Abstract
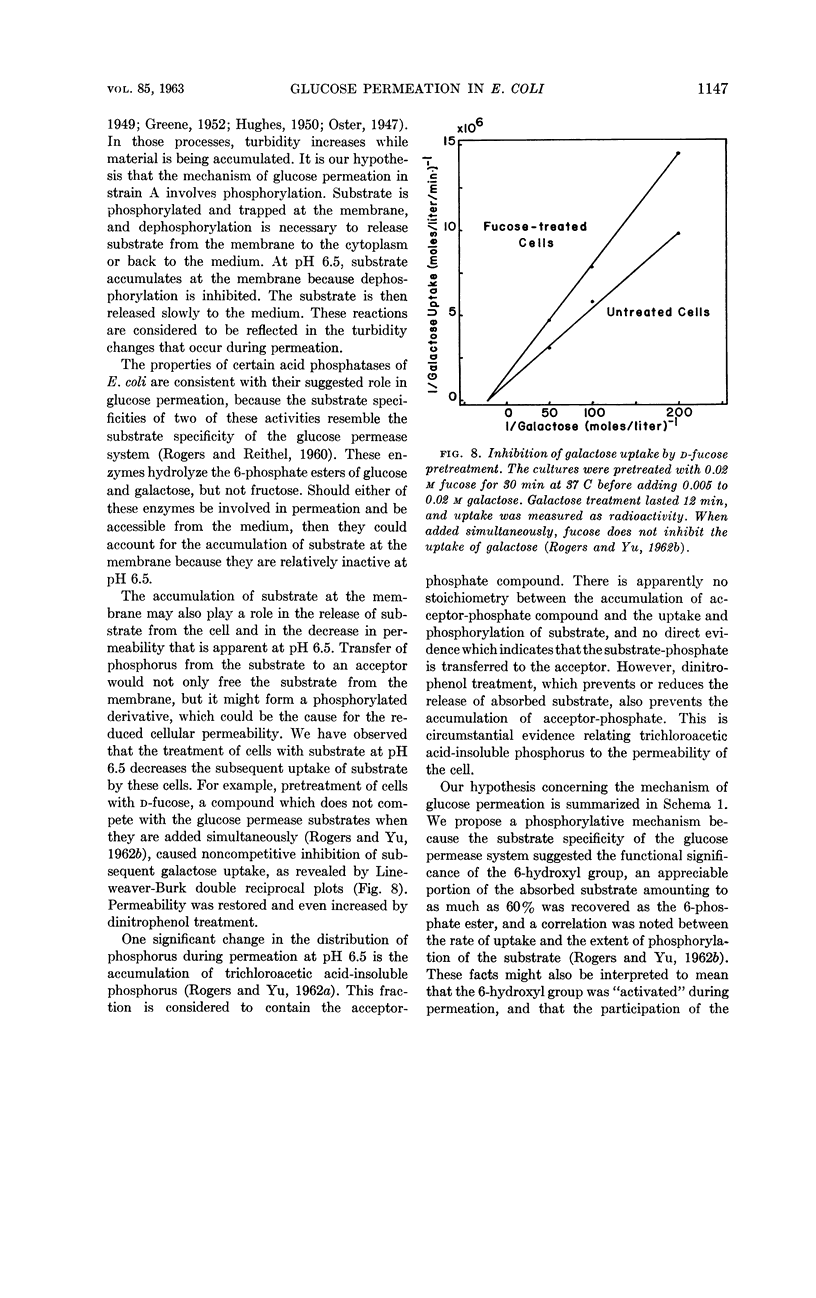
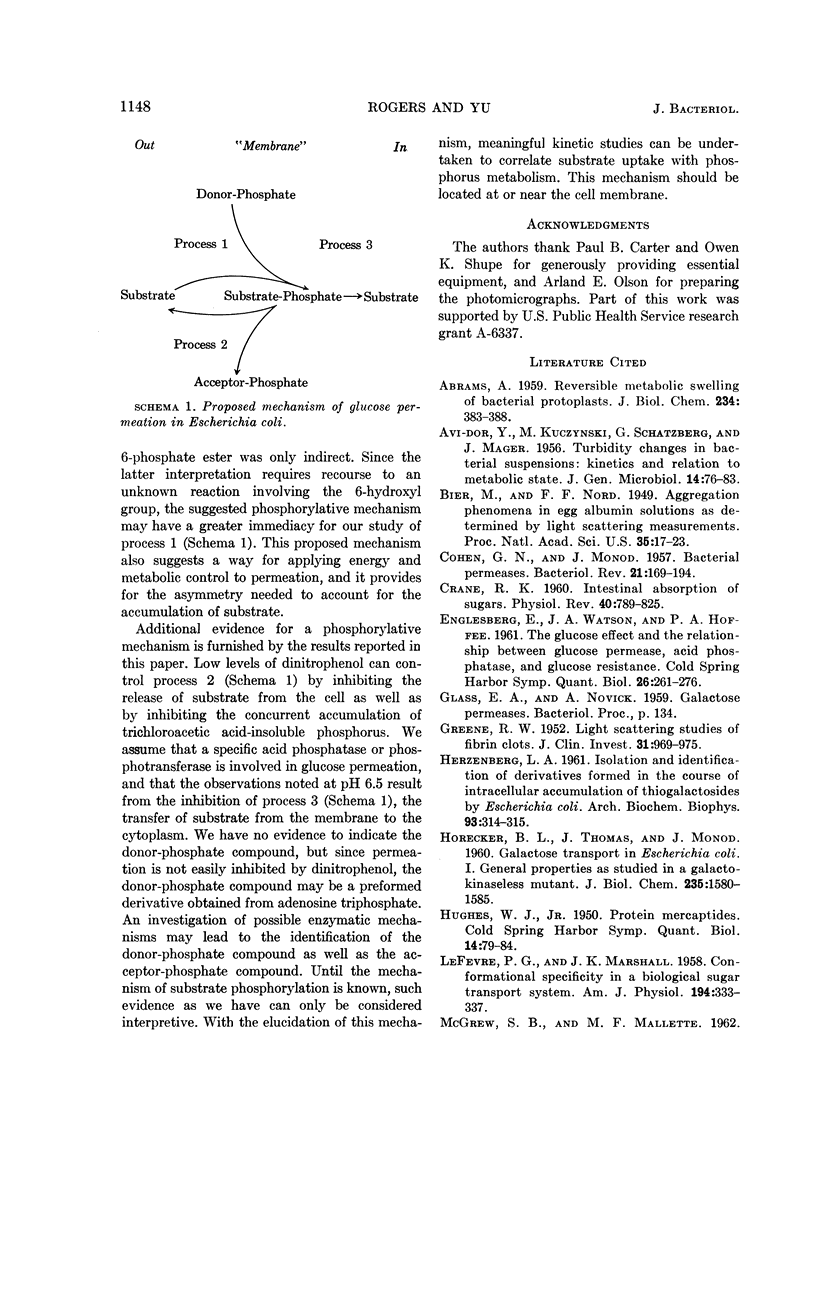
Rogers, Dexter (Utah State University, Logan) and Shon-Hua Yu. Turbidity change during glucose permeation in Escherichia coli. J. Bacteriol. 85:1141–1149. 1963.—In contrast to the normal turbidity decrease, which was observed at pH 5.5, the turbidity of suspensions of Escherichia coli strain A (Weigle) increased during uptake of glucose permease substrates when uptake was studied at pH 6.5. Although the turbidity increase at pH 6.5 was the reverse of the expected change, it correlated with the uptake of substrate. During the later phase of permeation at pH 6.5, both uptake of substrate and turbidity change were reversed. We suggest that the observed turbidity response indicated the accumulation of substrate at the cell membrane when the mechanism for releasing substrate to the cytoplasm was inhibited at pH 6.5. Under this condition, uptake of substrate did not cause swelling because the substrate was osmotically inactive, since it may have been bound to the membrane. Permeability was observed to be susceptible to modification by prior treatment with substrate, by aging in the presence of chloramphenicol, and by treatment with dinitrophenol. Permeation and turbidity change are discussed in terms of a proposed phosphorylative mechanism of glucose transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. Reversible metabolic swelling of bacterial protoplasts. J Biol Chem. 1959 Feb;234(2):383–388. [PubMed] [Google Scholar]
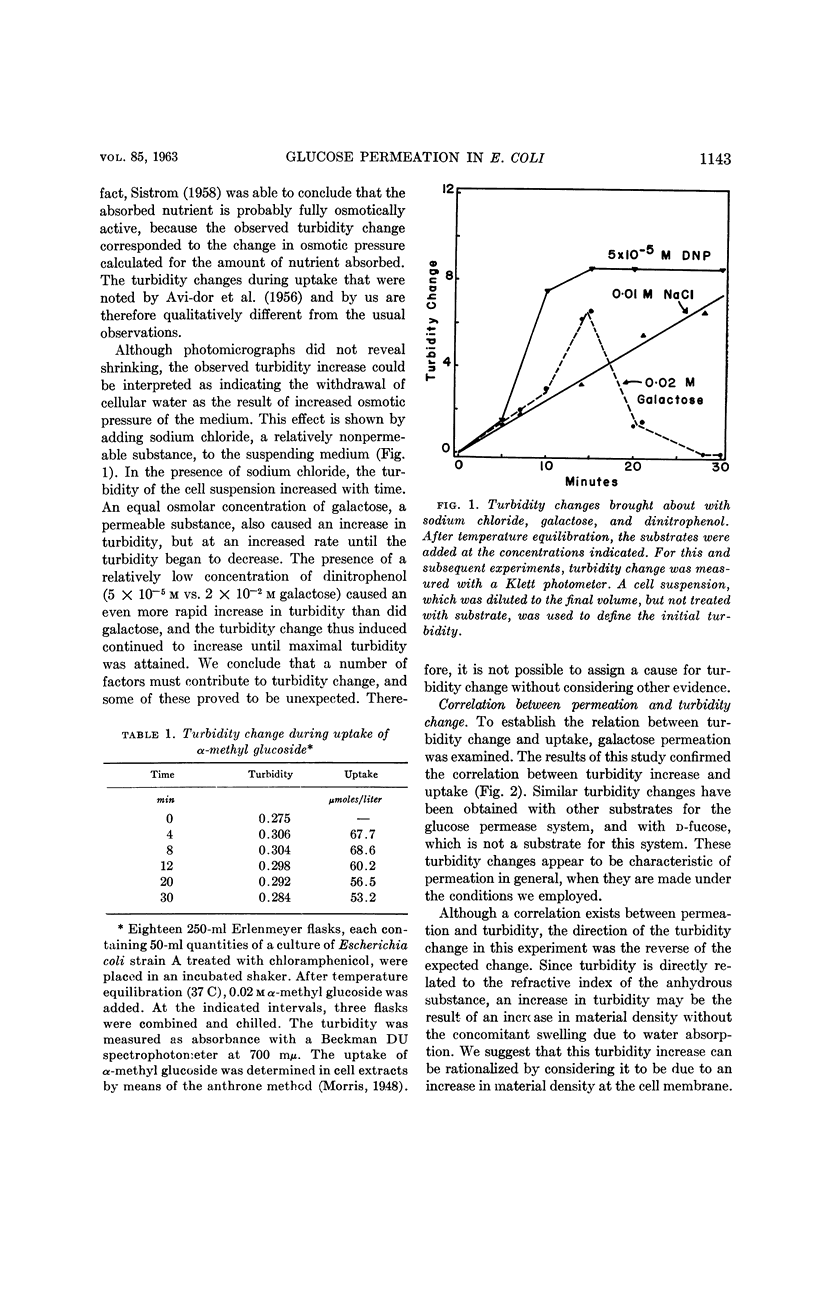
- AVI-DOR Y., KUCZYNSKI M., SCHATZBERG G., MAGER J. Turbidity changes in bacterial suspensions: kinetics and relation to metabolic state. J Gen Microbiol. 1956 Feb;14(1):76–83. doi: 10.1099/00221287-14-1-76. [DOI] [PubMed] [Google Scholar]
- BUTTIN G., COHEN G. N., MONOD J., RICKENBERG H. V. La galactoside-perméase d'Escherichia coli. Ann Inst Pasteur (Paris) 1956 Dec;91(6):829–857. [PubMed] [Google Scholar]
- Bier M., Nord F. F. Aggregation Phenomena in Egg Albumin Solutions as Determined by Light Scattering Measurements. Proc Natl Acad Sci U S A. 1949 Jan;35(1):17–23. doi: 10.1073/pnas.35.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K. Intestinal absorption of sugars. Physiol Rev. 1960 Oct;40:789–825. doi: 10.1152/physrev.1960.40.4.789. [DOI] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- GREENE R. W. Light scattering studies of fibrin clots. J Clin Invest. 1952 Nov;31(11):969–975. doi: 10.1172/JCI102689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZENBERG L. A. Isolation and identification of derivatives formed in the course of intracellular accumulation of thiogalactosides by Escherichia coli. Arch Biochem Biophys. 1961 May;93:314–315. doi: 10.1016/0003-9861(61)90270-3. [DOI] [PubMed] [Google Scholar]
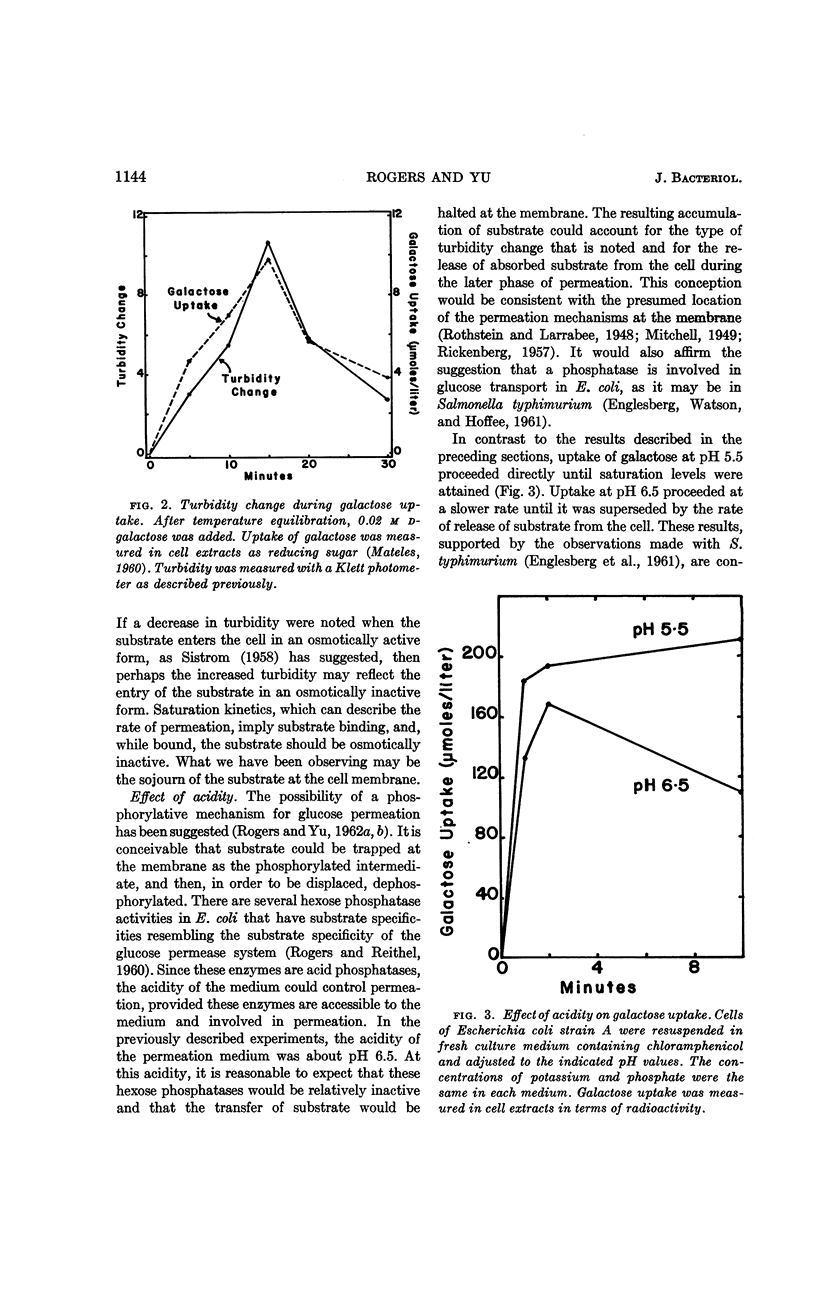
- HORECKER B. L., THOMAS J., MONOD J. Galactose transport in Escherichia coli. I. General properties as studied in a galactokinaseless mutant. J Biol Chem. 1960 Jun;235:1580–1585. [PubMed] [Google Scholar]
- HUGHES W. L., Jr Protein mercaptides. Cold Spring Harb Symp Quant Biol. 1950;14:79–84. doi: 10.1101/sqb.1950.014.01.011. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. Conformational specificity in a biological sugar transport system. Am J Physiol. 1958 Aug;194(2):333–337. doi: 10.1152/ajplegacy.1958.194.2.333. [DOI] [PubMed] [Google Scholar]
- MATELES R. I. Ferricyanide reduction method for reducing sugars. Nature. 1960 Jul 16;187:241–242. doi: 10.1038/187241a0. [DOI] [PubMed] [Google Scholar]
- McGrew S. B., Mallette M. F. ENERGY OF MAINTENANCE IN ESCHERICHIA COLI. J Bacteriol. 1962 Apr;83(4):844–850. doi: 10.1128/jb.83.4.844-850.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- PACKER L., PERRY M. Energy-linked light-scattering changes in Escherichia coli. Arch Biochem Biophys. 1961 Nov;95:379–388. doi: 10.1016/0003-9861(61)90163-1. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B. An inducible mechanism for accumulation of melibiose in Escherichia coli. J Bacteriol. 1957 Mar;73(3):376–385. doi: 10.1128/jb.73.3.376-385.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICKENBERG H. V. The site of galactoside-permease activity in Escherichia coli. Biochim Biophys Acta. 1957 Jul;25(1):206–207. doi: 10.1016/0006-3002(57)90449-3. [DOI] [PubMed] [Google Scholar]
- ROGERS D., REITHEL F. J. Acid phosphatases of Escherichia coli. Arch Biochem Biophys. 1960 Jul;89:97–104. doi: 10.1016/0003-9861(60)90018-7. [DOI] [PubMed] [Google Scholar]
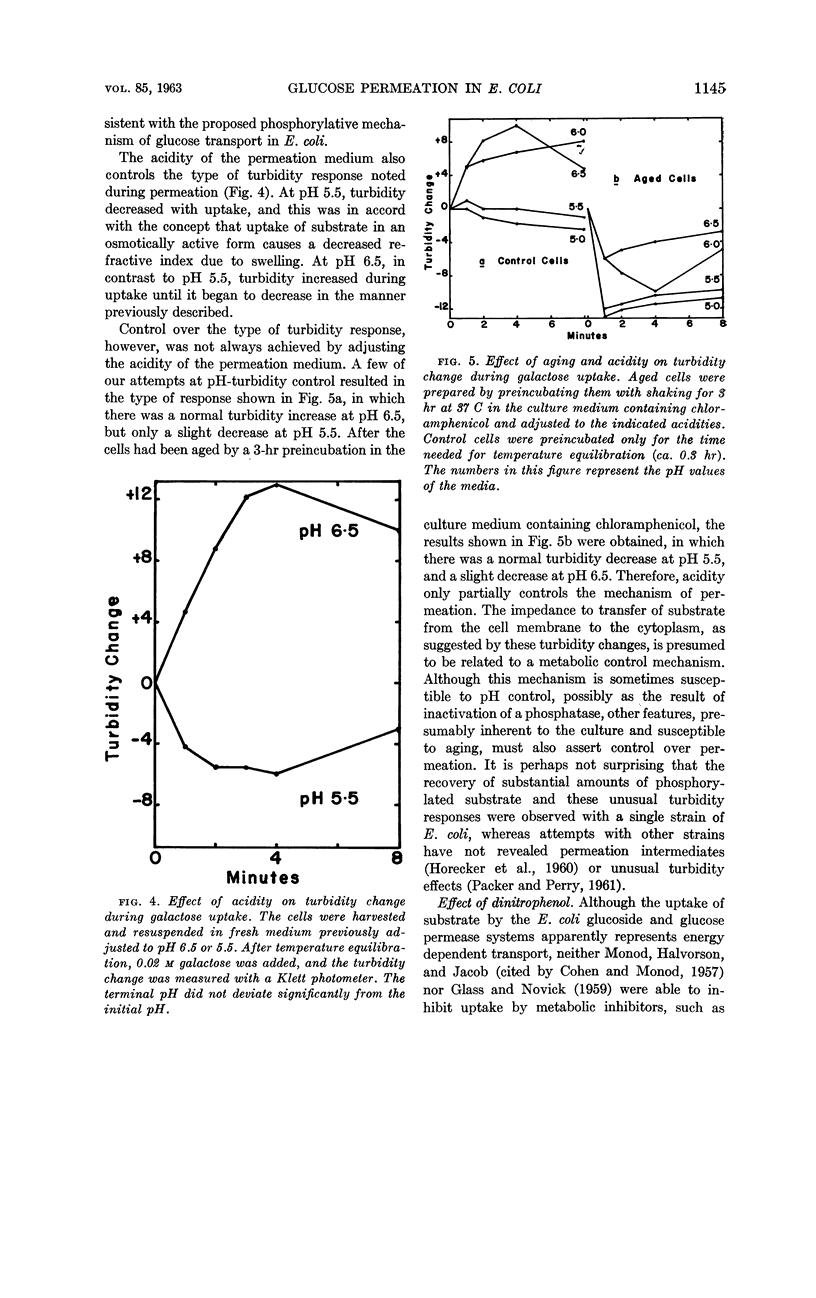
- ROGERS D., YU S. H. Substrate specificity of a glucose permease of Escherichia coli. J Bacteriol. 1962 Nov;84:877–881. doi: 10.1128/jb.84.5.877-881.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- ZABIN I., KEPES A., MONOD J. Thiogalactoside transacetylase. J Biol Chem. 1962 Jan;237:253–257. [PubMed] [Google Scholar]



