Abstract
Resection of a cerebral arteriovenous malformation (AVM), epileptic focus, or glioma, ideally has a prerequisite of microscopic delineation of the lesion borders in relation to the normal gray and white matter that mediate critical functions. Currently, Wada testing and functional magnetic resonance imaging (fMRI) are used for preoperative mapping of critical function, whereas electrical stimulation mapping (ESM) is used for intraoperative mapping. For lesion delineation, MRI and positron emission tomography (PET) are used preoperatively, whereas microscopy and histological sectioning are used intraoperatively. However, for lesions near eloquent cortex, these imaging techniques may lack sufficient resolution to define the relationship between the lesion and language function, and thus not accurately determine which patients will benefit from neurosurgical resection of the lesion without iatrogenic aphasia.
Optical techniques such as intraoperative optical imaging of intrinsic signals (iOIS) show great promise for the precise functional mapping of cortices, as well as delineation of the borders of AVMs, epileptic foci, and gliomas. Here we first review the physiology of neuroimaging, and then progress towards the validation and justification of using intraoperative optical techniques, especially in relation to neurosurgical planning of resection AVMs, epileptic foci, and gliomas near or in eloquent cortex. We conclude with a short description of potential novel intraoperative optical techniques.
Keywords: intraoperative, optical imaging, intrinsic signal, BOLD signal, functional brain mapping, neurosurgery, tumor resection, language mapping, cerebral cortex
INTRODUCTION
Normal brain function and neurovascular coupling
The regulation of cerebral blood flow (CBF), metabolic rate of oxygen utilization (CMRO2), and glucose utilization (CMRglc) during brain activity requires neurons, astrocytes, and vascular cells to act in precise coordination. Specifically, neuronal activity leads to localized increased oxygen and glucose metabolism, which in turn generates chemical signals that act on glia, endothelial cells, pericytes, and smooth muscle cells which transduce these signals into changes in vascular tone that leads to functional hyperemia (Hawkins and Davis, 2005). Such cellular changes cause a direct spatial and temporal relationship between chemo-electrical, CMRO2, CMRglc, and CBF changes, collectively termed “neurovascular coupling” (Figures 1 and 3). In the following section, the physiologic principles of neurovascular coupling that underlie the technique of optical imaging of intrinsic signals (OIS) will be discussed, as well as the neurosurgical applications of intraoperative OIS (iOIS).
Figure 1. Snapshot of microanatomy of neurovascular coupling.
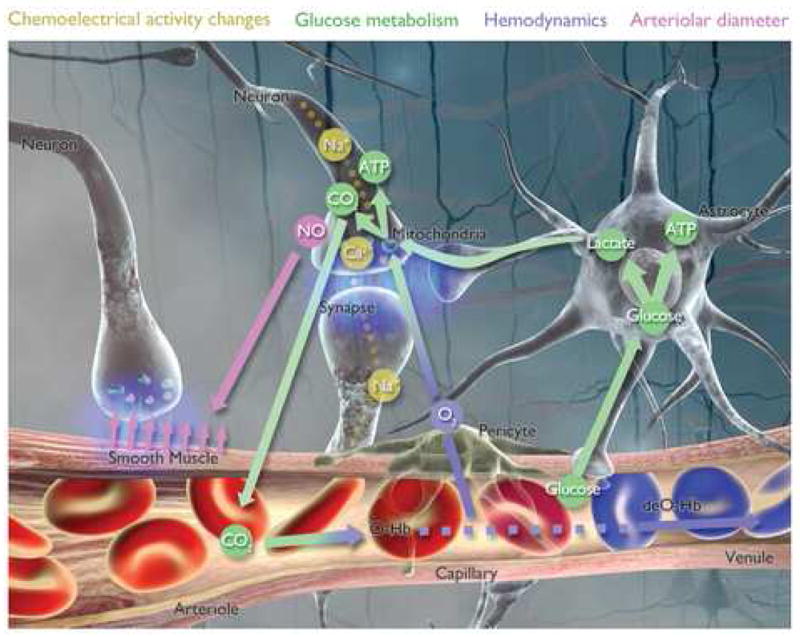
See text. Also, see video: http://www.loni.ucla.edu/SVG/index.php?vid=261.
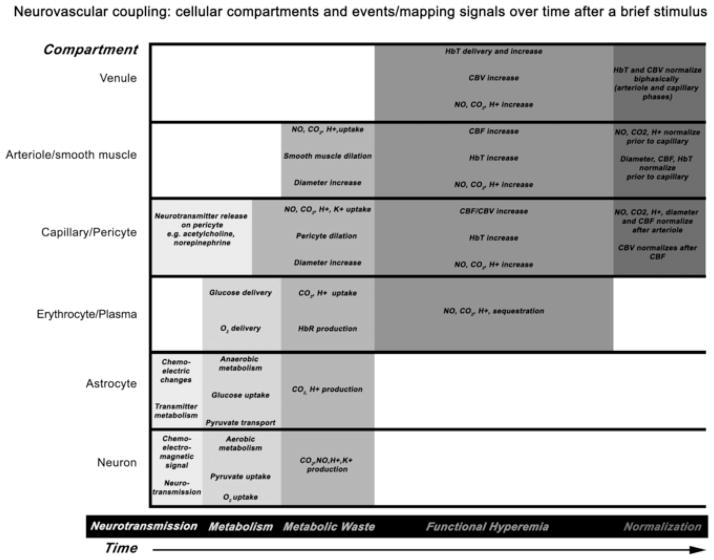
Figure 3. Neurovascular coupling events across cellular compartments.
Cellular compartments are depicted on the y-axis, time on the x-axis, and related events or mapping signals by the grayscale shading. Neurotransmission occurs within tens of milliseconds. Local metabolism increase and metabolic waste production occur within hundreds of milliseconds. Functional hyperemia and normalization occurs within seconds. There is a slight staggering in normalization of CBF and CBV changes. Prolonged stimulation may alter the timing, onset or offset of any of these events in the various compartments. AVMs primarily alter functional hyperemia, seizures alter all events but primarily neurotransmission, and gliomas primarily alter metabolism.
INTRAOPERATIVE OPTICAL IMAGING OF INTRINSIC SIGNALS
OIS is a brain mapping technique that can visualize brain compartments with micrometer and millisecond resolution. In its simplest configuration OIS is similar to fMRI, and maps changes in deoxyhemoglobin (HbR), and hence changes in CMRO2 and CBF can be inferred (Frostig et al., 1990; Malonek and Grinvald, 1997; Mayhew et al., 2000; Sheth et al., 2004b). OIS requires an optically visible brain, as such it has been used primarily in animals for neurovascular research (Frostig et al., 1990; Grinvald et al., 1991; Grinvald et al., 1986; Lieke, 1993; Prakash et al., 1996; Prakash et al., 2000), but also in humans intraoperatively (Cannestra et al., 1998a; Cannestra et al., 1996; Cannestra et al., 2000; Cannestra et al., 2001; Cannestra et al., 2004; Cannestra et al., 1998b; Haglund et al., 1992; Nariai et al., 2005; Pouratian et al., 2000; Pouratian et al., 2002b; Sato et al., 2002; Sato et al., 2005; Schwartz et al., 2004; Shoham and Grinvald, 2001; Toga et al., 1995). Specifically, iOIS has been demonstrated to be a potentially useful neurosurgical tool for both functional brain mapping (Cannestra et al., 2000; Cannestra et al., 2001; Haglund et al., 1992; Lin et al., 2001; Nariai et al., 2005; Schwartz, 2005; Toga et al., 1995) and lesion delineation (Cannestra et al., 2004; Popescu and Toms, 2006; Toms et al., 2005).
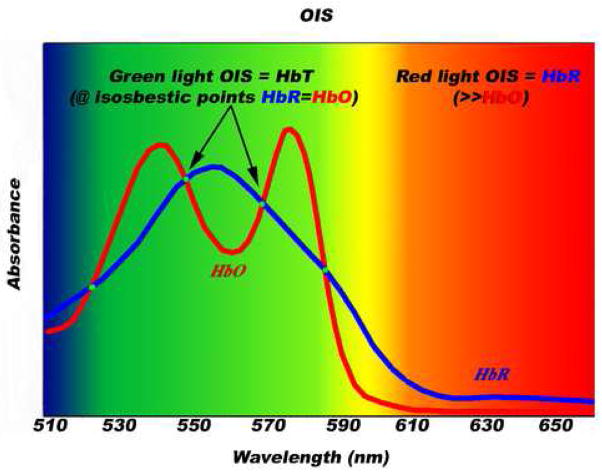
OIS exploits a trait that is inherent to vascularized tissues, especially the cerebral cortex—when illuminated with light, active cortex and its associated vasculature exhibit changes in light reflectance relative to inactive areas (Grinvald et al., 1986). Such activity-dependent light reflectance patterns occur without dyes or tracers, have characteristic spatiotemporal features, called “intrinsic signals.” There are three main sources of intrinsic signals, and each one can become dominant depending on the spectral composition of recorded light (Frostig et al., 1990; Malonek and Grinvald, 1996; Mayhew et al., 2000; Sheth et al., 2004a). With green-yellow light (~500–599 nm), total-hemoglobin (HbT) is the dominant source of the intrinsic signal (Figure 2). This signal is typically a monophasic response peaking in the capillary bed 3–5 s post stimulation, representing increased HbT from functional hyperemia (Frostig et al., 1990; Malonek and Grinvald, 1996; Mayhew et al., 2000; Sheth et al., 2004a). With red light (~600–699 nm), HbR is the dominant source of the intrinsic signal. This signal is typically a biphasic response in the capillary bed, with an early phase peaking 0.5–1.5 s post stimulation representing increased HbR (Frostig et al., 1990; Malonek and Grinvald, 1996; Mayhew et al., 2000; Sheth et al., 2004a) via increased CMRO2 (Thompson et al., 2003), followed by a late phase peaking 3–5 s post stimulation from functional hyperemia. Lastly, in the near infrared spectrum (~700–800 nm), hemoglobin minimally absorbs light, hence changes in cellular swelling, which causes light-scattering changes, are the dominant source of the intrinsic signal.
Figure 2. Green-light versus red-light OIS.
Hemoglobin is the major source of light absorption in the visible spectrum of exposed living brain. In the green spectrum, HbO and HbR have identical absorption at four different isosbestic points, hence maps with green-light OIS are largely maps of HbT changes. In the red spectrum, HbR absorbs light much more than HbO, hence maps with red-light OIS are largely maps of HbR changes.
OIS is usually described as an “invasive” imaging technique because it requires surgery to make the cortex visible. However, as iOIS is performed in the operating room by attaching a charge-coupled device (CCD) camera and optical filter(s) to the operating scope, it is non-invasive compared to the current “gold” standard intraoperative electrophysiological techniques, which require placing electrodes directly onto or into the brain. In essence, iOIS improves the neurosurgeon’s eyesight by extracting the most relevant information from reflected light arriving in the operating scope to create functional maps and to delineate cortical lesions. iOIS has been slowly evolving, as technical challenges have had to be overcome, most notably movement and noise reduction (reviewed in (Haglund and Hochman, 2004; Pouratian et al., 2003a; Pouratian et al., 2003b)). Additionally, iOIS requires a willing neurosurgeon, approval from the institutional review board, and consent of the patient. When these conditions are met, iOIS is performed in a traditional operating room environment following craniotomy, dural reflection, and positioning the CCD camera over the cortex. As sensory evoked potentials are robust, even in anesthetized brain, primary sensory cortical functional mapping with iOIS can be performed in fully anesthetized patients. However, language mapping requires a conscious patient, hence for patients with lesions near or in eloquent cortex intraoperative brain mapping (for any technique, including iOIS) requires the patient to be awoken under local anesthesia in the operating room in order to perform language mapping.
Presently, most iOIS studies have measured intrinsic signals from red light (Cannestra et al., 1998a; Cannestra et al., 1996; Cannestra et al., 2000; Cannestra et al., 2001; Cannestra et al., 2004; Cannestra et al., 1998b; Haglund et al., 1992; Nariai et al., 2005; Pouratian et al., 2000; Pouratian et al., 2002b; Sato et al., 2002; Sato et al., 2005; Schwartz et al., 2004; Shoham and Grinvald, 2001; Toga et al., 1995)—hence assessing changes in HbR in arterioles, capillaries, and venules—to map somatosensory evoked activity (Cannestra et al., 1998a; Cannestra et al., 1996; Cannestra et al., 2001; Cannestra et al., 1998b; Nariai et al., 2005; Sato et al., 2002; Sato et al., 2005; Schwartz et al., 2004; Shoham and Grinvald, 2001; Toga et al., 1995), tongue movements (Pouratian et al., 2002b), and language tasks (Cannestra et al., 2000; Cannestra et al., 2004; Pouratian et al., 2000). iOIS has also been used to delineate AVMs (Cannestra et al., 2004) and epileptiform after-discharges (Haglund et al., 1992). iOIS maps have been validated as accurate by comparison with “gold” standards of intraoperative electrocortical stimulation mapping (ESM), or cortical evoked potentials (EP). Additionally, a few studies have compared iOIS maps to preoperative fMRI, as well as intraoperative EP or ESM (Cannestra et al., 2001; Cannestra et al., 2004; Pouratian et al., 2002b).
These studies have demonstrated that iOIS is a useful intraoperative tool, however it still unproven that performing iOIS improves surgical outcome. From the neurophysiologic perspective, iOIS has provided insight into the spatiotemporal dynamics of human neurovascular coupling, which is outlined in the following section.
SPATIOTEMPORAL DYNAMICS ACROSS NEUROVASCULAR COMPARTMENTS
This section presents a conceptual model of the spatial and temporal evolution of neurovascular changes as they propagate through the various neurovascular compartments (figures 1 and 3). The model is based largely on animal OIS and human fMRI data, however, iOIS data has generally supported the hypotheses suggested from animal OIS studies (Andresen et al., 2006; Chen-Bee et al., 2007; Girouard and Iadecola, 2006; Zheng et al., 2005). This model has a few caveats: The exact temporal onset of some of the events is either not well described or studies have yielded contradictory results. Additionally, stimulus duration and intensity can drastically affect the timing and amplitude of different neurovascular events (Polley et al., 1999b), which may be a major source of contradictory data. To our knowledge, there are no in vivo studies of astrocyte function in humans (Koehler et al., 2006). Lastly, it is not precisely known how AVMs, seizure foci, and gliomas alter neurovascular coupling in the various cellular compartments.
Initial events (neurons, astrocytes, and capillaries)
Activated neurons release glutamate into the synaptic cleft, which increases glutamate uptake as well as glycolysis in nearby astrocytes. Glutamate is converted to glutamine, shuttled back to the neuron, and converted back to glutamate. Glycolysis in astrocytes results in excess lactate that is transported into the neurons. Neurons convert lactate into adenosine triphosphate via oxidative phosphorylation (Koehler et al., 2006; Magistretti, 2000). This leads to metabolic byproducts, such as nitric oxide (NO), protons, carbon dioxide, and potassium (Girouard and Iadecola, 2006).
During oxidative metabolism, local CMRO2 increases (Thompson et al., 2003), leading to transiently increased levels of deoxyhemoglobin (HbR) in the erythrocytes in local capillary beds (Berwick et al., 2005).
If the activating stimulus is strong enough, oxygen demand can exceed oxygen delivery at the basal blood flow rate. The activation leads to even more metabolic byproducts some of which are directly vasoactive, such as NO and potassium (Girouard and Iadecola, 2006). Additionally, a behaviorally relevant stimulus can lead to release of vasoactive neurotransmitters, such as acetylcholine or norepinephrine (Prakash and Frostig, 2005; Sandor, 1999). All these factors alter nearby endothelial cells, pericytes, and smooth muscle cells (Allt and Lawrenson, 2001), leading to localized increased HbT via increased CBF and CBV (functional hyperemia).
Functional hyperemia occurs just after increases in CMRO2 and leads to an overwhelming delivery of oxyhemoglobin (HbO). With a sufficiently strong stimulus, the rise of the HbO increase from functional hyperemia can blunt the initial HbR increase. This leads to a non-linearity of HbR relation to stimulus intensity
Upstream events (arterioles)
Endothelial cells, pericytes, and smooth muscle cells act together to change arteriolar diameter that increases CBF.
Local increases in blood flow (or AVMs) can “steal” blood from nearby arterioles (Cannestra et al., 1996), which limits how much CBF can increase over multiple, simultaneously activated areas, or for large magnitude stimuli. This leads to a loss of correlation between functional hyperemia and stimulus amplitude.
HbR increases are also transmitted upstream from capillary beds into arterioles (Berwick et al., 2005)
Downstream events (venules)
Increases in CBF in arterioles and capillaries are transmitted into venules, leading to a slightly delayed, increase in CBV and HbT.
Increased CMRO2 in capillary beds leads to higher HbR in the venules compared to arterioles.
In the following sections, we compare iOIS to other techniques and provide an overview of challenges for iOIS applied to resection of AVMs, seizure foci, and gliomas in or near eloquent cortex.
FUNCTIONAL MAPPING AND LESION DELINEATION
Language function mapping
Resection of non-primary sensorimotor and association cortices generally leaves minimal lasting neurological deficits, hence pre- or intra-operative functional mapping for patients with lesions within these areas is not essential. Conversely, primary sensorimotor cortices have predictable locations relative to cortical anatomical topography, and it is usually straightforward to confirm their locations, both with preoperative functional mapping and with intraoperative electrophysiological mapping techniques.
Language functions are more variable in their cortical topography, but language functions in the dominant frontal lobe and perisylvian regions (eloquent cortex) are the most crucial to preserve if surgery-induced aphasia is to be prevented. The preoperative functional brain mapping techniques of Wada testing and fMRI are currently approved for clinical use to determine the location of eloquent cortex (Abou-Khalil, 2007). Wada testing is currently the most widely used, although it carries some risk due to its invasiveness. It generally provides sufficient information about language and memory lateralization for a majority of patients. However a significant number of patients have complex topography of eloquent cortex, in which preoperative fMRI language mapping is quite useful (Bookheimer, 2007). However, preoperative language mapping with fMRI in the setting of AVMs, seizure foci, or gliomas present unique challenges and limitations:
AVMs have high blood flow and reduced vasomotor reactivity (Diehl et al., 1994), which frequently makes them invisible to conventional fMRI methodologies. Hence preoperative fMRI for patients with an AVM in or near eloquent cortex may be of limited utility in determining operability (Bookheimer, 2007; Cannestra et al., 2004).
Medically refractory epilepsy affects about 400,000 Americans. For many of these patients, their disability potentially may be completely eliminated by surgical intervention (Engel, 1996; Wiebe et al., 2001). As the surgery becomes more widely accepted and as more centers become capable of offering it there is still a delay of up to many years of patients having the disorder. Chronic epilepsy can lead to significant, atypical reorganization of the language maps (Sveller et al., 2006), which necessitates precise preoperative language mapping (Bookheimer, 2007; Woermann et al., 2003). Moreover, pre-ictal (Federico et al., 2005), ictal (Aghakhani et al., 2004), and inter-ictal (Benar et al., 2002) activity can dramatically increase the fMRI BOLD response and potentially provide a falsely lateralized or falsely positive preoperative language map.
Gliomas can have a mass effect that decreases or reduces blood flow, which in turn can create a false negative preoperative fMRI map. If the patient has aphasia from the tumor, preoperative mapping may be impossible (Bookheimer, 2007).
Moreover, even the most accurate preoperative map quickly distorts once the cortex is exposed. Mapping cortical function and dysfunction requires a technique with micrometer spatial resolution and can accommodate intraoperative tissue distortions.
Lesion delineation
Three surgically resectable lesions—AVMs, seizure foci, and tumors—derive from different cellular components and hence have different effects on neurovascular coupling. These differential effects make each of these lesions unique in respects to our current ability for pre- and intra-operative delineation.
Vascular lesions such as cavernous malformations or AVMs typically are easily delineated by preoperative imaging techniques, such as MRI or conventional angiography. Visually identifying the lesions is also typically straightforward intraoperatively, as they are readily visible with standard operating room optics. However, AVMs do pose challenges for preoperative functional mapping, as described above.
-
Seizures can arise from a focus of abnormal cortex that has excessive synchronous activity that dramatically alters neurovascular coupling during both ictal (Rowe et al., 1991) and interictal periods (Gaillard et al., 1995). Currently preoperative electroencephalography (EEG), magnetoencephalography (MEG), single photon emission computed tomography (SPECT), PET are all used to approximate the location of seizure foci. All these techniques have only centimeter resolution that may not enable precise localization. In cases of ambiguous localization, prior to resective surgery, electrocorticography (ECoG) using cortical strip, surface, or depth electrodes may further aid in more precisely defining seizure focus (Behrens et al., 1994). However, this delineation method then, requires a second surgery and associated risks while the patient has the electrodes in place, or unnecessarily puts the patients under surgical risk if the lesion is deemed inoperable.
Currently ECoG using grid, strip, or depth electrodes is routinely performed for intraoperative delineation of seizure foci (Abraham and Roland, 2003; Hidenori et al., 2007; Miller et al., 2007). While ECoG is considered the “gold” standard, it is invasive and has a restricted field of view and limited spatial resolution. Novel electrical techniques under development, such as EEG source imaging, may overcome these problems (Ding et al., 2007). Alternatively, iOIS techniques potentially could be used alone or in conjunction with ECoG, as interictal cortex is generally hypoperfused (Gaillard et al., 1995; Liu et al., 2001; Shariff et al., 2006) and has increased deoxyhemoglobin levels within the epileptic focus (Shariff et al., 2006; Suh et al., 2006). iOIS has already been demonstrated to be capable of providing high-resolution maps that provide localizing information about normal cortical functions in epileptic patients, as well as ictal and interictal epilepsy foci (Haglund and Hochman, 2004; Haglund and Hochman, 2005; Haglund et al., 1992; Schwartz, 2005; Suh et al., 2006).
-
Gliomas infiltrate the normal brain parenchyma, alter cellular metabolism, and subtly affect neurovascular coupling (Ravi and James, 2004). Low-grade gliomas present a major surgical challenge as most inevitably progress to high-grade tumors. Currently, gross total resection of a low-grade glioma is the treatment of choice, if possible. However, the surgical risks and benefits are carefully assessed because the resection itself can result in permanent neurological deficits. This is especially true for gliomas in eloquent cortex where the risk of aphasia is high, hence in such cases, there is a need for precise tumor delineation and functional mapping (Grier and Batchelor, 2006).
5-aminolevulinic acid ((5AL); an optical dye) is metabolized preferentially by tumor cells and induces intraoperative tumor fluorescence. Using intraoperative fluorescent optical imaging of 5AL enables more complete resections, leading to improved progression-free survival in patients with malignant gliomas (Stummer et al., 2006).
Another technique, optical coherence tomography (OCT) (Chen et al., 1999) has also recently been demonstrated to intraoperatively distinguish white matter and gray matter and potentially distinguish tumor from normal tissue within glioma resection cavities (Giese et al., 2006). Both 5AL imaging and OCT may be compatible with iOIS to allow essentially simultaneous, functional mapping and tumor delineation.
Functional mapping: BOLD fMRI versus red-light OIS
Blood oxygenation level dependent (BOLD) fMRI is commonly used for preoperative language mapping (Bookheimer, 2007). However, the exact etiology of the BOLD signal is still debated (see (Logothetis and Pfeuffer, 2004) for recent review). Similar to red-light OIS, it is generally assumed that the relative HbR concentration changes due to increased CBF, cerebral blood volume (CBV), and CMRO2 in active cortex gives rise to the positive BOLD signal (Hathout et al., 1999; Logothetis and Pfeuffer, 2004; Pouratian et al., 2002b; Seiyama et al., 2004; Yamamoto and Kato, 2002); although solely changes in CBV can also change the BOLD signal (Steinbrink et al., 2006). Similarly, the red-light OIS signal is not derived purely from HbR changes, as it also contains weak signals from HbO and light-scattering changes. Hence, interpretation of both BOLD fMRI and red-light OIS signals maps can be complex in depending on various physiologic conditions. However, newly developed variants of OIS, such as 2-dimesional optical spectroscopy (2DOS) (Berwick et al., 2005; Dunn et al., 2005; Prakash et al., 2007; Sheth et al., 2005), measure multiple wavelengths in the optical spectrum simultaneously, and hence eliminate signal contamination issues and allow for quantitative mapping of HbO, HbR, and HbT.
As outlined above, AVMs, seizures and gliomas all can alter neurovascular coupling and hence decrease the accuracy of pre-operative fMRI maps. iOIS offers several potential advantages over preoperative fMRI language mapping. Spatial resolution for iOIS is one to two orders of magnitude superior to fMRI and hence the lesion and functional cortex may be easily distinguished by the surgeon on iOIS images (Cannestra et al., 2004). Intraoperative mapping is inherently more accurate as the lesion and functional maps may change after pre-operative images are obtained, either due to lesion evolution and cortical plasticity, or also due to tissue displacement due to operative procedures.
Intraoperative electrical versus optical maps
Animal studies of neurovascular coupling demonstrate that within capillary beds, evoked HbT, HbR, CBF changes and electrical activity are topographically correlated (Brett-Green et al., 2001; Hyder et al., 2001; Masino, 2003; Peterson et al., 1998; Polley et al., 1999a; Polley et al., 2004). In humans, the spatial topography of responses detected by OIS, fMRI, ESM, and EPs are generally consistent with each other. However, as expected, there are some differences. For instance, an ESM and OIS language mapping study showed a close overlap and spatial correlation in Broca’s and Wernicke’s areas. However, red-light OIS maps also contained regions adjacent to, but just outside the ESM-defined maps (Cannestra et al., 2000). Thus, either 1) ESM did not map the language areas in their entirety, or 2) red-light OIS maps also detected cortical regions that were non-essential for language tasks. ESM is a technique that maps brain by disrupting normal function; hence, the second possibility may be more correct, as disrupting non-essential areas may not block the ability to perform language tasks.
Controversies in mapping signals
The correlation between the spatial topography and amplitude of different functional brain maps is still an area of research and debate. Part of the disagreement is methodological: techniques have different spatiotemporal resolution, signals, and data acquisition and analysis methods. For example, most OIS and iOIS studies use brief stimuli, whereas most fMRI studies use a monophasic response model. For these reasons, many fMRI and OIS maps of HbR changes are not directly comparable (Cannestra et al., 2001; Menon et al., 1995; Pouratian et al., 2002a; Pouratian et al., 2002b).
Map topography dependence on mapping signal
In addition to methodological differences, the topography of functional maps is also dependent on which mapping signal is used. For example, although red-light OIS and EP maps have been found to be in excellent spatial registration around the central sulcus (Cannestra et al., 2001; Sato et al., 2002; Shoham and Grinvald, 2001), a study that mapped EP, iOIS and fMRI responses in a single patient (Cannestra et al., 2001) found the fMRI BOLD map using a traditional monophasic response model (~CBF/CBV), was strongest within the vein in the central sulcus. However, when the initial negative BOLD signal (~HbR) was used all three spatial maps were virtually identical.
Overall, these studies suggested that maps derived from electrical activity and HbR/CMRO2 within the capillary beds are topographically correlated, whereas HbT, CBF, or CBV maps may be skewed due to maximal changes in downstream venules and veins.
Map intensity dependence on mapping signal
Besides map topography, another important feature for accurate comparison of intraoperative maps is the correlation of the magnitudes of the mapping signals. Animal OIS studies suggest that for relatively brief stimuli (as compared to prolonged stimuli, such as from the monophasic response model (Jones et al., 2002), the magnitude of late-HbR (Devor et al., 2003; Hewson-Stoate et al., 2005; Sheth et al., 2004b), HbT (Devor et al., 2003; Hewson-Stoate et al., 2005; Sheth et al., 2004b), CBF (Jones et al., 2004; Ngai et al., 1999; Sheth et al., 2004b), and HbO changes (Devor et al., 2003; Hewson-Stoate et al., 2005; Sheth et al., 2004b) appear linearly related to evoked electrical activity over a narrow range, but become non-linear if the stimulus is very weak or very strong (Devor et al., 2003; Hewson-Stoate et al., 2005; Jones et al., 2004; Ngai et al., 1999; Polley et al., 1999b; Sheth et al., 2003; Sheth et al., 2004b). With large evoked electrical activity the relationships are better described by a threshold or power law (Devor et al., 2003; Hewson-Stoate et al., 2005; Sheth et al., 2004b). However, in contrast to late-HbR, HbT, CBF, and HbO changes, early-HbR (CMRO2) changes (Buxton, 2001; Chen-Bee et al., 2007; Fox and Raichle, 1986; Menon et al., 1995; Obrig and Villringer, 2003; Raichle et al., 1976; Thompson et al., 2003; Valabregue et al., 2003) are more correlated to the topography of electrical activity but less correlated to the magnitude of tactile (Polley et al., 1999b) or electrical stimulus (Sheth et al., 2004b).
Similar to OIS studies, some fMRI studies show linear relations between the stimulus intensity and positive BOLD signal (Boynton et al., 1996), as well as BOLD signal and the amplitude of somatosensory EPs (Arthurs and Boniface, 2003). However, other studies suggest that there are also non-linearities between stimulus duration and BOLD signal (Birn et al., 2001), topographic and temporal differences between the positive and negative BOLD signal (Harel et al., 2002; Ugurbil et al., 1999), and differences in signal linearity across brain regions (Soltysik et al., 2004).
Improvements for magnitude mapping
As noted above, the late-HbR (red-light iOIS and positive BOLD fMRI) signal has been the most commonly used for iOIS. OIS using green-light (figure 2) detects HbT changes; and perfusion-based fMRI (such as with arterial spin labelling (ASL)) (Silva, 2005) detects CBF changes. Also noted above, CBF and HbT changes may correlate better with magnitude changes in neuronal activity compared to early-HbR or late-HbR changes. Indeed, in one of the few iOIS studies using green-light (Haglund and Hochman, 2005), the spatial extent of both electrical activity and HbT changes in epileptic cortex was reduced by treatment with mannitol or furosemide, but the magnitude of electrical activation and HbT changes near the stimulating electrode was not. Using H215O PET and BOLD-fMRI in auditory cortex, it is has been shown that changes in CBF are more linearly correlated than changes in HbR to word presentation rate (Rees et al., 1997). These studies suggest that using perfusion-based methodologies may provide better magnitude maps of brain function.
Which mapping signal is best?
In summary, functional mapping studies suggest that weak electrical, CMRO2 (Thompson et al., 2003), and early-HbR changes are topographically correlated, but CMRO2/early-HbR changes occur 0.5–2.5 s after electrical changes. In contrast, the magnitude of changes in HbT, CBF, and CBV are more linearly correlated to stronger electrical changes, but are less tightly related topographically and temporally, as they are transmitted downstream from arterioles and capillaries to venules and veins in 3–5 s (Cannestra et al., 2001; Woolsey et al., 1996). This implies that HbR/CMRO2 changes are topographically related to small electrical changes, but that functional hyperemia is more linearly related to increases in electrical activity (Buxton et al., 2004). However, a study of humans with neocortical epilepsy confuses the matter in that HbT was found to be better than HbR at topographically localizing epileptic foci (Haglund and Hochman, 2004). This further demonstrates the need to understand how different pathologies, such as epileptic foci, alter the relationships of mapping signals of neurovascular coupling.
Overall, these studies suggest that using both oximetric and perfusion signals for brain mapping may provide more accurate topographic and magnitude maps than either one alone. However, the choice of which mapping signal to use is also technique dependent, because OIS using either red or green light can easily distinguish surface arteries, veins, and capillaries; whereas fMRI using either BOLD or ASL cannot, and thus are more prone to volume averaging errors. Hence, iOIS tuned for both HbR and HbT changes should provide maps with the most relevant magnitude and topography. Moreover, AVMs, epileptic foci, and gliomas all affect the mapping signals of neurovascular coupling. Accounting for these effects is important when using iOIS or fMRI for surgical planning.
FUTURE OF INTRAOPERATIVE OPTICAL IMAGING
Beyond OIS: 2DOS
Precise topographic maps of HbR, HbO, and HbT have recently been quantified using novel 2DOS methods in animals (Berwick et al., 2005; Devor et al., 2003; Prakash et al., 2007; Sheth et al., 2005). 2DOS could also be used in humans to create three intraoperative functional maps of HbR, HbO and HbT changes. Additionally, although still controversial, light-changes in the near infrared spectrum (Gratton et al., 1995; Rector et al., 2001; Steinbrink et al., 2005) may derive from light-scattering changes which are more directly related to chemo-electrical changes in neurons and astrocytes. Using 2DOS acquisition hardware and software properly tuned to detect fast optical signals, theoretically, may provide an additional fourth intraoperative functional map of chemo-electrical changes.
Alternatively, the same four intraoperative functional maps could theoretically be derived by combining 2DOS with a future, clinically approved voltage sensitive dye. Moreover, both fluorescence spectroscopy (Koljenovi et al., 2002; Lin et al., 2001; Toms et al., 2005) and tumor-specific dyes (Jackson et al., 2007; Stummer et al., 2006) show great promise for sensitive and specific tumor delineation. Combining 2DOS with fluorescence spectroscopy or tumor-specific dyes may allow simultaneous functional mapping and tumor delineation.
Laser mapping
New laser-based techniques show great promise. For example, laser speckle imaging (LSI) has excellent spatiotemporal resolution for surface imaging of CBF (Briers, 2001; Dunn et al., 2001; Liu et al., 2005). OCT (Giese et al., 2006) and optical Doppler tomography (ODT) (Chen et al., 1999) have exquisite three-dimensional spatial resolution. OCT has recently been validated for intraoperative detection of residual tumor during resection of human gliomas (Giese et al., 2006).
Although only currently used in animals, 2-(or multi-)photon microscopy can delineate real-time changes in hemodynamics with cellular resolution (Shi et al., 1999). 2-photo microscopy has recently been combined laminar optical tomography (LOT) (Hillman et al., 2007; Hillman et al., 2006) for depth-resolved functional imaging of the vascular compartment dynamics in rat brain.
Overall, laser techniques show the greatest promise for ultra-high resolution functional mapping and tumor delineation.
Glial imaging
Calcium appears to play a crucial role in astrocyte function (Fields and Stevens-Graham, 2002). Current techniques that assess metabolic and chemo-electric changes, cannot spatially distinguish astrocyte from neuron (Hirase, 2005; Hopwood et al., 2005; Ido et al., 2001; Vlassenko et al., 2006). Whereas, optical techniques that can distinguish cell types are either too technically challenging to perform in vivo, or cannot measure metabolic function.
Advances in in vivo calcium imaging and development of glial-specific optical dyes of may eventually improve our understanding of astrocyte function and dysfunction (Kerr et al., 2005). Moreover, further development of dyes such as 5AL, will continue to improve the accuracy of intraoperative glioma delineation.
CONCLUSIONS
iOIS shows great promise for micrometer intraoperative functional brain mapping and lesion delineation. iOIS dramatically improves a neurosurgeon’s eyesight by creating functional and lesion maps from images from the surgical microscope. More precise individualized intraoperative maps may potentially improve surgical outcomes, especially resections of AVMs, seizure foci, and gliomas that lay in or near eloquent cortex.
Table 1.
Summary of iOIS papers.
| First Author | Year | Functional Map | Lesion delineated (or surgical indication) | Technique(s) | Pertinent Findings |
|---|---|---|---|---|---|
| Haglund (24) | 1992 | M1-Tongue | Epileptiform after discharges | 695 nm OIS | First iOIS paper |
| Toga (25) | 1995 | S1 | (tumors) | 610 nm OIS | Late-HbR signal begins at 1s, peaks at 3s, and ends by 9s |
| Cannestra (14) | 1996 | S1 | (tumors) | 610 nm OIS | Human/rodent comparison. Late-HbR signal peaks by 2–3s and ends by 6s |
| Cannestra (26) | 1998 | S1 | (tumors or AVMs) | 610 nm OIS | Early-HbR map more accurate than late-HbR map |
| Cannestra (27) | 1998 | S1/A1 | (tumors) | 610 nm OIS | Refractory period of late-HbR signal with repetitive stimulation |
| Cannestra (28) | 2000 | Language | (tumors or cavernous malformations or AVMs) | 610 nm OIS ESM | Broca’s cortex has “boxcar” shaped late-HbR signal. Wernicke’s cortex has more dynamic signal. |
| Pouratian (29) | 2000 | Bilingual Language | (astrocytoma) | 610 nm OIS | Spanish and English maps have both overlapping and distinct regions |
| Cannestra (30) | 2001 | S1 | (tumors or AVMs) | fMRI 610 nm OIS EP | Positive BOLD map contains vein, iOIS map more precise |
| Shoham (31) | 2001 | S1 | (tumors or AVMs) | 605 nm OIS EP | iOIS map of S1 hand representation |
| Pouratian (32) | 2002 | Tongue-M1 | (tumors or cavernous malformations or AVMs) | fMRI 610 nm OIS ESM | iOIS map larger than BOLD map, but signals of similar time course. BOLD map localized on vein, not as well correlated to ESM map as gyral components of iOIS map |
| Sato (33) | 2002 | S1, S2 | (tumors) | 605 nm OIS EP | iOIS map of S1 more topographic than S2 map |
| Cannestra (37) | 2004 | Language | AVMs | Wada fMRI 610 nm OIS ESM | iOIS helps for Spetzler-Martin eloquence score for AVM resection |
| Schwartz (34) | 2004 | S1 | (epilepsy) | 640 nm OIS | Cortical magnification of face is similar to other non-human primates |
| Nariai (35) | 2005 | S1 | (tumors) | 605 nm OIS | iOIS useful for delineating S1 borders during glioma resection |
| Sato (36) | 2005 | S1 | (epilepsy or tumors) | 605 nm OIS | iOIS useful for delineating S1 borders during epilepsy surgery |
| Haglund (47) | 2005 | None | Epileptiform after- discharges | 535 nm OIS | Mannitol and furosemide, decreased extent of epileptiform after-discharges, but not amplitude of stimulus |
Table 2.
Major variants of optical brain mapping techniques
| Optical Brain Mapping Technique | Mapping Signal | Validation in Animal or Human Brain | Required Hardware, | Advantages | Disadvantages | ~Spatial Resolution | ~Temporal Resolution | ~Field of View |
|---|---|---|---|---|---|---|---|---|
| Optical Imaging of Intrinsic Signals (OIS) (44–46) | HbR, HbT, light- scattering | Both | CCD, visible light source, lens/microscope, optical filter. | Relatively simple, used intraoperatively already | Requires exposed brain. Mapping signals have mixed sources. | 10−5 m | 10−2 s | Surface, ~cm2, larger FOV with poorer spatial resolution |
| Diffuse optical Tomography (DOT, NIRS) (92, 121–123) | HbR, HbO, HbT, light- scattering | Both | Fiber-optic near- infrared light source and detector array. | Non-invasive, feasibility demonstrated in human subjects, compatible with fMRI | Low resolution and low signal-to- noise ratio | 10−2 m | 10−2 s | Can be hemispheric or whole brain, ~2 cm depth |
| Optical Spectrosocopy (39, 40, 124, 125) | HbR, HbO, HbT, light- scattering, tumor auto- fluorescence | Both | Visible light spectroscopic device, 337nm laser. | Tumor and radiation necrosis delineation | May require contact of probe onto brain | 10−5 m | 10−3 s | Probe tip |
| 2D-Optical Spectroscopy (2DOS) (10, 72–74) | HbR, HbO, HbT, light- scattering | Animal | CCD, light source, lens/microscope, filterwheel or image splitter. | Best overall resolutions, may be ideal intraoperative technique | Requires exposed brain. | 10−5 m | 10−2 s | Surface, ~cm2, larger FOV with poorer spatial resolution |
| Laser Doppler flowmetry/imaging (LDF/LDI) (16, 87, 126) | CBF | Animal (LDI in human skin) | Laser, detector | Clinical device is available. | Requires exposed brain | 10−3 m | ~10−1 s (dependent on field of view) | Surface, 2D with scanning |
| Laser Speckle Imaging (LSI) (109–111, 127) | CBF | Animal (LSI in human skin) | Laser, microscope, CCD | Real-time 2D imaging of CBF | Requires exposed brain | 10−5 m | 10−3 s | Surface, ~cm2, larger FOV with poorer spatial resolution |
| Optical Coherence/Doppler Tomography (OCT/ODT) (65, 66, 128) | CBF | OCT in both, ODT in animal | Laser, detector | 3D imaging of blood flow, clinical OCT device is available. | Requires exposed brain | 10−6 m | ~10−1 s (dependent on field of view) | 3D with scanning, ~2mm depth |
| Laminar Optical Tomography (LOT) (114) | HbR, HbO, HbT | Animal | Photodiode array, lens/optics, 473 + 532nm lasers | 3D imaging | Requires exposed brain | 10−4 m | ~10−1 s (dependent on field of view) | Planar, 3D with scanning, ~2mm depth |
| Dye Imaging, e.g. Voltage-Sensitive Dyes (VSD; (129- 131)) or 5-amino levilinic acid (5- AL (64)) | VSD: Voltage, (as well as intrinsic signals, 5-AL: gliomas | VSD in animal, 5-AL in both | CCD or Photodiode Array, Dye | VSD maps subthreshold electrical changes. 5-AL maps gliomas borders | Toxicity of dyes, and bleaching of dyes. Requires exposed brain | 10−5 m | 10−3 s | ~mm2, larger FOV with poorer spatial resolution |
| Multi-Photon Microscopy (113, 119, 132, 133) | CBF, HbT, vessel diameter | Animal | Ti:Sapphire Laser, microscope | Microscopic resolution | Requires exposed brain, limited depth, tracer injection | 10−8 m | 10−3 s | ~0.3 mm2, ~0.3 mm depth |
Acknowledgments
This work was supported by NIH: MH52083. We thank Dr. Morgenstern, Dr. Steinmeier and the University Clinic Dresden for their financial support of Falk Uhlemann. We thank Kim Hager and Amanda Hammond for their assistance in creation of figure 1 and the video. We thank Jeremy Theriot for help in creation of figure 4.
Figure 4. Comparison of iOIS and i2DOS.
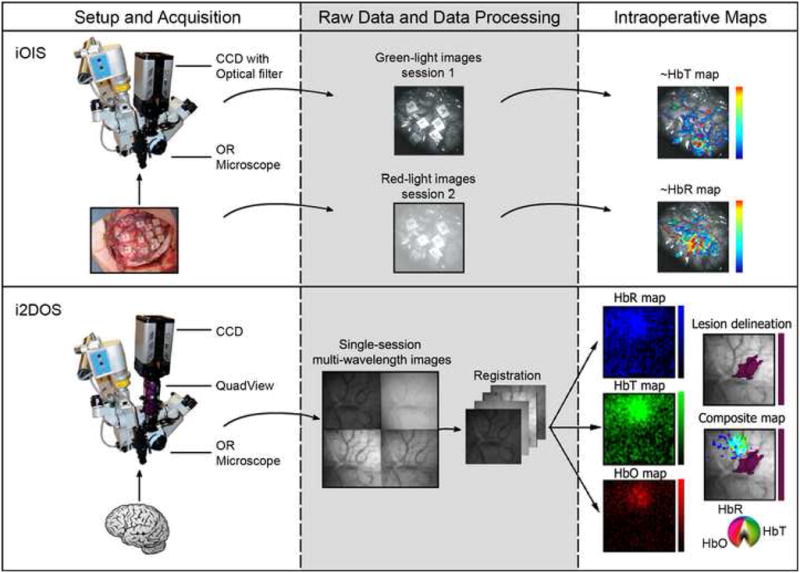
iOIS (top) is performed by using a CCD and optical filter attached to a standard neurosurgical operating microscope. Anesthetized sensory stimulation or awake language mapping (as in this case) sessions are performed with red-light or green-light filters to generate ~HbR or ~HbT maps. i2DOS (bottom) is a variant of i2DOS in which a QuadView optical filter replaces the single optical filter. Four simultaneous images are obtained from up to four different wavelengths; after registration algorithms are performed, maps of HbR, HbT and HbO are generated (mouse somatosensory maps are shown derived from (Prakash et al., 2007)). A hypothetical lesion delineation is shown in purple (this be from the same optical data or generated from other imaging modalities) and a composite map shown that would help guide the neurosurgical resection (composite color scale from (Sheth et al., submitted)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Khalil B. An Update on Determination of Language Dominance in Screening for Epilepsy Surgery: The Wada Test and Newer Noninvasive Alternatives. Epilepsia. 2007;48:442–455. doi: 10.1111/j.1528-1167.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- Abraham K, Roland F. Intraoperative electrocorticography in epilepsy surgery: useful or not? Seizure: the journal of the British Epilepsy Association. 2003;12:577–584. doi: 10.1016/s1059-1311(03)00095-5. [DOI] [PubMed] [Google Scholar]
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Pericytes: Cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Boniface SJ. What aspect of the fMRI BOLD signal best reflects the underlying electrophysiology in human somatosensory cortex? Clin Neurophysiol. 2003;114:1203–1209. doi: 10.1016/s1388-2457(03)00080-4. [DOI] [PubMed] [Google Scholar]
- Behrens E, Zentner J, van Roost D, Hufnagel A, Elger CE, Schramm J. Subdural and depth electrodes in the presurgical evaluation of epilepsy. Acta Neurochirurgica. 1994;128:84–87. doi: 10.1007/BF01400656. [DOI] [PubMed] [Google Scholar]
- Benar CG, Gross DW, Wang Y, Petre V, Pike B, Dubeau F, Gotman J. The BOLD response to interictal epileptiform discharges. Neuroimage. 2002;17:1182–1192. doi: 10.1006/nimg.2002.1164. [DOI] [PubMed] [Google Scholar]
- Berwick J, Johnston D, Jones M, Martindale J, Redgrave P, McLoughlin N, Schiessl I, Mayhew JEW. Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. European Journal of Neuroscience. 2005;22:1655–1666. doi: 10.1111/j.1460-9568.2005.04347.x. [DOI] [PubMed] [Google Scholar]
- Birn RM, Saad ZS, Bandettini PA. Spatial Heterogeneity of the Nonlinear Dynamics in the FMRI BOLD Response. Neuroimage. 2001;14:817–826. doi: 10.1006/nimg.2001.0873. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Pre-Surgical Language Mapping with Functional Magnetic Resonance Imaging. Neuropsychology Review. 2007;17:145–155. doi: 10.1007/s11065-007-9026-x. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett-Green BA, Chen-Bee CH, Frostig RD. Comparing the functional representations of central and border whiskers in rat primary somatosensory cortex. J Neurosci. 2001;21:9944–9954. doi: 10.1523/JNEUROSCI.21-24-09944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiological Measurement. 2001:R35–R66. doi: 10.1088/0967-3334/22/4/201. [DOI] [PubMed] [Google Scholar]
- Buxton RB. The elusive initial dip. Neuroimage. 2001;13:953–958. doi: 10.1006/nimg.2001.0814. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Black KL, Martin NA, Cloughesy T, Burton JS, Rubinstein E, Woods RP, Toga AW. Topographical and temporal specificity of human intraoperative optical intrinsic signals. Neuroreport. 1998a;9:2557–2563. doi: 10.1097/00001756-199808030-00024. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Blood AJ, Black KL, Toga AW. The evolution of optical signals in human and rodent cortex. Neuroimage. 1996;3:202–208. doi: 10.1006/nimg.1996.0022. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Bookheimer SY, Pouratian N, O’Farrell A, Sicotte N, Martin NA, Becker D, Rubino G, Toga AW. Temporal and topographical characterization of language cortices using intraoperative optical intrinsic signals. Neuroimage. 2000;12:41–54. doi: 10.1006/nimg.2000.0597. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Pouratian N, Bookheimer SY, Martin NA, Beckerand DP, Toga AW. Temporal spatial differences observed by functional MRI and human intraoperative optical imaging. Cereb Cortex. 2001;11:773–782. doi: 10.1093/cercor/11.8.773. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Pouratian N, Forage J, Bookheimer SY, Martin NA, Toga AW. Functional magnetic resonance imaging and optical imaging for dominant-hemisphere perisylvian arteriovenous malformations. Neurosurgery. 2004;55:804–812. doi: 10.1227/01.neu.0000137654.27826.71. discussion 812–804. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Pouratian N, Shomer MH, Toga AW. Refractory periods observed by intrinsic signal and fluorescent dye imaging. J Neurophysiol. 1998b;80:1522–1532. doi: 10.1152/jn.1998.80.3.1522. [DOI] [PubMed] [Google Scholar]
- Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The Triphasic Intrinsic Signal: Implications for Functional Imaging. J Neurosci. 2007;27:4572–4586. doi: 10.1523/JNEUROSCI.0326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhao Y, Srinivas S, Nelson SJ, Prakash N, Frostig RD. Optical doppler tomography. IEEE Journal of Selected Topics in Quantum Electronics. 1999;5:1134–1141. doi: 10.1109/2944.796347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron. 2003;39:353–359. doi: 10.1016/s0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Diehl RR, Henkes H, Nahser HC, Kuhne D, Berlit P. Blood flow velocity and vasomotor reactivity in patients with arteriovenous malformations. A transcranial Doppler study. Stroke. 1994;25:1574–1580. doi: 10.1161/01.str.25.8.1574. [DOI] [PubMed] [Google Scholar]
- Ding L, Wilke C, Xu B, Xu X, van Drongelen W, Kohrman M, He B. EEG Source Imaging: Correlating Source Locations and Extents With Electrocorticography and Surgical Resections in Epilepsy Patients. Journal of Clinical Neurophysiology. 2007;24:130. doi: 10.1097/WNP.0b013e318038fd52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage. 2005;27:279–290. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Engel J. Surgery for Seizures. N Engl J Med. 1996;334:647–653. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- Federico P, Abbott DF, Briellmann RS, Harvey AS, Jackson GD. Functional MRI of the pre-ictal state. Brain. 2005;128:1811–1817. doi: 10.1093/brain/awh533. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. NEUROSCIENCE: New Insights into Neuron-Glia Communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Fazilat S, White S, Malow B, Sato S, Reeves P, Herscovitch P, Theodore WH. Interictal metabolism and blood flow are uncoupled in temporal lobe cortex of patients with complex partial epilepsy. Neurology. 1995;45:1841–1847. doi: 10.1212/wnl.45.10.1841. [DOI] [PubMed] [Google Scholar]
- Giese A, Böhringer HJ, Leppert J, Kantelhardt SR, Lankenau E, Koch P, Birngruber R, Hüttmann G. Non-invasive intraoperative optical coherence tomography of the resection cavity during surgery of intrinsic brain tumors. Proceedings of SPIE. 2006;6078:60782Z. [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gratton G, Fabiani M, Friedman D, Franceschini MA, Fantini S, Corballis P, Gratton E. Rapid changes of optical parameters in the human brain during a tapping task. Journal of Cognitive Neuroscience. 1995;7:446–456. doi: 10.1162/jocn.1995.7.4.446. [DOI] [PubMed] [Google Scholar]
- Grier JT, Batchelor T. Low-Grade Gliomas in Adults. Oncologist. 2006;11:681–693. doi: 10.1634/theoncologist.11-6-681. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Frostig RD, Siegel RM, Bartfeld E. High-resolution optical imaging of functional brain architecture in the awake monkey. Proc Natl Acad Sci U S A. 1991;88:11559–11563. doi: 10.1073/pnas.88.24.11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Hochman DW. Optical imaging of epileptiform activity in human neocortex. Epilepsia 45 Suppl. 2004;4:43–47. doi: 10.1111/j.0013-9580.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Hochman DW. Furosemide and Mannitol Suppression of Epileptic Activity in the Human Brain. J Neurophysiol. 2005;94:907–918. doi: 10.1152/jn.00944.2004. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Ojemann GA, Hochman DW. Optical Imaging of Epileptiform and Functional Activity in Human Cerebral Cortex. Nature. 1992:358. doi: 10.1038/358668a0. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Hathout GM, Varjavand B, Gopi RK. The early response in fMRI: A modeling approach. Magnetic Resonance in Medicine. 1999;41:550–554. doi: 10.1002/(sici)1522-2594(199903)41:3<550::aid-mrm18>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hewson-Stoate N, Jones M, Martindale J, Berwick J, Mayhew J. Further nonlinearities in neurovascular coupling in rodent barrel cortex. Neuroimage. 2005;24:565–574. doi: 10.1016/j.neuroimage.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Hidenori S, Hiroyuki S, Shigeki S. Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal-lobe-mass lesions. Seizure: the journal of the British Epilepsy Association. 2007;16:120–127. doi: 10.1016/j.seizure.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM, Boas DA. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EMC, Devor A, Dunn AK, Boas DA. Laminar optical tomography: high-resolution 3D functional imaging of superficial tissues. Proceedings of SPIE. 2006;6143:61431M. [Google Scholar]
- Hirase H. A multi-photon window onto neuronal-glial-vascular communication. Trends in Neurosciences. 2005;28:217–219. doi: 10.1016/j.tins.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR in Biomedicine. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- Ido Y, Chang K, Woolsey TA, Williamson JR. NADH: sensor of blood flow need in brain, muscle, and other tissues. FASEB J. 2001 doi: 10.1096/fj.00-0652fje. 00-0652fje. [DOI] [PubMed] [Google Scholar]
- Jackson H, Muhammad O, Daneshvar H, Nelms J, Popescu A, Vogelbaum MA, Bruchez M, Toms SA. Quantum dots are phagocytized by macrophages and colocalize with experimental gliomas. Neurosurgery. 2007;60:524–530. doi: 10.1227/01.NEU.0000255334.95532.DD. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Mayhew J. Changes in blood flow, oxygenation, and volume following extended stimulation of rodent barrel cortex. Neuroimage. 2002;15:474–487. doi: 10.1006/nimg.2001.1000. [DOI] [PubMed] [Google Scholar]
- Jones M, Hewson-Stoate N, Martindale J, Redgrave P, Mayhew J. Nonlinear coupling of neural activity and CBF in rodent barrel cortex. Neuroimage. 2004;22:956–965. doi: 10.1016/j.neuroimage.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kerr JND, Greenberg D, Helmchen F. From The Cover: Imaging input and output of neocortical networks in vivo. PNAS. 2005;102:14063–14068. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koljenovi S, Choo-Smith L, Schut T, Kros J, Van Den Berge H, Puppels G. Discriminating Vital Tumor from Necrotic Tissue in Human Glioblastoma Tissue Samples by Raman Spectroscopy. Lab Invest. 2002;82:1265–1277. doi: 10.1097/01.lab.0000032545.96931.b8. [DOI] [PubMed] [Google Scholar]
- Lieke EE. Olfactory information processing in insects revealed by real-time optical imaging of intrinsic signals. In: Dirnagl Uea., editor. Optical Imaging of Brain Function and Metabolism. Plenum Press; New York: 1993. pp. 87–93. [DOI] [PubMed] [Google Scholar]
- Lin WC, Toms SA, Johnson M, Jansen ED, Mahadevan-Jansen A. In Vivo Brain Tumor Demarcation Using Optical Spectroscopy. Photochemistry and Photobiology. 2001;73:396–402. doi: 10.1562/0031-8655(2001)073<0396:ivbtdu>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Liu HL, Kochunov P, Hou J, Pu Y, Mahankali S, Feng CM, Yee SH, Wan YL, Fox PT, Gao JH. Perfusion-weighted imaging of interictal hypoperfusion in temporal lobe epilepsy using FAIR-HASTE: comparison with H (2)(15) O PET measurements. Magn Reson Med. 2001;45:431–435. doi: 10.1002/1522-2594(200103)45:3<431::aid-mrm1056>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang Z, Luo Q. Temporal clustering analysis of cerebral blood flow activation maps measured by laser speckle contrast imaging. Journal of Biomedical Optics. 2005;10:024019. doi: 10.1117/1.1891105. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magnetic Resonance Imaging. 2004;22:1517–1531. doi: 10.1016/j.mri.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Cellular bases of functional brain imaging: insights from neuron-glia metabolic coupling. Brain Research. 2000;886:108–112. doi: 10.1016/s0006-8993(00)02945-0. [DOI] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Vascular regulation at sub millimeter range. Sources of intrinsic signals for high resolution optical imaging. Adv Exp Med Biol. 1997;413:215–220. [PubMed] [Google Scholar]
- Masino SA. Quantitative comparison between functional imaging and single-unit spiking in rat somatosensory cortex. J Neurophysiol. 2003;89:1702–1712. doi: 10.1152/jn.00860.2002. [DOI] [PubMed] [Google Scholar]
- Mayhew J, Johnston D, Berwick J, Jones M, Coffey P, Zheng Y. Spectroscopic analysis of neural activity in brain: increased oxygen consumption following activation of barrel cortex. Neuroimage. 2000;12:664–675. doi: 10.1006/nimg.2000.0656. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, Ugurbil K. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med. 1995;33:453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- Miller KJ, denNijs M, Shenoy P, Miller JW, Rao RPN, Ojemann JG. Real-time functional brain mapping using electrocorticography. Neuroimage. 2007;37:504–507. doi: 10.1016/j.neuroimage.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Nariai T, Sato K, Hirakawa K, Ohta Y, Tanaka Y, Ishiwata K, Ishii K, Kamino K, Ohno K. Imaging of somatotopic representation of sensory cortex with intrinsic optical signals as guides for brain tumor surgery. J Neurosurg. 2005;103:414–423. doi: 10.3171/jns.2005.103.3.0414. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Jolley MA, D’Ambrosio R, Meno JR, Winn HR. Frequency-dependent changes in cerebral blood flow and evoked potentials during somatosensory stimulation in the rat. Brain Research. 1999;837:221–228. doi: 10.1016/s0006-8993(99)01649-2. [DOI] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Beyond the Visible--Imaging the Human Brain With Light. J Cereb Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Peterson BE, Goldreich D, Merzenich MM. Optical imaging and electrophysiology of rat barrel cortex. I. Responses to small single-vibrissa deflections. Cereb Cortex. 1998;8:173–183. doi: 10.1093/cercor/8.2.173. [DOI] [PubMed] [Google Scholar]
- Polley DB, Chen-Bee CH, Frostig RD. Two directions of plasticity in the sensory-deprived adult cortex. Neuron. 1999a;24:623–637. doi: 10.1016/s0896-6273(00)81117-4. [DOI] [PubMed] [Google Scholar]
- Polley DB, Chen-Bee CH, Frostig RD. Varying the degree of single-whisker stimulation differentially affects phases of intrinsic signals in rat barrel cortex. J Neurophysiol. 1999b;81:692–701. doi: 10.1152/jn.1999.81.2.692. [DOI] [PubMed] [Google Scholar]
- Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- Popescu MA, Toms SA. In vivo optical imaging using quantum dots for the management of brain tumors. Expert Review of Molecular Diagnostics. 2006;6:879–890. doi: 10.1586/14737159.6.6.879. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, O’Farrell AM, Sicotte NL, Cannestra AF, Becker D, Toga AW. Optical imaging of bilingual cortical representations. Case report. J Neurosurg. 2000;93:676–681. doi: 10.3171/jns.2000.93.4.0676. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J Neurosurg. 2002a;97:21–32. doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Sheth S, Bookheimer SY, Martin NA, Toga AW. Applications and limitations of perfusion-dependent functional brain mapping for neurosurgical guidance. Neurosurg Focus. 2003a;15:E2. doi: 10.3171/foc.2003.15.1.2. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Sheth SA, Martin NA, Toga AW. Shedding light on brain mapping: advances in human optical imaging. Trends Neurosci. 2003b;26:277–282. doi: 10.1016/S0166-2236(03)00070-5. [DOI] [PubMed] [Google Scholar]
- Pouratian N, Sicotte N, Rex D, Martin NA, Becker D, Cannestra AF, Toga AW. Spatial/temporal correlation of BOLD and optical intrinsic signals in humans. Magn Reson Med. 2002b;47:766–776. doi: 10.1002/mrm.10096. [DOI] [PubMed] [Google Scholar]
- Prakash N, Biag JD, Sheth SA, Mitsuyama S, Theriot J, Ramachandran C, Toga AW. Temporal profiles and 2-dimensional oxy-, deoxy-, and total-hemoglobin somatosensory maps in rat versus mouse cortex. Neuroimage. 2007;37:27–36. doi: 10.1016/j.neuroimage.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash N, Cohen-Cory S, Frostig RD. Rapid and opposite effects of BDNF and NGF on the functional organization of the adult cortex in vivo. Nature. 1996;381:702–706. doi: 10.1038/381702a0. [DOI] [PubMed] [Google Scholar]
- Prakash N, Frostig RD. What Has Intrinsic Signal Optical Imaging Taught Us About NGF-Induced Rapid Plasticity in Adult Cortex and Its Relationship to the Cholinergic System? Molecular Imaging and Biology. 2005;7:14–21. doi: 10.1007/s11307-005-0956-5. [DOI] [PubMed] [Google Scholar]
- Prakash N, Vanderhaeghen P, Cohen-Cory S, Frisen J, Flanagan JG, Frostig RD. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J Neurosci. 2000;20:5841–5847. doi: 10.1523/JNEUROSCI.20-15-05841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Grubb RL, Jr, Gado MH, Eichling JO, Ter-Pogossian MM. Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch Neurol. 1976;33:523–526. doi: 10.1001/archneur.1976.00500080001001. [DOI] [PubMed] [Google Scholar]
- Ravi DR, James CD. Altered molecular pathways in gliomas: An overview of clinically relevant issues. Seminars in oncology. 2004;31:595–604. doi: 10.1053/j.seminoncol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rector DM, Rogers RF, Schwaber JS, Harper RM, George JS. Scattered-Light Imaging in Vivo Tracks Fast and Slow Processes of Neurophysiological Activation. Neuroimage. 2001;14:977–994. doi: 10.1006/nimg.2001.0897. [DOI] [PubMed] [Google Scholar]
- Rees G, Howseman A, Josephs O, Frith CD, Friston KJ, Frackowiak RSJ, Turner R. Characterizing the Relationship between BOLD Contrast and Regional Cerebral Blood Flow Measurements by Varying the Stimulus Presentation Rate. Neuroimage. 1997;6:270–278. doi: 10.1006/nimg.1997.0300. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Berkovic SF, Austin MC, McKay WJ, Bladin PF. Patterns of postictal cerebral blood flow in temporal lobe epilepsy: Qualitative and quantitative analysis. Neurology. 1991;41:1096. doi: 10.1212/wnl.41.7.1096. [DOI] [PubMed] [Google Scholar]
- Sandor P. Nervous control of the cerebrovascular system: doubts and facts. Neurochemistry International. 1999;35:237–259. doi: 10.1016/s0197-0186(99)00067-4. [DOI] [PubMed] [Google Scholar]
- Sato K, Nariai T, Sasaki S, Yazawa I, Mochida H, Miyakawa N, Momose-Sato Y, Kamino K, Ohta Y, Hirakawa K, Ohno K. Intraoperative Intrinsic Optical Imaging of Neuronal Activity from Subdivisions of the Human Primary Somatosensory Cortex. Cereb Cortex. 2002;12:269–280. doi: 10.1093/cercor/12.3.269. [DOI] [PubMed] [Google Scholar]
- Sato K, Nariai T, Tanaka Y, Maehara T, Miyakawa N, Sasaki S, Momose-Sato Y, Ohno K. Functional representation of the finger and face in the human somatosensory cortex: intraoperative intrinsic optical imaging. Neuroimage. 2005;25:1292–1301. doi: 10.1016/j.neuroimage.2004.12.049. [DOI] [PubMed] [Google Scholar]
- Schwartz TH. The Application of Optical Recording of Intrinsic Signals to Simultaneously Acquire Functional, Pathological and Localizing Information and Its Potential Role in Neurosurgery. Stereotact Funct Neurosurg. 2005;83:36–44. doi: 10.1159/000085025. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Chen LM, Friedman RM, Spencer DD, Roe AW. Intraoperative optical imaging of human face cortical topography: a case study. Neuroreport. 2004;15:1527–1531. doi: 10.1097/01.wnr.0000131006.59315.2f. [DOI] [PubMed] [Google Scholar]
- Seiyama A, Seki J, Tanabe HC, Sase I, Takatsuki A, Miyauchi S, Eda H, Hayashi S, Imaruoka T, Iwakura T, Yanagida T. Circulatory basis of fMRI signals: relationship between changes in the hemodynamic parameters and BOLD signal intensity. Neuroimage. 2004;21:1204–1214. doi: 10.1016/j.neuroimage.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Shariff S, Suh M, Zhao M, Ma H, Schwartz TH. Recent developments in oximetry and perfusion-based mapping techniques and their role in the surgical treatment of neocortical epilepsy. Epilepsy and Behavior. 2006;8:363–375. doi: 10.1016/j.yebeh.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Sheth S, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Evaluation of coupling between optical intrinsic signals and neuronal activity in rat somatosensory cortex. Neuroimage. 2003;19:884–894. doi: 10.1016/s1053-8119(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Hageman N, Toga AW. Columnar Specificity of Microvascular Oxygenation and Volume Responses: Implications for Functional Brain Mapping. J Neurosci. 2004a;24:634–641. doi: 10.1523/JNEUROSCI.4526-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and Nonlinear Relationships between Neuronal Activity, Oxygen Metabolism, and Hemodynamic Responses. Neuron. 2004b;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou MW, Walker MA, Toga AW. Spatiotemporal evolution of functional hemodynamic changes and their relationship to neuronal activity. Journal of Cerebral Blood Flow & Metabolism. 2005;25:830–841. doi: 10.1038/sj.jcbfm.9600091. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Prakash N, Guiou M, Toga Arthur W. Validation and Visualization of Full-field Optical Spectroscopic Imaging of Cerebral Hemodynamics. Neuroimage. doi: 10.1016/j.neuroimage.2008.09.060. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid Spine Delivery and Redistribution of AMPA Receptors After Synaptic NMDA Receptor Activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shoham D, Grinvald A. The Cortical Representation of the Hand in Macaque and Human Area S-I: High Resolution Optical Imaging. J Neurosci. 2001;21:6820–6835. doi: 10.1523/JNEUROSCI.21-17-06820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC. Perfusion-based fMRI: Insights from animal models. Journal of Magnetic Resonance Imaging. 2005;22:745–750. doi: 10.1002/jmri.20461. [DOI] [PubMed] [Google Scholar]
- Soltysik DA, Peck KK, White KD, Crosson B, Briggs RW. Comparison of hemodynamic response nonlinearity across primary cortical areas. Neuroimage. 2004;22:1117–1127. doi: 10.1016/j.neuroimage.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Steinbrink J, Kempf FCD, Villringer A, Obrig H. The fast optical signal--Robust or elusive when non-invasively measured in the human adult? Neuroimage. 2005;26:996–1008. doi: 10.1016/j.neuroimage.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Steinbrink J, Villringer A, Kempf F, Haux D, Boden S, Obrig H. Illuminating the BOLD signal: combined fMRI-fNIRS studies. Magnetic Resonance Imaging. 2006;24:495–505. doi: 10.1016/j.mri.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncology. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- Suh M, Ma H, Zhao M, Sharif S, Schwartz TH. Neurovascular coupling and oximetry during epileptic events. Molecular Neurobiology. 2006;33:181–197. doi: 10.1385/MN:33:3:181. [DOI] [PubMed] [Google Scholar]
- Sveller C, Briellmann RS, Saling MM, Lillywhite L, Abbott DF, Masterton RAJ, Jackson GD. Relationship between language lateralization and handedness in left-hemispheric partial epilepsy. Neurology. 2006;67:1813–1817. doi: 10.1212/01.wnl.0000244465.74707.42. [DOI] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, Freeman RD. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science. 2003;299:1070–1072. doi: 10.1126/science.1079220. [DOI] [PubMed] [Google Scholar]
- Toga AW, Cannestra AF, Black KL. The temporal/spatial evolution of optical signals in human cortex. Cereb Cortex. 1995;5:561–565. doi: 10.1093/cercor/5.6.561. [DOI] [PubMed] [Google Scholar]
- Toms S, Lin WC, Weil R, Johnson M, Jansen E, Mahadevan-Jansen A. Intraoperative Optical Spectroscopy Identifies Infiltrating Glioma Margins with High Sensitivity. Neurosurgery. 2005;57:382–391. doi: 10.1227/01.neu.000176855.39826.2d. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Hu X, Chen W, Zhu XH, Kim SG, Georgopoulos A. Functional mapping in the human brain using high magnetic fields. Philos Trans R Soc Lond B Biol Sci. 1999;354:1195–1213. doi: 10.1098/rstb.1999.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valabregue R, Aubert A, Burger J, Bittoun J, Costalat R. Relation between cerebral blood flow and metabolism explained by a model of oxygen exchange. J Cereb Blood Flow Metab. 2003;23:536–545. doi: 10.1097/01.WCB.0000055178.31872.38. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Rundle MM, Raichle ME, Mintun MA. Regulation of blood flow in activated human brain by cytosolic NADH/NAD+ ratio. PNAS. 2006;103:1964–1969. doi: 10.1073/pnas.0510632103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M the Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study, G. A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko YE, Sui J, Wei L. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex. 1996;6:647–660. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kato T. Paradoxical correlation between signal in functional magnetic resonance imaging and deoxygenated haemoglobin content in capillaries: a new theoretical explanation. Physics in Medicine and Biology. 2002:1121–1141. doi: 10.1088/0031-9155/47/7/309. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Johnston D, Berwick J, Chen D, Billings S, Mayhew J. A three-compartment model of the hemodynamic response and oxygen delivery to brain. Neuroimage. 2005;28:925–939. doi: 10.1016/j.neuroimage.2005.06.042. [DOI] [PubMed] [Google Scholar]