Abstract
Choline was officially recognized as an essential nutrient by the Institute of Medicine (IOM) in 1998. There is a significant variation in the dietary requirement for choline that can be explained by common genetic polymorphisms. Because of its wide-ranging roles in human metabolism, from cell structure to neurotransmitter synthesis, choline-deficiency is now thought to have an impact on diseases such as liver disease, atherosclerosis and possibly neurological disorders. Choline is found in a wide variety of foods. Egg yolks are the most concentrated source of choline in the American diet, providing 680 milligrams per 100 grams. Mean choline intakes for older children, men, women and pregnant women are far below the Adequate Intake established by the IOM. Given the importance of choline in a wide range of critical functions in the human body, coupled with less than optimal intakes among the population, dietary guidance should be developed to encourage the intake of choline-rich foods.
Keywords: Choline, eggs, homocysteine, memory, methylation, methyl group, neural tube defects, phosphatidylcholine, pregnancy
Introduction
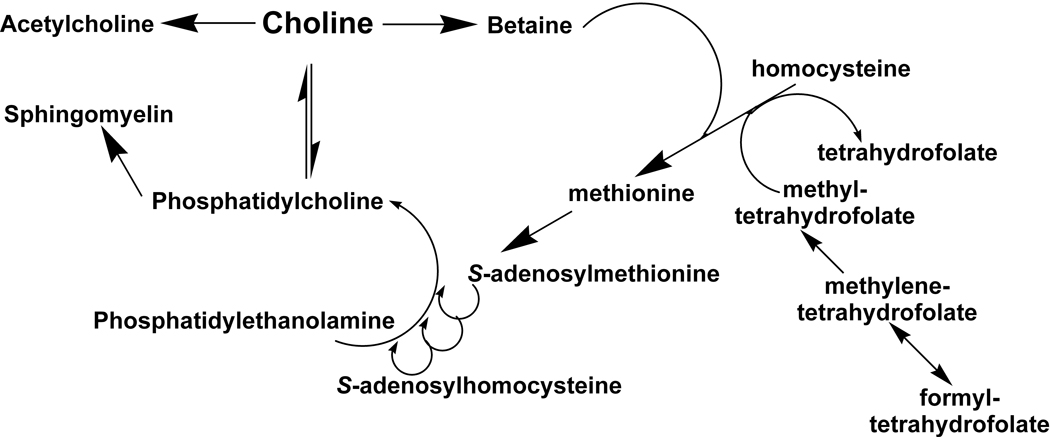
Choline was officially recognized as an essential nutrient by the Institute of Medicine in 1998.1 Its role in the body is complex. It is needed for neurotransmitter synthesis (acetylcholine), cell-membrane signaling (phospholipids), lipid transport (lipoproteins), and methyl-group metabolism (homocysteine reduction).2 It is the major dietary source of methyl groups via the synthesis of S-adenosylmethionine (AdoMet).3 At least 50 AdoMet-dependent reactions have been identified in mammals, and it is likely that the number is much higher.3 Such methylation reactions play major roles in biosynthesis of lipids, the regulation of several metabolic pathways, and detoxification in the body.3 Choline is required to make the phospholipids phosphatidylcholine, lysophosphatidylcholine, choline plasmalogen, and sphingomyelin—essential components for all membranes.4 It plays important roles in brain and memory development in the fetus and appears to decrease the risk of the development of neural tube defects.5,6
The importance of choline in the diet extends into adulthood and old age. In a study of healthy adult subjects deprived of dietary choline, 77% of the men and 80% of the postmenopausal women developed signs of subclinical organ dysfunction (fatty liver or muscle damage). Less than half of premenopausal women developed such signs.7 Ten percent of the subjects studied developed fatty liver, muscle damage, or both when they consumed the Adequate Intake (AI) of choline. The damage was reversed when they consumed a high-choline diet. Plasma choline concentration has been found to vary in response to diet, decreasing approximately 30 percent in humans fed a choline-deficient diet for 3 weeks.4 Based on estimated dietary intakes and studies reporting liver damage with lower choline intakes, the Institute of Medicine, Food and Nutrition Board set the AI for choline at 425 milligrams/per day for women aged 19 and older, and 550 milligrams/per day for men aged 19 and older.
Choline can be acquired from the diet and via de novo biosynthesis through the methylation of phosphatidylethanolamine (PE) to phosphatidylcholine (PC). However, de novo synthesis of choline alone is not sufficient to meet human requirements.1 Dietary choline from a variety of choline-containing foods is absorbed by the intestine and uptake is mediated by choline transporters.8 The major fate of choline is conversion to PC (also known as lecithin), which occurs in all nucleated cells.8 PC is the predominant phospholipid (>50%) in most mammalian membranes.9 Recent studies indicate that choline is recycled in the liver and redistributed from kidney, lung, and intestine to liver and brain when choline supply is low.8 Upon entry into the cell, choline is immediately phosphorylated to phosphocholine, or oxidized to betaine in some cell types such as hepatocytes.8 Betaine is important because of its role in the donation of methyl groups to homocysteine to form the essential amino acid methionine.10 While there are metabolic pathways for the interconversion of choline, phophatidylcholine, glycerophosphocholine, phosphocholine and sphingomyelin, the conversion of choline to betaine is irreversible.10
Any consideration of the requirements for choline and methionine needs to consider the close interrelationships with other methyl donors. Choline, methionine, and folate metabolism interact at the point that homocysteine is converted to methionine.1 Perturbing the metabolism of any one of these methyl donors reveals the complex interactions of the metabolic pathways, as compensatory changes occur in the enzymes and vitamin co-factors involved in the reactions.1,10 In rats, severe folate deficiency causes secondary hepatic choline deficiency.4 Humans fed with total parenteral nutrition solutions devoid of choline, but adequate for methionine and folate, develop fatty liver and liver damage.1 In healthy humans consuming adequate folate and methionine, inadequate choline intake can result in fatty liver or muscle damage that resolves when a source of dietary choline is provided. 4,7 Because of its wide-ranging role in human metabolism, from cell structure to neurotransmitter synthesis, choline deficiency is now thought to have an impact on diseases such as nonalcoholic fatty liver disease, atherosclerosis (via lipoprotein secretion), and possibly neurological disorders.8 Therefore, getting adequate choline in the diet is important throughout life for optimal health.
Pregnancy and Lactation
Pregnancy and lactation are times when demand for choline is especially high and the supply of choline is critical. The recommended Adequate Intake (AI) for pregnant women is 450 mg/d; 550 mg/d for lactating women. Large amounts of choline are delivered to the fetus across the placenta and choline concentration in amniotic fluid is 10-fold greater than that present in maternal blood.9 Plasma or serum choline concentrations are significantly higher in pregnant women, compared to nonpregnant women (10.7 microM of free choline and 2,780 microM of bound choline in nonpregnant women, compared to 16.5 microM and 3,520 microM at 36 to 40 weeks pregnancy) 11 and are 6- to7-fold higher in the fetus and newborn than they are in adults.12 The transport of choline from mother to fetus depletes maternal plasma choline in humans.13 Thus, despite enhanced capacity to synthesize choline during pregnancy, the demand for this nutrient exceeds the supply and stores can be depleted. Because human milk is rich in choline, lactation further increases maternal demand, resulting in extended depletion of tissue stores.14
Neural Tube Defects
Several nutritional factors have been implicated in the occurrence of neural tube defects (NTDs). Foremost among those factors has been the role of periconceptional intake of folic acid.5 Similar to folic acid, choline is involved in the methylation of homocysteine to methionine. Some research indicates that choline and methionine intakes may be factors in reducing risk of NTDs as well, independent of folate intake from food and supplements.5,6 The inhibition of choline uptake and metabolism in mouse embryos results in NTDs.15
Shaw et al.5 found that women in the lowest quartile for dietary choline intake had four times the risk of giving birth to a child with a neural tube defect, compared with women in the highest quartile of intake. Decreased risks of NTD-affected pregnancies were found for higher periconceptional intakes of choline for all NTDs as well as for spina bifida and anencephaly separately. The association remained strong after adjusting for maternal pre-pregnancy weight, height, education, race, ethnicity, periconceptional vitamin use, dietary folate intake, dietary methionine intake and total energy intake. Since choline and folate metabolism intersect at the pathway for methyl-group donation, it is reasonable to hypothesize that methylation reactions are the mechanism they share in common that influence neural tube closure.9
Memory Development
Recent studies show that choline supplementation during critical periods of neonatal development can have long-term beneficial effects on memory. (See Table 1) During later periods of pregnancy, the memory center of the brain (the hippocampus) is developing. In rodents, choline supplementation or depletion during this critical phase causes lifelong changes in brain structure and function. 9,13 Adult rodents typically experience a decline in memory as they age. Offspring exposed to extra choline in utero did not show this change with age.16
Table 1.
Effect of Maternal Choline Supplementation on Brain Function in Animals
| Reference | Animal model | Choline Dose | Measurements | Length | Outcome | Significance |
|---|---|---|---|---|---|---|
| Glenn et al. (2008)Error! Bookmark not defined.16 | 32 Male and female rats | Maternal diet supplemented with 5 g/kg vs control (1.1 g/kg) | Response to novel environments/objects ;hippocampal plasticity in adulthood | Tested at 1 and 24 months of age | Increase in exploration behavior in prepubertal females and an attenuation in the decline in exploration with age. Evidence of long-lasting effects of prenatal choline supplementation on markers of hippocampal plasticity. | ρ<0.05 |
| Stevens et al. (2008)Error! Bookmark not defined.17 | 34 Male and female rats | Maternal diet supplemented with 5 g/kg vs normal (1.1g/kg) vs deficient (0g/kg | Sensory inhibition in offspring as assessed by five sensory inhibition parameters | Tested at 11 to 12 months of age | Rats gestated on deficient choline showed abnormal sensory inhibition in 2 out of 5 parameters when tested at adulthood; rats gestated on normal or supplemented choline showed normal sensory inhibition. | ρ<0.01 |
| Stevens et al. (2008)Error! Bookmark not defined.18 | 27 Male and female mice | Maternal diet supplemented with 5 g/kg vs normal (1.1 g/kg) | Sensory inhibition in offspring as assessed by three sensory inhibition parameters | Tested at 10 to 12 weeks of age | Gestational choline supplementation produced permanent improvement in sensory inhibition in two out of three parameters tested in adult offspring. | ρ<0.001 |
| Cheng et al. (2008)Error! Bookmark not defined.19 | 32 Male and female rats | Maternal diet supplemented with 5 g/kg vs normal (1.1 g/kg) during days 12–17 of gestation | Animals were trained in a series of differential reinforcement schedules and their responses evaluated. Two time periods were selected and compared. | Tested at 7 months | Gestational choline supplementation at days 12–17, resulted in long-lasting effects exhibited by the establishment of more precise and enduring memories of the training procedures | ρ<0.01 |
| Cheng et al. (2008)Error! Bookmark not defined.20 | 12 Male rats | Maternal diet supplemented with 3.5 g/kg vs control (1.1 g/kg) during days 12–17 of gestation | Animals were trained and tested on auditory and visual signals at two different time intervals and two levels of intensity | Tested at 20 months | Prenatal choline supplementation enhanced auditory and visual responses in aged rats | <0.01 |
| Wong-Goodrich et al. (2008)Error! Bookmark not defined.21 | Experiment 160 Male rats Experiment 234 Male rats | Maternal diets were either choline deficient, sufficient (1.1g/kg) or supplemented (5 g/kg) on gestation days 12–17. Experiment 1 Offspring were divided into 2 groups (one group received a choline-deficient diet; another received 5.0g/kg of choline). The animals were returned to a 1.1 g/kg choline diet and given two additional retraining sessions. Experiment 2 One group of offspring was given a control diet (1.1 g/kg); the other group was given a choline supplemented diet for 12 weeks. | Experiment 1 Animals were trained and tested for hippocampally mediated spatial navigation, memory and plasticity at three time intervals. Experiment 2 Tested for spatial memory, dentate cell proliferation and neurogenesis. | Experiment 1 Trained at 70 days of age for 24 days. After 10 days retrained for 14 days. After 10 more days, animals were retrained again for 14 days. Experiment 2 At 15 months of age, animals were trained for 4 days on a water-maze. | In utero availability of choline affects cognitive and hippocampal responses when offspring are exposed to changes in the choline supply as adults. | <0.05 |
Pregnant rats supplemented with choline experience multiple modifications in the developmental patterns of gene expression known to influence learning and memory.17 The likely mechanism for these effects of choline involves DNA methylation, altered gene expression, and associated changes in stem cell proliferation and differentiation.18 In humans, the hippocampus continues to develop after birth, and it closely resembles the adult structure by 4 years of age. Extrapolating from rodent data, human sensitivity to the developmental effects of choline would occur in utero and continue up to age 4 years.18 A study of pregnant women and their children found no association between choline concentrations in maternal and cord blood and intelligence levels in their children at 5 years of age.19 Currently, there are no published studies in humans examining whether choline supplementation during pregnancy enhances memory performance in offspring.
A study of rat pups exposed to ethanol during the neonatal brain growth spurt, a developmental period that would be equivalent to postnatal development in humans, and treated with choline chloride, found that choline supplementation reduced the severity of fetal alcohol effects even after the alcohol exposure.20 The findings suggest that early dietary intervention with choline may reduce the severity of some fetal alcohol effects. Moreover, the ability of choline to attenuate ethanol’s effects was evident months after choline treatment, suggesting that choline’s effects are long lasting.20
The second half of pregnancy is characterized by a strong inverse relation between plasma betaine and homocysteine.21 Elevated maternal homocysteine concentrations are a risk factor for several adverse pregnancy events, including preeclampsia, prematurity and very low birth weight, and have been suggested to have an important role as a marker of pregnancy complications and adverse pregnancy outcomes.22 Though most studies have found a clear inverse association between maternal choline levels and homocysteine, Molloy et al.23 found a highly significant positive association between maternal choline levels and maternal homocysteine levels, suggesting that the high fetal demand for choline stimulates de novo synthesis of choline in the maternal liver with a resultant increase in homocysteine. Regardless of the specific mechanism that raises homocysteine, the recommendation remains the same—to increase choline intake by diet or supplementation to attenuate de novo synthesis and reduce homocysteine levels.23 These findings may indicate a need for intervention to ensure optimal choline intakes during pregnancy.23
Heart Disease
When choline stores are inadequate, there is a diminished capacity to methylate homocysteine to methionine, and plasma levels of homocysteine increase.24 Elevated levels of homocysteine have been associated with greater risk for several chronic diseases and conditions including cardiovascular disease,25 cancer,26 cognitive decline27 and bone fractures.28 Intakes of choline and betaine have been associated with lower homocysteine levels, whether intake of each nutrient is considered independently or in combination and whether the source is from food or supplements.29,30 Homocysteine can also be methylated to form methionine via another pathway involving vitamins B12 and folic acid. Methionine can, in turn, be converted to S-adenosyl methionine. Deficiency of either vitamin B12 or folic acid can result in elevated plasma homocysteine concentrations and increased risk for chronic disease.31
Olthof et al. found in a group of men, aged 50–71, with elevated homocysteine levels, that a high daily dose of choline (2.6 g), supplemented as phosphatidylcholine, lowered fasting as well as plasma homocysteine concentrations after a methionine-loading test.32 The choline supplementation was as effective as folic acid in lowering fasting homocysteine levels. Olthof suggested that if elevated homocysteine concentration causes cardiovascular disease, choline intake may reduce cardiovascular disease risk.
Inflammation
Findings from the ATTICA study indicated that subjects whose diets were rich in choline and betaine had the lowest levels of several inflammatory markers, including C-reactive protein (CRP), homocysteine, interleukin-6 and tumor necrosis factor.33 These findings were significant after adjusting for various sociodemographic, lifestyle and clinical characteristics of the participants. Higher combined dietary intakes of choline and betaine were associated with lower concentrations of all of the inflammatory markers measured in the study. This is similar to the findings of Cho et al.34 who found that among 1,960 participants in the Framingham Offspring Study, the combined dietary intakes of choline and betaine were associated with lower homocysteine concentrations. Most recently, findings from the Nurses’ Health Study revealed that those with the highest consumption of dietary choline had improved plasma levels of biomarkers for inflammation, including adiponectin, high-molecular-weight adiponectin, resistin and CRP.35 Elevated CRP has recently been recognized by the National Heart, Lung and Blood Institute as a useful marker for cardiovascular disease.36
The associations found between cardiovascular disease risk factors and typical dietary intakes of choline and betaine have not translated into increased risk from cardiovascular disease.37,38 Analysis of findings from the PROSPECT-EPIC cohort of 16,165 women in the Netherlands, aged 49–70, suggested that regular dietary intakes of betaine and choline were not associated with cardiovascular disease risk.38 However, even those in the highest quartile of choline intake fell below the current AI of 425 mg/d for women and the range of intake was small.
Breast Cancer
Choline deficiency in cell and rodent models is associated with DNA damage and apoptosis.39,40 Cells grown in choline-deficient medium have greater membrane fragility than cells grown in control medium.39 Induced choline deficiency in a group of 51 men and women aged 18–70 for 42 days also showed increased DNA damage and apoptosis in lymphocytes.400 High dietary intakes of choline have recently been associated with a decreased risk for breast cancer. In the first study to examine the association between choline and breast cancer, Xu et al.41 found that breast cancer risk was reduced 24% among women with high dietary intakes of choline. The association did not vary substantially with menopausal status or cancer type (invasive vs in situ).
Dietary Recommendations And Genetic Variations That Affect Choline Requirements
The recommended adequate intake (AI) for choline has been set at 425 mg/d for women, 450 mg/d for pregnant women, 550 mg/d for lactating women and 550 mg/d for men.1 However, an Estimated Average Requirement (EAR) has not been set because of a lack of sufficient human data.1,7
The potential public health implications of not consuming enough of this essential nutrient have only recently begun to be examined. The development of a database of the choline content of foods now makes it possible to track the dietary choline intakes of populations and correlate intakes with the incidence of disease.42
There is a significant variation in the dietary requirement for choline that can be explained by common genetic polymorphisms.41,45,43 Understanding dietary choline requirement and its modulation by genetic polymorphism has public health significance, especially in regards to its role in brain development.13 Current recommended intakes do not take into consideration these genetic variations as a modulator of dietary requirement. When the AI for choline was established in 1998, it was assumed that less than 5% of the population would be affected. It is now clear that as much as 50% of the population may have genetic polymorphisms that increase dietary methyl requirements, of which choline is a major source, leaving them susceptible to choline deficiency.44,45
When fed a choline-deficient diet, some men and women developed fatty liver and liver and muscle damage, whereas others did not. Premenopausal women who are carriers of a very common single nucleotide polymorphism (SNP) have been found to be 15 times as likely as non-carriers to develop signs of choline deficiency on a low-choline diet.45 Studies are underway to identify other genetic differences that contribute to individual variability in dietary requirements for choline.46
DNA methylation influences the expression of some genes and depends upon the availability of methyl groups from AdoMet. Alterations in DNA methylation, with resulting changes in gene expression, can have important consequences for embryogenesis and carcinogenesis; dietary choline availability during pregnancy influences the development of the brain in the fetus via choline-mediated alterations in the birth, migration and death of cells in the brain.44 Research is ongoing to identify genetic polymorphisms in choline related genes that affect their function, be it via changes in the regulation of the gene, which in turn could increase the requirement for choline in the diet. Identification of common polymorphisms that affect dietary requirements for choline could allow for the identification of individuals, especially pregnant women, for whom choline needs may exceed current recommendations. 18
Dietary choline requirements are an excellent example of nutrigenomics (the science of molecular-level interactions between nutrients and other dietary bioactives and the response of genes). Endogenous biosynthesis of choline is regulated by estrogen via gene estrogen response elements. DNA methylation is influenced by the availability of choline, and common genetic polymorphisms have major effects on the dietary requirement for this nutrient. These interactions have important health implications.46
Prevalence of Suboptimal Choline Intakes
At the time the AI for choline was established in 1998, it wasn’t known whether there were significant numbers of people who were choline deficient. It was known, however, that there were many who were folate deficient,1 which can impact choline status. There is now evidence that current choline recommendations may be suboptimal for a large percentage of the population. Women appear to depend upon a combination of dietary choline intake and high rates of endogenous estrogen-induced biosynthesis of choline to sustain normal pregnancy.13 There is, however, a real possibility that women in the U.S. may not get enough choline during pregnancy.5,13 The AI set by the Institute of Medicine for choline during pregnancy is 450 mg/d and for lactation 550 mg/d. Dietary choline intake among women varies from <300 mg/d to >500 mg/day. Intakes at the lower end of that range could increase the risk of having a baby with a neural tube birth defect.5,13
In a subset of subjects from the Nurses’ Health Study, the cutoff for the 95th percentile of choline intake was 411 mg/d, which suggests that most of women within this population were not meeting the recommended intake.29 Though many foods contain choline, there is at least a twofold variation in dietary intake in humans.13 A study of 16 adult women and 16 adult men, ages 18 – 67, found that only 6 of the 16 women met or exceeded the AI for choline.47 A recent analysis of data from NHANES 2003–2004 revealed that for older children, men, women and pregnant women, mean choline intakes are far below the AI. Ten percent or fewer had usual choline intakes at or above the AI.48
Additional analysis of NHANES 2003–2004 data found that choline intake decreases with age and that adults ages 71 and older consumed an average of about 264 milligrams per day, about one-half of the AI for choline.49
Food Sources of Choline
Choline is found in a wide variety of foods. (Table 2) The U.S Department of Agriculture recently released an updated version of its first database of choline content in foods, including more than 630 foods.42 Among the most concentrated sources of dietary choline are liver, eggs, and wheat germ. In foods, choline is found in free and esterified form (such as phosphocholine, glycerophosphocholine, phosphatidylcholine, and sphingomyelin).18 Human milk is rich in choline compounds. Soy-derived infant formulas have lower total choline concentrations than do human milk and bovine milk-derived formulas.50 Foods rich in the related compound, betaine, include wheat bran, wheat germ, quinoa, beets, spinach and spaghetti.42
Table 2.
Selected Food Sources of Choline (milligrams per serving)
| Food | Choline |
|---|---|
| Chicken, liver, cooked (3 oz) | 247 |
| Soy flour, defatted (1 cup) | 201 |
| Salmon, sockeye, smoked (3 oz) | 187 |
| Egg, whole, raw, fresh (1 large) | 125 |
| Quinoa, uncooked (1/2 cup) | 60 |
| Chicken, broilers or fryers, meat and skin, roasted (3 oz) | 56 |
| Turkey sausage, cooked (3 oz) | 55 |
| Wheat germ, toasted, plain (2 tbsp) | 50 |
| Milk, nonfat, fluid, with added vitamin A (8 ounces) | 38 |
| Cauliflower, cooked, boiled (1/2 cup) | 24 |
| Peas, green, frozen, cooked, drained (1/2 cup) | 22 |
| Bacon, pork, cured, cooked (2 pieces) | 20 |
| Almonds (1 oz) | 15 |
| Broccoli, cooked, boiled, drained (1/2 cup) | 15 |
| Frankfurter, beef (1) | 15 |
| Oat bran, raw (1/2 cup) | 15 |
| Pecans (1 oz) | 15 |
| Tomato paste, canned (2 tbsp) | 12 |
| Flaxseed (2 tbsp) | 11 |
Source: USDA Database for the Choline Content of Common Foods, Release Two, January 2008; USDA National Nutrient Database for Standard Reference, Release 20.
Dietary information obtained from the Nurses’ Health Study and the Nurses’ Health Study 2 revealed that animal products, including eggs, milk, chicken, beef, and pork to be the biggest contributors of choline in the diets of the female subjects.29
Though eggs are a more concentrated source of choline42, in the Nurses’ Health Study and Nurses’ Health Study 2, milk provided the largest percentage of dietary choline, because it was consumed more frequently. However, NHANES 2003–2004 data showed that eggs contributed a relatively higher share of total choline intake for those whose intake was at or above the AI, compared to others.48 Eggs also provide more choline per kilocalorie compared to most other foods, including milk. To get the same amount of choline found in a single egg (125 mg/72 calories; most of the choline is in the egg yolk – 680 mg/100g), one would need to consume 3 ¼ cups of nonfat milk (270 calories) or 3 ½ ounces of wheat germ (366 calories). In addition, adding an egg to the diet each day would increase the number of pregnant women meeting the AI from 10% to more than 50% and for older men and women from 5% to 20%.
Health Professional Awareness and Dietary Recommendations for Choline Intake
Given the importance of choline in a wide range of critical functions in the human body, coupled with less than optimal intakes among the population and evidence that as much as 50% of the population carry genetic variations that make it necessary to consume choline at levels greater than the AI, it is advised that dietary guidance be developed to encourage the intake of choline-rich foods. However, there are currently two major impediments to this goal.
Current dietary guidance from a variety of health organizations recommends limiting intake of cholesterol to less than 300 milligrams day; less than 200 milligrams a day for those with existing cardiovascular disease,51 which de facto limits consumption of eggs, one of the richest sources of choline (and cholesterol) in the American diet. One large egg contains 212 milligrams of cholesterol and 125 milligrams of choline.42,52 Secondly, health professionals, including physicians and registered dietitians, have limited knowledge about the biological importance of choline and are relatively unaware of the best dietary sources. A recent survey of health professionals found that of several nutrients, including calcium, vitamin D, protein, folate, iron, and vitamins E and A, choline was the least likely to be recommended. Only about 10% of those surveyed said that they were likely to recommend foods containing choline to their patients. Among OB/GYNs, who care for the population with the greatest choline needs, only about 6% were likely to recommend foods containing choline for healthy pregnant women.53 It is evident that the current lack of awareness, knowledge and education among both health professionals and the public regarding the important role of choline in the diet may have negative health consequences.
Conclusions
There is an immediate need to increase awareness among health professionals and consumers of choline as an essential, but currently suboptimal, nutrient, and further, to highlight the critical role it plays throughout life, especially for pregnant and lactating women. New analysis of NHANES data indicates that for the majority of the population choline consumption is far below current dietary recommendations. Increasing awareness of the pervasiveness of suboptimal choline intakes must become the focus of public health efforts in order to promote optimal health. Education regarding the richest food sources of choline can assist in reaching this goal.
Figure 1.
Metabolic pathway of choline
Acknowledgement
The authors would like to thank Densie Webb, Ph.D., R.D. for assistance in preparing this article.
Funding
Preparation of the article was made possible with an unrestricted education grant from the Egg Nutrition Center. The authors have no funding to declare.
Contributor Information
Steven H. Zeisel, Nutrition Research Institute, Department of Nutrition, School of Public Health and School of Medicine, University of North Carolina at Chapel Hill, CB#7461, Chapel Hill, NC 27599, (919) 843-4731, Steven_zeisel@unc.edu
Kerry-Ann da Costa, Department of Nutrition, School of Public health and School of Medicine, The University of North Carolina at Chapel Hill, CB#7461, Chapel Hill, NC 27599, (919) 966-7346, kdacosta@unc.edu
References
- 1.Food and Nutrition Board, Institute of Medicine. Washington, D.C.: National Academy of Sciences; Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B-6, Vitamin B012, Pantothenic Acid, Biotin, and Choline. 1998:390–422.
- 2.Penry J, Manore M. Choline: an important micronutirent for maximal endurance-exercise performance? Int J Sport Nutr Exerc Metab. 2008;18:191–203. doi: 10.1123/ijsnem.18.2.191. [DOI] [PubMed] [Google Scholar]
- 3.Stead L, Brosnan J, Brosnan M, Vance D, Jacobs R. Is it time to reevaluate methyl balance in humans. Am J Clin Nutr. 2006;83:5–10. doi: 10.1093/ajcn/83.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Zeisel S. Choline, an essential nutrient for humans. FASEB. 1991;5:2093–2098. [PubMed] [Google Scholar]
- 5.Shaw G, Carmichael S, Yang W, Selvin S, Schaffer D. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 6.Rees W, Wilson F, Maloney C. Sulfur amino acid metabolism in pregnancy: the impact of methionine in the maternal diet. J Nutr. 2006;136:1701S–1705S. doi: 10.1093/jn/136.6.1701S. [DOI] [PubMed] [Google Scholar]
- 7.Fischer L, da Costa K, Kwock L, Stewart P, Lu T, Stabler S, Allen R, Zeisel S. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85:1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Vance D. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Zeisel S. Perinatal choline influences brain structure and function. Nutr Rev. 2006;64:197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisel S, Mar MH, Howe J, Holden J. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 11.Ozarda I, Uncu G, Ulus I. Free and phospholipid-bound choline concentrations in serum during pregnancy, after deliver and in newborns. Arch Physiol Biochem. 2002;110:393–399. doi: 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- 12.Zeisel S. Developmental changes in rat blood choline concentration. Biochem J. 1981;198:565–570. doi: 10.1042/bj1980565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeisel S. Choline: Critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steegers-Theunissen R, Boers G, Trijbels F, et al. Maternal hyperhomocysteinemia: a risk factor for neural-tube defects? Metabolism. 1994;4:1475–1480. doi: 10.1016/0026-0495(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 15.Fisher M, Zeisel S, Mar M, Sadler T. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology. 2001;64:114–122. doi: 10.1002/tera.1053. [DOI] [PubMed] [Google Scholar]
- 16.Meck W, Williams C. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 17.Mellott T, Follettie M, Diesl V, Hill A, Lopez-Coviella I, Blusztago J. Prenatal choline availability modulated hippocampal and cerebral cortical gene expression. FASEB J. 2007;21:1311–1323. doi: 10.1096/fj.06-6597com. [DOI] [PubMed] [Google Scholar]
- 18.Zeisel S. The fetal origins of memory: the role of dietary choline in optimal brain development. J Pediatr. 2006;149:S131–S136. doi: 10.1016/j.jpeds.2006.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signore C, Ueland P, Troendle J, Mills J. Choline concentrations in human maternal and cord blood and intelligence at 5 yr of age. Am J Clin Nutr. 2008;87:896–902. doi: 10.1093/ajcn/87.4.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas J, Biane J, OBryan K, O'Neill T, Dominguez H. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- 21.Velzing-Aarts F, Holm P, Fokkema M, van der Dijs F, Ueland P, Muskiet F. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am J Clin Nutr. 2005;81:1283–1289. doi: 10.1093/ajcn/81.6.1383. [DOI] [PubMed] [Google Scholar]
- 22.Vollset S, Refsum H, Irgens L, et al. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine Study. Am J Clin Nutr. 2000;71:962–968. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- 23.Molloy A, Mills J, Cox C, et al. choline and homocysteine interrelations in umbilical cord and maternal plasma at delivery. Am J Clin Nutr. 2005;82:836–842. doi: 10.1093/ajcn/82.4.836. [DOI] [PubMed] [Google Scholar]
- 24.da Costa KA, Gaffney C, Fischer L, Zeisel S. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81:440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Homocysteine Studies Collaboration, 2002. Homocysteine and risk of ischemic heart disease and stroke. J Am Med Assoc. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Wu J. Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin Chim Acta. 2002;322:21–28. doi: 10.1016/s0009-8981(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 27.Seshadri S, Beiser A, Selhub J, Jacques P, Rosenberg I, D’Agostino R, Wilson P, Wolf P. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 28.van Meurs J, Dhonukshe-Rutten, Pluijm S, van der Klift M, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 29.Chiuve S, Giovannucci E, Hankinson S, et al. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am J Clin Nutr. 2007;86:1073–1081. doi: 10.1093/ajcn/86.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson W, Elmslie J, Lever M, Chambers S, George P. Dietary and supplementary betaine: acute effects on plasma betaine and homocysteine concentrations under standard and postmethionine load conditions in healthy male subjects. Am J Clin Nutr. 2008;87:577–585. doi: 10.1093/ajcn/87.3.577. [DOI] [PubMed] [Google Scholar]
- 31.Jacques P, Bostom A, Wilson P, Rich S, Rosenberg I, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. 2001;73:613–621. doi: 10.1093/ajcn/73.3.613. [DOI] [PubMed] [Google Scholar]
- 32.Olthof M, Brink E, Katan M, Verhoef P. Choline supplemented as phosphatidylcholine decreases fasting and postmethionine-loading plasma homocysteine concentrations in healthy men. Am J Clin Nutr. 2005;82:111–117. doi: 10.1093/ajcn.82.1.111. [DOI] [PubMed] [Google Scholar]
- 33.Detopoulou P, Panaglotakos B, Antonopoulou S, Pittsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in health adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–430. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- 34.Cho E, Zeisel S, Jacques P, et al. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma totoal homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2008;83:905–911. doi: 10.1093/ajcn/83.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fargnoli JL, Fung TT, Olencauk DM, Chamberland JP, Hu FB, Mantzoros CS. Adherence to healthy eating patterns is associated with higher circulating total and high-molecular-weight adiponectin and lower resistin concentrations in women from the Nurses’ Health Study. Am J Clin Nutr. 2008;88:1213–1324. doi: 10.3945/ajcn.2008.26480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Department of health and Human Services, National Institutes of Health, National, Heart, Lung and Blood Institute. Statement from Elizabeth G. Nabel, M.D., Director, National Heart, Lung, and Blood Institute on New Findings on the Role of Inflammation in Prevention of Coronary Heart Disease. [Accessed November 13, 2008]; www.nih.gov/news/health/nov2008/nhlbi-`10.htm.
- 37.Bidulescu A, Chambless L, Siega-Riz A, Zeisel S, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. BMC Cardiovasc Disord. 2007;7:20. doi: 10.1186/1471-2261-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalmeijer G, Olthof M, Verhoef P, bots M, van der Schouw Y. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr. 2008;62:386–394. doi: 10.1038/sj.ejcn.1602725. [DOI] [PubMed] [Google Scholar]
- 39.da Costa K, Badea M, Fischer L, Zeisel S. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. 2004;80:163–170. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- 40.da Costa K, Niculescu M, Craciunescu, Fischer L, Zeisel S. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. 2006;84:88–94. doi: 10.1093/ajcn/84.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, Gammon M, Zeisel S, et al. Choline metabolism and risk of breast cancer in a population-based study. FASEB J. 2008;22:1–8. doi: 10.1096/fj.07-101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. [Accessed July 11, 2008]; http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choln02.pdf.
- 43.da Costa K, Kozyreva O, Song J, Galanko J, Fischer L, Zeisel S. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niculescu M, Zeisel S. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 45.Kohlmeier M, da Costa K, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeisel S. Gene response elements, genetic polymorphisms and epigenetics influence the human dietary requirement for choline. IUBMB Life. 2007;59:380–387. doi: 10.1080/15216540701468954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer L, Scearce J, Mar M-H, et al. Ad libitum choline intake in healthy individuals meets or exceeds the proposed adequate intake level. J Nutr. 2005;135:826–829. doi: 10.1093/jn/135.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen H, Batres-Marques, Carriquiry A, Schalinske K. Choline in the diets of the U.S,. Population: NHANES, 2003–2004; Presented at the National Nutrient Data Bank Conference; 2007. [Google Scholar]
- 49.Keast D. Food sources of choline in the diets of US older adults: NHANES, 2999-2004; Presented at the National Nutrient Data Bank Conference; 2007. [Google Scholar]
- 50.Holmes-McNary M, Cheng WL, Mar MH, Fussel S, Zeisel S. Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr. 1996;64:572–576. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]
- 51.National Heart, Lung, and Blood Institute. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Bethesda, Maryland: US Department of Health and Human Services, Public Health Service, National Institutes of Health; 2002 publication no. (NIH) 02–5215.
- 52. [Accessed July 11, 2008]; http://www.nal.usda.gov/fnic/foodcomp/search.
- 53.StrategyOne Health Professionals Survey. 2007 April