Abstract
OBJECTIVE
Exposure to acrylamide in foodstuffs and smoking has become a worldwide concern. The effect of acrylamide on glucose homeostasis is not known. The goal of the present study was to test the hypothesis that trace acrylamide exposure might be independently associated with both reduced blood insulin and reduced insulin resistance.
RESEARCH DESIGN AND METHODS
We examined 1,356 participants with reliable measures of glucose homeostasis and Hb adducts of acrylamide (HbAA) and glycidamide from the National Health and Nutrition Examination Survey, 2003–2004. Glucose homeostasis was assessed by the measurement of plasma glucose, serum insulin, and the homeostasis model assessment of insulin resistance (HOMA-IR).
RESULTS
In a linear regression model, a 1-unit increase in log HbAA was associated with a decrease in serum insulin (β coefficient = −0.20 ± 0.05, P = 0.001) and HOMA-IR (β coefficient = −0.23 ± 0.05, P < 0.001). After HbAA concentrations were divided into quartiles in the fully adjusted models, the adjusted serum insulin level and HOMA-IR significantly decreased across quartiles of HbAA (Ptrend < 0.001 for both). In subgroup analysis, the association of HbAA levels with HOMA-IR and insulin levels was stronger in subjects who were white or had ever smoked or in subjects with a lower education level or a BMI <25 or >30 kg/m2.
CONCLUSIONS
Acrylamide is associated with reduced serum insulin levels in adults. Further clinical and animal studies are warranted to clarify the putative causal relationship.
Acrylamide, a highly reactive α,β-unsaturated carbonyl compound, is widely used to synthesize polymers for industrial applications such as soil conditioning, wastewater treatment, research applications, cosmetics, and the paper and textile industries (1). Besides industrial and laboratory uses, acrylamide in foodstuffs has become a worldwide concern because of its generation in a variety of fried and oven-baked foods during cooking. Attention was drawn to this fact initially by Tareke et al. (2) in 2002, and subsequent biochemical analysis showed that heating of free amino acids (asparagines) and sugars during food processing at 248°F (120°C) resulted in the formation of acrylamide concentrations of up to 1 mg/kg food (3). According to recent data, the mean daily workplace inhalation exposure is 1.4–18.6 μg/kg body wt (4), whereas human consumption via food is about 1 μg · kg body wt−1 · day−1 in the general population and 4 μg · kg body wt−1 · day−1 in high consumers (5). Moreover, acrylamide is a component of cigarette smoke. Its content has been estimated at 1.1–2.34 μg/cigarette, which is clearly an important source of acrylamide exposure (6).
The majority of acrylamide is conjugated with glutathione in the human body, and some (about 15%) is activated by cytochrome P450 CYP2E1 to a reactive epoxy compound, glycidamide. Glycidamide is subsequently metabolized by hydrolysis and conjugated with glutathione. Acrylamide and glycidamide form adducts with the NH2-terminal NH2 group of the valine residue of Hb (7). Hb adducts reflect an integrated concentration over 4 months (8); therefore, these adducts are useful as predictors of toxicity. Hb adducts of acrylamide (HbAA) and glycidamide (HbGA) have been used as biomarkers of exposure and to estimate the internal dose from workplace exposure as well as in the general population (9).
The biological consequences of acrylamide exposure have chiefly centered on neurotoxicity ever since this effect was observed in humans exposed to this compound in the workplace (9). Subsequently, experimental exposure of rodents to acrylamide has shown impaired development, reproductive toxicity, genotoxicity, and carcinogenicity. However, epidemiological studies in the workplace and general population have not established an unambiguous link between acrylamide exposure and human cancer (9–11). Acrylamide was classified by the International Agency for Research on Cancer as a probable human carcinogen (12). Given the lack of dose-response data for human neurotoxicity, the risk assessment was based on rodent studies and supported by primate studies of acrylamide neuropathy. Based on these data, the World Health Organization in 2005 concluded that the “no observed effect level” for acrylamide neuropathy is 0.2 mg · kg body wt−1 · day−1 (5). This provides a margin between exposure and the no observed effect level of 200. Therefore, it is not likely that acrylamide-induced neurotoxicity will be detected in the general population.
A recent study, using an animal model, showed both lower insulin and triglyceride concentrations after continuous intake of trace acrylamide in a dose of 0.28 ppm similar to that for the general population (13). In another study, rats treated with acrylamide at relatively low doses demonstrated diminished appetitive motivation (14). In a recent cross-sectional epidemiology study in Europe, HbAA was inversely associated with BMI in smokers (15). Based on these studies, we hypothesized that trace acrylamide exposure might be independently associated with reduced insulin resistance and improved serum lipid profiles. Because the homeostasis model assessment for insulin resistance (HOMA-IR) is a reliable surrogate measure of insulin resistance (16), we can use fasting glucose and insulin concentrations to accurately estimate insulin resistance status. The goal of the present study was to test this hypothesis by examining data from the National Health and Nutrition Examination Survey (NHANES) collected from 2003 to 2004.
RESEARCH DESIGN AND METHODS
Data were from NHANES 2003–2004. The NHANES is a population-based survey designed to collect information on the health and nutrition of the U.S. household population and to obtain a representative sample of the noninstitutionalized civilian U.S. population. The survey data are released every 2 years. Detailed survey operations manuals, consent documents, and brochures of the NHANES 2003–2004 are available on the NHANES Web site (17). We limited our analyses to the 6,990 participants aged at least 20 years who had a test for HbAA and HbGA. Among these subjects, only 3,169 had a morning examination and measurements of fasting plasma glucose and serum insulin. Of these participants, 1,356 subjects without missing data were included in the final analyses.
Anthropometric and biochemical data
According to the statements on the NHANES Web site, data were collected at all study sites by trained personnel using standardized procedures. Sociodemographic and medical information such as age, sex, race/ethnicity, education level, household income, and medication usage were collected during the household interview. Alcohol intake was recorded and determined by the questionnaire and categorized into three levels (<12 drinks, <144 drinks, and ≥144 drinks per year). Smoking status was categorized as active smoker, former smoker, and nonsmoker by a questionnaire on smoking (18). We also quantified smoking status by serum cotinine levels (<0.1, 0.1–10, or ≥10 ng/ml) in the same manner as in a previous study (19). BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured at the iliac crest to the nearest 0.1 cm. Laboratory measurements were performed in a mobile examination center. For serum markers, blood specimens were collected by glass tubes (red-top Vacutainer), whereas for plasma glucose, blood specimens were collected in a vacuum tube containing sodium fluoride (gray-top Vacutainer). Blood specimens were processed locally and then were stored and shipped to central laboratories for analysis. Levels of serum total cholesterol and triglyceride were measured enzymatically. Levels of HDL cholesterol were measured after precipitation of other lipoproteins on a Hitachi model 704 analyzer (Roche Diagnostics, Indianapolis, IN). Plasma glucose was measured by the hexokinase enzymatic method (Cobas Mira Chemistry System; Roche Diagnostic Systems, Montclair, NJ). Serum C- reactive protein levels were measured by latex-enhanced nephelometry (Dade Behring Nephelometer II Analyzer System; Dade Behring Diagnostic, Somerville, NJ). Cotinine was measured in serum samples using isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (Hewlett-Packard model 1090L high-performance liquid chromatograph and PE-Sciex API III triple quadrupole mass spectrometer) (20). GHb was measured by a boronate affinity high-performance liquid chromatography system and converted to A1C levels (automated high-performance liquid chromatography system, model CLC330; Primus, Kansas City, MO.). Serum insulin was determined by immunoenzymatic assay with Tosoh AIA-PACK immunoreactive insulin. The HOMA-IR, the product of basal glucose and insulin levels divided by 22.5, was used as a reliable surrogate measure of insulin resistance (16).
Assessment of HbAA and HbGA
Human whole blood or erythrocytes were used to measure HbAA and HbGA. Specifically, the reaction products with the NH2-terminal valine of the Hb protein chains (N-[2-carbamoylethyl] valine and N-[2-hydroxycarbamoyl-ethyl] valine for acrylamide and glycidamide adducts, respectively) were measured. This method is based on a modified Edman reaction, which uses the formation of Edman products from N-alkylated amino acids in neutral or alkaline conditions rather than the acidic conditions required in the conventional Edman reaction (21). This optimized method was further refined and modified to increase the sensitivity and enable automation. HbAA and HbGA were quantified using octapeptides with the same amino acid sequence as the NH2 terminus of the β-chain of Hb and with acrylamide and glycidamide attached at the valine residue (AA-VHLTPEEK, GA-VHLTPEEK); the corresponding stable isotope-labeled AA-Val(13C5 15N)-HLTPEEK was used as an internal standard. Total Hb was measured using calibrators provided with the manufacturer's assay kit (22). The limit of detection was 2 pmol/g Hb for HbAA and 3 pmol/g Hb for HbGA. For concentrations below the detection limits (only 0.07 and 2.2% of blood samples, respectively), a value was assigned by NHANES that we used in our analyses.
Statistics
Data are expressed as means ± SE. Continuous variables were compared between groups by an unpaired Student t test, whereas categorical variables were compared by a χ2 test. The strength of the associations between the concentrations of HbAA, HbGA, and plasma glucose homeostatic markers was tested using linear regression models.
For linear regression models, we used an extended model approach for covariates to adjust for potential confounders. Model 1 was adjusted for age, sex, and race; model 2 was adjusted for age, sex, race, smoking status, alcohol intake, education levels, household income, BMI, A1C, and insulin/glucose/HOMA-IR. When the plasma glucose was treated as an independent variable, serum insulin was entered as a dependent variable and vice versa. When the independent variables were the derivatives of the plasma glucose and the serum insulin, both the plasma glucose and the serum insulin were not adjusted in the model. The HOMA-IR was adjusted in the final model if the independent variable was not an arithmetic combination of the plasma glucose and the serum insulin. The relative contribution of each dependent variable to predict the variation of dependent variable was determined by partial R2. Log transformation was performed for variables with significant deviation from a normal distribution before further analyses.
We performed three sets of sensitivity analyses to test the robustness of our findings. First, to explore whether the associations between certain serum markers and HbAA and HbGA might be mediated by different foods (e.g., greater consumption of high-fat, high-calorie foods might result in high HbAA and HbGA and insulin resistance), we examined the associations after including daily nutrient intake (daily calories, carbohydrate, total fat, cholesterol, and fiber) from NHANES dietary interview data (23). Second, because medications such as antihyperglycemic, antihypertensive, and antihyperlipidemic agents might confound associations between HbAA concentrations and other glucose homeostatic markers, we performed our analyses again, including data on self-reported current medication usage. Third, because smoking is a major source of acrylamide exposure, we adjusted the smoking status both by questionnaire data and serum cotinine levels to avoid possible recall bias.
Sampling weights that account for unequal probabilities of selection, oversampling, and nonresponse were applied for all analyses using the Complex Sample Survey module of SPSS (version 13.0 for Windows XP; SPSS, Chicago, IL).
RESULTS
The study sample included 1,356 participants (Table 1). The weighted geometric mean HbAA concentrations were adjusted for age, sex, and race/ethnicity. As shown in Table 1, the mean HbAA concentrations were higher in young adults, men, active smokers, and subjects with a lower education level, lower annual household income, and lower BMI. Weighted mean HbGA concentrations were higher in young adults, active smokers, and subjects with a lower education level. The mean ± SE values for plasma glucose, serum insulin, and HOMA-IR were 5.61 ± 0.05 mmol/l, 64.7 ± 2.9 pmol/l, and 2.89 ± 0.13, respectively. The linear associations between the HbAA levels and markers of glucose homeostasis in sample subjects are shown in Table 2. A 1-unit increase in log HbAA was associated with a decrease in the serum insulin (β coefficient = −0.20 ± 0.05, P = 0.001) and HOMA-IR (β coefficient = −0.23 ± 0.05, P < 0.001). R2 values in the regression models were 0.363 and 0.418, respectively. The five most important determinants of serum insulin (partial R2) were BMI (0.231), acrylamide (0.013), smoking status (0.010), age (0.007), and alcohol intake (0.006). For HOMA-IR, the five most important determinants were BMI (0.198), A1C (0.074), acrylamide (0.013), smoking status (0.009), and age (0.008). HbAA levels were not associated with plasma glucose concentrations, A1C levels, lipid metabolism, C-reactive protein levels, and waist circumference. The results were similar after subjects with diabetes were excluded (data not shown). There were no associations between HbGA levels and any of the measured parameters (data not shown).
Table 1.
Acrylamide adducts and glycidamide adducts concentrations by participant characteristics
| No. (weighted %) | HbAA (pmol/g Hb) | P * | HbGA (pmol/g Hb) | P * | |
|---|---|---|---|---|---|
| Overall | 1,356 (100) | 61.6 (57.4–66.0) | 57.4 (54.0–61.6) | ||
| Age | 0.002 | <0.001 | |||
| 20–39 years | 494 (39.3) | 66.7 (61.0–73.0) | 63.4 (59.2–68.7) | ||
| 40–59 years | 401 (40.2) | 62.8 (56.3–70.1) | 59.2 (55.7–62.8) | ||
| ≥60 years | 461 (20.5) | 51.4 (47.5–55.2) | 45.2 (39.7–51.9) | ||
| Sex | 0.003 | 0.551 | |||
| Men | 660 (48.7) | 65.4 (59.7–71.5) | 57.4 (54.1–61.0) | ||
| Women | 696 (51.3) | 58.0 (54.6–62.2) | 58.0 (53.0–62.8) | ||
| Race | 0.065 | 0.712 | |||
| Mexican American | 279 (8.1) | 63.4 (59.7–75.2) | 61.6 (55.2–68.7) | ||
| Non-Hispanic white | 732 (73.2) | 62.2 (57.4–67.4) | 58.0 (53.0–62.8) | ||
| Non-Hispanic black | 245 (9.9) | 66.7 (58.6–75.2) | 56.3 (51.4–61.6) | ||
| Others | 100 (8.8) | 50.9 (42.1–61.6) | 54.1 (48.4–60.3) | ||
| Smoking | <0.001 | <0.001 | |||
| Nonsmoker | 888 (63.0) | 49.4 (46.5–52.0) | 48.4 (45.2–51.9) | ||
| Former smoker | 189 (13.3) | 52.5 (45.6–59.7) | 48.9 (41.7–56.8) | ||
| Active smoker | 279 (23.7) | 124.0 (108.9–141.2) | 98.5 (89.1–110.0) | ||
| Cotinine | <0.001 | <0.001 | |||
| <0.1 ng/ml | 736 (50.1) | 47.5 (45.2–49.9) | 47.0 (43.0–50.9) | ||
| 0.1–10 ng/ml | 264 (20.2) | 50.4 (44.3–56.8) | 49.9 (43.4–56.8) | ||
| ≥10 ng/ml | 356 (29.7) | 110.0 (99.5–121.5) | 90.0 (83.1–97.5) | ||
| Alcohol drinking status | 0.053 | 0.601 | |||
| <12 drinks/year | 681 (44.9) | 56.8 (51.9–62.2) | 57.4 (52.5–62.8) | ||
| <144 drinks/year | 325 (26.8) | 61.0 (54.6–67.4) | 58.0 (51.4–64.7) | ||
| ≥144 drinks/year | 350 (28.3) | 70.8 (64.1–78.3) | 58.0 (53.5–62.2) | ||
| Education levels | 0.001 | 0.005 | |||
| <High school | 366 (17.1) | 65.4 (58.6–73.0) | 62.2 (57.4–67.4) | ||
| High school | 345 (27.2) | 73.0 (62.8–83.9) | 65.4 (56.8–75.9) | ||
| >High school | 645 (55.6) | 55.7 (52.5–59.7) | 53.0 (48.9–57.4) | ||
| Annual household income (USD) | 0.001 | 0.244 | |||
| <25,000 | 420 (22.2) | 65.4 (59.7–71.5) | 58.0 (51.9–64.1) | ||
| 25,000–55,000 | 460 (32.3) | 64.1 (59.7–68.7) | 59.7 (53.5–66.7) | ||
| >55,000 | 476 (45.5) | 58.6 (53.0–64.1) | 56.3 (53.0–60.3) | ||
| BMI | 0.040 | 0.888 | |||
| <25 kg/m2 | 418 (33.4) | 68.7 (62.2–75.9) | 58.0 (53.0–63.4) | ||
| 25–30 kg/m2 | 490 (35.1) | 59.2 (53.5–65.4) | 56.3 (49.4–64.7) | ||
| ≥30 kg/m2 | 446 (31.4) | 57.4 (53.0–62.8) | 58.6 (55.2–62.2) |
Data are weighted geometric means (range) unless indicated otherwise. *Model adjusted for age, sex, and race.
Table 2.
Linear regression coefficients with 1-unit increase in log acrylamide adducts in sample subjects
| Model 1 | P | Model 2 | P | |
|---|---|---|---|---|
| Glucose homeostasis* | ||||
| Glucose | −0.13 (0.08) | 0.144 | −0.09 (0.08) | 0.262 |
| Log A1C | 0.00 (0.01) | 0.626 | 0.01 (0.01) | 0.253 |
| Log insulin | −0.19 (0.06) | 0.004 | −0.20 (0.05) | 0.001 |
| Log HOMA-IR | −0.21 (0.06) | 0.005 | −0.23 (0.05) | <0.001 |
| Lipids* | ||||
| Cholesterol | 0.04 (0.07) | 0.620 | 0.02 (0.08) | 0.839 |
| HDL cholesterol | −0.02 (0.03) | 0.391 | −0.05 (0.03) | 0.072 |
| Log triglycerides | −0.02 (0.04) | 0.517 | −0.01 (0.03) | 0.742 |
| Log C-reactive protein* | −0.10 (0.08) | 0.228 | −0.12 (0.12) | 0.350 |
| Waist circumference† | −2.44 (1.13) | 0.047 | 0.44 (1.11) | 0.697 |
Data are β coefficients (SEM). *Model 1 was adjusted for age, sex, and race/ethnicity; model 2 was adjusted for age, sex, race/ethnicity, smoking status, alcohol intake, education level, and household income, BMI, A1C, and insulin/glucose/HOMA.
†BMI was not adjusted for in model 2.
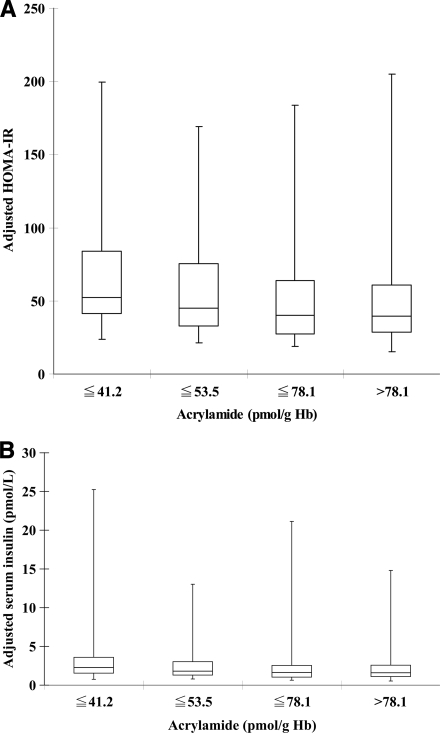
Linear regression coefficients (SEM) between HbAA, serum insulin, and HOMA-IR levels in different subgroups of subjects are shown in Table 3. The associations between HbAA and HOMA-IR were all significant in each age-group, whereas the insulin levels were not significant in older age (≥60 years). Moreover, the HbAA levels were associated with HOMA-IR and insulin levels in each sex but were more significant in subjects who were white, ever smokers, or had a lower education level or a BMI <25 or ≥30 kg/m2. We divided the HbAA levels into quartiles in the fully adjusted models, and the adjusted serum insulin levels and HOMA-IR are shown in Fig. 1. The trends of the serum insulin levels and HOMA-IR across quartiles of the HbAA levels were significant (P < 0.001 for both).
Table 3.
Linear regression coefficients between acrylamide adducts, plasma insulin, and HOMA-IR in different subgroups
| n | Log HOMA-IR* | P | Log insulin* | P | |
|---|---|---|---|---|---|
| Age | |||||
| 20–39 years | 494 | −0.23 (0.10) | 0.038 | −0.20 (0.09) | 0.049 |
| 40–59 years | 401 | −0.22 (0.05) | <0.001 | −0.22 (0.03) | <0.001 |
| ≥60 years | 461 | −0.25 (0.08) | 0.006 | −0.17 (0.10) | 0.100 |
| Sex | |||||
| Men | 660 | −0.20 (0.07) | 0.010 | −0.19 (0.06) | 0.008 |
| Women | 696 | −0.29 (0.07) | 0.001 | −0.24 (0.06) | 0.002 |
| Race | |||||
| White | 732 | −0.28 (0.05) | <0.001 | −0.24 (0.04) | <0.001 |
| Others | 624 | −0.12 (0.11) | 0.273 | −0.11 (0.11) | 0.334 |
| Smoking | |||||
| Nonsmoker | 888 | −0.19 (0.09) | 0.053 | −0.14 (0.09) | 0.143 |
| Ever smoker | 468 | −0.16 (0.04) | 0.002 | −0.15 (0.04) | 0.002 |
| Cotinine | |||||
| <0.1 ng/ml | 736 | −0.15 (0.11) | 0.183 | −0.08 (0.11) | 0.469 |
| ≥0.1 ng/ml | 620 | −0.16 (0.04) | 0.002 | −0.14 (0.04) | 0.003 |
| Alcohol drinking status | |||||
| <12 drinks/year | 681 | −0.26 (0.07) | 0.001 | −0.23 (0.07) | 0.004 |
| ≥12 drinks/year | 675 | −0.22 (0.07) | 0.004 | −0.20 (0.06) | 0.006 |
| Education levels | |||||
| ≤ High school | 711 | −0.31 (0.07) | 0.001 | −0.29 (0.07) | 0.001 |
| High school | 645 | −0.14 (0.06) | 0.025 | −0.11 (0.05) | 0.058 |
| BMI | |||||
| <25 kg/m2 | 418 | −0.29 (0.09) | 0.004 | −0.21 (0.09) | 0.025 |
| 25–30 kg/m2 | 490 | −0.12 (0.09) | 0.165 | −0.09 (0.08) | 0.281 |
| ≥30 kg/m2 | 446 | −0.33 (0.09) | 0.003 | −0.34 (0.09) | 0.001 |
Data are β coefficients (SEM). *Adjusted for the full model.
Figure 1.
Box and whisker plots of HOMA-IR (A) and adjusted serum insulin levels (B) across quartiles of the HbAA adducts in the fully adjusted model (age, sex, race, smoking status, alcohol intake, education level, household income, BMI, and plasma glucose or serum insulin). The trends of plasma insulin levels and HOMA-IR across quartiles of HbAA adducts were significant (P < 0.001 for both).
For sensitivity analyses, we first included daily nutrient intake (log-transformed daily calories, carbohydrate, total fat, cholesterol, and fiber) in our models. The inverse associations between HbAA concentrations and serum insulin (β coefficient = −0.25 ± 0.05, P < 0.001) and HOMA-IR (β coefficient = −0.27 ± 0.06, P = 0.001) remained significant in ever smokers. Second, after adjustment for medication usage (antihyperlipidemic, antihyperglycemic, and antihypertensive agents), the HbAA concentrations remained significantly associated with serum insulin levels (β coefficient = −0.24 ± 0.05, P < 0.001) and HOMA-IR (β coefficient = −0.26 ± 0.05, P < 0.001). Finally, the results were similar whether smoking status was obtained by questionnaire or by serum cotinine levels (data not shown).
CONCLUSIONS
Although there are several studies on the relationship between acrylamide exposure and health outcome, the relationship of acrylamide levels to diseases and laboratory abnormalities in a representative sample from a national population has never been explored. In the present study, we first showed that increased HbAA was associated with a decrease in blood insulin and insulin resistance status in adults.
There has been a great deal of progress in the last few years in understanding the toxicology and distribution of acrylamide in animals and in humans. However, the effects of acrylamide on glucose homeostasis and lipid metabolism were addressed in only one animal study (13). In that study, male Wistar rats were fed with acrylamide, formed from frying oil heated for 20 h at 180°C, for 12 weeks. The amount of acrylamide ingested was estimated to be similar to daily human intake (0.28 ppm). All of the rats grew well, and no gross abnormalities attributable to the experimental oil were observed, except that the acrylamide-fed rats had significantly lower insulin and triglyceride levels. These findings are in accord with ours, but the causal biochemical mechanisms are not clear. Because chronic smoking is associated with insulin resistance and high insulin levels (24), it is interesting that acrylamide in cigarette smoke might counterbalance the effect of smoking on glucose homeostasis. From subgroup analyses in the present study, increased acrylamide concentrations were generally associated with decreased serum insulin levels, particularly in subjects who were active smokers and had a low education level and high BMI, all of which are associated with high insulin resistance. Perhaps the association between acrylamide and insulin is more obvious in subjects with high acrylamide or high insulin resistance.
In a recent animal study, rats treated with acrylamide at doses of 5.0 mg · kg−1 · day−1) showed measurable decrements of food-motivated behavior when tested at 6–12 weeks of age. The lack of a body weight difference between treatment groups in these animals during the course of testing suggests that acrylamide may be capable of producing a direct effect on appetitive motivation in rats (14). However, the dosage used was far higher than exposure of the general human population. According to a recent cross-sectional epidemiology study in Europe, HbAA is inversely associated with BMI in smokers without adjustment for potential confounders (15). In our study, we also showed that HbAA is inversely associated with BMI and waist circumference with simple adjustment. However, after adjustment for smoking status, the association was not significant. Because smoking is an important source of acrylamide exposure and smoking could lead to weight loss, the association is probably confounded by smoking status.
Nutritional status, particularly amino acid balance, has an important effect on glutathione homeostasis (25), which may be relevant in detoxification. The adequate provision of sulfur-containing amino acids as well as glutamate and glycine is critical for the maximization of glutathione synthesis. Although there was no information about amino acid intake, the daily caloric and protein intakes were estimated to be 2,307.0 ± 44.1 kcal and 1.09 ± 0.03 g/kg, respectively, which are unlikely to lead to glutathione deficiency and therefore have little or no effect on acrylamide conjugation.
Measurements of HbAA and HbGA are expensive and difficult but well established. The modified Edman reaction method used in NHANES was successfully applied to adducts produced by acrylamide and glycidamide. This optimized method has been refined and modified in-house to increase sensitivity and enable automation. It has been widely used in several studies (8,21) and makes measurement of acrylamide exposure possible in population studies.
Our study has several limitations. First, the cross-sectional design does not permit any causal inferences. Second, exposure to acrylamide also might be an indicator of exposure to multiple chemicals, including other endocrine disruptors in smokers. If the associations reported here are confirmed in independent studies, more work will be needed to identify the mechanisms of action linking long-term, low-dose acrylamide exposure to health outcomes in humans.
In summary, we present the first report that acrylamide exposure is associated with both reduced blood insulin and insulin resistance. Because exposure to acrylamide in foodstuffs and smoking has become a worldwide concern, further longitudinal clinical and in vitro studies are urgently needed to elucidate the putative causal relationship.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
We thank the many individuals who contributed to the NHANES data we examined, including all of the anonymous participants in the study. We are particularly grateful to those who performed the laboratory assays of HbAA at the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int J Toxicol 2005; 24( Suppl. 2): 21– 50 [DOI] [PubMed] [Google Scholar]
- 2. Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 2002; 50: 4998– 5006 [DOI] [PubMed] [Google Scholar]
- 3. Sharp D. Acrylamide in food. Lancet 2003; 361: 361– 362 [DOI] [PubMed] [Google Scholar]
- 4. Center for Evaluation of Risks to Human Reproduction (CERHR). NTP-CERHR monograph on the potential human reproductive and developmental effects of acrylamide [article online], 2005. National Toxicology Program, 2004 (DHHS NIH publ. no. 05-4472). Available from http://cerhr.niehs.nih.gov/chemicals/acrylamide/Acrylamide_Monograph.pdf. Accessed 20 January 2009
- 5. World Health Organization/International Programme on Chemical Safety. Summary and conclusions of the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Rome, 8–17 February 2005 [article online], 2006. Available from http://www.who.int/entity/ipcs/food/jecfa/summaries/summary_report_64_final.pdf. Accessed 20 January 2009 [Google Scholar]
- 6. Boettcher MI, Schettgen T, Kutting B, Pischetsrieder M, Angerer J. Mercapturic acids of acrylamide and glycidamide as biomarkers of the internal exposure to acrylamide in the general population. Mutat Res 2005; 580: 167– 176 [DOI] [PubMed] [Google Scholar]
- 7. Fennell TR, Sumner SC, Snyder RW, Burgess J, Spicer R, Bridson WE, Friedman MA. Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol Sci 2005; 85: 447– 459 [DOI] [PubMed] [Google Scholar]
- 8. Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B Anal Technol Biomed Life Sci 2002; 778: 279– 308 [DOI] [PubMed] [Google Scholar]
- 9. Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C. Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol 2006; 36: 481– 608 [DOI] [PubMed] [Google Scholar]
- 10. Larsson SC, Akesson A, Wolk A. Long-term dietary acrylamide intake and breast cancer risk in a prospective cohort of Swedish women. Am J Epidemiol 2009; 169: 376– 381 [DOI] [PubMed] [Google Scholar]
- 11. Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol Biomarkers Prev 2007; 16: 2304– 2313 [DOI] [PubMed] [Google Scholar]
- 12. International Agency for Research on Cancer (IARC). Some Industrial Chemicals. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 60. Lyon, France, International Agency for Research on Cancer, 1994 [Google Scholar]
- 13. Totani N, Yawata M, Ojiri Y, Fujioka Y. Effects of trace acrylamide intake in Wistar rats. J Oleo Sci 2007; 56: 501– 506 [DOI] [PubMed] [Google Scholar]
- 14. Garey J, Paule MG. Effects of chronic low-dose acrylamide exposure on progressive ratio performance in adolescent rats. Neurotoxicology 2007; 28: 998– 1002 [DOI] [PubMed] [Google Scholar]
- 15. Vesper HW, Slimani N, Hallmans G, Tjønneland A, Agudo A, Benetou V, Bingham S, Boeing H, Boutron-Ruault MC, Bueno-de-Mesquita HB, Chirlaque D, Clavel-Chapelon F, Crowe F, Drogan D, Ferrari P, Johansson I, Kaaks R, Linseisen J, Lund E, Manjer J, Mattiello A, Palli D, Peeters PH, Rinaldi S, Skeie G, Trichopoulou A, Vineis P, Wirfält E, Overvad K, Strömberg U. Cross-sectional study on acrylamide hemoglobin adducts in subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. J Agric Food Chem 2008; 56: 6046– 6053 [DOI] [PubMed] [Google Scholar]
- 16. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487– 1495 [DOI] [PubMed] [Google Scholar]
- 17. The National Health and Nutrition Examination Surveys (NAHNES) [online article]. Available from http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/current_nhanes_03_04.htm. Accessed 20 January 2009
- 18. Weitzman M, Cook S, Auinger P, Florin TA, Daniels S, Nguyen M, Winickoff JP. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation 2005; 112: 862– 869 [DOI] [PubMed] [Google Scholar]
- 19. Navas-Acien A, Silbergeld E, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 2008; 300: 814– 822 [DOI] [PubMed] [Google Scholar]
- 20. Jacob P, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: Absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Specrom 1991; 20: 247– 252 [DOI] [PubMed] [Google Scholar]
- 21. Vesper HW, Ospina M, Meyers T, Ingham L, Smith A, Gray JG, Myers GL. Automated method for measuring globin adducts of acrylamide and glycidamide at optimized Edman reaction conditions. Rapid Commun Mass Spectrom 2006; 20: 959– 964 [DOI] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention, National Center for Health Statistics. Documentation, codebook, and frequencies: acrylamide and glycidamide laboratory data [online article], 2008. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06age_c.pdf. Accessed 20 January 2009
- 23. Centers for Disease Control and Prevention, National Center for Health Statistics. Documentation, codebook, and frequencies exam component: dietary interview—total nutrient intakes data [online article], 2006. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/dr1tot_c.pdf and http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/dr2tot_c.pdf. Accessed 20 January 2009
- 24. Ronnemaa T, Ronnemaa EM, Puukka P, Pyorala K, Laakso M. Smoking is independently associated with high plasma insulin levels in nondiabetic men. Diabetes Care 1996; 19: 1229– 1232 [DOI] [PubMed] [Google Scholar]
- 25. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 2004; 134: 489– 492 [DOI] [PubMed] [Google Scholar]