Abstract
Objectives
Late-life depression (LLD) is associated with persistent cognitive impairment in a subset of individuals. The purpose of this study was to 1) examine the frequency and characteristics of cognitive diagnoses (Mild Cognitive Impairment [MCI], dementia) among remitted elderly depressed subjects and 2) to compare the prevalence rate and correlates of cognitive diagnoses with those of comparison subjects.
Design
Crosssectional.
Setting
Outpatient geriatric mental health clinic.
Participants
The authors examined cognitive diagnoses among 109 subjects age 65 and older, after depression treatment response and 65 never-depressed, age- and education-equated comparison subjects.
Measurements
Cognitive diagnoses were independently established by the University of Pittsburgh’s Alzheimer’s Disease Research Center. Bivariate and multivariate analyses were conducted to examine the role of specific risk factors for cognitive diagnosis among depressed subjects.
Results
Relative to comparison subjects, nearly twice as many depressed subjects were diagnosed with MCI or dementia (48% versus 28%). Of the 109 depressed subjects, 38% were diagnosed with MCI (63% amnestic, 37% nonamnestic). The majority of amnestic MCI subjects (85%) had the multiple domain subtype. Age, but not age of onset or lifetime depression duration, predicted cognitive diagnosis.
Conclusions
Despite adequate depression treatment response, 48% of remitted depressed subjects had a cognitive diagnosis. Of the 38% diagnosed with MCI, there was high representation among both the amnestic and the nonamnestic subtypes, suggesting heterogeneity in cognitive course and outcomes in LLD.
Keywords: Late-life depression, mild cognitive impairment, dementia, diagnosis
Late-life depression (LLD) may be associated with persistent cognitive impairment in some individuals after effective treatment of depressive symptoms (e.g., Refs. 1–4). Some studies report that LLD may be associated with subsequent Mild Cognitive Impairment (MCI) and dementia (e.g., Refs. 5–8), including Alzheimer disease9–12 and vascular dementia.13,14 It is not clear whether depression represents a risk factor for or occurs in the prodromal stage of dementia (e.g., Refs. 15, 16). Thus, the nature of the relationship between depression and persistent cognitive impairment after resolution remains unclear and warrants further investigation.
Two longitudinal epidemiologic studies found that 13%–20% of subjects with moderate to high depressive symptoms subsequently develop MCI over an average follow-up of 3–6 years.17,18 Lyketsos et al.19 reported that 20% of individuals with MCI had concurrent depressive symptoms. However, these studies examined depressive symptoms and did not use formal research diagnostic criteria for mood disorders.
To date, no study has examined the rate of clinically diagnosed MCI and its subtypes in clinically diagnosed and treated subjects with late-life major depression using state-of-the art research diagnostic criteria. This is a particularly salient issue raised by Steffens et al.20 in the proceedings from the 2003 National Institute of Mental Health conference “Perspectives on Depression, MCI, and Cognitive Decline.” They called for increased collaboration among depression and memory disorders investigators and use of common depression and cognition assessment measures sensitive to mood and cognitive change in future studies of depression and cognition.20
Characterizing the MCI subtypes for which individuals with LLD are at risk after treatment response is important for several reasons. First, multiple mechanisms have been proposed as possible links between LLD, MCI, and dementia (e.g., Refs. 20, 21). Second, early identification and long-term follow-up of patients with MCI may provide useful information regarding course and etiology of future dementia outcomes (e.g., Refs. 7 and 21–25). Finally, as more efficacious therapies are developed, identifying MCI subtypes may also provide valuable information regarding appropriate or personalized therapeutic intervention.
Within this context, we sought to examine the frequency and characteristics of cognitive diagnoses (MCI, dementia) among clinically diagnosed and treated LLD patients using formal research diagnostic criteria. We utilized the classification system for MCI specified by Petersen,22,26 currently dictated by the National Alzheimer’s Coordinating Center,27 and in use in a host of dementia-related studies. This system includes four MCI subtypes: MCI-amnestic-single domain, MCI-amnestic-multiple domain, MCI-nonamnestic-single domain, and MCI-nonamnestic-multiple domain. On the basis of our previous studies,1–3 we predicted that the remitted depressed subjects would be more likely to be diagnosed with MCI than the comparison group. Because of the multiple, varied potential mechanisms and pathways between LLD and cognitive impairment, we hypothesized that after treatment response, approximately half of our subjects diagnosed with MCI would meet criteria for both the amnestic and nonamnestic forms of MCI.
METHODS
Subjects
After successful depression treatment response, we evaluated cognitive functioning of 109 consecutive elderly subjects without previous formal dementia diagnosis, who previously met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for a current episode of unipolar major depression (nonpsychotic). These subjects were required to have had a 17-item Hamilton Depression Rating Scale (HDRS28) score of ≤10 for 3 consecutive weeks to be included in the study as treatment responders. We also examined the cognitive functions of 65 age- and education-equated comparison subjects with no psychiatric history. We have previously reported on the cognitive outcomes, but not official cognitive diagnoses, of 40 of these comparison subjects.1
Depressed subjects were drawn from an ongoing maintenance intervention trial conducted within the Advanced Center for Intervention and Services Research Center for Late-Life Mood Disorders at the University of Pittsburgh. Depressed subjects were recruited through primary care physician referral for suspected depression. Comparison subjects were recruited from a variety of sources, including institutional review board-approved advertisements, community presentations, word of mouth, physician/clinician referral, and partnerships with community agencies. Depressed subjects’ cognitive data have not been presented elsewhere. Briefly, these studies include English-speaking subjects ≥65 years meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for a major depressive episode (nonpsychotic and nonbipolar) and a HDRS-17 score ≥15, with an available informant to provide corroborating information. Exclusion criteria include prior formal dementia diagnosis at study entry, substance abuse/dependence within the last year, electro-convulsive therapy within the previous 6 months, and a history of nontolerance to selective serotonin reuptake inhibitor pharmacotherapy. Comparison subjects met the same exclusion criteria and had no lifetime history of Axis I psychiatric disorder.
All depressed subjects were taking escitalopram 10–20 mg/day (N = 76), duloxetine 60–120 mg/day (N = 26), or venlaflaxine 75–300 mg/day (N = 6), and one subject was taking both escitalopram (20 mg) and venlaflaxine (225 mg/day) at the time of assessment. A substantial minority of our subjects were also taking benzodiazepines (N = 35; 33%). There was no significant difference in benzodiazepine usage between LLD treatment responders diagnosed with a cognitive disorder and those without a cognitive disorder (χ2 [1, N = 109] = 2.43, p = 0.12).
After treatment response, subjects were administered a comprehensive neuropsychological evaluation assessing multiple domains of cognitive functioning, including delayed recall, executive functioning, information processing speed, visual-spatial ability, and language ability (see Ref. 29). The median delay between meeting eligibility requirements (HAM-D ≤10 for 3 consecutive weeks) and the time of neuropsychological testing was 2.9 weeks (mean: 3.7 weeks; SD: 2.8; range: 0.1 – 13.0 weeks). Moreover, all subjects had to have a HDRS score of ≤10 on the day of the neuropsychological assessment to minimize the influence of residual depressive symptoms on cognitive performance.
To objectively assess instrumental activities of daily living performance, we administered the Performance Assessment of Self-Care Skills (PASS). The PASS is a performance-based, criterion-referenced tools designed to measure the disability in 26 basic and instrumental activities of daily living.30–32 Performance on each activity is observed and rated for independence, safety, and adequacy on a scale ranging from 0 to 3. Higher scores indicated greater independence, safety, and adequacy. The level of assistance provided to initiate, continue, and complete an activity are also recorded. The types of assistance range from 0 to 9, with higher levels indicating more intrusive/powerful assistance (e.g., 1 = verbal encouragement, 9 = total assist). The PASS has demonstrated adequate reliability and validity in various medical populations.30–32
Neuropsychological and magnetic resonance imaging data, clinical history, and PASS results were then forwarded to the University of Pittsburgh’s Alzheimer’s Disease Research Center (ADRC) for independent consensus adjudication of cognitive status. In brief, to diagnose MCI, the ADRC does not adhere to specific cutoff scores on neuropsychological measures, although most subjects’ raw score performance was between 1 and 2 standard deviations below that of healthy ADRC age and education-equated comparison subjects, with no accompanying functional decline. All subjects had an informant who provided information about cognitive and functional abilities and all clinicians completed the Clinical Dementia Rating (CDR) based on this interview. Lastly, all subjects in the study participated in an objective in-home instrumental activities of daily living assessment conducted by an occupational therapist. All of this information, in addition to the magnetic resonance imaging scan, was reviewed by ADRC clinicians during the diagnostic adjudication process and before conferring cognitive diagnosis. All depressed and comparison subjects were adjudicated with one of six possible classifications according to the National Alzheimer’s Coordinating Center Uniform Data Set criteria:27 No Cognitive Disorder, MCI-Amnestic-Single Domain, MCI-Amnestic-Multiple Domain, MCI Nonamnestic-Single Domain, MCI Nonamnestic-Multiple Domain, and Dementia. ADRC raters were not blind to whether they were examining data for depressed or comparison subjects. The University of Pittsburgh’s ADRC has a long history of adjudicating cognitive diagnoses with a high degree of accuracy (from 1990 to 2000, sensitivity = 98%, and specificity = 88%).33
RESULTS
There were no differences between LLD and comparison subjects in age or race; however, the depressed group had a higher percentage of women. The depressed subjects had more pretreatment depressive symptoms, more residual, subsyndromal posttreatment depressive symptoms, greater medical burden (Cumulative Illness Rating Scale-Geriatrics,34 and lower Mini Mental Status Examination [MMSE35] scores than did comparison subjects) (Table 1). Six depressed (four No Cognitive Disorder, one MCI amnestic multiple domain, and one MCI nonamnestic multiple domain) and two comparison subjects (both No Cognitive Disorder) had neuroimaging evidence of minor lacunar or larger infarcts. Forty-one percent of depressed subjects had early onset depression (first lifetime episode ≤ age 60) and 59% had late-onset depression (first lifetime episode >60; 20% recurrent).
TABLE 1.
Demographic and Clinical Measures by Diagnostic Group
| Treatment Responders (N = 109) | Controls (N = 65) | T or χ2 | df | p | |
|---|---|---|---|---|---|
| Age | 74.7 (5.6) | 74.0 (5.6) | −0.65 | 172 | 0.52 |
| Men, % (n) | 21 (23) | 35 (23) | 4.27 | 1 | 0.039 |
| White, % (n) | 89 (97) | 83 (54) | 1.24 | 1 | 0.27 |
| Education in years | 13.7 (2.6) | 13.9 (2.6) | 0.57 | 172 | 0.57 |
| CIRS-G total | 10.3 (3.2) | 6.5 (3.4) | −7.18 | 158 | 0.0001 |
| Initial HDRS (T1) | 18.5 (3.2) | 2.4 (1.8) | −36.46 | 171 | 0.0001 |
| Posttreatment HDRS (T2) | 5.8 (2.5) | 2.5 (1.9) | −9.28 | 171 | 0.0001 |
| Late onset, % (n) | 58.7 (64) | — | — | — | — |
| MMSE | 28.2 (1.9) | 28.9 (1.2) | 2.77 | 171 | 0.006 |
Notes: Comparison of LLD treatment responders with the comparison group on basic demographic and clinical variables. CIRS-G: Cumulative Illness Rating Scale for Geriatrics; HDRS: Hamilton Depression Rating Scale; MMSE: Mini Mental Status Examination.
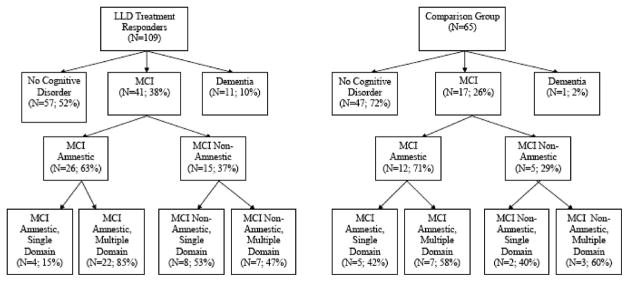
A greater percentage of LLD subjects (52 of 109; 48%) than comparison subjects (18 of 65; 28%) had a cognitive diagnosis after adjusting for differences in gender distribution (Cochran-Mantel-Haenszel [CMH] χ2 [1, N = 174] = 9.06, p ≤0.003; Cohen’s h effect size = 0.42). Thirty-eight percent (41 of 109) of depressed and 26% (17 of 65) of comparison subjects had MCI, and 10% (11 of 109) of depressed versus 2% (1 of 65) of comparison subjects were adjudicated as demented (Cochran-Mantel-Haenszel χ2 [3, N = 174] = 11.08, p ≤0.013) (Fig. 1).
FIGURE 1.
Flow Chart of ADRC Cognitive Classification
Sixty-three percent (26 of 41) of depressed subjects with MCI had the amnestic form of MCI, whereas 37% (15 of 41) were nonamnestic. Among those with the amnestic form, 85% (22 of 26) had the multiple-domain subtype. There was a more equal split among those depressed subjects with nonamnestic MCI; 47% (7 of 15) had the multiple-domain subtype and 53% (8 of 15) had the single-domain subtype.
In the comparison group, 72% (47 of 65) of subjects were cognitively normal. Of those with MCI, 71% (12 of 17) had the amnestic form and 29% (5 of 17) had the nonamnestic form.
Of the 11 depressed subjects diagnosed with dementia, 82% (9 of 11) met criteria for possible or probable Alzheimer disease (AD), and the only comparison subject with dementia (1 of 65; 2%) met criteria for probable AD.
In bivariate analysis, we found that older (>74 years) subjects (65%; 35 of 54) were more likely to be diagnosed with MCI or dementia than younger (≤ 74 years) subjects (31%; 17 of 55) (χ2 [1, N = 109] = 13.73, p ≤ 0.01) and subjects with late-onset depression were more likely to be diagnosed with MCI or dementia (56%; 36 of 64) than those with early onset LLD (35%; 16 of 45)(χ2 [1, N = 109] = 4.54, p ≤ 0.05). A majority of the depressed subjects meeting criteria for dementia (82%; 9 of 11) had late-onset depression. There was no difference between depressed subjects with shorter (≤ 10 years) and longer (>10 years) lifetime duration of depression (χ2 [1, N = 109] = 2.70, p >0.05).
We performed a logistic regression analysis to better understand the independent role of specific risk factors (age, age of onset, lifetime duration of depression) in predicting cognitive diagnosis (MCI or dementia) in LLD. In separate models, the age by age-of-onset interaction or the age by lifetime duration of depression interaction did not independently predict diagnosis. In a reduced model entering age, age of onset and lifetime duration of depression, analyses revealed that only older age predicted cognitive diagnosis (Wald χ2 [1, N = 109] = 9.26, p ≤ 0.01; odds ratio = 3.72; 95%CI = 1.60–8.68).
We compared LLD subjects classified with No Cognitive Disorder (N = 57) to those classified with MCI-Amnestic (N = 26) and MCI Nonamnestic (N = 15) subtypes, on relevant demographic and clinical variables. No Cognitive Disorder subjects were significantly younger (F[2, 95] = 5.78, p ≤ 0.005) and had higher MMSE scores (F[2, 95] = 3.95, p ≤ 0.005) than either the MCI-Amnestic or MCI Nonamnestic subjects. The three groups did not differ on education, coexisting medical burden, age of lifetime onset of depression, percent of subjects with a recurrent depressive episodes, severity of index depressive episode, or severity of residual depressive symptoms (Table 2).
TABLE 2.
Demographic and Clinical Measures in LLD Treatment Responders by Cognitive Classification
| MCI-Amnestic (N = 26) | MCI-Nonamnestic (N = 15) | Cognitively Normal (N = 57) | F or χ2 | df | p | |
|---|---|---|---|---|---|---|
| Age | 76.4 (5.5) | 76.7 (7.4) | 72.3 (5.9) | 5.78 | 2.95 | 0.004 |
| Men, % (n) | 23 (6) | 20 (3) | 21 (12) | 0.06 | 2 | 0.97 |
| White, % (n) | 88 (23) | 73 (11) | 95 (54) | 6.00 | 2 | 0.049 |
| Education in years | 12.8 (2.0) | 13.2 (2.5) | 14.1 (2.8) | 2.48 | 2.95 | 0.090 |
| CIRS-G total | 10.3 (2.5) | 11.1 (3.1) | 10.3 (3.5) | 0.38 | 2.89 | 0.69 |
| Initial HDRS | 19.0 (3.2) | 18.5 (3.0) | 18.2 (3.3) | 0.55 | 2.95 | 0.58 |
| Residual HDRS | 6.2 (2.4) | 6.1 (2.1) | 5.3 (2.7) | 1.36 | 2.95 | 0.26 |
| Recurrent, % (n) | 48 (10) | 38 (5) | 64 (30) | 338 | 2 | 0.18 |
| Age of onset | 58.5 (20.0) | 63.3 (24.3) | 54.2 (18.9) | 1.34 | 2.95 | 0.27 |
| Late onset, % (n) | 58 (15) | 80 (12) | 49 (28) | 4.63 | 2 | 0.10 |
| MMSE | 28.2 (1.3) | 28.1 (1.5) | 28.9 (1.2) | 3.95 | 2 | 0.02 |
Notes: Comparison of LLD patients diagnosed with No Cognitive disorder, MCI-Amnestic, and MCI-Nonamnestic, after treatment response, on basic demographic and clinical variables. The dementia group was excluded due to small sample size.
CIRS-G: Cumulative Illness Rating Scale for Geriatrics; HDRS: Hamilton Depression Rating Scale; MMSE: Mini Mental Status Examination.
DISCUSSION
The purpose of this study was to describe the patterns of clinically diagnosed MCI in clinically diagnosed and treated elderly depressed subjects. Our findings revealed that 1) approximately 50% of depressed subjects had a cognitive diagnosis when compared with 28% of comparison subjects and 2) although there were high rates of both, relatively more depressed subjects were diagnosed with the amnestic than the nonamnestic subtype of MCI. Of those with memory impairment, the majority met criteria for the multiple domain subtype. Previous clinical dementia diagnosis was an exclusion criterion for this study; nonetheless, based on our evaluation, 10% of depressed subjects were ultimately diagnosed with dementia relative to 2% of comparison subjects.
An important remaining challenge is to examine the longitudinal course of cognitive impairment. An extensive literature suggests that some forms of LLD may represent either an independent risk factor for AD (e.g., Ref. 6) or the prodromal stage of dementia (e.g., Ref. 16). Moreover, a recent neuropathological study from our research group revealed that among elderly patients treated for depression who subsequently developed dementia, AD predominated,36 although a large proportion of subjects had cooccurring cerebrovascular disease and Lewy bodies.
The present study, similar to reports in the MCI and dementia literature, suggests that older age is the best predictor of cognitive diagnosis in LLD. Moreover, our data revealed that among those depressed subjects clinically diagnosed with dementia, AD predominated (9 of 11; 82%).
Other literature suggests that elders with depression, particularly those with later lifetime onset of depressive symptoms, may be at risk for vascular dementia or that vascular lesions may hasten the presentation of a preexisting, but not yet manifest dementia, such as AD (e.g., Refs. 13, 14 and 37). Although six depressed subjects in the current study had neuroimaging evidence of minor lacunar infarcts, no subjects, including those with late-onset depression, were formally adjudicated with a diagnosis of vascular dementia.
In the current study, bivariate analyses revealed that subjects with late lifetime onset of depressive symptoms were more likely to be diagnosed with MCI or dementia than those with early onset, recurrent depression. However, when entered together into a logistic regression analyses with age and lifetime duration of depression, only age, and not age of onset or lifetime duration of depression predicted cognitive diagnosis.
Consistent with this finding, more specific analyses at the MCI subtype level revealed that only age, and neither differences in age of onset of depressive symptoms nor the percentage of subjects with late-onset depression, differentiated depressed subjects diagnosed with amnestic MCI, nonamnestic MCI, or No Cognitive Disorder. Thus, individuals with early versus late onset LLD seem to have comparable rates of cognitive impairment after resolution of depressive symptoms. We were unable to determine a specific pattern of cognitive impairment that differentiated early from late-onset subjects, crosssectionally.
We are planning to examine whether the cognitive course of subjects with amnestic versus nonamnestic impairment differs over time. One recent study found that while subjects with either amnestic or nonamnestic impairment converted to AD, amnestic subjects converted faster and those with nonamnestic impairment were more likely to remain cognitively stable over a 30-month follow-up.25
It will also be important to examine the longitudinal cognitive course of those depressed subjects diagnosed with No Cognitive Disorder. Some may cognitively decline over time, whereas others will likely remain cognitively normal. Elucidating the factors that influence preservation of cognitive ability in this subgroup will be particularly important.
Although LLD subjects had achieved treatment response for a median of 2.9 weeks before assessment in the current study, we followed an independent, nonoverlapping group of remitted LLD patients for 1 year in a previous study and reported significant persistent cognitive impairment compared with nondepressed comparison subjects.1 Because the cognitive symptoms associated with LLD seem to be persistent in a subgroup of patients, and may be progressive, follow-up studies will allow us to examine future course of cognitive outcomes and the predictive value of MCI subtypes. A subgroup of our patients may experience cognitive improvement with continued and sustained remission of their depressive symptoms and may revert to a diagnosis of No Cognitive Disorder in the future, as occurs at relatively high rates among nondepressed community dwelling elderly (e.g., Ref. 38). However, in our previous report,1 we found that very few depressed individuals went from cognitively impaired to cognitively normal over 1 year follow-up, especially compared with the relatively large proportion that went from cognitively normal to cognitively impaired. Nevertheless, we will carefully monitor how MCI rates change over time in these subjects and will report on the rates of conversion and reversion in subsequent studies. Together, these studies will have important implications for treatment and intervention.
Finally, it is important to note that a large proportion (26%) of our comparison subjects was diagnosed with MCI. This rate is greater than that reported in most epidemiological studies. The Cardiovascular Health Study Cognition Study reported 19% prevalence of MCI in the community and 22% in the Pittsburgh, PA, sample. This rate increased to 28% when considering subjects older than 75 years in the Pittsburgh, PA, sample. However, this elevated MCI rate was largely attributable to the higher rate of MCI among individuals aged 80–84 and 85 and older.39 The mean age of our comparison subjects was younger (74 years) and cannot completely explain the high rate of MCI among our comparison subjects. It is possible that the methods for recruiting the comparison subjects may have inadvertently led to the high rate of MCI diagnoses. Many of our comparison subjects were recruited for a study involving neuropsychological functioning in a university-based depression research center. It is possible that many of these subjects already had concerns about their cognitive functioning before participation in our study. Also, many of our comparison subjects were recruited from partnerships with primary care physicians who may have had concerns about the subjects’ cognitive abilities, reflecting a potential sampling bias issue. It is noteworthy that our depressed subjects were recruited from the same sources and may have also had concerns about their cognitive functioning. This is highlighted by the finding that although formal diagnosis of dementia was an exclusion criterion, 10% of our depressed subjects seem to have had undiagnosed dementia despite overall posttreatment mean MMSE scores of 28.2 for the group (mean posttreatment MMSE score for theses 11 subjects was 24.8). Nonetheless, the ADRC used the same objective adjudication process for all subjects in the current study, and we are confident that, although high relative to other reports in the literature, the rate of MCI accurately reflects the cognitive difficulties observed in our comparison subjects.
Strengths of this study include subject recruitment from primary care settings, rigorous depression diagnosis, protocolized depression treatment to complete response, and independent adjudication of cognitive status by the ADRC. Limitations include the cross-sectional, clinic-based nature of the design; we are continuing to follow these subjects and will report on their future cognitive outcomes. In particular, the cross-sectional nature of our study precludes us from making statements about the temporal relationship between the onset of the depressive episode and cognitive impairment in these subjects (i.e., whether the cognitive impairment developed before or concurrently with the depressive episode). The relative lack of racial diversity in our sample limits the generalizability of our findings. The subjects in this study were recruited from primary care offices and were identified by their physicians as likely depressed. Subjects’ cognitive functioning was not a factor related to recruitment. Nonetheless, it should be noted that our results relate specifically to elderly depressed individuals who agreed to participate in a study conducted within a university-based geriatric mental health specialty clinic.
In summary, despite adequate depression treatment response, LLD subjects were more likely to be diagnosed with MCI or dementia than comparison subjects. Approximately 40% of our elderly depressed group was diagnosed with MCI. Of these, a significant proportion had nonamnestic MCI. Of those with amnestic MCI, the majority met criteria for the multiple cognitive domain subtype. Larger, prospective studies, using cutting-edge neurodiagnostic tools (e.g., amyloid imaging, neuropathologic analyses of vascular lesions) and longitudinal follow-up may elucidate the course and pathways between LLD and both MCI and future dementia.
Acknowledgments
The authors thank Drs. Judith A. Saxton, William Klunk, Robert Sweet, and David Wolk of the ADRC for their assistance in the adjudication of subjects. They also thank the ACISR/LLMD clinicians for providing relevant clinical history and the staff of the Geriatric Neuropsychology Research Program for providing relevant information required for ADRC adjudication.
This work was supported in part by USPHS grants P30 MH071944, R37 MH43832, R01 MH 072947, T32 MH19986, and P50 AG05133.
Footnotes
Data in this manuscript were presented at the 35th Annual Meeting of the International Neuropsychological Society, Portland, Oregon, February 2007 and the Annual Meeting of the American Association of Geriatric Psychiatry, New Orleans, Louisiana, March 2007. Dr. Becker serves as a consultant for Grifols. Dr. Lopez has received honoraria and serves as a consultant for Forest Laboratories, Grifols, Servier, and Eisai/Pfizer. Dr. Reynolds has received pharmaceutical supplies from GlaskoSmithKline, Forest Laboratories, Pfizer, Eli Lilly, and Bristol-Myers Squibb. Dr. DeKosky receives research support from Elan, Myriad, Neurochem, and ONO. He serves on the advisory boards of AstraZeneca, Baxter, Myriad, NeuroMedix, NeuroPharma, and Cephalon. Dr. DeKosky also serves as a consultant for Eisai, Forest Laboratories, GlaskoSmithKline, Eli Lilly, Merck, Pfizer, and Servier. Drs. Bhalla, Butters, Aizenstein, and Snitz have no conflicts of interest to disclose.
References
- 1.Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 2.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 3.Nebes RD, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 4.Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. Am J Geriatr Psychiatry. 2004;12:50–56. [PubMed] [Google Scholar]
- 5.Steffens DC, Welsh-Bohmer KA, Burke JR, et al. Methodology and preliminary results from the neurocognitive outcomes of depression in the elderly study. J Geriatr Psychiatry Neurol. 2004;17:202–211. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- 6.Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabryelewicz T, Styczynska M, Luczywek E, et al. The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry. 2007;22:563–567. doi: 10.1002/gps.1716. [DOI] [PubMed] [Google Scholar]
- 8.Jean L, Simard M, van Reekum R, et al. Differential cognitive impairment in subjects with geriatric depression who will develop Alzheimer’s disease and other dementias: a retrospective study. Int Psychogeriatr. 2005;17:289–301. doi: 10.1017/s1041610205001511. [DOI] [PubMed] [Google Scholar]
- 9.Speck CE, Kukull WA, Brenner DE, et al. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995;6:366–369. doi: 10.1097/00001648-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 11.Green RC, Cupples LA, Kurz A, et al. Depression as a risk for Alzheimer’s disease: the mirage study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 12.Steffens DC, Plassman BL, Helms MJ, et al. A twin study of late-onset depression and apolipoprotein E epsilon 4 as risk factors for Alzheimer’s disease. Biol Psychiatry. 1997;41:851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 15.Schweitzer I, Tuckwell V, O’Brien J, et al. Is late-onset depression a prodrome to dementia? Int J Geriatr Psychiatry. 2002;17:997–1005. doi: 10.1002/gps.525. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Ganguli M, Mulsant BH, et al. The temporal relationship between depressive symptoms and dementia: a community-based prospective study. Arch Gen Psychiatry. 1999;56:261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- 17.Barnes DE, Alexopoulos GS, Lopez OL, et al. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63:273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 18.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 19.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 20.Steffens DC, Otey E, Alexopoulos GS, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63:130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- 21.Butters MA, Young JB, Lopez OL, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 23.Busse A, Hensel A, Guhne U, et al. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 24.Bozoki A, Giordani B, Heidebrink J, et al. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 25.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 26.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 27.Washington: University of Washington; 2006. National Alzheimer’s Coordinating Center: NACC Uniform Data Set Coding Guide. [Google Scholar]
- 28.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 29.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 30.Holm MB, Rogers JC. Performance assessment of self-care skills (PASS) In: Thorofare Hemphill-Pearson B., editor. Assessments in Occupational Therapy Mental Health. 2. NJ: SLACK; 2008. pp. 101–110. [Google Scholar]
- 31.Rogers JC, Holm MB, Beach S, et al. Task independence, safety, and adequacy among nondisabled and osteoarthritis-disabled older women. Arthritis Rheum. 2001;45:410–418. doi: 10.1002/1529-0131(200110)45:5<410::aid-art359>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 32.Raina KD, Rogers JC, Holm MB. Influence of the environment on activity performance in older women with heart failure. Disabil Rehabil. 2007;29:545–557. doi: 10.1080/09638280600845514. [DOI] [PubMed] [Google Scholar]
- 33.Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades: I. Neurology. 2000;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- 34.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SW, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Sweet RA, Hamilton RL, Butters MA, et al. Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology. 2004;29:2242–2250. doi: 10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- 37.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Ganguli M, Du Y, Dodge HH, et al. Depressive symptoms and cognitive decline in late-life: a prospective epidemiological study. Arch Gen Psychiatry. 2006;63:153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- 39.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]