Abstract
Recent work on the long-term stability of memory and synaptic plasticity has identified a potentially critical role for protein kinase Mzeta (PKMζ). PKMζ is a constitutively active, atypical isoform of protein kinase C that is believed to maintain long term potentiation at hippocampal synapses in vitro. In behaving animals, local inhibition of PKMζ disrupts spatial memory in the hippocampus and conditioned taste aversion memory in the insular cortex. The role of PKMζ in context fear memory is less clear. This study examined the role of PKMζ in amygdala and hippocampal neurons following a standard fear conditioning protocol. The results indicate that PKMζ inhibition in the amygdala, but not in the hippocampus, can disrupt fear memory. This suggests that PKMζ may only maintain select forms of memory in specific brain structures and does not participate in a universal memory storage mechanism.
Keywords: Protein Kinase Mzeta, Amygdala, Hippocampus, Fear Conditioning, ZIP
Long-term potentiation (LTP) is a persistent, activity-dependent increase in synaptic strength that is believed to be a neural substrate for some forms of memory formation and storage (Martin, Grimwood, & Morris, 2000; Moser, Krobert, Moser, & Morris, 1998; Whitlock, Heynen, Shuler, & Bear, 2006). While the induction of LTP is fairly well characterized, the maintenance of established LTP has historically received less attention. Recent work indicates that protein kinase Mzeta (PKMζ), an atypical isoform of protein kinase C, is both necessary and sufficient to maintain hippocampal LTP (Ling et al., 2002; Sajikumar, Navakkode, Sacktor, & Frey, 2005). PKMζ consists of a catalytic domain that is independent of the regulatory domain that accompanies all other PKC isoforms, giving it the unique ability to constantly maintain LTP by increasing the number of active postsynaptic AMPA receptors (Hernandez et al., 2003; Ling, Bernado, & Sacktor, 2006; Ling et al., 2002; Muslimov et al., 2004; Serrano, Yao, & Sacktor, 2005). To the extent that LTP represents a physiological substrate for long term memory in the behaving animal, inhibition of this kinase would be expected to disrupt the retention or storage of memory.
In vivo studies have demonstrated that inhibition of PKMζ appears to erase certain forms of established memories (Pastalkova et al., 2006; Shema, Hazvi, Sacktor, & Dudai, 2009; Shema, Sacktor, & Dudai, 2007). Spatial memory can be disrupted if PKMζ is inhibited in the hippocampus, a brain area known to play a role in spatial and contextual learning (Pastalkova et al., 2006; Serrano et al., 2008). Similarly if PKMζ is inhibited in the insular cortex, which plays a critical role in the learning of conditioned taste aversion, 1-month and 3-month-old taste aversion memories can be effectively erased (Shema et al., 2007; 2009). It is critically important to determine precisely which forms of memory require PKMζ activity. Recently, it was suggested that PKMζ is necessary to maintain specific associations but is not required for general contextual or procedural memory (Serrano et al., 2008). Consistent with this hypothesis, inhibition of PKMζ activity in the dorsal hippocampus disrupted the maintenance of specific spatial memory in the radial arm maze and water maze but was not effective in disrupting working memory or procedural strategies in these tasks (Serrano et al., 2008). Spatial memory in inhibitory avoidance and active avoidance tasks requiring similar contextual cues, however, were fully impaired by PKMζ inhibition (Pastalkova et al., 2006, Serrano et al., 2008). The distinction between spatial memories that require PKMζ and those that are independently maintained remains unclear.
The role of PKMζ in the maintenance of context fear associations is central to this debate. There is currently general agreement that the acquisition and long-term retention of Pavlovian fear conditioning, in which neutral cues are arranged to predict an aversive outcome such as foot shock, critically depends on processes occurring within the amygdala (Fanselow & LeDoux, 1999; Helmstetter, Parsons, & Gafford, 2008; Maren, 2001). Exposing rats to the training protocol during fear conditioning results in altered gene expression in amygdala neurons (Levenson et al., 2004; Ressler, Paschall, Zhou, & Davis, 2002; Stork, Stork, Pape & Obata, 2001), induction of LTP at local synapses (Rogan & LeDoux, 1995) and the activation of intracellular signaling pathways involved in long–term synaptic modification (Parsons, Gafford, & Helmstetter, 2006). If amygdala neurons are prevented from making new mRNA or protein during the period immediately after training, no new memories are formed (Bailey, Kim, Sun, Thompson, & Helmstetter, 1999; Parsons et al., 2006). Disruption of local protein synthesis in the amygdala during the period after memory retrieval is also sufficient to disrupt the “reconsolidation” of that memory (Nader, Schafe, & LeDoux, 2000).
Contextual fear conditioning requires involvement of the hippocampus in addition to the amygdala. It is believed that the hippocampus is responsible for providing a configural representation of individual context cues to the amygdala, where it becomes associated with the footshock (Matus-Amat, Higgins, Barrientos, & Rudy, 2004; Rudy & O'Reilly, 1999, 2001). Posttraining lesions of the hippocampus can prevent the recall of recent contextual fear without disrupting fear to a discrete CS, such as a tone (Kim & Fanselow, 1992). The involvement of the hippocampus in contextual but not cued fear learning allows for the study of two memories for the same training experience in a single animal.
Both auditory and contextual fear memory formation can be disrupted by inhibiting general kinase activity or protein synthesis in the amygdala (Bailey et al., 1999; Goosens, Holt, & Maren, 2000; Maren, Ferrario, Corcoran, Desmond, & Frey, 2003; Parsons et al., 2006; Schafe & Le Doux, 2000). Injections of similar inhibitors in the hippocampus are effective in disrupting context fear memory formation without affecting fear to the discrete auditory CS (Fischer, Sananbenesi, Schrick, Spiess, & Radulovic, 2004; Gafford, Parsons, & Helmstetter, 2005). Importantly, these inhibitors are only effective if applied within a few hours of the acquisition trial (Schafe & LeDoux, 2000) and thus affect memory formation, rather than memory storage. PKMζ inhibition, however, is uniquely able to reverse some forms of established memory after the period of consolidation has passed (Pastalkova et al., 2006; Sacktor, 2008; Shema et al., 2007; Serrano et al., 2008). Based on this past research, it might be expected that PKMζ inhibition in the amygdala would disrupt both contextual and auditory fear memory while inhibition of PKMζ in the hippocampus would selectively impair contextual fear memory. Surprisingly, initial work indicates that inhibiting PKMζ in the hippocampus fails to disrupt context fear memory while its inhibition in the amygdala following a slightly different training protocol is sufficient to impair both context and auditory fear memories (Serrano et al., 2008).
The purpose of this study was to further investigate the role of PKMζ in the maintenance of context and auditory fear memory using a standard Pavlovian fear conditioning procedure for all animals. Both the training context and an auditory cue were used as conditional stimuli (CS) and electric shocks were used as the biologically significant outcome. After training but before memory retrieval we applied the selective PKMζ inhibitor, ζ-pseudosubstrate inhibitory peptide (ZIP) (Ling et al., 2002; Pastalkova et al., 2006) to the basolateral complex of the amygdala or the dorsal hippocampus. Animals were tested in the training context 2 hours after ZIP injection to assess the strength of their context fear memory. Following this initial test, a number of follow-up tests were performed to ensure that any observed memory deficits were long-lasting and not attributable to tissue damage. These results will indicate whether long-term memory storage mechanisms in the hippocampus and amygdala use similar or different intra-cellular principles to maintain an identical associative fear memory.
Materials and Methods
Subjects and Surgery
The subjects were 71 male Long-Evans rats (300-375 g) obtained from Harlan (Madison, WI) and housed individually in shoebox cages with free access to water and rat chow. The colony room was maintained under a 14:10-h light/dark cycle and all behavioral tests were conducted during the light portion of this cycle. All procedures were approved by the Institutional Animal Care and Use Committee.
All animals were adapted to handling and transportation for three consecutive days before surgery. Before surgery, each rat was anesthetized with an intraperitoneal (IP) injection of sodium pentobarbital (1.5 mg/rat) followed by a second IP injection of ketamine hydrochloride (100 mg/kg). Animals were then prepared with bilateral stainless steel 26-gauge cannulae (Plastics One, Roanoke, VA) aimed at either the basolateral nucleus of the amygdala (BLA) using sereotaxic coordinates (n=49; 2.8 mm posterior, ±5.0 mm lateral, 3.5 mm ventral) or the dorsal hippocampus (n=22; −3.5 mm posterior, ±2.6 mm lateral, −3.0 mm ventral) relative to bregma (Paxinos & Watson, 1998). Cannulae were secured to the skull with stainless steel screws and epoxy. Following surgery, the incision site was swabbed with a lidocaine and prilocaine solution (2.5%/2.5%) to minimize discomfort during the recovery period. Stainless steel obdurators remained in the cannulae when rats were not being injected to prevent occlusion. Each rat was given a recovery period of at least 7 days before behavioral testing.
Apparatus
Fear conditioning was conducted in a set of four Plexiglas and stainless-steel chambers housed within sound-attenuating boxes (Context A). The floor was composed of stainless steel rods spaced 1.5 cm apart through which footshocks were delivered. Each chamber was illuminated by an overhead 7.5-W bulb and was connected to its own shock generator-scrambler (Grason-Stadler, West Concord, MA). Ventilation fans provided constant background noise (approximately 60 dB). Chambers were cleaned with a solution of 5% ammonium hydroxide between animals.
Fear reactions to the auditory CS were independently tested in a second set of chambers (Context B) that was distinct from Context A in a number of ways to result in maximum discriminibility including the use of infrared illumination, a solid Plexiglas floor, and a different odor and cleaning solution (2% acetic acid). Ventilation fans provided 62-64 dB of background noise.
The main behavioral dependent variable was the amount of time the rats spent engaged in freezing behavior. Freezing was defined as the absence of all bodily movement except that which is required for respiration. All other behavior was scored as general activity. A computer based digital video observation system (FreezeScan 1.0, CleverSys. Inc., Reston, VA, USA) continuously scored each rat as freezing or active throughout each session. Freezing during all behavioral sessions was analyzed as a percentage of each minute. Group differences were assessed using a one-way ANOVA, and Bonferroni post hoc tests where appropriate. In all cases, p < 0.05 was considered significant.
Infusions and Testing
All rats received bilateral infusions (0.5 μl/side) into the basolateral amygdala (n=49) or dorsal hippocampus (n=22). The volume was given over a 60s (amygdala) or 120s (hippocampi) period after which the injection cannulae remained in place for an additional 90 s to ensure proper diffusion. Prior work with local microinjection with these parameters indicates that they produce coverage throughout the amygdala and hippocampi bilaterally (Gafford et al., 2005; Parsons et al., 2006). The injection cannulae were cut to extend ~0.5 mm past the guide cannulae. Directly after infusions, rats were returned to their home cages. Myristoylated ZIP (myr-SIYRRGARRWRKL-OH, Invitrogen, Carlsbad, CA) or a scrambled, inactive version of the ZIP peptide (scrZIP, myr-RLYRKRIWRSAGR-OH, Sigma Genosys, St. Louis, MO) was dissolved in sterile saline to create the final concentration of 10 nmol/μl (Pastalkova et al., 2006).
All subjects were exposed to the restraint and injection procedure for the three days preceding training. Each rat was transported to the laboratory, wrapped in a towel, and gently restrained by hand for several minutes while the infusion pump was activated to allow rats to habituate to its noise. The obdurators were temporarily removed at this time and the scalp was cleaned with Betadine. Immediately following restraint handling, rats were returned to their homecages.
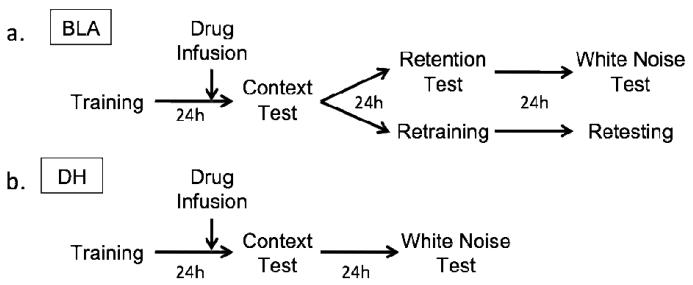
Figure 1 depicts the behavioral procedure for BLA-injected and DH-injected animals. Training consisted of a 6-minute baseline followed by four white noise (72 dB; 10s) - shock (1mA/1s) pairings separated by a 90s interval. Shocks were followed by a 4-minute post shock period after which the animals were removed and transported back to their home cages. Twenty-two hours after the training session, rats were infused with either ZIP in the BLA (n=16) or DH (n=7) saline in the BLA (n=17) or DH (n=7), or scrZIP in the BLA (n=16) or DH (n=8) as described above. Two hours after infusions (24 hours after the initial training session), all rats were placed back in Context A for a 15-minute retention test, after which they were returned to their home cages.
Figure 1.
Experimental timeline for BLA-injected (a) and DH-injected (b) animals. Drug infusions (arrow) occurred 2h prior to the initial context test.
24 hours after the initial context test, all of the animals with hippocampal cannulae were placed in Context B and and given an auditory CS retention test. This test consisted of a 6-minute baseline followed by 5 minutes of continuous exposure to the white noise CS. After a 4-minute post-exposure period, rats were removed from Context B and returned to their homecages. We chose to conduct the context and auditory fear memory tests on separate days specifically to minimize potential interactions between the test sessions. The animals with amygdala cannulae were divided into two groups following the initial context test. Half of the saline animals (n=9), half of the scrZIP animals (n=8), and half of the ZIP animals (n=8) received a context retention test 24 hours after the initial context test to determine whether any observed deficits were permanent. The retention test consisted of a second 15-minute exposure to Context A. Twenty-four hours later these rats were placed in Context B and given an auditory CS test as described above.
A second subset of animals, composed of 8 rats from each drug condition (n=24) was retrained in Context A 24 hours after the initial context test using the same parameters as the initial training session. Twenty-four hours after retraining, these rats were given a second 15-minute context test.
After behavioral testing was complete, animals were killed by an overdose of isoflurane and transcardially perfused with saline followed by a 10% buffered formalin solution. Heads were removed and submerged in buffered formalin for at least 24 hours. Brains were then removed and soaked in a 30% sucrose formalin solution for a minimum of 24 hours. Frozen 40 μm sections were then collected throughout the amygdala, mounted on slides, and stained with cresyl violet. Injection sites were determined with the aid of a rat brain atlas (Paxinos & Watson, 1998). Animals with injection sites outside the basolateral subdivision of the amygdala or the dorsal portion of the hippocampus were not included in the analysis.
Results
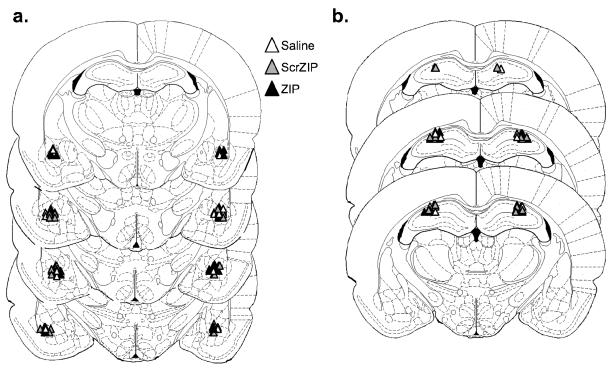
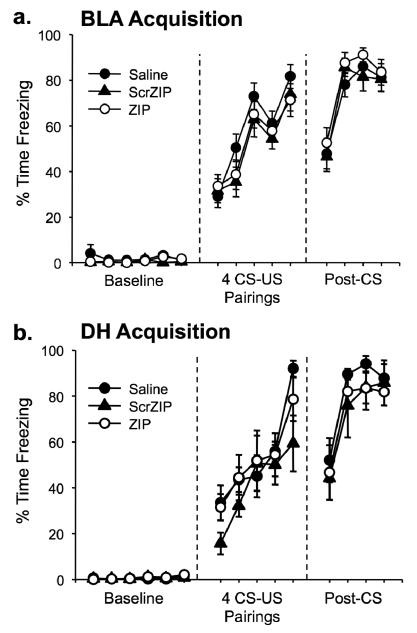
One rat was excluded from analysis in the amygdala group due to a misplaced cannula. The cannulae placements for the remaining 71 rats were deemed acceptable and were therefore included in the subsequent analyses. Figure 2 displays the target areas for acceptable injection locations. Training consisted of a 6 min baseline followed by 4 CS-US pairings. All rats showed normal behavior during the training session and no postshock freezing differences between groups were observed (Figure 3).
Figure 2.
Cannulae placements with acceptable injection locations. All cannulae were aimed at the basolateral nucleus of the amygdala (a) or at the dorsal hippocampus (b). White triangles represent infusions of the vehicle; gray triangles represent scr-ZIP infusions; black triangles represent ZIP infusions.
Figure 3.
Animals show normal acquisition of fear conditioning before drug infusion into the amygdala (a) or hippocampus (b). No significant differences were observed between groups throughout either session. Mean percentage of time spent freezing (±SEM) is shown for each minute of the acquisition trial.
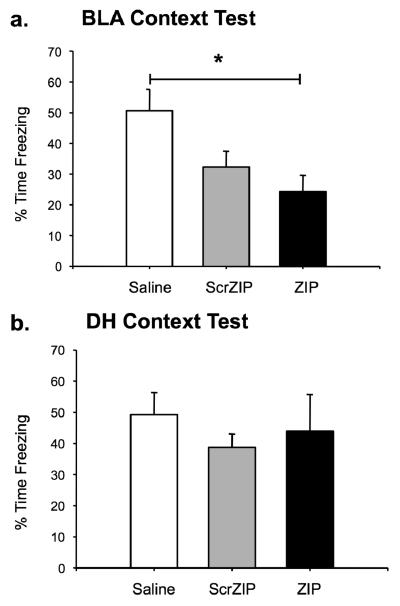
Figure 4 shows the effects of ZIP, scrZIP, and saline infusions into the BLA (Figure 4a) or the DH (Figure 4b) two hours before the context test. In both structures, saline animals showed normal retention of conditioned fear to the context. An one-way ANOVA indicated there was a significant main effect for drug in the BLA, F(2,46)=5.24, p=.009. Bonferroni post hoc tests determined that animals receiving ZIP infusions in the BLA showed a significant reduction in freezing compared to saline controls (p=.009), consistent with a role of PKMζ in the maintenance of context fear memory in the amygdala. Animals receiving infusions of scrZIP displayed an intermediate amount of context fear and did not differ significantly from either the vehicle or ZIP groups (Figure 4a). No significant effects were observed in animals receiving hippocampal injections (Figure 4b; F(2,19)=0.433, p=.655), consistent with other published work (Serrano et al., 2008). Thus, PKMζ inhibition in the amygdala is effective in disrupting the same context fear memory that is immune to PKMζ inhibition in the hippocampus.
Figure 4.
Infusions of ZIP in the amygdala or hippocampus have different effects on context fear memory. a. Infusions of ZIP into the amygdala disrupt a day-old context fear memory. ZIP animals spent significantly less time freezing than saline animals when tested in the training context. ScrZIP animals were not significantly different from ZIP or saline animals. b. Infusions of ZIP in the hippocampus do not disrupt a day-old context fear memory. No significant effect for drug was observed in the hippocampus. All data are presented as mean percentage of time spent freezing (±SEM) during the entire 15-minute context test. *p<0.05.
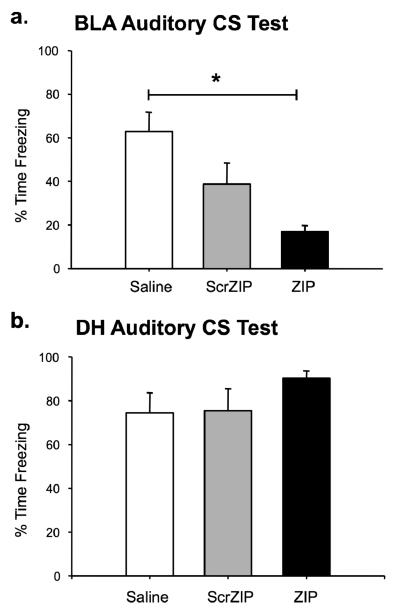
It is possible that the memory deficit observed for the ZIP animals was a performance artifact caused by residual drug in the amygdala during the time of testing. To assess whether this memory deficit was permanent or transient, a subset of the amygdala animals received a second context test 24 hours later (data not shown) followed by an auditory CS test at 48h post-injection. During the second context test, all groups showed a slight reduction in freezing, resulting in no significant differences between groups, F(2,22)=1.68, p=.210. A significant effect reappeared during the auditory CS test, however (Figure 5a; F(2,22)=8.582, p=.002). Post hoc analyses showed a significant decrement in freezing for ZIP animals relative to saline controls during the white noise presentation, p=.001. The scrZIP group again froze at an intermediate level that was not significantly different from either the vehicle or ZIP groups. Thus, the injection of ZIP into the amygdala was sufficient to impair memory of the white noise cue even 2 days later. These results indicate that the effects of ZIP are long-lasting (Pastalkova et al., 2006; Serrano et al., 2008; Shema et al., 2007) and cannot be explained by residual ZIP in the amygdala.
Figure 5.
Infusions of ZIP in the amygdala or hippocampus have different effects on auditory fear memory. a. ZIP animals (n=8) froze significantly less than saline controls (n=9) to the auditory cue presented in a novel context. ScrZIP animals (n=8) were not significantly different from ZIP or saline animals. b. Infusions of ZIP into the hippocampus do not disrupt freezing to the auditory CS. No significant effect for drug was observed in the hippocampus. All data are presented as mean percentage of time spent freezing (±SEM) during the 5-minute CS presentation in a novel context. *p<0.05.
As a control for nonspecific effects of the drug, long-term auditory fear memory was also assessed for DH animals at 24 hours post-injection (Figure 5b). The purpose of the context retention test given to BLA-injected animals was to assess the permanence of any observed context memory deficits in the initial test. Because no context memory impairment was observed for DH-injected animals, a second context retention test was deemed unnecessary and was not conducted. Consistent with other published work exploring hippocampal manipulations (Fischer et al., 2004; Gafford et al., 2005; Kim & Fanselow, 1992), ZIP injections in the DH did not reduce freezing to the auditory cue, F(2,19)=1.86, p=.183. Thus, PKMζ inhibition in the hippocampus is ineffective in disrupting auditory fear memory, as predicted.
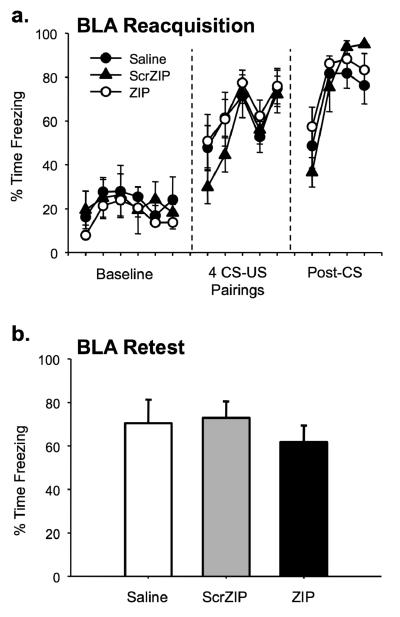
To ensure that the observed effects were not due to permanent amygdala damage, the remaining rats from the BLA injection group were retrained and retested following the initial context test (Figure 6). No significant difference persisted between groups during the retraining session, as indexed by equivalent levels of freezing during the postshock period of retraining (Figure 6a, F(2,21)=0.417, p=.665). A context test the following day demonstrated similar results; there were no significant differences between groups during the testing session (Figure 6b, F(2,21)=0.444, p=.647). This indicates that ZIP and scrZIP infusions do not permanently damage the amygdala, a result consistent with past work that demonstrates normal learning after ZIP infusions (Pastalkova et al., 2006; Serrano et al., 2008; Shema et al., 2007). If permanent damage to the amygdala was sustained, one would expect a disruption of normal context fear learning (Goosens & Maren, 2001; Nader et al., 2001; Phillips & LeDoux, 1992), which was not seen in the ZIP-injected or scr-ZIP injected animals.
Figure 6.
Context memory disruption is not from permanent damage to the amygdala. Half of the original animals were retrained and retested in the training context following the initial context test. a. Reacquisition occurred normally for ZIP and ScrZIP animals. No significant differences between drug conditions were observed in the post-CS period. b. Retesting occurred normally for ZIP and ScrZIP animals. Data are shown as mean percentage of time spent freezing (±SEM) for each minute (a) or for the entire context test (b).
Discussion
We found that inhibition of PKMζ in the amygdala was able to disrupt a day-old contextual fear memory while its inhibition in the dorsal hippocampus was ineffective in disrupting context fear memory, consistent with the results of at least one recent study (Serrano et al., 2008). Serrano and colleagues similarly demonstrated that ZIP injections in the amygdala one day after fear conditioning can disrupt both auditory and contextual fear cues while ZIP in the hippocampus following a slightly different training procedure had no effect. Importantly, the current study used the same training procedure for all animals. This removes the possibility that the fear conditioning protocol itself affects whether a memory is stored through PKMζ activity. Thus, the maintenance of an identical context fear memory seems to require PKMζ activity in the amygdala but not in the hippocampus, indicating that the structural requirements for PKMζ in context fear conditioning are dissociable between these structures, regardless of the specific fear conditioning procedure used
Follow-up tests demonstrated that the ZIP-induced memory reversal in the amygdala was long-lasting, not due to residual drug in the amygdala, and not a result of permanent amygdala damage. Taken together, these results suggest that contextual fear memories are sustained over time through constitutive action by PKMζ in the amygdala. Our results also indicate that the memory of the discrete white noise CS can also be disrupted by a local inhibition of PKMζ in the amygdala. Previous work has ruled out hyperactivity as an explanation for the freezing deficits observed following ZIP infusions in the amygdala (Serrano et al., 2008).
A number of explanations exist to explain the lack of effect of PKMζ inhibition in the hippocampus. It is possible that context fear memories in the hippocampus are not maintained by PKMζ but rely on some other molecular cascade for preservation. Alternatively, this finding could imply that the dorsal hippocampus is not an essential component in context fear storage, despite much evidence to the contrary (Kim & Fanselow, 1992; Matus-Amat et al., 2004; Rudy & O'Reilly, 2001). In light of the numerous studies that have established the hippocampus as key structure in context fear memory storage, it seems most plausible that the null effect observed following ZIP infusions in the hippocampus indicates that PKMζ activity is not a key mechanism in the hippocampal storage of context fear memory. Other mechanisms suggested to sustain context fear memory in the hippocampus include long-term depression and changes in neural excitability (Serrano et al., 2008). An additional possibility is a second autonomously active kinase that remains undiscovered. Further research is necessary to determine why context fear memory does not require hippocampal PKMζ activity while a number of other hippocampal dependent tasks, including the 8-arm radial arm maze, the water maze, and active place avoidance, have a clear requirement for hippocampal PKMζ activity.
It is also possible that hippocampal ZIP injections are able to disrupt context memory but our procedure was not sufficiently sensitive to detect this effect. For example, it could be argued that the injection volume used in the current study (0.5 μl/side) was not sufficient to bilaterally cover the hippocampus, providing only weak or partial PKMζ inhibition. Unpublished work from our lab indicates that this is not the case. We have found that injecting a volume of 1.0 μl of ZIP in each hippocampus is still unable to produce context memory deficits. This is consistent with the work of Serrano and colleagues, who also failed to see an effect with hippocampal ZIP injections of 1.0 μl/side (2008). Another possibility is that context memory disruption following a hippocampal ZIP injection appears more slowly in comparison to the memory deficit observed following BLA injections. If this is the case, perhaps our single context test at 2h following DH injections was not sufficient to detect this memory disruption. We find this explanation unlikely for two reasons. First, studies using ZIP to erase LTP have consistently shown a complete reversal of potentiation in the hippocampus within two hours following ZIP application (Ling et al., 2002; Pastalkova et al, 2006; Sajikumar et al., 2005;Serrano et al., 2005). If LTP is an analog for memory, then memory should also be fully disrupted at this time point. Second, all other published studies showing memory erasure following ZIP injection have been able to demonstrate this disruption at the 2h post-injection time point (Pastalkova et al., 2006; Shema et al., 2007; Serrano et al., 2008). This includes other behavioral tasks requiring the hippocampus (Pastalkova et al., 2006; Serrano et al., 2008). In fact, Serrano and colleagues demonstrated that a context test at a later time point, 26h after a hippocampal ZIP injection, similarly revealed no memory disruption relative to controls (2008). Taken together, these lines of evidence indicate that our null hippocampal effect is not a product of a slower time course of ZIP-mediated disruption.
Animals receiving an infusion of the scrambled ZIP control peptide into the BLA showed a slight, non-significant decrease in freezing behavior throughout the testing sessions compared to saline controls. This indicates that the scrambled peptide itself may weaken the memory to a slight degree. Any effects of the scrZIP peptide cannot be explained from amygdala damage because the scrZIP group retrained and retested normally. The deficit seen in the ZIP group was both stronger and more persistent than any impairment seen with the scrambled peptide, indicating that the effects of ZIP cannot be simply explained as an artifact of the peptide itself. It is possible that the scrambled peptide was able to weakly bind and inactivate some PKMζ molecules, due to the nearly-palindromic basic sequence of the pseudosubstrate peptide. The effects of scrZIP matched the pattern of deficits observed with ZIP, except for being muted in comparison to the active peptide. Importantly, this pattern included the selective disruption of a consolidated memory during the maintenance phase, an effect that has only been obtained to date through inhibition of PKMζ (Pastalkova et al, 2006; Serrano et al., 2008; Shema et al., 2007). Thus, the effects of the scrambled peptide are likely the result of weak inhibition of PKMζ. The saline condition may be a more appropriate control for assessing the effects of PKMζ inhibition, as the saline animals routinely demonstrated normal retention of conditioned fear, with an average freezing difference of only 1.4% between experiments (compare Figures 4a and 4b).
While it is possible that a lower dose of both ZIP and scrZIP would diminish the nonspecific effects of the scrambled peptide, further research indicates that 10 nmol/μl is close to the lowest fully-effective dose of ZIP. This dose was chosen to match published studies that used the two compounds, none of which demonstrated a nonspecific effect for scrZIP (Pastalkova et al., 2006; Serrano et al., 2008; Shema et al., 2007). Further work in our lab has demonstrated that a reduced injection of 4 nmol/μl of ZIP in the amygdala was not able to disrupt context fear memory when tested 2h later. Further, a recent study by Shema and colleagues (2009) indicates that a dose of 10 nmol/μl but not 3.3 nmol/μl of ZIP in the insular cortex is sufficient to erase a conditioned taste aversion memory. Future research should be conducted to identify the absolute lowest fully-effective dose of ZIP to prevent these nonspecific effects of the scrambled peptide.
One cannot exclude the possibility that the memory reversal observed in this study is only temporary, despite the strong disruption of the memory for 48 hours post-infusion. Previous work has demonstrated that ZIP-imposed memory disruptions had not recovered when tested one week after (Pastalkova et al., 2006; Shema et al., 2007) and one month after infusion (Shema et al., 2007). Despite this strong evidence that the effect of ZIP is permanent, longer time points have not yet been reported and, thus, the possibility of a transient effect cannot be entirely excluded.
This study demonstrates that although both context and auditory fear memories are maintained through the constitutive action of PKMζ in the amygdala, context fear memory in the hippocampus seems to utilize an alternative maintenance mechanism that has not yet been identified. These results, consistent with those of Serrano et al., 2008, indicate that PKMζ activity is necessary for the maintenance of certain forms of memory but is not essential for the storage of all forms of memory throughout the brain. Future research will be necessary to identify the molecular components responsible for maintaining PKMζ-independent memory, such as hippocampal-dependent context fear.
Acknowledgments
We thank Ryan G. Parsons for technical assistance. This work was supported by National Institute of Mental Health grants MH069558 (FJH) and MH060668 (FJH).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behavioral Neuroscience. 1999;113:276–282. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. Journal of Neuroscience. 2004;24:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Effects of post training hippocampal injections of midazolam on fear conditioning. Learning & Memory. 2005;12:573–578. doi: 10.1101/lm.51305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Holt W, Maren S. A role for amygdaloid PKA and PKC in the acquisition of long-term conditional fear memories in rats. Behavioural Brain Research. 2000;114:145–152. doi: 10.1016/s0166-4328(00)00224-2. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning & Memory. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiology of Learning and Memory. 2008;89:324–337. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, et al. Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase Cζ catalytic domain. Journal of Biological Chemistry. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Choi S, Lee SY, Cao YA, Ahn HJ, Worley KC, et al. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-rel. Journal of Neuroscience. 2004;24:3933–3943. doi: 10.1523/JNEUROSCI.5646-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, et al. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nature Neuroscience. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Ling DS, Bernardo LS, Sacktor TC. Protein kinase Mzeta enhances synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. European Journal of Neuroscience. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annual Review of Neuroscience. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. Journal of Neuroscience. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Muslimov IA, Nimmrich V, Hernandez AI, Tcherepanov A, Sacktor TC, Tiedge H. Dendritic transport and localization of protein kinase Mζ mRNA: Implications for molecular memory consolidation. Journal of Biological Chemistry. 2004;279:52613–52622. doi: 10.1074/jbc.M409240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. Journal of Neuroscience. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. Journal of Neuroscience. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, Affective, & Behavioral Neuroscience. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: The role of protein kinase Mzeta in maintaining long-term potentiation but not long-term depression. Journal of Neuroscience. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. The Journal of Neuroscience. 2000;20(RC96):1–5. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, et al. PKMζ maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biology. 2008;6:2698–2705. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. Journal of Neuroscience. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Hazvi S, Sacktor TC, Dudai Y. Boundary conditions for the maintenance of memory by PKMζ in neocortex. Learning & Memory. 2009;16:122–128. doi: 10.1101/lm.1183309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKMζ. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Stork O, Stork S, Pape HC, Obata K. Identification of genes expressed in the amygdala during the formation of fear memory. Learning & Memory. 2001;8:209–219. doi: 10.1101/lm.39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]