Abstract
Objective
This report describes the design and methodology of the Convergence Insufficiency Treatment Trial (CITT), the first large-scale, placebo-controlled, randomized clinical trial evaluating treatments for convergence insufficiency (CI) in children. We also report the clinical and demographic characteristics of patients.
Methods
We prospectively randomized children 9 to 17 years of age to one of four treatment groups: 1) home-based pencil push-ups, 2) home-based computer vergence/accommodative therapy and pencil push-ups, 3) office-based vergence/accommodative therapy with home reinforcement, 4) office-based placebo therapy. Outcome data on the Convergence Insufficiency Symptom Survey (CISS) score (primary outcome), near point of convergence (NPC), and positive fusional vergence were collected after 12 weeks of active treatment and again at 6 and 12 months post-treatment.
Results
The CITT enrolled 221 children with symptomatic CI with a mean age of 12.0 years (SD = +2.3). The clinical profile of the cohort at baseline was 9Δ exophoria at near (+/− 4.4) and 2Δ exophoria (+/−2.8) at distance, CISS score = 30 (+/−9.0), NPC = 14 cm (+/− 7.5), and near positive fusional vergence break = 13 Δ (+/− 4.6). There were no statistically significant nor clinically relevant differences between treatment groups with respect to baseline characteristics (p > 0.05).
Conclusion
Hallmark features of the study design include formal definitions of conditions and outcomes, standardized diagnostic and treatment protocols, a placebo treatment arm, masked outcome examinations, and the CISS score outcome measure. The baseline data reported herein define the clinical profile of those enrolled into the CITT.
Introduction
Convergence insufficiency (CI) is a common binocular vision disorder characterized by exophoria greater at near than at distance, a receded near point of convergence, and reduced positive fusional vergence (convergence amplitudes) at near.1–4 In studies that used standardized definitions of CI, investigators have reported a prevalence of 4.2% to 6% in school and clinic settings.2–4 Common symptoms of CI include discomfort, eyestrain, headaches, blurred vision, diplopia, sleepiness, difficulty concentrating, movement of print, and loss of comprehension after short periods of reading or performing close activities.5–11 Thus, CI may negatively impact health-related quality of life, potentially interfering with reading and near work performed for school, work, and/or leisure.
Considerable uncertainty exists regarding the best treatment for CI. Prescribed treatments include: base-in prism reading glasses, home-based pencil push-ups, home-based vision therapy/orthoptics, and office-based vergence/accommodative therapy.1,12–21 Recent studies surveying the eye care community regarding treatment patterns for persons with symptomatic CI suggest that home-based pencil push-ups is the most commonly prescribed treatment by both ophthalmologists and optometrists.19,20,22 The clinical popularity of the technique is most likely related to its simplicity and perceived cost effectiveness.
In spite of the wide-spread use of pencil push-ups for the treatment of CI, there have been few studies evaluating the effectiveness of this treatment.18,23 In the only randomized clinical trial studying the effectiveness of pencil push-ups in children with symptomatic CI, pencil push-ups were found to be no more effective than placebo vision therapy/orthoptics.23
Base-in prism reading glasses also represent a potentially cost-effective and easy to administer treatment. However, few studies regarding the effectiveness of this treatment have been published. In the only randomized trial incorporating a placebo treatment group and masked outcome examinations, base-in prism reading glasses were found to be no more effective than placebo reading glasses for the treatment of children with symptomatic CI.24
Although office-based vision therapy has been more extensively evaluated than either home-based pencil push-ups or base-in prism reading glasses, most studies have methodologic limitations such as: lack of clear definitions of CI or a successful outcome, retrospective design, failure to use masked examiners for outcome measures, small sample size, or no control group.21 In the only randomized trial of treatments for CI in children incorporating a placebo group, office-based vision therapy/orthoptics (i.e., office-based vergence/accommodative therapy with home reinforcement) was found to be more effective than home-based pencil push-ups or placebo vision therapy/orthoptics in reducing symptoms and improving signs of CI in children 9–18 years of age.23 Neither home-based pencil push-ups nor placebo therapy was effective in improving either symptoms or signs associated with CI.
Given the paucity of well-designed studies in this field, there is a need to further evaluate the effectiveness of treatments for patients with symptomatic CI. The Convergence Insufficiency Treatment Trial (CITT) is a randomized clinical trial designed to meet this need. The purpose of this report is to present the design and methodology of the first large-scale randomized clinical trial evaluating treatments for CI, including formal definitions, standardized diagnostic and treatment protocols, use of a reliable and valid symptom survey as the primary outcome measure, masked outcome examinations, and the development of a placebo vision therapy/orthoptics treatment arm. We also report the demographic and clinical characteristics of patients enrolled.
Methods
The tenets of the Declaration of Helsinki were followed throughout the study. The institutional review boards of all participating centers approved the protocol and informed consent forms. The parent or guardian (subsequently referred to as “parent”) of each study patient gave written informed consent and each patient gave assent to participation. Health Insurance Portability and Accountability Act (HIPAA) authorization was obtained from the parent. Study oversight was provided by an independent data and safety monitoring committee (listed in Appendix). This study is registered at ClinicalTrials.gov as the Convergence Insufficiency Treatment Trial (CITT).25
Study Design and Aims
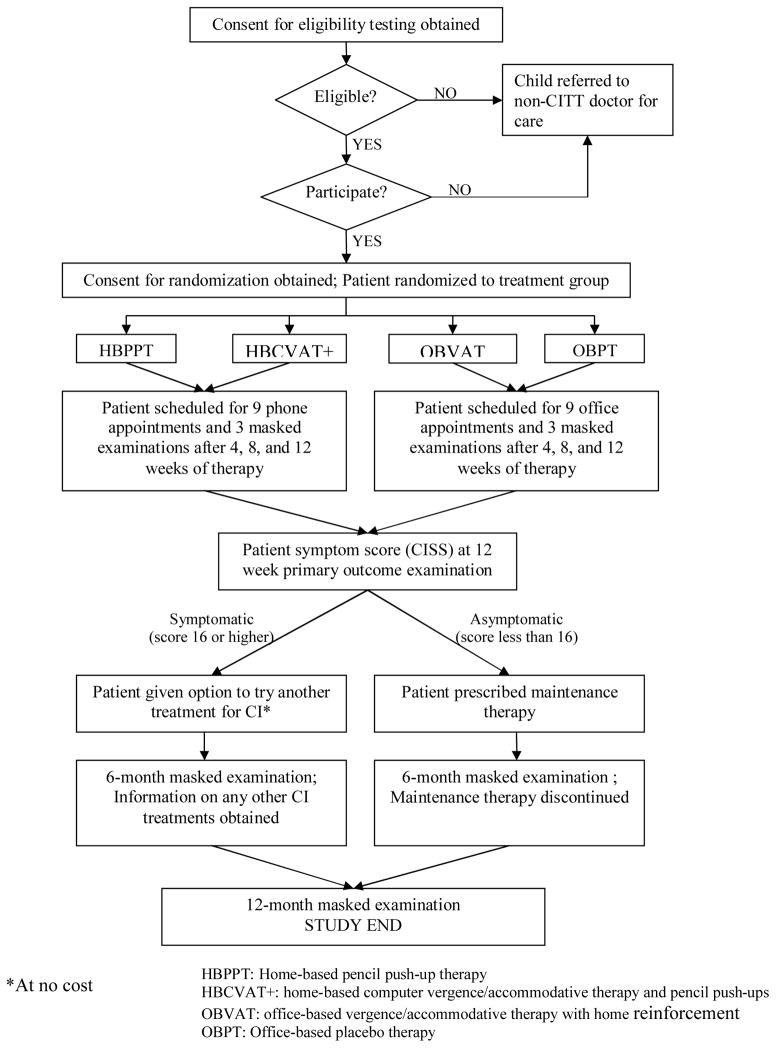
The CITT is a single-masked, placebo-controlled, multicenter, randomized clinical trial. Patients between the ages of 9 and 17 years (inclusive) were randomized to 1 of 4 interventions: 1) home-based pencil push-ups, 2) home-based computer vergence/accommodative therapy and pencil push-ups, 3) office-based vergence/accommodative therapy with home reinforcement, or 4) office-based placebo therapy. Patients in each treatment group received 12 weeks of treatment. Figure 1 provides an overview of the study design.
Figure 1. Study Design.
The primary goal of the study is to determine if any of the 3 treatments is more effective than placebo therapy in improving symptoms and signs in children with symptomatic CI and if there are differences among the three treatments in improving the affected child’s symptoms and signs. The primary outcome measure is the Convergence Insufficiency Symptom Survey (CISS) score (see Figure 2). Secondary outcome measures are the near point of convergence and positive fusional vergence at near.
Figure 2. CI Symptom Survey.
Clinician instructions: Read the following subject instructions and then each item exactly as written. If subject responds with “yes”-please qualify with frequency choices. Do not give examples.
Subject instructions: Please answer the following questions about how your eyes feel when reading or doing close work.
Selection of Primary Outcome Measure
Because symptoms are commonly reported by patients with CI6–11 measurement of symptoms and their severity is important for evaluating the effectiveness of treatments. Hence, the CITT Investigator group conducted a series of studies to develop a symptom questionnaire for use as the primary outcome measure in the CITT studies.26–29 This questionnaire, the CISS, is the first standardized instrument that has been shown to be valid and reliable for measuring the type and frequency of symptoms before and after treatment for patients with CI.28,29
CITT Study Organization
The CITT represents a collaborative effort involving: 1) a study chairman’s office at the Pennsylvania College of Optometry, 2) a data coordinating center (DCC) housed within the Optometry Coordinating Center at The Ohio State University College of Optometry, and 3) nine clinical sites across the United States, six optometric sites, two ophthalmology centers, and one site with an optometrist and ophthalmologist as co-principal investigators. The sites and investigators are listed in the appendix.
Description of Investigator Roles/Certification
All CITT personnel were trained and certified to perform their specific study tasks and were required to update their certification on a yearly basis. Before study enrollment commenced, investigators attended a 2-day training and certification meeting. Prior to this meeting, all investigators reviewed the CITT Manual of Procedures and completed a written examination. The written examination assessed the investigator’s knowledge of human subject protection issues, CITT study design, and topics specific to their role in the study. Therapists also reviewed CITT video demonstrations of the vision therapy/orthoptic procedures. The training meeting consisted of role-specific training sessions and practical examinations. Members of the CITT Executive Committee administered the training and testing. Prior to enrolling patients or collecting any CITT data, each site was required to have at least two individuals certified to serve in each of the following roles: site coordinator, unmasked examiner, masked examiner, and therapist.
Investigators who joined a CITT site subsequent to the initial training and certification meeting were certified for appropriate study tasks at the local level. New investigators were required to pass a written examination and to demonstrate knowledge of proper study procedures to a certified investigator in the same position. During the first year of the study, the CITT Study Chair and Principal Investigator of the Data Coordinating Center also observed investigators performing their specific study tasks on an enrolled patient during one or more site visit to each clinical center
Patient Selection and Definition of CI
Major eligibility criteria included children age 9 to 17 years of age (inclusive) who met the study definition of symptomatic CI: (1) a symptomatic score (average score of 16 or higher) on the CISS; (2) exophoria at near at least 4 prism diopters (△) greater than at distance; (3) a receded near point of convergence of ≥ 6 cm break, and (4) insufficient positive fusional vergence (i.e., failing Sheard’s criterion30 or positive fusional vergence ≤ 15 △ base-out) at near. Complete inclusion and exclusion criteria are listed in Table 1. Eligibility was initially evaluated by an eligibility examiner and then independently confirmed by the DCC via a web-based computer algorithm prior to randomization.
Table 1.
Eligibility Criteria
Inclusion Criteria
|
Exclusion Criteria
|
Eligibility Examination/Protocol
The following measurements were taken at the eligibility examination and at all masked examinations (listed in order of administration): CISS, cover/uncover (unilateral cover) test and alternate cover test with prism neutralization at distance and near, negative fusional vergence (blur, break, and recovery) at near, positive fusional vergence (blur, break, and recovery) at near, near point of convergence break and recovery, push-up accommodative amplitude (right eye only), accommodative facility (right eye only) with +2.00/−2.00 lenses, medication use survey, and a second administration (the average of the first and second scores were used for analysis) of CISS. All testing at near was performed at 40 cm. The examination procedures are described in detail in the CITT Manual of Procedures, which can be accessed at http://optometry.osu.edu/research/CITT/4363.cfm.
An academic behavior survey was completed by the child’s parent during the eligibility examination. This six-question survey (Table 2) was developed because previous studies have shown that parents tend to report a higher frequency of behaviors related to poor school performance in children with CI.26,31 The possible response options for each of the survey items are “Never”, “Infrequently”, “Sometimes”, “Fairly often”, or “Always” and are scored from zero to four.
Table 2.
Academic Behavior Survey Items
|
Other Clinical Testing
Testing at the eligibility examination also included best-corrected visual acuity at distance and near (test distance 40 cm), versions, stereopsis, pupil testing, an anterior segment examination, and administration of a questionnaire that screened for neurological deficits. A cycloplegic refraction (using 1% cyclopentolate) was performed if it had not been completed within 2 months prior to the initial eligibility examination and a posterior segment examination was also performed if not done within 12 months of the initial eligibility examination.
Protocol for Primary and Secondary Outcome Measures
Primary Outcome Measure
The CISS
The CISS was administered to the child before any other testing and then repeated after all testing was completed. Each response was scored as 0 to 4 points, with 4 representing the highest frequency of symptom occurrence (i.e., always). The 15 items were summed to obtain the total CISS score. The lowest possible score (least symptoms) was 0 and the highest was 60 (most symptomatic). For all analyses, the average score from the two administrations of the CISS will be used. A symptom score ≥ 16 has been found to differentiate children with symptomatic CI from those with normal binocular vision.28
Secondary Outcome Measure
Near Point of Convergence Break Value
The near point of convergence was measured three times with the Astron International (ACR/21) Accommodative Rule (Gulden Ophthalmics, Elkins Park, PA) with a printed Gulden fixation target consisting of a single column of 20/30 letters at 40 cm. The edge of rule was placed at the center of the patient’s forehead just above the level of the brow. The target was slowly (1–2cm/sec) moved toward the patient. When diplopia was reported, movement was stopped and the patient was asked “Does it stay two or does it come back into one?” If the patient recovered single vision within 1–2 seconds, the target was again moved toward the patient until the patient was unable to regain fusion.
Secondary Outcome Measure
Positive Fusional Vergence (Convergence Amplitudes) at Near
Positive fusional vergence was measured three times with a horizontal prism bar (Gulden B-16 horizontal prism bar with prismatic levels from 1Δ to 45Δ, Gulden Ophthalmics, Elkins Park, PA) while the patient fixated a hand-held fixation target (Gulden fixation Stick # 15302) with a single column of letters of 20/30 equivalent at a distance of 40 cm.. The patient was asked to report when the letters became blurred or double as prism was introduced at approx 2Δ/sec, pausing at each prism to confirm that the target was “single and clear.”
Randomization
Randomization was achieved using a secure website created and managed by the DCC. The website generated the patient’s group assignment and assigned the patient a unique study identification number using a pre-determined list generated by the DCC. Access to the list was limited to the programmer and PI of the DCC. The randomization algorithm assigned patients to the four treatment groups with equal probability using a randomized permuted block design so investigators could not predict the sequence of treatment assignments. To ensure approximately equal numbers of patients in each treatment arm, randomization was performed separately for each clinical site.
Treatment
The treatment prescribed was either home-based or office-based therapy involving a sequence of activities to develop efficient visual skills. The initial treatment session was scheduled within one month of randomization. At this initial treatment visit, the therapist instructed the patient how to perform in-office therapy procedures (for the office-based groups only) and the home therapy procedures associated with the assigned treatment arm (for all treatment groups). Some home-based therapy procedures required the use of a computer software vision therapy program. If a patient did not have access to a home computer, the study provided a loaner computer. This was necessary for 20 patients.
Home-based Treatment Groups
Patients in the home-based treatment groups were prescribed therapy to be performed at home 5 days per week. In addition, they were scheduled for weekly telephone appointments with the therapist during which time the home therapy log was reviewed and the therapist verbally motivated the patient in an attempt to maximize compliance with treatment. Each patient was also scheduled for a monthly in-office session with the therapist (after each masked examination). Although this protocol is more rigorous than that used in current clinical practice, it was designed to maximize retention and adherence to the home-based therapy protocols.
Home-based Pencil Push-ups
The CITT pencil push-ups procedure used a pencil with 20/60 size letters and a white index card placed in the background to provide a suppression check by using physiological diplopia awareness. The goal of the procedure was to move the pencil to within 2 to 3 cm of the brow, just above the nose on each push-up. Patients were instructed to perform the pencil push-ups procedure 15 minutes per day, 5 days per week. They maintained home therapy log forms, recording the closest distance that they could maintain fusion after each 5 minutes of therapy
Home-based Computer Vergence/Accommodative Therapy and Pencil Push-ups
The home-based computer vergence/accommodative therapy and pencil push-ups group practiced the same pencil push-up procedure as the home-based pencil push-ups group. In addition, they performed fusional vergence and accommodative therapy procedures with the Home Therapy System (HTS) computer software (Home Therapy Systems, Gold Canyon, AZ). The vergence base in, vergence base out, auto-slide vergence, and jump ductions vergence programs using random dot stereopsis targets were used for fusional vergence training and the accommodative rock program was used for accommodative therapy. Therapy was prescribed to be performed at home for 20 minutes per day (15 minutes for HTS and 5 minutes for pencil push-ups), 5 days per week. Patients maintained a home therapy log form and recorded the closest distance to which they could converge and maintain fusion for pencil push-up therapy and the level completed for each session of computer therapy.
Office-based Treatment Groups
Patients in the office-based treatment groups were scheduled for weekly in-office appointments with the therapist lasting approximately 60 minutes per visit during which time in-office procedures were performed, home therapy procedures were demonstrated, the home therapy log was reviewed, and the therapist verbally motivated the patient in an attempt to maximize adherence. Patients in the office-based treatment groups were prescribed 15 minutes of home therapy procedures to be completed 5 days per week. To enhance compliance we asked patients to log their home therapy activities including minutes spent on therapy and therapy goals achieved.
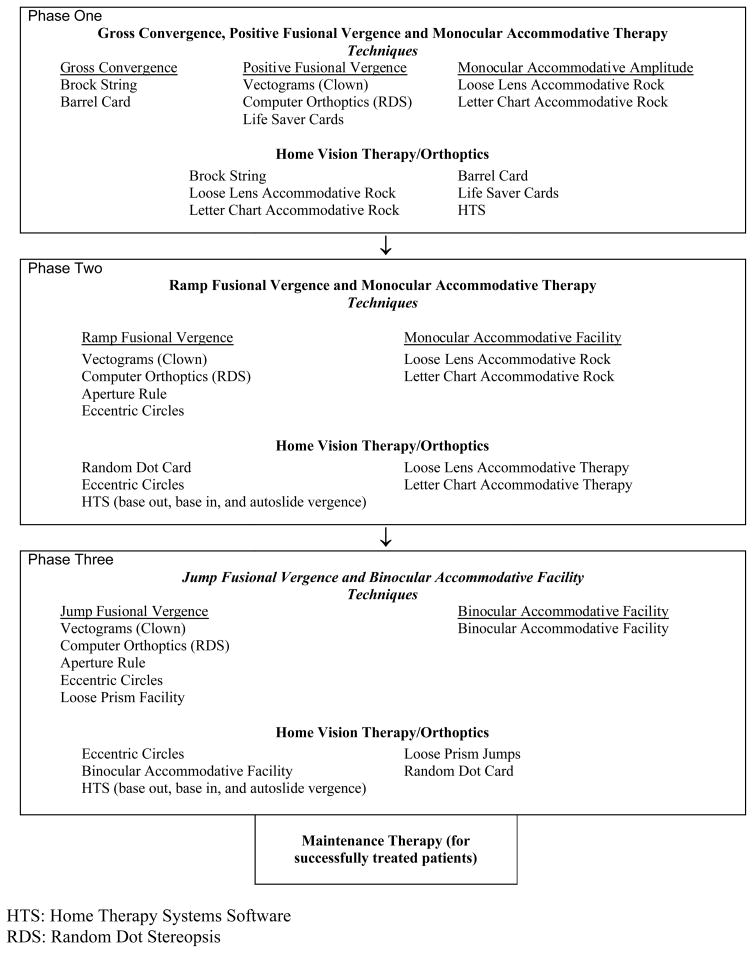
Office-based Vergence/Accommodative Therapy with Home Reinforcement
Office-based vergence/accommodative therapy with home reinforcement was administered by a therapist on an individual basis combined with procedures to perform at home. The treatment program consisted of 3 phases. Within each phase there were a number of subcategories wherein therapy procedures were arranged sequentially from easiest to most difficult. The therapy program is summarized in Table 3 and described in detail in Chapter 8 of the CITT Manual of Procedures that can be accessed at http://optometry.osu.edu/research/CITT/4363.cfm.
Table 3.
Office-Based Vergence/Accommodative Therapy With Home Reinforcement Protocol
 |
Patients were scheduled weekly visits lasting approximately 60 minutes per visit and were prescribed 15 minutes of home therapy procedures to be completed 5 days per week. To enhance compliance we asked patients to log their home therapy activities including minutes spent on therapy and therapy goals achieved.
Office-based Placebo Therapy
The office-based placebo therapy program included 18 procedures which were designed to look like real vision therapy/orthoptics yet they did not stimulate vergence, accommodation or eye movement skills beyond normal viewing conditions.32 The therapy program was comprised of modified traditional procedures (e.g. modified to be monocular rather than binocular) as well as testing procedures that would not require significant demand on the vergence, accommodative or eye movement systems. Like real vision therapy/orthoptics, patients frequently wore filter glasses and were told that the glasses were important to ensure that both eyes were being used. Similar to the real therapy procedures, objectives and goals were established for each placebo procedure to motivate patients to engage in the therapy activities. These procedures are detailed in the CITT Manual of procedures that can be accessed at http://optometry.osu.edu/research/CITT/4363.cfm. Therapists were asked to maintain the same level of enthusiasm as they did for actual vision therapy/orthoptics procedures.
There were differences among the groups in the amount of time spent doing vision therapy or interacting with the therapist, ranging from 135 minutes per week in the 2 office-based groups to 90 minutes for home-based pencil push-ups and 115 minutes for the home-based computer vergence/accommodative therapy and pencil push-ups group. This study was not designed to equalize the therapy time, rather, it was designed as an effectiveness study investigating these clinical treatments as typically provided in clinical practice.
Follow-Up and Primary Outcome Examination
Follow-up masked examinations were scheduled at 4 and 8 weeks with the primary outcome examination occurring at 12 weeks. Two long-term follow-up visits were scheduled 6 and 12 months after completion of the 12-week primary outcome visit.
Study Protocol at Completion of the 12 Weeks of Treatment
Patients who scored <16 on the CISS at the outcome examination were placed on maintenance therapy for 6 months. The maintenance therapy was specific for the treatment group with therapy prescribed to be performed at home for 15 minutes per week. Patients from any treatment group who scored ≥16 on the CISS (and thus were considered still symptomatic) were referred to a non-CITT clinician for alternative treatment at no cost. All patients were scheduled for follow-up visits at 6 and 12 months from completion of the 12-week treatment program.
Patient and Investigator Masking
While it was not feasible to mask patients to whether they were assigned to a home- or office-based treatment, office-based patients were masked regarding their group assignment (i.e., real or placebo therapy). While it was not feasible to mask the CITT therapists responsible for treating the patients, the investigators responsible for obtaining the outcome measures were masked to patient treatment assignment.
Statistical Analyses
Sample Size Considerations
All sample size calculations were performed using PASS 2000 software assuming a two-sided test with 90% power. For a given outcome measure, the common standard deviation obtained from the CITT pilot study23 was used as an estimate of variability. To control for multiple comparisons (4 groups compared two-at-a-time = 6 pair-wise comparisons), the alpha level used for determining sample size was set at 0.05/6 = 0.0083.
Clinically relevant differences between any two treatment groups were pre-determined by the CITT Executive Committee as 10 units on the CISS, 4 cm in near point of convergence and 10Δ in positive fusional vergence. The sample size of 208 (52 per treatment arm) was determined by finding the maximum required sample size among the three outcome variables which was then adjusted for 10% loss to follow-up.;
Data Entry and Quality Control
Personnel at the CITT DCC performed all data management and analyses. To access the CITT website for enrollment and subject tracking, an authorized user was assigned a unique username and password. All website passwords are changed every 6 months and users are locked out after 3 failed attempts to log in. The CITT project coordinator and programmer at the DCC assigned all usernames and passwords.
Site personnel with authority to enroll patients had 24-hour access to the website. Eligibility information was entered for all patients evaluated for enrollment regardless of eligibility status. This protocol allowed the DCC to track both testing and enrollment statistics at each clinical site on a monthly basis. The website was also used to schedule patients for their study visits and to indicate when a study visit had been completed. Listings of patients due or overdue for a study visit along with a personalized calendar of scheduled visits were available to both the DCC and each clinical site. Information from the scheduling module was used by the DCC to track and report retention on a monthly basis.
The Visual Basic Application (VBA) with SQL database backend was also used for data management and double data entry. Upon completion of the second entry of each data form, the application identified any required data edits which were sent to the appropriate clinical site for reconciliation. Data management tools included the ability to list 1) data forms not transmitted to the DCC; 2) outstanding data edits; 3) data forms not yet entered and 4) visits occurring outside of the acceptable window. A manual data audit comparing the data in the VBA to that on the form was performed on 10% of all forms received each month with additional data audits if the error rate exceeded 1%.
Data Analysis
All data analyses will be performed using the SAS software system version 9.1. Unless specifically stated otherwise, an α-level of 0.05 will be used to assess statistical significance. All analyses will follow the intention-to-treat principle.
The primary aim of the study is to compare each of the three outcome measures between the four treatment groups. A 4 group by 3 time period repeated measures analysis of covariance will be used to obtain an estimate of the Mean Square Error for use in group comparisons. The analysis will also include the interaction of group and time. This analysis method uses all of the longitudinal data collected, is robust to missing data, and is flexible enough to allow valid inferences concerning a variety of questions. The measure obtained at the eligibility examination will be used as a covariate because our initial pilot data showed a strong correlation between this value and all subsequent values. If the analysis indicates some group differences, Tukey’s method of adjustment for multiple comparison will be used to hold the overall error rate at α=0.05 while performing the six pair-wise hypothesis tests.
Data obtained at the 6- and 12-month masked examinations from patients who scored less than 16 on the CI Symptom Survey at week 12 will be used to study the long-term effects of treatment. Regression modeling will be used to determine the demographic and clinical measures from the eligibility examination and/or the 12-week visit, which predict long-term effectiveness of treatment for each treatment group. A 95% confidence interval will be constructed to describe the mean change in outcome from week 12 to both the 6- and 12 month follow-up visits for each treatment group. Because of the anticipated small numbers in some of the groups, we will not specifically compare the changes between treatment groups.
Results
Enrollment began in July 2005 and ended on October 31, 2006. Clinic enrollment ranged from 14 to 35 patients. Eligibility examinations were performed on 424 children ages 9 to 17 years (inclusive) with 232 (54.7%) deemed eligible and 221 (95.3% of those eligible) agreeing to participate. One eligible patient tested at the Miami, FL site initially decided not to participate because of transportation issues. The patient was then referred to the Ft. Lauderdale, FL site, was retested and decided to participate in the study. The patient is counted as an eligible patient at each site in the recruitment statistics. As shown in Table 4, non-participants were slightly older, had slightly lower symptom levels, a better near point of convergence, and a poorer accommodative amplitude. Reasons for non-participation included unwillingness of parents to have their children randomized, parent’s feeling that the study required too much time, transportation issues, and unwillingness of children to complete the at-home therapy procedures.
Table 4.
Summary statistics for variables measured at the eligibility examination, by enrollment status
| Characteristic | Participants (n=221) | Non-Participants (n=10) |
|---|---|---|
| Mean (std) Age (years) | 11.8 (2.3) | 12.8 (2.5) |
| Mean (std) CISS score | 29.9 (8.9) | 25.9 (6.8) |
| Mean (std) Near Point of Convergence (cm) | 14.3 (7.6) | 11.0 (4.9) |
| Mean (std) Positive Fusional Vergence Break (Δ) | 12.6 (4.7) | 12.0 (1.7) |
| Mean (std) Negative Fusional Vergence Break (Δ) | 14.2 (5.2) | 15.3 (4.4) |
| Mean (std) Monocular Accommodative Amplitude (cm) | 11.6 (4.2) | 10.0 (3.1) |
| % with Accommodative Insufficiency* | 55% | 50% |
| Mean (std) Monocular Accommodative Facility (cpm) | 6.9 (5.2) | 8.3 (3.9) |
| Mean (std) Near Phoria (Δ) | 9.3 exo (4.4) | 9.3 exo (4.2) |
| Mean (std) Distance Phoria (Δ) | 1.9 exo (2.8) | 2.1 exo (2.3) |
| Mean (std) Spherical Equivalent - Right Eye (D) | −0.09 (1.46) | −0.38 (1.38) |
| Gender: % Female | 60% | 40% |
| Race | ||
| % American Indian / Alaskan Native | 5% | 0% |
| % Asian / Pacific Islander | 2% | 20% |
| % Black or African American | 29% | 40% |
| % White | 53% | 40% |
| % Other | 11% | 0% |
| Ethnicity | ||
| % Hispanic or Latino | 34% | 30% |
| % Not Hispanic or Latino | 64% | 70% |
| % Missing | 2% | 0% |
| Attention Deficit Attention Disorder | ||
| % Yes | 15% | 0% |
| % No | 80% | 70% |
| % Missing | 5% | 30% |
Defined as participant’s accommodative amplitude less than Hoffstetter’s minimum (15–1/4 age) accommodative amplitude criteria minus 2.0 D
= prism diopters
cpm = cycles per minute
cm = centimeters
D = Diopters
Std = standard deviation
Fifty-four children were randomly assigned to the home-based pencil push-up group, 53 to the home-based computer vergence/accommodative therapy and pencil push-ups, 60 to the office-based vergence/accommodative therapy with home reinforcement group, and 54 to the office-based placebo therapy group. Table 5 displays the eligibility data by treatment group. Using analysis of variance to compare the groups, none of the differences in CISS, near point of convergence, or positive fusional vergence at near were clinically meaningful or statistically significant. Similarly, a chi-square test found no differences in the percentage with accommodative insufficiency across the four groups.
Table 5.
Summary statistics for variables measured at the eligibility examination, by treatment group
| Characteristic | HBPPT n=54 | HBCVAT n=53 | OBVT n=60 | OBPVT n=54 |
|---|---|---|---|---|
| Mean (std) Age (years) | 11.9 (2.2) | 11.6 (2.3) | 12.0 (2.6) | 11.8 (2.2) |
| Mean (std) CISS score | 27.6 (7.6) | 31.4 (9.1) | 30.0 (9.9) | 29.6 (8.9) |
| Mean (std) Near Point of Convergence (cm) | 14.7 (8.4) | 14.4 (7.5) | 13.3 (6.6) | 14.4 (7.8) |
| Mean (std) Base-out Break (Δ) | 13.0 (4.8) | 12.2 (4.8) | 12.7 (4.6) | 13.1 (4.4) |
| Mean (std) Base-in Break (Δ) | 15.9 (5.6) | 13.8 (4.7) | 13.5 (5.9) | 13.8 (4.4) |
| Mean (std) Monocular Accommodative Amplitude (D) | 10.1 (3.8) | 10.1 (4.5) | 9.9 (4.0) | 9.4 (3.0) |
| % with Accommodative Insufficiency* | 50% | 57% | 60% | 52% |
| Mean (std) Monocular Accommodative Facility (cpm) | 7.2 (4.8) | 6.2 (5.4) | 6.9 (4.7) | 7.4 (5.8) |
| Mean (std) Near Phoria (Δ) | 9.9 exo (5.0) | 9.4 exo (4.5) | 8.8 exo (3.7) | 9.0 exo (4.5) |
| Mean (std) Distance Phoria (Δ) | 2.4 exo (3.4) | 2.0 exo (3.0) | 1.7 exo (2.2) | 1.8 exo (2.5) |
| Mean (std) Spherical Equivalent - Right Eye (D) | −0.34 (1.5) | 0.08 (1.5) | −0.20 (1.3) | 0.15 (1.5) |
| % Female | 50% | 58% | 68% | 60% |
| Race | ||||
| % American Indian / Alaskan Native | 0% | 6% | 3% | 9% |
| % Asian / Pacific Islander | 4% | 0% | 3% | 0% |
| % Black or African American | 34% | 23% | 24% | 34% |
| % White | 57% | 55% | 58% | 45% |
| % Other | 6% | 17% | 12% | 9% |
| Ethnicity | ||||
| % Hispanic or Latino | 22% | 45% | 41% | 31% |
| % Not Hispanic or Latino | 78% | 55% | 59% | 69% |
| % Missing | ||||
| Attention Deficit Hyperactivity Disorder | ||||
| % Yes | 11% | 17% | 12% | 23% |
| % No | 83% | 79% | 85% | 73% |
| % Missing | 6% | 4% | 3% | 4% |
| Glasses wearer? | ||||
| % Yes | 44% | 30% | 27% | 37% |
| % No | 56% | 70% | 73% | 63% |
| Medication use | ||||
| Number (%) reporting use | 8 (15%) | 16 (30%) | 16 (27%) | 23 (43%) |
| % using psychotropic medications | 38% | 31% | 25% | 39% |
| % using pulmonary medications | 50% | 56% | 25% | 70% |
| % using allergy medications | 13% | 44% | 44% | 48% |
| Academic Performance Survey | ||||
| How often does child | ||||
| Have difficulty completing assignments? | ||||
| Mean score (std) | 1.74 (1.2) | 2.11 (1.3) | 1.55 (1.3) | 1.89 (1.3) |
| Median score | 2.0 | 2.0 | 1.0 | 2.0 |
| Have difficulty completing homework? | ||||
| Mean score (std) | 1.70 (1.2) | 1.98 (1.4) | 1.45 (1.3) | 1.76 (1.3) |
| Median score | 2.0 | 2.0 | 1.0 | 2.0 |
| Avoid reading or close work? | ||||
| Mean score (std) | 2.24 (1.3) | 2.32 (1.4) | 2.13 (1.3) | 2.11 (1.3) |
| Median score | 2.0 | 2.0 | 2.0 | 2.0 |
| Make careless mistakes in school/homework? | ||||
| Mean score (std) | 2.28 (1.2) | 2.36 (1.1) | 2.02 (1.3) | 2.20 (1.1) |
| Median score | 2.5 | 2.0 | 2.0 | 2.0 |
| Appear inattentive during reading or close work? | ||||
| Mean score (std) | 2.39 (1.2) | 2.42 (1.1) | 2.12 (1.2) | 2.26 (1.2) |
| Median score | 2.5 | 2.0 | 2.0 | 2.5 |
| How often does parent worry about school performance? | ||||
| Mean score (std) | 2.69 (1.3) | 2.81 (1.4) | 2.48 (1.5) | 2.48 (1.5) |
| Median score | 3.0 | 3.0 | 3.0 | 3.0 |
Defined as monocular accommodative amplitude less than Hoffstetter’s minimum accommodative amplitude criteria minus 2.0 D
HBPPT: Home-based pencil push-up therapy
HBCVAT+: home-based computer vergence/accommodative therapy and pencil push-ups
OBVAT: office-based vergence/accommodative therapy with home reinforcement
OBPT: Office-based placebo therapy
= prism diopters
cpm = cycles per minute
cm = centimeters
D = Diopters
Std = standard deviation
Discussion
The Convergence Insufficiency Treatment Trial is the first large-scale, randomized clinical trial to evaluate active treatments for symptomatic CI in children. Identification of the most effective treatment regimen is important because of the high prevalence of this binocular vision disorder and its potential effect on reading and other near visual activities. The results of the CITT will determine if any of the forms of active therapy are effective for symptomatic CI in children. The CITT is a carefully designed study which is providing necessary standardized diagnostic and treatment protocols. The eligibility criteria for defining CI were somewhat restrictive in that both the near point of convergence and positive fusional vergence at near had to be outside of normal values in patients exhibiting at least 4 more prism diopters of exophoria at near than at far. This was to ensure that those who enrolled had a definite diagnosis of CI. Because asymptomatic patients are generally not motivated to pursue treatment, only patients who exhibited a certain degree of symptoms (≥ 16 on the CISS) were eligible to participate. Patients who had previously undergone CI therapy were excluded because of potential unmasking and because their response to treatment may differ from that of untreated CI patients. The development of the placebo therapy arm is a novel aspect of the study and will provide a control against which other treatments can be compared and allow us to ensure that any treatment effects found are not merely due to placebo effect or regression to the mean21,33.
The CITT Investigator group has completed two other randomized clinical trials with symptomatic CI children.23,24 The baseline characteristics of the current study cohort are comparable to the cohorts recruited in the previous studies. Mean patient age, CISS score, near exophoria magnitude, and positive fusional vergence values at near were similar between the patients enrolled in this trial and our previous trials. The mean CISS score of 30.2 (std=9.0) for 119 patients enrolled in our previous studies is similar to the 29.9 (std=8.9) for this study. Positive fusional vergence differed by only 1.5Δ (previous group mean=11.1, std=3.8; CITT group mean=12.6, std=4.7). Slight differences were found in the mean near point of convergence. The mean near point of convergence was 16.0 (std=6.6) for those in our previous studies compared to 14.3 (std=7.6) for this study. These differences are not clinically significant and represent random variation. The proportion of females enrolled in the current study (60%) was comparable to that in previous studies (56%). While our study enrollment was diverse, it is not population-based and should not be used to suggest demographic variation in the prevalence of CI between genders or between ethnic or racial groups.
It is also important to consider the study sample’s external generalizability or the ability of the cohort of participating children to be representative of other CI patients in the population. Given the geographical distribution of the participating clinical sites, the inclusion of both optometry and ophthalmology clinics, and the ethnic and racial diversity of the participants, it is not unrealistic to assume that the CI patients enrolled in this study are representative of other children with symptomatic CI (using our study definition).
Conclusion
The Convergence Insufficiency Treatment Trial is the first, large-scale, randomized clinical trial to evaluate different forms of active therapy treatments for symptomatic CI in children.
Hallmark features of the study design include formal definitions, standardized diagnostic and treatment protocols, use of the CISS score as the primary outcome measure, masked outcome examinations, and an office-based placebo therapy treatment arm. The baseline data reported herein define the clinical profile of the children enrolled into the CITT and will be useful for interpreting the results of this randomized trial.
Acknowledgments
Supported by the National Eye Institute of the National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland
The Convergence Insufficiency Treatment Trial Investigator Group
Writing Committee
Lead Authors: Mitchell Scheiman, OD, G. Lynn Mitchell, MAS, Susan Cotter, OD, MS, Marjean Kulp, OD, MS, Michael Rouse, OD, MSEd, Richard Hertle, MD, Susanna Tamkins, OD. Additional Writing Committee members (alphabetical): Jeffrey Cooper, OD, MS, David Granet, MD, Brian Mohney, MD, Rachel Coulter, OD, Michael Gallaway, OD.
Clinical Sites
Sites are listed in order of the number of patients enrolled in the study with the number of patients enrolled is listed in parentheses preceded by the site name and location. Personnel are listed as (PI) for principal investigator, (SC) for coordinator, (E) for examiner, and (VT) for therapist.
Study Center: Bascom Palmer Eye Institute (35)
Susanna Tamkins, OD (PI); Hilda Capo, MD (E); Mark Dunbar, OD (E); Craig McKeown, MD (CO-PI); Arlanna Moshfeghi, MD (E); Kathryn Nelson, OD (E); Vicky Fischer, OD (VT); Adam Perlman, OD (VT); Ronda Singh, OD (VT); Eva Olivares (SC); Ana Rosa (SC); Nidia Rosado (SC); Elias Silverman (SC)
Study Center: SUNY College of Optometry (28)
Jeffrey Cooper, OD (PI); Ida Chung, OD (E); Steven Ritter, OD (E); Audra Steiner, OD (E, CoPI); Lyndon Wong, OD (E); Lily Zhu, OD (E); Marta Brunelli (VT); Stacy Friedman, OD (VT); Kaity Colon (SC)
Study Center: UAB School of Optometry (28)
Kristine Hopkins, OD (PI); Marcela Frazier, OD (E); Janene Sims, OD (E); Marsha Swanson, OD (E); Katherine Weise, OD (E); Adrienne Broadfoot, MS, OTR/L (VT, SC); Michelle Anderson, OD (VT); Catherine Baldwin (SC)
Study Center: NOVA Southeastern University (27)
Rachel Coulter, OD (PI); Deborah Amster, OD (E); Gregory Fecho, OD (E); Tanya Mahaphon, OD (E); Jacqueline Rodena, OD (E); Mary Bartuccio, OD (VT); Yin Tea, OD (VT); Annette Bade, OD (SC)
Study Center: Pennsylvania College of Optometry (25)
Michael Gallaway, OD (PI); Brandy Scombordi, OD (E); Ruth Shoge, OD (E); Lily Zhu, OD (E); Mark Boas, OD (VT); Tomohiko Yamada, OD (VT); Ryan Langan (SC)
Study Center - The Ohio State University College of Optometry (24)
Marjean Kulp, OD, MS (PI); Michelle Buckland, OD (E); Michael Earley, OD, PhD (E); Gina Gabriel, OD, MS (E); Aaron Zimmerman, OD (E); Kathleen Reuter, OD (VT); Andrew Toole, OD, MS (VT); Molly Biddle, MEd (SC); Nancy Stevens, MS, RD, LD (SC)
Study Center: Southern California College of Optometry (23)
Susan Cotter, OD, MS (PI); Eric Borsting, OD, MS (E); Michael Rouse, OD, MSEd, (E); Carmen Barnhardt, OD, MS (VT); Raymond Chu, OD (VT); Rebecca Bridgeford (SC); Jamie Morris (SC); Susan Parker (SC); Javier Villalobos (SC)
Study Center: University of CA San Diego: Ratner Children’s Eye Center (17)
David Granet, MD (PI); Lara Hustana, OD (E); Shira Robbins, MD (E); Erica Castro (VT); Cintia Gomi, MD (SC)
Study Center: Mayo Clinic (14)
Brian Mohney, MD (PI); Jonathan Holmes, MD (E); Melissa Rice, OD (VT); Virginia Karlsson, BS, CO (VT); Becky Nielsen (SC); Jan Sease, COMT/BS (SC); Tracee Shevlin (SC)
CITT Study Chair
Mitchell Scheiman, OD (Study Chair); Karen Pollack (Study Coordinator); Susan Cotter, OD, MS (Vice Chair); Richard Hertle, MD (Vice Chair); Marjean Taylor-Kulp, OD (Vice Chair); Michael Rouse, OD, MSEd (Consultant)
CITT Data Coordinating Center
Gladys Lynn Mitchell, MAS, (PI); Tracy Kitts, (Project Coordinator); Melanie Bacher (Programmer); Linda Barrett (Data Entry); Loraine Sinnott, PhD (Biostatistician); Kelly Watson (Student worker); Pam Wessel (Office Associate)
National Eye Institute, Bethesda, MD
Paivi Miskala, PhD
CITT Executive Committee
Mitchell Scheiman, OD; G. Lynn Mitchell, MAS; Susan Cotter, OD, MS; Richard Hertle, MD; Marjean Kulp, OD, MS; Paivi Miskala, PhD; Michael Rouse, OD, MSEd
Data and Safety Monitoring Committee
Marie Diener-West, PhD, Chair, Rev. Andrew Costello, CSsR, William V. Good, MD, Ron D. Hays, PhD, Argye Hillis, PhD (Through March 2006), Ruth Manny, OD, PhD
Footnotes
A listing of the writing committee and investigators who participated in the study appears in the Appendix
References
- 1.Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative and Eye Movement Disorders. Philadelphia: Lippincott, Williams and Wilkins; 2002. [Google Scholar]
- 2.Scheiman M, Gallaway M, Coulter R, et al. Prevalence of vision and ocular disease conditions in a clinical pediatric population, in review. J Am Optom Assoc. 1996;67:193–202. [PubMed] [Google Scholar]
- 3.Rouse MW, Hyman L, Hussein M, Solan H CIRS group. Frequency of convergence insufficiency in optometry clinic settings. Optom Vis Sci. 1998;75:88–96. doi: 10.1097/00006324-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Rouse MW, Borsting E, Hyman L, et al. Frequency of convergence insufficiency among fifth and sixth graders. Optom Vis Sci. 1999;76:643–49. doi: 10.1097/00006324-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Daum KM. Convergence insufficiency. Am J Optom Physiol Opt. 1984;61:16–22. doi: 10.1097/00006324-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kent PR, Steeve JH. Convergence insufficiency: incidence among military personnel and relief by orthoptic methods. Military Surgeon. 1953;112:202–205. [PubMed] [Google Scholar]
- 7.Hirsch MJ. A study of forty-eight cases of convergence insufficiency at the near point. Am J Optom Arch Am Acad Optom. 1943;20:52–58. [Google Scholar]
- 8.Mazow ML. The convergence insufficiency syndrome. J Ped Ophthalmol. 1971;8:243–44. [Google Scholar]
- 9.Duke-Elder S, Wybar K. Ocular Motility and Strabismus. In: Duke-Elder S, editor. System of Ophthalmology. St Louis: Mosby; 1973. pp. 547–551. [Google Scholar]
- 10.Pickwell LD, Hampshire R. The significance of inadequate convergence. Ophthal Physiol Opt. 1981;1:13–18. [PubMed] [Google Scholar]
- 11.Scheiman M, Gallaway M. The long-term effectiveness of vision therapy for the treatment of convergence insufficiency. Optom Vis Sci. 1997;74:S 167. [Google Scholar]
- 12.von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 5. St Louis: Mosby; 1996. [Google Scholar]
- 13.Pratt-Johnson JA, Tillson G. Management of Strabismus and Amblyopia. 2. New York: Thieme Medical Publishers; 2001. [Google Scholar]
- 14.von Noorden G, Helveston E. Strabismus: A decision making approach. St. Louis: Mosby-Year Book; 1994. [Google Scholar]
- 15.Griffin JR, Grisham JD. Binocular Anomalies: Diagnosis and Vision Therapy. 4. Boston: Butterworth-Heinemann; 2002. [Google Scholar]
- 16.Press LJ. Applied Concepts in Vision Therapy. St. Louis: Mosby-Year Book; 1997. [Google Scholar]
- 17.Hugonnier R, Clayette-Hugonnier C. Strabismus, Heterophoria and Ocular Motor Paralysis. St. Louis: CV Mosby; 1969. [Google Scholar]
- 18.Gallaway M, Scheiman M, Malhotra K. The effectiveness of pencil pushups treatment for convergence insufficiency: a pilot study. Optom Vis Sci. 2002;79:265–67. doi: 10.1097/00006324-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Chin FH, Faibish B, Hisaka C, Thal L, Tsuda K. A survey of the treatment of convergence insufficiency. J Beh Optom. 1995;6:91–92. 109. [Google Scholar]
- 20.Scheiman M, Cooper J, Mitchell GL, et al. A survey of treatment modalities for convergence insufficiency. Opt Vis Sci. 2002;79:151–57. doi: 10.1097/00006324-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Grisham JD. Visual therapy results for convergence insufficiency: a literature review. Am J Optom Physiol Opt. 1988;65:448–54. doi: 10.1097/00006324-198806000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Scheiman M, Mitchell GL, Cotter S, et al. In Reply: Convergence Insufficiency Randomized Clinical Trial. Arch Ophthalmol. 2005;123:1760–1761. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 23.Scheiman M, Mitchell GL, Cotter S, et al. A randomized trial of the effectiveness of treatments for convergence insufficiency in children. Arch Ophthalmol. 2005;123:14–24. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 24.Scheiman M, Cotter S, Rouse M, et al. Randomised clinical trial of the effectiveness of base-in prism reading glasses versus placebo reading glasses for symptomatic convergence insufficiency in children. Br J Ophthalmol. 2005;89:1318–23. doi: 10.1136/bjo.2005.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CITT Investigator Group. Convergence Insufficiency Treatment Trial. 2004 identifier NCT00338611. [Google Scholar]
- 26.Borsting E, Rouse MW, De Land PN CIRS Group. Prospective comparison of convergence insufficiency and normal binocular children on CIRS symptom surveys. Optom Vis Sci. 1999;76:221–28. doi: 10.1097/00006324-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Borsting E, Rouse MW, Deland PN, et al. Association of symptoms and convergence and accommodative insufficiency in school-age children. Optometry. 2003;74(1):25–34. [PubMed] [Google Scholar]
- 28.Borsting EJ, Rouse MW, Mitchell GL, et al. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9–18 years. Optom Vis Sci. 2003;80:832–838. doi: 10.1097/00006324-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Rouse MW, Borsting E, Mitchell GL, et al. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthal Physiol Opt. 2004;24:384–390. doi: 10.1111/j.1475-1313.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 30.Sheard C. Zones of ocular comfort. Am J Optom. 1930;7:9–25. [Google Scholar]
- 31.Borsting E, Rouse M, Chu R. Measuring ADHD behaviors in children with symptomatic accommodative dysfunction or convergence insufficiency: a preliminary study. Optometry. 2005;76:588–92. doi: 10.1016/j.optm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Kulp MT, Borsting E, Mitchell GL, et al. Feasibility of using placebo vision therapy in a clinical trial. Optom Vis Sci. 2002;79:S 12. doi: 10.1097/OPX.0b013e318169288a. [DOI] [PubMed] [Google Scholar]
- 33.Cooper J, Duckman R. Convergence insufficiency: incidence, diagnosis, and treatment. J Am Optom Assoc. 1978;49:673–80. [PubMed] [Google Scholar]