SUMMARY
Radiotherapy is generally used to treat a localized target that includes cancer. Mounting evidence indicates that radiotherapy also recruits biological effectors outside the treatment field, and has systemic effects. The implications of this aspect are discussed in this review, in the context of understanding the role of the host’s immune system in cooperating with standard cytotoxic treatments. Since effects from both chemotherapy and radiotherapy are sensed by the immune system, their combination with immunotherapy presents a new therapeutic opportunity. Radiotherapy carries the advantage of directly interfering with the primary tumor site, and potentially reverting some of the established immuno-supressive barriers present within the tumor microenvironment, ideally recovering the role of the primary tumor as an effective immunogenic hub. Local radiation also triggers systemic effects that can be harnessed in combination with immunotherapy to induce responses outside the radiation field. This review will cover some of the preclinical and clinical evidence in this regard.
INTRODUCTION
Ionizing radiation has been harnessed for over a century to treat cancer based largely on the rationale that rapidly proliferating cancer cells are more sensitive than normal tissues to DNA damage induced by radiation. The 5Rs of radiation biology, Repair, Reassortment, Repopulation, Reoxygenation, and Radiosensitivity, have traditionally recapitulated the therapeutic principles to maximize the anti-cancer effects while minimizing the toxic effects on the normal tissue included in the field of treatment [1]. Intrinsic radiosensitivity of different cancer cells has been extensively studied, but the mechanisms underlying this important variable remain incompletely understood. Most investigations of the determinants of radiosensitivity have focused on DNA damage and repair capacity of cells hit by radiation [2], whereas the critical experimental observation that the radiosensitivity of tumors in vivo is profoundly affected by the immunocompetence of the host, remained generally ignored for almost thirty years [3]. Recently, novel molecular pathways activated during cell stress were discovered to contribute to a type of cell death that is “immunogenic”, enabling important signaling to the immune system [4]. The relevance of this peculiar type of cell death induced by radiation is beginning to be understood, together with its potential clinical implications [5]. Importantly, an increased appreciation of the complex relationship between dying cells, their microenvironment, and the host immunological habitus is emerging [6]. This knowledge supports the consideration of a paradigm shift to interpret the response to radiotherapy in cancer patients, by acknowledging an active role of the patient’s immune system. Figure 1 shows the lymphocitic infiltration of a locally advanced breast cancer in a patient treated with a protocol of concurrent chemo-radiation. If successful, this process results in the acquisition of a tumor-specific immunity able to attack both the original tumor site as well as the metastatic sites, impacting patient’s survival. This novel perspective opens new clinical avenues to exploit the indirect effects of radiation within and outside the field of treatment and to explore combinations with established forms of immunotherapy.
Figure 1.
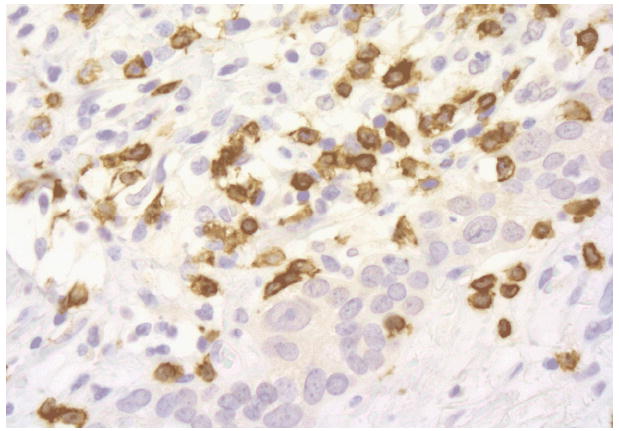
Cytotoxic T lymphocytes (brown) infiltrating residual breast cancer post-radiotherapy
This review includes the preliminary clinical evidence that radiation effects can be harnessed to result in inhibitory effects on tumor growth outside the treatment field, which cannot be explained only by direct damage to cancer cells. These effects appear to occur over a wide range of radiation doses and fractionation. Importantly, since clinical radiotherapy to a localized field inevitably exposes the rest of the organism to some dose of radiation (through internally scattered dose and from leakage from the collimated radiation source), it is relevant to distinguish these effects from systemic consequences of the stress response to ionizing radiation. For the scope of this review, we have grouped these effects of radiation into four sections: responses of un-irradiated cells to signals from irradiated cells; consequences of dose exposure to the rest of the body, after localized radiotherapy; radiation effects on the tumor microenvironment that result in systemic effects; and abscopal effects, from the latin ab scopus, consequences of radiotherapy that manifest away from the irradiated target [7]. While the first two types directly depend on exposure to a radiation dose, radiation-induced abscopal effects refer to those occurring far away from the irradiated site, independently from any direct exposure to radiation.
Responses of un-irradiated cells to signals from irradiated cells
Recent experimental strategies underscored radiation effects on cells adjacent to the irradiated cells, either within the radiation field or in its proximity, in a lower dose range of tissue dose exposure. In 1992 Nagasawa and Little reported the finding that, despite the experimental conditions in which only 1% of the cell population had been traversed by a densely ionizing particle, 30% of the population displayed chromosome damage in the form of sister chromatid exchanges [8]. Similarly, studies in rodent pulmonary epithelial cells demonstrated p53 induction in a much larger fraction of cells than that exposed to radiation. These effects occurring in cells that were not themselves directly irradiated were defined as “bystander effects” [9], and ranged from induction of genomic instability and gene mutations to cell-kill. Bystander effects transmission to non-irradiated cells has been demonstrated in experimental conditions that enable to irradiate only the cytoplasm of cells [10]. The report of chromatid breaks in the grafted marrow from his donor sister in a man rescued after lethal exposure to radiation from a nuclear accident in Japan is a classic example of bystander effects of radiation [11]. Partial lung radiation experiments in rats demonstrated effects in the shielded lung volume adjacent but external to the targeted field, where increased expression of Tumor Necrosis Factor alpha (TNF-α), Interleukin-1 alpha (IL-1α), Interleukin-1 beta (IL-1β), Interleukin-6 (IL-6), and Transforming Growth Factor beta (TGF- β) were also detected [12-14]. The nature of these signals and their role in radiation-induced second malignancies have been previously reviewed [2, 15]. Importantly, these non-targeted effects of radiotherapy involve the innate immune system and can occur outside the field of radiation.
Effects outside the field of local radiotherapy
Clinical radiotherapy for cancer inevitably exposes the rest of the body of the patient to some radiation dose, mediated by cellular and microenvironmental signaling [16, 17]. Importantly, in vivo experiments have demonstrated that cells of the innate immune system can be activated by ionizing radiation to produce the pro-inflammatory mediators of genomic instability [18, 19]. In a model of radiation-induced leukemogenesis, recognition of the apoptotic spleen cells by macrophages following 4 Gy irradiation was responsible for their activation [18]. The activated macrophages induced chromosomal instability in non-irradiated hematopoietic cells via the production of TNFα and reactive nitrogen species [19].
The intrinsic specificity of the experimental model chosen and of the genetic and immunological background of the irradiated animal appear to govern the type of responses detected after ionizing radiation. For instance, macrophages from the CBA/Ca strain susceptible to radiation-induced acute myeloid leukemia show an M1-like (pro-inflammatory) phenotype, whereas in the leukemia-resistant strain the macrophages have a predominantly M2-like (pro-wound-healing) phenotype [20]. Radiation induces “danger signals” that trigger inflammation; however, the interpretation of this process by the innate immune system appears dependent on the genetic background of the host [21]. Similarly, the outcome of the inflammatory response triggered by radiation can be a desirable or undesirable one depending upon the context. In the model of carcinogenesis discussed above, the M1 activation of the macrophages promoted genomic instability, potentially increasing the risk for leukemia development [20], whereas in most malignances it is the M2 type macrophages that have been associated with pro-tumorigenic activity, while the M1 type macrophages have anti-tumor activity, mediated directly by the ability to kill tumor cells, as well as indirectly by the activation of adaptive anti-tumor immunity [22].
In the clinic, the interplay between the host’s genetic background and the response to radiation exposure is elegantly exemplified by the work of Flint-Richter and Sadetzki. Based on the established risk for meningioma after radiation treatment for tinea capitis, they studied 525 families with a history of radiation exposure for this condition and whose members were classified based on the disease status and treatment. Eleven percent of the families with members diagnosed with a radiation-associated meningioma had additional first degree relatives with the condition, compared to one percent of the families without cases of radiation-associated meningioma (p-0.04) [23]. These results highlight the role of genetic susceptibility as a determinant of carcinogenesis risk after radiation exposure.
A similar genetic predisposition to hamper an immunogenic cell death was reported in an analysis of 280 node-positive breast cancer patients who were treated with doxorubicin-based adjuvant chemotherapy and local radiation. Both chemotherapy and radiotherapy cell killing generate signals from dying cells that target Toll-like receptor 4 (TLR4) on dendritic cells (DC), to enable efficient processing of antigens and their cross-presentation. Carriers of a polymorphism of the TLR4Asp299Gly had a significantly inferior disease-free survival when compared to the patients who had the normal allele (p=0.003) [5]. Both individual inherited radio-sensitivity [24] and immunity contribute to the explanation of the wide range of response observed after radiation.
The dose of radiation has been demonstrated to be associated with different effects. At radiation doses <0.5 Gy, generally too low to directly induce cell death, irradiated cells release oxygen and nitrogen radicals that activate innate immune cells, such as macrophages, to release cytokines. Depending on the environment and genetic background, this process can result in chronic inflammation that causes genetic alterations and cell death as a secondary event. It is in this setting that the immune-modulating effects of radiation promote mostly a pro-tumorigenic role of the immune system [20, 25]. Conversely, at doses sufficient to directly provoke significant cell death, radiation induces specific signals of danger that are sensed by innate immune cells such as dendritic cells, and lead to the activation of an adaptive immune response [4]. In the case of cancer radiotherapy this process can promote anti-tumor immunity.
Therefore, the immune-modulating effects of radiation are influenced by the dose, the type of signals generated by irradiated and non-irradiated cells, and by the activation of different types of innate immune cells. The tissue microenvironment and the presence of other immune modulators -- e.g., infectious agents -- regulate, together with the genetic background, the outcome of this process.
Radiation on the tumor microenvironment: the irradiated tumor as an immunogenic hub
Experimental evidence has unequivocally demonstrated that the immune system is an active participant in tumor progression, exerting both pro-tumorigenic and anti-tumor activities [26, 27]. Clinically apparent tumors have successfully undergone a selection process called “immuno-editing” and are resistant to immune rejection [27]. The mechanisms of resistance are multiple and complex and include production of immunosuppressive cytokines, down-regulation of antigenic molecules on the cancer cells, recruitment within tumors of immunoregulatory myeloid and lymphoid cells, and dysfunction of dendritic cells (DC) [28, 29]. Importantly, many of these factors hinder the success of immunotherapy. Radiation effects go far beyond the expected reduction in viable cancer cells, resulting in modifications of the tumor microenvironment that can interfere with its resistance to immune rejection.
Figure 2 graphically displays the hypothesis that the tumor and its microenvironment can be modified by treatment with ionizing radiation to enable the generation of a tumor-specific immune response. The microenvironment of solid tumors (central panel) includes, besides the neoplastic cancer cells, an atypical vascular network that results from angiogenesis and vasculogenesis, fibroblasts, and a variety of inflammatory cells. Among the latter, tumor-associated macrophages promote tumor progression by secreting a variety of factors, including matrix metalloproteinases and immunosuppressive cytokines [22]. Other myeloid cells, such as myeloid-derived suppressor cells (MDSC) contribute to angiogenesis and vasculogenesis and, together with regulatory T cells, are powerful suppressors of anti-tumor effector T cells [30, 31]. DC are present, but tumor and stromal secretion of vascular endothelial growth factor (VEGF), interleukin (IL)-10 and TGF- β inhibits their maturation into effective antigen-presenting cells [32]. Panel A and B schematically describe some of the recently discovered effects of ionizing radiation at the site of the irradiated tumor. Radiation can produce an immunogenic death of the most radiosensitive subset of cancer cells [33]. The recent identification of two mediators of this process, calreticulin (CRT) and high-mobility group protein B1 (HMGB1), has shed light on the mechanism by which the irradiated tumor becomes a robust source of antigens for an in situ auto-vaccination [5, 34]. Translocation of CRT to the surface of dying cancer cells promotes their uptake by DC and release of antigens that can be efficiently presented by DC. Release of HMGB1 by the dying cancer cells provides a danger signal that activates DC through the TLR4 pathway.
Figure 2.
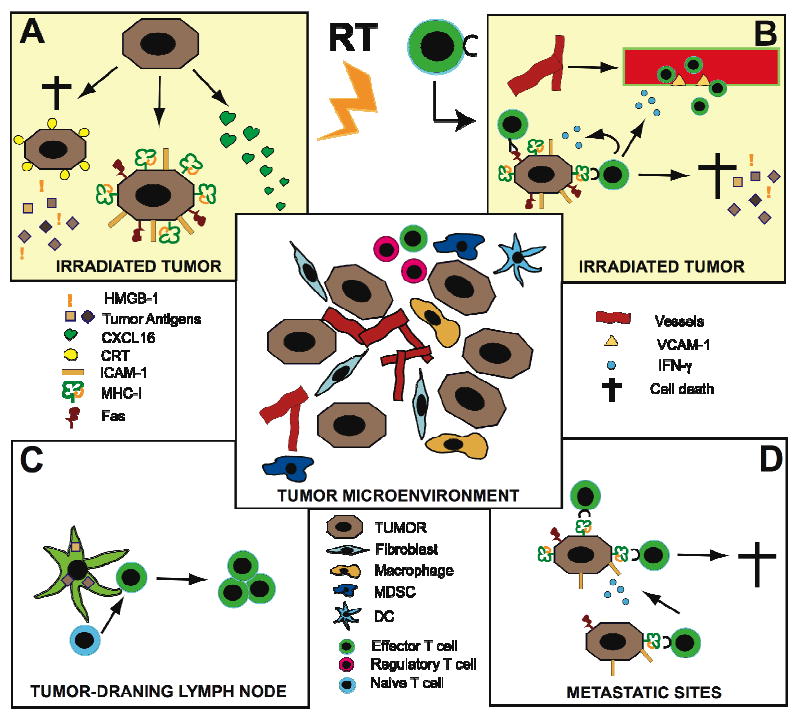
Changes in irradiated tumor (A) promote rejection by effector T cells (B). Tumor antigen-loaded DC migrate to lymph nodes and activate T cells (C) that inhibit metastases (D).
When the damage of radiation is not sufficient to induce death, survivin cancer cells display enhanced expression of adhesion molecules such as intercellular adhesion molecule (ICAM)-1, death receptor Fas, and major histocompatibility complex class I (MHC-I) antigen-presenting molecules, providing the tools for an improved recognition and killing by anti-tumor T cells [35]. Several hours after radiation exposure, protein translation is activated through the mTOR pathway, with the release of radiation-specific peptides potentially capable to induce a tumor-specific immune-response [36]. A similar effect was reported on the cells at the invasive edge of the irradiated cancer in a model of syngeneic murine brain tumor [37]. Other pro-immunogenic mechanisms are triggered by ionizing radiation. For instance, immune cells trafficking is enhanced through induction of chemokines, like CXCL16, capable of attracting effector T cells to the irradiated tumor site [38]. These changes are likely to reflect on clinical responses: for instance, CXCL16 induction was demonstrated to be a critical component of the successful combination of radiotherapy and CTLA4 blockade in a syngeneic mouse model of metastatic breast cancer [38, 39].
The activated DC loaded with tumor-derived antigens migrate to the tumor-draining lymph nodes (panel C). If the lymph nodes are outside of the treatment field, naïve T cells of the right specificity can be activated by interaction with the migrated DC, expand, and acquire effector functions. The effector T cells traffic to the irradiated tumor, attracted by the chemokine gradient, and this process is amplified by enhanced expression of vascular cell adhesion molecule (VCAM)-1 on tumor endothelium, which is mediated by IFN-γ produced by the T cells [40] (panel B). IFN-γ also enhances MHC-I expression on the cancer cells sustaining and extending the initial effect of radiation to secure efficient recognition and killing by the T cells [40]. Killing of cancer cells by effector T cells via Fas-induced apoptosis or by cytotoxic granules releases a new wave of tumor antigens that can boost the immune response [41]. This acquired anti-tumor immunity is now available to the host.
The radiation-induced up-regulation of MHC-I and other pro-immunogenic effects described occur at the irradiated site. However, if sufficient T cell effectors are generated, they can infiltrate the metastases and recognize cancer cells. Release of IFN-γ enhances this process, leading to effective elimination of tumor cells in the metastases, outside the radiation field (panel D). In summary, the generation of a sustained anti-tumor immune response at the irradiated tumor site, the “hub”, will not only determine the overall response of the irradiated tumor but also mediate an “abscopal effect” on the tumor sites outside of the treatment field.
Clinical evidence in support of this hypothesis is rapidly emerging. For instance, Nesslinger et al compared immune responses before and after radiation versus surgery in patients with prostate cancer [42]. Fourteen percent of patients who were treated with external-beam radiation therapy and 25% of those receiving brachytherapy acquired tumor-specific immune responses as demonstrated by new antibody formation measured by Western blot, compared to none of the patients who were treated by radical prostatectomy. In another study, Schaue et al measured T cell responses to the cancer antigen survivin in colorectal and prostate cancer patients after radiotherapy alone or in combination with chemotherapy [43]. Increases in survivin-specific CD8 cells were seen after therapy in most patients, more commonly in colorectal cancer patients whose tumor responded well to treatment. Of note, some patients also showed increases in regulatory T cells in peripheral blood.
Abscopal effects
In preclinical murine models surgical removal of the primary tumor accelerated the growth of metastatic foci, as measured by labeling index (LI), supporting the hypothesis of a crosstalk between primary tumor and metastases. These experiments provided preliminary evidence of a serum mediator of these effects [44]. Interestingly, in the same model pre-surgical treatment with radiation abrogated the accelerated metastatic growth induced by subsequent surgery [45].
However, in other models enhancement of distant metastases growth after irradiation of the primary tumor was observed, but the mechanisms involved and the relevance to clinical experience remain unclear [46]. More recently, irradiation of mouse lung carcinoma growing in the leg of syngeneic mice with doses that caused a complete response of the irradiated tumor (one dose of 30 to 40 Gy or five fractions of 10 Gy) resulted in enhanced growth of lung metastases: decreased levels of tumor-derived angiostatin were implicated in this effect [47]. In another model, the mouse MMTV/PyVmT transgenic model of breast cancer, induction of TGF-β1 by local radiation at a dose of 10 Gy was shown to mediate the enhancement of distant metastases [48]. Consistently, the effect was abrogated in tumors lacking the type II TGF-β Receptor. Radiation-increased serum TGF-β levels were also detected in tumor-free mice.
In contrast to the systemic, tumor-enhancing effects discussed above, experimental and clinical evidence in favor of an inhibitory role of local radiation on distant tumor growth has also been reported. It is referred to as the abscopal effect from the original definition by R. J. Mole [7]. Although the term “abscopal effect” has been used to refer to other types of local therapy that have systemic effects [49], it will be used here exclusively in its original meaning to indicate the effects at sites distant from the locally irradiated site. Clinical reports of an abscopal effect after radiotherapy are not numerous, but this phenomenon was detected in several different tumor types, including lymphoma, melanoma, and a variety of carcinoma [50-53].
However, only a few studies have investigated the possible mechanisms of the abscopal effect in experimental animal models. Camphausen et al. showed that irradiation of the leg of immunocompetent mice bearing a syngeneic tumor (lung carcinoma or fibrosarcoma) injected at a mid-line dorsal site resulted in inhibition of tumor growth [54]. The effect was radiation dose-dependent and was more pronounced when higher dose per fraction were used (10 Gy given five times versus 2 Gy given twelve times). Importantly, the effect was not tumor-specific and required intact function of the p53 pathway in the irradiated tissue, while p53 status did not affect the degree of response of the tumor [54]. The discovery that radiation activates p53-dependent pathways that induce the production of growth inhibitors by some human cells in vitro and some (but not all) mouse tissues in vivo provides a possible mechanism for the abscopal effect observed in the above study [55]. Although these findings are intriguing, the relevance to the abscopal effects seen in cancer patients remains unclear.
In another study employing a human pancreatic carcinoma implanted in T cell-deficient (nude) mice at two separate sites, irradiation of one tumor resulted in a slight growth enhancement of the non-irradiated tumor [56]. If radiation was given with concomitant capecitabine (an anticancer prodrug of 5-fluorouracil), the non-irradiated tumor was markedly inhibited, whereas capecitabine alone had no significant effect. The mechanisms of this abscopal effect were not investigated, but since the mice used lacked T cells, this effect could not be mediated by a specific anti-tumor response but rather by cytokines or other innate immune mechanisms.
In marked contrast to the above study, T cells were required for the abscopal effect obtained with the combination of local radiation and Flt-3 Ligand (Flt-3L) in a mouse model of mammary carcinoma [57]. Flt3-L is a growth factor for DC, which are a population of bone marrow-derived professional antigen-presenting cells capable of activating T cells [58]. Tumor cells were injected at two separate sites into syngeneic immunocompetent mice and when one tumor received radiation, the growth of the non-irradiated tumor was not altered. Administration of Flt-3L with radiation but not with mock treatment caused a significant inhibition also of the tumor outside of the radiation field, and the effect was tumor-specific. Development of tumor-specific cytolytic T cells (CTL) in the treated mice further supported the interpretation that the abscopal effect was mediated by anti-tumor T cells activated by the combination of local radiation and Flt3-L [57].
T cells were also implicated as mediators of the abscopal effect induced by the combination of radiation and ECI301, a recombinant variant of the chemokine macrophage inflammatory protein-1α (MIP1α) in mouse models of carcinoma and sarcoma [59]. Using the same experimental design described above, one tumor site was irradiated and the other served as a read-out of the abscopal effect. Radiation and ECI301 as single modalities did not inhibit the growth of the non-irradiated tumor, whereas in combination they had a significant effect which, depending on the tumor type, required CD4, CD8 T cells or natural killer (NK) cells [59]. Importantly, irradiation of the normal tissue in the leg of mice did not induce the abscopal effect, suggesting that radiation-induced death or stress response of the tumor cells was required to trigger an anti-tumor immune response.
Overall, these data indicate that local radiation can trigger complex tissue responses that can have systemic effects on tumor growth. Radiation dose and tissue type influence the local response. The models chosen, with different degrees of immuno-competence of the host and the intrinsic characteristics of the cancer cells, influence the translation of this response into a systemic anti- or pro-tumor effect. Opposing effects are likely to coexist, explaining the reasons why radiation as a single modality seldom results in clinically significant abscopal effects. However, preclinical as well as recent clinical studies suggest that these effects on the irradiated tissue could be harnessed to enhance the response to different types of cancer immunotherapy [60].
Clinical evidence of interaction between ionizing radiation and immune response
The concept of harnessing some of the effect of radiotherapy on the primary tumor to recover an efficient systemic immune response invites the combination with already established immunotherapies. The first clinical trial combined a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer [61].
This was a randomized phase II study, with patients receiving local definitive radiation with or without vaccine. Primary endpoint of the trial was immunologic response, with secondary endpoints of safety and clinical response. A total of 30 patients entered the study. Patients in the combination arm received a priming vaccine of recombinant vaccinia (rV) expressing prostate-specific antigen (PSA) (rV-PSA) admixed with rV expressing the co-stimulatory molecule B7-1 (rV-B7-1), followed by monthly booster vaccines with recombinant fowlpox (rF)-PSA. The vaccines were given with local granulocyte-macrophage colony-stimulating factor (GM-CSF, Leukine) and low dose systemic IL-2. While no detectable increases in PSA-specific T cells were seen in the radiotherapy-only arm, the 13 patients who completed the vaccination and radiation course had at least 3-fold increase (P < .0005). There was also evidence of de novo generation of T cells to prostate-associated antigens not present in the vaccine, a phenomenon described as “antigen cascade”, among the patients treated in the combination arm, providing indirect evidence of immune-mediated tumor-killing.
After radiation exposure, the role of dying tumor cells in priming DCs was tested in a phase I clinical trial of 14 patients with hepatoma [62]. A single dose of 8 Gy of external-beam radiation therapy to the tumor was followed by an intratumoral injection of immature DCs, delivered on days 2 and 24. Twelve of fourteen patients had a partial response, and most patients had increases in alpha-fetoprotein-specific immune responses by cytokine-release assay and ELISPOT.
In neither trial, however, was it possible to demonstrate a direct link between the induction of an immune response and the clinical outcome. More clinical research is warranted to prove efficacy of combinations of radiotherapy and immunotherapy.
Rationale for combining radiotherapy and GM-CSF
The use of cytokines promoting the growth and differentiation of DC such as granulocyte-macrophage colony-stimulating factor (GM-CSF) is one of the most promising approaches in cancer immunotherapy [63, 64]. The rationale is that GM-CSF increases the mobilization, differentiation, and function of DC [65, 66] resulting in potential reversal of the host’s immune tolerance its own tumor-associated antigens [67, 68].
Because of these properties, current cancer vaccine trials often incorporate GM-CSF, either used concomitantly by subcutaneous administration or through the infusion of cells engineered to over-express it [69]. The use of GM-CSF as a single immunotherapy strategy has also shown promise.
A beneficial effect of GM-CSF with chemotherapy was discovered by investigators from the Amsterdam University Hospital who reported the clinical results of a phase II study in patients with locally advanced breast cancer (LABC) treated with neo-adjuvant chemotherapy plus GM-CSF. Six cycles of neo-adjuvant chemotherapy were given with the primary tumor and draining lymph nodes in situ for a prolonged period with GM-CSF administered as a hematopoietic growth factor. Remarkably good response and survival were seen, warranting further exploration of this strategy [70]. Similarly, in patients with high-risk, resected melanoma, one-year treatment with GM-CSF appeared to improve overall survival by almost three-fold compared with matched historical controls [71]. A recent paper on immune-monitoring of a similar series of patients demonstrated that melanoma patients showing a GM-CSF-induced transient increase of DC are more likely to remain in remission [72]. The Eastern Cooperative Oncology Group is currently conducting a randomized trial of GM-CSF in patients with high-risk melanoma: the trial has completed accrual and results are pending.
Encouraged by our preclinical experience of inducing an abscopal effect with the combination of radiotherapy and Flt-3 ligand [57], we designed a “proof-of principle” clinical trial, aimed at detecting an abscopal response, i.e. a response outside the radiation field after GM-CSF in metastatic cancer patients. Eligible were patients with at least 3 measurable lesions, who had experienced stable disease or progression during systemic chemotherapy. The same systemic therapy was maintained but radiation therapy was added to one lesion, at a dose of 3.5 Gy X 10 daily fractions, over 2 weeks. Starting on day seven (after one week of radiation) GM-CSF, 125 micrograms/m2, was given s.c., and repeated daily for 14 days. Abscopal response was defined as a measurable response in any of the lesions outside the radiation field. Assessment was performed by PET-CT.
Currently 14 patients have accrued to this trial. Tumor histology was: lung cancer (6), poorly differentiated thymic carcinoma (2), breast carcinoma (4), bladder carcinoma (1), eccrine carcinoma (1). Median age was 62 (range 41-89). A total of 26 cycles of therapy were administered (10 patients received 2 cycles) during chemotherapy. Grade 3 toxicity encountered consisted of lymphopenia in two cases, and thrombocytopenia, anemia or nausea/vomiting in one case each. Most common toxicity consisted of fatigue: grade 3 in one case and grade 1-2 in ten. Two patients suffered syncopal episodes, possibly related to EDTA in the formulation of GM-CSF used in this trial. At the time of publication, twelve patients could be evaluated for response (i.e. had completed treatment and data from PET/CT before and following therapy were available): four achieved an abscopal response (30%) classified as a partial response of at least one target lesion outside the treatment field. Figures 3 and 4 exemplify an abscopal response. In five patients a decrease in Standardized Uptake Value (SUV) of non-irradiated lesions was observed on PET scan. In three patients the response was preceded by a “flare” effect at PET, possibly corresponding to a robust inflammatory response [73].
Figure 3.
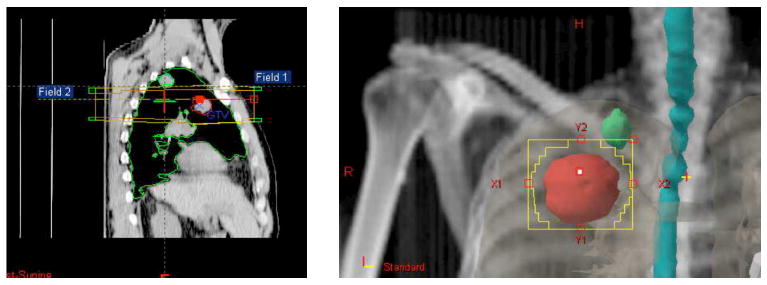
Sagittal (3a) and coronal (3b) views of two metastatic lesions in a case of poorly differentiated thymic carcinoma. Two parallel opposed radiation fields treated the most caudal metastasis, deliberately excluding the apical one.
Figure 4.
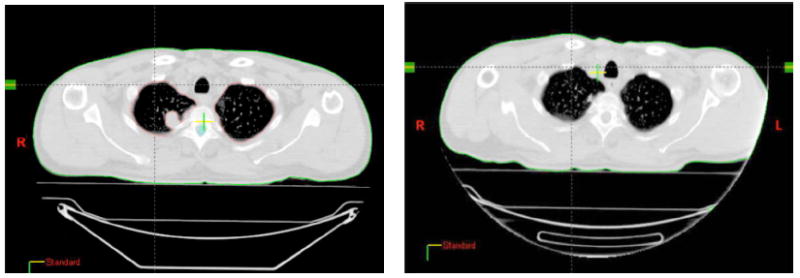
4a. CT cut of the original apical lesion that was not included in the radiation field (see 3a,b). 4b. The same lesion two months after treatment of a different, caudal metastasis with radiation and GM-CSF.
While the preliminary data confirms feasibility of harnessing the local radiotherapy effects to synergize with immune therapy, this trial continues accrual to the planned target number of 29 patients. All patients accrued to this trial had metastatic disease and were heavily pre-treated. Because of these characteristics, it is unlikely for this study to establish whether abscopal responses change the clinical outcome. Adding immune monitoring to detect the immunological response that is associated with the abscopal responses could provide important information and possibly justify testing this combination in an earlier clinical setting.
Conclusions
Multiple lines of evidence suggest that the application of ionizing radiation to a target that encompasses the tumor elicits effects that exceed cell killing per se, and include specific and effective signals to the immune system of the host. The understanding of these signals and their consequences has opened a novel area of research that is based on the acknowledgement that clinical radiotherapy might impact systemic disease through the immune system. While the evidence is rapidly accumulating, more investigation is needed to determine how best to harness these properties of ionizing radiation.
Similarly, the knowledge of what is the ideal combination with immune-therapy remains elusive. Preclinical data suggests that improved activation of effector T cells by CTLA-4 blocking antibodies might enhance the pro-immunogenic effects of radiotherapy [39]. The pro-immunogenic effects of radiotherapy, however, are likely to be counteracted by immune-suppressive ones, for example mediated by induction of TGF-β that suppresses the expression of cytotoxic mediators in CD8 cells, and promotes the generation of regulatory T cells [74]. Nevertheless, if the irradiated tumor could be used to generate a vaccine, some adjustment of the standard clinical practice will be required: for instance, irradiation of draining nodes that are not involved by metastases should be avoided since it will deplete the radiosensitive naïve T cells, which are a potential source of tumor-reactive T cells.
In conclusion, the application of clinical radiotherapy as a partner to immune-therapy is opening a new field of investigations.
Acknowledgments
SCF is supported by Department of Defense Center of Excellence Award BC030282, The Breast Cancer Research Foundation, and Core Grant NIH 5P30CA016087-27
SD is supported by NIH R01 CA113851, Research Scholar award RSG-05-145-01-LIB from the American Cancer Society, and by a grant from The Chemotherapy Foundation
The Authors are grateful to Dr. Mary Helen Barcellos Hoff, Associate Professor of Radiation Oncology and Cell Biology, New York University School of Medicine, for her critical reading of the final manuscript.
Footnotes
Conflicts of interests: The authors declare that they have no competing interests.
Search Strategy and Selection Criteria Data for this Review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms abscopal, radiation, tumor immunity, bystander. Only papers published in English between 1953 and 2008 were included.
References
- 1.Steel GG, McMillan TJ, Peacock JH. The 5Rs of radiobiology. Int J Radiat Biol. 1989;56(6):1045–8. doi: 10.1080/09553008914552491. [DOI] [PubMed] [Google Scholar]
- 2.Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6(7):520–8. doi: 10.1016/S1470-2045(05)70246-1. [DOI] [PubMed] [Google Scholar]
- 3.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63(5):1229–35. [PubMed] [Google Scholar]
- 4.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14(7):1237–43. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 5.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 6.Zeh H, Jr, Lotze MT. Addicted to death: invasive cancer and the immune response to unscheduled cell death. J Immunother. 2005;28:1–9. doi: 10.1097/00002371-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Mole RJ. Whole body irradiation - radiology or medicine? Br J Radiol. 1953;26:234–41. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 8.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer research. 1992 Nov 15;52(22):6394–6. [PubMed] [Google Scholar]
- 9.Hickman AW, Jaramillo RJ, Lechner JF, Johnson NF. Alpha-particle-induced p53 protein expression in a rat lung epithelial cell strain. Cancer research. 1994 Nov 15;54(22):5797–800. [PubMed] [Google Scholar]
- 10.Shao C, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc Natl Acad Sci U S A. 2004 Sep 14;101(37):13495–500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiba S, Saito A, Ogawa S, Takeuchi K, Kumano K, Seo S, et al. Transplantation for accidental acute high-dose total body neutron- and gamma-radiation exposure. Bone Marrow Transplant. 2002 Jun;29(11):935–9. doi: 10.1038/sj.bmt.1703568. [DOI] [PubMed] [Google Scholar]
- 12.Khan MA, Van Dyk J, Yeung IW, Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol. 2003 Jan;66(1):95–102. doi: 10.1016/s0167-8140(02)00325-0. [DOI] [PubMed] [Google Scholar]
- 13.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006 May;79(2):231–8. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005 Dec;81(12):887–99. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- 15.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006 May 1;65(1):1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–75. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 17.Hall EJ. Radiobiology fot the radiologist. Philadelphia, PA: J. B. Lippincott; 1994. [Google Scholar]
- 18.Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 2001;20(48):7085–95. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- 19.Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer research. 2008;68(19):8122–6. doi: 10.1158/0008-5472.CAN-08-0698. [DOI] [PubMed] [Google Scholar]
- 20.Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer research. 2008;68(2):450–6. doi: 10.1158/0008-5472.CAN-07-3050. [DOI] [PubMed] [Google Scholar]
- 21.McBride WH, Chiang C-S, Olson JL, Wang C-C, Hong J-H, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 22.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 23.Flint-Richter P, Sadetzki S. Genetic predisposition for the development of radiation-associated meningioma: an epidemiological study. Lancet Oncol. 2007 May;8(5):403–10. doi: 10.1016/S1470-2045(07)70107-9. [DOI] [PubMed] [Google Scholar]
- 24.Hall EJ, Brenner DJ, Worgul B, Smilenov L. Genetic susceptibility to radiation. Adv Space Res. 2005;35(2):249–53. doi: 10.1016/j.asr.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 25.Wright EG, Coates PJ. Untargeted effects of ionizing radiation: implications for radiation pathology. Mutat Res. 2006;597:119–32. doi: 10.1016/j.mrfmmm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 28.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–9. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 29.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 30.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 31.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008 Mar;13(3):193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008 Sep;29(3):372–83. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007 Oct;14(10):1848–50. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 34.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer research. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 36.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006 May 15;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newcomb EW, Demaria S, Lukyanov Y, Shao Y, Schnee T, Kawashima N, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12:4730–7. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demaria S, Kawashima N, Yang AM, Devitt M-L, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases following treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 40.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008 Mar 1;180(5):3132–9. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 41.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 42.Nesslinger NJ, Sahota RA, Stone B, Johnson K, Chima N, King C, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007 Mar 1;13(5):1493–502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 43.Schaue D, Comin-Anduix B, Ribas A, Zhang L, Goodglick L, Sayre JW, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008 Aug 1;14(15):4883–90. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher B, Gunduz N, Coyle J, Rudock C, Saffer E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer research. 1989 Apr 15;49(8):1996–2001. [PubMed] [Google Scholar]
- 45.Fisher B, Saffer E, Rudock C, Coyle J, Gunduz N. Effect of local or systemic treatment prior to primary tumor removal on the production and response to a serum growth-stimulating factor in mice. Cancer research. 1989 Apr 15;49(8):2002–4. [PubMed] [Google Scholar]
- 46.von Essen CF. Radiation enhancement of metastasis: a review. Clin Exp Metastasis. 1991;9(2):77–104. doi: 10.1007/BF01756381. [DOI] [PubMed] [Google Scholar]
- 47.Camphausen K, Moses MA, Beecken WD, Khan MK, Folkman J, O’Reilly MS. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer research. 2001;61(5):2207–11. [PubMed] [Google Scholar]
- 48.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117(5):1305–13. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev. 2005;31(3):159–72. doi: 10.1016/j.ctrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol. 1973;46:220–2. doi: 10.1259/0007-1285-46-543-220. [DOI] [PubMed] [Google Scholar]
- 51.Rees GJ, Ross CM. Abscopal regression following radiotherapy for adenocarcinoma. Br J Radiol. 1983 Jan;56(661):63–6. doi: 10.1259/0007-1285-56-661-63. [DOI] [PubMed] [Google Scholar]
- 52.Antoniades J, Brady LW, Lightfoot DA. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int J Radiat Oncol Biol Phys. 1977;2:141–7. doi: 10.1016/0360-3016(77)90020-7. [DOI] [PubMed] [Google Scholar]
- 53.Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998 October;43(4):575–7. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camphausen K, Moses MA, Menard C, Sproull M, Beecken W-D, Folkman J, et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990–3. [PubMed] [Google Scholar]
- 55.Komarova EA, Diatchenko L, Rokhlin OW, Hill JE, Wang ZJ, Krivokrysenko VI, et al. Stress-induced secretion of growth inhibitors: a novel tumor suppressor function of p53. Oncogene. 1998;17(9):1089–96. doi: 10.1038/sj.onc.1202303. [DOI] [PubMed] [Google Scholar]
- 56.Blanquicett C, Saif MW, Buchsbaum DJ, Eloubeidi M, Vickers SM, Chhieng DC, et al. Antitumor efficacy of capecitabine and celecoxib in irradiated and lead-shielded, contralateral human BxPC-3 pancreatic cancer xenografts: clinical implications of abscopal effects. Clin Cancer Res. 2005;11(24):8773–81. doi: 10.1158/1078-0432.CCR-05-0627. [DOI] [PubMed] [Google Scholar]
- 57.Demaria S, Ng B, Devitt M-L, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 58.O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–46. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 59.Shiraishi K, Ishiwata Y, Nakagawa K, Yokochi S, Taruki C, Akuta T, et al. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin Cancer Res. 2008;14(4):1159–66. doi: 10.1158/1078-0432.CCR-07-4485. [DOI] [PubMed] [Google Scholar]
- 60.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gulley JL, Arlen PM, Bastian N, Morin N, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 62.Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005 Mar-Apr;28(2):129–35. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 63.Borrello I, Pardoll D. GM-CSF-based cellular vaccines: a review of the clinical experience. Cytokine Growth Factor Rev. 2002 Apr;13(2):185–93. doi: 10.1016/s1359-6101(01)00034-x. [DOI] [PubMed] [Google Scholar]
- 64.Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008 Apr;222:287–98. doi: 10.1111/j.1600-065X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 65.Cairo MS. Review of G-CSF and GM-CSF. Effects on neonatal neutrophil kinetics. Am J Pediatr Hematol Oncol. 1989 Summer;11(2):238–44. [PubMed] [Google Scholar]
- 66.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006 Feb;16(2):126–33. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 67.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001 Nov;94(4):459–73. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 68.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 69.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002 Oct;188:147–54. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 70.Pinedo HM, Buter J, Luykx-de Bakker SA, Pohlmann PR, van Hensbergen Y, Heideman DA, et al. Extended neoadjuvant chemotherapy in locally advanced breast cancer combined with GM-CSF: effect on tumour-draining lymph node dendritic cells. Eur J Cancer. 2003 May;39(8):1061–7. doi: 10.1016/s0959-8049(03)00131-x. [DOI] [PubMed] [Google Scholar]
- 71.Spitler LE, Grossbard ML, Ernstoff MS, Silver G, Jacobs M, Hayes FA, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000 Apr;18(8):1614–21. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 72.Daud AI, Mirza N, Lenox B, Andrews S, Urbas P, Gao GX, et al. Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2008 Jul 1;26(19):3235–41. doi: 10.1200/JCO.2007.13.9048. [DOI] [PubMed] [Google Scholar]
- 73.Formenti SC, Friedman K, Chao K, Adams S, Fenton-Kerimian M, Donach ME, et al. Abscopal Response in Irradiated Patients: Results of a Proof of Principle Trial. International Journal of Radiation Oncology*Biology*Physics. 2008;72(1 Supplement 1):S6–S7. [Google Scholar]
- 74.Wan YY, Flavell RA. ‘Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev. 2007 Dec;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


