Abstract
Background
Amodiaquine (AQ) is paired with artesunate (AS) or sulfadoxine-pyrimethamine (SP) in recommended antimalarial regimens. It is unclear how readily AQ resistance will be selected with combination chemotherapy.
Methods
We collected 61 Plasmodium falciparum samples from a cohort of Ugandan children randomized to treatment with AQ/SP, AS/AQ, or artemether-lumefantrine (AL) for uncomplicated malaria. In vitro sensitivity to monodesethylamodiaquine (MDAQ) was measured with a histidine rich protein-2-based ELISA, and potential resistance-mediating polymorphisms pfmdr-1were evaluated.
Results
Parasites from subjects previously treated with AQ/SP or AS/AQ within 12 weeks were less sensitive to MDAQ (n=18; mean IC50 62.9 nM; range 12.7–158.3 nM) than parasites from those not treated within 12 weeks (n=43; mean IC50 37.5 nM; range 6.3–184.7 nM; p=0.0085) or only those in the treatment arm that did not contain AQ (n=20; mean IC50 28.8 nM; range 6.3–121.8 nM; p=0.0042). The proportion of strains with polymorphisms expected to mediate diminished response to AQ (pfmdr-1 86Y and 1246Y) increased after prior AQ therapy, although differences were not significant.
Conclusions
Prior therapy selected for diminished response to MDAQ, suggesting that AQ-containing regimens may rapidly lose efficacy in Africa. The mechanism of diminished MDAQ response is not fully explained by known mutations in pfmdr-1.
Keywords: Malaria, antimalarial, Plasmodium falciparum, amodiaquine, drug resistance, in vitro sensitivity
INTRODUCTION
The control of Plasmodium falciparum malaria is seriously challenged by resistance to many available drugs [1]. Among drugs with diminished antimalarial activity are the 4-aminoquinolines, notably chloroquine (CQ) and amodiaquine (AQ). However, despite very high levels of resistance to CQ in Africa, AQ often offers effective antimalarial treatment, and it remains an important part of our antimalarial drug portfolio [2]. With increasing resistance, there is now a clear consensus that uncomplicated falciparum malaria should be treated with drug combinations, ideally artemisinin-based combination therapy (ACT) [3]. ACT regimens all rely on an artemisinin component and a longer-acting partner drug [4]. AQ plays a key role in current treatment strategies. When partnered with artesunate (AS), AQ has shown excellent antimalarial efficacy in multiple studies in Africa, and this combination is now available as a co-formulated drug [5–9]. In addition, the older combination of AQ plus sulfadoxine-pyrimethamine (SP) has shown good efficacy in many areas [5, 10, 11], and is recommended by the World Health Organization to treat uncomplicated malaria when the efficacy of the individual components is acceptable and ACTs are not available [2].
With all ACTs there is a risk of selection of resistance to artemisinin partner drugs, which circulate long after elimination of the short-acting artemisinin component [12]. This is a particular concern for AQ, as resistance to this drug is already well established in parts of Africa [8, 13, 14]. AQ appears to act principally after conversion to an active metabolite, monodesethylamodiaquine (MDAQ) [15]; parasites are ~3-fold less sensitive in vitro to MDAQ than to the parent compound [16]. Parasites with a range of in vitro sensitivity to MDAQ have been described in Africa [17–21]. In general >90% of isolates from different sites in Africa were classified as sensitive to MDAQ (using the IC50 cut-off of 60 nM MDAQ), although a recent study from Kenya reported that 26% of baseline isolates had an IC50 for MDAQ >60 nM [21]. In South America and Asia, resistance to MDAQ or AQ has been more common [16, 22–25]. Relatively little information on P. falciparum sensitivity to MDAQ is available from East Africa, the location of our study, and an area where resistance to AQ is quite common.
The mechanism of P. falciparum resistance to AQ or MDAQ is incompletely understood; mutations in genes encoding two putative drug transporters, pfcrt and pfmdr1, appear to play important roles. The pfcrt 76T mutation is the principal mediator of resistance to CQ [26]. In areas where parasites with wild type pfcrt still circulate, the 76T mutation predicted poor response to therapy with AQ [27–29], and this mutation was selected in parasites that caused recurrent infections after treatment with AQ [11, 28–31]. The pfcrt 76T mutation was also associated with decreased in vitro sensitivity to MDAQ in field isolates [32] and in genetically altered laboratory strains [33]. Polymorphisms in a second putative transporter, pfmdr1, impact upon the sensitivity of malaria parasites to a number of drugs [34]. The pfmdr1 86Y polymorphism predicted poor AQ treatment outcome in most [28, 29], but not all [27] studies from Africa and was selected by prior therapy with AQ in a number of studies [11, 28–31, 35]. However, prevalence of the pfmdr1 86Y mutation was not associated with in vitro drug sensitivity in a study from Africa [32], and in samples from Colombia the pfmdr1 86Y mutation was seen in only ~10% of strains with in vitro resistance to MDAQ [22]. Taken together, recent studies suggest that the molecular basis of resistance to AQ is complex, with apparent contributions of polymorphisms in pfcrt and pfmdr1, and likely additional factors contributing to the resistant phenotype.
As we move to routine treatment of malaria with combination regimens, it is unclear how readily resistance to AQ will be selected by treatment with AQ-containing combinations. To gain insight in this area, we evaluated the in vitro activity of MDAQ against 61 clinical samples from Ugandan children treated with one of three combination regimens, two of which included AQ. We then searched for associations between in vitro drug sensitivity, clinical outcomes, prior drug use, and known molecular mediators of resistance. Most notably, we found that prior use of AQ-containing combinations selected for subsequent infections with diminished responsiveness to MDAQ.
METHODS
Clinical trial
All samples were from a cohort of 601 children randomly selected from a community in Kampala, Uganda, aged 1–10 years at enrollment in 2004–05, as previously described [9, 36]. Upon presentation with the first episode of uncomplicated malaria, participants were randomly assigned to receive AQ/SP, AS/AQ, or AL, which they received thereafter for each episode of uncomplicated malaria. With each treatment, efficacy was assessed following WHO guidelines [37], with genotyping to distinguish recrudescence and new infection after therapy, all as previously described [9]. For this study we analyzed a subset of samples, collected between August, 2006 and March, 2007 for which parasites were successfully grown in short-term culture to allow determination of in vitro drug sensitivity. The clinical trial was approved by the Uganda National Council of Science and Technology and the Institutional Review Boards of Makerere University and the University of California, San Francisco.
In vitro sensitivity to MDAQ
Upon diagnosis of uncomplicated malaria, and before the initiation of therapy, blood was collected into heparinized tubes and delivered within 30 minutes to our laboratory. Specimens were centrifuged, supernatant and buffy coat were removed, erythrocytes were washed twice in RPMI 1640 medium pre-warmed to 37°C, samples were diluted to 0.05% parasitemia and 2% hematocrit, and parasites were assessed for drug sensitivity under sterile conditions in RPMI 1640 supplemented with 0.5% Albumax and 100 µg/ml gentamicin in 96-well cell culture plates that had been coated with 7 serial dilutions of MDAQ (6.25–400 nM, each in duplicate) and dried overnight. Wells without drug served as controls. For each sample, 200 µl of culture was added to each well, with duplicate wells for each concentration of MDAQ. Plates were incubated for 72 hours at 37°C in a candle jar, and samples were then frozen (−20°C overnight) and thawed before analysis. Parasite growth was assayed by comparing levels of histidine rich protein-2 (HRP-2) in treated and control cultures [38]. HRP-2 was quantified using a commercial ELISA test kit (Malaria Ag Celisa, Cellabs). Samples were diluted (1:4–1:10; the same dilution for each sample in an experiment) in water, and 100 µl of each hemolyzed sample preparation was added to an ELISA plate pre-coated with anti-HRP-2 and incubated at room temperature for 1 h. Plates were then washed four times with the kit washing solution, 100 µl of secondary antibody was added to each well for1 h at room temperature, plates were again washed 4 times, 100 µl of chromogen substrate was added to each well, plates were incubated for 15 min in the dark, and 50 µl of stopping solution was added. Absorbance (450 nm) was then measured for each well with a SpectraMAX 340 spectrophotometer (Molecular Devices). Optical density values were fitted to normal curves based on serial dilutions of HRP-2 standards and a four-parameter curve model (Softmax Pro 2.1.1, Molecular Devices), and IC50s were calculated based on a nonlinear regression model, with attention to test validity based on adequate readings above background, as previously described [39].
Genetic polymorphisms in pfmdr-1
Upon diagnosis of uncomplicated malaria blood spots were also dried on filter paper, which was stored with dessicant in sealed plastic bags at room temperature. DNA was subsequently extracted with chelex and previously characterized polymorphisms in pfcrt and pfmdr-1 were analyzed by nested PCR followed by mutation-specific restriction endonuclease (ApoI, AflIII, DraI, DdeI, AseI and EcoRV) cleavage, as previously described for the pfcrt K76T and pfmdr-1 N86Y, Y184F, S1034C, N1042D, and D1246Y polymorphisms, respectively [40]. DNA fragments were separated by agarose gel electrophoresis, and electrophoretic band patterns were categorized as wild type, mixed, or mutant genotypes by visual inspection of gels and comparison with DNA from control strains 7G8 and FCR3, obtained from the Malaria Research and Reference Reagent Resource Center (MR4).
Statistical analyses
Clinical data were entered and verified with Access (Microsoft Corporation). Statistical analyses were performed with Stata, version 10 (StataCorp). Clinical outcomes were assessed as previously described [9]. Values for in vitro drug sensitivity of parasite samples were not normally distributed, and were assessed using the Kruskal-Wallis rank test. Prevalences of mutations were compared with Fisher’s exact test. For all assessments, p values <0.05 were considered statistically significant.
RESULTS
In vitro drug sensitivity of fresh P. falciparum samples
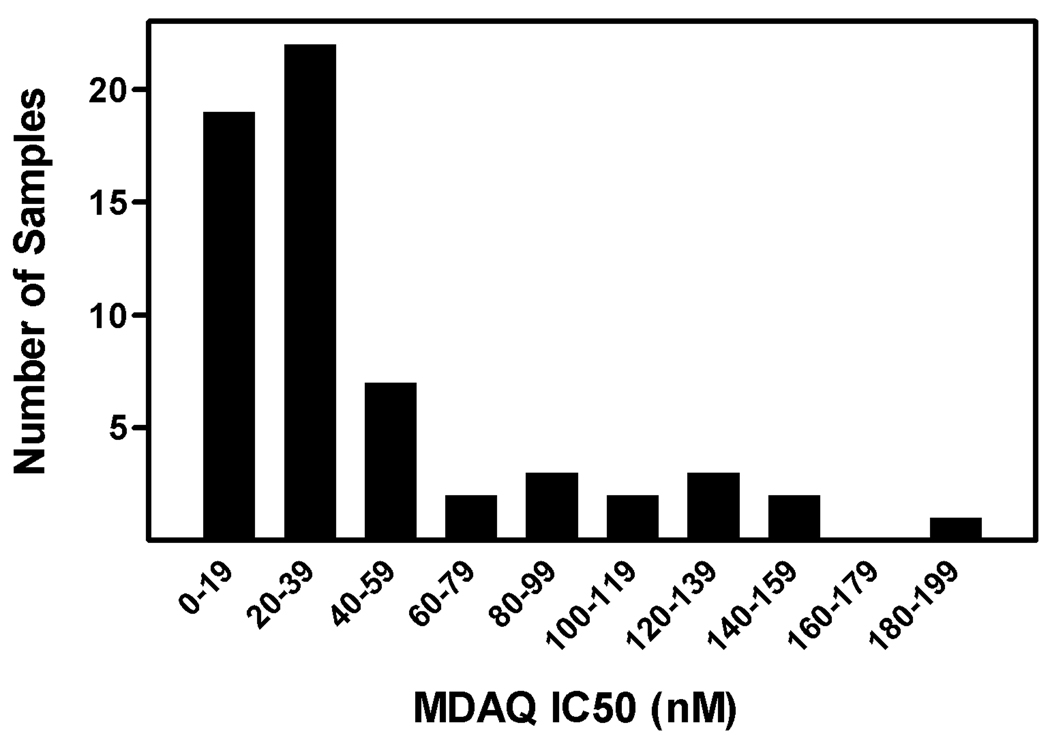
Blood was collected from children presenting with uncomplicated falciparum malaria, and the in vitro sensitivity of infecting parasites to MDAQ, the principal active metabolite of AQ, was determined. Of 72 samples cultured during the time frame of the study, 61 had successful in vitro analyses, with a wide range of in vitro drug sensitivity (figure 1, supplemental table). Samples that could not be evaluated included 1 that was contaminated with bacteria, 4 that did not grow in culture, and 6 that failed to provide an adequate dose response curve for analysis. Using a previously assigned cut-off for resistance of IC50 >60 nM [17], 13 (21.3%) of the parasite samples were categorized as resistant to MDAQ, and 8 (13.1%) had IC50 >100 nM. Control parasite lines (reference numbers from MR4 are provided) yielded IC50 results of 30.9 nM for D6 (MRA-285), 29.8 nM for HB3 (MRA-155), 267.7 nM for W2 (MRA-157), and 262.1 nM for K1 (MRA-159); the first two strains are considered sensitive, and the last two resistant to CQ.
Figure 1. Sensitivity of clinical samples to MDAQ.
Parasites were placed into culture and sensitivities were assessed by comparing levels of HRP-2 with those of untreated controls with an ELISA assay.
Association of in vitro drug sensitivity and clinical treatment failure
The three combination regimens included in our clinical trial were all quite efficacious, and so few recrudescences occurred after therapy [9]. Only 7 of the 61 samples (11.5%) were from episodes of malaria classified as recrudescent. For these samples, and for both the AQ-containing treatment arms and the AL arm, recrudescence was equally likely in those with parasites sensitive and resistant to MDAQ (table 1). Thus, although sample size was small for this analysis, we saw no clear association between MDAQ sensitivity and treatment failure.
Table 1.
Clinical outcomes stratified by in vitro MDAQ sensitivity.
| Study regimen |
MDAQ sensitivity (IC50, nM) |
Number with indicated sensitivity |
Number with recrudescent outcomes (%) |
|---|---|---|---|
| AQ/SP | < 60 | 10 | 2 (20) |
| > 60 | 5 | 1 (20) | |
| AQ/AS | < 60 | 18 | 1 (5.6) |
| > 60 | 6 | 0 | |
| AL | < 60 | 20 | 2 (10) |
| > 60 | 2 | 1 (50) |
Parasites with diminished sensitivity to MDAQ were selected by prior therapy with AQ
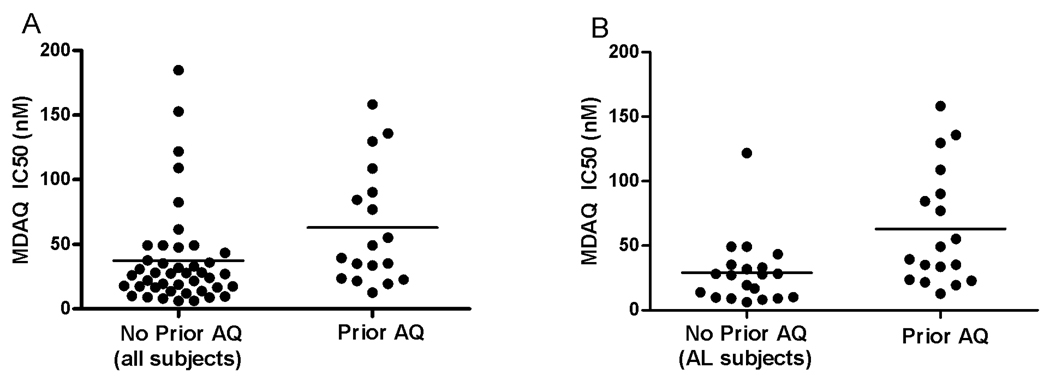
To assess the impact on drug sensitivity of recent prior therapy, we compared the MDAQ sensitivity of parasites from subjects that were previously treated with AQ/SP or AS/AQ within 12 weeks with those from patients not treated with these drugs within this interval and with only those from patients in the AL treatment arm, and so not treated with AQ during the course of the study. Parasites from subjects who were previously treated with AQ/SP or AS/AQ within 12 weeks were less sensitive to AQ (n=18; mean IC50 62.9 nM; range 12.7–158.3 nM) compared to parasites from those not treated with an AQ-containing regimen within 12 weeks (n=43; mean IC50 37.5 nM; p=0.0085; range 6.3–184.7 nM) or compared to parasites from those in the treatment arm that did not contain AQ (AL subjects; n=20; mean IC50 28.8 nM; p=0.0042; range 6.3–121.8 nM) (figure 2). Similar associations were seen when drug sensitivities were compared only for parasites causing new infections (not shown) and for infections occurring within 6, 8, or 10 weeks of prior therapy, although with intervals ≤6 weeks differences in drug sensitivities were not statistically significant (table 2).
Figure 2. Selection of parasites with diminished response to AQ.
Sensitivities were determined as described in Methods, and results are plotted for parasites from all patients that did not receive an AQ-containing regimen within 12 weeks (No Prior AQ; A) or only patients in the AL treatment arm, who did not receive an AQ-containing regimen for the course of the study (No Prior AQ; B), in both cases compared to results for subjects who did receive an AQ-containing regimen within 12 weeks prior to the time of this analysis (Prior AQ). Means are indicated by horizontal lines. Differences for both comparisons were significant, as described in the text.
Table 2.
MDAQ sensitivity of parasites causing infections soon after prior infections
| Time since prior treatment with AQ |
Average MDAQ IC50, nM | n | p value1 |
|---|---|---|---|
| 6 weeks | 46.4 | 11 | 0.076 |
| 8 weeks | 54.5 | 14 | 0.019 |
| 10 weeks | 57.3 | 17 | 0.0073 |
| 12 weeks | 62.9 | 18 | 0.0042 |
Mean IC50 values were compared with those for samples from patients in the AL treatment arm (n=20; mean IC50 28.8 nM) by the Kruskal-Wallis test.
Association of parasite genetic polymorphisms with prior therapy with AQ
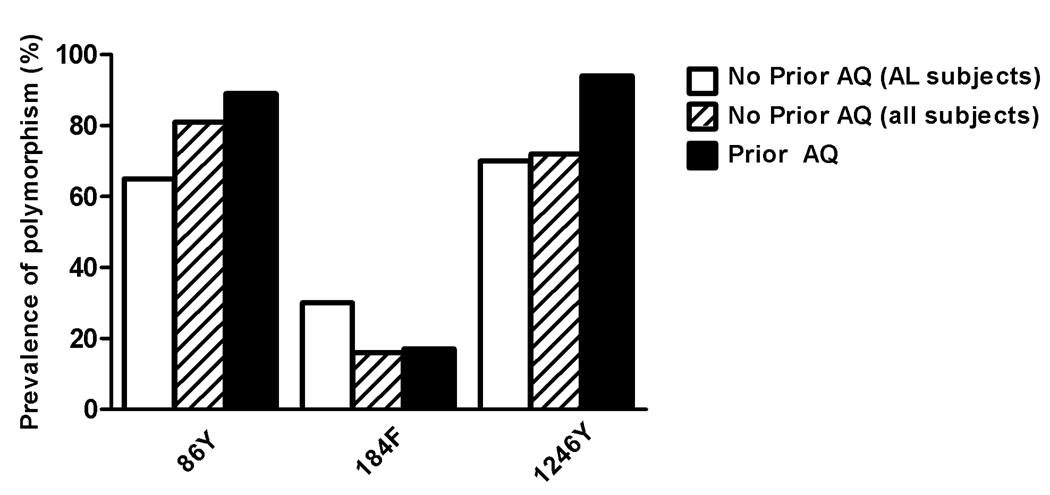
We also characterized genetic polymorphisms that have previously been identified in the pfcrt and pfmdr1 genes. Consistent with other results from Kampala [41], all 61 samples that provided in vitro sensitivity results had only the mutant pfcrt allele (76T) that has previously been associated with CQ resistance [26]. As seen previously [11, 28–31, 35], prior treatment with AQ (within 12 weeks) was associated with two pfmdr1 polymorphisms, 86Y and 1246Y, that have been associated with decreased responsiveness to this drug, although baseline prevalences of these polymorphisms were high, and differences were not statistically significant (figure 3). Another polymorphism, 184F, was not selected by prior therapy with AQ, and two other mutations, 1034C and 1042D, were not seen in any samples. Of note, the lower prevalence of pfmdr1 86Y in subjects from the AL treatment group was probably due, in part, to selection of the wild type sequence by AL, as has been shown previously.
Figure 3. Selection of pfmdr1 alleles.
The proportions of samples with mutations at the alleles indicated are shown for the two comparisons described in figure 2.
Association of parasite genetic polymorphisms and MDAQ sensitivity
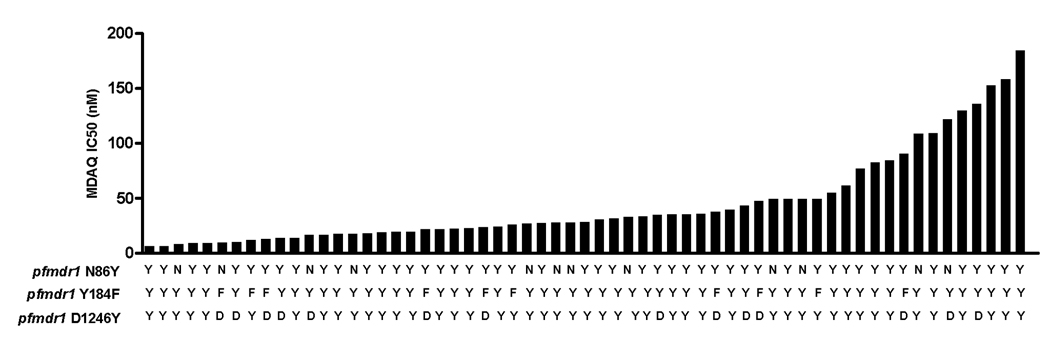
We also attempted to identify associations between pfmdr1 polymorphisms and in vitro sensitivity to MDAQ. Straightforward correlations were not seen (figure 4). The prevalence of the mutant allele was slightly higher in parasites categorized as resistant for N86Y (prevalence 86Y 38/48 (79.2%) in sensitive vs. 11/13 (84.6%) in resistant), prevalences were the same for 1246Y (prevalence 1246Y 37/48 (77.1%) in sensitive vs. 10/13 (76.9%) in resistant), and the prevalence of the mutant allele was lower in resistant parasites for Y184F (prevalence 184F 9/48 (18.8%) in sensitive vs. 1/13 (7.7%) in resistant). No differences in allele prevalence were statistically significant.
Figure 4. Correlation of in vitro sensitivity to MDAQ with pfmdr1 genotypes.
For each sample studied, the sequence at the three alleles of interest and the in vitro sensitivity to MDAQ are shown.
DISCUSSION
ACT is now the international standard for the treatment of P. falciparum malaria, as multiple new combination regimens offer excellent antimalarial efficacy [4]. However, there is concern that, since all ACTs include a short-acting artemisinin and long-acting partner drug, the regimens will select for resistance to partner drugs, especially in areas where reinfection after treatment is common. This concern is arguably greatest for AQ-containing combinations, as resistance to this drug is already fairly common. AQ is rapidly metabolized to MDAQ [15], an active metabolite which has a half life of about 2 weeks [12]. Thus, after therapy with a regimen containing AQ, MDAQ circulates at decreasing levels for many weeks. We hypothesized that children treated with AQ-containing regimens would be at increased risk of AQ-resistance in subsequent infections. To test this hypothesis, we studied sensitivity to MDAQ in P. falciparum parasites causing uncomplicated malaria in a cohort of children in Kampala who received AQ/SP, AS/AQ, or AL for each episode of uncomplicated malaria. Kampala is known to have a fairly high prevalence of AQ-resistant malaria [42], and parasites that caused malaria in our cohort demonstrated a wide range of sensitivities to MDAQ. Importantly, parasites that caused infections within 12 weeks of a prior treatment with AQ had decreased sensitivity to MDAQ compared to those that caused infections in individuals not recently treated with AQ. Thus, as we hypothesized, prior treatment with AQ selected for parasites with diminished drug sensitivity. This result suggests that resistance to AQ may quite rapidly be selected by treatment of malaria with combinations including AQ.
We identified a broad range of sensitivities to MDAQ in parasites causing uncomplicated malaria in our cohort of Ugandan children. Resistant parasites (based on a cut-off of MDAQ IC50 >60 nM) were seen in 21% of infections, consistent with prior clinical trials in Kampala showing frequent treatment failures with AQ monotherapy [42]. These results and recent similar findings from Kenya (26% of isolates had MDAQ IC50 >60 nM) [21] suggest a more extensive problem with AQ resistance in East Africa than in some other regions of Africa, where >90% of parasites were sensitive to MDAQ in vitro in recent studies in Madagascar [43], Ghana [44], Cameroon [17], Congo [20], Senegal [18], Rwanda [45] and in a collection of isolates from different countries [19]. Older studies that considered sensitivity to AQ, rather than MDAQ, also generally reported high levels of sensitivity, although 16% of samples were reported to demonstrate in vitro resistance in a study from the Central African Republic [46]. In our study, MDAQ-resistant parasites caused infections in those with and without recent prior therapy with AQ; selective pressure from circulating AQ was not required for infection with a resistant strain. However, prior therapy with AQ led to an increased predilection for infection with MDAQ-resistant parasites.
It was also of interest to determine if the in vitro sensitivity of parasites to MDAQ was associated with clinical outcomes. However, of the 61 parasite samples studied, only 7 represented recrudescent infections as determined by multi-locus genotyping, and only 4 of these were in subjects in an AQ-containing treatment arm. Therefore we lacked power to assess associations between in vitro sensitivity to MDAQ and treatment outcomes. Nonetheless, recrudescences occurred after infection with parasites sensitive and resistant to MDAQ, arguing against any simple association between in vitro drug sensitivity and clinical response. Similarly, in studies in Gabon [47] and Kenya [21] in vitro sensitivity to MDAQ did not correlate with clinical response to therapy with AQ. Indeed, it is likely that clinical responses to AQ-containing combination regimens are complex, with effects of varied pharmacokinetics [15], pharmacogenomics [48], host immunity, and other factors in addition to the drug responsiveness of parasites. Nonetheless, parasite drug sensitivity is clearly an important component of a successful treatment response. A better appreciation of mediators of parasite resistance to AQ will be of value in optimizing utilization of available drug regimens.
We also searched for associations between in vitro drug sensitivity and polymorphisms that have previously been linked to altered sensitivity to AQ. The 76T polymorphism in the putative drug transporter pfcrt, which is the primary mediator of resistance to chloroquine, also predicts poor response to AQ [27–29] and is selected in new infections following therapy with AQ [11, 28–31]. The pfcrt 76T mutation was also associated with decreased in vitro sensitivity to MDAQ in field isolates from the Central African Republic [32] and in genetically altered laboratory strains [33]. Considering our results, it is noteworthy that, even with 100% prevalence of 76T in our set of parasites, 79% of samples demonstrated sensitive in vitro responses to MDAQ. Thus, the common pfcrt polymorphism, although predictive of decreased response to MDAQ, does not by itself dictate MDAQ resistance. Polymorphisms in a second putative drug transporter, pfmdr1, have not been as clearly linked to AQ treatment outcome, but in some studies from Africa the pfmdr1 86Y polymorphism predicted poor AQ treatment outcomes [28, 29]. Also, as seen in this study, pfmdr1 86Y was selected by prior therapy with AQ in a number of studies [11, 28–31, 35]. However, prevalence of the pfmdr1 86Y mutation was not associated with in vitro drug sensitivity in samples from the Central African Republic [32], from Colombia [22], or in our study from Uganda. Taken together, our results are consistent with those from other areas, suggesting contributions of polymorphisms in both pfcrt and pfmdr1 to AQ resistance, but likely involvement of additional host (e.g. genetics, pharmacokinetics, immunity) and parasite (e.g. additional polymorphisms) factors in high level AQ resistance.
Our study had some limitations. Our evaluations of in vitro sensitivity of clinical samples were necessarily limited to parasites capable of growing in short term culture to allow measurement of in vitro drug sensitivity. Exclusion of parasites that did not grow in culture may have introduced bias. Infections in Kampala are commonly polyclonal, and both complexity of infection [49] and key polymorphisms in pfmdr1 [50] have been seen to change after in vitro culture. Most of our in vitro sensitivity measurements were thus based on assessment of a mixed population of parasites in which competition between sensitive and resistant strains might obscure results for parasites capable of mediating resistant outcomes. Comparisons with results from other studies must take into account the fact that a number of different assays have been used to measure in vitro drug sensitivity; comparisons of values between studies may be misleading. In vitro measurements leave potential for error, as, since they are measured only during the first life cycle after parasite collection, they cannot be repeated. Thus, for any single measurement, there is the possibility that human error or other factors led to misrepresentation of the true results. Despite these limitations, considering our large set of evaluated samples, our results strongly suggest that prior therapy with AQ selects for decreased sensitivity to the drug.
Our results add concern regarding the long-term prospects for AQ-containing combination therapies for the treatment of P. falciparum malaria. Selection of resistant parasites was readily apparent after prior therapy with AQ. These results suggest diminishing efficacy of AQ-containing combination regimens as they are increasingly used in Africa. The results further highlight the need for continued scrutiny of the efficacies of current antimalarial regimens, as efficacies will likely diminish over time, and for continued efforts to develop new antimalarial treatments that circumvent drug resistance.
Supplementary Material
Acknowledgments
We thank the participants in the clinical trial from which samples were collected, their parents and guardians, and our clinical study team. We thank Harald Noedl, Medical University of Vienna, for valuable advice regarding drug sensitivity assays and Grant Dorsey and Bryan Greenhouse, University of California, San Francisco, for expert assistance with statistical analyses. Control P. falciparum strains were from the Malaria Research and Reference Reagent Resource Center.
Financial support
This research was supported by grants from the Fogarty International Center (TW01506) and National Institute of Allergy and Infectious Diseases (AI52142), National Institutes of Health, and from the Doris Duke Charitable Foundation, for which PJR is a Distinguished Clinical Scientist.
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
This work was presented in part at the 57th annual meeting of the American Society of Tropical Medicine and Hygiene, New Orleans, LA, December, 2008; Abstract #598.
References
- 1.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva: World Health Organization; Guidelines for the Treatment of Malaria. 2006
- 3.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 4.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77:181–192. [PubMed] [Google Scholar]
- 5.Staedke SG, Mpimbaza A, Kamya MR, Nzarubara BK, Dorsey G, Rosenthal PJ. Combination treatments for uncomplicated falciparum malaria in Kampala, Uganda: randomised clinical trial. Lancet. 2004;364:1950–1957. doi: 10.1016/S0140-6736(04)17478-3. [DOI] [PubMed] [Google Scholar]
- 6.Yeka A, Banek K, Bakyaita N, et al. Artemisinin versus nonartemisinin combination therapy for uncomplicated malaria: randomized clinical trials from four sites in Uganda. PLoS Med. 2005;2:e190. doi: 10.1371/journal.pmed.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martensson A, Stromberg J, Sisowath C, et al. Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis. 2005;41:1079–1086. doi: 10.1086/444460. [DOI] [PubMed] [Google Scholar]
- 8.Mutabingwa TK, Anthony D, Heller A, et al. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet. 2005;365:1474–1480. doi: 10.1016/S0140-6736(05)66417-3. [DOI] [PubMed] [Google Scholar]
- 9.Dorsey G, Staedke S, Clark TD, et al. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA. 2007;297:2210–2219. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 10.Mockenhaupt FP, Ehrhardt S, Dzisi SY, et al. A randomized, placebo-controlled, double-blind trial on sulfadoxine-pyrimethamine alone or combined with artesunate or amodiaquine in uncomplicated malaria. Trop Med Int Health. 2005;10:512–520. doi: 10.1111/j.1365-3156.2005.01427.x. [DOI] [PubMed] [Google Scholar]
- 11.Zongo I, Dorsey G, Rouamba N, et al. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet. 2007;369:491–498. doi: 10.1016/S0140-6736(07)60236-0. [DOI] [PubMed] [Google Scholar]
- 12.Stepniewska K, White NJ. Pharmacokinetic determinants of the window of selection for antimalarial drug resistance. Antimicrob Agents Chemother. 2008;52:1589–1596. doi: 10.1128/AAC.00903-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zongo I, Dorsey G, Rouamba N, et al. Amodiaquine, sulfadoxine-pyrimethamine, and combination therapy for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. Am J Trop Med Hyg. 2005;73:826–832. [PubMed] [Google Scholar]
- 14.Nsimba B, Guiyedi V, Mabika-Mamfoumbi M, et al. Sulphadoxine/pyrimethamine versus amodiaquine for treating uncomplicated childhood malaria in Gabon: a randomized trial to guide national policy. Malar J. 2008;7:31. doi: 10.1186/1475-2875-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishna S, White NJ. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clin Pharmacokinet. 1996;30:263–299. doi: 10.2165/00003088-199630040-00002. [DOI] [PubMed] [Google Scholar]
- 16.Childs GE, Boudreau EF, Milhous WK, et al. A comparison of the in vitro activities of amodiaquine and desethylamodiaquine against isolates of Plasmodium falciparum. Am J Trop Med Hyg. 1989;40:7–11. doi: 10.4269/ajtmh.1989.40.7. [DOI] [PubMed] [Google Scholar]
- 17.Basco LK, Ringwald P. Molecular epidemiology of malaria in Cameroon. XXIV. Trends of in vitro antimalarial drug responses in Yaounde, Cameroon. Am J Trop Med Hyg. 2007;76:20–26. [PubMed] [Google Scholar]
- 18.Agnamey P, Brasseur P, de Pecoulas PE, Vaillant M, Olliaro P. Plasmodium falciparum in vitro susceptibility to antimalarial drugs in Casamance (southwestern Senegal) during the first 5 years of routine use of artesunate-amodiaquine. Antimicrob Agents Chemother. 2006;50:1531–1534. doi: 10.1128/AAC.50.4.1531-1534.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaddouri H, Nakache S, Houze S, Mentre F, Le Bras J. Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from africa by using a Plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob Agents Chemother. 2006;50:3343–3349. doi: 10.1128/AAC.00367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pradines B, Hovette P, Fusai T, et al. Prevalence of in vitro resistance to eleven standard or new antimalarial drugs among Plasmodium falciparum isolates from Pointe-Noire, Republic of the Congo. J Clin Microbiol. 2006;44:2404–2408. doi: 10.1128/JCM.00623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasi P, Abdulrahaman A, Mwai L, et al. In vivo and In vitro efficacy of amodiaquine against Plasmodium falciparum in an area of continued use of 4-aminoquinolines in East Africa. J Infect Dis. 2009;199:1575–1582. doi: 10.1086/598862. [DOI] [PubMed] [Google Scholar]
- 22.Echeverry DF, Holmgren G, Murillo C, et al. Short report: polymorphisms in the pfcrt and pfmdr1 genes of Plasmodium falciparum and in vitro susceptibility to amodiaquine and desethylamodiaquine. Am J Trop Med Hyg. 2007;77:1034–1038. [PubMed] [Google Scholar]
- 23.Long GW, Watt G, Sy N, Buck RL, Sangalang RP, Jr, Ranoa CP. In vitro drug response of Plasmodium falciparum in the Philippines: increased resistance to amodiaquine. Southeast Asian J Trop Med Public Health. 1987;18:202–206. [PubMed] [Google Scholar]
- 24.Restrepo-Pineda E, Arango E, Maestre A, Do Rosario VE, Cravo P. Studies on antimalarial drug susceptibility in Colombia, in relation to Pfmdr1 and Pfcrt. Parasitology. 2008;135:547–553. doi: 10.1017/S0031182008004307. [DOI] [PubMed] [Google Scholar]
- 25.Yang HL, Liu DQ, Yang YM, et al. In vitro sensitivity of Plasmodium falciparum to eight antimalarials in China-Myanmar and China-Lao PDR border areas. Southeast Asian J Trop Med Public Health. 1997;28:460–464. [PubMed] [Google Scholar]
- 26.Fidock DA, Nomura T, Talley AK, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochong EO, van den Broek IV, Keus K, Nzila A. Short report: association between chloroquine and amodiaquine resistance and allelic variation in the Plasmodium falciparum multiple drug resistance 1 gene and the chloroquine resistance transporter gene in isolates from the upper Nile in southern Sudan. Am J Trop Med Hyg. 2003;69:184–187. [PubMed] [Google Scholar]
- 28.Dokomajilar C, Lankoande ZM, Dorsey G, Zongo I, Ouedraogo JB, Rosenthal PJ. Roles of specific Plasmodium falciparum mutations in resistance to amodiaquine and sulfadoxine-pyrimethamine in Burkina Faso. Am J Trop Med Hyg. 2006;75:162–165. [PubMed] [Google Scholar]
- 29.Happi CT, Gbotosho GO, Folarin OA, et al. Association between mutations in Plasmodium falciparum chloroquine resistance transporter and P. falciparum multidrug resistance 1 genes and in vivo amodiaquine resistance in P. falciparum malaria-infected children in Nigeria. Am J Trop Med Hyg. 2006;75:155–161. [PubMed] [Google Scholar]
- 30.Holmgren G, Gil JP, Ferreira PM, Veiga MI, Obonyo CO, Bjorkman A. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infect Genet Evol. 2006;6:309–314. doi: 10.1016/j.meegid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Djimde AA, Fofana B, Sagara I, et al. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am J Trop Med Hyg. 2008;78:455–461. [PubMed] [Google Scholar]
- 32.Menard D, Yapou F, Manirakiza A, Djalle D, Matsika-Claquin MD, Talarmin A. Polymorphisms in pfcrt, pfmdr1, dhfr genes and in vitro responses to antimalarials in Plasmodium falciparum isolates from Bangui, Central African Republic. Am J Trop Med Hyg. 2006;75:381–387. [PubMed] [Google Scholar]
- 33.Sidhu AB, Verdier-Pinard D, Fidock DA. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298:210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 35.Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob Agents Chemother. 2007;51:3023–3025. doi: 10.1128/AAC.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis JC, Clark TD, Kemble SK, et al. Longitudinal study of urban malaria in a cohort of Ugandan children: description of study site, census and recruitment. Malar J. 2006;5:18. doi: 10.1186/1475-2875-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World HO. Geneva: World Health Organization; Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. 2003
- 38.Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg. 2006;75:1205–1208. [PubMed] [Google Scholar]
- 39.Basco L. Geneva: World Health Organization; Field application of in vitro assays for the sensitivity of human malaria parasites to antimalarial drugs. 2007
- 40.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol. 2000;108:13–23. doi: 10.1016/s0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 41.Dorsey G, Kamya MR, Singh A, Rosenthal PJ. Polymorphisms in the Plasmodium falciparum pfcrt and pfmdr-1 genes and clinical response to chloroquine in Kampala, Uganda. J Infect Dis. 2001;183:1417–1420. doi: 10.1086/319865. [DOI] [PubMed] [Google Scholar]
- 42.Staedke SG, Kamya MR, Dorsey G, et al. Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: a randomised trial. Lancet. 2001;358:368–374. doi: 10.1016/S0140-6736(01)05557-X. [DOI] [PubMed] [Google Scholar]
- 43.Rason MA, Andrianantenaina HB, Ariey F, Raveloson A, Domarle O, Randrianarivelojosia M. Prevalent pfmdr1 n86y mutant Plasmodium falciparum in Madagascar despite absence of pfcrt mutant strains. Am J Trop Med Hyg. 2007;76:1079–1083. [PubMed] [Google Scholar]
- 44.Quashie NB, Duah NO, Abuaku B, Koram KA. The in-vitro susceptibilities of Ghanaian Plasmodium falciparum to antimalarial drugs. Ann Trop Med Parasitol. 2007;101:391–398. doi: 10.1179/136485907X176553. [DOI] [PubMed] [Google Scholar]
- 45.Tinto H, Rwagacondo C, Karema C, et al. In-vitro susceptibility of Plasmodium falciparum to monodesethylamodiaquine, dihydroartemisinin and quinine in an area of high chloroquine resistance in Rwanda. Trans R Soc Trop Med Hyg. 2006;100:509–514. doi: 10.1016/j.trstmh.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Menard D, Djalle D, Manirakiza A, et al. Drug-resistant malaria in Bangui, Central African Republic: an in vitro assessment. Am J Trop Med Hyg. 2005;73:239–243. [PubMed] [Google Scholar]
- 47.Aubouy A, Mayombo J, Keundjian A, Bakary M, Le Bras J, Deloron P. Short report: lack of prediction of amodiaquine efficacy in treating Plasmodium falciparum malaria by in vitro tests. Am J Trop Med Hyg. 2004;71:294–296. [PubMed] [Google Scholar]
- 48.Parikh S, Ouedraogo JB, Goldstein JA, Rosenthal PJ, Kroetz DL. Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin Pharmacol Ther. 2007;82:197–203. doi: 10.1038/sj.clpt.6100122. [DOI] [PubMed] [Google Scholar]
- 49.Nsobya SL, Kiggundu M, Joloba M, Dorsey G, Rosenthal PJ. Complexity of Plasmodium falciparum clinical samples from Uganda during short-term culture. J Infect Dis. 2008;198:1554–1557. doi: 10.1086/592506. [DOI] [PubMed] [Google Scholar]
- 50.Purfield A, Nelson A, Laoboonchai A, et al. A new method for detection of pfmdr1 mutations in Plasmodium falciparum DNA using real-time PCR. Malar J. 2004;3:9. doi: 10.1186/1475-2875-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.