Abstract
Wnt signaling regulates neural stem cell (NSC) function and development throughout an individual's lifetime. Intriguingly, adult hippocampal progenitors (AHPs) produce several Wnts, and the intracellular machinery necessary to respond to them, creating the potential for an active autocrine-signaling loop within this stem cell niche. However, the standard luciferase-based Wnt assay failed to detect this signaling loop. This assay is inherently less temporally sensitive to activity among a population of unsynchronized proliferating cells because it relies on the rapidly degrading reporter luciferase. We circumvented this limitation using a promoter assay that employs green fluorescent protein (GFP), as a relatively long-lived reporter of canonical Wnt activity. We found that at baseline, AHPs secreted functional Wnt that self-stimulates low-level canonical Wnt signaling. Elimination baseline Wnt activity, via application of an extracellular Wnt antagonist promoted neurogenesis, based on a combination of unbiased gene expression analysis and cell-fate analysis. A detailed clonal analysis of progenitors transduced with specific intracellular antagonists of canonical signaling, either Axin or truncated cadherin (β-catenin sequestering), revealed that loss of baseline signaling depletes the population of multipotent precursors, thereby driving an increasing fraction to assume a committed cell fate (i.e., unipotent progenitors). Similarly, baseline Wnt signaling repressed differentiation of human NSCs. Although the specific Wnts produced by neural precursors vary with age and between species, their effects remain remarkably consistent. In sum, this study establishes that autonomous Wnt signaling is a conserved feature of the neurogenic niche that preserves the delicate balance between NSC maintenance and differentiation.
Keywords: Wnt, Neurogenesis, Clonal analysis, Human, Neural stem cell, Autocrine
Introduction
A few, privileged brain regions contain neural stem cell (NSC) that continue to produce new neurons throughout adulthood. Advanced age, inflammation, or stress decreases that rate of new neuron production in either hippocampus or subventricular zone (SVZ) [1–5], whereas exercise, antidepressants, or mood stabilizers (e.g., lithium) enhance neurogenesis [3, 6–9]. These slowly dividing undifferentiated neurons exist as individuals cells or small clusters [10, 11], within a microenvironmental niche that contains many signals capable of driving their maturation. How NSCs resist these surrounding differentiation forces remains poorly understood.
Autocrine signaling is one strategy that can render uncommitted progenitors partially insensitive to differentiating effects of their environment. In the nervous system, such autocrine signaling promotes the survival of diverse neuronal populations via secretion of trophic factors like brain derived neurotrophic factor or leukemia inhibitory factor (LIF) [12, 13]. More recently, it was shown that autocrine PDGF signaling regulates NSC proliferation and development [14]. The most conservative definition of autocrine signaling requires proof that a specific cell is responding to the very same molecules of neurotrophin that it just released into the extracellular milieu and not to molecules released by an adjoining cell (i.e., paracrine). However, this definition may not be the most biologically relevant to the understanding of how NSCs interact with their environment. As these primitive cells exist as small multipotent islands surrounded by a sea of more mature cell types they constitute a single compartment of the more complex neurogenic niche within the adult brain, so we refer to this process as within-niche signaling [15–17].
Recent studies identified Wnt, present in the NSC microenvironment (i.e., niche), as a potent regulator of adult neurogenesis [18, 19]. Wnts transduce numerous signaling cascades, but the best studied (canonical), ultimately down-regulating the kinase GSK-3β, leading to β-catenin/TCF signaling in nucleus. This pathway was recently shown to regulate differentiation in the stem cell niche of several adult tissues, including skin, muscle, colon, marrow [20–27], and to regulate maintenance of adult hippocampal and SVZ neural progenitors [18, 19]. Intriguingly, cells that respond to Wnt often express Wnt, raising the possibility that Wnt functions as an autocrine signal in these cells. Wnt is particularly well suited to autocrine signaling because it is highly lipophilic and remain primarily localized at the secreting cell membrane [28, 29]. Recently, such an autocrine Wnt signaling loop was shown to promote proliferation of breast cancer cells and [20, 26] and mesenchymal stem cells [30], though its specific function appears both cell and context dependent [31–34].
The possible role of autocrine Wnt signaling in adult neural progenitors is relatively unexplored. A few studies show that Wnt regulates adult neural progenitor development [18, 35–37]; however, none observed autocrine or within niche signaling. Lie et al. reported evidence that adult rat hippocampal progenitors (AHPs) express message for Wnts known to activate the canonical pathways, along with all the cellular machinery to respond to it; yet, they failed to detect autocrine activity among untreated AHPs [18]. We hypothesized that Wnt, a highly lipophilic secreted ligand, is a likely candidate for autocrine signal among NSCs because its range of action is often highly restricted, [28, 29] and has already demonstrated an autocrine activity in other cell types [38]. For example, an autocrine Wnt signaling loop preserves the self-renewal capacity of hematopoietic stem cells [27, 39, 40] and promotes the continued proliferation of poorly differentiated cancer cells, which share many properties with normal stem cells [41]. Moreover, mouse embryonic stem (ES) cells and ES-derived NSCs express an array of different Wnts and Wnt receptors, whereas antagonism of Wnt activity enhances neurogenesis, suggesting the presence of autocrine signaling [29, 42]. Recent studies demonstrate that canonical Wnt signaling directly regulates adult hippocampal progenitor [AHPs] development [18] and may explain the clinical efficacy of lithium [9]. Lie et al. found substantial baseline canonical activity in the dentate of Wnt (LEF/TCF-LacZ) reporter mice, in vivo, but not in proliferating AHPs in vitro, leading them to argue against the presence of an autocrine Wnt signaling loop in these progenitors. We undertook the current study to more carefully explore the possible roles of AHP derived Wnt.
Here, using a novel green fluorescent protein (GFP)-based assay, our data argue that previous failures to detect autocrine Wnt function reflect a limitation of the standard assay rather than an absence of biological activity in rat AHPs. Using clonal analysis, we further demonstrate that inhibiting autocrine Wnt signaling increases the number of neurons formed and leads to a loss of multipotency among AHPs. Finally, we show that a functionally similar autocrine loop is active in human neural progenitors (hNPs), emphasizing the relevance of these findings to human health and disease.
Experimental Procedures
Primary Cell Culture
Rat AHPS
Hippocampal progenitors from adult female Fisher 344 rats were prepared as previously described [1, 43] and maintained in minimal survival medium (MSM) which contained Dulbecco's modified Eagle's medium (DMEM):F12 (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) + N2 supplement (Invitrogen) + FGF2 (Peprotech, Rocky Hill, NJ, http://www.peprotech.com; 1 ng/ml), expanded in MSM supplemented with FGF2 (20 ng/ml), then differentiated by supplementation with 1% fetal calf serum (FCS; Hyclone, Logan, UT, http://www.hyclone.com) and either 500 ng/ml all trans-retinoic acid (Sigma) and/or FGF withdrawal, as noted.
Human NSCs
Human fetal neural progenitors were previously described [44, 45] and propagated in human NSC medium (HNM) consisting of Neurobasal A, BIT9500 (10%; Stem Cell Technology, Vancouver, CA), FGF2 (20 ng/ml), EGF (20 g/ml), LIF (10 ng/ml), heparin (2 μg/ml), and differentiated as above.
Human ES Cells
The HSF-1 (University of California, San Francisco) hES cell line was maintained on irradiated mouse embryonic fibroblasts (MEFs) and propagated as follows. MEFs were derived from 13-day embryos of CF-1 mice and dosed with 40 Gy of gamma irradiation. Irradiated MEFs were plated onto 0.1% gelatin coated six-well plates at 2 × 105 cells per well. hES culture medium consisted of DMEM/F12 (Invitrogen Corp.) supplemented with 20% knockout (KO) serum replacer, 1 mM l-glutamine, 0.1 mM β-mercaptoethanol, 1% nonessential amino acids, and 10 ng/ml FGF2. Cells were grown as clustered colonies in six-well plates and passaged after enzymatic treatment. Briefly, once the cultures reached confluence (∼4–5 days), they were washed once with phosphate-buffered saline (PBS) to remove residual culture media and incubated with collagenase IV (200 U/mL) and dispase (1 U/mL) in hES media for 15–20 minutes at 37°C. Cells were mechanically broken into small clumps (∼100–500 cells per clump), washed once with hES medium, and seeded at 1:5 to 1:10 dilutions (5 × 104 to 1 × 105 cells per cm2) on new 0.1% gelatin-coated culture dishes seeded with new irradiated MEFs. hES culture medium was changed daily to maintain undifferentiated state. For NSC generation, nearly confluent human ES cells (hESCs) were first split with trypsin 0.05%/0.5 mM ethylenediaminetetraacetic acid) and plated onto poly-ornithine/laminin coated glass coverslips at a density to yield approximately 100 NSC colonies per coverslip. These hESC-derived NSCs were differentiated by switching from HNM to medium consisting of neurobasal A, B27 (2×), heparin (2.5 μg/ml), FGF2 (5 ng/ml), all-trans retinoic acid (500 ng/ml). Cultures, regardless of type, were regularly checked for trypan blue exclusion before fixation and never did nonviable (i.e., non-excluding) cells exceed 1% of total cell number.
Immunochemistry
At the end of the appropriate differentiation-protocol, the wells were rinsed, fixed, and blocked as previously described [9], using the following primary antibodies mouse anti-Tuj1 (Mouse IgG2a 1:1,000; Covance, Princeton, NJ, http://www.covance.com), guinea pig anti-GFAP (1:1,000; Advanced Immunochemical), cleaved caspase-3 (17 kDa) rabbit polyclonal antibody (1:100; Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com), mouse anti-human Ki67 (1:150; BD), rabbit anti-Ki67 (1:2,000; Novocastra Ltd., Newcastle upon Tyne, U.K., http://www.novocastra.co.uk), rabbit anti-human nestin (1:1,000; Chemicon, Temecula, CA, http://www.chemicon.com), mouse anti-human nestin (1:500; Chemicon), mouse anti-rat nestin (1:500; Chemicon), Rip (1:100; DHSB), oligodendrocyte O4, (1:100; Chemicon). Primary antibody labeling was visualized using anti-species specific secondary antibodies conjugated to either Alexa-350, Alexa-488 Alexa-594, or Alexa-633 (Invitrogen), as indicated.
Viral Preparation and Infection
Retroviral vectors were transfected into HEK293-based packaging cell line 293GPG (gift from Dr. R. Mulligan) in the absence of tetracycline to produce active VSV-G pseudotyped MoMLV retrovirus and after 48 hours the medium was completely changed. Lentivirus was produced by triple transfection of HEK293T cells with plasmids CMVdR8.7 (packaging), pMDG2 (VSV-G envelope), and the viral vector (e.g., pRRL-cPPT-TOP-GFP-WPRE). In both cases, 24–48 hours after either transfection (lentivirus) or tetracycline induction (retrovirus) were collected supplemented with polybrene (4 μg/ml; Sigma), spun for 3 minutes at 3,000g, and filtered through 0.45 μm sterile syringe filter to remove floating 293 cells or debris. Clarified supernatants were concentrated by ultracentrifugation at 50,000g × 90 minutes at 4°C and then resuspended in Optimem (Invitrogen) supplemented with polybrene (Sigma) 4–8 μg/ml. Final viral titers ≥1 × 108 ml−1 were obtained consistently. AHPs were infected on day 0, then after 3 days in vitro (DIV 3), sufficient time to allow for complete reverse transcription, viral integration, and transgene expression. AHPs were pulsed with BrdU, lysed for subsequent protein analysis, or allowed to differentiate. The membrane and intracellular domain of Xenopus N-Cadherin [46] was PCR amplified as EcoR1/Sal1, then shuttled into EcoR1/Xho1 sites of pWZL-I-Blast. The Fz8CRD-Fc was PCR amplified as Sfi1 fragment and cloned into retrovirus pRCIPG-ccdB.
Proliferation Assays
Cultures were pulsed with BrdU (2.5 μM) for 4 hours (Rat AHPs) or 12 hours (Human ESCs and human NPs). Cells were fixed in paraformaldehyde (4%) then denatured with 100 Kunitz units of DNase1 (Sigma D-5025; Bovine Pancreas) in TRIS-buffered saline + 1 mM MgCl2 + 1 mM β-mercaptoethanol pH 8.0 for 30 minutes at 37°C, to make the BrdU epitope more accessible to antibody labeling. BrdU was detected with anti-BrdU antibody (1:250; Accurate).
Clonal Analysis
Monolayer cultures of AHPs were infected with retrovirus containing either Axin-MSCV-IRES-GFP or iCad-WZL-IRES-Blast, replated after 72 hours, and allowed to proliferate for 1–4 weeks in MSM. After allowing proliferation, cells were replated at clonal density [47, 48] and allowed to differentiate for 1 weeks then fixed at −20°C for 30 minutes in ethanol, washed to remove residual GFP, and refixed in paraformaldehyde 2% for 15 minutes at room temperature, then analyzed by standard immunofluorescent techniques as described [9]. Each clone was scored for total number of young neurons (Tuj1), glia (GFAP), and total number of cells (DAPI). Fewer than 1% of cells were oligodendrocytes (RIP or O4) to visualize O4 or Rip, cell surface epitopes were preserved by labeling cells with primary antibody for 30 minuets at room temperature before fixation with acid–ethanol.
Expression Analysis
RNA was extracted from cultured AHPs (RNAeasy, Qiagen, Hilden, Germany, http://www1.qiagen.com) and cDNA was synthesized by reverse transcription (Superscript II First strand Kit; Invitrogen) according to the manufacturers instructions. Gene-specific PCR primers were designed for mouse Wnt homologs, and specificity was tested using plasmid cDNA clones (gifts as footnoted later) and validated for use with rat using cDNA pooled from Fisher 344 E10, E14 whole embryos and E18, P1 brains. RT-PCR was performed using platinum-Taq (Invitrogen) by standard methods. The number of cycles (i.e., end-point) for each primer pair was chosen to produce submaximal product (i.e., subsaturating reaction conditions).
Microarray Analysis
RNA was harvested as above from AHPs treated with Fz8CRD for 24 hours and untreated controls were collected in three separate experiments, then pooled by group (Fz8CRD vs. Control). RNA concentrations were determined by spectrophotometry (Nanodrop; A260/280-1.8) and RNA quality was further evaluated using an Agilent Bio-Analyzer. Two hundred nanogram total RNA was then amplified, labeled, and hybridized to RatRef-12 Expression BeadChip (Illumina, San Diego, CA) by the UCLA microarray core. Human progenitor RNA was analyzed using HumanRef-8 Expression BeadChip (Illumina). Ontology analysis of genes showing greater than 1.5-fold (1,975 genes) or twofold (1,018 genes) expression differences between Fz8CRD versus control, using DAVID software http://david.abcc.ncifcrf.gov. Significance of overrepresentation was adjusted for multiple comparisons using the conservative Benjamini-Hockberg correction. All level 3&4 Gene Ontology categories that remained significantly overrepresented (p < .01) were reported.
Luciferase Assays Using TOPFLASH and FOPFLASH
These were performed according to standard protocols [49]. Briefly, adult NSCs (used as reporters) were plated at 50,000 cells per well in 24-well plates and transfected with pTOPFLASH or pFOPFLASH in combination with other plasmids, such as hDkk-1 as indicated, using Lipofectamine 2000 (Invitrogen). Wnt3a or control medium was added following transfection for 48 hours before harvesting the cells in lysis buffer containing 100 mM potassium phosphate buffer (pH 7.5), 1 mM DTT, and 1% Triton X-100, then cells were frozen at −80°C. Lysates were assayed in buffer conatining 25 mM glycylglycine, 15 mM magnesium sulfate (pH 7.8), 1 mg/ml bovine serum albumin, 5 mM ATP, and 0.4 mM luciferin, using a Turner TD-20/20 Luminometer.
OTP-GFP Reporter Assays
HEK293 were infected with a lentivirus containing an enhanced TOP promoter (OTP) driving eGFP expression (OTG293 cells) [27, 46]. As GFP has a half-life of >24 hours [50, 51] accumulated GFP fluorescence should integrate Wnt signaling over a period of hours to days. To validate this new assay system, we plated at 25,000 OTG293 cells per 24-well plate. When these reached 75% confluence growth was supplemented 1:1 either Wnt-conditioned medium from LWnt3A cells (ATCC#CRL-2647) or control medium from native L9 cells medium, then resupplemented every other day for 7 days, sufficient time for Wnt induced eGFP expression to reach 95% of steady state. We found that Wnt3a, but not control conditioned medium, substantially raised GFP expression in OTG293 cells. These data show that OTG293 cells can function as Wnt sensors. To further validate this assay, we evaluated the dose-response relationship using purified Wnt3a (0.1–200 ng/ml; R&D Systems Inc., Minneapolis, http://www.rndsystems.com). This revealed an EC-5,50,95 of 1,20,100 ng/ml, respectively (data not shown).
In later experiments, OTG293 cells were similarly plated and allowed to reach 75% confluence. At this time, L9 fibroblasts (negative control), L9-Wnt3a fibroblasts (positive control), or AHPs were cocultured on top of the OTG293 cells for 7 days. Fields of cells were imaged and analyzed with Scion Image or Metamorph.
Wnt Quantification (DALIA: Dual Affinity Ligand ImmunoAssay)
Capture plates were prepared by adsorbing heparin (100 μg/ml; Sigma) onto Falcon Primaria 96-well tissue culture plates overnight. After rinsing, plates were incubated with AHP-CM or purified Wnt3a for 2 hours at room temperature. Medium was removed and rinsed twice with Dulbecco's Phosphate Buffered Saline (DPBS), then the plates were incubated for 2 hours at room temperature with FZ8CRD-Fc or FZ8CRD-fc + FZ5CRD-fc (1–100 ng/ml; R&D Systems). After rinsing with DPBS, FZ8CRD was detected with HRP-conjugated anti-human secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) and colorometric development, as described earlier for ELISA assays. The detection sensitivity was observed to be <10 pg/ml of commercially purified Wnt3a.
Partially Purified and Conditioned Wnt Medium
Partially Purified medium (PPM) was prepared according to Castelo-Branco et al. [52], from Rb1a cells infected with pLNCX-Wnt3,3a,5a,7a-HA, infected with RCIPG-Fz8CRDFc or transiently transected with pSPORT-Wnt8b (gift of Jahn Mason) in DMEM/F12 ± heparin (2 g/ml), B27 (Invitrogen), bovine serum albumin (Fraction V; 10%), and Defined Lipid Concentrate (1×). In some experiments, conditioned medium was depleted of bulk proteins (BSA, transferrin), and Wnt concentrated by methods adapted from [28, 53]. Briefly, CMs were mixed 1:1 with CHAPS (2%, Sigma) then run over a 1 ml Hi-TRAP heparin column (GE Healthcare), washed with PBS + 1% CHAPS then eluted in a single step with NaCl 1 M + PBS/CHAPS 1%. The eluate was desalted and concentrated 100-fold using centrifugal concentrator (Amicon 30,000 mw) and medium consisting of PBS/BSA (100 μg/ml)/CHAPS (1%), stored at 4°C, then rediluted 1:100 immediately before use. Conditioned Wnt Media (CM): Control CM and Wnt3a CM were collected from Wnt3aL9 and L9-Wnt3 fibroblasts (gift of Roel Nusse) by previously published methods [54], except cells were grown in DMEM:F12 (1:1) + 5% heat inactivated FCS + Heparin (2 μg/ml). Dkk-1-CM was collected from L9 cells transfected with pcDNA3-Dkk-1. PPMs and CMs were applied to AHPs diluted 1:1 in MSM, unless otherwise noted. Commercially purified Wnt3a, Fz8CRD-Fc, and Dkk-1 were obtained from R&D Systems. In experiments using purified Fz8CRD-Fc (R&D Systems), Fz8CRD-Fc was added at 5 μg/ml every 24 hours. Similarly, recombinant human Wnt 1 (Peprotech) and Wnt3a (R&D Systems) were added to media at 100 ng/ml and replenished every 24 hours. All Wnts were maintained as stock supplemented with BSA (Fraction V, Sigma) as a carrier.
All experiments contained herein were performed according to all appropriate guidelines governing use of recombinant DNA, animal welfare, and use of human embryonic stem cells, set forth by the National Institutes of Health and the author's institution where experiments were performed, either UCLA or Stanford University, respectively.
Results
We first sought to replicate the previous findings of Lie et al. [18] using independently generated AHP lines. Under maintenance conditions, these progenitors constitute a uniform appearing population (Fig. 1A) where nearly all cells are actively proliferating (i.e., nearly 100% traverse S-phase during a 24 hours window), as assessed by BrdU incorporation, and less than 0.1% express neuronal or glial lineage-specific markers (Tuj1, MAP2, GFAP, S100b, O4, RIP, OX-42/CD11b). Using these AHP lines, we employed standard non-saturating RT-PCR to assess whether they possessed necessary components to generate an active canonical autocrine loop. We observed that AHPs expressed mRNA for a remarkable variety of Wnts and Wnt receptors (Fig. 1B), consistent with published in vivo adult dentate expression [55, 56].
Figure 1.
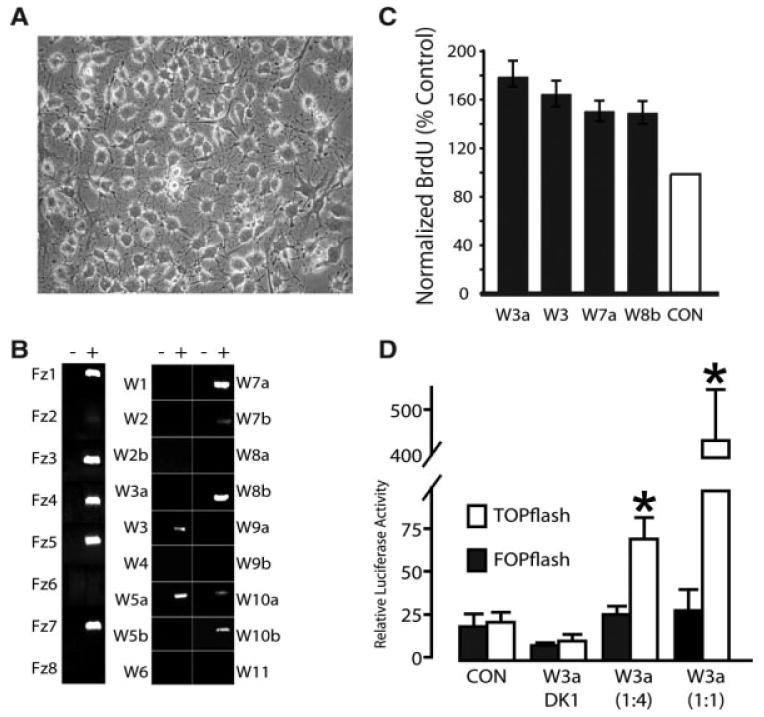
Novel canonical assay detects baseline Wnt activity. (A): Phase contrast micrographs of adult hippocampal progenitors (AHPs) plated a density of 104 per cm2 into expansion medium. (B): RT-PCR confirms Wnt and Frizzled expression in proliferating AHPs. PCR analysis of Wnts and Frizzleds in AHPs (+) RT, (−) RT control. cDNA pooled from Fisher 344 E10, E14 whole embryos and E18, P1 brains was used for positive controls (not shown). (C): Native Wnts enhance AHP proliferation. Partially purified Wnt 3a,3,7a and 8b were applied to AHP for 48 hours, pulsed with BrdU for 2 hours then fixed and proliferation assessed. Bars represent number of Wnt treated cells that incorporated BrdU (i.e., traversing s-phase of the cell cycle), expressed as percent of untreated also incorporating BrdU (mean ± s.e.m.). Control bar (100% by definition) is included as a visual reference. (D): TOPflash Luciferase assay of Wnt-induced LEF/TCF activation fails to detect baseline activity. Wnt3a conditioned medium (W3a), applied to AHPs at dilutions shown, significantly enhances activity of the TOPFLASH (open bar) luciferase reporter containing functional TCF/LEF-1-binding motifs, whereas it does not activate FOPFLASH reporter (closed bar), containing mutant binding sites. Similarly, Dickkopf-1 conditioned medium (DK1) inhibits Wnt3a-dependent canonical signaling activity (i.e., no significant difference in activation between DK1 + W3a and control). Under nonstimulated conditions the TOP and FOP reporters are equally active, revealing no baseline canonical activity. Abbreviations: BrdU, 5-bromo-2-deoxyuridine; CON, control.
We functionally evaluated a selection of these expressed Wnts by applying partially purified Wnt 7a, Wnt8b, Wnt3, and Wnt3a (200 ng/ml) to AHP cultures for 48 hours and measured their effects on proliferation using the standard BrdU incorporation assay. Wnt7a and Wnt8b, like Wnt3/3a [18] significantly increased AHP proliferation (Fig. 1C). Together these expression and proliferation studies demonstrate the functionality of the Wnt signaling machinery present in vitro and in vivo.
As these AHPs are capable of responding to high concentrations of the same Wnts they natively produce we sought to determine there was any detectable canonical Wnt activity at baseline. To assay this we compared luciferase activity in AHPS transfected with TOPflash, a LEF/TCF reporter of canonical activity, versus FOPflash, the nearly identical reporter in which the LEF/TCF recognitions sequences have been scrambled to prevent transcription factor binding (Fig. 1D). Exogenous Wnt3a (positive control) stimulated reporter activity; yet, we did not observe any canonical activity in unstimulated AHPS (i.e., TOPflash and FOPflash activity were similar at baseline), consistent with previous reports [18].
Autonomous Wnt Signaling Among Neural Progenitors
Our data demonstrate that AHPs can respond to the same canonical Wnts that they express, but the most widely accepted assay fails to detect such activity, at baseline. This leaves open the possibility that these Wnts may still form an autocrine, or within-niche loop, but signal via, as yet undefined, noncanonical pathways. If an active within-niche Wnt loop exists among AHPs then inhibiting Wnt signaling should change patterns of gene expression, independent of which Wnt pathways are active. As AHPs potentially secrete such a variety of different Wnts, we inhibited endogenous Wnt signaling by extracellularly applying a broadly efficacious antagonist, Fz8CRD-Fc [27, 57]. This widely used synthetic Wnt antagonist is a fusion of the Frizzled8-CRD (Wnt binding) domain and the Fc region of human immunoglobulin. After applying this broad-specificity Wnt antagonist for 24 hours, we assayed gene expression using rat-specific oligonucleotide arrays and compared it with the resting profile of gene expression in the absence of Wnt blockade. We performed an ontology analysis of the 1,018 most changed genes (more than or equal to twofold change) as a means of identifying possible roles for autocrine Wnt production (supporting information Table 1). This unbiased, exploratory genome-wide analysis revealed that Wnt inhibition upregulated genes involved in neuronal differentiation and downregulated those that maintain progenitors in the cell cycle. Surprisingly, the observed pattern is most consistent with the presence of an active canonical, rather than noncanonical signaling. Specifically, these data provide circumstantial support for the existence of an autocrine canonical loop within the AHP niche that maintains undifferentiated AHPs in the cell cycle.
So far, we had observed that inhibiting endogenous Wnt signaling produced a gene expression pattern that is consistent with active canonical signaling; yet, none was detected by the standard TOP/FOPflash assay. To address this apparent paradox, we hypothesized that Wnt is only secreted by AHPs during specific phases of the cell cycle. As a result, autocrine signaling among an unsynchronized population of AHPs induces low levels of canonical activation that are undetectable by the TOPflash assay. To test this model, we developed a cell-based reporter system capable of reading-out a summation of canonical activity over a protracted time-course (i.e., longer than the average length of the cell cycle in AHPs). We generated a reporter cell line (OTG293) that stably expresses an enhanced TOP promoter (OTP) driving eGFP, which has a half-life measured in days, rather than hours (luciferase).
We cultured progenitors directly onto OTG293 reporter, in NSC medium, cells for 7 days to test whether AHP produced active Wnt. We observed that the AHP-induced fluorescence was twofold higher than when OTG cells are cocultured with native fibroblasts (i.e., negative control), but less than half the fluorescence induced by Wnt-3a over-expressing fibroblasts (Fig. 2A–2D). Using a novel ELISA-like, dual affinity ligand assay (DALIA: see Experimental Procedures Section) that has a detection sensitivity of ≤200 pg/ml we were unable to detect the presence of Wnt in serum-free AHP conditioned medium. Similarly, daily application of serum-free AHP conditioned medium to OTP-GFP reporter cell did not induce detectable Wnt activity (data not shown), suggesting that AHP derived Wnt is not widely diffusible under out culture conditions. These data demonstrate that AHPs secrete Wnt that is active close to the secreting cell, lending further support to our hypothesis that Wnt is an autocrine signal.
Figure 2.
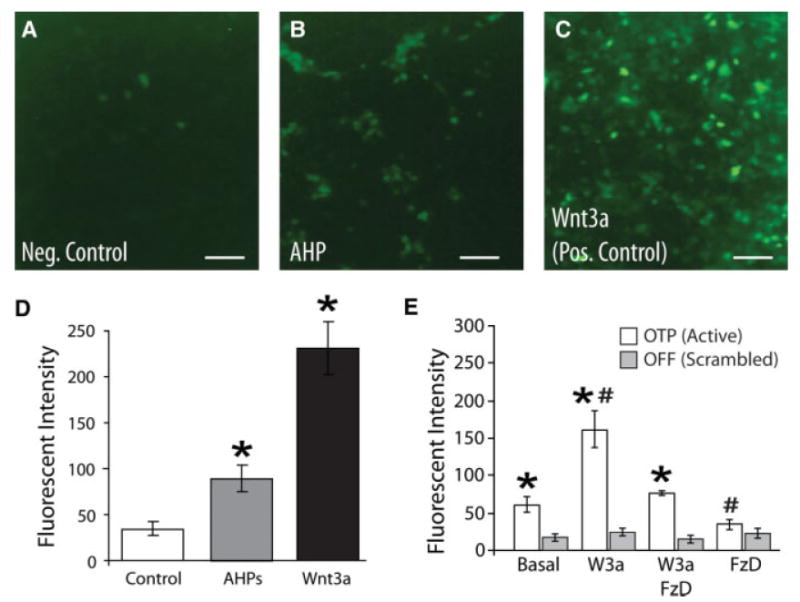
Cell autonomous Wnt signaling in AHPs. (A–D): OTP-GFP assay detects AHP secreted Wnt. HEK293 cells stably expressing LEF/TCF-GFP Wnt reporter (OTG293) were generated as described in Experimental Procedures Section. Either naïve fibroblasts as a negative control (A: Control), AHPs (B: AHP), or fibroblasts engineered to overexpress Wnt3a as positive controls, (C: Wnt-3a) were plated onto established monolayers of OTG reporter cells and the overall green fluorescent protein (GFP) fluorescence measured at 7 days in vitro (DIV). (Insets) Fluorescent micrographs of LEF/TCF-GFP Wnt reporter cells imaged using equal camera exposures (4 seconds). As summarized (D), Wnt3a production by L9-Wnt3a cells induces a substantial increase in GFP fluorescence among OTG293 cells, whereas native L9 cells do not produce any significant increase in GFP expression, demonstrating the sensitivity and specificity of this assay. As summarized, AHPs cocultured on OTG293 cells induce a significant, but submaximal increase in GFP expression, demonstrating that AHPs secrete active Wnt. (E): Basal AHP Wnt activity. AHPs were lentivirally transduced with either the OTP-GFP Wnt reporter (open bar) or the OFF-GFP negative control (gray bar) that contains nonfunctional LEF binding sites (similar to FOPflash above) and the fluorescence measured after 7 DIV. These AHPs were cultured in either control medium (base) or in the presence of the Wnt antagonist Fz8CRD (1 μg/ml), Wnt3a (300 ng/ml), or a combination of the two. At baseline, AHPs exhibit significant canonical activity, whereas application of saturating recombinant Wnt3a (for 7 days) induced a larger, significant increase in reporter activity. In contrast, application of Fz8CRD effectively blocked both endogenous (i.e., baseline) and exogenous (i.e., bath applied) Wnt activity. Together, these data indicate the presence of baseline canonical Wnt activity and validate the specificity of the antagonist. Mean ± s.e.m.; (*OTP vs. OFF activity, #Drug vs. Baseline OTP activity, p < .05; ANOVA with Tukey post hoc test). Abbreviations: AHPs, adult hippocampal progenitors.
To verify that Fz8CRD-Fc was specifically revealing active baseline canonical signaling, we lentivirally transduced AHPs with the fluorescent canonical reporter OTP-GFP [27] or scrambled binding site control, OFF-GFP. We observed that application of Fz8CRD-Fc to OTP-GFP infected AHPs reduced measured Wnt activity by nearly half (Fig. 2E) to the level seen in OFF-GFP transduced cells. In contrast, AHPs at baseline, displayed a twofold to threefold greater fluorescence than either similarly infected fibroblasts or AHPs infected with OFF-GFP. Together, these experiments indicate the presence of baseline canonical Wnt activity and validate the specificity of the antagonist.
Autocrine Wnt Function
We explored the functional role for the AHP-derived Wnt effects by blocking basal autocrine/paracrine Wnt activity. We found that application of the antagonist Fz8CRD-Fc to proliferating AHPs, produced a small, but statistically significant reduction in BrdU labeling, yet significantly increased the total number of neurons formed (Fig. 3). These data suggest that proliferating AHPs exhibit constitutive, low-level canonical Wnt activity that represses neuronal differentiation. To test this hypothesis, we sought to blunt β-catenin accumulation, a key step in canonical signaling. We retrovirally transduced proliferating AHPs with an intracellular fragment of N-cadherin (iCad) that contains the β-catenin binding domain [46] [58] and resistance to the antibiotic blasticidin (blast). Overexpression of this cadherin fragment functions like a buffer to sequester fluxes of β-catenin, thereby antagonizing β-catenin. We investigated the effect of antagonizing β-catenin by plating these infected AHPs as adherent monolayers at clonal density and then differentiating them. We observed that iCad transduction significantly reduced the size of colonies and reduced by half (Fig. 4A), the fraction of colonies that contained both neurons and glia (Fig. 4B), suggesting a loss of multipotent precursors.
Figure 3.
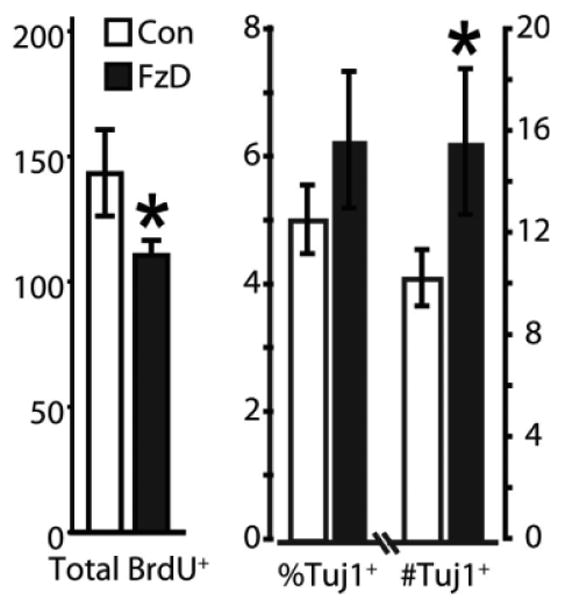
Wnt antagonism increase neuron formation. Fz8CRD (1 μg/ml) applied to adult hippocampal progenitors (AHPs) to inhibit baseline Wnt signaling caused a modest, yet significant reduction in proliferation, as measured by BrdU incorporation (BrdU+ per mm2). In contrast, Fz8CRD produced a significant increase in the number of neurons formed and trend toward an increase in the fraction of AHPs becoming neurons. Values are mean ± s.e.m.; Student's t test, *p < .05. Abbreviation: BrdU, 5-bromo-2-deoxyuridine.
Figure 4.

Sequestering β-catenin reduces adult hippocampal progenitor (AHP) multipotency. AHPs were retrovirally transduced with a truncated N-cadherin (iCad) that possesses an intact β-catenin binding domain, thereby sequestering β-catenin (i.e., dominant negative β-catenin). Transduced AHPs proliferated for 3 weeks postinfection then were replated at clonal density, differentiated, and scored for total number of young neurons (Tuj1), glia (glial fibrillary acidic protein), and total number of cells (4′,6-diamidino-2-phenylindole). Overexpression of iCad significantly decreased both the size of each clone and the number of colonies formed by multipotent progenitors (i.e., most clones contained a single cells type). Mean ± s.e.m.; (*p < .05; ANOVA with Tukey post hoc test).
We interpret these data to show that loss of β-catenin/canonical activity reduces the proportion of proliferating AHPs that remain multipotent. However, an alternative explanation is that our construct, functioning as a dominant negative cadherin, reduced potency by interfering with intercellular cadherin signaling, independent of β-catenin [58, 59]. To address this possibility, we used a similar experimental design and retrovirally transduced AHPs with axin, an inhibitory component of the canonical pathway that is upstream of β-catenin and uninvolved with cadherin signaling. Therefore, overexpression of axin should prevent canonical activation, independent of the presence of extracellular Wnt. We observed that over-expressing axin in proliferating rat AHPS significantly increases the fraction that differentiate into neurons (Fig. 5A), similar to what we observed when we blocked Wnt extracellularly (Fig. 3A). When analyzed on a clone-by-clone basis, axin overexpression for either 1 or 4 weeks before differentiation, equally reduced the size of colonies formed (Fig. 5B), but did not affect AHPs ability to form neurospheres. Moreover, over-expression for 4 weeks, but not 1 week, before differentiation significantly reduced the fraction of clones that arose from a multipotent progenitor (Fig. 5C). The data demonstrate that inhibition of the canonical Wnt pathways depletes multipotent progenitors from the AHP population.
Figure 5.
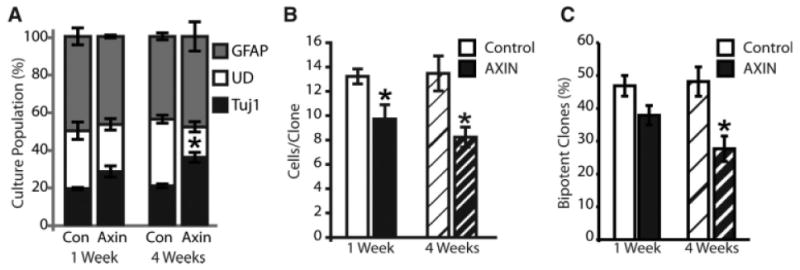
Loss of canonical signaling diminishes the multipotent progenitor pool. AHPs were transduced with Axin to specifically interfere with intracellular canonical signaling. Transduced cells were proliferated for 1 or 4 weeks, differentiated and labeled, and individually scored for expression of either Tuj1 (immature neurons) or GFAP (glia). (A): Bulk properties of axin-transduced AHPs. Axin significantly increased the fraction of progenitors differentiating into neuron (Tuj1; black bars) without affecting the glial (GFAP; gray bars) fraction, and decreased the fraction remaining UD (open bars). (B, C): Clonal analysis. Axin overexpression equally reduced colony size at 1 (plain bars) and 4 weeks (hatched bars), but significantly reduced multipotency after 4 weeks of proliferation, only. Values are means ± s.e.m., asterisks indicate significant differences from control at p ≤ .05 by two way ANOVA with Tukey post hoc test. Abbreviations: GFAP, glial fibrillary acidic protein; UD, undifferentiated.
Autocrine Wnt Signaling Across Species
Our data demonstrate that interruption of an endogenous Wnt signaling loop in rat AHPs slowly reduces the population of multipotent progenitors. This finding is consistent with previous reports that Wnt inhibition preferentially induces neuronal differentiation of mouse embryonic stem cells [31, 33]. We investigated whether this autocrine loop that is present in adult rat neural progenitors and mouse progenitors [31, 33, 58] is a general feature across species, and thus functionally relevant to human neurogenesis. First, we explored this question using hESCs. We propagated HSF-1 cells, a 46XX hESC line, then allowed them to differentiate in the presence of Fz8CRD-Fc, Wnt1, or control NSC medium for 8 days. This relatively brief time-course analysis produced Tuj1+ neurons at various stages of development in all three conditions. These Tuj1+ cells exhibited morphologies ranging from the least mature with a glial-like appearance to the more mature neurons with small round soma and extended processes (Fig. 6). We found that inhibiting Wnt with Fz8CRD increased both the total number of Tuj1+ cells per colony, the number of more mature, process bearing neurons and most prominently, increased the number of colonies that contained these more mature cell types. In contrast, Wnt activation with recombinant Wnt1 significantly repressed neuronal differentiation. These data suggest that Wnt expands the pool of uncommitted progenitors at the expanse of neuronal differentiation.
Figure 6.
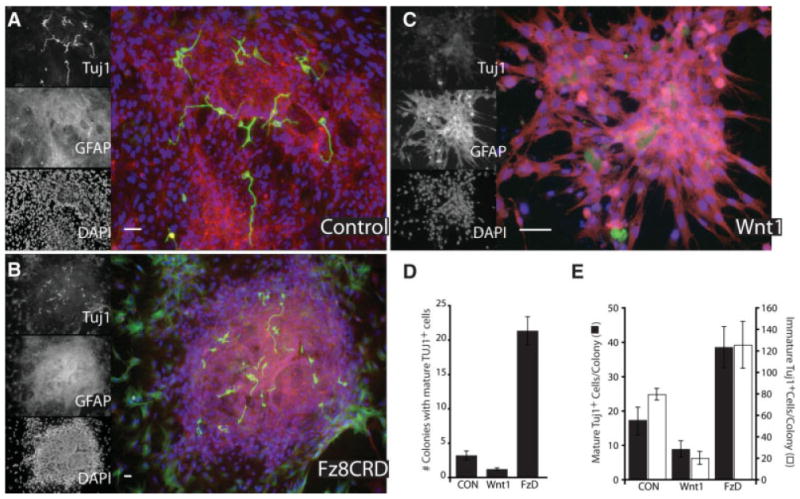
Wnt antagonism promotes neuronal fate among human embryonic stem cells. Human embryonic stem (HSF-1) cells were treated with Wnt-1 (200 ng/ml), Fz8CRD (5 μg/ml), or control and then labeled for immature neurons (Tuj1; green), glia (GFAP; red), or nuclei (DAPI; blue). (A–C): Fluorescent micrographs of an individual differentiated colony, illustrating a reduced number of mature (process bearing) neurons in Wnt1 treated cultures and increased neuron production with Wnt antagonism (Fz8CRD). Scale bars = 30 μm (A), 50 μm (B), and 20 μm (C). (D, E): Distributions of more or less mature young neurons (Tuj1+) among differentiated colonies demonstrate that Wnt antagonism significantly increased the number of colonies that produced more mature neurons (D) and increased the overall number of both mature and immature neurons per colony (E), whereas Wnt1 had an opposite effect. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein.
Our findings evince the presence of an autocrine-signaling loop in early hNPs. However, because we used hES cells, it is formally possible that Wnt inhibition was acting on a more primitive ectodermal precursor. Therefore, we validated these findings using defined multipotent hNPs. As with our initial experiments with rat AHPs, we first examined baseline gene expression to verify that they express Wnt and all the signaling components necessary to form an autocrine loop. Microarray-based expression profiling of three separately derived hNP lines (i.e., three different individuals) reveals that these precursors consistently express Wnt3 and Wnt 7a, whereas hNPs expresses Wnt 5b I lieu of Wnt8b (data not shown). Next, using our standard in vitro paradigm, we applied Fz8CRD or Wnt1 to adherent monolayer cultures of hNPs for 48 hours, briefly pulsed (4 hours) with BrdU, and then continued culturing the hNPs in the presence of Fz8CRD or Wnt1 for 3 weeks while allowing cells to differentiate. We observed that Wnt inhibition significantly increased the number of neurons formed (Fig. 7B) without changing the mitotic cell fraction, whereas Wnt1 more than doubled the mitotic index (Fig. 7A). In contrast, strong canonical activation with recombinant Wnt1 significantly increased the number of GFAP positive cells, whereas concomitantly decreasing the number of neurons formed, likely representing an expansion of the pool of undifferentiated GFAP-positive stem cells [60, 61].
Figure 7.
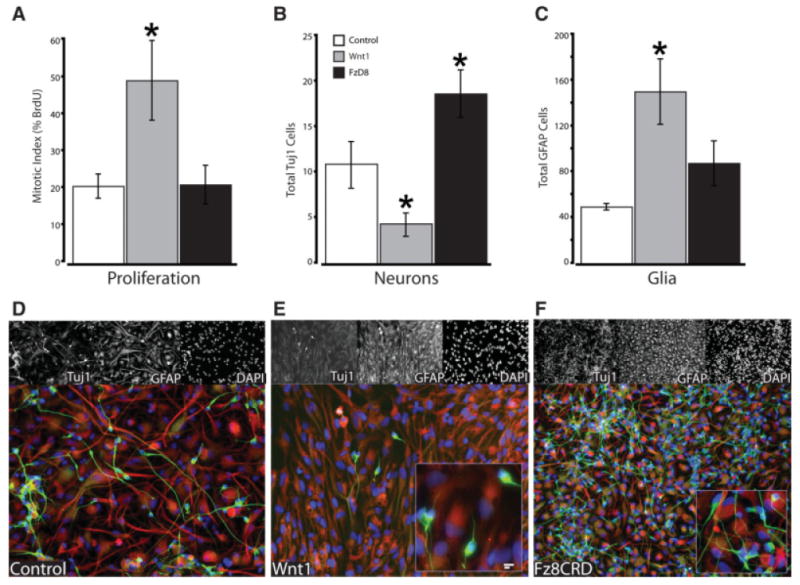
Autocrine signaling in human neural progenitors (hNPs) represses differentiation. (A): hNPs were cultured in presence of either Wnt1 or the antagonist Fz8CRD for 48 hours then pulsed with BrdU for 12 hours. Summary of BrdU labeling of AHPs showing that Wnt1 activation significantly increased proliferation, whereas antagonism had no effect. (B–F): hNPs were differentiated in presence of Wnt1 or the antagonist Fz8CRD for 1 month then scored for neuronal (Tuj1) and glia cell fate (GFAP). (D–F): Representative micrograph of hNPs differentiated under control conditions (D, ×20), Wnt1 (E, ×20; inset ×40), or Fz8CRD (F, ×10; inset ×40). Wnt antagonism only modestly increased glia (C, F), but significantly increased neuron formation (B, F). In contrast, Wnt1 significantly increased glia formation (C) and significantly decreased neuron formation (B). Often, the neurons formed exhibited a less mature appearance with fewer processes (E, inset) than in control or when Wnt was blocked (F, inset). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein.
Discussion
We investigated the hypothesis that within-niche Wnt signaling regulates the differentiation and proliferation of rodent and hNPs. Using a novel reporter system, we detected significant, but subsaturating levels of secreted Wnt activity among cultured AHPs, and the presence of baseline canonical signaling in the AHPs themselves. Based on previous observations that Wnt-3/3a are neurogenic [18, 35], one might have reasonably predicted that AHP-derived Wnt would act similarly and form the basis of an autocrine positive feedback loop that enhances the production of new neurons. However, we observed just the opposite. Using both Wnt agonists and antagonists, antagonizing endogenous Wnt signaling consistently enhanced neuron formation. This effect was independent of species, developmental age of precursors, or method of antagonizing Wnt, but also independent of proliferation. These data support a model in which within-niche, autocrine Wnt signaling helps maintain the stem cell character of AHPs by concomitant enhancement of LEF/TCF-dependent cell-cycle reentry and repression of differentiation.
This study demonstrates that low-level autocrine Wnt signaling preserves the multipotency of rodent and hNPs. However, among the interesting questions raised by this study, several deserve further discussion: (a) Why did previous reports fail to detect autocrine Wnt signaling among adult neural progenitors? (b) What is the native role of neural progenitor-derived Wnt signaling versus Wnt derived from mature glia or neurons? (c) Why should different canonical Wnts (i.e., Wnt1 and Wnt3/3a) have such divergent effects and how can both Wnt agonism and antagonism enhance neurogenesis (e.g., Wnt3a vs. Fz8CRD)?
Detecting Basal Canonical Wnt Signaling
We found that adult rat hippocampal progenitors in vitro, express a wide complement of Wnts/frizzled receptors. Although these Wnts should activate canonical signaling [62–64], we were unable to detect baseline activity using the widely accepted TOP/FOPflash assay, consistent with previously reports [18]. We hypothesized hat the standard TOPflash assay has two inherent shortcomings: (a) it is insensitive to small, but persistent levels of canonical activation and (b) poorly suited to detecting cyclic changes of canonical activation among an unsynchronized population of AHPs. These problems arise because the TOPflash assay relies on expression of luciferase, which has a relatively short half-life (i.e., less than the AHPs' cell cycle duration, approximately 2 hours). When canonical activity is actively downregulated during certain phases of the cell cycle, only a fraction of asynchronously proliferating AHPs will exhibit significant canonical activity at baseline. This is a notable limitation because such cyclic canonical pathway activity has been proven essential for proper development of other types of dividing progenitors [65–67]. To circumvent these limitations, we developed an improved assay based on coculturing AHPs with a reporter cell line that is capable of integrating and reporting canonical activity over a much longer time window. Using this system, we were able to detect significant, but subsaturating levels of Wnt activity among cultured AHPs, and the presence of baseline canonical signaling in the AHPs themselves.
Autocrine Wnt Preserves Mulitpotency
We explored the functional role of autocrine/paracrine Wnt signaling by inhibiting this activity in otherwise normal progenitors. These progenitors express such a variety of potentially redundant Wnts that many common approaches like knockouts or even RNA interference are impractical. We circumvented this difficulty by inhibiting endogenous Wnt signaling with a broadly specific extracellular Wnt antagonist Fz8CRD. Although effective, this approach presents two important interpretational confounds. First, different Wnts can potentially transduce multiple (i.e., noncanonical) pathways; inhibiting all these Wnts with Fz8CRD could have produced an effect independent of the canonical pathway. Second, the observed effects may be paracrine, rather than autocrine (i.e., Wnt released from partially differentiated neighbors in the culture dish). Paracrine effects are less likely because (a) intracellular antagonism of canonical signaling produced similar neurogenic effects to extracellular Wnt blockade. (b) AHP pools are homogeneous with few nondifferentiated progenitors, at baseline. (c) Diffusion of Wnt in culture medium is generally very limited [28, 29]. Furthermore, in our NPC culture systems, conditioned medium from neural precursors fails to activate Wnt–β-catenin signaling activity as detected by Wnt/β-catenin luciferase or GFP reporter assays.
We targeted two key components of canonical signaling, β-catenin and axin, a means of specifically, intracellularly antagonizing this pathway. We blocked β-catenin signaling via overexpression of the N-cadherin intracellular domain (iCad). This fragment contains a β-catenin binding domain and thus buffers rises in intracellular β-catenin; however, it also functions as a dominant-negative regulator of intercellular cadherin signaling [58, 59]. Therefore, we must consider the possibility that loss of cadherin signaling might ultimately be responsible for the observed burnout (i.e., loss of potency) of AHPs. However, loss of cadherin signaling should not compromise the interpretation of our clonal analysis because (a) previous reports that overexpression of both full length and truncated cadherin have similar effects on cell fate among embryonic neural progenitors, independent of cell–cell adhesion [58], and (b) overexpression of axin, another Wnt antagonist that is independent of cadherin, produced the same neurogenic effect.
How does autocrine Wnt signaling preserve multipotency? Autocrine canonical (i.e., β-catenin-LEF/TCF) Wnt signaling may directly or indirectly maintain neural precursors in their slowly proliferative, multipotential state, just as it does in maintaining the pluripotency of ES cells [31]. First, because dividing neural precursors tend to remain undifferentiated; elevated β-catenin-LEF/TCF signaling could indirectly block differentiation by encouraging AHPs to re-enter into the cell cycle (e.g., in G0 phase), as suggested by our microarray expression data. Second, autocrine canonical Wnt may be signaling through β-catenin binding to HMG-box containing transcription factors other than LEF/TCF. For example, β-catenin binds to SOXB1 transcription factors like Sox2 [68, 69] and overexpression of Sox2 inhibits neuronal differentiation and maintains NSC character [70]. This suggests a model in which low-level, autocrine Wnt signaling helps maintain the stem cell character of AHPs by simultaneously enhancing LEF/TCF-dependent proliferation and repressing differentiation via SOX activation.
Wnt Signaling in Neurogenesis
Wnt1, 3, 3a, 7a, and 8b are often grouped together on the basis of their ability to activate the canonical pathway and transform mammary cells [63]; however, this system inadequately reflects their biology in AHPs. Although each Wnt exhibits a general preference for one specific signaling pathway, it is the receptor complement that actually determines which second messenger cascade(s) is activated. For example, Wnt5a can activate opposing canonical and noncanonical pathways in the same cells, depending on whether the coreceptor ROR2 is expressed [71]. Moreover, it is well documented that different “canonical” Wnts exert divergent neurogenic effects [52, 72].
These data suggest a model of Wnt-stimulated AHP function in which proliferation, cell cycle recruitment, and neuronal fate induction are separable processes, regulated by canonical and noncanonical signals. In this model, robust canonical signaling drives proliferation, whereas low-level activity concomitantly represses differentiation (supporting information Fig. 1). This is consistent with our previous work showing that β-catenin over-expression, a surrogate for pure canonical activation, increased both gliogenesis and neurogenesis from AHPs [9]. In contrast, other signals, like noncanonical Wnts (e.g., Wnt5a) or other factors (e.g., TGFβ) are responsible for specifying cell fate [73]. As a result, enhanced Wnt signaling increases neurogenesis by expanding the pool of multipotent precursors, which under the appropriately favorable differentiation conditions will generate more neurons. Similarly, inhibiting Wnt will allow a greater fraction of these multipotent precursors to differentiate, resulting in an apparent increase in neurons, which is what was observed here.
Conclusion
In conclusion, autocrine Wnt signaling provides one mechanism for maintaining the multipotent NSC pool throughout an individual's lifetime. Unfortunately, a variety of pathologic conditions, from normal aging to cranial radiation, irreversibly deplete this population. Therefore, our findings may facilitate future gene or somatic cell-based therapies, though further in vivo experimentation will be required to validate this strategy.
Supplementary Material
Acknowledgments
We wish to thank Xiang Yu, Karl Deisseroth, and John Mason for access to their unpublished data; Pedro Zomarano, Tanishtha Reya, Jan Kitejewski, Andreas Kispert, Jen-Chin Hsieh, Ami Okada, Roel Nusse, Christoff Niehrs, Eric Fearon, Angela Barth, John Mason, Gregory Shakleford, Gary Nolan, Richard Mulligan, Laurie Ailles, Irving Weissman, Dritan Agalliu, and Anna Kenney for gifts of plasmids; Hiroki Toda for assistance with ELISA assays; and Rachael Simonoff, Brett Abrahams, Michele Monje, Brandy Ormerod, and Akiko Mori for helpful discussions. This work was supported by Sierra Pacific MIRECC/VA, and NIH-T32MH1993808 fellowships, NARSAD, APA/Wyeth Young Investigator Awards, John Douglas French Alzheimer's Foundation, and NIMH K08MH74362 (E.M.W.), R01MH065756 (H.I.K.), R01MH060233 (D.H.G.), R21NS045015 (T.D.P.).
Footnotes
Disclosure of Potential Conflicts of Interest: The authors indicate no potential conflicts of interest.
Author contributions: E.M.W.: conception and design, financial support, collection and/or assembly of data, manuscript writing, final approval of manuscript; A.P.: collection and/or assembly of data; H.I.K.: financial support, provision of study material; T.D.P.: conception and design, financial support; D.H.G.: conception and design, financial support, manuscript writing.
See www.StemCells.com for supporting information available online.
References
- 1.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 2.Iosif RE, Ekdahl CT, Ahlenius H, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Praag H, Shubert T, Zhao C, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Rajkowska G, Du F, et al. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 8.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 9.Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2008;13:285–292. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- 10.Filippov V, Kronenberg G, Pivneva T, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nature Rev Neurosci. 2001;2:287. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 12.Acheson A, Conover JC, Fandl JP, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JG, Patterson PH. LIF is an autocrine factor for sympathetic neurons. Mol Cell Neurosci. 1997;9:372–380. doi: 10.1006/mcne.1997.0635. [DOI] [PubMed] [Google Scholar]
- 14.Erlandsson A, Enarsson M, Forsberg-Nilsson K. Immature neurons from CNS stem cells proliferate in response to platelet-derived growth factor. J Neurosci. 2001;21:3483. doi: 10.1523/JNEUROSCI.21-10-03483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wurmser AE, Palmer TD, Gage FH. Neuroscience. Cellular interactions in the stem cell niche Science. 2004;304:1253–1255. doi: 10.1126/science.1099344. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Palmer TD. Adult neurogenesis and the vascular Nietzsche. Neuron. 2002;34:856. doi: 10.1016/s0896-6273(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 18.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 19.Adachi K, Mirzadeh Z, Sakaguchi M, et al. β-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 20.Bafico A, Liu G, Goldin L, et al. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15:701–707. [PubMed] [Google Scholar]
- 22.Hall CL, Kang S, MacDougald OA, et al. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97:661. doi: 10.1002/jcb.20735. [DOI] [PubMed] [Google Scholar]
- 23.Chim CS, Pang R, Fung TK, et al. Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia. 2007;21:2527–2536. doi: 10.1038/sj.leu.2404939. [DOI] [PubMed] [Google Scholar]
- 24.DeAlmeida VI, Miao L, Ernst JA, et al. The soluble wnt receptor Friz-zled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67:5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- 25.Fodde R, Brabletz T. Wnt/β-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Schlange T, Matsuda Y, Lienhard S, et al. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res. 2007;9:R63. doi: 10.1186/bcr1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 28.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 29.Coudreuse D, Korswagen HC. The making of Wnt: New insights into Wnt maturation, sorting and secretion. Development. 2007;134:3–12. doi: 10.1242/dev.02699. [DOI] [PubMed] [Google Scholar]
- 30.Etheridge SL, Spencer GJ, Heath DJ, et al. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- 31.Aubert J, Dunstan H, Chambers I, et al. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Natbiotechnol. 2002;20:1240. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- 32.Otero JJ, Fu W, Kan L, et al. β-Catenin signaling is required for neural differentiation of embryonic stem cells. Development. 2004;131:3545. doi: 10.1242/dev.01218. [DOI] [PubMed] [Google Scholar]
- 33.Verani R, Cappuccio I, Spinsanti P, et al. Expression of the Wnt inhibitor Dickkopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem. 2007;100:242–250. doi: 10.1111/j.1471-4159.2006.04207.x. [DOI] [PubMed] [Google Scholar]
- 34.Davidson KC, Jamshidi P, Daly R, et al. Wnt3a regulates survival, expansion, and maintenance of neural progenitors derived from human embryonic stem cells. Mol Cell Neurosci. 2007;36:408–415. doi: 10.1016/j.mcn.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Yu JM, Kim JH, Song GS, et al. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol Cell Biochem. 2006;288:17–28. doi: 10.1007/s11010-005-9113-3. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch C, Campano LM, Wohrle S, et al. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp Cell Res. 2007;313:572–587. doi: 10.1016/j.yexcr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Yin ZS, Zhang H, Wang W, et al. Wnt-3a protein promote neuronal differentiation of neural stem cells derived from adult mouse spinal cord. Neurol Res. 2007;29:847–854. doi: 10.1179/016164107X223539. [DOI] [PubMed] [Google Scholar]
- 38.Parkin NT, Kitajewski J, Varmus HE. Activity of Wnt-1 as a transmembrane protein. Genes Dev. 1993;7:2181. doi: 10.1101/gad.7.11.2181. [DOI] [PubMed] [Google Scholar]
- 39.Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suda T, Arai F. Wnt signaling in the niche. Cell. 2008;132:729–730. doi: 10.1016/j.cell.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Gilbertson RJ, Rich JN. Making a tumour's bed: Glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 42.Colombo E, Giannelli SG, Galli R, et al. Embryonic stem-derived versus somatic neural stem cells: A comparative analysis of their developmental potential and molecular phenotype. Stem Cells. 2006;24:825. doi: 10.1634/stemcells.2005-0313. [DOI] [PubMed] [Google Scholar]
- 43.Palmer TD, Markakis EA, Willhoite AR, et al. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Svendsen CN, ter Borg MG, Armstrong RJ, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 45.Palmer TD, Schwartz PH, Taupin P, et al. Cell culture. Progenitor cells from human brain after death Nature. 2001;411:42. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Malenka RC. β-Catenin is critical for dendritic morphogenesis. Natneurosci. 2003;6:1169. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- 47.Barraud P, Thompson L, Kirik D, et al. Isolation and characterization of neural precursor cells from the Sox1-GFP reporter mouse. Eur J Neurosci. 2005;22:1555–1569. doi: 10.1111/j.1460-9568.2005.04352.x. [DOI] [PubMed] [Google Scholar]
- 48.Barraud P, Stott S, Mollgard K, et al. In vitro characterization of a human neural progenitor cell coexpressing SSEA4 and CD133. J Neurosci Res. 2007;85:250–259. doi: 10.1002/jnr.21116. [DOI] [PubMed] [Google Scholar]
- 49.van de Wetering M, Cavallo R, Dooijes D, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 50.Day RN, Kawecki M, Berry D. Dual-function reporter protein for analysis of gene expression in living cells. Biotechniques. 1998;25:848. doi: 10.2144/98255bt02. [DOI] [PubMed] [Google Scholar]
- 51.Corish P, Tyler-Smith C. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng. 1999;12:1035. doi: 10.1093/protein/12.12.1035. [DOI] [PubMed] [Google Scholar]
- 52.Castelo-Branco G, Wagner J, Rodriguez FJ, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, And Wnt-5a. Proc Natl Acad Sci USA. 2003;100:12747. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen G, Birnbaum RS, Yablonka-Reuveni Z, et al. Separation of mouse crushed muscle extract into distinct mitogenic activities by heparin affinity chromatography. J Cell Physiol. 1994;160:563–572. doi: 10.1002/jcp.1041600320. [DOI] [PubMed] [Google Scholar]
- 54.Shibamoto S, Higano K, Takada R, et al. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 55.Shimogori T, VanSant J, Paik E, et al. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473:496. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- 56.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh JC, Kodjabachian L, Rebbert ML, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 58.Noles SR, Chenn A. Cadherin inhibition of β-catenin signaling regulates the proliferation and differentiation of neural precursor cells. Mol Cell Neurosci. 2007;35:549–558. doi: 10.1016/j.mcn.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Nieman MT, Kim JB, Johnson KR, et al. Mechanism of extracellular domain-deleted dominant negative cadherins. J Cell Sci. 1999;112(Part 10):1621. doi: 10.1242/jcs.112.10.1621. [DOI] [PubMed] [Google Scholar]
- 60.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 61.Garcia AD, Doan NB, Imura T, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Natneurosci. 2004;7:1233. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 62.Kelly GM, Greenstein P, Erezyilmaz DF, et al. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development. 1995;121:1787. doi: 10.1242/dev.121.6.1787. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu H, Julius MA, Giarre M, et al. Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ. 1997;8:1349. [PubMed] [Google Scholar]
- 64.Sumanas S, Strege P, Heasman J, et al. The putative wnt receptor Xenopus frizzled-7 functions upstream of β-catenin in vertebrate dorsoventral mesoderm patterning. Development. 2000;127:1981. doi: 10.1242/dev.127.9.1981. [DOI] [PubMed] [Google Scholar]
- 65.Aulehla A, Wehrle C, Brand-Saberi B, et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 66.Galceran J, Miyashita-Lin EM, Devaney E, et al. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127:469. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- 67.Lee SM, Tole S, Grove E, et al. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 68.Kan L, Israsena N, Zhang Z, et al. Sox1 acts through multiple independent pathways to promote neurogenesis. Dev Biol. 2004;269:580. doi: 10.1016/j.ydbio.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Zorn AM, Barish GD, Williams BO, et al. Regulation of Wnt signaling by Sox proteins: XSox17 α/β and XSox3 physically interact with β-catenin. Mol Cell. 1999;4:487. doi: 10.1016/s1097-2765(00)80200-2. [DOI] [PubMed] [Google Scholar]
- 70.Graham V, Khudyakov J, Ellis P, et al. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 71.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. Plos Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 73.Falk S, Wurdak H, Ittner LM, et al. Brain area-specific effect of TGFβ signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell. 2008;2:472–483. doi: 10.1016/j.stem.2008.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


