Abstract
Lipoic Acid is a disulfhydryl-containing compound used in clinical medicine and in experimental models as an antioxidant. We developed a stable isotope dilution capillary gas chromatography/mass spectrometry assay for lipoic acid. We assayed a panel of the metabolites of transmethylation and transsulfuration 30 minutes after injecting lipoic acid 100 mg/kg in a rat model. Lipoic acid values rose 1000-fold in serum and 10-fold in liver. A methylated metabolite of lipoic acid was also detected but not quantitated. Lipoic acid injection caused a massive increase in serum S-adenosylhomocysteine and marked depletion of liver S-adenosylmethionine. Serum total cysteine was depleted but liver cysteine and glutathione were maintained. Serum total homocysteine doubled with increases also in cystathionine, N, N-dimethylglycine and alpha-aminobutyric acid. In contrast, after injection of 2-mercaptoethanesulfonic acid (MESNA), serum total cysteine and homocysteine were markedly depleted and there were no effects on serum S-adenosylmethionine or S-adenosylhomocysteine. We conclude that large doses of lipoic acid both displace sulfhydryls from binding sites resulting in depletion of serum cysteine but also pose a methylation burden with severe depletion of liver S-adenosylmethionine and massive release of S-adenosylhomocysteine. These changes may have previously unrecognized deleterious effects that should be investigated in both human disease and experimental models.
Keywords: homocysteine, methionine, cysteine, transmethylation, transsulfuration
Introduction
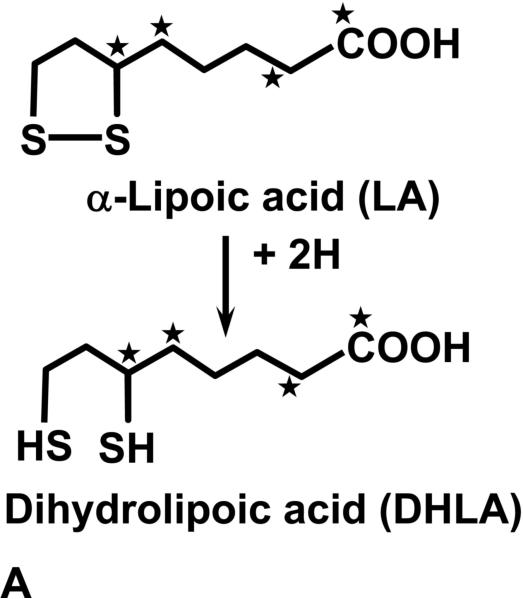
There is extensive interest in alpha-lipoic acid (LA) as a therapeutic agent for diabetic neuropathy (1) and as an antioxidant to counteract diseases of aging (2, 3). It is also being explored in many experimental models as a treatment for oxidant damage including ischemia-perfusion injuries (4), toxic applications (5) and in models of nervous system degenerative diseases (7) and atherosclerosis (8, 9). LA is available without a prescription in the United States from vitamin and supplement suppliers and is also stocked in food stores and pharmacies. LA, as the lipoamide form, is a naturally occurring factor for the mitochondrial multienzyme complexes which catalyze oxidative decarboxylation of alpha-keto acids (2). However, large therapeutic doses of LA are intended to exploit the reducing power of its two sulfhydryl groups (2). LA contains an intramolecular disulfide as shown in Figure 1a. Intracellular reduction of the disulfide takes place after ingestion and dihydrolipoic acid (DHLA) is formed, which is considered to be the important antioxidant (2, 10). The metabolism of exogenous DHLA proceeds both by S-methylation of the sulfhydryl groups and beta oxidation of the side chain (11, 12) (Figure 1b). In fact, it has been shown that large amounts of methylated metabolites are excreted in the urine after oral doses in humans (12). It would not be surprising that large doses of LA might perturb the balance of the sulfur containing amino acids such as cysteine and homocysteine as well as other metabolites in the pathways of methionine metabolism. In fact, investigations in tissue culture models have shown that cells exposed to LA excrete excess homocysteine into the media (13).
Figure 1a.
The structures of alpha-lipoic acid (LA) and the reduced form dihydrolipoic acid (DHLA) are shown. The asterisks demonstrate the site of the stable isotope 13C labels in the molecule.
Figure 1b.
Two of the major metabolites of lipoic acid are shown; 4, 6-bismethylthiohexanoic acid (BMHA) and 2, 4-bismethylthio-butanoic acid (BMBA). The asterisks demonstrate expected sites of the stable isotope 13C labels after metabolism/catabolism of labeled LA.
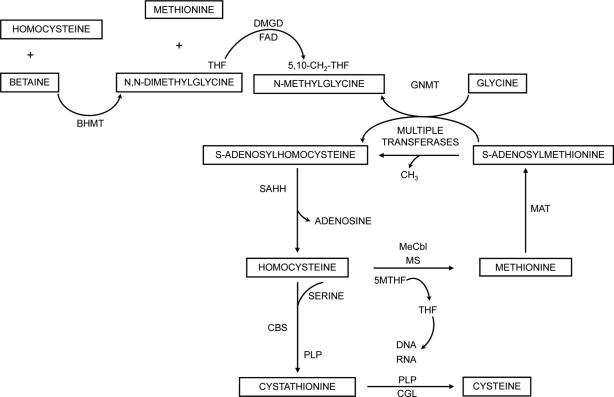
Pathways of methionine and homocysteine metabolism are shown in Figure 2. Homocysteine is at a branch point where it can be remethylated to methionine, which is the precursor for S-adenosylmethionine (SAM), the methyl-donor for important transmethylation reactions, including the synthesis of creatine, acetylcholine and many small molecules such as neurotransmitters and also DNA methylation (14). S-adenosylhomocysteine (SAH) is generated by such reactions and is an inhibitor of most transmethylation reactions. It is cleared by S-adenosylhomocysteine hydrolase (SAHH) to homocysteine and adenosine. Homocysteine is cleared by condensation with serine by cystathionine beta synthase (CBS) to cystathionine, which is cleaved to form cysteine. SAM is an activator of CBS so that in conditions of methionine excess more homocysteine is shunted to cystathionine and cysteine (14). Some experimental work suggests that CBS is also activated under conditions of oxidant stress when the need for cysteine is high since the latter is the rate limiting substrate for glutathione synthesis (15, 16). Liver SAM concentrations are partially controlled by the synthesis of N-methylglycine by glycine N-methyltransferase, a folate-binding enzyme that is not inhibited by SAH (17). The recent description of individuals with an inborn error of SAHH demonstrates how devastating interruption of transmethylation reactions can be (18). These individuals have hypermethioninemia, massive serum SAH accumulation and severe myopathy, liver disease and developmental delay. Biochemical changes showed that acetylcholine and creatine synthesis were impaired and the cause of many of the clinical manifestations because oral replacement of choline and creatine was beneficial (18).
Figure 2.
The metabolic pathways of remethylation, transmethylation and transsulfuration are shown (14). Homocysteine can be methylated by either betaine to produce methionine and N, N-dimethylglycine by betaine homocysteine methyltransferase (BHMT) or by 5-methyltetrahydrofolate (5MTHF) by methionine synthase, a reaction dependent on folate and methylcobalamin (MECbl). Methionine is activated to S-adenosylmethionine (SAM) by methionine adenosyltransferase (MAT). SAM is the methyl donor for many transmethylation reactions that produce S-adenosylhomocysteine (SAH). SAH is hydrolysed by S-adenosylhomocysteine hydrolase (SAHH) to homocysteine and adenosine. When methionine is abundant then excess homocysteine is cleared by condensing with serine by cystathionine beta synthase (CBS), a pyridoxal phosphate (PLP)-dependent reaction to form cystathionine and alpha-ketobutyrate. Cystathionine can be cleared by cystathionine gamma lyase (CGL), also PLP-dependent, to form cysteine. Not shown is further metabolism of alpha-ketoglutyrate to alpha -aminobutyric acid. When SAM is in excess, glycine N-methyltransferase (GNMT) methylates glycine to form N-methylglycine.
We developed a specific stable isotope dilution gas chromatography/mass spectrometry assay for lipoic acid and studied the changes in methionine pathway metabolites in rats after IP injection of LA. We also compared those changes with the effects of an injection of 2-mercaptoethanesulfonic acid (MESNA), an agent used clinically to modulate thiols (19).
Methods
Gas chromatography/Mass Spectrometry Assay Lipoic Acid
Racemic unlabeled LA, dihydrolipoic acid, MESNA and other chemicals were obtained from Sigma Aldrich. Stable isotope labeled LA containing 4 13C molecules (DL alpha lipoic acid 1, 2, 5, 6, 13C > 99 atom %) was custom synthesized by Isotech, Miamisburg, OH. Pure labeled or unlabeled LA was dissolved in methanol, dried and derivitized with t- butyldimethylsilyltrifluoroacetamide in acetonitrile and subjected to gas chromatography/mass spectrometry. Consistent with a previous report, the achieved spectrum was compatible with derivitization of the carboxyl group with one t- butyldimethylsilyl group (20). The largest peak abundance was found for the M-57 ion thus m/z 263 was used to monitor the unlabeled LA, and m/z 267 for the stable isotope labeled form. There was contamination of unlabeled LA of about 1%, comparing the area of ion m/z 263 to the m/z 267 peak area when the derivitized internal standard was chromatographed. When unlabeled LA was chromatographed the natural isotope abundance run-over of m/z 267 was 1.4% of the peak area of m/z 263. An assay for body fluids was developed as follows: test tubes containing 8.8 nmol 13C labeled LA, 1000 ul H20, up to 400 ul of sample, 10 nmol dithiothreital and 10 umol NaOH, were incubated at 40° C for 30 minutes, then applied to 200 mg columns of YMC, GEL ODSA 12NMS-15 uM from YMC Company, Kyoto, Japan that have been pre-washed with 1 mL methanol followed with 3 mL H20. The loaded columns were washed with 3 mL H20 twice and the sample eluted with 1 mL methanol into autosampler vials, which were taken to dryness with a Savant evaporative centrifuge. Dried samples were derivitized with 30 uL of a solution containing 10 uL N-methyl-N (tert-butyldimethylsilyl) trifluoroacetamide (Pierce Chemical, Rockford, IL) and 20 uL acetonitrile. After incubation at 90° C for 30 minutes in sealed autosampler vials, 0.5 uL was analyzed by gas chromatography/mass spectrometry using a Supelco SPB-1 capillary column 20.0 n × 250 uM film thickness and internal diameter 0.30. Instrumentation included an Agilent 6890 Series GC system (PLUS +) equipped with an Agilent 7683 Series injector and Agilent 5973 network Mass Selective Detector. The initial column temperature was 80° C held for 0.5 minutes after the sample injection and increased to 320° C at a rate of 30° C/minute. The Mass Selective Detector was operated in the selected ion monitoring mode in which ion, m/z 263 was used to monitor endogenous LA and ion, m/z 267 was monitored for the labeled internal standard. A single peak was detected at 6.8 minutes under these conditions. Figure 3a shows the results of assay of 400 ul of control rat serum in which it can be seen that there were no interfering peaks for either ion. When rat serum was assayed without internal standard no peaks of m/z 267 were found in the relevant time window used to quantitate LA. Pooled human serum was spiked with racemic LA at a concentration of 2 umol/L and used to determine the precision of the assay. In other experiments discarded pooled human serum was spiked with varying concentrations of racemic LA for determination of the sensitivity of the assay. The 1% contamination of m/z 263 peak area in the internal standard contributed approximately 170 nmol/L to the value obtained for control rat or human serum and was subtracted from the values reported. However, this was under the sensitivity limits of the assay as described in Results and was of little practical importance.
Figure 3a.
Gas chromatography/mass spectrometry chromatograms of LA are shown for an assay of 400 uL of control rat serum, as described in Methods. The m-57 ions were monitored. The internal standard ion m/z 267 is shown above and m/z 263 below for the endogenous LA. The calculated result was 0.67 umol/L.
Putative methylated metabolites of LA were assayed by searching chromatographic runs of rat serum obtained after injection of labeled and/or unlabeled LA for the expected spectra of derivitized fragment based on published literature (11, 12). Liver tissue was assayed by homogenizing 50-200 mg of flash frozen rat liver with 8.8 nmol of 13C LA in 1 cc of water and 0.5 cc normal saline with a polytron, (Kinematica AG Litale Lucerne, Switzerland) for 15 seconds. The samples were centrifuged for 15 minutes at 5000 × g and processed as described for body fluids above.
Assay of Dihydrolipoic Acid
Dihydrolipoic acid (D, L-6, 8-thioctic acid, reduced form) was dissolved in methanol and derivitized as described for LA in body fluid assays of DHLA the same assay developed for LA was followed, except that DTT was omitted. Analysis of the spectrum showed that DHLA derivitized with three t- butyldimethylsilyl groups thus the M-57 fragment was monitored at ion m/z 493. The M-57 fragment of m/z 497 was detected and monitored in experiments showing conversion of 13C labeled LA to DHLA.
Assay of Methionine Metabolites
Total homocysteine (tHcy), cystathionine, methionine, total cysteine, N-methylglycine, N, N-dimethylglycine and alpha-aminobutyric acid were quantitated in serum and liver tissue by capillary stable isotope dilution gas chromatography/mass spectrometry as previously described (21-23). SAM and SAH were quantitiated by stable isotope dilution liquid chromatography/mass spectrometry as previously described (24). Liver glutathione and oxidized glutathione were assayed as previously described (25).
Rat Studies
The rat studies were approved by the Institutional Animal Care and Use Committee. The University of Colorado animal facility is veterinarian supervised and accredited. Male Sprague Dawley rats, 300-400 g size were obtained from animal supply companies and kept under quarantine for at least a week. They were maintained on routine lab chow in a 12 hour day/night cycle in the animal care facility. They were not fasted prior to experimentation. Controls and experimentals were injected IP under manual restraint and sacrificed by exsanguination after 30 minutes under terminal anesthesia using Isoflurane inhalation. Ten mL of whole blood was obtained by cardiac puncture and allowed to clot for 15 minutes at room temperature. The blood samples were then placed on ice and centrifuged within 30 minutes. Small pieces of rat liver were flash frozen on dry ice or dropped into liquid nitrogen and kept continuously frozen including during weighing, until homogenization.
Preparation of Lipoic Acid for Injection
Racemic LA was dissolved in equal parts of 5% sodium bicarbonate and phosphate buffered saline and the pH adjusted to approximately 7.5. The control solution was made similarly and adjusted with HCl as necessary to match the pH and osmotic load to be given to rats. The final solution was 50 mg/mL so that the rats received between 0.5 and 1 mL IP to deliver 100 mg/kg rat body weight. This dose was chosen because of the large number of published reports using similar doses and route of administration (25). Groups of rats were injected with L-methionine 10 mg/kg in combination with LA 100 mg/kg and compared to LA only. DHLA was dissolved in Chelex treated 95% ethanol prior to mixing with buffers as described for LA. MESNA was dissolved in water and injected IP (150 mg/kg). Rats were sacrificed 60 minutes after MESNA injection.
In order to determine whether 4, 6-bismethylthiohexanoic acid (BMHA), a methylated metabolite of LA, was formed in vivo, a rat was injected with 30 mg of 13C labeled LA, sacrificed after 30 minutes and serum was assayed for LA, and BMHA. It was expected that BMHA would retain 2 of the 13C labeled molecules (Figure 1) and would derivitize with one t- BDMS group. Therefore, the ions of m/z 267 for M-57, 309 for M-15 and 324 for M+ respectively were monitored for labeled BMHA as compared to endogenous BMHA with a spectrum-demonstrating ions m/z 265, 307 and 322. Once the peak of BMHA was identified in rat body fluids, then the peak area of m/z 265 was compared to that of LA internal standard peak m/z 267 in the same chromatographic run giving a gross approximation of quantitation.
Statistical Methods
Statistical evaluations were performed with a commercial statistics program SSPS for Windows Base 15.0 from SPSS, Chicago, Illinois. The differences between 2 groups were analyzed by T-tests with Levene's test for equality of variances to account for skewing of the data. Two-tailed tests with a significance level of p < 0.05 was used. Correlations between variables were analyzed with the Pearson correlation coefficient reported.
Results
Assay of LA
Pooled human serum did not have a detectable peak of LA or DHLA. When sufficient racemic LA was added to raise the concentration to 2 umol/L the coefficient of variation for intraassay variation was a mean of 7.5% performed on 3 different occasions. Pooled human serum was spiked with increasing concentrations of racemic LA and it was determined that the lower limit of the assay was 500 nmol/L. It was often necessary to assay samples at much lower volumes as shown in Figure 3b, since the value for lipoic acid in injected rats ranged from 10 to 1000 fold increased over the baseline values. Thus when endogenous LA exceeded greatly the amount of added internal standard the 1.4% contribution of m/z 267 peak area resulting from natural isotope abundance was subtracted from the internal standard peak, however this problem was minimized by assaying small serum amounts. Varying amounts of DHLA were converted to LA when pure DHLA was dried and derivitized therefore, further attempts to quantitate serum DHLA were not pursued.
Figure 3b.
The gas chromatography/mass spectrometry chromatograms are shown for assay of 20 uL of rat serum 30 minutes after LA 100 mg/kg was injected IP and assayed as described in Fig.4a. The calculated value for serum LA was 20 umol/L.
Rat Serum Lipoic Acid after IP Injection
Baseline (control) rat serum contained small quantities of lipoic acid detectable as shown in Figure 3a. Injection of 100 mg/kg IP of LA into 26 rats resulted in a massive rise in serum LA as shown in Table 1 (Figure 3b). There was a 1000-fold increase of LA in the serum and about a 10-fold increase in liver LA at 30 minutes. Serum total homocysteine doubled accompanied by a 10-20 fold increase in serum SAH. Liver tHcys was not significantly different but the liver SAH tripled in those injected with LA. The serum SAM was not changed yet liver SAM decreased markedly to 22% of the control animals values. The serum total cysteine dropped markedly to 32% of the control value yet was unchanged in liver. Methionine was increased in serum and unchanged in liver in contrast to the marked depletion in SAM. Cystathionine values were modestly increased in serum and in liver with an accompanying increase in alpha-aminobutyric acid in serum. N, N-dimethylglycine was increased in serum in the injected animals.
Table 1.
Rat Serum and Liver Metabolites 30 minutes after LA (100 mg/kg) (n = 26) vs. Control IP Injection (n = 15)
| Serum (uM or nM*) |
Liver (nmol/g) |
|||||
|---|---|---|---|---|---|---|
| Metabolite | LA | Control | LA | Control | ||
| Mean (SD) |
Mean (SD) |
p |
Mean (SD) |
Mean (SD) |
p |
|
| Lipoic Acid | 266 (294) | 0.2 (0.4) | 0.002 | 51.9 (52.0)† | 5.8 (11.3)† | 0.001 |
| tHcys | 7.3 (1.9) | 3.4 (1.1) | <0.001 | 42.1 (18.0) | 31.4 (18.5) | 0.120 |
| SAH | 507 (757) | 33 (12) | 0.002 | 69.6 (13.7) | 20.3 (10.5) | <0.001 |
| SAM | 177 (86) | 205 (71) | 0.323 | 12.8 (8.9) | 56.7 (16.0) | <0.001 |
| Cystathionine | 935 (186) | 773 (261) | 0.026 | 8.5 (3.5) | 5.5 (3.5) | 0.025 |
| Cysteine | 70 (22) | 216 (28) | <0.001 | 1120 (522) | 1280 (496) | 0.408 |
| Methionine | 81.9 (21.7) | 63.7 (18.3) | 0.009 | 648 (268) | 745 (214) | 0.302 |
| N, N-Dimethylglycine | 10.8 (3.2) | 7.5 (4.1) | 0.006 | 97.2 (32.2) | 78.4 (21.1) | 0.091 |
| N-Methylglycine | 3.4 (0.9) | 2.9 (1.4) | 0.236 | 42.1 (14.8) | 35.0 (21.8) | 0.260 |
| Alpha-aminobutyric acid | 6.3 (1.5) | 3.9 (0.6) | <0.001 | 62.5 (40.5) | 41.4 (24.6) | 0.124 |
All serum metabolites are shown as umol/L except SAH, SAM and cystathionine, which are nmol/L
N = 19 for LA rats and 8 for control rats for liver lipoic acid
Mean (SEM) liver glutathione concentrations were not different between LA treated (N = 3) 4.1 (0.09) vs. control (N = 4) 4.2 (0.36) mmol/g, (p = 0.096). Oxidized liver glutathione (GSSG) was also unchanged 47.4 (2.1) vs. 54.7 (4.3), (p = 0.306).
A group of 5 rats was injected with LA alone and compared to 5 rats that were injected with LA and methionine 10 mg/kg. There was no difference between the two groups in any of the measured metabolites, except for a trend in increased serum methionine mean (SD) 113.1 (29.4) vs. 79.9 (4.4) umol/L, p = 0.064. Table 2 shows the metabolite values obtained when a group of 6 rats was injected with LA 100 mg/kg then compared to 5 rats injected with DHLA 100 mg/kg IP. There was no difference in the serum or liver concentration of LA. Serum and liver tHcy values were significantly higher in the LA injected rats. However, there was no difference in the marked depletion of serum cysteine although liver cysteine trended lower in the rats injected with LA as compared to DHLA. The serum SAH trended lower in those who received LA but the reverse was true in the liver. Serum SAM and N-methylglycine were lower in the LA treated group but liver SAM trended higher as compared to those who received DHLA.
Table 2.
Rat serum and liver metabolites 30 minutes after LA (100 mg/kg) (N=6) vs. DHLA 100 mg/kg (N=5) IP injection
| Serum (uM or nM*) | Liver (nmol/g) | |||||
|---|---|---|---|---|---|---|
| LA | DHLA | LA | DHLA | |||
| Metabolite | ||||||
| Mean (SD) | Mean (SD) | p | Mean (SD) | Mean (SD) | p | |
| Lipoic Acid | 243 (85) | 196 (44) | 0.272 | 114 (46) | 77 (15) | 0.107 |
| tHcys | 9.4 (1.2) | 6.8 (1.5) | 0.011 | 35.1 (5.6) | 24.0 (4.5) | 0.006 |
| SAH* | 233 (58) | 409 (156) | 0.083 | 82 (7) | 73 (9) | 0.076 |
| SAM* | 136 (15) | 188 (39) | 0.014 | 9 (2) | 7 (2) | 0.051 |
| Cysteine | 69 (5) | 72 (16) | 0.708 | 770 (57) | 845 (65) | 0.070 |
| Methionine | 80.0 (9.6) | 86.3 (7.1) | 0.257 | 427 (35) | 483 (52) | 0.062 |
| N-methylglycine | 3.1 (0.9) | 4.2 (0.5) | 0.040 | 49.6 (12.4) | 71.4 (10.3) | 0.012 |
SAH and SAM are nmol/L
The internal standard 13C LA was readily detected in serum and urine in a rat after IP injection. The putative, methylated metabolite, BMHA containing two 13C molecules was detected in serum, liver and urine with a retention time of 5.77 minutes using conditions developed for assay of LA. The major derivitized fragments, M-57, M-15 and M+, m/z 267, 324 and 309, respectively were present and the ions co-eluted. When chromatograms were compared to a rat injected with unlabeled LA, a similar pattern of ions were found with m/z 265, 322 and 307 at 5.75 minutes. The lack of an available stable isotope labeled internal standard prevented quantitation of the methylated metabolites. However, in liver homogenates assayed for LA after unlabeled LA injection, the peak area of m/z 265 at 5.75 minutes (retention time for BMHA) was compared to the LA internal standard peak area of m/z 267 at 6.3 minutes demonstrating a mean recovery of 26% BMHA as compared to LA with a wide range of 1.6-81.8% in 8 rats. Therefore, a gross approximation for mean (S.D.) liver BMHA after injection was 27.5 (40.0) nmol/g. This estimated liver BMHA correlated with liver LA (Pearson correlation −0.73 (p=0.039) and inversely with serum cysteine −0.93 (p=0.001). The other metabolite BMBA, was not detected in rat serum or other fluids under our assay conditions.
Serum LA was inversely correlated with serum cysteine −0.725 ( p < 0.001), serum methylglycine −0.549 (p = 0.001), serum methionine −0.405 (p = 0.019) and serum DMG −0.403 (p = 0.02) and directly correlated with liver SAM 0.356 (p = 0.05) and liver tHcys 0.636 (p < 0.001). Serum LA was not correlated with serum SAM, SAH or tHcy however. Serum cysteine correlated directly with serum methionine 0.316 (p = 0.033), serum MG 0.425 (p = 0.012) and indirectly with liver LA −0.562 (0.12) and liver tHcy −0.440 (0.17).
MESNA is a sulfhydryl containing compound commonly used as a uroprotective agent and is known to decrease serum cysteine and homocysteine. In order to determine whether the effects of lowering cysteine (by LA) were independent of the effects on SAH, MESNA, 150 mg/kg, was injected in 5 rats and compared to 6 injected with vehicle alone. Table 3 shows that serum cysteine was decreased to 17% of control but tHcy was also markedly decreased in contrast to the increase seen with LA. Serum SAM and SAH were not significantly changed from control. The liver tHcy, cysteine and other metabolites were unchanged.
Table 3.
Rat Serum and liver metabolites 60 minutes after Mesna (150 mg/kg) (N=5) vs. Control (N=6) IP injection
| Serum (uM or nM*) | Liver (nmol/g)† | |||||
|---|---|---|---|---|---|---|
| Mesna | Control | Mesna | Control | |||
| Mean (SD) | Mean (SD) | p. | Mean (SD) | Mean (SD) | p. | |
| tHcys | 0.9 (0.1) | 2.9 (0.8) | 0.002 | 23.4 (12.1) | 19.2 (4.1) | 0.485 |
| SAH* | 128 (99) | 69 (9.2) | 0.253 | NDa | ND | |
| SAM* | 252 (44) | 234 (24) | 0.459 | ND | ND | |
| Cysteine | 40 (8) | 234 (46) | 0.001 | 1003 (153) | 963 (197) | 0.725 |
| Methionine | 60.0 (9.5) | 63.0 (6.4) | 0.678 | 248 (38) | 257 (42) | 0.743 |
| Cystathionine | 587 (76) | 872 (181) | 0.010 | 13.6 (7.1) | 12.2 (5.9) | 0.746 |
Serum SAH, SAM and cystathionine are nmol/L
N = 5 (Mesna) and N = 5 (Control) for liver values
ND = Not Determined
Discussion
There were massive changes in concentration of some of the metabolites of transmethylation and transsulfuration pathways within 30 minutes, after a commonly used experimental dose of LA was injected into rats. Serum SAH increased 20-fold and serum cysteine decreased by 60%. Accompanying the rise in serum and liver SAH was a massive depletion of liver SAM with sparing of serum SAM. Despite the extreme drop in serum cysteine, liver cysteine was unchanged as was liver methionine, and glutathione. Accompanying the rise in tHcys was an increase in N, N-dimethylglycine concentrations, possibly due to methylation of homocysteine by liver betaine homocysteine methyltransferase (22). The rise in serum and liver cystathionine and alpha-aminobutyric acid probably reflect an increase in transsulfuration flux which is somewhat surprising given the marked SAM depletion in the liver since SAM is an activator of cystathionine beta-synthase (14). SAH may also activate CBS thus, there may have been further depletion of liver SAM because of loss of homocysteine to transsulfuration (14). In contrast, MESNA injection only induced serum cysteine and tHcy depletion.
The metabolic changes summarized above may be due to either shifts in sulfhydryl binding caused by the disulfhydryl LA and/or an induced methylation burden due to the metabolism of LA/DHLA. It is believed that absorbed LA is enzymatically converted to the reduced form, DHLA by mitochondrial dihydrolipoamide dehydrogenase, cytosolic glutathione reductase and thioredoxin reductase (2). It is likely that when large amounts of DHLA are present then other sulphur containing compounds such as homocysteine and cysteine may be released from sulfhydryl binding to albumin and other proteins and compounds. Two other sulfhydryl containing therapeutics, MESNA and N-acetylcysteine, have been shown to cause decreases in plasma cysteine and homocysteine similar to what occurred in this study (19, 26, 27). The release of free cysteine may promote its uptake by tissues and in this study despite marked serum depletion of cysteine, there was maintenance of hepatic cysteine and no change in glutathione. Previous investigators have found increased glutathione concentrations after LA treatment of rodents (3, 9, 26, 28-30) and in tissue culture (31-33), possibly because of the displacement of bound cysteine and its increased tissue availability for synthesis of glutathione. This mobilization of cysteine and increased production of glutathione may be part of the beneficial antioxidant effect that has been reported in many experiments. The need for reduction of lipoic acid by the organism would seem to negate some of the antioxidant effect. However, we found comparable changes in metabolites when we compared injections of LA and DHLA which suggests that using the already reduced compound has little benefit.
Detailed investigations of the pharmacokinetics and metabolism of lipoic acid have been reported (11, 12). LA/DHLA is metabolized by beta hydroxylation of the carbon side chain, and S-methylation (11, 12). The major metabolite of LA found in healthy volunteers was BMHA (12) (see Figure 1b) along with smaller quantities of other methylated metabolites. We were also able to find serum and liver BMHA after injection of labeled or unlabeled LA in rats. The reported studies in humans showed that approximately 60% of the recovered amount in urine after oral dosing was BMHA and only 1.6% the parent compound (12). We estimated that hepatic BMHA was about 25% of the simultaneous hepatic LA concentration. Thus, it appears that most administered LA is methylated, which could be the cause of the marked depletion of liver SAM in the rats injected in this investigation.
There is an estimated transmethylation flux of 220-260 umol/kg per day for humans (34). The dose of lipoic acid we injected in rats was 500 umol/kg, thus likely a major consumer of SAM with resulting massive production of SAH. Since MESNA depleted serum cysteine without significantly raising SAH or homocysteine, the latter effects are likely not due to the sulfhydryl reducing capability of LA. Inhibition of SAHH with adenosine-dialdehyde was reported to decrease the extracellular homocysteine in cell cultures exposed to LA (13) suggesting that production of homocysteine rather than displacement might be the cause of the elevated levels seen in our rats. Two other commonly used drugs, niacin and L-dopa, which are used in comparably large doses have also been shown to increase total homocysteine in humans (35, 36). This has been attributed to their requirement for methylation. An inhibitor of L-dopa methylation, tolcapone, decreased plasma SAH and homocysteine concentrations in patients treated with L-dopa (37).
Commonly used doses of LA in human disease range from 600-1800 mg/day (1), which for a 70 kg person will range from roughly 9-26 mg/kg, thus 4-10-fold less than in our rat model. However, at the higher doses it is likely that LA might pose a methylation burden and human subjects should be investigated for changes in the transmethylation and transsulfuration metabolites. A recent report suggests that an improved formulation of R-(+)-LA has significantly higher bioavailability and thus, might be expected to have more effect on methylation status (38). Placebo controlled trials in diabetic neuropathy have shown clear benefit for LA with few demonstrated side effects (1). Fortunately, there is not a dose response, with lower doses equally effective, which would likely have less potential to cause hyperhomocysteinemia. In Parkinson's disease treatment with L-Dopa, the hyperhomocysteinemia presumptively attributed to the methylation burden has been correlated with vascular disease (35). Therefore some concern may be warranted if large doses of LA are used in human medicine. An interesting difference in LA dose toxicity has been described in cats as compared to humans and dogs, which could be investigated for methylation defects (39). LA is being investigated in cell models as a cancer chemotherapeutic agent since high media concentrations (100 umol/L – 5 mmol/L) have been reported to induce apoptosis in a variety of cell lines (40-43). The impact of probable SAM depletion and SAH induced transmethylation inhibition should be investigated in these systems.
In summary, we have demonstrated that a common experimental dose of LA causes massive depletion of liver SAM, elevation of serum SAH and increased tHcy in rats. Similar depletion of serum cysteine with MESNA was not accompanied by changes in SAM and SAH suggesting that the former effects are due to different aspects of LA metabolism/catabolism. Further studies in humans may be interesting.
Acknowledgements
This work has been supported by National Institutes of Health/National Institute on Aging: AG-09834 (SPS) and National Institute of Environmental Health Sciences U45 ES-015678 (SPS, CWW). Expert technical assistance was provided by Carla Ray, Linda Farb and Bev Raab.
Abbreviations
- LA
Lipoic acid
- DHLA
dihydrolipoic acid
- SAM
S-adenosylmethionine
- SAH
S-adenosylhomocysteine
- SAHH
S-adenosylhomocysteine hydrolase
- CBS
Cystathionine B-synthase
- DTT
dithiothreitol
- tHcy
total homocysteine
- IP
intraperitoneal
- MESNA
Sodium 2-mercaptoethanesulfonate
- BMHA
4, 6-bismethylthio-hexanoic acid
- BMBA
2, 4-bismethylthio-butanoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29:2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 2.Packer L, Witt EH, Tritschler HJ. Alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 3.Suh JH, Wang H, Liu RM, Liu J, Hagen TM. (R)-alpha-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for GSH synthesis. Arch. Biochem. Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dünschede F, Erbes K, Kircher A, Westermann S, Seifert J, Schad A, Oliver K, Kiemer AK, Theodor J. Reduction of ischemia reperfusion injury after liver resection and hepatic inflow occlusion by alpha-lipoic acid in humans. World J. Gastroenterol. 2006;12:6812–6817. doi: 10.3748/wjg.v12.i42.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lateef H, Aslam MN, Stevens MJ, Varani J. Pretreatment of diabetic rats with lipoic acid improves healing of subsequently-induced abrasion wounds. Arch. Dermatol. Res. 2005;297:75–83. doi: 10.1007/s00403-005-0576-6. [DOI] [PubMed] [Google Scholar]
- 6.Caylak E, Aytekin M, Halifeoglu I. Antioxidant effects of methionine, alpha-lipoic acid, N-acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. Exp. Toxicol. Pathol. 2008;60:289–294. doi: 10.1016/j.etp.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Milgram NW, Araujo JA, Hagen TM, Treadwell BV, Ames BN. Acetyl-L-carnitine and alpha-lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J. 2007;21:3756–3762. doi: 10.1096/fj.07-8531com. [DOI] [PubMed] [Google Scholar]
- 8.Wollin SD, Jones PJ. Alpha-lipoic acid and cardiovascular disease. J. Nutr. 2003;133:3327–3330. doi: 10.1093/jn/133.11.3327. [DOI] [PubMed] [Google Scholar]
- 9.Yi X, Maeda N. Alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006;55:2238–2244. doi: 10.2337/db06-0251. [DOI] [PubMed] [Google Scholar]
- 10.Brown SE, Ross MF, Sanjuan-Pla A, Manas AR, Smith RA, Murphy MP. Targeting lipoic acid to mitochondria: synthesis and characterization of a triphenylphosphonium-conjugated alpha-lipoyl derivative. Free Radic. Biol. Med. 2007;42:1766–1780. doi: 10.1016/j.freeradbiomed.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Schupke H, Hempel R, Peter G, Hermann R, Wessel K, Engel J, Kronbach T. New metabolic pathways of alpha-lipoic acid. Drug Metab. Dispos. 2001;29:855–862. [PubMed] [Google Scholar]
- 12.Teichert J, Hermann R, Ruus P, Preiss R. Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers. J Clin. Pharmacol. 2003;43:1257–1267. doi: 10.1177/0091270003258654. [DOI] [PubMed] [Google Scholar]
- 13.Hultberg B. Elimination of high amounts of extracellular homocysteine in human cell lines. Clin. Chim. Acta. 2005;356:117–124. doi: 10.1016/j.cccn.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin. Thromb. Hemost. 2000;26:219–225. doi: 10.1055/s-2000-8466. [DOI] [PubMed] [Google Scholar]
- 15.Panayiotidis MI, Stabler SP, Allen RH, Ahmad A, White CW. Cigarette smoke extract increases S-adenosylmethionine and cystathionine in human lung epithelial-like (A549) cells. Chem. Biol. Interact. 2004;147:87–97. doi: 10.1016/j.cbi.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Prudova A, Bauman Z, Braun A, Vitvitsky V, Lu SC, Banerjee R. S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc. Natl. Acad. Sci. USA. 103:6489–6494. doi: 10.1073/pnas.0509531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo EJ, Wagner C. Tissue distribution of glycine N-methyltransferase, a major folate-binding protein of liver. Proc Natl. Acad. Sci. USA. 1994;91:210–214. doi: 10.1073/pnas.91.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barić I, Cuk M, Fumić K, Vugrek O, Allen RH, Glenn B, Maradin M, Pazanin L, Pogribny I, Rados M, Sarnavka V, Schulze A, Stabler S, Wagner C, Zeisel SH, Mudd SH. S-Adenosylhomocysteine hydrolase deficiency: a second patient, the younger brother of the index patient, and outcomes during therapy. J. Inherit. Metab. Dis. 2005;28:885–902. doi: 10.1007/s10545-005-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urquhart BL, Freeman DJ, Spence JD, House AA. MESNA as a nonvitamin intervention to lower plasma total homocysteine concentration: implications for assessment of the homocysteine theory of atherosclerosis. J. Clin. Pharmacol. 2007;47:991–997. doi: 10.1177/0091270007303767. [DOI] [PubMed] [Google Scholar]
- 20.Kataoka H. Chromatographic analysis of lipoic acid and related compounds. J. Chromatogr. B. Biomed. Sci. Appl. 1998;717:247–262. doi: 10.1016/s0378-4347(97)00628-2. [DOI] [PubMed] [Google Scholar]
- 21.Stabler SP, Marcell PD, Podell ER, Allen RH, Savage DG, Lindenbaum J. Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J. Clin. Invest. 1988;81:466–474. doi: 10.1172/JCI113343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stabler SP, Lindenbaum J, Savage DG, Allen RH. Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood. 1993;81:3404–3413. [PubMed] [Google Scholar]
- 23.Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism. 1993;42:1448–1460. doi: 10.1016/0026-0495(93)90198-w. [DOI] [PubMed] [Google Scholar]
- 24.Stabler SP, Allen RH. Quantification of serum and urinary S-adenosylmethionine and S-adenosylhomocysteine by stable-isotope-dilution liquid chromatography-mass spectrometry. Clin. Chem. 2004;50:365–372. doi: 10.1373/clinchem.2003.026252. [DOI] [PubMed] [Google Scholar]
- 25.Shaik IH, Mehvar R. Rapid determination of reduced and oxidized glutathione levels using a new thiol-masking reagent and the enzymatic recycling method: application to the rat liver and bile samples. Anal. Bioanal. Chem. 2006;385:105–113. doi: 10.1007/s00216-006-0375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dulundu E, Ozel Y, Topaloglu U, Sehirli O, Ercan F, Gedik N, Sener G. Alpha-lipoic acid protects against hepatic ischemia-reperfusion injury in rats. Pharmacology. 2007;79:163–170. doi: 10.1159/000098953. [DOI] [PubMed] [Google Scholar]
- 27.Doshi S, McDowell I, Goodfellow J, Stabler S, Boger R, Allen R, Newcombe R, Lewis M, Moat S. Relationship between S-adenosylmethionine, S-adenosylhomocysteine, asymmetric dimethylarginine, and endothelial function in healthy human subjects during experimental hyper- and hypohomocysteinemia. Metabolism. 2005;54:351–360. doi: 10.1016/j.metabol.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Mervaala E, Finckenberg P, Lapatto R, Müller DN, Park JK, Dechend R, Ganten D, Vapaatalo H, Luft FC. Lipoic acid supplementation prevents angiotensin II-induced renal injury. Kidney Int. 2003;64:501–508. doi: 10.1046/j.1523-1755.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnsen-Soriano S, Garcia-Pous M, Arnal E, Sancho-Tello M, Garcia-Delpech S, Miranda M, Bosch-Morell F, Diaz-Llopis M, Navea A, Romero FJ. Early lipoic acid intake protects retina of diabetic mice. Free. Radic. Res. 2008;42:613–617. doi: 10.1080/10715760802206791. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM, Farrag MM. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008;243:261–270. doi: 10.1016/j.tox.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Han D, Handelman G, Marcocci L, Sen CK, Roy S, Kobuchi H, Tritschler HJ, Flohé L, Packer L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6:321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- 32.Hultberg B, Andersson A, Isaksson A. Lipoic acid increases glutathione production and enhances the effect of mercury in human cell lines. Toxicology. 2002;175:103–110. doi: 10.1016/s0300-483x(02)00060-4. [DOI] [PubMed] [Google Scholar]
- 33.Jia Z, Hallur S, Zhu H, Li Y, Misra HP. Potent upregulation of glutathione and NAD(P)H:quinone oxidoreductase 1 by alpha-lipoic acid in human neuroblastoma SH-SY5Y cells: protection against neurotoxicant-elicited cytotoxicity. Neurochem. Res. 2008;33:790–800. doi: 10.1007/s11064-007-9496-5. [DOI] [PubMed] [Google Scholar]
- 34.Mudd SH, Brosnan JT, Brosnan ME, Jacobs RL, Stabler SP, Allen RH, Vance DE, Wagner C. Methyl balance and transmethylation fluxes in humans. Am. J. Clin. Nutr. 2007;85:19–25. doi: 10.1093/ajcn/85.1.19. [DOI] [PubMed] [Google Scholar]
- 35.Dierkes J, Westphal S. Effect of drugs on homocysteine concentrations. Semin. Vasc. Med. 2005;5:124–139. doi: 10.1055/s-2005-872398. [DOI] [PubMed] [Google Scholar]
- 36.Stead LM, Jacobs RL, Brosnan ME, Brosnan JT. Methylation demand and homocysteine metabolism. Adv. Enzyme Regul. 2004;44:321–333. doi: 10.1016/j.advenzreg.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Müller T, Kuhn W. Tolcapone decreases plasma levels of S-adenosyl-L-homocysteine and homocysteine in treated Parkinson's disease patients. Eur. J. Clin. Pharmacol. 2006;62:447–450. doi: 10.1007/s00228-006-0132-0. [DOI] [PubMed] [Google Scholar]
- 38.Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern. Med. Rev. 2007;12:343–351. [PubMed] [Google Scholar]
- 39.Hill AS, Werner JA, Rogers QR, O'Neill SL, Christopher MM. Lipoic acid is 10 times more toxic in cats than reported in humans, dogs or rats. J. Anim. Physiol. Anim. Nutr. (Berl) 2004;88:150–156. doi: 10.1111/j.1439-0396.2003.00472.x. [DOI] [PubMed] [Google Scholar]
- 40.Simbula G, Columbano A, Ledda-Columbano GM, Sanna L, Deidda M, Diana A, Pibiri M. Increased ROS generation and p53 activation in alpha-lipoic acid-induced apoptosis of hepatoma cells. Apoptosis. 2007;12:113–123. doi: 10.1007/s10495-006-0487-9. [DOI] [PubMed] [Google Scholar]
- 41.Shi DY, Liu HL, Stern JS, Yu PZ, Liu SL. Alpha-lipoic acid induces apoptosis in hepatoma cells via the PTEN/Akt pathway. FEBS. Lett. 2008;582:1667–1671. doi: 10.1016/j.febslet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 42.Selvakumar E, Hsieh TC. Regulation of cell cycle transition and induction of apoptosis in HL-60 leukemia cells by lipoic acid: role in cancer prevention and therapy. J. Hematol. Oncol. 2008;1:4. doi: 10.1186/1756-8722-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamasaki M, Kawabe A, Nishimoto K, Madhyastha H, Sakakibara Y, Suiko M, Okamoto T, Suda T, Uehira K, Nishiyama K. Dihydro-alpha-lipoic acid has more potent cytotoxicity than alpha-lipoic acid. In. Vitro. Cell. Dev. Biol. Anim. 2009 Jan 1; doi: 10.1007/s11626-008-9164-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]