Abstract
Background
3-Bromopyruvate is an alkylating agent with antitumor activity. It is currently believed that blockade of ATP production from glycolysis and mitochondria is the primary mechanism responsible for this antitumor effect. The present studies have uncovered a new and novel mechanism for the antitumor activity of 3-bromopyruvate.
Methods
Transport of 3-bromopyruvate via SLC5A8, a tumor suppressor and a Na+-coupled electrogenic transporter for short-chain monocarboxylates, was studied using a mammalian cell expression and the Xenopus laevis oocyte expression systems. The effect of 3-bromopyruvate on histone deacetylases (HDACs) was monitored using the lysate of the human breast cancer cell line MCF7 and human recombinant HDAC isoforms as the enzyme sources. Cell viability was monitored by FACS analysis and colony formation assay. Acetylation status of histone H4 was evaluated by Western blot.
Results
3-Bromopyruvate is a transportable substrate for SLC5A8, with the transport process being Na+-coupled and electrogenic. MCF7 cells do not express SLC5A8 and are not affected by 3-bromopyruvate. However, when transfected with SLC5A8 or treated with inhibitors of DNA methylation, these cells undergo apoptosis in the presence of 3-bromopyruvate. This cell death is associated with inhibition of HDAC1/HDAC3. Studies with different isoforms of human recombinant HDACs identify HDAC1 and HDAC3 as the targets for 3-bromopyruvate.
Conclusions
3-Bromopyruvate is transported into cells actively via the tumor suppressor SLC5A8 and the process is energized by an electrochemical Na+ gradient. Ectopic expression of the transporter in MCF7 cells leads to apoptosis, and the mechanism involves inhibition of HDAC1/HDAC3.
Tumor cells are unique in that they derive most of their metabolic energy from glycolysis rather than from oxidative phosphorylation.1-6 Since glycolysis itself generates only 2 moles of ATP per glucose while oxidative phosphorylation within mitochondria generates 30 moles of ATP per glucose, tumor cells have to upregulate glycolysis 20 to 30-fold to meet their energy requirements. This is achieved by several means. Glucose entry into tumor cells is enhanced, expression of most of the enzymes involved in glycolysis is upregulated, and lactate dehydrogenase isoenzymes are expressed differentially such that pyruvate is effectively converted into lactate and NAD+ is regenerated to maintain optimal activity of glyceraldehyde 3-phosphate dehydrogenase.7 In normal cells, conversion of pyruvate into lactate is facilitated only when oxygen supply becomes limited whereas in tumor cells this process occurs in the presence of oxygen. This metabolic shift from oxidative phosphorylation to glycolysis in tumor cells as the primary source of ATP underlies the Warburg hypothesis, which states that tumor cells exhibit elevated levels of glycolysis in the presence of oxygen.8
Since tumor cells rely heavily on glycolysis for ATP production, inhibition of this metabolic pathway provides a logical target for cancer treatment. With this rationale, 3-bromopyruvate was developed as a potential antitumor agent.9, 10 Being a potent alkylating agent, 3-bromopyruvate has several molecular targets. The most important among them that is relevant to tumor cell metabolism is hexokinase, the first enzyme in the glycolytic pathway. Hexokinase II, one of the four isoforms of the enzyme, is expressed at very low levels in most normal cells, but upregulated several-fold in tumor cells.11 This enzyme is inactivated by 3-bromopyruvate, thus resulting in the blockade of the first step in glycolysis. 3-Bromopyruvate also inhibits pyruvate kinase, which catalyzes the conversion of phosphoenolpyruvate into pyruvate.12 In addition, 3-bromopyruvate enters the mitochondria, inhibits oxidative phosphorylation, and prevents mitochondrial ATP production.11 It is currently believed that the blockade of energy production is the primary mechanism for the antitumor activity of 3-bromopyruvate.5, 10, 11 The antitumor activity of 3-bromopyruvate has been demonstrated in several models of cancer.13-17
Recently, a plasma membrane transporter, known as SLC5A8, has been identified as a tumor suppressor in colon cancer.18, 19 The transporter is expressed in normal colon but silenced in colon cancer via DNA methylation. It is a Na+-coupled electrogenic transporter for short-chain fatty acids such as acetate, propionate, and butyrate, and also other monocarboxylates such as lactate and pyruvate20-22 and the B-complex vitamin nicotinate.23, 24 The ability of SLC5A8 to transport butyrate, a bacterial fermentation product and an inhibitor of histone deacetylases (HDACs), underlies its tumor-suppressive effect in the colon.25, 26 Surprisingly, the transporter is silenced not only in colon cancer but also in tumors of a variety of non-colonic tisues.19 Butyrate is not relevant to any of these tissues. Therefore, it was puzzling why the transporter is silenced in these tumors. Our recent studies have provided a likely mechanism for this phenomenon.27, 28 Pyruvate, an ubiquitous metabolite, is a substrate for SLC5A8, and is as potent as butyrate as an HDAC inhibitor. Pyruvate and butyrate specifically inhibit HDAC1 and HDAC3, the two isoenzymes which are upregulated in cancer. The ability of SLC5A8 to transport pyruvate actively into cells with resultant intracellular inhibition of HDAC1/HDAC3 underlies the tumor-suppressive effect of the transporter in non-colonic tissues. 3-Bromopyruvate is structurally related to pyruvate. Therefore, we asked whether SLC5A8 and HDAC1/HDAC3 play any role in the tumor-suppressive effect of 3-bromopyruvate. Here we show that 3-bromopyruvate is indeed a transportable substrate for SLC5A8 and that it is a potent inhibitor of HDAC1 and HDAC3. Ectopic expression of SLC5A8 in breast cancer cells leads to accumulation of 3-bromopyruvate inside the cells with subsequent inhibition of HDAC1/HDAC3, resulting in cell death. These findings uncover a new and novel molecular mechanism for the tumor-suppressive activity of 3-bromopyruvate.
Materials and Methods
Materials
[14C]Nicotinate was purchased from American Radiolabeled Chemicals (St. Louis, MO). Pyruvate and 3-bromopyruvate were obtained from Sigma (St. Louis, MO). Human SLC5A8 and mouse Slc5a8 were originally cloned from human intestine and mouse kidney, respectively.20, 22 Rat Slc5a8 was cloned by RT-PCR using liver mRNA as the template.
Functional Expression of SLC5A8 cDNAs in Human Retinal Pigment Epithelial (HRPE) Cells
Human, mouse, and rat SLC5A8 cDNAs were expressed in HRPE cells using the vaccinia virus expression technique.20, 22 Uptake of [14C]-nicotinate (15 μM) was measured with a 15-min incubation. HRPE cells transfected with empty vector were used to measure background transport activity. Uptake measurements in vector- and cDNA- transfected cells were made in parallel, and the SLC5A8-specific transport was determined by subtracting the transport values measured in vector-transfected cells from the transport values measured in cDNA-transfected cells. The uptake buffer was 25 mM Hepes/Tris (pH 7.5), containing 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose. Dose-response studies were done to determine the IC50 values (i.e., concentration necessary to cause 50% inhibition) for the inhibition of nicotinate uptake by pyruvate and 3-bromopyruvate. Experiments were repeated three times, and in each experiment measurements were made in duplicate or triplicate. Results are given as means ± S. E.
Functional Expression of Human SLC5A8 in X. laevis Oocytes
Capped cRNA from human SLC5A8 cDNA (cloned in pGH19, a X. laevis oocyte expression vector) was synthesized using the mMESSAGE-mMACHINE kit (Ambion, Austin, TX). Mature oocytes from X. laevis were injected with 50 ng of cRNA. Water-injected oocytes served as controls. Electrophysiological studies were performed by the two-microelectrode voltage-clamp method20. Oocytes were perifused with a NaCl-containing buffer (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, pH 7.5), followed by the same buffer containing pyruvate or 3-bromopyruvate. The membrane potential was clamped at -50 mV. The differences between the steady-state currents measured in the presence and absence of substrates were considered as the substrate-induced currents. In the analysis of the saturation kinetics, K0.5 (i.e., substrate concentration necessary for induction of half-maximal current) was calculated by fitting the Michaelis-Menten equation to the values of the substrate-induced currents. The Na+-activation kinetics was analyzed by measuring the substrate-specific currents in the presence of increasing concentrations of Na+. The data for the Na+-dependent currents were analyzed according to the Hill equation to determine the Hill coefficient (h, the number of Na+ ions involved in the activation process) and K0.5 for Na+ (i.e., concentration of Na+ necessary for half-maximal activation). Since expression levels varied significantly from oocyte to oocyte, kinetic analyses were done by normalizing the expression levels. This was done by taking the maximally induced SLC5A8-specific current in each kinetic experiment in individual oocytes as 1. To investigate the current-voltage (I-V) relationship, step changes in membrane potential were applied, each for 100 ms in 20-mV increments. Each experiment was repeated with four different oocytes.
Ectopic Expression of SLC5A8 in the Mammary Cancer Cell Line MCF7
Cells were seeded either in 10-cm (for protein) or in 35-mm (for FACS) culture dishes and cultured in the absence of pyruvate. Cells were transfected with pcDNA or human SLC5A8 cDNA using Fugene 6 and Opti-MEM. pEGFP-N1 was used for co-transfection to determine transfection efficiency. After 24 h, cells were treated with or without pyruvate or 3-bromopyruvate (1 mM) for 24 h. Cells were collected, centrifuged and washed twice with PBS for preparation of protein lysates. For FACS analysis, cells were fixed in 50% ethanol, treated with 0.1% sodium citrate, 1 mg/ml RNase A, and 50 μg/ml propidium iodide, and then subjected to fluorescence-activated cell sorting (FACS; FACS Caliber, Becton Dickinson) analysis.
siRNA-Mediated Silencing of HDAC Isoforms in MCF7 cells
MCF7 cells were seeded in 6-well plates and cultured in DMEM medium. After 24 h, cells were transfected with HDAC1 siRNA, HDAC2 siRNA or HDAC3 siRNA (Santa Cruz Biotechnology; catalogue numbers: sc-29343 for HDAC1 siRNA; sc-29345 for HDAC2 siRNA; sc-35538 for HDAC3 siRNA) according to the manufacturer's instructions. Scrambled siRNA (Santa Cruz Biotechnology; catalogue number: sc-37007) was used as a negative control. After 48 h transfection, cells were treated with 3-bromopyruvate for 48 h; cells were collected and processed for RNA extraction and FACS analysis.
Effect of 3-Bromopyruvate on MCF7 Cell Apoptosis following Treatment with DNA Methylation Inhibitors
MCF7 cells were plated (50, 000 cells/dish) in 10 cm dish; 24 h later, cells were treated with 5′-azacytidine or 5′-aza-deoxycytidine (2 μg/ml) for 72 h. Medium was changed every 24 h with the DNA methylation inhibitors added fresh each time. After 72 h, cells were treated with 3-bromopyruvate (1 mM) for 48 h. Cells were then collected and processed for RNA extraction and FACS analysis.
RT-PCR
For the analysis of the expression of the HDAC isoforms HDAC1-3 in MCF7 cells, RNA prepared from the cells was used for RT-PCR. Gene-specific PCR primers were designed based on the nucleotide sequences available in GenBank. GAPDH mRNA was used as an internal control.
Western Blot Analysis
Fifty μg of protein was fractionated by SDS-PAGE and then transferred to a nitrocellulose membrane (Schleicher & Schull). Membranes were blocked with 2% bovine serum albumin and then hybridized with anti-histone H4 and anti-acetylated histone H4-Lys16 (both from Upstate Biotechnology) antibody at 4°C overnight, followed by treatment with appropriate secondary antibody at room temperature for 1 h. Proteins were visualized by ECL Super Signal Western System (GE Healthcare).
Colony Formation Assay
MCF7 cells were transfected in 10-cm dishes with pcDNA3.1 or SLC5A8 expression construct, along with pEGFP-N1 to check the transfection efficiency. The following day, cells were trypsinized and seeded into 6-well plates (10, 000 cells/well) in DMEM medium without pyruvate. After 24 h, cells were exposed to 750 μg/ml G418 and different concentrations of pyruvate or 3-bromopyruvate for two weeks, changing medium every three days. Cells were washed with PBS and fixed in 100% methanol for 30 min followed by staining with KaryoMax Giemsa stain for 1 h. The wells were washed with water and dried overnight at room temperature. Finally, the contents in the wells were dissolved with 1% SDS in 0.2N NaOH for 1 h and the optical density of the released dye was measured at 630 nm.
Measurement of HDAC Activity
A commercially available kit (BioVision) was used to determine HDAC activity. MCF7 cells were transfected with pcDNA or SLC5A8 cDNA followed by treatment with or without pyruvate or 3-bromopyruvate (1 mM) for 24 h. Cells were lysed, and 100 μg of protein in the lysate was used for the enzyme assay according to the manufacturer's instructions. The activity of recombinant human HDAC isoforms was also measured using the same kit. The recombinant HDAC isoforms were purchased from Cayman Chemical Company.
Results
Interaction of 3-Bromopyruvate with SLC5A8 in a Mammalian Cell Expression System
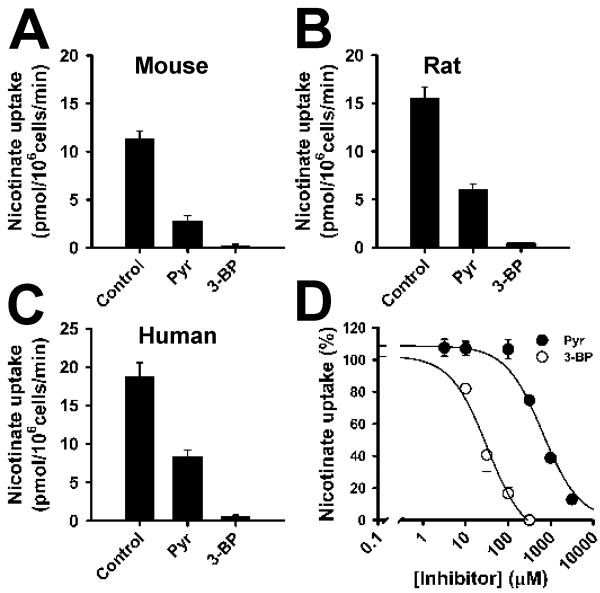
We compared the abilities of pyruvate and 3-bromopyruvate to compete with nicotinate for uptake via mouse, rat, and human SLC5A8 in a mammalian cell expression system using HRPE cells (Fig. 1A-C). The transport function of the heterologously expressed transporter was monitored by the uptake of [14C] nicotinate (15 μM; 15-min incubation) in the presence of NaCl. Under control conditions, nicotinate uptake in cDNA-transfected cells was 7 to 15-fold higher than in vector-transfected cells. With rat Slc5a8, the uptake was inhibited 60% with 1 mM pyruvate and 97% with 1 mM 3-bromopyruvate. With mouse Slc5a8 and human SLC5A8, the corresponding values for inhibition were 75% and 98%, and 55% and 96%, respectively. The IC50 value for the inhibition of human SLC5A8 was 652 ± 139 μM for pyruvate and 32 ± 9 μM for 3-bromopyruvate (Fig. 1D). These data show that 3-bromopyruvate interacts with the transporter in all three species with high affinity. However, these data do not show whether or not 3-bromopyruvate is a transportable substrate for SLC5A8. 3-Bromopyruvate may be a transportable substrate, which competes with nicotinate for the transport process. Alternatively, 3-bromopyruvate may function as a blocker of the transporter by interacting with the substrate-binding site without itself being translocated across the membrane. In both cases, 3-bromopyruvate can cause inhibition of nicotinate uptake. The approach used here with the mammalian expression system cannot differentiate between these two possibilities.
FIGURE 1.
Interaction of 3-bromopyruvate with SLC5A8 in a mammalian cell expression system. Mouse (A), rat (B), and human (C) SLC5A8 cDNAs were expressed in HRPE cells, and the transport function was monitored by the uptake of nicotinate (15 μM) in the presence of Na+. Uptake values from vector-transfected cells were subtracted from uptake values from cDNA-transfected cells to determine SLC5A8-specific uptake. (A-C) SLC5A8-specific uptake of nicotinate in the absence (control) or presence of pyruvate (1 mM) or 3-bromopyruvate (1 mM). (D) Dose-response for the inhibition of SLC5A8-mediated nicotinate uptake by pyruvate and 3-bromopyruvate. Data represent means ± S. E. (n = 6-9). Pyr, pyruvate; 3-BP, 3-bromopyruvate.
Direct Evidence for the Transport of 3-Bromopyruvate via SLC5A8
SLC5A8 from rat, mouse, and human tissues is a Na+-coupled electrogenic transporter. Transport of substrates via the transporter is associated with membrane depolarization, which can be detected by electrophysiological means. If a compound is a blocker and not a transportable substrate, it will not cause membrane depolarization. The X. laevis oocyte expression system is useful to differentiate between a transportable substrate and a blocker for any electrogenic transporter.29, 30 To determine whether 3-bromopyruvate is a transportable substrate or blocker of SLC5A8, we expressed human SLC5A8 in X. laevis oocytes by injection of SLC5A8 cRNA, and monitored its transport function electrophysiologically using the two-microelectrode voltage-clamp technique. Since SLC5A8-mediated transport process is electrogenic associated with membrane depolarization, it can be detected with this technique in the form of inward currents. We first used pyruvate as a positive control. Perifusion of SLC5A8-expressing oocytes with 1 mM pyruvate induced inward currents in the presence of NaCl. The currents were not detectable in the absence of Na+ (Fig. 2A), indicating the obligatory nature of Na+ for the process. Perifusion of the oocytes with 1 mM 3-bromopyruvate also induced inward currents in a Na+-dependent manner, showing unequivocally that this compound is a transportable substrate for SLC5A8 (Fig. 2A). At a concentration of 1 mM, the current induced by 3-bromopyruvate is approximately 50% of the current induced by pyruvate (Fig. 2B).
FIGURE 2.
Direct evidence for SLC5A8-mediated transport of 3-bromopyruvate. Human SLC5A8 was expressed in X. laevis oocytes and the transport function was monitored electrophysiologically using the two-microelectrode voltage-clamp technique. (A) Inward currents induced by pyruvate (1 mM) and 3-bromopyruvate (1 mM) in the presence (NaCl) or absence (NMDG chloride) of Na+. (B) Data from four different oocytes. In each oocyte, the current induced by pyruvate was taken as 100%. The value for pyruvate-induced currents in these four oocytes was 215 ± 45 nA.
We then characterized the kinetic features of SLC5A8-mediated 3-bromopyruvate transport. Similar to pyruvate, transport of 3-bromopyruvate was also influenced by membrane potential (Fig. 3A). The inward current induced by 3-bromopyruvate increased as the membrane potential was increased (hyperpolarization) and decreased as the membrane potential was decreased (depolarization). This is an expected feature of an electrogenic transport process via a solute carrier. This suggests that the transport process is energized not only by the transmembrane concentration gradient of Na+ but also by the membrane potential. The currents induced by pyruvate and 3-bromopyruvate were saturable with increasing concentrations of these compounds (Fig. 3B, C). The K0.5 value was 312 ± 16 μM for pyruvate and 495 ± 25 μM for 3-bromopyruvate. The Na+-activation kinetics of 3-bromopyruvate-induced currents was sigmoidal, indicating that more than one Na+ was involved in the activation process (Fig. 3D). The Hill coefficient for the activation of transport by Na+ was 1.4 ± 0.2, suggesting that 3-bromopyruvate is transported by SLC5A8 with a Na+:3-bromopyruvate stoichiometry of 2:1. The K0.5 value for Na+ to activate the transport process was 32 ± 4 mM. Since 3-bromopyruvate is a monocarboxylate with a single negative charge, simultaneous transport of two Na+ with one 3-bromopyruvate will result in the transfer of one net positive charge into the oocyte. This provides the molecular basis for membrane depolarization associated with the transport process.
FIGURE 3.
Characteristics of SLC5A8-mediated transport of 3-bromopyruvate. (A) Relationship between substrate-induced currents and membrane potential. Concentration of pyruvate and 3-bromopyruvate was 1 mM. (B) Saturation kinetics for pyruvate-induced currents. (C) Saturation kinetics for 3-bromopyruvate-induced currents. (D) Na+-activation kinetics for 3-bromopyruvate-induced currents. Inset: Hill plot.
Relevance of SLC5A8-mediated Transport and Subsequent Inhibition of HDACs to the Tumor-suppressive Function of 3-Bromopyruvate
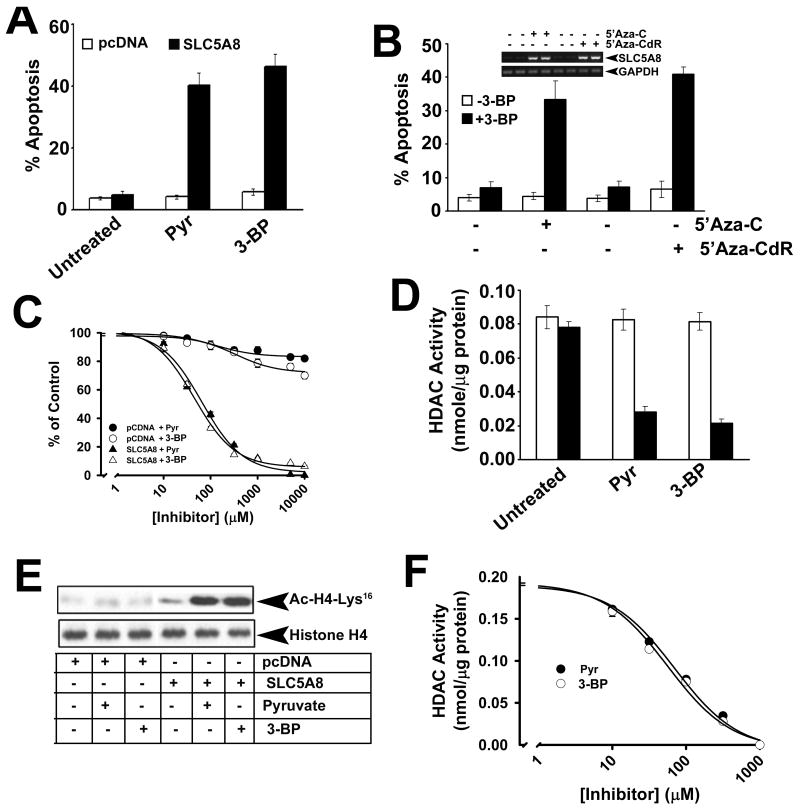
MCF7 cells (a human breast cancer cell line) were not affected when cultured in the presence of pyruvate (1 mM) or 3-bromopyruvate (1 mM). This is evident from the lack of any change in apoptosis under these conditions compared to control (i. e., in the absence of pyruvate or 3-bromopyruvate) in vector-transfected cells (Fig. 4A). SLC5A8 is a tumor suppressor, which is silenced in cancer. As in most tumor cells, the transporter is not expressed in MCF7 cells.27 When SLC5A8 was expressed ectopically in these cells, pyurvate and 3-bromopyruvate induced marked apoptosis, demonstrating that SLC5A8 is necessary for the tumor-suppressive effect of 3-bromopyruvate in these cells. We used an alternative approach to confirm these findings. Instead of the ectopic expression of SLC5A8, we induced the expression of endogenous SLC5A8 by treating the cells with the DNA methylation inhibitors 5′-azacytidine or 5′-azadeoxycytidine, followed by treatment with pyruvate and 3-bromopyruvate. Again, pyruvate (data not shown) and 3-bromopyruvate induced apoptosis only with treatment of the cells with the DNA methylation inhibitors (Fig. 4B). We then used the colony formation assay to determine the IC50 values for pyruvate and 3-bromopyruvate to interfere with cell viability in MCF7 cells (Fig. 4C). Vector-transfected cells were affected only minimally in the presence of increasing concentrations of these two compounds. In contrast, when SLC5A8 was expressed ectopically, the cells failed to form colonies in a dose-dependent manner with increasing concentrations of pyruvate (IC50 value, 65 ± 12 μM) and 3-bromopyruvate (IC50 value, 45 ± 5 μM). The induction of cell death in MCF7 cells by pyruvate and 3-bromopyruvate was associated with inhibition of HDACs (Fig. 4D). When vector-transfected MCF7 cells were cultured in the presence of 1 mM pyruvate or 3-bromopyruvate, there was no change in HDAC activity. But, when the cells transfected with SLC5A8 cDNA were cultured in the presence of 1 mM pyruvate or 3-bromopyruvate, the activity of HDACs was reduced by 70-80%. This corroborated with changes in the acetylation status of histone H4-Lys16 (Fig. 4E). The acetylation status of this lysine residue increased in SLC5A8-expressing MCF7 cells when cultured in the presence of pyruvate or 3-bromopyruvate. Dose-response studies with a cell-free system using MCF7 cell lysate as the source of HDAC activity showed that pyurvate and 3-bromopyruvate inhibited HDACs in these cells with comparable IC50 values (69 ± 12 μM for pyruvate and 56 ± 11 μM for 3-bromopyruvate) (Fig. 4F).
FIGURE 4.
Apoptosis induced in MCF7 cells by 3-bromopyruvate is dependent on SLC5A8 and is associated with inhibition of HDACs. (A) MCF7 cells were transfected with either vector alone or human SLC5A8 cDNA, and then cultured in the absence or presence of pyruvate (1 mM) or 3-bromopyruvate (1 mM) for 48 h. Apoptosis was monitored by FACS. (B) MCF7 cells were treated with 5′-azacytidine (5′-Aza-C) or 5′-azadeoxycytidine (5′-Aza-CdR) for 72 h and then cultured in the absence or presence of 3-bromopyruvate as described in (A) and apoptosis was monitored by FACS. RNA prepared from these cells was used for RT-PCR to monitor SLC5A8 expression. (C) Dose-response for growth arrest and/or cell death induced by pyruvate and 3-bromopyruvate in vector-transfected and SLC5A8 cDNA-transfected MCF7 cells. Cell viability was monitored by colony formation assay. (D) Cells were treated as described for (A) and the cell lysates were used as the source of HDAC activity. (E) Cells were treated as described for (A) and the cell lysates were used for Western blot to determine the acetylation status of histone H4-Lys16. (F) Dose-response for the inhibition of HDACs from MCF7 cells by pyruvate and 3-bromopyruvate. Data are means ± S. E. (n = 4).
Isoform Specificity of 3-Bromopyruvate-mediated HDAC Inhibition
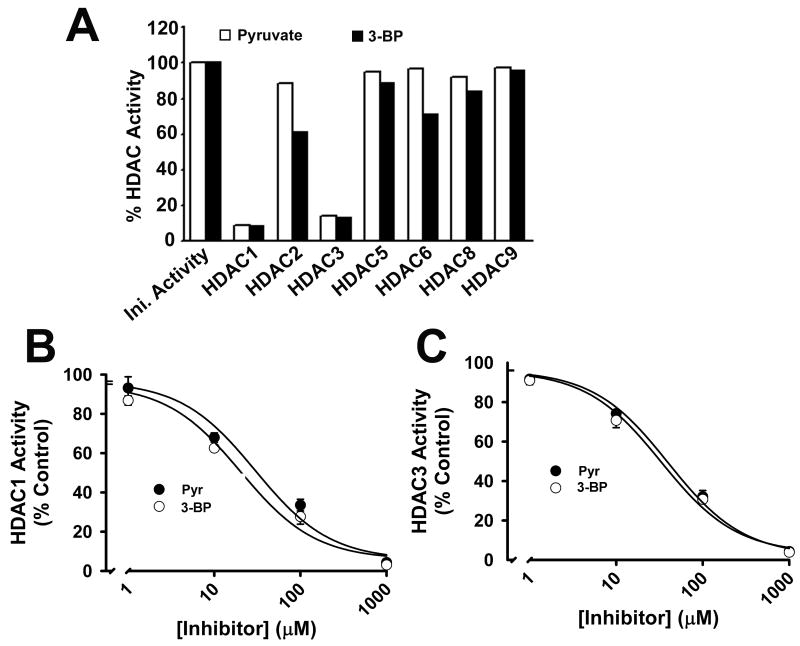
Since there are several isoforms of HDACs, we used recombinant human HDAC isoforms to determine the isoform specificity of HDAC inhibition by 3-bromopyruvate (Fig. 5A). At a concentration of 1 mM, 3-bromopyruvate caused marked inhibition of HDAC1 and HDAC3. HDAC2 and HDAC6 were also inhibited to a lesser, but significant, extent. There was no effect on HDAC5, HDAC8 and HDAC9. Under similar conditions, pyruvate also inhibited HDAC1 and HDAC3 preferentially. HDAC1 and HDAC3 were inhibited by pyruvate and 3-bromopyruvate with similar potency. The IC50 values for the inhibition of HDAC1 by pyruvate and 3-bromopyruvate were 30 ± 14 μM and 20 ± 9 μM, respectively (Fig. 5B). The corresponding values for the inhibition of HDAC3 were 40 ± 11 μM and 33 ± 11 μM (Fig. 5C).
FIGURE 5.
Inhibition of human recombinant HDAC isoforms by pyruvate and 3-bromopyruvate. (A) Activity of individual HDAC isoforms was measured in the absence or presence of pyruvate (1 mM) or 3-bromopyruvate (1 mM). (B, C) Dose-response for inhibition of HDAC1 and HDAC3 by pyruvate and 3-bromopyruvate. Data are means ± S. E. (n = 3).
Relevance of Inhibition of HDAC1/HDAC3 to 3-Bromopyrovate-induced Apoptosis in MCF7 Cells
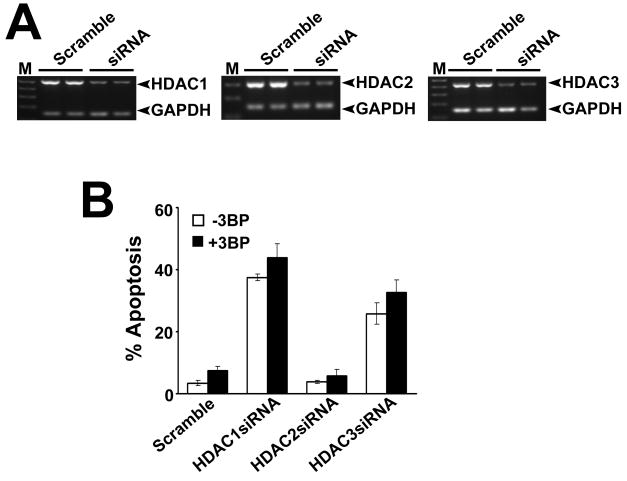
If 3-bromopyruvate induces apoptosis in cancer cells by inhibiting HDAC1/HDAC3, silencing of these two isoforms should have a similar effect. We treated MCF7 cells with siRNAs specific for HDAC1, HDAC2, or HDAC3, and then exposed the cells to 3-bromopyruate. We confirmed the silencing of the siRNA targets by RT-PCR (Fig. 6A). A scrambled siRNA was used as a negative control. Silencing of either HDAC1 or HDAC3 itself induced significant apoptosis in MCF7 cells (Fig. 6B); subsequent treatment with 3-bromopyruvate did not have any added effect. In contrast, silencing of HDAC2 did not cause apoptosis in the presence or absence of 3-bromopyruvate. These results suggest that 3-bromopyruvate-induced apoptosis in MCF7 cells is most likely mediated through inhibition of HDAC1/HDAC3.
FIGURE 6.
Relevance of inhibition of HDAC1 and HDAC3 to 3-bromopyruvate-induced apoptosis in MCF7 cells. MCF7 cells were transfected with HDAC1-, HDAC2- and HDAC3-specific siRNAs, and then incubated with or without 3-bromopyruvate (1 mM) for 48 h. A scrambled siRNA was used as a negative control. (A) RNA was extracted from these cells, and expression of HDAC1, HDAC2 and HDAC3 was monitored by RT-PCR using isoform-specific primers. GAPDH was used as a loading control. (B) Cells were treated as described in (A) and then used for FACS analysis to monitor apoptosis. Data are means ± S. E. (n = 3).
Discussion
The present studies delineate a novel mechanism for the antitumor activity of 3-bromopyruvate. Here we showed that 3-bromopyruvate is a transportable substrate for SLC5A8, a plasma membrane transporter with tumor-suppressive function. The transport process is electrogenic and is obligatorily dependent on Na+. The Na+:3-bromopyruvate stoichiometry of 2:1 suggests that the transport process is associated with the transfer of one net positive charge into cells. This provides the basis for the electrogenic nature of the transport process, which is supported by the inward currents induced by 3-bromopyruvate in SLC5A8-expressing oocytes in the presence of Na+ under voltage-clamp conditions. Thus, SLC5A8 is able to mediate the concentrative entry of 3-bromopyruvate into cells. We also showed that 3-bromopyruvate is a potent inhibitor of HDACs. HDAC1 and HDAC3 are the specific targets. It is important to note that these are the two isoforms, which are upregulated in cancer. We have provided evidence for increased expression of these two isoforms in colon cancer 28 and in breast cancer (data not shown). The transport of 3-bromopyruvate via SLC5A8 in MCF7 cells and subsequent intracellular inhibition of HDAC1/HDAC3 underlies the ability of this compound to cause cell death in these cells. The findings that siRNA-mediated silencing of HDAC1 or HDAC3 also induces apoptosis in breast cancer cells are consistent with these conclusions.
It is currently believed that 3-bromopyruvate exerts its antitumor effect by depleting ATP specifically in tumor cells.5, 10, 11 The targets responsible for energy depletion are the metabolic pathways associated with glycolysis and oxidative phosphorylation. Since these pathways operate in normal cells as well as in tumor cells, the question arises as to the mechanism responsible for the tumor cell-specific effect of 3-bromopyruvate. It has been speculated that, being a structurally related analog of pyruvate, 3-bromopyruvate may enter cells via monocarboxyalte transporters (MCTs).5, 10, 11 Tumor cell metabolism yields large amounts of lactate, and therefore tumor cells upregulate some isoforms of MCTs as a means to export lactate, thereby protecting themselves from undue intracellular acidification.7 MCT-mediated entry of 3-bromopyruvate may thus explain the tumor cell-specific effect of this compound. Interestingly, we did not find any significant cell death in vector-transfected MCF7 cells when cultured in the presence of 3-bromopyruvate. These cells do not express SLC5A8 due to silencing of the gene by DNA methylation27; therefore, the entry of 3-bromopyruvate into these cells must depend on MCTs. However, the expression levels of MCTs in this cell line are very low even though it is a malignant cell line.31 This could be the reason why these cells are resistant to 3-bromopyruvate. The same cells become highly susceptible to cell death induced by 3-bromopyruvate when SLC5A8 is expressed ectopically because this provides an active mechanism for the entry of the compound into cells. Previous studies have demonstrated the antitumor activity of 3-bromopyruvate in liver and pancreatic cancer cell lines, but there is no information available on the levels of expression of MCTs or SLC5A8 in these cells.14, 16, 17 Obviously, these cells must have a mechanism for 3-bromopyruvate entry, but the identity of the transporter responsible for the process is not known.
The present studies also show that 3-bromopyruvate is a potent HDAC inhibitor with selectivity towards HDAC1 and HDAC3. HDAC inhibitors have been recognized as potential antitumor agents; several such compounds have shown potential in clinical trials.32, 33 The findings that 3-bromopyruvate is an HDAC inhibitor are not all that surprising based on our previous findings that pyruvate is a potent inhibitor of HDAC1 and HDAC3.27, 28 However, there may be significant differences between pyruvate and 3-bromopyruvate in the mechanism of inhibition. 3-Bromopyruvate is an alkylating agent whereas pyruvate is not. It is therefore possible that 3-bromopyruvate causes irreversible inhibition of HDAC1/HDAC3 by covalently modifying the enzyme. This may not be the case with pyruvate. Irrespective of the mechanism, inhibition of HDACs is likely to contribute to the antitumor activity of 3-bromopyruvate.
Tumor cells will be susceptible to 3-bromopyruvate only if a transport mechanism exists for the entry of this compound. SLC5A8 is not expressed in most tumor cells. We speculate that tumor cells silence SLC5A8 to prevent cell death caused by pyruvate-induced inhibition of HDACs. SLC5A8 is an influx transporter due to its coupling to a transmembrane electrochemical Na+ gradient whereas MCTs are facilitative monocarboxylate transporters. MCTs are upregulated in tumor cells to facilitate the elimination of lactate from the cells. It is the MCT4 isoform which is induced in cancer.31, 34 The in vitro efficacy of 3-bromopyruvate as an anticancer agent in tumor cell lines will therefore depend on the level of expression of MCT4. The silencing of SLC5A8 in tumor cells and in primary tumors occurs by an epigenetic mechanism involving DNA methylation. Treatment of tumor cells with DNA methylation inhibitors re-activates the expression of the gene.18, 26, 27 Several DNA methylation inhibitors have shown promise for the treatment of solid tumors and lymphoma.35 If inhibition of DNA methylation in vivo induces re-expression of SLC5A8 in tumors, 3-bromopyruvate and DNA methylation inhibitors may synergize to improve efficacy in cancer treatment.
Acknowledgments
Funded by: National Institutes of Health grant CA131402
Footnotes
There are no financial disclosures from the authors.
References
- 1.Stubbs M, McSheehy PMJ, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 2.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 3.Ristow M. Oxidative metabolism in cancer growth. Curr Opin Clin Nutr Metab Care. 2006;9:339–345. doi: 10.1097/01.mco.0000232892.43921.98. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen PL. The cancer cell's “power plants” as promising therapeutic targets: An overview. J Bioenerg Biomembr. 2007;39:1–12. doi: 10.1007/s10863-007-9070-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–274. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 7.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 9.Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001;173:83–91. doi: 10.1016/s0304-3835(01)00667-x. [DOI] [PubMed] [Google Scholar]
- 10.Geschwind JF, Georgiades CS, Ko YH, Pedersen PL. Recently elucidated energy catabolism pathways provide opportunities for novel treatments in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2004;4:449–457. doi: 10.1586/14737140.4.3.449. [DOI] [PubMed] [Google Scholar]
- 11.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: Cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acan NL, Ozer N. Modification of human erythrocyte pyruvate kinase by an active site-directed reagent: bromopyruvate. J Enzyme inhib. 2001;16:457–464. doi: 10.1080/14756360109162395. [DOI] [PubMed] [Google Scholar]
- 13.Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002;62:3909–3913. [PubMed] [Google Scholar]
- 14.Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, et al. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004;324:269–275. doi: 10.1016/j.bbrc.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Vali M, Liapi E, Kowalski J, Hong K, Khwaja A, et al. Intraarterial therapy with a new potent inhibitor of tumor metabolism (3-bromopyruvate): Identification of therapeutic dose and method of injection in an animal model of liver cancer. J Vasc Interv Radiol. 2007;18:95–101. doi: 10.1016/j.jvir.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Kim W, Yoon JH, Jeong JM, Cheon GJ, Lee TS, et al. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther. 2007;6:2554–2562. doi: 10.1158/1535-7163.MCT-07-0115. [DOI] [PubMed] [Google Scholar]
- 17.Cao X, Bloomston M, Zhang T, Frankel WL, Jia G, et al. Synergistic antipancreatic tumor effect by simultaneously targeting hypoxic cancer cells with HSP90 inhibitor and glycolysis inhibitor. Clin Cancer Res. 2008;14:1831–1839. doi: 10.1158/1078-0432.CCR-07-1607. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganapathy V, Thangaraju M, Gopal E, Itagaki S, Miyauchi S, Prasad PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–199. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J Biol Chem. 2004;279:13293–13296. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 21.Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, et al. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol. 2004;557:719–731. doi: 10.1113/jphysiol.2004.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal E, Fei YJ, Sugawara M, Miyauchi S, Zhuang L, et al. Expression of slc5a8 in kidney and its role in Na+-coupled transport of lactate. J Biol Chem. 2004;279:44522–44532. doi: 10.1074/jbc.M405365200. [DOI] [PubMed] [Google Scholar]
- 23.Gopal E, Fei YJ, Miyauchi M, Zhuang L, Prasad PD, Ganapathy V. Sodium-coupled and electrogenic transport of B-complex vitamin nicotinic acid by slc5a8, a member of the Na/glucose co-transporter gene family. Biochem J. 2005;388:309–316. doi: 10.1042/BJ20041916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gopal E, Miyauchi S, Martin PM, Ananth S, Roon P, et al. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res. 2007;24:575–584. doi: 10.1007/s11095-006-9176-1. [DOI] [PubMed] [Google Scholar]
- 25.Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor-suppressive function of the transporter. Life Sci. 2006;78:2419–2425. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Thangaraju M, Cresci G, Itagaki S, Mellinger J, Browning DD, et al. Sodium-coupled transport of the short-chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg. 2008;12:1773–1782. doi: 10.1007/s11605-008-0573-0. [DOI] [PubMed] [Google Scholar]
- 27.Thangaraju M, Gopal E, Martin PM, Ananth S, Smith SB, et al. SLC5A8 triggers tumor cell apoptosis through pyruvate-dependent inhibition of histone deacetylases. Cancer Res. 2006;66:11560–11564. doi: 10.1158/0008-5472.CAN-06-1950. [DOI] [PubMed] [Google Scholar]
- 28.Thangaraju M, Carswell KN, Prasad PD, Ganapathy V. Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition of HDAC1/HDAC3. Biochem J. 2009;417:379–389. doi: 10.1042/BJ20081132. [DOI] [PubMed] [Google Scholar]
- 29.Itagaki S, Gopal E, Zhuang L, Fei YJ, Miyauchi S, et al. Interaction of ibuprofen and other structurally related NSAIDs with the sodium-coupled monocarboxylate transporter SMCT1 (SLC5A8) Pharm Res. 2006;23:1209–1216. doi: 10.1007/s11095-006-0023-1. [DOI] [PubMed] [Google Scholar]
- 30.Karunakaran S, Umapathy NS, Thangaraju M, Hatanaka T, Itagaki S, et al. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem J. 2008;414:343–355. doi: 10.1042/BJ20080622. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB231. Cancer Res. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 32.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 33.Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 34.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J Biol Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 35.Goffin J, Eisenhauer E. DNA methyltransferase inhibitors – state of the art. Ann Oncol. 2002;13:1699–1716. doi: 10.1093/annonc/mdf314. [DOI] [PubMed] [Google Scholar]