Abstract
Changes in the state of CREB phosphorylation and in LTP in the hippocampus have been associated with learning and memory. Here we show that galanin, the neuropeptide released in the hippocampal formation from cholinergic and noradrenergic fibers, that has been shown to produce impairments in memory consolidation in the Morris water maze task inhibits both LTP and CREB phosphorylation in the rat hippocampus in-vivo. While there are many transmitters regulating CREB phosphorylation none has been shown to suppress behaviorally-induced hippocampal CREB phosphorylation as potently as galanin. The in-vivo inhibition of dentate gyrus-LTP and of CREB phosphorylation by the agonist occupancy of GalR1 and GALR2-type galanin receptors provides strong in-vivo cellular and molecular correlates to galanin-induced learning deficits and designates galanin as a major regulator of the memory consolidation process.
Galanin is a 29 amino acid neuropeptide shown to co-exist in the hippocampus with septal cholinergic and locus coeruleus (LC) noradrenergic inputs (Melander, Staines, and Rokaeus, 1986). Galanin inhibits the release of glutamate, norepinephrine, serotonin, and acetylcholine (Kinney, Emmerson, & Miller, 1998; Ogren et al., 1998; Robinson, et al., 1996; Wang, et. al., 1999; Fisone et al., 1991). Galanin modulates signal transduction mechanisms by inhibiting adenylyl cyclase activity and activating phosphatidylinositol hydrolysis (Iismaa & Shine, 1999; Karelson &Langel, 1998; Palazzi et al., 1991). Three galanin receptor subtypes (GalR1, GalR2, and GalR3) have been identified, all belonging to the G-protein coupled receptor (GPCR) family. GalR1 and GalR2 are found more prevalently in the CNS and both GalR1 and GalR2 are expressed in the hippocampus (Burazin et al., 2000). GalR1 signaling is mediated via Gi type G-proteins while GalR2 receptor is a Gq11/Gi coupled receptor (McDermott and Sharp, 1995; Karelson and Langel, 1998). Galanin binding in-vitro initially results in an inhibitory action on adenylate cyclase (AC) (through GalR1) and in a delayed activation of MAP kinase (through GalR2) (Chen et al., 1992; Valkna et al., 1995; Iismaa and Shine, 1999). Endogenous galanin protects neurons from excitotoxicity via inhibition of CREB phosphorylation (Mazarati et al., 2000), and this inhibitory effect on CREB phosphorylation may be a rapid means of feedback regulating expression of the GalR1 type galanin receptor (Zachariou et al., 2001).
Galanin administered into the lateral ventricles or hippocampus produces deficits in acquisition or working memory on several learning and memory tasks in rodents, including trace fear conditioning and the Morris water maze (McDonald, et al., 1998; Ogren, Kehr, & Schoott, 1996; Ogren et al., 1998; Ogren, et al., 1999; Kinney et al., 2002). We have recently demonstrated that a specific learning and memory deficit produced by ICV galanin administration in performance of the Morris water maze was the impairment in memory consolidation (Kinney et al., 2003). It was also shown that the galanin induced learning and memory impairment was due to a galanin-induced inhibition of adenylate cyclase activity, as pre-treatment of the animals with forskolin, a non-specific adenylate cyclase activator rescued the galanin-induced cognitive impairment.
Several studies have established that regulation of adenylate cyclase activity plays a crucial role in learning and memory and long term potentiation (LTP) through regulation of cAMP levels and subsequent PKA-catalyzed CREB phosphorylation (Abel et al., 1998). In the present study we investigated the extent to which administration of galanin following behavioral training, shown to produce cognitive impairment (Kinney et al., 2003), affects the hippocampal levels of CREB phosphorylation. We have also utilized the GalR1/GalR2 agonist galanin 1–29 and galanin 2–11, which is a more selective agonist for GalR2 than GalR1, to determine which of the two hippocampal galanin receptors mediates the effects on phosphorylation of CREB. Since GalR2 activation has been shown to lead to increases in intracellular calcium and activation of the extracellular regulated kinases (ERKs), which in turn can phosphorylate CREB (Thiels & Klann, 2001), we have also followed the phosphorylation of ERKs in response to behavioral training and to galanin receptor agonists.
A second series of experiments was designed to investigate the effect of galanin 1–29 and galanin 2–11 on in-vivo hippocampal long term potentiation. Previous studies have demonstrated that in hippocampal slices galanin impairs CA1/CA3 LTP in rat and mice (Coumis and Davies, 2002), and dentate gyrus LTP (Badieh et. al., 2003). In addition, galanin knockout mice exhibited an increase in LTP induction and maintenance, whereas galanin over-expressing mice displayed a more rapid decay in LTP (Mazarati et al, 2000) which are congruent with behavioral studies reporting that i.c.v. administration of galanin impaired performance in spatial memory tasks (McDonald et al 1998; Wrenn and Crawley 2001). Badieh et. al., 2005 have explored the effects of galanin (2–11) and galanin (1–29) on slices and demonstrated that both caused a transient attenuation of LTP maintenance when applied at 21 min post-HFTs stimulation for 15 min with a larger effect exhibited by superfusion of galanin (2–11). Although application of galanin (1–29) or galanin (2–11) did not affect the PPF response indicating that the effect of the receptor(s) agonist is predominantly postsynaptic. However, as the entire collection of inputs and outputs of the hippocampus are intact, the nature of in-vivo LTP differs from that obtained in slice preparations. We have thus investigated the effects of galanin 1–29 and galanin 2–11 on the induction of hippocampal LTP in-vivo and compared the results with those obtained for the effects of galanin 1–29 and galanin 2–11 on cognition and on CREB phosphorylation in the hippocampus. We report a greater GalR1 receptor-mediated inhibition of CREB phosphorylation in-vivo that is likely to be the mechanism underlying the inhibition of LTP and memory consolidation.
Materials/Methods
Behavior
Subjects
Forty-five adult male Sprague-Dawley rats (Taconic, Germantown NY), approximately 200 grams in weight at the start of the experiment were used. Rats were group housed until the time of surgery and individually housed following the surgery. Rats were housed in a vivarium maintained at 22° C on a 12-h light-dark cycle (0800 light onset) with continuous access to rat chow and water in the home cage. All behavioral tests were conducted during the light phase of the daily cycle. All procedures were performed in accordance with The NIH guidelines for the care and use of laboratory animals, and approved by The Scripps Research Institute Animal Care and Use Committee.
Surgery
Rats underwent aseptic stereotaxic surgery under ketamine (100 mg/kg) xylazine (10 mg/kg) anesthesia. The ketamine/xylazine cocktail was administered at 1.52 mg/Kg animal body weight. A guide cannula 1.4 cm in length, of 26-gauge stainless steel hypodermic tubing (Plastics One, St. Louis, MO) was implanted into the right lateral ventricle at coordinates 0.5 mm posterior, 1.0 mm lateral to bregma, and 3.5 mm ventral to the surface of the skull (Paxinos and Watson, 1986). The cannula was secured to the skull using stainless steel screws and dental acrylic. A 31-gauge stylet was secured to the guide cannula following the surgery. Rats were then administered an analgesic (Ketoprofen, 5 mg/kg) to minimize post-operative pain. After surgery rats were given at least 8 days recovery before the start of behavioral testing.
Morris Water Maze
The Morris water maze was conducted in a circular tank, 1.4 m in outer diameter and 60 cm in height. Tap water, 48 cm deep, was maintained at a temperature of 25° C and made opaque by the addition of white non-toxic paint, and changed daily. The escape platform placed in the center of one of the 4 quadrants (target quadrant) was a 10 cm2 white plastic platform, placed 40 cm from the inside wall of the maze and 1 cm below the surface of the water.
Behavioral Testing
Subjects were taken individually from the colony room to a dedicated testing room that contained the water maze and large geometric shapes positioned on the interior walls that served as distal spatial cues. Four training trials per day were conducted. The rat was placed into the maze at one of three randomized locations, in the center of a quadrant that does not contain the escape platform (non-target quadrant). The animal was allowed to swim in the maze until it reached the hidden platform and placed its forepaws on the platform. If after sixty seconds the animal had not located the hidden platform, it was guided to the platform by the experimenter. Each rat was given fifteen seconds on the platform to allow orientation to distal extra-maze cues in reference to the hidden platform. Following the fifteen seconds on the platform, the rat was removed and placed under a heat lamp for 15 seconds, after which the next trial was initiated. Three additional trials were conducted in an identical fashion, for a total of 4 training trials per day. The training trials for the hidden platform were conducted for 2 successive days. An additional non-trained control group (n=5) was utilized that underwent the stereotaxic surgery and were administered saline but were not given training trials in the Morris water maze.
Drug treatments
Rats were administered one of the following drug treatments thirty minutes following completion of training trials in the Morris water maze: A) 0.9% physiological saline, 3µl (n=10). B) Rat galanin 1–29 (Bachem Bioscience Inc., King of Prussia, PA) dissolved in saline vehicle at a concentrations of; 3 nmol/3 µl (n=10) or C) 5 nmol/3 µl (n=10). D) Rat galanin 2–11 (n=10) (3 nmol/3 µl; Vulpes Ltd., Tallinn, Estonia). Microinjections were performed with a 10 µl Hamilton syringe connected via Becton Dickinson polyethylene tubing (PE20) to a 1.5 cm injector (Plastics One) fabricated from 31 gauge hypodermic tubing. The rat was gently restrained to remove the 31 gauge stylet, and the 1.5 cm injector was inserted into the guide cannula. The animal was then allowed to freely explore a small cage during the infusion of 3 µl over 1.5 minutes, with each 1 µl separated by ten seconds, and an additional sixty seconds before the injector was withdrawn from the guide cannula. Following the removal of the injector, the 31 gauge stylet was reinserted into the guide cannula.
Tissue collection
Rats were euthanized via CO2 asphyxiation 15 minutes following drug administration on the second day of training. Tissue collected for the immunohistochemistry (n=5 per group) was first fixed utilizing trans-cardiac perfusion methods with 4% paraformaldehyde, followed by immediate removal of the brains. Tissue was then sectioned at a thickness of 40µm for the IHC experiments. Hippocampi from separate rats (n=5 per group) were collected for the SDS-PAGE experiments by immediately dissecting out the hippocampi and freezing the tissue at −80.
SDS-PAGE and Western Blots: pCREB/CREB and pMAPK/MAPK ratios
Hippocampi were homogenized in a non-denaturing lysis buffer consisting of 20 mM HEPES pH 7.5, 10 mM EGTA pH 8.0, 40 mM β-glycerophosphate, 1% NP-40, 2.5 mM MgCl2, 2 mM orthovanadate, 1 mM DTT, 1mM phenylmethylsulfonyl fluoride (PMSF), 20 µg/ml aprotinin, and 20 µg/ml leupeptin. Lysates were centrifuged at 15,000 × g for 5 minutes at four degrees and the supernatant was then sonicated and a protein assay to determine concentration was performed using the biciconinic acid method (BCA, Pierce). Samples (40 µg) were separated on 10 % SDS-PAGE gels according to the method of Laemmli (1970). Proteins were then electro-transferred to nitropure 45 micron nitrocellulose membranes. Membranes were blocked in 5% milk in Tris-buffered saline 0.05% Tween (TBST) overnight. Membranes were incubated first with primary antibodies against pCREB (1:500 dilution; Cell Signaling) followed by redetection with anti-CREB antibody (1:500 dilution; Cell Signaling), or with anti-pERK antibody (1:1000 dilution)followed by antiERK antibody (1:1000 dilution) in TBST-5% milk for 2 hours at room temperature. Detection of specific binding was performed by incubation with HRP-conjugated secondary antibodies (1:5000, Vector) for 1 hour at room temperature. Specific signal was detected with Super Signal west pico chemiluminescent substrate (Pierce, Rockford, IL), and exposure to Kodak biomax ML film. Images were scanned for densitometry, using Quantity One software package and average intensity was used for each sample. Ratios were determined for intensity of pCREB/CREB and pERK/ERK. All data were then analyzed via the Sigmastat statistical analysis software package.
Immunohistochemistry
Whole brains were placed in 8% paraformaldehyde for 48 hours followed by being placed in 30% sucrose with 0.05% sodium azide for 2 days. Brains were then sectioned at a thickness of 40µm in a cryostat. Sections were stored at 4° C in 5% sucrose in PBS until the immunohistochemistry (IHC) experiments. Sections were placed in ceramic wells and remained free floating until the completion of the IHC procedure. Sections were initially washed three times in TBS (0.5M pH 7.4 + 0.05% Tween) for 5 minutes each followed by being placed in a 5% goat serum PBS (0.3% Tween) solution with primary antibodies directed against pCREB and microtubule associated protein (MAP-2). Sections were incubated with the primary antibody overnight at room temperature. Sections were then rinsed 3 times in an excess of TBS (0.5M pH 7.4 + 0.05% Tween) with gentle rotation. Following the wash, sections were incubated at room temperature for 45 minutes with biotinylated secondary antibodies (Elite Vectastain ABC kit, Burlingame, CA). Sections were then rinsed again 3 times for 5 minutes with TBS. Sections were then incubated for 45 minutes at room temperature in ABC reaction (Elite Vectastain ABC kit, Burlingame, CA) followed by another 3 washes in TBS for five minutes each. The sections were then given a ten minute incubation in TSA solution (TSA fluorescent system 1:100 dilution, Perkin-Elmer, Boston, MA). Sections were then rinsed 3 times for 10 minutes in PBS and mounted on slides for imaging.
Confocal images were collected of the dentate gyrus utilizing an Olympus Fluoview microscope system with a 20× objective. Images were scanned utilizing the Fluoview programming application (Olympus of America). The images collected represent scanning lasers sensitive to 488 nm consistent with the secondary fluorescent antibodies utilized.
A second series of sections were treated in an identical fashion, however, following the ABC reaction sections were incubate for 4 minutes in a filtered solution of PBS and 3, 3’- Diaminobenzidine tetrahydro-chloride dihydrate (DAB, Aldrich, St Louis MO). Sections were immediately rinsed 3 times in PBS and mounted on slides. The DAB treated sections were imaged and captured with a digital camera (Axiovision software; Axiocam, Zeiss).
Electrophysiology
Animals and surgical preparation
Male Sprague-Dawley rats (250–400 g) were anesthetized with halothane (3.0–4.0%), tracheotomized, and placed into a stereotaxic apparatus. Halothane anesthesia was adjusted to 0.9–1.1 % upon completion of surgery and maintained at that level throughout the duration of the experiment. Body temperature was maintained at 37.0 ± 0.5 °C by a feedback regulated heating pad. All care and procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The skull was exposed and holes were drilled to accommodate placement of stimulating and recording electrodes. The dura was opened over recording and stimulation sites to prevent breakage of micropipettes and to mitigate brain trauma. Halothane anesthesia was maintained at 1% following surgery.
Extracellular recordings
Evoked field-potentials were recorded with single 3.0 M NaCl filled micropipettes (6–11 mΩ; 1–2 µm i.d.) stereotaxically placed into the cellular region of the dentate gyrus (coordinates: 4.0 mm posterior and 2.5 mm lateral relative to bregma, 2.6–3.6 mm ventral from dura) [Paxinos and Watson, 1986]. Field potentials were preamplified with an Axon Instruments Multiclamp 700A amplifier and displayed on a Tektronix (Beaverton, OR) digital oscilloscope, filtered (0.1–10 kHz (−3dB)), digitized (20 kHz and 12-bit resolution) and processed on- and off-line by customized National Instruments LabVIEW software on MacIntosh computers. Hippocampal electroencephalographic (EEG) and evoked field potential at 5,000× and 100× and filtered at 0.1–50 Hz and 0.1 Hz–10 kHz (−3 dB), respectively. Extracellular potentials were digitized with National Instruments (Austin, TX) NB-MIO-16 data acquisition boards on MacIntosh computers at 200 Hz for EEG activity and 20 kHz for evoked responses at 12-bit resolution. Extracellularly recorded action potentials were discriminated with a World Precision Instruments WP-121 Spike Discriminator (Sarasota, FL) and converted to computer-level pulses. Discriminated spikes and stimulation events were also captured by National Instruments NB-MIO-16 data acquisition boards in Macintosh computers.
Stimulation
Square-wave constant current pulses (0.3–1.5 mA; 0.15 ms duration; average frequency, 0.1 Hz) were generated by a Grass PSIU6 isolation unit controlled by a MASTER 8 Pulse Generator. Field-potentials were elicited in the DG by stimulation of the perforant path with insulated, bipolar stainless steel (130 µm) electrodes located in the angular bundle (coordinates: 8.1 mm AP, 4.2 mm L, 3.0 mm V) [Paxinos and Watson, 1986]. Recordings made from the cellular levels of the dentate yielded a Population spike (PS) superimposed on the positive-polarity pEPSP/pIPSP complex. PS amplitudes were determined by a median filter and peak detection algorithm. Stimulus/response curves were generated before and 5 min after drug treatment at selected afferent stimulus levels: threshold, 50% maximum and maximum PS amplitude. Paired-pulse curves were generated by testing various intervals (10–160 ms) of paired stimuli applied to the perforant path at 50% maximum PS amplitude. To induce long-term potentiation (LTP) we delivered a high frequency stimulation (HFS) that consisted of ten trains of stimuli (each consisting of five pulses at 400 Hz). Stimulus pulses intensity was adjusted to give 40–50% maximal PS amplitude every 30 seconds. A baseline recording for 10 minutes was followed by HFS delivery and 60 minutes post HFS.
Drug administration
A stainless steel guide cannula (14 mm length, 24 ga) was implanted aseptically into the right lateral ventricle (coordinates: 0.5 mm posterior, 1.0 mm lateral to bregma, and 3.5 mm ventral from dura) [Paxinos and Watson, 1986]. After a completion of stimulus-response curve at three response levels: threshold, 50% maximum and maximum PS amplitude and paired-pulse curves, baseline recording was conducted for 20 minutes, then rats were administered one of the following drug treatments: A) 0.9% physiological saline, 3µl n=5. B) Rat galanin 1–29 (3 nmol/3 µl), n=5 or C) Rat galanin 2–11 (3 nmol/3 µl) n=5. Microinjections were performed with a 10 µl Hamilton syringe connected via Becton Dickinson polyethylene tubing (PE20) to a 1.5 cm injector. The infusion of 3 µl over 1.5 minutes, with each 1 µl separated by ten seconds, and an additional sixty seconds before the injector was withdrawn from the guide cannula. After 5 minutes a second completion of stimulus-response curve at three response levels of PS amplitude and paired-pulse curves were conducted followed by 10 minutes baseline recording and then HFS was delivered into the perforant path. Field potentials were recorder for additional 60 minutes after HFS. In addition a total of twelve (4 saline, 4 galanin 1–29 and 4 galanin 2–11 treated rats) animals were euthanized 30 minutes following the tetanization in order to remove the hippcampi and perform westerns for CREB and pCREB.
Statistical Analysis
All data were analyzed utilizing the Sigmastat statistical application (Chicago, IL). Behavioral data were analyzed via Repeated Measures Analysis of Variance (RMANOVA) and Tukey post-hoc comparisons were utilized where appropriate. Data from the Western Blots and the electrophysiology experiments were analyzed by Analysis of Variance (ANOVA) and Tukey post-hoc comparisons.
Results
Behavior
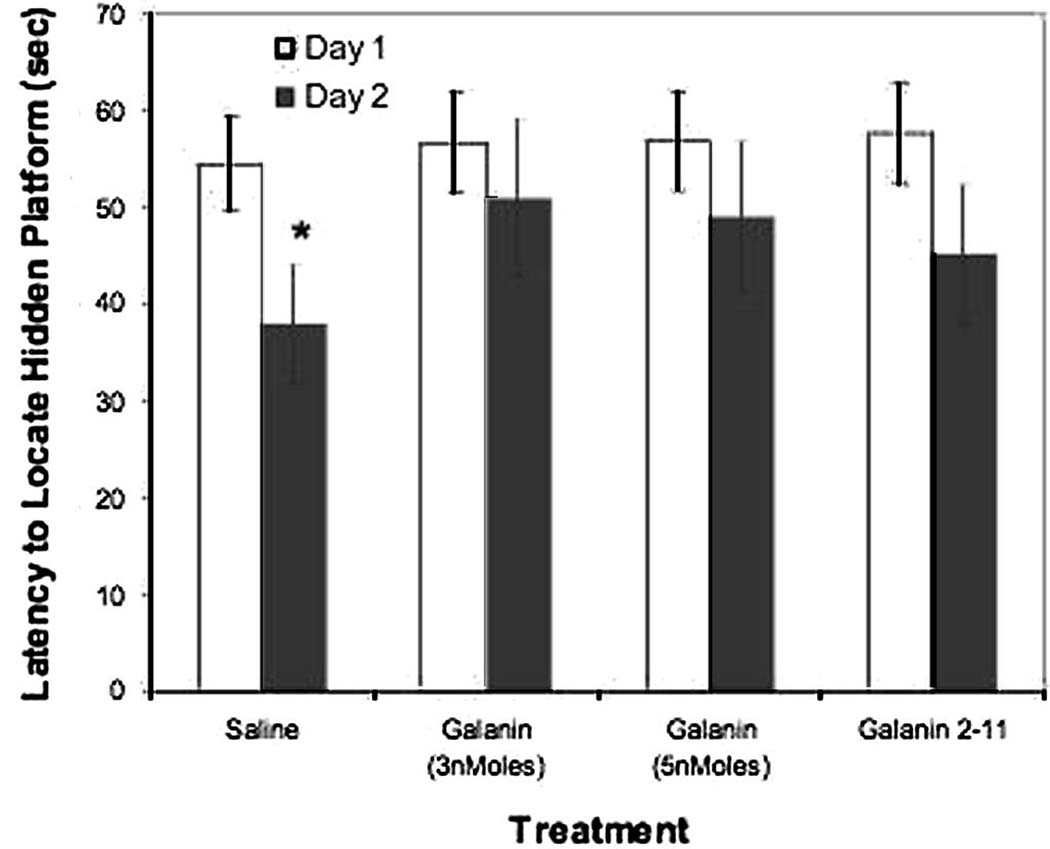
Rats trained in the Morris water maze for two days and treated with saline vehicle showed a significant improvement in the latency to locate the hidden platform across training days, whereas rats treated with 3 nmol/3µl or 5 nmol/3µl galanin1–29 (activating GalR1 and GalR2) or galanin 2–11 3 nmol/3µl (activating preferentially GalR2) displayed no improvement across training days (see Figure 1). Tukey post-hoc comparisons revealed there were no significant differences between day one and day two in groups treated with galanin 1–29 3nmol/3µl (p=0.34) 5 nmol/3µl (p=0.28), and galanin 2–11 (p=0.13). Rats injected with saline displayed a significant reduction in escape latency between training days (p=0.02).
Figure 1.
Latency to locate the hidden platform over two successive training days. Each training day consisted of four trials and the mean latency of all trials is displayed. Galanin or saline vehicle (n=10 per group) was administered ICV to rats, 30 minutes after the start of the training trials, consistent with previous findings that galanin impairs consolidation (Kinney et al., 2003). Significant effects were detected for treatment (F(3,20) = 4.46 p=0.015), training day (F(1,20) = 66.81 p<0.01), and the treatment × day interaction (F(3,20) = 3.47 p=0.035). Subjects administered saline displayed significantly lower latencies to locate the hidden platform (* p<0.05) between days one and two. No significant differences were observed for rats treated with galanin 1–29 or galanin 2–11, indicating no significant improvement in performance across the two training days.
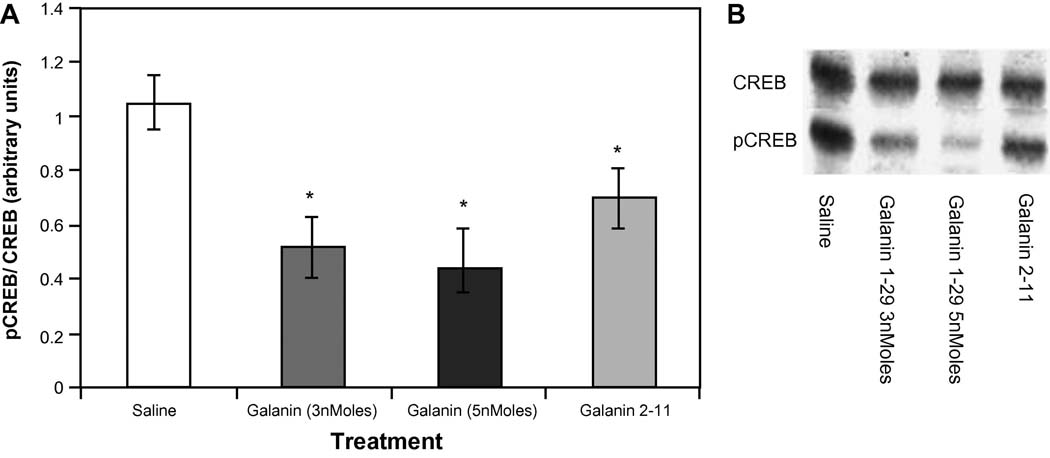
Training in the Morris water maze task induced an increase in the levels of CREB phosphorylation compared to the non-trained controls (data not shown). As can be seen in Figure 2 ICV (Intracerebroventricular) administration of galanin receptor agonists: galanin 1–29 or galanin 2–11 significantly attenuated this effect, as determined by the ratio obtained for pCREB/CREB for each group compared to the ratio attained in the vehicle trained group (F(3,12)= 3.629 p<0.023) (Figure 2). Post-hoc comparisons revealed a reduction in CREB phosphorylation following the infusion of galanin 1–29 (3 and 5 nmol/3µl (p=0.015 and p=0.011, respectively) and galanin 2–11 (p=0.024) compared to saline controls. The data indicate that galanin administration reduced the extent of CREB phosphorylation by 30%. No differences in the phosphorylation state of ERKs (F(3,12)= 3.629 p=0.32) were observed following the administration of any of the galanin receptor ligands to animals trained in the Morris water maze (data not shown).
Figure 2.
A) pCREB/CREB ratios from hippocampi taken from each of the behaviorally tested groups (n=5 per group) administered saline or galanin receptor ligands as well as the non-trained control group. Significant reductions in CREB phosphorylation were obtained for groups administered galanin 1–29 (3nmoles and 5nmoles) and galanin 2–11 compared to saline controls (*p<.05 versus saline treatment group). All treatment groups exhibited a significant increase in CREB phosphorylation compared to the non-trained group (#p<.05 versus all treatment groups). B) Representative Western Blot images for each of the treatment groups.
In-vivo Electrophysiology
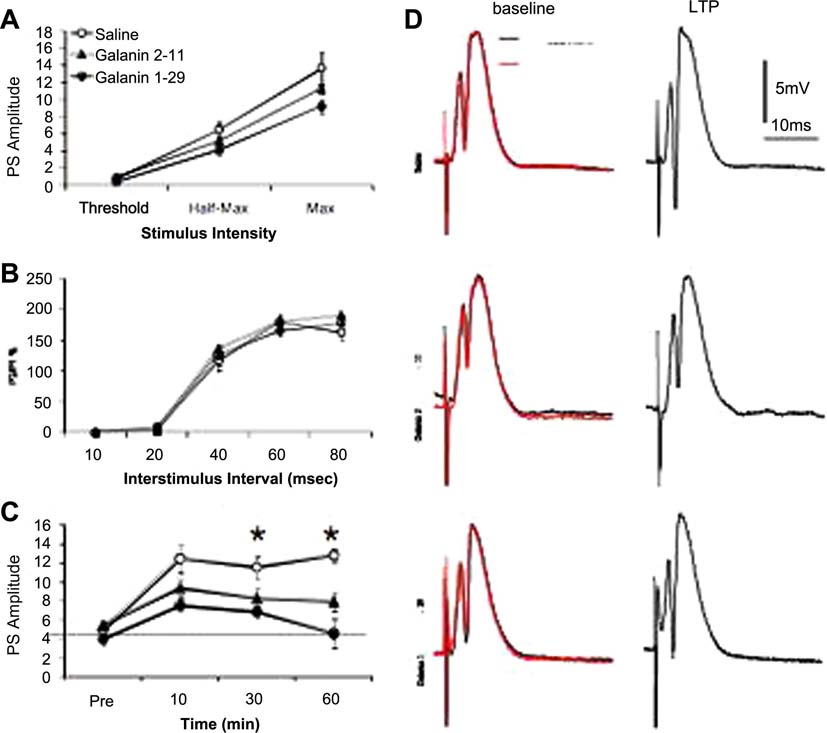
We recorded extracellular neuronal field potentials in the dentate gyrus (DG) in-vivo. Stimulation of the perforant path evoked field potentials in the stratum granulosum whose waveforms were characterized by a relatively fast negative-going population spike (PS) superimposed on the positive-going field EPSP/IPSP complex (3D). The PS amplitude was monotonically related to stimulus intensity tested at three response levels: threshold, half-maximum and maximum (Figures 3A and 3A’). Galanin 2–11 and galanin 1–29 had no effect on stimulus-response curves at any stimulus strength before and after ICV treatment (Figure 3A and 3A’). Galanin 1–29 caused a moderate reduction in the amplitude of PS recorded in the dentate gyrus following a single test stimulus at half-maximal or maximal intensities. Although galanin 1–29 caused a slight reduction in PS amplitudes, the level of attenuation was not statistically significant (P>0.1, n=6). Neither did infusion of the GalR2 agonist; galanin 2–11 modified the PS amplitude responses at the various stimulus intensities after ICV treatment. These inhibitory synaptic changes most likely reflect the hyperpolarizing effect of galanin (Pieribone et al., 1995) mediated by opening of K+-channels (reviewed in Bartfai, 1995). It is important to point out that although there were differences in amplitude no statistical differences were observed. To characterize the effects of galanin on presynaptic function in the DG region of the hippocampus in-vivo we used the paired-pulse (PP) paradigm to measure short-term facilitation. We used a PP stimulation protocol with interstimulus intervals (ITIs) ranging from 10 to 80 ms. Paired equipotent stimulation of the perforant path (50–70% maximum field response amplitude) resulted in interval-dependent response profiles. PP responses in the DG were characterized by a period of PP inhibition (PPI) during the initial 20 msec and a period of PP facilitation (PPF), a form of short-term plasticity (Zucker, 1989) from 40 to 60 ms. Compared to saline treated rats, application of neither galanin 1–29 nor galanin 2–11 induced a significant change in the PP response at different time intervals (10–80ms) (Figure 3B and 3B’; p>0.05) before and after ICV treatment. These data suggest that galanin has no effect on presynaptic function in the DG.
Figure 3.
Effects of galanin on dentate physiology in-vivo. (A) No significant differences in the PS amplitudes across stimulus levels was found; (B) Saline, galanin 2–11 or galanin 1–29 had no significant effect on PP responses; (C) High-frequency afferent stimulation (HFS) resulted in prolonged synaptic enhancement of PS amplitudes in the DG in saline-treated (□), but not in galanin 2–11-treated (▲) or galanin 1–29-treated (●) rats at 30 min (F(2,21)=3.55, p=0.0.046; n=6–10) and 60 min (F(2,21)=3.94, p=0.404; post HFS (saline vs galanin 2–11 and galanin 1–29). Pre-HFS values were not different between groups (F(2,21)=0.39, p=0.67; n=6–10) and at 10 min post HFS (F(2,21)=2.62, p=0.0.096; n=6–10), 60 min (F(2,21)=3.94, p=0.404. Each group represents the mean ± s.e.m. *, p < 0.05 (ANOVA). (D) Representative samples waveforms were characterized by a relatively fast negative-going population spike (PS) superimposed on the positive-going field EPSP/IPSP complex. At baseline, superimposed recording during pretreatment (black) and post treatment (red) show no differences in the amplitude of the PS in galanin 2–11 and galanin 1–29 treated rats. Samples of recordings at 60 minutes after HFS indicate a significant enhancement only in the control group whereas galanin 2–11 and galanin 1–29 impaired LTP maintenance.
We then determined whether galanin affects LTP induction in the DG in-vivo (Figure 3C). High frequency stimulation (10 trains of 5 pulses at 400 Hz) in saline-treated rats elicited a large potentiation of PS amplitudes that lasted for 60 minutes (Figure 3C and 3D; p>0.05). In contrast, galanin 2–11 and galanin1–29 significantly attenuated LTP induction (Figure 3C; p < 0.05; Figure 3D). The level of attenuation was higher in the group treated with galanin 1–29 although the PS amplitude remained statistically significant above the baseline level at certain time points (1–13 min pos HFS, P<0.01, figure 3C) returning to baseline by 40 minutes.
Administration of the GalR2 agonist, galanin 2–11 attenuated the HFS-mediated LTP in DG (figure 3C). The post-tetanic potentiation was significantly (P<0.05) above the baseline level but reduced compared to saline control. This level of potentiation was maintained during 30 minutes and after that the level of potentiation reached the lowest level however still above baseline (figure 3C and 3D).
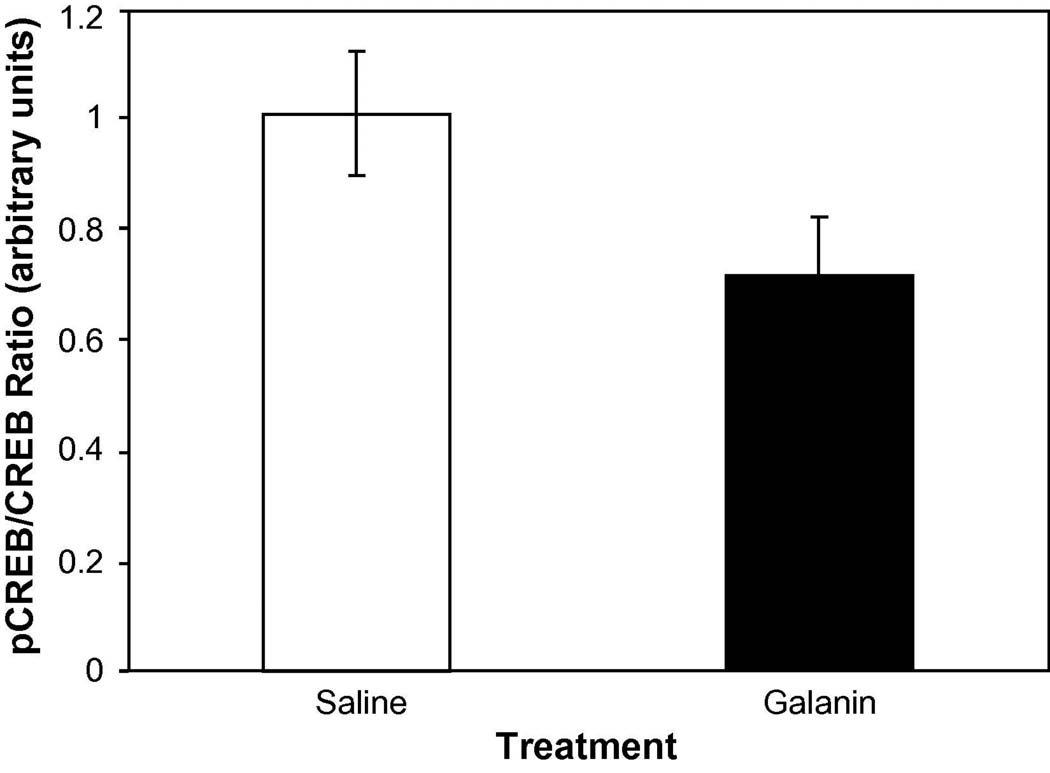
In-vivo LTP induces phosphorylation of CREB in the hippocampus similarly to behavioral training. We examined the phosphorylation of CREB using Western blots, similarly as for the behaviorally tested rats. Data from the Western Blots indicated that the administration of galanin 1–29 prior to induction of LTP produced a significant (F(1,6) = 9.58 p=0.021) reduction in the phosphorylated form of CREB (Figure 4).
Figure 4.
pCREB/CREB ratios for hippocampi from subjects administered saline (n=4) or galanin 1–29 (3nmoles/3µl; n=4) prior to the induction of LTP. Hippocampi were dissected out 30 minutes following the induction of LTP and westerns were performed in the hippocampus that recordings were taken from. A significant reduction in CREB phosphorylation was obtained between treatments (* p<.05).
Immunohistochemistry
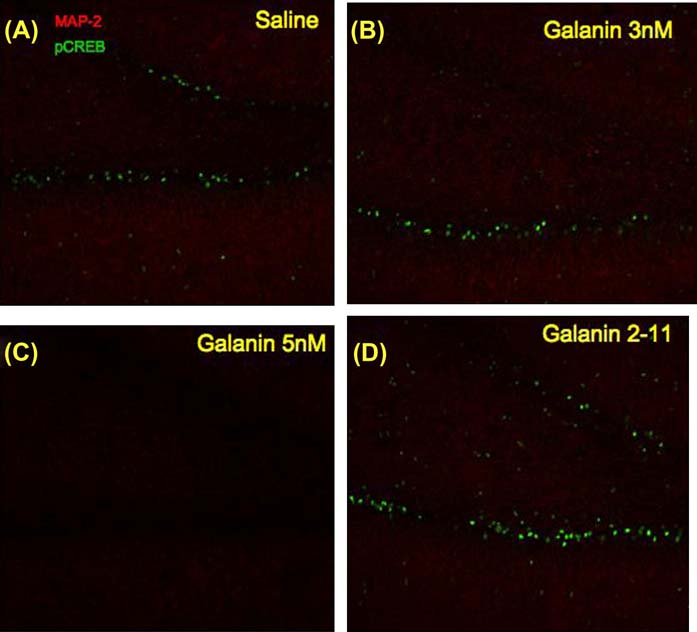
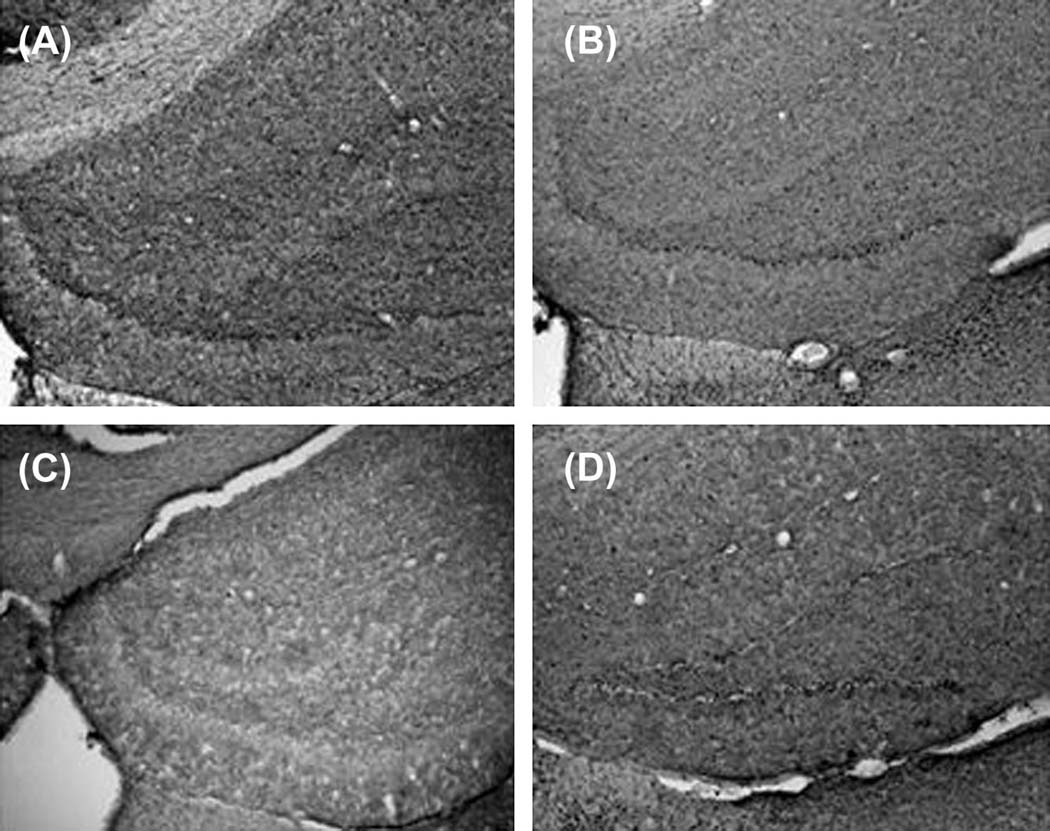
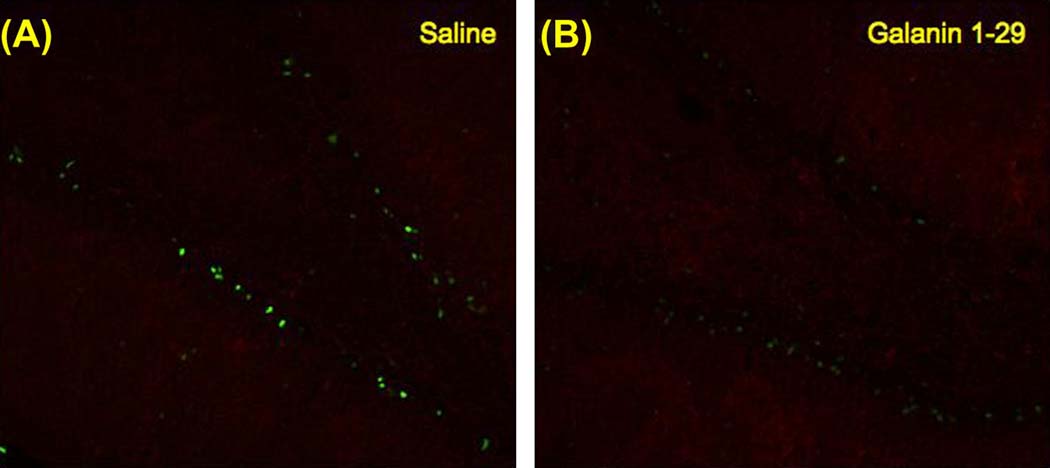
Hippocampal sections from the behaviorally tested groups in the Morris water maze task were processed for immunohistochemistry (IHC) and imaged to verify the changes observed in the Western Blots. A higher training-induced pCREB immunoreactivity was observed in the dentate gyrus of saline treated rats (Figure 5A) compared to subjects treated with galanin 1–29 at 5 nmol/3µl concentration (Figure 5C). The IHC failed to detect a large change in pCREB levels in subjects administered galanin 1–29 at 3 nmol/3µl (Figure 5B) or following galanin 2–11 (3 nmol/3µl) administration (Figure 5D). In addition, DAB stained sections displayed a similar profile of increased pCREB immunoreactivity in the subjects administered saline versus any of the remaining treatment groups (Figure 6). Sections processed in an identical fashion taken from the in-vivo LTP studies indicated higher levels of CREB phosphorylation in the DG taken from subjects administered saline (Figure 7A) versus those administered galanin 1–29 (3 nmol/3µl) (Figure 7B).
Figure 5.
Representative images from the IHC experiments for each of the behaviorally tested groups (tissue examined from 5 subjects per group consistently displayed differences depicted in above representative images). Images were captured of the dentate gyrus at 20× magnification utilizing antibodies directed against pCREB (488nm) and MAP-2 (594nm) for rats treated with A) saline, B) galanin 1–29 3nMoles/3µl, C) galanin 1–29 5nMoles/3µl, and D) galanin 2–11. Sections from the rats treated with galanin 1–29 5nMoles/3µl displayed a large reduction in pCREB immunoreactivity.
Figure 6.
Representative photomicrographs of rat hippocampal sections stained for phospho-CREB using DAB, taken from animals exposed to Morris water maze and treated 30 min after training with saline (A), galanin 1–29 3nmols/3µl (B), galanin 1–29 5nmols/3µl galanin (C), and galanin 2–11 3nmol/3µl (D). Sections from the saline treated grouped evinced more intense staining throughout the dorsal hippocampus and a greater number of immunoreactive cell bodies in the dentate gyrus and CA3/CA1 region (tissue examined from 5 subjects per group consistently displayed differences depicted in above representative images).
Figure 7.
Representative images of pCREB and MAP-2 immunoreactivity in the dentate gyrus of rats treated with A) saline or B) galanin 1–29 30 minutes after the induction of in-vivo LTP. Sections collected from rats administered saline prior to induction of LTP displayed greater intensity of pCREB compared to galanin administered rats.
Discussion
The data in the present study provides the first documentation of a specific molecular mechanism by which galanin may affect spatial learning processes in-vivo. Galanin has been shown to mediate a variety of neurological functions relevant to synaptic plasticity and learning and memory (Wrenn and Crawley, 2001). Previous studies demonstrated that galanin impairs spatial learning and memory (Kinney et al., 2003) as well as in-vitro LTP (Sakurai et al., 1996). However, no previous study demonstrated downstream targets of galanin receptor activation that may produce these cognitive deficits in intact organisms. Further, the data from the current project provide support for a mechanism by which galanin impairs consolidation in agreement with previous studies (Kinney et al., 2003) in which forskolin (non-selective adenylate cyclase activator) was able to rescue the galanin induced deficit. The data in the current study demonstrate that galanin receptor agonists administered following training trials in a spatial learning and memory task cause a spatial learning and memory deficit, consistent with previous data, as well as significantly reduce CREB phosphorylation within the hippocampus. These findings connect much of the in-vivo data on galanin effects in rodents with data on the role of CREB phosphorylation in cognition (cf Viola et. al., 2000 for review).
Galanin receptor activation has previously been shown to inhibit adenylyl cyclase activity via G-protein coupled GalR1 type galanin receptors (cf Kinney et. al., 2003). In-vitro studies demonstrated that GALR1 activation results in a decrease in adenylate cyclase activation (Karelson and Langel, 1998), and thus a likely reduction in cAMP-dependent protein kinase A (PKA) activation and downstream phosphorylation of CREB. Several studies have demonstrated that inhibition or elimination of second messengers that stimulate CREB phosphorylation results in dramatic deficits in spatial learning and memory (Abel et al., 1997; Rotenberg et al., 2000), as well as other associative forms of memory (Goosens et al., 2000).
We found no galanin effects on the phosphorylation state of another key integrator of intracellular signaling, ERKs. This could be due to the time course of ERK activation, the schedule of drug administration, and collection of tissue in the present study. Any rapid alteration in the phosphorylation state of ERKs produced by galanin may have been missed, since we scheduled the administration of galanin and the collection of tissue to observe changes in pCREB, which has been shown to require 30 minutes to reach peak activation (Buxbaum and Dudai, 1989; Bourtchouladze et al., 1998). It is likely that any changes in the phosphorylation state of ERKs due to behavioral training and/or due to galanin receptor activation would have returned to baseline well before the tissue was collected.
Our data also indicate that galanin impairs in-vivo LTP. These findings are consistent with the observed memory impairment in-vivo as well as with the previous reports on galanin impairing in-vitro LTP in hippocampal slices. These studies have established that galanin impairs both CA1/CA3 LTP (Coumis and Davies, 2002) and DG LTP (Badieh et. al., 2003) in hippocampal slices. The in-vivo data presented here indicate that the ICV administration of galanin 1–29 or galanin 2–11 produced an important deficit in the induction of LTP. It is particularly interesting to note that the in-vivo paired-pulse facilitation experiments do not indicate a galanin induced deficit suggesting that the impairment observed in LTP is not a result of presynaptic effects of galanin. The activity of dentate granule cells is modified, in part, by local circuit GABAergic interneurons within the dentate subfield and extrahippocampal inputs from medial septum and brainstem structures, including the locus coeruleus and median raphe (Bronzino et al 1997; Bekenstein and Lothman, 1991). Stimulation of the perforant path releases glutamate, which simultaneously activates the dentate granule cells and the basket cell interneurons (feed forward inhibition). Depolarization of granule cells activates the mossy fibers and the axon collaterals, which in turn activate the basket cell interneurons again (feedback or recurrent inhibition) (Bronzino et al 1997). The paired-pulse index is used as an effective measure of the modulation of hippocampal dentate granule cells (DiScenna and Teyler, 1994). The regulation of excitatory synaptic drive onto GABAergic interneurons is essential for GABAergic inhibition onto granule cells. Postsynaptic GABAA receptors reportedly produce fast synaptic inhibition (Stephenson, 1988), while presynaptic GABAB receptors are highly sensitive in controlling endogenous transmitter release (Bowery, 1993). Glutamatergic postsynaptic responses and collaterally evokes GABA release from the GABA interneuron, which acts on GABAA receptors for at least 25–50 ms, but not for more than 100 ms for the feed forward inhibition (Kawashima et al., 2006; Takita et al., 2007) and either galanin 1–29 or galanin 2–11 does not affect presynaptic modulation (figure 3B and 3B’). Rather, the reduction in potentiation observed as well as the impaired maintenance of the potentiation may be attributable to the reduction in CREB phosphorylation.
Results from the Western Blots of hippocampal tissue from the in-vivo electrophysiology experiments further support this interpretation, as the amount of phospho-CREB in the hippocampi of galanin treated rats was reduced compared to saline controls.
Our data establish an important reduction in intracellular signaling involving pCREB, due to galanin receptor (GalR1 and GalR2) activation in-vivo. It is interesting to speculate on the biological importance of endogenous galanin altering CREB phosphorylation. Several neuronal functions rely on CREB-dependent transcription and it is surprising that a single neuropeptide can have such a dramatic effect. The establishment of a molecular link in a mammalian system of learning and memory between impairment of performance and occupancy of neuropeptide receptor provides a pharmacological target in the galaninergic system for modification of cognitive performance.
The selectivity of galanin 2–11 to bind to GalR2 with substantially higher affinity than to GalR1 has been evaluated by studying [125I]-galanin (1–29) displacement from GalR1 or GalR2 expressing cell lines (Mazarati et al., 2004) or GalR3 in transfected cell lines (Lu et al., 2005), however the affinity of galanin 2–11 for GalR3 is higher than for GalR1. Similarly to galanin 2–11, galanin 1–29 has a high affinity to GalR3 to the same degree as its affinity to GalR2 (Branchek et al 1998 and Branchek et al 2000). Based on Badie-Mahdavi et al (2005) study and since the level of GalR3 is very low in mice hippocampal formation, we consider the effect of galanin 2–11 to be through activation of GalR2. Therefore, the GalR1 and GalR2 are present at nearly the same concentrations in the DG (Badie-Mahdavi, 2005) and results must be interpreted by taking into account the contributions of both GalR types (GalR1 and GalR2) as galanin 1–29 has similar affinity for them.
This is the first in vivo study that investigates the effect of the activation of the GalR types, GalR1 and GalR2, on synaptic transmission and plasticity in DG. Galanin 1–29, a mixed GalR1/GalR2 agonist, significantly attenuated LTP induction in DG (at 51 min post-HFTs stimulation) compared with saline-treated rats. The data on GalR2 agonist, galanin 2–11, are the first to examine the contribution of GalR2 activation to hippocampal synaptic plasticity without the simultaneous activation of GalR1, as is the case in studies using galanin 1–29. Application of galanin 2–11 produced an attenuation of LTP induction, however less robust and durable compared with galanin 1–29 treated rats, which is the opposite in Badie-Mahdavi, 2005 study where 2–11 produced higher attenuation of LTP induction compared to galanin 1–29 and difference could be explained because Badie-Mahdavi used slices rather than the complete brain. The present study seems to suggest that the effect of GalR2 activation is less effective in attenuating LTP induction and maintenance than simultaneous activation of GalR1 and GalR2 by galanin 1–29 although the effects of GalR1 activation alone on DG synaptic plasticity are difficult to examine pharmacologically due to lack of a GalR1 selective agonist or antagonist.
Given that galanin was not administered in the behavioral experiments until 30 m post-training, and for in vivo electrophysiology experiments galanin was chose to be administered prior to LTP induction was based on; 1) the assumption that galanin is a selective drug for plasticity and not for normal information processing in the brain, and that galanin would knock out a critical step on plasticity and 2) Badie-Mahdavi et al (2005) demonstrated that in slices superfusion of Galanin 2–11 or Galanin 1–29 at 20 min post-LTP induction caused a transient reduction in the level of LTP however the level pf potentiation was partially restored after superfusion. The first assumption was accepted based on demonstration of selective effects of drugs such as AP5 (n- 2-amino- 5-phosphonopentanoate) that block the NMDA receptor, preventing LTP while sparing normal hippocampal synaptic transmission (e.g., Bliss and Lynch 1988). To the extent that the role of the NMDA receptor is fully selective for plasticity, one could predict these drugs could prevent new learning and still not affect non learning performance or retention of learning normally accomplished before drug treatment. Consistent with these predictions, some of the earliest and strongest evidence supporting a connection between LTP and memory came from demonstrations that a drug-induced blockade of NMDA receptors prevents new learning (Morris et al. 1986). In addition drugs that enhance the induction of NMDA-dependent LTP also facilitate learning and memory (Staubli et al. 1994; Mondadori et al. 1989; Weiskrantz and Mondadori 1991).
Induction of LTP in DG is predominantly a post-synaptic phenomenon (Lynch, 2004). Increase in the intracellular Ca2+ through activation of NMDA receptor is the main mechanism underlying the DG LTP initiation. The expression and maintenance of LTP, however, has been linked to several signaling events, such as activation of CaMKII, cAMP-dependent protein kinases as well as synaptic modification. In this study we investigated the contribution of hippocampal GalR1 and/or GalR2 to regulation of the level of phosphorylation of CREB during Galanin 2–11 or galanin 1–29-induced attenuation of LTP. These data indicate that activation of GalR1 and/or GalR2-mediated attenuation of LTP in DG could be occurring through reduction of pCREB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus- based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Research Brain Research Review. 1998;26:360–378. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- Badie-Mahdavi H, Lu X, Behrens MM, Tallent M, Bartfai T. Society for Neuroscience Annual Meeting, poster #255.15. New Orleans, LA: 2003. GALR1 or GALR2 activation attenuates the level of LTP in dorsal CA1 region of hippocampus: Possible involvement of CREB-P. [Google Scholar]
- Badie-Mahdavi H, Lu X, Behrens MM, Bartfai T. Role of galanin receptor 1 and galanin receptor 2 activation in synaptic plasticity associated with 3',5'-cyclic AMP response element-binding protein phosphorylation in the dentate gyrus: studies with a galanin receptor 2 agonist and galanin receptor 1 knockout mice. Neuroscience. 2005;133(2):591–604. doi: 10.1016/j.neuroscience.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Bartfai T. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press Ltd.; 1995. A neuropeptide with important central nervous system actions (chapter 50) [Google Scholar]
- Beal MF, MacGarvey U, Swartz KJ. Galanin immunoreactivity is increased in the nucleus basalis of Meynert in Alzheimer's disease. Annals of Neurology. 1990;28:157–161. doi: 10.1002/ana.410280207. [DOI] [PubMed] [Google Scholar]
- Bekenstein JW, Lothman EW. Electrophysiological characterization of associational pathway terminating on dentate gyrus granule cells in the rat. Hippocampus. 1991 Oct;1(4):399–404. doi: 10.1002/hipo.450010408. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lynch MA. Long term potentiation of synaptic transmission in the hippocampus: Properties and mechanisms. In: Landfield PW, Deadwyler SA, editors. Long term potentiation: From biophysics to behavior. New York, NY: Alan R. Liss; 1988. pp. 3–12. [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learning & Memory. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptor pharmacology. Annu. Rev. Pharmacol. Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- Bowser R, Kordower JH, Mufson EJ. A confocal microscopic analysis of galaninergic hyperinnervation of cholinergic basal forebrain neurons in Alzheimer's disease. Brain Pathology. 1997;7:723–730. doi: 10.1111/j.1750-3639.1997.tb01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchek T, Smith KE, Walker MW. Molecular biology and pharmacology of galanin receptors. Ann N Y Acad Sci. 1998;863:94–107. doi: 10.1111/j.1749-6632.1998.tb10687.x. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Bronzino JD, Blaise JH, Morgane PJ. The paired-pulse index: a measure of hippocampal dentate granule cell modulation. Ann Biomed Eng. 1997 Sep-Oct;25(5):870–873. doi: 10.1007/BF02684171. [DOI] [PubMed] [Google Scholar]
- Burazin TC, Larm JA, Ryan MC, Gundlach AL. Galanin-R1 and -R2 receptor mRNA expression during the development of rat brain suggests differential subtype involvement in synaptic transmission and plasticity. Eur J Neurosci. 2000;12(8):2901–2917. doi: 10.1046/j.1460-9568.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Dudai Y. A quantitative model for the kinetics of cAMP-dependent protein kinase (type II) activity. Long-term activation of the kinase and its possible relevance to learning and memory. Biological Chemistry. 1989;264:9344–9351. [PubMed] [Google Scholar]
- Chen Y, Laburthe M, Amiranoff B. Galanin inhibits adenylate cyclase of rat brain membranes. Peptides. 1992;13:339–341. doi: 10.1016/0196-9781(92)90118-m. [DOI] [PubMed] [Google Scholar]
- Coumis U, Davies CH. The effects of galanin on long-term synaptic plasticity in the CA1 area of rodent hippocampus. Neuroscience. 2002;112(1):173–182. doi: 10.1016/s0306-4522(02)00007-6. [DOI] [PubMed] [Google Scholar]
- DiScenna PG, Teyler TJ. Development of inhibitory and excitatory synaptic transmission in the rat dentate gyrus. Hippocampus. 1994 Oct;4(5):569–576. doi: 10.1002/hipo.450040506. [DOI] [PubMed] [Google Scholar]
- Givens BS, Olton DS, Crawley JN. Galanin in the medial septal area impairs working memory. Brain Research. 1992;582:71–77. doi: 10.1016/0006-8993(92)90318-4. [DOI] [PubMed] [Google Scholar]
- Gleason TC, Dreiling JL, Crawley JN. Rat strain differences in response to galanin on the Morris water task. Neuropeptides. 1999;33:265–270. doi: 10.1054/npep.1999.0044. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Holt W, Maren S. A role for amygdaloid PKA and PKC in the acquisition of long-term conditional fear memories in rats. Behavioral Brain Research. 2000;114:145–152. doi: 10.1016/s0166-4328(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Iismaa TP, Shine J. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Results and Problems in Cell Differentiation. 1999;26:257–291. doi: 10.1007/978-3-540-49421-8_12. [DOI] [PubMed] [Google Scholar]
- Karelson E, Langel Ü. Galaninergic signaling and adenylate cyclase. Neuropeptides. 1998;32:197–210. doi: 10.1016/s0143-4179(98)90038-5. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Izaki Y, Grace AA, Takita M. Cooperativity between hippocampal–prefrontal short-term plasticity through associative long-term potentiation. Brain Res. 2006;1109:37–44. doi: 10.1016/j.brainres.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Emmerson PJ, Miller RJ. Galanin receptor-mediated inhibition of glutamate release in the arcuate nucleus of the hypothalamus. Journal of Neuroscience. 1998;18:3489–3500. doi: 10.1523/JNEUROSCI.18-10-03489.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Starosta G, Holmes A, Wrenn CC, Yang RJ, Harris AP, Long KC, Crawley JN. Deficits in trace cued fear conditioning in galanin-treated rats and galanin-overexpressing transgenic mice. Learn Mem. 2002;9(4):178–190. doi: 10.1101/m.49502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Starosta G, Crawley JN. Central Galanin Administration Blocks Consolidation of Spatial Learning. Neurobiology of Learning and Memory. 2003;80:42–54. doi: 10.1016/s1074-7427(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu X, Lundström L, Bartfai T. Galanin (2–11) binds to GalR3 in transfected cell lines: limitations for pharmacological definition of receptor subtypes. Neuropeptides. 2005 Jun;39(3):165–167. doi: 10.1016/j.npep.2004.12.013. Epub 2005 Feb 1. [DOI] [PubMed] [Google Scholar]
- Lynch M. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, Wynick D, Wasterlain CG. Modulation of hippocampal excitability and seizures by galanin. J Neuroscience. 2000;20(16):6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Lu X, Kilk K, Langel U, Wasterlain C, Bartfai T. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur J Neurosci. 2004;19:3235–3244. doi: 10.1111/j.0953-816X.2004.03449.x. [DOI] [PubMed] [Google Scholar]
- Melander T, Staines WA, Rokaeus A. Galanin-like immunoreactivity in hippocampal afferents in the rat, with special reference to cholinergic and noradrenergic inputs. Neuroscience. 1986;19(1):223–240. doi: 10.1016/0306-4522(86)90017-5. [DOI] [PubMed] [Google Scholar]
- McDermott AM, Sharp GW. Gi2 and Gi3 proteins mediate the inhibition of adenylyl cyclase by galanin in the RINm5f cell. Diabetes. 1995;44:453–459. doi: 10.2337/diab.44.4.453. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Crawley JN. Galanin receptor antagonist M40 blocks galanin-induced choice accuracy deficits on a delayed nonmatching-to-position task. Behavioral Neuroscience. 1996;110:1025–1032. doi: 10.1037//0735-7044.110.5.1025. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Crawley JN. Galanin-acetylcholine interactions in rodent memory tasks and Alzheimer's disease. Journal of Psychiatry and Neuroscience. 1997;22:303–317. [PMC free article] [PubMed] [Google Scholar]
- McDonald MP, Wenk GL, Crawley JN. Analysis of galanin and the galanin antagonist M40 on delayed non matching to position performance in rats lesioned with the cholinergic immunotoxin 192 IgG-saporin. Behavioral Neuroscience. 1997;111:552–563. doi: 10.1037//0735-7044.111.3.552. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Gleason TC, Robinson JK, Crawley JN. Galanin inhibits performance on rodent memory tasks. Annals of the New York Academy of Sciences. 1998;863:305–322. doi: 10.1111/j.1749-6632.1998.tb10704.x. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long term potentiation by an N-methyI-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mondadori C, Weiskrantz L, Buerki H, Petschke F, Fagg GE. NMDA receptor antagonists can enhance or impair learning performance in animals. Exp. Brain Res. 1989;75:449–456. doi: 10.1007/BF00249896. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Cochran E, Benzing W, Kordower JH. Galaninergic innervation of the cholinergic vertical limb of the diagonal band (Ch2) and bed nucleus of the stria terminalis in aging, Alzheimer's disease and Down's syndrome. Dementia. 1993;4:237–250. doi: 10.1159/000107329. [DOI] [PubMed] [Google Scholar]
- Ögren SO, Kehr J, Schött PA. Effects of ventral hippocampal galanin on spatial learning and on in-vivo acetylcholine release in the rat. Neuroscience. 1996;75:1127–1140. doi: 10.1016/0306-4522(96)00215-1. [DOI] [PubMed] [Google Scholar]
- Ögren SO, Schött PA, Kehr J, Yoshitake T, Misane I, Mannstrom P, Sandin J. Modulation of acetylcholine and serotonin transmission by galanin. Relationship to spatial and aversive learning. Annals of the New York Academy of Sciences. 1998;863:342–363. doi: 10.1111/j.1749-6632.1998.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Ögren SO, Schött PA, Kehr J, Misane I, Razani H. Galanin and learning. Brain Research. 1999;848:174–182. doi: 10.1016/s0006-8993(99)01973-3. [DOI] [PubMed] [Google Scholar]
- Palazzi E, Felinska S, Zambelli M, Fisone G, Bartfai T, Consolo S. Galanin reduces carbachol stimulation of phosphoinositide turnover in rat ventral hippocampus by lowering Ca2+ influx through voltage-sensitive Ca2+ channels. Journal of Neurochemistry. 1991;56:739–747. doi: 10.1111/j.1471-4159.1991.tb01986.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Second Edition. Orlando, FL: Academic Press; 1986. [Google Scholar]
- Pieribone VA, Xu Z, Zhang X, Grillner S, Bartfai T, Hokfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64:861–874. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- Robinson JK, Zocchi A, Pert A, Crawley JN. Galanin microinjected into the medial septum inhibits scopolamine-induced acetylcholine overflow in the rat ventral hippocampus. Brain Research. 1996;709:81–87. doi: 10.1016/0006-8993(95)01307-5. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Abel T, Hawkins RD, Kandel ER, Muller RU. Parallel instabilities of long-term potentiation, place cells, and learning caused by decreased protein kinase A activity. Journal of Neuroscience. 2000;20:8096–8102. doi: 10.1523/JNEUROSCI.20-21-08096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai E, Maeda T, Kaneko S, Akaike A, Satoh M. Galanin inhibits long-term potentiation at Schaffer collateral-CA1 synapses in guinea-pig hippocampal slices. Neurosci Letters. 1996;212(1):21–24. doi: 10.1016/0304-3940(96)12772-5. [DOI] [PubMed] [Google Scholar]
- Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc. Natl. Acad. Sci. 1994;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson FA. Understanding the GABAA receptor: a chemically gated ion channel. Biochem J. 1988 Jan 1;249(1):21–32. doi: 10.1042/bj2490021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takita M, Kuramochi M, Izaki Y, Ohtomi M. In vivo temporal property of GABAergic neural transmission in collateral feed-forward inhibition system of hippocampal-prefrontal pathway. Brain Res. 2007 May 30;1150:69–73. doi: 10.1016/j.brainres.2007.02.063. Epub 2007 Mar 2. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Klann E. Extracellular signal-regulated kinase, synaptic plasticity, and memory. Rev Neurosci. 2001;12(4):327–345. doi: 10.1515/revneuro.2001.12.4.327. [DOI] [PubMed] [Google Scholar]
- Valkna A, Jureus A, Karelson E, Zilmer M, Bartfai T, Langel Ü. Differential regulation of adenylate cyclase activity in rat ventral and dorsal hippocampus by rat galanin. Neuroscience Letters. 1995;187:75–78. doi: 10.1016/0304-3940(95)11340-1. [DOI] [PubMed] [Google Scholar]
- Wang HY, Wild KD, Shank RP, Lee DH. Galanin inhibits acetylcholine release from rat cerebral cortex via a pertussis toxin-sensitive G(i) protein. Neuropeptides. 1999;33:197–205. doi: 10.1054/npep.1999.0024. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Mondadori C. MK-801 can facilitate passive avoidance memory when retention is not present in control animals, and can fail to facilitate when it is present. Psychopharmacology. 1991;105:145–150. doi: 10.1007/BF02244300. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Crawley JN. Pharmacological evidence supporting a role for galanin in cognition and affect. Progress in Neuropsychopharmacology and Biological Psychiatry. 2001;25:283–299. doi: 10.1016/s0278-5846(00)00156-1. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Kansal L, Merriam P, Picciotto MR. Galanin receptor 1 gene expression is regulated by cyclic AMP through a CREB-dependent mechanism. J Neurochem. 2001;76(1):191–200. doi: 10.1046/j.1471-4159.2001.00018.x. [DOI] [PubMed] [Google Scholar]