Abstract
Background
Human Immunodeficiency Virus and Simian Immunodeficiency Virus infections are characterized by a severe loss of Th-17 cells (IL-17+CD4+ T cells) that has been associated with disease progression and systemic dissemination of bacterial infections. Anti-retroviral therapy (ART) has led to repopulation of CD4+ T cells in peripheral tissues with little sustainable repopulation in mucosal tissues. Given the central importance of Th-17 cells in mucosal homeostasis, it is not known if the failure of ART to permanently repopulate mucosal tissues is associated with a failure to restore Th-17 cells that are lost during infection.
Methods
Dynamics of α4+β7hi CD4+ T cells in peripheral blood of SIV infected rhesus macaques were evaluated and compared to animals that were treated with ART. The frequency of Th-17 and Tc-17 cells was determined following infection and after therapy. Relative expression of IL-21, IL-23, and TGFβ was determined using Taqman PCR.
Results
Treatment of SIV infected rhesus macaques with anti-retroviral therapy was associated with a substantial repopulation of mucosal homing α4+β7hi CD4+ T cells in peripheral blood. This repopulation, however, was not accompanied by a restoration of Th-17 responses. Interestingly, SIV infection was associated with an increase in Tc-17 responses (IL-17+CD8+ T cells) suggesting to a skewing in the ratio of Th-17 : Tc-17 cells from a predominantly Th-17 phenotype to a predominantly Tc-17 phenotype. Surprisingly, Tc-17 responses remained high during the course of therapy suggesting that ART failed to correct the imbalance in Th-17 : Tc-17 responses induced following SIV infection.
Conclusions
ART was associated with substantial repopulation of α4+β7hi CD4+ T cells in peripheral blood with little or no rebound of Th-17 cells. On the other hand, repopulation of α4+β7hi CD4+ T cells was accompanied by persistence of high levels of Tc-17 cells in peripheral blood. The dysregulation of Th-17 and Tc-17 responses likely plays a role in disease progression.
Keywords: HIV, SIV, simian, immunodeficiency, Mucosa, CD4, Gut, Intestine, ART, PMPA, FTC, Tenofovir
Introduction
Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections display a significant loss of CD4+ T cells(3, 11, 15, 17, 20, 21, 30) very early during the course of infection. The cells lost are memory cells that are critical for the generation of secondary immune responses against previously encountered pathogens or vaccines. Hence, the severe, and early loss of memory CD4 T cells is thought to play a role in the early onset of immunodeficiency.
Mucosal tissues are a major site for viral replication and dissemination(1, 3, 5, 9-12, 15, 17, 20, 21, 24, 25, 27, 28, 30). Recent studies have shown that the severity of changes in the mucosa during HIV and SIV infections is associated with loss of T-helper-17 (Th-17) cells that are important for maintaining immune homeostasis at these sites(2, 4, 8, 19, 29). Th-17 deficiency has been associated with systemic dissemination of Salmonella(29). We have previously shown(14) that the primary producers of IL-17 were the mucosal homing memory CD4+ T cells that expressed the α4+β7hi phenotype, and these cells were rapidly destroyed after SIV infection skewing the T helper responses towards a Th-1 phenotype. Others have shown that the balance between Th-17 and Th-1 cells in mucosal tissues was significantly altered during infection and was associated with progression of disease(4). Likewise, a number of studies have shown that HIV and SIV infections were associated with loss of Th-17 responses(2, 4, 8, 19, 29).
Little is known about the repopulation of Th-17 responses in patients undergoing highly activated anti-retroviral therapy (HAART). Macal et al(19) showed that restoration of mucosal CD4+ T cells was associated with repopulation of Th-17 cells, whereas others have reported little or no repopulation of Th-17 responses in HIV infected patients undergoing HAART(2). Likewise, not much is known about the role of IL-17+CD8+ T cells (Tc-17) in HIV infection.
The primary objective of these studies were to determine if anti-retroviral therapy (ART) leads to the repopulation of mucosal homing CD4+ T cells in the periphery, and if this repopulation was associated with the restoration of Th-17 responses. To address this question, we evaluated the dynamics of mucosal homing (α4+β7hi) and non-homing (β7−) CD4+ T cells in peripheral blood after SIV infection and compared them to animals undergoing ART. α4+β7hi and β7− CD4+ T cells were delineated based on the expression of β7 and CD95, with both α4+β7hi and β7− CD4+ T cells being CD95+. We have previously shown(14) that mucosal homing CD4+ T cells could be delineated based on the expression of β7 that is primarily coexpressed with the α4 integrin, and costains with the ACT-1 clone that recognizes an epitope on the α4β7 heterodimer. To determine if ART restored Th-17 cells, we evaluated the ability of α4+β7hi and β7− CD4+ T cell subsets to produce IL-17. Our results demonstrate that ART is associated with a substantial repopulation of mucosal homing α4+β7hi CD4+ T cells in the periphery. However, this repopulation was not accompanied by a corresponding restoration of Th-17 responses. Rather, we observed a significant increase in Tc-17 responses that remained persistently high during the course of therapy. Overall, these results suggest that ART fails to effectively restore the Th-17 : Tc-17 imbalance seen during chronic SIV infection.
Materials and Methods
Animals, infection & samples
Rhesus macaques (Macaca mulatta) housed at Advanced Bioscience Laboratories Inc., MD were used in this study. Animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care guidelines and were sero-negative for SIV, simian retrovirus and simian T-cell leukemia virus type-1. All the animals were infected with 100 animal-infectious doses of uncloned pathogenic SIVmac251 intravenously. Four animals were treated continuously with PMPA and FTC daily starting at 13 weeks pi. PMPA and FTC were obtained from Gilead Sciences, Inc. (Foster City, CA). Peripheral blood was collected prior to, and at various time points after challenge. Additionally, peripheral blood was obtained from uninfected animals from an unrelated study.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation. Plasma viral loads were determined by real-time PCR (ABI Prism 7500 sequence detection system, Applied Biosystems) using reverse-transcribed viral RNA as templates using methods previously described(6). CD4 T-cell-associated viral DNA was measured by a quantitative PCR assay for SIV gag using a Perkin-Elmer ABI 7500 (Applied Biosystems) instrument as previously described(7, 20) using SIV gag primers and probe as described by Lifson et al(18). The assay was calibrated using a cell line that carried a single copy of proviral SIV DNA as described previously(20).
Relative expression of IL-21, IL-23 and TGFβ mRNA were determined in total PBMC from uninfected, SIV infected (week 28 pi) and SIV infected treated (week 28 pi; 15 weeks ART) animals using the dCt method with previously described primers/probes(13) using β2M(7, 20) as endogenous control on a Perkin-Elmer ABI 7500 instrument. Data are shown relative to uninfected PBMC.
Antibodies and flow cytometry
All antibodies used in this study except for IL-17 were obtained from BD Biosciences (San Diego, CA), and titrated using rhesus macaque PBMC. For phenotypic analysis and sorting of CD4 T cell subsets cells were labeled simultaneously with the following combinations of antibodies: CD3-Cy7APC, CD8-Alexa-700, CD4-APC, CD95-FITC, Integrin β7 (Fib 504 clone)-Cy5-PE. Labeled cells were fixed in 0.5% paraformaldehyde, and analyzed using a Becton Dickinson Aria sorter. IL-17 production in CD4 T cell subsets were determined after stimulation with Phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO) at 10 ng / ml and Ionomycin (Sigma-Aldrich, St. Louis, MO ) at 500 ng /ml in the presence of Brefeldin-A (BD Biosciencs) for 4 hours. After stimulation cells were labeled with anti-CD3-Cy7APC, CD8-Alexa-700, CD4-PE, CD95-FITC, Integrin β7-Cy5-PE. Cells were fixed in Cytofix/perm buffer (BD Biosciences), and labeled with anti-IL-17-APC (e-Biosciences). Labeled cells were fixed in 0.5% paraformaldehyde, and analyzed using a modified Becton Dickinson Aria sorter.
Data analysis
Flow cytometric data was analyzed using FlowJo version 8.6 (Tree Star, Inc., Ashland, OR). Statistical analysis was performed with GraphPad Prism Version 4.0 software (GraphPad Prism Software, Inc. San Diego, CA).
Results
ART significantly suppressed viral loads leading to repopulation of α4+β7hiCD4+ T cells
To determine if ART suppressed viral infection, we evaluated the level of plasma viremia, and SIV infection in memory CD4 T cells during ART and compared them to untreated control animals. Our results show that ART significantly suppressed plasma viral loads to below detection (<30 copies / ml of plasma; Fig. 1a), and significantly reduced the frequency of SIV infected memory CD4 T cells (Fig. 1b) as compared to untreated animals.
Figure 1. ART leads to significant repopulation of both α4+β7hi and β7− CD4+ T cells in peripheral blood.
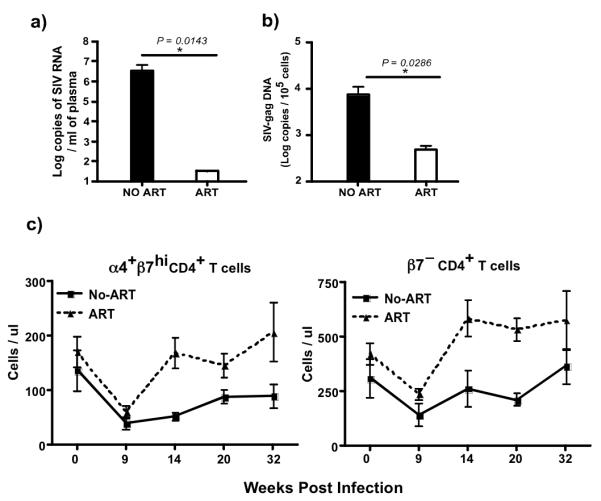
(a) Plasma and (b) memory CD4+ T cells associated viral loads at week 28 pi. (c) Absolute counts of α4+β7hi and β7− CD4+ T cells in treated animals (n = 4) as compared to untreated animals (n = 4). α4+β7hi and β7− CD4+ T cells were delineated based on the expression of β7 and CD95, with both α4+β7hi and β7− CD4+ T cells being CD95+. ART was initiated at 13 weeks pi in treated group of animals.
Next we evaluated if suppression of viremia was associated with repopulation of memory CD4 T cells that home to mucosal tissues. We have previously shown(14) that total memory CD4+ T cells in peripheral blood comprised of two major subsets namely, α4+β7hi and β7−CD4+ T cells with ~ 25% of memory T cells expressing the α4+β7hi phenotype and the rest expressing the β7− phenotype. Both the subsets expressed CD95, a marker expressed by memory T cells(26). We observed a substantial increase in the absolute numbers of both α4+β7hi and β7−CD4+ T cells in peripheral blood of treated animals as compared to untreated animals.
Th-17 cells fail to repopulate whereas Tc-17 cells are increased during ART
To evaluate if the repopulation of mucosal homing memory CD4 T cells was accompanied by the restoration of Th-17 responses, we evaluated the ability of α4+β7hi and β7−CD4+ and CD8+T cells in peripheral blood to produce IL-17 following therapy and compared them to untreated animals.
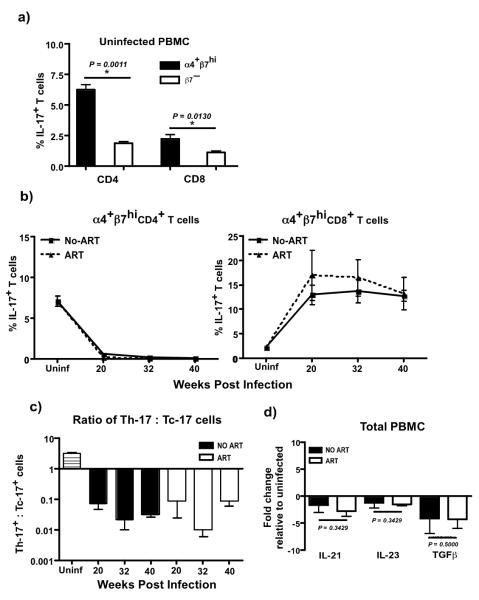
Our results demonstrated that the major source of Th-17 and Tc-17 responses in healthy animals was found to be predominantly within α4+β7hiCD4+ and α4+β7hiCD8+T cells (Fig. 2a). Interestingly, healthy rhesus macaques harbored ~ 3 × more Th-17 cells as compared to Tc-17 cells indicating that the homeostatic balance between Th-17 and Tc-17 cells likely plays an important role in maintaining mucosal integrity and function.
Figure 2. Th-17 cells fail to repopulate after therapy.
(a) α4+β7hiCD4+ and CD8+T cells harbor significantly higher frequencies of IL-17 producing cells in healthy animal’s (n = 4). α4+β7hi and β7− CD4+ and CD8+T cells were delineated based on the expression of β7 and CD95. Gates were set on CD95+ cells to delineate α4+β7hi and β7− memory T cells(14). (b) Frequency of IL-17 producing α4β7hiCD4+ and CD8+T cells in untreated SIV infected (n = 4) and SIV infected treated animals (n = 4). (c) Ratio of Th-17 : Tc-17 responses in uninfected (n = 4), untreated SIV infected (n = 4) and treated animals (n = 4). (d) Relative expression of IL-21, IL-23 and TGFβ mRNA in total PBMC from untreated SIV infected (n = 4) and treated SIV infected animals at 28 weeks pi (n = 4). ART was initiated at 13 weeks pi in treated group of animals. Data are shown relative to uninfected animals (n = 4).
SIV infection was associated with a near total depletion of Th-17 responses (Fig. 2b). In contrast to Th-17 responses, we observed a dramatic increase in the frequency of Tc-17 cells that stayed high through the course of infection. Surprisingly, there was little or no repopulation of Th-17 cells during the course of continuous ART suggesting that ART failed to reverse the viral infection associated loss of Th-17 responses in peripheral blood. Likewise, ART failed to suppress the increased levels of Tc-17 responses seen in untreated animals. Taken together these data suggests that chronic infection is associated with a significant shift in the balance of Th-17 : Tc-17 responses from a Th-17 phenotype to a predominantly Tc-17 phenotype (Fig. 2c), a dysregulation that could not be reversed with ART even after effective suppression of viral replication.
To determine if the inability to repopulate Th-17 responses was associated with loss of IL-21, IL-23 and TGFβ responses, we evaluated the expression of IL-21, IL-23 and TGFβ mRNA in treated animals and compared them to untreated animals. Previous studies(16, 31) have shown that IL-21, IL-23 and TGFβ play an important role in the generation and maintenance of Th-17 responses. Our results showed that there was a ~2-4 fold down regulation of IL-21, IL-23 and TGFβ mRNA in both treated and untreated animals as compared to uninfected animals. Interestingly, there was no difference in the level of IL-21, IL-23 and TGFβ expression between treated and untreated animals.
Discussion
Th-17 cells have been shown to play an important role in immune homeostasis in mucosal tissues. These cells are severely depleted very early during the course of infection leading to an imbalance in Th-17 : Th-1 responses that is thought to contribute to disease progression(2, 4, 8, 14, 19). Loss of Th-17 cells have been associated with translocation of bacterial products and systemic dissemination of bacteria such as Salmonella leading to chronic immune activation and disease progression(29). Unlike pathogenic SIV infection, natural hosts such as SIV infected Sooty mangabeys preserve Th-17 cells and do not exhibit immune activation observed in pathogenic SIV infections(2).
It is interesting that repopulation of mucosal homing CD4+ T cells was not accompanied by the restoration of Th-17 responses or a decrease in Tc-17 responses suggesting that pathogenic SIV infection is associated with a significant dysregulation of the IL-17 pathway that involves both CD4+ and CD8+ T cells. The significance of this dysregulation, and the failure of ART to restore the homeostatic balance that existed prior to infection are not clear. It is possible that the dysregulation in Th-17 : Tc-17 responses likely contributes to the eventual failure of ART to control viral replication. IL-17 is a proinflammatory cytokine, and the increased levels of Tc-17 responses even after therapy suggests that the proinflammatory environment persists even after viral replication is effectively suppressed with therapy. The exact role of Tc-17 responses in HIV pathogenesis is not clear, and additional studies are needed to better understand the role Tc-17 cells play in disease progression. Ndhlovu et al(23) reported that suppression of HIV-1 plasma viral loads below detection preserved IL-17 producing T cells in HIV-1 infection. However, this study measured IL-17 responses in total PBMC, and it is possible that the IL-17 responses observed in these HIV infected subjects undergoing HAART was due to IL-17 production by Tc-17 cells.
Studies(22) have shown that CD4 T cells either fail to or only transiently repopulate mucosal tissue during long-term HAART. It is possible that that skewing of the Th-17 : Tc-17 responses from a Th-17 to a predominantly Tc-17 phenotype even after long-term ART likely sustains that loss of mucosal homeostasis, and contributes to the lack of mucosal repopulation. Previous studies(19) have shown that effective repopulation of CD4 T cells in the gut associated lymphoid tissues in patients undergoing HAART was associated with enhanced Th-17 cells.
It is not clear why Th-17 cells failed to repopulate after therapy. It is possible that Th-17 deficiency is accompanied by a deficiency in Th-17 promoting cytokines such as IL-21, IL-23 and TGFβ that are required for the development and differentiation of Th-17 cells(16, 31). In fact, we saw a down regulation of the IL-21, IL-23 and TGFβ following SIV infection that could not be reversed with ART suggesting that the dysregulation of the Th-17 pathway likely involves other cytokines that are necessary for the development and differentiation of Th-17 cells. Though the deficiency in these IL-17 promoting cytokines may explain the lack of repopulation of Th-17 cells, it does not explain the significant increase in Tc-17 cells suggesting to other mechanisms playing a role in this process. Note that we measured IL-21, IL-23 and TGFβ expression in total PBMC. It is possible that in secondary lymphoid tissues such as the mucosa where most of the Tc-17 cells originate, these IL-17 promoting cytokines are being expressed at much higher levels that likely contribute to the increase in Tc-17 cells. It is also important to point out that we evaluated IL-17 production following PMA/Ionomycin stimulation that measures the potential to make IL-17 rather than actual in vivo responses, and it is possible that other stimuli such as bacterial or fungal products may induce Th-17 responses in the animals we studied. PMA/ionomycin, however, are potent mitogenic activators of cytokine responses. Hence, it was surprising that little or no Th-17 responses could be detected at the time points we evaluated, whereas higher frequencies of Tc-17 cells were detectable at the same time points.
In conclusion, our results show that long-term therapy fails to effectively repopulate Th-17 cells and restore the homeostatic balance between Th-17 : Tc-17 responses that existed prior to infection. It is possible that the failure to restore Th-17 cells or suppress Tc-17 cells during therapy likely contributes to the eventual failure of the immune system to control viral infection during ART.
Acknowledgments
We thank Nancy Miller at the SVEU of NIAID for help with the animals; Karen Wolcott at the Biomedical Instrumentation Core facility at USUHS for help with flow cytometry; Dr. Deborah Weiss and Jim Treece at ABL, Inc, Rockville, MD for expert assistance with the animals.
The described project was supported by Grant Number K22AI07812 from the National Institutes of Allergy and Infectious diseases (NIAID) & Grant number R21DE018339 from the National Institute of Dental and Craniofacial Research (NIDCR) to JJM. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID or NIDCR or the National Institutes of Health.
M.K and S.B performed the experiments and helped in the analysis and interpretation of data, and preparation of the manuscript. M.P and JL helped with plasma viral load assays. J.J M designed, helped with preparation of the manuscript, and supervised the study.
Footnotes
Competing Interests The authors declare they have no competing financial interests.
References
- 1.Belmonte L, Olmos M, Fanin A, Parodi C, Bare P, Concetti H, Perez H, de Bracco MM, Cahn P. The intestinal mucosa as a reservoir of HIV-1 infection after successful HAART. Aids. 2007;21:2106–8. doi: 10.1097/QAD.0b013e3282efb74b. [DOI] [PubMed] [Google Scholar]
- 2.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecchinato V, Trindade CJ, Laurence A, Heraud JM, Brenchley JM, Ferrari MG, Zaffiri L, Tryniszewska E, Tsai WP, Vaccari M, Parks RW, Venzon D, Douek DC, O’Shea JJ, Franchini G. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–88. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, Ward DJ, Kovacs JA, Mannon PJ, Fauci AS. Persistence of HIV in Gut-Associated Lymphoid Tissue despite Long-Term Antiretroviral Therapy. J Infect Dis. 2008 doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 6.Cline AN, Bess JW, Piatak M, Jr., Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 7.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 8.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 10.Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, Dunham RM, Paiardini M, Klucking S, Danesh A, Strobert EA, Apetrei C, Pandrea IV, Kelvin D, Douek DC, Staprans SI, Sodora DL, Silvestri G. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–34. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heise C, Miller CJ, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–20. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 13.Huang D, Qiu L, Wang R, Lai X, Du G, Seghal P, Shen Y, Shao L, Halliday L, Fortman J, Shen L, Letvin NL, Chen ZW. Immune gene networks of mycobacterial vaccine-elicited cellular responses and immunity. J Infect Dis. 2007;195:55–69. doi: 10.1086/509895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kader M, Wang X, Piatak M, Jr., Lifson JD, Roederer M, Veazey RS, Mattapallil JJ. a4+b7hi CD4+ Memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009 doi: 10.1038/mi.2009.90. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, Stahl-Hennig C, Kaup FJ, Stallmach A, Zeitz M. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–23. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 16.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 18.Lifson JD, Rossio JL, Piatak M, Jr., Parks T, Li L, Kiser R, Coalter V, Fisher B, Flynn BM, Czajak S, Hirsch VM, Reimann KA, Schmitz JE, Ghrayeb J, Bischofberger N, Nowak MA, Desrosiers RC, Wodarz D. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001;75:10187–99. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, Dandekar S. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–88. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 20.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 21.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndhlovu LC, Chapman JM, Jha AR, Snyder-Cappione JE, Pagan M, Leal FE, Boland BS, Norris PJ, Rosenberg MG, Nixon DF. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. Aids. 2008;22:990–2. doi: 10.1097/QAD.0b013e3282ff884e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paiardini M, Frank I, Pandrea I, Apetrei C, Silvestri G. Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev. 2008;10:36–46. [PubMed] [Google Scholar]
- 25.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr., Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Poles MA, Boscardin WJ, Elliott J, Taing P, Fuerst MM, McGowan I, Brown S, Anton PA. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr. 2006;43:65–8. doi: 10.1097/01.qai.0000230524.71717.14. [DOI] [PubMed] [Google Scholar]
- 28.Poles MA, Elliott J, Taing P, Anton PA, Chen IS. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J Virol. 2001;75:8390–9. doi: 10.1128/JVI.75.18.8390-8399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008 doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]