Abstract
Liver re-transplantation surgery has a high rate of allograft failure due to patient co-morbidities and technical demands of the procedure. Success of liver re-transplantation could depend on surgeon experience and processes of care that relate to center volume. We performed a retrospective cohort study of adult liver re-transplantation procedures performed from 1/1/1996 through 12/31/2005 using registry data from the Organ Procurement Transplantation Network. The primary outcome was one-year allograft failure. Liver transplant centers were categorized as small, intermediate or high volume by dividing overall liver transplants into three tertiles of approximately equal size. Mean annual volume of overall liver transplants was <50 for low volume centers, 50 – 88 for intermediate volume centers, and >88 for high volume centers. The primary analysis consisted of 3977 liver re-transplantation patients. The unadjusted risk of one-year allograft failure was 37.8%. In multivariable logistic regression, the risk of one-year allograft failure was not significantly different between low (reference), intermediate (O.R. 0.86, CI 0.72–1.03, p=0.11) and high volume centers (O.R. 0.88, CI 0.74–1.04, p=0.14). Results were similar when the analysis was limited to re-transplantation performed >160 days after initial transplantation. Center volume is an imprecise surrogate measure for one-year outcomes after liver re-transplantation.
Introduction
Over the past two decades, a number of investigators have detected an association between increased hospital volume of specific surgical procedures and improved outcomes.(5, 10, 3, 7, 18, 27, 25) Liver transplantation has high morbidity and mortality and requires an experienced multi-disciplinary care team, but prior studies have not consistently found an association between center volume and allograft survival after liver transplant surgery.(13, 2, 24) These studies did not specifically examine outcomes after liver re-transplantation, a procedure that carries increased risk due to severity of patient illness and also due to technical challenges related to scarring and repeat anastomoses when substantial time has passed since the initial surgery.(21)
Patient and allograft survival after liver transplantation surgery depend on diverse donor, recipient and allograft factors, as well as characteristics of the center and the transplant staff.(15, 21, 17) An association between higher transplant volume and improved outcomes might be related to numerous center characteristics, such as the technical skill of individual surgeons, physician judgment in selecting patients and allografts, or improved processes of care, such as prevention of iatrogenic complications.(4) Notably, however, these center characteristics could vary independently of center volume of transplantations. For instance, surgeons who perform liver transplantation may also perform other surgeries including kidney or pancreas transplantation that enhance their technical skills.(14, 9) Likewise, high quality processes of care, such as prevention of infection in the intensive care unit, may not be specific to liver transplant recipients alone.(9) Additionally, high center volume could negatively impact survival if center staff and resources were not adequate to meet the clinical demands needed to care for patients after transplantation.
An examination of center volume and outcomes after liver re-transplantation also requires consideration of the effect of Model for End-Stage Liver Disease (MELD) system for allograft allocation. Survival on the liver transplantation wait-list has improved since the institution of MELD, and some data suggest that patient and allograft survival after liver re-transplantation have also improved.(12, 17) A study of center volume and outcomes for patients undergoing liver transplantation in the MELD era, however, failed to find an association between higher volume and patient mortality.(24)
The aims of this study were to examine whether outcomes after liver re-transplantation are associated with overall volume of liver transplants at a center, and to examine whether outcomes for re-transplantation have changed since the institution of the Model for End-Stage Liver Disease (MELD) system for allocation in February 2002.
Methods
Using registry data from the Organ Procurement and Transplantation Network, we performed a retrospective cohort study of adult liver re-transplantations in the United States between 1/1/1996 and 12/31/2005. The University of Pennsylvania Institutional Review Board approved the study.
Primary outcome
Allograft failure by one-year after liver transplantation.
Center volume
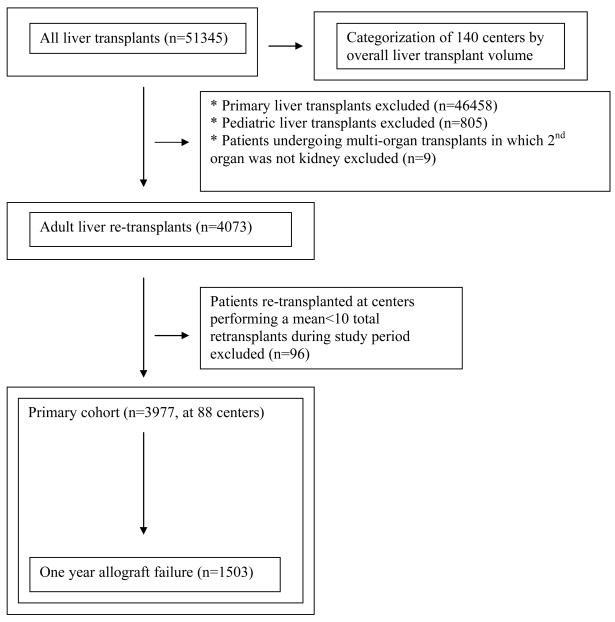
Similar to prior authors, we categorized transplant centers as small, intermediate or large volume by dividing the dataset of overall liver transplants into three tertiles of approximately equal size.(2) Our primary analysis, however, was restricted to adult patients undergoing liver re-transplantation. See Figure 1.
Figure 1.
Generation of cohort of patients undergoing liver re-transplantation
Inclusion criteria
Adults undergoing repeat liver transplantation.
Exclusion criteria
The following patients were excluded from our primary analysis – patients undergoing initial liver transplantation, recipients aged <18 years, and recipients at centers performing <10 liver re-transplants during the study period.
Statistical analysis
Analyses were performed using Stata software (Stata 10.0, Stata Corporation, College Station, Texas).
Primary and secondary analyses
Two primary analyses with the outcome of allograft survival were performed: one that included patients undergoing re-transplantation in both the pre- and post-MELD eras (referred to as the ‘combined analysis’), and another corresponding to the period after the institution of the MELD system on February 27, 2002.
The following secondary analyses were performed: an analysis of allograft survival among liver re-transplants performed prior to the institution of the MELD system, an analysis of allograft survival restricted to re-transplants >160 days after initial liver transplantation during the combined period, an unadjusted analysis of allograft survival restricted to patients with high MELD score, and an analysis of patient survival after liver re-transplantation during the combined period.
Independent variables
On the basis of published literature, we identified variables that would require adjustment in examining the relationship of center volume to one-year allograft failure. Donor variables included age, gender, race, height, cause of death, and donation after cardiac death. Recipient variables included age, gender, race, Status 1 designation, life support, hepatitis C infection, diabetes, simultaneous kidney transplant, MELD era (a binary variable corresponding to whether or not transplant occurred after 2/27/2002), and time between initial transplant and re-transplant.
Allograft variables included cold ischemia, warm ischemia, split liver, and UNOS Share type. Share type refers to the allograft allocation, in which 3=local, 4=regional, 5=national, and 6=foreign. For simplicity, we generated a binary variable corresponding to local or regional versus national or foreign allograft sharing.(21, 15, 23)
Missing data
Re-transplantation patients in our primary cohort had missing data on 5 variables considered relevant to the outcomes. These variables were: allograft warm ischemia (n=831; 20.9% of cohort), allograft cold ischemia (n=577; 14.5% of cohort), donor height (n=45; 1.1% of cohort), time between first transplant and re-transplant (n=14; 0.3% of cohort) and donor age (n=4; 0.1% of cohort). For these variables, the mean value was imputed for multivariable analyses of allograft failure. We examined the effect that this imputation had on the point estimates of the association of center volume and outcomes by repeating the analyses without imputation.
Testing the assumption of linearity in the logit
We graphically assessed the relationship of our continuous variables to the binary outcome of one year allograft failure (lincheck command in Stata).(16) Three variables had non-linear relationships to the outcome: cold ischemia, warm ischemia and time between transplants. Therefore, we created categorical variables (xtile command in Stata). Each variable was divided into quartiles by dividing the primary cohort into 4 equal groups on the basis of that variable.
Univariate analysis
For univariate comparisons of recipient, donor and allograft characteristics to center volume category, we used the Anova test to compare the means of continuous variables. The chi-square test was used to compare categorical variables. Alpha level for univariate and multivariable analysis was set at p≤0.05.
Multivariable logistic regression for the outcomes of one-year allograft failure and one-year patient mortality
We chose logistic regression instead of Cox regression because a multivariable Cox model did not satisfy the proportional-hazards assumption based on Schoenfeld residuals. Using the multivariable logistic model, the hypothesis of good fit was not rejected for the Hosmer-Lemeshow goodness of fit test (p=0.38).
The multivariable models for one-year allograft failure and patient mortality included all recipient, donor and allograft attributes listed in the univariate methods section, except as follows: MELD score was only put in the model in the secondary analysis restricted to post-MELD patients, because for patients transplanted prior to 2/27/2002, the majority were missing data required to calculate MELD score.
Detectable difference
Using an allograft failure rate of 38%, we calculated that our analysis would have 80% power to detect a 5% difference in the outcome between two center volume categories, i.e. of 33% versus 38%, based on a ratio of patients in each category of 1.6 to 1.
Results
Categorization of centers by overall liver transplant volume
During the 10-year period, 51,345 total adult liver transplants were performed in the US at 140 transplant centers. This group of all liver patients was used to categorize centers on the basis of volume.
We categorized centers as low, intermediate and high volume by dividing the 51,345 liver transplant patients into 3 tertiles of approximately equal size. The 100 centers in the low volume category performed a median of 20 liver transplants each year, with a range of 0.1 – 49.1 transplants. The 27 centers in the intermediate volume category performed a median of 62 liver transplants each year, with a range of 50 – 88 transplants. The 13 centers in the high volume category performed a median of 138 liver transplants each year, with a range of 88.1 – 210.4 transplants. These data are presented in Table 1.
Table 1.
Transplant centers stratified by category of overall liver transplant volume (n=51345)
| Low volume | Intermediate volume | High volume | |
|---|---|---|---|
| Number of centers | 100 | 27 | 13 |
| Number of liver transplants overall | 17160 | 17276 | 16909 |
| Median annual volume of liver transplants/yr at individual centers | 20 | 62 | 138 |
| Range of mean annual volume of liver transplants/yr at individual centers | 0.1 – 49.1 | 50.0 – 88.0 | 88.1 – 210.4 |
| Number of re-transplants (% of overall) |
1430 (8.3) |
1515 (8.8) |
1942 (11.5) |
| Mean center-specific annual volume of re-transplants | 1.6 | 5.6 | 14.9 |
| Intra-quartile range | (0.5 – 2.7) | (4.1 – 6.8) | (9.3 – 22.3) |
Liver re-transplantation patients
During the 10-year period, 4,887 patients underwent re-transplantation. The mean center-specific ratio of re-transplants/total transplants was 9.0%. For low volume centers, 8.3% of total transplants were re-transplants, compared to 8.8% for intermediate volume centers and 11.5% for high volume centers. The correlation between the overall rate of transplantation at a center and the rate of re-transplantation was 0.92 (p<0.01).
Primary cohort
A total of 3,977 adult patients (81.4% of total re-transplants) met our inclusion criteria. Generation of the primary cohort is displayed in Figure 1. For re-transplant patients and centers in the primary cohort, the mean center-specific ratio of re-transplants/total transplants was 9.6%.
For the primary cohort, the risk of one-year allograft failure was 37.8% and the risk of one-year patient death was 28.8% after liver re-transplantation. The mean age at re-transplantation was 47.6 years. 2987 (75.1%) recipients were white, 429 (10.8%) were Hispanic and 394 (9.9%) were black. 2,477 (62.3%) recipients were male.
A number of recipient, donor and allograft characteristics differed by volume category. Low volume centers had a higher proportion of white recipients (77.9%) compared to intermediate (73.1%) and high (75.0%) volume centers (p=0.03). We also identified a statistically significant interaction between center volume and MELD era on the outcome of ethnicity of the recipient. Specifically, the proportion of white patients at high volume centers grew substantially more post-MELD than at small and medium volume centers (p<0.01 for interaction term.) In contrast, the proportion of Hispanic patients was greatest (14.3%) at high volume centers pre-MELD, but post-MELD, high volume centers had the lowest proportion of Hispanic patients (10.0%) (p<0.01 for interaction term).
At low volume centers, 37.0% of recipients had hepatitis C infection, compared to 32.5% at intermediate volume centers and 28.5% at high volume centers (p<0.01). Large centers used liver allografts with a higher risk of predicted failure as measured by the donor risk index (DRI).(15) The median DRI at high volume centers was 1.39, compared to 1.28 for small volume centers and 1.27 for intermediate volume centers (p<0.01). These data are presented in Tables 2 and 3.
Table 2.
Characteristics of the primary cohort of liver re-transplantation recipients, donors and allografts *
| Overall (n=3977) |
Low (n=997) |
Intermediate (n=1291) |
High (n=1689) |
p-value | |
|---|---|---|---|---|---|
| RECIPIENT | |||||
| Mean age in years (s.d.) | 47.6 (11.3) | 47.0 (11.1) | 47.3 (11.3) | 48.1 (11.4) | <0.01 |
| Ethnicity (%) | |||||
| White | 2987 (75.1) | 777 (77.9) | 944 (73.1) | 1266 (75.0) | 0.03 |
| Hispanic | 429 (10.8) | 92 (9.2) | 123 (9.5) | 214 (12.7) | <0.01 |
| Black | 394 (9.9) | 96 (9.6) | 173 (13.4) | 125 (7.4) | <0.01 |
| Asian | 126 (3.2) | 30 (3.0) | 34 (2.6) | 62 (4.0) | 0.26 |
| Amer Indian | 19 (0.5) | 0 (0.0) | 8 (0.6) | 11 (0.7) | 0.04 |
| Hawaiian | 6 (0.2) | 0 (0.0) | 3 (0.2) | 3 (0.2) | 0.34 |
| Multi | 16 (0.4) | 2 (0.2) | 6 (0.5) | 8 (0.5) | 0.51 |
| Unknown | 0 | 0 | 0 | 0 | NA |
| Male (%) | 2477 (62.3) | 646 (64.8) | 791 (61.3) | 1040 (61.6) | 0.17 |
| Diabetic (%) | 759 (19.1) | 199 (20.0) | 237 (18.4) | 323 (19.1) | 0.63 |
| Hepatitis C (%) | 1270 (31.9) | 369 (37.0) | 420 (32.5) | 383 (28.5) | <0.01 |
| Status 1 (%) | 1482 (37.3) | 361 (36.2) | 475 (36.8) | 646 (38.3) | 0.52 |
| Life support (%) | 1253 (31.5) | 322 (32.3) | 348 (27.0) | 583 (34.5) | <0.01 |
| Live donor | 38 (1.0) | 13 (1.3) | 13 (1.0) | 12 (0.7) | 0.30 |
| Simultaneous kidney transplant | 297 (7.5) | 90 (9.0) | 107 (8.3) | 100 (5.9) | <0.01 |
| Mean MELD (s.d.) ** | 28.0 (8.3) | 28.3 (8.4) | 27.8 (7.9) | 27.8 (8.6) | 0.39 |
| Late re-transplants (%) *** | 1986 (49.9) | 516 (51.8) | 642 (49.7) | 828 (49.0) | 0.39 |
| DONOR | |||||
| Mean age in years (s.d.) | 37.1 (17.0) | 36.0 (16.8) | 36.4 (16.4) | 38.2 (17.4) | <0.01 |
| Ethnicity (%) | |||||
| White | 2842 (71.5) | 750 (75.2) | 951 (73.7) | 1141 (67.6) | <0.01 |
| Black | 548 (13.8) | 112 (11.2) | 212 (16.4) | 224 (13.3) | <0.01 |
| Hispanic | 450 (11.3) | 111 (11.1) | 87 (6.7) | 252 (14.9) | <0.01 |
| Asian | 78 (2.0) | 17 (1.7) | 23 (1.8) | 38 (2.3) | 0.53 |
| AmerIndian | 6 (0.2) | 1 (0.1) | 2 (0.2) | 3 (0.2) | 0.88 |
| Hawaiian | 8 (0.2) | 2 (0.2) | 3 (0.2) | 3 (0.2) | 0.95 |
| Multi | 31 (0.8) | 2 (0.2) | 5 (0.5) | 22 (1.3) | <0.01 |
| Unknown | 14 (0.4) | 2 (0.2) | 6 (0.5) | 6 (0.4) | 0.57 |
| Male (%) | 2278 (57.3) | 587 (58.9) | 724 (56.1) | 967 (57.3) | 0.41 |
| CVA (%) | 1654 (41.6) | 390 (39.1) | 529 (41.0) | 735 (43.5) | 0.07 |
| DCD (%) | 59 (1.5) | 13 (1.3) | 19 (1.5) | 27 (1.6) | 0.83 |
| Height centimeters (s.d.) | 168.4 (19.1) | 168.2 (20.8) | 169.2 (18.0) | 168.0 (18.9) | 0.63 |
| Median Donor Risk Index **** | 1.33 | 1.28 | 1.27 | 1.39 | <0.01 |
| ALLOGRAFT | |||||
| Mean hours of cold ischemia (s.d) | 8.2 (4.6) | 8.2 (4.7) | 8.0 (4.7) | 8.4 (4.4) | 0.14 |
| Mean minutes of warm ischemia (s.d.) | 44.0 (21.2) | 46.0 (25.6) | 45.0 (24.7) | 42.3 (15.1) | <0.01 |
| Share type (%) ***** | |||||
| 3 | 2388 (60.1) | 652 (65.4) | 874 (67.7) | 862 (51.0) | <0.01 |
| 4 | 1254 (31.5) | 290 (29.1) | 335 (26.0) | 629 (37.2) | <0.01 |
| 5 | 333 (8.4) | 55 (5.5) | 82 (6.4) | 196 (11.6) | <0.01 |
| 6 | 2 (0.05) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 0.30 |
| Split liver (%) | 79 (2.0) | 20 (2.0) | 23 (1.8) | 36 (2.1) | 0.79 |
Limited to liver transplants meeting study criteria
Model for End-Stage Liver Disease; reported here only for post-MELD era patients (not available for most pre-MELD patients.)
Re-transplants occurring >160 days after the primary liver transplant
A measure of allograft failure risk calculated from donor and allograft characteristics: donor age, cause of death, cardiac death status, race, height, allograft split liver status, allograft share type, and allograft cold ischemia time (15)
Share type refers to the allograft allocation, in which 3=local, 4=regional, 5=national, and 6=foreign.
Table 3.
Liver re-transplantation and unadjusted outcomes, by center volume category (n=3977)
| Overall | Low volume (n=997) |
Intermediate volume (n=1291) |
High volume (n=1689) |
p value | |
|---|---|---|---|---|---|
| Unadjusted allograft failure by one-year (%) | 37.8 | 40.0 | 35.9 | 37.9 | 0.14 |
| Unadjusted patient mortality by one-year (%) | 28.8 | 31.2 | 28.0 | 28.0 | 0.15 |
Center volume and unadjusted outcomes
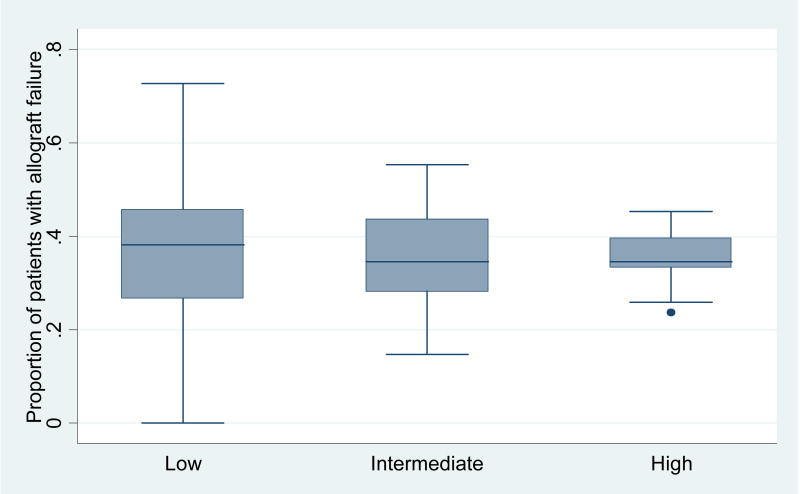
The one-year unadjusted risk of allograft failure for liver re-transplants did not differ by center volume for the combined period (p=0.14). These results are displayed in Figure 2. The unadjusted risk of patient mortality by one-year also did not differ by center volume (p=0.15). These data are presented in Table 3.
Figure 2.
Distribution of unadjusted center-specific allograft failure at 1 year after liver re-transplantation by volume category*
*p=0.14
We also performed an unadjusted analysis of one-year allograft survival for post-MELD patients with high MELD scores (high score defined as >28, the median MELD). This secondary analysis also did not show better results at intermediate (OR 1.01, CI 0.68–1.48, p=0.97) or high volume centers (OR 1.27, CI 0.89–1.82, p=0.19).
Center volume and outcomes using multivariable logistic regression
As shown in Table 4, results from multivariable logistic regression showed that the risk of one-year allograft failure was not significantly different between low (reference), intermediate (OR 0.86, CI 0.72–1.03, p=0.11) and high volume centers (OR 0.88, CI 0.74–1.04, p=0.14). This multivariable analysis was repeated without imputation; the point estimates of the associations between center volume and the outcome remained similar and p-values remained non-significant.
Table 4.
Multivariable logistic regression analysis of characteristics associated with one-year allograft failure after liver re-transplantation *
| Combined (n=3977) |
Pre-MELD (n=2374) |
Post-MELD (n=1603) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics of interest | O.R. | C.I. | p | O.R. | C.I. | p | O.R. | C.I. | p |
| Intermediate center volume | 0.86 | 0.72–1.03 | 0.11 | 0.74 | 0.58–0.93 | 0.01 | 1.09 | 0.82–1.46 | 0.55 |
| High center volume | 0.88 | 0.74–1.04 | 0.14 | 0.79 | 0.63–0.98 | 0.03 | 1.05 | 0.79–1.40 | 0.72 |
| Recipient characteristics | O.R. | C.I. | p | O.R. | C.I. | p | O.R. | C.I. | p |
| Age (per ten years) | 1.09 | 1.03–1.17 | <0.01 | 1.11 | 1.02–1.20 | 0.02 | 1.07 | 0.96–1.18 | 0.22 |
| Male | 0.95 | 0.83–1.10 | 0.49 | 0.94 | 0.78–1.12 | 0.48 | 1.00 | 0.79–1.27 | 0.98 |
| Black ethnicity | 1.08 | 0.86–1.36 | 0.51 | 0.95 | 0.68–1.31 | 0.74 | 1.19 | 0.85–1.67 | 0.31 |
| Diabetes | 1.15 | 0.97–1.36 | 0.12 | 0.95 | 0.75–1.19 | 0.63 | 1.48 | 1.14–1.93 | <0.01 |
| Hepatitis C | 1.24 | 1.07–1.44 | <0.01 | 1.14 | 0.94–1.37 | 0.19 | 1.41 | 1.11–1.78 | <0.01 |
| Life support | 1.56 | 1.31–1.87 | <0.01 | 1.57 | 1.25–1.97 | <0.01 | 1.47 | 1.08–2.00 | 0.01 |
| Status 1 | 1.19 | 0.97–1.47 | 0.10 | 1.30 | 1.01–1.68 | 0.04 | 1.05 | 0.70–1.57 | 0.83 |
| MELD score ** | NA | NA | NA | NA | NA | NA | 1.02 | 1.01–1.04 | <0.01 |
| Dialysis | 1.71 | 1.42–2.07 | <0.01 | 1.69 | 1.31–2.17 | <0.01 | 1.51 | 1.09–2.08 | 0.01 |
| Simultaneous kidney transplant | 0.77 | 0.58–1.02 | 0.07 | 0.79 | 0.53–1.17 | 0.24 | 0.76 | 0.50–1.15 | 0.19 |
| Quartile of time between transplants *** | Reference | Reference | Reference | ||||||
| 2nd quartile | 1.35 | 1.08–1.68 | <0.01 | 1.41 | 1.07–1.84 | 0.01 | 1.25 | 0.83–1.89 | 0.28 |
| 3rd quartile | 1.94 | 1.48–2.53 | <0.01 | 2.86 | 2.06–3.96 | <0.01 | 0.99 | 0.60–1.64 | 0.97 |
| 4th quartile | 1.25 | 0.94–1.66 | 0.12 | 1.49 | 1.04–2.13 | 0.03 | 0.90 | 0.54–1.50 | 0.69 |
| Donor characteristics | O.R. | C.I. | p | O.R. | C.I. | p | O.R. | C.I. | P |
| Age (per 10 years) | 1.16 | 1.10–1.22 | <0.01 | 1.17 | 1.10–1.25 | <0.01 | 1.16 | 1.07–1.26 | <0.01 |
| Male | 0.94 | 0.81–1.09 | 0.39 | 0.88 | 0.73–1.06 | 0.16 | 1.08 | 0.82–1.42 | 0.58 |
| Black ethnicity | 1.41 | 1.17–1.71 | <0.01 | 1.55 | 1.21–2.00 | <0.01 | 1.32 | 0.97–1.78 | 0.07 |
| Cause of death: CVA | 1.06 | 0.88–1.27 | 0.54 | 1.10 | 0.87–1.38 | 0.43 | 0.98 | 0.74–1.32 | 0.91 |
| Cause of death: Anoxia | 0.92 | 0.71–1.19 | 0.52 | 1.18 | 0.84–1.66 | 0.35 | 0.65 | 0.43–0.97 | 0.03 |
| Donation after cardiac death | 2.31 | 1.35–3.95 | <0.01 | 2.51 | 0.89–7.05 | 0.08 | 2.29 | 1.20–4.38 | 0.01 |
| Height (per 10 cm) | 0.92 | 0.88–0.95 | <0.01 | 0.93 | 0.89–0.97 | <0.01 | 0.84 | 0.74–0.94 | <0.01 |
| Allograft characteristics | O.R. | C.I. | p | O.R. | C.I. | p | O.R. | C.I. | P |
| Allograft shared beyond region | 1.25 | 0.98–1.59 | 0.08 | 1.19 | 0.89–1.59 | 0.24 | 1.39 | 0.87–2.24 | 0.18 |
| Split liver | 1.97 | 1.23–3.15 | <0.01 | 2.21 | 1.29–3.77 | <0.01 | 1.58 | 0.53–4.67 | 0.41 |
| Quartile of cold ischemia**** | Reference | Reference | Reference | ||||||
| 2nd quartile | 1.11 | 0.92–1.35 | 0.28 | 1.22 | 0.94–1.58 | 0.13 | 1.03 | 0.76–1.40 | 0.83 |
| 3rd quartile | 1.23 | 1.01–1.50 | 0.04 | 1.33 | 1.02–1.72 | 0.03 | 1.15 | 0.84–1.57 | 0.39 |
| 4th quartile | 1.34 | 1.10–1.63 | <0.01 | 1.42 | 1.10–1.83 | <0.01 | 1.25 | 0.90–1.73 | 0.18 |
| Quartile of warm ischemia ***** | Reference | Reference | Reference | ||||||
| 2nd quartile | 1.03 | 0.86–1.23 | 0.78 | 0.92 | 0.72–1.17 | 0.50 | 1.24 | 0.94–1.63 | 0.13 |
| 3rd quartile | 1.20 | 0.94–1.54 | 0.15 | 1.17 | 0.85–1.63 | 0.33 | 1.25 | 0.84–1.87 | 0.27 |
| 4th quartile | 1.26 | 1.04–1.53 | 0.02 | 1.33 | 1.04–1.69 | 0.03 | 1.10 | 0.78–1.53 | 0.59 |
O.R.: Odds ratio; C.I.: 95% Confidence interval; MELD: Model for End-Stage Liver Disease
Multivariable logistic model included variables listed in the table.
Score not available for the majority of patients during the pre-MELD period
Time between transplant: quartile 1 = 1–11 days, quartile 2 = 12–159 days, quartile 3 = 160 – 918 days, quartile 4 = 919 – 7281 days
Cold ischemia: quartile 1 = 4.5–1.40 hours, quartile 2 = 6.0–8.1 hours, quartile 3 = 8.2–9.3 hours, quartile 4 = 9.4–70.4 hours
Warm ischemia: quartile 1 = 0–35 minutes, quartile 2 = 36–44 minutes, quartile 3=45–49 minutes, quartile 4 = 50–237 minutes
In our secondary analysis, we found that higher center volume was associated with better one-year allograft outcomes in the pre-MELD but not the post-MELD period. In a multivariable regression analysis restricted to recipients of liver re-transplants in the pre-MELD period, center volume was associated with a lower risk of one-year allograft failure for patients at intermediate (O.R. 0.74, CI 0.58 – 0.93, p=0.01) and high volume centers (O.R. 0.79, CI 0.63–0.98, p=0.03). In the analysis restricted to patients in the post-MELD period, however, there was no difference in one-year allograft failure according to center volume.
A secondary analysis of the effect of center volume on one-year allograft failure was also performed using only late re-transplants (those occurring >160 days after the original transplant surgery). In the combined period, there was no significant difference in outcomes by center volume. Compared to low volume centers, intermediate volume centers had an odds ratio of allograft failure of 0.86 (C.I. 0.66 – 1.11, p=0.24) and high volume centers had an odds ratio of 0.87 (C.I. 0.68–1.12, p=0.29). (data not shown in tables).
MELD era and outcomes
We also examined changes in liver re-transplantation in the pre- and post-MELD periods. The proportion of liver re-transplants/total liver transplants fell from 10.4% pre-MELD to 8.4% post-MELD (p<0.01) (data not shown in tables). As shown in Table 5, in univariate analysis, the risk of one-year allograft failure after liver re-transplantation was lower in the post-MELD era (p<0.01). In multivariable analysis, the decrease in allograft failure post-MELD remained significant (O.R. 0.85, CI 0.74–0.98, p=0.03).
Table 5.
Patient and allograft survival after liver re-transplantation, by MELD era
| Pre-MELD (n=2374) |
Post-MELD (n=1603) |
p-value | |
|---|---|---|---|
| Allograft failure by one-year | 963 | 540 | |
| Proportion (%) | 40.6 | 33.7 | <0.01 |
| Patient mortality by one-year | 719 | 426 | |
| Proportion (%) | 30.3 | 26.6 | 0.01 |
| Odds ratio for allograft failure by one-year in multivariable analysis * | Reference | 0.85 | |
| Confidence interval | 0.74 – 0.98 | 0.03 | |
| Odds ratio for patient mortality by one-year in multivariable analysis * | Reference | 0.94 | |
| Confidence interval | 0.81 – 1.10 | 0.46 |
Results from multivariable logistic regression adjusted for recipient, donor and allograft characteristics listed in Table 4
Multivariable logistic regression analyses of center volume and patient mortality
High volume centers did have better one-year patient mortality after liver re-transplantation. Compared to low volume centers, intermediate volume centers had an odds ratio of 0.91 (C.I. 0.75–1.10, p=0.32) and high volume centers had an odds ratio of 0.83 (C.I. 0.69 – 1.00, p=0.048) for patient mortality in the combined period. These data are presented in Table 6.
Table 6.
Multivariable analysis of center volume and one-year patient survival after liver re-transplantation *
| Combined (n=3977) |
Pre-MELD (n=2374) |
Post-MELD (n=1603) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Center volume | O.R. | C.I. | p | O.R. | C.I. | p | O.R. | C.I. | p |
| Intermediate | 0.91 | 0.75–1.10 | 0.32 | 0.86 | 0.67–1.10 | 0.22 | 1.01 | 0.74–1.38 | 0.94 |
| High | 0.83 | 0.70–1.00 | 0.048 | 0.80 | 0.63–1.01 | 0.06 | 0.89 | 0.66–1.21 | 0.46 |
Results from multivariable logistic regression adjusted for recipient, donor and allograft characteristics listed in Table 4.
This analysis of one-year patient mortality was repeated without imputation; results were substantially changed. In the analysis without imputation, there was no significant difference in outcomes by center volume. Compared to low volume centers, intermediate volume centers had an odds ratio of patient mortality of 1.02 (C.I. 0.81 – 1.28, p=0.89) and high volume centers had an odds ratio of 0.95 (C.I. 0.76–1.18, p=0.63).
Discussion
Despite strong interest in number of transplants as a predictor of center quality, prior studies have not consistently demonstrated a relationship between overall liver transplant volume and patient outcomes.(13, 2, 24) Our study is the first to examine center volume and allograft and patient outcomes for liver re-transplantation, a procedure with elevated risk of allograft failure and recipient death. We found that larger centers accept allografts with a higher donor risk index, but the adjusted risk of one-year allograft failure did not differ by center volume. One-year patient mortality was improved at high volume centers, but this finding was not consistent when imputation was not performed. An examination of allograft failure and volume category shows that outcomes overlap considerably across each category.
Center volume of surgical procedures is a metric that has gained attention from health care services researchers, patients and payors.(14) Center volume has been associated with improved outcomes for diverse procedures including coronary artery bypass surgery, trauma surgery, prostatectomy, esophagectomy and pancreatectomy.(18, 27, 25, 11, 22) Prior authors have argued that with increasing number of procedures, surgeons may remain technically facile, post-operative complications may become more readily recognized and treated, and processes of care such as ventilator weaning may be improved.(5, 20, 6) In the case of organ transplantation, patients may easily obtain data on a center’s annual number of organ transplants through the continuously updated website of the Scientific Registry of Transplant Recipients.(1) Insurance corporations may also preferentially direct their covered patients to high volume institutions.(14, 8) Additionally, hospitals may draw attention to the volume of their organ transplantation programs because transplantation is a high visibility enterprise that generates prestige and income for medical centers.(19) In light of this attention to center volume, detailed studies of outcomes at centers of different volume are important.
Initial studies by Edwards et al. and Axelrod et al. showed that patients at higher volume centers had improved survival after liver transplantation.(13, 2, 24) Similar to our study, Axelrod et al. categorized liver transplant centers into low, intermediate and high volume by dividing overall liver transplants into 3 tertiles of approximately equal size.(2) Although the study by Axelrod et al. demonstrated that liver transplant recipients at intermediate and higher volume centers had improved patient survival at one-year, their study also showed that outcomes for centers in each volume category overlapped. Therefore, some low volume liver transplant centers had superior adjusted outcomes than some high volume centers.
A subsequent study by Northup et al. that examined data for patients after the institution of the MELD system of organ allocation did not find an association between volume and outcomes. Notably, mortality after liver transplantation decreased after the MELD score was incorporated into the US system of allocation. Northup et al. hypothesized that the lack of association between volume and outcomes in their analysis of post-MELD patients might be related to an improved ability to perform case-mix adjustment by incorporating MELD scores in their multivariable analysis.(24)
Given the proposed rationales for a relationship between center volume and organ transplantation outcomes, liver re-transplantation would seem like a procedure likely to demonstrate differences in patient and allograft survival if volume were an important predictor of a clinically meaningful improvement in survival. Patient undergoing liver re-transplantation typically have substantial co-morbidities. For late re-transplantation, scarring in the operative field also makes this procedure challenging.(21, 23) The unadjusted risk of allograft failure after liver re-transplantation is typically 30–40%, high enough that some authors have questioned whether liver re-transplantation represents an unethical use of organs that might otherwise be allocated to patients with better clinical prospects.(26)
We did not find a significant association between center volume and allograft survival after adult liver re-transplantation in our primary analysis. Additionally, center volume was not associated with improved allograft survival when restricted to patients undergoing re-transplantation >160 days after the initial transplant, when scarring may be more severe. Although larger centers did have improved allograft survival in the pre-MELD era, this difference was not present in the post-MELD era. Interestingly, in the post-MELD analysis, the odds ratio for allograft survival for intermediate and high volume centers actually reversed direction and became >1, although this difference across volume categories was not significant.
Center volume might be an imprecise predictor of one-year allograft failure after liver re-transplantation for a number of reasons. Center volume of liver transplants may not relate closely to surgical expertise if transplant surgeons regularly perform other procedures related to the liver and other abdominal organs.(14) Another possible explanation, given discordant findings in the pre- and post-MELD eras, is that center practices have changed over time. It is possible that lower volume centers are now more conservative in their selection of liver transplant recipients in ways not fully captured by multivariable analysis, or that the MELD system has resulted in better allocation of organs to appropriate candidates, resulting in better outcomes in centers generally. Advances in immunosuppression and treatment of complications such as infection could also have diminished advantages in experience that larger volume centers enjoyed in the past. Lastly, higher center volume might also confer disadvantages in some cases if staff are unable to adequately respond to the needs of liver transplant patients, who have multiple co-morbidities and are frequently clinically unstable.
In contrast to our other results, we did find that patients undergoing liver re-transplantation at high volume centers had a lower risk of mortality by one year. This association between higher volume and patient mortality, however, was not found when imputation was not performed. It is possible that the association between volume and patient mortality could only be detected with the larger sample size that was enabled by the imputation process. We must also consider the possibility that the process of imputation introduced bias into the analysis and that the association between high volume and improved patient survival is spurious. An additional problem is that presumably, processes of care related to volume that enhance patient survival should also enhance allograft survival.
This study has limitations that must be acknowledged. Our analysis included less than 4000 patients, a smaller sample than prior studies of overall liver transplantation. Thus, a true difference of <5% in allograft failure across volume categories might not have been detected. Another limitation is that registry data do not include all characteristics that might affect outcomes after liver re-transplantation. For instance, information about allograft quality (such as biopsy results) is limited. Therefore, differences in the populations of donors or recipients across volume categories might exist but not be adequately adjusted for in our multivariable analysis. We also lacked recipient MELD scores for recipients before February 2002, a limitation that could explain discordant results across the pre-MELD and post-MELD eras. Additionally, our dataset does not have information about the surgeon performing the procedure. Without these data, we are unable to examine whether volume of procedures by individual surgeons corresponded to improved outcomes. Lastly, the OPTN database does not include potential recipients who were evaluated but declined by individual programs due to co-morbidities or de-conditioning. This element of judgment by transplant clinicians could not be evaluated by our study.
Conclusions
Despite improvements in outcomes since the institution of the MELD system, liver re-transplantation remains a procedure with an elevated risk of allograft failure and patient mortality. In multivariable analysis, one-year patient survival was improved at high volume centers, but one-year allograft failure was not. Additionally, individual center outcomes overlapped substantially across volume categories, showing that annual volume of liver transplantation procedures was an imprecise surrogate measure for patient outcomes at a center. Instead of focusing attention on annual volume, patients and providers should instead take advantage of the ready availability of center-specific risk-adjusted outcomes when comparing transplant centers.
Acknowledgments
Funding Sources: Dr. Reese is supported by NIH Career Development Award, K23 - DK078688-01
UNOS Disclaimer: “This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.”
Contributor Information
Peter P. Reese, University of Pennsylvania; Renal, Hypertension and Electrolyte Division; 1 Founders, 3400 Spruce St, Philadelphia, PA, 19104
Heidi Yeh, Massachusetts General Hospital; Department of Surgery; WHT 503, 55 Fruit St, Boston, MA 02114
Arwin M. Thomasson, University of Pennsylvania; Center for Clinical Epidemiology and Biostatistics; 507 Blockley Hall; 423 Guardian Drive, Philadelphia, PA 19104
Justine Shults, University of Pennsylvania; Center for Clinical Epidemiology and Biostatistics; 610 Blockley Hall; 423 Guardian Drive, Philadelphia, PA 19104
James F. Markmann, Massachusetts General Hospital; Department of Surgery; WHT 503, 55 Fruit St, Boston, MA 02114
References
- 1.Scientific Registry of Transplant Recipients. Transplant Program and OPO-Specific Reports. 2008 doi: 10.1097/MOT.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod DA, Guidinger MK, McCullough KP, Leichtman AB, Punch JD, Merion RM. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4:920–7. doi: 10.1111/j.1600-6143.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 3.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg. 2004;198:626–32. doi: 10.1016/j.jamcollsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 6.Chang AC, Birkmeyer JD. The volume-performance relationship in esophagectomy. Thorac Surg Clin. 2006;16:87–94. doi: 10.1016/j.thorsurg.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Cowan JA, Jr, Dimick JB, Leveque JC, Thompson BG, Upchurch GR, Jr, Hoff JT. The impact of provider volume on mortality after intracranial tumor resection. Neurosurgery. 2003;52:48–53. doi: 10.1097/00006123-200301000-00005. discussion 53–4. [DOI] [PubMed] [Google Scholar]
- 8.Delbanco S. Surg Clin North Am. Vol. 87. 2007. Employers flex their muscles as health care purchasers; pp. 883–7.pp. vii [DOI] [PubMed] [Google Scholar]
- 9.Dimick JB, Staiger DO, Birkmeyer JD. Are mortality rates for different operations related?: implications for measuring the quality of noncardiac surgery. Med Care. 2006;44:774–8. doi: 10.1097/01.mlr.0000215898.33228.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimick JB, Stanley JC, Axelrod DA, Kazmers A, Henke PK, Jacobs LA, Wakefield TW, Greenfield LJ, Upchurch GR., Jr Variation in death rate after abdominal aortic aneurysmectomy in the United States: impact of hospital volume, gender, and age. Ann Surg. 2002;235:579–85. doi: 10.1097/00000658-200204000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimick JB, Wainess RM, Upchurch GR, Jr, Iannettoni MD, Orringer MB. Ann Thorac Surg. Vol. 79. 2005. National trends in outcomes for esophageal resection; pp. 212–6. discussion 217–8. [DOI] [PubMed] [Google Scholar]
- 12.Edwards EB, Harper AM. The impact of MELD on OPTN liver allocation: preliminary results. Clin Transpl. 2002:21–8. [PubMed] [Google Scholar]
- 13.Edwards EB, Roberts JP, McBride MA, Schulak JA, Hunsicker LG. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med. 1999;341:2049–53. doi: 10.1056/NEJM199912303412703. [DOI] [PubMed] [Google Scholar]
- 14.Epstein AM. Volume and outcome--it is time to move ahead. N Engl J Med. 2002;346:1161–4. doi: 10.1056/NEJM200204113461512. [DOI] [PubMed] [Google Scholar]
- 15.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 16.Gamma A. LINCHECK: Stata module to graphically assess the linearity of a continuous variable in a regression model. Boston College Department of Economics; 2005. [Google Scholar]
- 17.Ghabril M, Dickson R, Wiesner R. Improving outcomes of liver retransplantation: an analysis of trends and the impact of Hepatitis C infection. Am J Transplant. 2008;8:404–11. doi: 10.1111/j.1600-6143.2007.02082.x. [DOI] [PubMed] [Google Scholar]
- 18.Hannan EL, Siu AL, Kumar D, Kilburn H, Jr, Chassin MR. The decline in coronary artery bypass graft surgery mortality in New York State. The role of surgeon volume. Jama. 1995;273:209–13. [PubMed] [Google Scholar]
- 19.Howard RJ. The challenging triangle: balancing outcomes, transplant numbers and costs. Am J Transplant. 2007;7:2443–5. doi: 10.1111/j.1600-6143.2007.01961.x. [DOI] [PubMed] [Google Scholar]
- 20.Kraus TW, Buchler MW, Herfarth C. Relationships between volume, efficiency, and quality in surgery--a delicate balance from managerial perspectives. World J Surg. 2005;29:1234–40. doi: 10.1007/s00268-005-7988-5. [DOI] [PubMed] [Google Scholar]
- 21.Markmann JF, Gornbein J, Markowitz JS, Levy MF, Klintmalm GB, Yersiz H, Morrisey M, Drazan K, Farmer DG, Ghobrial RM, Goss J, Seu P, Martin P, Goldstein LI, Busuttil RW. A simple model to estimate survival after retransplantation of the liver. Transplantation. 1999;67:422–30. doi: 10.1097/00007890-199902150-00015. [DOI] [PubMed] [Google Scholar]
- 22.Nathens AB, Jurkovich GJ, Maier RV, Grossman DC, MacKenzie EJ, Moore M, Rivara FP. Relationship between trauma center volume and outcomes. Jama. 2001;285:1164–71. doi: 10.1001/jama.285.9.1164. [DOI] [PubMed] [Google Scholar]
- 23.Northup PG, Pruett TL, Kashmer DM, Argo CK, Berg CL, Schmitt TM. Donor factors predicting recipient survival after liver retransplantation: the retransplant donor risk index. Am J Transplant. 2007;7:1984–8. doi: 10.1111/j.1600-6143.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 24.Northup PG, Pruett TL, Stukenborg GJ, Berg CL. Survival after adult liver transplantation does not correlate with transplant center case volume in the MELD era. Am J Transplant. 2006;6:2455–62. doi: 10.1111/j.1600-6143.2006.01501.x. [DOI] [PubMed] [Google Scholar]
- 25.Rathore SS, Epstein AJ, Volpp KG, Krumholz HM. Hospital coronary artery bypass graft surgery volume and patient mortality, 1998–2000. Ann Surg. 2004;239:110–7. doi: 10.1097/01.sla.0000103066.22732.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ubel PA, Arnold RM, Caplan AL. Rationing failure. The ethical lessons of the retransplantation of scarce vital organs. Jama. 1993;270:2469–74. doi: 10.1001/jama.270.20.2469. [DOI] [PubMed] [Google Scholar]
- 27.Yao SL, Lu-Yao G. Population-based study of relationships between hospital volume of prostatectomies, patient outcomes, and length of hospital stay. J Natl Cancer Inst. 1999;91:1950–6. doi: 10.1093/jnci/91.22.1950. [DOI] [PubMed] [Google Scholar]