Abstract
Background
Progress in clinical medicine relies on the willingness of patients to take part in experimental clinical trials, particularly randomized controlled trials (RCTs). Before agreeing to enroll in clinical trials, patients require guarantees that they will not knowingly be harmed and will have the best possible chances of receiving the most favorable treatments. This guarantee is provided by the acknowledgment of uncertainty (equipoise), which removes ethical dilemmas and makes it easier for patients to enroll in clinical trials.
Methods
Since the design of clinical trials is mostly affected by clinical equipoise, the “clinical equipoise hypothesis” has been postulated. If the uncertainty requirement holds, this means that investigators cannot predict what they are going to discover in any individual trial that they undertake. In some instances, new treatments will be superior to standard treatments, while in others, standard treatments will be superior to experimental treatments, and in still others, no difference will be detected between new and standard treatments. It is hypothesized that there must be a relationship between the overall pattern of treatment successes and the uncertainties that RCTs are designed to address.
Results
An analysis of published trials shows that the results cannot be predicted at the level of individual trials. However, the results also indicate that the overall pattern of discovery of treatment success across a series of trials is predictable and is consistent with clinical equipoise hypothesis. The analysis shows that we can discover no more than 25% to 50% of successful treatments when they are tested in RCTs. The analysis also indicates that this discovery rate is optimal in helping to preserve the clinical trial system; a high discovery rate (eg, a 90% to 100% probability of success) is neither feasible nor desirable since under these circumstances, neither the patient nor the researcher has an interest in randomization. This in turn would halt the RCT system as we know it.
Conclusions
The “principle or law of clinical discovery” described herein predicts the efficiency of the current system of RCTs at generating discoveries of new treatments. The principle is derived from the requirement for uncertainty or equipoise as a precondition for RCTs, the precept that paradoxically drives discoveries of new treatments while limiting the proportion and rate of new therapeutic discoveries.
Introduction
In its article #4, the Declaration of Helsinki1 states: “Medical progress is based on research which ultimately must rest in part on experimentation involving human subjects.”
Indeed, progress and discoveries cannot occur without the willingness of patients to be enrolled in clinical trials. Clinical trials, particularly well-designed randomized controlled trials (RCTs), are widely considered to be the most important vehicle for generating evidence about successful treatments that can improve disease and patient outcomes.2 Before agreeing to enroll in clinical trials, patients require guarantees that they will not knowingly be harmed and will have an optimal chance of receiving the best available treatments. The patient knows that a researcher cannot guarantee favorable outcomes. Nevertheless, researchers usually have a premonition that one treatment will be more effective than the other; otherwise, they would never embark on the particular trial. However, researchers cannot test all their ideas in RCTs; they are constrained by ethical precepts and pragmatic limitations. A key ethical precept is that an RCT should be done only if the physicians and patients are uncertain about the relative effects of the new and standard treatments to be compared.3 This requirement for uncertainty4,5 represents a fundamental principle that protects patients from knowingly being exposed to inferior treatments, while at the same time drives therapeutic advances in clinical medicine, as discussed below.
Ethics of Uncertainty and Design of Clinical Trials
It is rare that ethicists agree on many difficult questions related to human experimentation, but it is interesting to note that there is overwhelming agreement among ethicists that before patients are invited to participate in clinical studies such as RCTs, the uncertainty requirement should be met, ie, patients should be enrolled in a trial only if there is a substantial uncertainty about which of the trial treatments would be more beneficial.6 Ethicists, however, continue to disagree about the relationship of uncertainty to other ethical principles,7,8 including the locus of uncertainty, ie, “Whose uncertainty is more morally relevant?”9 — that of the individual physician (theoretical equipoise),10–12 the patient (indifference principle),13,14 the treating physician and the patient (uncertainty principle),4,15 the community of expert practitioners (trialists) (clinical equipoise),5,12,16 or the community of patients, advocacy groups, and lay people (community equipoise)?17,18 From the perspective of the trial conduct and design, each of these loci of uncertainty bears a somewhat different relationship to treatment outcomes.
Clinical Equipoise
Clinical equipoise5 relates to the uncertainty of a group of experts who honestly disagree about which treatment is better (“product of professional consensus”). This reflects the collective uncertainty that is crucial for informing the design of clinical trials. In fact, clinical equipoise addresses the most important issue of a clinical trial: the choice of an adequate comparative control.3,6,19,20 This uncertainty is typically articulated during the process of the protocol approval, such as rigorous procedure for approval of a RCT through the National Cancer Institute cooperative group mechanism.21 The process ensures that highly biased proposals, such as the use of an inferior comparator or other methodological problems, are vetted by other members of this community of experts.
Individual Uncertainty
Fried22 originally termed this type of uncertainty as theoretical equipoise, reflecting the honest belief of individual physicians that one treatment is not preferred over another.10–12 During the last decade, individual uncertainty was often termed the uncertainty principle,4,15 according to which RCTs are ethically and scientifically permissible only when both the treating physician and the patient are substantially uncertain regarding the merits of treatments being offered. When uncertainty at an individual level concerns patients only, this is termed the principle of indifference.13,14,23,24
While adherence to these principles preserves personal values and trust between physicians and their patients, none of these principles is believed to affect the design of the trial itself.6 Rather, the adherence to these principles, or the lack thereof, affects the trial’s generalizibility and patient accrual. This is because, strictly speaking, physicians who accept equipoise as an entry criterion for a trial are expected to adhere to agreed-upon eligibility criteria. However, physicians who believe in the uncertainty principle may be uncomfortable with such agreed-upon clinical research protocols and/or some of the eligibility criteria, and therefore they may be tempted to exclude some patients or to offer treatment to patients who do not meet eligibility criteria under the equipoise requirement.6 Theoretically at least, the results obtained using these principles as the entry criteria to RCTs may have different applicability to nontrial patients.6 Nevertheless, we would expect that internal validity remains intact since both equipoise and the uncertainty principle relate to a prerandomization phase of the trial and do not affect the postrandomization phase of the trial.
Community Equipoise
This principle reflects uncertainty at the level of involvement of patients, advocacy groups, and lay people in clinical research on the basis of belief that medical knowledge is not confined to the profession of physicians and that physicians have ethical responsibility for shared decision-making.18 Occasionally the advocacy groups participate in the design of an RCT along with professional experts, but most often they influence research agenda and not design itself. It should be noted that the concepts discussed here overlap and are not mutually exclusive.25,26
Based on these arguments, the design of a clinical trial is mostly influenced by clinical equipoise, which in turn affects the results of the trial and, as discussed below, the proportion of discovery of new successful treatments.6
Acknowledgment of Uncertainty: Scientific and Ethical Foundation of Clinical Trials
A fundamental premise of clinical research is that investigators typically cannot predict the results of a clinical trial. The entire encounter is fraught with uncertainties as hoped-for benefits but unknown risks color the decision of patients to enroll in clinical trials.27 It is this uncertainty, or rather the need to clearly articulate uncertainties, that is at the heart of clinical research.3,6,28,29 Since different clinical situations and health care interventions are associated with different levels of uncertainty, a proposal has been made to develop a taxonomy of clinical uncertainties.6 The idea behind this proposal is that the choice of scientific method should be matched to the underlying uncertainties regarding treatment effects.6 According to this view, RCTs are used to resolve uncertainties about treatment effects of a few (typically two) therapeutic alternatives that, before the trial is conducted, are believed (but not known) to be about equal in terms of superiority to one another.6 It also follows that an RCT would not be considered the method of choice for addressing uncertainties in which there are firmly held beliefs that one particular treatment is dramatically superior to all other existing alternatives, as in the discovery of penicillin or insulin.6,30
Therefore, the entire purpose of undertaking an RCT is to address uncertainty about the relevant merits of competing treatment alternatives. If there were no uncertainties, there would be no need for RCTs.6,31 If indeed the researchers could know the results of trials in advance, the scientific and ethical value of such research would be seriously compromised. Scientifically, nothing new can be learned when the answer is already known, and ethically, investigators would expose at least some patients (50%, in a typical RCT) to inferior treatments.6,31 Fundamentally, this is where the theory of human experimentation is linked with the theory of rational choice6,32,33: enrollment in an RCT is ethically and scientifically justified only when there is a substantial uncertainty about which treatment will be more effective.3,6,11,25,29,31,34 The key concept behind this requirement is that the interests of patients as well as society (researchers) are best served if this prerequisite holds: acknowledgment of uncertainty provides the best guarantee to patients that they will not be willingly harmed while at the same time will have the optimal chance of obtaining superior treatments.3,6,11,27,31 The conclusion of the trial will provide tangible social value since future patients can be offered treatment that has now been shown to be superior.
Overall Pattern of Treatment of Success Can Be Predicted From the Level of Uncertainties Existing Before RCT Is Undertaken
The main hypothesis put forward here is that there must be a relationship between the overall pattern of treatment successes and the uncertainties that RCTs are designed to address.3,6 Since the design of a clinical trial is mostly affected by clinical equipoise, the clinical equipoise hypothesis has been postulated.3,6 When the uncertainty requirement holds, the investigators cannot predict what they are going to discover in any individual trial that they undertake — in some trials, new treatments will be superior to standard treatments, while in other trials, standard treatments will be superior, and sometimes no difference will be detected between new and standard treatments.3,6,20,35–37 Although the results cannot be predicted at the level of any single trial, the pattern of discovery should be predictable if the hypothesis of a relationship between equipoise and treatment outcome is correct. In fact, the equipoise hypothesis appears to have described the fundamental process (called the “principle or a law of clinical discovery”6,38), which can predict efficiency of our current system of RCTs at generating clinical discoveries.
Theoretically, treatment successes should follow a normal distribution.3,6 However, in an evaluation of the pattern of treatment successes in RCTs of cancer treatments that were completed during the last 50 years under the auspices of the National Cancer Institute (781 randomized comparisons in 624 trials that enrolled 261,451 patients), investigators at our institute found that the distribution of treatment successes slightly but significantly deviated from the normal dis-tribution.39 The analysis of the distribution of treatment successes in cancer is mostly consistent with the power law,40,41 indicating that, on average, new treatments would be more successful than standard treatments but with sufficient unpredictability in the results at the level of individual trials. This is an important result; as discussed above, if one treatment (experimental or standard) is known to be superior, neither the patient nor the researcher has an interest in randomization. Naturally, patients would be expected to request the treatments that are believed to be superior — and researchers would be inclined to offer these treatments — making the use of RCT design both undesirable and impossible. Under these circumstances, the entire system of clinical trials would cease to exist, thus making discoveries of new treatments impossible since patients would not be willing to be enrolled in RCTs. In fact, as discussed below, patients are willing to participate in trials only if the results cannot be predicted in advance. Provided the uncertainty about treatment effects is clearly acknowledged, there is no a priori reason to be cautious about participation in clinical trials since new treatments tend to be, on average, neither substantially better nor worse than standard therapies once they have reached the testing stage in RCTs.39
The crucial point here is that the evidence in a particular case depends on our accumulated experience — the proportion of successes across a series of trials. That is, our assessments of the prospects of treatment success at the level of a single trial depend on the existing evidence, ie, a pattern of discovery of successful treatments in a cohort of the trials (average results). As discussed above, the clinical trial system would cease to exist if the results across of all trials would predictably favor one treatment over another (eg, experimental over standard treatments). Hence, the equipoise requirement enables therapeutic discoveries by facilitating participation in clinical trials. If the track records of evaluating previous success in similar research indicated high probability that the results can be predicted, ie, there is no uncertainty about treatment results, people would refuse to participate in trials and the RCT system would come to halt. For example, Johnson et al42 showed that only 3% of people would enroll in an RCT if the estimated probability that new treatments are better than the standard regimens is 80%. If the estimated success rate is 90% to 100%, no one would participate in the RCT where the probability of random allocation is 50%:50%, although institutional review board (IRB) members would allow testing on rats under these conditions.43 This means that given these circumstances of predicted treatment success, few trials would ever be launched and thus no new therapeutic discovery would be possible. However, 50% of lay people would approve an RCT if the estimated success rate is 70% in favor of the new treatment (over 30% for the standard treatment).42 Also it has been shown that most IRB members would, on average, approve an RCT if the estimate of probability of success is 60%:40% in favor of one treatment over another.43 Indeed, Mills et al44 found that the acceptance of clinical equipoise was crucial to actual patients’ consent to randomization. Therefore, when the equipoise requirement is adhered to, both IRB members and individual patients find it easier to enroll in RCTs. As a result, this helps preservation of the clinical trial system, which in turn serves as a driver of advances in clinical medicine. Only when the results cannot be predicted in advance and when the overall distribution of successes of experimental therapies is about equal to the success of standard therapies, the most rational course of both patients and researchers is to randomize.6,39
However, researchers typically hope that the new treatments they are about to test will be better than standard treatments. For example, an examination of research protocols of large cohorts of cancer trials supported by the National Cancer Institute indicated that investigators are never in theoretical 50:50 equipoise; they almost always hope that the new treatments are better.39 The prior knowledge, beliefs, expertise, and experience that investigators bring to the design of the trials are most likely the reason why new therapies are, on average, slightly superior to standard treatments. Investigators and sponsors need to have some belief in the likely success of the new treatments they assess; otherwise, it would be difficult to maintain material and intellectual investment in lengthy and costly experiments such as RCTs, without which new treatment discoveries would not be possible. Nevertheless, for ethical and practical reasons, they cannot test every idea they have, particularly their “sure bets.” Researchers are sufficiently constrained by the uncertainty requirement. By requiring high uncertainty as a precondition for the trial, the probability of success is proportionally reduced. As a result, no more than 25% to 50% of treatments that reach the stage of testing in an RCT will prove to be successful.39,45,46 Thus, there appears to exist the “limits of discoverability”: the success rate cannot (and should not) be 100%. These limits of discoverability in clinical research are directly determined by the equipoise principle. The limits, however, refer to the proportion of successful testing that one can expect to obtain from RCT testing. The public can help increase the absolute number of discoveries of successful treatments by increasing willingness to participate in RCTs. Currently only 3% to 5% of eligible patients participate in clinical trials.47,48 If they understand how clinical research discoveries are made, it is possible that they would be more willing to participate in clinical trials. Ultimately, everyone has a moral responsibility to support research,1 and once the public is aware of the principles outlined here, this can be easier to accomplish.
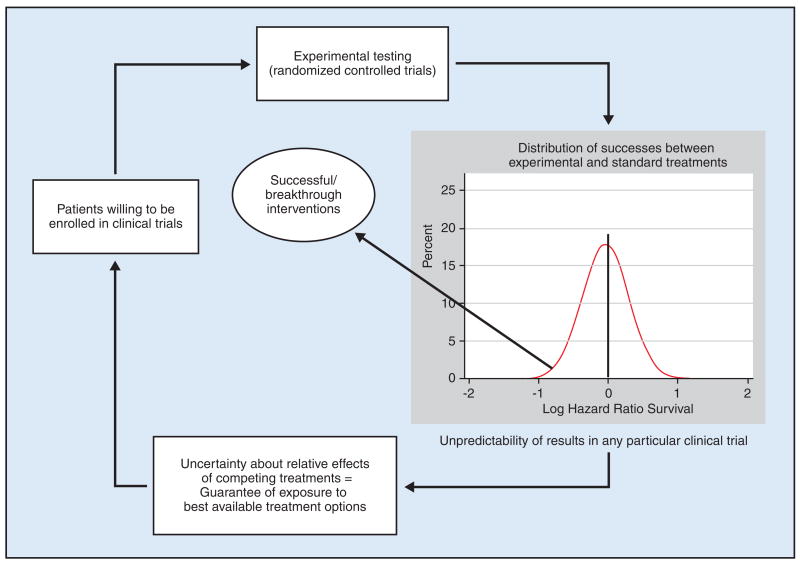
A recently proposed “law of clinical discoveries,” articulated in more detail here, states that it is this ethical requirement for uncertainty that determines advances in medicine6,38,49 (Figure), meaning that the pattern of observed successes is not an accident. There is a predictable relationship between the acknowledgment of uncertainty (the moral principle) on which trials are based and the ultimate outcomes of clinical trials.3
Figure.
A proposed model of clinical discoveries: how the ethical principle equipoise converges to become a scientific principle driving treatment progress. To be enrolled in clinical trials, patients require guarantees that they will not knowingly be harmed and will have an optimal chance of receiving the best available treatments. If the odds of treatment successes are too skewed in one direction or the other, patients would be reluctant to enroll in the clinical trials. As a result, clinical research would not be possible and no new treatments could be discovered. The best odds for patients to receive the optimal treatments are when the chance of success of the new treatment is about 50:50(1:1). These odds are best guaranteed by adherence to the equipoise principle. Under equipoise, it is easier for patients to enroll in the trial and institutional review boards to approve the study. With patient enrollment, discovery of a new successful treatment becomes possible. At the same time, equipoise limits the proportion of treatments that eventually will prove successful. This is the paradox of equipoise, which in turn helps maintain the clinical trials system. [The graph insert shows actual distribution of treatment success in randomized controlled trials conducted by the US National Cancer Institute Cooperative Groups from 1955 to 2006.] Data from Djulbegovic B, Kumar A, Soares HP, et al. Treatment success in cancer: new cancer treatment successes identified in phase 3 randomized controlled trials conducted by the National Cancer Institute-sponsored cooperative oncology groups, 1955 to 2006. Arch Intern Med. 2008;168(6):632–642. Figure adapted from Kumar A, Soares H, Wells R, et al. Are experimental treatments for cancer in children superior to established treatments? Observational study of randomised controlled trials by the Children’s Oncology Group. BMJ. 2005;331(7528):1295. Epub 2005 Nov 18. Reprinted with permission by BMJ Publishing Group Ltd.
Conclusions
Progress in clinical medicine rests heavily on the willingness of patients to take part in experimental clinical trials.1 Prior to enrolling in these trials, they require guarantees that they will not knowingly be harmed and will have an optimal chance of receiving the best available treatments. The best guarantee is provided by the acknowledgment of uncertainty, which removes ethical dilemma and makes it easier for patients to enroll in clinical trials.50 As a result of patient participation in RCTs, new treatments can be discovered. However, there are limits to how much can be discovered: ultimately, we will be successful in only about 50% of testings. Unlike speculation in businesses, where the investor seeks maximum certainty,51 the success rates in clinical research are both driven and constrained by the ethical principle of equipoise or requirement for uncertainty. These seemingly contradictory forces represent a paradox of equipoise.
Acknowledgments
Dr Djulbegovic receives grant/research support from the Research Program on Research Integrity, Office of Research Integrity, and National Institutes of Health (grants: No 1R01NS044417-01, 1 R01 NS052956-01, and 1R01CA133594-01 NIH/ORI). Funding agencies had no role in the preparation, review, or approval of this manuscript. Dr Djulbegovic reports no significant relationship with the companies/organizations whose products or services may be referenced in this article.
References
- 1.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284(23):3043–3045. [PubMed] [Google Scholar]
- 2.Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet. 2001;357(9253):373–380. doi: 10.1016/S0140-6736(00)03651-5. [DOI] [PubMed] [Google Scholar]
- 3.Djulbegovic B. Acknowledgment of uncertainty: a fundamental means to ensure scientific and ethical validity in clinical research. Curr Oncol Rep. 2001;3(5):389–395. doi: 10.1007/s11912-001-0024-5. [DOI] [PubMed] [Google Scholar]
- 4.Peto R, Baigent C. Trials: the next 50 years. Large scale randomised evidence of moderate benefits. BMJ. 1998;317(7167):1170–1171. doi: 10.1136/bmj.317.7167.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317(3):141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 6.Djulbegovic B. Articulating and responding to uncertainties in clinical research. J Med Philos. 2007;32(2):79–98. doi: 10.1080/03605310701255719. [DOI] [PubMed] [Google Scholar]
- 7.Miller PB, Weijer C. Trust based obligations of the state and physician-researchers to patient-subjects. J Med Ethics. 2006;32(9):542–547. doi: 10.1136/jme.2005.014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283(20):2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 9.Lilford RJ. Ethics of clinical trials from a bayesian and decision analytic perspective: whose equipoise is it anyway? BMJ. 2003;326(7396):980–981. doi: 10.1136/bmj.326.7396.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chard JA, Lilford RJ. The use of equipoise in clinical trials. Soc Sci Med. 1998;47(7):891–898. doi: 10.1016/s0277-9536(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 11.Edwards SJ, Lilford RJ, Braunholtz DA, et al. Ethical issues in the design and conduct of randomised controlled trials. Health Technol Assess. 1998;2(15):i–vi. 1–132. [PubMed] [Google Scholar]
- 12.Weijer C, Shapiro SH, Cranley Glass K. For and against: clinical equipoise and not the uncertainty principle is the moral underpinning of the randomised controlled trial. BMJ. 2000;321(7263):756–758. doi: 10.1136/bmj.321.7263.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veatch RM. Indifference of subjects: an alternative to equipoise in randomized clinical trials. Soc Philos Policy. 2002;19(2):295–323. doi: 10.1017/s0265052502192120. [DOI] [PubMed] [Google Scholar]
- 14.Veatch RM. The irrelevance of equipoise. J Med Philos. 2007;32(2):167–183. doi: 10.1080/03605310701255776. [DOI] [PubMed] [Google Scholar]
- 15.Warlow C. Advanced issues in the design and conduct of randomized clinical trials: the bigger the better? Stat Med. 2002;21(19):2797–2805. doi: 10.1002/sim.1283. [DOI] [PubMed] [Google Scholar]
- 16.Weijer C, Miller PB. When are research risks reasonable in relation to anticipated benefits? Nat Med. 2004;10(6):570–573. doi: 10.1038/nm0604-570. [DOI] [PubMed] [Google Scholar]
- 17.Gifford F. Community-equipoise and the ethics of randomized clinical trials. Bioethics. 1995;9(2):127–148. doi: 10.1111/j.1467-8519.1995.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 18.Karlawish JH, Lantos J. Community equipoise and the architecture of clinical research. Camb Q Healthc Ethics. 1997;6(4):385–396. doi: 10.1017/s0963180100008136. [DOI] [PubMed] [Google Scholar]
- 19.Djulbegovic B, Clarke M. Scientific and ethical issues in equivalence trials. JAMA. 2001;285(9):1206–1208. doi: 10.1001/jama.285.9.1206. [DOI] [PubMed] [Google Scholar]
- 20.Djulbegovic B, Lacevic M, Cantor A, et al. The uncertainty principle and industry-sponsored research. Lancet. 2000;356(9230):635–638. doi: 10.1016/S0140-6736(00)02605-2. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Therapy Evaluation Program. Bethesda, MD: National Cancer Institute, US National Institutes of Health; [Accessed May 15, 2009]. http://ctep.cancer.gov/ [Google Scholar]
- 22.Fried C. Medical Experimentation: Personal Integrity and Social Policy. North Holland/American Elsevier; Amsterdam/New York: 1974. [Google Scholar]
- 23.Hill AB. Medical ethics and controlled trials. BMJ. 1963;1(5337):1043–1049. doi: 10.1136/bmj.1.5337.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill AB. Clinical trials and the acceptance of uncertainty. BMJ. 1987;294(6584):1419. doi: 10.1136/bmj.294.6580.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djulbegovic B, Cantor A, Clarke M. The importance of preservation of the ethical principle of equipoise in the design of clinical trials: relative impact of the methodological quality domains on the treatment effect in randomized controlled trials. Account Res. 2003;10(4):301–315. doi: 10.1080/714906103. [DOI] [PubMed] [Google Scholar]
- 26.Lilford RJ, Djulbegovic B. Equipoise and “the uncertainty principle” are not mutually exclusive. BMJ. 2001;322:795. [PubMed] [Google Scholar]
- 27.Silverman WA. Gnosis and random allotment. Control Clin Trials. 1981;2(2):161–164. doi: 10.1016/0197-2456(81)90006-4. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers I. Well informed uncertainties about the effects of treatments. BMJ. 2004;328(7438):475–476. doi: 10.1136/bmj.328.7438.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halpern SD, Karlawish JH, Weijer C, et al. Informing patients of uncertainty in clinical trials. JAMA. 2001;285(21):2713–2714. doi: 10.1001/jama.285.21.2713-a. [DOI] [PubMed] [Google Scholar]
- 30.Glasziou P, Chalmers I, Rawlins M, et al. When are randomised trials unnecessary? Picking signal from noise. BMJ. 2007;334(7589):349–351. doi: 10.1136/bmj.39070.527986.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman WA, Chalmers I. Casting and drawing lots: a time honoured way of dealing with uncertainty and ensuring fairness. BMJ. 2001;323(7327):1467–1468. doi: 10.1136/bmj.323.7327.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill AB. The clinical trial. N Engl J Med. 1952;247(4):113–119. doi: 10.1056/NEJM195207242470401. [DOI] [PubMed] [Google Scholar]
- 33.Rawls J. A Theory of Justice. Cambridge, MA: Harvard University Press; 1999. Revised ed. [Google Scholar]
- 34.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. New York, NY: Springer Science + Business Media, LLC; 1998. [Google Scholar]
- 35.Halpern SD, Karlawish JH. Industry-sponsored research: University of Pennsylvania Research Ethics Working Group. Lancet. 2000;356(9248):2193. doi: 10.1016/s0140-6736(05)67270-4. [DOI] [PubMed] [Google Scholar]
- 36.Djulbegovic B, Bennet CL, Adams JR, et al. Industry-sponsored research. Lancet. 2000;356(9248):2193–2194. doi: 10.1016/S0140-6736(05)72986-X. [DOI] [PubMed] [Google Scholar]
- 37.Joffe S, Harrington DP, George SL, et al. Satisfaction of the uncertainty principle in cancer clinical trials: retrospective cohort analysis. BMJ. 2004;328(7454):1463. doi: 10.1136/bmj.38118.685289.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A, Soares H, Wells R, et al. Are experimental treatments for cancer in children superior to established treatments? Observational study of randomised controlled trials by the Children’s Oncology Group. BMJ. 2005;331(7528):1295. doi: 10.1136/bmj.38628.561123.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djulbegovic B, Kumar A, Soares HP, et al. Treatment success in cancer: new cancer treatment successes identified in phase 3 randomized controlled trials conducted by the National Cancer Institute-sponsored cooperative oncology groups, 1955 to 2006. Arch Intern Med. 2008;168(6):632–642. doi: 10.1001/archinte.168.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watts D. The “new” science of networks. Annu Rev Sociol. 2004;30:243–270. [Google Scholar]
- 41.Newman MEJ. Power laws, Pareto distributions and Zipf’s law. Contemp Phys. 2005;46(5):323–351. [Google Scholar]
- 42.Johnson N, Lilford RJ, Brazier W. At what level of collective equipoise does a clinical trial become ethical? J Med Ethics. 1991;17(1):30–43. doi: 10.1136/jme.17.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djulbegovic B, Bercu B. At what level of collective equipoise does a clinical trial become ethical for the IRB members?. Paper presented at the University of South Florida Third National Symposium - Bioethical Considerations in Human Subject Research; March 8–10, 2002; Clearwater, Florida.. [Google Scholar]
- 44.Mills N, Donovan JL, Smith M, et al. Perceptions of equipoise are crucial to trial participation: a qualitative study of men in the ProtecT study. Control Clin Trials. 2003;24(3):272–282. doi: 10.1016/s0197-2456(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 45.DiMasi JA, Grabowski HG. Economics of new oncology drug development. J Clin Oncol. 2007;25(2):209–216. doi: 10.1200/JCO.2006.09.0803. [DOI] [PubMed] [Google Scholar]
- 46.Chan JK, Ueda SM, Sugiyama VE, et al. Analysis of phase II studies on targeted agents and subsequent phase III trials: what are the predictors for success? J Clin Oncol. 2008;26(9):1511–1518. doi: 10.1200/JCO.2007.14.8874. [DOI] [PubMed] [Google Scholar]
- 47.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 48.Comis RL, Miller JD, Aldigé CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21(5):830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 49.Soares HP, Kumar A, Daniels S, et al. Evaluation of new treatments in radiation oncology: are they better than standard treatments? JAMA. 2005;293(8):970–978. doi: 10.1001/jama.293.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godlee F. In praise of uncertainty. BMJ. 2005;331(7528) 0-f. [Google Scholar]
- 51.Peters WP. Cooperative groups are not.... Arch Intern Med. 2008;168(19):2172–2173. doi: 10.1001/archinte.168.19.2172-b. author reply 2173–2174. [DOI] [PubMed] [Google Scholar]