Abstract
The ethyl acetate extract of the aerial parts of Ajuga turkestanica afforded 6 neo-clerodane diterpenes, including two novel compounds, 14, 15-dihydroajugachin B (1) and 14-hydro-15-methoxyajugachin B (2), in addition to the known diterpenoids chamaepitin (3), ajugachin B (4), ajugapitin (5) and lupulin A (6). Structures were established through exhaustive NMR spectroscopic analysis and chemical transformation in the case of 1. The full 1H and 13C NMR assignment of the C-15 R and S configurations of 14-hydro-15-methoxyajugachin B and chamaepitin were elucidated.
Keyword index: Ajuga turkestanica; Labiatae; neo-clerodane diterpenes; 14, 15-dihydroajugachin B; 14-hydro-15-methoxyajugachin B
1. Introduction
The genus Ajuga (Labiatae), comprised of more than 40 species widely distributed in temperate regions of both hemispheres (Hedge, 1992), has been a focus of interest due to significant medicinal and economic properties. Ajuga contains at least three classes of potentially bioactive compounds: clerodane diterpenes, phytoecdysteroids and iridoid glycosides. Clerodane diterpenes are known for their activity as insect alleochemicals (Camps and Coll, 1993; Klein Gebbinck et al., 2002), and are also recognized sources of antimicrobial, antiviral, antitumor, antibiotic and amoebicidal activities (Coll and Tandron, 2007). In addition, many Ajuga species are well known among phytoecdysteroid-producing plants because of the great variety of such compounds they contain. Phytoecdysteroids exhibit significant physiological activities in insects and also in mammals, which might correlate with the successful applications in folk medicine of the plants that produce them. Both the clerodane diterpenoids and the phytoecdysteroids with potential insect antifeedant and moulting hormone activities, respectively, may work interactively to potentiate the bioactivity of the Ajuga plants (Camps and Coll, 1993). Also, Ajuga is known as a source of iridoid glycosides, which have demonstrated cancer chemopreventive activity (Konoshima et al., 2000).
Ajuga turkestanica (Regel) Briq., a perennial herb growing mainly in Central Asia, is known as a rich source of bioactive substances and is used by local people to treat heart diseases, muscle and stomach aches (Mamatkhanov et al., 1998; Abdukadirov et al., 2004). Several phytoecdysteroids (turkesterone, 20-hydroxyecdysone, cyasterone, cyasterone 22-acetate, ajugalactone, ajugasterone B, α-ecdysone and ecdysone 2, 3-monoacetonide) have been reported in this species along with the iridoids harpagide and harpagide 8-acetate (Usmanov et al., 1971, 1973, 1975, 1978; Baltaev, 2000; Ramazanov, 2005).
In this paper, we report the isolation and structural elucidation of six neo-clerodane diterpenes, two of which are novel compounds. This is the first study to identify neo-clerodane diterpenes in A. turkestanica.
2. Results and discussion
The ethyl acetate extract from the aerial parts of Ajuga turkestanica yielded two new neo-clerodane diterpenoids: 14, 15-dihydroajugachin B (1) and 14-hydro-15-methoxyajugachin B (2), together with the known diterpenes: chamaepitin (14-hydro-15-hydroxyajugachin B) (3) (Camps et al., 1987), ajugachin B (4) (Boneva et al., 1990), ajugapitin (5) (Hernandez et al., 1982), and lupulin A (Hao et al., 1996). 1H and 13C NMR spectra of compounds 3 and 5 were in agreement with the published data for chamaepitin and lupulin. Previous reports did not provide the full set of assignments for the compounds with R and S configuration at C-15, however we report in this manuscript the full NMR assignments, and the characteristic NMR signals, for chamaepitin and lupulin A, respectively, with R and S configuration at C-15.
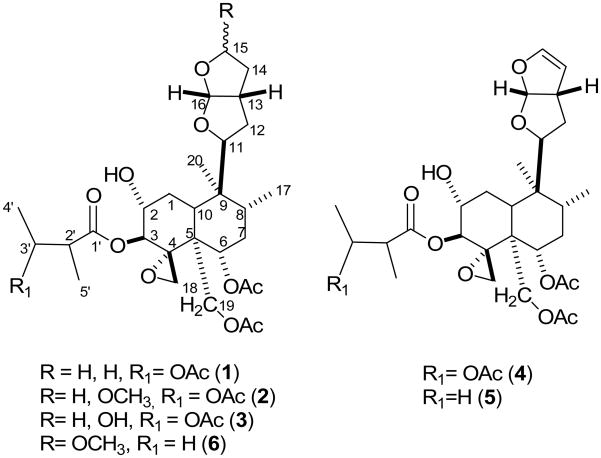
Compound 1 was obtained as an optically active white amorphous substance. The IR spectrum showed absorption bands for hydroxyl (3457 cm−1) and ester groups (1728 and 1245 cm−1). HR-ESI-MS of 1 showed a molecular ion peak [M+H]+ at m/z 611.3063, corresponding to the molecular formula C31H47O12. The 1H- and 13C NMR spectra (Tables 1 & 2) indicated clearly that the compound is a neo-clerodane diterpene and, furthermore, closely related to the known compounds 3 and 4, suggesting a neo-clerodane diterpene with the same 3′ acetoxy-2′-methylbutyryl functionality at C-3. This was clear from the downfield signal for H-3′ (δ 5.14, m) and the two methyl doublets at δ 1.20 and 1.11 (d, J = 7.1Hz, 4′ and 5′ CH3, respectively). The presence of this functionality at C-3 was confirmed by the downfield signal due to a proton on a carbon atom bearing an oxygen at δ 5.16 (1H, d, J = 9.5 Hz, H-3). The presence of three acetate groups was evident from the 1H NMR spectra (δ 2.10, 2.01 and 1.91) and 13C NMR spectra (δc 170.3, 171.3 and 171.4). Two of these acetate groups were attached at positions 6 and 18 and the third one was a substituent at position 3′ of the 2-methylbutyryloxy moiety. Likewise, the presence of an unsubstituted hexahydrofuran ring system was confirmed by the signals at δ 5.66 (1H, d, J = 5.1 Hz, H-16 β), 4.09 (1H, dd, J = 11.4, 5.2 Hz, H-11α), 3.85 (2H, m, H-15) and 2.87 (1H, m, H-13 β). Furthermore, the peak correlating signals at δ 3.65 (ddd, J = 9.5, 8.8, 4.9 Hz) and 5.16 (d, J = 9.5 Hz) in the 1H-1H COSY NMR spectrum of 1, as well as their proton coupling pattern, indicated that the hydroxyl and 2-methylbutyryloxy groups were located at C-2 and C-3, respectively. The relative configuration at H-2 and H-3 was deduced by comparing some neo-clerodane diterpenes previously isolated from Ajuga pseudoiva (Ben Jannet et al., 2000), and by considering the large coupling constant (J = 9.5 Hz) between H-2 and H-3. The hydroxyl group at C-2 was assigned as α-oriented, with the 2-methylbutyryloxy ester functionality β- oriented. This assignment was reinforced by NOE correlations of H-3 to the two diastereotopic protons at C-19. The 1H-1H COSY NMR spectra, as well as their proton coupling patterns, were sufficient to interpret all correlated protons. The structure of 1 was confirmed through catalytic hydrogenation of ajugachin B (4) (Barton et al., 1961) to afford 14, 15-dihydroajugachin B (1). The 1H and 13C NMR spectral data of the semi-synthetic and the isolated compound 1 are in total agreement, thereby supporting the structure assignment. From the above data, compound 1 was confirmed as 14, 15-dihydroajugachin B (1) with the structure shown in Fig 1.
Table 1.
1H NMR spectroscopic data δH m (J in Hz) for neo-clerodane diterpenes 1–3 (CDCl3, 500 MHz) resonance assignments for methylene groups are given as “a” and “b”, where a indicates the resonance with the lowest δ value.
| H | (1) | 2 (R) | 2 (S) | 3 (R) | 3 (S) |
|---|---|---|---|---|---|
| 1α | 1.75 m | 1.68m | 1.68 m | 1.70 m | 1.70 m |
| 1β | 2.58 m | 2.57m | 2.57 m | 2.56 m | 2.56 m |
| 2 β | 3.65 ddd (9.5, 8.8, 4.9) | 3.63m | 3.63 m | 3.60 m | 3.60 m |
| 3 α | 5.16 d (9.5) | 5. 13 d (9.0) | 5.14 d (9.5) | 5.11 d (9.5) | 5.11 d (9.5) |
| 6 β | 4.67 dd (11.9,4.5) | 4.66 dd (11.6,4.5) | 4.66 dd (11.8,4.5) | 4.64 dd (10.9,5.0) | 4.64 dd (10.9,5.0) |
| 7 α | 1.62 dd (11.8,4.5) | 1.60 m | 1.60 m | 1.57 m | 1.57m |
| 7 β | 1.46 m | 1.44 m | 1.44 m | 1.40 m | 1.40 m |
| 8 β | 1.46m | 1.50 m | 1.5 m | 1.48 m | 1.48 m |
| 10 β | 1.75 m | 1.78 m | 1.78 m | 1.70 m | 1.70 m |
| 11 α | 4.09 dd (11.4,5.2) | 3.97 dd (11.6,4.2) | 4.35 d (11.3) | 3.94 dd (11.2,4.2) | 4.55 dd (11.2,5.7) |
| 12a | 1.82 m | 1.60–1.75 m | 1.60–1.75 m | 1.52–1.78 m | 1.52–1.78 m |
| 12b | 1.62 m | 1.60–1.75 m | 1.60–1.75 m | 1.52–1.78 m | 1.52–1.78 m |
| 13 β | 2.87 m | 2.97 m | 2.77 m | 3.04 m | 2.80 m |
| 14a | 2.16 m | 2.16 m | 2.22 m | 2.22 m | 2.13 m |
| 14b | 1.75 m | 1.78 m | 1.61 m | 1.78 m | 1.68 m |
| 15 | 3.85 m | 5.07 d (4.9) | 4.94 d (5.4) | 5.58 d (4.2) | 5.47 d (5.7) |
| 16 β | 5.66 d (5.1) | 5.71 d (5.6) | 5.79 d (5.4) | 5.78 d (5.4) | 5.74 d (5.4) |
| 17 | 0.86 d (6.1) | 0.84 d (6.6) | 0.85 d (6.6) | 0.82 d (6.2) | 0.84 d (6.2) |
| 18b | 2.78 d (4.3) | 2.75 d (4.3) | 2.77 d (4.3) | 2.74 d (4.1) | 2.74 d (4.1) |
| 18a | 2.52 d (4.3) | 2.48 d (4.3) | 2.48 d (4.3) | 2.47 d (4.1) | 2.47 d (4.1) |
| 19a | 4.78 d (12.5) | 4.76 d (12.6) | 4.76 d (12.6) | 4.72 d (12.1) | 4.72 d (12.1) |
| 19b | 4.25 d (12.5) | 4.33 d (12.2) | 4.33 d (12.2) | 4.34 d (12.4) | 4.34 d (12.4) |
| 20 | 0.94 s | 0.92 s | 0.89 s | 0.89 s | 0.86 s |
| 2′ | 2.58 m | 2.57m | 2.57 m | 2.56 m | 2.56 m |
| 3′ | 5.14 m | 5.14 m | 5.14 m | 5.08 m | 5.08 m |
| 4′ | 1.20 d (7.1) | 1.18 d (6.6) | 1.18 d (6.6) | 1.16 d (6.4) | 1.16 d (6.4) |
| 5′ | 1.11 d (7.1) | 1.10 d (7.0) | 1.10 d (7.0) | 1.07 d (7.0) | 1.07 d (7.0) |
| OMe | - | 3.30 s | 3.30 s | - | - |
| acetate | 2.01 s | 2.00 s | 1.99 s | 1.96 s | 1.96 s |
| acetate | 1.91 s | 1.91 s | 1.89 s | 1.87 s | 1.87 s |
| acetate | 2.10 s | 2.10 s | 2.08 s | 2.06 s | 2.06 s |
Table 2.
13C NMR spectroscopic data (δc in ppm) for neo-clerodane diterpenes 1–3 (125 MHz, CDCl3)
| C | (1) | 2 (R) | 2 (S) | 3 (R) | 3 (S) |
|---|---|---|---|---|---|
| 1 | 29.8 | 30.5 | 29.8 | 30.0 | 29.9 |
| 2 | 72.9 | 73.1 | 73.2 | 71.6 | 71.6 |
| 3 | 71.6 | 71.6 | 71.6 | 72.8 | 72.8 |
| 4 | 63.0 | 63.0 | 63.1 | 63.0 | 63.0 |
| 5 | 46.0 | 45.8 | 45.7 | 45.7 | 45.7 |
| 6 | 71.2 | 71.2 | 71.4 | 71.1 | 71.1 |
| 7 | 33.2 | 33.3 | 33.4 | 33.2 | 33.4 |
| 8 | 35.8 | 36.1 | 36.0 | 35.9 | 35.7 |
| 9 | 42.3 | 40.6 | 40.7 | 41.1 | 41.1 |
| 10 | 43.4 | 43.8 | 43.6 | 43.6 | 43.5 |
| 11 | 84.8 | 83.1 | 82.9 | 83.4 | 83.0 |
| 12 | 32.7 | 32.6 | 32.8 | 32.3 | 32.9 |
| 13 | 40.5 | 40.1 | 40.1 | 40.1 | 40.2 |
| 14 | 32.7 | 38.2 | 39.7 | 38.9 | 39.9 |
| 15 | 68.5 | 105.0 | 105.0 | 98.7 | 98.5 |
| 16 | 107.9 | 107.4 | 109.3 | 107.5 | 109.4 |
| 17 | 16.6 | 16.6 | 16.5 | 16.8 | 16.6 |
| 18 | 42.1 | 42.3 | 42.4 | 43.5 | 43.6 |
| 19 | 61.3 | 61.3 | 61.4 | 61.3 | 61.4 |
| 20 | 13.9 | 13.9 | 14.0 | 13.9 | 14.0 |
| 1′ | 172.4 | 172.2 | 172.2 | 172.4 | 172.4 |
| 2′ | 45.7 | 46.4 | 46.4 | 46.0 | 46.0 |
| 3′ | 84.8 | 82.9 | 82.9 | 83.0 | 83.0 |
| 4′ | 17.3 | 17.6 | 17.6 | 17.3 | 17.3 |
| 5′ | 13.0 | 13.3 | 13.3 | 13.0 | 13.0 |
| CH3COO | 21.0 | 21.1 | 21.1 | 21.1 | 21.1 |
| CH3COO | 21.2 | 21.2 | 21.2 | 21.2 | 21.2 |
| CH3COO | 21.3 | 21.4 | 21.4 | 21.3 | 21.3 |
| CH3-O | - | 54.9 | 54.7 | - | - |
| CH3COO | 170.3 | 170.2 | 170.2 | 170.3 | 170.3 |
| CH3COO | 171.3 | 171.3 | 171.3 | 171.4 | 171.4 |
| CH3COO | 171.4 | 171.4 | 171.4 | 171.4 | 171.4 |
Fig. 1.
neo-Clerodane diterpenes (1–6) isolated from Ajuga turkestanica
Compound 2 was obtained as a 1:4, R to S, epimeric mixture of a 15-methoxy derivative of dihydroajugachin (1), as indicated from its 1H NMR and also the occurrence of pairs of signals in its 13C NMR spectrum. The epimeric mixture was obtained as an amorphous white material which was homogenous on TLC. The IR spectrum showed absorption bands for hydroxyl (3436 cm−1) and ester groups (1732 and 1245 cm−1). The HR-ESI-MS showed a molecular ion peak at m/z 641.3182 [M+1]+, corresponding to the molecular formula C32H48O13. The 1H NMR spectrum of 2 was very close to that of the isolated compound 3 (chamaepitin), with an additional methoxy group at δ 3.30 (s), suggesting a methylated derivative of 3. The presence of a non-equimolar mixture of C-15 epimers enabled us to assign the proton signals for both compounds depending on their integration values. In fact, H-11, H-13, H-15 and H-16 appeared as pairs of signals: H-11α at δ 3.97 and 4.35 (dd, J = 11.6, 4.2 Hz and 11.2 Hz); H-13 at δ 3.97 and 2.77 (m); H-15 at δ 5.07 and 4.94 (d, J = 4.9 Hz and 5.4 Hz); H-16 at δ 5.71 and 5.79 (d, J = 5.6 and 4.4 Hz), for R and S configurations, respectively. The resonance of H-11 in the 15 R configuration appeared at higher field than in the 15 S configuration.
In contrast, the resonance of H-13 occurred in the 15 R configuration at lower field than in the 15 S configuration. Similarly, several peaks in the 13C NMR displayed higher shift values for the 15 R configuration: C-12 and C-14 resonated at δc 32.62 (32.83, S) and 38.27 (39.70, S), respectively, whereas the C-15 resonated at slightly lower field (δc 105.04 for R and 105.02 for S). However, the greatest difference was observed for C-16 where the 15 R epimer resonated at δc 107.42 while the 15 S configuration resonated at a much lower field (δc 109.37). NMR data were in full agreement with those reported in Rosselli et al. (2004) for the R and S configuration of the 15-methoxy-hexahydrofuran neo-clerodane diterpenes (see Table 1 and 2). The presence of the 2-methylbutyryloxy functionality at C-3 was evident from the two methyl doublets at δ 1.18 and 1.10 (d, J = 6.6 and 7.0 Hz, for 4′ and 5′ CH3, respectively). The presence of this functionality was also confirmed by the downfield signal due to a proton on a carbon atom bearing an oxygen at δ 5.13 and 5.14 (1H each, d, J = 9.0 and 9.5 Hz, H-3) for the C-15, R and S configuration, respectively. The 1H-1H COSY NMR spectra, as well as their proton coupling patterns, were efficient in interpretation of all correlated protons. 13C NMR assignments were determined by DEPT spectra. On the basis of the above data, compound 2 was confirmed as a C-15 epimeric mixture of 14-hydro-15-methoxyajugachin B and was assigned the structure 2 shown in Fig 1.
Compound 3 was obtained as an amorphous white material. Its NMR spectral data showed that it was an equimolar mixture of the C-15 epimers. It has a molecular formula of C31H46O13, deduced from its HR-ESI-MS (m/z 627.3019 [M+1]+) and its IR showed absorption bands for free hydroxyl (3436 cm−1) and ester groups (1733 and 1250 cm−1). 1H and 13C NMR were in complete agreement with the data reported for chamaepitin (C-15 equimolar epimeric mixture) previously isolated from Ajuga chamaepitys (Camps et al. 1987). The 1H, 13C and 2D NMR enabled us to provide precise assignment for the protons and carbons of both the R and S configurations that were not fully assigned in the previously reported study. Our spectral data were in full agreement with the rules proposed by Rosselli et al. (2004) for R and S configurations of the 15-hydroxy hexahydrofuran neo-clerodane diterpenes (see Tables 1 and 2).
Compound 4 and 5 were identified according to their physical, chemical and spectral data, which were in full agreement with the reported data for ajugachin B (Boneva et al., 1990) and ajugapitin (Hernandez et al., 1982; Boneva et al., 1990).
Compound 6 was obtained as 1:2 mixture of the C-15 epimers (R and S, respectively) of the known neo-clerodane lupulin A previously isolated from Ajuga lupulina (Chen et al., 1996). The 1H NMR data for the mixture shows that H-11, -13, -15 and -16 appear as pairs which differ in their chemical shifts as observed with our isolated 14-hydro-15-methoxyajugachin B (2) and chamaepitin (3). Since the previously identified compound was the S epimer, it is worthy to report here the NMR signals that showed a great deal of difference between the two epimers: H-11 resonated at δ 4.03 and 4.41, H-13 at δ3.02 and 2.82, H-15 at δ 5.12 and 4.99 and H-16 at δ5.76 and 5.84 for the C-15, R and S -configurations, respectively. Likewise, in the 13C NMR: δc C-11 resonated at 83.06 and 82.4 and C-16 resonated at 107.09 and 109.1 for the R and S configurations, respectively. This assignment was in total agreement with the reported rule for the 15-methoxy-hexahydrofurofuran neo-clerodane diterpenes (Rosselli et al., 2004).
It is of interest to note that many of the 15-hydroxy and 15-methoxy derivatives of neo-clerodane diterpenes having a hexahydrofuran functionality have been reported in Ajuga species and other plants as epimeric mixtures (Coll and Tandron, 2007; Rosselli et al., 2004). The separation of individual configurations from these mixtures is difficult using ordinary chromatographic methods. Assignment of the NMR data of both epimers in the mixtures was successfully performed utilizing 2D NMR and following the reported rules for 15-hydroxy- and methoxy-hexahydrofuran neo-clerodane diterpenes. (Rosselli et al., 2004).
3. Experimental
3.1. Plant material
Shoots and leaves of A. turkestanica were harvested in July 2004 from the mountainous regions of Uzbekistan (UIUC; ICBG Central Asia, voucher UPL_00057, ILLS, MO). All biological materials were transferred according to the ICBG material transfer agreement signed between Rutgers University and Uzbekistan on August 30th, 2004 and approved by the National Institutes of Health.
3.2. Extraction and isolation
Dried powdered plant tissue of A. turkestanica (500 g) was extracted with MeOH (3 × 1 L). After evaporation of solvent and addition of water (200 mL), the aqueous suspension was partitioned with pet ether (3 × 0.5 L), then with EtOAc (4 × 0.5 L) to afford 18.5 g and 11.9 g, respectively. The EtOAc extract on purification over silica gel column using n-hexane and n-hexane-EtOAc step gradient yielded 5 (20 mg), 4 (350 mg), 1 (180 mg), 3 (100 mg), 2 (25 mg) and 6 (2.8 mg), with this sequence of separations. Later fractions eluted with a step gradient of MeOH in EtOAc afforded phytoecdysteroids and iridoid glycosides (Cheng et al., 2008).
14, 15-Dihydroajugachin B (1)
White amorphous powder, [α]20D -28 (CHCl3; c 0.19); IR vmax (CHCl3) 3457 (OH), 2971, 1728 and1245 (ester) groups, 1369, 1026, 752 cm−1. 1H and 13C NMR (see Tables 1 and 2, respectively). HR-ESI-MS m/z 611.363 (calc. for C31H47O12 611.3068) [M+1]+; ESI-MS m/z (rel. int.): 611 [M+1]+ (55), 593 (35), 575 (25), 551 (28), 515 (22), 433 (100), 391 (85), 313 (55). Hydrogenation of compound 4 over palletized charcoal (Barton et al., 1961) afforded 14, 15-Dihydroajugachin B. The optical rotation and NMR data observed for the obtained compound were identical to those data observed for the naturally isolated compound 1.
14-hydro-15-methoxyajugachin B (2)
White amorphous material, IR vmax (CHCl3) 3436 (OH), 2956, 1732 and1245 (ester) groups, 1372, 1080, 749 cm−1. 1H and 13C NMR see Tables 1 and 2, respectively. HR-ESI-MS m/z 641.3182 (calc. for C32H49O13 641.3173) [M+1]+; ESI-MS m/z (rel. int.): 658 [M+ H2O]+ (100), 641 [M+1]+ (65), 591 (40), 463 60), 421 (65), 389 (50), 371 (65), 329 (45), 311 (60).
Chamaepitin (14-hydro-15-hydroxyajugachin B) (3)
White amorphous material, IR, 1H and 13C NMR were in agreement with the published data (Camps et al., 1987). See Tables 1 and 2 for the R and S configurations. HR-ESI-MS m/z 627.3019 (calc. for C31H47O13 627.3017) [M+1]+; ESI-MS m/z (rel. int.): 644 [M+ H2O]+ (100), 627 [M+1]+ (50), 591 (35), 407 (70), 389 (85), 329 (60), 311 (50).
Acknowledgments
The authors would like to gratefully thank the Fogarty International Center of the National Institutes of Health (NIH) under U01 TW006674 for the International Cooperative Biodiversity Groups for funding this work. We appreciate the aid of Dr. Igor V. Belolipov, Tashkent State Agrarian University, Tashkent, Uzbekistan, for arranging for collection of Ajuga foliage.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdukadirov IT, Khodzhaeva MA, Turakhozhaev MT, Mamatkhanov AT. Carbohydrates from Ajuga turkestanica. Chem Nat Compd. 2004;40:85–86. [Google Scholar]
- Baltaev UA. Phytoecdysteroids: Structure, sources, and biosynthesis in plants. Russ J Bioorg Chem. 2000;26:799–831. doi: 10.1023/a:1026662505403. [DOI] [PubMed] [Google Scholar]
- Barton DHR, Cheng HT, Cross AD, Jackman LM. Diterpenoid bitter principles. Part III. The constitution of clerodin. J Chem Soc. 1961:5061–5073. [Google Scholar]
- Ben Jannet H, Chaari A, Bakhrouf A, Mighri Z. Structure- antibacterial activity relationship of secondary metabolites from Ajuga pseudoiva Rob. leaves Nat Prod Res. 2006;20:299–304. doi: 10.1080/14786410500129129. [DOI] [PubMed] [Google Scholar]
- Ben Jannet H, Harzalla-Skhiri F, Mighri Z, Simmonds MSJ, Blaney WM. Responses of Spodoptera littoralis larvae to Tunisian plant extracts and to neo-clerodane diterpenoids isolated from Ajuga pseudoiva leaves. Fitoterapia. 2000;71:105–112. doi: 10.1016/s0367-326x(99)00146-x. [DOI] [PubMed] [Google Scholar]
- Boneva IM, Mikhova BP, Malakov PY, Papanov GY, Duddeck H, Spassov SL. Neo-clerodane diterpenoids from Ajuga chamaepitys. Phytochemistry. 1990;29:2931–2933. [Google Scholar]
- Camps F, Coll J, Dargallo O, Rius J, Miravitlles C. Clerodane diterpenoids from Teucrium and Ajuga plants. Phytochemistry. 1987;26:1475–1479. [Google Scholar]
- Camps F, Coll J. Insect allelochemicals from Ajuga plants. Phytochemistry. 1993;32:1361–70. [Google Scholar]
- Chen H, Tan RX, Liu ZL, Zhang Y. Antibacterial neoclerodane diterpenoids from Ajuga lupulina. J Nat Prod. 1996;59:668–670. doi: 10.1021/np960385s. [DOI] [PubMed] [Google Scholar]
- Cheng DM, Yousef GG, Grace MH, Rogers RB, Gorelick-Feldman J, Raskin I, Lila MA. In vitro production of phytoecdysteroids from Ajuga turkestanica. Plant Cell Tiss Org Cult. 2008;93:73–83. [Google Scholar]
- Coll J, Tandron YA. neo -Clerodane diterpenoids from Ajuga: structural elucidation and biological activity. Phytochem Rev. 2008;7:25–49. [Google Scholar]
- Hedge IC. A global survey of the biogeography of the Labiatae. In: Harley RM, Reynolds T, editors. Advances in Labiate Science. Royal Botanic Gardens; Kew, Richmond, Surrey, UK: 1992. pp. 7–17. [Google Scholar]
- Hernandez A, Pascual C, Sanz J, Rodríguez B. Diterpenoids from Ajuga chamaepitys: Two neo-clerodane derivatives. Phytochemistry. 1982;21:2909–2911. [Google Scholar]
- Klein Gebbinck EA, Jansen BJM, de Groot A. Insect antifeedant activity of clerodane diterpenes and related model compounds. Phytochemistry. 2002;61:737–770. doi: 10.1016/s0031-9422(02)00174-7. [DOI] [PubMed] [Google Scholar]
- Konoshima T, Takasaki M, Tokuda H, Nishino H. Cancer chemopreventive activity of an iridoid glycoside, 8-acetylharpagide, from Ajuga decumbens. Cancer Lett. 2008;157:87–92. doi: 10.1016/s0304-3835(00)00479-1. [DOI] [PubMed] [Google Scholar]
- Mamatkhanov AU, Yakubova MR, Syrov VN. Isolation of turkesterone from the epigeal part of Ajuga turkestanica and its anabolic activity. Chem Nat Compd. 1998;34:150–154. [Google Scholar]
- Ramazanov NS. Phytoecdysteroids and other biologically active compounds from plants of the genus Ajuga. Chem Nat Compd. 2005;41:361–369. [Google Scholar]
- Rosselli S, Maggio A, Piozzi F, Bruno M. Assigning the C-15 configuration of 15-hydroxy-, 15-methoxy-, 15-ethoxy-hexahydrofurofuran neoclerodane diterpenoids. Tetrahedron. 2004;60:8791–8800. [Google Scholar]
- Usmanov VZ, Gorovits MB, Abubakirov NK. Phytoecdysones of Ajuga turkestanica. Chem Nat Compd. 1971;4:535–536. [Google Scholar]
- Usmanov VZ, Gorovits MB, Abubakirov NK. Phytoecdysones of Ajuga turkestanica II. Chem Nat Compd. 1973;1:125–126. [Google Scholar]
- Usmanov VZ, Gorovits MB, Abubakirov NK. Phytoecdysones of Ajuga turkestanica III. Chem Nat Compd. 1975;4:466–470. [Google Scholar]
- Usmanov VZ, Rashkes YV, Abubakirov NK. Phytoecdysones of Ajuga turkestanica VI. 22-acetylcyasterone. Chem Nat Compd. 1978;2:215–219. [Google Scholar]