Abstract
Proliferation of smooth muscle cells (SMCs) is the key event in the pathogenesis of intimal hyperplasia (IH) leading to coronary artery bypass graft (CABG) occlusion. The saphenous vein (SV) conduits are often affected by IH, while the internal mammary artery (IMA) conduits remain remarkably patent. SMC proliferation is mediated by the cell cycle, under the control of cyclin-dependent kinases (cdks), cdk-inhibitors and the retinoblastoma protein (Rb). Early passage of the SMCs through the cell cycle involves crossing the non-reversible G1 checkpoint, the restriction (R) point. In this study, we investigated the effect of mitogenic insulin-like growth factor (IGF)-1 stimulation on the R-point and its relationship with the phosphorylation of Rb protein and the cdk inhibitors p21 and p27 in SV and IMA SMCs. We observed no change in the R-point following IGF-1 activation in either SV or IMA SMCs. However, Rb-phosphorylation occurred much earlier and was quantitatively greater in SV SMCs than IMA. Overexpression of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) in SV SMCs followed by IGF-1 activation significantly decreased the expression of cyclin E and pRb and induced p27 expression in SV SMCs, while, pRb levels were markedly decreased and p27 levels were significantly increased in IMA SMCs. Silencing the PTEN gene by siRNA transfection of IMA SMCs significantly induced the expression of pRb and inhibited p27 expression, while, the expression levels of cyclin E, pRb, p21 and p27 were unaffected by the silencing of PTEN in SV SMCs. These results demonstrate that the PTEN plays a critical role in regulating cell cycle entry. Therefore, overexpression of PTEN possibly by means of gene therapy could be a viable option in regulating the cell cycle in SV SMCs in the treatment of vein graft disease.
Keywords: CABG, cell cycle, intimal hyperplasia, PTEN, R-point, saphenous vein, vein graft
Introduction
Vascular restenosis following coronary artery bypass graft (CABG) is a major clinical problem in the field of vascular biology. Restenosis is primarily due to intimal hyperplasia (IH) and secondary atheroma formation affects the saphenous vein (SV) conduit while the IMA is almost resistant to IH-associated fibroproliferative vascular pathology. Several inter-related processes are believed to be involved, which include pro-hyperplasia pathways, inflammatory cytokines, mitogenic growth factors all of which finally proceed to the progression of the cell cycle and leads to proliferation of the SMCs.
Mitogenic stimuli trigger different signaling pathways, which converge at the cell cycle. Quiescent cells are in the G0-phase and enter the cell cycle upon mitogenic stimulation. In the G1-phase, mitogenic and anti-mitogenic signals are integrated and the decision is made whether to continue, exit or pause the cell cycle. Hyperphosphorylation of the retinoblastoma protein (Rb) by cyclin E-dependent kinases is an important checkpoint which when overcome results in the commitment of the cells to further progress through the cell cycle, independent of extracellular stimuli [1]. Rb, which in its hypo-phosphorylated state becomes active, binds to promoter-bound members of the E2F family of transcription factors. Active Rb inhibits transcriptional activation of E2F-responsive genes including components of the replication machinery, such as cyclin E, DNA polymerase a and proliferating cell nuclear antigen (PCNA). Phosphorylation of Rb releases E2F from transcriptional restraint, which is initiated by cyclin D/cdk4/6 and completed by cyclin E/cdk2 [2]. Inhibitors of cyclin-dependent kinase (cdk), p21 and p27, inhibit cdk2 and cdk4/6, thereby inhibiting progression through G1 and S phases [3]. p27 inhibits cdk2 and cdk4/6 complexes and also inhibits cell cycle progression [3]. Phosphatase and tensin homologue deleted on chromosome 10 (PTEN), a dual lipid protein phosphatase, negatively controls the PI3K/PKB pathway and also exerts its influence on the cell cycle. Suppression of proliferation due to arrest in G1 phase of the cell cycle is associated with corresponding increases in the levels of cell cycle inhibitors such as p27 and decreased levels of Rb protein phosphorylation [4–6].
Mammalian cells are dependent upon external mitogens for cell cycle progression during G1. But this is only true until the cells reach a certain point, the restriction point (R point) [7]. The Rb pathway is a major regulatory pathway of the G1 phase. Phosphorylation of Rb by cyclin D-cdk4/6 and cyclin E-cdk2 regulates progression through G1 and is tightly controlled.
We have recently reported temporal PTEN inactivation causing increased proliferation of SV SMCs than in the IMA [8]. This suggests that the endogenous difference in the PTEN expression and activity in the SMCs of two bypass conduits could be responsible for IH in the vein grafts involving downstream cell cycle proteins. Therefore, the aim of this study was to study the proteins associated with the R-point and subsequent G1/S exit in SV and IMA SMCs. The hypothesis was that PTEN negatively regulates the cell cycle via the cell cycle regulatory proteins p21 and p27 leading to reduced phosphorylation of Rb protein in IMA SMCs, which in part could explain the reduced rate of proliferation in IMA SMCs, as compared to that in SV SMCs.
Methods
Human tissue collection
The study protocol was approved by the Institutional Review Board of Creighton University and samples were collected anonymously. SV and IMA tissues, left over from CABG procedures in human beings, were placed in the University of Wisconsin (UW) solution and brought to the laboratory. We used the tissues from 38 patients (age 46–78 years with a median of 59.5 year). Paired venous and arterial samples from the same patient were preferred in order to nullify any inter-patient differences due to age, gender or clinical conditions. The tissues were collected in the UW solution, which is widely used for organ storage and transport and the viability of the tissues and the functional property of the cells are maintained for at least 24 hrs [9].
Cell Isolation
The tissue samples were processed under strict aseptic condition by the established method in our laboratory [10, 11]. Briefly, the vessels were stripped of the adventitia and the endothelial layer was removed by gentle blunt dissection. The tissue was minced into small pieces performed with sterile scalpel blades and then subjected to enzymatic digestion performed with a mixture of 2% elastase and 1% collagenase (Sigma Chemical Co., St. Louis, MO, USA) in Dulbecco's Modified Eagle's Medium (DMEM) over a period of 3 hrs. The cell digest was strained through 100 μm sterile cell strainer and the filtrate was centrifuged at 900 × g for 10 min. The cell pellet was resuspended in pre-warmed smooth muscle cell medium (ScienCell Research Laboratories, San Diego, CA, USA) supplemented with 10% fetal bovine solution (FBS) and plated in 25 cm2 culture flasks.
Western blotting
After appropriate experimental treatment and time course points, the cell monolayer was washed with ice cold phosphate-buffered saline (PBS) and the cells were detached by trypsinization and centrifuged at 300 × g for 5 min. Cell lysis was done by the addition of 50 μl of ice-cold RIPA (radioim-munoprecipitation) buffer [0.05 min Tris buffer (pH 7.2), 0.15 min NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate (SDS)] and the cell extracts were kept on ice and vortexed periodically for a total duration of 30 min. The lysates were sonicated and transferred to eppendorf microcentrifuge tubes and centrifuged at 14,000 ×g for 10 min at 4°C. The pellet was discarded, and the protein lysates were stored at −70°C for analysis of protein expression [8, 10, 11].
The lysates were separated by gel electrophoresis and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were then blocked and probed with a specific primary antibody. The following antibodies were used in this study at the stated dilution: cyclin E (mouse, 1:500, Cell Signaling Technology Beverly, MA, USA), pRb (ser 608) (Rabbit, 1:500, Cell Signaling), p21 (mouse, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and p27 (Rabbit, 1:500, Santa Cruz).
Overexpression of PTEN
SMCs were transiently transfected using the pORF9-hPTEN expression vector containing the human open reading frame from InvivoGen (San Diego, CA, USA) according to the established method in our laboratory [8]. The cells were plated on the day before the transfection at a density of 110,000 cells per well in a 6-well plate with 2 ml of smooth muscle cell growth medium (SMGM) supplemented with 5% FBS. The cells were grown for 24 hrs at 37°C in a CO2 incubator till they reached 70–80% confluence. For each well, 2μg of DNA was diluted with 0.5 ml of Gibco OPTI-MEM I (Invitrogen Corporation, Carlsbad, CA, USA) medium without serum. For each well, 10 μl of Lipofectamine (Invitrogen Corporation, Carlsbad, CA, USA) reagent was diluted in 0.5 ml of OPTI-MEM 1 medium without serum. The diluted DNA and Lipofectamine reagent were mixed and incubated at room temperature for 20 min. The media from the cells was aspirated and the cell monolayer was washed once with pre-warmed DMEM without serum. The cells were overlaid with the DNA-Lipofectamine mixture (1 ml per well) and incubated at 37°C and 5% CO2 for 5 hrs. Following incubation, 1 ml DMEM containing 20% FBS was added to each well without removing the transfection mixture. Incubate further 18–24 hrs. Cell extracts were assayed for gene activity at 24 and 48 hrs after transfection using Western blotting for PTEN protein expression. To check for toxicity and cell viability, trypan blue dye exclusion was carried out in isolated cell suspension [8, 10, 11].
PTEN gene silencing
Gene silencing was used to inhibit the expression of PTEN gene in IMA and SV SMCs performed with a siRNA duplex against the PTEN gene [8]. The siRNA duplex sequence shown below was purchased from Integrated DNA Technologies, Inc. (Coralville, IA, USA).
5′-GGAAUAUCUAGUACUUACUUUAACA-3′ (sense)
3′- UCCUUAUAGAUCAUGAAUGAAAUUGU-5′ (antisense)
Using 6-well plates, cells (1 × 105/well) were seeded in 2 ml of antibiotic-free SMGM medium supplemented with 10%FBS, 24 hrs before the transfection. The cells were incubated at 37°C in a CO2 incubator until cells reached about 60–70% confluence which was ideal for transfection and was usually attained after overnight incubation. Transfection was done using siPORT amine (Ambion, Austin, TX, USA). SiPORT Amine was diluted in Opti-MEM 1 serum-free medium. For a 6-well plate set up, approx 8 μl of the SiPORT Amine was diluted in 100 μl of the media. The oligonucleotide RNA was also diluted in Opti-MEM 1 serum-free medium (25 μl of RNA + 100 μl media was sufficient for a 6-well plate transfection). The diluted SiPORT Amine and diluted RNA were mixed together and allowed to incubate at room temperature for 10 min in order to equilibrate. To each of the wells, equal volumes of the SiPORT Amine and RNA mixture was added and gently rocked back and forth to ensure adequate mixing. The control well was not treated with the RNA, but only with SiPORT Amine and Opti-MEM. The culture plates were returned to the incubator and assayed for target gene activity 24–72 hrs after transfection. Cell toxicity/death was assessed using trypan blue exclusion and gene knockdown was assessed using Western blotting against the target gene.
Statistical analysis
All data are represented as mean ± SEM. Data were analyzed using the one-way anova with Tukey's after test for analysis of inter- and intra-sample groups. P < 0.05 was considered as significant.
Results
The R-point and phosphorylation of Rb protein
The R-point is the G1 checkpoint beyond which cells are not influenced by growth factors for cell cycle progression. The R-point was measured by the accumulation of cyclin E, which occurs at the point of G1 to S phase exit. Immunoblotting of cyclin E and densitometric analysis revealed that the R-point in SV SMCs was at 3 hrs, as indicated by a significant (P < 0.001) rise in cyclin E expression in response to insulin-like growth factor (IGF)-1 (100 ng/ml). In IMA SMCs, the R-point reached at 4 hrs (Fig. 1).
1.
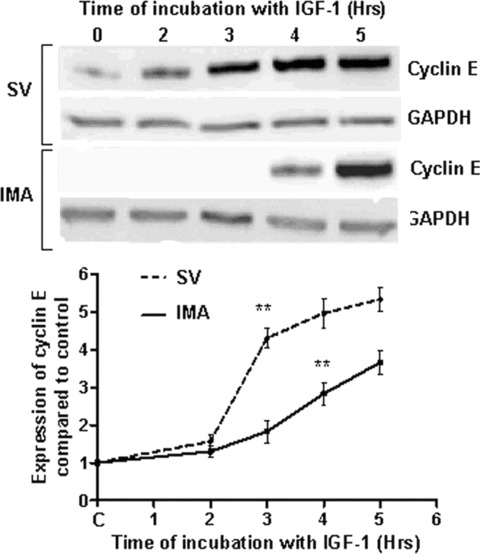
Effect of IGF-1 stimulation on the determination of R-point based on cyclin E expression. Cyclin E expression marks the R-point, which occurs at G1/S transition. Thus, cyclin E accumulation is used to assess the R-point. SV (left panel) and IMA (right panel) SMCs were stimulated with IGF-1 (100 ng/ml) over a time course of 0–5 hrs. Cyclin E levels were determined by Western blotting and the results are graphically represented as densitometric analysis of five individual samples of SV and IMA cells. (**P < 0.001)
The phosphorylation of Rb protein was distinct from the R-point and occurred at a time-point downstream of the R-point (Fig. 2). In SV SMCs, there was a significant (P < 0.001) rise in Rb phosphorylation at about 4 hrs and remained at significant levels till 12 hrs after which it declines to control levels. However, in the IMA SMCs, Rb phosphorylation was downstream of the R-point and reached significant levels at 8 hrs (P < 0.05) with peak levels at 12 hrs (P < 0.001) before returning to control levels (Fig. 2). There was a lag of about 4 hrs between the R-point and significant increase in Rb-phosphorylation in IMA SMCs as compared to about 1 hr in SV SMCs. The degree of phosphorylation observed in IMA SMCs was quantitatively far less than that seen in SV SMCs (Fig. 2).
2.
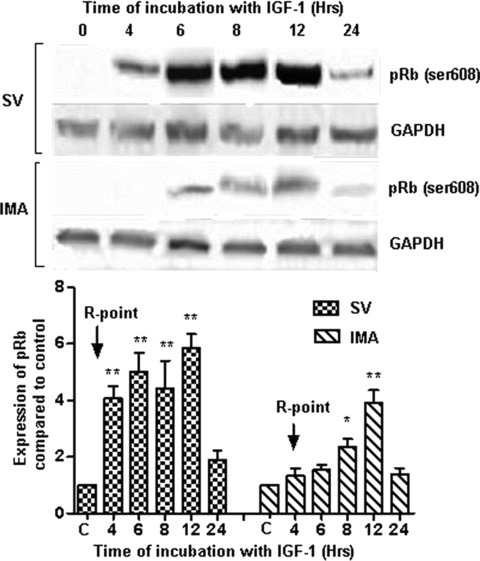
Phosphorylation of Retinoblastoma protein (ser 608) following IGF-1 activation. SV and IMA SMCs were stimulated with IGF-1 (100 ng/ml) over a time course of 0–24 hrs. Phosphorylation of retinoblastoma protein was determined by Western blotting and densitometric analysis of five individual samples of SV and IMA cells is represented graphically. The phosphorylation of Rb is shown with relation to the restriction (R) point. (*P < 0.001; *P < 0.05)
Expression of Cdk inhibitors, p21 and p27
The cell cycle inhibitors p21 and p27 block cell cycle progression by specifically inhibiting cyclin E, D and A-associated cdk complexes. The quiescent SMCs of both SV and IMA showed the expression of p21 (Fig. 3). IGF-1 (100 ng/ml) stimulation did not significantly alter their levels in either of the cell groups at least up to 24 hrs. The p27 expression was also present in both SV and IMA quiescent cells and in both cases the levels did not reach statistically significant levels up to 24 hrs following insulin-like growth factor (IGF)-1 stimulation (Fig. 4).
3.
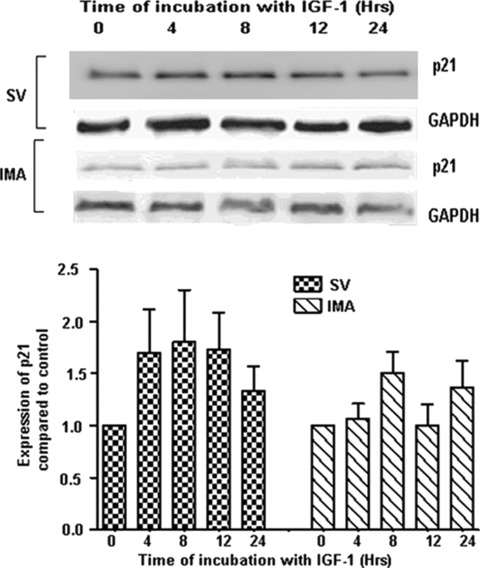
Effect of IGF-1 activation on the expression of cdk inhibitor p21. SV and IMA SMCs were treated with IGF-1 (100 ng/ml) over a time-period extending from 0–24 hrs. p21 was determined by Western blotting and densitometric analysis of five individual samples of SV and IMA cells is represented graphically. GAPDH was used as loading control. There was no significant difference between any of the time points.
4.
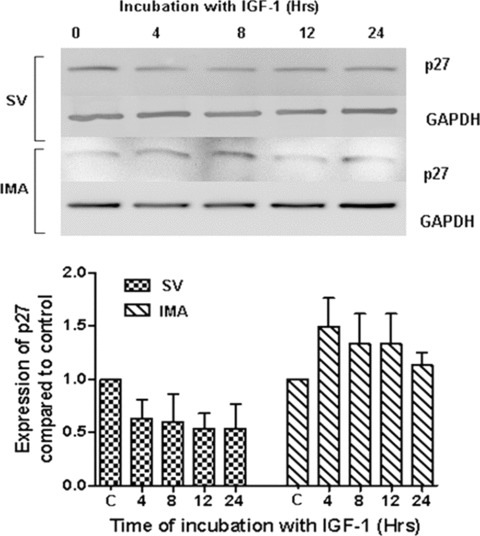
Effect of IGF-1 activation on the expression of cdk inhibitor p27. SV and IMA SMCs were treated with IGF-1 (100 ng/ml) over a time-period extending from 0–24 hrs. p27 was determined by Western blotting and the densitometric analysis of five individual samples of SV and IMA cells is represented graphically. GAPDH was used as loading control. There was no significant difference between any of the time-points.
Expression of cyclin E, pRb, p21 and p27 in pORF9-hPTEN transfected SV and IMA SMCs
We observed maximum effect of IGF-1 on the expression of cyclin E at 5 hrs and on the expression of pRb, p27 and p21 at 12 hrs. Therefore, we further examined the influence of PTEN on the expression of the proteins related to the cell cycle and R-point transition. SV SMCs were transfected to overexpress PTEN using the pORF9-hPTEN vector. The cells were mitogenically stimulated with IGF-1 (100 ng/ml) and the expression of cyclin E, pRb, p21 and p27 was assessed using immunoblotting and densitometric analysis. In SV SMCs, overexpression of PTEN significantly decreased the expression of cyclin E and pRb and induced p27 expression. The p21 levels were unaffected by the overexpression of PTEN (Fig. 5). In the IMA SMCs, overexpression of PTEN significantly decreased the expression of only pRb and induced p27 expression (Fig. 6). The cyclin E and p21 levels were unaffected by the overexpression of PTEN in IMA SMCs (Fig. 6).
5.
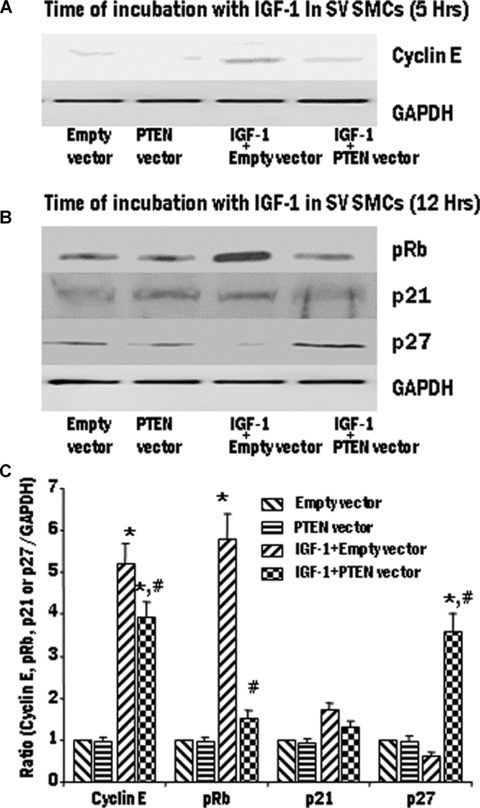
The effect of IGF-1 activation on cell cycle proteins in pORF9-hPTEN-transfected SV SMCs. SV cells were transiently transfected with PTEN vector to overexpress the protein. Transfected SV SMCs were treated with IGF-1 (100 ng/ml) for 5 hr or 12 hrs. Expression levels of cyclin E (A), pRb, p21 and p27 (B) were determined by Western blotting and the densitometric analysis of five individual samples of SV cells (C) is represented graphically. (*P < 0.01 compared with control group. #P < 0.01 compared with IGF-1 + empty vector).
6.
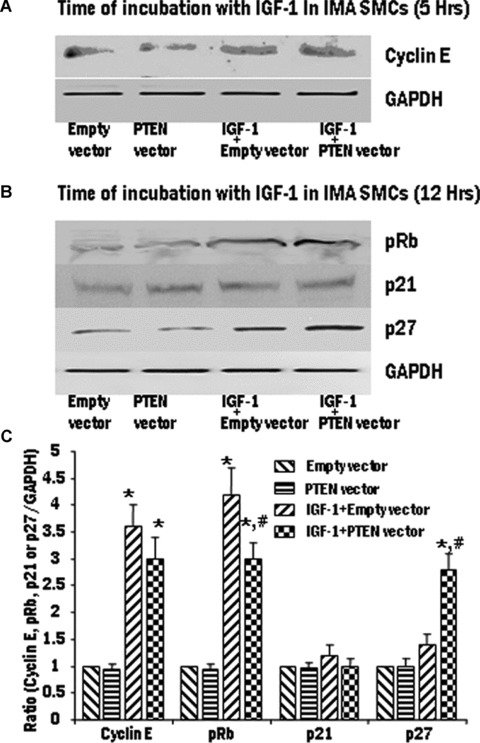
The effect of IGF-1 activation on cell cycle proteins in pORF9-hPTEN-transfected IMA SMCs. IMA cells were transiently transfected with PTEN vector to overexpress the protein. Transfected IMA SMCs were treated with IGF-1 (100 ng/ml) for 5 hrs or 12 hrs. Expression levels of cyclin E (A), pRb, p21 and p27 (B) were determined by Western blotting and the densitometric analysis of five individual samples of IMA cells (C) is represented graphically. (*P < 0.01 compared with control group. #P < 0.01 compared with IGF-1 + empty vector).
Expression of cyclin E, pRb, p21 and p27 in PTEN gene silenced SV and IMA SMCs
The effect of loss of PTEN functioning was assessed using siRNA-mediated PTEN gene silencing in SV and IMA SMCs. Vascular smooth muscle cells (VSMCs) were transfected with siRNA for PTEN and non-silencing siRNA and the protein expression of PTEN was determined by Western blot analysis. A marked reduction in PTEN protein expression was observed at 48 hrs and 72 hrs following siRNA treatment (data not shown). PTEN-silenced IMA and SV SMCs were exposed to IGF-1 (100 ng/ml) and the expression of the various proteins was analyzed by immunoblotting and densitometry analysis. In SV SMCs, The expression of cyclin E, pRb, p21 and p27 levels were unaffected by silencing of PTEN (Fig. 7). However, In the IMA SMCs, siRNA for PTEN significantly induced the expression of pRb and inhibited p27 expression (Fig. 8). The expression levels of cyclin E and p21 were unaffected by the overexpression of PTEN (Fig. 8).
7.
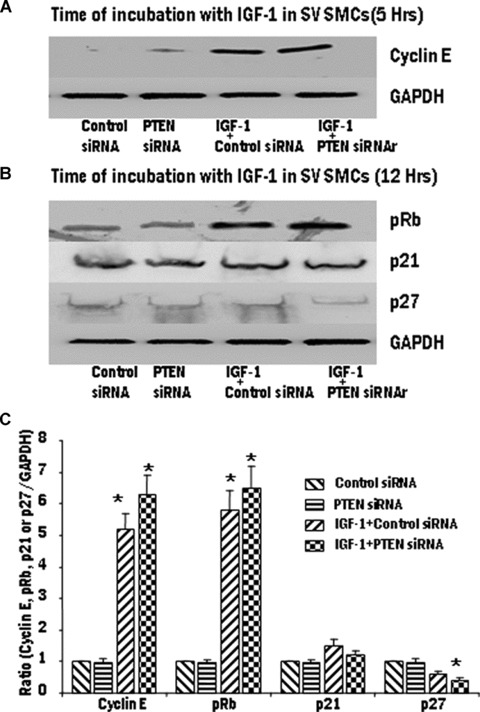
Effect of PTEN gene silencing on the expression of cyclin E, phosphorylation of Rb protein, p21 and p27 in SV SMCs. The transfected cells were stimulated with IGF-1 (100 ng/ml). Expression levels of cyclin E (A) and pRb, p21 and p27 (B) were determined by Western blotting and the densitometric analysis of five individual samples of SV cells (C) is represented graphically. (*P < 0.01 compared with control group. #P < 0.01 compared with IGF-1 + empty vector).
8.
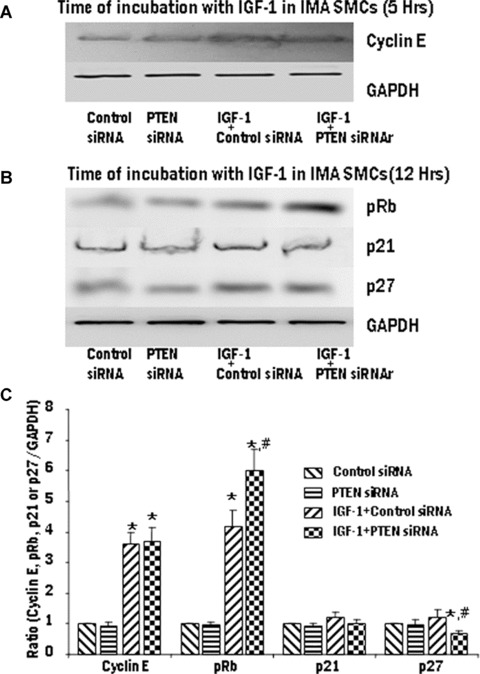
Effect of PTEN gene silencing on the expression of cyclin E, phosphorylation of Rb protein, p21 and p27 in IMA SMCs. The transfected cells were stimulated with IGF-1 (100 ng/ml). Expression levels of cyclin E (A), pRb, p21 and p27 (B) were determined by Western blotting and the densitometric analysis of five individual samples of IMA cells (C) is represented graphically. (*P < 0.01 compared with control group. #P < 0.01 compared with IGF-1 + empty vector).
Discussion
Mitogenic stimuli trigger different signalling pathways, but all finally converge at the cell cycle. In the G1-phase of the cell cycle, mitogenic and anti-mitogenic signals are integrated and the decision is made whether to continue, exit or pause the cell cycle, beyond the R-point, the cells are committed to further progress through the cell cycle, independent of extracellular stimuli [1]. Reports have shown that phosphorylation of Rb is not the molecular mechanism behind the R-point transition [12, 13]. Results from our study support this conclusion since we observed that phosphorylation of Rb occurred downstream of the R-point and was in no way affected by the phosphorylation state of Rb.
Vascular injury results in hyperphosphorylation of Rb protein mediated by the PI3K-PKB pathway, which can be prevented by the use of statins and inhibitors of the Rho/Rho kinase pathway [14]. This suggests an alternative pathway to Rb protein hyper-phosphorylation. Vascular endothelial growth factor (VEGF) also appears to have an indirect action on Rb protein phosphorylation and this could be as a result of the cross-talk, which is commonly seen between receptors involved in the triggering of the pathway leading to the development of IH [15]. Retinoids attenuate growth factor-induced down-regulation of p27, which is a major negative regulator of Rb phosphorylation and thus exerts an anti-proliferative action on SMCs.
Cell cycle proteins and their patterns of expression have been extensively studied and increased levels have been documented during lesion formation in the vessel wall [16]. c-fos, c-jun and c-myc mRNAs are seen to rise within hours of injury but return to baseline after a relatively short period followed by a second peak at or around the 7th day after injury during which time maximal proliferation activity occurs [17, 18]. p27, which is normally at relatively high levels in the quiescent cells, fall rapidly soon after the onset of injury [19]. Conversely, p21 levels, which are normally low in quiescent SMCs, rise after 7 days and reach a peak after 2 weeks [20]. This seems to suggest that p21 has a delayed mechanism of action after arterial injury and is not an immediate acting cdk inhibitor [19]. Passage through the R-point requires suppression of the normally high levels of p27 expression present in quiescent cells [21]. Although p27 is a known early inhibitor of the cdks, its exact mode of action still remains unclear. Recent studies have shown an association between p21 and MCM7, which is a DNA-replicating protein, to inhibit DNA replication prior to S phase [22].
Even though the cell cycle is known to be the final convergent point of all proliferative pathways and is the watershed of proliferation, there seems to be still many unanswered questions. The R-point appears to be under multiple controls. The majority of inhibitory control mechanisms such as PTEN, p53, p21 p27 are all upstream of the R-point. Although some researchers have reported a second R-point as well as some forms of short-circuit mechanisms should the R-point mechanism fail, their existence is still under study. Mutagenesis of important negative regulators of the cell cycle has been seen in cancerous tissues. In the case of IH, however, it does not appear to be mutagenesis or any kind of gene disruption, but dysregulation of one or more inhibitory/regulatory proteins upstream of the R-point.
Recently, we reported temporal inactivation of PTEN in SV SMCs, whereas in the IMA SMCs PTEN remains active for longer period [8]. We also observed increased proliferation in IGF-1-stimulated SV SMCs than in the IMA [8]. Therefore, the observed difference in rate of rise in cyclin E expression and thus the R-point between SV and IMA SMCs could be due to difference in the endogenous levels of total and activated PTEN. Furthermore, as shown in this study, the effect of the overexpression of PTEN on decrease in cyclin E expression was predominant in the SMCs of SV, with no significant effect in the IMA SMCs. Interestingly, silencing the PTEN gene had no effect on the expression of cyclin E, pRb, p21 and p27 levels in SV SMCs. However, in the IMA SMCs, siRNA for PTEN significantly induced the expression of pRb and inhibited p27 expression. This further supports the role of PTEN in regulating cell cycle proteins, especially cyclin E, and the R-point. Thus, the difference in the endogenous levels of activated and inactivated PTEN in the SMCs of the two CABG conduits could explain, at least in part, the pathophysiology of vein-graft disease.
Thus, the cell cycle provides a promising target for therapeutic intervention. Although radiotherapy and paclitaxel are able to reduce IH [23, 24], so far, the most promising agent seems to be rapamycin (Sirolimus) which selectively inhibits mTor and exerts its antiproliferative properties by reduction of translation and stabilization of p27 [25]. Gene therapy despite the poor results in clinical trials remains a viable option. Our studies have shown that PTEN gene therapy could be explored in the prevention and/or treatment of IH in vein-graft disease.
Acknowledgments
This study was supported by National Institutes of Health grants R01HL070885 and R01HL07349 (to D.K.A.) and Creighton University LB692 Nebraska Tobacco Settlement Grant (to D.K.A.). We gratefully acknowledge the contributions of The Nebraska Heart Hospital, Lincoln, NE, for providing the tissue samples for our study.
References
- 1.Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–77. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 3.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–91. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 4.Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002–8. [PubMed] [Google Scholar]
- 5.Li H, Lu S, Fong L. [Study on the status of methylation of Rb gene promoter in human esophageal cancer and effect of NMBzA on Rb gene promoter in monkey esophageal epithelium] Zhonghua Zhong Liu Za Zhi. 1998;20:412–4. [PubMed] [Google Scholar]
- 6.Simpson MT, MacLaurin JG, Xu D, Ferguson KL, Vanderluit JL, Davoli MA, Roy S, Nicholson DW, Robertson GS, Park DS, Slack RS. Caspase 3 deficiency rescues peripheral nervous system defect in retinoblastoma nullizygous mice. J Neurosci. 2001;21:7089–98. doi: 10.1523/JNEUROSCI.21-18-07089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA. 1974;71:1286–90. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra AK, Jia G, Gangahar DM, Agrawal DK. Temporal inactivation causes proliferation of saphenous vein smooth muscle cells of human CABG conduits. J Cell Mol Med. 2008;12:1–13. doi: 10.1111/j.1582-4934.2008.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abebe W, Cavallari N, Agrawal DK, Rowley J, Thorpe PE, Hunter WJ, Edwards JD. Functional and morphological assessment of rat aorta stored in University of Wisconsin and Eurocollins solutions. Transplantation. 1993;56:808–16. doi: 10.1097/00007890-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Jia G, Mitra AK, Cheng G, Gangahar DM, Agrawal DK. Angiotensin II and IGF-1 regulate connexin43 expression via ERK and p38 signaling pathways in vascular smooth muscle cells of coronary artery bypass conduits. J Surg Res. 2007;142:137–42. doi: 10.1016/j.jss.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Jia G, Cheng G, Gangahar DM, Agrawal DK. Involvement of connexin 43 in angiotensin II-induced migration ad proliferation of saphenous vein smooth muscle cells via the MAPK-AP-1-signaling pathway. J Mol Cell Cardiol. 2008;44:882–90. doi: 10.1016/j.yjmcc.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graff P, Amellem O, Seim J, Stokke T, Pettersen EO. The role of p27 in controlling the oxygen-dependent checkpoint of mammalian cells in late G1. Anticancer Res. 2005;25:2259–67. [PubMed] [Google Scholar]
- 13.Martinsson HS, Zickert P, Starborg M, Larsson O, Zetterberg A. Changes in cell shape and anchorage in relation to the restriction point. J Cell Physiol. 2005;203:27–34. doi: 10.1002/jcp.20204. [DOI] [PubMed] [Google Scholar]
- 14.Kozai T, Eto M, Yang Z, Shimokawa H, Luscher TF. Statins prevent pulsatile stretch-induced proliferation of human saphenous vein smooth muscle cells via inhibition of Rho/Rho-kinase pathway. Cardiovasc Res. 2005;68:475–82. doi: 10.1016/j.cardiores.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Dorafshar AH, Angle N, Bryer-Ash M, Huang D, Farooq MM, Gelabert HA, Freischlag JA. Vascular endothelial growth factor inhibits mitogen-induced vascular smooth muscle cell proliferation. J Surg Res. 2003;114:179–86. doi: 10.1016/s0022-4804(03)00254-3. [DOI] [PubMed] [Google Scholar]
- 16.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–9. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 17.Miano JM, Vlasic N, Tota RR, Stemerman MB. Smooth muscle cell immediate-early gene and growth factor activation follows vascular injury. A putative in vivo mechanism for autocrine growth. Arterioscler Thromb. 1993;13:211–9. doi: 10.1161/01.atv.13.2.211. [DOI] [PubMed] [Google Scholar]
- 18.Mann C, Boccara G, Fabre JM, Grevy V, Colson P. The detection of carbon dioxide embolism during laparoscopy in pigs: a comparison of transesophageal Doppler and end-tidal carbon dioxide monitoring. Acta Anaesthesiol Scand. 1997;41:281–6. doi: 10.1111/j.1399-6576.1997.tb04680.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang ZY, Simari RD, Perkins ND, San H, Gordon D, Nabel GJ, Nabel EG. Role of the p21 cyclin-dependent kinase inhibitor in limiting intimal cell proliferation in response to arterial injury. Proc Natl Acad Sci USA. 1996;93:7905–10. doi: 10.1073/pnas.93.15.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hitomi M, Yang K, Guo Y, Fretthold J, Harwalkar J, Stacey DW. p27Kip1 and cyclin dependent kinase 2 regulate passage through the restriction point. Cell Cycle. 2006;5:2281–9. doi: 10.4161/cc.5.19.3318. [DOI] [PubMed] [Google Scholar]
- 21.Nallamshetty S, Crook M, Boehm M, Yoshimoto T, Olive M, Nabel EG. The cell cycle regulator p27Kip1 interacts with MCM7, a DNA replication licensing factor, to inhibit initiation of DNA replication. FEBS Lett. 2005;579:6529–36. doi: 10.1016/j.febslet.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 23.Grise MA, Massullo V, Jani S, Popma JJ, Russo RJ, Schatz RA, Guarneri EM, Steuterman S, Cloutier DA, Leon MB, Tripuraneni P, Teirstein PS. Five-year clinical follow-up after intracoronary radiation: results of a randomized clinical trial. Circulation. 2002;105:2737–40. doi: 10.1161/01.cir.0000018126.87045.e0. [DOI] [PubMed] [Google Scholar]
- 24.Vinals F, Pouyssegur J. Confluence of vascular endothelial cells induces cell cycle exit by inhibiting p42/p44 mitogen-activated protein kinase activity. Mol Cell Biol. 1999;19:2763–72. doi: 10.1128/mcb.19.4.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakurai R, Bonneau HN, Honda Y, Fitzgerald PJ. Intravascular ultrasound findings in ENDEAVOR II and ENDEAVOR III. Am J Cardiol. 2007;100:71M–6M. doi: 10.1016/j.amjcard.2007.08.025. [DOI] [PubMed] [Google Scholar]


