Introduction
Pancreatic cancer is the fourth leading cause of cancer related deaths in the United States, resulting in 30,000 deaths each year, in part because it is one of the most lethal cancers with a death to incidence ratio of 0.99 1. Lethality of pancreatic cancer is largely due to late stage of diagnosis and resistance to current therapies. Greater than 80% of patients are unresectable and most present with metastatic disease due to aggressive localized invasion and a high incidence of early metastasis 2. Worldwide, the incidence of death from pancreatic cancer has increased, which, together with the lack of current therapies, emphasizes the need to determine mechanisms of pancreatic cancer progression and better targets for diagnosis and treatment.
The current model of pancreatic cancer progression includes mutations that activate Kras, followed by inactivating mutations or loss of expression of tumor suppressor genes including p53, p16INK4A and SMAD4 3. Sonic hedgehog (SHH) and other proteins downstream of the hedgehog pathway were recently detected in precursor lesions and samples of primary tumors from patients with pancreatic adenocarcinoma, which implicates this pathway in the initiation and progression of pancreatic cancer given its absence in normal adult pancreas 4, 5. Transgenic mouse models in which SHH was expressed in mouse pancreatic epithelium, in concert with activated Ras signaling, enhanced formation of pancreatic intraepithelial neoplasia and accelerated lethality, further supporting a role for hedgehog as an early contributor to this lethal disease 6.
We investigated the contribution of SHH to pancreatic cancer initiation and progression by expressing SHH in a transformed primary ductal-derived epithelial cell line from the human pancreas, transformed hTert-HPNE (T-HPNE), which was described previously 7. This cell line has been documented to represent an appropriate and important model system to delineate signaling mechanisms for Ras-mediated oncogenesis and growth of pancreatic ductal epithelial cells8, and as such represents a reasonable system to evaluate the superimposition of SHH expression on pancreatic cells with a defined and limited set of transforming insults. We also directly inhibited the activity of SHH in vivo using the Capan-2 pancreatic cancer cell line in an orthotopic model, which is known to express SHH and form moderately-differentiated tumors in nude mice. Our data provide evidence that expression of SHH contributes to the formation of desmoplasia in pancreatic cancer. We show further that SHH affects the differentiation and motility of human pancreatic stellate cells and fibroblasts. These data implicate SHH as a mediator of the desmoplastic response in pancreatic cancer.
Materials and Methods
Cell lines, culture conditions
The cell line we used for these experiments was hTert-HPNE. The cells were originally isolated from the ductal structure of a human pancreas and immortalized by expressing the catalytic subunit of telomerase (h-Tert), along with subsequent transductions with retroviral constructs to introduce a Kras mutation, the human papillomavirus E6 and E7 peptides and the SV40 small t antigen, as described previously7. The hTert-HPNE cells and were maintained in Medium D containing one volume of M3 base (InCell Corp., San Antonio, TX, USA), three volumes of glucose-free DMEM, 5% FBS, 5.5mM glucose, 10ng/ml EGF, and 50μg/ml gentamycin. The cells were cultured at 37°C in humidified atmosphere containing 5% CO2.
For overexpression of SHH, the cDNA of SHH was cloned into the pWZL-blast retroviral vector (courtesy Dr. Wade Bushman, University of Wisconsin Medical School and Dr. Ouellete, Eppley Institute). Clones expressing SHH were isolated by serial dilutions and selection using Blastocidine. A control vector was tranduced into the control cell lines.
The Capan-2 pancreatic cancer cell line is maintained in McCoy's 5A media and are cultured at 37°C in humidified atmosphere containing 5% CO2. Pancreatic stellate cells and fibroblasts were isolated from an islet transplant program at UNMC and are maintained in RPMI medium at 37°C in humidified atmosphere containing 5% CO2.
Western blot analysis
Whole cell lysates were prepared by adding 1.5 ml cell lysis buffer [10 nmol/L Tris-HCl (pH 8.0), 150mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride and 1% Triton X-100] to cells grown to 80% confluence in T175 tissue culture flasks. Sixty micrograms of protein were loaded onto 4-20% Novex Tris-Glycine gradient denaturing polyacrylamide gels (Invitrogen) in a 1× SDS-PAGE running buffer, containing 1g/L SDS, 3g/L Tris base, and 14.4 g/L glycine. The proteins were transferred onto polyvinylpyrolidine diflouride membranes by electrophoresis. Once transferred, the membranes were blocked in Blotto [5% dry milk resuspended in 1× TBS (0.9% NaCl, 10mmol/L Tris pH 7.4, and 0.5%MgCl)] overnight at 4°C. Anti-SHH primary antibody (Santa Cruz, CA, USA) was incubated at a 1:200 dilution at room temperature for 2 hours. The membrane was washed 3 times, 10 minutes each time, before the addition of the secondary antibody (goat anti-rabbit HRP) at a 1:5000 dilution for 50 minutes. Immune complexes were identified using ECL Western blotting detection reagents. Membranes were stripped at subsequently blotted with anti β-actin antibody as a loading control.
Invasion Assay
In vitro invasive potential of cells overexpressing SHH was assayed using the Biocoat Matrigel Invasion Chamber (Becton Dickinson Labware, Bedford, MA). The invasion assay with pancreatic myofibroblasts included an initial seeding of 50,000, 250,000 or 500,000 T-HPNE or T-HPNE+SHH cells in the lower chambers. The cells were allowed to adhere for 8 hours. The media was then removed and replaced with RPMI medium containing 7% FBS. In the upper chambers, 250,000 pancreatic myofibroblasts were seeded and then allowed to incubate for 22 hours at 37°C and 5% CO2. For invasion with the pancreatic fibroblasts with rhSHH, 250,000 cells were seeded in the upper chamber in RPMI medium containing 7% FBS. The lower chambers included control RPMI with 7% FBS, 0.1ug/ml recombinant SHH (R&D systems, Minneapolis, MN) and 1.0 ug/ml recombinant SHH. These chambers were also allowed to sit for 22 hours at 37°C and 5% CO2. For analyses of all invasion assays, cells on the upper portion of the matrix membrane were wiped off with cotton tipped swab and cells on the bottom of this membrane were fixed and stained with Diff-Quick staining kit (Allegiance) and observed by a light microscopy. The number of invading cells was quantified by counting the membranes at 200× magnification.
Subcutaneous Injections in Athymic Nude Mice
Three million cells were injected subcutaneously in athymic nude mice. Prior to injection, the cells were trypsinized, counted, washed twice in 1×PBS and resuspended at a density of 3 × 106 cells/ 30μl. The cells were injected between the scapulae. Tumor growth was monitored every two days by measuring diameters using a caliper. The mice were euthanized when the tumor volume reached 1,800mm3.
Orthotopic implantation in athymic nude mice
One million T-HPNE and T-HPNE.SHH were each injected into the pancreas of 12 or 13 mice respectively to analyze tumor desmoplasia in response to overexpression of SHH. Preparation of cells was as described above for the subcutaneous injection model. Orthotopic implantation of T-HPNE cell lines was performed as described previously9.
SHH Inhibition in Orthotopic Models
To inhibit SHH in the Capan-2 orthotopic models, we administered the 5E1 antibody using intraperitoneal injections at a dosage of 500ug/mouse once per week for10 weeks. An IgG control antibody, 4E11, was also administered to a separate group of mice at a concentration of 500ug/mouse and administered once per week.
Confocal and Immunohistochemical analysis
To analyze cell morphology in the orthotopic tumors, primary tumors were resected and placed in formalin for 24 hours before being fixed for staining. Staining with Hematoxylin and Eosin allowed for the visualization of cells within the tumor sections. Fibroblasts were visually differentiated from tumor cells on the basis of cellular morphology and by staining with the markers described below.
To identify SHH in the primary human tumor sections, SHH (1:200) (Santa Cruz, CA, USA) anti-rabbit primary antibody (Santa Cruz, CA, USA) was incubated on paraffin-embedded tissue sections for 2 hours at 37° C. The secondary antibody incubation (anti-rabbit, 1:1000) was incubated for 50 minutes at 37°C. Confirmation of desmoplasia included analysis of reactivity with α-smooth muscle actin (1:400) (Sigma,) collagen I (1:50) (Abcam); and fibronectin (1:50) (Abcam). The secondary antibody (anti-mouse 1:1000) was incubated for 60 minutes. Staining was develpped by Vectastain ABC reagent and DAB substrate.
For confocal microscopy, anti-ribonuclear protein (1:20) (Chemicon) and anti-smooth muscle actin (1:500) (Sigma) were incubated on paraffin-embedded tissue sections for 1 hour at room temp. Secondary reagents, anti-mouse IgM-Alexa 647 and anti-mouse IgG-Alexa 488 (1:2000) were incubated for 1 hour.
Primary Cell Culture
Primary human pancreatic stellate cells and myofibroblasts were isolated as described previously7, 10. Cells were monitored for three weeks and were identified by cellular morphology and by analysis with markers described above as cells of mesenchymal lineage, or pancreatic myofibroblasts.
In vitro Wound Healing Assays
Wound healing assays were performed with the pancreatic myofibroblasts. 30,000 cells were seeded in 24-well plates and grown into a monolayer. A pipette was used to make a scratch through the monolayer and media was replaced. RPMI containing 7% FBS was used as a control. RPMI containing 7% FBS and 0.1ug/ml rhSHH along with RPMI containing 7%FBS, 0.1ug/ml rhSHH and 0.1ug/ml 5E1 (Iowa hybridoma bank) served as the experimental conditions.
Statistical analysis
The in vitro colony forming assays, wound healing assays and invasion assays were analyzed using a one-way Anova test as part of the Prizm statistical analysis package. Significance was determined at the 95% confidence interval.
Results
Inhibition of SHH signaling decreases desmoplasia
We evaluated the contribution of SHH to the growth properties of pancreatic tumor cells by administering monoclonal antibody 5E1 11, a blocking antibody to SHH, to mice challenged orthotopically with Capan-2, which expresses moderately high levels of SHH and forms moderately-differentiated pancreatic tumors with desmoplasia 12. One million Capan-2 cells were implanted orthotopically into the pancreas of athymic nude mice and 500 μg of 5E1 was administered once per week. An isotype control IgG antibody, 4E11, was administered to a control group of mice. Treatment with 5E1 resulted in a decreased tumor volume from an average of ∼ 0.45cm3 (Capan-2 controls) to ∼ 0.1cm3 (5E1- treated mice).
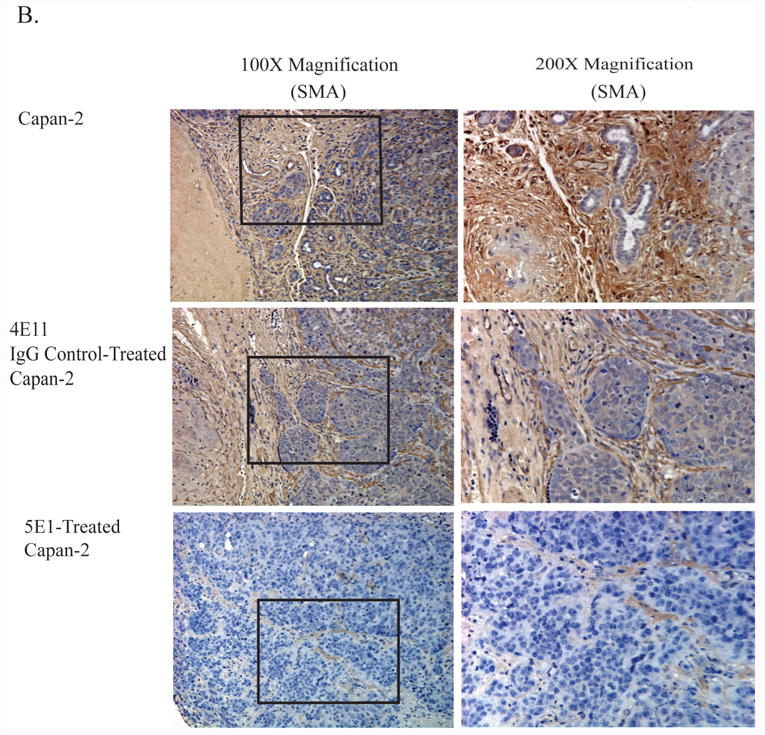
Histological and immunohistological analysis of tumor sections revealed that inhibiting SHH with 5E1 significantly decreased the degree of desmoplasia. Figure 1 shows staining of tumor sections with hematoxylin and eosin and with the mesenchymal marker for differentiated fibroblasts, α-smooth muscle actin, (SMA). The images in Figure 1A are representative of the desmoplastic reaction in tumors derived from the Capan-2 cell line with and without inhibition of SHH by treatment with antibody 5E1. Tumors in animals treated with the control antibody, 4E11, showed dense desmoplastic reactions. Tumors derived from mice treated with the 5E1 showed minimal evidence of desmoplasia. We confirmed that areas of desmoplasia contained myofibroblasts by staining the tumor sections with SMA (Figure 1B). Morphometric analysis revealed a significant decrease in the number of SMA+ cells between the control tumors and the tumors from mice treated with 5E1 (Figure 1C).
Figure 1. Inhibition of SHH signaling decreases desmoplasia in Capan-2 orthotopic tumors.
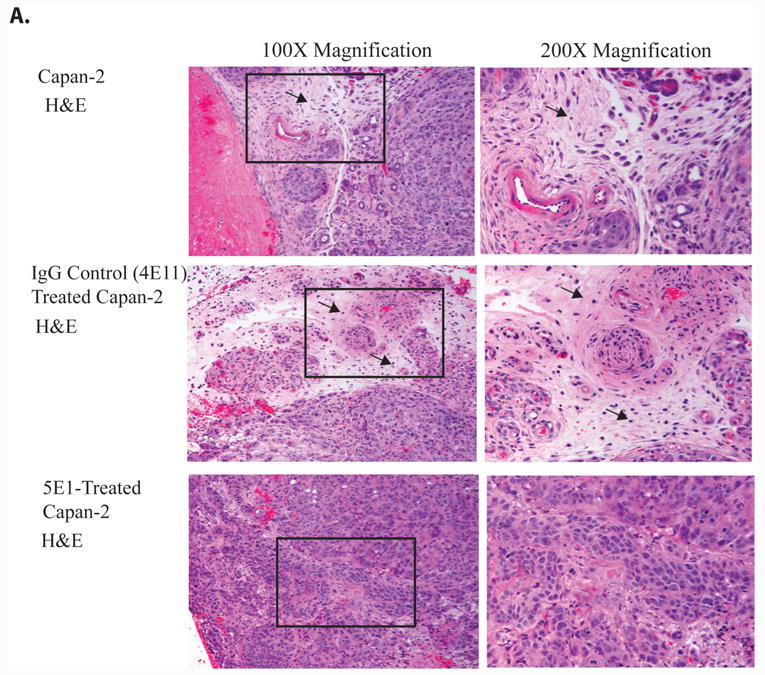
A. Histological analysis of tumors derived following orthotopic challenge with Capan-2 and treatment with a blocking antibody for SHH (5E1), an isotype control (4E11) or no treatment (Capan-2). 100× magnification of H&E staining showing reactive stroma and desmoplasia in the Capan-2 and control 4EII- treated Capan-2 tumors (see black arrows). The 5E1 treated Capan-2 tumors showed little or no evidence of desmoplasia by histological examination.
B. Immunohistochemistry using the mesenchymal marker SMA. Paraffin-embedded sections of tumors that arose from orthopic implantation of Capan-2 cells and treatment with a blocking antibody for SHH (5E1), an isotype control (4E11) or no treatment (Capan-2). Positive staining for SMA is indicated by the brown coloration. Counterstaining was performed using hematoxylin (blue).
C. Morphometric quantification of the relative amount of SMA+ desmoplasia in the Capan-2 control, 4E11 and 5E1-treated tumors. The 5E1 tumors showed a significant decrease in the amount of SMA+ desmoplasia. Relative SMA+ areas on the tumor sections were quantified by calcuating the total cellular content of the section, and expressing the results as a percentage of SMA+ desmoplasia/all cell types in total area ×100. Two representing results of 5E1-treated Capan-2 primary tumors showed some staining with SMA, but the areas of staining were individualized fibroblasts representing less than 20% of the section. Inhibition of SHH significantly decreased the amount of SMA+ desmoplasia in Capan-2 tumors (n=5/group).
SHH expression promotes a desmoplastic reaction when overexpressed in Transformed HPNE cells
To further investigate the role of SHH in the production of pancreatic cancer desmoplasia, we overexpressed SHH in a transformed primary pancreatic epithelial cell line, T-HPNE. SHH overexpression in this cell line was confirmed by western blot (Supplemental Figure 1). T-HPNE and T-HPNE cells expressing SHH (T-HPNE.SHH) were implanted orthotopically into the pancreas of athymic nude mice. Both T-HPNE cells and T-HPNE.SHH formed poorly-differentiated tumors at the orthotopic site. Expression of SHH in the T-HPNE cells resulted in an increase in mean tumor weight from ∼ 1g (T-HPNE) to ∼ 3g (T-HPNE.SHH). There was dense desmoplasia in tumors produced by the T-HPNE.SHH cell line, while desmoplasia was minimal in tumors derived from T-HPNE cells not expressing SHH, as evidenced by morphological evaluation, and immunohistochemical analysis of the orthotopic tumors derived from the T-HPNE.SHH cell line with SMA to confirm that cells in the desmoplastic reaction were myofibroblasts. Our analysis showed a significant increase in cells that stained positive for this mesenchymal marker within the tumor and at the periphery (Figure 2A and B). Tumors derived from the T-HPNE cell line showed minimal reactivity with α-smooth muscle actin (Figure 2A), and reactivity was restricted to the area around pancreatic ducts, which have been shown previously to stain positive. Figure 2C shows morphometric analysis of tumors derived from the T-HPNE and T-HPNE.SHH cells and indicates a significant increase in desmoplasia in the tumors derived from the T-HPNE.SHH cells.
Figure 2. SHH promotes desmoplasia in an orthotopic model of pancreatic cancer.


A and B. Histological analysis of orthotopic pancreatic tumors derived from the T-HPNE.SHH cell line. Tumor sections were stained for the marker SMA to determine if there was a difference in the amount of SMA+ desmoplasia in the SHH expressing tumor cells. Positive staining for SMA is indicated in brown. (A) 40× magnification of peripheral sections of T-HPNE and T-HPNE.SHH pancreatic tumors representing the large desmoplastic reaction in the periphery of the T-HPNE.SHH orthotopic tumors (note black arrows). Infiltrating SMA+ cells are also indicated with a black arrow. (B). 200× magnification. Immunohistochemical analysis using SMA to stain for myofibroblasts in the T-HPNE and T-HPNE.SHH tumor sections. Positive staining is indicated in brown and SMA+ cells are shown with the black arrows.
C. Morphometric analysis of desmoplasia in T-HPNE vs. T-HPNE.SHH orthotopic tumors. Analysis was done by quantifying SMA+ areas on the tumor sections relative to the total cellular content, and expressing the results as a percentage: SMA+ desmoplasia/total area ×100. Tumors derived from the T-HPNE.SHH cell line showed significantly more desmoplasia than the tumors derived from the T-HPNE cells (n=13/ group).
We stained adjacent sections with collagen I and fibronectin to determine if the myofibroblasts synthesized these two well-characterized components of desmoplasia. Figure 3A shows IHC staining of tumors derived from the T-HPNE.SHH cell line and confirms that the area containing myofibroblasts and desmoplsia also stained positive for fibronectin and collagen I. Morever, there were few myofibroblasts in the tumors derived from the T-HPNE cell line (Figure 3A) and immunohistochemical analysis of these sections showed an absence of collagen I and fibronectin.
Figure 3. Expression of Collagen 1 and Fibronectin in orthotopic tumor sections.
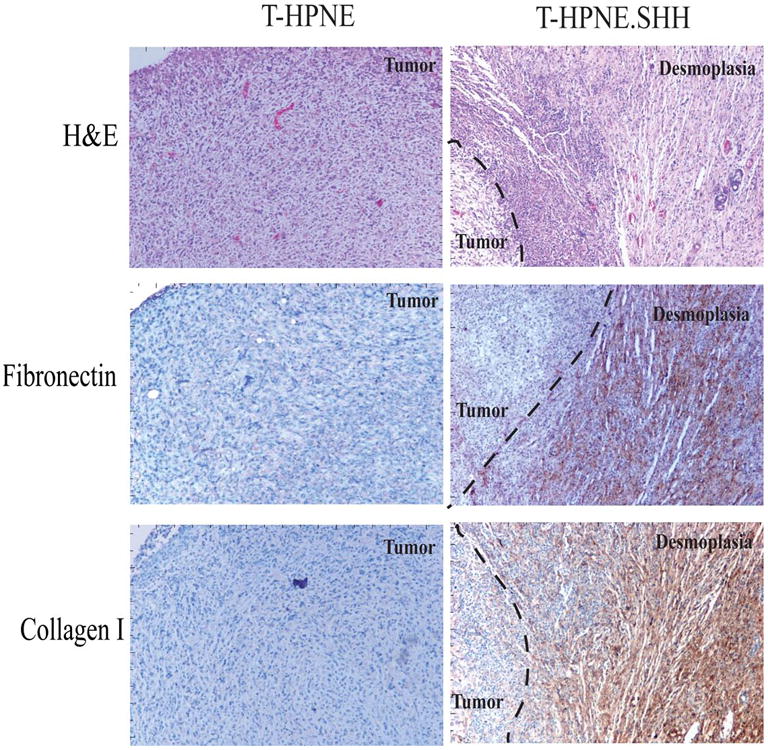

A. Representative immunohistochemical analysis of T-HPNE and T-HPNE.SHH tumor sections to determine if Fibronectin and Collagen 1 are expressed in either of the tumor sections. Areas of desmoplasia were identified using staining for SMA (described in Figures 1 and 2). Evidence that the SMA+ areas were expressing fibronectin and collagen 1 is indicated by brown staining in the areas of the tumor sections designated desmoplasia in the T-HPNE.SHH tumor sections. We did not observe positive staining in tumors derived from the T-HPNE cell line. H&E staining of the tumor sections is also shown.
B. Representative IHC staining for Collagen 1 and Fibronectin in the Capan-2 orthotopic tumors with no treatment, treatment with 4E11 or treatment with 5E1. There was a decrease in the intensity and amount of staining for both Collagen 1 and Fibronectin in the tumor sections treated with 5E1 when compared to the controls.
In Figure 3B, we confirmed the presence of collagen 1 and fibronectin using IHC to stain for these components in tumors derived from untreated mice challenged with the Capan-2 cell line. There is a decrease in both the intensity and quantity of both collagen 1 and fibronectin staining in the Capan-2 tumors derived from the mice treated with 5E1.
Myofibroblasts express Gli-1
We sought to determine if SHH was stimuating myofibroblasts by a paracrine mechanism. Figure 4A shows IHC analysis of human pancreatic cancer tissue obtained from autopsy, demonstrating that tumor cells stain positive for SHH, while the surrounding fibroblasts and areas of desmoplasia were positive for Gli-1, whose expression is known to be induced following stimulation with SHH. These data support the hypothesis that fibroblasts that make up the desmoplastic reaction in human pancreatic cancer show evidence of paracrine signaling in response to SHH produced by human pancreatic cancer cells.
Figure 4. SHH paracrine signaling between tumor and stroma.


A. Representative immunohistochemical analysis of primary pancreatic ductal adenocarcinoma tissue sections from patients with pancreatic cancer. Positive staining for Gli-1 or SHH is indicated by the brown color on the tissue sections. We observed positive staining for SHH in the tumor cells, while there was minimal staining in the tumor cells for Gli-1 (note black arrows showing positive staining; white arrows indicate negative staining). We observed minimal staining for Gli-1 in the tumor cells and observed reactivity with the Gli-1 antibody in areas of desmoplasia.
B. Immunohistochemical analysis of T-HPNE.SHH tumors. 200× magnification showing both SMA and Gli-1 staining. The brown staining in adjacent sections indicates the SMA+ myofibroblasts infiltrating the T-HPNE.SHH tumor section are expressing Gli-1 (n=3).
We also analyzed adjacent sections of T-HPNE.SHH tumors with antibodies to SMA and Gli-1, which showed evidence that SMA+ myofibroblasts expressed Gli-1, supporting the hypothesis that these cells were responding to SHH produced by the T-HPNE-SHH cells in a paracrine manner (Figure 4B).
Myofibroblasts and SMA+ cells are of murine origin
We used a human-specific antibody to a ribonuclear protein and an antibody that binds to both murine and human SMA to determine if the SMA+ cells were of human or murine origin. Figure 5A presents confocal analysis of expression of these markers in orthotopic tumors derived from human T-HPNE, T-HPNE.SHH, Capan-2 and 5E1-treated Capan-2 tumors. The majorities of these cells were clearly of murine origin and showed morphology of myofibroblasts.
Figure 5. SHH enhances pancreatic stellate cell proliferation and differentiation and enhances pancreatic myofibroblast invasion.


A. Confocal microscopy of T-HPNE and T-HPNE.SHH tumors (as indicated) using anti-human ribonuclear protein (blue) and anti-SMA (green). DIC plus overlay shows that in the T-HPNE.SHH tumors, there are cells positive for SMA but not human ribonuclear protein, which indicates that the majority of the myofibroblasts are of murine origin. Confocal analysis of anti-ribonuclear protein (RNP) or SMA expression on Capan-2 control tumors and tumors treated with the 5E1 inhibitor (as indicated). The anti-human ribonuclear protein is shown in blue and SMA is in green. In the 5E1-teated Capan-2 tumors, isolated SMA+ cells can be identified as green in the overlay.
B. Serial sections showing SMA and Cyclin D1 staining of SMA+ myofibroblasts in Capan-2 tumors (brown stain).
C. Real time PCR analysis on markers of mesenchymal and epithelial differentiation performed on pancreatic stellate cells after 24 hour stimulation with recombinant human SHH at either 1ug/ml or 10ug/ml. SHH stimulation increased the expression of Desmin, Vimentin and Smooth muscle actin and decreased the expression of Cytokeratin 19.
SHH promotes the differentiation and proliferation of pancreatic stellate cells
Confocal analysis of the 5E1-treated Capan-2 tumors (Figure 5A) revealed single SMA+ cells embedded in the human tumors, raising the possibility that these were stellate cells that were progenitors to the myofibroblasts observed in Capan-2 tumors when SHH signaling was not inhibited (Figure 5A). Capan-2 tumors were stained for cyclin D1, which was detected in the myofibroblasts, supporting the hypothesis that these represented stellate cells within the tumor that were proliferating in response to SHH stimulation (Figure 5B).
We isolated pancreatic stellate cells from the human pancreas, as was described previously 7, 10, and confirmed their lineage by using PCR to identify markers of both epithelial and mesenchymal lineage. Consistent with the anticipated phenotype for pancreatic stellate cells, the isolated cells expressed higher levels of SMA and vimentin and displayed a spindle-like morphology (Supplemental Figure 2). We stimulated these cells with rhSHH for 24 hours at 1ug/ml and 10ug/ml, and observed an increase in the mesenchymal markers SMA, vimentin and desmin and a decrease in the epithelial marker CK19 (Figure 5C). These data are the first to suggest that SHH induces differentiation of pancreatic stellate cells into myofibroblasts.
Sonic hedgehog in vitro promotes the invasion of human pancreatic myofibroblasts
We analyzed the ability of human myofibroblasts from the pancreas to respond to SHH in matrigel invasion assays, to determine if SHH contributes to increased desmoplasia by enhancing the motility and invasive capacity of these cells. Human pancreatic myofibroblasts, which express the markers vimentin and SMA and lack expression of CK19 (Supplemental Figure 3A) were isolated and shown to express Gli-1 when stimulated with SHH (Supplemental Figure 3B). Figure 6A shows results of a matrigel invasion assay, in which increasing numbers of T-HPNE and T-HPNE.SHH cells were seeded in the lower chamber and myofibroblasts were seeded in the upper chamber. For cultures containing 250,000 and 500,000 T-HPNE cells expressing SHH, there was a significant increase in the invasive capacity of myofibroblasts as compared to T-HPNE controls. We repeated this experiment with recombinant SHH in the lower chamber (rhSHH-R&D systems, Minneapolis, MN). Figure 6B shows that there was a concentration-dependent and significant increase in the ability of the myofibroblasts to invade through a matrigel membrane in response to exposure to rhSHH.
Figure 6. SHH increases the in vitro invasive capacity of primary cells from the human pancreas.

A. Invasion assay in matrigel invasion chambers. T-HPNE cells or T-HPNE.SHH cells were seeded in the lower chambers at densities of 50,000 cells/ well, 250,000 cells/ well and 500,000 cells/ well. With 250,000 and 500,000 cells, SHH secreted from the T-HPNE.SHH cell line significantly increased the invasive potential of an immortalized human pancreatic myofibroblast cell line (*p<0.05). Quantitative analysis of numbers of invasive cells for each cell line is represented in the bar graph ± SE (n=3).
B. Invasion assay in matrigel invasion chambers. Recombinant SHH in the lower chamber of the invasion assay significantly increased the invasive potential of an immortalized human pancreatic myofibroblast cell line (**p< 0.01). Quantitative analysis of numbers of invasive cells for each cell line is represented in the bar graph ± SE (n=3).
C and D. In vitro wound healing assay. C. Pictures of initial time point controls and 48hour time points showing the affects of SHH-stimulation on the pancreatic myofibroblasts. D. Quantitative analysis of the in vitro wound healing assay (% closure). SHH-stimulated immortalized human pancreatic myofibroblasts showed a significant increase in their ability to recover from the wound (*p=0.05). The addition of the 5E1 blocking antibody significantly inhibited the SHH-mediated wound-healing ability back to control levels (*p=0.05). Values are shown in the bar graph ±SD for each cell line.
We also performed an in vitro wound-healing assay to determine if SHH increased the ability of the myofibroblasts in a monolayer to migrate into a wound. Figure 6 C and D show the visual and quantitative results from this experiment. After 48 hours, SHH significantly increased the ability of the myofibroblasts to recover from the wound (90% recovery with SHH vs. 45% recovery in the control cells and cells with SHH and the 5E1 SHH inhibitor). These experiments support the hypothesis that SHH increases the migration and invasion of human pancreatic myofibroblasts in vitro.
Discussion
Hedgehog signaling was first identified and characterized in Drosophila and has been shown to play a role in organogenesis and differentiation. During vertebrate patterning, hedgehog signaling induces differentiation of the skeleton, central nervous system, early gut endoderm, limb axes and ventral somite buds 13, 14. Interestingly, SHH has not been shown to contribute to normal differentiation of the pancreas, and ectopic expression in the epithelia of the embryonic pancreatic mesoendoderm leads to malformation of the pancreatic mesoderm and an increase in the number of mesenchymal cells in the developing pancreatic compartment 4.
There are three known human hedgehog family members: Sonic (SHH), Indian (Ihh) and Desert (Dhh). After translation of the 45kD protein, the hedgehog proteins undergo an autocatalytic cleavage facilitated by a cholesterol modification, an event that yields a 19 kD N-terminal protein that is secreted, and 25 kD C-terminal protein that remains associated with the cell 15, 16. The secreted ligand binds to a 12-pass transmembrane protein, Patched (Ptch), which relieves the inhibitory effect of Patched on another transmembrane protein, Smoothened (SMO) 15-17, which in turn enables hedgehog signaling. The complex signaling cascade downstream of SMO activation is not well characterized, yet ultimately SHH transduces a signal that induces nuclear translocation of Gli, a target transcription factor, and the transcriptional activation of hedgehog target genes, including Ptch, Gli and hedgehog interaction protein, Hhip 18, 19
Several reports have implicated the misregulation of the hedgehog signaling pathway in the initiation and progression of pancreatic cancer 5, 20. Mutations in this pathway are associated with cancer initiation and progression, as mutations in the tumor suppressor Patched are characterized in basal cell carcinoma, meduloblastoma and rhabdomyosarcoma, while oncogenic mutations in the downstream transcription factor Gli have been identified in glioblastoma and basal cell carcinoma 21, 22. Pancreatic cancer presents a different oncogenic insult within the hedgehog pathway, as SHH is upregulated in the tumor cells but absent in the normal pancreas.
We observed that expression of SHH was correlated with desmoplasia in two cell lines grown orthotopically in nude mice. Desmoplasia, a common and well noted histological feature of pancreatic cancer, has been proposed to contribute to progression of the disease23; however, the molecular processes that cause the desmoplastic reaction are not well characterized. Previous experiments in prostate cancer have determined that SHH contributes to desmoplasia and tumor growth24; thus we propose SHH has a similar mechanistic role in pancreatic cancer.
We explored the requirement for SHH in the induction of desmoplasia by administering a SHH-neutralizing antibody, 5E1, to mice with Capan-2 cells injected orthotopically into their pancreas. Capan-2 tumors express SHH and produce a desmoplastic reaction when grown orthotopically in mice. Capan-2 tumors treated with the SHH-inhibiting antibody were smaller and did not elicit a desmoplastic response when compared with the control tumors derived from the Capan-2 cell line that received no treatment or an isotype-control antibody. Similarly, SHH expression in T-HPNE cells (T-HPNE.SHH) produced larger tumors that showed significant differences in the cellular morphology. Within the tumor sections of the T-HPNE.SHH cell line, we noted the presence of infiltrating fibroblasts as long, extended cells type with elongated nuclei. This fibrotic infiltration was accompanied by expression of collagen 1 and fibronectin, and represented further evidence of a desmoplastic reaction within tumors from the SHH overexpressing transformed cell line 25, 26. This reaction was not observed in the tumor sections from the T-HPNE cell line.
We furthered evaluated the effects of SHH signaling in vitro on primary pancreatic myofibroblasts and found that SHH acted as a chemoattractant. This result complements previous studies, which showed that cancer cells recruit fibroblasts, and provides the first reported evidence that SHH, secreted from transformed pancreatic epithelia, contributes to the recruitment and organization of fibroblasts to enhance tumor progression 27. We also show that SHH can induce the differentiation and proliferation of human pancreatic stellate cells, which can also be a mechanism whereby SHH induces desmoplasia.
In summary, our data are significant in that they identify SHH as a regulator of one critical pathological outcome of pancreatic ductal adenocarcinoma: the production of desmoplasia, or the infiltration of fibrous tissue into neoplasms, which results from the proliferation of fibroblasts and the increase in fibrosis within the stroma28-30. Desmoplasia is a common histological feature of many adenocarcinomas 31, 32. The stroma, or complex microenvironment surrounding tumor cells, includes inflammatory cells, fibroblasts, extracellular matrix and small blood and lymphatic vessels 33, 34. Previous experiments have shown that TGFβ is a mediator of fibrosis12 in pancreatic cancer. In data not shown, we performed microarray on the T-HPNE vs. T-HPNE.SHH cells and determined that the expression of SHH did not upregulate the expression of TGFβ. Thus, it is possible that SHH directly influences the desmoplasic reaction, but it remains possible that SHH acts by inducing signals that interact with TGFβ signaling pathways 35. We have also shown that SHH can act as a chemoattractant for myofibroblasts in invasion assays and in wound healing assays, where SHH increased the ability of pancreatic myofibroblasts to recover from a wound. These data suggest that SHH is a critical player in the tumor microenvironment. Further elucidation of its functions will lead to a better understanding of the pathogenesis of this lethal disease and the potential for therapeutic targeting of SHH to disrupt the microenvironment in pancreatic cancer.
Supplementary Material
Supplemental Figure 1. Western blot confirmation of SHH overexpression in the T-HPNE.SHH cell line. Levels were compared to B-actin, which served as the loading control for the cell lines.
Supplemental Figure 2. Isolated human pancreatic stellate cells. PCR analysis of both epithelial and mesenchymal markers. 100× magnification of the cells' morphology.
Supplemental Figure 3. A. Isolation of human pancreatic myofibroblasts.The cells have a characteristic differentiated myofibroblasts morphology.The cells also stain posisitive for the mesenchymal markers SMA and vimentin.
B. SHH paracrine stimulation of Gli1 in the pancreatic myofibroblasts. Both sections were stained for the presence of Gli1 after 12 hours of control supernatant or a 1:20 dilution of SHH-containing media. Positive staining is indicated by the dark brown coloration.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. doi: 10.1097/00000658-200209000-00012. discussion 366-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 4.Kayed H, Kleeff J, Osman T, Keleg S, Buchler MW, Friess H. Hedgehog signaling in the normal and diseased pancreas. Pancreas. 2006;32:119–129. doi: 10.1097/01.mpa.0000202937.55460.0c. [DOI] [PubMed] [Google Scholar]
- 5.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KM, Nguyen C, Ulrich AB, Pour PM, Ouellette MM. Immortalization with telomerase of the Nestin-positive cells of the human pancreas. Biochem Biophys Res Commun. 2003;301:1038–1044. doi: 10.1016/s0006-291x(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell PM, Groehler AL, Lee KM, Ouellette MM, Khazak V, Der CJ. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res. 2007;67:2098–2106. doi: 10.1158/0008-5472.CAN-06-3752. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsumida H, Swanson BJ, Singh PK, Caffrey TC, Kitajima S, Goto M, Yonezawa S, Hollingsworth MA. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12:2976–2987. doi: 10.1158/1078-0432.CCR-05-1197. [DOI] [PubMed] [Google Scholar]
- 10.Lawson T, Ouellette M, Kolar C, Hollingsworth M. Culture and immortalization of pancreatic ductal epithelial cells. Methods Mol Med. 2005;103:113–122. doi: 10.1385/1-59259-780-7:113. [DOI] [PubMed] [Google Scholar]
- 11.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 12.Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, Jesnowski R. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–555. [PubMed] [Google Scholar]
- 13.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 14.Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 15.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 16.Porter JA, Ekker SC, Park WJ, von Kessler DP, Young KE, Chen CH, Ma Y, Woods AS, Cotter RJ, Koonin EV, Beachy PA. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell. 1996;86:21–34. doi: 10.1016/s0092-8674(00)80074-4. [DOI] [PubMed] [Google Scholar]
- 17.Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 18.Lipinski RJ, Gipp JJ, Zhang J, Doles JD, Bushman W. Unique and complimentary activities of the Gli transcription factors in Hedgehog signaling. Exp Cell Res. 2006;312:1925–1938. doi: 10.1016/j.yexcr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 20.Kayed H, Kleeff J, Keleg S, Guo J, Ketterer K, Berberat PO, Giese N, Esposito I, Giese T, Buchler MW, Friess H. Indian hedgehog signaling pathway: expression and regulation in pancreatic cancer. Int J Cancer. 2004;110:668–676. doi: 10.1002/ijc.20194. [DOI] [PubMed] [Google Scholar]
- 21.Zedan W, Robinson PA, Markham AF, High AS. Expression of the Sonic Hedgehog receptor “PATCHED” in basal cell carcinomas and odontogenic keratocysts. J Pathol. 2001;194:473–477. doi: 10.1002/path.940. [DOI] [PubMed] [Google Scholar]
- 22.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 23.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:S84–86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm ML, Munoz A, Lipinski R, Thrasher JB, Bushman W. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145:3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 25.Hauptmann S, Zardi L, Siri A, Carnemolla B, Borsi L, Castellucci M, Klosterhalfen B, Hartung P, Weis J, Stocker G, et al. Extracellular matrix proteins in colorectal carcinomas. Expression of tenascin and fibronectin isoforms. Lab Invest. 1995;73:172–182. [PubMed] [Google Scholar]
- 26.Hanamura N, Yoshida T, Matsumoto E, Kawarada Y, Sakakura T. Expression of fibronectin and tenascin-C mRNA by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int J Cancer. 1997;73:10–15. doi: 10.1002/(sici)1097-0215(19970926)73:1<10::aid-ijc2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Ishii G, Sangai T, Ito T, Hasebe T, Endoh Y, Sasaki H, Harigaya K, Ochiai A. In vivo and in vitro characterization of human fibroblasts recruited selectively into human cancer stroma. Int J Cancer. 2005;117:212–220. doi: 10.1002/ijc.21199. [DOI] [PubMed] [Google Scholar]
- 28.Mollenhauer J, Roether I, Kern HF. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14–24. doi: 10.1097/00006676-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Kuniyasu H, Abbruzzese JL, Cleary KR, Fidler IJ. Induction of ductal and stromal hyperplasia by basic fibroblast growth factor produced by human pancreatic carcinoma. Int J Oncol. 2001;19:681–685. doi: 10.3892/ijo.19.4.681. [DOI] [PubMed] [Google Scholar]
- 30.Kloppel G, Lingenthal G, von Bulow M, Kern HF. Histological and fine structural features of pancreatic ductal adenocarcinomas in relation to growth and prognosis: studies in xenografted tumours and clinico-histopathological correlation in a series of 75 cases. Histopathology. 1985;9:841–856. doi: 10.1111/j.1365-2559.1985.tb02870.x. [DOI] [PubMed] [Google Scholar]
- 31.Hartel M, Di Mola FF, Gardini A, Zimmermann A, Di Sebastiano P, Guweidhi A, Innocenti P, Giese T, Giese N, Buchler MW, Friess H. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg. 2004;28:818–825. doi: 10.1007/s00268-004-7147-4. [DOI] [PubMed] [Google Scholar]
- 32.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 33.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 34.Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Western blot confirmation of SHH overexpression in the T-HPNE.SHH cell line. Levels were compared to B-actin, which served as the loading control for the cell lines.
Supplemental Figure 2. Isolated human pancreatic stellate cells. PCR analysis of both epithelial and mesenchymal markers. 100× magnification of the cells' morphology.
Supplemental Figure 3. A. Isolation of human pancreatic myofibroblasts.The cells have a characteristic differentiated myofibroblasts morphology.The cells also stain posisitive for the mesenchymal markers SMA and vimentin.
B. SHH paracrine stimulation of Gli1 in the pancreatic myofibroblasts. Both sections were stained for the presence of Gli1 after 12 hours of control supernatant or a 1:20 dilution of SHH-containing media. Positive staining is indicated by the dark brown coloration.


