Abstract
Background
Human parainfluenza virus type 1 (hPIV-1) causes serious respiratory tract infections, especially in children. This study investigated the efficacy of the novel hemagglutinin-neuraminidase (HN) inhibitor BCX 2798 in the prophylaxis of lethal and the treatment of non-lethal parainfluenza virus infection in mice.
Methods
In the prophylaxis model, 129x1/SvJ mice were inoculated with a 90% lethal dose of a recombinant Sendai virus, in which the HN gene was replaced with that of hPIV-1 (rSeV[hPIV-1HN]). The mice were intranasally treated either once or for 5 d with 1 or 10 mg/kg/d of BCX 2798, starting 4 h before infection. In the therapeutic model, mice were infected with 100 PFU per mouse of rSeV(hPIV-1HN) and treated intranasally with 0.1, 1 or 10 mg/kg/d of BCX 2798 for 5 d, starting 24 or 48 h after infection, or for 4 d starting 72 h after infection.
Results
Similar to multiple dosing, a single intranasal prophylaxis with 1 or 10 mg/kg of BCX 2798 protected approximately 40% or 90%, respectively, of mice from death by rSeV(hPIV-1HN) infection. BCX 2798 also significantly reduced virus lung titers (in a dose- and time-dependent manner) and histopathologic changes in the airways in non-lethally infected mice at multiple intranasal dosages in the therapeutic model, with the lowest effective dosage being 0.1 mg/kg/d administered 24 h after infection.
Conclusion
BCX 2798 was effective in the prophylaxis of lethal and in the therapy of non-lethal parainfluenza virus infection in mice, suggesting further consideration of BCX 2798 for clinical trials.
Introduction
Human parainfluenza virus type 1 (hPIV-1) belongs to the Paramyxoviridae family (a family of single-stranded negative-sense RNA viruses) [1]. hPIV-1 (together with other types of hPIVs; e.g., 2, 3 and 3) causes serious respiratory tract infections in infants, young children, the elderly and immunocompromised hosts [1]. It is responsible for approximately 6% of pediatric hospitalizations [2] and is one of the principal etiologic agents of croup [3]. Although hPIVs have been known for more than 50 years, we still do not have a specific, effective licensed therapeutic to manage these widespread pathogens. Due to the importance of the hemagglutinin-neuraminidase (HN) glycoprotein of parainfluenza virus in membrane attachment, penetration of the cell surface and spread [1, 4, 5], interest has focused on the development of selective inhibitors of this major surface glycoprotein.
Utilizing the three-dimensional structure of the HN protein of Newcastle disease virus (an avian paramyxovirus) with conservation of the amino acid residues found around the active site among all parainfluenza viruses [6, 7], a novel potent HN inhibitor, BCX 2798, was designed and proposed as an important advancement in the management of hPIV infections.
We previously demonstrated the high efficacy of BCX 2798 against hPIV-1 in in vitro assays and in the prophylaxis of a 90% lethal infection in mice with a recombinant Sendai virus, in which the HN gene was replaced with that of hPIV-1 (rSeV[hPIV-1HN]) [8]. The recombinant virus was designed specifically to develop an efficient read-out system to test BCX 2798 in vivo. Mice are poorly permissive to hPIVs, and infections of semipermissive laboratory animals (e.g., hamsters and cotton rats) with hPIVs result in minimal lung pathology and an asymptomatic course of disease [1, 9, 10, 11, 12]. Infection of mice with rSeV(hPIV-1HN) has led to severe illness and robust replication of the virus in the lungs.
Prophylaxis will be important for management of hPIV infections, especially in immunocompromised hosts (e.g., patients after bone marrow or solid organ transplantation or undergoing immunosuppressive therapy) because the level of mortality due to hPIVs in this group reaches 30% [16]. To optimize the prophylaxis dosage regimen in our rSeV(hPIV-1HN) mouse model, we determined the efficacy of various single intranasal dosages of BCX 2798 against lethal recombinant virus infection in mice.
We also evaluated the efficacy of various intranasal dosages of BCX 2798 in 24-, 48- and 72-hour delayed-treatment regimens against non-lethal rSeV(hPIV-1HN) infection. Our previously published findings showed that treatment of mice with BCX 2798 starting 24 h after 90% lethal infection with rSeV(hPIV-1HN) did not prevent death due to the peak of viral titers (~106 TCID50/ml) in the lungs at the start of treatment [8]. Because natural infections with hPIVs are unlikely to lead to death in immunocompetent patients [1, 13], we developed a model of non-lethal rSeV(hPIV-1HN) infection in mice that represents an infectious dose of hPIV during human-to-human transmission and mimics the pattern of virus replication in the respiratory tract of non-immunocompromised humans [14, 15].
To complete our evaluation of BCX 2798 in the rSeV(hPIV-1HN) mouse model, we assessed the applicability of oral, intramuscular, intraperitoneal and intravenous routes of BCX 2798 administration in the prophylaxis and therapy of rSeV(hPIV-1HN) infection in mice.
The results of our studies on the efficacy of the HN inhibitor BCX 2798 suggest that it is a promising candidate for clinical use in the effort to counter the effects of hPIVs that can cause severe respiratory diseases.
Materials and methods
HN inhibitor
BCX 2798 (BioCryst Pharmaceuticals, Inc., Birmingham, AL) is a derivative of Neu5Ac2en (2-deoxy-2,3-dehydro-N-acetylneuraminic acid) in which the O-4 hydroxyl group was replaced by an azido group, and the methyl group of the acetamino moiety at C-5 was replaced with an isopropyl group [8]. BCX 2798, provided as a lyophilized powder, was solubilized in phosphate-buffered saline (PBS) before use in the in vitro and in vivo experiments described herein.
Cell cultures and viruses
LLC-MK2 (monkey kidney epithelial) and 293T (human kidney epithelial; used for the rescue of rSeV[hPIV-1HN] ) cells were grown in Dulbecco’s modified Eagle’s medium that contained 10% fetal bovine serum.
The rSeV(hPIV-1HN), which contains the hPIV-1 HN gene in place of the HN gene of SeV, was rescued from the full-length SeV cDNA genome pSeV(+) by using a reverse-genetics process, as described previously [8]. The rescued virus was plaque-purified on LLC-MK2 cells and amplified in embryonated chicken eggs. Sequence analysis of the HN gene of rSeV(hPIV-1HN) revealed no mutations.
The infectivity of the rescued viruses was determined using plaque assays. Briefly, LLC-MK2 cells in six-well plates were inoculated with serial 10-fold virus dilutions. After incubation for 1 h at room temperature, the inoculum was removed, and 1× minimum essential medium containing 2% bovine serum albumin and acetylated trypsin (5 μg/ml) mixed with 1% agarose was added to the plates. After 5 d of incubation at 33°C, the second overlay, which consisted of minimal essential medium with 5% fetal bovine serum mixed with 1% neutral red and agarose, was added to the plates to help visualize plaques.
Drug studies in mice
Animal work was performed in a Biosafety Level 2+ facility at St. Jude Children’s Research Hospital and approved by the institution’s Animal Care and Use Committee. In the prophylaxis, 8-week-old female 129x1/SvJ mice (Jackson Laboratories, Bar Harbor, ME) were anesthetized via inhalation of isoflurane (2.5%; Baxter Healthcare Corporation, Deerfield, IL) and inoculated intranasally with 4.2 × 107 PFU per mouse of rSeV(hPIV-1HN (a 90% lethal dose) in 50 μl of sterile PBS. Single and multiple (twice a day for 5 d) intranasal treatments of mice with 1 or 10 mg/kg/d of BCX 2798 in 50 μl of PBS were initiated 4 h before infection. Control animals were infected but were treated with PBS only. The efficacy of the compound in the prophylactic model was evaluated on the basis of reduction of virus titer in the lungs, prevention of weight loss and death, and survival rate determined 21 d after infection.
In the therapeutic model, mice were infected with 100 PFU per mouse of rSeV(hPIV-1HN) and treated intranasally twice a day with 0.1, 1 or 10 mg/kg/d of BCX 2798, starting either 24 or 48 h after infection (treatment for five consecutive days), or 72 h after infection (treatment for four consecutive days). The virus and compound volumes were similar to those used in the prophylaxis model. Activity of the compound in the therapeutic model was evaluated on the basis of reduction of the virus titer in the mouse lungs as well as the histopathologic changes in the airways.
To control drug toxicity, five uninfected mice were given each tested dosage of compound and observed for survival, weight change and overt toxic effects for 21 d.
Assay of virus in mouse lungs
Lungs from 3 mice from each BCX 2798- or PBS-treated group infected either with 100 or 4.2 × 107 PFU per mouse of rSeV(hPIV-1HN) were harvested 3, 5 or 7 d after infection and analyzed to determine the virus growth. The procedure for lung suspension preparation was described in our previous report [8]. Virus titers (PFU/ml) were determined in LLC-MK2 cells using plaque assays as described in the preceding text. The mean titers ± the standard error of the mean (SEM) are presented.
Analysis of residual drug activity
Lungs from three uninfected mice intranasally treated with 10 mg/kg/d of BCX 2798 with single or multiple regimens (twice a day for 5 d) or PBS were collected 7 d later. The residual drug activity in the lung suspension was determined by hemagglutination inhibition (HI) and neuraminidase inhibition (NI) tests.
HI test
Lung suspensions were serially diluted (ratio, 1:2), and the dilutions were preincubated with a standard dose of rSeV(hPIV-1HN) equal to four hemagglutination units for 1 h at room temperature. The mixtures were then incubated for 1 h at 4°C with 0.5% chicken red blood cells. In a parallel HI test, activity of BCX 2798 against rSeV(hPIV-1HN) was evaluated. The concentration of the compound that showed 50% agglutination was considered the IC50.
NI test
The neuraminidase activity of rSeV(hPIV-1HN) was measured by a standard fluorometric assay using 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (Sigma Chemical Co., St. Louis, MO) as the substrate, as described previously (8). In the NI test, lung suspensions were serially diluted (ratio, 1:4), and 25 μl of each dilution was incubated for 1 h at room temperature with 25 μl of diluted virus at astandard amount of neuraminidase activity (200 relative fluorescenceunits). The reaction was initiated by the addition of substrate and stopped after 1 h of incubation at 37°C. In a parallel NI test, activity of BCX 2798 against rSeV(hPIV-1HN) was evaluated. The extent of NI was defined as the concentration of compound required to reduce the neuraminidase activity of the treated virus to 50% of that of the control virus. The IC50 values were calculated by plotting the percentage of fluorescence inhibition relative to the control versus the log concentrations of the compounds.
Histopathologic studies
Three mice from each BCX 2798- or PBS-treated group infected with 100 PFU per mouse of rSeV(hPIV-1HN) were euthanized 8 d after infection. The lungs were removed and processed for histopathologic analysis as described previously [8]. Pneumonia was scored by an experienced veterinary pathologist (KLB) blind to the composition of the groups. The histopathology score (0 to 4) was assigned based on the following criteria: 0, no pathology; 1, minimal peribronchiolar and perivascular infiltrates of lymphocytes, plasma cells and few neutrophils, rare extension of inflammation to alveoli; 2, mild peribronchiolar and perivascular leukocyte infiltration, mild airway epithelial hyperplasia with focal cell necrosis, localized intra-alveolar inflammation; 3, moderate peribronchiolar and perivascular leukocyte infiltration, moderate epithelial hyperplasia and necrosis medium and small airways, locally extensive intra-alveolar inflammatory cell infiltrates; and 4, diffuse bronchointerstitial pneumonia. The mean score (± SEM) for each group is presented.
Statistical analysis
The Kaplan-Meier method was used to estimate the probability of survival, and the log-rank test was used to compare survival estimates for groups that received different treatments (BCX 2798 or control [PBS]), that received treatment at different doses, and that received similar treatment using different regimens (single or multiple) over the period of 21 d. The mean days to death were estimated as the number of days that the mice survived after viral infection. If no death occurred during the observation period, the mean days to death was considered to be 21 d. An analysis of variance (ANOVA) model followed by Tukey’s multiple comparison test was used to estimate and compare the virus lung titers and weight changes in the treatment groups. A P value <0.05 was considered significant.
Results
Prophylactic efficacy of BCX 2798 in mice
We evaluated the prophylactic efficacy of the compound in a 90% lethal model of rSeV(hPIV-1HN) infection in mice.
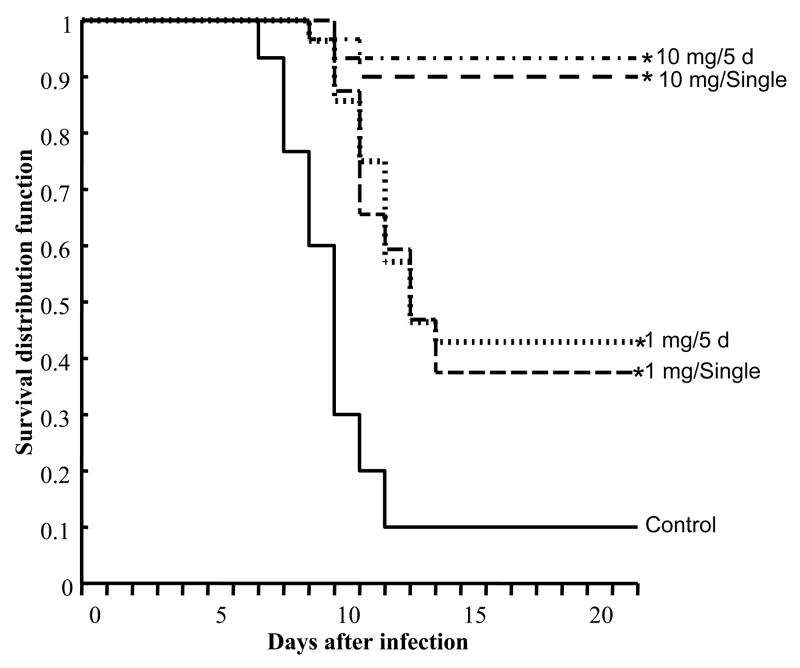
Comparison of the survival curves for infected mice intranasally treated with 1 or 10 mg/kg/d of BCX 2798 at single or multiple dosages against survival curves for infected PBS-treated mice showed that both dosages administered on either regimen were protective against lethal infection with rSeV(hPIV-1HN) (P<0.05) (Figure 1). However, the higher dosage of BCX 2798 was more effective than the lower one in terms of survival and weight loss (Table 1). Thus, 10 mg/kg/d of BCX 2798 protected 90% of the mice (given a single dose) and 93.3% of mice (given the compound for 5 d) from death, whereas in groups treated with 1 mg/kg/d, only 37.5% of mice in the single-dosage treatment group and 42.9% of mice in the multiple-dosage treatment group survived. Survival for the control group (infected but treated with PBS only) was 10%. Survival of mice appeared to correlate with weight loss. Mice that received 10 mg/kg/d of BCX 2798 on either regimen had higher survival rate (at 21 d after infection) and less weight loss than those treated with 1 mg/kg/d of compound (either regimen) or PBS 5, 7, and 9 d after infection (P<0.05). There were no statistically significant differences in survival and weight loss between single and multiple intranasal prophylactic regimens with BCX 2798 at either dosage of 1 or 10 mg/kg/d.
FIG. 1.
Effect of a single intranasal prophylactic dose of BCX 2798 on survival of mice inoculated with a 90% lethal dose of rSeV(hPIV-1HN). 129x1/SvJ mice were administered 1 or 10 mg/kg/d of BCX 2798 in single or multiple (twice daily for five consecutive days) regimens starting 4 h before lethal infection with 4.2 × 107 PFU of virus per mouse. Control infected mice were treated with PBS alone. The Kaplan-Meier method was used to estimate the probability of survival, which is expressed as survival distribution function. A value of 1 corresponds to 100% survival. *P<0.05 compared with the control group.
TABLE 1.
Effect of a single intranasal prophylactic dose of BCX 2798 against lethal rSeV(hPIV-1HN) infection in mice§
| Compound | Dosage (mg/kg/d) | Dosing schedule | Mean weight loss on day after infection (% ± SEM)†* |
Survivors, n/total (%) | Days to death (mean ± SEM)# | ||
|---|---|---|---|---|---|---|---|
| 5 | 7 | 9 | |||||
| BCX 2798 | 1 | single | −13.9±4.2 | −17.1±0.9 | −19.8±0.8 | 12/32 (37.5) | 15.2±0.8 |
| 5 d | −11.8±1.0 | −14.5±1.0 | − 21.8±1.2 | 12/28 (42.9) | 15.6±0.9 | ||
| 10 | single | −7.5±1.2 | −9.8±1.5 | − 11.6±1.4 | 27/30 (90) | 19.9±0.6 | |
| 5 d | −5.6±1.0 | −10.1±1.1 | − 13.8±1.1 | 28/30 (93.3) | 20.3±1.4 | ||
| PBS | N/A‡ | −18.6±0.9 | −24.8±0.8 | − 29.3±1.0 | 3/30 (10) | 10.7±0.8 | |
BCX 2798 was administered to 129x1/SvJ mice beginning 4 h before lethal infection with 4.2 × 107 PFU per mouse of rSeV(hPIV-1HN) in single or multiple (twice a day for five consecutive days) intranasal regimens. Control infected mice were treated with PBS alone. Weight change and survival of mice were monitored through 21 d after infection.
Loss or gain of weight was calculated for each mouse as a percentage of weight on day 0 before virus infection.
*Weight changes in all treatment groups differed significantly from those in the control group in a repeated-measures ANOVA test (P<0.05).
The mean days to death was the number of days of survival after the lethal challenge with the virus.
N/A, not applicable.
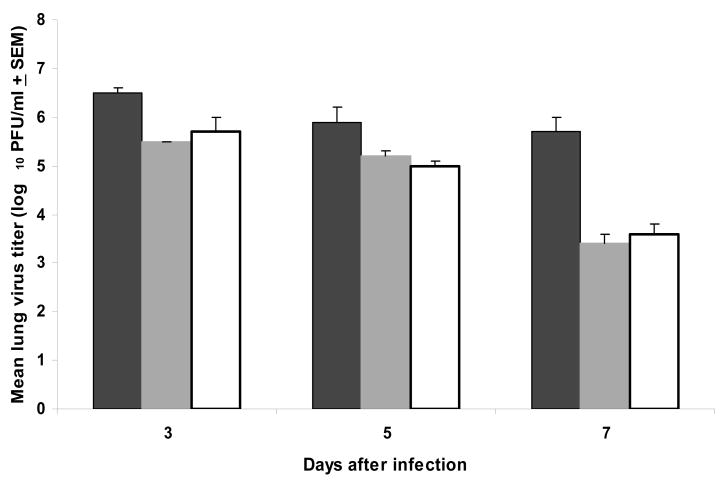
Our previously published data showed that the antiviral effect of prophylactically administered BCX 2798 (10 mg/kg/d, 5 d, intranasally) was associated with a significant reduction of rSeV(hPIV-1HN) in mouse lungs (8). Analysis of the rSeV(hPIV-1HN) titers in the mouse lungs prophylactically treated with a single dosage of 10 mg/kg/d of compound showed that, similar to multiple administration, a single dose also significantly lowered recombinant virus titers in lethally infected mice compared with control mice (infected but treated with PBS only) 3, 5 or 7 d after infection (P<0.05) (Figure 2). Comparison of the virus lung titers between single and multiple intranasal prophylactic regimens with BCX 2798 at this dosage did not reveal significant differences. Analysis of residual drug activity in the lungs of uninfected mice treated with 10 mg/kg/d of BCX 2798 with single or multiple regimens did not reveal any inhibitory activity in the lung suspensions on tested rSeV(hPIV-1HN) as compared to that of PBS-treated mice in HI and NI tests (data not shown). Prophylaxis of lethal rSeV(hPIV-1HN) infection with 1 mg/kg/d of BCX 2798 in either regimen did not result in a significant reduction of virus lung titers. Taken together, our data indicate that a single intranasal administration of BCX 2798 in the 4-hour prophylactic model had a similar protective effect as multiple dosing against lethal rSeV(hPIV-1HN) infection in mice.
FIG. 2.
Effect of a single intranasal prophylactic dose of BCX 2798 on virus titers in the lungs of mice infected with a 90% lethal dose of rSeV(hPIV-1HN). Mice were treated with 10 mg/kg/d of BCX 2798 in multiple (twice daily for five consecutive days; gray box) or single (white box) regimens starting 4 h before inoculation of 4.2 × 107 PFU of virus per mouse. Control infected mice were treated only with PBS (black box). Each data point represents the mean virus titer of three mice plotted with error bars indicating the SEM. Virus lung titers in both treatment groups differed significantly from those in the control group in a one-way ANOVA model (P<0.05).
To compare the efficacy of different routes of BCX 2798 administration, we treated mice intranasally, orally, intramuscularly, intraperitoneally or intravenously with 10 mg/kg/d of compound twice a day for 5 d, starting 4 h before a 90% lethal infection with rSeV(hPIV-1HN). Our data (not shown) indicated that none, other than the intranasal route of compound administration, had significant effects on lethal rSeV(hPIV-1HN) infection in mice in terms of protection from weight loss and death or extension of mean days to death.
Therapeutic efficacy of BCX 2798 in mice
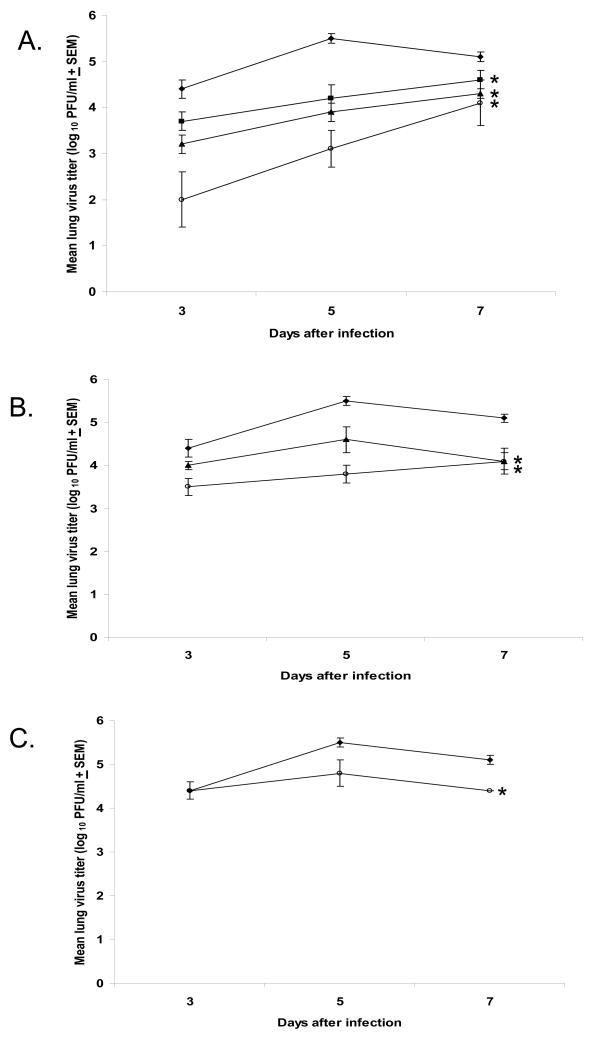
Our previously published data showed that the high level of viral titers (~106 TCID50/ml) in the lungs at the start of treatment was a reason for a lack of BCX 2798 efficacy against a 90% lethal dose of rSeV(hPIV-1HN) when treatment of mice was delayed for 24 hours [8]. Studies on experimental hPIV-1 infection in humans showed that the virus reaches the peak of its titer ~104.4 TCID50/ml in the human respiratory tract not earlier than 3 d after infection [15]. To develop a mouse model of parainfluenza virus infection that represents the course of hPIV-1 infection in humans, we determined the pathogenicity of the different doses (from 100 to 104 PFU) of rSeV(hPIV-1HN) in mice. Our data showed that infection of mice with 100 PFU of rSeV(hPIV-1HN) mirrors the kinetics of experimental hPIV-1 infection in humans with the highest level of rSeV(hPIV-1HN) titers in the mouse lungs of ~4.0 × 104 to 5.0 × 105 PFU/ml observed on days 3 to 7 after infection (Figure 3). The virus titers were lower than the assay’s detectable level (100 PFU/ml) at 1 and 9 d after infection (data not shown). The chosen rSeV(hPIV-1HN) dose also correlated with evidence from studies in adult volunteers that the infectious dose of hPIVs is very small (<100 virus particles) [14]. This represents the first established mouse model of non-lethal infection for the evaluation of the therapeutic efficacy of HN inhibitors.
FIG. 3.
Effect of treatment with BCX 2798 on virus titers in mice infected with 100 PFU of rSeV(hPIV-1HN). BCX 2798 at dosages of 0.1 (-■-), 1 (-▲-) and 10 (-○-) mg/kg/d was administered intranasally to 129x1/SvJ mice starting at either 24 h (A) or 48 h (B) after infection (treatment for five consecutive days) or 72 h (C) after infection (treatment for four consecutive days). Control infected mice were treated with PBS alone (- ◆ -). The mean virus lung titers from six to nine mice from at least two independent in vivo experiments are presented. The averages for each group are plotted with error bars indicating the SEM. *P<0.05 compared with the control group; one-way ANOVA model.
In this model, infected animals did not show signs of infection, such as weight loss or death. Thus, the virus lung titers and histopathologic changes in airways were the only parameters used for evaluation of activity of the compound in the therapeutic model.
Figure 3 shows the efficacy of the different multiple dosages of intranasally administered BCX 2798 on the reduction of mouse virus lung titers in a therapeutic model. Our data indicated that the titers of the virus in the lungs of mice treated with the compound were lower than those in the control group (treated with PBS only) in a dose-and time-dependent fashion.
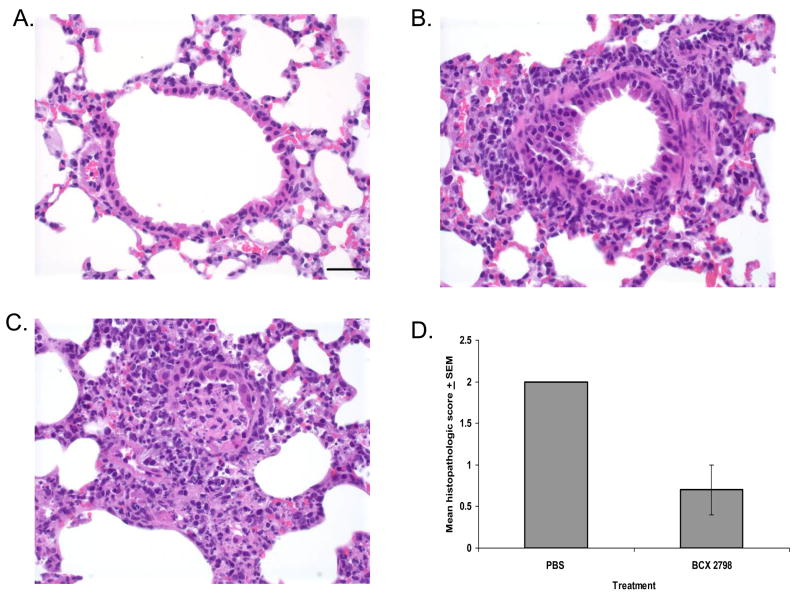
Treatment of rSeV(hPIV-1HN)-infected mice with BCX 2798 starting 24 h after infection significantly reduced the virus lung titers throughout the infection in all treated groups (Figure 3A) (P<0.05). The virus lung titers in infected untreated mice were from 11- to 24-fold, from 9- to 16-fold and from 5- to 13-fold higher than in the groups treated with 10, 1 or 0.1 mg/kg/d of compound, respectively, at the same time points (3, 5 or 7 d after infection). Examination of the histopathologic changes in the lungs of infected mice treated with PBS showed mild to moderate infiltrates of lymphocytes, plasma cells and macrophages around vessels and medium airways (Figure 4C) with a mean score of 2.0 (all mice scored 2 in this group) (Figure 4D). Focally extensive airway epithelial necrosis was observed in small and medium airways. In contrast, lungs from mice treated with 10 mg/kg/d of BCX 2798 had only minimal or no inflammatory response (Figure 4B) with a mean score of 0.7 (score range, 0 to 1) (Figure 4D). When leukocyte infiltration was observed in the lungs of mice from this group, it consisted of low numbers of lymphocytes and plasma cells in perivascular and peribronchiolar spaces. No signs of lung inflammation were seen in mice in the uninfected control groups that received PBS (Figure 4A) or BCX 2798 only (data not shown).
FIG. 4.
Effect of treatment with BCX 2798 on histopathologic changes in lungs of mice infected with 100 PFU of rSeV(hPIV-1HN). Infected mice (three per group) were intranasally treated with 10 mg/kg/d of compound or PBS for 5 d starting 24 h after infection. Lungs were removed 8 d after infection. The sections were stained with hematoxylin and eosin and examined microscopically. High-power view (400×) of the stained section is shown. Bar = 30 μm. (A) Uninfected mice treated with PBS; (B) Infected mice treated with BCX 2798 (note the mild peribronchiolar infiltrates of lymphocytes, plasma cells and neutrophils and absence of airway epithelial necrosis); (C) Infected mice treated with PBS (note a peribronchiolar cuffing of the distal airway by inflammatory cells; the airway lumen is plugged by a mixture of sloughed necrotic epithelial cells and inflammatory cells, and there is local extension of the inflammation and necrosis into the alveoli); (D) Histopathologic scoring of infected mouse lungs. The degree of histopathologic changes was graded on a scale of 0 (no change) to 4 (severe pneumonia). The averages for each group are plotted with error bars indicating the SEM.
Treatment of rSeV(hPIV-1HN)-infected mice with BCX 2798 starting 48 h after infection (Figure 3B) significantly reduced the virus lung titers throughout the infection only in the groups of mice treated with 1 or 10 mg/kg/d of the compound (P<0.05). The virus lung titers in untreated mice were from 10- to 17-fold higher than those in the group treated with 10 mg/kg/d of compound and from 4- to 10-fold higher than in mice receiving 1 mg/kg/d of BCX 2798.
Treatment with BCX 2798 starting 72 h after infection (Figure 3C) resulted in a significant reduction of virus lung titers only in mice treated with 10 mg/kg/d of the compound (P<0.05). The level of virus lung titer was about 7-fold higher in control mice than in treated mice both 5 and 7 d after infection.
Analysis of the efficacies of different dosages of BCX 2798 in the same therapeutic model showed that the higher dosage of the compound was more effective than the lower one in reducing mouse lung titers of rSeV(hPIV-1HN) (P<0.05).
Comparison of the efficacies of similar dosages of BCX 2798 administered 24, 48 or 72 h after infection showed that initiating treatment earlier after infection was more effective at lowering mouse lung titers of rSeV(hPIV-1HN) than delaying treatment (P<0.05).
Studies on the efficacy of the single intranasal or multiple (during 5 d) oral, intravenous, intraperitoneal and intramuscular administration of 10 mg/kg/d of the compound did not result in lower rSeV(hPIV-1HN) lung titers in infected mice in the 24-hour delayed-treatment model (data not shown). The results for BCX 2798 administered by other than intranasal route to mice in the therapeutic model was consistent with our data obtained for BCX 2798 in the 4-hour prophylactic model.
Thus, the results of our current study indicate that BCX 2798, when administered intranasally, was effective for both prophylaxis and therapy of parainfluenza virus infection in mice.
Discussion
BCX 2798 represents a new class of antiviral compounds targeting parainfluenza virus HN. In this study, we first assessed the effectiveness of a single intranasal dose of compound in the prophylaxis of lethal rSeV(hPIV-1HN) infection and multiple intranasal doses in the treatment of non-lethal infection in mice. Similar to our previously published results (8), BCX 2798 was well tolerated by mice during the treatment and post-treatment periods at all tested dosages (from 0.1 to 10 mg/kg/d). We found that single intranasal doses of 1 or 10 mg/kg were as efficacious as a multiple intranasal dosage regimen in preventing weight loss and death in infected mice. Previously, the high efficacy of a single dose of drug administration in reducing weight loss and death in lethally infected mice was reported for the influenza virus neuraminidase inhibitor peramivir [17].
A dosage of 10 mg/kg/d of BCX 2798 prophylactically administered to mice using either regimen also significantly reduced virus lung titers, but 1 mg/kg/d did not. The high level of variability within the group of mice treated with 1 mg/kg/d of BCX 2798 (60% of them died and 40% survived) might be why statistical significance was not achieved with the number of mice used in the virus lung titer analysis. When virus lung titer is the only parameter for evaluation of drug efficacy (as in our non-lethal model of rSeV[hPIV-1HN] infection), expansion of animal resources might be the solution. We tested prophylactic efficacy of 1 mg/kg/d of BCX 2798 (single vs. multiple regimen) in the lethal model of rSeV(hPIV-1HN) infection. Thus, in addition to the virus lung titers, the weight change and death parameters enabled us to conclude that 1 mg/kg/d was protective in this model and to determine that a single intranasal dose was as efficacious as a multiple intranasal regimen.
The therapeutic efficacy of BCX 2798 was tested in a non-lethal model of rSeV(hPIV-1HN) infection in mice. Due to the moderate level and steady rate of virus replication in the mouse lungs, weight loss and death did not occur in infected animals. Thus, our evaluation of BCX 2798 efficacy was limited to comparing the virus lung titers and histopathologic changes in airways of treated and untreated groups of mice.
Our data showed that only a multiple administration of BCX 2798 for five consecutive days significantly reduced virus lung titers in infected mice in the therapeutic model, with the lowest effective dosage being 0.1 mg/kg/d administered intranasally 24 h after infection. The greatest reduction of virus titers in the lung, accompanied by only minor or no histopathologic changes, was observed with the highest tested dosage of 10 mg/kg/d administered intranasally 24 h after infection. This indicates that the combination of a high dosage of the compound with an earlier start of treatment might be the most effective regimen in the HN inhibitor application.
We noted a similar level of virus lung titers (about 104 PFU/ml) in mice treated with different dosages in the same treatment model or similar dosages administered in different treatment models 7 d after infection (Figure 3). This phenomenon could be due to the involvement of the immune response (in addition to antiviral activity of the compound) in lowering virus lung titers at this time point; however, we do not address this issue here. Further studies are required to understand how the HN inhibitor and immune system cooperate during parainfluenza virus infection.
Our previous study focused on the efficacy of the intranasal route of BCX 2798 administration [8] because the respiratory tract is the site for parainfluenza virus replication. However, studies with influenza virus neuraminidase inhibitors indicate that respiratory infection can also be effectively inhibited by oral (oseltamivir) [18] or intramuscular (peramivir) [17, 19] drug administration. Our current study showed no efficacy, other than that of the intranasal route, of BCX 2798 delivery on rSeV(hPIV-1HN) infection in mice. This indicates that nasal administration might be the first choice for effective delivery of BCX 2798 in clinical trials.
Overall, the results reported here suggest that BCX 2798 is a potent parainfluenza virus HN inhibitor that showed high efficacy in the prophylaxis and therapy of rSeV(hPIV-1HN) infection in mice. In addition, our in vitro and in vivo studies on the isolation of parainfluenza virus variants with decreased susceptibility to BCX 2798 indicate that resistance to parainfluenza HN inhibitors does not develop easily in either cell culture or an animal model. Of the 100 virus clones isolated in LLC-MK2 cells from the lungs of the rSeV(hPIV-1HN)-infected mice prophylactically treated with BCX 2798, only one clone possessed a drug-resistant substitution (N173S) on its HN [20]. This indicates a low possibility of drug-resistant virus emergence and enhances the idea that HN inhibitors have clinical potential. Introduction of this novel class of antiviral compounds into clinical practice may significantly broaden resources available to control hPIV infections.
Acknowledgments
This work was supported by a research grant 160244010 from BioCryst Pharmaceuticals, Inc. (Birmingham, AL), the Cancer Center Support Grant CA 21765 from the US National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC). We thank Pamela Freiden for technical assistance and David Galloway, ELS, for editorial comments regarding the manuscript.
Footnotes
Conflict of interest
We do not have any conflicts of interest to disclose.
References
- 1.Karron RA, Collins PL. Parainfluenza Viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott/The Williams & Wilkins Co., a Wolters Kluwer Business; Philadelphia, Pa: 2007. pp. 1497–1526. [Google Scholar]
- 2.Counihan ME, Shay DK, Holman RC, Lowther SA, Anderson LJ. Human parainfluenza virus-associated hospitalizations among children less than five years of age in the United States. Pediatr Infect Dis. 2001;20:646–653. doi: 10.1097/00006454-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Marx A, Torok TJ, Holman RC, Clarke MJ, Anderson LJ. Pediatric hospitalizations for croup (laryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. J Infect Dis. 1997;176:1423–1427. doi: 10.1086/514137. [DOI] [PubMed] [Google Scholar]
- 4.Stone-Hulslander J, Morrison TG. Detection of an interaction between the HN and F proteins in Newcastle disease virus-infected cells. J Virol. 1997;71:6287–6295. doi: 10.1128/jvi.71.9.6287-6295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Q, Hu X, Compans RW. Association of the parainfluenza virus fusion and hemagglutinin-neuraminidase glycoproteins on cell surfaces. J Virol. 1997;71:650–656. doi: 10.1128/jvi.71.1.650-656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crennell S, Takimoto T, Portner A, Taylor G. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat Struct Biol. 2000;7:1068–1074. doi: 10.1038/81002. [DOI] [PubMed] [Google Scholar]
- 7.Takimoto T, Taylor GL, Crennell SJ, Scroggs RA, Portner A. Crystallization of Newcastle disease virus hemagglutinin-neuraminidase glycoprotein. Virology. 2000;270:208–214. doi: 10.1006/viro.2000.0263. [DOI] [PubMed] [Google Scholar]
- 8.Alymova IV, Taylor G, Takimoto T, et al. Efficacy of novel hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 against human parainfluenza viruses in vitro and in vivo. Antimicrob Agents Chemother. 2004;48:1495–1502. doi: 10.1128/AAC.48.5.1495-1502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascoli CC, Metzgar DP, Larson EJ, Fuscaldo AA, Gower TA. An animal model for studying infection and immunity to and attenuation of human parainfluenza viruses. Dev Biol Stand. 1975;28:414–421. [PubMed] [Google Scholar]
- 10.Collier AM, Clyde WA., Jr Model systems for studying the pathogenesis of infections causing bronchiolitis in man. Pediatr Res. 1977;11:243–246. [PubMed] [Google Scholar]
- 11.Murphy TF, Dubovi EJ, Clyde WA., Jr The cotton rat as an experimental model of human parainfluenza virus type 3 disease. Exp Lung Res. 1981;2:97–109. doi: 10.3109/01902148109052306. [DOI] [PubMed] [Google Scholar]
- 12.Tao T, Davoodi F, Cho CJ, et al. A live attenuated recombinant chimeric parainfluenza virus (PIV) candidate vaccine containing the hemagglutinin-neuraminidase and fusion glycoproteins of PIV1 and the remaining proteins from PIV3 induces resistance to PIV1 even in animal immune to PIV3. Vaccine. 2000;18:1359–1366. doi: 10.1016/s0264-410x(99)00406-5. [DOI] [PubMed] [Google Scholar]
- 13.Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith CB, Purcell RH, Bellanti JA, Chanock RM. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966;275:1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- 15.Murphy BR, Richman DD, Chalhub EG, et al. Failure of attenuated temperature-sensitive influenza A (H3N2) virus to induce heterologous interference in humans to parainfluenza type 1 virus. Infect Immun. 1975;12:62–68. doi: 10.1128/iai.12.1.62-68.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wendt CH, Weisdorf DJ, Jordan MC, et al. Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med. 1992;326:921–926. doi: 10.1056/NEJM199204023261404. [DOI] [PubMed] [Google Scholar]
- 17.Bantia S, Arnold CS, Parker CD, Upshaw R, Chand P. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antivir Res. 2006;69:39–45. doi: 10.1016/j.antiviral.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Roche Laboratories Inc. Tamiflu (oseltamivir phosphate) [Patient information] Available from http://www.rocheusa.com/products/tamiflu/ppi.pdf.
- 19.Yun NE, Linde NS, Zacks MA, et al. Injectable peramivir mitigates disease and promotes survival in ferrets and mice infected with the highly virulent influenza virus, A/Vietnam/1203/04 (H5N1) Virology. 2008;374:198–209. doi: 10.1016/j.virol.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alymova IV, Taylor G, Mishin VP, et al. Loss of the N-linked glycan at residue 173 on human parainfluenza virus type 1 hemagglutinin-neuraminidase exposes a second receptor-binding site. J Virology. 2008;82:8400–8410. doi: 10.1128/JVI.00474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]