Abstract
Iron neurotoxicity may contribute to the pathogenesis of intracerebral hemorrhage (ICH). The tetracycline derivative minocycline is protective in ICH models, due putatively to inhibition of microglial activation. Although minocycline also chelates iron, its effect on iron neurotoxicity has not been reported, and was examined in this study. Cortical cultures treated with 10 μM ferrous sulfate for 24h sustained loss of most neurons and an increase in malondialdehyde. Minocycline prevented this injury, with near-complete protection at 30 μM. Two other inhibitors of microglial activation, doxycycline and macrophage/microglia inhibitory factor, were ineffective. Oxidation of isolated culture membranes by iron was also inhibited by minocycline. Consistent with prior observations, minocycline chelated iron in a siderophore colorometric assay; at concentrations less than 100 μM, its activity exceeded that of deferoxamine. These results suggest that attenuation of iron neurotoxicity may contribute to the beneficial effect of minocycline in hemorrhagic stroke and other CNS injury models.
Keywords: cell culture, free radical, hemoglobin toxicity, inflammation, intracerebral hemorrhage, mouse, oxidative stress, stroke
Introduction
Tissue iron is increased within one day in the vicinity of an experimental intracerebral hemorrhage [1], and persists for at least three months [2]. A growing body of experimental evidence suggests that this iron may contribute to cell injury. Reducing heme breakdown and iron release with heme oxygenase (HO) inhibitors or HO gene knockout is beneficial in animal models [3,4], and protects neurons from hemoglobin toxicity in cell culture [5]. More specifically, post-hemorrhage treatment with the iron chelator deferoxamine reduces edema, oxidative injury markers, and neuronal loss, and also improves behavioral outcome [2,6].
A peri-hematomal inflammatory infiltrate, consisting of leukocytes and activated microglia, is observed within 24 hours of experimental intracerebral hemorrhage and also may contribute to secondary injury [7]. This inflammation hypothesis has recently been tested using the tetracycline derivative minocycline [8,9,10], which inhibits microglial activation and is beneficial in several ischemic stroke models [11], presumably due to its anti-inflammatory effect. However, as described by Grenier et al. [12], minocycline has strong iron-chelating activity, which has been of some clinical relevance. Its absorption after oral administration is greatly reduced when administered with iron or calcium supplements, consistent with its affinity for metal cations [13]. Skin hyperpigmentation, an adverse effect of long-term minocycline therapy, is a consequence of dermal precipitation of a minocycline-iron complex [14]. By depriving bacteria of an essential nutrient, iron chelation may also account in part for its antibiotic effect [12], although evidence supporting the physiologic relevance of this mechanism is limited.
The redox activity of iron is altered in a highly variable manner by chelator binding. Catalysis of hydroxyl radical generation via the Fenton reaction requires at least one of six iron coordination sites to be available, or occupied by a low-affinity ligand such as water [15]. A chelator that occupies fewer than six sites may not prevent oxidative injury, and may even increase it if it mobilizes iron from protein binding sites in a redox-active state [16]. Despite the recent interest in minocycline therapy for hemorrhagic stroke, its effect on iron-mediated oxidative neuronal injury has never been reported. The present study tested that hypothesis that minocycline attenuates the oxidative neurotoxicity of iron in primary cortical cell cultures.
Materials and Methods
Cortical cell cultures
All procedures on animals were reviewed and approved by the Thomas Jefferson University Institutional Animal Care and Use Committee (IACUC). Mixed cortical cell cultures, containing both neurons and glia were prepared from fetal B6129 mice (gestational age 13- to 15-days), as previously described [5]. The dissociated cell suspension was plated on glial feeder cultures (>90% GFAP+, approximately 2% microglia by tomato lectin staining [17]) in 24-well plates (Falcon, Becton Dickinson, Franklin Lakes, NJ), using a plating density of 0.12 hemisphere in 0.4 ml medium per well. Plating medium contained Minimal Essential Medium (MEM, Invitrogen, Carlsbad, CA), 5% equine serum (Hyclone, Logan, UT), 5% fetal bovine serum (Hyclone), 23 mM glucose, and 2 mM glutamine. On day 5-6 in vitro, two-thirds of the culture medium was aspirated and replaced with feeding medium, which was similar to plating medium except that it contained 10% equine serum and no fetal bovine serum. This procedure was repeated on day 9 or 10 and then daily beginning on day 11. Glial feeder cultures were prepared from postnatal day 1-3 mice, using plating medium similar to that described above, except that it was supplemented with 10 ng/ml epidermal growth factor (Sigma, St. Louis, MO, Product #E1247), 10% equine serum and 10% fetal bovine serum. Glial culture medium was partially changed twice weekly.
Iron exposure
Experiments were conducted at 12-16 days in vitro. At this time point, neurons are readily distinguished from glial cells in dissociated cultures by their phase-bright cell bodies and processes. Ferrous sulfate (FeSO4) was used exclusively since heme breakdown by the heme oxygenase enzymes, as occurs after hemorrhagic CNS injuries, releases ferrous iron [18]. Exposure to FeSO4 alone or with drugs was carried out in MEM containing 10 mM glucose (MEM10) at 37°C in a 5% CO2 atmosphere. Control cultures were included in each experiment and were subjected to medium exchanges only. Minocycline and doxycycline (both purchased from Sigma) were prepared as 10 mM stock solutions in cell culture-grade water, and were then diluted in MEM10. Macrophage/microglia inhibitory factor (MIF, Thr-Lys-Pro) was purchased from American Peptide Company, Sunnyvale, CA. A 20 mM stock solution was prepared in cell culture-grade water, aliquoted, and stored at -20°C until used. The FeSO4 concentration used in these experiments was determined from prior concentration-toxicity studies, which demonstrated that 24 h treatment with 10 μM FeSO4 killed almost all neurons, without significantly injuring glial cells in this culture system [5,19].
Cell injury assessment
Cell death was quantified by lactate dehydrogenase (LDH) release assay, which correlates well with cell counts after trypan blue staining in mixed cortical cultures [20], as previously described [21]. In order to compare results from experiments using cultures prepared in different platings, which have slightly different neuronal densities, all LDH values were normalized to those in cultures treated with 10 μM FeSO4. The mean LDH activity of control sister cultures treated with medium exchange only was subtracted from all values to quantify the signal that was specific to the cytotoxic exposure, following the protocol of Koh and Choi [20].
Malondialdehyde is a sensitive marker of iron-mediated neuronal injury in this system [21]. After sampling for LDH assay, cultures were harvested, proteins were precipitated with 4.5% trichloroacetic acid, and malondialdehyde was quantified as previously described [21]. Protein was assayed by the BCA method (Pierce, Rockford, IL); malondialdehyde values were expressed as nanomoles/milligram protein.
In vitro membrane oxidation assay
Iron-mediated lipid oxidation was assessed in a cell-free system using the method of Sadrzadeh et al. [22]. Glial cultures were used as a source of membranes for this assay due to their greater availability compared with post-mitotic primary neurons. Glial cells were harvested by scraping in physiologic saline, and were then sonicated. Membranes from two culture wells per sample (approximately 50 μg protein) were collected by centrifugation (8000 x g, 5 min). After the supernatant was discarded, FeSO4 (50 μM) was added in 375 μL 50 mM Tris buffer (pH 7.4), alone or with tetracyclines. Samples were incubated at room temperature for 1 hour. Malondialdehyde was then quantified as previously described [21].
Iron chelation assay
The universal siderophore assay of Schwyn and Neilands, as modified by Grenier et al. [12], was used to assess iron chelating activity. The indicator for this assay is a chrome azurol S/Fe3+/hexadecyltrimethylammonium bromide (HDTMA) complex that undergoes color change from blue to orange when iron is extracted by a strong chelator. The assay solution contained 150 μM chrome azurol S, 600 μM HDTMA, and 15 μM FeCl3 in piperazine buffer (pH 5.6). Dilutions of test compounds were incubated in the assay solution (total volume 0.5 ml) for 1 hour at room temperature; absorbance at 630 nm was then quantified.
Immunoblotting
Cells were lysed in buffer containing 210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EDTA, 0.1 % sodium dodecyl sulfate, and 0.1 % Triton X-100. After sonication and centrifugation, the protein concentration of the supernatant was quantified (BCA method, Pierce, Rockford, IL). Samples (20 μg protein each) were then boiled in buffer containing Tris-Cl 60 mM, β-mercaptoethanol 5%, sodium dodecyl sulfate 2%, glycerol 10%, and bromophenol blue 0.05% for 3 min. Proteins were separated on 12 % SDS polyacrylamide gels at 80-100 V. The gels were then soaked in transfer buffer (glycine 39mM, Tris-CL 48mM, SDS 0.037% and methanol 20%), and were transferred to polyvinylidene difluoride membranes with a semidry transfer apparatus for 40 minutes at 20V. Completion of transfer was assessed by observing the transfer of the pre-stained protein marker (Bio-Rad Laboratories, Hercules, CA, Cat. No. 161-0375). After blocking with 5% nonfat dry milk, membranes were exposed overnight at 4°C to rabbit anti-horse spleen ferritin (Sigma, 1:250 dilution) combined with rabbit anti-actin antibody (1/1500, Sigma) as a gel loading control. After washing, they were then exposed to HRP-conjugated goat anti-rabbit IgG antibody (1:3500) for 1h at room temperature. Immunoreactive proteins were visualized using Super Signal West Femto Reagent (Pierce) and Kodak Gel Logic 2200.
Statistical Analysis
Data were analyzed with one-way analysis of variance. Differences between groups were then assessed with the Bonferroni Multiple Comparisons test.
Results
Minocycline protects cortical neurons from iron
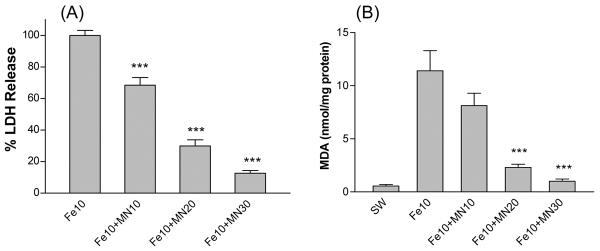
Consistent with prior observations using this model [5,23], cultures treated with 10 μM FeSO4 for 24 hours sustained widespread neuronal death, without injury to the feeder glial monolayer. Concomitant treatment with equimolar minocycline reduced neuronal death, as measured by LDH release assay, by approximately one-third at 24 hours (Fig 1A). Increasing the minocycline concentration to 30 μM reduced LDH release by 87.4±1.8%, which was similar to the neuroprotection provided by 30 μM deferoxamine (91.0±4.4% reduction). Malondialdehyde (MDA) was increased approximately twenty-fold by iron treatment (Fig 1B). Changes in mean MDA values associated with minocycline treatment correlated with injury reduction as assessed by LDH release.
Figure 1.
Minocycline protects cortical neurons from iron. Bars represent mean (± S.E.M, n = 6-12/condition) percentage lactate dehydrogenase (LDH) release (A) and malondialdehyde (MDA) levels (B) after 24 hour treatment with 10 μM ferrous sulfate (Fe) alone or with indicated concentrations (μM) of minocycline (MN). LDH values are scaled to that in cultures treated with 10 μM ferrous sulfate, which produces near-100% neuronal death, after subtraction of mean LDH in sister cultures subjected to wash and medium exchange only (SW, sham wash), in order to yield the LDH signal specific for neuronal injury. ***P<0.001. v. ferrous sulfate alone, Bonferroni multiple comparisons test.
Other microglial activation inhibitors have no effect on iron neurotoxicity
Doxycycline and the peptide Thr-Lys-Pro (microglia inhibitory factor) inhibit microglial activation in primary cultures. At concentrations previously determined to be effective in vitro [24,25], neither significantly reduced neuronal death or malondialdehyde levels in iron-treated cells (Table 1).
Table 1.
Effect of doxycycline and macrophage/microglia inhibitory factor on iron neurotoxicity
| LDH | MDA | |
|---|---|---|
| Sham Wash | 0.0 ± 4.5 | 0.4 ± 0.1 |
| Fe 10 | 100.0 ± 4.4 | 10.2 ± 0.9 |
| Fe10+DX10 | 87.1 ± 3.2 | 7.7 ± 1.7 |
| Fe10+DX30 | 88.8 ± 4.2 | 8.2 ± 1.8 |
| Fe10+MIF500 | 82.9 ± 9.5 | 9.2 ± 0.5 |
Percentage LDH release and culture malondialdehyde (MDA) content (nmol/mg protein, mean ± S.E.M, n = 6-12/condition) in cultures after 24 hour treatment with 10 μM ferrous sulfate (Fe) alone or with indicated concentrations (μM) of doxycycline (DX) or macrophage/microglia inhibitory factor (MIF).
Minocycline prevents lipid oxidation in isolated cell membranes
In order to determine if minocycline directly inhibited iron-catalyzed lipid oxidation, its effect on malondialdehyde production in isolated culture membranes was quantified. In samples treated with 50 μM FeSO4 for 1h, malondialdehyde was increased 4.5-fold compared with control samples incubated with Tris buffer only (Fig. 2). Equimolar minocycline reduced malondialdehyde to control levels, while equimolar doxycycline had no effect.
Figure 2.
Effect of minocycline and doxycycline on lipid peroxidation in isolated culture membranes. Malondialdehyde (mean ± S.E.M, n = 8/condition) in membranes incubated for 30 minutes with 50 μM ferrous sulfate (Fe) alone or with indicated concentrations (μM) of minocycline (MN) or doxycycline (DX). Control samples were incubated in Tris buffer alone. ***P<0.001. v. ferrous sulfate alone, Bonferroni multiple comparisons test.
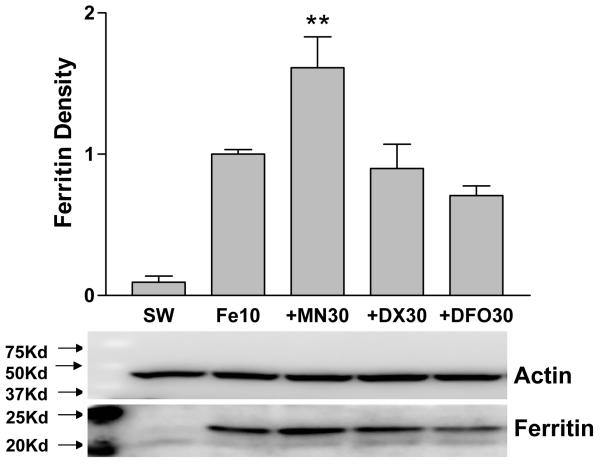
Minocycline increases ferritin expression in cultures treated with iron
Ferritin expression was increased tenfold in cultures treated with 10 μM FeSO4 for 24 h, compared with sham-washed controls (Fig. 3). In cultures treated with FeSO4 plus minocycline for 24h, the mean ferritin level was 17-fold higher than controls, and was significantly increased compared with the mean value in cultures treated with FeSO4 only. Minocycline treatment alone had no effect on ferritin expression (relative density values: 0.179 ± 0.041 v. 0.183 ± 0.036 for sham-wash). Doxycycline and deferoxamine did not alter ferritin induction by iron.
Figure 3.
Effect of minocycline, doxycycline, and deferoxamine on ferritin induction by ferrous sulfate. Bars represent mean ferritin band density (± S.E.M, n = 6-9/condition) in mixed neuron-glia cultures harvested after 24 hour incubation with ferrous sulfate (Fe) 10 μM alone or with 30 μM minocycline (MN), doxycycline (DX), or deferoxamine (DFO). Sham wash (SW) cultures were subjected to washes and medium exchange only. Representative immunoblot was immunstained with anti-horse spleen ferritin and anti-actin (gel loading control). **P<0.01. v. ferrous sulfate alone.
Comparison of chelating activities of minocycline and deferoxamine
Incubation with minocycline produced a rapid a decrease in absorbance at 630 nm of the chrome azurol S-based universal siderophore assay solution (Fig. 4), in agreement with prior observations [12]. Surprisingly, at concentrations below 100 μM, its iron chelating activity as assessed by this assay exceeded that of deferoxamine. Deferoxamine had greater activity at concentrations greater than 100 μM.
Figure 4.
Comparison of chelating activities of minocycline and deferoxamine. Absorbance (630 nm, mean ± S.E.M., n = 5/condition) of chrome azurol S/Fe3+/hexadecyltrimethylammonium bromide (HDTMA) complex after 1 hour incubation with indicated concentrations (μM) of minocycline or deferoxamine, scaled to absorbance of samples incubated with water vehicle only (=1.0). *P<0.05, ***P<0.001. v. minocycline absorbance.
Discussion
These results demonstrate that minocycline potently inhibits iron neurotoxicity in cortical cell cultures and iron-catalyzed lipid oxidation in isolated cell membranes. Furthermore, two other inhibitors of microglial activation, doxycycline and macrophage/microglia inhibitory factor (MIF) [24,25], provide no cytoprotection. Since iron neurotoxicity contributes to neuronal loss after experimental ICH [2,6], these data suggest that any therapeutic benefit of minocycline may be due at least in part to direct attenuation of this injury mechanism, rather than to a specific anti-inflammatory effect.
Minocycline concentrations in the low nanomolar range are sufficient to prevent microglial activation [26], which may be mediated by inhibition of poly (ADP-ribose) polymerase-1 (PARP-1) [27]. In contrast, low micromolar concentrations were required for neuroprotection in the present study. The effect of minocycline in ICH models resulted from treatment with 45-90 mg/kg/day [8,9,10]. Published pharmacokinetic data suggest that these high doses are quite likely to produce minocyline levels in the micromolar range. In a rat model of ischemic stroke, peak serum concentrations after intravenous injection of 3 or 10 mg/kg minocycline were 7 μM and 26 μM, respectively. Although brain levels were not measured, minocycline readily crosses the blood-brain barrier, and steady state brain concentrations are only slightly lower than those in serum. In uninjured dogs, Barza et al. [28] reported a venous serum concentration of 3.4 μg/ml (6.9 μM) and a brain concentration of 2.9 μg/ml (5.9 μM) after administration of a 5 mg/kg minocycline loading dose followed by continuous infusion of 1 mg/kg for three hours. Further studies testing minocycline at doses that are likely to produce low nanomolar brain concentrations may help to clarify its mechanism of action.
Doxycycline has also been reported to have iron chelating activity [12], but it provided no cytoprotection at effective minocycline concentrations. Two factors may account for this discrepancy. First, minocycline is considerably more lipid-soluble, with a chloroform-water partition coefficient of 30 at pH 7.4, versus 0.48 for doxycycline and 0.09 for tetracycline [28]. It may therefore accumulate at a higher concentration in cell compartments that are vulnerable to iron-catalyzed lipid oxidation, such as the hydrophobic interior of cell membranes. Second, minocycline also has a direct antioxidant effect, scavenging free radicals in the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay with an IC50 in of 3.1 μM, compared with 783 μM for doxycycline and 985 μM for tetracycline [29].
Contrary to our expectation that iron chelators would reduce ferritin induction by ferrous sulfate, deferoxamine and doxycycline had no effect, while minocycline increased ferritin levels. Ferritin expression in response to iron loading is regulated at the translational level by iron regulatory proteins (IRP's), which bind to an iron responsive element in the 5' untranslated region of ferritin mRNA and inhibit translation [30]. In this culture system and in the intact CNS, IRP2 is the primary regulator [31,32]; it undergoes ubiquitination and proteasomal degradation in iron-replete cells, enabling translation [33]. This process is initiated by iron binding at cysteine residues [34], and is apparently not significantly impaired by a threefold molar excess of deferoxamine or doxycycline. Moreover, it appears to be facilitated by minocycline, which may be due to increased iron delivery by the lipophilic minocycline-iron complex, although other mechanisms related to the efficiency of iron transfer from minocycline to IRP2 cannot be excluded. Since ferritin attenuates iron-mediated neuronal injury [35], its increased expression may also contribute to the neuroprotective effect of minocycline.
Injury to cells surrounding a hematoma may be mediated by a variety of mechanisms, including mechanical injury from clot expansion and retraction, toxicity of hemoglobin and thrombin, and inflammation [36]. It is unlikely that any single drug that specifically targets one mechanism will provide an optimal outcome. Minocycline appears to have multiple beneficial actions. Since it has been proven to be safe for human administration, it clearly seems worthy of further investigation. However, at doses that are likely to produce micromolar concentrations in the CNS, it has very limited utility as a pharmacologic probe in mechanistic studies. Its efficacy in hemorrhagic stroke models should not be considered compelling evidence that microglial activation plays a role in the pathogenesis of ICH.
Acknowledgements
Funding for this study was provided by grants from the National Institutes of Health (R01 NS042273-06) and the Great Rivers Affiliate of the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wu J, Hua Y, Keep RF, Nakemura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- [2].Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–12. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- [3].Gong Y, Tian H, Xi G, Keep RF, Hoff JT, Hua Y. Systemic zinc protoporphyrin administration reduces intracerebral hemorrhage-induced brain injury. Acta Neurochir Suppl. 2006;96:232–6. doi: 10.1007/3-211-30714-1_50. [DOI] [PubMed] [Google Scholar]
- [4].Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J. Neurosurg. 2007;106:428–35. doi: 10.3171/jns.2007.106.3.428. [DOI] [PubMed] [Google Scholar]
- [5].Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Rad. Biol. Med. 2003;35:872–881. doi: 10.1016/s0891-5849(03)00431-3. [DOI] [PubMed] [Google Scholar]
- [6].Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–8. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- [7].Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- [8].Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, Yong VW, Peeling J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53:731–42. doi: 10.1002/ana.10553. [DOI] [PubMed] [Google Scholar]
- [9].Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol. 2007;207:227–37. doi: 10.1016/j.expneurol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- [10].Wu J, Yang S, Xi G, Fu G, Keep RF, Hua Y. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res. 2009;31:183–8. doi: 10.1179/174313209X385680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–21. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grenier D, Huot MP, Mayrand D. Iron-chelating activity of tetracyclines and its impact on the susceptibility of Actinobacillus actinomycetemcomitans to these antibiotics. Antimicrob Agents Chemother. 2000;44:763–6. doi: 10.1128/aac.44.3.763-766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Leyden JJ. Absorption of minocycline hydrochloride and tetracycline hydrochloride. Effect of food, milk, and iron. J Am Acad Dermatol. 1985;12:308–12. doi: 10.1016/s0190-9622(85)80041-4. [DOI] [PubMed] [Google Scholar]
- [14].Gordon G, Sparano BM, Iatropoulos MJ. Hyperpigmentation of the skin associated with minocycline therapy. Arch Dermatol. 1985;121:618–23. [PubMed] [Google Scholar]
- [15].Graf E, Mahoney JR, Bryant RG, Eaton JW. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984;259:3620–4. [PubMed] [Google Scholar]
- [16].Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; Oxford: 1999. pp. 54–55. [Google Scholar]
- [17].Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yoshida T, Migita CT. Mechanism of heme degradation by heme oxygenase. J Inorg Biochem. 2000;82:33–41. doi: 10.1016/s0162-0134(00)00156-2. [DOI] [PubMed] [Google Scholar]
- [19].Chen J, Regan RF. Heme oxygenase-2 gene deletion increases astrocyte vulnerability to hemin. Biochem. Biophys. Res. Commun. 2004;318:88–94. doi: 10.1016/j.bbrc.2004.03.187. [DOI] [PubMed] [Google Scholar]
- [20].Koh JY, Choi DW. Vulnerability of cultured cortical neurons to damage by excitotoxins: Differential susceptibility of neurons containing NADPH-diaphorase. J. Neurosci. 1988;8:2153–2163. doi: 10.1523/JNEUROSCI.08-06-02153.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Regan RF, Jasper E, Guo YP, Panter SS. The effect of magnesium on oxidative neuronal injury in vitro. J. Neurochem. 1998;70:77–85. doi: 10.1046/j.1471-4159.1998.70010077.x. [DOI] [PubMed] [Google Scholar]
- [22].Sadrzadeh SMH, Anderson DK, Panter SS, Hallaway PE, Eaton JW. Hemoglobin potentiates central nervous system damage. J. Clin. Invest. 1987;79:662–664. doi: 10.1172/JCI112865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen-Roetling J, Regan RF. Effect of heme oxygenase-1 on the vulnerability of astrocytes and neurons to hemoglobin. Biochem Biophys Res Commun. 2006;350:233–7. doi: 10.1016/j.bbrc.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–66. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lai AY, Todd KG. Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia. 2006;53:809–16. doi: 10.1002/glia.20335. [DOI] [PubMed] [Google Scholar]
- [26].Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006;103:9685–90. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Barza M, Brown RB, Shanks C, Gamble C, Weinstein L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother. 1975;8:713–20. doi: 10.1128/aac.8.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kraus RL, Pasieczny R, Lariosa-Willingham K, Turner MS, Jiang A, Trauger JW. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem. 2005;94:819–27. doi: 10.1111/j.1471-4159.2005.03219.x. [DOI] [PubMed] [Google Scholar]
- [30].Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–16. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- [31].Meyron-Holtz EG, Ghosh MC, Iwai K, LaVaute T, Brazzolotto X, Berger UV, Land W, Ollivierre-Wilson H, Grinberg A, Love P, Rouault TA. Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. Embo J. 2004;23:386–95. doi: 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Regan RF, Li Z, Chen M, Zhang X, Chen-Roetling J. Iron regulatory proteins increase neuronal vulnerability to hydrogen peroxide. Biochem Biophys Res Commun. 2008;375:6–10. doi: 10.1016/j.bbrc.2008.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Iwai K, Drake SK, Wehr NB, Weissman AM, LaVaute T, Minato N, Klausner RD, Levine RL, Rouault TA. Iron-dependent oxidation, ubiquitination, and degradation of iron regulatory protein 2: Implications for degradation of oxidized proteins. Proceedings of the National Academy of Sciences. 1998;95:4924–4928. doi: 10.1073/pnas.95.9.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kang DK, Jeong J, Drake SK, Wehr NB, Rouault TA, Levine RL. Iron regulatory protein 2 as iron sensor. Iron-dependent oxidative modification of cysteine. J Biol Chem. 2003;278:14857–64. doi: 10.1074/jbc.M300616200. [DOI] [PubMed] [Google Scholar]
- [35].Regan RF, Chen M, Li Z, Zhang X, Benvenisti-Zarom L, Chen-Roetling J. Neurons lacking iron regulatory protein-2 are highly resistant to the toxicity of hemoglobin. Neurobiol Dis. 2008;31:242–249. doi: 10.1016/j.nbd.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]