Abstract
OBJECTIVE
To determine whether pharmacological treatment of depression in low-income minorities with diabetes improves A1C and quality of life (QOL).
RESEARCH DESIGN AND METHODS
This was a 6-month, randomized, double-blind, placebo-controlled trial. Patients were screened for depression using Whooley's two-question tool at a county diabetes clinic. Depression was confirmed (or not) with the Computerized Diagnostic Interview Survey (CDIS) software program, and the severity of depression was assessed monthly by the Hamilton Depression Scale (HAM-D). Depressed subjects with A1C levels ≥8.0% were randomly assigned to receive either sertraline or placebo. Diabetes care was provided by nurses following detailed treatment algorithms who were unaware of therapy for depression.
RESULTS
A total of 150 subjects answered positively to at least one question on Whooley's questionnaire. The positive predictive value for depression diagnosed by CDIS was 69, 67, and 84% for positive answers to question 1 only, question 2 only, or both, respectively. Of the 89 subjects who entered the study, 75 completed. An intention-to-treat analysis revealed significant differences between baseline and 6 months in HAM-D and pain scores, QOL, and A1C and systolic blood pressure levels in both groups, with no differences between groups for the first three but a significantly greater decrease with sertraline in A1C and systolic blood pressure levels. Changes in HAM-D scores and A1C levels were significantly correlated in all subjects (P = 0.45 [P < 10−6]).
CONCLUSIONS
In this low-income minority population, pharmacological treatment of depression significantly improved A1C and systolic blood pressure levels compared with placebo.
The prevalence of depression among people with diabetes is more than twice that of the general population (1). Coexistence of depression in persons with diabetes is associated with worse glycemic control (2), which may be due to less adherence to self-care behaviors and medications (3). Eventually, there is increased morbidity (4) and mortality (5) and higher medical costs (6).
The prevalence of untreated depression in people with diabetes is higher in minorities (1). Yet, screening for and treating depression are less common in this population (7). Very little research has been published on diabetes and depression with a focus on minority populations, who have significant disparities in outcomes (8), such as higher A1C levels (9), increased rates of complications (10), and more severe depression (8).
Depression is associated with worse glycemic control (2). Some studies have evaluated whether treatment of depression will improve A1C levels (11–20). However, these drug studies were open label, were of short duration, and/or were conducted in highly educated (more than high school education) Caucasian populations. Most showed that although depression was improved, A1C levels were not. We sought to determine whether use of antidepressants in a minority population with uncontrolled diabetes improved their A1C levels, quality of life (QOL), and depression compared with placebo.
RESEARCH DESIGN AND METHODS
This was a 6-month randomized, double-blind, placebo-controlled study. Patients in a Los Angeles County diabetes clinic were screened for depression with Whooley's two-question tool (21) (question 1: “During the past month, have you often been bothered by feeling down, depressed or hopeless?”; question 2: “During the past month, have you often been bothered by having little interest or pleasure in doing things?”). Patients with positive answers to one or both of these questions who stated an interest in participating in a study of depression and diabetes had depression confirmed (or not) with the Computerized Diagnostic Interview Survey (CDIS) software program. If results were positive, the severity of depression was assessed by the Hamilton Depression (HAM-D) survey, a 21-question survey that is the most widely used outcome measure for evaluating depression severity (22). Exclusion criteria were current use of antidepressants, A1C levels <8%, pregnancy, dialysis, liver disease by history or liver enzyme levels elevated three times greater than normal, blood pressure >160 mmHg systolic or >95 mmHg diastolic, a history of severe depression (as determined by previous hospitalization or suicide attempts), and a positive answer to the suicide question on the HAM-D survey on the initial evaluation.
Once depression was positively diagnosed, subjects were randomly assigned by a computer program to receive either sertraline or placebo. The subject, study coordinator, and investigator were unaware of the study group to which a given patient was assigned. Study subjects continued their diabetes care in the county diabetes clinic where it was provided by nurses following detailed treatment algorithms (23) who were unaware of the therapy for depression. Study visits were conducted monthly, at which time the study coordinator evaluated the patient using the HAM-D score. Blood was drawn for measurement of sertraline levels, pill counts were done before new medication was issued, and pain was assessed using a visual numeric analog scale from 1 to 10 at each visit. At each study visit, the coordinator discussed the laboratory results and encouraged subjects to take both their diabetes and study medications as ordered. Sertraline was started at a dose of 50 mg (one pill), and, if at the monthly follow-up subjects' depression scores on the HAM-D questionnaire did not improve (i.e., their scores did not decrease), their medication was increased to two pills, either placebo or 100 mg of sertraline. If a subject's answer to the suicide question on the HAM-D questionnaire was positive, the psychiatry urgent care clinic was paged and study personnel took the subject to the mental health urgent care center located one floor below in the same building.
A1C levels were measured every 2 months. QOL was assessed at baseline and at the end of the study by the Diabetes-39 questionnaire (24). All subjects were seen in group sessions monthly for an American Diabetes Association–approved diabetes education program given by the study coordinator, in which adherence to medications was also stressed. At the last visit, subjects met with the study psychiatrist who unblinded them and determined what further depression treatment the subject might need.
The primary outcome variable was the change in A1C levels between baseline and 6 months. The major secondary outcome variable was the change in QOL at 6 months compared with baseline. The other secondary outcomes were the other two outcome measures of diabetes, i.e., LDL cholesterol and blood pressure levels, the lowering of which is causally related to decreased diabetes complications, especially macrovascular disease. The data were subjected to a test for normality based on the skewness and kurtosis of the underlying frequency distribution. If results of this test were significant, a nonparametric test was then used on the data. An ANCOVA model or its nonparametric equivalent (Wilcoxon's rank-sum test based on the differences from baseline) was used to assess differences between the two arms at 6 months; the two-tailed Student's t test or its nonparametric equivalent (Wilcoxon's rank-sum test) was used to assess pairwise between-group differences. In addition, the within-group change from baseline to 6 months was assessed using Wilcoxon's signed-rank test. Secondary clinical outcomes also included depression and pain scores. The results were reported based on an intent-to-treat analysis (last observation carried forward) for both depression and diabetes outcomes. To compare baseline characteristics of study subjects, the χ2 test was used for qualitative data and either the two-sample t or Wilcoxon's rank-sum test was used for quantitative end points.
The study was approved by the Charles Drew University Institutional Review Board. Subjects signed an informed consent form before the CDIS evaluation.
RESULTS
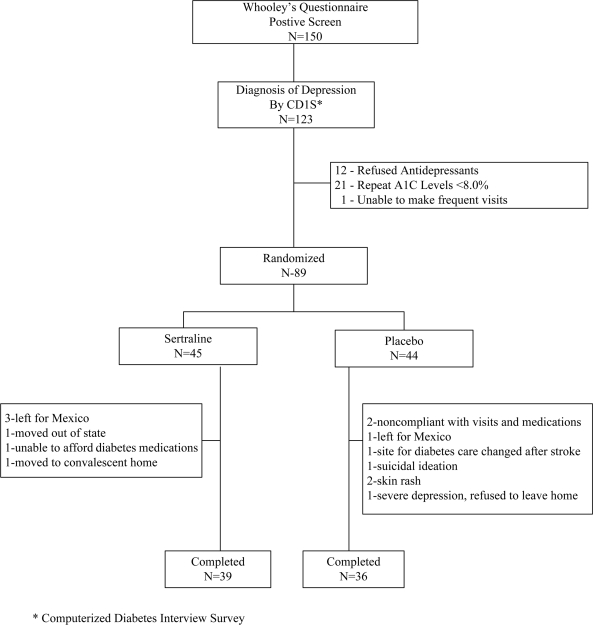
For the study, 150 subjects answered yes to at least one of the two questions on Whooley's questionnaire, and all subjects provided informed consent for further evaluation. Of these, 123 tested positive for depression on the CDIS for an overall positive predictive value (PPV) for Whooley's questionnaire of 82%. The PPVs for positivity to question 1 only, to question 2 only, or to both were 69, 67, and 84%, respectively. Of the 123 subjects who tested positive for depression with the CDIS, 12 decided against taking antidepressants, 21 had repeat A1C levels <8%, and 1 could not make the frequent visits. Therefore, 89 subjects whose baseline characteristics are summarized in Table 1 were randomly assigned; of these, 75 completed the study. There were no significant differences in baseline characteristics between the two groups. Fourteen subjects (whose baseline characteristics were similar to those of the 75 completers) were withdrawn from the study for the reasons listed in Fig. 1.
Table 1.
Baseline demographics
| Sertraline group | Placebo group | |
|---|---|---|
| n | 45 | 44 |
| Women | 33 | 32 |
| Age (years) | 52 ± 8 | 53 ± 10 |
| Hispanic | 39 | 39 |
| African American | 5 | 5 |
| Other | 1 | |
| Years of diabetes | 13 ± 7 | 12 ± 7 |
| Type 2/type 1 diabetes | 45/0 | 42/2 |
| Pain scale | 6 ± 2 | 6 ± 3 |
| HAM-D* | 19 ± 5 | 20 ± 6 |
| A1C (%) | 10.0 ± 1.8 | 9.7 ± 1.6 |
| LDL cholesterol (mg/dl) | 101 ± 29 | 99 ± 34 |
| Systolic blood pressure (mmHg) | 137 ± 13 | 137 ± 14 |
| Diastolic blood pressure (mmHg) | 73 ± 9 | 75 ± 12 |
| Weight (lbs) | 181.6 ± 40.0 | 188.1 ± 61.7† |
Data are means ± SD or n.
*21-question survey to evaluate degree of depression.
†n = 43 (one patient in wheelchair and weight not measured).
Figure 1.
CONSORT diagram depicting subject flow.
The response to treatment is shown in Table 2. A1C levels fell significantly in both groups, but the decrease in the sertraline group was more than twice as great as that in the placebo group (−2.0% ± 2.1 vs. −0.9% ± 2.0, P = 0.003). Measurement of blood sertraline levels revealed that 15 of the 45 subjects who were assigned to take the drug were not taking it. However, the results were not changed appreciably when these noncompliant subjects were omitted from the analysis of the sertraline group. Systolic blood pressure fell significantly in both groups, and again the fall in the sertraline group was significantly greater than that in the placebo group (−15 ± 18 vs. −6 ± 15 mmHg, P = 0.003). HAM-D scores fell significantly in both groups with no difference between the groups. There was a significant (P < 10−6) correlation of 0.45 between the changes in A1C levels and HAM-D scores in the entire group of subjects. There were significant differences between baseline and end of study in both groups but no differences between the two groups for pain scores (Table 2) and QOL (Table 3). Diastolic blood pressure, LDL cholesterol concentrations, and weight did not change significantly in either group. The results were similar when only the completers were analyzed.
Table 2.
Response to treatment
| Sertraline | Placebo | P | |
|---|---|---|---|
| A1C levels (%) | |||
| Baseline | 10.0 ± 1.8 | 9.7 ± 1.6 | NS |
| 6 months | 8.0 ± 1.4 | 8.8 ± 1.9 | <0.01 |
| P | <0.001 | <0.01 | |
| Systolic blood pressure (mmHg) | |||
| Baseline | 137 ± 13 | 137 ± 14 | NS |
| 6 months | 122 ± 15 | 131 ± 14 | =0.003 |
| P | <10−5 | =0.01 | |
| Diastolic blood pressure (mmHg) | |||
| Baseline | 73 ± 9 | 75 ± 12 | NS |
| 6 months | 72 ± 10 | 72 ± 11 | NS |
| P | NS | NS | |
| LDL cholesterol concentration (mg/dl) | |||
| Baseline | 101 ± 29 | 99 ± 34 | NS |
| 6 months | 91 ± 28 | 93 ± 30 | NS |
| P | NS | NS | |
| Weight (lbs) | |||
| Baseline | 181.6 ± 40.0 | 188.1 ± 61.7† | NS |
| 6 months | 181.5 ± 40.2 | 188.7 ± 58.8† | NS |
| P | NS | NS | |
| HAM-D scores* | |||
| Baseline | 19 ± 5 | 20 ± 6 | NS |
| 6 months | 11 ± 6 | 13 ± 8 | NS |
| P | <0.001 | <0.001 | |
| Pain scale | |||
| Baseline | 6 ± 2 | 6 ± 2 | NS |
| 6 months | 4 ± 3 | 4 ± 3 | NS |
| P | <0.001 | =0.01 |
Data are means ± SD.
*Depression score: 0–7, none; 8–13, mild; 14–18, moderate; 19–22, severe; >22, very severe.
†n = 43 (one patient in wheelchair and weight not measured). NS, nonsignificant (P > 0.05).
Table 3.
QOL subscales and scores
| Sertraline |
Placebo |
|||
|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |
| Subscales* | ||||
| Diabetes control | 69.6 ± 13.4 | 49.8 ± 22.0 | 66.7 ± 18.6 | 55.7 ± 17.7 |
| Anxiety and worry | 78.8 ± 12.6 | 57.1 ± 21.8 | 76.7 ± 16.5 | 61.9 ± 22.1 |
| Social burden | 68.2 ± 17.3 | 43.8 ± 25.8 | 63.7 ± 25.2 | 50.4 ± 23.8 |
| Sexual function | 67.8 ± 28.2 | 56.7 ± 31.2 | 66.6 ± 32.4 | 61.1 ± 33.2 |
| Energy and mobility | 67.6 ± 16.2 | 44.6 ± 21.1 | 63.1 ± 20.1 | 49.7 ± 22 |
| Scores† | ||||
| Overall QOL | 3.5 ± 3 | 50.0 ± 3 | 3.0 ± 2 | 4.0 ± 2 |
| Diabetes severity | 6.0 ± 3.0 | 5.0 ± 2.0 | 6.0 ± 3.0 | 5.0 ± 2.0 |
*Data are means ± SD. P < 0.05 for all subscales for both groups except for sexual function in the placebo group; no difference between groups.
†Data are medians ± interquartile range. Improvement is an increase in overall QOL and a decrease in diabetes severity. P < 0.05 for both scores but no difference between groups.
CONCLUSIONS
Sertraline-treated patients had greater improvement in A1C and systolic blood pressure levels than control patients, despite equivalent improvement in depression as measured by HAM-D. Thus, depression and pain scores (Table 2) and QOL (Table 3) improved significantly in patients receiving either sertraline or placebo, but there were no differences between the two groups. In contrast, although A1C levels fell significantly in both groups, the decrease in patients receiving sertraline was more than twofold greater than in those receiving the placebo, and this difference between groups was statistically significant. However, there was a very significant (P < 10−6) correlation of 0.45 between changes in depression and A1C levels in all of the subjects. A placebo effect to explain the significant fall in the control group would not be unexpected in this situation and may have been enhanced by the twice a month interaction with the study coordinator. The interaction with the study coordinator might also explain the similar improvements in depression, QOL, and pain scores between the two groups. These questionnaires were administered by the coordinator who often had to provide verbal explanations to the subjects about them. Perhaps the subjects did not want to “disappoint” her.
These robust positive effects of sertraline to significantly lower A1C levels in this study stand in contrast with most of the literature concerning treatment of depression in people with diabetes. In all of the randomized trials (11–16,18,20), depression scores significantly improved. However, pharmacological treatment alone (11,13) or psychological plus pharmacological treatment (15) did not affect A1C levels. In one study (16), patients were first treated in an open-label fashion with an antidepressant, and the 43% who responded were randomly assigned to continue either pharmacological treatment or to receive a placebo in a maintenance phase. Although recurrence of depression was significantly delayed by the active drug, the improvement in A1C levels during the open-label phase was maintained with no difference between the two groups during the maintenance phase. In a mildly depressed group of diabetic patients, A1C levels significantly decreased at 3 months, but there was no difference at 6 months between pharmacological and placebo treatment (18). In a study evaluating cognitive behavior therapy, A1C levels were similar to those in a control group receiving no specific antidepressant therapy at the end of the 12-week treatment period but were significantly lower 6 months later (12). However, these levels remained high in both groups (9.5% vs. 10.9%). Finally, in a randomized clinical trial in which depressed patients received a combination of pharmacological and psychological treatments compared with usual care, there was no difference in A1C levels when the entire groups were analyzed (15). However, in the active treatment group, A1C levels fell significantly in those who had high depression scores compared with those with low scores. This difference was not found in the usual care group.
Conflicting results were seen in two open-label studies. In one, in which depression was treated with an antidepressant, A1C levels were significantly decreased in those whose depression improved but not in those who did not show a remission (17). In the other one in which treatment was by group cognitive behavior therapy, depression significantly improved but there was no change in A1C levels (20).
The PPVs for yes answers to question 1 only, to question 2 only, or to both on Whooley's questionnaire were 69, 67, and 84%, respectively. To the best of our knowledge, this is the first study to evaluate the PPVs of the responses to Whooley's questionnaire using an objective measure for the diagnosis of depression, the CDIS. These results suggest that this simple two-question screening tool could be an effective way to identify depressed patients in a busy office practice, especially if both questions were answered in the affirmative.
Because depression is significantly associated with treatment nonadherence (25), it is likely that the improvement in A1C and systolic blood pressure levels in both groups was due to better adherence to the treatment recommendations of the nurses. One interpretation of these results is that increased contact with a sympathetic questioner (and listener) helps patients with depression, leading to better medication adherence, but pharmacological treatment of the underlying depression still yields an incremental benefit.
These results suggest an effective approach to the time constraints hindering primary care physicians caring for patients with poor glycemic control in whom depression is suspected, especially in low-income, minority populations. Whooley's screening questionnaire could be used liberally in those patients, and if results were positive (especially if both questions were answered in the affirmative), an antidepressant should be considered. These patients can be difficult to treat successfully, but, in this manner, both depression and uncontrolled diabetes and systolic blood pressure may be improved.
Acknowledgments
This research was supported by UCLA/DREW Project EXPORT, the National Center on Minority Health and Health Disparities (PD20MD000148 and P20D000182), and the National Institutes of Health (Grant U54-RR-014616).
No potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT00624013, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Anderson S, Freedland K, Clouse R, Lustman P: The prevalence of co-morbid depression in adults with diabetes. Diabetes Care 2001; 24: 1069– 1077 [DOI] [PubMed] [Google Scholar]
- 2. Lustman P, Anderson R, Freedland K, Groot MD, Carney R, Clouse R: Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000; 23: 934– 942 [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez J, Safran S, Cagliero E, Wexler D, Delahanty L, Wittenberg E, Blais M, Meigs J, Grant R: Depression, self care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care 2007; 30: 2222– 2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black SA, Markides KS, Ray LA: Depression predicts increased incidence of adverse health outcomes in Mexican Americans with type 2 diabetes. Diabetes Care 2003; 26: 2822– 2828 [DOI] [PubMed] [Google Scholar]
- 5. Xuanping Z, Norris S, Gregg E, Cheng Y, Beckles G, Kahn H: Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol 2005; 161: 652– 660 [DOI] [PubMed] [Google Scholar]
- 6. Simon G, Katon W, Lin E, Ludman E, Von Korff M, Ciechanowski P, Young B: Diabetes and depression as predictors of health care costs. Gen Hosp Psychiatry 2005; 27: 344– 351 [DOI] [PubMed] [Google Scholar]
- 7. Wagner J, Tsimikas J, Abbot G, De Groot M, Heapy A: Racial and ethnic differences in diabetic patient-reported depression symptoms, diagnosis, and treatment. Diabetes Res Clin Pract 2007; 75: 119– 122 [DOI] [PubMed] [Google Scholar]
- 8. Black SA: Increase in Health Burden Associations with co-morbid depression in older diabetic Mexican Americans: results from the Hispanic Established Population for the Epidemiologic Study of the Elderly survey. Diabetes Care 1999; 22: 56– 64 [DOI] [PubMed] [Google Scholar]
- 9. Kirk J, Passmore L, Bell R, Narayan KM, D'Agostino R, Arcury T, Quandt S: Disparities in A1C levels between Hispanic and non-Hispanic white adults with diabetes: a meta analytic analysis. Diabetes Care 2008; 31: 240– 246 [DOI] [PubMed] [Google Scholar]
- 10. Roy M, Roy A, Affouf M: Depression is a risk factor for poor glycemic control and retinopathy in African Americans with type 1 diabetes. Psychosom Med 2007; 69: 537– 542 [DOI] [PubMed] [Google Scholar]
- 11. Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, Carney RM, McGill JB: Effects of nortriptyline on depression and glucose regulation in diabetes: results of a double blind, placebo controlled trial. Psychosom Med 1997; 59: 241– 250 [DOI] [PubMed] [Google Scholar]
- 12. Lustman P, Griffith L, Freedland K, Kissel S, Clouse R: Cognitive behavior therapy for depression in type 2 diabetes: a randomized, controlled trial. Ann Intern Med 1998; 129: 613– 621 [DOI] [PubMed] [Google Scholar]
- 13. Lustman P, Freedland K, Griffith L, Clouse R: Fluoxetine for depression in diabetes: a randomized double-blind placebo controlled trial. Diabetes Care 2000; 23: 618– 623 [DOI] [PubMed] [Google Scholar]
- 14. Williams JW, Katon W, Lin EHB, Noel PH, Worchel J, Cornell J, Harpole L, Fultz BA, Hunkeler E, Mika VS, Unutzer J. IMPACT Investigators: The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann Intern Med 2004; 140: 1054– 1056 [DOI] [PubMed] [Google Scholar]
- 15. Katon WJ, van Korff M, Lin EHB, Simon G, Ludman E, Russo J, Ctechanowski P, Walker E, Bush T: The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004; 61: 1042– 1049 [DOI] [PubMed] [Google Scholar]
- 16. Lustman PJ, Clouse RE, Hirsh IB, Gelenberg AJ, Nix BD, Freedland KE: Sertraline for the prevention of depression recurrence in diabetes: a randomized, double-blind, placebo controlled trial. Arch Gen Psychiatry 2006; 63: 521– 529 [DOI] [PubMed] [Google Scholar]
- 17. Lustman P, Williams M, Sayuk G, Nix B, Clouse R: Factors influencing glycemic control in type 2 diabetes during acute-and maintenance phase treatment of major depressive disorder with bupropion. Diabetes Care 2007; 30: 459– 466 [DOI] [PubMed] [Google Scholar]
- 18. Paile-Hyvarinen M, Wahlbeck K, Eriksson J: Quality of life and metabolic status in mildly depressed patients with type 2 diabetes treated with paroxetine: a double-blind randomized placebo-controlled 6 month trial. BMC Fam Pract 2007; 8: 34– 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Georgiades A, Zucker N, Friedman KE, Mosunic CJ, Applegate K, Lane JD, Feinglos MN, Surwit RS: Changes in depressive symptoms and glycemic control in diabetes mellitus. Psychosom Med 2007; 69: 235– 241 [DOI] [PubMed] [Google Scholar]
- 20. Snoek FJ, van der Ven NCW, Twisk JWR, Hogenelst MHE, Tromp-Wever AME, van der Ploeg HM, Heine RJ: Cognitive behavior therapy (CBT) compared with blood glucose awareness training (BGAT) in poorly controlled type 1 diabetic patients: long term effects on HbA1c moderated by depression: a randomized controlled trial. Diabetic Med 2008; 25: 1337– 1342 [DOI] [PubMed] [Google Scholar]
- 21. Whooley MA, Avins AL, Miranda J: Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med 1997; 12: 439– 445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamilton M: A rating scale for depression. J Neuro Neurosurg Psychiatry 1960; 23: 56– 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davidson MB, Castellanos M, Duran P, Karlan V: Effective diabetes care by a registered nurse following treatment algorithms in a minority population. Am J Manag Care 2006; 12: 226– 232 [PubMed] [Google Scholar]
- 24. Boyer JG, Earp JA: The development of an instrument for assessing the quality of life of people with diabetes: Diabetes-39. Med Care 1997; 35: 440– 453 [DOI] [PubMed] [Google Scholar]
- 25. Gonzalez JS, Peyrot M, McCarl LAM, Collins EM, Serpa L, Mimiaga MJ, Safren S: Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 2008; 31: 2398– 2403 [DOI] [PMC free article] [PubMed] [Google Scholar]