Abstract
Attachment of tumor cells to endothelial cells is critical for migration of tumor cells out of the vascular system to establish metastases. We have studied human melanoma cell (C8161) adhesion and migration in response to stimulation by soluble collagen IV (CIV) using a modified Boyden chamber. In this modified chamber, shear flow can be introduced over the cell-substrate interface affecting tumor cell chemotactic migration through a microporous filter. Our results suggest that transmigration of C8161 cells under flow conditions can be influenced by PMNs, mediated by Mac-1 and ICAM-1 adhesive interactions and enhanced by altered cytokine production.
Keywords: Melanoma, Neutrophil, Shear Stress, Signaling, Extravasation
2. INTRODUCTION
Active tumor cell adhesion and migration have long been recognized as necessary for invasion and metastasis (1, 2). To metastasize, tumor cells must shed into the blood stream (intravasation) directly by invasion into the tumor-derived vasculature or indirectly by lymphatic drainage, survive in the circulation, arrest on vascular endothelium via adhesion, and finally migrate through vessel barrier (extravasation) and proliferate in the target organs (Figure 1). Among those steps, tumor cell adhesion and migration play a key role in tumor metastasis. However, the precise behavior and mechanisms by which tumor cells transmigrate through endothelium remain unclear.
Figure 1.
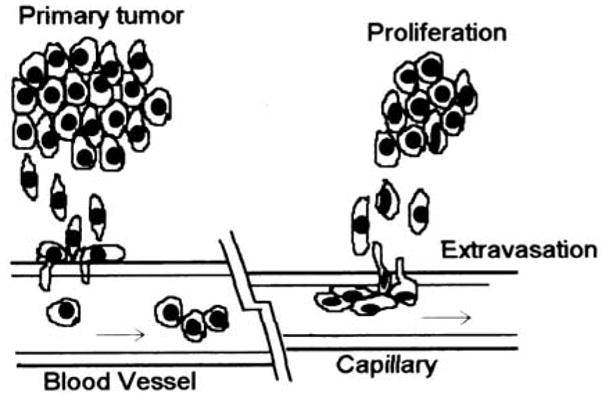
Spread of cancer from the primary site to the secondary site. Tumor cells are shown crossing the wall of a blood vessel.
Cell adhesion in shear flow is important in understanding the mechanism of cellular interaction with a vessel wall (3). Significant progress has been made in the past decade toward understanding the receptor-mediated basis of cell adhesion. In vivo experimental studies using intravital microscopy have suggested that adhesion molecules of the selectin family mediate the initial rolling of circulating cells on the endothelium, whereas firm adhesion is mediated by interactions between integrin receptors and immunoglobulin super family (4). Conventional flow chambers are often used to study cell interactions with different surface (5, 6).
Metastasizing tumor cells are likely to encounter both soluble and insoluble extracellular matrix (ECM) components as they traverse vascular walls and basement membranes. Some chemotactic factors, including ECM molecules, have been shown to stimulate the intrinsic motility of tumor cells in vitro (7, 8). These factors are believed to influence both the extent and the direction of tumor cell movement in vivo to specific target organs. Tumor cells exhibit an amoeboid movement similar to that of human polymorphonuclear (PMN) leukocytes, characterized by pseudopod protrusion at the leading edge of the cell, followed by directional locomotion toward a chemotactic source derived from the target tissue. Many in vitro methods, such as micropipette (9) and migration chamber (10, 11), have been used routinely to characterize cellular chemotaxis -- the directional migration of cells along a gradient of soluble attractant stimulation.
Metastatic inefficiency has largely been considered the result of a massive destruction of cancer cells within the dynamic circulation, due to the immune system and/or hemodynamic shear forces. To further simulate tumor cell migration within the circulation, conventional flow assay or micropipette technique will not be applicable. Therefore, we have developed a novel in vitro flow-migration system. It consists of a porous substrate that contains soluble collagen IV (CIV) proteins as chemoattractant. Using a peristaltic pump, circulating tumor cells can then be introduced into the flow channel in a well-defined flow field. The total number of transmigrated tumor cells can be microscopically quantified in terms of various parameters including shear stress, chemoattractant concentration and modulated cell adhesion.
3. MATERIALS AND METHODS
3.1. Materials
Soluble type IV collagen (CIV) was obtained from Collaborative Research (Bedford, MA). For chemotactic assays, the collagen was diluted to various concentrations in Dulbecco’s modified Eagle’s medium (DMEM) (Biofluids, MD) with 0.1% bovine serum albumin (BSA) (Sigma, St. Louis, MO), and the solution was brought to a pH of 7.4 and an osmolality of 300 mmol/kg. Monoclonal antibodies to human CD11a (LFA-1), CD11b (MAC-1) and ICAM-1 were purchased from CalTag Laboratories (CA).
3.2. Cell Culture
C8161 human melanoma cells were cultured in DMEM-F12 supplemented with 10% FBS. Tumor cells for assays were detached while subconfluent by brief exposure to 0.05% trypsin/0.02% EDTA and allowed to regenerate for 1 hr in a tissue culture medium containing DMEM/10% FBS.
Neonatal melanocytes (NHEM; Bio Whittaker-Clonetics) cells were maintained in manufacturer’s EGM-2-MV medium. Prior to each experiment the cells were detached and rocked (8 rpm) for one hour in culture medium at 37°C and then for an additional hour in RPMI 1640 with 0.1%w/v BSA. The viability of detached NHEM cells has been tested; approximately 80% of the detached NHEM cells were found to be viable both immediately after detachment and after 2 hours of preparatory rocking.
Fibroblast L-cells that had been transfected to express human ICAM-1 (EI cells; provided by Dr. Scott Simon, UC Davis) were maintained in culture as described elsewhere (12). The level of ICAM-1 expression on EI cells (with an average mean fluorescence intensity 2.81 ±0.39) was comparable to that expressed on HUVECs (with an average fluorescence intensity 3.28 ±0.35) stimulated with cytokine interleukin IL-1β for 4 hr (12). EI cells were used as a substrate for cell adhesion and a model endothelial cell in this study.
3.3. Neutrophil Isolation
Fresh blood was obtained from healthy adults under informed consent as approved by The Pennsylvania State University IRB. Neutrophils (PMNs) were isolated and enriched using Ficoll-Hypaque gradient (Sigma). The isolated PMN layer was first suspended in 0.1% human serum albumin (HSA; Baxter Healthcare Corp.) in DPBS and washed. The cell pellet was then suspended in ACK lysis buffer (0.15M NH4Cl, 10.0mM KHCO3, 0.1 mM Na2EDTA in distilled H2O) for 5 minutes to remove red cells. Finally, the cells were washed and resuspended in 0.1%HSA/DPBS at a concentration of 1×106 cells/ml. The PMNs were rocked (8 rpm) at 37°C until they were assayed, usually within 2 hours. To activate cells, PMNs were also treated with IL-8 (10ng/ml, 1 hour). Mac-1 was functionally blocked by treating the cells with 5μg of IgG anti-human Mac-1 (CalTag Laboratories) per 1×106 cells in blocking buffer for 30 minutes at 37°C. Fixed cells were prepared by suspending cells in 4% paraformaldehyde and incubated at 4°C for 1 hour. The cells were then washed and resuspended in media in a concentration appropriate for the experiment. Cell death was verified using trypan blue.
3.4. Flow-Migration Chamber Assay
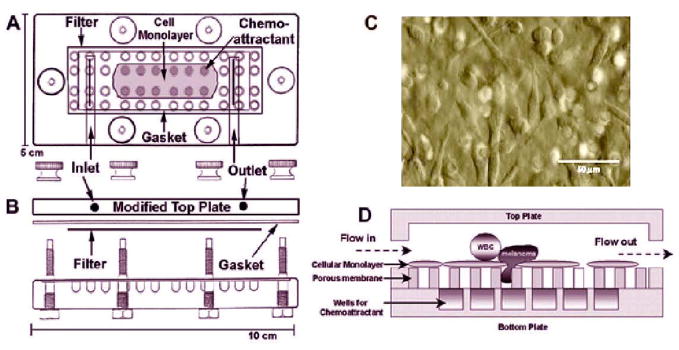
In order to simulate the in vivo tumor extravasation process, a chemotaxis flow-migration system was developed (Figure 2).
Figure 2.
Flow migration chamber from the top (A) and the side views (B). Micrograph of an EI cell monolayer that was grown on the filter (C). EI cells provide a consistent cell substrate with a stable level of ICAM-1 expression. Circulating flow provides a model of a microvascular environment where a cell monolayer acts as a barrier that cells must migrate through in response to chemotactic stimulation from the bottom wells (D).
In brief, the chamber consists of a top and bottom plate separated by a gasket. The bottom plate has 48 centered chemotactic wells and screws around the perimeter to affix the top plate. The top plate has an inlet and outlet allowing for circulating flow through the chamber. The gasket is an 11cm × 5.5cm piece of 0.02 inch-thick silicon (PharmElast), in which a 7cm × 2cm opening was cut from the center for the flow field. The wall shear stress (τw) is related to the volumetric flow rate (Q) by τw=6μQ/wh2, where μ is the fluid viscosity, h is height and w is width of the flow field. PVP-free polycarbonate filters (8-μm pore size; NeuroProbe) were sterilized and coated with fibronectin (30μg/ml, 3 hour) (Collaborative Biomedical Products). Prior to each experiment, a monolayer of EI cells was grown nearly to 100% confluence on prepared filters (typically 36–48 hours after seeding with cells).
The chamber’s center 12 wells were filled with soluble CIV (100μg/ml in RPMI 1640/0.1%BSA) (Collaborative Biomedical Products) and control wells were filled with medium (RPMI 1640/0.1%BSA). The flow loop was primed with warmed medium to eliminate bubbles in the system. The chamber was infused with the cells of interest (C8161 only; PMN only; or C8161+PMN together) into the flow field on the monolayer substrate. An equal number of each cell type was introduced to the chamber, 5×105 cells, in the case of C8161+PMN a total of 1×106 cells. When the assay was completed, the flow chamber was removed from the flow loop and disassembled and the filter was gently removed and imaged either by fluorescence microscopy and then stained with Diff-Quik (Dade Behring Inc.) or immediately stained. The cells on the bottom side of the filter were imaged using an inverted microscope and captured via NIH Image (v. β4.0.2) on a PC. Three images of each migration filter were quantified and averaged for each data point. For each data point, at least three filters were analyzed. Random PMN and C8161 migration were subtracted from each sample and no C8161 cells were found in the chemoattractant wells after 4 hours of migration.
4. RESULTS
Dynamic tumor cell extravasation was characterized to study the process of tumor cell adhesion to vascular endothelium under flow conditions and subsequent transendothelial migration in response to chemotactic stimulation from interstitial space (Figure 1). To model this extravasation process, a novel in vitro flow-migration system was used. C8161 cells were selected to characterize this new extravasation assay because they are invasive and metastatic melanoma tumor cells, which have been known to be highly motile. C8161 cell migration was quantified under a range of shear stresses, chemoattractant gradients and time lengths to determine standard assay parameters, and to examine how those parameters influence tumor cell extravasation in terms of cell adhesion and motility.
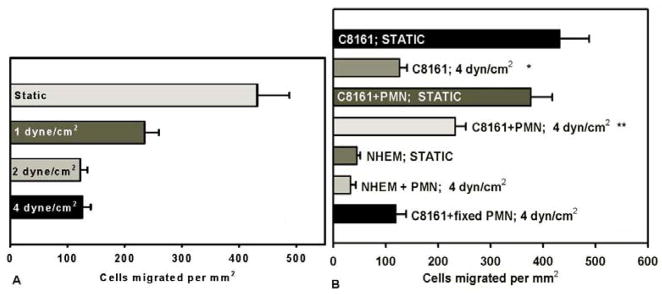
Tumor cell extravasation would normally occur in the microvasculature where fluid flows are steady and relatively slow. To model these in vivo conditions, flow-migration assays were run at a range of low shear stresses from 0–4 dyne/cm2. Figure 3A shows that shear stresses can modulate the extent of C8161 cell migration. There was a significantly initial drop from the static migration case (0 dyne/cm2; 431.8±56.1 cells per mm2) to 1 dyne/cm2 (234.83±25.1 cells per mm2), and a second decrease in migration from 1 to 2 dyne/cm2 (122.2±12.8 cells per mm2).
Figure 3.
(A) Migration assays under static and flow conditions. C8161 cells migrated to CIV (100μg/ml) over 4 hours. Each bar represents at least 4 trials and the error bar represents the SEM. (B) The number of migrated C8161 cells under 4 dyn/cm2 of shear stress (C8161; 4dyn/cm2) was compared with the migration under static condition (* p=0.002 with respect to “C8161; Static” case), while the presence of PMNs does not affect C8161 migration under static conditions (C8161+PMN; Static). C8161 cell migration is significantly higher in the presence of PMNs (C8161+PMN; 4dyn/cm2) than those C8161 cells without PMNs under flow conditions (**p<0.01 with respect to “C8161; 4dyn/cm2” case). Melanocytes (NHEM) and fixed PMNs were used as negative controls.
When exposed to a shear flow (4 dyn/cm2), nearly 3.5 times fewer C8161 cells migrated toward CIV than those under the no flow condition (Figure 3B). The static migration assays showed that C8161 chemotaxis was virtually the same in the presence or absence of PMNs (Figure 3B; p=0.47). The addition of PMNs to the C8161 suspension significantly enhanced C8161 migration to CIV under shear stress (Figure 3B; p<0.01). As negative controls, NHEM (melanocytes) migration was tested under the static condition and NHEM cells were co-suspended with PMN using the flow-migration assay to test for cell migration under shear conditions. Melanocyte chemotaxis was found to be nearly the same as background level in each case (Figure 3B). Fixed PMNs were used to account for the increase in cell number while not introducing biological affects. Results shown in Figure 3B indicate that when fixed PMNs are present, C8161 cells migrate at a similar level to C8161 cells alone, suggesting that doubling the cell number in the system would not change the cell migratory behavior.
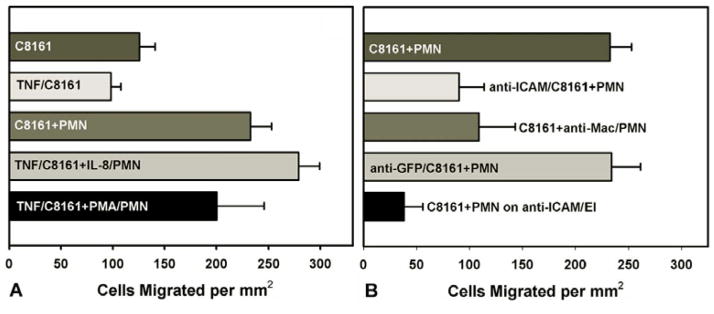
Adhesion molecules involved in PMN-C8161 interaction were characterized using the flow migration chamber. Under the influence of IL-8-activated PMNs, TNF-α-stimulated C8161 cells resulted in higher chemotaxis than TNF-α-stimulated C8161 cells alone (p>0.002) (Figure 4A). PMA-activated PMNs produced a less significant increase in C8161 chemotactic migration (Figure 4A). This suggests that Mac-1 on PMNs may be involved in PMN-mediated C8161 adhesion and migration. To investigate ICAM-1 involvement in PMN–influenced C8161 migration, adhesion was tested by blocking ICAM-1 on C8161 cells with antibody treatment. Antibody (anti-ICAM-1) treated C8161 cells migrated at a significantly lower level than untreated C8161 cells in the presence of PMNs (Figure 4B), showing a similar level of migration of untreated C8161 cells in the absence of PMNs (Figure 4A). These results suggest that PMNs could mediate C8161 cell adhesion via an ICAM-1-dependant mechanism, hence enhancing melanoma migration. To test this hypothesis, untreated C8161 cells were added to anti-Mac-1 treated PMNs. Blocking Mac-1 on PMNs reduced C8161 chemotactic migration (Figure 4B). In addition, ICAM-1 molecules were blocked on the EI cell monolayer, preventing cell adhesion to the substrate. This even more dramatically reduced C8161 migration and brought it to nearly background levels. To test for non-specific antibody blocking, C8161 cells treated with anti-GFP were found to migrate at the same level as untreated cells (Figure 4B). Together, these results suggest PMN-mediated C8161 adhesion and migration under flow conditions could be via an ICAM-1/Mac-1 dependent mechanism.
Figure 4.
(A) Migration of TNFα-activated C8161 cells (TNF/C8161) cells was found to be the same as unstimulated C8161 cells. In contrast, the migration of TNF/C8161 in the presence of IL-8-stimulated PMN (TNF/C8161+IL-8/PMN) increases C8161 migration by 20% compared to “C8161+PMN”. PMA-activated PMNs (TNF/C8161+PMA/PMN) do not increase melanoma cell migration significantly above “C8161+PMN”. (B) PMN-influenced C8161 cell (C8161+PMN) migration is significantly reduced by blocking either ICAM-1 on C8161 (anti-ICAM/C8161+PMN; p<0.02), Mac-1 on PMNs (C8161+anti-Mac/PMN; p<0.04), or ICAM-1 on the EI monolayer (C8161+PMN on anti-ICAM/EI; p<0.001). All p values are with respect to “C8161+PMN”. Nonspecific antibody treatment was found to have no effect on the tumor cells migration (anti-GFP/C8161+PMN). ICAM-1 antibody blocked C8161 cells were found to have similarly low migration to CIV, with or without PMNs (data not shown). All results were obtained under 100μg/ml CIV and 4dyn/cm2 shear stress over 4 hours.
An adhesion flow assay (5) was used to examine possible heterotypic cell aggregation between C8161 and PMNs, which could mediate C8161 cell adhesion to the EI monolayer under shear stress. As seen from Figure 5, TNF-α-stimulated C8161 cells that aggregated with IL-8-activated PMNs result in stronger tumor cell adhesion to an EI cell surface than unstimulated C8161–PMN pairs. TNF-α-stimulated C8161 cells were found to have similar adhesion characteristics as unstimulated C8161 cells. Antibody blocking either ICAM-1 on C8161 cells or Mac-1 on PMNs, respectively, reduced C8161–PMN aggregation and decreased C8161 adhesion to EI cell monolayer to a similar low level as C8161 cells alone without PMNs. This data suggests that improved cell adhesion to an ICAM-1 expressing monolayer is one of the primary mechanisms by which PMNs can influence C8161 migration.
Figure 5.
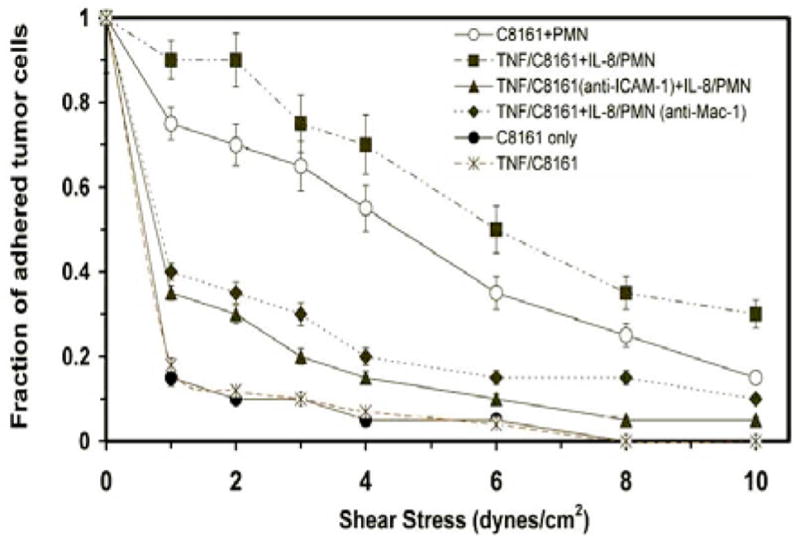
Adhesion assay examines heterotypic C8161-PMN aggregation that results in successful C8161 arrest on ICAM-1 expressing EI cell substrate under increasing shear stresses. Only the cases where a C8161 cell was paired with one or two PMNs were counted. Results are reported as the fraction of C8161 or C8161-PMN pairs that remain adhered under a given shear stress over 20 seconds, normalized by the cell number initially adhered to the substrate under no-flow condition. Single type C8161 cells (●) and TNF/C8161 cells (X) show the weakest adherence to EI cells at very low shear stresses. C8161-PMN cell pairs (O) are able to withstand significantly higher shear stresses. C8161 cell adhesion to EI substrate is further strengthened with cytokine activated C8161 and PMNs (TNF/C8161 and IL-8/PMN; ■). The doublet adhesion is ablated when antibodies were used to block either ICAM-1 on TNF/C8161 cells (▲) or Mac-1 on IL-8/PMNs (◆). Lines connecting data points are included for clarity and do not imply curve fitting.
5. DISCUSSION
Tumor cell extravasation requires a coordination of many cellular events and processes. Development of an in vitro assay that characterizes these processes allows the ability to isolate and characterize factors that contribute to successful or inhibited extravasation. In the case of tumor cells, shear-resistant adhesion and migration are important steps in cancer metastasis formation. No prior existing apparatus allowed both steps to be studied simultaneously. We have developed a novel flow-migration assay that allows cell-substrate adhesion and subsequent migration to be characterized and quantified in a controlled in vitro environment.
Endothelial cells transiently express ICAM-1, the ligand for β2-integrins. It is through this receptor-ligand interaction that firm adhesion of PMNs to the blood vessel wall is achieved. However, adhesion-mediated tumor cell arrest differs from the leukocyte adhesion and migration cascade (1). For example, tumor cells do not exhibit “leukocyte-like rolling” adhesive interaction with the endothelium for initial microvascular arrest. We have found very low levels of β2-integrins but relatively high levels of functional ICAM-1 on C8161 cells, as well as on another human melanoma cell line A2058 (data not shown). The relatively low surface expression of β2-integrins by C8161 cells makes melanoma cell adhesion to endothelial ICAM-1 improbable under physiological flow conditions. A potential mechanism for allowing melanoma cell adhesion to the endothelium under shear forces could be elicitation of β2-integrin expressing host cells in the blood circulation, e.g., PMNs, to act as a binding mediator between the tumor cell and the endothelium.
Migration of C8161 cells was characterized under static and shear conditions (4 dyn/cm2). Under static conditions, C8161 cells do not have any difficulty migrating through the cell monolayer due to their invasive nature. Obviously, there are no fluid shear forces challenging the cell-substrate adhesion during tumor cell migration. In this scenario, the addition of PMNs to the tumor cell suspension does not result in any increase in C8161 migration. When a shear flow is present, C8161 migration decreases significantly, indicating that C8161 cell adhesion by itself to an ICAM-1 cellular monolayer is less efficient for successful cell migration under physiological shear forces.
Results show that C8161-PMN aggregates adhere more strongly to ICAM-1 expressing EI cell substrate than single-type C8161 cells. PMNs that highly express Mac-1 and LFA-1 could possibly form a bridge and bind to both the ICAM-1 expressing C8161 and the EI cell monolayer. Antibody blocking of ICAM-1 on C8161 cells or blocking Mac-1 on PMNs dramatically reduces the C8161-PMN aggregation and subsequent C8161 adhesion to EI cells under increasing shear stresses, although ICAM-1 blocking appears to have more profound influence on C8161-PMN adhesion than Mac-1 blocking. Another corroborating piece of data is that blocking the ICAM-1 on the EI monolayer, thereby preventing all adhesion to the monolayer, reduces C8161 migration more than five-fold. These assays give experimental evidence that implicate β2-integrin and ICAM-1 interactions in regulating C8161/PMN conjugate adhesion to the endothelial surface.
The nature of PMNs raises questions to their effect on C8161 cells in terms of cytotoxicity. Viability studies verify that within the time frame of the flow-migration assay (4 hours) the vast majority (greater than 90%) of C8161 cells and PMNs are alive (data not shown).
Studies on many cell types have shown that chemotactic signals can elicit a series of biochemical events, which are regulated in a coordinated manner to result in a controlled cell motion. At the biochemical level, the mechanism of migration used by tumor cells may parallel, or be similar to, that used by many nonmalignant motile cells. Such studies are currently being developed in our laboratory to provide insights into the ability and effectiveness of tumor cell extravasation, and respective mechanisms that underlie the diversity of the tumor metastatic processes.
Acknowledgments
This work was supported by grants from NSF-BES-0138474 and NIH-CA-97306.
References
- 1.Liotta LA. Cancer cell invasion and metastasis. Sci Am. 1992;266:54–63. doi: 10.1038/scientificamerican0292-54. [DOI] [PubMed] [Google Scholar]
- 2.Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 3.Dong C, Cao JC, Stuble EJ, Lipowsky HH. Mechanics of leukocyte deformation and adhesion to endothelium in shear flow. Ann Biomed Eng. 1999;27:298–312. doi: 10.1114/1.143. [DOI] [PubMed] [Google Scholar]
- 4.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 5.Cao J, Donell B, Deaver DR, Lawrence MB, Dong C. In vitro imaging technique and analysis of human T-Leukemic cell adhesion to ICAM-1 in shear flow. Microvasc Res. 1998;55:124–137. doi: 10.1006/mvre.1997.2064. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence MB, McIntire LV, Eskin SG. Effects of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 7.Aznavoorian S, Stracke ML, Krutzsch HC, Schiffmann E, Liotta LA. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J Cell Biol. 1990;110:1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong C, Aznavoorian S, Liotta LA. Two phases of pseudopod protrusion in tumor cells revealed by a micropipette. Microvasc Res. 1994;47:55–67. doi: 10.1006/mvre.1994.1005. [DOI] [PubMed] [Google Scholar]
- 9.You J, Mastro AM, Dong C. Application of the dual-micropipet technique to the measurement of tumor cell locomotion. Exp Cell Res. 1999;248:160–171. doi: 10.1006/excr.1999.4388. [DOI] [PubMed] [Google Scholar]
- 10.Zicha D, Dunn GA, Brown AF. A New Direct-Viewing Chemotaxis Chamber. J Cell Sci. 1991;99:769–775. doi: 10.1242/jcs.99.4.769. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond SH. Ability of Polymorphonuclear Leukocytes to Orient in Gradient of Chemotactic Factors. J Cell Biol. 1977;75:606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopalan PK, Smith CW, Lu H, Berg E, Mcintire LV, Simon SI. PMN CD18-dependent arrest on ICAM-1 in shear flow can be activated through L-selectin. J Immunol. 1997;158:367–375. [PubMed] [Google Scholar]