Abstract
Background
Women with clinical findings suggestive of ischemia but without findings of obstructive coronary artery disease (CAD) on angiography represent a frequent clinical problem; predicting prognosis is challenging.
Methods
The Women’s Ischemia Syndrome Evaluation (WISE) study examined symptomatic women referred for clinically indicated coronary angiography and followed up for a mean 5.2 years. The St James Women Take Heart (WTH) Project enrolled asymptomatic, community-based women with no history of heart disease who were followed up for 10 years. We compared cardiovascular events (ie, myocardial infarction, stroke, and hospitalization for heart failure) and death in 540 WISE women with suspected ischemia but no angiographic evidence of obstructive CAD with those from a cohort of 1000 age- and race-matched WTH women.
Results
Compared with the WISE women, asymptomatic WTH women had a lower prevalence of obesity, family history of CAD, hypertension, and diabetes mellitus (P<.001). Five-year annualized event rates for cardiovascular events were 16.0% in WISE women with nonobstructive CAD (stenosis in any coronary artery of 1%–49%), 7.9% in WISE women with normal coronary arteries (stenosis of 0% in all coronary arteries), and 2.4% in asymptomatic WTH women (P≤.002), after adjusting for baseline CAD risk factors. The cardiovascular events were most frequent in women with 4 or more cardiac risk factors, with the 5-year annualized cardiovascular event rate being 25.3% in women with nonobstructive CAD, 13.9% in WISE women with normal coronary arteries, and 6.5% in asymptomatic women (P=.003).
Conclusion
Women with symptoms and signs suggestive of ischemia but without obstructive CAD are at elevated risk for cardiovascular events compared with asymptomatic community-based women.
Symptoms of angina in the absence of clinically significant coronary artery disease (CAD) constitute a relatively common clinical scenario in women and remain a challenge for physicians caring for such patients. Until recently, the prognosis of women with signs and symptoms suggestive of myocardial ischemia in the absence of obstructive CAD was thought to be benign,1–3 and such women have been offered little more than reassurance that they do not have heart disease, despite signs and symptoms that have required them to undergo coronary angiography.4 More recently, the notion of its benign nature has been challenged, with evidence showing that women with chest pain in the setting of normal or nonobstructive coronary arteries have a high risk of future cardiac events.5–9 To our knowledge, there have been no prospective studies examining the implications of chest pain in the absence of obstructive CAD in women, relative to a population of asymptomatic women.
Chest pain and other equivalent cardiac symptoms that are suggestive of myocardial ischemia, even in the absence of obstructive CAD, have important functional and economic implications to women and society. A recent retrospective study of men and women with suspected ischemia resulting in referral for angiography showed that women were more often found to have angiographically normal coronary arteries. These same women were 4 times more likely to be readmitted for chest pain or for acute coronary syndrome within the next 180 days.9 Data from the Women’s Ischemia Syndrome Evaluation (WISE) study estimate that, among the approximately 500 000 US women who undergo coronary angiography annually, half will have no obstructive lesions (where obstruction is considered ≥50% narrowing in any coronary artery) in contrast to 7% to 17% of men who undergo angiography.9–13
Given this high rate of nonobstructive coronary angiograms, until recently such women were usually offered little in terms of treatment, despite recurrent symptoms requiring hospitalization, repeated procedures, functional disabilities, and future cardiac events that translate to a heavy economic burden with average lifetime costs estimated to be greater than $750 000.14 Despite being a common clinical scenario with important public health consequences, the prevalence of chest pain in the absence of obstructive CAD has not declined since it was first reported.15–18
We sought to investigate the prognostic implications of cardiac symptoms in women with nonobstructive CAD compared with community-dwelling women without cardiac symptoms. We investigated this prospectively in a cohort of symptomatic women referred for clinically indicated coronary angiography and compared them with a cohort of asymptomatic women free of known CAD at baseline.
METHODS
SYMPTOMATIC WOMEN
The symptomatic women were enrolled in the WISE study, sponsored by the National Institutes of Health and National Heart, Lung, and Blood Institute, a prospective study that enrolled women at 4 clinical centers. The aim of the WISE study was to improve diagnostic testing strategies and advance new hypotheses related to the pathophysiologic processes of cardiovascular disease in women and has been described in full elsewhere. 19 The study cohort included 936 women of 7603 screened who presented with chest pain symptoms or suspected myocardial ischemia and underwent diagnostic coronary angiography. Women with a diagnosis of CAD or a previous coronary event (ie, myocardial infarction, stroke, or revascularization) were excluded from this study.
All participants underwent a baseline physical examination that included blood pressure and physical assessments. Demographic data, medical history, and symptoms were collected at the initial evaluation. Cardiac risk was assessed using a self-report questionnaire inquiring about the patient’s history of diabetes mellitus, dyslipidemia, and hypertension and family history of CAD and other cardiac risk factors. All women underwent coronary angiography at enrollment, and these angiograms were quantitatively and qualitatively evaluated for the presence and extent of CAD by the WISE angiographic core laboratory (blinded to historical data) as previously described. 20 Obstructive CAD was defined as 50% or greater stenosis in any epicardial coronary artery, and only the 540 women with normal coronary arteries (defined as 0% stenosis in all coronary arteries) or nonobstructive CAD (defined as 1%–49% stenosis in any coronary artery) were included in this analysis.
Outcome data were collected by telephone interview at 6 weeks after the initial assessment and annually thereafter. Death certificates were reviewed blindly to determine the cause of death. The mean follow-up time was 5.2 years. Study procedures and follow-up methods were approved by each center’s institutional review board. Informed consent was received from all WISE participants.
ASYMPTOMATIC WOMEN
The asymptomatic population came from the St James Women Take Heart (WTH) Project. The aim of the WTH was to evaluate the role of exercise stress testing in asymptomatic women. The WTH cohort has been described in full previously.21,22 Briefly, in 1992 a call for volunteers from the greater Chicago metropolitan area resulted in a cohort of 5932 asymptomatic women. Inclusion criteria were being 35 years or older, having no active cardiovascular disease, and being able to walk on a treadmill at a moderate pace. Women were excluded if they were pregnant, had experienced typical anginal symptoms or myocardial infarction within the previous 3 months, weighed more than 147 kg, or had blood pressure of 170/110 mm Hg or higher before initiating the stress test.
All participants underwent a physical examination. During the recording of the baseline resting electrocardiography, supine blood pressure was measured by technicians using standard clinical procedures.23
For this analysis, the WTH study-specific exclusion criteria were (1) performance of the modified Bruce protocol (n=109); (2) presence of any cardiac disease, including previous myocardial infarction, documented cardiovascular disease, heart failure, or valvular heart disease (n=91); and (3) incomplete data concerning cardiac risk factors (n=11). In 2001, a clinical follow-up was performed. Questionnaires were used to document new cardiovascular outcomes since the baseline evaluation. All-cause mortality was determined using a National Death Index search to identify deaths and causes of death from the baseline evaluation in 1992 through the end of 2000.
Study procedures and follow-up methods were approved by the institutional review boards of the study’s research centers. Informed consent was received from all WTH participants.
CARDIOVASCULAR END POINTS
The cardiovascular end point collected in both cohorts included myocardial infarction, hospitalization for heart failure, stroke, cardiac mortality, and all-cause mortality. The primary composite end point consisted of cardiovascular events, defined as the composite of myocardial infarction, hospitalization for heart failure, stroke, or cardiac mortality. The secondary composite end point consisted of myocardial infarction, hospitalization for heart failure, stroke, or all-cause mortality.
STATISTICAL ANALYSIS
For the 540 WISE women without obstructive CAD, we randomly selected 1000 age- and race-matched WTH women without previous evidence of CAD. Matching was accomplished by oversampling among minority and older WTH women. The WISE women were further stratified by the presence vs absence of obstructive stenosis, with normal coronary arteries defined as 0% stenosis (normal coronary arteries) (n=318), and nonobstructive coronary arteries defined as 1% to 49% luminal stenosis (n=222). Descriptive analyses of all variables were examined. Population characteristics between the symptomatic (WISE) and asymptomatic (WTH) women were compared using χ2 tests or Fisher exact measures for categorical variables or using the t test or nonparametric rank sum test (2 sided), where appropriate, for continuous variables. Because time-to-event data were unavailable in the WTH women, standard survival analysis methods could not be used. Instead, we calculated average 5-year event rates that were then entered as the number of events per subgroup into χ2 analysis. To calculate risk-adjusted P values, adjusted event rates were generated by general linear methods and reconverted to 5-year risk-adjusted number of events per subgroup for χ2 analysis.
RESULTS
COMPARISON OF POPULATION CHARACTERISTICS
The WISE and WTH women were well matched, with a mean (SD) age of 55.7 (10.8) vs 55.0 (10.3) years (P=.20) and racial minorities constituting 17.6% vs 17.5% (P=.96). Within the symptomatic WISE cohort of 936 women, a total of 396 (42.3%) had 50% or greater stenosis in 1 or more coronary arteries. Among those without obstructive lesions, 318 (58.9%) had normal coronary arteries and 222 (41.1%) had nonobstructive CAD. Baseline characteristics of the WISE and WTH populations are summarized in Table 1. As expected, most traditional cardiac risk factors were more prevalent in the symptomatic compared with the asymptomatic women, with the highest prevalence in those with nonobstructive CAD (P<.001). The only exceptions were that the asymptomatic women had lower mean high-density lipoprotein (HDL-C) and higher mean low-density lipoprotein (LDL-C) cholesterol levels compared with the symptomatic cohort, regardless of the degree of stenosis. Diastolic blood pressure was also higher in the asymptomatic women (P<.001). Family history of CAD was highest in those with symptoms of angina, but there was no difference in the prevalence in those with normal coronary arteries compared with those with nonobstructive CAD. The use of aspirin, antihypertensive medication, and medications to lower lipid levels was significantly greater in the symptomatic women, particularly those with nonobstructive CAD (P<.001) (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristicsa
| Symptomatic Women (WISE) | ||||
|---|---|---|---|---|
| Asymptomatic Women (WTH) (n=1000) |
Normal Coronary Arteriesb (n=318) |
Nonobstructive CADc (n=222) |
Age-Adjusted P Value |
|
| Age, mean (SD), y | 54.8 (10.3) | 53.6 (10.4) | 58.7 (10.6) | .02 |
| BMI, median (IQR) | 26.0 (23.4–29.9) | 29.1 (25.6–34.6) | 28.9 (24.7–33.2) | <.001 |
| Race | .85 | |||
| White | 82.2 | 81.8 | 83.3 | |
| African American | 12.3 | 17.3 | 16.2 | |
| Other | 5.5 | 0.9 | 0.4 | |
| Postmenopausal | 68.1 | 73.2 | 85.9 | <.001 |
| Family history of CAD | 43.6 | 66.2 | 64.5 | <.001 |
| Hypertensiond | 17.6 | 50.2 | 60.8 | <.001 |
| Diabetes mellituse | 5.0 | 14.2 | 19.8 | <.001 |
| Metabolic syndromef | 35.5 | 49.8 | 59.5 | <.001 |
| Smoking history | 17.2 | 45.6 | 57.7 | <.001 |
| SBP, mean (SD), mm Hg | 129.7 (19.1) | 134.8 (19.8) | 136.5 (21.4) | <.001 |
| DBP, mean (SD), mm Hg | 82.2 (10.2) | 76.8 (10.5) | 76.9 (10.8) | <.001 |
| HDL-C level, mean (SD), mg/dL | 52.4 (15.3) | 55.7 (12.3) | 54.3 (13.8) | .003 |
| LDL-C level, mean (SD), mg/dL | 136.0 (34.8) | 111.3 (36.2) | 113.8 (39.6) | <.001 |
| Use of medications to lower lipid levels | 2.7 | 11.7 | 36.5 | <.001 |
| Antihypertensive medication use | 12.3 | 40.7 | 47.8 | <.001 |
| Aspirin use | 23.9 | 44.3 | 62.0 | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAD, coronary artery disease; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; WISE, Women’s Ischemia Syndrome Evaluation; WTH, St James Women Take Heart Project.
SI conversion factor: To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
Unless otherwise indicated, data are expressed as percentage of patients.
Indicates 0% stenosis.
Indicates 1% to 49% stenosis.
Based on a self-reported history of hypertension (treated or untreated).
Based on a self-reported history of diabetes mellitus and/or fasting blood glucose level of greater than 140 mg/dL or nonfasting blood glucose level of greater than 200 mg/dL (to convert glucose to millimoles per liter, multiply by 0.0555).
Defined by the Third Adult Treatment Panel criteria, with at least 3 of 5 of the stated criteria.
5-YEAR CARDIOVASCULAR EVENT RATES
Rates of adverse cardiovascular events were highest for the symptomatic women with nonobstructive CAD compared with the symptomatic women with normal coronary arteries (P<.001) (Table 2). However, symptomatic women with normal coronary arteries had approximately 3-fold higher rates of the primary and secondary composite end points compared with the asymptomatic cohort of women (P=.002 and P=.008, respectively), even after adjusting for age, race, body mass index (calculated as weight in kilograms divided by height in meters squared), history of hypertension and diabetes mellitus, education, employment, family history of CAD, menopausal status, smoking history, and presence of the metabolic syndrome (Table 2). In addition, further analyses were performed that adjusted for LDL-C and HDL-C levels and history of hypertension, and no significant differences were found compared with the reported analysis; however, these covariates were not available for all women and are not reported herein. The increased rates of events in symptomatic women with normal coronary arteries were largely accounted for by increased incidence of hospitalization for heart failure (P=.002) and stroke (P=.004). Although the rate of cardiovascular death was more than twice as high as the rate in the control WTH (asymptomatic) women, this was not statistically significant.
Table 2.
Five-Year Rates of Cardiovascular Outcomes in Asymptomatic Women Compared With Symptomatic Women With Normal and Nonobstructive CAD
| Symptomatic Women (WISE) | |||||
|---|---|---|---|---|---|
| Asymptomatic Women (WTH) (n=1000) |
Normal Coronary Arteriesa (n=318) |
Nonobstructive CADb (n=222) |
Adjusted P Valuec |
Adjusted P Valuec,d |
|
| MI, % | 0.7 | 0.9 | 3.9 | .07 | .31 |
| Hospitalization for CHF, % | 0.3 | 3.3 | 5.6 | <.001 | .002 |
| Stroke, % | 1.0 | 2.4 | 5.2 | .002 | .004 |
| Death due to CV, % | 0.6 | 1.5 | 4.4 | .11 | .82 |
| All-cause mortality, % | 2.1 | 3.0 | 8.2 | .04 | .74 |
| Primary composite end point, %e | 2.4 | 7.9 | 16.0 | <.001 | .002 |
| Secondary composite end point, %f | 3.9 | 9.1 | 19.1 | <.001 | .008 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CV, cardiovascular causes; MI, myocardial infarction; WISE, Women’s Ischemia Syndrome Evaluation; WTH, St James Women Take Heart Project.
Indicates 0% stenosis.
Indicates 1% to 49% stenosis.
Adjusted for age, race, body mass index, systolic blood pressure, diabetes mellitus, education, employment, family history of CAD, smoking history, and the metabolic syndrome.
Compares the WTH cohort with the WISE cohort who had normal coronary arteries.
Consists of MI, hospitalization for heart failure, stroke, or cardiovascular death.
Consists of MI, hospitalization for heart failure, stroke, or death due to any cause.
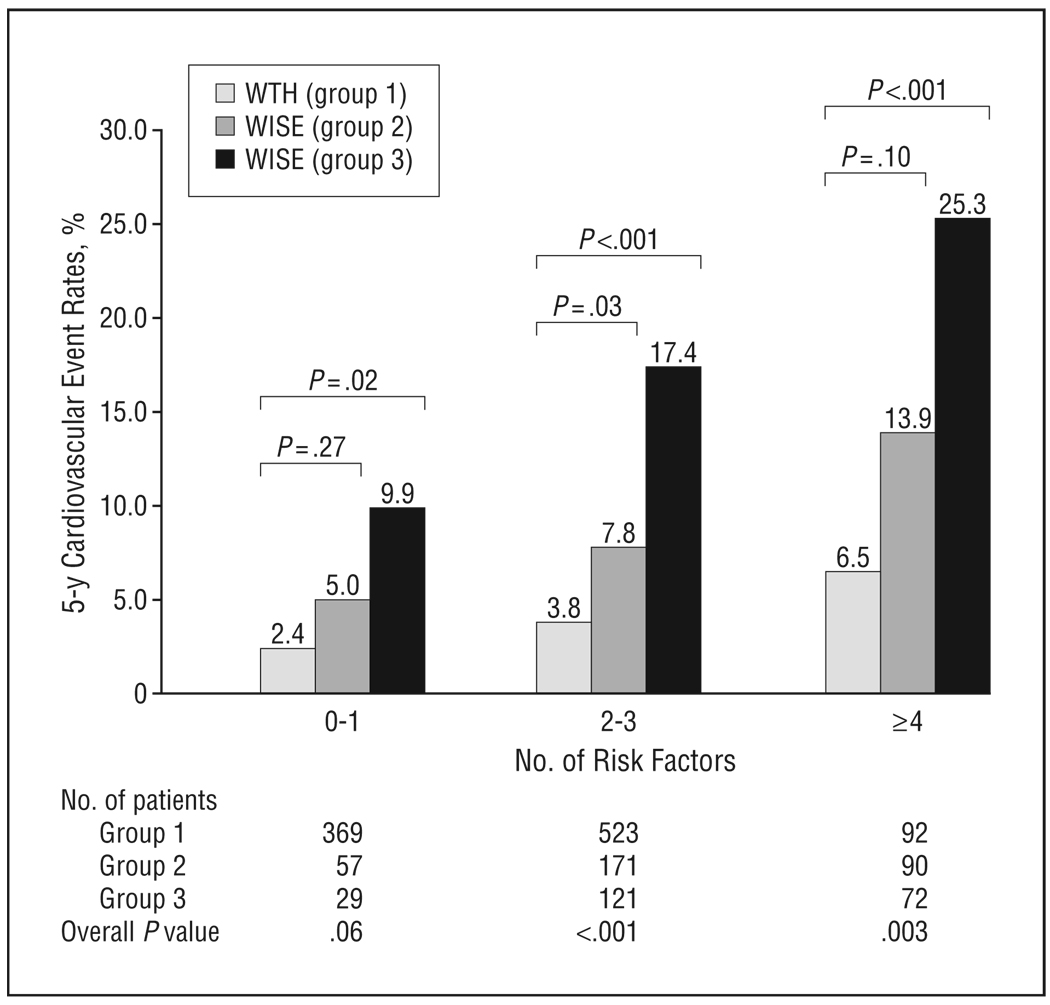
When categorized by the number of cardiovascular risk factors, which included smoking history, diabetes, LDL-C level of greater than130mg/dL,HDL-Clevel of greater than 50 mg/dL (to convert cholesterol levels to millimoles per liter, multiplyby0.0259), family history of CAD, body mass index of 30 or greater, and systolic blood pressure higher than 140 mm Hg, within each population of women there was an increased rate of the primary composite end point with an increasing number of cardiac risk factors (Figure1). The WISE women had higher rates of the primary composite end point within all risk categories compared with the WTH women. Among those with 4 or more cardiovascular risk factors, WISE women with nonobstructive CAD were more than 3 times as likely to experience the primary composite end point than asymptomatic women( 25.3%vs6.5%, respectively) (P<.001).
Figure 1.
Five-year primary composite event rate according to risk factor category. Rates are presented for asymptomatic women (group 1) compared with symptomatic women with normal coronary arteries (0% stenosis) (group 2) and nonobstructive coronary artery disease (stenosis in any coronary artery of 1%–49%) (group 3). Cardiovascular risk factors include smoking, diabetes mellitus, low-density lipoprotein cholesterol level of greater than 130 mg/dL, high-density lipoprotein cholesterol level of less than 50 mg/dL, family history of coronary artery disease, body mass index (calculated as weight in kilograms divided by height in meters squared) of 30 or more, and systolic blood pressure higher than 140 mm Hg. Primary composite event consists of nonfatal myocardial infarction, hospitalization for congestive heart failure, stroke, or cardiovascular death. WISE indicates Women’s Ischemia Syndrome Evaluation; WTH, St James Women Take Heart Project. To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
The relationship between age and cardiovascular events differed by subgroups (Figure 2). In asymptomatic women, age was an important risk factor with respect to outcome. Specifically, before age 62 years, the event rate increased slightly by age. After 62 years of age, the event rate underwent a sharp increase (P<.001 for trend), becoming similar to the event rate experienced by symptomatic women without noticeable stenosis. In symptomatic women with completely normal-appearing coronary arteries, there was a slight trend toward increasing event rates with age. However, this trend is not statistically significant (P=.55 for trend). Despite similar event rates in both of these groups after 62 years of age, the overall rates differ significantly, as demonstrated in Figure 2 (P=.003). Finally, age is a significant factor in symptomatic women with nonobstructive CAD (P=.047 for trend), with most of the difference from the other 2 groups emerging after 54 years of age (Figure 2).
Figure 2.
Five-year primary composite event rate according to age. Rates are presented for asymptomatic women (group 1) compared with symptomatic women with normal coronary arteries (0% stenosis) (group 2) and nonobstructive coronary artery disease (stenosis in any coronary artery of 1%–49%) (group 3). Primary composite event rate consists of nonfatal myocardial infarction, hospitalization for congestive heart failure, stroke, or cardiovascular death. WISE indicates Women’s Ischemia Syndrome Evaluation; WTH, St James Women Take Heart Project.
COMMENT
Women with cardiac symptoms but without obstructive CAD constitute a relatively common clinical scenario and, until recently, have been considered to be a low-risk population based on early cohort studies.2,3,24 Despite evidence of myocardial ischemia that is demonstrated after presenting with cardiac symptoms, an angiogram that is reportedly normal or shows nonobstructive CAD will ultimately result in little medical treatment, aside from reassurance, despite the presence of cardiac symptoms. This approach is of concern to clinicians and patients because many of these women will continue to have symptoms that will lead to rehospitalization, repeated diagnostic testing, and little treatment or relief of the presenting symptoms. Our study demonstrates for the first time, to our knowledge, that community-dwelling women with symptoms of myocardial ischemia have an increased risk of future cardiovascular events, even in the absence of obstructive CAD by coronary angiography or when results of an angiogram are considered completely normal compared with asymptomatic women. The risk of nonfatal and fatal cardiovascular events for symptomatic women with nonobstructive CAD during the next 5 years is almost twice that of symptomatic women with normal coronary arteries and 4-fold greater than that of asymptomatic women. This is the first study to date demonstrating that normal coronary arteries associated with cardiac symptoms do not carry a benign prognosis when compared with those of asymptomatic women, even after controlling for traditional cardiac risk factors.
Only 1 previous study in women shows that the presence of ischemic symptoms may be associated with increased cardiovascular events.25 The Women’s Health Initiative Observational Study followed up a large cohort of postmenopausal women free of heart disease at baseline. The authors demonstrated that women who were hospitalized with a diagnosis of nonspecific chest pain were at an increased risk for a future cardiovascular event after the hospitalization (11.0% compared with 9.5% in those without such a hospitalization). These women with nonspecific chest pain were older and had more cardiac risk factors, which is confirmed by our findings. However, that study included only women who were hospitalized overnight and thus did not include women who may have had an outpatient workup for similar symptoms; importantly, no data were provided regarding whether angiography was performed or what the results of such testing were. Nonetheless, results of that study and ours support the notion that symptoms suggestive of ischemia may be an independent predictor of cardiovascular events.
We hypothesize that the reason for the poor prognosis in symptomatic women in the absence of obstructive CAD is the fact that an appreciable number of women with cardiac symptoms have myocardial ischemia due to endothelial dysfunction, as previously shown in a subset of women from the WISE Study.5 Therefore, the appearance of normal coronary arteries may be misleading because many women may have myocardial ischemia related to atherosclerotic disease reflected by endothelial dysfunction that is not seen by results of traditional coronary angiography. Endothelial dysfunction reflects an abnormal coronary arteriole response to myocardial oxygen demand. In normal coronary arteries, coronary flow reserve can increase 2.5- to 5-fold in response to a metabolic or pharmacologic stress.26,27 An impaired response reflects ischemia that may be occurring during periods of increased myocardial oxygen demand.28
It has been hypothesized that this abnormal endothelial response may be the earliest marker of vascular disease, representing a critical step in atherosclerosis.29,30 The mechanism of this impaired endothelial response appears to be due to imbalance between endothelium-derived vasodilators that have antithrombotic and antimitogenic properties and endothelium-derived vasoconstricting factors with proatherogenic activity. 31–33 Endothelial dysfunction can be detected by coronary flow response during cardiac catheterization using intracoronary Doppler ultrasonography velocity with provoking agents such as acetylcholine.6,34 It can also be assessed using single-photon emission computed tomography, 35 positron emission tomography,26 and magnetic resonance imaging,35,36 where myocardial perfusion is assessed at the endothelial level.
Previous studies, including studies from the WISE cohort, have demonstrated that women without angiographic CAD but with persistent anginal symptoms have a worse prognosis if there is evidence of endothelial dysfunction. 5–7,37,38 In the WISE study, 74 of the women with no CAD on angiography underwent magnetic resonance spectroscopy to detect myocardial ischemia with handgrip stress, and 14 of these had an abnormal result. Women with no obstructive CAD and a normal magnetic resonance spectroscopy finding had a 13% cardiovascular event rate during the next 3 years. Women with no obstructive CAD but an abnormal magnetic resonance spectroscopy finding had a cardiovascular event rate of 43% during the next 3 years, which was similar to the event rate in the reference WISE women with obstructive CAD (48%) (P=.42).5 Another study that followed up 42 women with chest pain, angiographically normal coronary arteries, and evidence of severe endothelial dysfunction demonstrated that 30% of these women developed CAD during the next 10 years.6 Similar findings were shown in a study by Suwaidi et al,37 in which 157 men and women with nonobstructive CAD underwent testing for endothelial dysfunction. Those with evidence of severe endothelial dysfunction had an increase in cardiovascular events.
Our present study also demonstrated that, although the number of cardiac risk factors was directly associated with cardiac events, the presence of ischemic symptoms in these women was an independent predictor of future cardiac events, despite their angiographically normal or nonobstructive coronary arteries. Previous studies have shown that endothelial dysfunction is associated with traditional cardiac risk factors known to predispose to atherosclerosis. 39,40 Although the relative importance of each cardiac risk factor is difficult to determine in the small studies to date, several studies have demonstrated that the treatment of cardiovascular risk factors known to result in endothelial dysfunction is associated with an improvement in endothelial response.41–44 It has been hypothesized by others that endothelial dysfunction may be thought of as the integrated risk of cardiac risk factors and may serve as a sensitive marker for their functional significance,33,45 which may explain why in the symptomatic women the effect of cardiac risk factors on future cardiac events was greatest in the women with nonobstructive CAD compared with symptomatic women with normal coronary arteries and was least in the asymptomatic women. The risk factor burden seems to be greatest in those with some visible atherosclerotic disease, despite its nonobstructive nature. Nonetheless, women with normal-appearing coronary arteries who are symptomatic have a poorer prognosis, even with the same risk factor burden, possibly because their symptoms reflect endothelial dysfunction due to the effect of traditional cardiac risk factors on these women.
STUDY LIMITATIONS
The symptomatic WISE women included only women who were referred for a coronary angiogram if clinically indicated based on signs and symptoms suggestive of myocardial ischemia. This group would include both appropriate and potentially inappropriate referrals, but such a referral pattern reflects what is seen in most angiographic laboratories. The WISE cohort was accessed through 1 of 4 hospital systems and, as such, this cohort reflects women who have access to health care.
The asymptomatic WTH cohort is a volunteer cohort of women who were asked to undergo an exercise stress test as part of the study and is subject to the usual effect of volunteer bias.
Cardiovascular events were self-reported in both cohorts and, as a result, were subject to recall bias. Only deaths in the WISE cohort were confirmed by medical records and adjudicated for cause. The WTH cohort was reexamined and interviewed for cardiovascular events at 10 years in contrast to the annual collection of events by the WISE investigators, possibly introducing more recall bias into the WTH event rates. All of the women undergoing assessment in both cohorts came from different geographical areas with different rates of cardiovascular disease, and this may be a confounder in the analysis. A subset of the WISE cohort underwent assessment of endothelial function, and we based our hypothesis on this and the findings of other studies.
Another limitation of this study was our inability to adjust for left ventricular hypertrophy on electrocardiography because this was not available for the full WISE cohort. Evidence of left ventricular hypertrophy has been shown to be associated with increased cardiovascular events.46 Nonetheless, we adjusted for systolic blood pressure, which is often associated with left ventricular hypertrophy.47
CLINICAL IMPLICATIONS
Based on our findings reported herein and the findings of others5–7,34,37,38 linking endothelial dysfunction and future cardiovascular events, we recommend that all women with symptoms suggestive of ischemia undergo initial evaluation for obstructive CAD. If there is no evidence of obstructive CAD, such women need further assessment for endothelial dysfunction. If endothelial dysfunction is identified by coronary vascular function studies, targeted treatment to improve endothelial function is recommended. 4 Women with symptoms but without endothelial dysfunction need aggressive risk factor modification. 4 Asymptomatic women should have routine risk factor management, as outlined by the American Heart Association guidelines for heart disease prevention in women (Figure 3).48
Figure 3.
Suggested algorithm for the treatment of women with and without symptoms suggestive of ischemia. AHA indicates American Hospital Association; CAD, coronary artery disease; MRI, magnetic resonance imaging; PET, positron emission tomography; and SPECT, single-photon emission computed tomography. Adapted and modified with permission from Bugiardini and Bairey Merz.4
CONCLUSIONS
Women with signs and symptoms of myocardial ischemia but normal coronary arteries or a nonobstructive finding on a coronary angiogram historically have been told that they do not have heart disease. Nonetheless, these women often have persistent symptoms that require re-hospitalization and repeated cardiac testing and that pose great difficulty to clinicians caring for such women.14 We have demonstrated that such symptomatic women with normal and nonobstructive CAD are at a relatively high risk of future cardiac events, particularly symptomatic women with 1 or more cardiac risk factors. Given that CAD remains the leading cause of death in women in the United States and that this clinical scenario of cardiac symptoms in the absence of obstructive CAD is relatively common, future investigations need to expand on the mechanisms and pathophysiologic processes of vascular dysfunction in symptomatic women. In addition, future research needs to focus on randomized clinical trials to determine the effectiveness of treatment both for symptoms and for endothelial dysfunction. Nonetheless, symptomatic women with normal coronary arteries or nonobstructive CAD do not have a benign prognosis and, as such, should have cardiac risk factors aggressively treated and possibly should be considered for further testing for endothelial dysfunction.
Acknowledgment
Funding/Support: This study was supported by contracts N01-HV-68161, N01-HV-68162, N01-HV- 68163, and N01-HV-68164 from the National Heart, Lung, and Blood Institute; General Clinical Research Center grant MO1-RR00425 from the National Center for Research Resources; and grants from the Gustavus and Louis Pfeiffer Research Foundation, the Women’s Guild of Cedars-Sinai Medical Center, and the Ladies Hospital Aid Society of Western Pennsylvania.
Footnotes
Author Contributions: Study concept and design: Gulati, Cooper-DeHoff, Shaw, Handberg, Zineh, Kelsey, Arnsdorf, Black, Pepine, and Bairey Merz. Acquisition of data: Gulati, Shaw, Arnsdorf, Black, Pepine, and Bairey Merz. Analysis and interpretation of data: Gulati, Cooper-DeHoff, McClure, Johnson, Shaw, Handberg, Kelsey, Arnsdorf, Black, and Bairey Merz. Drafting of the manuscript: Gulati, Cooper-DeHoff, McClure, Shaw, and Handberg. Critical revision of the manuscript for important intellectual content: Cooper-DeHoff, Johnson, Shaw, Handberg, Zineh, Kelsey, Arnsdorf, Black, Pepine, and Bairey Merz. Statistical analysis: Gulati, McClure, Johnson, Shaw, and Kelsey. Obtained funding: Gulati, Black, and Bairey Merz. Administrative, technical, and material support: Gulati, Black, and Bairey Merz. Study supervision: Johnson, Kelsey, Black, and Bairey Merz.
Financial Disclosure: None reported.
Additional Contributions: The women who participated and continue to participate in the WTH and WISE study made an immeasurable contribution to the study of heart disease in women.
References
- 1.Bemiller CR, Pepine CJ, Rogers AK. Long-term observations in patients with angina and normal coronary arteriograms. Circulation. 1973;47(1):36–43. doi: 10.1161/01.cir.47.1.36. [DOI] [PubMed] [Google Scholar]
- 2.Kemp HG, Kronmal RA, Vlietstra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986;7(3):479–483. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 3.Lichtlen PR, Bargheer K, Wenzlaff P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol. 1995;25(5):1013–1018. doi: 10.1016/0735-1097(94)00519-v. [DOI] [PubMed] [Google Scholar]
- 4.Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293(4):477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BD, Shaw LJ, Buchthal SD, et al. National Institutes of Health-National Heart, Lung, and Blood Institute. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(24):2993–2999. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 6.Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation. 2004;109(21):2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. [DOI] [PubMed] [Google Scholar]
- 7.von Mering GO, Arant CB, Wessel TR, et al. National Heart, Lung, and Blood Institute. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 8.Bugiardini R, Manfrini O, De Ferrari GM. Unanswered questions for management of acute coronary syndrome: risk stratification of patients with minimal disease or normal findings on coronary angiography. Arch Intern Med. 2006;166(13):1391–1395. doi: 10.1001/archinte.166.13.1391. [DOI] [PubMed] [Google Scholar]
- 9.Humphries KH, Pu A, Gao M, Carere RG, Pilote L. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J. 2008;155(2):375–381. doi: 10.1016/j.ahj.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Pepine CJ, Balaban RS, Bonow RO, et al. American College of Cardiology Foundation. Women’s Ischemic Syndrome Evaluation: current status and future research directions: report of the National Heart, Lung and Blood Institute Workshop, October 2–4, 2002, section I: diagnosis of stable ischemia and ischemic heart disease. Circulation. 2004;109(6):e44–e46. doi: 10.1161/01.CIR.0000116206.77324.5B. [DOI] [PubMed] [Google Scholar]
- 11.Hochman JS, McCabe CH, Stone PH, et al. TIMI Investigators. Outcome and profile of women and men presenting with acute coronary syndromes: a report from TIMI IIIB: Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1997;30(1):141–148. doi: 10.1016/s0735-1097(97)00107-1. [DOI] [PubMed] [Google Scholar]
- 12.Hochman JS, Tamis JE, Thompson TD, et al. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med. 1999;341(4):226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 13.Glaser R, Herrmann HC, Murphy SA, et al. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA. 2002;288(24):3124–3129. doi: 10.1001/jama.288.24.3124. [DOI] [PubMed] [Google Scholar]
- 14.Shaw LJ, Merz CN, Pepine CJ, et al. Women’s Ischemia Syndrome Evaluation (WISE) Investigators. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114(9):894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 15.Kemp HG, Elliott WC, Gorlin R. The anginal syndrome with normal coronary arteriography. Trans Assoc Am Physicians. 1967;80:59–70. [PubMed] [Google Scholar]
- 16.Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med. 1967;276(19):1063–1066. doi: 10.1056/NEJM196705112761904. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy JW, Killip T, Fisher LD, Alderman EL, Gillespie MJ, Mock MB. The clinical spectrum of coronary artery disease and its surgical and medical management, 1974–1979: the Coronary Artery Surgery Study. Circulation. 1982;66(5 pt 2):III16–III23. [PubMed] [Google Scholar]
- 18.Sullivan AK, Holdright DR, Wright CA, Sparrow JL, Cunningham D, Fox KM. Chest pain in women: clinical, investigative, and prognostic features. BMJ. 1994;308(6933):883–886. doi: 10.1136/bmj.308.6933.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) Study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33(6):1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 20.Sharaf BL, Williams DO, Miele NJ, et al. A detailed angiographic analysis of patients with ambulatory electrocardiographic ischemia: results from the Asymptomatic Cardiac Ischemia Pilot (ACIP) Study angiographic core laboratory. J Am Coll Cardiol. 1997;29(1):78–84. doi: 10.1016/s0735-1097(96)00444-5. [DOI] [PubMed] [Google Scholar]
- 21.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108(13):1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 22.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353(5):468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 23.Recommendations for human blood pressure determination by sphygmomanometers. Circulation. 1988;77(2):501A–504A. [PubMed] [Google Scholar]
- 24.Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function: long-term follow-up study. J Am Coll Cardiol. 1995;25(4):807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 25.Robinson JG, Wallace R, Limacher M, et al. Elderly women diagnosed with nonspecific chest pain may be at increased cardiovascular risk. J Womens Health (Larchmt) 2006;15(10):1151–1160. doi: 10.1089/jwh.2006.15.1151. [DOI] [PubMed] [Google Scholar]
- 26.Reis SE, Holubkov R, Conrad Smith AJ, et al. WISE Investigators. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141(5):735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 27.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 28.Sheps DS, Kaufmann PG, Sheffield D, et al. Sex differences in chest pain in patients with documented coronary artery disease and exercise-induced ischemia: results from the PIMI Study. Am Heart J. 2001;142(5):864–871. doi: 10.1067/mhj.2001.119133. [DOI] [PubMed] [Google Scholar]
- 29.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108(17):2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 31.Lerman A, Burnett JC., Jr. Intact and altered endothelium in regulation of vasomotion. Circulation. 1992;86(6) suppl:III12–III19. [PubMed] [Google Scholar]
- 32.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9(11):1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 33.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111(3):363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 34.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 35.Doyle M, Fuisz A, Kortright E, et al. The impact of myocardial flow reserve on the detection of coronary artery disease by perfusion imaging methods: an NHLBI WISE study. J Cardiovasc Magn Reson. 2003;5(3):475–485. doi: 10.1081/jcmr-120022263. [DOI] [PubMed] [Google Scholar]
- 36.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346(25):1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 37.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 38.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr., Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107(22):2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 39.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24(6):1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 40.Vita JA, Treasure CB, Nabel EG, et al. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81(2):491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 41.Gokce N, Vita JA, McDonnell M, et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95(2):266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Celermajer DS. Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Ann Intern Med. 1999;130(7):578–581. doi: 10.7326/0003-4819-130-7-199904060-00017. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson BE, Tyni-Lenne R, Svedenhag J, et al. Physical training in syndrome X: physical training counteracts deconditioning and pain in syndrome X. J AmColl Cardiol. 2000;36(5):1619–1625. doi: 10.1016/s0735-1097(00)00931-1. [DOI] [PubMed] [Google Scholar]
- 44.Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes: the RECIFE (Reduction of Cholesterol in Ischemia and Function of the Endothelium) Trial. Circulation. 1999;99(25):3227–3233. doi: 10.1161/01.cir.99.25.3227. [DOI] [PubMed] [Google Scholar]
- 45.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 46.Ang D, Lang C. The prognostic value of the ECG in hypertension: where are we now? J Hum Hypertens. 2008;22(7):460–467. doi: 10.1038/jhh.2008.24. [DOI] [PubMed] [Google Scholar]
- 47.Elias MF, Sullivan LM, Elias PK, et al. Left ventricular mass, blood pressure, and lowered cognitive performance in the Framingham offspring. Hypertension. 2007;49(3):439–445. doi: 10.1161/01.HYP.0000256361.68158.24. [DOI] [PubMed] [Google Scholar]
- 48.Mosca L, Banka CL, Benjamin EJ, et al. Expert Panel/Writing Group; American Heart Association; American Academy of Family Physicians; American College of Obstetricians and Gynecologists; American College of Cardiology Foundation; Society of Thoracic Surgeons; American Medical Women’s Association; Centers for Disease Control and Prevention; Office of Research on Women’s Health; Association of Black Cardiologists; American College of Physicians; World Heart Federation; National Heart, Lung, and Blood Institute; American College of Nurse Practitioners. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115(11):1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]