Abstract
Red-green colour blindness, which results from the absence of either the long- (L) or middle- (M) wavelength-sensitive visual photopigments, is the most common single locus genetic disorder. Here, the possibility of curing colour blindness using gene therapy was explored in experiments on adult monkeys that had been colour blind since birth. A third type of cone pigment was added to dichromatic retinas, providing the receptoral basis for trichromatic colour vision. This opened a new avenue to explore the requirements for establishing the neural circuits for a new dimension of colour sensation. Classic visual deprivation experiments1 have led to the expectation that neural connections established during development would not appropriately process an input that was not present from birth. Therefore, it was believed that treatment of congenital vision disorders would be ineffective unless administered to the very young. Here, however, addition of a third opsin in adult red-green colour-deficient primates was sufficient to produce trichromatic colour vision behaviour. Thus, trichromacy can arise from a single addition of a third cone class and it does not require an early developmental process. This provides a positive outlook for the potential of gene therapy to cure adult vision disorders.
Gene therapy was performed on adult squirrel monkeys (Saimiri sciureus) that were missing the L opsin gene. In this species, some females have trichromatic colour vision while males are red-green colour blind2. Serotype 2/5 recombinant adeno-associated virus (rAAV) containing a human L-opsin gene under control of the L/M opsin enhancer and promoter (Fig. 1a) was delivered to the photoreceptor layer via subretinal injections (see Full Methods online). Transcriptional regulatory elements were chosen to direct expression preferentially in M cones, but not short- (S) wavelength-sensitive cones or rods 3. To provide the receptoral basis for trichromacy, animals received three 100 μL injections (containing a total of 2.7×1013 viral particles) in each eye which produced a relatively uniform, third submosaic of approximately 15–36% of M cones that coexpressed the transgene (Fig. 1e, f).
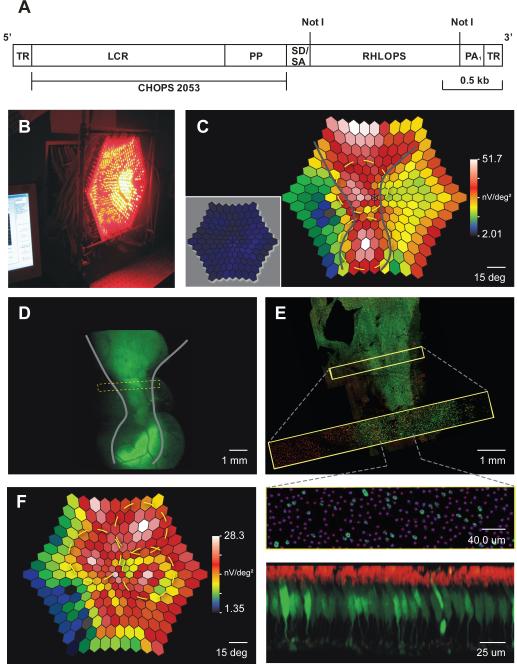
Fig. 1.
rAAV2/5 vector produced functional L-opsin in primate retina. a, Molecular map; TR = terminal repeats; LCR = locus control region; PP = proximal promoter; SD/SA = splice donor/acceptor; RHLOPS = recombinant human L opsin cDNA; PA1 = polyadenylation signal. b, Red light mf-ERG stimulus. c, Mf-ERG 40 weeks after two injections (yellow circles) of a mixture of L-opsin- and GFP-coding viruses. Grey lines show borders of highest response; for comparison, inset = mfERG 16 weeks post-injection; there was no reliable signal from L-opsin, unchanged from baseline. High responses in far peripheral retina were measured reliably and may have originated from offshoot of one of the injections. d, Fluorescence photographs from a similar retinal area as c; grey lines from c were copied in d. e, Confocal microscopy revealed a mosaic pattern of GFP expression in 5–12% of cones. Because GFP-coding virus was diluted to 1/3 compared to L-opsin virus, an estimated 15–36% of cones in behaviourally tested animals express L-opsin. f, Mf-ERG from a behaviourally tested animal 70 weeks after 3 injections of L-opsin virus.
Prior to treatment, monkeys were trained to perform a computer-based colour vision test, the Cambridge Colour Test4,5, which was modified for use with animals6 (Fig. 2a). Dichromats who are missing either the L- or M-photopigment fail to distinguish from grey: colours near the so-called “spectral neutral point” located in the blue-green region of colour space (near dominant wavelength (DW) 490 nm) and complementary colours near the “extra-spectral neutral point,” in the red-violet region (near DW = −499 nm). While trichromats have four main hue percepts – blue, yellow, red, and green – dichromats have only two percepts, nominally blue and yellow. Before treatment, two dichromatic monkeys completed three colour vision tests consisting of 16 hues (Fig. 2b, c). Four-to-six months was required to test all 16 hues; thus, baseline results represent testing conducted for more than a year. As predicted, prior to treatment monkeys had low thresholds (averaging < 0.03 units in u', v' colour space) for colours that represent blues and yellows to their eyes, but always failed to discriminate the blue-green (DW = 490 nm) and red-violet hues (DW = −499 nm) with thresholds extrapolated from psychometric functions being orders of magnitude higher (Fig. 2b, c). Results were highly repeatable, with no improvement between the first and third tests, making us confident that animals would not spontaneously improve in the absence of treatment.
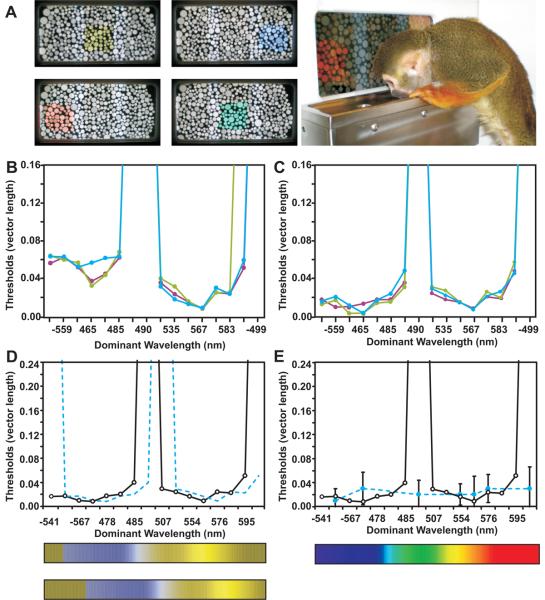
Fig. 2.
Pre-therapy colour vision and possible treatment outcomes. a, Colour vision stimuli examples. b, Pre-therapy results, monkey 1. Hues tested are represented as dominant wavelengths (DWs) rather than u', v' coordinates. If a hue could not be reliably distinguished at even the highest saturation, the extrapolated threshold approached infinity. c, Pre-therapy results, monkey 2. de, Possible experimental outcomes: Monkeys could have a relative increase in long-wavelength sensitivity, but remain dichromatic (dashed lines, d); theoretical colour spectrum appearances for a dichromat and a possible “spectral shift” are shown. Alternatively, dichromatic monkeys could become trichromatic. Results from a trichromatic female control monkey are plotted (dashed line, e; error bars = SEM and n varied from 7–11).
Co-expressing the L-opsin transgene within a subset of endogenous M-cones shifted their spectral sensitivity to respond to long wavelength light, thus producing two distinct cone types absorbing in the middle-to-long wavelengths, as required for trichromacy. The spectral sensitivity shift was readily detected using a custom-built wide-field colour multifocal electroretinogram (mf-ERG) system (Fig. 1b, c, f) (see ref. 7 for details). In preliminary experiments, validity of the colour mf-ERG was tested using an animal that had received a mixture of the L-opsin-coding virus plus an identical virus, except that a green fluorescent protein (GFP) gene replaced the L-opsin gene. As reported previously, faint GFP fluorescence was first detected at 9 weeks post-injection, and it continued to increase in area and intensity through 24 weeks8. While faint signs of GFP were first detectable at 9 weeks, L-opsin levels sufficient to produce suprathreshold mf-ERG signals were still not present at 16 weeks post-injection (Fig. 1c, inset). After GFP fluorescence became robust, the red light mf-ERG, which indicates responses from the introduced L-opsin, showed highly elevated response amplitudes in two areas (Fig. 1c) corresponding to locations of subretinal injections (Fig. 1d).
The two dichromatic monkeys who participated in behavioural tests of colour vision were treated with only L-opsin-coding virus. While the elongated pattern produced by two injections in Fig. 1c and d allowed mf-ERG validation, the treatment goal was to produce a homogeneous region, as resulted from 3 injections shown in f, where the highest mf-ERG response covered about 80° of central retina, roughly the area for which humans have good red-green discrimination. These results demonstrate that gene therapy changed the spectral sensitivity of a subset of the cones. A priori, there were two possibilities for how a change in spectral sensitivity might change colour vision behaviour: 1) animals may have an increase in sensitivity to long-wavelength light, but if the neural circuitry for extracting colour information from the nascent “M+L cone” submosaic was absent, they would remain dichromatic, the hallmark of which is having two hues that are indistinguishable from grey (Fig. 2d). The spectral neutral point for individuals that have only S- and M-cones, (e.g. monkeys 1 and 2 pre-therapy), occurs near dominant wavelength (DW) = 495 nm. At the limit, an increase in spectral sensitivity would shift the monkeys' neutral point toward that of individuals with only S and L cones, near DW = 505 nm (dashed blue lines, Fig. 2d). 2) The second, more engaging possibility was that treatment would be sufficient to expand sensory capacity in monkeys, providing them with trichromatic vision. In this case, the animals' post-therapy results would appear similar to Fig. 2e, obtained from a trichromatic female control monkey.
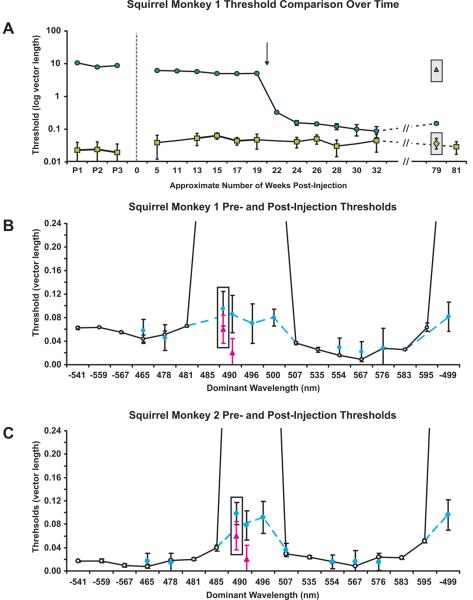
Daily testing continued after treatment. After about 20 weeks post-injection (arrow, Fig. 3a), the trained monkeys' thresholds for blue-green and red-violet (DWs = 490 and −499nm, respectively, Fig. 3b, c) improved, reducing to an average of 0.08 units in u', v' colour space, indicating that they gained trichromatic vision. This time point corresponded to the same period in which robust levels of transgene expression were reported in the squirrel monkey8. A trichromatic female monkey and untreated dichromatic monkeys were tested in parallel. As expected, the female had low thresholds for all colours, averaging < 0.03 units in u', v' colour space, but the untreated dichromats always failed to discriminate DWs = 490 nm (triangle, Fig. 3a) and −499 nm, indicating a clear difference between treated and untreated monkeys.
Fig. 3.
Gene therapy produced trichromatic colour vision. a, Time course of thresholds for the blue-green confusion colour, DW = 490 nm (circles), and a yellowish colour, DW = 554 nm (squares). A logarithmic scale was used to fit high thresholds for DW = 490 nm; significant improvement occurred after 20 weeks. Enclosed data points = untreated dichromatic monkey thresholds, DW = 490 nm (triangle) and DW = 554 nm (diamond). b–c, Comparison of pre-therapy (open circles, solid line) and post-therapy thresholds (solid dots, dashed line). Enclosed data points are DW = 490 nm thresholds when tested against a red-violet background (DW = −499 nm); pink triangles = trichromatic female control thresholds. Error bars = SEM; n varied from 7–11.
Early experiments in which we obtained negative results served as “sham controls,” demonstrating that acquiring a new dimension of colour vision requires a shift in spectral sensitivity that results from expression of an L pigment in a subset of M cones. Using similar subretinal injection procedures, we delivered fewer viral particles of an L-opsin-coding rAAV2/5 virus with an extra 146 base pair (bp) segment near the splice donor/acceptor site that had been carried over from the cloning vector and that was absent in the GFP-coding rAAV2/5 virus. The 146 bp segment contained an ATG and a duplicate mRNA start site that may have interfered with expression (see Full Methods online). Three monkeys received injections of this vector, containing an average of 1.7×1012 virus particles per eye, and no reliable changes in spectral sensitivity were measured using the ERG. One animal was also tested behaviourally and his colour vision was unchanged from baseline 1 year after injection. In subsequent experiments reported here, we removed the extra 146 bp segment and also increased the amount of viral particles delivered per eye by approximately 16-fold, to 2.7×1013. Negative results from earlier injections demonstrated that the subretinal injection procedure itself does not produce changes in the ERG or in colour vision.
The change in spectral sensitivity measured with the mf-ERG is necessary but not sufficient to produce a new colour vision capacity. For example, individuals with L but no M cones (termed deuteranopes) have a relatively enhanced sensitivity to red light but they are still as dichromatic as individuals with M but no L cones (protanopes), in that they are unable to distinguish particular “colours” from grey. To verify that the behavioural change observed in animals expressing the L pigment transgene was not purely a shift in spectral sensitivity (see Fig. 2d), monkey 1 was also tested on DWs = 496 and 500 nm, and monkey 2 was tested on DWs 496 and 507 nm. Together, these DWs span the possible confusion points for deuteranopes and protanopes and for any intermediate dichromatic forms that could arise from expressing combinations of L and M pigments. As shown in Fig. 3b and c, both monkeys' measured thresholds for these additional hues were similar to their thresholds for DW = 490 nm, demonstrating they now lacked a spectral neutral point and have become truly trichromatic. Furthermore, treated monkeys were able to discriminate blue-green (DW = 490 nm) when it was tested against a red-violet background (DW = −499 nm), instead of the grey background, indicating that the monkeys' newly-acquired “green” and “red” percepts were distinct from one another. The treated monkeys' improvement in colour vision has remained stable for over 2 years and we plan to continue testing the animals to evaluate long term treatment effects.
Classic experiments in which visual deprivation of one eye during development caused permanent vision loss1 led to the idea that inputs must be present during development for the formation of circuits to process them. From the clear change in behaviour associated with treatment, compared both between and within subjects, we conclude that adult monkeys gained new colour vision capacities because of gene therapy. These startling empirical results provide insight into the evolutionary question of what changes in the visual system are required for adding a new dimension of colour vision. Previously, it seemed possible that a transformation from dichromacy to trichromacy would require evolutionary/developmental changes, in addition to acquiring a third cone type. For example, L and M opsin-specific genetic regulatory elements might have been required to direct the opsins into distinct cone types9 that would be recognized by L and M cone-specific retinal circuitry10, and to account for cortical processing, multi-stage circuitry11 might have evolved specifically for the purpose of trichromacy. However, our results demonstrate that trichromatic colour vision behaviour requires nothing more than a third cone type. As an alternative to the idea that the new dimension of colour vision arose by acquisition of a new L vs. M pathway, it is possible that it exploited the pre-existing blue-yellow circuitry. For example, if addition of the third cone class split the formerly S vs. M receptive fields into two types with differing spectral sensitivities, this would obviate the need for neural rewiring as part of the process of adopting new colour vision.
Some form of inherent plasticity in the mammalian visual system can be inferred from the acquisition of novel colour vision, as was also demonstrated in genetically engineered mice12; however, the point has been made that such plasticity need not imply that any rewiring of the neural circuitry has occurred 13. Similarly, given the fact that new colour vision behaviour in adult squirrel monkeys corresponded to the same time interval as the appearance of robust levels of transgene expression, we conclude that rewiring of the visual system was not associated with the change from dichromatic to trichromatic vision.
Treated adult monkeys unquestionably respond to colours previously invisible to them. The internal experiences associated with the dramatic change in discrimination thresholds measured here cannot be determined; therefore, we cannot know whether the animals experience new internal sensations of “red” and “green.” Nonetheless, we do know that evolution acts on behaviour, not on internalized experiences, and we suggest that gene therapy recapitulated what occurred during evolution of trichromacy in primates. These experiments demonstrate that a new colour vision capacity, as defined by new discrimination abilities, can be added by taking advantage of pre-existing neural circuitry and, internal experience aside, full colour vision could have evolved in the absence of any other change in the visual system except the addition of a third cone type.
Gene therapy trials are underway for Leber's congenital amaurosis14–16. Thus far, treatment has been administered to individuals who have suffered retinal degeneration from the disease. The experiments reported here are the first to use gene therapy in primates to address a vision disorder in which all photoreceptors are intact and healthy, making it possible to assess the full potential of gene therapy to restore visual capacities. Treatment allowing monkeys to see new colours in adulthood provides a striking counter-example to what occurs under conditions of monocular deprivation. For instance, it is impossible to restore vision in an adult who had grown up with a unilateral cataract. Future technologies will allow many opportunities for functions to be added or restored in the eye. While some changes may produce outcomes analogous to monocular deprivation, we predict that others, like gene therapy for red-green colour blindness, will provide vision where there was previously blindness.
Methods Summary
Confocal Microscopy
The animal in Fig. 1 c and d succumbed to respiratory illness, unrelated to gene therapy, approximately 2 years and 3 months post-injection. The retina was fixed in 4% paraformaldehyde in phosphate buffered saline (PBS), and rinsed in PBS with 10% and 30% sucrose. It was sequentially incubated with 10% Normal Donkey Serum, rabbit monoclonal antibody to M/L opsin (Chemicon AB5405), and a Cy3 (red) conjugated donkey anti-rabbit antibody (Jackson Immunoresearch). Confocal images were analyzed using ImageJ (rsbweb.nih.gov). In the middle panel of Fig. 1e, magenta dots mark cone locations, and the red anti-M/L-opsin antibody staining was removed to show GFP-expressing (green) cells more clearly.
Behavioural Colour Vision Assessment
A three-alternative forced-choice paradigm in which position and saturation of the stimulus was randomized between trials was used. Monkeys had to discriminate the location of a coloured patch of dots that varied in size and brightness, surrounded by similarly varying grey dots. When animals touched the coloured target, a positive tone sounded and a juice reward was given; the next stimulus appeared immediately. (The squirrel monkey shown in Fig. 2c is drinking a reward from a previous trial.) If the wrong position was chosen, a negative tone sounded, and a 2–3 sec “penalty time” occurred before the next trial.
For each hue, monkeys were tested on up to 11 different saturations ranging from 0.01 to 0.11 in u', v' colour space (CIE 1976) and a threshold was calculated, which was taken as the saturation required to reach a criterion of 57% correct, the value determined to be significantly greater than chance (33% correct, P = 0.05); see ref. 6 for full details. All procedures were conducted in accordance with the guidelines of the U.S. NIH regarding the care and use of animals.
Methods
Viral vector
CHOPS2053 was a 2.1 kb fragment containing the locus control region (LCR) and proximal promoter (PP) upstream of the human X-chromosome opsin gene array9,17. These elements (also known as pR2.1) have been shown to target transgene expression to mammalian L/M cones3,18. RHLOPS was a 1.2 kb fragment containing recombinant human L opsin cDNA. A clone of the human L opsin cDNA19, known as hs7, was generously provided by J. Nathans. The QuickChange kit (Stratagene) was used to convert codon 180 so that it would encode a human L pigment maximally sensitive to 562 nm20. The virus was made using the genome from rAAV serotype 2 and the capsid from serotype 5, and the preparation had 9×1013 DNase-resistant vector genome containing particles per mL. To prevent vector aggregation, 0.014% Tween 20 was added to the final vector preparation. A total of 2.7×1013 viral particles was injected per eye.
An earlier version of the L-opsin coding rAAV2/5 used in previous unsuccessful experiments contained an extra 146 base pair segment between the splice donor/acceptor site and the translational start codon of the L-opsin gene that had been carried over from the cloning vector. Because we were concerned that this fragment may have interfered with transgene expression, a second version of L-opsin rAAV2/5 in which the extra 146 bp had been removed was used in later experiments described here. In addition to modifying the vector, we also increased the amount of viral particles delivered per eye by approximately 16-fold, from 1.7×1012 to 2.7×1013. Thus, we cannot conclude from this set of experiments what exact titer of viral particles was required to produce the effects on color vision behaviour, or exactly what effects, if any, the extra 146 bp had on transgene expression in earlier unsuccessful attempts.
The single-stranded DNA genome of conventional rAAV vectors, including rAAV2/5 used here, is devoid of Rep coding sequences. Thus, the vector genome is stabilized predominantly in an episomal form; however, the potential for integration exists21. According to NIH guidelines, the viral vector used here is rated biosafety level 1 (BSL1), and animal biosafety level 1(ABSL1) meaning no special precautions were required in handling the virus or animals treated with the virus. Following treatment, squirrel monkeys had an increase in AAV antibody titers, ranging from 4–12 fold. Antibody titers remained unchanged in untreated control animals who were housed with treated animals.
Subretinal Injections
Subretinal injections were performed by a vitreo-retinal surgeon (T. B. C.) using a KDS model 210 syringe pump under a stereomicroscope. A 500 μL Hamilton Gastight (# 1750TTL) Luer Lock syringe was connected to 88.9 cm of 30 gauge teflon tubing with male Luer Lock adapters at both ends (Hamilton 30TF double hub), which was then connected to a 30 gauge Becton Dickinson Yale regular bevel cannula (ref# 511258) that was manually bent to produce a 135° angle 1.5 mm from the tip. All components were sterilized prior to use. The syringe and tubing were filled with sterile lactated Ringers solution to produce a dead volume of approximately 210 μL. Just prior to injection, 300 μL of rAAV was withdrawn using a rate of 100 μL/min.
Squirrel monkeys were anesthetized using intramuscular injections of ketamine (15 mg/kg) and xylazine (2 mg/kg); atropine (0.05 mg/kg) was also given to reduce airway secretions. The eye was dilated with 2–3 drops of tropicamide (1%) and treated with 1 drop each of betadine (5%), vigamox (0.5%), and proparacaine (1%). Subconjunctival injection of 0.1 mL of lidocaine (2%) was given and the anterior portion of the eye was exposed by performing a temporal canthotomy followed by limited conjuntival peritomy. Eyelids were held open with a speculum designed for premature infants. A temporal sclerotomy was made 1 mm posterior to the limbus with a 27 gauge needle, through which the injection cannula was inserted. Three subsequent 100 μL injections were made at different subretinal locations using an infusion rate of 1060 μL/min. Post-procedure, 0.05mL each of decadron (10 mg/mL), kenalog (40 mg/mL), and cephazolin (100 mg/mL) were injected subconjunctivaly; 1 drop each of betadine (5%) and vigamox (0.5%) and a 0.6 cm strip of tobradex (0.3% tobramycin, 0.1% dexamethasone) ointment were applied topically; 10–20 mL of subcutaneous fluids (sterile lactated Ringers) were also given. Subsequent administration of steroids and analgesics were administered as needed post-procedure for potential inflammation or discomfort.
Acknowledgments
This work was supported by the National Institutes of Health grants R01EY016861 (M.N.) and R01EY11123 (W.W.H.); Research Training Program in Vision Science Grant T32EY014537; NEI Core Grants for Vision Research P30EY01931, P30EY01730, and P30EY08571; the Harry J. Heeb Foundation, the Posner Foundation, Macular Vision Research Foundation, Foundation Fighting Blindness, Hope for Vision, and Research to Prevent Blindness. The authors would like to thank V. Chiodo, S. Boye, D. Conklyn, P. M. Summerfelt, K. Chmielewski, and K.L. Gunther for technical assistance. J. N. is the Bishop Professor in Ophthalmology, M. N. is the Ray Hill Professor in Ophthalmology, and W.W.H. is Rybaczki-Bullard Professor of Ophthalmology.
Footnotes
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
References
- 1.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs GH. A perspective on color vision in platyrrhine monkeys. Vision Res. 1998;38:3307–3313. doi: 10.1016/s0042-6989(97)00405-7. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Timmers AM, Guy J, Pang J, Hauswirth WW. Cone-specific expression using a human red opsin promoter in recombinant AAV. Vision Res. 2007;48 doi: 10.1016/j.visres.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 4.Reffin JP, Astell S, Mollon JD. Colour Vision Deficiencies X. Kluwer Academic Publishers; Dordrecht: 1991. Trials of a computer-controlled colour vision test that preserves the advantages of pseudo-isochromatic plates; pp. 69–76. [Google Scholar]
- 5.Regan BC, Reffin JP, Mollon JD. Luminance noise and the rapid determination of discrimination ellipses in colour deficiency. Vision Res. 1994;34:1279–1299. doi: 10.1016/0042-6989(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 6.Mancuso K, Neitz M, Neitz J. An adaptation of the Cambridge Colour Test for use with animals. Vis. Neurosci. 2006 doi: 10.1017/S0952523806233364. [DOI] [PubMed] [Google Scholar]
- 7.Kuchenbecker J, Sahay M, Tait DM, Neitz M, Neitz J. Topography of the long- to middle-wavelength sensitive cone ratio in the human retina assessed with a wide-field color multifocal electroretinogram. Vis. Neurosci. 2008;25:301–306. doi: 10.1017/S0952523808080474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancuso K, et al. Recombinant adeno-associated virus targets passenger gene expression to cones in primate retina. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2007;24:1411–1416. doi: 10.1364/josaa.24.001411. [DOI] [PubMed] [Google Scholar]
- 9.Nathans J, Piantanida TP, Eddy RL, Shows TB, Hogness DS. Molecular genetics of inherited variation in human color vision. Science. 1986;232:203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- 10.Shapley R. Specificity of cone connections in the retina and color vision. Focus on “Specificity of cone inputs to macaque retinal ganglion cells”. J. Neurophysiol. 2006;95:587–588. doi: 10.1152/jn.01054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeValois RL, DeValois KK. A multi-stage color model. Vision Res. 1993;33:1053–1065. doi: 10.1016/0042-6989(93)90240-w. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs GH, Williams GA, Cahill H, Nathans J. Emergence of Novel Color Vision in Mice Engineered to Express a Human Cone Photopigment. Science. 2007;315:1723–1725. doi: 10.1126/science.1138838. [DOI] [PubMed] [Google Scholar]
- 13.Makous W. Comment on “Emergence of Novel Color Vision in Mice Engineered to Express a Human Cone Photopigment”. Science. 2007;318:196. doi: 10.1126/science.1146084. [DOI] [PubMed] [Google Scholar]
- 14.Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bainbridge JW, Ali RR. Success in sight: The eyes have it! Ocular gene therapy trials for LCA look promising. Gene Ther. 2008;15:1191–1192. doi: 10.1038/gt.2008.117. [DOI] [PubMed] [Google Scholar]
- 16.Cideciyan AV, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, et al. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- 18.Mauck MC, Mauncuso K, Kuchenbecher J, Connor TB, Hauswirth WW, Neitz J, Neitz M. Longitudinal evaluation of expression of virally delivered transgenes in gerbil cone photoreceptors. Vis. Neurosci. 2008;25:273–282. doi: 10.1017/S0952523808080577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986;232:193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- 20.Neitz M, Neitz J, Jacobs GH. Spectral tuning of pigments underlying red-green color vision. Science. 1991;252:971–974. doi: 10.1126/science.1903559. [DOI] [PubMed] [Google Scholar]
- 21.Büning H, Perabo L, Coutelle O, Quadt-Humme S, Hallek M. Recent developments in adeno-associated virus vector technology. J. Gene Med. 2008;10:717–733. doi: 10.1002/jgm.1205. [DOI] [PubMed] [Google Scholar]