Abstract
OBJECTIVE
Gestational diabetes is a risk factor for large-for-gestational-age (LGA) newborns, but many LGA babies are born to mothers with normal glucose tolerance. We aimed to clarify the association of maternal glycemia across the whole distribution with birth weight and risk of LGA births in mothers with normal glucose tolerance.
RESEARCH DESIGN AND METHODS
We undertook a population-based gestational diabetes screening in an urban area of Hungary in 2002–2005. All singleton pregnancies of mothers ≥18 years of age, without known diabetes or gestational diabetes (World Health Organization criteria) and data on a 75-g oral glucose tolerance test at 22–30 weeks of gestation, were included (n = 3,787, 78.9% of the target population). LGA was determined as birth weight greater than the 90th percentile using national sex- and gestational age–specific charts.
RESULTS
Mean ± SD maternal age was 30 ± 4 years, BMI was 22.6 ± 4.0 kg/m2, fasting blood glucose was 4.5 ± 0.5 mmol/l, and postload glucose was 5.5 ± 1.0 mmol/l. The mean birth weight was 3,450 ± 476 g at 39.2 ± 1.2 weeks of gestation. There was a U-shaped association of maternal fasting glucose with birth weight (Pcurve = 0.004) and risk of having an LGA baby (lowest values between 4 and 4.5 mmol/l, Pcurve = 0.0004) with little change after adjustments for clinical characteristics. The association of postload glucose with birth weight (P = 0.03) and the risk of an LGA baby (P = 0.09) was weaker and linear.
CONCLUSIONS
Both low and high fasting glucose values at 22–30 weeks of gestation are associated with increased risk of an LGA newborn. We suggest that the excess risk related to low glucose reflects the increased use of nutrients by LGA fetuses that also affects the mothers' fasting glucose.
The presence of a large fetus (either defined by a weight cutoff or as large for gestational age [LGA]) is associated with multiple risks for both the mother and the newborn. Short-term risks include a two- to threefold increase in intrauterine death rate, a higher probability of operative delivery and several neonatal morbidities (e.g., shoulder dystocia and brachial plexus injuries), and increased risk of maternal injuries (i.e., perineal laceration). There are also long-term risks for the newborn, such as diabetes later in life, obesity, and the development of metabolic syndrome (1). Maternal uncontrolled pregestational diabetes and gestational diabetes mellitus are important risk factors for macrosomia and LGA newborns (1,2), and evidence from randomized controlled trials on the treatment of gestational diabetes mellitus supports a causal link between maternal hyperglycemia and risk of macrosomia (3,4).
Recently, some reports have raised the possibility that the association of fasting glucose and 2-h postload glucose with birth weight and risk of macrosomia may actually be U-shaped such that both high and low levels of maternal glucose are related to elevated risk of having large babies (5–7). To date, however, evidence to support this curvilinear association is weak. The largest observational study addressing this question, the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, was unable to prove any significant deviation from linearity in the association between fasting and postload glucose and the risk of LGA babies. However, in this multicenter study the level of significance was corrected for multiple testing potentially, increasing risk of false null findings (8). Most other population-based studies did not formally test nonlinearity in the blood glucose-macrosomia association (5–7,9–15). Moreover, many studies were based on nonfasting maternal glucose measurements, which allow analysis of the high end of the glucose distribution but cannot detect any true effect of low glucose levels on the risk of macrosomia. To clarify the association between maternal glucose and newborn size, we set out to investigate in detail the association of fasting and 2-h postload glucose with birth weight and the risk of LGA newborns in women with normal glucose tolerance drawn from a general population.
RESEARCH DESIGN AND METHODS
In 1999 a universal, population-based screening program for gestational diabetes mellitus was launched at the Szent Imre Teaching Hospital in Budapest, Hungary. Screening was performed using a 75-g oral glucose tolerance test (OGTT) according to World Health Organization criteria (16). The program was approved by the Szent Imre Hospital Ethics Committee. The institute is located in Budapest and serves an urbanized population of ∼200,000. After the first 2 years of the screening program, the ascertainment rate was >99% of all pregnant women delivered in the hospital (17,18).
The screening database captured 5,335 pregnancies between 1 January 2002 and 30 April 2005. Our target population did not include women aged <18 years at the time of delivery (n = 8), women with twin pregnancies (n = 115), or women with known pregestational diabetes or gestational diabetes mellitus (n = 411), leaving a total of 4,801 pregnancies for further analysis. We excluded 641 women with an early or late OGTT (<22 or >30 weeks of gestation at the time of analysis), 45 women because of missing birth weight data or delivery before 24 weeks of gestation, 266 because of missing or incomplete blood glucose data, and 62 because of missing data on covariates. Thus, the final analytic sample consisted of 3,787 women (78.9% of the women in the target population).
Measurements
All 75-g OGTTs were performed in the morning hours (before 10 a.m.) between 22 and 30 weeks of gestation. Women were instructed to follow an unrestricted diet for at least 3 days before the test and to come for the investigation after at least 8 h of fasting. After the collection of fasting blood samples, women were asked to drink 75 g of anhydrous glucose in 250–300 ml of water. Blood samples, also taken 2 h after the glucose load, were collected into fluoride-containing tubes. Venous plasma glucose was measured in the central laboratory of the hospital using a glucose-oxidase–based method. Gestational diabetes mellitus was diagnosed if either the fasting venous plasma glucose was ≥7.0 mmol/l or the 2-h postload venous plasma glucose was ≥7.8 mmol/l.
Data on birth weight and sex of the newborn were extracted from the discharge documentation. LGA was determined as a birth weight greater than the 90th percentile on sex- and gestational age–specific national charts (19).
Gestational age was primarily based on the date of the last menstrual period and was substituted by the result of a first-trimester ultrasound examination (∼8–12 weeks of gestation) if information on the last menstrual period was missing or if there was a >2-week discrepancy between the two estimates.
At the time of the OGTT, anthropometric measurements (height and weight) were performed and BMI was calculated. Systolic and diastolic blood pressures were determined by a trained nurse using a standard mercury sphygmomanometer. The mean arterial pressure was calculated as (2 × diastolic blood pressure + systolic blood pressure)/3. After delivery a detailed questionnaire was completed during a face-to-face interview by a trained researcher (A.P.). Family history of diabetes among first-degree relatives, age of the mother, smoking status during pregnancy (yes or no), ethnicity (Caucasian or other), and parity (0, 1, or ≥2) were recorded.
Statistical analysis
Descriptive statistics are given as means ± SD for continuous variables and percentages for categorical variables. After investigation of crude frequencies of LGA babies according to fasting and 2-h postload glucose categories, we computed binary logistic regression models with LGA as the outcome and glucose categories as dummy variables (fasting glucose ≤3.5, 3.51–4.0, 4.01–4.5, 4.51–5.0, 5.01–5.5, and ≥5.51 mmol/l and 2-h postload glucose ≤4.0, 4.01–4.8, 4.81–5.6, 5.61–6.4, 6.41–7.2, and ≥7.21 mmol/l), the third category being the reference category in both analyses. Odds ratios (ORs) and 95% CIs were adjusted for ethnicity, parity of the mother, and sex of the newborn (model 1) and then were further adjusted for maternal age, BMI, smoking, family history of diabetes, gestational age at the OGTT, mean arterial blood pressure, and height (model 2). Similar generalized linear models using logit link were computed with continuous fasting and postload blood glucose and their squared terms (to test for nonlinearity) as independent variables instead of glucose categories and to estimate probabilities and their CIs on continuous glucose scales.
Further analyses applying general linear modeling examined associations of maternal glucose with birth weight describing crude and adjusted birth weights (and SEM) in the above glucose categories. Then we applied similar models to test for linear and curvilinear trends in these associations with linear and quadratic glucose terms (as required). We estimated birth weights from these models after adjusting for covariates (model 1 and model 2) as described above. All statistical analyses were done using SPSS for Windows 16.0. Two-tailed P < 0.05 was considered statistically significant.
RESULTS
Characteristics of the mothers and newborns and pregnancy outcomes are summarized in Table 1. The mean age of the subjects was 29.7 years, and the mean fasting and 2-h postload glucose levels were 4.5 and 5.5 mmol/l, respectively. There were 12 stillbirths and no maternal deaths in the target population.
Table 1.
Characteristics of study subjects and their newborns
| % | Value | |
|---|---|---|
| Maternal | ||
| Age (years) | 29.7 ± 4.2 (27.1–32.3) | |
| BMI (kg/m2)* | 25.2 ± 4.0 (22.4–27.0) | |
| Caucasian (%) | 98.4 | |
| Mean arterial pressure (mmHg)* | 81.4 ± 9.1 (73.3–86.7) | |
| Blood glucose (mmol/l)* | ||
| Fasting | 4.5 ± 0.5 (4.2–4.8) | |
| 2-h | 5.5 ± 1.0 (4.8–6.2) | |
| Length of gestation at the time of OGTT (weeks) | 25.5 ± 1.8 (24–27) | |
| Any smoking during pregnancy (%) | 4.4 | |
| Diabetes among first-degree relatives (%) | 12.8 | |
| Nulliparous (%) | 56.9 | |
| Newborn | ||
| Gestational age at delivery (weeks) | 39.2 ± 1.2 (39–40) | |
| Birth weight (g) | 3,450 ± 476 (3,150–3,750) | |
| Male sex (%) | 51.6 | |
| LGA (%)† | 16.5 |
Data are % or means ± SD (interquartile range). n = 3,787.
*Age, BMI, and mean arterial pressure were obtained at the OGTT (22–30 weeks of gestation).
†LGA was determined as a birth weight greater than the 90th percentile on sex- and gestational age–specific national charts.
Risk of LGA newborns according to the fasting and 2-h postload glucose levels
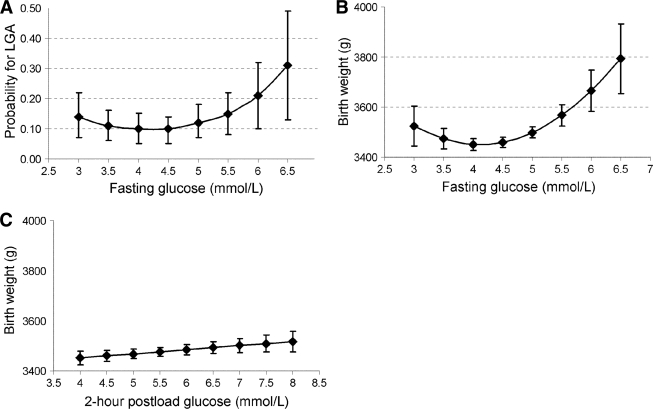
The frequency of LGA newborns across the predefined fasting and postload glucose categories is shown in Table 2. Fasting blood glucose showed a nonlinear relationship with the risk of LGA: compared with the reference group (4.01–4.5 mmol/l) both lower and higher maternal fasting glucose levels were associated with increased risk (Table 2). The curvilinear association was statistically significant in a model simultaneously including continuous fasting glucose and its square irrespective of adjustments (Pcurve = 0.004 for model 1 and Pcurve = 0.04 for model 2) (Fig. 1A).
Table 2.
Risk of LGA newborns by fasting and 2-h postload glucose categories in 3,787 singleton pregnancies
| LGA/total number | % LGA* | Model 1† | Model 2‡ | |
|---|---|---|---|---|
| Fasting glucose categories | ||||
| ≤3.5 mmol/l | 23/137 | 16.8 | 1.28 (0.79–2.06) | 1.33 (0.81–2.19) |
| 3.51–4.0 mmol/l | 84/489 | 17.2 | 1.33 (1.003–1.77) | 1.38 (1.03–1.86) |
| 4.01–4.5 mmol/l | 178/1,300 | 13.7 | 1 (referent) | 1 (referent) |
| 4.51–5.0 mmol/l | 230/1,357 | 16.9 | 1.27 (1.03–1.57) | 1.25 (1.00–1.56) |
| 5.01–5.5 mmol/l | 84/417 | 20.1 | 1.53 (1.15–2.05) | 1.36 (1.00–1.83) |
| ≥5.51 mmol/l | 25/87 | 28.7 | 2.45 (1.50–4.03) | 1.84 (1.08–3.15) |
| 2-h postload glucose categories | ||||
| ≤4.0 mmol/l | 42/275 | 15.3 | 1.00 (0.69–1.45) | 1.02 (0.69–1.50) |
| 4.01–4.8 mmol/l | 120/710 | 16.9 | 1.15 (0.89–1.49) | 1.16 (0.88–1.51) |
| 4.81–5.6 mmol/l | 164/1,106 | 14.8 | 1 (referent) | 1 (referent) |
| 5.61–6.4 mmol/l | 165/957 | 17.2 | 1.19 (0.94–1.51) | 1.21 (0.95–1.55) |
| 6.41–7.2 mmol/l | 94/550 | 17.1 | 1.18 (0.90–1.56) | 1.19 (0.89–1.59) |
| ≥7.21 mmol/l | 39/189 | 20.6 | 1.50 (1.02–2.23) | 1.43 (0.95–2.17) |
Data are ORs (95% CI).
*LGA was determined as a birth weight greater than the 90th percentile on sex- and gestational age–specific national charts.
†Model 1 is adjusted for ethnicity, parity of the mother, and sex of the newborn.
‡Model 2 is adjusted for ethnicity, parity of the mother, sex of the newborn, maternal age, BMI, smoking, family history of diabetes, gestational age at the OGTT, mean arterial blood pressure, and height.
Figure 1.
Estimated probability of an LGA newborn by continuous fasting blood glucose (A) and estimated birth weight according to continuous fasting (B) and 2-h postload (C) glucose levels adjusted for ethnicity, parity of the mother, and sex of the newborn. Error bars show 95% CI around the estimates.
Postload glucose showed a weaker association with the risk of LGA and only the highest maternal glucose category was related to elevated risk in a model adjusted for sex, ethnicity, and parity. However, this association was attenuated and became nonsignificant after further adjustments (Table 2). In the logistic regression analysis using continuous glucose values as predictors, the squared term was nonsignificant (Pcurve = 0.46) and the linear association between postload glucose and the risk of LGA was weak and only borderline significant (OR 1.08 [95% CI 0.99–1.17], per 1 mmol/l increase in postload glucose, P = 0.08 for model 1; 1.07 [0.98–1.17], per 1 mmol/l increase in postload glucose, P = 0.13 for model 2).
Birth weight according to the fasting and 2-h postload glucose levels
The crude birth weight showed a U-shaped association with the predefined fasting glucose categories with the lowest values in the third group (4.01–5.0 mmol/l) and higher values at both ends of the glucose distribution (Table 3). The association between continuous maternal fasting glucose levels and birth weight followed a quadratic function; the lowest birth weight values were between 4 and 4.5 mmol/l (Pcurve < 0.0001 for model 1) (Fig. 1B). This association was robust to adjustment for covariates (Pcurve = 0.01 for model 2; detailed data not shown).
Table 3.
Birth weight by fasting and 2-h postload glucose categories in 3,787 singleton pregnancies
| Crude birth weight (g) | Model 1* | Model 2† | |
|---|---|---|---|
| Fasting glucose categories | |||
| ≤3.5 mmol/l | 3,496 (3,417–3,576) | 3,525 (3,447–3,604 | 3,529 (3,451–3,606) |
| 3.51–4.0 mmol/l | 3,447 (3,405–3,489) | 3,477 (3,435–3,520) | 3,491 (3,449–3,532) |
| 4.01–4.5 mmol/l | 3,397 (3,371–3,423) | 3,423 (3,396–3,450) | 3,437 (3,410–3,464) |
| 4.51–5.0 mmol/l | 3,466 (3,440–3,491) | 3,492 (3,465–3,518) | 3,496 (3,470–3,522) |
| 5.01–5.5 mmol/l | 3,535 (3,490–3,581) | 3,553 (3,508–3,599) | 3,528 (3,483–3,572 |
| ≥5.51 mmol/l | 3,537 (3,437–3,637) | 3,559 (3,461–3,657) | 3,549 (3,452–3,646) |
| 2-h postload glucose categories | |||
| ≤4.0 mmol/l | 3,440 (3,383,3,496) | 3,459 (3,403–3,515) | 3,453 (3,398–3,508) |
| 4.01–4.8 mmol/l | 3,427 (3,392–3,462) | 3,451 (3,416–3,487) | 3,443 (3,409–3,478) |
| 4.81–5.6 mmol/l | 3,450 (3,422–3,478) | 3,479 (3,449–3,508) | 3,486 (3,458–3,515) |
| 5.61–6.4 mmol/l | 3,454 (3,424–3,485) | 3,479 (3,448–3,511) | 3,488 (3,458–3,519) |
| 6.41–7.2 mmol/l | 3,466 (3,426–3,506) | 3,498 (3,457–3,538) | 3,511 (3,471–3,550) |
| ≥7.21 mmol/l | 3,484 (3,416–3,552) | 3,521 (3,453–3,588) | 3,530 (3,465–3,595) |
Data are means (95% CI).
*Model 1 is adjusted for ethnicity, parity of the mother, and sex of the newborn.
†Model 2 is adjusted for ethnicity, parity of the mother, sex of the newborn, maternal age, BMI, smoking, family history of diabetes, gestational age at the OGTT, mean arterial blood pressure, and height.
There was a linear association between 2-h postload glucose and birth weight (P = 0.03 for model 1) with no evidence of curvilinearity (Table 3, Fig. 1C). Again, further adjustment had little effect on the models (P = 0.002 for model 2, detailed data not shown).
CONCLUSIONS
Short summary
In this population-based screening program of gestational diabetes mellitus using a 75-g OGTT, the risk of LGA newborns was nonlinearly related to fasting glucose levels among predominantly Caucasian women residing in an urban area of Hungary. Increased risk was found at both ends of the maternal fasting glucose distribution. The association between fasting glucose and birth weight of the newborns was similarly U-shaped with the lowest values found between 4 and 4.5 mmol/l. In contrast, 2-h postload glucose showed only a borderline relationship with the risk of LGA and the association with birth weight was linear and weaker than that for fasting glucose.
Comparison with other studies
Although there are several population-based screening studies for gestational diabetes mellitus, only a few reported fasting glucose values at the time of the screening (5,7–11,13,14,20). This is not surprising because the widely accepted American Diabetes Association Position Statement generally recommends prescreening with a glucose challenge test that is performed irrespective of fasting status. An OGTT is recommended only in the case of an abnormal result (21).
However, at least six previous studies had data on maternal fasting glucose. Two of the medium-sized studies did not report tests for nonlinearity or risk for categorical glucose groups and thus were not informative regarding the U-shaped association between fasting glucose and the risk of LGA (9,20). Two other studies reported formal tests of nonlinearity for the fasting glucose–LGA relationship: One showed no clear inflection point for these associations but observed an elevated risk in the lowest glucose category (7). The HAPO study found no significant deviation from linearity (8). Despite its very large sample size, some circumstances should be mentioned that might have reduced sensitivity to observe elevated LGA at the low end of maternal fasting glucose. First, the HAPO study involved a multinational, multiethnic population, leading to several tests for interactions in the analysis and thus limiting the power for showing a relationship that might only hold for certain ethnicities. Second, because of the excessive number of outcomes investigated, the HAPO investigators had to correct their P values for multiple testing, which may have increased the risk of a type I error (rejecting a null hypothesis when it is true). Third, in the categorical analysis of fasting glucose, their lowest group was defined as glucose levels <4.2 mmol/l, which does not differentiate the three lowest categories used in the present analysis (8). Furthermore, when we combined the first three groups into one category in our data, the relationship between fasting glucose categories and LGA seemed to be linear (data available on request). There is a further study that reports on macrosomia (birth weight over a certain cutoff value) as a function of fasting glucose levels and suggests no deviation from linearity (5).
Literature data are not much more detailed regarding the relationship between fasting glucose and birth weight. At least four studies reported positive correlations with various strengths between birth weight and fasting glucose without testing for nonlinearity (11,13,14,20). One of these investigated the shape of the fasting glucose–birth weight relationship and found that the linear association between fasting glucose and birth weight holds only for the middle ranges of the glucose distribution (7).
We did not observe a U-shaped association between postload glucose and size of newborn, and the linear trend found was only suggestive. In previous studies, a linear relationship between 2-h postload glucose and the risk of LGA or macrosomia was a frequent observation before adjustment for covariates (5,7–10,12,15). Whereas some studies reported a strong linear relationship after several adjustments (8,9), others, similarly to us, found weak or nonsignificant relationships between postload glucose and the risk of LGA or macrosomia (5,10). In our study, this observation was related to mothers with normal glucose tolerance; that is, those at the low and moderate but not at the high end of the 2-h postload glucose distribution (glucose levels >7.8 mmol/l). In general, 2-h postload glucose seems to be a weaker predictor of birth weight than fasting glucose, similarly to our findings with or without further adjustments (7,14,15).
Possible mechanisms
Elevated risk of an LGA newborn and an increased birth weight at the high end of the maternal glucose distribution supports the Pedersen (22) hypothesis, which suggesting that maternal hyperglycemia (even within the normal range) increases the levels of fetal insulin, leading to accelerated growth and macrosomia in the fetus. We propose fetal hyperinsulinemia as a plausible mechanism underlying the association between low fasting glucose and risk of LGA newborns. Fetal insulin is probably the most important growth hormone during intrauterine development, and increases in fetal insulin levels are related to accelerated growth (1,2). Fetal hyperinsulinemia may lead to lower maternal glucose levels by increasing fetoplacental siphoning of maternal glucose in normoglycemic mothers (2,23). In line with this reasoning, there is some evidence to show a decrease in glucose levels as gestation progresses in women with hyperinsulinemic newborns, whereas glucose levels are increasing in mothers with normoinsulinemic newborns (24). All the above together suggest a common mechanism (fetal hyperinsulinemia) linking lower fasting glucose levels and the risk of LGA or increased birth weight. Further studies are warranted to test this hypothesis.
Study strengths and limitations
Our study benefits from the universal use of the 75-g OGTT in an ethnically homogeneous population as this allowed us to reliably explore the low end of the glucose distribution. A similar shape of the association was confirmed for both categorical and continuous glucose levels as predictors, and we were able to adjust for a wide range of known confounders of macrosomia in our analysis. The women who were excluded based on missing data were more likely to be from ethnic minorities and to be smokers, but their general characteristics were very similar to those of the population investigated; thus, it is unlikely that this exclusion had a large effect on our findings (supplemental data, available at http://care.diabetesjournals.org/cgi/content/full/dc09-1088/DC1). Although the exclusion of women meeting the World Health Organization criteria for gestational diabetes mellitus reduced the high end of the glucose distribution, this is unlikely to have selectively removed the smaller babies from the lowest categories or the larger babies from the middle ranges, so we think that our findings of a nonlinear association of fasting glucose with birth weight and macrosomia are valid. We used the latest published percentile charts for the definition of LGA newborns; however, it obviously does not correspond to our target population, given the 16.5% rate of LGA in this study (19). This result is in agreement with an overall increase in the prevalence of large babies worldwide (1). We believe that a general increase in birth weight would not invalidate our findings on the risk of LGA and definitely would not affect the associations found for birth weight. However, we investigated a surrogate pregnancy outcome, and thus it remains unclear whether the LGA babies of mothers with decreased fasting glucose have an otherwise poor obstetric outcome compared with normal-weight babies.
Implications
In a large-scale study we found that the prediction of a LGA newborn and birth weight may be improved by adding the square of fasting glucose value to the known predictors. Further research is needed to determine whether our findings are generalizable to other populations and to examine fetal hyperinsulinemia and other potential underlying mechanisms for the excess risk of LGA babies among mothers with low fasting glucose.
Supplementary Material
Acknowledgments
The research was supported by the Hungarian Scientific Medical Council (ETT 254/2000).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gynecol Scand 2008; 87: 134– 145 [DOI] [PubMed] [Google Scholar]
- 2. Langer O. Fetal macrosomia: etiologic factors. Clin Obstet Gynecol 2000; 43: 283– 297 [DOI] [PubMed] [Google Scholar]
- 3. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005; 352: 2477– 2486 [DOI] [PubMed] [Google Scholar]
- 4. Langer O, Rodriguez DA, Xenakis EM, McFarland MB, Berkus MD, Arrendondo F. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol 1994; 170: 1036– 1046 [DOI] [PubMed] [Google Scholar]
- 5. Sermer M, Naylor CD, Gare DJ, Kenshole AB, Ritchie JW, Farine D, Cohen HR, McArthur K, Holzapfel S, Biringer A. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. The Toronto Tri-Hospital Gestational Diabetes Project. Am J Obstet Gynecol 1995; 173: 146– 156 [DOI] [PubMed] [Google Scholar]
- 6. Clausen T, Burski TK, Oyen N, Godang K, Bollerslev J, Henriksen T. Maternal anthropometric and metabolic factors in the first half of pregnancy and risk of neonatal macrosomia in term pregnancies. A prospective study. Eur J Endocrinol 2005; 153: 887– 894 [DOI] [PubMed] [Google Scholar]
- 7. Sacks DA, Greenspoon JS, Abu-Fadil S, Henry HM, Wolde-Tsadik G, Yao JF. Toward universal criteria for gestational diabetes: the 75-gram glucose tolerance test in pregnancy. Am J Obstet Gynecol 1995; 172: 607– 614 [DOI] [PubMed] [Google Scholar]
- 8. HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991– 2002 [DOI] [PubMed] [Google Scholar]
- 9. Mello G, Parretti E, Cioni R, Lucchetti R, Carignani L, Martini E, Mecacci F, Lagazio C, Pratesi M. The 75-gram glucose load in pregnancy: relation between glucose levels and anthropometric characteristics of infants born to women with normal glucose metabolism. Diabetes Care 2003; 26: 1206– 1210 [DOI] [PubMed] [Google Scholar]
- 10. Moses RG, Calvert D. Pregnancy outcomes in women without gestational diabetes mellitus related to the maternal glucose level. Is there a continuum of risk? Diabetes Care 1995; 18: 1527– 1533 [DOI] [PubMed] [Google Scholar]
- 11. Langhoff-Roos J, Wibell L, Gebre-Medhin M, Lindmark G. Placental hormones and maternal glucose metabolism: a study of fetal growth in normal pregnancy. Br J Obstet Gynaecol 1989; 96: 320– 326 [DOI] [PubMed] [Google Scholar]
- 12. Tallarigo L, Giampietro O, Penno G, Miccoli R, Gregori G, Navalesi R. Relation of glucose tolerance to complications of pregnancy in nondiabetic women. N Engl J Med 1986; 315: 989– 992 [DOI] [PubMed] [Google Scholar]
- 13. Farmer G, Russell G, Hamilton-Nicol DR, Ogenbede HO, Ross IS, Pearson DW, Thom H, Kerridge DF, Sutherland HW. The influence of maternal glucose metabolism on fetal growth, development and morbidity in 917 singleton pregnancies in nondiabetic women. Diabetologia 1988; 31: 134– 141 [DOI] [PubMed] [Google Scholar]
- 14. Breschi MC, Seghieri G, Bartolomei G, Gironi A, Baldi S, Ferrannini E. Relation of birthweight to maternal plasma glucose and insulin concentrations during normal pregnancy. Diabetologia 1993; 36: 1315– 1321 [DOI] [PubMed] [Google Scholar]
- 15. Little RR, McKenzie EM, Shyken JM, Winkelmann SE, Ramsey LM, Madsen RW, Goldstein DE. Lack of relationship between glucose tolerance and complications of pregnancy in nondiabetic women. Diabetes Care 1990; 13: 483– 487 [DOI] [PubMed] [Google Scholar]
- 16. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539– 553 [DOI] [PubMed] [Google Scholar]
- 17. Kerényi Z, Péterfalvi A, Bosnyák Z, Madarász E, Tabák ÁG, Szánthó J, Rákóczi I, Tamás G. Incidence of gestational diabetes mellitus: results of a validated universal screening (Abstract). Diabetologia 2004; 47: A104 [Google Scholar]
- 18. Tabák Á, Tamás G, Péterfalvi A, Bosnyák Z, Madarász E, Rákóczi I, Kerényi Z. The effect of paternal and maternal history of diabetes mellitus on the development of gestational diabetes mellitus. J Endocrinol Invest. 15 May 2009. [ Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19. Joubert K. Magyar születéskori testtö- meg—és testhossz-standardok az 1990–96: évi országos élveszületési adatok alapján. Magy Noorv Lapja 2000; 63: 155– 163 [Google Scholar]
- 20. Ong KK, Diderholm B, Salzano G, Wingate D, Hughes IA, MacDougall J, Acerini CL, Dunger DB. Pregnancy insulin, glucose, and BMI contribute to birth outcomes in nondiabetic mothers. Diabetes Care 2008; 31: 2193– 2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008; 31: S12– S54 [DOI] [PubMed] [Google Scholar]
- 22. Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954; 16: 330– 342 [DOI] [PubMed] [Google Scholar]
- 23. Nolan CJ, Proietto J. The feto-placental glucose steal phenomenon is a major cause of maternal metabolic adaptation during late pregnancy in the rat. Diabetologia 1994; 37: 976– 984 [DOI] [PubMed] [Google Scholar]
- 24. Weiss PA, Scholz HS, Haas J, Tamussino KF. Effect of fetal hyperinsulinism on oral glucose tolerance test results in patients with gestational diabetes mellitus. Am J Obstet Gynecol 2001; 184: 470– 475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.