Abstract
Alcohol use disorders are common in the world. However, the development of novel drugs to prevent alcohol-induced brain damage is based upon an improved neurobiological understanding on the cellular changes that take place in the brain. We previously reported that ethanol exposure lowered cell proliferation and increased cell apoptosis in all cell types, but affects brain cell lines the most, while ethanol and the anti-depressant drug deprenyl, an monoamine oxidase B (MAO B) inhibitor, exposure in unison increases cell viability. Here we investigated the molecular mechanism of the neuroprotective effect of deprenyl (0.25 nM) on ethanol (75 mM)-induced harmful effect. Transforming growth factor-beta-inducible early gene 2 (TIEG2) is an activator for MAO B. MAO B levels increase has been shown to contribute to neuronal cell death. This study uses the neuronal cell line to address whether ethanol induced cell death is through the activation of TIEG2-MAO B apoptotic pathway, and whether deprenyl protects cells from the effects of alcohol through the inhibition of this pathway. We have found that ethanol exposure increases the levels of mRNA and protein/catalytic activity for both TIEG2 and MAO B, while ethanol and deprenyl exposure in unison reduce the expression of both TIEG2 and MAO B, however it increases cell viability. Additionally, TIEG2-overexpressed cells display more cellular death-induced by ethanol than control cells. In summary, this study demonstrates the role of TIEG2 in ethanol induced cell death. The inhibition of the TIEG2-MAO B pathway may be one of the mechanisms for the neuroprotective effect of deprenyl.
Keywords: alcohol, neuroprotection, transforming growth factor-beta-inducible early gene 2, monoamine oxidase B, cell viability
Introduction
Alcohol use disorders are common around the world and also have a high correlation between alcohol use disorders and other psychiatric problems, such as major depression (1). Although short-term alcohol drinking causes euphoric and stress-relieving effects, numerous clinical and experimental studies have shown that alcohol use is a major risk factor for neurobehavioural diseases, inflammation disorders and enhanced susceptibility to bacterial infection (2–4). In particular, it affects the brain and alters its normal function (5). This includes altering the effects of neurotransmitters, suppressing nerve signals and causing cell death (6). In rodents, ethanol exposure during development significantly reduces the size of the brain as well as brain/body weight ratios (7). There are many adverse physical effects from long-term exposure to alcohol including the increased activity in the liver that causes cell death and chronic hepatic diseases (alcoholic fatty liver, alcoholic hepatitis and cirrhosis, etc) (8) (7) and an increase in the number of apoptotic cells in various brain areas (9).
Monoamine oxidase (MAO) metabolizes biogenic and dietary amines in the central nervous system and peripheral tissues, including monoamine neurotransmitters serotonin, norepinephrine, dopamine, and phenylethylamine. MAO plays important roles in several psychiatric and neurological disorders (10). MAO exists in two forms, MAO A and MAO B. Their catalytic activity generates H2O2 and nitrogen species, which are toxic products and may cause oxidative damage to mtDNA and have potential implications for apoptosis, aging, and neurodegenerative processes.
Deprenyl (selegiline), an irreversible inhibitor of monoamine oxidase B (MAO B), was synthesized as an antidepressant and used to treat Parkinson’s disease (11). Because MAO degrades serotonin and produces reactive oxygen that may cause cell death, an MAO inhibitor prevents cell apoptosis (12) (13). Deprenyl or related compounds may be neuroprotective in general through the inhibition of “death” signal transduction-mediated by MAO, induced by endogenous and environmental factors (11). Deprenyl in low concentrations that induce MAO B inhibition potently inhibits serum withdrawal induced apoptosis in tissue cultures of neuro-ectodermal origin (14). This report is consistent with our previous studies (15) that ethanol can induce apoptosis in neuronal cells, and deprenyl in a low concentration can protect cells from the harmful effects of ethanol.
Transforming growth factor-beta-inducible early gene 2 (TIEG2, also called KLF11--Kruppel-Like Factor 11) is a human transforming growth factor-beta-(TGF-β) inducible early gene. It is a recently identified human TGF-β-inducible zinc finger protein belonging to Sp1-like family of transcription factors. TIEG2 protein is a negative regulator of cell growth and induces apoptosis (16–18) by binding to GC-rich sequences (19) located in the promoter region of several genes including MAO B promoter. Ethanol has been shown to potentiate TGF-β1-mediated growth inhibition in the rat neuroblastoma cells (20), and ethanol exposure increases TGF-β1 signal(21) that may increase TIEG2 protein level and lead to apoptotic death of cells (22). Our previous data have shown that TIEG2 activates MAO B gene expression (23). This study investigates the neuroprotective effect of antidepressant drug (deprenyl) on ethanol-induced apoptosis possibly mediated by TIEG2 and MAO B.
Materials and Methods
Cell Lines, DNA plasmids and reagents
SH-SY5Y, a human neuroblastoma cell line, was purchased from The American Type Culture Collection (ATCC). SH-SY5Y was cultured in RPMI1640 supplemented with 10% fetal bovine serum and antibiotics. TIEG2-expression vector was a gift from Dr. Raul Urrutia, Mayo Clinic. TIEG2 coding sequence was cloned into pcDNA3.1 His A expression vector. MAO B inhibitor, selegiline (deprenyl), was purchased from Sigma-Aldrich USA. The antibodies used in this study were purchased from Santa Cruz Biotechnology, except that anti-TIEG2 antibody was from BD Transduction Laboratory.
TIEG2-stably transfected cell line
In generating the TIEG2-stable cell line, SH-SY5Y were plated at a density of 5 × 106 cells in a 10-cm dish. The next day the TIEG2 expression vector or pcDNA 3.1 was transfected into cells with a Superfect transfection reagent (Qiagen Inc). After 24h, cells were treated with Geneticin (G418; 600ug/ml). Resistant clones isolated into separate dishes after 6 days and cultured under continuous G418 selection (13).
Cell culture and treatments with ethanol and deprenyl
Before treatments, SH-SY5Y cells were seeded on 10-cm dishes or 6-well plates. After overnight culture in medium, the medium was replaced with new medium containing 75 mM of ethanol with or without 0.25 nM of deprenyl for three days. As ethanol is volatile, a closed chamber system was utilized to stabilize the ethanol concentration in the culture medium (24) (25). With this system, ethanol concentrations are maintained at steady ethanol levels (more than ~90% of the original concentration) for 3 days in medium. Briefly, cell culture dishes or 6-well plate containing SH-SY5Y cells were placed on a rack inside a plastic container that could be tightly sealed. A separate sealed container was used for each ethanol concentration. The bottom of each container was a reservoir that was filled with 200 ml of an aqueous solution with the same ethanol concentration that was present in the culture medium. A nonethanol control had a bath of water only. The underlying principle of this method is that the alcohol in the bath evaporates into the air inside the sealed container establishing a stable vapor pressure so that there is no net loss of ethanol from the culture medium. Before sealing the containers, a small amount (60 cc) of CO2 was injected into each container. The concentration of CO2 in the chamber was routinely tested and was determined to be stable at 5%. The containers was sealed and maintained in an incubator at 37°Cfor up to 3 days as needed (25).
The ethanol concentration we used (75 mM for examining the effect of deprenyl) was within the standard range of in vitro study (26). When a heavy drinker’s ethanol concentration in blood reaches ~50–100 mM, he probably shows slurred speech and unsteadiness (27). Therefore the ethanol concentration for this study is around the physiological effect of ethanol in alcoholics.
Real-time PCR (RT-PCR)
Total RNAs were extracted with Trizol from cultured cells. Reverse transcription was carried out with SuperScript first strand synthesis system for RT-PCR (Invitrogen Inc) following the manufacture’s instruction. Specific primers for the human MAO B and TIEG2 were designed as follows:
| MAO B | Sense, | 5′-GACCATGTGGGAGGCAGGACTTAC-3′ |
| Antisense, | 5′-CGCCCACAAATTTCCTCTCCTG-3′ | |
| TIEG2 | Sense, | 5′-CCTGTTGCGGATAAGACCCCTCAC-3′ |
| Antisense, | 5′-AAAGCCGGCAATCTGGAGTCTGGA-3′ |
The mRNA quantitative analyses for each group were performed by Real-Time PCR using a Bio-Rad iCycler system. The real-time PCR was performed with a SYBR supermix kit (Bio-Rad). The data were analyzed by the software from Bio-Rad as described previously (23).
MAO B catalytic activity assay
SH-SY5Y was grown to confluence, harvested, and washed with phosphate-buffered saline. One hundred micrograms of total proteins were incubated with 10μM 14C-labeled PEA (Amersham Biosciences) in the assay buffer (50 mM sodium phosphate buffer, pH 7.4) at 37°C for 20 min and terminated by the addition of 100 μl of 6 N HCl. The reaction products were then extracted with ethyl acetate/toluene (1:1) and centrifuged for 7 min. The organic phase containing the reaction product was extracted, and its radioactivity was obtained by liquid scintillation spectroscopy (28).
Western blot
Cells were cultured in medium with ethanol (75 mM) for 3 days, washed by PBS (pH 7.4), and sonicated in 500 μL of RIPA lysis buffer (10 mM Tris·HCl, pH 7.4/160 mM NaCl/1% Triton/1% Na dexycholate/0.1% SDS/1 mM EDTA/1 mM EGTA) supplemented with protease inhibitors (Sigma). The samples were then freeze thawed and centrifuged for 2min. at 12,500rpm. The supernatant was then kept and transferred to a new tube. Thirty micrograms (for TIEG2) of total proteins were separated by 10.5% SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes. After the transfer, membranes were blocked at room temperature for 2 h with 5% nonfat dry milk in TBS (10 mM Tris-HCl, pH 7.5, 150 mM NaCl). The membranes were then incubated with mouse anti-TIEG2 antibody (1:500) or mouse anti-actin antibody (1:1000) overnight at 4°C. After incubation with respective secondary antibody at room temperature for 2 h, the bands were visualized by hoseradish peroxidase (HRP) reaction using SuperSignal West Pico Chemiluminescent Substrate (PIERCE).
MTT assay for proliferation rate/cell viability evaluation
Cell viability and proliferation was measured by tetrazolium salt (MTT) (13) (15). The medium in excess of 2 ml (6-well plates) was removed and 40 μl of MTT dye (5mg/ml) in sterile PBS was added to 360 μl of medium or PBS depending on cell confluence. Plates were incubated for 4 to 5 hours, during which time the mitochondria in living cells converted the soluble yellow dye (MTT) into an insoluble purple formazan crystal. Cells and dye were then solubilized by the addition of 800 – 1000 μl of DMSO to the 6-well plates. Optical density of each well at 572 nm was determined using the NanoDrop Spectrophotometer.
Statistical analysis
All values are presented as means ± SD. A one-way ANOVA followed by a post hoc Bonferroni’s t-test was employed when three or more groups were to be compared. A paired t-test was performed for the statistical analysis of two groups. A P value less than 0.05 was considered to be statistically significant.
Results
Ethanol increases the MAO B mRNA level and enzymatic activity, but deprenyl reverses the effect of ethanol
SH-SY5Y cells were treated with 75 mM ethanol for three days. Then the cellular mRNA and MAO B activity were determined by real time-PCR and traditional 14C method assay. The ethanol concentration we used (75 mM) was clinically relevant, because the ethanol at 50–100 mM reflects blood ethanol levels in chronic alcoholics (29) (30). Therefore, the ethanol concentration in our study was within the levels that results in physiological effects observed in alcoholics.
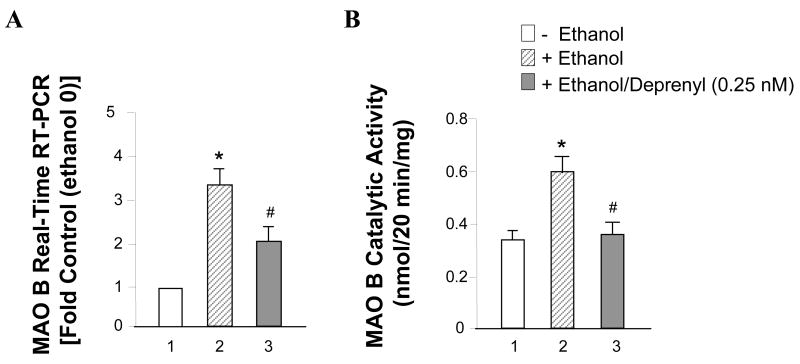
The results showed that ethanol induced cellular MAO B mRNA level increased about 3.5 times more than the control; this increase could be reduced by 0.25 nM deprenyl treatment (Figure 1A, lanes 2 vs. 1 and 3 vs. 1). At the same time, we found MAO B activity in ethanol treated cells increased 1.8 fold more than control cells (Figure 1B, lanes 2 vs. 1). However, deprenyl significantly decreased the MAO B catalytic activity (Figure 1B, lanes 3 vs. 2).
Figure 1.
Effects of ethanol on MAO B mRNA level and catalytic activity. SHSY5Y cells were treated with or without 75 mM ethanol or with ethanol plus 0.25 nM deprenyl for three days. (A) Cellular mRNA was extracted and quantitative real-time RT-PCR was performed. (B) MAO B enzyme activity was determined by enzymatic activity assay. Data represent the mean ± S.D. of three independent experiments. Controls were untreated cells (0 mM of ethanol) which were taken as 1. *P < 0.01 versus control cells and # < 0.05 versus cells treated with ethanol alone (one-way ANOVA followed by a post hoc Bonferroni’s t-test).
Ethanol increases the MAO B mRNA and protein levels, but deprenyl reverses the effect of ethanol
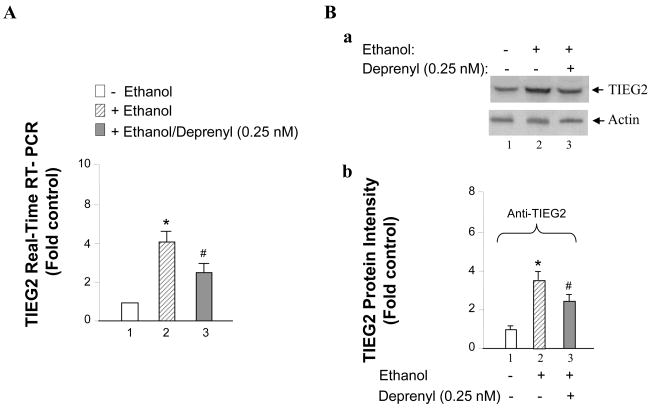
To test the possibility that TIEG2 may take part in the ethanol-induced cell death, TIEG2 mRNA levels and protein levels were detected with RT-PCR and western blot. The results show that ethanol could induce TIEG2 mRNA expression 4-fold more than that of the control cells (Figure 2A lanes 2 vs. 1), deprenyl plus ethanol could just increase 2.8-fold (Figure 2A, lanes 3 vs. 1). Similarly, TIEG2 protein expression level was increased around 3.5 times, however deprenyl could inhibit TIEG2 expression (Figure 2B, a and b, lanes 2 and 3 vs. 1).
Figure 2.
Effects of ethanol on TIEG2 mRNA and protein levels. SHSY5Y cells were treated with or without 75 mM ethanol or with ethanol plus 0.25 nM deprenyl for three days. (A) Cellular TIEG2 mRNA level was detected with quantitative real-time RT-PCR, and (B) TIEG2 protein was examined by western blot. (a) TIEG2 protein levels in cells treated with or without ethanol or with ethanol plus deprenyl were shown by western blot, and actin was used as the internal control. (b) The optical density analysis with western blot shows TIEG2 has about 3.5 fold expression in ethanol treated cells, whereas, deprenyl inhibited the TIEG2 expression. Data represent the mean ± S.D. of three independent experiments. *P < 0.01 versus control cells without ethanol treatment and # < 0.05 versus cells treated with ethanol (one-way ANOVA followed by a post hoc Bonferroni’s t-test).
TIEG2 enhances, but MAO B inhibitor (deprenyl) protects, cell death induced by ethanol
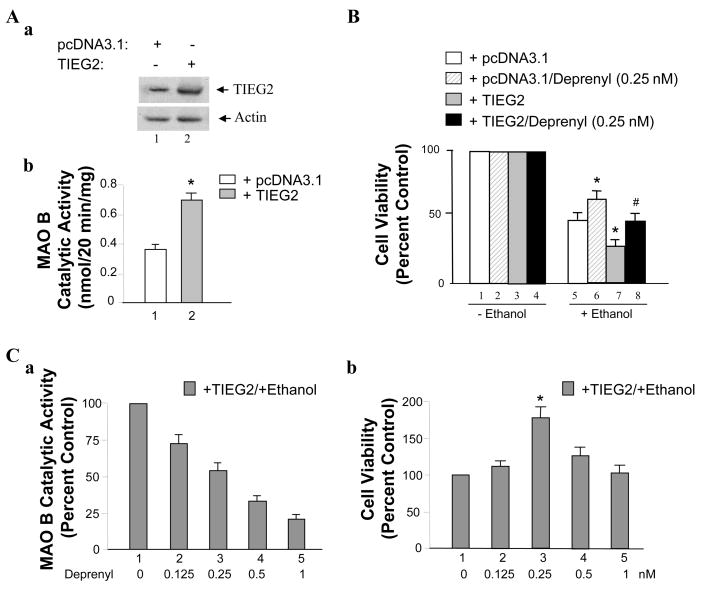
Ethanol has been found to increase the MAO B gene expression and catalytic activity in the human glioma 1242-MG cells (16). With our previous experiment, 75 mM ethanol treatment in conjunction with 0.25 nM deprenyl provided the most protection against apoptotic activity for brain cells SH-SY5Y and U-118 MG (31). Here, we use pcDNA and TIEG2 stably transfected SH-SY5Y cell lines (Figure 3Aa) to investigate the role of TIEG2 in ethanol-induced cell death and the neuroprotective effect of deprenyl. We have previously shown that TIEG2 is a transcriptional activator for MAO B (23). As shown in Figure 3Ab, the MAO B catalytic activity is increased by ~2-fold in TIEG2-overexpressed cells as compared to that in pcDNA3.1-transfected cells (Figure 3Ab, lanes 2 vs. 1).
Figure 3.
The effect of TIEG2 on ethanol-induced cell death. (A) Compare the expression of TIEG2 and MAO B catalytic activity in TIEG2/pcDNA stably transfected SH-SY5Y cell lines. (a) Western Blot analysis of the expression of TIEG2 in TIEG2/pcDNA overexpressed cell lines. (b) MAO B catalytic activity is about 2 times higher in TIEG2 stably transfected cells than pcDNA stably transfected cells. Values are expressed as means ± S.D. of at least three independent experiments._*P < 0.02 (paired t-test). (B) Effects of ethanol, ethanol plus deprenyl and TIEG2 on cell survival rates. pcDNA3.1 stably transfected cells or TIEG2 stably transfected cells were treated with 75 mM ethanol with/without 0.25 nM deprenyl for three days. Then the cell viability was determined by MTT assay. (C) Effects of different dosage of deprenyl on MAO B catalytic activity and the protection of ethanol induced cell death in TIEG2-stably transfected SH-SY5Y cell line. Cells were treated with or without 75 mM ethanol in the conjunction with 0.125, 0.25, 0.5 or 1 nM of deprenyl as indicated in the figure for three days. Then (a) the MAO B catalytic activity or (b) cell viability (MTT assay) was determined. All data are presented as the mean ± S.D. of at least three independent experiments. Controls were untreated cells (0 mM of ethanol in B and 0 nM of deprenyl in C) which were taken as 100%. *P < 0.05 versus control cells and # < 0.05 versus cells-stably expressing TIEG (one-way ANOVA followed by a post hoc Bonferroni’s t-test).
Next, cells were exposed to 75 mM ethanol in conjunction with 0.25 nM deprenyl for three days, and the cell viability (in survival rate) was observed in SH-SY5Y cells compared to that in cells treated with 75 mM ethanol alone. The results show that TIEG2-overexpression could induce more cell death with the presence of 75 mM ethanol than that in the control group which was stably transfected with empty pcDNA3.1 vector (Figure 3, lanes 7 vs. 5). However, deprenyl could protect cells from ethanol’s harmful effect (Figure 3, lanes 6 vs. 5 and 8 vs. 7).
Previously, we have shown that 0.25 nM of deprenyl produced the best neuroprotective effect on SH-SY5Y cells (15). In order to examine whether 0.25 nM deprenyl is also the most appropriate dosage in this study using TIEG2-overexpressed cells, the different concentrations (0, 0.125, 0.25, 0.5 and 1 nM) of deprenyl were used to test the inhibitory effects on MAO B catalytic activity and cell death. As shown in Figure 3Ca, the ethanol treatment in conjunction with deprenyl for three days exhibited the inhibition on MAO B catalytic activity in a concentration dependent manner. Furthermore, MTT assay was performed (Figure 3Cb) to determine the effects of different concentrations (0, 0.125, 0.25, 0.5 and 1 nM) of deprenyl on cell viability in TIEG2-overexpressed cells. The result showed that the ethanol treatment in conjunction with 0.25 nM deprenyl for three days increased the cell survival rate by 175% as compared to that of control cells (Figure 3Cb, lanes 3 vs. 1), suggesting that 0.25 nM of deprenyl has the most protection against apoptotic activity.
Discussion
An understanding of the molecular mechanisms of cellular apoptosis toward excessive alcohol consumption is crucial for the development of new treatments for alcoholism. In this study, we examine the TIEG2-MAO B role in the ethanol induced apoptosis, and the neuroprotective effect of deprenyl via inhibition of TIEG2-MAO B mediated cell death with SH-SY5Y cell line.
The MAO B gene is located on the Xp11.2–11.4 chromosome and consists of 15 exons with identical exon- intron organization (32), and its activity increases progressively in the brain throughout adult life (33) (34). An aberrant increase of MAO B activity has been implicated in several psychiatric and neurodegenerative disorders (35) (36). Thus one predicted mechanism for cell death is an abnormal increase in monoamine oxidase (37). Previously, the physiologically relevant concentration of ethanol has been found to increase the MAO gene expression and catalytic activity in the human glioma 1242-MG cells (31). The increased activity of MAO may thereby increase production of hydrogen peroxide (H2O2, a major source for oxidative stress) and cause apoptosis (38). The SH-SY5Y, a human neuroblastoma cell line, treated with 75 mM ethanol, could increase the expression of mRNA and protein, in particular, MAO B catalytic activity also increased. Previously, we showed that the level of Caspase 3, an apoptotic marker protein, was increased significantly by ethanol treatment, suggesting that ethanol-induced cell death is mediated at least partially by apoptotic pathway (15).
Transforming growth factor-beta-inducible early gene 2 (TIEG2) is an activator for MAO B through Sp1 overlapping sites (GC-rich sequence) located at the promoter region of MAO B. Sp1-like protein plays key roles in the regulation of MAO B gene expression (23). It has been reported that TIEG2 induces apoptosis in murine OLI-neu cells (39). With the ethanol treated SH-SY5Y system, we were able to show that the mRNA and protein levels for TIEG2 were increased significantly along with the increase in MAO B activity. Using TIEG2-overexpressed stable cell line, we further demonstrated that TIEG2 could increase the MAO B catalytic activity, and also enhance the cellular apoptosis triggered by ethanol, whereas, deprenyl, an MAO B inhibitor, could protect cell death induced by ethanol, because ethanol and deprenyl exposure in unison reduced the expression of both TIEG2 and MAO B.
Deprenyl is an irreversible inhibitor of MAO B which is an antidepressant drug, and is now also used in the treatment of Parkinson’s disease. Deprenyl in much lower concentrations needed to induce MAO B inhibition (less than ~1 nM) potently inhibits serum withdrawal (40) and nitric oxide (41) induced apoptosis. However, in high concentration, deprenyl induces apoptosis in cell cultures (14). Our findings suggest that 0.25 nM deprenyl and ethanol exposure in unison for three days is able to inhibit MAO B catalytic activity and produce the best neuroprotective effect comparing to other concentrations (0.125, 0.5 and 1 nM) of deprenyl. This may be due to the higher concentration (more than 0.25 nM) of deprenyl used to start to induce apoptosis in cell culture (14).
In summary, TIEG2-MAO B-mediated apoptotic pathway may contribute to ethanol induced neurotoxicity. The inhibition of this apoptotic signaling pathway may be one of the mechanisms for the neuroprotective effect of deprenyl.
Acknowledgments
This study was supported by Public Health Service Grants P20 RR17701, MH67996, and a NARSAD Young Investigator Award. The contribution by Dr. Raul Urrutia for providing TIEG2-pcDNA3.1 expression vector is also acknowledged. Shakevia Johnson and Shawna Tazik were supported by the Neuroscience Summer Scholars Program, Department of Psychiatry, University of Mississippi Medical Center.
Abbreviation
- MAO
monoamine oxidase
- MTT
3-(4, 5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bormide
References
- 1.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62(10):1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 2.Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Current neurology and neuroscience reports. 2003;3(1):40–45. doi: 10.1007/s11910-003-0035-8. [DOI] [PubMed] [Google Scholar]
- 3.Frank J, Witte K, Schrodl W, Schutt C. Chronic alcoholism causes deleterious conditioning of innate immunity. Alcohol and alcoholism (Oxford, Oxfordshire) 2004;39(5):386–392. doi: 10.1093/alcalc/agh083. [DOI] [PubMed] [Google Scholar]
- 4.Gullo L, Migliori M, Brunetti MA, Manca M. Alcoholic pancreatitis: new insights into an old disease. Current gastroenterology reports. 2005;7(2):96–100. doi: 10.1007/s11894-005-0046-5. [DOI] [PubMed] [Google Scholar]
- 5.Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci U S A. 2003;100(13):7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Q, Hu Y, Wu P, Cheng X, Li M, Yu D, Deng J. Prenatal alcohol exposure and the neuroapoptosis with long-term effect in visual cortex of mice. Alcohol and alcoholism (Oxford, Oxfordshire) 2007;42(4):285–290. doi: 10.1093/alcalc/agm032. [DOI] [PubMed] [Google Scholar]
- 8.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. Faseb J. 2001;15(8):1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 9.Pignataro L, Miller AN, Ma L, Midha S, Protiva P, Herrera DG, Harrison NL. Alcohol regulates gene expression in neurons via activation of heat shock factor 1. J Neurosci. 2007;27(47):12957–12966. doi: 10.1523/JNEUROSCI.4142-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama W, Naoi M. Neuroprotection by (−)-deprenyl and related compounds. Mech Ageing Dev. 1999;111(2–3):189–200. doi: 10.1016/s0047-6374(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 12.Tatton WG, Ju WY, Holland DP, Tai C, Kwan M. (−)-Deprenyl reduces PC12 cell apoptosis by inducing new protein synthesis. J Neurochem. 1994;63(4):1572–1575. doi: 10.1046/j.1471-4159.1994.63041572.x. [DOI] [PubMed] [Google Scholar]
- 13.Ou XM, Chen K, Shih JC. Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci U S A. 2006;103(29):10923–10928. doi: 10.1073/pnas.0601515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magyar K, Szende B. (−)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicology. 2004;25(1–2):233–242. doi: 10.1016/S0161-813X(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 15.Johnson S, Williams AN, Johnson C, Ou XM. The effects of antidepressant drug on ethanol-induced cell death. Drug Discov Ther. 2007;1(2):130–135. [PubMed] [Google Scholar]
- 16.Tachibana I, Imoto M, Adjei PN, Gores GJ, Subramaniam M, Spelsberg TC, Urrutia R. Overexpression of the TGFbeta-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J Clin Invest. 1997;99(10):2365–2374. doi: 10.1172/JCI119418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook T, Gebelein B, Mesa K, Mladek A, Urrutia R. Molecular cloning and characterization of TIEG2 reveals a new subfamily of transforming growth factor-beta-inducible Sp1-like zinc finger-encoding genes involved in the regulation of cell growth. J Biol Chem. 1998;273(40):25929–25936. doi: 10.1074/jbc.273.40.25929. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JS, Moncrieffe MC, Kaczynski J, Ellenrieder V, Prendergast FG, Urrutia R. A conserved alpha-helical motif mediates the interaction of Sp1-like transcriptional repressors with the corepressor mSin3A. Mol Cell Biol. 2001;21(15):5041–5049. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook T, Gebelein B, Belal M, Mesa K, Urrutia R. Three conserved transcriptional repressor domains are a defining feature of the TIEG subfamily of Sp1-like zinc finger proteins. J Biol Chem. 1999;274(41):29500–29504. doi: 10.1074/jbc.274.41.29500. [DOI] [PubMed] [Google Scholar]
- 20.Luo J, Miller MW. Transforming growth factor beta1-regulated cell proliferation and expression of neural cell adhesion molecule in B104 neuroblastoma cells: differential effects of ethanol. J Neurochem. 1999;72(6):2286–2293. doi: 10.1046/j.1471-4159.1999.0722286.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen CP, Kuhn P, Chaturvedi K, Boyadjieva N, Sarkar DK. Ethanol induces apoptotic death of developing beta-endorphin neurons via suppression of cyclic adenosine monophosphate production and activation of transforming growth factor-beta1-linked apoptotic signaling. Mol Pharmacol. 2006;69(3):706–717. doi: 10.1124/mol.105.017004. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn P, Sarkar DK. Ethanol induces apoptotic death of beta-endorphin neurons in the rat hypothalamus by a TGF-beta 1-dependent mechanism. Alcohol Clin Exp Res. 2008;32(4):706–714. doi: 10.1111/j.1530-0277.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 23.Ou XM, Chen K, Shih JC. Dual functions of transcription factors, transforming growth factor-beta-inducible early gene (TIEG)2 and Sp3, are mediated by CACCC element and Sp1 sites of human monoamine oxidase (MAO) B gene. J Biol Chem. 2004;279(20):21021–21028. doi: 10.1074/jbc.M312638200. [DOI] [PubMed] [Google Scholar]
- 24.Adickes ED, Mollner TJ, Lockwood SK. Closed chamber system for delivery of ethanol to cell cultures. Alcohol and alcoholism (Oxford, Oxfordshire) 1988;23(5):377–381. doi: 10.1093/oxfordjournals.alcalc.a044832. [DOI] [PubMed] [Google Scholar]
- 25.Luo J, Miller MW. Differential sensitivity of human neuroblastoma cell lines to ethanol: correlations with their proliferative responses to mitogenic growth factors and expression of growth factor receptors. Alcohol Clin Exp Res. 1997;21(7):1186–1194. [PubMed] [Google Scholar]
- 26.Ku BM, Joo Y, Mun J, Roh GS, Kang SS, Cho GJ, Choi WS, Kim HJ. Heme oxygenase protects hippocampal neurons from ethanol-induced neurotoxicity. Neurosci Lett. 2006;405(3):168–171. doi: 10.1016/j.neulet.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 27.Paton A. Alcohol in the body. Bmj. 2005;330(7482):85–87. doi: 10.1136/bmj.330.7482.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geha RM, Rebrin I, Chen K, Shih JC. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877–9882. doi: 10.1074/jbc.M006972200. [DOI] [PubMed] [Google Scholar]
- 29.Yao Z, Zhang J, Dai J, Keller ET. Ethanol activates NFkappaB DNA binding and p56lck protein tyrosine kinase in human osteoblast-like cells. Bone. 2001;28(2):167–173. doi: 10.1016/s8756-3282(00)00425-7. [DOI] [PubMed] [Google Scholar]
- 30.Henriksen JH, Gronbaek M, Moller S, Bendtsen F, Becker U. Carbohydrate deficient transferrin (CDT) in alcoholic cirrhosis: a kinetic study. J Hepatol. 1997;26(2):287–292. doi: 10.1016/s0168-8278(97)80043-8. [DOI] [PubMed] [Google Scholar]
- 31.Ekblom J, Zhu QS, Chen K, Shih JC. Monoamine oxidase gene transcription in human cell lines: treatment with psychoactive drugs and ethanol. J Neural Transm. 1996;103(6):681–692. doi: 10.1007/BF01271228. [DOI] [PubMed] [Google Scholar]
- 32.Lan NC, Heinzmann C, Gal A, Klisak I, Orth U, Lai E, Grimsby J, Sparkes RS, Mohandas T, Shih JC. Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics. 1989;4(4):552–559. doi: 10.1016/0888-7543(89)90279-6. [DOI] [PubMed] [Google Scholar]
- 33.Fowler CJ, Wiberg A, Oreland L, Marcusson J, Winblad B. The effect of age on the activity and molecular properties of human brain monoamine oxidase. J Neural Transm. 1980;49(1–2):1–20. doi: 10.1007/BF01249185. [DOI] [PubMed] [Google Scholar]
- 34.Saura J, Luque JM, Cesura AM, Da Prada M, Chan-Palay V, Huber G, Loffler J, Richards JG. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994;62(1):15–30. doi: 10.1016/0306-4522(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 35.Schneider G, Oepen H, von Wedel HR. Monoamine oxidase activity in brain regions and organs of patients with Parkinson’s disease and Huntington’s disease and serum MAO activity of patients with Huntington’s disease as compared with neurologically healthy individuals (author’s transl) Arch Psychiatr Nervenkr. 1981;230(1):5–15. doi: 10.1007/BF00343762. [DOI] [PubMed] [Google Scholar]
- 36.Mann JJ, Kaplan RD, Bird ED. Elevated postmortem monoamine oxidase B activity in the caudate nucleus in Huntington’s disease compared to schizophrenics and controls. J Neural Transm. 1986;65(3–4):277–283. doi: 10.1007/BF01249088. [DOI] [PubMed] [Google Scholar]
- 37.De Zutter GS, Davis RJ. Pro-apoptotic gene expression mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Proc Natl Acad Sci U S A. 2001;98(11):6168–6173. doi: 10.1073/pnas.111027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagatsu T, Sawada M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: possible implications of glial cells. Journal of neural transmission. 2006;71:53–65. doi: 10.1007/978-3-211-33328-0_7. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Spittau B, Behrendt M, Peters B, Krieglstein K. Human TIEG2/KLF11 induces oligodendroglial cell death by downregulation of Bcl-X(L) expression. J Neural Transm. 2007;114(7):867–875. doi: 10.1007/s00702-007-0635-6. [DOI] [PubMed] [Google Scholar]
- 40.Tatton WG, Chalmers-Redman RM, Ju WJ, Mammen M, Carlile GW, Pong AW, Tatton NA. Propargylamines induce antiapoptotic new protein synthesis in serum- and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther. 2002;301(2):753–764. doi: 10.1124/jpet.301.2.753. [DOI] [PubMed] [Google Scholar]
- 41.Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL, Dawson TM, Sawa A, Snyder SH. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci U S A. 2006;103(10):3887–3889. doi: 10.1073/pnas.0511321103. [DOI] [PMC free article] [PubMed] [Google Scholar]