]. The usefulness of the rat model to study varicella latency is difficult to evaluate because: (i) non-ganglionic tissues have not been analyzed; and (ii) VZV does not reactivate in vivo in rats.
Infectious VZV and virus proteins have been detected in CD4+ and CD8+ T cells up to 3 weeks after infection of human fetal thymus and liver implants under the kidney capsule or under the skin of severe combined immunodeficiency (SCID-hu) mice [136]. The lack of an intact immune system in these mice, together with external infection of the implanted tissue rather than by viremia, makes it a difficult model for the study of latency.
Human tissue xenografts in SCID mice have also been used to study VZV infection and latency. Human fetal dorsal root ganglia (DRG) implanted under the capsule of the kidney become vascularized and maintain organotypic features. VZV infection of DRG xenografts, either by injection of virus-infected fibroblasts into the xenograft or indirectly by inoculation of VZV-infected T cells into the tail vein, results in a burst of VZV replication in the DRG. Within two weeks, VZV DNA, VZV proteins and herpes virions are evident; importantly, cell fusion (syncytial formation) does not develop and DRG architecture is not destroyed. After acute ganglionic infection, VZV appears to become latent, as evidenced by a low abundance of VZV DNA, an absence of infectious virus and detection of only VZV gene 63 transcripts exclusively in neurons [137].
In contrast, a later study that used DRG xenografts to analyze the neuropathogenesis of VZV infection revealed VZV DNA, VZV proteins and nucleocapsids in both neurons and satellite cells, and numerous viral-induced syncytia [138]. Deletion of VZV glycoprotein I (gI) resulted in loss of neuronal/satellite cell fusion, reduced virus DNA replication and lack of transition from acute to latent infection, and mutational analysis of the VZV gI promoter region revealed neuronal-specific transcriptional regulation [139]. Taken together, DRG xenograft maintained in SCID mice provides a unique model to study VZV neuropathogenesis, latency and gene regulation in human neuronal cells.
Oral-nasal-conjunctival application of attenuated vaccine (Oka) strain of VZV in marmoset results in mild pneumonitis and production of VZV antibody without symptomatic disease [140]. Subcutaneous inoculation of Oka VZV (vaccine strain) into the breast of a chimpanzee produces viremia and a mild rash restricted to the inoculation site [141], but ganglionic and non-ganglionic tissues were not analyzed in that study.
Simian varicella virus (SVV)
SVV produces the primate counterpart of human chickenpox. Although the true host for natural SVV infection is unknown, epidemic outbreaks in different species of monkeys including African green, patas and macaques were reported in the 1960s at different primate centers in both the US and UK [142]. Clinical, pathological, immunological and virological features of SVV infection of nonhuman primates closely resemble those of human VZV infection [143,144]. After an incubation period of one or more weeks, SVV leads to fever and a papulovesicular rash of skin and mucous membranes in monkeys. Infectious virus can be recovered from blood MNCs [145.146]. Hemorrhagic necrosis, inflammation and eosinophilic intranuclear inclusions are detected in skin and visceral tissues [147]. SVV establishes latent infection in neurons of multiple ganglia [148] and reactivates under stress to cause zoster [145]. VZV and SVV glycoproteins share immunologic cross-reactivity as demonstrated by the protection of SVV-infected monkeys after VZV immunization [149]. The two virus genomes are similar in size, structure and the organization of genes [150,151]. Analysis of the complete nucleotide sequence of SVV has revealed an invertible 665-basepair leftward terminal element that is absent in the VZV genome [152–154].
SVV pathogenesis, latency and reactivation
SVV infection of primates has proved to be a useful model to study varicella pathogenesis, latency and reactivation. Two experimental models of varicella infection have been established. In the first model, intratracheal inoculation of African green or Cynomologous monkeys with 103–104 PFUs of SVV results in viremia, and infectious virus can be recovered from blood MNCs between 2 and 11 days post-inoculation [155,156]. Monkey ganglia, like other visceral organs, become infected with SVV before the appearance of rash [157]. Histopathological analysis reveals necrosis and intranuclear inclusions in lung, liver and spleen [158]. SVV-specific antigens and nucleic acid are present in liver, lung, spleen, adrenal gland, kidney, lymph node, bone marrow, and in ganglia at all levels of the neuraxis [143,155,158]. SVV-specific immediate-early, early and late transcripts are found in skin, lung, liver and ganglia of acutely infected (11–12 days post-infection) monkeys [158]. SVV DNA and RNA are present in multiple tissues, including ganglia, liver and blood MNCs, months to years after experimental intratracheal inoculation [159,160]. This model can serve in designing antiviral treatments that maintain the virus in the latent state in patients taking immunosuppressive drugs.
In the second model, simulated natural infection in African green or Cynomologous monkeys results in virus latency in ganglia. SVV-seronegative monkeys are exposed to other monkeys previously inoculated intratracheally with SVV [148]. Ten to 14 days later, monkeys exposed to intratracheally inoculated monkeys develop a mild rash, and detection of SVV DNA in skin scrapings of naturally infected monkeys confirms that SVV causes the rash. Occasionally, SVV DNA is also detected in blood MNCs of naturally infected monkeys. At 6 to 8 weeks after the resolution of rash, SVV DNA is present in multiple ganglia along the neuraxis, but not in lung or liver, indicating latent infection. Subclinical reactivation of latent SVV has been observed in an asymptomatic irradiated rhesus macaque, leading to disseminated varicella in a seronegative irradiated monkey from the same colony [161]. We recently demonstrated that latently infected monkeys subjected to stress and experimental immunosuppression undergo SVV reactivation, as confirmed by the development of zoster, the detection of VZV DNA in organs other than ganglia, the presence of SVV RNA specific for late capsid proteins (ORFs 40 and 9) in ganglia, and the presence of SVV antigens in skin, ganglia (including axons) and viscera [162].
This second model can be used to examine viral as well as cellular processes that play a role in the establishment, maintenance of and reactivation from latency. With respect to the role of cellular processes, it remains unclear why an age-related decline in T cell-mediated immunity in humans correlates strongly with zoster incidence, yet VZV-specific T cells are not essential in maintaining latency in ganglia [163]. Although VZV downregulates major histocompatibility complex (MHC) class I surface expression by its retention in the Golgi compartment [164–166], neurons generally do not express MHC class I genes, so that latency and reactivation are probably regulated by an innate immune response involving cytokines or chemokines. The identification of the SVV-specific T cell response and associated cytokines in ganglia during reactivation will help to identify potential targets for prevention of zoster in humans.
Future perspective
Continuing clinical, pathological and virological studies of patients suffering from the multiple neurological complications of VZV reactivation are needed. While VZV vaccination in healthy adults has proven to be highly effective, there is also a need for anti-viral drugs that block VZV reactivation in patients taking immunosuppressive drugs. Development of such reagents, as well as better treatments for patients suffering from complications of viral reactivation, awaits further studies to elucidate the still-unclear molecular mechanism that enable the virus to maintain life-long infection of a specific cell type and that trigger viral reactivation, in a manner presumably involving virus-induced modulation of the cellular immune system. Analyses of viral nucleic acid and gene expression in latently infected human ganglia and the development of a primate model of varicella latency are being used to address these issues.
Executive summary
Introduction
Varicella zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus.
Primary infection causes varicella (chickenpox), after which virus becomes latent in multiple cranial nerve ganglia, dorsal root ganglia and autonomic ganglia along the entire neuraxis.
A declining cell-mediated immunity to VZV with advancing age or immunosuppression (cancer, transplant recipients and AIDS patients) can lead to virus reactivation from latently infected human ganglia anywhere along the neuraxis.
Zoster and postherpetic neuralgia (PHN)
VZV reactivation produces zoster, often followed by chronic pain (PHN).
VZV reactivation also produces pain without rash (zoster sine herpete).
Antivirals (famciclovir, 500 mg orally three times daily or valacyclovir, 1 g three times daily for 7–10 days) speed healing of rash.
PHN is characterized by constant, severe, stabbing or burning, dysesthetic pain that persists for at least three months and sometimes years after resolution of rash. The cause and pathogenesis of PHN are unknown.
The detection of VZV DNA and proteins in blood mononuclear cells of many PHN patients and the favorable response of some PHN patients to antiviral treatment support the concept that PHN is produced by low-level ganglionitis.
Less frequent serious neurological complications of VZV reactivation
In addition to zoster and PHN, VZV reactivation may lead to myelopathy (paralysis and incontinence), vasculopathy (transient ischemic attacks or stroke), retinal disease (loss of vision) and cerebellitis (unsteadiness).
All neurological complications of VZV reactivation can occur without rash.
VZV vasculopathy results from productive virus infection in large or small cerebral arteries or both, and can be treated successfully with intravenous acyclovir.
Confirmation of VZV vasculopathy requires virological analysis to detect amplifiable VZV DNA or anti-VZV IgG antibodies or both in the CSF; the CSF does not always contain VZV DNA, but does contain anti-VZV IgG.
Vaccination to prevent zoster
Vaccination after age 60 years reduces the incidence of shingles by 51%, PHN by 66% and the burden of illness by 61%.
If every healthy adult over age 60 years in the United States were vaccinated, there would still be about 500,000 zoster cases annually, about 200,000 of whom will experience PHN.
Latency
VZV DNA is detected in latently infected human ganglia along the entire neuraxis.
Five VZV transcripts have been found in latently infected human ganglia as well as proteins corresponding to three of the transcripts.
The most prevalent and abundant transcript in latently infected human ganglia maps to the VZV open reading frame (ORF) 63.
VZV ORF 63 encodes an immediate-early protein.
Animal models
SVV infection of primates has proved to be a useful model to study varicella pathogenesis, latency and reactivation.
SVV becomes latent in monkey ganglionic neurons, and spontaneous or experimental reactivation produces zoster.
Bibliography
Papers of special note have been highlighted as of considerable interest (**) to readers.
- 1.Harnisch JP. Zoster in the elderly: Clinical, immunologic and therapeutic considerations. J Am Geriatr Soc. 1984;32:789–793. doi: 10.1111/j.1532-5415.1984.tb06298.x. [DOI] [PubMed] [Google Scholar]
- 2.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–1609. [PubMed] [Google Scholar]
- 3.Gilden DH, Cohrs RJ, Mahalingam R. Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol. 2003;16:243–258. doi: 10.1089/088282403322396073. [DOI] [PubMed] [Google Scholar]
- 4.Leppard B, Naburi AE. Herpes zoster: an early manifestation of HIV infection. Afr Health. 1998;21:5–6. [PubMed] [Google Scholar]
- 5.Kakourou T, Theodoridou M, Mostrou G, Syriopoulou V, Papadogeorgaki H, Constantopoulos A. Herpes zoster in children. J Am Acad Dermatol. 1998;39:207–210. doi: 10.1016/s0190-9622(98)70076-3. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher JG, Merigan TC. Prolonged herpes-zoster infection associated with immunosuppressive therapy. Ann Intern Med. 1979;91:842–846. doi: 10.7326/0003-4819-91-6-842. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal DT, Salzman KL, Baringer JR, Forghani B, Gilden DH. MRI abnormalities in chronic active varicella zoster infection. Neurology. 2004;63:1538–1539. doi: 10.1212/01.wnl.0000141855.67420.73. [DOI] [PubMed] [Google Scholar]
- 8.Carroll WM, Mastaglia FL. Optic neuropathy and ophthalmoplegia in herpes zoster oticus. Neurology. 1979;29:726–729. doi: 10.1212/wnl.29.5.726. [DOI] [PubMed] [Google Scholar]
- 9.Meenken C, van den Horn GJ, de Smet MD, van der Meer JT. Optic neuritis heralding varicella zoster virus retinitis in a patient with acquired immunodeficiency syndrome. Ann Neurol. 1998;43:534–536. doi: 10.1002/ana.410430420. [DOI] [PubMed] [Google Scholar]
- 10.Archambault P, Wise JS, Rosen J, Polomeno RC, Auger N. Herpes zoster ophthalmoplegia. Report of six cases. J Clin Neuroophthalmol. 1988;8:185–193. [PubMed] [Google Scholar]
- 11.Sodhi PK, Goel JL. Presentations of cranial nerve involvement in two patients with herpes zoster ophthalmicus. J Commun Dis. 2001;33:130–135. [PubMed] [Google Scholar]
- 12.Karmon Y, Gadath N. Delayed oculomotor nerve palsy after bilateral cervical zoster in an immunocompetent patient. Neurology. 2005;65:170. doi: 10.1212/01.wnl.0000167287.02490.76. [DOI] [PubMed] [Google Scholar]
- 13.Garty B-Z, Dinari G, Sarnat H, Cohen S, Nitzan M. Tooth exfoliation and osteonecrosis of the maxilla after trigeminal herpes zoster. J Pediatr. 1985;106:71–73. doi: 10.1016/s0022-3476(85)80469-8. [DOI] [PubMed] [Google Scholar]
- 14.Manz HJ, Canter HG, Melton J. Trigeminal herpes zoster causing mandibular osteonecrosis and spontaneous tooth exfoliation. South Med J. 1986;79:1026–1028. doi: 10.1097/00007611-198608000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Volvoikar P, Patil S, Dinkar A. Tooth exfoliation, osteonecrosis and neuralgia following herpes zoster of trigeminal nerve. Indian J Dent Res. 2002;13:11–14. [PubMed] [Google Scholar]
- 16.Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149–154. doi: 10.1136/jnnp.71.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asnis DS, Micic L, Giaccio D. Ramsay Hunt syndrome presenting as a cranial polyneuropathy. Cutis. 1996;57:421–424. [PubMed] [Google Scholar]
- 18.Robillard RB, Hilsinger RL, Jr, Adour KK. Ramsay Hunt facial paralysis: clinical analyses of 185 patients. Otolaryngol Head Neck Surg. 1986;95:292–297. doi: 10.1177/01945998860953P105. [DOI] [PubMed] [Google Scholar]
- 19.Furuta Y, Ohtani F, Aizawa H, Fukuda S, Kawabata H, Bergstrom T. Varicella-zoster virus reactivation is an important cause of acute peripheral facial paralysis in children. Pediatr Infect Dis J. 2005;24:97–101. doi: 10.1097/01.inf.0000151032.16639.9c. [DOI] [PubMed] [Google Scholar]
- 20.Merchet MP, Gruener G. Segmental zoster paresis of limbs. Electromyogr Clin Neurophysiol. 1996;36:369–375. [PubMed] [Google Scholar]
- 21.Yoleri O, Olmez N, Oztura I, Sengul I, Gunaydin R, Memis A. Segmental zoster paresis of the upper extremity: a case report. Arch Phys Med Rehabil. 2005;86:1492–1494. doi: 10.1016/j.apmr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JP, Keal EE. Cervical herpes zoster and diaphragmatic paralysis. Br J Dis Chest. 1969;63:222–226. doi: 10.1016/s0007-0971(69)80022-7. [DOI] [PubMed] [Google Scholar]
- 23.Tjandra J, Mansel RE. Segmental abdominal herpes zoster paresis. Aust N Z J Surg. 1986;56:807–808. doi: 10.1111/j.1445-2197.1986.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 24.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 25.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–1122. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor GR, Janney RP. In vivo testing confirms a blunting of the human cell-mediated immune mechanism during space flight. J Leukoc Biol. 1992;51:129–132. doi: 10.1002/jlb.51.2.129. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koelle DM, Benedetti J, Langenberg A, Corey L. Asymptomatic reactivation of herpes simplex virus in woman after the first episode of genital herpes. Ann Intern Med. 1992;116:433–437. doi: 10.7326/0003-4819-116-6-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold E. Serologic and virus-isolation studies of patients with varicella or herpes-zoster infection. N Engl J Med. 1966;274:181–185. doi: 10.1056/NEJM196601272740403. [DOI] [PubMed] [Google Scholar]
- 30.Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Denny-Brown D, Adams RD, Fitzgerald PJ. Pathologic features of herpes zoster: A note on “geniculate herpes”. Arch Neurol Psychiatry. 1944;51:216–231. [Google Scholar]
- 32.Cheatham WJ, Dolan TF, Jr, Dower JC, Weller TH. Varicella: report on two fatal cases with necropsy, virus isolation, and serologic studies. Am J Pathol. 1956;32:1015–1035. [PMC free article] [PubMed] [Google Scholar]
- 33.Esiri MM, Tomlinson AH. Herpes zoster: demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 34.Ghatak NR, Zimmerman HM. Spinal ganglion in herpes zoster. Arch Pathol. 1973;95:411–415. [PubMed] [Google Scholar]
- 35.Nagashima K, Nakazawa M, Endo H. Pathology of the human spinal ganglia in varicella-zoster virus infection. Acta Neuropathol. 1975;33:105–117. doi: 10.1007/BF00687537. [DOI] [PubMed] [Google Scholar]
- 36.Murakami S, Hato N, Horiuchi J, Honda N, Gyo K, Yanagihara N. Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: significance of early diagnosis and treatment. Ann Neurol. 1997;41:353–357. doi: 10.1002/ana.410410310. [DOI] [PubMed] [Google Scholar]
- 37.Furuta Y, Ohtani F, Fukuda S, Inuyama Y, Nagashima K. Reactivation of varicella-zoster virus in delayed facial palsy after dental treatment and oro-facial surgery. J Med Virol. 2000;62:42–45. [PubMed] [Google Scholar]
- 38.Furuta Y, Ohtani F, Mesuda Y, et al. Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy. Neurology. 2000;55:708–710. doi: 10.1212/wnl.55.5.708. [DOI] [PubMed] [Google Scholar]
- 39.Rogers RS, III, Tindall JP. Herpes zoster in the elderly. Postgrad Med. 1971;50:153–157. doi: 10.1080/00325481.1971.11697705. [DOI] [PubMed] [Google Scholar]
- 40.Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327–331. doi: 10.1016/s0885-3924(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 41.Vafai A, Wellish M, Gilden DH. Expression of varicella-zoster virus in blood mononuclear cells of patients with postherpetic neuralgia. Proc Natl Acad Sci USA. 1988;85:2767–2770. doi: 10.1073/pnas.85.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devlin ME, Gilden DH, Mahalingam R, Dueland AN, Cohrs R. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165:619–622. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]
- 43**.Mahalingam R, Wellish M, Brucklier J, Gilden DH. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol. 1995;1:130–133. doi: 10.3109/13550289509111018. The most extensive study that correlates postherpetic neuralgia with persistent VZV infection. [DOI] [PubMed] [Google Scholar]
- 44.Terada K, Niizuma T, Kawano S, Kataoka N, Akisada T, Orita Y. Detection of varicella-zoster virus DNA in peripheral mononuclear cells from patients with Ramsay Hunt syndrome or zoster sine herpete. J Med Virol. 1998;56:359–363. [PubMed] [Google Scholar]
- 45.Gilden DH, Cohrs RJ, Hayward AR, Wellish M, Mahalingam R. Chronic varicella zoster virus ganglionitis – a possible cause of postherpetic neuralgia. J Neurovirol. 2003;9:404–407. doi: 10.1080/13550280390201722. [DOI] [PubMed] [Google Scholar]
- 46.Quan D, Hammack BN, Kittelson J, Gilden DH. Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch Neurol. 2006;63:940–942. doi: 10.1001/archneur.63.7.noc60049. [DOI] [PubMed] [Google Scholar]
- 47.Dubinsky RM, Kabbani H, El-Chami Z, et al. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63:959–965. doi: 10.1212/01.wnl.0000140708.62856.72. [DOI] [PubMed] [Google Scholar]
- 48.Rowbotham MC, Manville NS, Ren J. Pilot tolerability and effectiveness study of levetiracetam for postherpetic neuralgia. Neurology. 2003;61:866–867. doi: 10.1212/01.wnl.0000079463.16377.07. [DOI] [PubMed] [Google Scholar]
- 49.Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348:1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- 50.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 51.Huff JC. Antiviral treatment in chickenpox and herpes zoster. J Am Acad Dermatol. 1988;18:204–206. doi: 10.1016/s0190-9622(88)70029-8. [DOI] [PubMed] [Google Scholar]
- 52.Beutner KR, Friedman DJ, Forszpaniak C, Anderson PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–1553. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia A randomized, double-blind, placebo-controlled trial Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89–96. doi: 10.7326/0003-4819-123-2-199507150-00002. [DOI] [PubMed] [Google Scholar]
- 54.Gilden DH, Mahalingam R, Cohrs RJ, Kleinschmidt-DeMasters BK, Forghani B. The protean manifestations of varicella-zoster virus vasculopathy. J Neurovirol. 2002;8:75–79. doi: 10.1080/13550280290167902. [DOI] [PubMed] [Google Scholar]
- 55.Fukumoto S, Kinjo M, Hokamura K, Tanaka K. Subarachnoid hemorrhage and granulomatous angiitis of the basilar artery: demonstration of the varicella-zoster-virus in the basilar artery lesions. Stroke. 1986;17:1024–1028. doi: 10.1161/01.str.17.5.1024. [DOI] [PubMed] [Google Scholar]
- 56.O’Donohue JM, Enzmann DR. Mycotic aneurysm in angiitis associated with herpes zoster ophthalmicus. Am J Neuroradiol. 1987;8:615–619. [PMC free article] [PubMed] [Google Scholar]
- 57.Bhayani N, Ranade P, Clark NM, McGuinn M. Varicella-zoster virus and cerebral aneurysm;case report and review of the literature. Clin Infect Dis. 2008;47:e1–3. doi: 10.1086/588842. [DOI] [PubMed] [Google Scholar]
- 58**.Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;11:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. The most extensive analysis to date of virologically verified VZV vasculopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgoon MP, Hammack BN, Owens GP, Maybach AL, Eikelenboom MJ, Gilden DH. Oligoclonal immunoglobulins in cerebrospinal fluid during varicella zoster virus (VZV) vasculopathy are directed against VZV. Ann Neurol. 2003;54:459–463. doi: 10.1002/ana.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60**.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology. 1996;47:1441–1446. doi: 10.1212/wnl.47.6.1441. The first demonstration of production VZV infection in arteries of a patient with VZV vasculopathy. [DOI] [PubMed] [Google Scholar]
- 61.Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–1073. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- 62.Hilt DC, Buchholz D, Krumholz A, Weiss H, Wolinsky JS. Herpes zoster ophthalmicus and delayed contralateral hemiparesis caused by cerebral angiitis: diagnosis and management approaches. Ann Neurol. 1983;14:543–553. doi: 10.1002/ana.410140509. [DOI] [PubMed] [Google Scholar]
- 63.Gilden DH, Lipton HL, Wolf JS, et al. Two patients with unusual forms of varicella-zoster virus vasculopathy. N Eng J Med. 2002;347:1500–1503. doi: 10.1056/NEJMoa020841. [DOI] [PubMed] [Google Scholar]
- 64.Pina MA, Ara JR, Capablo JL, Omeñaca M. Myelitis and optic neuritis caused by varicella. Rev Neurol. 1997;25:1575–1576. [PubMed] [Google Scholar]
- 65.Celik Y, Tabak F, Mert A, Celik AD, Aktuǧlu Y. Transverse myelitis caused by varicella. Clin Neurol Neurosurg. 2001;103:260–261. doi: 10.1016/s0303-8467(01)00166-4. [DOI] [PubMed] [Google Scholar]
- 66.Gilden DH, Beinlich BR, Rubinstien EM, et al. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994;44:1818–1823. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- 67.Kleinschmidt-DeMasters BK, Gilden DH. Varicella-zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–780. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 68.Devinsky O, Cho ES, Petito CK, Price RW. Herpes zoster myelitis. Brain. 1991;114:1181–1196. doi: 10.1093/brain/114.3.1181. [DOI] [PubMed] [Google Scholar]
- 69.de Silva SM, Mark AS, Gilden DH, et al. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–931. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- 70.Chua HC, Tjia H, Sitoh YY. Concurrent myelitis and Guillain-Barre syndrome after varicella infection. Int J Clin Pract. 2001;55:643–644. [PubMed] [Google Scholar]
- 71.Schvoerer E, Frechin V, Warter A, et al. Persistent multiple pulmonary nodules in a nonimmunocompromised woman after varicella-related myelitis treated with acyclovir. J Clin Microbiol. 2003;41:4904–4905. doi: 10.1128/JCM.41.10.4904-4905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orme HT, Smith G, Nagel MA, Bert RJ, Mickelson TS, Gilden DH. VZV spinal cord infarction identified by diffusion-weighted magnetic resonance imaging (DWI) Neurology. 2007;69:398–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- 73.Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol. 2005;20:155–160. doi: 10.1080/08820530500232027. [DOI] [PubMed] [Google Scholar]
- 74.Guex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies A spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5:259–265. doi: 10.3109/09273949709085066. [DOI] [PubMed] [Google Scholar]
- 75.Lewis JM, Nagae Y, Tano Y. Progressive outer retinal necrosis after bone marrow transplantation. Am J Ophthalmol. 1996;122:892–895. doi: 10.1016/s0002-9394(14)70391-5. [DOI] [PubMed] [Google Scholar]
- 76.Franco-Paredes C, Bellehemeur T, Merchant A, Sanghi P, DiazGranados C, Rimland D. Aseptic meningitis and optic neuritis preceding varicella-zoster progressive outer retinal necrosis in a patient with AIDS. AIDS. 2002;16:1045–1049. doi: 10.1097/00002030-200205030-00011. [DOI] [PubMed] [Google Scholar]
- 77.Menerath JM, Gerard M, Laurichesse H, et al. Bilateral acute retinal necrosis in a patient with acquired immunodeficiency syndrome. J Fr Ophtalmol. 1995;18:625–633. [PubMed] [Google Scholar]
- 78.Johnston WH, Holland GN, Engstrom RE, Jr, Rimmer S. Recurrence of presumed varicella-zoster virus retinopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;116:42–50. doi: 10.1016/s0002-9394(14)71742-8. [DOI] [PubMed] [Google Scholar]
- 79.Moorthy RS, Weinberg DV, Teich SA, et al. Management of varicella zoster virus retinitis in AIDS. Br J Ophthalmol. 1997;81:189–194. doi: 10.1136/bjo.81.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Austin RB. Progressive outer retinal necrosis syndrome: a comprehensive review of its clinical presentation, relationship to immune system status, and management. Clin Eye Vis Care. 2000;12:119–129. doi: 10.1016/s0953-4431(00)00052-7. [DOI] [PubMed] [Google Scholar]
- 81**.Gilden DH, Wright RR, Schneck SA, Gwaltney JM, Jr, Mahalingam R. Zoster sine herpete, a clinical variant. Ann Neurol. 1994;35:530–533. doi: 10.1002/ana.410350505. The first virological proof that VZV can cause chronic radicular pain without rash. [DOI] [PubMed] [Google Scholar]
- 82.Lewis GW. Zoster sine herpete. Br Med J. 1958;34:418–421. doi: 10.1136/bmj.2.5093.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi M. Effectiveness of live varicella vaccine. Expert Opin Biol Ther. 2004;4:199–216. doi: 10.1517/14712598.4.2.199. [DOI] [PubMed] [Google Scholar]
- 84.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 85.Ku CC, Besser J, Abendroth A, Grose C, Arvin AM. Varicella-zoster virus pathogenesis and immunobiology: new concepts emerging from investigations with the SCIDhu mouse model. J Virol. 2005;79:2651–2658. doi: 10.1128/JVI.79.5.2651-2658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohrs RJ, Hurley MP, Gilden DH. Array analysis of viral gene transcription during lytic infection of cells in tissue culture with varicella zoster virus. J Virol. 2003;77:11718–11732. doi: 10.1128/JVI.77.21.11718-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kennedy PGE, Grinfeld E, Craigon M, et al. Transcriptional analysis of varicella-zoster virus infection using long oligonucleotide-based microarrays. J Gen Virol. 2005;86:2673–2684. doi: 10.1099/vir.0.80946-0. [DOI] [PubMed] [Google Scholar]
- 88**.Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. VZV was the first human herpesvirus to be sequenced. [DOI] [PubMed] [Google Scholar]
- 89.Tyler SD, Peters GA, Grose C, et al. Genomic cartography of varicella-zoster virus: a complete genome-based analysis of strain variability with implications for attenuation and phenotypic differences. Virology. 2007;359:447–458. doi: 10.1016/j.virol.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 90.Muir WB, Nichols R, Breuer J. Phylogenetic analysis of varicella-zoster virus: evidence of intercontinental spread of genotypes and recombination. J Virol. 2002;76:1971–1979. doi: 10.1128/JVI.76.4.1971-1979.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gordon YJ. Pathogenesis and latency of herpes simplex virus type 1 (HSV-1): an ophthalmologist’s view of the eye as a model for the study of the virus-host relationship. Adv Exp Med Biol. 1990;278:205–209. doi: 10.1007/978-1-4684-5853-4_21. [DOI] [PubMed] [Google Scholar]
- 92.Margolis TP, Elfman FL, Leib D, et al. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J Virol. 2007;81:11069–11074. doi: 10.1128/JVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Willey DE, Trousdale MD, Nesburn AB. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Invest Ophthalmol Vis Sci. 1984;25:945–950. [PubMed] [Google Scholar]
- 94.Sawtell NM, Thompson RL. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Birmanns B, Reibstein I, Steiner I. Characterization of an in vivo reactivation model of herpes simplex virus from mice trigeminal ganglia. J Gen Virol. 1993;74:2487–2491. doi: 10.1099/0022-1317-74-11-2487. [DOI] [PubMed] [Google Scholar]
- 96.Tenser RB, Hyman RW. Latent herpesvirus infections of neurons in guinea pigs and humans. Yale J Biol Med. 1987;60:159–167. [PMC free article] [PubMed] [Google Scholar]
- 97.Dueland AN, Ranneberg-Nilsen T, Degre M. Detection of latent varicella zoster virus DNA and human gene sequences in human trigeminal ganglia by in situ amplification combined with in situ hybridization. Arch Virol. 1995;140:2055–2066. doi: 10.1007/BF01322692. [DOI] [PubMed] [Google Scholar]
- 98.Mahalingam R, Wellish M, Cohrs R, et al. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci USA. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kennedy PG, Grinfeld E, Gow JW. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci USA. 1998;95:4658–4662. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LaGuardia JJ, Cohrs RJ, Gilden DH. Prevalence of varicella-zoster virus DNA in dissociated human trigeminal ganglion neurons and nonneuronal cells. J Virol. 1999;73:8571–8577. doi: 10.1128/jvi.73.10.8571-8577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levin MJ, Cai GY, Manchak MD, Pizer LI. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol. 2003;77:6979–6987. doi: 10.1128/JVI.77.12.6979-6987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang K, Lau TY, Morales M, Mont EK, Straus SE. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J Virol. 2005;79:14079–14087. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kinchington PR, Reinhold WC, Casey TA, Straus SE, Hay J, Ruyechan WT. Inversion and circularization of the varicella-zoster virus genome. J Virol. 1985;56:194–200. doi: 10.1128/jvi.56.1.194-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clarke P, Beer T, Cohrs R, Gilden DH. Configuration of latent varicella-zoster virus DNA. J Virol. 1995;69:8151–8154. doi: 10.1128/jvi.69.12.8151-8154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gary L, Gilden DH, Cohrs RJ. Epigenetic regulation of VZV ORF 62 and 63 in latently infected human trigeminal ganglia. J Virol. 2006;80:4921–4926. doi: 10.1128/JVI.80.10.4921-4926.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–10518. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kennedy PG, Grinfeld E, Bell JE. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J Virol. 2000;74:11893–11898. doi: 10.1128/jvi.74.24.11893-11898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mahalingam R, Kennedy PGE, Gilden DH. The problems of latent varicella zoster virus in human ganglia: precise cell location and viral content. J NeuroVirol. 1999;5:445–448. doi: 10.3109/13550289909045372. [DOI] [PubMed] [Google Scholar]
- 110.Croen KD, Ostrove JM, Dragovic LJ, Straus SE. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc Natl Acad Sci USA. 1988;85:9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meier JL, Holman RP, Croen KD, Smialek JE, Straus SE. Varicella-zoster virus transcription in human ganglia. Virology. 1993;193:193–200. doi: 10.1006/viro.1993.1115. [DOI] [PubMed] [Google Scholar]
- 112**.Cohrs RJ, Barbour M, Gilden DH. Varicella zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J Virol. 1996;70:2789–2796. doi: 10.1128/jvi.70.5.2789-2796.1996. The multiple transcripts present in latently infected human ganglia were identified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PGE. Varicella zoster virus gene 66 transcription and translation in latently infected human ganglia. J Virol. 2003;77:6660–6665. doi: 10.1128/JVI.77.12.6660-6665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114**.Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed VZV genes in human ganglia. J Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. Transcripts encoded by VZV gene 63 were shown to be the most prevalent and abundant in latently infected human ganglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grinfeld E, Kennedy PG. Translation of varicella-zoster virus genes during human ganglionic latency. Virus Genes. 2004;29:317–319. doi: 10.1007/s11262-004-7434-z. [DOI] [PubMed] [Google Scholar]
- 117.Theil D, Derfuss T, Paripovic I, et al. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Theil D, Paripovic I, Derfuss T, et al. Dually infected (HSV-1/VZV) single neurons in human trigeminal ganglia. Ann Neurol. 2003;54:678–682. doi: 10.1002/ana.10746. [DOI] [PubMed] [Google Scholar]
- 119.Hufner K, Derfuss T, Herberger S, et al. Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J Neuropathol Exp Neurol. 2006;65:1022–1030. doi: 10.1097/01.jnen.0000235852.92963.bf. [DOI] [PubMed] [Google Scholar]
- 120.Sadzot-Delvaux C, Thonard P, Schoonbroodt S, Piette J, Rentier B. Varicella-zoster virus induces apoptosis in cell culture. J Gen Virol. 1995;76:2875–2879. doi: 10.1099/0022-1317-76-11-2875. [DOI] [PubMed] [Google Scholar]
- 121.König A, Hömme C, Hauröder B, Dietrich A, Wolff MH. The varicella-zoster virus induces apoptosis in vitro in subpopulations of primary human peripheral blood mononuclear cells. Microbes Infect. 2003;4:879–889. doi: 10.1016/s1286-4579(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 122.Hood C, Cunningham AL, Slobedman B, Boadle RA, Abendroth A. Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J Virol. 2003;77:12852–12864. doi: 10.1128/JVI.77.23.12852-12864.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hood C, Cunningham AL, Slobedman B, et al. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J Virol. 2006;80:1025–1031. doi: 10.1128/JVI.80.2.1025-1031.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aubert M, Rice SA, Blaho JA. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J Virol. 2001;75:1013–1030. doi: 10.1128/JVI.75.2.1013-1030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 126.Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Ann Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- 127.Myers MG, Stanberry LR, Edmond BJ. Varicella-zoster virus infection of strain 2 guinea pigs. J Infect Dis. 1985;151:106–113. doi: 10.1093/infdis/151.1.106. [DOI] [PubMed] [Google Scholar]
- 128.Wroblewska Z, Devlin M, Reilly K, van Trieste H, Wellish M, Gilden DH. The production of varicella zoster virus antiserum in laboratory animals. Arch Virol. 1982;74:233–238. doi: 10.1007/BF01314717. [DOI] [PubMed] [Google Scholar]
- 129.Myers MG, Connelly BL, Stanberry LR. Varicella in hairless guinea pigs. J Infect Dis. 1991;163:746–751. doi: 10.1093/infdis/163.4.746. [DOI] [PubMed] [Google Scholar]
- 130.Lowry PW, Sabella C, Koropchak CM, et al. Investigation of the pathogenesis of varicella-zoster virus infection in guinea pigs by using polymerase chain reaction. J Infect Dis. 1993;167:78–83. doi: 10.1093/infdis/167.1.78. [DOI] [PubMed] [Google Scholar]
- 131.Sadzot-Delvaux C, Merville-Louis MP, Delree P, et al. An in vivo model of varicella-zoster virus latent infection of dorsal root ganglia. J Neurosci Res. 1990;26:83–89. doi: 10.1002/jnr.490260110. [DOI] [PubMed] [Google Scholar]
- 132.Brunell PA, Ren LC, Cohen JI, Straus SE. Viral gene expression in rat trigeminal ganglia following neonatal infection with varicella-zoster virus. J Med Virol. 1999;58:286–290. doi: 10.1002/(sici)1096-9071(199907)58:3<286::aid-jmv15>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 133.Annunziato P, LaRussa P, Lee P, et al. Evidence of latent varicella-zoster virus in rat dorsal root ganglia. J Infect Dis. 1998;178 (Suppl 1):S48–S51. doi: 10.1086/514261. [DOI] [PubMed] [Google Scholar]
- 134.Debrus S, Sadzot-Delvaux C, Nikkels AF, Piette J, Rentier B. Varicella-zoster virus gene 63 encodes an immediate-early protein that is abundantly expressed during latency. J Virol. 1995;69:3240–3245. doi: 10.1128/jvi.69.5.3240-3245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kennedy PG, Grinfeld E, Bontems S, Sadzot-Delvaux C. Varicella-zoster virus gene expression in latently infected rat dorsal root ganglia. Virology. 2001;289:218–223. doi: 10.1006/viro.2001.1173. [DOI] [PubMed] [Google Scholar]
- 136.Moffat JF, Stein MD, Kaneshima H, Arvin AM. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–5242. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zerboni L, Ku CC, Jones CD, Zehnder JL, Arvin AM. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc Natl Acad Sci USA. 2005;102:6490–6495. doi: 10.1073/pnas.0501045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reichelt M, Zerboni L, Arvin AM. Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J Virol. 2008;82:3971–3983. doi: 10.1128/JVI.02592-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zerboni L, Reichelt M, Jones CD, Zehnder JL, Ito H, Arvin AM. Aberrant infection and persistence of varicella-zoster virus in human dorsal root ganglia in vivo in the absence of glycoprotein 1. Proc Natl Acad Sci USA. 2007;104:14086–14091. doi: 10.1073/pnas.0706023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Provost PJ, Keller PM, Banker FS, et al. Successful infection of the common marmoset (Callithrix jacchus) with human varicella-zoster virus. J Virol. 1987;61:2951–2955. doi: 10.1128/jvi.61.10.2951-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cohen JI, Moskal T, Shapiro M, Purcell RH. Varicella in chimpanzees. J Med Virol. 1996;50:289–292. doi: 10.1002/(SICI)1096-9071(199612)50:4<289::AID-JMV2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 142.Gray WL. Simian varicella: a model for human varicella-zoster virus infections. Rev Med Virol. 2004;14:363–381. doi: 10.1002/rmv.437. [DOI] [PubMed] [Google Scholar]
- 143.Roberts ED, Baskin GB, Soike K, Gibson SV. Pathologic changes of experimental simian varicella (Delta herpesvirus) infection in African green monkeys (Cercopithecus aethiops) Am J Vet Res. 1984;45:523–530. [PubMed] [Google Scholar]
- 144.Wenner HA, Abel D, Barrick S, Seshumurty P. Clinical and pathogenetic studies of Medical Lake macaque virus infections in cynomolgus monkeys (simian varicella) J Infect Dis. 1977;135:611–622. doi: 10.1093/infdis/135.4.611. [DOI] [PubMed] [Google Scholar]
- 145.Soike KF, Rangan SR, Gerone PJ. Viral disease models in primates. Adv Vet Sci Comp Med. 1984;28:151–199. doi: 10.1016/b978-0-12-039228-5.50011-5. [DOI] [PubMed] [Google Scholar]
- 146.Gray WL. Pathogenesis of simian varicella virus. J Med Virol. 2003;70 (Suppl 1):S4–S8. doi: 10.1002/jmv.10312. [DOI] [PubMed] [Google Scholar]
- 147.Wolf RH, Smetana HF, Allen WP, Felsenfeld AD. Pathology and clinical history of Delta herpesvirus infection in patas monkeys. Lab Anim Sci. 1974;24:218–221. [PubMed] [Google Scholar]
- 148**.Mahalingam R, Traina-Dorge V, Wellish M, Smith J, Gilden DH. Naturally acquired simian varicella virus infection in African green monkeys. J Virol. 2002;76:8548–8550. doi: 10.1128/JVI.76.17.8548-8550.2002. The demonstration that simian varicella virus becomes latent in ganglia after naturally acquired infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Soike KF, Keller PM, Ellis RW. Immunization of monkeys with varicella-zoster virus glycoprotein antigens and their response to challenge with simian varicella virus. J Med Virol. 1987;22:307–313. doi: 10.1002/jmv.1890220403. [DOI] [PubMed] [Google Scholar]
- 150.Pumphrey CY, Gray WL. The genomes of simian varicella virus and varicella zoster virus are colinear. Virus Res. 1992;26:255–266. doi: 10.1016/0168-1702(92)90017-4. [DOI] [PubMed] [Google Scholar]
- 151.White TM, Mahalingam R, Kolhatkar G, Gilden DH. Identification of simian varicella virus homologues of varicella zoster virus genes. Virus Genes. 1997;15:265–269. doi: 10.1023/a:1007940822747. [DOI] [PubMed] [Google Scholar]
- 152.Gray WL, Starnes B, White MW, Mahalingam R. The DNA sequence of the simian varicella virus genome. Virology. 2001;284:123–130. doi: 10.1006/viro.2001.0912. [DOI] [PubMed] [Google Scholar]
- 153.Mahalingam R, White T, Wellish M, Gilden DH, Soike K, Gray WL. Sequence analysis of the leftward end of simian varicella virus (EcoRI-I fragment) reveals the presence of an 8-bp repeat flanking the unique long segment and an 881-bp open-reading frame that is absent in the varicella zoster virus genome. Virology. 2000;274:420–428. doi: 10.1006/viro.2000.0465. [DOI] [PubMed] [Google Scholar]
- 154.Mahalingam R, Gray WL. The simian varicella virus genome contains an invertible 665 base pair terminal element that is absent in the varicella zoster virus genome. Virology. 2007;366:387–393. doi: 10.1016/j.virol.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dueland AN, Martin JR, Devlin ME, et al. Acute simian varicella infection Clinical, laboratory, pathologic, and virologic features. Lab Invest. 1992;66:762–773. [PubMed] [Google Scholar]
- 156.Iltis JP, Arrons MC, Castellano GA, et al. Simian varicella virus (delta herpesvirus) infection of patas monkeys leading to pneumonia and encephalitis. Proc Soc Exp Biol Med. 1982;169:266–279. doi: 10.3181/00379727-169-41342. [DOI] [PubMed] [Google Scholar]
- 157.Mahalingam R, Wellish M, Soike K, White T, Kleinschmidt-DeMasters BK, Gilden DH. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- 158.Gray WL, Mullis L, Soike KF. Viral gene expression during acute simian varicella virus infection. J Gen Virol. 2002;83:841–846. doi: 10.1099/0022-1317-83-4-841. [DOI] [PubMed] [Google Scholar]
- 159.White TM, Mahalingam R, Traina-Dorge V, Gilden DH. Persistence of simian varicella virus DNA in CD4(+) and CD8(+) blood mononuclear cells for years after intratracheal inoculation of African green monkeys. Virology. 2002;303:192–198. doi: 10.1006/viro.2002.1664. [DOI] [PubMed] [Google Scholar]
- 160.White TM, Mahalingam R, Traina-Dorge V, Gilden DH. Simian varicella virus DNA is present and transcribed months after experimental infection of adult African green monkeys. J Neurovirol. 2002;8:191–203. doi: 10.1080/13550280290049705. [DOI] [PubMed] [Google Scholar]
- 161.Kolappaswamy K, Mahalingam R, Traina-Dorge V, et al. Disseminated simian varicella virus infection in an irradiated rhesus macaque (Macaca mulatta) J Virol. 2007;81:411–415. doi: 10.1128/JVI.01825-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mahalingam R, Traina-Dorge V, Wellish M, et al. Simian varicella virus reactivation in cynomolgus monkeys. Virology. 2007;368:50–59. doi: 10.1016/j.virol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 163.Verjans GM, Hintzen RQ, van Dun JM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci USA. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–9049. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abendroth A, Lin I, Slobedman B, Ploegh H, Arvin AM. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J Virol. 2001;75:4878–4888. doi: 10.1128/JVI.75.10.4878-4888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cohen JI. Infection of cells with varicella-zoster virus down-regulates surface expression of class I major histocompatibility complex antigens. J Infect Dis. 1998;177:1390–1393. doi: 10.1086/517821. [DOI] [PubMed] [Google Scholar]
Websites
- 202.U.S. Census Bureau. US interim projects by age, sex, race, and hispanic origin. 2004 http://www.census.gov/ipc/www/usinterimproj/natprojtab02a.pdf.
- 203.National Center for Biotechnology Information (NCBI) 2008 http://www.ncbi.nlm.nih.gov/sites/entrez.