Abstract
OBJECTIVE
To evaluate the time course of leptin, adiponectin, and resting energy expenditure (REE) responses in overweight elderly males after acute resistance exercise protocols of various intensity configurations.
RESEARCH DESIGN AND METHODS
Forty inactive men (65–82 years) were randomly assigned to one of four groups (n = 10/group): control, low-intensity resistance exercise, moderate-intensity resistance exercise, and high-intensity resistance exercise. Exercise energy cost, REE, leptin, adiponectin, cortisol, insulin, lactate, glucose, nonesterified fatty acids (NEFAs), and glycerol were determined at baseline, immediately after exercise, and during a 72-h recovery period.
RESULTS
Exercise energy cost was lower in high-intensity than in low-intensity and moderate-intensity groups (221.6 ± 8.8 vs. 295.6 ± 10.7 and 281.6 ± 9.8 kcal, P < 0.001). Lactate, glucose, NEFAs, and glycerol concentrations increased (P < 0.001) after exercise and returned to baseline thereafter in all groups. REE increased (P < 0.001) in all groups at 12 h in an intensity-dependent manner (P < 0.05). REE reached baseline after 48 h in the low- and moderate-intensity groups and after 72 h in the high-intensity group. Cortisol peaked in all active groups after exercise (P < 0.001) and remained elevated (P < 0.001) for 12 h. After adjustment for plasma volume shifts, leptin remained unaltered. Adiponectin concentration increased after 12 h and remained elevated for 24 h only in the high-intensity group (P < 0.001).
CONCLUSIONS
Resistance exercise does not alter circulating leptin concentration but does increase REE and adiponectin in an intensity-dependent manner for as long as 48 and 24 h, respectively, in overweight elderly individuals. It appears that resistance exercise may represent an effective approach for weight management and metabolic control in overweight elderly individuals.
Aging is characterized by progressive impairment of carbohydrate intolerance and is usually accompanied by physical inactivity and obesity, which may induce hyperinsulinemia, insulin resistance, and cardiovascular disease (1). Obesity, a growing health concern, is characterized by low-grade inflammation, which is associated with insulin resistance and metabolic diseases such as diabetes (2).
Leptin and adiponectin, adipose tissue–derived cytokine proteins, are involved in insulin resistance and inflammation (3). Leptin improves fatty acid oxidation in muscle, regulates short-term carbohydrate intake, mediates energy balance and body weight, and upregulates resting energy expenditure (REE) (3). Adiponectin is inversely related to body fatness and type 2 diabetes risk in healthy adults (4), has an anti-inflammatory action, and is involved in substrate metabolism (5,6). Aging increases body fat and leptin levels probably owing to an upregulation of leptin gene expression and is characterized by a negative association between adiponectin and body fat distribution (4).
Exercise is believed to extend life-span by reducing the incidence of cardiovascular and other degenerative diseases and increases functional performance in older individuals (7). Regular exercise seems to create energy deficits that help to regulate body weight and fat on a long-term basis in older individuals (1). Chronic resistance exercise increases muscle tissue, reduces aging-related sarcopenia (8), and alters adipokine responses in elderly individuals (7). Acute resistance exercise may elevate lipid mobilization in subcutaneous adipose tissue, energy expenditure, and neuroendocrine responses of obese subjects (9). Although the American Diabetes Association endorses resistance exercise as a means of improving body composition and metabolic control in obesity and diabetes (2), limited information exists regarding the acute effects of resistance exercise on adipokine and REE responses in aging. Therefore, the purpose of the present investigation was to explore 1) the time course of leptin, adiponectin, and REE responses after a single resistance exercise bout in elderly individuals and 2) whether resistance exercise intensity represents an important factor in adipokine and REE responses in elderly individuals.
RESEARCH DESIGN AND METHODS
Forty apparently healthy, older sedentary men (recruited from a volunteer database, by word of mouth and fliers sent to local medical practitioners, physiotherapists, and nursing homes) participated in a four-group randomized, repeated-measures, controlled trial. Written informed consent was obtained, and procedures were performed in accordance with the Declaration of Helsinki and institutional review board. Subjects' physical characteristics are shown in Table 1. Inclusion criteria were 1) complete inactivity (≥10 years): maximal oxygen consumption (Vo2peak) <25 ml · kg−1 min−1 and a score <9.0 on the Modified Baecke Questionnaire for Older Adults (7); 2) weight stability (±2 kg) before entry (≥6 months); 3) absence of restraining orthopedic/neuromuscular diseases; 4) resting blood pressure <160/100 mmHg; 5) no use of tobacco, aspirin, alcohol-containing beverages, and medications that may affect lipid metabolism or body composition; and 6) no history of diabetes or glucose intolerance.
Table 1.
Physical characteristics, physical activity levels, strength performance of the subjects, as well as average intensity and average number of repetitions of the exercise protocols in each group
| Control group | Low-intensity group | Moderate-intensity group | High-intensity group | |
|---|---|---|---|---|
| Age (years) | 71.7 ± 2.0 | 72.4 ± 1.6 | 71.0 ± 1.2 | 71.1 ± 1.5 |
| Height (m) | 1.67 ± 0.01 | 1.65 ± 0.02 | 1.68 ± 0.03 | 1.65 ± 0.01 |
| Weight (kg) | 81.3 ± 2.7 | 80.3 ± 3.3 | 82.0 ± 2.8 | 78.3 ± 1.7 |
| BMI (kg/m2) | 29.1 ± 0.6 | 29.4 ± 0.9 | 29.0 ± 0.4 | 28.7 ± 0.3 |
| Skinfold sum (mm) | 126.4 ± 3.3 | 124.8 ± 4.1 | 125.1 ± 2.7 | 124.6 ± 4.4 |
| Waist-to-hip ratio | 0.95 ± 0.07 | 0.94 ± 0.06 | 0.93 ± 0.09 | 0.96 ± 0.07 |
| Vo2peak (ml · kg−1 · min−1) | 16.1 ± 0.6 | 16.7 ± 0.4 | 16.3 ± 0.5 | 17.0 ± 0.4 |
| Activity level* | 8.15 ± 0.3 | 8.34 ± 0.4 | 7.89 ± 0.2 | 8.21 ± 0.2 |
| Trunk strength (kg)† | 37.7 ± 3.4 | 38.9 ± 3.9 | 36.8 ± 2.9 | 38.3 ± 4.2 |
| Lower limb strength (kg)‡ | 69.6 ± 5.2 | 72.3 ± 5.8 | 68.7 ± 4.5 | 73.1 ± 5.9 |
| HOMAIR index | 2.92 ± 0.7 | 3.30 ± 0.6 | 3.23 ± 0.5 | 2.88 ± 0.4 |
| Exercise energy cost (kcal) | NA | 295.6 ± 10.7 | 281.6 ± 9.8 | 221.6 ± 8.8§ |
| Repetitions | NA | 14.6 ± 0.3 | 9.7 ± 0.2 | 6.9 ± 0.1 |
| Average intensity (% 1RM) | NA | 46.1 ± 0.4 | 64.9 ± 0.9 | 85.2 ± 0.6 |
Data are means ± SEM.
*According to Baecke physical activity questionnaire.
†One maximal repetition measured in chest press.
‡One maximal repetition measured in leg press.
§Significant difference between the high-intensity group and the other groups (P < 0.05). HOMAIR, homeostasis model assessment of insulin resistance; NA, not applicable.
After they were medically screened and had their REE and anthropometric profile measured, participants underwent a diagnostic treadmill test to exhaustion to evaluate their Vo2peak and were given 5-day diet recall forms to complete. After familiarization, participants had their maximal strength (one repetition maximal [1RM]) measured in each exercise used in the subsequent resistance exercise protocol (for resistance adjustment) and were randomly assigned to one of four groups (n = 10/group): control, low-intensity resistance exercise, moderate-intensity resistance exercise, and high-intensity resistance exercise. Blood sampling and REE measurement were performed at baseline, immediately after exercise, and at 12, 24, 48, and 72 h of recovery.
Preliminary measurements
Body weight, height, waist-to-hip circumferences, and skinfold thickness were measured as described previously (7). Vo2peak was determined during exercise to exhaustion on a treadmill using a modified version of the Bruce protocol by open-circuit spirometry (VmaxST; SensorMedics, Yorba Linda, CA) with an automated online pulmonary gas-exchange system via breath-by-breath analysis as described (7). 1RM was determined using standard procedures (7). The intraclass correlation coefficient for repeated 1RM measurements ranged between 0.87 and 0.96 for all exercises. Group differences in habitual dietary intakes were analyzed with commercially available software (Science Technologies, Athens, Greece).
REE
REE was determined in a semirecumbent position in the morning for 45 min after an overnight fast. Vo2/Vco2 production rates were measured from expired air samples collected via a ventilated hood system (Vmax29c; SensorMedics). After a 10-min stabilization period, 20 consecutive 1-min measurements were taken and averaged. REE was calculated by the Weir equation and expressed per 24 h (7).
Exercise intervention
After a brief warmup, participants performed three sets on resistance equipment selected to stress the major muscles (chest press, leg extension, shoulder press, leg curls, latissimus pull down, leg press, arm curls, triceps extension, abdominal curls, and back extensions) using standard techniques during a 60-min session (7). Intensity (percent 1RM) and rest intervals between sets were set at 45–50% and 2 min, 60–65% and 4 min, and 80–85% and 6 min in the low-, moderate-, and high-intensity groups, respectively (Table 1) (7). Throughout exercise (and in between-sets rest intervals), breath-by-breath inhaled Vo2 to exhaled Vco2 exchange was recorded via a portable metabolic analyzer (VmaxST) as described previously (9). The test-retest intraclass correlation coefficient of the exercise energy cost measurement was 0.91.
Blood sampling and analysis
Blood was drawn from an antecubital vein into evacuated tubes containing either SST Gel (for serum) or EDTA (for plasma). The tubes were placed on ice immediately and centrifuged (4°C, 1,500g, 15 min). Collected serum or plasma was stored in multiple aliquots at −75°C until assayed (in duplicate).
Blood lactate, plasma glucose, serum NEFAs, and serum glycerol were determined spectrophotometrically (Hitachi UV 2001) with commercially available kits. Serum leptin was determined with a commercially available ELISA kit (DRG Diagnostics, Marburg/Lahn, Germany) with a sensitivity of 0.5 ng/ml. Plasma adiponectin was analyzed with a commercially available radioimmunoassay kit (Linco Research, St. Charles, MO), with a sensitivity of 0.5 ng/ml. Insulin was measured with an immunoassay (Access Immunoassay System; Beckman Coulter, Fullerton, CA), with a sensitivity of 0.5 μIU/ml. Cortisol was analyzed with a commercially available ELISA kit (DRG Diagnostics). Insulin resistance was calculated using the homeostasis assessment model (HOMA) method. Concentrations of variables were corrected for plasma volume shifts during exercise on the basis of hematocrit and hemoglobin values. Intra- and interassay variability ranged from 4.3 to 5.8% and from 3.5 to 7.9%, respectively, for all parameters measured.
Statistical analysis
Results are expressed as means ± SEM. Between-group resting differences were estimated by one-way ANOVA with Bonferroni correction for multiple comparisons. Time-effect and within-group differences were estimated by repeated-measures two-way ANOVA (group × time) for repeated measures on time with Bonferroni correction for multiple comparisons. All tests were two-tailed, and P < 0.05 was considered significant.
RESULTS
Mean intensity and repetition number performed differed (P < 0.001) among groups (Table 1). Age, BMI, waist-to-hip ratio, skinfold sum, activity level, trunk and lower limb strength, HOMA, REE, and Vo2peak (Table 1) were comparable among groups. Subjects in all groups could be classified as overweight or obese based on their BMI, skinfold sum, and waist-to-hip ratio. Mean exercise energy cost was lower in the high-intensity group than in the low- and moderate-intensity groups (221.6 ± 8.8 vs. 295.6 ± 10.7 and 281.6 ± 9.8 kcal, respectively, P < 0.001) (Table 1).
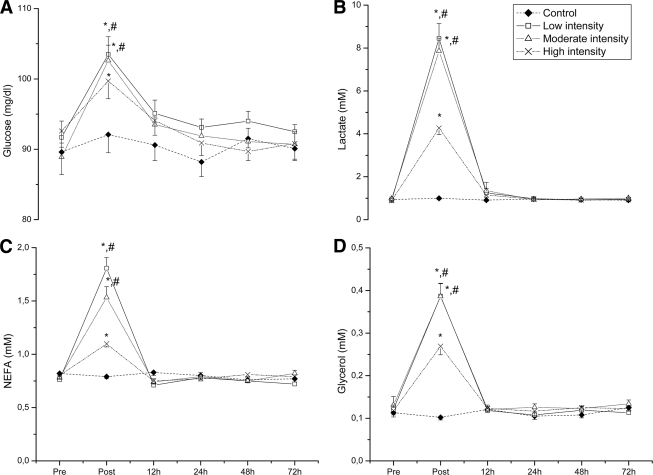
Glucose, lactate, NEFA, and glycerol concentrations (Fig. 1) increased (P < 0.001) immediately after exercise and returned to baseline thereafter in all groups. The low- and moderate-intensity groups induced a greater (P < 0.001) response than the high-intensity group.
Figure 1.
The effect of acute resistance exercise on concentrations of glucose (A), lactate (B), NEFA (C), and glycerol (D). Error bars represent SE. *Significantly different from the respective baseline (P < 0.05); #significant difference between the high-intensity group and the other groups (P < 0.05).
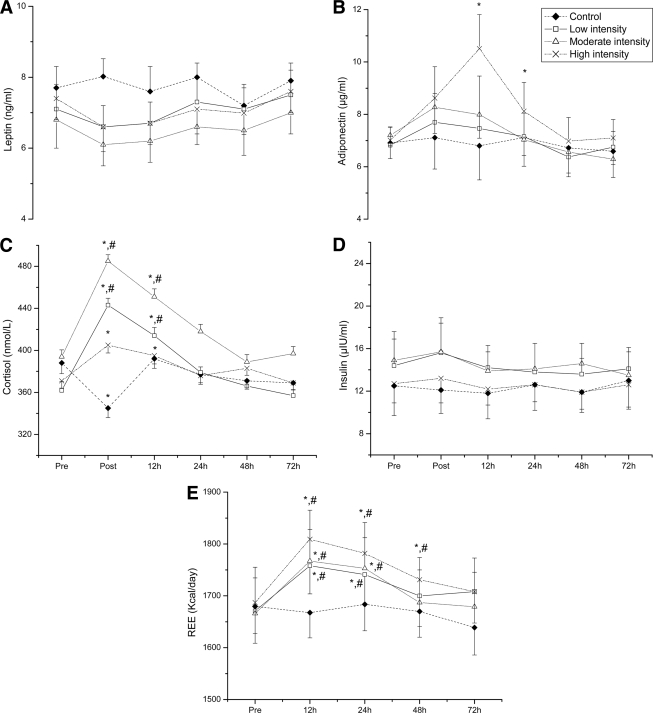
Insulin, cortisol, leptin, and adiponectin concentrations (Fig. 2) were comparable among groups at baseline. HOMA (not shown) and insulin remained unaffected by exercise during recovery. In the control group, cortisol levels exhibited the expected circadian rhythm of cortisol secretion (P < 0.001), thus being significantly lower at 12 h compared with baseline and 1 day after the control session (P < 0.001). In contrast, cortisol peaked in all exercise groups immediately after exercise (P < 0.001) and remained elevated (P < 0.001) for 12 h in the low- and moderate-intensity groups compared with baseline morning cortisol levels. In the high-intensity group, 12-h cortisol levels were marginally increased compared with baseline (P = 0.05). Cortisol concentration was normalized within 24 h of recovery.
Figure 2.
The effect of acute resistance exercise on concentrations of leptin (A), adiponectin (B), and cortisol (C), insulin (D), and REE (E). Error bars represent SE. *Significantly different from the respective baseline (P < 0.05); #significant difference between the high-intensity group and the other groups (P < 0.05).
REE (Fig. 2) was similar in all groups at baseline. REE increased (P < 0.001) in all exercise groups 12 h within recovery, with the high-intensity group eliciting a greater (P < 0.05) response than the other groups. REE returned to baseline at 48 h in the low- and moderate-intensity groups and at 72 h in the high-intensity group.
Although leptin declined after exercise (P = 0.011), adjustment for changes in plasma volume distribution eliminated leptin postexercise variation (P = 0.412). Adiponectin concentration increased immediately after exercise in the moderate- (P = 0.003) and high-intensity (P < 0.001) groups and remained elevated for 12 and 24 h after exercise in the moderate- (P = 0.08) and high-intensity (P < 0.05) groups, respectively. After adiponectin values were corrected for hemoconcentration, changes in the moderate-intensity group were eliminated and remained elevated only in the high-intensity group at 12 (P < 0.05) and 24 (marginally nonsignificant, P = 0.09) h of recovery.
CONCLUSIONS
Resistance exercise is an essential element of exercise prescription for older individuals on the basis of various position statements endorsed by the American Diabetes Association and other organizations. In the present study, we monitored circulating leptin, adiponectin, and REE for as long as 72 h after a resistance exercise bout of different intensity configurations in overweight elderly individuals. The novel findings of this study are that in older men acute resistance exercise 1) does not alter leptin responses, 2) augments circulating adiponectin levels only if intensity is of a sufficient magnitude, 3) increases REE for 24–48 h in an intensity-dependent manner, and 4) raises energy expenditure.
Leptin baseline values were higher than the corresponding values in lean young males but were lower than those observed in human obesity and insulin-resistant states (10,11) because our sample had lower BMI and insulin sensitivity within the normal range as estimated by HOMA, which was used only for descriptive reasons. Leptin absolute values declined after resistance exercise, but these changes were abolished after their correction for hemoconcentration. Human studies that used cardiovascular exercise reported an acute leptin decline after high-intensity protocols but not after mild exercise protocols (11), suggesting that the exercise program configuration (intensity, duration, and total volume) could explain discrepancies in previous findings. Our results in older men coincide with previous observations in young lean males, indicating that the circulating leptin concentration remains unaffected by variations in resistance exercise intensity (10).
Leptin seems to respond mainly to extreme exercise conditions, resulting in substantial hormonal perturbations and exercise energy cost (>800 kcal) (11). In this study, leptin remained unaltered despite pronounced metabolic and hormonal perturbations (NEFAs, glycerol, glucose, and cortisol increases) independent of intensity. Although increased glucose uptake by peripheral tissues in the presence of lactic acidosis may decrease blood leptin (12), the increase in blood lactate (four- to ninefold) in the present study (indicative of increased glucose uptake) was not accompanied by a leptin decline. It is possible that these protocols did not induce adequate glucose uptake and utilization capable of reducing serum leptin.
Another plausible explanation for the absence of leptin changes is previous observations of leptin mRNA upregulation by hexosamines and inhibition of leptin decline by glucose infusion (13). An 8–15% increase in glucose levels as observed in this study and a possible increase in hexosamine due to increased muscle glycolysis as reflected by the increased lactate concentrations may have inhibited a leptin decrease despite marked metabolic disturbances.
It is possible that the exercise energy cost magnitude is a more important factor than intensity for exercise-induced leptin responses. Leptin demonstrated a decline only when the exercise energy deficit was >800 kcal (11). Although there are no studies with older adults, leptin in young adults failed to change with resistance exercise or aerobic work that produced low to moderate energy deficits (150–500 kcal) (10). Resistance exercise in this study induced a 222- to 296-kcal deficit, which is well below the 800-kcal threshold. Others proposed a delayed (9–48 h) leptin decline after exercise (11). Although we monitored leptin responses for 72 h, no changes were recorded. Therefore, an exercise energy cost threshold may be a prerequisite for exercise-induced leptin changes. Although leptin has been implicated in sarcopenic states such as aging (14), an anabolic intervention such as RE does not seem to alter it.
Adiponectin resting levels were close to the lower limit of its normal range (5–20 μg/ml), which coincides with its corresponding values reported in human obesity (∼6 μg/ml), suggesting possible reduced protein secretion with increasing fatness (3). Adiponectin data after acute resistance exercise in aging are not available. Adiponectin appears to remain unaltered in young individuals after cardiovascular exercise after correction for hemoconcentration (15).
Acute plasma adiponectin perturbations are rarely noticed despite its substantial increase in subcutaneous tissue interstitial space during exercise because it requires a marked increase of adiponectin secretion rate due to its relatively large pool in plasma. Postexercise adiponectin changes have been measured only for short periods (<24 h). After exercise, adiponectin may need 24–72 h to increase because perturbations in substrate metabolism require 24–48 h to develop. In this study, adiponectin increased in the high-intensity group only after 12 h and remained marginally elevated for 24 h, confirming previous observations of a delayed postexercise increase (16). Cardiovascular exercise seems to upregulate adiponectin levels only after high-intensity protocols (15–17). The adiponectin elevation in this study cannot be attributed to a circadian rhythm because the control group did not demonstrate any changes and adiponectin may not exhibit diurnal variations (18).
Adiponectin is related to increased insulin sensitivity, which is mainly attributed to increased fat oxidation and inhibition of hepatic glucose production, whereas exercise enhances insulin sensitivity (3). However, acute exercise studies reported a dissociation between adiponectin concentration and insulin sensitivity changes (19), whereas moderate-intensity resistance exercise does not appear to alter insulin sensitivity acutely (9). Given that adiponectin in this study increased in the high-intensity group and insulin sensitivity remained unaffected, one would suggest that in overweight elderly individuals, adiponectin is not associated with insulin sensitivity after exercise and its rise is related to other factors.
Adiponectin is negatively associated with insulin resistance, whereas acute high-intensity resistance may induce hyperglycemia and transient hyperinsulinemia (9). Furthermore, insulin may inhibit adiponectin gene expression (20). However, adiponectin concentration increased in the absence of any discernible insulin changes, suggesting that its increase may not be mediated by insulin. Adiponectin concentration may be regulated acutely by NEFAs (19). However, adiponectin failed to change in the low- and moderate-intensity groups despite a marked NEFA elevation.
Low- and moderate-intensity resistance exercise resulted in a substantially greater metabolic stress than high-intensity resistance exercise based on greater lactate and cortisol responses, coinciding with earlier findings (21). Protocols eliciting the greatest cortisol response also elicit the greatest acute growth hormone and lactate response (22). The lactate increase also coincides with a cortisol increase (22), suggesting that lactate may be a factor contributing to the cortisol increase seen with resistance exercise. It appears that cortisol responses to resistance exercise depend on metabolic requirements, which appear to be higher in the low- to moderate-intensity protocols, based on the lactate response and energy cost of these protocols. The greater lactate response in the low- and moderate-intensity groups is mainly attributed to the shorter resting intervals used by these groups, thereby allowing a lower lactate clearance (23). Therefore, adiponectin responses may be related to factors other than substrate metabolism during exercise because it remained unaltered in low- and moderate-intensity groups and increased only in the high-intensity group.
Based on previous observations of different adiponectin responses between trained and untrained individuals (16) and adiponectin elevation only after high-intensity resistance exercise in this study, we hypothesize that the amount of muscle tissue recruited may affect adiponectin responses. The reduced hepatic gluconeogenesis and muscle triglyceride levels after adiponectin therapy suggest that adiponectin may be implicated in the communication between adipose and muscle tissue (5). Moreover, adiponectin upregulates fat oxidation in skeletal muscle by activating AMP-activated protein kinase, which is related to muscle mass (6). These findings suggest that recruitment of greater muscle mass by high-intensity protocols may lead to a more pronounced adiponectin response in an attempt to regulate substrate utilization.
Aging decreases REE owing to age-associated sarcopenia, physical inactivity, and reduced energy intake (1). Disproportionate REE reductions relative to daily energy intake may predispose to age-related increases in body fatness. Chronic resistance exercise may help to increase or maintain REE and add muscle mass during weight loss programs in older individuals (7). Although a prolonged REE elevation (24–72 h) after resistance exercise has been documented in young adults (17), there is limited information for overweight elderly individuals. Our results indicate that low- and moderate-intensity resistance exercise elevates REE for 24 h after exercise, whereas high-intensity resistance exercise induces a more prolonged (48-h) response. REE elevation has been attributed mainly to homeostatic perturbations that require energy to repair exercise-induced muscle trauma and support tissue hypertrophy. Maximal rates of muscle protein turnover may last 48 h after heavy resistance exercise (8). Because the energy cost of protein turnover may account for as much as 20% of resting metabolism, the energy utilization during the 48-h recovery period in this study may have been reflected in REE elevations. It is characteristic that the high-intensity protocol induced the greatest REE response and the lowest lipolytic response as reflected by glycerol and free fatty acid levels. The higher REE in the high-intensity scheme may be explained by the higher degree of muscle damage induced by the high-intensity resistance exercise protocols compared with protocols of lower intensity (24). Furthermore, muscle damage after exercise is accompanied by temporary decreased lipid oxidation (25).
Resistance exercise prescription for the older, overweight population, is based on acute program variables, i.e., intensity, frequency (session/week), resting intervals, and type and number of exercises. To our knowledge, this is the first study to address the effect of resistance exercise intensity and frequency on various metabolic responses, suggesting that the residual effect of a single resistance exercise session is largely dependent on the intensity level of the protocol. The high-intensity protocol has a residual effect on REE and adipokines of 2 days and 1 day, respectively. In contrast, low- and moderate-intensity protocols demonstrate a shorter residual effect (12–24 h). Therefore, for frail elderly individuals, we may prescribe moderate- to low-intensity schemes of higher frequency (daily or every other day), whereas for more advanced individuals, we can prescribe higher intensities with lower frequency (i.e., twice/week). Furthermore, it appears that even the lowest resistance exercise intensity may alter REE for several hours after exercise, an important finding for those who cannot tolerate intense loads.
It seems that high-intensity resistance exercise elicits a lower metabolic stress compared with lower-intensity schemes, suggesting that it may be suited for older populations. Because of a lower response of cortisol, a known catabolic hormone, high-intensity resistance exercise may provide a greater hypertrophic stimulus, which is crucial in the effort to offset aging-associated sarcopenia. The longer resting intervals adopted during high-intensity resistance exercise may better accommodate the older population with respect to lactate clearance in between work periods because less lactate accumulation has been associated with fatigue delay.
Our data show that acute resistance exercise induces an energy expenditure of ∼220–300 kcal/session for overweight elderly men, suggesting that resistance exercise may be an important factor in overall weight management programs in elderly individuals. These numbers may aid personal trainers, physicians, and dietitians in estimating the day-to-day energy expenditure when resistance exercise is included in a weight loss program.
Chronic resistance exercise studies using different intensity levels are scarce. The most relevant study (7) showed a leptin decline and an adiponectin and REE increase by moderate- to high-intensity resistance training. In the present study, we found an acute decrease in leptin levels only in the noncorrected values, although a trend for lower leptin values in the corrected leptin levels also remained during the first 12 h of postexercise recovery. Our data also show an acute increase only after acute high-intensity resistance exercise, even though higher, but nonsignificant, levels of adiponectin were detected after low- and moderate-intensity exercise within 12 h of recovery. Similarly, a REE rise was also observed in this acute study. It appears that acute resistance exercise elicits similar responses of these variables compared with chronic training in this population group.
In summary, resistance exercise does not alter circulating leptin concentration but increases REE and adiponectin in an intensity-dependent manner for as long as 48 and 24 h, respectively, in overweight elderly individuals. These findings indicate that overweight older men may benefit from training with a frequency of two to three resistance exercise sessions per week. In addition, it appears that all resistance exercise schemes induce marked energy expenditure.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
We thank all the subjects for their participation and commitment to the study and Ioannis Galanis for his technical assistance with diet analysis. We acknowledge the assistance of Dr. M. Manousaki in the hormone measurements.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. Philadelphia, Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 2. Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD: Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006; 29: 1433– 1438 [DOI] [PubMed] [Google Scholar]
- 3. Simpson KA, Fiatarone-Singh MA: Effects of exercise on adiponectin: a systematic review. Obesity 2008; 16: 241– 256 [DOI] [PubMed] [Google Scholar]
- 4. Tsuchida A, Yamauchi T, Ito Y: Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 2004; 279: 30817– 30822 [DOI] [PubMed] [Google Scholar]
- 5. Berg AH, Combs TP, Du X, Brownlee M, Scherer PE: The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001; 7: 947– 953 [DOI] [PubMed] [Google Scholar]
- 6. Tomas E, Kelly M, Xiang X, Tsao TS, Keller C, Keller P, Luo Z, Lod H, Saha AK, Unger R, Ruderman NB: Metabolic and hormonal interactions between muscle and adipose tissue. Proc Nutr Soc 2004; 63: 381– 385 [DOI] [PubMed] [Google Scholar]
- 7. Fatouros IG, Tournis S, Leontsini D, Jamurtas AZ, Sxina M, Thomakos P, Manousaki M, Douroudos I, Taxildaris K, Mitrakou M: Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity-related. J Clin Endocrinol Metab 2005; 90: 5970– 5977 [DOI] [PubMed] [Google Scholar]
- 8. Chesley A, MacDougall JO, Tarnopolsky MA, Atkinse SA, Smith K: Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 1992; 73: 1383– 1388 [DOI] [PubMed] [Google Scholar]
- 9. Chatzinikolaou A, Fatouros I, Petridou A, Jamourtas A, Avloniti A, Douroudos I, Mastorakos G, Lazaropoulou C, Papassotiriou I, Tournis S, Mitrakou A, Mougios V: Adipose tissue lipolysis is upregulated in lean and obese men during acute resistance exercise. Diabetes Care 2008; 31: 1397– 1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zafeiridis A, Smilios I, Considine RV, Tokmakidis SP: Serum leptin responses after acute resistance exercise protocols. J Appl Physiol 2003; 94: 591– 597 [DOI] [PubMed] [Google Scholar]
- 11. Nindl BC, Kraemer WJ, Arciero PJ, Samatallee N, Leone CD, Mayo MF, Hafeman DL: Leptin concentrations experience a delayed reduction after resistance exercise in men. Med Sci Sports Exerc 2002; 34: 608– 613 [DOI] [PubMed] [Google Scholar]
- 12. Teta D, Bevington A, Brown J, Throssell D, Harris KPG, Walls J: Effects of acidosis on leptin secretion from 3T3–L1 adipocytes and on serum leptin in the uraemic rat. Clin Sci (Lond) 1999; 97: 363– 368 [PubMed] [Google Scholar]
- 13. Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L: A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998; 393: 684– 688 [DOI] [PubMed] [Google Scholar]
- 14. Waters DL, Qualls CR, Dorin RI, Veldhuis JD, Baumgartner RN: Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J Gerontol Med Sci, 2008; 63: 536– 541 [DOI] [PubMed] [Google Scholar]
- 15. Kraemer RR, Aboudehen KS, Carruth AK, Durand RJ, Acevedo EO, Hebert EP, Johnson LG, Castracane VD: Adiponectin responses to continuous and progressively intense intermittent exercise. Med Sci Sports Exerc 2003; 35: 1320– 1325 [DOI] [PubMed] [Google Scholar]
- 16. Jurimae J, Purge P, Jurimae T: Adiponectin is altered after maximal exercise in highly trained male rowers. Eur J Appl Physiol 2005; 93: 502– 505 [DOI] [PubMed] [Google Scholar]
- 17. Jamurtas AZ, Theocharis V, Koukoulis G, Stakias N, Fatouros IG, Kouretas D, Koutedakis Y: The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur J Appl Physiol 2006; 97: 122– 126 [DOI] [PubMed] [Google Scholar]
- 18. Hotta K, Funahashi T, Arita T, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y: Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000; 20: 1595– 1599 [DOI] [PubMed] [Google Scholar]
- 19. Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM: The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 2002; 51: 2968– 2974 [DOI] [PubMed] [Google Scholar]
- 20. Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R: Hormonal regulation of adiponectin gene expression in 3T3–L1 adipocytes. Biochem Biophys Res Commun 2002; 290: 1084– 1089 [DOI] [PubMed] [Google Scholar]
- 21. Hoffman JR, Kang JJ, Maresh CM, Kraemer WJ, French D, Nioka S, Kime R, Rundell KW, Ratamess NA, Faigenbaum AD, Chance B: Comparison of low and high-intensity resistance exercise on lipid peroxidation: role of muscle oxygenation. J Strength Cond Res 2007; 21: 118– 122 [DOI] [PubMed] [Google Scholar]
- 22. Kraemer WJ, Ratamess NA: Hormonal responses and adaptations to resistance exercise and training. Sports Med 2005; 35: 339– 361 [DOI] [PubMed] [Google Scholar]
- 23. Ratamess NA, Falvo MJ, Mangine GT, Hoffman JR, Faigenbaum AD, Kang J: The effect of rest interval length on metabolic responses to the bench press exercise. Eur J Appl Physiol 2007; 100: 1– 17 [DOI] [PubMed] [Google Scholar]
- 24. Nosaka K, Newton M: Difference in the magnitude of muscle damage between maximal and submaximal eccentric loading. J Strength Cond Res 2002; 16: 202– 208 [PubMed] [Google Scholar]
- 25. Krishnan RK, Evans WJ, Kirwan JP: Impaired substrate oxidation in healthy elderly men after eccentric exercise. J Appl Physiol 2003; 94: 716– 723 [DOI] [PubMed] [Google Scholar]